Design, Characterization and Pharmacokinetic–Pharmacodynamic Evaluation of Poloxamer and Kappa-Carrageenan-Based Dual-Responsive In Situ Gel of Nebivolol for Treatment of Open-Angle Glaucoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Method for Analysis of NEB
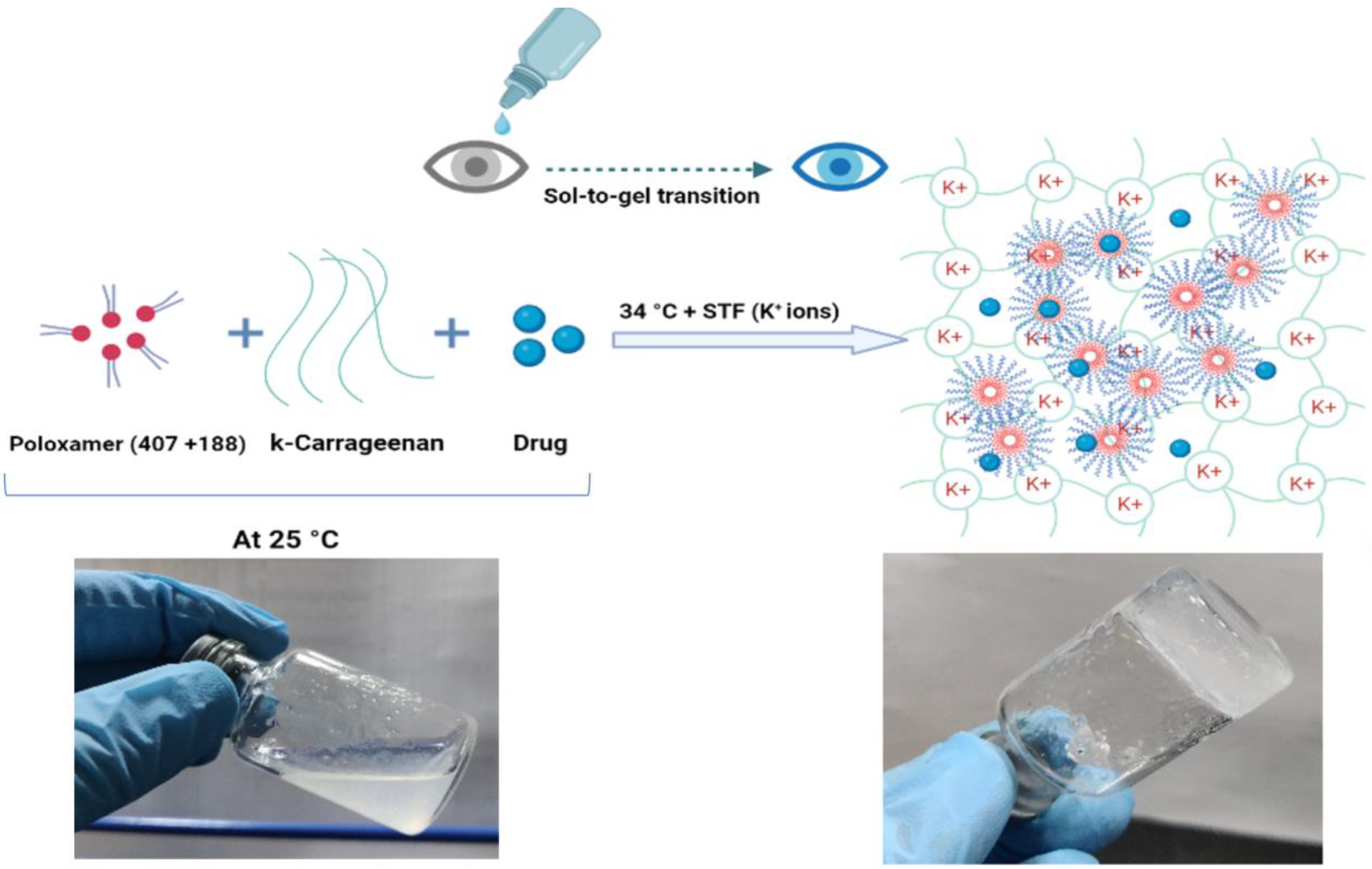
2.3. Preparation and Optimization of NEB-Loaded Dual-Responsive In Situ Gel
2.3.1. Preparation of NEB-Loaded Dual-Responsive In Situ Gel
2.3.2. Optimization of NEB-Loaded Dual-Responsive In Situ Gel
2.4. Characterization of Blank and NEB-Loaded Dual-Responsive In Situ Gels
2.4.1. Determination of Gelling Temperature and Solution State Viscosity of the In Situ Gels
2.4.2. Physical Appearance, pH and Drug Content of Optimized NEB-Loaded Dual-Responsive In Situ Gel
2.5. Rheological Study of Blank and NEB-Loaded Dual-Responsive In Situ Gels
2.6. Mucoadhesion Studies of NEB-Loaded In Situ Gels
2.7. In Vitro Drug Release Studies of NEB-Loaded In Situ Gels
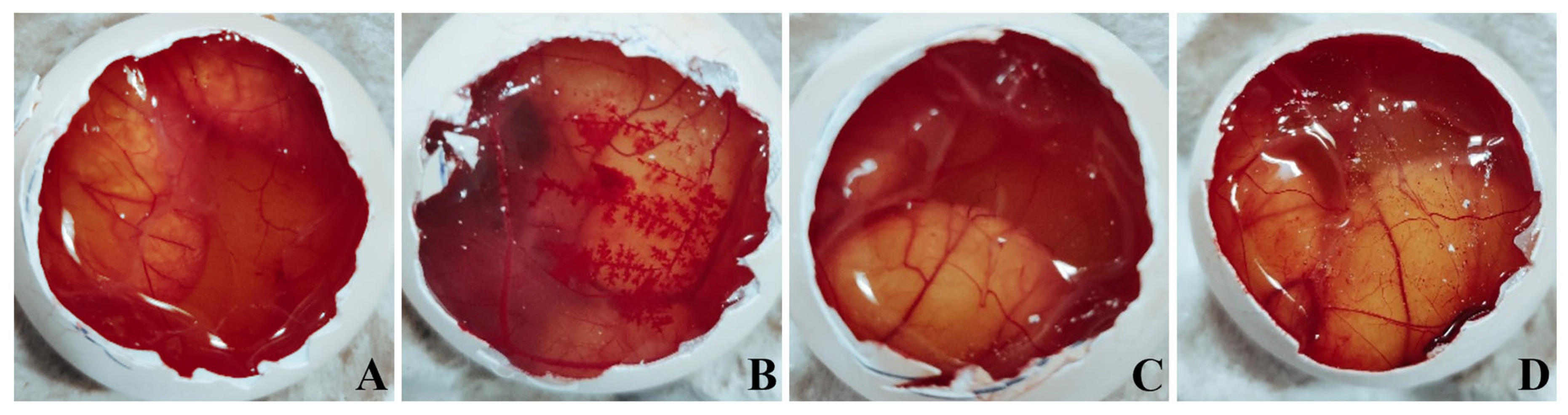
2.8. Ex Vivo Ocular Irritation Test (HET-CAM) of the Optimized In Situ Gels
2.9. Hemolysis Study of the Optimized In Situ Gels
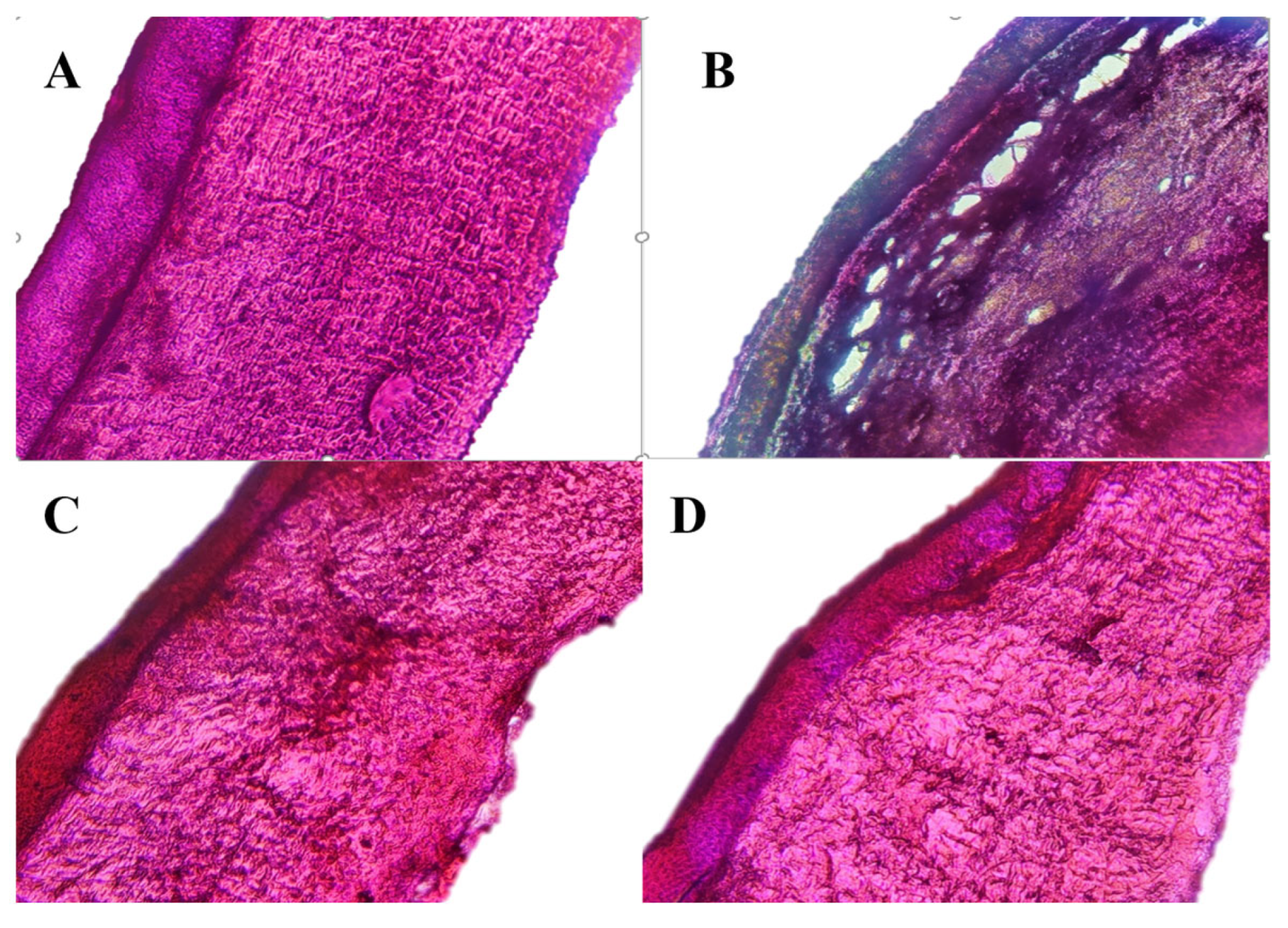
2.10. Ocular Histopathology Studies of In Situ Gels
2.11. In Vivo Studies of the Optimized NEB-Loaded Dual-Responsive In Situ Gel
2.11.1. Ocular Pharmacokinetic Studies
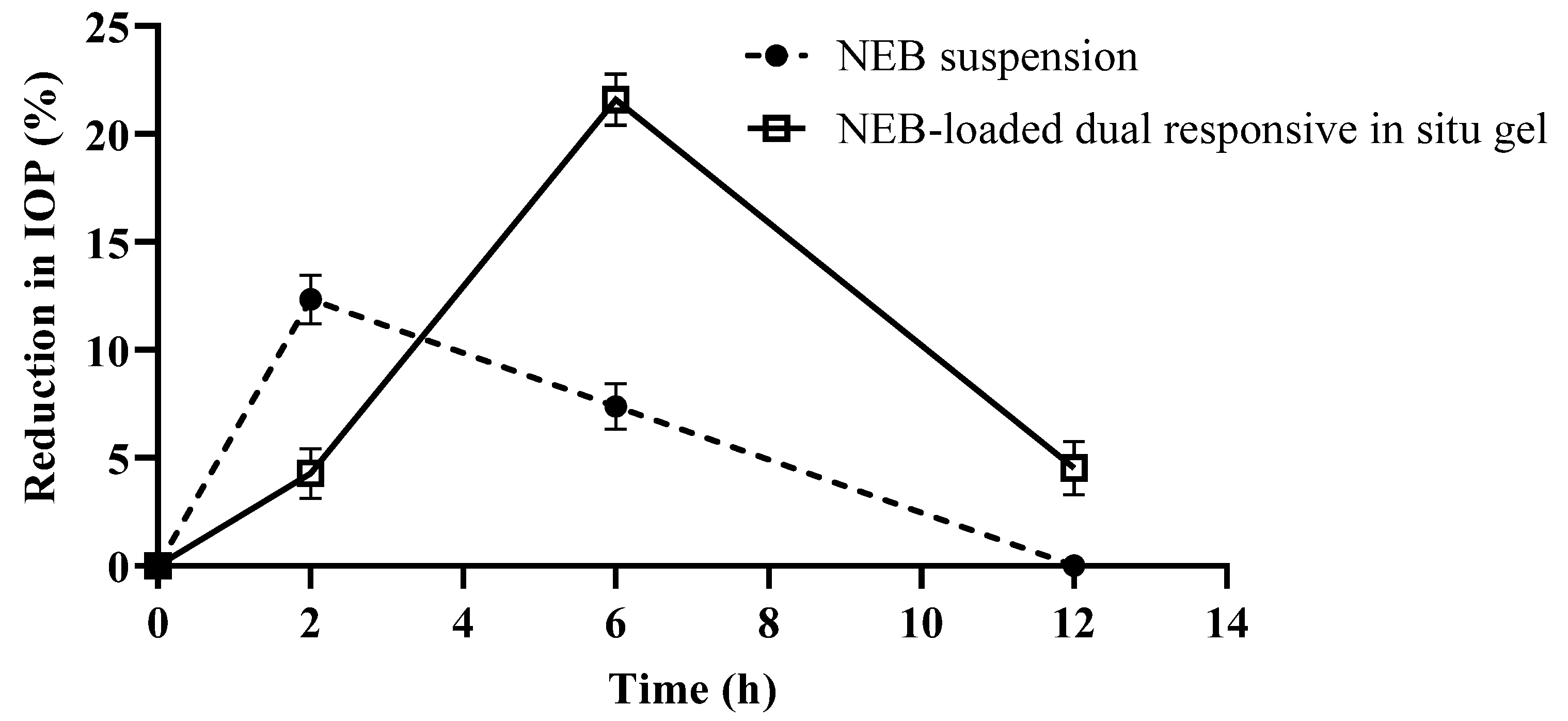
2.11.2. Pharmacodynamic Studies
3. Results
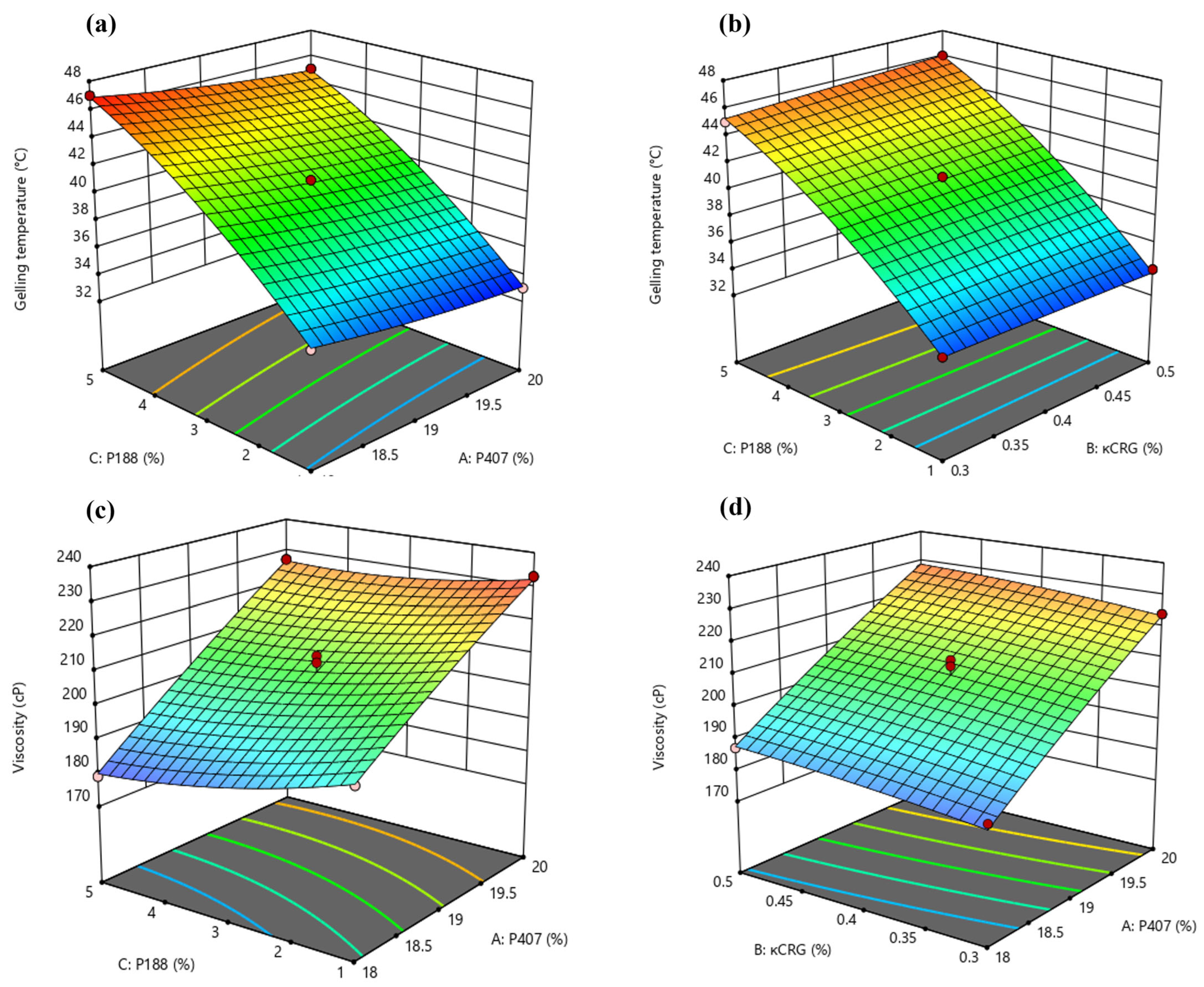
3.1. Optimization of Dual-Responsive In Situ Gels Using BBD
3.1.1. Effect of Critical Formulation Factors on the Gelling Temperature (Y1) of In Situ Gels
3.1.2. Effect of Critical Formulation Factors on the Solution State Viscosity () of In Situ Gels
3.1.3. Identification of Optimized Conditions Using Desirability Function
3.2. Characterization of Optimized NEB-Loaded Dual-Responsive In Situ Gel
3.2.1. Gelling Temperature and Solution State Viscosity
3.2.2. Physical Appearance, pH, Osmolarity and Drug Content of the Optimized Dual-Responsive In Situ Gels
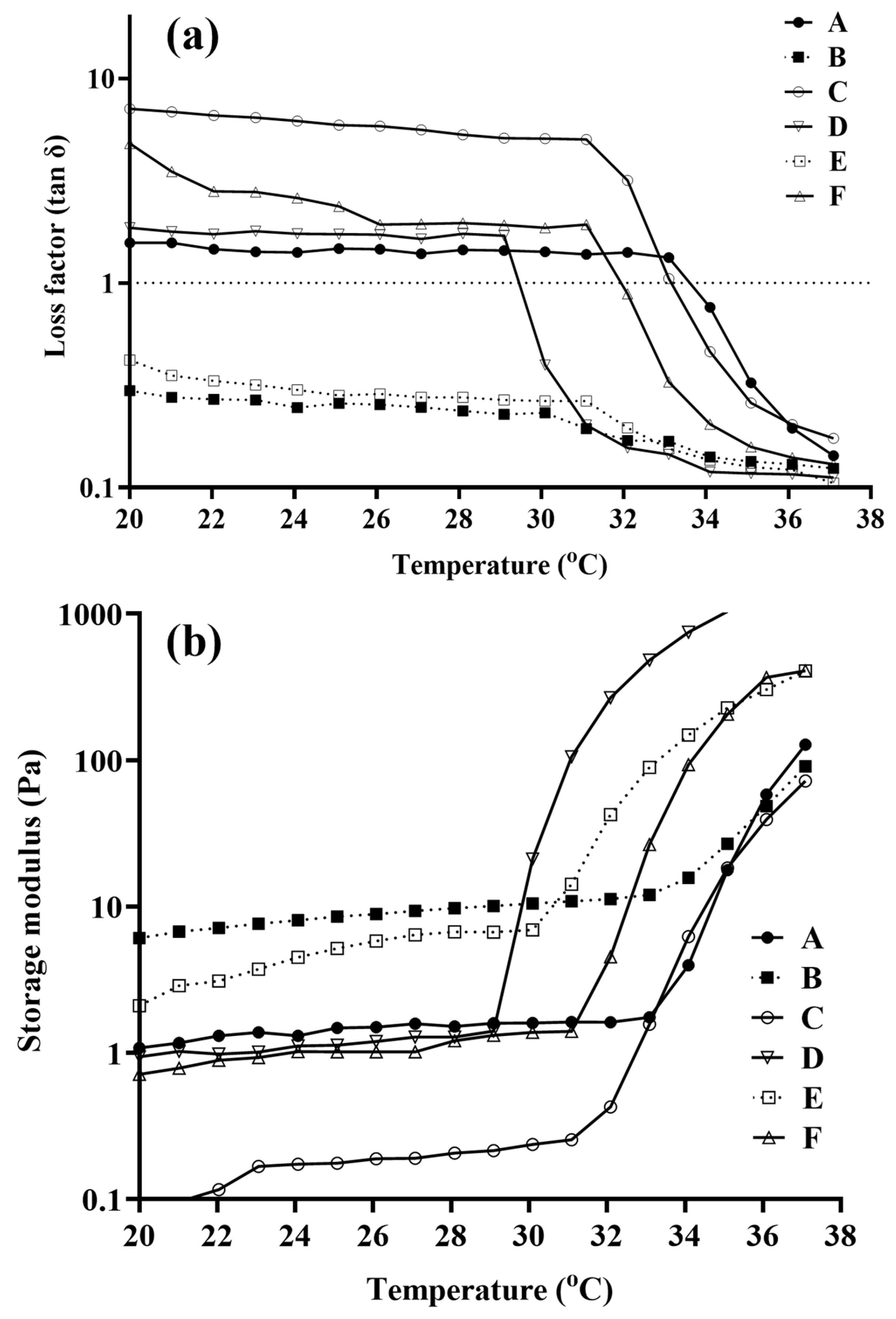
3.3. Rheological Studies of Blank and NEB-Loaded Dual-Responsive In Situ Gels
3.4. Mucoadhesion Study of the Blank In Situ Gels
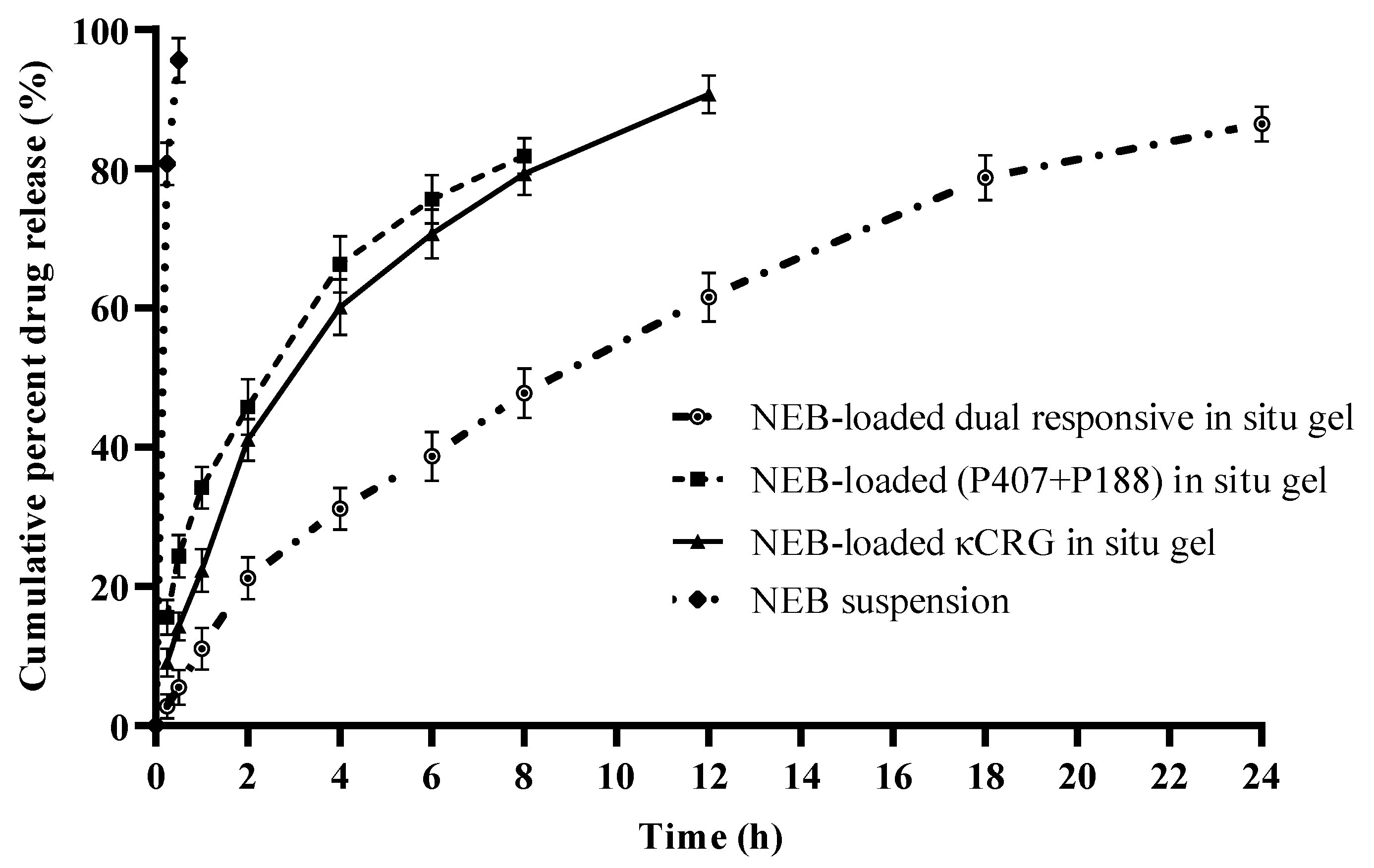
3.5. In Vitro Drug Release Studies of NEB-Loaded In Situ Gels
3.6. Ex Vivo Ocular Irritation Test (HET-CAM) of the Optimized In Situ Gels
3.7. Hemolysis Study of the Optimized In Situ Gels
3.8. Ocular Histopathology Studies of the Optimized In Situ Gels
3.9. In Vivo Studies of the Optimized NEB-Loaded Dual-Responsive In Situ Gel
3.9.1. Pharmacokinetic Study
3.9.2. Pharmacodynamic Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma: A Review. Jama 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gazzard, G.; Foster, P.J.; Devereux, J.G.; Oen, F.; Chew, P.; Khaw, P.T.; Seah, S. Intraocular Pressure and Visual Field Loss in Primary Angle Closure and Primary Open Angle Glaucomas. Br. J. Ophthalmol. 2003, 87, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Toris, C.B.; Gagrani, M.; Ghate, D. Current Methods and New Approaches to Assess Aqueous Humor Dynamics. Expert Rev. Ophthalmol. 2021, 16, 139–160. [Google Scholar] [CrossRef]
- Anne, M.V.; Gillies, W.E. Ocular β-Blockers in Glaucoma Management. Drugs Aging 1992, 2, 208–221. [Google Scholar]
- Taniguchi, T.; Kitazawa, Y. The Potential Systemic Effect of Topically Applied Beta-Blockers in Glaucoma Therapy. Curr. Opin. Ophthalmol. 1997, 8, 55–58. [Google Scholar] [CrossRef]
- Buckley, M.M.-T.; Goa, K.L.; Clissold, S.P. Ocular Betaxolol. Drugs 1990, 40, 75–90. [Google Scholar] [CrossRef]
- Szumny, D.; Szeląg, A. The Influence of New Beta-Adrenolytics Nebivolol and Carvedilol on Intraocular Pressure and Iris Blood Flow in Rabbits. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 917–923. [Google Scholar] [CrossRef] [Green Version]
- Wareham, L.K.; Buys, E.S.; Sappington, R.M. The Nitric Oxide-Guanylate Cyclase Pathway and Glaucoma. Nitric Oxide 2018, 77, 75–87. [Google Scholar] [CrossRef]
- Qi, H.; Chen, W.; Huang, C.; Li, L.; Chen, C.; Li, W.; Wu, C. Development of a Poloxamer Analogs/Carbopol-Based in Situ Gelling and Mucoadhesive Ophthalmic Delivery System for Puerarin. Int. J. Pharm. 2007, 337, 178–187. [Google Scholar] [CrossRef]
- Gupta, H.; Jain, S.; Mathur, R.; Mishra, P.; Mishra, A.K.; Velpandian, T. Sustained Ocular Drug Delivery from a Temperature and PH Triggered Novel in Situ Gel System. Drug Deliv. 2007, 14, 507–515. [Google Scholar] [CrossRef]
- Nagarwal, R.C.; Kant, S.; Singh, P.N.; Maiti, P.; Pandit, J.K. Polymeric Nanoparticulate System: A Potential Approach for Ocular Drug Delivery. J. Control. Release 2009, 136, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Chowhan, A.; Giri, T.K. Polysaccharide as Renewable Responsive Biopolymer for in Situ Gel in the Delivery of Drug through Ocular Route. Int. J. Biol. Macromol. 2020, 150, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Langer, R.; Lendlein, A.; Lahann, J. From Advanced Biomedical Coatings to Multi-functionalized Biomaterials. J. Macromol. Sci. Part C Polym. Rev. 2006, 46, 347–375. [Google Scholar] [CrossRef]
- Nastyshyn, S.; Stetsyshyn, Y.; Raczkowska, J.; Nastishin, Y.; Melnyk, Y.; Panchenko, Y.; Budkowski, A. Temperature-Responsive Polymer Brush Coatings for Advanced Biomedical Applications. Polymers 2022, 14, 4245. [Google Scholar] [CrossRef]
- Goel, M.; Picciani, R.G.; Lee, R.K.; Bhattacharya, S.K. Aqueous Humor Dynamics: A Review. Open Ophthalmol. J. 2010, 4, 52. [Google Scholar] [CrossRef] [Green Version]
- Fabiani, C.; Li Voti, R.; Rusciano, D.; Mutolo, M.G.; Pescosolido, N. Relationship between Corneal Temperature and Intraocular Pressure in Healthy Individuals: A Clinical Thermographic Analysis. J. Ophthalmol. 2016, 2016, 3076031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chassenieux, C.; Tsitsilianis, C. Recent Trends in PH/Thermo-Responsive Self-Assembling Hydrogels: From Polyions to Peptide-Based Polymeric Gelators. Soft Matter 2016, 12, 1344–1359. [Google Scholar] [CrossRef]
- Carlfors, K.E.J.; Petersson, R. Rheological Evaluation of Poloxamer as an in Situ Gel for Ophthalmic Use. Eur. J. Pharm. Sci. 1998, 6, 105–112. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A Review of Poloxamer 407 Pharmaceutical and Pharmacological Characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Swain, G.P.; Patel, S.; Gandhi, J.; Shah, P. Development of Moxifloxacin Hydrochloride Loaded In-Situ Gel for the Treatment of Periodontitis: In-Vitro Drug Release Study and Antibacterial Activity. J. Oral Biol. Craniofacial Res. 2019, 9, 190–200. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A Review on Synthesis, Properties and Applications of Natural Polymer Based Carrageenan Blends and Composites. Int. J. Biol. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef]
- Bhowmick, B.; Sarkar, G.; Rana, D.; Roy, I.; Saha, N.R.; Ghosh, S.; Bhowmik, M.; Chattopadhyay, D. Effect of Carrageenan and Potassium Chloride on an in Situ Gelling Ophthalmic Drug Delivery System Based on Methylcellulose. RSC Adv. 2015, 5, 60386–60391. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Wei, G.; Lu, W.-Y. Effect of Carrageenan on Poloxamer-Based in Situ Gel for Vaginal Use: Improved in Vitro and in Vivo Sustained-Release Properties. Eur. J. Pharm. Sci. 2009, 37, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, C.; Liu, Z.; Li, Q.; Yan, X.; Liu, Y.; Lu, W. Enhancement in Bioavailability of Ketorolac Tromethamine via Intranasal in Situ Hydrogel Based on Poloxamer 407 and Carrageenan. Int. J. Pharm. 2014, 474, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.S.; Ravi, P.R.; Kaswan, L.; Raghuvanshi, R.S. Development and Validation of a Bio-Analytical Method for Simultaneous Quantification of Nebivolol and Labetalol in Aqueous Humor and Plasma Using LC-MS/MS and Its Application to Ocular Pharmacokinetic Studies. J. Chromatogr. B 2020, 1136, 121908. [Google Scholar] [CrossRef]
- Hirun, N.; Kraisit, P.; Tantishaiyakul, V. Thermosensitive Polymer Blend Composed of Poloxamer 407, Poloxamer 188 and Polycarbophil for the Use as Mucoadhesive In Situ Gel. Polymers 2022, 14, 1836. [Google Scholar] [CrossRef]
- Vijaya, C.; Goud, K.S. Ion-Activated in Situ Gelling Ophthalmic Delivery Systems of Azithromycin. Indian J. Pharm. Sci. 2011, 73, 615. [Google Scholar] [CrossRef] [Green Version]
- Xiong, W.; Gao, X.; Zhao, Y.; Xu, H.; Yang, X. The Dual Temperature/PH-Sensitive Multiphase Behavior of Poly (N-Isopropylacrylamide-Co-Acrylic Acid) Microgels for Potential Application in in Situ Gelling System. Colloids Surf. B Biointerfaces 2011, 84, 103–110. [Google Scholar] [CrossRef]
- Mandal, S.; Thimmasetty, M.K.M.J.; Prabhushankar, G.L.; Geetha, M.S. Formulation and Evaluation of an in Situ Gel-Forming Ophthalmic Formulation of Moxifloxacin Hydrochloride. Int. J. Pharm. Investig. 2012, 2, 78. [Google Scholar] [CrossRef] [Green Version]
- Okur, N.Ü.; Yozgatli, V.; Okur, M.E. In Vitro–in Vivo Evaluation of Tetrahydrozoline-loaded Ocular in Situ Gels on Rabbits for Allergic Conjunctivitis Management. Drug Dev. Res. 2020, 81, 716–727. [Google Scholar] [CrossRef]
- Kaur, H.; Ioyee, S.; Garg, R. Formulation and Evaluation of In-Situ Ocular Gel of Gatifloxacin. Int. J. Pharm. Res. Heal. Sci. 2016, 4, 1365–1370. [Google Scholar] [CrossRef]
- Ceulemans, J.; Ludwig, A. Optimisation of Carbomer Viscous Eye Drops: An in Vitro Experimental Design Approach Using Rheological Techniques. Eur. J. Pharm. Biopharm. 2002, 54, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Gratieri, T.; Gelfuso, G.M.; Rocha, E.M.; Sarmento, V.H.; de Freitas, O.; Lopez, R.F.V. A Poloxamer/Chitosan in Situ Forming Gel with Prolonged Retention Time for Ocular Delivery. Eur. J. Pharm. Biopharm. 2010, 75, 186–193. [Google Scholar] [CrossRef]
- Destruel, P.-L.; Zeng, N.; Maury, M.; Mignet, N.; Boudy, V. In Vitro and in Vivo Evaluation of in Situ Gelling Systems for Sustained Topical Ophthalmic Delivery: State of the Art and Beyond. Drug Discov. Today 2017, 22, 638–651. [Google Scholar] [CrossRef]
- Weng, L.; Chen, X.; Chen, W. Rheological Characterization of in Situ Crosslinkable Hydrogels Formulated from Oxidized Dextran and N-Carboxyethyl Chitosan. Biomacromolecules 2007, 8, 1109–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woertz, C.; Preis, M.; Breitkreutz, J.; Kleinebudde, P. Assessment of Test Methods Evaluating Mucoadhesive Polymers and Dosage Forms: An Overview. Eur. J. Pharm. Biopharm. 2013, 85, 843–853. [Google Scholar] [CrossRef]
- Kiss, E.L.; Berkó, S.; Gácsi, A.; Kovács, A.; Katona, G.; Soós, J.; Csányi, E.; Gróf, I.; Harazin, A.; Deli, M.A. Development and Characterization of Potential Ocular Mucoadhesive Nano Lipid Carriers Using Full Factorial Design. Pharmaceutics 2020, 12, 682. [Google Scholar] [CrossRef]
- Lin, H.-R.; Sung, K.C. Carbopol/Pluronic Phase Change Solutions for Ophthalmic Drug Delivery. J. Control. Release 2000, 69, 379–388. [Google Scholar] [CrossRef]
- Abrego, G.; Alvarado, H.; Souto, E.B.; Guevara, B.; Bellowa, L.H.; Parra, A.; Calpena, A.; Garcia, M.L. Biopharmaceutical Profile of Pranoprofen-Loaded PLGA Nanoparticles Containing Hydrogels for Ocular Administration. Eur. J. Pharm. Biopharm. 2015, 95, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Peppas, N.A.; Sahlin, J.J. A Simple Equation for the Description of Solute Release. III. Coupling of Diffusion and Relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Moosa, R.M.; Choonara, Y.E.; du Toit, L.C.; Tomar, L.K.; Tyagi, C.; Kumar, P.; Carmichael, T.R.; Pillay, V. In Vivo Evaluation and In-Depth Pharmaceutical Characterization of a Rapidly Dissolving Solid Ocular Matrix for the Topical Delivery of Timolol Maleate in the Rabbit Eye Model. Int. J. Pharm. 2014, 466, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Batista-Duharte, A.; Jorge Murillo, G.; Pérez, U.M.; Tur, E.N.; Portuondo, D.F.; Martínez, B.T.; Téllez-Martínez, D.; Betancourt, J.E.; Pérez, O. The Hen’s Egg Test on Chorioallantoic Membrane: An Alternative Assay for the Assessment of the Irritating Effect of Vaccine Adjuvants. Int. J. Toxicol. 2016, 35, 627–633. [Google Scholar] [CrossRef]
- Terreni, E.; Chetoni, P.; Burgalassi, S.; Tampucci, S.; Zucchetti, E.; Chipala, E.; Alany, R.G.; Al-Kinani, A.A.; Monti, D. A Hybrid Ocular Delivery System of Cyclosporine-A Comprising Nanomicelle-Laden Polymeric Inserts with Improved Efficacy and Tolerability. Biomater. Sci. 2021, 9, 8235–8248. [Google Scholar] [CrossRef] [PubMed]
- Arruda, I.R.S.; Albuquerque, P.B.S.; Santos, G.R.C.; Silva, A.G.; Mourão, P.A.S.; Correia, M.T.S.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Structure and Rheological Properties of a Xyloglucan Extracted from Hymenaea Courbaril Var. Courbaril Seeds. Int. J. Biol. Macromol. 2015, 73, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Kaur, G.; Singh, D.; Brar, V. Bioadhesive Okra Polymer Based Buccal Patches as Platform for Controlled Drug Delivery. Int. J. Biol. Macromol. 2014, 70, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Kimna, C.; Winkeljann, B.; Hoffmeister, J.; Lieleg, O. Biopolymer-Based Nanoparticles with Tunable Mucoadhesivity Efficiently Deliver Therapeutics across the Corneal Barrier. Mater. Sci. Eng. C 2021, 121, 111890. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Liu, L.; Cai, C.; Xin, H.; Liu, W. Design and Evaluation of a Brinzolamide Drug-Resin in Situ Thermosensitive Gelling System for Sustained Ophthalmic Drug Delivery. Chem. Pharm. Bull. 2014, c14-00451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diwan, R.; Ravi, P.R.; Agarwal, S.I.; Aggarwal, V. Cilnidipine Loaded Poly (ε-Caprolactone) Nanoparticles for Enhanced Oral Delivery: Optimization Using DoE, Physical Characterization, Pharmacokinetic, and Pharmacodynamic Evaluation. Pharm. Dev. Technol. 2021, 26, 278–290. [Google Scholar] [CrossRef]
- Reddy, I.K.; Vaithiyalingam, S.R.; Khan, M.A.; Bodor, N.S. Intraocular Pressure-Lowering Activity and in Vivo Disposition of Dipivalyl Terbutalone in Rabbits. Drug Dev. Ind. Pharm. 2001, 27, 137–141. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, N.; Hua, H.; Liu, T.; Tang, Y.; Fu, L.; Yang, Y.; Ma, X.; Zhao, Y. Preparation, Pharmacokinetics and Pharmacodynamics of Ophthalmic Thermosensitive in Situ Hydrogel of Betaxolol Hydrochloride. Biomed. Pharmacother. 2016, 83, 107–113. [Google Scholar] [CrossRef]
- Bron, A.J.; Willshire, C. Tear Osmolarity in the Diagnosis of Systemic Dehydration and Dry Eye Disease. Diagnostics 2021, 11, 387. [Google Scholar] [CrossRef] [PubMed]
- Yermak, I.M.; Davydova, V.N.; Kravchenko, A.O.; Chistyulin, D.A.; Pimenova, E.A.; Glazunov, V.P. Mucoadhesive Properties of Sulphated Polysaccharides Carrageenans from Red Seaweed Families Gigartinaceae and Tichocarpaceae. Int. J. Biol. Macromol. 2020, 142, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K. Managing Adverse Effects of Glaucoma Medications. Clin. Ophthalmol. 2014, 8, 903. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Factors | Levels Used | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| Independent Variables | |||
| X1 = P407 concentration (% w/v) | 18% | 19% | 20% |
| X2 = κCRG concentration (% w/v) | 0.3% | 0.4% | 0.5% |
| X3 = P188 concentration (% w/v) | 1% | 3% | 5% |
| Dependent variables | Constraints | ||
| Y1 = Gelling temperature | In range of 33–35 °C | ||
| Y2 = Solution state viscosity at 25 °C | Minimize | ||
| Run | Critical Factors | Response | |||
|---|---|---|---|---|---|
| P407 Concentration (X1, %w/v) | κCRG Concentration (X2, %w/v) | P188 Concentration (X3, %w/v) | Gelling Temperature (Y1, °C) | Sol State Viscosity (Y2, cP) | |
| 1 | 19 | 0.4 | 3 | 41 | 212 |
| 2 | 19 | 0.4 | 3 | 41 | 205 |
| 3 | 19 | 0.5 | 1 | 34 | 217 |
| 4 | 18 | 0.3 | 3 | 42 | 183 |
| 5 | 19 | 0.5 | 5 | 46 | 209 |
| 6 | 19 | 0.3 | 5 | 45 | 199 |
| 7 | 20 | 0.4 | 5 | 45 | 227 |
| 8 | 18 | 0.4 | 1 | 35 | 195 |
| 9 | 19 | 0.4 | 3 | 41 | 204 |
| 10 | 19 | 0.3 | 1 | 34 | 211 |
| 11 | 20 | 0.4 | 1 | 33 | 233 |
| 12 | 18 | 0.4 | 5 | 47 | 179 |
| 13 | 20 | 0.5 | 3 | 40 | 227 |
| 14 | 19 | 0.4 | 3 | 40 | 210 |
| 15 | 19 | 0.4 | 3 | 41 | 205 |
| 16 | 18 | 0.5 | 3 | 42 | 187 |
| 17 | 20 | 0.3 | 3 | 40 | 224 |
| Source | Gelling Temperature (Y1, °C) | Sol State Viscosity at 25 °C (Y2, cP) | ||||||
|---|---|---|---|---|---|---|---|---|
| Sum of Squares | DF | F-Value | p-Value | Sum of Squares | DF | F-Value | p-Value | |
| Model | 289.07 | 9 | 214.12 | <0.0001 | 3835.69 | 91 | 46.22 | <0.0001 |
| X1 | 8.00 | 1 | 53.33 | 0.0002 | 3486.13 | 1 | 378.05 | <0.0001 |
| X2 | 0.125 | 1 | 0.833 | 0.3917 | 66.13 | 1 | 7017 | 0.0316 |
| X3 | 276.13 | 1 | 1840.83 | <0.0001 | 220.50 | 1 | 23.91 | 0.0018 |
| X1 X2 | 0.000 | 1 | 0.000 | 1.000 | 0.25 | 1 | 0.0271 | 0.8739 |
| X1 X3 | 0.000 | 1 | 0.000 | 1.000 | 25 | 1 | 2.71 | 0.1436 |
| X2 X3 | 0.250 | 1 | 1.67 | 0.2377 | 4.16 | 1 | 0.433 | 0.5312 |
| X12 | 0.213 | 1 | 1.42 | 0.2721 | 6.32 | 1 | 0.6852 | 0.4351 |
| X22 | 0.0026 | 1 | 0.0175 | 0.8984 | 2.21 | 1 | 0.240 | 0.6392 |
| X32 | 4.42 | 1 | 29.49 | 0.0010 | 26.84 | 1 | 2.91 | 0.1317 |
| Residual | 1.05 | 7 | 64.45 | 7 | ||||
| Lack of fit | 0.2500 | 3 | 0.4167 | 0.751 | 13.75 | 3 | 0.3609 | 0.7856 |
| Pure error | 0.800 | 4 | 50.80 | 4 | ||||
| Total | 290.12 | 16 | 3900.24 | 16 | ||||
| Formulation | Experimental Condition Used in Rheological Study | ||
|---|---|---|---|
| Only Temp Ramp | Temp Ramp in Presence of DI Water | Temp Ramp in Presence of STF | |
| Blank dual-responsive in situ gel | tan δ > 1 in the range of 20–33 °C and tan δ = 1 at 34 °C | tan δ >> 1 in the range of 20–33 °C and tan δ = 1 at 34 °C | tan δ < 1 in the range of 20–30 °C and tan δ << 1 in the range of 30–37 °C |
| NEB-loaded dual-responsive in situ gel | 20–30 tan δ > 1 At 32 °C tan δ = 1 | tan δ >> 1 in the range of 20–32 °C and tan δ = 1 at 33 °C | tan δ < 1 in the range of 20–31 °C and tan δ << 1 in the range of 31–37 °C |
| Biological Matrix | Parameters | Units | Treatments | |
|---|---|---|---|---|
| NEB Suspension | NEB In Situ Gel | |||
| Aqueous humor | Cmax | ng/mL | 28.2 ± 3.1 | 35.14 ± 2.25 * |
| Tmax | h | 2 | 4 | |
| AUC0–24 | ng/mL * h | 189.0 ± 13.14 | 364.1 ± 16.76 *** | |
| AUC0-∞ | ng/mL * h | 194.9 ± 12.17 | 381.8 ± 18.32 *** | |
| MRT0-∞ | h | 6.12 ± 0.178 | 8.11 ± 0.12 *** | |
| Plasma | Cmax | ng/mL | 1.8 ± 0.01 | 0.6 ± 0.01 *** |
| Tmax | h | 0.5 | 1 | |
| AUC0–24 | ng/mL * h | 20.2 ± 2.7 | 4.1 ± 0.2 *** | |
| AUC0-∞ | ng/mL * h | 33.2 ± 2.1 | 8.0 ± 0.43 *** | |
| MRT0-∞ | h | 25.8 ± 1.5 | 11.01 ± 0.6 *** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rawat, P.S.; Ravi, P.R.; Mir, S.I.; Khan, M.S.; Kathuria, H.; Katnapally, P.; Bhatnagar, U. Design, Characterization and Pharmacokinetic–Pharmacodynamic Evaluation of Poloxamer and Kappa-Carrageenan-Based Dual-Responsive In Situ Gel of Nebivolol for Treatment of Open-Angle Glaucoma. Pharmaceutics 2023, 15, 405. https://doi.org/10.3390/pharmaceutics15020405
Rawat PS, Ravi PR, Mir SI, Khan MS, Kathuria H, Katnapally P, Bhatnagar U. Design, Characterization and Pharmacokinetic–Pharmacodynamic Evaluation of Poloxamer and Kappa-Carrageenan-Based Dual-Responsive In Situ Gel of Nebivolol for Treatment of Open-Angle Glaucoma. Pharmaceutics. 2023; 15(2):405. https://doi.org/10.3390/pharmaceutics15020405
Chicago/Turabian StyleRawat, Pradeep Singh, Punna Rao Ravi, Shahid Iqbal Mir, Mohammed Shareef Khan, Himanshu Kathuria, Prasanna Katnapally, and Upendra Bhatnagar. 2023. "Design, Characterization and Pharmacokinetic–Pharmacodynamic Evaluation of Poloxamer and Kappa-Carrageenan-Based Dual-Responsive In Situ Gel of Nebivolol for Treatment of Open-Angle Glaucoma" Pharmaceutics 15, no. 2: 405. https://doi.org/10.3390/pharmaceutics15020405
APA StyleRawat, P. S., Ravi, P. R., Mir, S. I., Khan, M. S., Kathuria, H., Katnapally, P., & Bhatnagar, U. (2023). Design, Characterization and Pharmacokinetic–Pharmacodynamic Evaluation of Poloxamer and Kappa-Carrageenan-Based Dual-Responsive In Situ Gel of Nebivolol for Treatment of Open-Angle Glaucoma. Pharmaceutics, 15(2), 405. https://doi.org/10.3390/pharmaceutics15020405







