Synthesis and Characterization of Supermagnetic Nanocomposites Coated with Pluronic F127 as a Contrast Agent for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Chemicals, and Apparatus
2.2. Synthesis of the Nanocomposites
2.3. Characterization
2.4. Formulation of MNP-F127-DOX Core-Shell Nanocomposites
2.5. In Vitro DOX Release under Conditions Involving Various pH
2.6. Cytotoxicity Assay
2.7. Cellular Uptake
2.8. Statistical Analyses
3. Results and Discussion
3.1. Characterization of MNP and MNP-F127
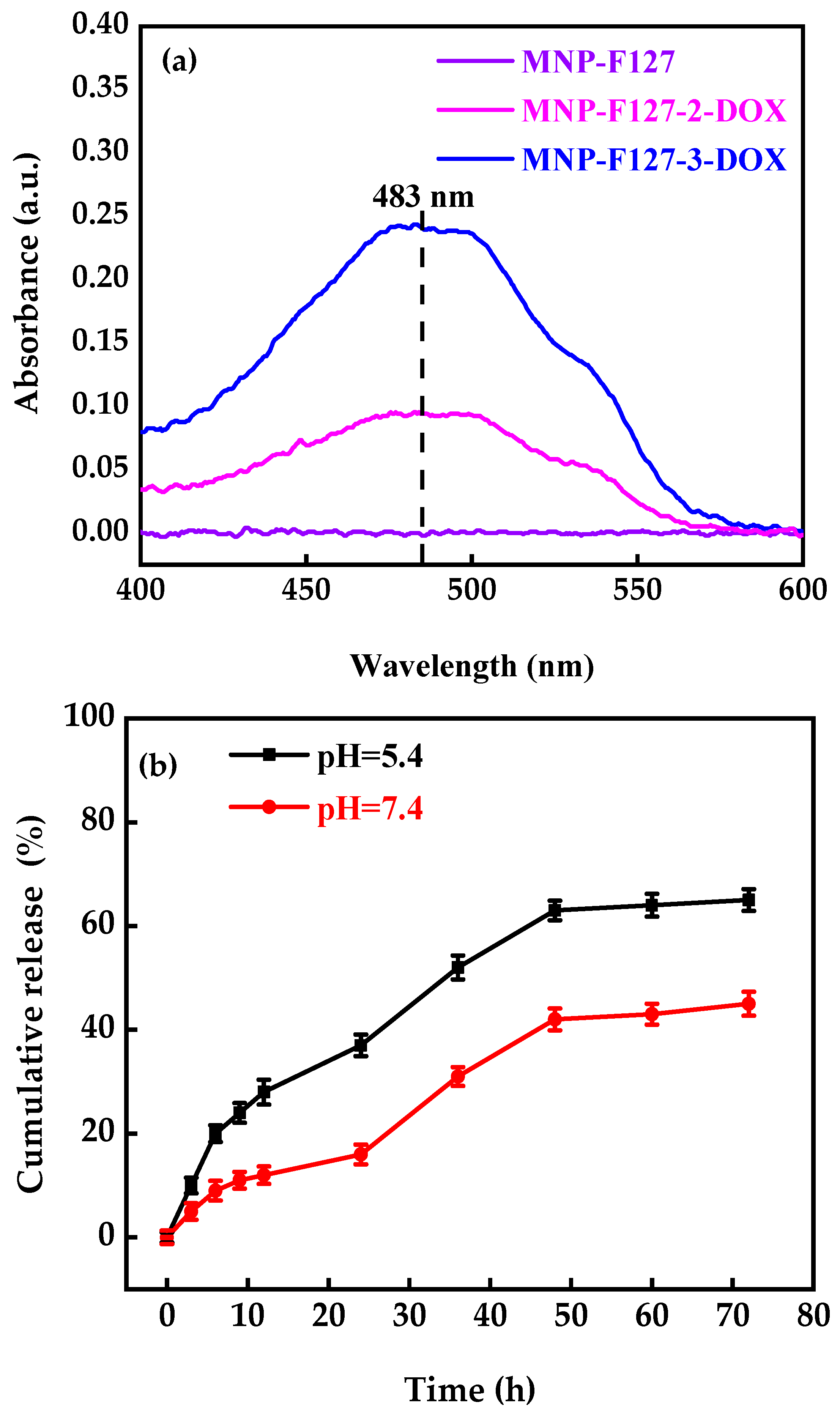
3.2. DOX Encapsulation and Release
3.3. In Vitro Cytotoxicity Studies
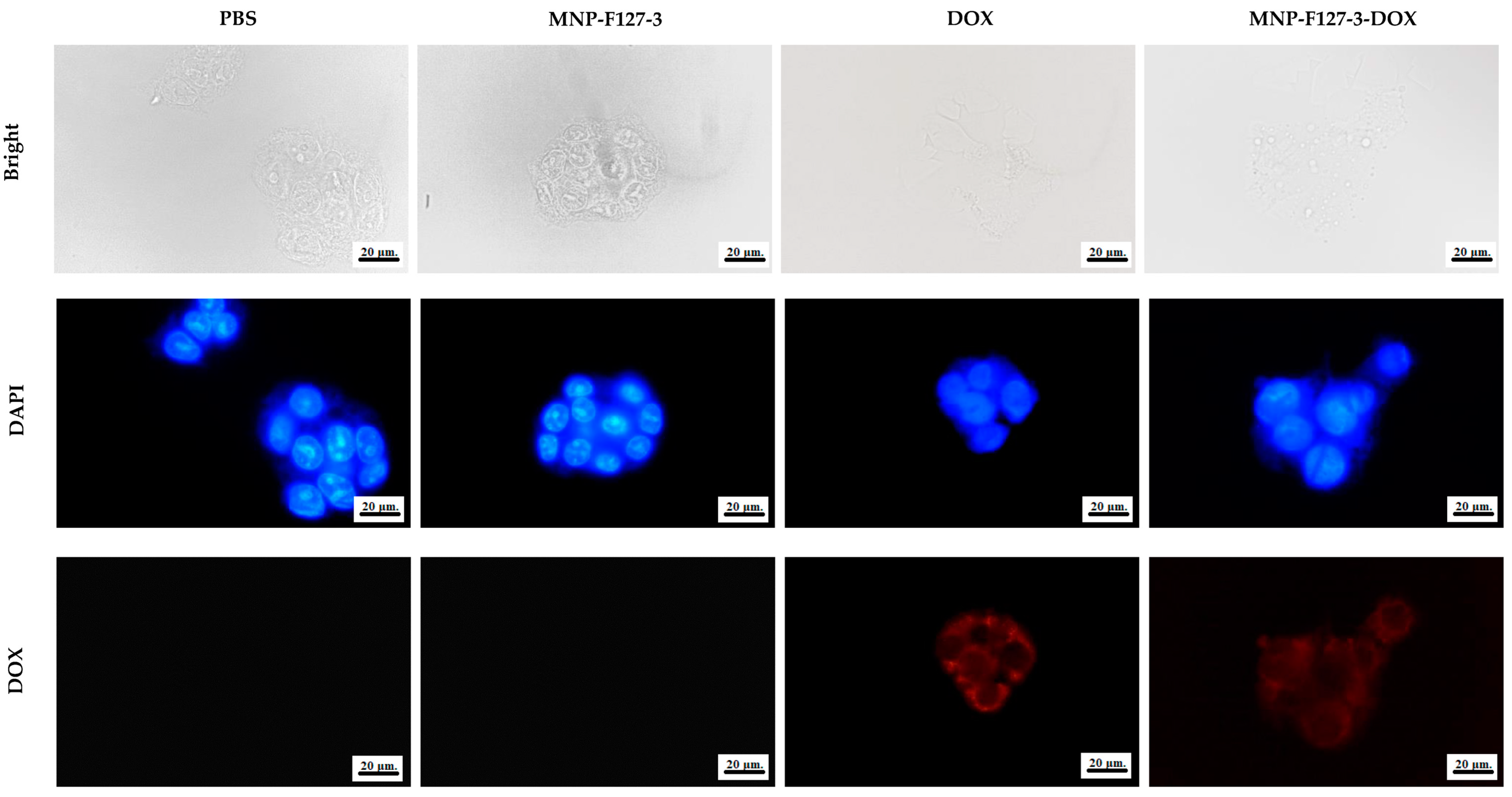
3.4. Cellular Uptake
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhi, D.; Yang, T.; Yang, J.; Fu, S.; Zhang, S. Targeting strategies for superparamagnetic iron oxide nanoparticles in cancer therapy. Acta Biomater. 2020, 102, 13–34. [Google Scholar] [CrossRef]
- Zugazagoitia, J.; Guedes, C.; Ponce, S.; Ferrer, I.; Molina-Pinelo, S.; Paz-Ares, L. Current challenges in cancer treatment. Clin. Ther. 2016, 38, 1551–1566. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, Y.; Jia, F.; Liu, W.; Zhou, D.; Jin, Q.; Ji, J. Tailoring supramolecular prodrug nanoassemblies for reactive nitrogen species-potentiated chemotherapy of liver cancer. ACS Nano 2021, 15, 8663–8675. [Google Scholar] [CrossRef]
- Zong, Y.; Friedman, J.R. Liver development. Dig. Liver Dis. 2014, 4, 1–813. [Google Scholar] [CrossRef]
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Soerjomataram, I. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Rumgay, H.; Ferlay, J.; de Martel, C.; Georges, D.; Ibrahim, A.S.; Zheng, R.; Soerjomataram, I. Global, regional and national burden of primary liver cancer by subtype. Eur. J. Cancer 2022, 161, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Moreno, M.; Silva-Gomez, J.A.; Lucano-Landeros, S.; Santos, A.; Monroy-Ramirez, H.C.; Armendariz-Borunda, J. Liver cancer: Therapeutic challenges and the importance of experimental models. Can. J. Gastroenterol. Hepatol. 2021, 2021, 8837811. [Google Scholar] [CrossRef] [PubMed]
- Gravante, G.; Carino, N.D.L.; Overton, J.; Manzia, T.M.; Orlando, G. Primary carcinoids of the liver: A review of symptoms, diagnosis and treatments. Dig. Surg. 2008, 25, 364–368. [Google Scholar] [CrossRef]
- Thorgeirsson, S.S.; Grisham, J.W. Molecular pathogenesis of human hepatocellular carcinoma. Nat. Genet. 2002, 31, 339–346. [Google Scholar] [CrossRef]
- Brenner, D.J.; Doll, R.; Goodhead, D.T.; Hall, E.J.; Land, C.E.; Little, J.B.; Zaider, M. Cancer risks attributable to low doses of ionizing radiation: Assessing what we really know. Proc. Natl. Acad. Sci. USA 2003, 100, 13761–13766. [Google Scholar] [CrossRef]
- Gross, C.E.; Frank, R.M.; Hsu, A.R.; Diaz, A.; Gitelis, S. External beam radiation therapy for orthopaedic pathology. JAAOS-J. Am. Acad. Orthop. Surg. 2015, 23, 243–252. [Google Scholar] [CrossRef]
- Dobson, J. Magnetic nanoparticles for drug delivery. Drug Dev. Res. 2006, 67, 55–60. [Google Scholar] [CrossRef]
- Corrie, P.G. Cytotoxic chemotherapy: Clinical aspects. Medicine 2008, 36, 24–28. [Google Scholar] [CrossRef]
- Sinha, R.; Kim, G.J.; Nie, S.; Shin, D.M. Nanotechnology in cancer therapeutics: Bioconjugated nanoparticles for drug delivery. Mol. Cancer Ther. 2006, 5, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, C.; Zhang, X.; Chen, G.; Hu, Q.; Li, H.; Gu, Z. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 2019, 14, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, J.; Zhao, N.; Wang, C.; Kamar, S.; Zhou, Y.; He, Z.; Yang, J.; Sun, B.; Shi, X.; et al. Progress in the chemotherapeutic treatment of osteosarcoma. Oncol. Lett. 2018, 16, 6228–6237. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.K.; Liu, Y.P.; Ho, J.H.; Hsu, S.C.; Lee, O.K. Amine-surface-modified super- paramagnetic iron oxide nanoparticles interfere with differentiation of human mesenchymal stem cells. J. Orthop. Res. 2012, 30, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Karp, J.M.; Langer, R.; Joshi, N. The future of drug delivery. Chem. Mater. 2023, 35, 359–363. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Kohestanian, M.; Streb, C. pH and thermal dual-responsive poly (NIPAM-co-GMA)-coated magnetic nanoparticles via surface-initiated RAFT polymerization for controlled drug delivery. Mater. Sci. Eng. C 2020, 108, 110418. [Google Scholar] [CrossRef] [PubMed]
- Vallabani, N.V.; Singh, S.; Karakoti, A.S. Magnetic nanoparticles: Current trends and future aspects in diagnostics and nanomedicine. Curr. Drug Metab. 2019, 20, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, J.A.; Chilkoti, A. Nanomaterials for drug delivery. Science 2012, 337, 303–305. [Google Scholar] [CrossRef]
- Liang, R.; Wei, M.; Evans, D.G.; Duan, X. Inorganic nanomaterials for bioimaging targeted drug delivery and therapeutic. Chem. Commun. 2014, 50, 14071–14081. [Google Scholar] [CrossRef] [PubMed]
- El-Boubbou, K. Magnetic iron oxide nanoparticles as drug carriers: Clinical relevance. Nanomedicine 2018, 13, 953–971. [Google Scholar] [CrossRef]
- Mou, X.; Ali, Z.; Li, S.; He, N. Applications of magnetic nanoparticles in targeted drug delivery system. J. Nanosci. Nanotechnol. 2015, 15, 54–62. [Google Scholar] [CrossRef]
- Wang, X.Q.; Zhang, Q. pH-sensitive polymeric nanoparticles to improve oral bioavailability of peptide/protein drugs and poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2012, 82, 219–229. [Google Scholar] [CrossRef]
- Jones, M.C.; Ranger, M.; Leroux, J.C. pH-sensitive unimolecular polymeric micelles: Synthesis of a novel drug carrier. Bioconjug. Chem. 2003, 14, 774–781. [Google Scholar] [CrossRef]
- Kong, S.D.; Luong, A.; Manorek, G.; Howell, S.B.; Yang, J. Acidic hydrolysis of N- ethoxybenzylimidazoles (NEBIs): Potential applications as pH-sensitive linkers for drug delivery. Bioconjug. Chem. 2007, 18, 293–296. [Google Scholar] [CrossRef]
- Bromberg, L.; Alakhov, V.Y.; Hatton, T.A. Enhanced transfection of polyplexes based on pluronic-polypropylenimine dendrimer for gene transfer. Curr. Opin. Colloid Interface Sci. 2006, 11, 217–223. [Google Scholar] [CrossRef]
- Liang, W.Q.; Gong, H.Y.; Yin, D.F.; Lu, S.Y.; Fu, Q. Biomedical applications of biodegradable polymers. Chem. Pharm. Bull. 2011, 59, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.M.; Zhang, X.; Li, B.J.; Sun, X.; Zhang, S. pH-induced shape-memory polymers. Macromol. Rapid Commun. 2011, 32, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Fusco, S.; Borzacchiello, A.; Netti, P.A. Thermoresponsive poly(ε-caprolactone)-poly(ethylene/propylene glycol) copolymers as injectable hydrogels for cell therapies. J. Bioact. Compat. Polym. 2006, 21, 149–164. [Google Scholar] [CrossRef]
- Weinand, C.; Pomerantseva, I.; Neville, C.M.; Gupta, R.; Weinberg, E.; Madisch, I.; Shapiro, F.; Abukawa, H.; Troulis, M.J.; Vacanti, J.P. Thermoresponsive hydrogels in biomedical applications-a review. Bone 2006, 38, 555–563. [Google Scholar] [CrossRef]
- Rahdar, A.; Hajinezhad, M.R.; Sargazi, S.; Barani, M.; Karimi, P.; Velasco, B.; Zarei, S. Pluronic F127/carfilzomib-based nanomicelles as promising nanocarriers: Synthesis, characterization, biological, and in silico evaluations. J. Mol. Liq. 2022, 346, 118271. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Xiao, M.; Wang, D.; Qu, Y.; Zou, L.; Zhang, J. Oral colon-targeted mucoadhesive micelles with enzyme-responsive controlled release of curcumin for ulcerative colitis therapy. Chin. Chem. Lett. 2022, 33, 4924–4929. [Google Scholar] [CrossRef]
- Li, J.; Zhao, J.; Tan, T.; Liu, M.; Zeng, Z.; Zeng, Y.; Xie, T. Nanoparticle drug delivery system for glioma and its efficacy improvement strategies: A comprehensive review. Int. J. Nanomed. 2020, 15, 2563. [Google Scholar] [CrossRef]
- Sun, S.J.; Deng, P.; Peng, C.E.; Ji, H.Y.; Mao, L.F.; Peng, L.Z. Selenium-modified chitosan induces Hepg2 cell apoptosis and differential protein analysis. Cancer Manag. Res. 2022, 14, 3335–3345. [Google Scholar] [CrossRef]
- Sargazi, S.; Hajinezhad, M.R.; Barani, M.; Mukhtar, M.; Rahdar, A.; Baino, F.; Pandey, S. F127/cisplatin microemulsions: In vitro, in vivo and computational studies. Appl. Sci. 2021, 11, 3006. [Google Scholar] [CrossRef]
- Lin, J.J.; Chen, J.S.; Huang, S.J.; Ko, J.H.; Wang, Y.M.; Chen, T.L.; Wang, L.F. Folic acid–Pluronic F127 magnetic nanoparticle clusters for combined targeting, diagnosis, and therapy applications. Biomaterials 2009, 30, 5114–5124. [Google Scholar] [CrossRef]
- Oh, S.H.; Kim, J.H.; Song, K.S.; Jeon, B.H.; Yoon, V.; Seo, T.B.; Namgung, U.; Lee, I.W.; Lee, J.H. Peripheral nerve regeneration within an asymmetrically porous PLGA/Pluronic F127 nerve guide conduit. Biomaterials 2008, 29, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, J.H.; Oh, S.H.; Kim, S.J.; Hah, Y.S.; Park, B.W.; Kim, D.R.; Rho, G.J.; Maeng, G.H.; Jeon, R.H.; et al. Tissue-engineered bone formation using periosteal-derived cells and polydioxanone/pluronic F127 scaffold with pre-seeded adipose tissue-derived CD146 positive endothelial-like cells. Biomaterials 2011, 32, 5033–5045. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Li, L.; Dong, H.Q.; Cai, X.J.; Ren, T.B. Pluronic F127 nanomicelles engineered with nuclear localized functionality for targeted drug delivery. Mater. Sci. Eng. C 2013, 33, 2698–2707. [Google Scholar] [CrossRef] [PubMed]
- Alakhov, V.Y.; Moskaleva, E.Y.; Batrakova, E.V.; Kabanov, A.V. Hypersensitization of multidrug resistant human ovarian carcinoma cells by pluronic P85 block copolymer. Bioconjug. Chem. 1996, 7, 209–216. [Google Scholar] [CrossRef]
- Arcamone, F.; Cassinelli, G.; Fantini, G.; Grein, A.; Orezzi, P.; Pol, C. Adriamycin, 14-hydroxydaunomycin: A new antitumor antibiotic from S. peucetius var. caesius. Biotechnol Bioeng. 1969, 11, 1101–1110. [Google Scholar] [CrossRef]
- Cortes-Funes, H.; Coronado, C. Role of anthracyclines in the era of targeted therapy. Cardiovasc. Toxicol. 2007, 7, 56–60. [Google Scholar] [CrossRef]
- Zhao, N.; Woodle, M.C.; Mixson, A.J. Advances in delivery systems for doxorubicin. J. Nanomed. Nanotechnol. 2018, 9, 1–22. [Google Scholar] [CrossRef]
- Espinoza, M.J.C.; Lin, K.S.; Weng, M.T.; Kunene, S.C.; Liu, S.Y.; Lin, Y.S. In vivo and in vitro studies of magnetic silica nanocomposites decorated with Pluronic F127 for controlled drug delivery system. J. Ind. Eng. Chem. 2022, 115, 510–520. [Google Scholar] [CrossRef]
- Rahdar, A.; Hajinezhad, M.R.; Barani, M.; Sargazi, S.; Zaboli, M.; Ghazy, E.; Pandey, S. Pluronic F127/Doxorubicin microemulsions: Preparation, characterization, and toxicity evaluations. J. Mol. Liq. 2022, 345, 117028. [Google Scholar] [CrossRef]
- Espinoza, M.J.C.; Lin, K.S.; Weng, M.T.; Kunene, S.C.; Lin, Y.S.; Lin, Y.T. Synthesis and characterization of silica nanoparticles from rice ashes coated with chitosan/cancer cell membrane for hepatocellular cancer treatment. Int. J. Biol. Macromol. 2023, 228, 487–497. [Google Scholar] [CrossRef]
- Xu, B.; Yuan, L.; Hu, Y.; Xu, Z.; Qin, J.J.; Cheng, X.D. Synthesis, characterization, cellular uptake, and in vitro anticancer activity of fullerenol-doxorubicin conjugates. Front. Pharmacol. 2021, 11, 598155. [Google Scholar] [CrossRef] [PubMed]
- Bekaroğlu, M.G.; Kiriş, A.; Başer, H.N.; İşçi, S. Stabilizer effect of tumor-targeting ligands on the drug delivering Fe3O4 nanoparticles. Appl. Phys. A 2023, 129, 182. [Google Scholar] [CrossRef]
- Ansari, A.A.; Hasan, T.N.; Syed, N.A.; Labis, J.P.; Alshatwi, A.A. In-vitro cytotoxicity and cellular uptake studies of luminescent functionalized core-shell nanospheres. Saudi J. Biol. Sci. 2017, 24, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Z.; Peng, Y.; Ding, J.; Zhou, W. A smart pH-sensitive delivery system for enhanced anticancer efficacy via paclitaxel endosomal escape. Saudi J. Biol. Sci. 2019, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zang, F.; Wu, H.; Li, J.; Xie, J.; Ma, M.; Zhang, Y. Using PEGylated magnetic nanoparticles to describe the EPR effect in tumor for predicting therapeutic efficacy of micelle drugs. Nanoscale 2018, 10, 1788–1797. [Google Scholar] [CrossRef] [PubMed]
- Shanina, B.D.; Konchits, A.A.; Krasnovyd, S.V.; Shevchenko, Y.B.; Petranovs’ ka, A.L.; Rieznichenko, L.S. Magnetic nanoparticle ensembles with promising biophysical applications: An EPR study. J. Appl. Phys. 2022, 132, 163905. [Google Scholar] [CrossRef]
- Osada, K.; Christie, R.J.; Kataoka, K. Polymeric micelles from poly (ethylene glycol)–poly (amino acid) block copolymer for drug and gene delivery. J. R. Soc. Interface 2009, 6, S325–S339. [Google Scholar] [CrossRef]
- Lee, J.; Choa, Y.H.; Kim, J.; Kim, K.H. Comparison of the magnetic properties for the surface-modified magnetite nanoparticles. IEEE Trans. Magn. 2011, 47, 2874–2877. [Google Scholar] [CrossRef]
- Homogen, M. Synthesis and physicochemical properties of magnetite nanoparticles (Fe3O4) as potential solid support for homogeneous catalysts. Malays. J. Anal. Sci. 2018, 22, 768–774. [Google Scholar] [CrossRef]
- Zahoor, M.; Ullah, A.; Alam, S.; Muhammad, M.; Hendroko Setyobudi, R.; Zekker, I.; Sohail, A. Novel magnetite nanocomposites (Fe3O4/C) for efficient immobilization of ciprofloxacin from aqueous solutions through adsorption pre-treatment and membrane processes. Water 2022, 14, 724. [Google Scholar] [CrossRef]
- Fan, X.; Xie, L.; Liang, J.; Ren, Y.; Zhang, L.; Yue, L.; Sun, X. In situ grown Fe3O4 particle on stainless steel: A highly efficient electrocatalyst for nitrate reduction to ammonia. Nano Res. 2022, 15, 3050–3055. [Google Scholar] [CrossRef]
- Shagholani, H.; Ghoreishi, S.M.; Mousazadeh, M. Improvement of interaction between PVA and chitosan via magnetite nanoparticles for drug delivery application. Int. J. Biol. Macromol. 2015, 78, 130–136. [Google Scholar] [CrossRef]
- Al Kayal, T.; Panetta, D.; Canciani, B.; Losi, P.; Tripodi, M.; Burchielli, S.; Soldani, G. Evaluation of the effect of a gamma irradiated DBM-pluronic F127 composite on bone regeneration in Wistar rat. PLoS ONE 2015, 10, e0125110. [Google Scholar] [CrossRef]
- Wang, T.; Bai, J.; Jiang, X. Cellular uptake of nanoparticles by membrane penetration: A study combining confocal microscopy with FTIR spectroelectrochemistry. ACS Nano 2012, 6, 1251–1259. [Google Scholar] [CrossRef]
- Mapukata, S.; Osifeko, O.L.; Nyokong, T. Dual phototransformation of the pollutants methyl orange and Cr (VI) using phthalocyanine-cobalt ferrite based magnetic nanocomposites. Heliyon 2019, 5, e01509. [Google Scholar] [CrossRef] [PubMed]
- Salatin, S.; Yari Khosroushahi, A. Overviews on the cellular uptake mechanism of polysaccharide colloidal nanoparticles. J. Cell. Mol. Med. 2017, 21, 1668–1686. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Khatri, P.K.; Ganguly, S.K.; Jain, S.L. Magnetic silica beads functionalized with cobalt phthalocyanine for the oxidation of mercaptans in an alkali free aqueous medium. RSC Adv. 2014, 4, 29124–29130. [Google Scholar] [CrossRef]
- Giannaccini, M.; Giannini, M.; Calatayud, M.P.; Goya, G.F.; Cuschieri, A.; Dente, L.; Raffa, V. Magnetic nanoparticles as intraocular drug delivery system to target retinal pigmented epithelium (RPE). Int. J. Mol. Sci. 2014, 15, 1590–1605. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.A.; Vanhecke, D.; Michen, B. Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages. Beilstein J. Nanotechnol. 2014, 5, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Madani, F.; Lindberg, S.; Langel, Ü. Mechanisms of cellular uptake of cell-penetrating peptides. J. Biophys. 2011, 2011, 414729. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Lu, F. A study on the hemocompatibility of dendronized chitosan derivatives in red blood cells. Drug Des. Develop. Therapy 2015, 9, 2635. [Google Scholar] [CrossRef]









| Kinetics Model | Parameter | pH 5.4 | pH 7.4 |
|---|---|---|---|
| a First-order | R2 | 0.786 | 0.897 |
| b Korsmeyer–Peppas | R2 | 0.971 | 0.966 |
| c Higuchi | R2 | 0.979 | 0.938 |
| d Weibull | R2 | 0.981 | 0.960 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrera Espinoza, M.J.; Lin, K.-S.; Weng, M.-T.; Kunene, S.C.; Lin, Y.-S.; Wu, C.-M. Synthesis and Characterization of Supermagnetic Nanocomposites Coated with Pluronic F127 as a Contrast Agent for Biomedical Applications. Pharmaceutics 2023, 15, 740. https://doi.org/10.3390/pharmaceutics15030740
Carrera Espinoza MJ, Lin K-S, Weng M-T, Kunene SC, Lin Y-S, Wu C-M. Synthesis and Characterization of Supermagnetic Nanocomposites Coated with Pluronic F127 as a Contrast Agent for Biomedical Applications. Pharmaceutics. 2023; 15(3):740. https://doi.org/10.3390/pharmaceutics15030740
Chicago/Turabian StyleCarrera Espinoza, Maria Janina, Kuen-Song Lin, Meng-Tzu Weng, Sikhumbuzo Charles Kunene, You-Sheng Lin, and Chun-Ming Wu. 2023. "Synthesis and Characterization of Supermagnetic Nanocomposites Coated with Pluronic F127 as a Contrast Agent for Biomedical Applications" Pharmaceutics 15, no. 3: 740. https://doi.org/10.3390/pharmaceutics15030740
APA StyleCarrera Espinoza, M. J., Lin, K.-S., Weng, M.-T., Kunene, S. C., Lin, Y.-S., & Wu, C.-M. (2023). Synthesis and Characterization of Supermagnetic Nanocomposites Coated with Pluronic F127 as a Contrast Agent for Biomedical Applications. Pharmaceutics, 15(3), 740. https://doi.org/10.3390/pharmaceutics15030740







