The Delivery and Activation of Growth Factors Using Nanomaterials for Bone Repair
Abstract
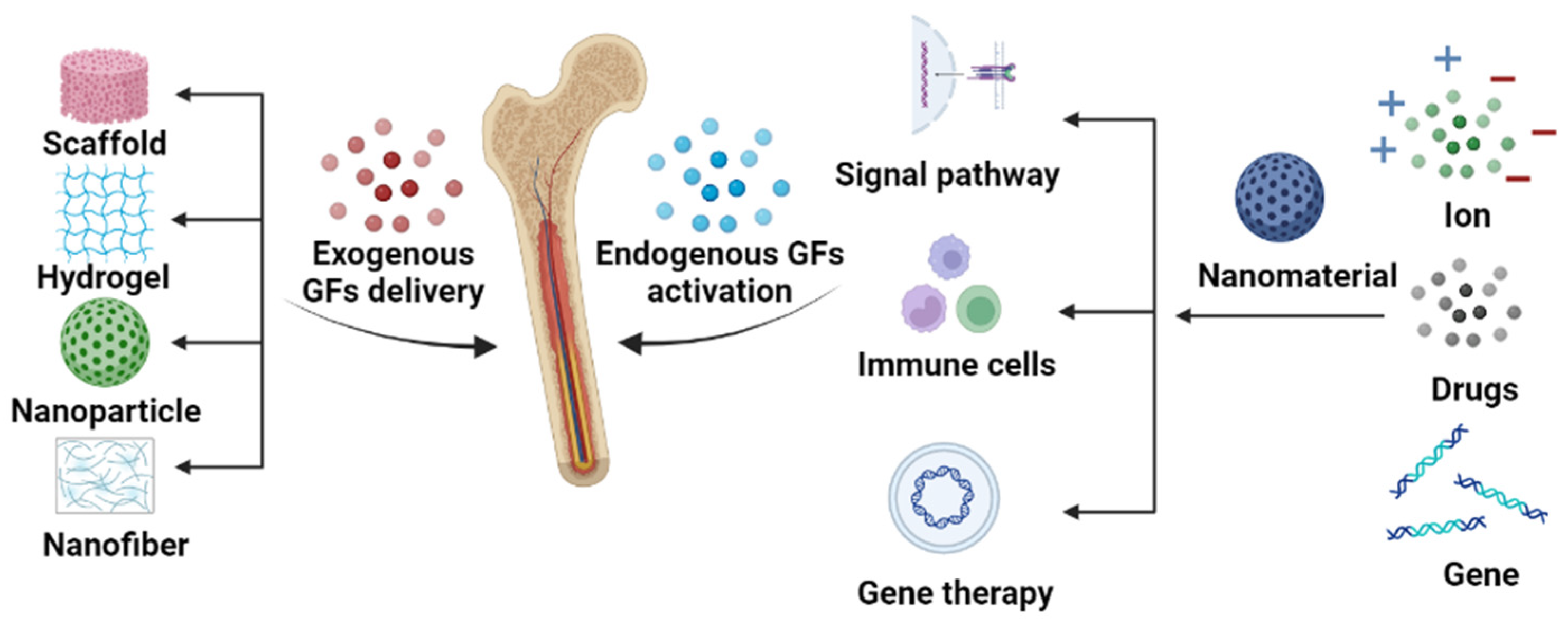
1. Introduction
2. Exogenous Growth Factor Delivery
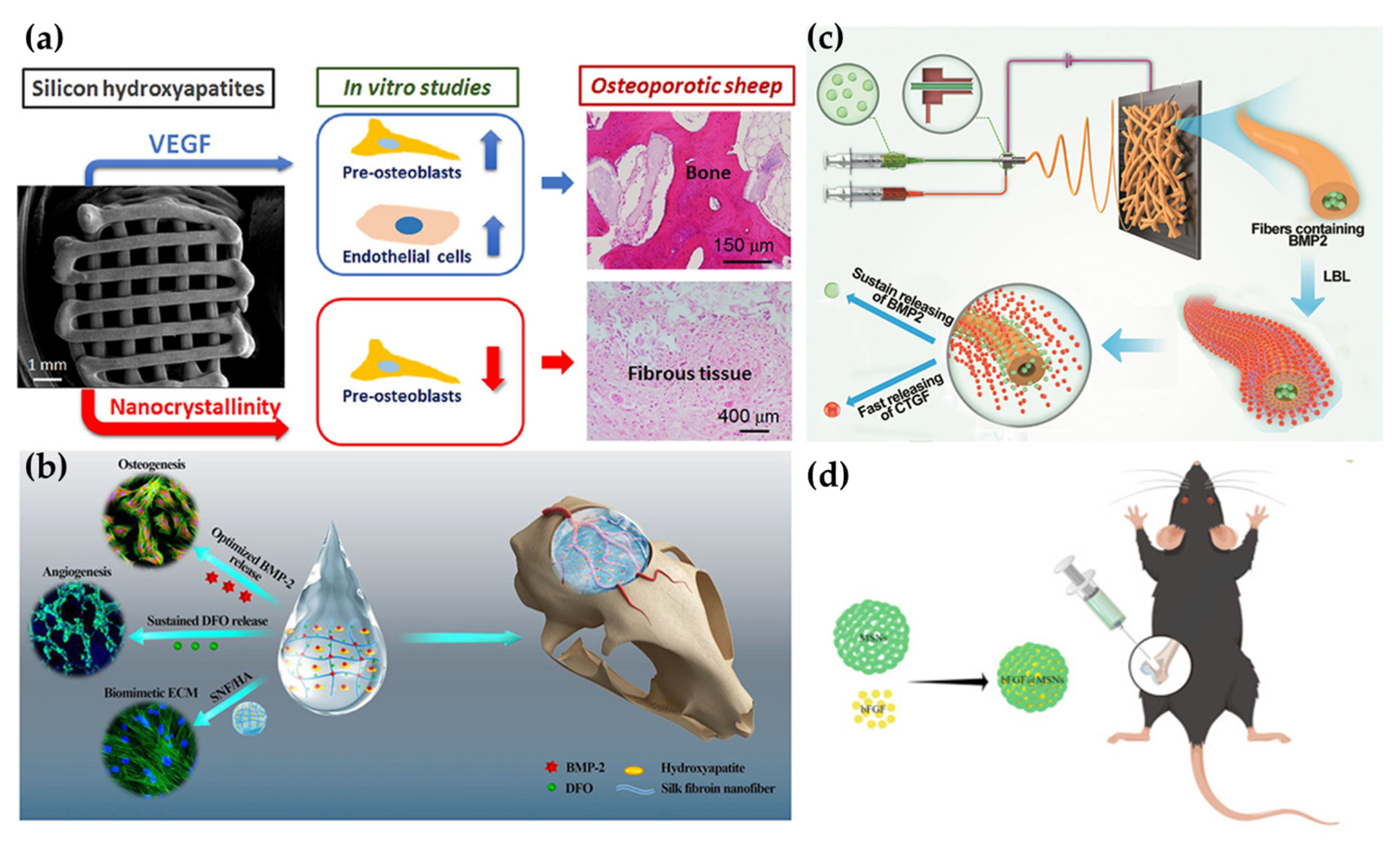
2.1. Scaffolds
2.2. Hydrogels
2.3. Nanofibers
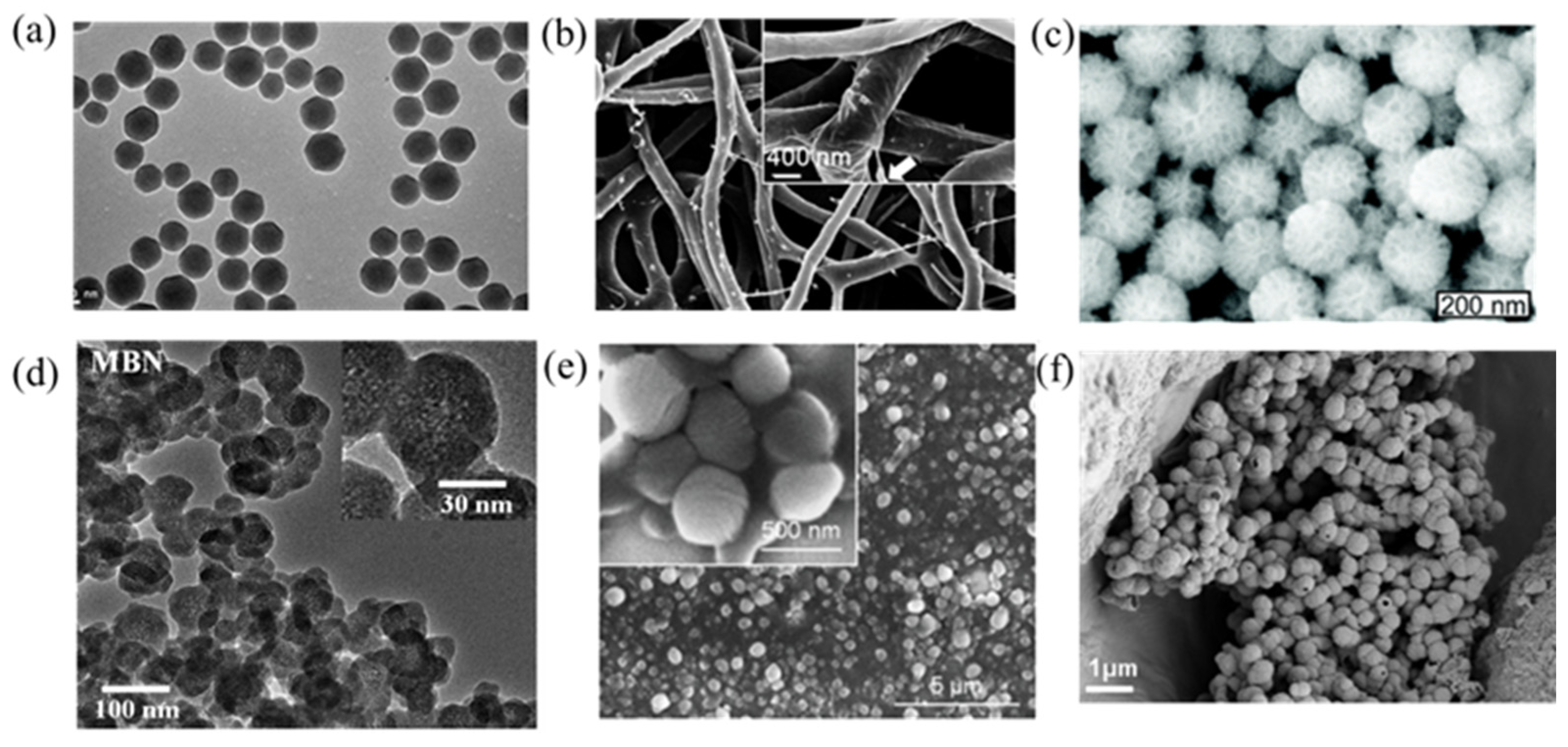
2.4. Nanoparticles
3. Endogenous Growth Factor Activation
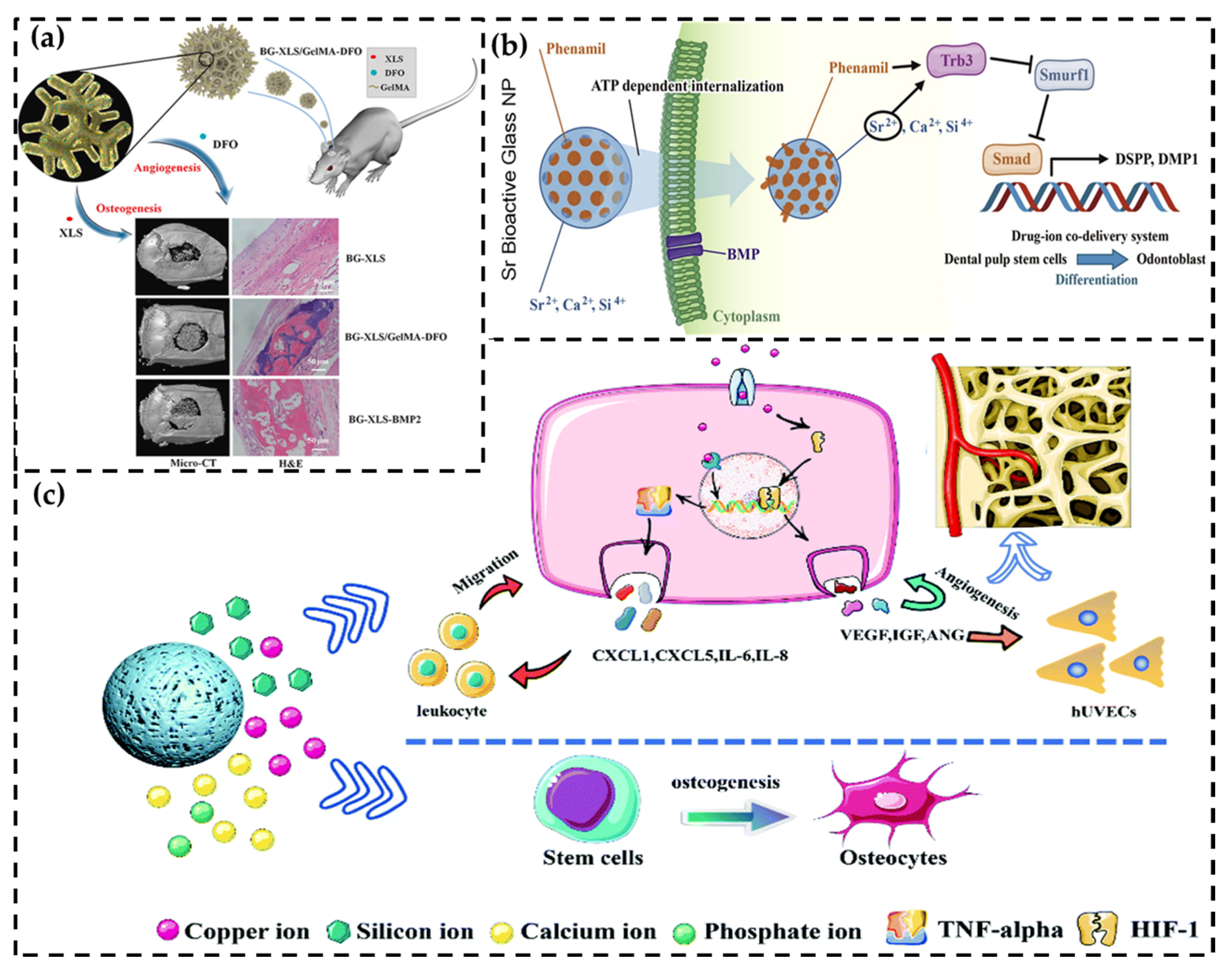
3.1. Signal Pathway Activation
3.2. Immune System Stimulation
3.2.1. Macrophage
3.2.2. Monocytes and T Cells
3.3. Gene Therapy
3.3.1. RNA
3.3.2. Plasmid
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verrier, S.; Alini, M.; Alsberg, E.; Buchman, S.R.; Kelly, D.; Laschke, M.W.; Menger, M.D.; Murphy, W.L.; Stegemann, J.P.; Schutz, M.; et al. Tissue engineering and regenerative approaches to improving the healing of large bone defects. Eur. Cell Mater. 2016, 32, 87–110. [Google Scholar] [CrossRef] [PubMed]
- Archunan, M.W.; Petronis, S. Bone Grafts in Trauma and Orthopaedics. Cureus 2021, 13, e17705. [Google Scholar] [CrossRef] [PubMed]
- Research&Market, L. Global Cranial Implants Market (2019–2025)–Research and Markets. 2019. Available online: https://www.researchandmarkets.com/reports/4803302/global-cranial-implants-market-2019-2025 (accessed on 10 March 2023).
- Martin, V.; Bettencourt, A. Bone regeneration: Biomaterials as local delivery systems with improved osteoinductive properties. Mat. Sci. Eng. C Mater. 2018, 82, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Pape, H.C.; Evans, A.; Kobbe, P. Autologous bone graft: Properties and techniques. J. Orthop. Trauma 2010, 24 (Suppl. S1), S36–S40. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Ebied, M.; Xu, J.; Zreiqat, H. Current Approaches to Bone Tissue Engineering: The Interface between Biology and Engineering. Adv. Health. Mater. 2018, 7, e1701061. [Google Scholar] [CrossRef]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46, 92–102. [Google Scholar] [CrossRef]
- Bohner, M. Resorbable biomaterials as bone graft substitutes. Mater Today 2010, 13, 24–30. [Google Scholar] [CrossRef]
- Gong, T.; Xie, J.; Liao, J.F.; Zhang, T.; Lin, S.Y.; Lin, Y.F. Nanomaterials and bone regeneration. Bone Res. 2015, 3, 15029. [Google Scholar] [CrossRef]
- Bal, Z.; Korkusuz, F.; Ishiguro, H.; Okada, R.; Kushioka, J.; Chijimatsu, R.; Kodama, J.; Tateiwa, D.; Ukon, Y.; Nakagawa, S.; et al. A novel nano-hydroxyapatite/synthetic polymer/bone morphogenetic protein-2 composite for efficient bone regeneration. Spine J. 2021, 21, 865–873. [Google Scholar] [CrossRef]
- Balagangadharan, K.; Chandran, S.V.; Arumugam, B.; Saravanan, S.; Venkatasubbu, G.D.; Selvamurugan, N. Chitosan/nano-hydroxyapatite/nano-zirconium dioxide scaffolds with miR-590-5p for bone regeneration. Int. J. Biol. Macromol. 2018, 111, 953–958. [Google Scholar] [CrossRef]
- Casarrubios, L.; Gomez-Cerezo, N.; Sanchez-Salcedo, S.; Feito, M.J.; Serrano, M.C.; Saiz-Pardo, M.; Ortega, L.; de Pablo, D.; Diaz-Guemes, I.; Fernandez-Tome, B.; et al. Silicon substituted hydroxyapatite/VEGF scaffolds stimulate bone regeneration in osteoporotic sheep. Acta Biomater. 2020, 101, 544–553. [Google Scholar] [CrossRef]
- Wang, J.; Wang, M.L.; Chen, F.Y.; Wei, Y.H.; Chen, X.N.; Zhou, Y.; Yang, X.; Zhu, X.D.; Tu, C.Q.; Zhang, X.D. Nano-Hydroxyapatite Coating Promotes Porous Calcium Phosphate Ceramic-Induced Osteogenesis Via BMP/Smad Signaling Pathway. Int. J. Nanomed. 2019, 14, 7987–8000. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Lian, R.X.; Liu, L.; Liu, T.; Bi, C.; Hong, K.; Zhang, S.Q.; Ren, J.Z.; Wang, H.K.; Ouyang, N.J.; et al. Biomimetic Hydroxyapatite Nanorods Promote Bone Regeneration via Accelerating Osteogenesis of BMSCs through T Cell-Derived IL-22. Acs Nano 2022, 16, 755–770. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.A.A.; Pack, S.P. Biomimetic and bioinspired silicifications: Recent advances for biomaterial design and applications. Acta Biomater. 2021, 120, 38–56. [Google Scholar] [CrossRef]
- Peng, Z.L.; Zhao, T.S.; Zhou, Y.Q.; Li, S.H.; Li, J.J.; Leblanc, R.M. Bone Tissue Engineering via Carbon-Based Nanomaterials. Adv. Health. Mater. 2020, 9, e1901495. [Google Scholar] [CrossRef]
- Shadjou, N.; Hasanzadeh, M.; Khalilzadeh, B. Graphene based scaffolds on bone tissue engineering. Bioengineered 2018, 9, 38–47. [Google Scholar] [CrossRef]
- Daneshmandi, L.; Barajaa, M.; Rad, A.T.; Sydlik, S.A.; Laurencin, C.T. Graphene-Based Biomaterials for Bone Regenerative Engineering: A Comprehensive Review of the Field and Considerations Regarding Biocompatibility and Biodegradation. Adv. Health. Mater. 2021, 10, e202001414. [Google Scholar] [CrossRef] [PubMed]
- Babuska, V.; Kasi, P.B.; Chocholata, P.; Wiesnerova, L.; Dvorakova, J.; Vrzakova, R.; Nekleionova, A.; Landsmann, L.; Kulda, V. Nanomaterials in Bone Regeneration. Appl. Sci. 2022, 12, 6793. [Google Scholar] [CrossRef]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef]
- Dhawan, U.; Jaffery, H.; Salmeron-Sanchez, M.; Dalby, M.J. An ossifying landscape: Materials and growth factor strategies for osteogenic signalling and bone regeneration. Curr. Opin. Biotechnol. 2022, 73, 355–363. [Google Scholar] [CrossRef]
- Phillips, A.M. Overview of the fracture healing cascade. Injury 2005, 36 (Suppl. S3), S5–S7. [Google Scholar] [CrossRef] [PubMed]
- Janssens, K.; ten Dijke, P.; Janssens, S.; Van Hul, W. Transforming growth factor-beta1 to the bone. Endocr. Rev. 2005, 26, 743–774. [Google Scholar] [CrossRef] [PubMed]
- Salazar, V.S.; Gamer, L.W.; Rosen, V. BMP signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol. 2016, 12, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaz, A.; Jayasuriya, A.C. Osteogenic differentiation cues of the bone morphogenetic protein-9 (BMP-9) and its recent advances in bone tissue regeneration. Mat. Sci. Eng. C-Mater. 2021, 120, 111748. [Google Scholar] [CrossRef]
- Takeuchi, T.; Yoshida, H.; Tanaka, S. Role of interleukin-6 in bone destruction and bone repair in rheumatoid arthritis. Autoimmun. Rev. 2021, 20, 102884. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Marie, P.J. Fibroblast growth factor signaling in skeletal development and disease. Gene Dev. 2015, 29, 1463–1486. [Google Scholar] [CrossRef] [PubMed]
- Glass, G.E.; Chan, J.K.; Freidin, A.; Feldmann, M.; Horwood, N.J.; Nanchahal, J. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc. Natl. Acad. Sci. USA 2011, 108, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Byun, H.; Madhurakkat Perikamana, S.K.; Lee, S.; Shin, H. Current Advances in Immunomodulatory Biomaterials for Bone Regeneration. Adv. Health. Mater. 2019, 8, e1801106. [Google Scholar] [CrossRef]
- Pfeilschifter, J.; Oechsner, M.; Naumann, A.; Gronwald, R.G.; Minne, H.W.; Ziegler, R. Stimulation of bone matrix apposition in vitro by local growth factors: A comparison between insulin-like growth factor I, platelet-derived growth factor, and transforming growth factor beta. Endocrinology 1990, 127, 69–75. [Google Scholar] [CrossRef]
- Hollinger, J.O.; Hart, C.E.; Hirsch, S.N.; Lynch, S.; Friedlaender, G.E. Recombinant human platelet-derived growth factor: Biology and clinical applications. J. Bone Joint. Surg. Am. 2008, 90 (Suppl. S1), 48–54. [Google Scholar] [CrossRef]
- Steed, D.L. The role of growth factors in wound healing. Surg. Clin. N. Am. 1997, 77, 575–586. [Google Scholar] [CrossRef]
- Lauzon, M.A.; Daviau, A.; Marcos, B.; Faucheux, N. Nanoparticle-mediated growth factor delivery systems: A new way to treat Alzheimer’s disease. J. Control. Release 2015, 206, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhao, J.; Brochmann, E.J.; Wang, J.C.; Murray, S.S. Bone morphogenetic protein-2 and tumor growth: Diverse effects and possibilities for therapy. Cytokine Growth Factor Rev. 2017, 34, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Carragee, E.J.; Chu, G.; Rohatgi, R.; Hurwitz, E.L.; Weiner, B.K.; Yoon, S.T.; Comer, G.; Kopjar, B. Cancer Risk After Use of Recombinant Bone Morphogenetic Protein-2 for Spinal Arthrodesis. J. Bone Jt. Surg. -Am. Vol. 2013, 95a, 1537–1545. [Google Scholar] [CrossRef]
- Lo, K.W.H.; Ulery, B.D.; Ashe, K.M.; Laurencin, C.T. Studies of bone morphogenetic protein-based surgical repair. Adv. Drug Deliver Rev. 2012, 64, 1277–1291. [Google Scholar] [CrossRef]
- Kuroda, Y.; Kawai, T.; Goto, K.; Matsuda, S. Clinical application of injectable growth factor for bone regeneration: A systematic review. Inflamm. Regen. 2019, 39, 20. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.H.R.W.; Mills, L.; Noble, B. The role of growth factors and related agents in accelerating fracture healing. J. Bone Jt. Surg. Br. 2006, 88b, 701–705. [Google Scholar] [CrossRef]
- Ordikhani, F.; Zandi, N.; Mazaheri, M.; Luther, G.A.; Ghovvati, M.; Akbarzadeh, A.; Annabi, N. Targeted nanomedicines for the treatment of bone disease and regeneration. Med. Res. Rev. 2021, 41, 1221–1254. [Google Scholar] [CrossRef]
- Cui, Y.; Li, H.R.; Li, Y.X.; Mao, L.X. Novel insights into nanomaterials for immunomodulatory bone regeneration. Nanoscale Adv. 2022, 4, 334–352. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.Y.; Li, Q.T.; Gao, H.C.; Yao, L.T.; Lin, Z.F.; Li, D.G.; Zhu, S.L.; Liu, C.; Yang, Z.; Wang, G.; et al. 3D printing of Cu-doped bioactive glass composite scaffolds promotes bone regeneration through activating the HIF-1 alpha and TNF-alpha pathway of hUVECs. Biomater. Sci. 2021, 9, 5519–5532. [Google Scholar] [CrossRef]
- Hu, M.; Xiao, F.; Ke, Q.F.; Li, Y.; Chen, X.D.; Guo, Y.P. Cerium-doped whitlockite nanohybrid scaffolds promote new bone regeneration via SMAD signaling pathway. Chem. Eng. J. 2019, 359, 1–12. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, J.S.; Niu, K.; Li, W.Y.; Jiang, N.; Li, J.L.; Yu, Q.S.; Wu, C.A. Electrospun artificial periosteum loaded with DFO contributes to osteogenesis via the TGF-beta 1/Smad2 pathway. Biomater. Sci. 2021, 9, 2090–2102. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.Y.; Jiang, X.; Zhou, X.W.; Wang, O.R.; Wu, Q.Z.; Ren, L.; Zhu, J.X.; Zhu, S.S.; Tebon, P.; Sun, W.J.; et al. Stimuli-Responsive Delivery of Growth Factors for Tissue Engineering. Adv. Health. Mater. 2020, 9, e1901714. [Google Scholar] [CrossRef] [PubMed]
- De Witte, T.M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, Z.; Shi, Y.; Dong, L.; Wang, C. Modulating macrophage activities to promote endogenous bone regeneration: Biological mechanisms and engineering approaches. Bioact. Mater. 2021, 6, 244–261. [Google Scholar] [CrossRef]
- Lyons, J.G.; Plantz, M.A.; Hsu, W.K.; Hsu, E.L.; Minardi, S. Nanostructured Biomaterials for Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 922. [Google Scholar] [CrossRef]
- Shen, M.K.; Wang, L.L.; Feng, L.; Gao, Y.; Li, S.J.; Wu, Y.L.; Xu, C.Y.; Pei, G.X. bFGF-Loaded Mesoporous Silica Nanoparticles Promote Bone Regeneration Through the Wnt/?-Catenin Signalling Pathway. Int. J. Nanomed. 2022, 17, 2593–2608. [Google Scholar] [CrossRef]
- Cheng, G.; Yin, C.C.; Tu, H.; Jiang, S.; Wang, Q.; Zhou, X.; Xing, X.; Xie, C.Y.; Shi, X.W.; Du, Y.M.; et al. Controlled Co-delivery of Growth Factors through Layer-by-Layer Assembly of Core-Shell Nanofibers for Improving Bone Regeneration. Acs. Nano 2019, 13, 6372–6382. [Google Scholar] [CrossRef]
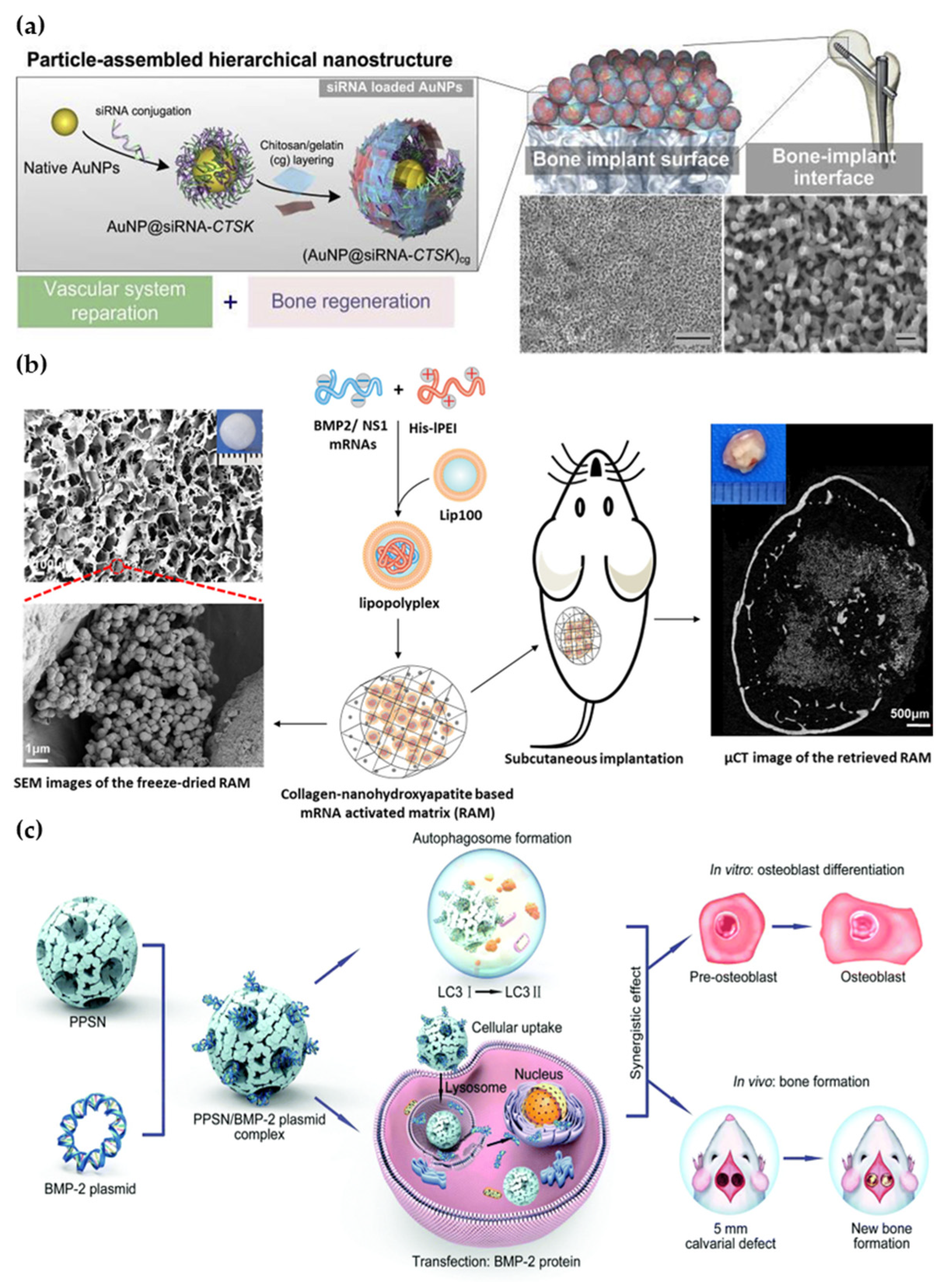
- Xu, X.; Sun, M.; Wang, D.; Bu, W.; Wang, Z.; Shen, Y.; Zhang, K.; Zhou, D.; Yang, B.; Sun, H. Bone formation promoted by bone morphogenetic protein-2 plasmid-loaded porous silica nanoparticles with the involvement of autophagy. Nanoscale 2019, 11, 21953–21963. [Google Scholar] [CrossRef]
- Lee, J.H.; Mandakhbayar, N.; El-Fiqi, A.; Kim, H.W. Intracellular co-delivery of Sr ion and phenamil drug through mesoporous bioglass nanocarriers synergizes BMP signaling and tissue mineralization. Acta Biomater. 2017, 60, 93–108. [Google Scholar] [CrossRef]
- Zhang, J.; Tong, D.; Song, H.; Ruan, R.; Sun, Y.; Lin, Y.; Wang, J.; Hou, L.; Dai, J.; Ding, J.; et al. Osteoimmunity-Regulating Biomimetically Hierarchical Scaffold for Augmented Bone Regeneration. Adv. Mater. 2022, 34, e2202044. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.P.; Perche, F.; Midoux, P.; Cabral, C.S.D.; Malard, V.; Correia, I.J.; EI-Hafci, H.; Petite, H.; Logeart-Avramoglou, D.; Pichon, C. In Vivo bone tissue induction by freeze-dried collagen-nanohydroxyapatite matrix loaded with BMP2/NS1 mRNAs lipopolyplexes. J. Control. Release 2021, 334, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, H.S.; Pashkuleva, I. Biomimetic supramolecular designs for the controlled release of growth factors in bone regeneration. Adv. Drug. Deliver. Rev. 2015, 94, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fan, T.T.; Chen, J.D.; Su, J.C.; Zhi, X.; Pan, P.P.; Zou, L.; Zhang, Q.Q. Magnetic bioinspired micro/nanostructured composite scaffold for bone regeneration. Colloid Surf. B 2019, 174, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Feng, X.D.; Wang, Y.; Wang, X.S.; He, Y. Biomimetic and immunomodulatory baicalin-loaded graphene oxide-demineralized bone matrix scaffold for in vivo bone regeneration. J. Mater. Chem. B 2021, 9, 9720–9733. [Google Scholar] [CrossRef]
- Zhu, L.S.; Luo, D.; Liu, Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int. J. Oral. Sci. 2020, 12, 6. [Google Scholar] [CrossRef]
- Nel, A.E.; Madler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Cheng, L.C.; Jiang, X.; Wang, J.; Chen, C.; Liu, R.S. Nano-bio effects: Interaction of nanomaterials with cells. Nanoscale 2013, 5, 3547–3569. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, R.; Chen, C. The Nano-Bio Interactions of Nanomedicines: Understanding the Biochemical Driving Forces and Redox Reactions. Acc. Chem. Res. 2019, 52, 1507–1518. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Santos-Ruiz, L.; Becerra, J.; Feito, M.J.; Fernandez-Villa, D.; Serrano, M.C.; Diaz-Guemes, I.; Fernandez-Tome, B.; Enciso, S.; Sanchez-Margallo, F.M.; et al. Synergistic effect of Si-hydroxyapatite coating and VEGF adsorption on Ti6Al4V-ELI scaffolds for bone regeneration in an osteoporotic bone environment. Acta Biomater. 2019, 83, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, K.; Shen, L.; Yu, L.; Ding, T.; Ma, B.; Ge, S.; Li, J. Metal Phenolic Nanodressing of Porous Polymer Scaffolds for Enhanced Bone Regeneration via Interfacial Gating Growth Factor Release and Stem Cell Differentiation. ACS Appl. Mater. Interfaces 2022, 14, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Ding, Z.; Zheng, X.; Lu, Q.; Kong, X.; Zhou, X.; Lu, G.; Kaplan, D.L. Injectable hydrogel systems with multiple biophysical and biochemical cues for bone regeneration. Biomater. Sci. 2020, 8, 2537–2548. [Google Scholar] [CrossRef]
- Kuttappan, S.; Mathew, D.; Jo, J.I.; Tanaka, R.; Menon, D.; Ishimoto, T.; Nakano, T.; Nair, S.V.; Nair, M.B.; Tabata, Y. Dual release of growth factor from nanocomposite fibrous scaffold promotes vascularisation and bone regeneration in rat critical sized calvarial defect. Acta Biomater. 2018, 78, 36–47. [Google Scholar] [CrossRef]
- Wang, B.; Guo, Y.W.; Chen, X.F.; Zeng, C.; Hu, Q.K.; Yin, W.; Li, W.; Xie, H.; Zhang, B.Y.; Huang, X.C.; et al. Nanoparticle-modified chitosan-agarose-gelatin scaffold for sustained release of SDF-1 and BMP-2. Int. J. Nanomed. 2018, 13, 7395–7408. [Google Scholar] [CrossRef]
- Murugan, S.; Parcha, S.R. Fabrication techniques involved in developing the composite scaffolds PCL/HA nanoparticles for bone tissue engineering applications. J. Mater. Sci. Mater. M 2021, 32, 93. [Google Scholar] [CrossRef] [PubMed]
- Naskar, D.; Ghosh, A.K.; Mandal, M.; Das, P.; Nandi, S.K.; Kundu, S.C. Dual growth factor loaded nonmulberry silk fibroin/carbon nanofiber composite 3D scaffolds for in vitro and in vivo bone regeneration. Biomaterials 2017, 136, 67–85. [Google Scholar] [CrossRef]
- Dong, Y.S.; Sun, X.; Zhang, Z.L.; Liu, Y.F.; Zhang, L.; Zhang, X.Y.; Huang, Y.; Zhao, Y.H.; Qi, C.X.; Midgley, A.C.; et al. Regional and sustained dual -release of growth factors from biomimetic tri-layered scaffolds for the repair of large-scale osteochondral defects. Appl. Mater. Today 2020, 19, 100548. [Google Scholar] [CrossRef]
- Sun, B.; Lian, M.; Han, Y.; Mo, X.; Jiang, W.; Qiao, Z.; Dai, K. A 3D-Bioprinted dual growth factor-releasing intervertebral disc scaffold induces nucleus pulposus and annulus fibrosus reconstruction. Bioact. Mater. 2021, 6, 179–190. [Google Scholar] [CrossRef]
- Yao, Q.Q.; Liu, Y.X.; Selvaratnam, B.; Koodali, R.T.; Sun, H.L. Mesoporous silicate nanoparticles/3D nanofibrous scaffold-mediated dual-drug delivery for bone tissue engineering. J. Control. Release 2018, 279, 69–78. [Google Scholar] [CrossRef]
- Czekanska, E.M.; Geng, J.; Glinka, M.; White, K.; Kanczler, J.; Evans, N.D.; Oreffo, R.O.C.; Bradley, M. Combinatorial delivery of bioactive molecules by a nanoparticle-decorated and functionalized biodegradable scaffold. J. Mater. Chem. B 2018, 6, 4437–4445. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef] [PubMed]
- Saroia, J.; Wang, Y.E.; Wei, Q.H.; Zhang, K.; Lu, T.L.; Zhang, B. A review on biocompatibility nature of hydrogels with 3D printing techniques, tissue engineering application and its future prospective. Bio.-Des. Manuf. 2018, 1, 265–279. [Google Scholar] [CrossRef]
- Saldin, L.T.; Cramer, M.C.; Velankar, S.S.; White, L.J.; Badylak, S.F. Extracellular matrix hydrogels from decellularized tissues: Structure and function. Acta Biomater. 2017, 49, 1–15. [Google Scholar] [CrossRef]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef]
- Xavier, J.R.; Thakur, T.; Desai, P.; Jaiswal, M.K.; Sears, N.; Cosgriff-Hernandez, E.; Kaunas, R.; Gaharwar, A.K. Bioactive nanoengineered hydrogels for bone tissue engineering: A growth-factor-free approach. Acs Nano 2015, 9, 3109–3118. [Google Scholar] [CrossRef]
- Arno, M.C.; Inam, M.; Weems, A.C.; Li, Z.H.; Binch, A.L.A.; Platt, C.I.; Richardson, S.M.; Hoyland, J.A.; Dove, A.P.; O’Reilly, R.K. Exploiting the role of nanoparticle shape in enhancing hydrogel adhesive and mechanical properties. Nat. Commun. 2020, 11, 1420. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.; Fang, L.; Ma, C.; Zhao, Y.; Liu, H.; Che, S.; Zvyagin, A.V.; Yang, B.; Lin, Q. Hydrogel Composites with Different Dimensional Nanoparticles for Bone Regeneration. Macromol. Rapid Commun. 2021, 42, e2100362. [Google Scholar] [CrossRef]
- Mi, L.; Liu, H.Q.; Gao, Y.; Miao, H.; Ruan, J.P. Injectable nanoparticles/hydrogels composite as sustained release system with stromal cell-derived factor-1 alpha for calvarial bone regeneration. Int. J. Biol. Macromol. 2017, 101, 341–347. [Google Scholar] [CrossRef]
- Yuan, Q.; Li, L.; Peng, Y.; Zhuang, A.; Wei, W.; Zhang, D.; Pang, Y.; Bi, X. Biomimetic nanofibrous hybrid hydrogel membranes with sustained growth factor release for guided bone regeneration. Biomater. Sci. 2021, 9, 1256–1271. [Google Scholar] [CrossRef]
- Miao, Y.L.; Chen, Y.H.; Luo, J.S.; Liu, X.; Yang, Q.; Shi, X.T.; Wang, Y.J. Black phosphorus nanosheets-enabled DNA hydrogel integrating 3D-printed scaffold for promoting vascularized bone regeneration. Bioact. Mater. 2023, 21, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Armstrong, J.P.K.; Pence, I.J.; Kit-Anan, W.; Puetzer, J.L.; Carreira, S.C.; Moore, A.C.; Stevens, M.M. Glycosylated superparamagnetic nanoparticle gradients for osteochondral tissue engineering. Biomaterials 2018, 176, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Casanova, S.; Martin-Saavedra, F.M.; Escudero-Duch, C.; Falguera Uceda, M.I.; Prieto, M.; Arruebo, M.; Acebo, P.; Fabiilli, M.L.; Franceschi, R.T.; Vilaboa, N. Local delivery of bone morphogenetic protein-2 from near infrared-responsive hydrogels for bone tissue regeneration. Biomaterials 2020, 241, 119909. [Google Scholar] [CrossRef]
- Shen, H.; Lin, H.; Sun, A.X.; Song, S.J.; Wang, B.; Yang, Y.H.; Dai, J.W.; Tuan, R.S. Acceleration of chondrogenic differentiation of human mesenchymal stem cells by sustained growth factor release in 3D graphene oxide incorporated hydrogels. Acta Biomater. 2020, 105, 44–55. [Google Scholar] [CrossRef]
- Pacelli, S.; Acosta, F.; Chakravarti, A.R.; Samanta, S.G.; Whitlow, J.; Modaresi, S.; Ahmed, R.P.H.; Rajasingh, J.; Paul, A. Nanodiamond-based injectable hydrogel for sustained growth factor release: Preparation, characterization and in vitro analysis. Acta Biomater. 2017, 58, 479–491. [Google Scholar] [CrossRef]
- Chen, M.J.; Zhang, Y.H.; Zhang, W.A.; Li, J. Polyhedral Oligomeric Silsesquioxane-Incorporated Gelatin Hydrogel Promotes Angiogenesis during Vascularized Bone Regeneration. Acs Appl. Mater. Interfaces 2020, 12, 22410–22425. [Google Scholar] [CrossRef] [PubMed]
- Cidonio, G.; Alcala-Orozco, C.R.; Lim, K.S.; Glinka, M.; Mutreja, I.; Kim, Y.H.; Dawson, J.I.; Woodfield, T.B.F.; Oreffo, R.O.C. Osteogenic and angiogenic tissue formation in high fidelity nanocomposite Laponite-gelatin bioinks. Biofabrication 2019, 11, 035027. [Google Scholar] [CrossRef]
- Ingavle, G.C.; Leach, J.K. Advancements in Electrospinning of Polymeric Nanofibrous Scaffolds for Tissue Engineering. Tissue Eng. Part B Rev. 2014, 20, 277–293. [Google Scholar] [CrossRef]
- Wu, L.; Gu, Y.; Liu, L.; Tang, J.; Mao, J.; Xi, K.; Jiang, Z.; Zhou, Y.; Xu, Y.; Deng, L.; et al. Hierarchical micro/nanofibrous membranes of sustained releasing VEGF for periosteal regeneration. Biomaterials 2020, 227, 119555. [Google Scholar] [CrossRef]
- Qu, D.; Zhu, J.P.; Childs, H.R.; Lu, H.H. Nanofiber-based transforming growth factor-beta 3 release induces fibrochondrogenic differentiation of stem cells. Acta Biomater. 2019, 93, 111–122. [Google Scholar] [CrossRef]
- Udomluck, N.; Lee, H.; Hong, S.; Lee, S.H.; Park, H. Surface functionalization of dual growth factor on hydroxyapatite-coated nanofibers for bone tissue engineering. Appl. Surf. Sci. 2020, 520, 146311. [Google Scholar] [CrossRef]
- Sun, H.; Dong, J.; Wang, Y.Y.F.; Shen, S.Y.; Shi, Y.; Zhang, L.; Zhao, J.; Sun, X.L.; Jiang, Q. Polydopamine-Coated Poly(L-lactide) Nanofibers with Controlled Release of VEGF and BMP-2 as a Regenerative Periosteum. Acs Biomater. Sci. Eng. 2021, 7, 4883–4897. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.Y.; Wu, J.H.; Xia, Y. Loading BMP-2 on nanostructured hydroxyapatite microspheres for rapid bone regeneration. Int. J. Nanomed. 2019, 14, 2753–2754, Erratum in Int. J. Nanomed. 2018, 13, 4083. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Z.; Du, B.; Tan, S.Y.; Wang, Q.; Li, Y.; Zhou, L. Vertical Guided Bone Regeneration in the Rabbit Calvarium Using Porous Nanohydroxyapatite Block Grafts Coated with rhVEGF(165) and Cortical Perforation. Int. J. Nanomed. 2020, 15, 10059–10073. [Google Scholar] [CrossRef]
- Kanniyappan, H.; Venkatesan, M.; Panji, J.; Ramasamy, M.; Muthuvijayan, V. Evaluating the inherent osteogenic and angiogenic potential of mesoporous silica nanoparticles to augment vascularized bone tissue formation. Micropor. Mesopor. Mat. 2021, 319, 111083. [Google Scholar] [CrossRef]
- Mao, S.C.; Wang, S.A.; Niu, Y.T.; Wu, J.L.; Jia, P.P.; Zheng, J.X.; Dong, Y.M. Induction of Cartilage Regeneration by Nanoparticles Loaded with Dentin Matrix Extracted Proteins. Tissue Eng. Part A 2022, 28, 807–817. [Google Scholar] [CrossRef]
- Shen, M.; Wang, L.; Feng, L.; Xu, C.; Gao, Y.; Li, S.; Wu, Y.; Pei, G. Cefazolin/BMP-2-Loaded Mesoporous Silica Nanoparticles for the Repair of Open Fractures with Bone Defects. Oxid. Med. Cell. Longev. 2022, 2022, 8385456. [Google Scholar] [CrossRef]
- Zhou, X.J.; Feng, W.; Qiu, K.X.; Chen, L.; Wang, W.Z.; Nie, W.; Mo, X.M.; He, C.L. BMP-2 Derived Peptide and Dexamethasone Incorporated Mesoporous Silica Nanoparticles for Enhanced Osteogenic Differentiation of Bone Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2015, 7, 15777–15789. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Maleki, A.; de la Guardia, M.; Bani, M.S.; Chenab, K.K.; Pashazadeh-Panahi, P.; Baradaran, B.; Mokhtarzadeh, A.; Hamblin, M.R. Carbon based nanomaterials for tissue engineering of bone: Building new bone on small black scaffolds: A review. J. Adv. Res. 2019, 18, 185–201. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, G.; Wang, X.; Jiang, L.; Jiang, F.; Li, G.; Zhang, Z.; Jiang, X. Magnetically Controlled Growth-Factor-Immobilized Multilayer Cell Sheets for Complex Tissue Regeneration. Adv Mater. 2017, 29, 1703795. [Google Scholar] [CrossRef]
- Zhong, C.; Feng, J.; Lin, X.J.; Bao, Q. Continuous release of bone morphogenetic protein-2 through nano-graphene oxide-based delivery influences the activation of the NF-kappa B signal transduction pathway. Int. J. Nanomed. 2017, 12, 1215. [Google Scholar] [CrossRef]
- Li, X.L.; Liu, M.H.; Cheng, H.X.; Wang, Q.; Miao, C.N.; Ju, S.M.; Liu, F. Development of ionic liquid assisted-synthesized nano-silver combined with vascular endothelial growth factor as wound healing in the care of femoral fracture in the children after surgery. J. Photoch. Photobio. B 2018, 183, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Zhang, C.L.; Liu, K.M.; Zhu, X.; Liu, F.; Ge, X.F. Biologically synthesized titanium oxide nanostructures combined with morphogenetic protein as wound healing agent in the femoral fracture after surgery. J. Photoch. Photobio. B 2018, 182, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.L.; Jiang, N.; Liang, S.; Chen, F.L.; Fang, L.; Wang, X.; Wang, J.J.; Chen, L.H. Functionalization of a clustered TiO2 nanotubular surface with platelet derived growth factor-BB covalent modification enhances osteogenic differentiation of bone marrow mesenchymal stem cells. Biomaterials 2020, 230, 119650. [Google Scholar] [CrossRef]
- Bruno, M.C.; Cristiano, M.C.; Celia, C.; d’Avanzo, N.; Mancuso, A.; Paolino, D.; Wolfram, J.; Fresta, M. Injectable Drug Delivery Systems for Osteoarthritis and Rheumatoid Arthritis. Acs Nano 2022, 16, 19665–19690. [Google Scholar] [CrossRef]
- Wang, Z.M.; Wang, Z.F.; Lu, W.W.; Zhen, W.X.; Yang, D.Z.; Peng, S.L. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017, 9, e435. [Google Scholar] [CrossRef]
- Marquez, L.; de Abreu, F.A.; Ferreira, C.L.; Alves, G.D.; Miziara, M.N.; Alves, J.B. Enhanced bone healing of rat tooth sockets after administration of epidermal growth factor (EGF) carried by liposome. Injury 2013, 44, 558–564. [Google Scholar] [CrossRef]
- Dirzu, N.; Lucaciu, O.; Dirzu, D.S.; Soritau, O.; Cenariu, D.; Crisan, B.; Tefas, L.; Campian, R.S. BMP-2 Delivery through Liposomes in Bone Regeneration. Appl. Sci. 2022, 12, 1373. [Google Scholar] [CrossRef]
- Lee, C.S.; Hsu, G.C.; Sono, T.; Lee, M.; James, A.W. Development of a Biomaterial Scaffold Integrated with Osteoinductive Oxysterol Liposomes to Enhance Hedgehog Signaling and Bone Repair. Mol. Pharm. 2021, 18, 1677–1689. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.C.; Zheng, C.P.; Li, Y.Q.; Bian, S.Q.; Pan, H.B.; Zhao, X.L.; Lu, W.W. Bone Targeted Delivery of SDF-1 via Alendronate Functionalized Nanoparticles in Guiding Stem Cell Migration. ACS Appl. Mater. Interfaces 2018, 10, 23700–23710. [Google Scholar] [CrossRef]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural Polymeric Scaffolds in Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 474. [Google Scholar] [CrossRef] [PubMed]
- De Witte, T.M.; Wagner, A.M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Degradable Poly(Methyl Methacrylate)-co-Methacrylic Acid Nanoparticles for Controlled Delivery of Growth Factors for Bone Regeneration. Tissue Eng. Part A 2020, 26, 1226–1242. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, R.; Hwang, M.P.; Van, S.Y.; Do, S.H.; Park, H.; Lee, K.; Kim, S.H.; Yun, K.; Park, K. Osteogenic/Angiogenic Dual Growth Factor Delivery Microcapsules for Regeneration of Vascularized Bone Tissue. Adv. Health. Mater. 2015, 4, 1982–1992. [Google Scholar] [CrossRef]
- Parchen, G.P.; Jacumazo, J.; Koop, H.S.; Biscaia, S.M.P.; Trindade, E.S.; Silveira, J.L.M.; de Freitas, R.A. Modulation of Epidermal Growth Factor Release by Biopolymer-Coated Liposomes. J. Pharm. Sci. 2020, 109, 2294–2301. [Google Scholar] [CrossRef]
- Wei, D.X.; Qiao, R.R.; Dao, J.W.; Su, J.; Jiang, C.M.; Wang, X.C.; Gao, M.Y.; Zhong, J. Soybean Lecithin-Mediated Nanoporous PLGA Microspheres with Highly Entrapped and Controlled Released BMP-2 as a Stem Cell Platform. Small 2018, 14, e1800063. [Google Scholar] [CrossRef] [PubMed]
- Gholami, L.; Nooshabadi, V.T.; Shahabi, S.; Jazayeri, M.; Tarzemany, R.; Afsartala, Z.; Khorsandi, K. Extracellular vesicles in bone and periodontal regeneration: Current and potential therapeutic applications. Cell Biosci. 2021, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yu, H.; Sun, M.; Yang, P.; Hu, X.; Ao, Y.; Cheng, J. The tissue origin effect of extracellular vesicles on cartilage and bone regeneration. Acta Biomater. 2021, 125, 253–266. [Google Scholar] [CrossRef]
- Ren, S.; Tang, X.; Liu, L.; Meng, F.; Yang, X.; Li, N.; Zhang, Z.; Aimaijiang, M.; Liu, M.; Liu, X.; et al. Reinforced Blood-Derived Protein Hydrogels Enable Dual-Level Regulation of Bio-Physiochemical Microenvironments for Personalized Bone Regeneration with Remarkable Enhanced Efficacy. Nano Lett. 2022, 22, 3904–3913. [Google Scholar] [CrossRef]
- Cheng, G.; Ma, X.; Li, J.M.; Cheng, Y.E.; Cao, Y.; Wang, Z.M.; Shi, X.W.; Du, Y.M.; Deng, H.B.; Li, Z.B. Incorporating platelet-rich plasma into coaxial electrospun nanofibers for bone tissue engineering. Int. J. Pharmaceut. 2018, 547, 656–666. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Moraschini, V.; Zhang, Y.F.; Gruber, R.; Wang, H.L. Efficacy of platelet-rich fibrin on bone formation, part 1: Alveolar ridge preservation. Int. J. Oral. Impl. 2021, 14, 181–194. [Google Scholar]
- Choi, S.Y.; Song, M.S.; Ryu, P.D.; Lam, A.T.N.; Joo, S.W.; Lee, S.Y. Gold nanoparticles promote osteogenic differentiation in human adipose-derived mesenchymal stem cells through the Wnt/beta-catenin signaling pathway. Int. J. Nanomed. 2015, 10, 4383–4392. [Google Scholar] [CrossRef]
- Sanchez-Duffhues, G.; Hiepen, C.; Knaus, P.; ten Dijke, P. Bone morphogenetic protein signaling in bone homeostasis. Bone 2015, 80, 43–59. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, Z.Y.; Ding, L.; Zhang, P.; Liu, C.; Chen, D.F.; Zhao, F.J.; Wang, G.; Chen, X.F. Self-Adhesive Hydrogel Biomimetic Periosteum to Promote Critical-Size Bone Defect Repair via Synergistic Osteogenesis and Angiogenesis. ACS Appl. Mater. Interfaces 2022, 14, 36395–36410. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Z.; Sun, Y.; Shen, J.J.; Min, H.S.; Xu, J.; Chai, Y.M. Strontium doped mesoporous silica nanoparticles accelerate osteogenesis and angiogenesis in distraction osteogenesis by activation of Wnt pathway. Nanomed. Nanotechnol. 2022, 41, 102496. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.F.; Wang, Z.L. Involvement of FAK/P38 Signaling Pathways in Mediating the Enhanced Osteogenesis Induced by Nano-Graphene Oxide Modification on Titanium Implant Surface. Int. J. Nanomed. 2020, 15, 4659–4676. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, X.R.; Wang, Y.T.; Liu, Y.X.; Pan, Y.N.; Li, Y.J.; Ji, M.; Zhao, X.Q.; Huang, S.B.; Yao, Q.Q. Hypoxia-mimicking 3D bioglass-nanoclay scaffolds promote endogenous bone regeneration. Bioact. Mater. 2021, 6, 3485–3495. [Google Scholar] [CrossRef]
- Huang, C.C.; Kang, M.; Lu, Y.; Shirazi, S.; Diaz, J.I.; Cooper, L.F.; Gajendrareddy, P.; Ravindran, S. Functionally engineered extracellular vesicles improve bone regeneration. Acta Biomater. 2020, 109, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Zolnik, B.S.; Gonzalez-Fernandez, A.; Sadrieh, N.; Dobrovolskaia, M.A. Nanoparticles and the immune system. Endocrinology 2010, 151, 458–465. [Google Scholar] [CrossRef]
- Mahon, O.R.; Browe, D.C.; Gonzalez-Fernandez, T.; Pitacco, P.; Whelan, I.T.; Von Euw, S.; Hobbs, C.; Nicolosi, V.; Cunningham, K.T.; Mills, K.H.G.; et al. Nano-particle mediated M2 macrophage polarization enhances bone formation and MSC osteogenesis in an IL-10 dependent manner. Biomaterials 2020, 239, 119833. [Google Scholar] [CrossRef]
- Jin, S.S.; He, D.Q.; Luo, D.; Wang, Y.; Yu, M.; Guan, B.; Fu, Y.; Li, Z.X.; Zhang, T.; Zhou, Y.H.; et al. A Biomimetic Hierarchical Nanointerface Orchestrates Macrophage Polarization and Mesenchymal Stem Cell Recruitment To Promote Endogenous Bone Regeneration. Acs Nano 2019, 13, 6581–6595. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak, P.; Koziol, M.; Przekora, A. The Chitosan/Agarose/NanoHA Bone Scaffold-Induced M2 Macrophage Polarization and Its Effect on Osteogenic Differentiation In Vitro. Int. J. Mol. Sci. 2021, 22, 1109. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Jin, C.; Ma, L.; Feng, X.B.; Deng, X.Y.; Wu, S.L.; Liu, X.M.; Yang, C. Accelerated Bone Regeneration by Gold-Nanoparticle-Loaded Mesoporous Silica through Stimulating Immunomodulation. ACS Appl. Mater. Interfaces 2019, 11, 41758–41769. [Google Scholar] [CrossRef] [PubMed]
- Toita, R.; Kang, J.H.; Tsuchiya, A. Phosphatidylserine liposome multilayers mediate the M1-to-M2 macrophage polarization to enhance bone tissue regeneration. Acta Biomater. 2022, 154, 583–596. [Google Scholar] [CrossRef]
- Nakazaki, M.; Morita, T.; Lankford, K.L.; Askenase, P.W.; Kocsis, J.D. Small extracellular vesicles released by infused mesenchymal stromal cells target M2 macrophages and promote TGF-beta upregulation, microvascular stabilization and functional recovery in a rodent model of severe spinal cord injury. J. Extracell Vesicles 2021, 10, e12137. [Google Scholar] [CrossRef]
- Huang, Q.L.; Ouyang, Z.X.; Tan, Y.N.; Wu, H.; Liu, Y. Activating macrophages for enhanced osteogenic and bactericidal performance by Cu ion release from micro/nano-topographical coating on a titanium substrate. Acta Biomater. 2019, 100, 415–426. [Google Scholar] [CrossRef]
- Song, Y.; Wu, H.; Gao, Y.; Li, J.; Lin, K.; Liu, B.; Lei, X.; Cheng, P.; Zhang, S.; Wang, Y.; et al. Zinc Silicate/Nano-Hydroxyapatite/Collagen Scaffolds Promote Angiogenesis and Bone Regeneration via the p38 MAPK Pathway in Activated Monocytes. ACS Appl. Mater. Interfaces 2020, 12, 16058–16075. [Google Scholar] [CrossRef]
- Yin, C.C.; Zhao, Q.; Li, W.; Zhao, Z.F.; Wang, J.Y.; Deng, T.; Zhang, P.; Shen, K.L.; Li, Z.B.; Zhang, Y.F. Biomimetic anti-inflammatory nano-capsule serves as a cytokine blocker and M2 polarization inducer for bone tissue repair. Acta Biomater. 2020, 102, 416–426. [Google Scholar] [CrossRef]
- Li, M.T.; Wei, F.; Yin, X.Q.; Xiao, L.; Yang, L.; Su, J.H.; Weng, J.; Feng, B.; Xiao, Y.; Zhou, Y.H. Synergistic regulation of osteoimmune microenvironment by IL-4 and RGD to accelerate osteogenesis. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 109, 110508. [Google Scholar] [CrossRef]
- Bordoni, V.; Reina, G.; Orecchioni, M.; Furesi, G.; Thiele, S.; Gardin, C.; Zavan, B.; Cuniberti, G.; Bianco, A.; Rauner, M.; et al. Stimulation of bone formation by monocyte-activator functionalized graphene oxide in vivo. Nanoscale 2019, 11, 19408–19421. [Google Scholar] [CrossRef]
- Singhatanadgit, W.; Toso, M.; Pratheepsawangwong, B.; Pimpin, A.; Srituravanich, W. Titanium dioxide nanotubes of defined diameter enhance mesenchymal stem cell proliferation via JNK- and ERK-dependent up-regulation of fibroblast growth factor-2 by T lymphocytes. J. Biomater. Appl. 2019, 33, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, K.H.; Kim, S.; Lee, Y.M.; Seol, Y.J. BMP-2 Gene Delivery-Based Bone Regeneration in Dentistry. Pharmaceutics 2019, 11, 393. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guo, Z.; Tian, H.; Chen, X. Production and clinical development of nanoparticles for gene delivery. Mol. Ther. Methods Clin. Dev. 2016, 3, 16023. [Google Scholar] [CrossRef] [PubMed]
- Yau, W.W.Y.; Rujitanaroj, P.O.; Lam, L.; Chew, S.Y. Directing stem cell fate by controlled RNA interference. Biomaterials 2012, 33, 2608–2628. [Google Scholar] [CrossRef]
- Geng, Z.; Wang, X.G.; Zhao, J.; Li, Z.Y.; Ma, L.L.; Zhu, S.L.; Liang, Y.Q.; Cui, Z.D.; He, H.Y.; Yang, X.J. The synergistic effect of strontium-substituted hydroxyapatite and microRNA-21 on improving bone remodeling and osseointegration. Biomater. Sci. 2018, 6, 2694–2703. [Google Scholar] [CrossRef]
- Li, D.H.; Yang, Z.Y.; Luo, Y.; Zhao, X.; Tian, M.; Kang, P.D. Delivery of MiR335-5p-Pendant Tetrahedron DNA Nanostructures Using an Injectable Heparin Lithium Hydrogel for Challenging Bone Defects in Steroid-Associated Osteonecrosis. Adv. Health. Mater. 2022, 11, 2101412. [Google Scholar] [CrossRef]
- Raimondo, S.; Urzi, O.; Conigliaro, A.; Bosco, G.L.; Parisi, S.; Carlisi, M.; Siragusa, S.; Raimondi, L.; Luca, A.; Giavaresi, G.; et al. Extracellular Vesicle microRNAs Contribute to the Osteogenic Inhibition of Mesenchymal Stem Cells in Multiple Myeloma. Cancers 2020, 12, 449. [Google Scholar] [CrossRef]
- Guo, S.; Gu, J.; Ma, J.; Xu, R.; Wu, Q.; Meng, L.; Liu, H.; Li, L.; Xu, Y. GATA4-driven miR-206-3p signatures control orofacial bone development by regulating osteogenic and osteoclastic activity. Theranostics 2021, 11, 8379–8395. [Google Scholar] [CrossRef]
- Wei, F.; Li, M.; Crawford, R.; Zhou, Y.; Xiao, Y. Exosome-integrated titanium oxide nanotubes for targeted bone regeneration. Acta Biomater. 2019, 86, 480–492. [Google Scholar] [CrossRef]
- Zhuang, Y.; Cheng, M.; Li, M.; Cui, J.; Huang, J.; Zhang, C.; Si, J.; Lin, K.; Yu, H. Small extracellular vesicles derived from hypoxic mesenchymal stem cells promote vascularized bone regeneration through the miR-210-3p/EFNA3/PI3K pathway. Acta Biomater. 2022, 150, 413–426. [Google Scholar] [CrossRef]
- Chen, S.; Tang, Y.; Liu, Y.; Zhang, P.; Lv, L.; Zhang, X.; Jia, L.; Zhou, Y. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. 2019, 52, e12669. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.G.; Guo, B.S.; Li, Q.; Peng, J.; Yang, Z.J.; Wang, A.Y.; Li, D.; Hou, Z.B.; Lv, K.; Kan, G.H.; et al. miR-214 targets ATF4 to inhibit bone formation. Nat. Med. 2013, 19, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.L.; Lan, Y.; Feng, Z.A.; Feng, L.B.; Yang, J.J.; Liu, Y.; Bian, L.M.; Tan, J.L.; Lai, R.F.; Guo, R. Functionalization of SF/HAP Scaffold with GO-PEI-miRNA inhibitor Complexes to Enhance Bone Regeneration through Activating Transcription Factor 4. Theranostics 2019, 9, 4525–4541. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.N.; Chen, X.; Zhang, Z.P.; Zhang, X.J.; Saunders, L.; Zhou, Y.S.; Ma, P.X. Nanofibrous Spongy Microspheres To Distinctly Release miRNA and Growth Factors To Enrich Regulatory T Cells and Rescue Periodontal Bone Loss. Acs Nano 2018, 12, 9785–9799. [Google Scholar] [CrossRef]
- Xing, H.L.; Wang, X.; Xiao, G.; Zhao, Z.M.; Zou, S.Q.; Li, M.; Richardson, J.J.; Tardy, B.L.; Xie, L.X.; Komasa, S.; et al. Hierarchical assembly of nanostructured coating for siRNA-based dual therapy of bone regeneration and revascularization. Biomaterials 2020, 235, 119784. [Google Scholar] [CrossRef]
- Malek-Khatabi, A.; Javar, H.A.; Dashtimoghadam, E.; Ansari, S.; Hasani-Sadrabadi, M.M.; Moshaverinia, A. In situ bone tissue engineering using gene delivery nanocomplexes. Acta Biomater. 2020, 108, 326–336. [Google Scholar] [CrossRef]
- Tenkumo, T.; Saenz, J.R.V.; Nakamura, K.; Shimizu, Y.; Sokolova, V.; Epple, M.; Kamano, Y.; Egusa, H.; Sugaya, T.; Sasaki, K. Prolonged release of bone morphogenetic protein-2 in vivo by gene transfection with DNA-functionalized calcium phosphate nanoparticle-loaded collagen scaffolds. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018, 92, 172–183. [Google Scholar] [CrossRef]
- Jalal, A.R.; Dixon, J.E. Efficient Delivery of Transducing Polymer Nanoparticles for Gene-Mediated Induction of Osteogenesis for Bone Regeneration. Front. Bioeng. Biotech. 2020, 8, 849. [Google Scholar] [CrossRef]
- Chakka, J.L.; Acri, T.; Laird, N.Z.; Zhong, L.; Shin, K.; Elangovan, S.; Salem, A.K. Polydopamine functionalized VEGF gene-activated 3D printed scaffolds for bone regeneration. Rsc. Adv. 2021, 11, 13282–13291. [Google Scholar] [CrossRef]
- Zha, Y.; Li, Y.W.; Lin, T.Y.; Chen, J.; Zhang, S.M.; Wang, J.L. Progenitor cell-derived exosomes endowed with VEGF plasmids enhance osteogenic induction and vascular remodeling in large segmental bone defects. Theranostics 2021, 11, 397–409. [Google Scholar] [CrossRef]
- Cai, Y.; Wu, C.X.; Ou, Q.H.; Zeng, M.H.; Xue, S.; Chen, J.L.; Lu, Y.; Ding, C.H. Enhanced osteoarthritis therapy by nanoengineered mesenchymal stem cells using biomimetic CuS nanoparticles loaded with plasmid DNA encoding TGF-beta 1. Bioact. Mater. 2023, 19, 444–457. [Google Scholar] [CrossRef]
- Raftery, R.M.; Mencia-Castano, I.; Sperger, S.; Chen, G.; Cavanagh, B.; Feichtinger, G.A.; Redl, H.; Hacobian, A.; O’Brien, F.J. Delivery of the improved BMP-2-Advanced plasmid DNA within a gene-activated scaffold accelerates mesenchymal stem cell osteogenesis and critical size defect repair. J. Control. Release 2018, 283, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.P.; Raftery, R.M.; Murphy, R.; Chen, G.; Heise, A.; O’Brien, F.J.; Cryan, S.A. Gene activated scaffolds incorporating star-shaped polypeptide-pDNA nanomedicines accelerate bone tissue regeneration in vivo. Biomater. Sci. 2021, 9, 4984–4999. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Fang, J.; Zhong, C.X.; Wang, M.; Ren, F.Z. Spatiotemporal Delivery of pBMP2 and pVEGF by a Core-Sheath Structured Fiber-Hydrogel Gene-Activated Matrix Loaded with Peptide-Modified Nanoparticles for Critical-Sized Bone Defect Repair. Adv. Health. Mater. 2022, 11, 2201096. [Google Scholar] [CrossRef] [PubMed]
- Moncal, K.K.; Aydin, R.S.T.; Godzik, K.P.; Acri, T.M.; Heo, D.N.; Rizk, E.; Wee, H.; Lewis, G.S.; Salem, A.K.; Ozbolat, I.T. Controlled Co-delivery of pPDGF-B and pBMP-2 from intraoperatively bioprinted bone constructs improves the repair of calvarial defects in rats. Biomaterials 2022, 281, 121333. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Xu, C.; Lei, C. The Delivery and Activation of Growth Factors Using Nanomaterials for Bone Repair. Pharmaceutics 2023, 15, 1017. https://doi.org/10.3390/pharmaceutics15031017
Li Y, Xu C, Lei C. The Delivery and Activation of Growth Factors Using Nanomaterials for Bone Repair. Pharmaceutics. 2023; 15(3):1017. https://doi.org/10.3390/pharmaceutics15031017
Chicago/Turabian StyleLi, Yiwei, Chun Xu, and Chang Lei. 2023. "The Delivery and Activation of Growth Factors Using Nanomaterials for Bone Repair" Pharmaceutics 15, no. 3: 1017. https://doi.org/10.3390/pharmaceutics15031017
APA StyleLi, Y., Xu, C., & Lei, C. (2023). The Delivery and Activation of Growth Factors Using Nanomaterials for Bone Repair. Pharmaceutics, 15(3), 1017. https://doi.org/10.3390/pharmaceutics15031017







