Application of Machine-Learning Algorithms for Better Understanding the Properties of Liquisolid Systems Prepared with Three Mesoporous Silica Based Carriers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
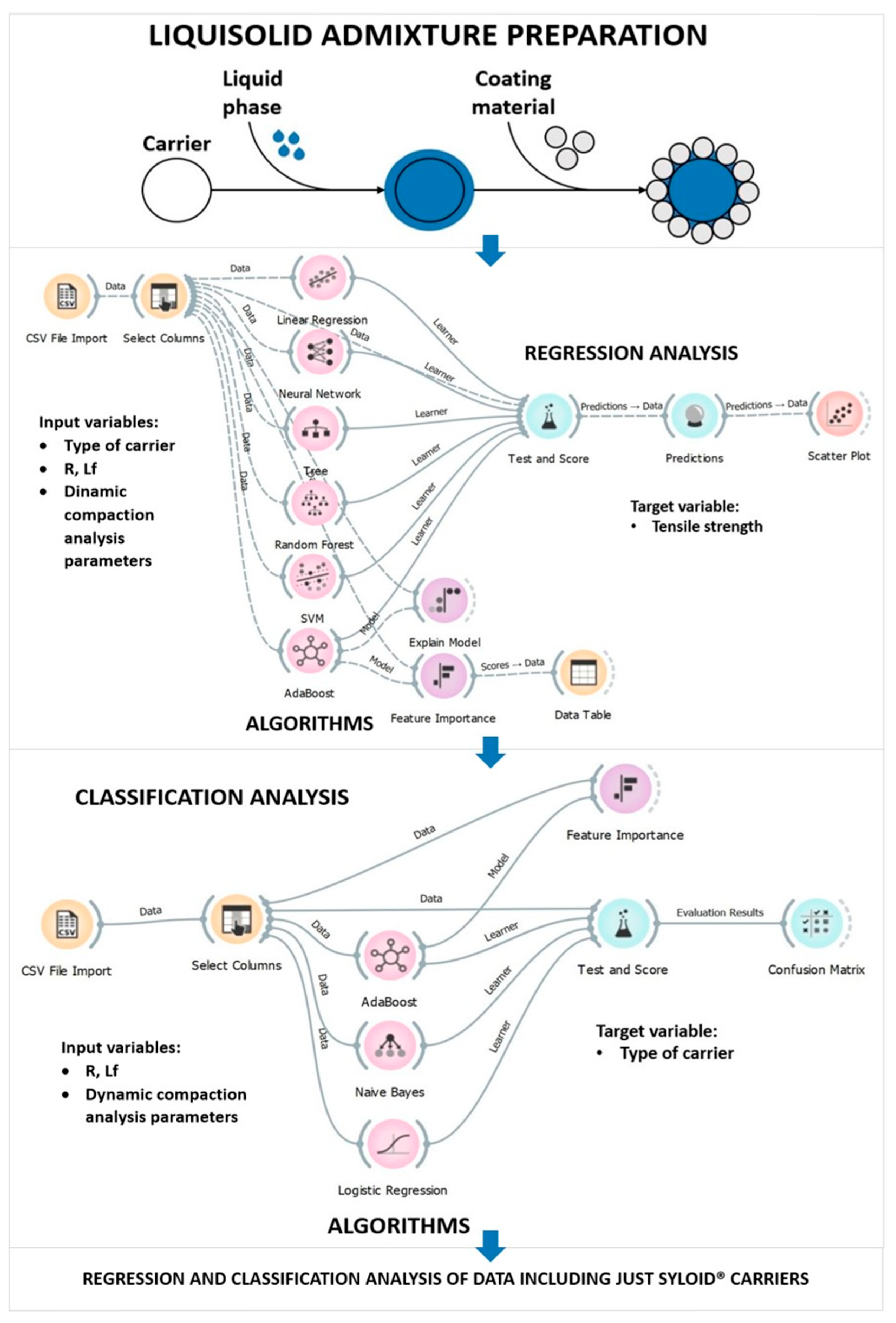
2.2. Preparation of LS Admixtures
2.3. LS Admixtures’ Flowability
2.4. Dynamic Compaction Analysis
2.5. Data Analysis
3. Results and Discussion
3.1. Flowability of LS Admixtures
3.2. Compaction Behavior of LSS
3.2.1. Regression Analysis
3.2.2. Classification Analysis
3.2.3. Analysis of Differences between Syloid® Carriers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodriguez-Aller, M.; Guillarme, D.; Veuthey, J.L.; Gurny, R. Strategies for formulating and delivering poorly water-soluble drugs. J. Drug Deliv. Sci. Technol. 2015, 30, 342–351. [Google Scholar] [CrossRef]
- Bertoni, S.; Hasa, D.; Albertini, B.; Perissutti, B.; Grassi, M.; Voinovich, D.; Passerini, N. Better and greener: Sustainable pharmaceutical manufacturing technologies for highly bioavailable solid dosage forms. Drug Deliv. Transl. Res. 2022, 12, 1843–1858. [Google Scholar] [CrossRef] [PubMed]
- Nokhodchi, A.; Hentzschel, C.M.; Leopold, C.S. Drug release from liquisolid systems: Speed it up, slow it down. Expert Opin. Drug Deliv. 2011, 8, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Spireas, S. Liquisolid Systems and Methods for Preparing Same. U.S. Patent 6,423,339 B1, 23 July 2002. [Google Scholar]
- Hentzschel, C.M.; Sakmann, A.; Leopold, C.S. Suitability of various excipients as carrier and coating materials for liquisolid compacts. Drug Dev. Ind. Pharm. 2011, 37, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Spireas, S.; Bolton, S.M. Liquisolid Systems and Methods for Preparing Same. U.S. Patent 5,800,834, 1 September 1998. [Google Scholar]
- Tayel, S.A.; Soliman, I.I.; Louis, D. Improvement of dissolution properties of carbamazepine through application of the liquisolid tablet technique. Eur. J. Pharm. Biopharm. 2008, 69, 342–347. [Google Scholar] [CrossRef]
- Vittal, G.V.; Deveswaran, R.; Bharath, S.; Basavaraj, B.V.; Madhavan, V. Formulation and characterization of ketoprofen liquisolid compacts by Box-Behnken design. Int. J. Pharm. Investig. 2012, 2, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Azharshekoufeh, L.B.; Shokri, J.; Adibkia, K.; Javadzadeh, Y. Liquisolid technology: What it can do for NSAIDs delivery? Colloids Surf. B Biointerfaces 2015, 136, 185–191. [Google Scholar] [CrossRef]
- Vranikova, B.; Gajdziok, J.; Vetchy, D. Modern evaluation of liquisolid systems with varying amounts of liquid phase prepared using two different methods. BioMed Res. Int. 2015, 2015, 608435. [Google Scholar] [CrossRef]
- Hani, U.; Suresh Babu, M.; Fatima, A.; Tauqeer, S. Formulation and evaluation of liquisolid compacts of flunarizine hydrochloride. Int. J. Pharm. Technol. 2015, 6, 7115–7130. [Google Scholar]
- Gong, W.; Wang, Y.; Sun, L.; Yang, J.; Shan, L.; Yang, M.; Gao, C. Development of itraconazole liquisolid compact: Effect of polyvinylpyrrolidone on the dissolution properties. Curr. Drug Deliv. 2016, 13, 452–461. [Google Scholar] [CrossRef]
- Badawy, M.A.; Kamel, A.O.; Sammour, O.A. Use of biorelevant media for assessment of a poorly soluble weakly basic drug in the form of liquisolid compacts: In vitro and in vivo study. Drug Deliv. 2016, 23, 818–827. [Google Scholar] [CrossRef]
- Khames, A. Investigation of the effect of solubility increase at the main absorption site on bioavailability of BCS class II drug (risperidone) using liquisolid technique. Drug Deliv. 2017, 24, 328–338. [Google Scholar] [CrossRef] [Green Version]
- Khames, A. Formulation and Characterization of Eplerenone Nanoemulsion Liquisolids, An Oral Delivery System with Higher Release Rate and Improved Bioavailability. Pharmaceutics 2019, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.; Doddapaneni, R.; Patki, M.; Sekar, V.; Bagde, A.; Singh, M. Erlotinib-valproic acid liquisolid formulation: Evaluating oral bioavailability and cytotoxicity in erlotinib-resistant non-small cell lung cancer cells. AAPS PharmSciTech 2019, 20, 135. [Google Scholar] [CrossRef]
- Jyoti, J.; Anandhakrishnan, N.K.; Singh, S.K.; Kumar, B.; Gulati, M.; Gowthamarajan, K.; Kumar, R.; Yadav, A.K.; Kapoor, B.; Pandey, N.K.; et al. A three-pronged formulation approach to improve oral bioavailability and therapeutic efficacy of two lipophilic drugs with gastric lability. Drug Deliv. Transl. Res. 2019, 9, 848–865. [Google Scholar] [CrossRef]
- Jhaveri, M.; Nair, A.B.; Shah, J.; Jacob, S.; Patel, V.; Mehta, T. Improvement of oral bioavailability of carvedilol by liquisolid compact: Optimization and pharmacokinetic study. Drug Deliv. Transl. Res. 2020, 10, 975–985. [Google Scholar] [CrossRef]
- Sheta, N.M.; Elfeky, Y.A.; Boshra, S.A. Cardioprotective efficacy of silymarin liquisolid in isoproterenol prompted myocardial infarction in rats. AAPS PharmSciTech 2020, 21, 81. [Google Scholar] [CrossRef]
- Kurek, M.; Woyna-Orlewicz, K.; Khalid, M.H.; Jachowicz, R. Optimization of furosemide liquisolid tablets preparation process leading to their mass and size reduction. Acta Pol. Pharm. 2016, 73, 1325–1331. [Google Scholar]
- Lu, M.; Xing, H.; Yang, T.; Yu, J.; Yang, Z.; Sun, Y.; Ding, P. Dissolution enhancement of tadalafil by liquisolid technique. Pharm. Dev. Technol. 2017, 22, 77–89. [Google Scholar] [CrossRef]
- Jaipakdee, N.; Limpongsa, E.; Sripanidkulchai, B.; Piyachaturawat, P. Preparation of Curcuma comosa tablets using liquisolid techniques: In vitro and in vivo evaluation. Int. J. Pharm. 2018, 553, 157–168. [Google Scholar] [CrossRef]
- Suliman, A.S.; Anderson, R.J.; Elkordy, A.A. Preparation of novel optimum liquisolid compacts via incorporating water granulation process to enhance the powder characterizations and dissolution behavior of a poorly soluble drug: Norfloxacin. Powder Technol. 2019, 354, 259–270. [Google Scholar] [CrossRef]
- Aleksić, I.; German Ilić, I.; Cvijić, S.; Parojčić, J. An investigation into the influence of process parameters and formulation variables on compaction properties of liquisolid systems. AAPS PharmSciTech 2020, 21, 242. [Google Scholar] [CrossRef] [PubMed]
- Aleksić, I.; Glišić, T.; Cvijić, S.; Parojčić, J. Liquisolid systems: Evaluation of the influence of formulation variables on the optimum liquid load. Arh. Farm. 2022, 72, 61–76. [Google Scholar] [CrossRef]
- Vraníková, B.; Svačinová, P.; Marushka, J.; Brokešová, J.; Holas, O.; Tebbens, J.D.; Šklubalová, Z. The importance of the coating material type and amount in the preparation of liquisolid systems based on magnesium aluminometasilicate carrier. Eur. J. Pharm. Sci. 2021, 165, 105952. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, N.R.; Irny, P.V.; Patil, U.S. Solid state behavior of progesterone and its release from Neusilin US2 based liquisolid compacts. J. Drug Deliv. Sci. Technol. 2017, 38, 97–106. [Google Scholar] [CrossRef]
- Garg, A.; Mago, V. Role of machine learning in medical research: A survey. Comput. Sci. Rev. 2021, 40, 100370. [Google Scholar] [CrossRef]
- Sallam, N.M.; Saleh, A.I.; Arafat Ali, H.; Abdelsalam, M.M. An Efficient Strategy for Blood Diseases Detection Based on Grey Wolf Optimization as Feature Selection and Machine Learning Techniques. Appl. Sci. 2022, 12, 10760. [Google Scholar] [CrossRef]
- Sallam, N.M.; Saleh, A.I.; Arafat Ali, H.; Abdelsalam, M.M. An Efficient EGWO Algorithm as Feature Selection for B-ALL Diagnoses and its Subtypes Classification Using Peripheral Blood Smear Image. Alex. Eng. J. 2023, 68, 39–66. [Google Scholar] [CrossRef]
- Philip, A.; Shahiwala, A.; Rashid, M.; Faiyazuddin, M. A Handbook of Artificial Intelligence in Drug Delivery, 1st ed.; Elsevier Science: Cambridge, MA, USA, 2022. [Google Scholar]
- Castro, B.M.; Elbadawi, M.; Ong, J.J.; Pollard, T.; Song, Z.; Gaisford, S.; Perez, G.; Basit, A.W.; Cabalar, P.; Goyanes, A. Machine learning predicts 3D printing performance of over 900 drug delivery systems. J. Control. Release 2021, 337, 530–545. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, M.; Liu, Y.; Chen, M.; Wu, S.; Wang, M.; Wang, L.; Gao, Z.; Han, D.; Liu, L.; et al. Design of spherical crystallization of active pharmaceutical ingredients via a highly efficient strategy: From screening to preparation. ACS Sustain. Chem. Eng. 2021, 9, 9018–9032. [Google Scholar] [CrossRef]
- Amasya, G.; Aksu, B.; Badilli, U.; Onay-Besikci, A.; Tarimci, N. QbD guided early pharmaceutical development study: Production of lipid nanoparticles by high pressure homogenization for skin cancer treatment. Int. J. Pharm. 2019, 563, 110–121. [Google Scholar] [CrossRef]
- Roggo, Y.; Jelsch, M.; Heger, P.; Ensslin, S.; Krumme, M. Deep learning for continuous manufacturing of pharmaceutical solid dosage form. Eur. J. Pharm. Biopharm. 2020, 153, 95–105. [Google Scholar] [CrossRef]
- Bourquin, J.; Schmidli, H.; van Hoogevest, P.; Leuenberger, H. Comparison of artificial neural networks (ANN) with classical modelling techniques using different experimental designs and data from a galenical study on a solid dosage form. Eur. J. Pharm. Sci. 1998, 6, 287–300. [Google Scholar] [CrossRef]
- Takagaki, K.; Arai, H.; Takayama, K. Creation of a tablet database containing several active ingredients and prediction of their pharmaceutical characteristics based on ensemble artificial neural networks. J. Pharm. Sci. 2010, 99, 4201–4214. [Google Scholar] [CrossRef]
- Aksu, B.; De Matas, M.; Cevher, E.; Özsoy, Y.; Güneri, T.; York, P. Quality by design approach for tablet formulations containing spray coated ramipril by using artificial intelligence techniques. Int. J. Drug Deliv. 2012, 4, 11. [Google Scholar] [CrossRef]
- Khalid, M.H.; Tuszyński, P.K.; Kazemi, P.; Szlek, J.; Jachowicz, R.; Mendyk, A. Transparent computational intelligence models for pharmaceutical tableting process. Comp. Adapt. Syst. Model. 2016, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Akseli, I.; Xie, J.; Schultz, L.; Ladyzhynsky, N.; Bramante, T.; He, X.; Deanne, R.; Horspool, K.R.; Schwabe, R. A Practical framework toward prediction of breaking force and disintegration of tablet formulations using machine learning tools. J. Pharm. Sci. 2017, 106, 234–247. [Google Scholar] [CrossRef] [Green Version]
- Djuris, J.; Cirin-Varadjan, S.; Aleksic, I.; Djuris, M.; Cvijic, S.; Ibric, S. Application of machine-learning algorithms for better understanding of tableting properties of lactose co-processed with lipid excipients. Pharmaceutics 2021, 13, 663. [Google Scholar] [CrossRef]
- Floryanzia, S.; Ramesh, P.; Mills, M.; Kulkarni, S.; Chen, G.; Shah, P.; Lavrich, D. Disintegration testing augmented by computer Vision technology. Int. J. Pharm. 2022, 619, 121668. [Google Scholar] [CrossRef]
- Iwata, H.; Hayashi, Y.; Hasegawa, A.; Terayama, K.; Okuno, Y. Classification of scanning electron microscope images of pharmaceutical excipients using deep convolutional neural networks with transfer learning. Int. J. Pharm. X 2022, 4, 100135. [Google Scholar] [CrossRef]
- Barmpalexis, P.; Grypioti, A.; Eleftheriadis, G.K.; Fatouros, D.G. Development of a new aprepitant liquisolid formulation with the aid of artificial neural networks and genetic programming. AAPS PharmSciTech 2017, 19, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Pharmaceuticals & Excipients, Neusilin®. Available online: http://www.fujichemical.co.jp/english/medical/medicine/neusilin/neusilin_brochure.pdf (accessed on 30 September 2022).
- Syloid® Silica Brochure. Available online: https://grace.com/products/syloid-silica/ (accessed on 30 September 2022).
- European Pharmacopoeia (Ph. Eur. MMXVII), 9th ed.; European Pharmacopoeia Commission: Strasbourg, France, 2017.
- The United States Pharmacopeia, 44th ed.; US Pharmacopeia Convention: Rockville, MD, USA, 2021.
- Newton, J.M.; Rowley, G.; Fell, J.T.; Peacock, D.G.; Ridgway, K. Computer analysis of the relation between tablet strength and compaction pressure. J. Pharm. Pharmacol. 1971, 23, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Demsar, J.; Curk, T.; Erjavec, A.; Gorup, C.; Hocevar, T.; Milutinovic, M.; Mozina, M.; Polajnar, M.; Toplak, M.; Staric, A.; et al. Orange: Data Mining Toolbox in Python. J. Mach Learn Res. 2013, 14, 2349–2353. [Google Scholar]
- Maulud, D.; Abdulazeez, A.M. A review on linear regression comprehensive in machine learning. J. Appl. Sci. Technol. Trends 2020, 1, 140–147. [Google Scholar] [CrossRef]
- Kruse, R.; Mostaghim, S.; Borgelt, C.; Braune, C.; Steinbrecher, M. Multi-layer perceptrons. In Computational Intelligence: A Methodological Introduction, 1st ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 53–124. [Google Scholar]
- Netrapalli, P. Stochastic Gradient Descent and Its Variants in Machine Learning. J. Indian Inst. Sci. 2019, 99, 201–213. [Google Scholar] [CrossRef]
- Costa, V.G.; Pedreira, C.E. Recent advances in decision trees: An updated survey. Artif. Intell. Rev. 2022, 1–36. [Google Scholar] [CrossRef]
- Mäki-Lohiluoma, E.; Säkkinen, N.; Palomäki, M.; Winberg, O.; Ta, H.X.; Heikkinen, T.; Kiljunen, E.; Kauppinen, A. Use of machine learning in prediction of granule particle size distribution and tablet tensile strength in commercial pharmaceutical manufacturing. Int. J. Pharm. 2021, 609, 121146. [Google Scholar] [CrossRef]
- Taser, P.Y. Application of Bagging and Boosting Approaches Using Decision Tree-Based Algorithms in Diabetes Risk Prediction. Proceedings 2021, 74, 6. [Google Scholar] [CrossRef]
- Cervantes, J.; Garcia-Lamont, F.; Rodríguez-Mazahua, L.; Lopez, A. A comprehensive survey on support vector machine classification: Applications, challenges and trends. Neurocomputing 2020, 408, 189–215. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.T.; Mao, J.; Shen, C.; Xie, R.L.; Mu, B. Development of novel in silico prediction model for drug-induced ototoxicity by using naïve Bayes classifier approach. Toxicol. Vitr. 2020, 65, 104812. [Google Scholar] [CrossRef]
- Altarazi, S.; Allaf, R.; Alhindawi, F. Machine Learning Models for Predicting and Classifying the Tensile Strength of Polymeric Films Fabricated via Different Production Processes. Materials 2019, 12, 1475. [Google Scholar] [CrossRef] [Green Version]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Kostelanská, K.; Prudilová, B.B.; Holešová, S.; Vlček, J.; Vetchý, D.; Gajdziok, J. Comparative Study of Powder Carriers Physical and Structural Properties. Pharmaceutics 2022, 14, 818. [Google Scholar] [CrossRef]
- Mura, P.; Valleri, M.; Fabianelli, E.; Maestrelli, F.; Cirri, M. Characterization and evaluation of different mesoporous silica kinds as carriers for the development of effective oral dosage forms of glibenclamide. Int. J. Pharm. 2019, 563, 43–52. [Google Scholar] [CrossRef]
- Vraníková, B.; Gajdziok, J.; Vetchý, D. Determination of flowable liquid retention potential of aluminometasilicate carrier for liquisolid systems preparation. Pharm. Dev. Technol. 2015, 20, 839–844. [Google Scholar] [CrossRef]
- Mamidi, H.K.; Mishra, S.M.; Rohera, B.D. Determination of maximum flowable liquid-loading potential of Neusilin® US2 and investigation of compressibility and compactibility of its liquisolid blends with PEG (400). J. Drug Deliv. Sci. Technol. 2019, 54, 101285. [Google Scholar] [CrossRef]
- Goh, H.P.; Heng, P.W.S.; Liew, C.V. Comparative evaluation of powder flow parameters with reference to particle size and shape. Int. J. Pharm. 2018, 547, 133–141. [Google Scholar] [CrossRef]
- Principal Components Analysis (PCA). Available online: http://strata.uga.edu/8370/handouts/pcaTutorial.pdf (accessed on 2 December 2022).
- Mašinsko Učenje. Available online: http://ml.matf.bg.ac.rs/readings/ml.pdf (accessed on 2 December 2022).
- Pitt, K.G.; Heasley, M.G. Determination of the tensile strength of elongated tablets. Powder Technol 2013, 238, 169–175. [Google Scholar] [CrossRef]
- Gumaste, S.G.; Pawlak, S.A.; Dalrymple, D.M.; Nider, C.J.; Trombetta, L.D.; Serajuddin, A.T.M. Development of solid sedds, iv: Effect of adsorbed lipid and surfactant on tableting properties and surface structures of different silicates. Pharm. Res. 2013, 30, 3170–3185. [Google Scholar] [CrossRef] [Green Version]
- Nagy, B.; Galata, D.L.; Farkas, A.; Nagy, Z.K. Application of Artificial Neural Networks in the Process Analytical Technology of Pharmaceutical Manufacturing—A Review. AAPS J. 2022, 24, 70. [Google Scholar] [CrossRef]
- McCormick, D. Evolutions in direct compression. Pharm. Technol. 2005, 17, 52–62. [Google Scholar]








| Carrier | R 1 | Lf 2 | PEG (%) | Flow Rate (g/s) | CI 3 (%) | HR 4 |
|---|---|---|---|---|---|---|
| Neusilin® US2 | 10 * | 0.7 | 38.9 | 1.81 ± 0.09 | 14.6 ± 0.6 | 1.17 ± 0.01 |
| 10 * | 0.8 | 42.1 | 1.81 ± 0.03 | 15.0 ± 1.4 | 1.18 ± 0.02 | |
| 10 * | 1.0 | 47.6 | 2.40 ± 0.03 | 13.9 ± 1.7 | 1.16 ± 0.02 | |
| 10 | 1.2 | 52.2 | 2.30 ± 0.06 | 14.2 ± 1.4 | 1.17 ± 0.02 | |
| 10 | 1.3 | 54.2 | 2.05 ± 0.11 | 13.9 ± 1.0 | 1.16 ± 0.01 | |
| 20 * | 0.7 | 40.0 | 1.23 ± 0.03 | 18.3 ± 0.2 | 1.22 ± 0.00 | |
| 20 * | 0.8 | 43.2 | 1.30 ± 0.03 | 18.4 ± 0.8 | 1.23 ± 0.01 | |
| 20 * | 1.0 | 48.8 | 2.22 ± 0.06 | 15.2 ± 1.8 | 1.18 ± 0.03 | |
| 20 | 1.2 | 53.3 | 2.68 ± 0.04 | 11.4 ± 0.1 | 1.13 ± 0.00 | |
| 20 | 1.3 | 55.3 | 2.43 ± 0.05 | 14.0 ± 0.9 | 1.16 ± 0.01 | |
| 30 * | 0.7 | 40.4 | 2.14 ± 0.11 | 12.7 ± 1.4 | 1.15 ± 0.02 | |
| 30 * | 0.8 | 43.6 | 1.17 ± 0.02 | 18.2 ± 0.8 | 1.22 ± 0.01 | |
| 30 * | 1.0 | 49.2 | 2.02 ± 0.08 | 14.8 ± 1.8 | 1.17 ± 0.03 | |
| 30 | 1.2 | 53.7 | 2.42 ± 0.14 | 12.2 ± 1.1 | 1.14 ± 0.01 | |
| 30 | 1.3 | 55.7 | 2.32 ± 0.04 | 14.5 ± 2.8 | 1.17 ± 0.04 | |
| Syloid® XDP 3150 | 10 * | 0.6 | 35.3 | 4.48 ± 0.49 | 15.0 ± 1.0 | 1.17 ± 0.01 |
| 10 * | 0.7 | 38.9 | 3.46 ± 0.20 | 15.2 ± 1.8 | 1.18 ± 0.02 | |
| 10 | 0.8 | 42.1 | 3.30 ± 0.26 | 14.2 ± 1.0 | 1.17 ± 0.01 | |
| 10 | 1.0 | 47.6 | 4.75 ± 0.37 | 17.0 ± 0.0 | 1.21 ± 0.00 | |
| 20 * | 0.6 | 35.3 | 4.50 ± 0.68 | 10.0 ± 0.0 | 1.11 ± 0.00 | |
| 20 * | 0.7 | 38.9 | 3.31 ± 0.19 | 14.0 ± 2.0 | 1.17 ± 0.03 | |
| 20 | 0.8 | 43.2 | 3.28 ± 0.39 | 13.2 ± 0.2 | 1.15 ± 0.00 | |
| 20 | 0.9 | 46.2 | 4.74 ± 0.49 | 11.0 ± 0.0 | 1.13 ± 0.00 | |
| 30 * | 0.6 | 36.7 | 5.25 ± 0.50 | 11.0 ± 1.0 | 1.13 ± 0.01 | |
| 30 * | 0.7 | 40.4 | 3.93 ± 0.54 | 13.0 ± 1.0 | 1.16 ± 0.02 | |
| 30 | 0.8 | 43.6 | 2.96 ± 0.26 | 12.7 ± 1.1 | 1.15 ± 0.01 | |
| Syloid® XDP 3050 | 10 | 0.5 | 31.3 | 2.43 ± 0.32 | 19.0 ± 1.0 | 1.23 ± 0.02 |
| 10 * | 0.6 | 35.3 | 2.77 ± 0.23 | 18.0 ± 0.0 | 1.22 ± 0.01 | |
| 10 * | 0.7 | 38.9 | 2.40 ± 0.22 | 17.0 ± 0.0 | 1.21 ± 0.00 | |
| 10 * | 0.8 | 42.1 | 1.60 ± 0.04 | 16.2 ± 0.7 | 1.19 ± 0.01 | |
| 10 | 1.0 | 47.6 | 2.98 ± 0.19 | 15.0 ± 1.0 | 1.18 ± 0.01 | |
| 20 * | 0.6 | 35.3 | 1.95 ± 0.24 | 17.0 ± 0.0 | 1.20 ± 0.00 | |
| 20 * | 0.7 | 38.9 | 1.80 ± 0.01 | 16.0 ± 2.0 | 1.20 ± 0.04 | |
| 20 * | 0.8 | 43.2 | 1.85 ± 0.02 | 15.7 ± 1.0 | 1.19 ± 0.01 | |
| 20 * | 0.9 | 46.2 | 2.53 ± 0.10 | 15.0 ± 1.0 | 1.17 ± 0.01 | |
| 30 * | 0.6 | 36.7 | 2.54 ± 0.36 | 15.0 ± 2.0 | 1.16 ± 0.01 | |
| 30 * | 0.7 | 40.4 | 2.16 ± 0.04 | 17.0 ± 1.0 | 1.21 ± 0.02 | |
| 30 * | 0.8 | 43.6 | 2.05 ± 0.09 | 15.0 ± 2.0 | 1.18 ± 0.03 |
| Model | MSE 1 | RMSE 2 | MAE 3 | R2 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Training Data | Test Data | Training Data | Test Data | Training Data | Test Data | Training Data | Test Data | |
| AdaBoost | 0.00015201 | 0.06507389 | 0.01232917 | 0.25509584 | 0.00312455 | 0.17468138 | 0.99986102 | 0.94050451 |
| SVM 5 | 0.03555609 | 0.06479570 | 0.18856323 | 0.25454999 | 0.12102410 | 0.16794962 | 0.96749192 | 0.94075885 |
| Linear Regression | 0.06839292 | 0.08120252 | 0.26152040 | 0.28496056 | 0.19852719 | 0.21471604 | 0.93746999 | 0.92575848 |
| Neural networks | 0.05815805 | 0.07246392 | 0.24115981 | 0.26919124 | 0.18134422 | 0.20151931 | 0.94682749 | 0.93374798 |
| Tree | 0.00749373 | 0.08475951 | 0.08656636 | 0.29113486 | 0.06079310 | 0.19475213 | 0.99314866 | 0.92250642 |
| Random forest | 0.06050200 | 0.08642901 | 0.24597155 | 0.29398810 | 0.18556466 | 0.23168898 | 0.94468447 | 0.92098003 |
| Model | AUC 1 | CA 2 | Precision | Recall | ||||
|---|---|---|---|---|---|---|---|---|
| Training Data | Test Data | Training Data | Test Data | Training Data | Test Data | Training Data | Test Data | |
| AdaBoost | 1.00000000 | 0.92381536 | 1.00000000 | 0.90000000 | 1.00000000 | 0.90213558 | 1.00000000 | 0.92381536 |
| Naïve Bayes | 0.97528595 | 0.9620098 | 0.87333333 | 0.86666667 | 0.88611765 | 0.87748252 | 0.87333333 | 0.9620098 |
| Logistic Regression | 0.92279412 | 0.90767974 | 0.78666667 | 0.78666667 | 0.78529412 | 0.78529412 | 0.78666667 | 0.90767974 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glišić, T.; Djuriš, J.; Vasiljević, I.; Parojčić, J.; Aleksić, I. Application of Machine-Learning Algorithms for Better Understanding the Properties of Liquisolid Systems Prepared with Three Mesoporous Silica Based Carriers. Pharmaceutics 2023, 15, 741. https://doi.org/10.3390/pharmaceutics15030741
Glišić T, Djuriš J, Vasiljević I, Parojčić J, Aleksić I. Application of Machine-Learning Algorithms for Better Understanding the Properties of Liquisolid Systems Prepared with Three Mesoporous Silica Based Carriers. Pharmaceutics. 2023; 15(3):741. https://doi.org/10.3390/pharmaceutics15030741
Chicago/Turabian StyleGlišić, Teodora, Jelena Djuriš, Ivana Vasiljević, Jelena Parojčić, and Ivana Aleksić. 2023. "Application of Machine-Learning Algorithms for Better Understanding the Properties of Liquisolid Systems Prepared with Three Mesoporous Silica Based Carriers" Pharmaceutics 15, no. 3: 741. https://doi.org/10.3390/pharmaceutics15030741






