Potential of Mesenchymal Stromal Cell-Derived Extracellular Vesicles as Natural Nanocarriers: Concise Review
Abstract
:1. Introduction
2. Mesenchymal Stromal Cells (MSCs)
3. Mesenchymal Stromal Cell-Derived Extracellular Vesicles (MSC-Derived EVs)
4. Mesenchymal Stromal Cell-Derived Extracellular Vesicles (MSC-Derived EVs) for Drug Delivery Systems (DDS)
5. The Isolation of Extracellular Vesicles
| Methods | Mechanisms | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Ultracentrifugation | Gold standard method based on sedimentation coefficient. Several centrifugation steps. | Large number of EVs with high purity. Simple and low cost. Isolation from large volumes. | Expensive. Specific infrastructure needed. Time-consuming. Low recovery rate. Potential EV damage. | [79,87] |
| Density gradients | Separation based on EV density and size. | Highly purified EVs. Preserved integrity. | Multistep procedure. Complex. Costly and time-consuming. Potential EV aggregation. | [72,88] |
| Polymer-based precipitation | Changes in EV solubility and aggregation using water-excluding polymers. | Fast and easy to use. Minimal cost. Suitable for large volume. No specialized equipment. | Low purity. Residual polymers and coprecipitation of contaminants. | [76,89] |
| Ultrafiltration | Size-based method. Membrane filters with specific size exclusion limits. | Simple and efficient. High purity and high productivity. No volume limitation. Can be associated with other methods. | EV deformation. Filter plugging. Loss of EVs via membrane attachment. Protein contamination. | [90,91] |
| Size-exclusion-based chromatography | Size-based separation. Columns filled with polymers with heterogeneous pores. | Simple and efficient. High purity and high quality. No EV damage. Separation of large and small molecules. | Long running time. Costly. Limitations on sample volume. Needs further enrichment. | [82,92] |
| Immunoaffinity | Based on specific interactions between immobilized antibodies and ligands on the EV surface. | High purity. High specificity. Isolation of EV subtypes. High recovery and good integrity. Can be combined with other methods. | High reagent costs. Not for large-scale purification. | [85,93] |
6. Methods for Loading Drugs into EVs
| Methods | Mechanisms | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Electroporation | Creation of pores under short and high voltage pulses | Wide applicability Simple and fast method RNAs and hydrophilic compounds | Aggregation Low loading capacity Morphological changes Special equipment | [58,104,105] |
| Sonication | Mechanical shear force produced using ultrasound probe compromises the integrity of the EV membrane, which permits drug encapsulation | High loading capacity Applicable for small RNAs | Destruction of membrane integrity Potential drug adhesion to the membrane affecting release | [98] |
| Extrusion | EVs are mixed with a drug and the mixture is loaded into a syringe-based lipid extruder with 100–400 nm porous membranes under a controlled temperature | High loading efficiency | Changes in EV membrane properties | [15] |
| Freeze/thaw cycles | Drug are incubated with EVs and at least 3 cycles of freeze/thawing (using −80 °C or liquid nitrogen) | Simple to perform Lower loading than sonication or extrusion Potential membrane fusion | EV aggregation Size increase | [14,109] |
| Saponin treatment | Pore formation in EV lipid bilayers via removal of cholesterol | High loading efficiency | Toxicity Loss of membrane integrity | [15,110] |
| Dialysis | Formation of drug transmembrane channels using osmotic pressure | Small molecular substances High drug-loading efficiency | EV size and charge changes | [15] |
7. Loaded EVs for Therapy in Preclinical Studies
7.1. In Vitro Studies
| Diseases | Cell Lines | EV Sources | Active Pharmaceutical Ingredient (API) | API Loading Method (before/after EV Isolation) | Main Results | References |
|---|---|---|---|---|---|---|
| (a) | ||||||
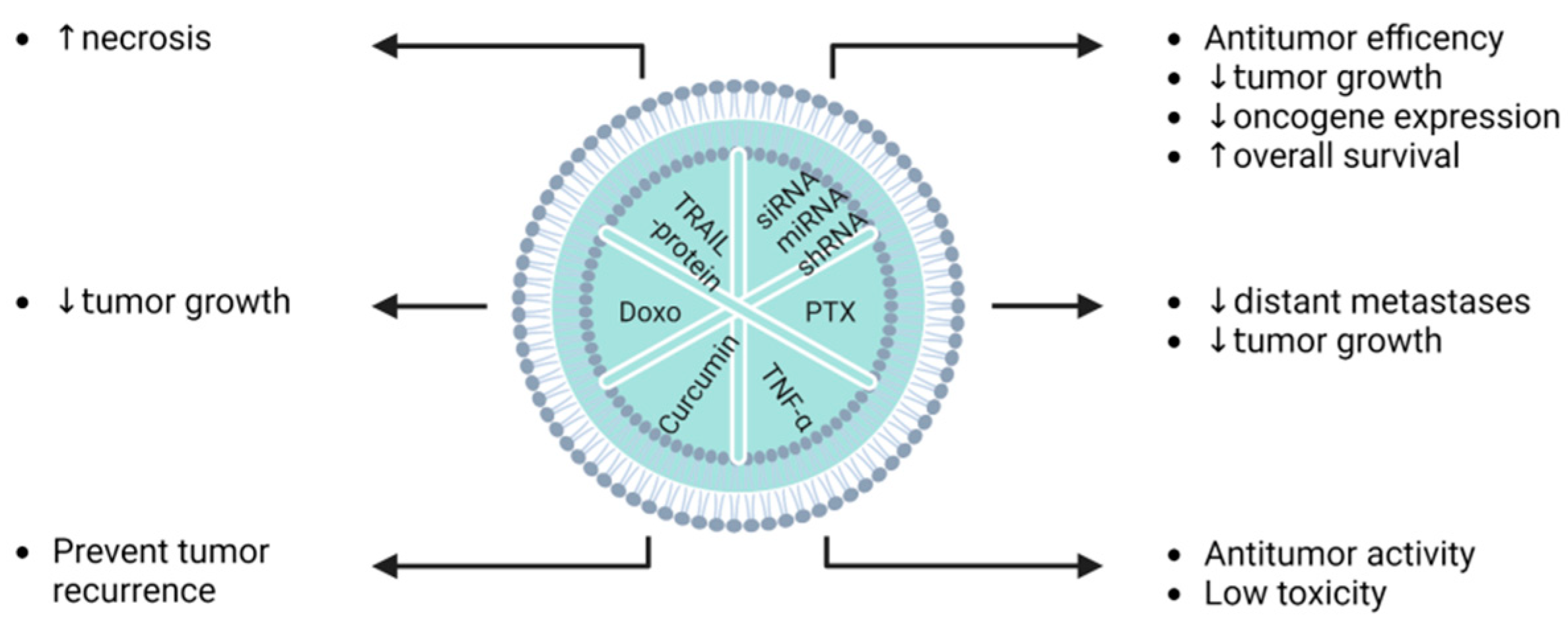
| Glioma | U87MG C6 HEB | BM-MSCs | Indocyanine green and curcumin | Electroporation (after isolation) | Exos-CUR + ICG caused cell inhibition by inducing apoptosis and cell arrest in G2/M phase, while a NIR-induced photothermal effect was synergistic with chemo-phototherapy, directly causing cell necrosis to achieve superior anticancer effects. | [113] |
| Glioma | GSC267 GSC20 GSC6-27 GSC8-11 GSC2-14 | MSCs | miRNA-124a and PTEN-mRNA | Transfection (plasmid-based/before isolation) | Exo-miR124 reduced the viability and clonogenicity of GSCs compared with controls. | [118] |
| Glioblastoma multiforme | 9L | MSCs | miRNA-146b | Transfection (plasmid-based/before isolation) | 9L glioma cells treated by M146-exo showed a decrease in EGFR and NF-kB protein levels. | [112] |
| Breast cancer | TUBO 4T1 | BM-MSCs | Doxorubicin | Electroporation (after isolation) | More efficient binding of LAMP2b-DARP in protein-exosomes to HER2-positive TUBO cells was observed, compared to HER2-negative 4T1 cells. | [120] |
| Breast cancer | A549 SK-OV3 MDA-hyb1 | MSCs | Paclitaxel | Incubation (before isolation) | More efficient tumour-targeting properties were observed with drug-loaded Exos. | [119] |
| Breast cancer | TUBO 4T1 | MSCs | miRNA-142-3p | Electroporation (after isolation) | anti-miR-142-3p-loaded Exos reduced the miR-142-3p and miR-150 levels, and increased the transcription of APC and P2X7R. | [121] |
| Colorectal cancer | MCF7 C26 | MSCs | Doxorubicin | Electroporation (after isolation) | DOXO@exosomes-apt suppressed C26 and MCF7 cell growth. | [122] |
| Hepatocellular carcinoma | HCC Huh7 SMMC-7721 PLC/PRF HL-7702 | MSCs | miRNA-199a | Transfection (lentivirus-based/before isolation) | Exo-199a delivery to HCC cells sensitized them to doxorubicin by targeting and inhibiting the mTOR pathway. | [123] |
| Hepatocellular carcinoma | HepG2 | MSCs | Doxorubicin | Ultrasonication (after isolation) | Doxorubicin loaded in desialylated MSC-derived EVs as drug delivery system to target hepatoma cell lines. | [124] |
| Melanoma | MCF7 A549 Colo201 HCM HUVEC HKC L929 | MSCs | TNF-α | Transfection (plasmid-based/before isolation) | CTNF-α-exosome-SPIONs enhanced tumour cell growth inhibition via the TNFR I-mediated apoptotic pathway. | [125] |
| Melanoma | B16F0 | MSCs | TRAIL protein | Transfection (plasmid-based/before isolation) | Exo-TRAIL induced 2.5× more cell death (apoptosis level) compared to exosomes from non-treated B16F0 cells. | [126] |
| Osteosarcoma | MG63 HOS 143B H9C2 | MSCs | Doxorubicin | Incubation (after isolation) | Osteosarcoma cell proliferation and migration were suppressed by Exo-Doxo. | [117] |
| Pancreatic cancer | PANC1 BxPC3 | BM-MSCs | siKRASG12D | Electroporation (after isolation) | siKrasG12D iExo upregulated genes associated with proteasome, lysosome, and phagosome pathways in Panc-1 cells. | [129] |
| Pancreatic cancer | PANC1 BxPC3 MIA-Capa21 Capan1 | BM-MSCs | siKRASG12D and pLKO.1-shKRASG12D | Electroporation (after isolation) | KRASG12D mRNA and phosphorylated-ERK protein levels were reduced by iExosomes (with siRNA or shRNA targeting KRASG12D) in human Panc-1 cells. | [128] |
| Pancreatic cancer | HPDEC Capan1 CFPAC-1 BxPC3 | hucMSCs | miRNA-145-5p | Transfection reagent (after isolation) | 145-exo treatment resulted in the downregulation of Smad3, N-cadherin and Bcl-2 expression and upregulation of the E-cadherin and Bax genes in PDAC cells. | [130] |
| (b) | ||||||
| Acute myocardial infarction | H9C2 EPCs | ADSC | miRNA-126 | Transfection (miRNA-based/before isolation) | miR-126-exosomes prevented myocardial damage from inflammation, apoptosis, or fibrosis, and promoted angiogenesis. | [114] |
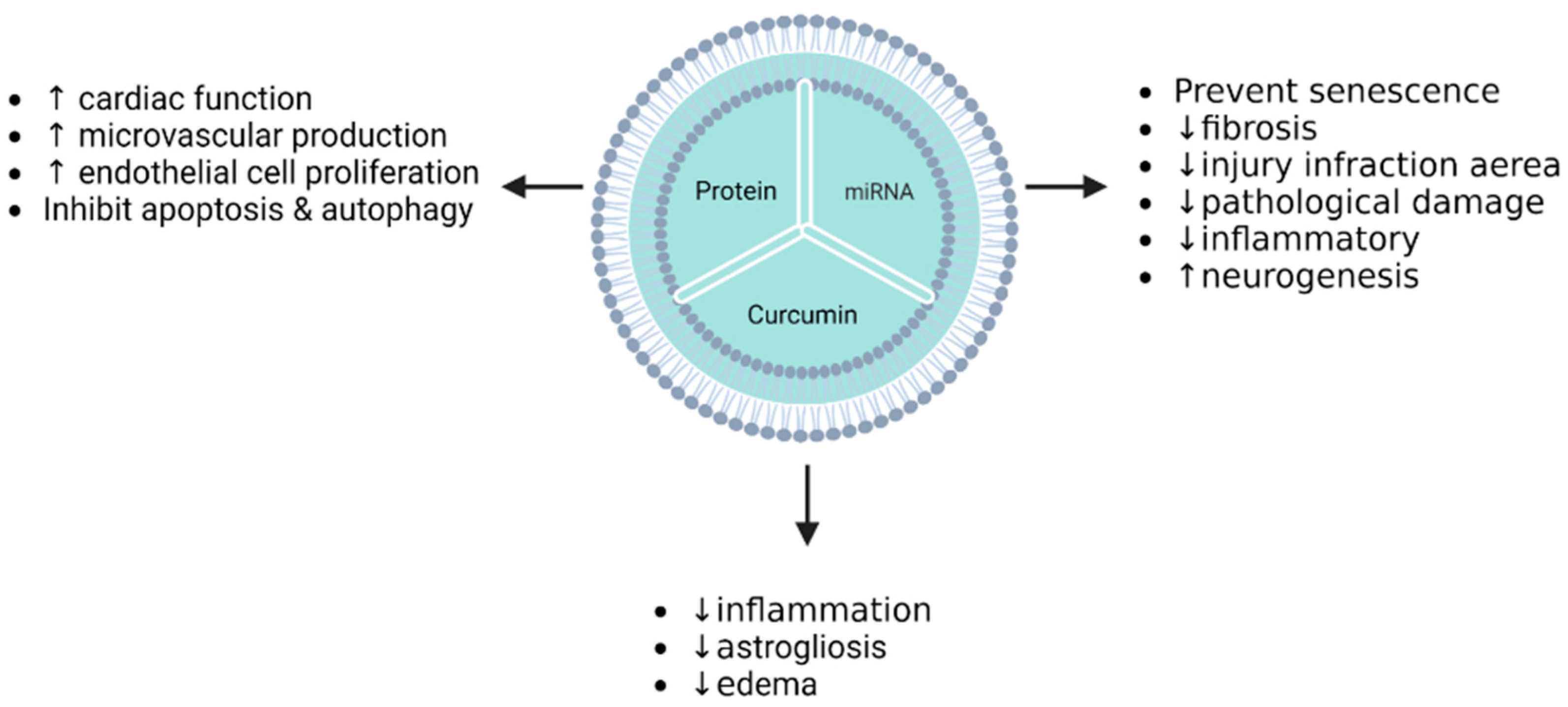
| Acute myocardial infarction | H9C2 EAhy926 | MSCs | Akt | Transfection (adenovirus-based/before isolation) | Endothelial cell proliferation, migration, and tube-like structure formation were promoted by Akt-Exo. | [131] |
| Acute myocardial infarction | CFs H9C2 HUVEC | uc-MSCs | TIMP2 protein | Transfection (lentivirus-based/before isolation) | Exosomes derived from TIMP2-modified ucMSCs repaired the ischemia injuries by inhibiting apoptosis and promoting angiogenesis, and ECM remodeling in cardiomyocytes. | [116] |
| Acute myocardial infarction | Myocardial and endothelial cells (“homemade” isolation) | MSCs | Stromal-derived factor 1 (SDF1) | Transfection (plasmid-based/before isolation) | Autophagy and apoptosis were inhibited in myocardial cells via SDF1 overexpression mediated by EVs. Moreover, EVs promoted the microvascular regeneration of cardiac endothelial cells. | [115] |
| Myocardial ischemia reperfusion injury | Cardiomyocytes (“homemade” isolation) | BMSCs | miRNA-125b | Transfection (miRNA-based/before isolation) | I/R myocardium cells treated with BMSC-Exo-125b showed inhibition of apoptosis and inflammation, and an increase in cell viability. | [132] |
| Cerebral ischemia | BV-2 | MSCs | miRNA-223-3p | Transfection (lentivirus-based/before isolation) | Exosomal miR-223-3p increased M2 microglia transformation into M1 microglia induced by NMLTC4 in a concentration-dependent manner, and decreased mRNA and protein expression of CysLT2R. | [133] |
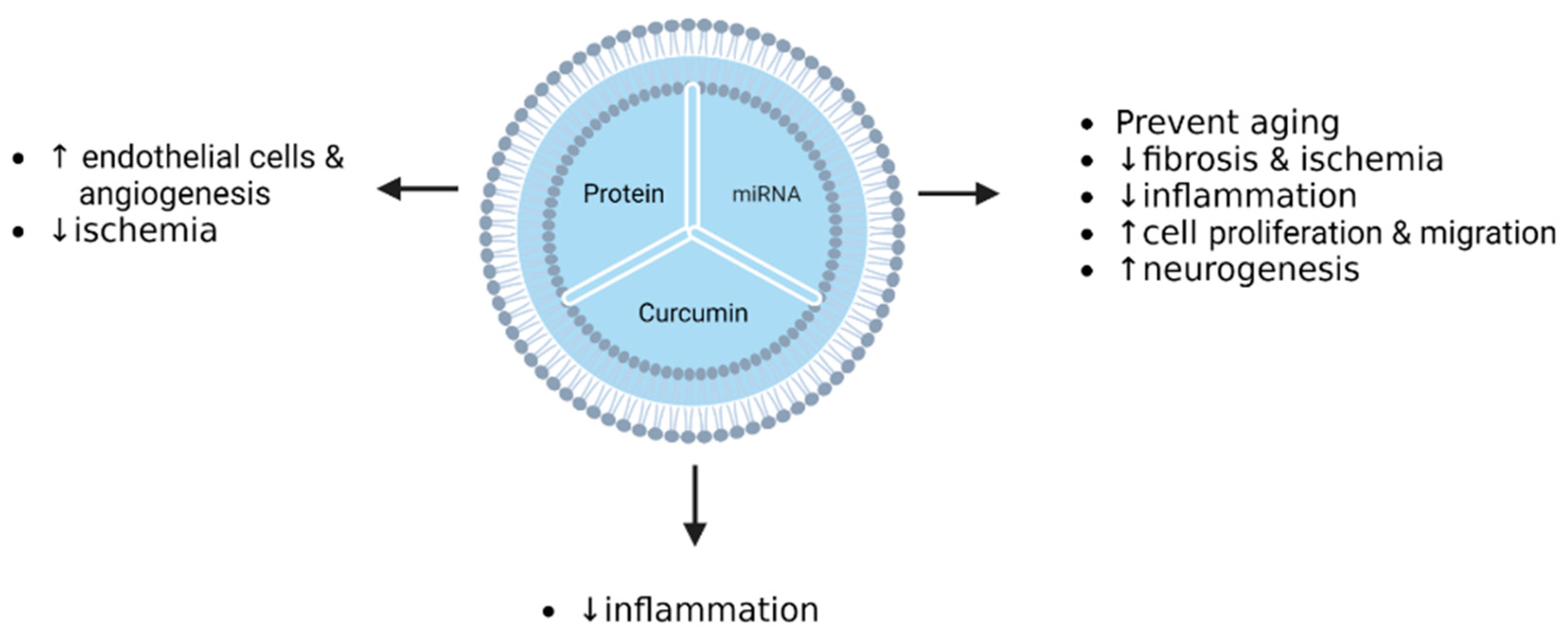
| Cerebral ischemia | HeLa U87 | BMSCs | Curcumin | Incubation (after isolation) | cRGD-Exo exhibited high affinity/specificity to cells expressing integrin avb3. | [134] |
| Ageing-induced vascular dysfunction | H9C2 | ucMSCs | miRNA-675 | Transfection (miRNA-based/before isolation) | miR-675 delivered by exosomes inhibited cell senescence. miR-675 mimic could inhibit ageing-related β-gal staining and promote cell proliferation in ageing cardiomyocytes. | [135] |
| Osteoarthritis | Chondrocytes (“homemade” isolated) | SMSCs | miRNA-140-5p | Transfection (lentivirus-based/before isolation) | The proliferation and migration of ACs were enhanced by SMSC-140-Exos without damaging ECM secretion. | [136] |
| Rheumatoid arthritis | HUVEC | MSCs | miRNA-150-5p | Transfection (plasmid-based/before isolation) | Exo-150 downregulated tube formation of HUVECs via MMP14 and VEGF pathways. | [137] |
| Intestinal fibrosis | IEC-6 | BMSCs | miRNA-200b | Transfection (lentivirus-based/before isolation) | MiR-200b-MVs reversed the morphology in TGF-β1-treated IEC-6 cells. | [138] |
7.2. In Vivo Animal Studies
8. Loaded EVs for Therapy in Clinical Trials
9. Challenges
Author Contributions
Funding
Conflicts of Interest
References
- Ratajczak, J.; Wysoczynski, M.; Hayek, F.; Janowska-Wieczorek, A.; Ratajczak, M.Z. Membrane-Derived Microvesicles: Important and Underappreciated Mediators of Cell-to-Cell Communication. Leukemia 2006, 20, 1487–1495. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Batsali, A.K.; Georgopoulou, A.; Mavroudi, I.; Matheakakis, A.; Pontikoglou, C.G.; Papadaki, H.A. The Role of Bone Marrow Mesenchymal Stem Cell Derived Extracellular Vesicles (MSC-EVs) in Normal and Abnormal Hematopoiesis and Their Therapeutic Potential. J. Clin. Med. 2020, 9, 856. [Google Scholar] [CrossRef]
- Salybekov, A.A.; Kunikeyev, A.D.; Kobayashi, S.; Asahara, T. Latest Advances in Endothelial Progenitor Cell-Derived Extracellular Vesicles Translation to the Clinic. Front. Cardiovasc. Med. 2021, 8, 734562. [Google Scholar] [CrossRef]
- el Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J.A. Extracellular Vesicles: Biology and Emerging Therapeutic Opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.M.; Kroemer, G.; Zitvogel, L. Extracellular Vesicles: Masters of Intercellular Communication and Potential Clinical Interventions. J. Clin. Investig. 2016, 126, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Escudé Martinez de Castilla, P.; Tong, L.; Huang, C.; Sofias, A.M.; Pastorin, G.; Chen, X.; Storm, G.; Schiffelers, R.M.; Wang, J.W. Extracellular Vesicles as a Drug Delivery System: A Systematic Review of Preclinical Studies. Adv. Drug Deliv. Rev. 2021, 175, 113801. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular Vesicles as a Next-Generation Drug Delivery Platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- Gimona, M.; Pachler, K.; Laner-Plamberger, S.; Schallmoser, K.; Rohde, E. Manufacturing of Human Extracellular Vesicle-Based Therapeutics for Clinical Use. Int. J. Mol. Sci. 2017, 18, 1190. [Google Scholar] [CrossRef]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and Challenges of Extracellular Vesicle-Based Drug Delivery System: Considering Cell Source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, W.; Du, Y.; Zhang, C.; Pan, F.; Yao, Y.; Zhang, T.; Peng, Q. Exosomes: The next Generation of Endogenous Nanomaterials for Advanced Drug Delivery and Therapy. Acta Biomater. 2019, 86, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, T.N.; Jay, S.M. Production of Extracellular Vesicles Loaded with Therapeutic Cargo. Methods Mol. Biol. 2018, 1831, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lu, S.; Liang, X.; Cao, B.; Wang, S.; Jiang, J.; Luo, H.; He, S.; Lang, J.; Zhu, G. ΓδTDEs: An Efficient Delivery System for MiR-138 with Anti-Tumoral and Immunostimulatory Roles on Oral Squamous Cell Carcinoma. Mol. Ther. Nucleic Acids 2019, 14, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Bosch, S.; de Beaurepaire, L.; Allard, M.; Mosser, M.; Heichette, C.; Chrétien, D.; Jegou, D.; Bach, J.M. Trehalose Prevents Aggregation of Exosomes and Cryodamage. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active Loading into Extracellular Vesicles Significantly Improves the Cellular Uptake and Photodynamic Effect of Porphyrins. J. Control. Release 2015, 205, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.; Huang, D.; Sang, C.; Zhong, T.; Zhang, Z.; Tang, Z. Advances in Mesenchymal Stem Cell-Derived Exosomes as Drug Delivery Vehicles. Front. Bioeng. Biotechnol. 2022, 9, 1411. [Google Scholar] [CrossRef]
- Rezaie, J.; Nejati, V.; Mahmoodi, M.; Ahmadi, M. Mesenchymal Stem Cells Derived Extracellular Vesicles: A Promising Nanomedicine for Drug Delivery System. Biochem. Pharmacol. 2022, 203, 115167. [Google Scholar] [CrossRef]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016, 25, 829–848. [Google Scholar] [CrossRef]
- Dominici, M.; le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Bouland, C.; Philippart, P.; Dequanter, D.; Corrillon, F.; Loeb, I.; Bron, D.; Lagneaux, L.; Meuleman, N. Cross-Talk Between Mesenchymal Stromal Cells (MSCs) and Endothelial Progenitor Cells (EPCs) in Bone Regeneration. Front. Cell Dev. Biol. 2021, 9, 674084. [Google Scholar] [CrossRef]
- Thanaskody, K.; Jusop, A.S.; Tye, G.J.; Wan Kamarul Zaman, W.S.; Dass, S.A.; Nordin, F. MSCs vs. IPSCs: Potential in Therapeutic Applications. Front. Cell Dev. Biol. 2022, 10, 1005926. [Google Scholar] [CrossRef]
- Wen, D.; Peng, Y.; Liu, D.; Weizmann, Y.; Mahato, R.I. Mesenchymal Stem Cell and Derived Exosome as Small RNA Carrier and Immunomodulator to Improve Islet Transplantation. J. Control. Release 2016, 238, 166–175. [Google Scholar] [CrossRef]
- Bruno, S.; Kholia, S.; Deregibus, M.C.; Camussi, G. The Role of Extracellular Vesicles as Paracrine Effectors in Stem Cell-Based Therapies. Adv. Exp. Med. Biol. 2019, 1201, 175–193. [Google Scholar] [CrossRef]
- Wu, K.; Xing, F.; Wu, S.Y.; Watabe, K. Extracellular Vesicles as Emerging Targets in Cancer: Recent Development from Bench to Bedside. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 538–563. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhao, Y.; Sun, X.; Xing, Y.; Wang, X.; Yang, Q. Immunomodulation of MSCs and MSC-Derived Extracellular Vesicles in Osteoarthritis. Front. Bioeng. Biotechnol. 2020, 8, 575057. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Feng, G.; Jiang, L.; Chen, Z.; Xin, W.; Zhang, X. The Emerging Role of Extracellular Vesicles from Mesenchymal Stem Cells and Macrophages in Pulmonary Fibrosis: Insights into MiRNA Delivery. Pharmaceuticals 2022, 15, 1276. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P. The Nature and Significance of Platelet Products in Human Plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Leijendekker, R.; Harding, C.V.; Melief, C.J.M.; Geuze, H.J. B Lymphocytes Secrete Antigen-Presenting Vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of Established Murine Tumors Using a Novel Cell-Free Vaccine: Dendritic Cell-Derived Exosomes. Nat. Med. 1998, 4, 594–600. [Google Scholar] [CrossRef]
- Guo, Q.; Yan, J.; Song, T.; Zhong, C.; Kuang, J.; Mo, Y.; Tan, J.; Li, D.; Sui, Z.; Cai, K.; et al. MicroRNA-130b-3p Contained in MSC-Derived EVs Promotes Lung Cancer Progression by Regulating the FOXO3/NFE2L2/TXNRD1 Axis. Mol. Ther. Oncolytics 2020, 20, 132–146. [Google Scholar] [CrossRef]
- Saari, H.; Lázaro-Ibáñez, E.; Viitala, T.; Vuorimaa-Laukkanen, E.; Siljander, P.; Yliperttula, M. Microvesicle- and Exosome-Mediated Drug Delivery Enhances the Cytotoxicity of Paclitaxel in Autologous Prostate Cancer Cells. J. Control. Release 2015, 220, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Gatti, S.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Cantaluppi, V.; Tetta, C.; Camussi, G. Microvesicles Derived from Human Adult Mesenchymal Stem Cells Protect against Ischaemia-Reperfusion-Induced Acute and Chronic Kidney Injury. Nephrol. Dial. Transplant. 2011, 26, 1474–1483. [Google Scholar] [CrossRef]
- Ibrahim, A.G.E.; Cheng, K.; Marbán, E. Exosomes as Critical Agents of Cardiac Regeneration Triggered by Cell Therapy. Stem Cell Rep. 2014, 2, 606–619. [Google Scholar] [CrossRef]
- Xin, H.; Li, Y.; Cui, Y.; Yang, J.J.; Zhang, Z.G.; Chopp, M. Systemic Administration of Exosomes Released from Mesenchymal Stromal Cells Promote Functional Recovery and Neurovascular Plasticity after Stroke in Rats. J. Cereb. Blood Flow Metab. 2013, 33, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Liu, S.; Wang, C.; Wang, Y.; Wan, M.; Liu, F.; Gong, M.; Yuan, Y.; Chen, Y.; Cheng, J.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Mitochondrial Damage and Inflammation by Stabilizing Mitochondrial DNA. ACS Nano 2021, 15, 1519–1538. [Google Scholar] [CrossRef]
- Miyaki, S.; Lotz, M.K. Extracellular Vesicles in Cartilage Homeostasis and Osteoarthritis. Curr. Opin. Rheumatol. 2018, 30, 129–135. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; El Andaloussi, S.; Vader, P. Extracellular Vesicles as Drug Delivery Systems: Why and How? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Zhou, X.; Xie, F.; Wang, L.; Zhang, L.; Zhang, S.; Fang, M.; Zhou, F. The Function and Clinical Application of Extracellular Vesicles in Innate Immune Regulation. Cell. Mol. Immunol. 2020, 17, 323–334. [Google Scholar] [CrossRef] [PubMed]
- van Balkom, B.W.M.; Gremmels, H.; Giebel, B.; Lim, S.K. Proteomic Signature of Mesenchymal Stromal Cell-Derived Small Extracellular Vesicles. Proteomics 2019, 19, 1800163. [Google Scholar] [CrossRef]
- Alonso-Alonso, M.L.; García-Posadas, L.; Diebold, Y. Extracellular Vesicles from Human Adipose-Derived Mesenchymal Stem Cells: A Review of Common Cargos. Stem Cell Rev. Rep. 2022, 18, 854–901. [Google Scholar] [CrossRef]
- Donoso-Quezada, J.; Ayala-Mar, S.; González-Valdez, J. The Role of Lipids in Exosome Biology and Intercellular Communication: Function, Analytics and Applications. Traffic 2021, 22, 204–220. [Google Scholar] [CrossRef]
- Yamamoto, A.; Yasue, Y.; Takahashi, Y.; Takakura, Y. Determining The Role of Surface Glycans in The Pharmacokinetics of Small Extracellular Vesicles. J. Pharm. Sci. 2021, 110, 3261–3267. [Google Scholar] [CrossRef]
- Mathew, B.; Ravindran, S.; Liu, X.; Torres, L.; Chennakesavalu, M.; Huang, C.C.; Feng, L.; Zelka, R.; Lopez, J.; Sharma, M.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles and Retinal Ischemia-Reperfusion. Biomaterials 2019, 197, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chen, J.; Chen, L. The Roles of Mesenchymal Stem Cell-Derived Exosomes in Diabetes Mellitus and Its Related Complications. Front. Endocrinol. 2022, 13, 1027686. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, P.; Raman, S.; Glynn, A.; Barry, F.; Murphy, J.M. Mesenchymal Stem Cell Therapy for Osteoarthritis: The Critical Role of the Cell Secretome. Front. Bioeng. Biotechnol. 2019, 7, 9. [Google Scholar] [CrossRef]
- Tang, J.; Cui, X.; Zhang, Z.; Xu, Y.; Guo, J.; Soliman, B.G.; Lu, Y.; Qin, Z.; Wang, Q.; Zhang, H.; et al. Injection-Free Delivery of MSC-Derived Extracellular Vesicles for Myocardial Infarction Therapeutics. Adv. Healthc. Mater. 2022, 11, 2100312. [Google Scholar] [CrossRef]
- Cosenza, S.; Toupet, K.; Maumus, M.; Luz-Crawford, P.; Blanc-Brude, O.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cells-Derived Exosomes Are More Immunosuppressive than Microparticles in Inflammatory Arthritis. Theranostics 2018, 8, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Qiu, S.; Xu, J.; Kang, Q.; Chai, Y. Exosomes Secreted by Young Mesenchymal Stem Cells Promote New Bone Formation During Distraction Osteogenesis in Older Rats. Calcif. Tissue Int. 2020, 106, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Yuana, Y.; Sturk, A.; Nieuwland, R. Extracellular Vesicles in Physiological and Pathological Conditions. Blood Rev. 2013, 27, 31–39. [Google Scholar] [CrossRef]
- Khan, M.; Nickoloff, E.; Abramova, T.; Johnson, J.; Verma, S.K.; Krishnamurthy, P.; Mackie, A.R.; Vaughan, E.; Garikipati, V.N.S.; Benedict, C.; et al. Embryonic Stem Cell-Derived Exosomes Promote Endogenous Repair Mechanisms and Enhance Cardiac Function Following Myocardial Infarction. Circ. Res. 2015, 117, 52–64. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current Trends in the Use of Liposomes for Tumor Targeting. Nanomedicine 2013, 8, 1509–1528. [Google Scholar] [CrossRef]
- Nogueira, E.; Gomes, A.C.; Preto, A.; Cavaco-Paulo, A. Design of Liposomal Formulations for Cell Targeting. Colloids Surf. B Biointerfaces 2015, 136, 514–526. [Google Scholar] [CrossRef]
- Srivatsav, A.T.; Kapoor, S. The Emerging World of Membrane Vesicles: Functional Relevance, Theranostic Avenues and Tools for Investigating Membrane Function. Front. Mol. Biosci. 2021, 8, 640355. [Google Scholar] [CrossRef] [PubMed]
- le Roux, D.M.; Nicol, M.P.; Myer, L.; Vanker, A.; Stadler, J.A.M.; von Delft, E.; Zar, H.J. Lower Respiratory Tract Infections in Children in a Well-Vaccinated South African Birth Cohort: Spectrum of Disease and Risk Factors. Clin. Infect. Dis. 2019, 69, 1588–1596. [Google Scholar] [CrossRef]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.G. A Novel Nanoparticle Drug Delivery System: The Anti-Inflammatory Activity of Curcumin Is Enhanced When Encapsulated in Exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef]
- Ren, J.; He, W.; Zheng, L.; Duan, H. From Structures to Functions: Insights into Exosomes as Promising Drug Delivery Vehicles. Biomater. Sci. 2016, 4, 910–921. [Google Scholar] [CrossRef]
- Hallal, S.; Tűzesi, Á.; Grau, G.E.; Buckland, M.E.; Alexander, K.L. Understanding the Extracellular Vesicle Surface for Clinical Molecular Biology. J. Extracell. Vesicles 2022, 11, e12260. [Google Scholar] [CrossRef] [PubMed]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal Stem Cells: Immune Evasive, Not Immune Privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Jhan, Y.Y.; Prasca-Chamorro, D.; Palou Zuniga, G.; Moore, D.M.; Arun Kumar, S.; Gaharwar, A.K.; Bishop, C.J. Engineered Extracellular Vesicles with Synthetic Lipids via Membrane Fusion to Establish Efficient Gene Delivery. Int. J. Pharm. 2020, 573, 118802. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering Exosomes as Refined Biological Nanoplatforms for Drug Delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.M.; Wiklander, P.B.O.; Nordin, J.Z.; Al-Shawi, R.; Wood, M.J.; Vithlani, M.; Schapira, A.H.V.; Simons, J.P.; El-Andaloussi, S.; Alvarez-Erviti, L. Systemic Exosomal SiRNA Delivery Reduced Alpha-Synuclein Aggregates in Brains of Transgenic Mice. Mov. Disord. 2014, 29, 1476–1485. [Google Scholar] [CrossRef]
- Nemati, M.; Singh, B.; Mir, R.A.; Nemati, M.; Babaei, A.; Ahmadi, M.; Rasmi, Y.; Golezani, A.G.; Rezaie, J. Plant-Derived Extracellular Vesicles: A Novel Nanomedicine Approach with Advantages and Challenges. Cell Commun. Signal. 2022, 20, 1–16. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Q.; Wang, S.; Weng, W.; Jing, Y.; Su, J. Bacterial Extracellular Vesicles as Bioactive Nanocarriers for Drug Delivery: Advances and Perspectives. Bioact. Mater. 2021, 14, 169–181. [Google Scholar] [CrossRef]
- Rizzo, J.; Rodrigues, M.L.; Janbon, G. Extracellular Vesicles in Fungi: Past, Present, and Future Perspectives. Front. Cell. Infect. Microbiol. 2020, 10, 346. [Google Scholar] [CrossRef]
- Garaeva, L.; Kamyshinsky, R.; Kil, Y.; Varfolomeeva, E.; Verlov, N.; Komarova, E.; Garmay, Y.; Landa, S.; Burdakov, V.; Myasnikov, A.; et al. Delivery of Functional Exogenous Proteins by Plant-Derived Vesicles to Human Cells in Vitro. Sci. Rep. 2021, 11, 6489. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Ma, J.; Zhou, Y.; Lu, R. Focusing on Future Applications and Current Challenges of Plant Derived Extracellular Vesicles. Pharmaceuticals 2022, 15, 708. [Google Scholar] [CrossRef]
- Gardiner, C.; di Vizio, D.; Sahoo, S.; Théry, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques Used for the Isolation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey. J. Extracell. Vesicles 2016, 5, 32945. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef]
- Iwai, K.; Minamisawa, T.; Suga, K.; Yajima, Y.; Shiba, K. Isolation of Human Salivary Extracellular Vesicles by Iodixanol Density Gradient Ultracentrifugation and Their Characterizations. J. Extracell. Vesicles 2016, 5, 30829. [Google Scholar] [CrossRef]
- Taylor, D.D.; Shah, S. Methods of Isolating Extracellular Vesicles Impact Down-Stream Analyses of Their Cargoes. Methods 2015, 87, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Andreu, Z.; Rivas, E.; Sanguino-Pascual, A.; Lamana, A.; Marazuela, M.; González-Alvaro, I.; Sánchez-Madrid, F.; de la Fuente, H.; Yáñez-Mó, M. Comparative Analysis of EV Isolation Procedures for MiRNAs Detection in Serum Samples. J. Extracell. Vesicles 2016, 5, 31655. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Palaniyandi, K.; Ramalingam, S.; Sahabudeen, S.; Raja, N.S. Exosomes Isolated from Two Different Cell Lines Using Three Different Isolation Techniques Show Variation in Physical and Molecular Characteristics. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183490. [Google Scholar] [CrossRef]
- Deregibus, M.C.; Figliolini, F.; D’Antico, S.; Manzini, P.M.; Pasquino, C.; de Lena, M.; Tetta, C.; Brizzi, M.F.; Camussi, G. Charge-Based Precipitation of Extracellular Vesicles. Int. J. Mol. Med. 2016, 38, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Li, B.; Fang, C.; Liang, X.; Xie, Y.; Sun, X.; Wang, W.; Zheng, L.; Wang, D. Extracellular Vesicles of Mesenchymal Stem Cells Are More Effectively Accessed through Polyethylene Glycol-Based Precipitation than by Ultracentrifugation. Stem Cells Int. 2022, 2022, 3577015. [Google Scholar] [CrossRef] [PubMed]
- Parimon, T.; Garrett, N.E.; Chen, P.; Antes, T.J. Isolation of Extracellular Vesicles from Murine Bronchoalveolar Lavage Fluid Using an Ultrafiltration Centrifugation Technique. J. Vis. Exp. 2018, 2018, 141. [Google Scholar] [CrossRef]
- Lobb, R.J.; Becker, M.; Wen, S.W.; Wong, C.S.F.; Wiegmans, A.P.; Leimgruber, A.; Möller, A. Optimized Exosome Isolation Protocol for Cell Culture Supernatant and Human Plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Lamparski, H.G.; Metha-Damani, A.; Yao, J.Y.; Patel, S.; Hsu, D.H.; Ruegg, C.; le Pecq, J.B. Production and Characterization of Clinical Grade Exosomes Derived from Dendritic Cells. J. Immunol. Methods 2002, 270, 211–226. [Google Scholar] [CrossRef]
- Böing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.W.; Sturk, A.; Nieuwland, R. Single-Step Isolation of Extracellular Vesicles by Size-Exclusion Chromatography. J. Extracell. Vesicles 2014, 3, 23430. [Google Scholar] [CrossRef]
- Guan, S.; Guan, S.; Yu, H.; Yu, H.; Yan, G.; Gao, M.; Sun, W.; Zhang, X. Characterization of Urinary Exosomes Purified with Size Exclusion Chromatography and Ultracentrifugation. J. Proteome Res. 2020, 19, 2217–2225. [Google Scholar] [CrossRef] [PubMed]
- Benedikter, B.J.; Bouwman, F.G.; Vajen, T.; Heinzmann, A.C.A.; Grauls, G.; Mariman, E.C.; Wouters, E.F.M.; Savelkoul, P.H.; Lopez-Iglesias, C.; Koenen, R.R.; et al. Ultrafiltration Combined with Size Exclusion Chromatography Efficiently Isolates Extracellular Vesicles from Cell Culture Media for Compositional and Functional Studies. Sci. Rep. 2017, 7, 15297. [Google Scholar] [CrossRef] [PubMed]
- Greening, D.W.; Xu, R.; Ji, H.; Tauro, B.J.; Simpson, R.J. A Protocol for Exosome Isolation and Characterization: Evaluation of Ultracentrifugation, Density-Gradient Separation, and Immunoaffinity Capture Methods. Methods Mol. Biol. 2015, 1295, 179–209. [Google Scholar] [CrossRef] [PubMed]
- Zarovni, N.; Corrado, A.; Guazzi, P.; Zocco, D.; Lari, E.; Radano, G.; Muhhina, J.; Fondelli, C.; Gavrilova, J.; Chiesi, A. Integrated Isolation and Quantitative Analysis of Exosome Shuttled Proteins and Nucleic Acids Using Immunocapture Approaches. Methods 2015, 87, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.Q.; Almughlliq, F.B.; Vaswani, K.; Peiris, H.N.; Mitchell, M.D. Exosome Enrichment by Ultracentrifugation and Size Exclusion Chromatography. Front. Biosci. 2018, 23, 865–874. [Google Scholar] [CrossRef]
- Cvjetkovic, A.; Lötvall, J.; Lässer, C. The Influence of Rotor Type and Centrifugation Time on the Yield and Purity of Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 23111. [Google Scholar] [CrossRef]
- Momen-Heravi, F. Isolation of Extracellular Vesicles by Ultracentrifugation. Methods Mol. Biol. 2017, 1660, 25–32. [Google Scholar] [CrossRef]
- Weng, Y.; Sui, Z.; Shan, Y.; Hu, Y.; Chen, Y.; Zhang, L.; Zhang, Y. Effective Isolation of Exosomes with Polyethylene Glycol from Cell Culture Supernatant for In-Depth Proteome Profiling. Analyst 2016, 141, 4640–4646. [Google Scholar] [CrossRef]
- Zeng, X.; Yi, X.; Chen, L.; Zhang, H.; Zhou, R.; Wu, J.; Chen, Y.; Huang, W.; Zhang, L.; Zheng, J.; et al. Characterization and Bioassays of Extracellular Vesicles Extracted by Tangential Flow Filtration. Regen. Med. 2022, 17, 141–154. [Google Scholar] [CrossRef]
- Davies, R.T.; Kim, J.; Jang, S.C.; Choi, E.J.; Gho, Y.S.; Park, J. Microfluidic Filtration System to Isolate Extracellular Vesicles from Blood. Lab. Chip 2012, 12, 5202–5210. [Google Scholar] [CrossRef]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef]
- Logozzi, M.; di Raimo, R.; Mizzoni, D.; Fais, S. Immunocapture-Based ELISA to Characterize and Quantify Exosomes in Both Cell Culture Supernatants and Body Fluids. Methods Enzymol. 2020, 645, 155–180. [Google Scholar] [CrossRef]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome Delivered Anticancer Drugs across the Blood-Brain Barrier for Brain Cancer Therapy in Danio Rerio. Pharm. Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine Milk-Derived Exosomes for Drug Delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef]
- Hettich, B.F.; Bader, J.J.; Leroux, J.C. Encapsulation of Hydrophilic Compounds in Small Extracellular Vesicles: Loading Capacity and Impact on Vesicle Functions. Adv. Healthc. Mater. 2022, 11, 2100047. [Google Scholar] [CrossRef] [PubMed]
- Mehryab, F.; Rabbani, S.; Shahhosseini, S.; Shekari, F.; Fatahi, Y.; Baharvand, H.; Haeri, A. Exosomes as a Next-Generation Drug Delivery System: An Update on Drug Loading Approaches, Characterization, and Clinical Application Challenges. Acta Biomater. 2020, 113, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of Exosome-Encapsulated Paclitaxel to Overcome MDR in Cancer Cells. Nanomedicine 2016, 12, 655–664. [Google Scholar] [CrossRef]
- Wei, H.; Chen, J.; Wang, S.; Fu, F.; Zhu, X.; Wu, C.; Liu, Z.; Zhong, G.; Lin, J. A Nanodrug Consisting Of Doxorubicin And Exosome Derived From Mesenchymal Stem Cells For Osteosarcoma Treatment In Vitro. Int. J. Nanomed. 2019, 14, 8603–8610. [Google Scholar] [CrossRef]
- Gaurav, I.; Thakur, A.; Iyaswamy, A.; Wang, X.; Chen, X.; Yang, Z. Factors Affecting Extracellular Vesicles Based Drug Delivery Systems. Molecules 2021, 26, 1544. [Google Scholar] [CrossRef]
- Pascucci, L.; Coccè, V.; Bonomi, A.; Ami, D.; Ceccarelli, P.; Ciusani, E.; Viganò, L.; Locatelli, A.; Sisto, F.; Doglia, S.M.; et al. Paclitaxel Is Incorporated by Mesenchymal Stromal Cells and Released in Exosomes That Inhibit in Vitro Tumor Growth: A New Approach for Drug Delivery. J. Control. Release 2014, 192, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, D.; Nam, H.; Moon, S.; Kwon, Y.J.; Lee, J.B. Engineered Extracellular Vesicles and Their Mimetics for Clinical Translation. Methods 2020, 177, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhang, B.; Ocansey, D.K.W.; Xu, W.; Qian, H. Extracellular Vesicles: A Bright Star of Nanomedicine. Biomaterials 2021, 269, 120467. [Google Scholar] [CrossRef] [PubMed]
- Abas, B.I.; Demirbolat, G.M.; Cevik, O. Wharton Jelly-Derived Mesenchymal Stem Cell Exosomes Induce Apoptosis and Suppress EMT Signaling in Cervical Cancer Cells as an Effective Drug Carrier System of Paclitaxel. PLoS ONE 2022, 17, e0274607. [Google Scholar] [CrossRef]
- Kooijmans, S.A.A.; Stremersch, S.; Braeckmans, K.; de Smedt, S.C.; Hendrix, A.; Wood, M.J.A.; Schiffelers, R.M.; Raemdonck, K.; Vader, P. Electroporation-Induced SiRNA Precipitation Obscures the Efficiency of SiRNA Loading into Extracellular Vesicles. J. Control. Release 2013, 172, 229–238. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Gudbergsson, J.M.; Skov, M.N.; Christiansen, G.; Gurevich, L.; Moos, T.; Duroux, M. Evaluation of Electroporation-Induced Adverse Effects on Adipose-Derived Stem Cell Exosomes. Cytotechnology 2016, 68, 2125–2138. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Zhu, Y.; Ali, D.J.; Tian, T.; Xu, H.; Si, K.; Sun, B.; Chen, B.; Xiao, Z. Engineered Exosomes for Targeted Co-Delivery of MiR-21 Inhibitor and Chemotherapeutics to Reverse Drug Resistance in Colon Cancer. J. Nanobiotechnol. 2020, 18, 10. [Google Scholar] [CrossRef]
- Won Lee, G.; Thangavelu, M.; Joung Choi, M.; Yeong Shin, E.; Sol Kim, H.; Seon Baek, J.; Woon Jeong, Y.; Eun Song, J.; Carlomagno, C.; Miguel Oliveira, J.; et al. Exosome Mediated Transfer of MiRNA-140 Promotes Enhanced Chondrogenic Differentiation of Bone Marrow Stem Cells for Enhanced Cartilage Repair and Regeneration. J. Cell. Biochem. 2020, 121, 3642–3652. [Google Scholar] [CrossRef]
- Sato, Y.T.; Umezaki, K.; Sawada, S.; Mukai, S.A.; Sasaki, Y.; Harada, N.; Shiku, H.; Akiyoshi, K. Engineering Hybrid Exosomes by Membrane Fusion with Liposomes. Sci. Rep. 2016, 6, 21933. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as Drug Delivery Vehicles for Parkinson’s Disease Therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Li, Y.; Wang, Q.; Cai, N.; Wu, L.; Yan, X. Single-Particle Assessment of Six Different Drug-Loading Strategies for Incorporating Doxorubicin into Small Extracellular Vesicles. Anal. Bioanal. Chem. 2022. [Google Scholar] [CrossRef]
- Katakowski, M.; Buller, B.; Zheng, X.; Lu, Y.; Rogers, T.; Osobamiro, O.; Shu, W.; Jiang, F.; Chopp, M. Exosomes from Marrow Stromal Cells Expressing MiR-146b Inhibit Glioma Growth. Cancer Lett. 2013, 335, 201–204. [Google Scholar] [CrossRef]
- Li, G.; Wang, J.; Xu, M.; Zhang, H.; Tu, C.; Yang, J.; Chen, X.; Yao, Q.; Lan, P.; Xie, M. Engineered Exosome for NIR-Triggered Drug Delivery and Superior Synergistic Chemo-Phototherapy in a Glioma Model. Appl. Mater. Today 2020, 20, 100723. [Google Scholar] [CrossRef]
- Luo, Q.; Guo, D.; Liu, G.; Chen, G.; Hang, M.; Jin, M. Exosomes from MiR-126-Overexpressing Adscs Are Therapeutic in Relieving Acute Myocardial Ischaemic Injury. Cell. Physiol. Biochem. 2017, 44, 2105–2116. [Google Scholar] [CrossRef]
- Gong, X.H.; Liu, H.; Wang, S.J.; Liang, S.W.; Wang, G.G. Exosomes Derived from SDF1-Overexpressing Mesenchymal Stem Cells Inhibit Ischemic Myocardial Cell Apoptosis and Promote Cardiac Endothelial Microvascular Regeneration in Mice with Myocardial Infarction. J. Cell. Physiol. 2019, 234, 13878–13893. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Liu, X.; Yin, Y.; Zhang, P.; Xu, Y.W.; Liu, Z. Exosomes Derived from TIMP2-Modified Human Umbilical Cord Mesenchymal Stem Cells Enhance the Repair Effect in Rat Model with Myocardial Infarction Possibly by the Akt/Sfrp2 Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 1958941. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Chen, F.; Chen, J.; Lin, H.; Wang, S.; Wang, Y.; Wu, C.; Lin, J.; Zhong, G. Mesenchymal Stem Cell Derived Exosomes as Nanodrug Carrier of Doxorubicin for Targeted Osteosarcoma Therapy via SDF1-CXCR4 Axis. Int. J. Nanomed. 2022, 17, 3483–3495. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.M.; Hossain, A.; Gumin, J.; Momin, E.N.; Shimizu, Y.; Ledbetter, D.; Shahar, T.; Yamashita, S.; Parker Kerrigan, B.; Fueyo, J.; et al. Mesenchymal Stem Cells as Natural Biofactories for Exosomes Carrying MiR-124a in the Treatment of Gliomas. Neuro-Oncology 2018, 20, 380–390. [Google Scholar] [CrossRef]
- Melzer, C.; Rehn, V.; Yang, Y.; Bähre, H.; von der Ohe, J.; Hass, R. Taxol-Loaded MSC-Derived Exosomes Provide a Therapeutic Vehicle to Target Metastatic Breast Cancer and Other Carcinoma Cells. Cancers 2019, 11, 798. [Google Scholar] [CrossRef]
- Gomari, H.; Moghadam, M.F.; Soleimani, M.; Ghavami, M.; Khodashenas, S. Targeted Delivery of Doxorubicin to HER2 Positive Tumor Models. Int. J. Nanomed. 2019, 14, 5679–5690. [Google Scholar] [CrossRef] [Green Version]
- Naseri, Z.; Oskuee, R.K.; Jaafari, M.R.; Moghadam, M.F. Exosome-Mediated Delivery of Functionally Active MiRNA-142-3p Inhibitor Reduces Tumorigenicity of Breast Cancer in Vitro and in Vivo. Int. J. Nanomed. 2018, 13, 7727–7747. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, E.; Abnous, K.; Farzad, S.A.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Targeted Doxorubicin-Loaded Mesenchymal Stem Cells-Derived Exosomes as a Versatile Platform for Fighting against Colorectal Cancer. Life Sci 2020, 261, 118369. [Google Scholar] [CrossRef] [PubMed]
- Lou, G.; Chen, L.; Xia, C.; Wang, W.; Qi, J.; Li, A.; Zhao, L.; Chen, Z.; Zheng, M.; Liu, Y. MiR-199a-Modified Exosomes from Adipose Tissue-Derived Mesenchymal Stem Cells Improve Hepatocellular Carcinoma Chemosensitivity through MTOR Pathway. J. Exp. Clin. Cancer Res. 2020, 39, 4. [Google Scholar] [CrossRef]
- Yang, C.; Guan, Z.; Pang, X.; Tan, Z.; Yang, X.; Li, X.; Guan, F. Desialylated Mesenchymal Stem Cells-Derived Extracellular Vesicles Loaded with Doxorubicin for Targeted Inhibition of Hepatocellular Carcinoma. Cells 2022, 11, 2642. [Google Scholar] [CrossRef]
- Zhuang, M.; Chen, X.; Du, D.; Shi, J.; Deng, M.; Long, Q.; Yin, X.; Wang, Y.; Rao, L. SPION Decorated Exosome Delivery of TNF-α to Cancer Cell Membranes through Magnetism. Nanoscale 2019, 12, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Shamili, F.H.; Bayegi, H.R.; Salmasi, Z.; Sadri, K.; Mahmoudi, M.; Kalantari, M.; Ramezani, M.; Abnous, K. Exosomes Derived from TRAIL-Engineered Mesenchymal Stem Cells with Effective Anti-Tumor Activity in a Mouse Melanoma Model. Int. J. Pharm. 2018, 549, 218–229. [Google Scholar] [CrossRef]
- Chandana, S.R.; Babiker, H.M.; Mahadevan, D. Therapeutic Trends in Pancreatic Ductal Adenocarcinoma (PDAC). Expert Opin. Investig. Drugs 2019, 28, 161–177. [Google Scholar] [CrossRef]
- Kamerkar, S.; Lebleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes Facilitate Therapeutic Targeting of Oncogenic KRAS in Pancreatic Cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef]
- Mendt, M.; Kamerkar, S.; Sugimoto, H.; McAndrews, K.M.; Wu, C.C.; Gagea, M.; Yang, S.; Blanko, E.V.R.; Peng, Q.; Ma, X.; et al. Generation and Testing of Clinical-Grade Exosomes for Pancreatic Cancer. JCI Insight 2018, 3, 99263. [Google Scholar] [CrossRef]
- Ding, Y.; Cao, F.; Sun, H.; Wang, Y.; Liu, S.; Wu, Y.; Cui, Q.; Mei, W.T.; Li, F. Exosomes Derived from Human Umbilical Cord Mesenchymal Stromal Cells Deliver Exogenous MiR-145-5p to Inhibit Pancreatic Ductal Adenocarcinoma Progression. Cancer Lett. 2019, 442, 351–361. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, Y.; Sun, L.; Sun, X.; Zhao, X.; Sun, X.; Qian, H.; Xu, W.; Zhu, W.; Sun, L.I. Exosomes Derived from Akt-Modified Human Umbilical Cord Mesenchymal Stem Cells Improve Cardiac Regeneration and Promote Angiogenesis via Activating Platelet-Derived Growth Factor, D. Stem Cells Transl. Med. 2017, 6, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, Y.; Ding, X.; Li, Q.; Qiu, F.; Wang, M.; Shen, Z.; Zheng, H.; Fu, G. Bone Marrow Mesenchymal Stem Cell-Secreted Exosomes Carrying MicroRNA-125b Protect against Myocardial Ischemia Reperfusion Injury via Targeting SIRT7. Mol. Cell. Biochem. 2020, 465, 103–114. [Google Scholar] [CrossRef]
- Zhao, Y.; Gan, Y.; Xu, G.; Hua, K.; Liu, D. Exosomes from MSCs Overexpressing MicroRNA-223-3p Attenuate Cerebral Ischemia through Inhibiting Microglial M1 Polarization Mediated Inflammation. Life Sci. 2020, 260, 118403. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhang, H.X.; He, C.P.; Fan, S.; Zhu, Y.L.; Qi, C.; Huang, N.P.; Xiao, Z.D.; Lu, Z.H.; Tannous, B.A.; et al. Surface Functionalized Exosomes as Targeted Drug Delivery Vehicles for Cerebral Ischemia Therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef]
- Han, C.; Zhou, J.; Liu, B.; Liang, C.; Pan, X.; Zhang, Y.; Zhang, Y.; Wang, Y.; Shao, L.; Zhu, B.; et al. Delivery of MiR-675 by Stem Cell-Derived Exosomes Encapsulated in Silk Fibroin Hydrogel Prevents Aging-Induced Vascular Dysfunction in Mouse Hindlimb. Mater. Sci. Eng. C 2019, 99, 322–332. [Google Scholar] [CrossRef]
- Tao, S.-C.; Yuan, T.; Zhang, Y.-L.; Yin, W.-J.; Guo, S.-C.; Zhang, C.-Q. Exosomes Derived from MiR-140-5p-Overexpressing Human Synovial Mesenchymal Stem Cells Enhance Cartilage Tissue Regeneration and Prevent Osteoarthritis of the Knee in a Rat Model. Theranostics 2017, 7, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, H.; Xia, Y.; Yan, F.; Lu, Y. Therapeutic Potential of Mesenchymal Cell–Derived MiRNA-150-5p–Expressing Exosomes in Rheumatoid Arthritis Mediated by the Modulation of MMP14 and VEGF. J. Immunol. 2018, 201, 2472–2482. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, C.Z.; Zhu, R.; Fan, H.; Liu, X.X.; Duan, X.Y.; Tang, Q.; Shou, Z.X.; Zuo, D.M. MiR-200b-Containing Microvesicles Attenuate Experimental Colitis Associated Intestinal Fibrosis by Inhibiting Epithelial-Mesenchymal Transition. J. Gastroenterol. Hepatol. 2017, 32, 1966–1974. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Chen, X.; Wang, L.; Yang, G. Exosome Mediated Delivery of MiR-124 Promotes Neurogenesis after Ischemia. Mol. Ther. Nucleic Acids 2017, 7, 278–287. [Google Scholar] [CrossRef]
- Kalani, A.; Chaturvedi, P.; Kamat, P.K.; Maldonado, C.; Bauer, P.; Joshua, I.G.; Tyagi, S.C.; Tyagi, N. Curcumin-Loaded Embryonic Stem Cell Exosomes Restored Neurovascular Unit Following Ischemia-Reperfusion Injury. Int. J. Biochem. Cell Biol. 2016, 79, 360–369. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.J.O.; Floriano, J.F.; Nicastro, L.; Emanueli, C.; Catapano, F. Novel Applications of Mesenchymal Stem Cell-Derived Exosomes for Myocardial Infarction Therapeutics. Biomolecules 2020, 10, 707. [Google Scholar] [CrossRef] [PubMed]
- Kordelas, L.; Rebmann, V.; Ludwig, A.K.; Radtke, S.; Ruesing, J.; Doeppner, T.R.; Epple, M.; Horn, P.A.; Beelen, D.W.; Giebel, B. MSC-Derived Exosomes: A Novel Tool to Treat Therapy-Refractory Graft-versus-Host Disease. Leukemia 2014, 28, 970–973. [Google Scholar] [CrossRef]
- Nassar, W.; El-Ansary, M.; Sabry, D.; Mostafa, M.A.; Fayad, T.; Kotb, E.; Temraz, M.; Saad, A.N.; Essa, W.; Adel, H. Umbilical Cord Mesenchymal Stem Cells Derived Extracellular Vesicles Can Safely Ameliorate the Progression of Chronic Kidney Diseases. Biomater. Res. 2016, 20, 21. [Google Scholar] [CrossRef] [PubMed]
- Pirisinu, M.; Pham, T.C.; Zhang, D.X.; Hong, T.N.; Nguyen, L.T.; Le, M.T. Extracellular Vesicles as Natural Therapeutic Agents and Innate Drug Delivery Systems for Cancer Treatment: Recent Advances, Current Obstacles, and Challenges for Clinical Translation. Semin. Cancer Biol. 2022, 80, 340–355. [Google Scholar] [CrossRef]
- Munoz, J.L.; Bliss, S.A.; Greco, S.J.; Ramkissoon, S.H.; Ligon, K.L.; Rameshwar, P. Delivery of Functional Anti-MiR-9 by Mesenchymal Stem Cell-Derived Exosomes to Glioblastoma Multiforme Cells Conferred Chemosensitivity. Mol. Ther. Nucleic Acids 2013, 2, e126. [Google Scholar] [CrossRef] [PubMed]
- IExosomes in Treating Participants with Metastatic Pancreas Cancer with KrasG12D Mutation—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03608631?term=NCT03608631&draw=2&rank=1 (accessed on 1 December 2022).
- Exosome-Based Nanoplatform for Ldlr MRNA Delivery in FH—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05043181?term=NCT05043181&draw=2&rank=1 (accessed on 1 December 2022).
- Allogenic Mesenchymal Stem Cell Derived Exosome in Patients with Acute Ischemic Stroke—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03384433?term=NCT03384433&draw=2&rank=1 (accessed on 1 December 2022).

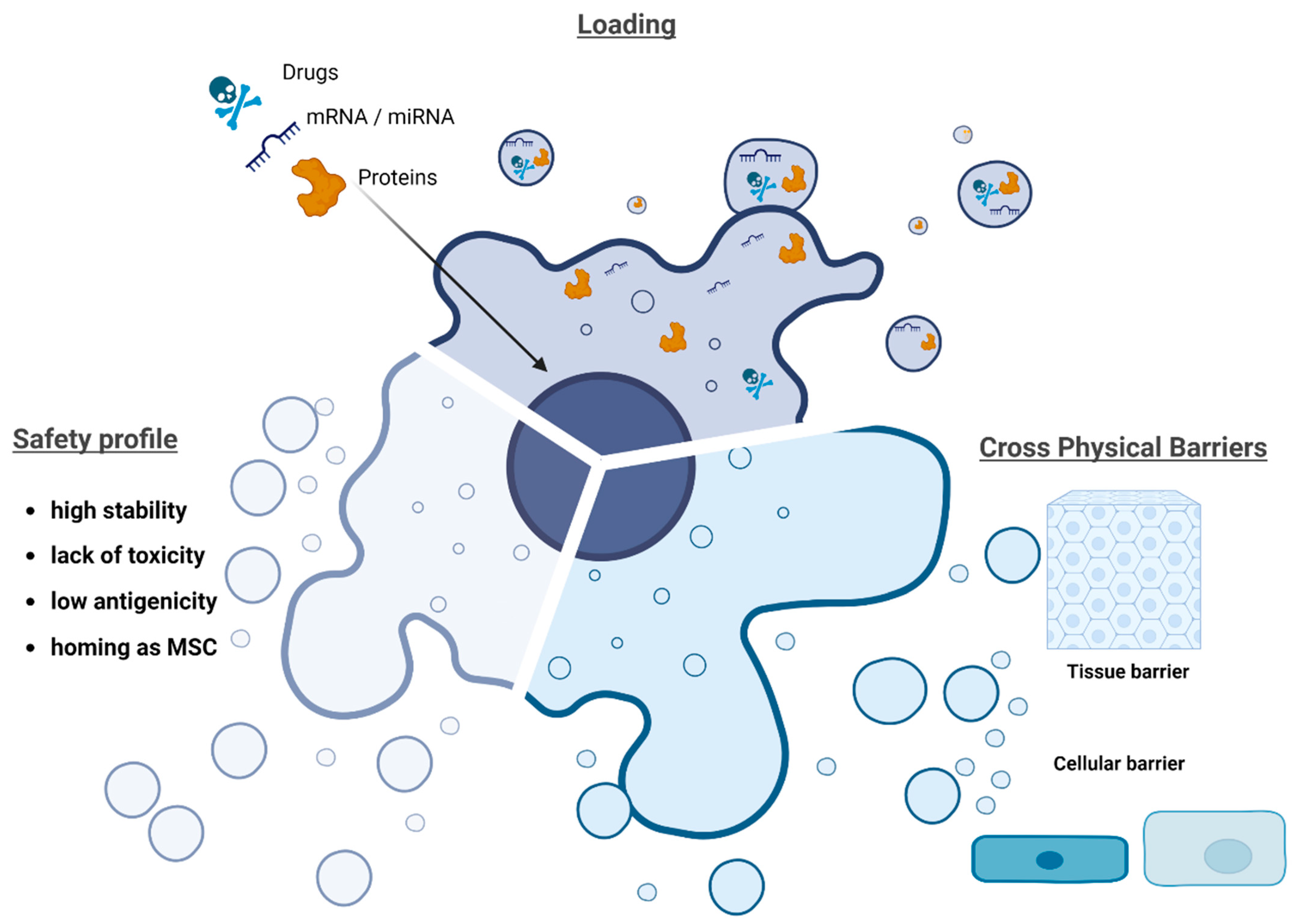


| Disease | Model | EV Sources | Active Pharmaceutical Ingredient (API) | API Loading Method (before/after EVs Isolation) | Main Results | References |
|---|---|---|---|---|---|---|
| (a) | ||||||
| Glioma | Mouse | BM-MSCs | Indocyanine green and curcumin | Electroporation (after isolation) | Exos-based combined therapy drastically abrogated glioma and increased the prevention of rapid tumour recurrence following transient phototherapy and total tumour remission in a mouse model. | [113] |
| Glioma | Mouse | MSCs | miRNA-124a and PTEN-mRNA | Transfection (plasmid-based/before isolation) | Exosomes loaded with a supraphysiological level of miR124a inhibited the growth of GSCs in mice with an intracranial GSC xenograft. | [118] |
| Glioblastoma multiforme | Rat | MSCs | miRNA-146b | Transfection (plasmid-based/before isolation) | The injection of miR146 expressing MSC-derived exosomes inside the tumour decreased the growth of tumours in rats with a glioma xenograft. | [112] |
| Breast cancer | Mouse | BM-MSCs | Doxorubicin | Electroporation (after isolation) | Targeted doxorubicin-loaded exosomes showed specific delivery to the target tissues in a murine breast cancer model, and reduced the tumour growth rate, compared to free drug or untargeted exosomes. | [120] |
| Breast cancer | Mouse | MSCs | Paclitaxel | Incubation (before isolation) | Systemic IV injection of MSC-derived Taxol exosomes reduced by 60% the subcutaneous primary tumour, and distant organ metastases in NODscid mice with metastatic MDA-hyb1 breast cancer. | [119] |
| Breast cancer | Mouse | MSCs | miRNA-142-3p | Electroporation (after isolation) | LNA-anti-miR-142-3p MSCs-derived exosomes reduced the expression level of miR-1423p and miR150 in tumour-bearing mice. | [121] |
| Colorectal cancer | Mouse | MSCs | Doxorubicin | Electroporation (after isolation) | Ectopic model of C26 in BALB mice showed that a single IV injection of targeted DOXO@exosomes-apt significantly suppressed the tumour growth compared to free DOXO. | [122] |
| Hepatocellular carcinoma | Mouse | MSCs | miRNA-199a | Transfection (lentivirus-based/before isolation) | AMSCs-Exo-199a could be used to distribute miR199a to tumour tissue. Moreover, they increased the chemotherapeutic effects of doxorubicin by targeting and inhibiting the mTOR pathway. | [123] |
| Hepatocellular carcinoma | Mouse | MSCs | Doxorubicin | Ultrasonication (after isolation) | Doxorubicin loaded in desialylated MSC-derived EVs as a drug delivery system targeted hepatoma cells in mouse model. | [124] |
| Melanoma | Mouse | MSCs | TNF-α | Transfection (plasmid-based/before isolation) | Coupled SPIONs and CTNF-α anchored exosomes delivered peptide drugs to the cytomembrane better than to the cytoplasm, and resulted in an increase in antitumour activity and lower toxicity. | [125] |
| Melanoma | Mouse | MSCs | TRAIL protein | Transfection (plasmid-based/before isolation) | Homing ability to Exo-TRAIL reduced tumour progression by enhancing necrosis in cancer cells following multidose administration in both in vivo and in vitro models. | [126] |
| Osteosarcoma | Mouse | MSCs | Doxorubicin | Incubation (after isolation) | Exo-DOXO displayed higher cytotoxicity than free drug, and was efficient as a drug delivery system. | [117] |
| Pancreatic cancer | Mouse | MSCs | siKRASG12D | Electroporation (after isolation) | Both BM-MSCs- and BJ-MSCs-derived exosomes loaded with siKRASG12D showed a robust antitumour efficiency in PDAC models. | [129] |
| Pancreatic cancer | Mouse | MSCs | siKRASG12D and pLKO.1-shKRASG12D | Electroporation (after isolation) | Exosomes derived from mouse skin fibroblast were used as a nanocarrier to specifically target pancreatic cancer cells in multiple mouse models of pancreatic cancer. EV injection drastically increased OS. | [128] |
| Pancreatic cancer | Mouse | hucMSCs | miRNA-145-5p | Transfection reagent (after isolation) | Intratumour injection of miR145-5p UC-MSCs-derived exosomes reduced xenograft tumour growth in a BALB/c mouse model of Panc-1 cells. | [130] |
| (b) | ||||||
| Acute myocardial infarction | Rat | Adipose stem cells | miRNA-126 | Transfection (miRNA-based/before isolation) | The treatment of AMI rats with miR-126-enriched exosomes decreased the infarction area in myocardial injury, inflammatory cytokine expression, and cardiac fibrosis. | [114] |
| Acute myocardial infarction | Rat | MSCs | Akt | Transfection (adenovirus-based/before isolation) | Exosomes derived from Akt-modified hucMSCs promoted angiogenesis, in which PDGF-D was involved in Akt-Exo-mediated angiogenesis. Additionally, they improved cardiac function in rats with AMI induced by LAD ligation. | [131] |
| Acute myocardial infarction | Rat | MSCs | TIMP2 protein | Transfection (lentivirus-based/before isolation) | Exosomes derived from huc-MSCs via the Akt/Sfrp2 pathway inhibited apoptosis in cardiomyocytes and promoted angiogenesis and ECM remodeling in ischemic myocardium. | [116] |
| Acute myocardial infarction | Mouse | MSCs | Stromal-derived factor 1 | Transfection (plasmid-based/before isolation) | Inhibition of ischemic myocardial cell autophagy and microvascular production of endothelial cells were promoted in MI mice treated with Exo-SDF10. | [115] |
| Myocardial ischemia–reperfusion injury | Rat | BMSCs | miRNA-125b | Transfection (miRNA-based/before isolation) | Injection of BM-MSCs-Exo-125b reduced pathological damage and decreased SIRT7 level expression in I/R rats model tissues. | [132] |
| Cerebral ischemia | Rat | MSCs | miRNA-223-3p | Transfection (lentivirus-based/before isolation) | Ischemic cortex and hippocampus MCAO/R surgery-mediated injury were treated by miR-223-3p-MSC-derived exosomes. | [133] |
| Cerebral ischemia | Mouse | BMSCs | Curcumin | Incubation (after isolation) | cRGD-Exo-cur suppressed inflammation by targeting NF-κB. | [134] |
| Cerebral ischemia | Mouse | BM-MSCs | miRNA-124 | Electroporation (after isolation) | Cortical neural progenitors were promoted by systemic administration of RVG-exosomes miR-124. Ischemia injury was attenuated by stimulating neurogenesis. | [139] |
| Cerebral ischemia–reperfusion injury | Mouse | MSCs | Curcumin | Incubation and freeze/thaw cycle (after isolation) | IR-injury mice treated by MESC-exocur showed a reduction in neurological score, oedema, astrogliosis, NDMAR1 expression, and inflammation. | [140] |
| Ageing-induced vascular dysfunction | Mouse | UMSCs | miRNA-675 | Transfection (miRNA-based/before isolation) | Targeting the TGF-β1/p21 pathway by miR-675 UC-MSCs exosomes prevented senescence, ischemic legs, and muscle ageing. | [135] |
| Osteoarthritis | Rat | SMSCs | miRNA-140-5p | Transfection (lentivirus-based/before isolation) | OA rat model treated with sMSC-140-Exos showed delayed early-stage OA progression by promoting chondrocyte proliferation and migration via the inhibition of SOX9 and ECM. | [136] |
| Rheumatoid arthritis | Mouse | MSCs | miRNA-150-5p | Transfection (plasmid-based/before isolation) | Inhibition of MMP-14 and TNF, driven by Exo-150-5p, decreased synovial inflammatory and joint damage in a CIA mouse model. | [137] |
| Intestinal fibrosis | Rat | BMSCs | miRNA-200b | Transfection (lentivirus-based/before isolation) | EMT remodeling and the target protein ZEB1/2 alleviated colon fibrosis via treatment of a rat model with miR-200-MVs. | [138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Draguet, F.; Bouland, C.; Dubois, N.; Bron, D.; Meuleman, N.; Stamatopoulos, B.; Lagneaux, L. Potential of Mesenchymal Stromal Cell-Derived Extracellular Vesicles as Natural Nanocarriers: Concise Review. Pharmaceutics 2023, 15, 558. https://doi.org/10.3390/pharmaceutics15020558
Draguet F, Bouland C, Dubois N, Bron D, Meuleman N, Stamatopoulos B, Lagneaux L. Potential of Mesenchymal Stromal Cell-Derived Extracellular Vesicles as Natural Nanocarriers: Concise Review. Pharmaceutics. 2023; 15(2):558. https://doi.org/10.3390/pharmaceutics15020558
Chicago/Turabian StyleDraguet, Florian, Cyril Bouland, Nathan Dubois, Dominique Bron, Nathalie Meuleman, Basile Stamatopoulos, and Laurence Lagneaux. 2023. "Potential of Mesenchymal Stromal Cell-Derived Extracellular Vesicles as Natural Nanocarriers: Concise Review" Pharmaceutics 15, no. 2: 558. https://doi.org/10.3390/pharmaceutics15020558
APA StyleDraguet, F., Bouland, C., Dubois, N., Bron, D., Meuleman, N., Stamatopoulos, B., & Lagneaux, L. (2023). Potential of Mesenchymal Stromal Cell-Derived Extracellular Vesicles as Natural Nanocarriers: Concise Review. Pharmaceutics, 15(2), 558. https://doi.org/10.3390/pharmaceutics15020558






