Recent Progress in Chitosan-Based Nanomedicine for Its Ocular Application in Glaucoma
Abstract
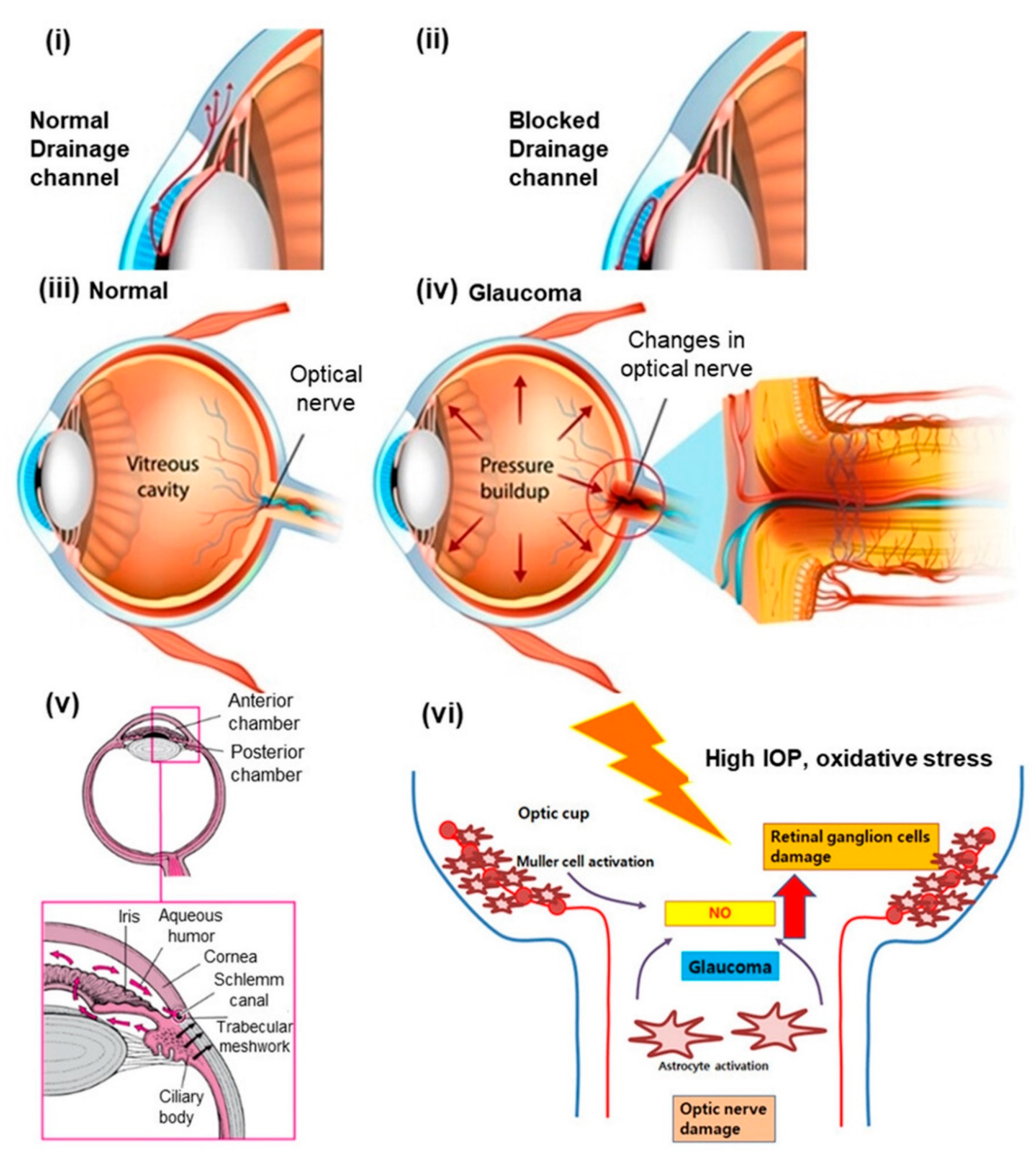
:1. Introduction
2. Significance of Chitosan-Based Nanomedicine to Overcome Drug Delivery Challenges in Glaucoma
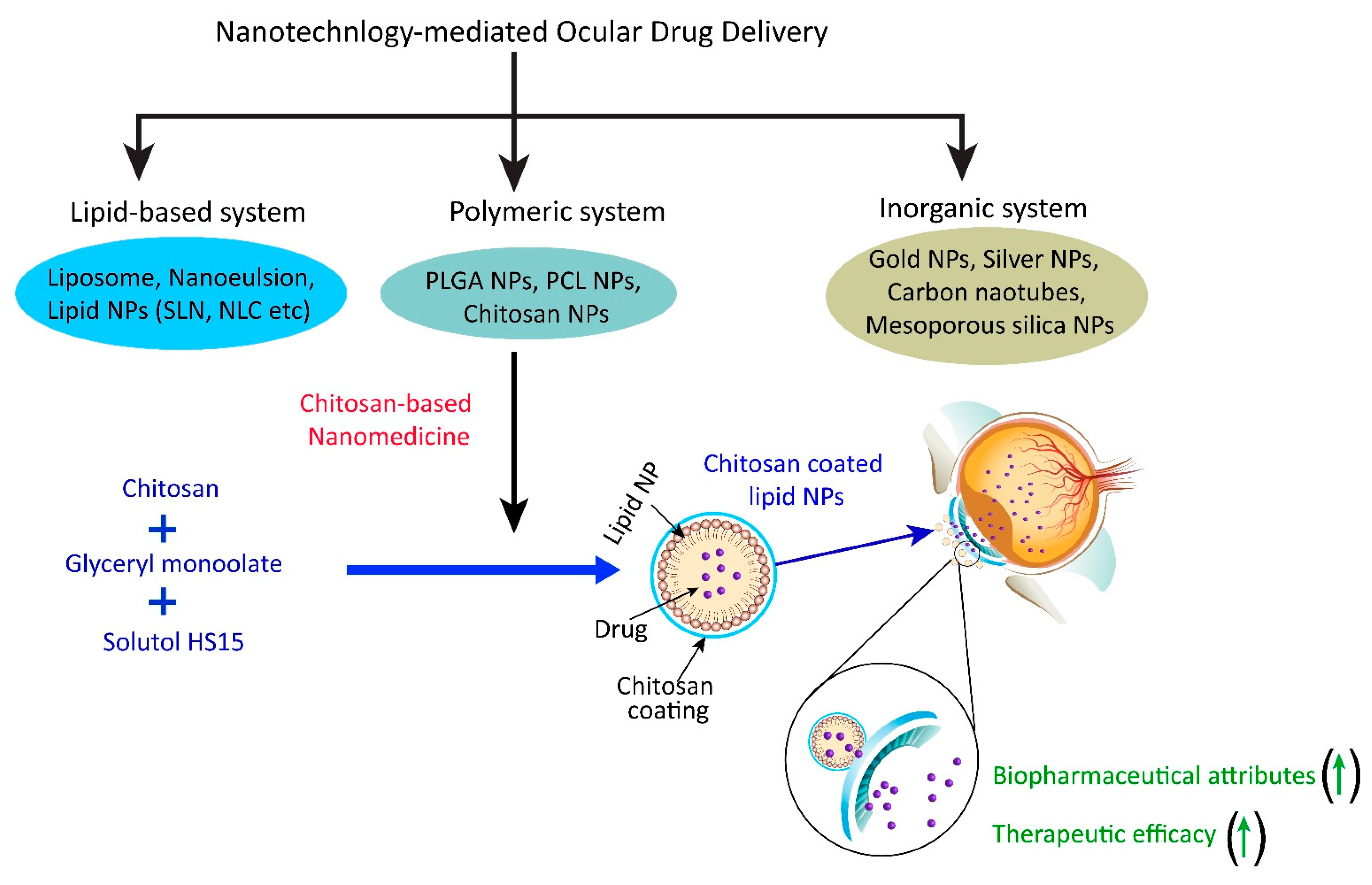
3. Different Types of Chitosan-Based Nanomedicine for Ocular Application
3.1. Chitosan Nanoparticles
3.2. Chitosan Coated Nanoformulation System
3.3. Chitosan-Based Hybrid Nanoparticles
4. Chitosan-Based Nanomedicine for Ocular Application in Glaucoma: Contemporary Research
4.1. Improvement in Biopharmaceutical Attributes of Loaded Drugs
4.2. Improvement in the Therapeutic Efficacy of Loaded Drugs
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wagner, I.V.; Stewart, M.W.; Dorairaj, S.K. Updates on the Diagnosis and Management of Glaucoma. Mayo Clin. Proc. Innov. Qual. Outcomes 2022, 6, 618–635. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zeng, W.; Wu, S.; Chen, X.; Zheng, T.; Ke, M. Measurement of retinal changes in primary acute angle closure Glaucoma under different durations of symptoms. J. Ophthalmol. 2019, 2019, 540983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Vargas JL, C.; Di Polo, A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef] [PubMed]
- Kumara, B.N.; Shambhu, R.; Prasad, K.S. Why chitosan could be apt candidate for glaucoma drug delivery–An overview. Int. J. Biol. Macromol. 2021, 176, 47–65. [Google Scholar] [CrossRef]
- Sim, R.H.; Sirasanagandla, S.R.; Das, S.; Teoh, S.L. Treatment of glaucoma with natural products and their mechanism of action: An update. Nutrients 2022, 14, 534. [Google Scholar] [CrossRef]
- Wadhwa, A.; Jadhav, C.; Yadav, K.S. Bimatoprost: Promising novel drug delivery systems in treatment of glaucoma. J. Drug Deliv. Sci. Technol. 2022, 69, 103156. [Google Scholar] [CrossRef]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.; Karla, P.K.; Boddu, S.H. Ocular drug delivery barriers—Role of nanocarriers in the treatment of anterior segment ocular diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Lanier, O.L.; Manfre, M.G.; Bailey, C.; Liu, Z.; Sparks, Z.; Kulkarni, S.; Chauhan, A. Review of approaches for increasing ophthalmic bioavailability for eye drop formulations. AAPS Pharmscitech 2021, 22, 107. [Google Scholar] [CrossRef]
- Gómez-Garzón, M.; Martínez-Ceballos, M.A.; Gómez-López, A.; Rojas-Villarraga, A. Application of nanotechnology in ophthalmology: Where are we? Charact. Appl. Nanomater. 2022, 5, 66–78. [Google Scholar] [CrossRef]
- Patel, K.D.; Silva, L.B.; Park, Y.; Shakouri, T.; Keskin-Erdogan, Z.; Sawadkar, P.; Kim, H.W. Recent advances in drug delivery systems for glaucoma treatment. Mater. Today Nano 2022, 18, 100178. [Google Scholar] [CrossRef]
- Sahu, T.; Ratre, Y.K.; Chauhan, S.; Bhaskar LV, K.S.; Nair, M.P.; Verma, H.K. Nanotechnology based drug delivery system: Current strategies and emerging therapeutic potential for medical science. J. Drug Deliv. Sci. Technol. 2021, 63, 102487. [Google Scholar] [CrossRef]
- Khiev, D.; Mohamed, Z.A.; Vichare, R.; Paulson, R.; Bhatia, S.; Mohapatra, S.; Biswal, M.R. Emerging nano-formulations and nanomedicines applications for ocular drug delivery. Nanomaterials 2021, 11, 173. [Google Scholar] [CrossRef]
- Jain, A.; Prajapati, S.K.; Kumari, A.; Mody, N.; Bajpai, M. Engineered nanosponges as versatile biodegradable carriers: An insight. J. Drug Deliv. Sci. Technol. 2020, 57, 101643. [Google Scholar] [CrossRef]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Struct. Nano-Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Buse, J.; El-Aneed, A. Properties, engineering and applications of lipid-based nanoparticle drug-delivery systems: Current research and advances. Nanomedicine 2010, 5, 1237–1260. [Google Scholar] [CrossRef]
- Kraft, J.C.; Freeling, J.P.; Wang, Z.; Ho, R.J. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J. Pharm. Sci. 2014, 103, 29–52. [Google Scholar] [CrossRef] [Green Version]
- Ojea-Jimenez, I.; Comenge, J.; Garcia-Fernandez, L.; Megson, Z.A.; Casals, E.; Puntes, V.F. Engineered inorganic nanoparticles for drug delivery applications. Curr. Drug Metab. 2013, 14, 518–530. [Google Scholar] [CrossRef] [Green Version]
- Vaneev, A.; Tikhomirova, V.; Chesnokova, N.; Popova, E.; Beznos, O.; Kost, O.; Klyachko, N. Nanotechnology for topical drug delivery to the anterior segment of the eye. Int. J. Mol. Sci. 2021, 22, 12368. [Google Scholar] [CrossRef]
- Han, X.; Wang, C.; Liu, Z. Red blood cells as smart delivery systems. Bioconj. Chem. 2018, 29, 852–860. [Google Scholar] [CrossRef]
- Li, Y.; Raza, F.; Liu, Y.; Wei, Y.; Rong, R.; Zheng, M.; Qiu, M. Clinical progress and advanced research of red blood cells based drug delivery system. Biomaterials 2021, 279, 121202. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Irimia, T.; Ghica, M.V.; Popa, L.; Anuţa, V.; Arsene, A.L.; Dinu-Pîrvu, C.E. Strategies for improving ocular drug bioavailability and corneal wound healing with chitosan-based delivery systems. Polymers 2018, 10, 1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burhan, A.M.; Klahan, B.; Cummins, W.; Andrés-Guerrero, V.; Byrne, M.E.; O’reilly, N.J.; Hughes, H. Posterior segment ophthalmic drug delivery: Role of muco-adhesion with a special focus on chitosan. Pharmaceutics 2021, 13, 1685. [Google Scholar] [CrossRef] [PubMed]
- Fong, D.; Hoemann, C.D. Chitosan immunomodulatory properties: Perspectives on the impact of structural properties and dosage. Future Sci. OA 2018, 4, FSO225. [Google Scholar] [CrossRef] [Green Version]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E.; King, M.W. Chitosan based bioadhesives for biomedical applications: A review. Carbohydr. Polym. 2022, 282, 119100. [Google Scholar] [CrossRef]
- Silva, B.; São Braz, B.; Delgado, E.; Gonçalves, L. Colloidal nanosystems with mucoadhesive properties designed for ocular topical delivery. Int. J. Pharm. 2021, 606, 120873. [Google Scholar] [CrossRef]
- Sun, X.; Sheng, Y.; Li, K.; Sai, S.; Feng, J.; Li, Y.; Tian, B. Mucoadhesive phenylboronic acid conjugated chitosan oligosaccharide-vitamin E copolymer for topical ocular delivery of voriconazole: Synthesis, in vitro/vivo evaluation, and mechanism. Acta Biomater. 2022, 138, 193–207. [Google Scholar] [CrossRef]
- Jhaveri, J.; Raichura, Z.; Khan, T.; Momin, M.; Omri, A. Chitosan nanoparticles-insight into properties, functionalization and applications in drug delivery and theranostics. Molecules 2021, 26, 272. [Google Scholar] [CrossRef]
- Zamboulis, A.; Nanaki, S.; Michailidou, G.; Koumentakou, I.; Lazaridou, M.; Ainali, N.M.; Bikiaris, D.N. Chitosan and its derivatives for ocular delivery formulations: Recent advances and developments. Polymers 2020, 12, 1519. [Google Scholar] [CrossRef]
- Luo, L.J.; Huang, C.C.; Chen, H.C.; Lai, J.Y.; Matsusaki, M. Effect of deacetylation degree on controlled pilocarpine release from injectable chitosan-g-poly (N-isopropylacrylamide) carriers. Carbohydr. Polym. 2018, 197, 375–384. [Google Scholar] [CrossRef]
- Luo, Q.; Zhao, J.; Zhang, X.; Pan, W. Nanostructured lipid carrier (NLC) coated with Chitosan Oligosaccharides and its potential use in ocular drug delivery system. Int. J. Pharm. 2011, 403, 185–191. [Google Scholar] [CrossRef]
- Yanat, M.; Schroën, K. Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React. Funct. Polym. 2021, 161, 104849. [Google Scholar] [CrossRef]
- Mohamed, H.B.; Attia Shafie, M.A.; Mekkawy, A.I. Chitosan Nanoparticles for Meloxicam Ocular Delivery: Development, In Vitro Characterization, and In Vivo Evaluation in a Rabbit Eye Model. Pharmaceutics 2022, 14, 893. [Google Scholar] [CrossRef]
- Ricci, F.; Racaniello, G.F.; Lopedota, A.; Laquintana, V.; Arduino, I.; Lopalco, A.; Denora, N. Chitosan/sulfobutylether-β-cyclodextrin based nanoparticles coated with thiolated hyaluronic acid for indomethacin ophthalmic delivery. Int. J. Pharm. 2022, 622, 121905. [Google Scholar] [CrossRef]
- Kalam, M.A. Development of chitosan nanoparticles coated with hyaluronic acid for topical ocular delivery of dexamethasone. Int. J. Biol. Macromol. 2016, 89, 127–136. [Google Scholar] [CrossRef]
- Nagarwal, R.C.; Kumar, R.; Pandit, J.K. Chitosan coated sodium alginate–chitosan nanoparticles loaded with 5-FU for ocular delivery: In vitro characterization and in vivo study in rabbit eye. Eur. J. Pharm. Sci. 2012, 47, 678–685. [Google Scholar] [CrossRef]
- Wang, F.Z.; Zhang, M.W.; Zhang, D.S.; Huang, Y.; Chen, L.; Jiang, S.M.; Li, R. Preparation, optimization, and characterization of chitosan-coated solid lipid nanoparticles for ocular drug delivery. J. Biomed. Res. 2018, 32, 411. [Google Scholar]
- Badran, M.M.; Alomrani, A.H.; Almomen, A.; Bin Jardan, Y.A.; Abou El Ela, A.E.S. Novel Metoprolol-Loaded Chitosan-Coated Deformable Liposomes in Thermosensitive In Situ Gels for the Management of Glaucoma: A Repurposing Approach. Gels 2022, 8, 635. [Google Scholar] [CrossRef]
- Roy, S.; Goh, K.L.; Verma, C.; Dasgupta Ghosh, B.; Sharma, K.; Maji, P.K. A Facile Method for Processing Durable and Sustainable Superhydrophobic Chitosan-Based Coatings Derived from Waste Crab Shell. ACS Sustain. Chem. Eng. 2022, 10, 4694–4704. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Yao, C.H.; Lue, S.J.; Yang, C.J.; Su, Y.H.; Huang, C.C.; Lai, J.Y. Amination-mediated nano eye-drops with enhanced corneal permeability and effective burst release for acute glaucoma treatment. Chem. Eng. J. 2023, 451, 138620. [Google Scholar] [CrossRef]
- Jiao, J.; Li, X.; Zhang, S.; Liu, J.; Di, D.; Zhang, Y.; Wang, S. Redox and pH dual-responsive PEG and chitosan-conjugated hollow mesoporous silica for controlled drug release. Mater. Sci. Eng. C 2016, 67, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Hirst, S.M.; Karakoti, A.S.; Tyler, R.D.; Sriranganathan, N.; Seal, S.; Reilly, C.M. Anti-inflammatory properties of cerium oxide nanoparticles. Small 2009, 5, 2848–2856. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.; Li, F.; Chen, H.; Wang, Y.; Zhu, Y.; Yang, X.; Zhuo, Y. Caspase-8 promotes NLRP1/NLRP3 inflammasome activation and IL-1β production in acute glaucoma. Proc. Natl. Acad. Sci. USA 2014, 111, 11181–11186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richard, S.; Boucher, M.; Saric, A.; Herbet, A.; Lalatonne, Y.; Petit, P.X.; Motte, L. Optimization of pegylated iron oxide nanoplatforms for antibody coupling and bio-targeting. J. Mater. Chem. B 2017, 5, 2896–2907. [Google Scholar] [CrossRef]
- Radwan SE, S.; El-Moslemany, R.M.; Mehanna, R.A.; Thabet, E.H.; Abdelfattah EZ, A.; El-Kamel, A. Chitosan-coated bovine serum albumin nanoparticles for topical tetrandrine delivery in glaucoma: In vitro and in vivo assessment. Drug Deliv. 2022, 29, 1150–1163. [Google Scholar] [CrossRef]
- Raj, R.; Wairkar, S.; Sridhar, V.; Gaud, R. Pramipexole dihydrochloride loaded chitosan nanoparticles for nose to brain delivery: Development, characterization and in vivo anti-Parkinson activity. Int. J. Biol. Macromol. 2018, 109, 27–35. [Google Scholar] [CrossRef]
- Piazzini, V.; Landucci, E.; D’Ambrosio, M.; Fasiolo, L.T.; Cinci, L.; Colombo, G.; Bergonzi, M.C. Chitosan coated human serum albumin nanoparticles: A promising strategy for nose-to-brain drug delivery. Int. J. Biol. Macromol. 2019, 129, 267–280. [Google Scholar] [CrossRef]
- Khan, N.; Khanna, K.; Bhatnagar, A.; Ahmad, F.J.; Ali, A. Chitosan coated PLGA nanoparticles amplify the ocular hypotensive effect of forskolin: Statistical design, characterization and in vivo studies. Int. J. Biol. Macromol. 2018, 116, 648–663. [Google Scholar] [CrossRef]
- Jiang, P.; Jacobs, K.M.; Ohr, M.P.; Swindle-Reilly, K.E. Chitosan–polycaprolactone core–shell microparticles for sustained delivery of bevacizumab. Mol. Pharm. 2020, 17, 2570–2584. [Google Scholar] [CrossRef]
- Silva, B.; Marto, J.; São Braz, B.; Delgado, E.; Almeida, A.J.; Gonçalves, L. New nanoparticles for topical ocular delivery of erythropoietin. Int. J. Pharm. 2020, 576, 119020. [Google Scholar] [CrossRef]
- Saha, M.; Saha, D.R.; Ulhosna, T.; Sharker, S.M.; Shohag, M.H.; Islam, M.S.; Reza, H.M. QbD based development of resveratrol-loaded mucoadhesive lecithin/chitosan nanoparticles for prolonged ocular drug delivery. J. Drug Deliv. Sci. Technol. 2021, 63, 102480. [Google Scholar] [CrossRef]
- Hafner, A.; Lovrić, J.; Voinovich, D.; Filipović-Grčić, J. Melatonin-loaded lecithin/chitosan nanoparticles: Physicochemical characterisation and permeability through Caco-2 cell monolayers. Int. J. Pharm. 2009, 381, 205–213. [Google Scholar] [CrossRef]
- Tan, Q.; Liu, W.; Guo, C.; Zhai, G. Preparation and evaluation of quercetin-loaded lecithin-chitosan nanoparticles for topical delivery. Int. J. Nanomed. 2011, 6, 1621. [Google Scholar]
- Liu, L.; Zhou, C.; Xia, X.; Liu, Y. Self-assembled lecithin/chitosan nanoparticles for oral insulin delivery: Preparation and functional evaluation. Int. J. Nanomed. 2016, 11, 761. [Google Scholar] [CrossRef] [Green Version]
- Özcan, İ.; Azizoğlu, E.; Şenyiğit, T.; Özyazıcı, M.; Özer, Ö. Enhanced dermal delivery of diflucortolone valerate using lecithin/chitosan nanoparticles: In-vitro and in-vivo evaluations. Int. J. Nanomed. 2013, 8, 461. [Google Scholar] [CrossRef] [Green Version]
- Chu, X.Y.; Huang, W.; Wang, Y.L.; Meng, L.W.; Chen, L.Q.; Jin, M.J.; Gao, C.S. Improving antitumor outcomes for palliative intratumoral injection therapy through lecithin–chitosan nanoparticles loading paclitaxel–cholesterol complex. Int. J. Nanomed. 2019, 14, 689. [Google Scholar] [CrossRef] [Green Version]
- Katiyar, S.; Pandit, J.; Mondal, R.S.; Mishra, A.K.; Chuttani, K.; Aqil, M.; Sultana, Y. In situ gelling dorzolamide loaded chitosan nanoparticles for the treatment of glaucoma. Carbohydr. Polym. 2014, 102, 117–124. [Google Scholar] [CrossRef]
- Wadhwa, S.; Paliwal, R.; Paliwal, S.R.; Vyas, S.P. Hyaluronic acid modified chitosan nanoparticles for effective management of glaucoma: Development, characterization, and evaluation. J. Drug Target. 2010, 18, 292–302. [Google Scholar] [CrossRef]
- Li, J.; Tian, S.; Tao, Q.; Zhao, Y.; Gui, R.; Yang, F.; Hou, D. Montmorillonite/chitosan nanoparticles as a novel controlled-release topical ophthalmic delivery system for the treatment of glaucoma. Int. J. Nanomed. 2018, 13, 3975. [Google Scholar] [CrossRef] [Green Version]
- Abruzzo, A.; Giordani, B.; Miti, A.; Vitali, B.; Zuccheri, G.; Cerchiara, T.; Bigucci, F. Mucoadhesive and mucopenetrating chitosan nanoparticles for glycopeptide antibiotic administration. Int. J. Pharm. 2021, 606, 120874. [Google Scholar] [CrossRef]
- Li, W.; Yang, C.; Lu, J.; Huang, P.; Barnstable, C.J.; Zhang, C.; Zhang, S.S. Tetrandrine protects mouse retinal ganglion cells from ischemic injury. Drug Des. Dev. Ther. 2014, 8, 327–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Jin, X.; Zhang, L.; Yang, Y.; Liu, R.; Li, Z. Comparison of different chitosan lipid nanoparticles for improved ophthalmic tetrandrine delivery: Formulation, characterization, pharmacokinetic and molecular dynamics simulation. J. Pharm. Sci. 2020, 109, 3625–3635. [Google Scholar] [CrossRef] [PubMed]
- Wenling, C.; Duohui, J.; Jiamou, L.; Yandao, G.; Nanming, Z.; Xiufang, Z. Effects of the degree of deacetylation on the physicochemical properties and Schwann cell affinity of chitosan films. J. Biomater. Appl. 2005, 20, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.C.; Jabrail, F.H. Effects of degree of deacetylation and cross-linking on physical characteristics, swelling and release behavior of chitosan microspheres. Carbohydr. Polym. 2006, 66, 43–54. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Rubenicia AM, L.; Cubillan, L.D.; Sicam VA, D.; Macabeo AP, G.; Villaflores, O.B.; Castillo, A.L. Intraocular pressure reduction effect of 0.005% latanoprost eye drops in a hyaluronic acid-chitosan nanoparticle drug delivery system in albino rabbits. Transl. Vis. Sci. Technol. 2021, 10, 2. [Google Scholar] [CrossRef]
- Silva, B.; Gonçalves, L.M.; Braz, B.S.; Delgado, E. Chitosan and Hyaluronic Acid Nanoparticles as Vehicles of Epoetin Beta for Subconjunctival Ocular Delivery. Mar. Drugs 2022, 20, 151. [Google Scholar] [CrossRef]
- Shahab, M.S.; Rizwanullah, M.; Alshehri, S.; Imam, S.S. Optimization to development of chitosan decorated polycaprolactone nanoparticles for improved ocular delivery of dorzolamide: In vitro, ex vivo and toxicity assessments. Int. J. Biol. Macromol. 2020, 163, 2392–2404. [Google Scholar] [CrossRef]
- Dubey, V.; Mohan, P.; Dangi, J.S.; Kesavan, K. Brinzolamide loaded chitosan-pectin mucoadhesive nanocapsules for management of glaucoma: Formulation, characterization and pharmacodynamic study. Int. J. Biol. Macromol. 2020, 152, 1224–1232. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hoseini, S.J.; Hamzehlou, S.; Darroudi, M.; Verdi, J.; Mozafari, M. Biomedical applications of nanoceria: New roles for an old player. Nanomedicine 2018, 13, 3051–3069. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.Y.; Song, S.Y.; Go, S.H.; Sohn, H.S.; Baik, S.; Hyeon, T. Synergistic oxygen generation and reactive oxygen species scavenging by manganese ferrite/ceria co-decorated nanoparticles for rheumatoid arthritis treatment. ACS Nano 2019, 13, 3206–3217. [Google Scholar] [CrossRef]
- Lese, I.; Graf, D.A.; Tsai, C.; Taddeo, A.; Matter, M.T.; Constantinescu, M.A.; Olariu, R. Bioactive nanoparticle-based formulations increase survival area of perforator flaps in a rat model. PLoS ONE 2018, 13, e0207802. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Chung, S.J.; Nafiujjaman, M.; Hill, M.L.; Siziba, M.E.; Contag, C.H.; Kim, T. Ceria-based nanotheranostic agent for rheumatoid arthritis. Theranostics 2020, 10, 11863. [Google Scholar] [CrossRef]
- Lai, J.L.; Liu, Y.H.; Liu, C.; Qi, M.P.; Liu, R.N.; Zhu, X.F.; Hu, C.M. Indirubin inhibits LPS-induced inflammation via TLR4 abrogation mediated by the NF-kB and MAPK signaling pathways. Inflammation 2017, 40, 1–12. [Google Scholar] [CrossRef]
- Lee, J.C.; Kassis, S.; Kumar, S.; Badger, A.; Adams, J.L. p38 mitogen-activated protein kinase inhibitors—Mechanisms and therapeutic potentials. Pharmacol. Ther. 1999, 82, 389–397. [Google Scholar] [CrossRef]
- Doucette, L.P.; Walter, M.A. Prostaglandins in the eye: Function, expression, and roles in glaucoma. Ophthalmic Genet. 2017, 38, 108–116. [Google Scholar] [CrossRef]
- Bensinger, R.; Shin, D.H.; Kass, M.A.; Podos, S.M.; Becker, B. Pilocarpine ocular inserts. Investig. Ophthalmol. Vis. Sci. 1976, 15, 1008–1010. [Google Scholar]
- Huang, P.; Xu, Y.; Wei, R.; Li, H.; Tang, Y.; Liu, J.; Zhang, C. Efficacy of tetrandrine on lowering intraocular pressure in animal model with ocular hypertension. J. Glaucoma 2011, 20, 183–188. [Google Scholar] [CrossRef]
- Abdelmonem, R.; Elhabal, S.F.; Abdelmalak, N.S.; El-Nabarawi, M.A.; Teaima, M.H. Formulation and characterization of acetazolamide/carvedilol niosomal gel for glaucoma treatment: In vitro, and in vivo study. Pharmaceutics 2021, 13, 221. [Google Scholar] [CrossRef]
- Amal El Sayeh, F.; El Khatib, M.M. Formulation and evaluation of new long acting metoprolol tartrate ophthalmic gels. Saudi Pharm. J. 2014, 22, 555–563. [Google Scholar]
- Popova, E.V.; Tikhomirova, V.E.; Beznos, O.V.; Chesnokova, N.B.; Grigoriev, Y.V.; Klyachko, N.L.; Kost, O.A. Chitosan-covered calcium phosphate particles as a drug vehicle for delivery to the eye. Nanomed. Nanotechnol. Biol. Med. 2022, 40, 102493. [Google Scholar] [CrossRef] [PubMed]
- Gounani, Z.; Asadollahi, M.A.; Pedersen, J.N.; Lyngsø, J.; Pedersen, J.S.; Arpanaei, A.; Meyer, R.L. Mesoporous silica nanoparticles carrying multiple antibiotics provide enhanced synergistic effect and improved biocompatibility. Colloids Surf. B Biointerfaces 2019, 175, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhang, M.; Huang, X.; Chen, B.; Ding, Y.; Zhang, Y.; Kim, I. Smart l-borneol-loaded hierarchical hollow polymer nanospheres with antipollution and antibacterial capabilities. Mater. Today Chem. 2022, 26, 101252. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, W.; Lu, Y.; Xu, Y.; Wang, C.; Yu, D.G.; Kim, I. Recent advances in poly (α-L-glutamic acid)-based nanomaterials for drug delivery. Biomolecules 2022, 12, 636. [Google Scholar] [CrossRef]








| Conventional Ocular Drug Delivery | Nanotechnology-Mediated Ocular Drug Delivery |
|---|---|
| Limited aqueous solubility | Improved aqueous solubility |
| Limited ocular/corneal permeability | Improved ocular/corneal permeability |
| Immediate effects | Sustained/prolong effects |
| Nonspecific | Specific |
| Low bioavailability and intersubject variability | Improved bioavailability and minimized intersubject variability |
| Limited drug efficacy | Improved drug efficacy |
| Possibility of untoward effects | Minimized possibility of untoward effects |
| Type of Nanomedicine | Therapeutics | Composition | Biopharmaceutical Attributes | Ref. |
|---|---|---|---|---|
| Chitosan-coated NPs | Metoprolol | Chitosan, phosphatidylcholine, cholesterol |
| [38] |
| Chitosan-coated NPs | Pilocarpine | Chitosan, silica, ethylene glycol, cerium nitrate |
| [40] |
| Chitosan-coated NPs | Tetrandrine | Chitosan, bovine serum albumin, glutaraldehyde |
| [45] |
| Chitosan-coated NPs | Tetrandrine | Chitosan, glyceryl monooleate, poloxamer 407, kolliphor® HS 15 |
| [62] |
| Chitosan-based hybrid NPs | Latanoprost | Chitosan, hyaluronic acid, sodium tripolyphosphate |
| [66] |
| Chitosan-based hybrid NPs | Epoetin beta (EPOβ) | Chitosan and hyaluronic acid |
| [67] |
| Chitosan-based hybrid NPs | Dorzolamide | Chitosan, polycaprolactone, polyvinyl alcohol |
| [68] |
| Chitosan-based hybrid NPs | Brinzolamide | Chitosan, pectin, Tween 80 |
| [69] |
| Type of Nanomedicine | Therapeutics | In Vivo Model | Pharmacodynamics Performance | Ref. |
|---|---|---|---|---|
| Chitosan-coated NPs | Metoprolol | Albino rabbits | Developed system exhibited a 73.6% decrease in IOP compared to a 54.7% decrease in IOP by the pure drug in a thermosensitive in situ gel after 6 h of ocular administration. | [38] |
| Chitosan-coated NPs | Pilocarpine | Acute glaucoma rabbit model | Developed system highly effective in decreasing the extremely high IOP (92 mmHg) to a normal level (20 mmHg) until 4 h of instillation. | [40] |
| Chitosan-coated NPs | Tetrandrine | Rabbits | Developed system exhibited a 49.35% decrease in IOP compared to a 25.1% decrease in IOP by a pure drug after 4 h of ocular administration. | [45] |
| Chitosan-based hybrid NPs | Latanoprost | Normotensive albino rabbits | A developed system is more effective in reducing the IOP than by a drug alone.IOP reduction during the treatment period was 27.3% by the developed chitosan-based system compared to 19.3% and 20.3% for the plain latanoprost and marketed product (Xalatan), respectively. | [66] |
| Chitosan-based hybrid NPs | Brinzolamide | Albino rabbits | Developed system exhibited significant improvement in % decrease in IOP and prolonged IOP lowering effect compared to the marketed product. | [69] |
| Chitosan-based hybrid NPs | Enalaprilat | Normotensive rabbits | Chitosan-calcium phosphate hybrid system exhibited a significant decrease in IOP after single instillation compared to enalaprilat in solution. | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albarqi, H.A.; Garg, A.; Ahmad, M.Z.; Alqahtani, A.A.; Walbi, I.A.; Ahmad, J. Recent Progress in Chitosan-Based Nanomedicine for Its Ocular Application in Glaucoma. Pharmaceutics 2023, 15, 681. https://doi.org/10.3390/pharmaceutics15020681
Albarqi HA, Garg A, Ahmad MZ, Alqahtani AA, Walbi IA, Ahmad J. Recent Progress in Chitosan-Based Nanomedicine for Its Ocular Application in Glaucoma. Pharmaceutics. 2023; 15(2):681. https://doi.org/10.3390/pharmaceutics15020681
Chicago/Turabian StyleAlbarqi, Hassan A., Anuj Garg, Mohammad Zaki Ahmad, Abdulsalam A. Alqahtani, Ismail A. Walbi, and Javed Ahmad. 2023. "Recent Progress in Chitosan-Based Nanomedicine for Its Ocular Application in Glaucoma" Pharmaceutics 15, no. 2: 681. https://doi.org/10.3390/pharmaceutics15020681
APA StyleAlbarqi, H. A., Garg, A., Ahmad, M. Z., Alqahtani, A. A., Walbi, I. A., & Ahmad, J. (2023). Recent Progress in Chitosan-Based Nanomedicine for Its Ocular Application in Glaucoma. Pharmaceutics, 15(2), 681. https://doi.org/10.3390/pharmaceutics15020681







