Novel Approach for the Approximation of Vitamin D3 Pharmacokinetics from In Vivo Absorption Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Liposomal Vitamin D3 Formulation
2.3. Characterization of Liposomal Vitamin D Formulation
2.4. Cryogenic Transmission Electron Microscopy (TEM) Imaging
2.5. Clinical Studies and Quantification of Calcidiol in Serum
2.6. The Reconstruction of Pharmacokinetic Curve for Calcidiol
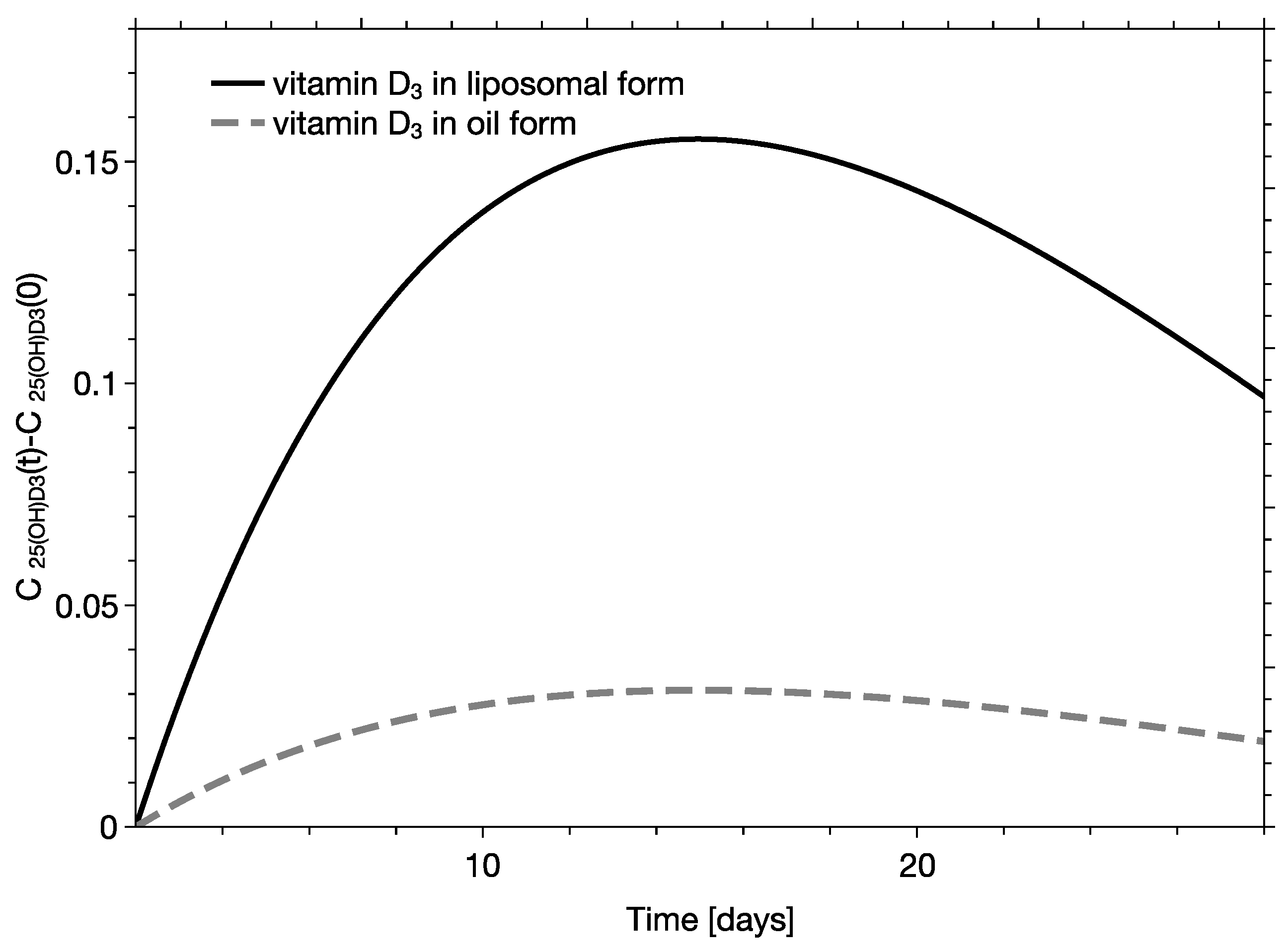
3. Results and Discussion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramakrishnan, V.; Yang, Q.J.; Quach, H.; Cao, Y.; Chow, E.; Mager, D.; Pang, S. Physiologically-Based Pharmacokinetic-Pharmacodynamic Modeling of 1 alpha,25-Dihydroxyvitamin D-3 in Mice. Drug Metab. Dispos. 2016, 44, 189–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawyer, M.E.; Tran, H.T.; Evans, M.V. A physiologically based pharmacokinetic model of vitamin D. J. Appl. Toxicol. 2017, 37, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Łukawski, M.; Dałek, P.; Borowik, T.; Foryś, A.; Langner, M.; Witkiewicz, W.; Przybyło, M. New oral liposomal vitamin C formulation: Properties and bioavailability. J. Liposome Res. 2019, 30, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C Pharmacokinetics: Implications for Oral and Intravenous Use. Ann. Intern. Med. 2004, 140, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Shane, B. Folate and vitamin B12 metabolism: Overview and interaction with riboflavin, vitamin B6, and polymorphisms. Food Nutr. Bull. 2008, 29 (Suppl. S2), S5–S16; discussion S17–S19. [Google Scholar] [CrossRef]
- Wilson, L.; Tripkovic, L.; Hart, K.; Lanham-New, S. Vitamin D deficiency as a public health issue: Using vitamin D-2 or vitamin D-3 in future fortification strategies. Proc. Nutr. Soc. 2017, 76, 392–399. [Google Scholar] [CrossRef] [Green Version]
- Pilz, S.; Zittermann, A.; Trummer, C.; Theiler-Schwetz, V.; Lerchbaum, E.; Keppel, M.H.; Grübler, M.R.; März, W.; Pandis, M. Vitamin D testing and treatment: A narrative review of current evidence. Endocr. Connect. 2019, 8, R27–R43. [Google Scholar] [CrossRef] [Green Version]
- Carlberg, C. Nutrigenomics of Vitamin D. Nutrients 2019, 11, 676. [Google Scholar] [CrossRef] [Green Version]
- Anderson, P.H. Vitamin D Activity and Metabolism in Bone. Curr. Osteoporos. Rep. 2017, 15, 443–449. [Google Scholar] [CrossRef]
- Moulas, A.N.; Vaiou, M. Vitamin D fortification of foods and prospective health outcomes. J. Biotechnol. 2018, 285, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Maurya, V.K.; Aggarwal, M. Factors influencing the absorption of vitamin D in GIT: An overview. J. Food Sci. Technol. 2017, 54, 3753–3765. [Google Scholar] [CrossRef] [PubMed]
- Denburg, M.R.; Bhan, I. Vitamin D-Binding Protein in Health and Chronic Kidney Disease. Semin. Dial. 2015, 28, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Jorde, R. The Role of Vitamin D Binding Protein, Total and Free 25-Hydroxyvitamin D in Diabetes. Front. Endocrinol. 2019, 10, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.; Behera, C.; Paudwal, G.; Rawat, N.; Baldi, A.; Gupta, P.N. Recent Advances in Formulation Strategies for Efficient Delivery of Vitamin D. AAPS PharmSciTech 2018, 20, 11. [Google Scholar] [CrossRef]
- Rezhdo, O.; Speciner, L.; Carrier, R. Lipid-associated oral delivery: Mechanisms and analysis of oral absorption enhancement. J. Control. Release 2016, 240, 544–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, M.; Farrell, C.-J.L.; Pusceddu, I.; Fabregat-Cabello, N.; Cavalier, E. Assessment of vitamin D status—A changing landscape. Clin. Chem. Lab. Med. 2017, 55, 3–26. [Google Scholar] [CrossRef] [Green Version]
- Corstens, M.N.; Berton-Carabin, C.; De Vries, R.; Troost, F.J.; Masclee, A.A.M.; Schroen, K. Food-grade micro-encapsulation systems that may induce satiety via delayed lipolysis: A review. Crit. Rev. Food Sci. Nutr. 2015, 57, 2218–2244. [Google Scholar] [CrossRef]
- Guo, Q.; Bellissimo, N.; Rousseau, D. The Physical State of Emulsified Edible Oil Modulates Its in Vitro Digestion. J. Agric. Food Chem. 2017, 65, 9120–9127. [Google Scholar] [CrossRef]
- Borel, P.; Caillaud, D.; Cano, N.J. Vitamin D Bioavailability: State of the Art. Crit. Rev. Food Sci. Nutr. 2015, 55, 1193–1205. [Google Scholar] [CrossRef]
- Hayes, A.; Cashman, K.D. Food-based solutions for vitamin D deficiency: Putting policy into practice and the key role for research. Proc. Nutr. Soc. 2016, 76, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Wang, P.-W.; Alalaiwe, A.; Lin, Z.-C.; Fang, J.-Y. Use of Lipid Nanocarriers to Improve Oral Delivery of Vitamins. Nutrients 2019, 11, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, L.; Wang, D.; Liu, F.; Gao, Y. Emulsion design for the delivery of beta-carotene in complex food systems. Crit. Rev. Food Sci. Nutr. 2018, 58, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B.; Argin, S.; Ozilgen, M.; McClements, D.J. Nanoemulsion delivery systems for oil-soluble vitamins: Influence of carrier oil type on lipid digestion and vitamin D3 bioaccessibility. Food Chem. 2015, 187, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Desmarchelier, C. Bioavailability of Fat-Soluble Vitamins and Phytochemicals in Humans: Effects of Genetic Variation. Annu. Rev. Nutr. 2018, 38, 69–96. [Google Scholar] [CrossRef] [Green Version]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, F.; Fan, W.; Jiang, S.; Ma, Y.; Lu, Y.; Qi, J.; Ahmad, E.; Dong, X.; Zhao, W.; Wu, W. Size-Dependent Translocation of Nanoemulsions via Oral Delivery. ACS Appl. Mater. Interfaces 2017, 9, 21660–21672. [Google Scholar] [CrossRef]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef]
- Bornhorst, G.M.; Gouseti, O.; Wickham, M.S.; Bakalis, S. Engineering Digestion: Multiscale Processes of Food Digestion. J. Food Sci. 2016, 81, R534–R543. [Google Scholar] [CrossRef] [Green Version]
- Porter, C.; Pouton, C.; Cuine, J.; Charman, W. Enhancing intestinal drug solubilization using lipid-based delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 673–691. [Google Scholar] [CrossRef]
- Doskocz, J.; Dałek, P.; Foryś, A.; Trzebicka, B.; Przybyło, M.; Mesarec, L.; Iglič, A.; Langner, M. The effect of lipid phase on liposome stability upon exposure to the mechanical stress. Biochim. Biophys. Acta-Biomembr. 2020, 1862, 183361. [Google Scholar] [CrossRef] [PubMed]
- Doskocz, J.; Dałek, P.; Przybyło, M.; Trzebicka, B.; Foryś, A.; Kobyliukh, A.; Iglič, A.; Langner, M. The Elucidation of the Molecular Mechanism of the Extrusion Process. Materials 2021, 14, 4278. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Lu, Y.; Qi, J. Oral delivery of liposomes. Ther. Deliv. 2015, 6, 1239–1241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chang, D.; Yang, Y.; Zhang, X.; Tao, W.; Jiang, L.; Liang, X.; Tsai, H.; Huang, L.; Mei, L. Systematic investigation on the intracellular trafficking network of polymeric nanoparticles. Nanoscale 2017, 9, 3269–3282. [Google Scholar] [CrossRef]
- Raikos, V.; Ranawana, V. Designing emulsion droplets of foods and beverages to enhance delivery of lipophilic bioactive components—A review of recent advances. Int. J. Food Sci. Technol. 2016, 52, 68–80. [Google Scholar] [CrossRef]
- Dałek, P.; Drabik, D.; Wołczańska, H.; Foryś, A.; Jagas, M.; Jędruchniewicz, N.; Przybyło, M.; Witkiewicz, W.; Langner, M. Bioavailability by design—Vitamin D3 liposomal delivery vehicles. Nanomed. Nanotechnol. Biol. Med. 2022, 43, 102552. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.K.; Sobkowiak, P.; Drzymała-Czyż, S.; Krzyżanowska-Jankowska, P.; Sapiejka, E.; Skorupa, W.; Pogorzelski, A.; Nowicka, A.; Wojsyk-Banaszak, I.; Kurek, S.; et al. Fat-Soluble Vitamin Supplementation Using Liposomes, Cyclodextrins, or Medium-Chain Triglycerides in Cystic Fibrosis: A Randomized Controlled Trial. Nutrients 2021, 13, 4554. [Google Scholar] [CrossRef]
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Asp. Med. 2012, 33, 209–290. [Google Scholar] [CrossRef]
- Sazali, N.H.; Alshishani, A.; Saad, B.; Chew, K.Y.; Chong, M.M.; Miskam, M. Salting-out assisted liquid–liquid extraction coupled with high-performance liquid chromatography for the determination of vitamin D3 in milk samples. R. Soc. Open Sci. 2019, 6, 190952. [Google Scholar] [CrossRef] [Green Version]
- Ibarguren, M.; Alonso, A.; Tenchov, B.G.; Goñi, F.M. Quantitation of cholesterol incorporation into extruded lipid bilayers. Biochim. Biophys. Acta-Biomembr. 2010, 1798, 1735–1738. [Google Scholar] [CrossRef] [Green Version]
- Jamei, M.; Turner, D.; Yang, J.; Neuhoff, S.; Polak, S.; Rostami-Hodjegan, A.; Tucker, G. Population-Based Mechanistic Prediction of Oral Drug Absorption. AAPS J. 2009, 11, 225–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armas, L.A.G.; Hollis, B.W.; Heaney, R.P. Vitamin D2 is much less effective than vitamin D3 in humans. J. Clin. Endocrinol. Metab. 2004, 89, 5387–5391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, T.L.; Lock, J.Y.; Carrier, R.L. Engineering the Mucus Barrier. Annu. Rev. Biomed. Eng. 2018, 20, 197–220. [Google Scholar] [CrossRef] [PubMed]
- Julve, J.; Martín-Campos, J.M.; Escolà-Gil, J.C.; Blanco-Vaca, F. Chylomicrons: Advances in biology, pathology, laboratory testing, and therapeutics. Clin. Chim. Acta 2016, 455, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Chun, R.F.; Peercy, B.E.; Orwoll, E.S.; Nielson, C.M.; Adams, J.S.; Hewison, M. Vitamin D and DBP: The free hormone hypothesis revisited. J. Steroid Biochem. Mol. Biol. 2013, 144, 132–137. [Google Scholar] [CrossRef] [Green Version]
- Kuai, R.; Li, D.; Chen, Y.E.; Moon, J.J.; Schwendeman, A. High-Density Lipoproteins: Nature’s Multifunctional Nanoparticles. ACS Nano 2016, 10, 3015–3041. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.C.; Furlanetto, T.W. Intestinal absorption of vitamin D: A systematic review. Nutr. Rev. 2017, 76, 60–76. [Google Scholar] [CrossRef]
- Ocampo-Pelland, A.S.; Gastonguay, M.R.; French, J.F.; Riggs, M. Model-based meta-analysis for development of a population-pharmacokinetic (PPK) model for Vitamin D3 and its 25OHD3 metabolite using both individual and arm-level data. J. Pharmacokinet. Pharmacodyn. 2016, 43, 191–206. [Google Scholar] [CrossRef]
- Ocampo-Pelland, A.S.; Gastonguay, M.R.; Riggs, M. Model-based meta-analysis for comparing Vitamin D2 and D3 parent-metabolite pharmacokinetics. J. Pharmacokinet. Pharmacodyn. 2017, 44, 375–388. [Google Scholar] [CrossRef]

| Liposome | Oil | |
|---|---|---|
| The outcome of the homogenization in stomach | predictable | unpredictable |
| Stability of the dispersion with respect to droplet size | high | low |
| Uniformity of droplet population | high | low |
| Stable droplet size distribution during digestion | medium | low |
| Ability to cross the mucus barrier | high | limited |
| Parameter | Liposome Formulation | Oil Formulation |
|---|---|---|
| Cmax | 0.335 [ng/L] | 0.067 [ng/L] |
| Tmax | 14 days | 14 days |
| t1/2 | 43 days | 43 days |
| AUC1day | 0.135 [day·ng/L] | 0.027 [day·ng/L] |
| AUC2days-30day | 8.646 [day·ng/L] | 1.718 [day·ng/L] |
| AUC1day-30day | 8.511 [day·ng/L] | 1.692 [day·ng/L] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żurek, G.; Przybyło, M.; Witkiewicz, W.; Langner, M. Novel Approach for the Approximation of Vitamin D3 Pharmacokinetics from In Vivo Absorption Studies. Pharmaceutics 2023, 15, 783. https://doi.org/10.3390/pharmaceutics15030783
Żurek G, Przybyło M, Witkiewicz W, Langner M. Novel Approach for the Approximation of Vitamin D3 Pharmacokinetics from In Vivo Absorption Studies. Pharmaceutics. 2023; 15(3):783. https://doi.org/10.3390/pharmaceutics15030783
Chicago/Turabian StyleŻurek, Grzegorz, Magdalena Przybyło, Wojciech Witkiewicz, and Marek Langner. 2023. "Novel Approach for the Approximation of Vitamin D3 Pharmacokinetics from In Vivo Absorption Studies" Pharmaceutics 15, no. 3: 783. https://doi.org/10.3390/pharmaceutics15030783
APA StyleŻurek, G., Przybyło, M., Witkiewicz, W., & Langner, M. (2023). Novel Approach for the Approximation of Vitamin D3 Pharmacokinetics from In Vivo Absorption Studies. Pharmaceutics, 15(3), 783. https://doi.org/10.3390/pharmaceutics15030783





