Overview of Side-Effects of Antibacterial Fluoroquinolones: New Drugs versus Old Drugs, a Step Forward in the Safety Profile?
Abstract
:1. Introduction
2. Materials and Methods
3. Relevant FQs Used in Therapy
3.1. The Main FQs with Clinical Importance
| QNs/FQs | 1st Generation | 2nd Generation | 3rd Generation | 4th Generation |
|---|---|---|---|---|
| Nalidixic Acid | Ciprofloxacin, Nadifloxacin 1, Norfloxacin, Ofloxacin, Pefloxacin | Gatifloxacin 2, Levofloxacin | Besifloxacin 2, Delafloxacin, Finafloxacin 3, Moxifloxacin | |
| Antibacterial spectrum | Enterobacteria. No activity against Gram-positive bacteria. | Enterobacteriaceae; some atypical pathogens; Pseudomonas aeruginosa (only Ciprofloxacin); some Gram-positive bacteria (including Streptococcus pneumoniae), moderate activity against Staphylococcus aureus (Ciprofloxacin, Norfloxacin, Ofloxacin, Pefloxacin) Staphylococcus aureus ((MRSA) and coagulase-negative staphylococci), aerobic Gram-negative and anaerobic pathogens (Nadifloxacin 1) | Broad-spectrum, including Staphylococcus aureus, Streptococcus species, and Gram-negative pathogens (Gatifloxacin 2) Enterobacteriaceae; Atypical pathogens; Streptococcus pneumoniae, penicillin-resistant (Levofloxacin) | Streptococcus pneumoniae, Staphylococcus epidermidis, Staphylococcus aureus, Hemophilus influenzae, Moraxella catarrhalis, Corynebacterium spp. (Besifloxacin 2) Broad-spectrum (including methicillin-resistant Staphylococcus aureus) (Delafloxacin) Broad-spectrum activity (Finafloxacin 3) Enterobacteriaceae; atypical pathogens; Pseudomonas aeruginosa; Streptococci; Staphylococcus aureus methicillin-sensitive; anaerobic pathogens (Moxifloxacin) |
| Indications | Uncomplicated urinary tract infections (UTI) | Uncomplicated and complicated UTI, pyelonephritis, sexually transmitted diseases, prostatitis, respiratory tract infections, skin, soft tissues, bones, and joint infections (Ciprofloxacin, Norfloxacin, Ofloxacin, Pefloxacin) Acne vulgaris and other skin infections (Nadifloxacin 1). | Bacterial conjunctivitis due to susceptible pathogens (Gatifloxacin 2) Acute and chronic bronchitis, exacerbated forms, acquired pneumonia (nosocomial) (Levofloxacin) | Bacterial conjunctivitis (Besifloxacin 2) Bacterial skin and skin structure infections (Delafloxacin) Acute otitis externa (Finafloxacin 3) Sexually transmitted diseases, prostatitis, skin and tissue infections, acute and chronic bronchitis, exacerbated forms, acquired pneumonia (nosocomial), intra-abdominal infections, and gynecological infections (Moxifloxacin) |
| References | [2,42] | [1,43] | [44,45,46] | [27,44,47] |
3.2. Essential Chemical Characteristics
3.3. Mechanism of Action
3.4. Safety Warnings concerning Emerging Serious AEs
| No. | Year | Regulatory Entity | Document | Title of Document | Targeted AEs | The Formulations/Administration Concerned | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 2008 | FDA | FDA alert (8 July 2008) | Information for Healthcare Professionals: Fluoroquinolone Antimicrobial Drugs Black Boxed Warning | Increased risk of tendinitis and tendon rupture | Formulations for systemic use (except ophthalmic or otic formulations) | [71,72,73] |
| 2 | 2011 | FDA | FDA alert (February 2011) | Information for Healthcare Professionals: Fluoroquinolone Antimicrobial Drugs Black Boxed Warning? | Worsening symptoms of patients with myasthenia gravis | Formulations for systemic use | [32,74] |
| 3 | 2013 | FDA | FDA Drug Safety Communication (15 August 2013) | FDA requires label changes to warn of the risk for possibly permanent nerve damage from antibacterial fluoroquinolone drugs taken by mouth or by injection | Side-effects of peripheral neuropathy | Formulations for systemic use except for ophthalmic or otic formulations | [75] |
| 4 | 2016 | FDA | FDA Drug Safety Communication (12 May 2016) | FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side-effects that can occur together | Side-effects concerning tendons, muscles, joints, nerves, and CNS | Formulations for systemic use | [76] |
| 5 | 2016 | FDA | FDA Drug Safety Communication (26 July 2016) | FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side-effects (safety labeling changes) | Side-effects involving nerves, the CNS, tendons, muscles, and joints | Formulations for systemic use | [32] |
| 6 | 2018 | FDA | FDA (10 July 2018) | FDA reinforces safety information about serious low blood sugar levels and mental health side-effects with fluoroquinolone antibiotics; requires label changes (warnings) | Serious risk of blood sugar drop and negative impact on mental health | Formulations for systemic use | [68] |
| 7 | 2018 | FDA | FDA Drug Safety Communication (20 December 2018) | FDA warns about the increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolone antibiotics in certain patients (safety announcement) | Higher risk of aortic dissections or ruptures of an aortic aneurysm | Formulations for systemic use | [77] |
| 8 | 2018 | EMA | EMA/668915/2018 (5 October 2018) | Fluoroquinolone and quinolone antibiotics: PRAC recommends new restrictions on use following a review of disabling potentially long-lasting side-effects available online | Long-term adverse effects affecting tendons, bones, and the nervous system | Formulations for systemic and inhalation route | [34] |
| 9 | 2019 | EMA | EMA/175398/2019 (11 March 2019) | Disabling and potentially permanent side-effects lead to suspension or restrictions of quinolone and fluoroquinolone antibiotics | Side-effects involving the CNS, bones, muscles, joints, and tendons | Formulations for systemic and inhalation route | [35] |
| 10 | 2020 | EMA | EMA/Direct Healthcare Professional Communication (DHPC) (29 November 2020) | DHPC: Systemic and inhaled FQs: risk of heart valve regurgitation/incompetence | Risk of heart valve regurgitation/incompetence | Formulations for systemic and inhalation route | [78] |
3.5. Withdrawal of Some FQs over Time
4. The Modern FQs
Recently Approved FQs
5. Side-Effects of FQs and Underlying Mechanisms
5.1. Aortic Aneurysm and Aortic Dissection
5.1.1. Underlying Mechanisms of Aortic Aneurysm and Aortic Dissection
5.1.2. Reported Aortic Aneurysm and Aortic Dissection Associated with Modern FQs
5.2. Tendinopathy/Tendon Rupture
5.2.1. Underlying Mechanisms of Tendinopathy/Tendon Rupture
5.2.2. Tendinopathy/Tendon Rupture Associated with Modern FQs
5.3. Retinal Detachment
5.3.1. Underlying Mechanisms of Retinal Detachment
5.3.2. Retinal Detachment Associated with Modern FQs
5.4. Peripheral Neuropathy
5.4.1. Underlying Mechanisms of Peripheral Neuropathy
5.4.2. Reported Peripheral Neuropathy Associated with Modern FQs
5.5. Neuropsychiatric Toxicity
5.5.1. Underlying Mechanisms of Neuropsychiatric Toxicity
5.5.2. Reported Neuropsychiatric Toxicity Associated with Modern FQs
5.6. Seizures
5.6.1. Underlying Mechanisms of Seizures
5.6.2. Seizures Associated with Modern FQs
5.7. Exacerbation of Myasthenia Gravis
5.7.1. Underlying Mechanisms of Exacerbation of Myasthenia Gravis
5.7.2. Reported Exacerbation of Myasthenia Gravis Associated with Modern FQs
5.8. Cutaneous Side-Effects, Hypersensitivity Reactions, Anaphylaxis
5.8.1. Underlying Mechanism of Cutaneous Side-Effects, Hypersensitivity Reactions, and Anaphylaxis
5.8.2. Cutaneous Side-Effects, Hypersensitivity Reactions, and Anaphylaxis Associated with Modern FQs
5.9. Phototoxicity
5.9.1. Underlying Mechanisms of Phototoxicity
5.9.2. Reported Phototoxicity Associated with Modern FQs
5.10. Clostridium difficile Infection
5.10.1. Underlying Mechanisms of Clostridium difficile Infection
5.10.2. Reported Clostridium difficile Infections Associated with Modern FQs
5.11. QT Prolongation (Fatal Arrhythmia) and Torsade de Pointes
5.11.1. Underlying Mechanisms of QT Prolongation (Fatal Arrhythmia) and Torsade de Pointes
5.11.2. QT Prolongation (Fatal Arrhythmia) and Torsade de Pointes Associated with Modern FQs
5.12. Dysglycemia/Hypoglycemia and Hyperglycemia
5.12.1. Underlying Mechanisms of Dysglycemia
5.12.2. Reported Dysglycemia and Hypoglycemia Associated with Modern FQs
5.13. Hepatotoxicity
5.13.1. Underlying Mechanisms of Hepatotoxicity
5.13.2. Reported Hepatotoxicity Associated with Modern FQs
5.14. Genotoxicity
5.14.1. Underlying Mechanism of Genotoxicity
5.14.2. Genotoxicity Associated with Modern FQs
5.15. Severe Hemolytic-Uremic Syndrome
5.15.1. Underlying Mechanisms of Severe Hemolytic-Uremic Syndrome
5.15.2. Severe Hemolytic-Uremic Syndrome Associated with Modern FQs
5.16. Acute Renal Failure
5.16.1. Underlying Mechanisms of Acute Renal Failure
5.16.2. Acute Renal Failure Associated with Modern FQs
6. Management of Side-Effects
- Discontinuation of the regimen
- 2.
- Rest and decrease of physical load on the tendon alongside physical therapy
- 3.
- Avoiding FQs regimens in patients with pre-existing conditions of aortic aneurysm or aortic dissection
- 4.
- Reducing the duration of FQs’ administration may decrease the risk of peripheral neuropathy side-effect occurrence
- 5.
- Monitoring the serum folate level and supplementation in FQ-induced neuropathy
- 6.
- Avoiding the FQs therapy in patients with a history of seizures
- 7.
- Collaboration between the health professional team to prevent and treat hypersensitive syndrome to FQs
- 8.
- Avoiding exposure to sunlight
- 9.
- Increase the Clostridium difficile infection control measures
- 10.
- Reversing severe induced hypoglycemia by FQs
- 11.
- Replacing the culprit drug for the elevation of hepatic transaminases
- 12.
- Combating potential crystalluria produced by FQs in the kidney
- 13.
- Avoiding the co-administration of FQs and renin–angiotensin system blockers
- 14.
- Lower doses of FQs in older patients with advanced chronic kidney disease
7. Rational Use and Caution of Modern FQs
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beale, J.M., Jr.; Block, J.H. (Eds.) Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, 12th ed.; Wolters Kluwer Health: Baltimore, MD, USA, 2010; ISBN 978-0-7817-7929-6. [Google Scholar]
- Ball, P. Quinolone Generations: Natural History or Natural Selection? J. Antimicrob. Chemother. 2000, 46, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Bush, N.G.; Diez-Santos, I.; Abbott, L.R.; Maxwell, A. Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance. Molecules 2020, 25, 5662. [Google Scholar] [CrossRef] [PubMed]
- Rusu, A.; Lungu, I.-A.; Moldovan, O.-L.; Tanase, C.; Hancu, G. Structural Characterization of the Millennial Antibacterial (Fluoro)Quinolones—Shaping the Fifth Generation. Pharmaceutics 2021, 13, 1289. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, G.; Chew, N.S.Y. The History of Quinolones. In Fluoroquinolone Antibiotics; Ronald, A.R., Low, D.E., Eds.; Milestones in Drug Therapy; Birkhäuser: Basel, Switzerland, 2003; pp. 1–10. ISBN 978-3-0348-8103-6. [Google Scholar]
- Lesher, G.Y.; Froelich, E.J.; Gruett, M.D.; Bailey, J.H.; Brundage, R.P. 1,8-Naphthyridine Derivatives. A New Class of Chemotherapeutic Agents. J. Med. Pharm. Chem. 1962, 91, 1063–1065. [Google Scholar] [CrossRef]
- MacDougall, C. Sulfonamides, Trimethoprim-Sulfamethoxazole, Quinolones, and Agents for Urinary Tract Infections. In Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 13th ed.; Brunton, L.L., Hilal-Dandan, R., Knollmann, B.C., Eds.; McGraw-Hill Education: New York, NY, USA, 2017. [Google Scholar]
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone Antibiotics. Medchemcomm 2019, 10, 1719–1739. [Google Scholar] [CrossRef] [PubMed]
- Tillotson, G.S. Quinolones: Structure-Activity Relationships and Future Predictions. J. Med. Microbiol. 1996, 44, 320–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, L.R. Quinolone Molecular Structure-Activity Relationships: What We Have Learned about Improving Antimicrobial Activity. Clin. Infect. Dis. 2001, 33, S180–S186. [Google Scholar] [CrossRef] [PubMed]
- Domagala, J.M.; Hagen, S.E. Structure-Activity Relationships of the Quinolone Antibacterials in the New Millennium: Some Things Change and Some Do Not. In Quinolone Antimicrobial Agents, 3rd ed.; American Society of Microbiology Press: Washington, DC, USA, 2003; pp. 3–18. [Google Scholar] [CrossRef]
- Srinivasan, S.; Beema Shafreen, R.M.; Nithyanand, P.; Manisankar, P.; Pandian, S.K. Synthesis and in Vitro Antimicrobial Evaluation of Novel Fluoroquinolone Derivatives. Eur. J. Med. Chem. 2010, 45, 6101–6105. [Google Scholar] [CrossRef] [PubMed]
- Asif, M. Study of Antimicrobial Quinolones and Structure Activity Relationship of Anti-Tubercular Compounds. Res. Rev. J. Chem. 2015, 4, 28–70. [Google Scholar]
- Brighty, K.E.; Gootz, T.D. Chapter 2—Chemistry and Mechanism of Action of the Quinolone Antibacterials. In The Quinolones, 3rd ed.; Andriole, V.T., Ed.; Academic Press: San Diego, CA, USA, 2000; pp. 33–97. ISBN 978-0-12-059517-4. [Google Scholar]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of Quinolone Action and Resistance. Biochemistry 2014, 53, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.C. Mechanisms of Action and Resistance of Older and Newer Fluoroquinolones. Clin. Infect. Dis. 2000, 31, S24–S28. [Google Scholar] [CrossRef]
- Hooper, D.C.; Jacoby, G.A. Topoisomerase Inhibitors: Fluoroquinolone Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025320. [Google Scholar] [CrossRef] [Green Version]
- Correia, S.; Poeta, P.; Hébraud, M.; Capelo, J.L.; Igrejas, G. Mechanisms of Quinolone Action and Resistance: Where Do We Stand? J. Med. Microbiol. 2017, 66, 551–559. [Google Scholar] [CrossRef]
- Blondeau, J.M. Fluoroquinolones: Mechanism of Action, Classification, and Development of Resistance. Surv. Ophthalmol. 2004, 49, S73–S78. [Google Scholar] [CrossRef]
- Fàbrega, A.; Madurga, S.; Giralt, E.; Vila, J. Mechanism of Action of and Resistance to Quinolones. Microb. Biotechnol. 2009, 2, 40–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocsis, B.; Gulyás, D.; Szabó, D. Delafloxacin, Finafloxacin, and Zabofloxacin: Novel Fluoroquinolones in the Antibiotic Pipeline. Antibiotics 2021, 10, 1506. [Google Scholar] [CrossRef] [PubMed]
- Polat, H.K.; Pehlivan, S.B.; Ozkul, C.; Calamak, S.; Ozturk, N.; Aytekin, E.; Firat, A.; Ulubayram, K.; Kocabeyoglu, S.; Irkec, M.; et al. Development of Besifloxacin HCl Loaded Nanofibrous Ocular Inserts for the Treatment of Bacterial Keratitis: In Vitro, Ex Vivo and in Vivo Evaluation. Int. J. Pharm. 2020, 585, 119552. [Google Scholar] [CrossRef]
- Thakare, R.; Singh, S.; Dasgupta, A.; Chopra, S. Lascufloxacin Hydrochloride to Treat Bacterial Infection. Drugs Today 2020, 56, 365–376. [Google Scholar] [CrossRef]
- Hagihara, M.; Kato, H.; Shibata, Y.; Sakanashi, D.; Asai, N.; Suematsu, H.; Yamagishi, Y.; Mikamo, H. In Vivo Pharmacodynamics of Lascufloxacin and Levofloxacin against Streptococcus Pneumoniae and Prevotella Intermedia in a Pneumonia Mixed-Infection Mouse Model. Anaerobe 2021, 69, 102346. [Google Scholar] [CrossRef] [PubMed]
- Totoli, E.G.; Nunes Salgado, H.R. Besifloxacin: A Critical Review of Its Characteristics, Properties, and Analytical Methods. Crit. Rev. Anal. Chem. 2018, 48, 132–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeCory, H.H.; Sanfilippo, C.M.; Proskin, H.M.; Blondeau, J.M. Characterization of Baseline Polybacterial versus Monobacterial Infections in Three Randomized Controlled Bacterial Conjunctivitis Trials and Microbial Outcomes with Besifloxacin Ophthalmic Suspension 0.6%. PLoS ONE 2020, 15, e0237603. [Google Scholar] [CrossRef]
- McKeage, K. Finafloxacin: First Global Approval. Drugs 2015, 75, 687–693. [Google Scholar] [CrossRef]
- Poole, R.M. Nemonoxacin: First Global Approval. Drugs 2014, 74, 1445–1453. [Google Scholar] [CrossRef]
- Chang, L.-W.; Hsu, M.-C.; Zhang, Y.-Y. Nemonoxacin (Taigexyn®): A New Non-Fluorinated Quinolone; IntechOpen: London, UK, 2019; ISBN 978-1-78984-473-3. [Google Scholar]
- Cheng, S.-L.; Wu, R.-G.; Chuang, Y.-C.; Perng, W.-C.; Tsao, S.-M.; Chang, Y.-T.; Chang, L.-W.; Hsu, M.-C. Integrated Safety Summary of Phase II and III Studies Comparing Oral Nemonoxacin and Levofloxacin in Community-Acquired Pneumonia. J. Microbiol. Immunol. Infect. 2019, 52, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Bryant, E.E. Quinolones. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Office of the Commissioner FDA Updates Warnings for Fluoroquinolone Antibiotics. Available online: https://www.fda.gov/news-events/press-announcements/fda-updates-warnings-fluoroquinolone-antibiotics (accessed on 12 August 2021).
- Gatti, M.; Bianchin, M.; Raschi, E.; De Ponti, F. Assessing the Association between Fluoroquinolones and Emerging Adverse Drug Reactions Raised by Regulatory Agencies: An Umbrella Review. Eur. J. Intern. Med. 2020, 75, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Francisco, E.M. Fluoroquinolone and Quinolone Antibiotics: PRAC Recommends New Restrictions on Use Following Review of Disabling Potentially Long-Lasting Side Effects. Available online: https://www.ema.europa.eu/en/news/fluoroquinolone-quinolone-antibiotics-prac-recommends-new-restrictions-use-following-review (accessed on 12 August 2021).
- Francisco, E.M. Disabling and Potentially Permanent Side Effects Lead to Suspension or Restrictions of Quinolone Fluoroquinolone Antibiotics. Available online: https://www.ema.europa.eu/en/news/disabling-potentially-permanent-side-effects-lead-suspension-restrictions-quinolone-fluoroquinolone (accessed on 12 August 2021).
- Dassault Systèmes BIOVIA Draw for Academics. Available online: https://discover.3ds.com/biovia-draw-academic (accessed on 1 November 2022).
- Rubinstein, E. History of Quinolones and Their Side Effects. CHE 2001, 47, 3–8. [Google Scholar] [CrossRef]
- Lungu, I.-A.; Moldovan, O.-L.; Biriș, V.; Rusu, A. Fluoroquinolones Hybrid Molecules as Promising Antibacterial Agents in the Fight against Antibacterial Resistance. Pharmaceutics 2022, 14, 1749. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.L.; Singh, A.; Kumar, S.; Majumdar, D.K. Besifloxacin the fourth generation fluoroquinolone: A review. J. Drug Deliv. Ther. 2014, 4, 39–44. [Google Scholar] [CrossRef]
- Tanaka, K.; Vu, H.; Hayashi, M. In Vitro Activities and Spectrum of Lascufloxacin (KRP-AM1977) against Anaerobes. J. Infect. Chemother. 2021, 27, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, A.; Mastim, M.; Shah, M.; Gutte, R.; Joshi, P.; Kumbhar, D.; Periasamy, H.; Palwe, S.R.; Chavan, R.; Bhagwat, S.; et al. Efficacy and Safety of a Novel Broad-Spectrum Anti-MRSA Agent Levonadifloxacin Compared with Linezolid for Acute Bacterial Skin and Skin Structure Infections: A Phase 3, Openlabel, Randomized Study. J. Assoc. Physicians India 2020, 68, 30–36. [Google Scholar]
- Sweetman, S.C. (Ed.) Martindale: The Complete Drug Reference, 36th ed.; Pharmaceutical Press: London, UK; Chicago, IL, USA, 2009; ISBN 978-0-85369-840-1. [Google Scholar]
- Narayanan, V.; Motlekar, S.; Kadhe, G.; Bhagat, S. Efficacy and Safety of Nadifloxacin for Bacterial Skin Infections: Results from Clinical and Post-Marketing Studies. Dermatol. Ther. 2014, 4, 233–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limberakis, C. Quinolone Antibiotics: Levofloxacin (Levaquin®), Moxifloxacin (Avelox®), Gemifloxacin (Factive®), and Garenoxacin (T-3811). In The Art of Drug Synthesis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 39–69. ISBN 978-0-470-13497-9. [Google Scholar]
- Cervantes, L.J.; Mah, F.S. Clinical Use of Gatifloxacin Ophthalmic Solution for Treatment of Bacterial Conjunctivitis. Clin. Ophthalmol. 2011, 5, 495–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drug Approval Package: Zymar (Gatifloxacin) NDA #021493. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/021493_Zymar.cfm (accessed on 5 August 2022).
- Mah, F.S.; Sanfilippo, C.M. Besifloxacin: Efficacy and Safety in Treatment and Prevention of Ocular Bacterial Infections. Ophthalmol. Ther. 2016, 5, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domagala, J.M. Structure-Activity and Structure-Side-Effect Relationships for the Quinolone Antibacterials. J. Antimicrob. Chemother. 1994, 33, 685–706. [Google Scholar] [CrossRef]
- Blum, M.D.; Graham, D.J.; McCloskey, C.A. Temafloxacin Syndrome: Review of 95 Cases. Clin. Infect. Dis. 1994, 18, 946–950. [Google Scholar] [CrossRef]
- Mogle, B.T.; Steele, J.M.; Thomas, S.J.; Bohan, K.H.; Kufel, W.D. Clinical Review of Delafloxacin: A Novel Anionic Fluoroquinolone. J. Antimicrob. Chemother. 2018, 73, 1439–1451. [Google Scholar] [CrossRef] [Green Version]
- Bassetti, M.; Hooper, D.; Tillotson, G. Analysis of Pooled Phase 3 Safety Data for Delafloxacin in Acute Bacterial Skin and Skin Structure Infections. Clin. Infect. Dis. 2019, 68, S233–S240. [Google Scholar] [CrossRef] [Green Version]
- Suaifan, G.A.R.Y.; Mohammed, A.A.M. Fluoroquinolones Structural and Medicinal Developments (2013–2018): Where Are We Now? Bioorganic Med. Chem. 2019, 27, 3005–3060. [Google Scholar] [CrossRef]
- Lu, T.; Zhao, X.; Li, X.; Drlica-Wagner, A.; Wang, J.-Y.; Domagala, J.; Drlica, K. Enhancement of Fluoroquinolone Activity by C-8 Halogen and Methoxy Moieties: Action against a Gyrase Resistance Mutant of Mycobacterium Smegmatis and a Gyrase-Topoisomerase IV Double Mutant of Staphylococcus Aureus. Antimicrob. Agents Chemother. 2001, 45, 2703–2709. [Google Scholar] [CrossRef] [Green Version]
- Thomas, G. Medicinal Chemistry: An Introduction, 2nd ed.; Wiley: Chicester, UK, 2008. [Google Scholar]
- Haas, W.; Sanfilippo, C.M.; Hesje, C.K.; Morris, T.W. Contribution of the R8 Substituent to the in Vitro Antibacterial Potency of Besifloxacin and Comparator Ophthalmic Fluoroquinolones. Clin. Ophthalmol. 2013, 7, 821–830. [Google Scholar] [CrossRef] [Green Version]
- Drlica, K.; Hiasa, H.; Kerns, R.; Malik, M.; Mustaev, A.; Zhao, X. Quinolones: Action and Resistance Updated. Curr. Top Med. Chem. 2009, 9, 981–998. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Hao, H.; Dai, M.; Liu, Z.; Yuan, Z. Antibacterial Action of Quinolones: From Target to Network. Eur. J. Med. Chem. 2013, 66, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Redgrave, L.S.; Sutton, S.B.; Webber, M.A.; Piddock, L.J.V. Fluoroquinolone Resistance: Mechanisms, Impact on Bacteria, and Role in Evolutionary Success. Trends Microbiol. 2014, 22, 438–445. [Google Scholar] [CrossRef]
- Malik, M.; Zhao, X.; Drlica, K. Lethal Fragmentation of Bacterial Chromosomes Mediated by DNA Gyrase and Quinolones. Mol. Microbiol. 2006, 61, 810–825. [Google Scholar] [CrossRef]
- Hong, Y.; Zeng, J.; Wang, X.; Drlica, K.; Zhao, X. Post-Stress Bacterial Cell Death Mediated by Reactive Oxygen Species. Proc. Natl. Acad. Sci. USA 2019, 116, 10064–10071. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.; Li, Q.; Gao, Q.; Xie, J.; Huang, H.; Drlica, K.; Zhao, X. Reactive Oxygen Species Play a Dominant Role in All Pathways of Rapid Quinolone-Mediated Killing. J. Antimicrob. Chemother. 2020, 75, 576–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Rosado, A.I.; Valencia, E.Y.; Rodríguez-Rojas, A.; Costas, C.; Galhardo, R.S.; Blázquez, J.; Rodríguez-Beltrán, J. Reactive Oxygen Species Are Major Contributors to SOS-Mediated Mutagenesis Induced by Fluoroquinolones. bioRxiv 2018, 428961. [Google Scholar] [CrossRef]
- Michalak, K.; Sobolewska-Włodarczyk, A.; Włodarczyk, M.; Sobolewska, J.; Woźniak, P.; Sobolewski, B. Treatment of the Fluoroquinolone-Associated Disability: The Pathobiochemical Implications. Oxidative Med. Cell. Longev. 2017, 2017, e8023935. [Google Scholar] [CrossRef] [Green Version]
- Roberts, J.R. InFocus: Fluoroquinolone Side Effects Just Got Scarier. Emerg. Med. News 2018, 40, 26–27. [Google Scholar] [CrossRef]
- EMA. Quinolone- and Fluoroquinolone-Containing Medicinal Products. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/quinolone-fluoroquinolone-containing-medicinal-products (accessed on 12 August 2021).
- Center for Drug Evaluation and Research FDA Drug Safety Communication: FDA Updates Warnings for Oral and Injectable Fluoroquinolone Antibiotics Due to Disabling Side Effects. FDA 2019. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-updates-warnings-oral-and-injectable-fluoroquinolone-antibiotics (accessed on 16 September 2022).
- Aschenbrenner, D.S. The FDA Revises Boxed Warning For Fluoroquinolones-Again. Am. J. Nurs. 2016, 116, 22–23. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research. FDA Reinforces Safety Information about Serious Low Blood Sugar Levels and Mental Health Side Effects with Fluoroquinolone Antibiotics; Requires Label Changes. FDA 2018. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-reinforces-safety-information-about-serious-low-blood-sugar-levels-and-mental-health-side (accessed on 20 September 2022).
- Office of the Commissioner FDA. In Brief: FDA Warns That Fluoroquinolone Antibiotics Can Cause Aortic Aneurysm in Certain Patients. FDA 2019. Available online: https://www.fda.gov/news-events/fda-brief/fda-brief-fda-warns-fluoroquinolone-antibiotics-can-cause-aortic-aneurysm-certain-patients (accessed on 19 September 2022).
- EMA. Meeting Highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 1–4 October 2018. Available online: https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-1-4-october-2018 (accessed on 20 September 2022).
- Tanne, J.H. FDA Adds “Black Box” Warning Label to Fluoroquinolone Antibiotics. BMJ 2008, 337, 135. [Google Scholar] [CrossRef] [Green Version]
- Waknine, Y. Fluoroquinolones Earn Black Box Warning for Tendon-Related Adverse Effects. Available online: https://www.medscape.com/viewarticle/577302 (accessed on 21 September 2022).
- Center for Drug Evaluation and Research Postmarket Drug Safety Information for Patients and Providers—Information for Healthcare Professionals: Fluoroquinolone Antimicrobial Drugs [Ciprofloxacin (Marketed as Cipro and Generic Ciprofloxacin), Ciprofloxacin Extended-Release (Marketed as Cipro XR and Proquin XR), Gemifloxacin (Marketed as Factive), Levofloxacin (Marketed as Levaquin), Moxifloxacin (Marketed as Avelox), Norfloxacin (Marketed as Noroxin), and Ofloxacin (Marketed as Floxin)]. Available online: http://wayback.archive-it.org/7993/20161022101528/http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm126085.htm (accessed on 21 September 2022).
- Jones, S.C.; Sorbello, A.; Boucher, R.M. Fluoroquinolone-Associated Myasthenia Gravis Exacerbation. Drug Saf. 2011, 34, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Center for Drug Evaluation and Research Drug Safety and Availability—FDA Drug Safety Communication: FDA Requires Label Changes to Warn of Risk for Possibly Permanent Nerve Damage from Antibacterial Fluoroquinolone Drugs Taken by Mouth or by Injection. Available online: http://wayback.archive-it.org/7993/20161022101530/http://www.fda.gov/Drugs/DrugSafety/ucm365050.htm (accessed on 21 September 2022).
- Center for Drug Evaluation and Research. FDA Drug Safety Communication: FDA Advises Restricting Fluoroquinolone Antibiotic Use for Certain Uncomplicated Infections; Warns about Disabling Side Effects That Can Occur Together. FDA 2016. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-advises-restricting-fluoroquinolone-antibiotic-use-certain (accessed on 21 September 2022).
- FDA. Drug Safety Communication FDA Warns about Increased Risk of Ruptures or Tears in the Aorta Blood Vessel with Fluoroquinolone Antibiotics in Certain Patients. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-increased-risk-ruptures-or-tears-aorta-blood-vessel-fluoroquinolone-antibiotics (accessed on 10 October 2022).
- EMA. Systemic and Inhaled Fluoroquinolones: Risk of Heart Valve Regurgitation/Incompetence. Available online: https://www.ema.europa.eu/en/medicines/dhpc/systemic-inhaled-fluoroquinolones-risk-heart-valve-regurgitationincompetence (accessed on 20 September 2022).
- Etminan, M.; Sodhi, M.; Ganjizadeh-Zavareh, S.; Carleton, B.; Kezouh, A.; Brophy, J.M. Oral Fluoroquinolones and Risk of Mitral and Aortic Regurgitation. J. Am. Coll. Cardiol. 2019, 74, 1444–1450. [Google Scholar] [CrossRef]
- Guzzardi, D.G.; Teng, G.; Kang, S.; Geeraert, P.J.; Pattar, S.S.; Svystonyuk, D.A.; Belke, D.D.; Fedak, P.W.M. Induction of Human Aortic Myofibroblast-Mediated Extracellular Matrix Dysregulation: A Potential Mechanism of Fluoroquinolone-Associated Aortopathy. J. Thorac. Cardiovasc. Surg. 2019, 157, 109–119.e2. [Google Scholar] [CrossRef] [PubMed]
- Systemic and Inhaled Fluoroquinolones: Small Risk of Heart Valve Regurgitation; Consider Other Therapeutic Options First in Patients at Risk. Available online: https://www.gov.uk/drug-safety-update/systemic-and-inhaled-fluoroquinolones-small-risk-of-heart-valve-regurgitation-consider-other-therapeutic-options-first-in-patients-at-risk (accessed on 19 July 2022).
- Strange, J.E.; Holt, A.; Blanche, P.; Gislason, G.; Torp-Pedersen, C.; Christensen, D.M.; Hansen, M.L.; Lamberts, M.; Schou, M.; Olesen, J.B.; et al. Oral Fluoroquinolones and Risk of Aortic or Mitral Regurgitation: A Nationwide Nested Case-Control Study. Eur. Heart J. 2021, 42, 2899–2908. [Google Scholar] [CrossRef] [PubMed]
- Tandan, M.; Cormican, M.; Vellinga, A. Adverse Events of Fluoroquinolones vs. Other Antimicrobials Prescribed in Primary Care: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Antimicrob. Agents 2018, 52, 529–540. [Google Scholar] [CrossRef] [Green Version]
- Hook, E.W.; Golden, M.R.; Taylor, S.N.; Henry, E.; Tseng, C.; Workowski, K.A.; Swerdlow, J.; Nenninger, A.; Cammarata, S. Efficacy and Safety of Single-Dose Oral Delafloxacin Compared With Intramuscular Ceftriaxone for Uncomplicated Gonorrhea Treatment: An Open-Label, Noninferiority, Phase 3, Multicenter, Randomized Study. Sex Transm Dis 2019, 46, 279–286. [Google Scholar] [CrossRef]
- Kurono, Y.; Kawauchi, H.; Hori, S.; Tateda, K.; Totsuka, K.; Asano, M.; Suzuki, K. Phase III Double-Blind Comparative Study of Lascufloxacin versus Levofloxacin in Patients with Sinusitis. Jpn. J. Chemother. 2020, 68, 68–80. [Google Scholar]
- Health Canada Summary Safety Review—Oral Fluoroquinolones—Assessing the Potential Risk of Retinal Detachment. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/safety-reviews/summary-safety-review-oral-fluoroquinolones-assessing-potential-risk-retinal.html (accessed on 5 October 2022).
- Health Canada Summary Safety Review—Fluoroquinolones—Assessing the Potential Risk of Persistent and Disabling Side Effects. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/safety-reviews/summary-safety-review-fluoroquinolones-assessing-potential-risk-persistent-disabling-effects.html (accessed on 5 October 2022).
- Medicines and Healthcare products Regulatory Agency Fluoroquinolone Antibiotics: New Restrictions and Precautions for Use Due to Very Rare Reports of Disabling and Potentially Long-Lasting or Irreversible Side Effects. Available online: https://www.gov.uk/drug-safety-update/fluoroquinolone-antibiotics-new-restrictions-and-precautions-for-use-due-to-very-rare-reports-of-disabling-and-potentially-long-lasting-or-irreversible-side-effects (accessed on 5 October 2022).
- Cheng, A.C.; Turnidge, J.; Collignon, P.; Looke, D.; Barton, M.; Gottlieb, T. Control of Fluoroquinolone Resistance through Successful Regulation, Australia. Emerg. Infect. Dis. 2012, 18, 1453–1460. [Google Scholar] [CrossRef]
- Therapeutic Goods Administration (TGA) Fluoroquinolone Antibiotics and Risk of Aortic Aneurysm/Dissection. Available online: https://www.tga.gov.au/news/safety-updates/fluoroquinolone-antibiotics-and-risk-aortic-aneurysmdissection (accessed on 5 October 2022).
- Therapeutic Goods Administration (TGA) Update—Fluoroquinolone Antibiotics and Adverse Events. Available online: https://www.tga.gov.au/news/safety-updates/update-fluoroquinolone-antibiotics-and-adverse-events (accessed on 5 October 2022).
- Outterson, K.; Powers, J.H.; Seoane-Vazquez, E.; Rodriguez-Monguio, R.; Kesselheim, A.S. Approval and Withdrawal of New Antibiotics and Other Antiinfectives in the U.S., 1980–2009. J. Law. Med. Ethics 2013, 41, 688–696. [Google Scholar] [CrossRef] [Green Version]
- Bowie, W.R.; Willetts, V.; Jewesson, P.J. Adverse Reactions in a Dose-Ranging Study with a New Long-Acting Fluoroquinolone, Fleroxacin. Antimicrob. Agents Chemother. 1989, 33, 1778–1782. [Google Scholar] [CrossRef] [Green Version]
- Geddes, A.M. Safety of Fleroxacin in Clinical Trials. Am. J. Med. 1993, 94, 201S–203S. [Google Scholar] [CrossRef]
- Kimura, N.; Miyazaki, E.; Matsuno, O.; Abe, Y.; Tsuda, T. Drug-induced pneumonitis with eosinophilic infiltration due to tosufloxacin tosilate. J. Jpn. Respir. Soc. 1998, 36, 618–622. [Google Scholar]
- Choi, M.K.; Woo, H.Y.; Heo, J.; Cho, M.; Kim, G.H.; Song, G.A.; Kim, M.B. Toxic Epidermal Necrolysis Associated with Sorafenib and Tosufloxacin in a Patient with Hepatocellular Carcinoma. Ann. Dermatol. 2011, 23, S404–S407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, R.C., Jr.; Ambrose, P.G. Antimicrobial Safety: Focus on Fluoroquinolones. Clin. Infect. Dis. 2005, 41, S144–S157. [Google Scholar] [CrossRef]
- Aronson, J.K. Meyler’s Side Effects of Drugs: The International Encyclopedia of Adverse Drug Reactions and Interactions; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 978-0-444-53716-4. [Google Scholar]
- Mandell, L.A.; Ball, P.; Tillotson, G. Antimicrobial Safety and Tolerability: Differences and Dilemmas. Clin. Infect. Dis. 2001, 32, S72–S79. [Google Scholar] [CrossRef]
- Finch, R.G. The Withdrawal of Temafloxacin. Drug Saf. 1993, 8, 9–11. [Google Scholar] [CrossRef]
- Young, A.R.; Fakouhi, T.D.; Harrison, G.I.; Roniker, B.; Swabb, E.A.; Hawk, J.L.M. The UVR Wavelength Dependence for Lomefloxacin Photosensitization of Human Skin. J. Photochem. Photobiol. B Biol. 1996, 32, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.J.; Fakouhi, T.D.; Stern, R.S.; Bourget, T.; Roniker, B.; Swabb, E.A. Photoreactions with a Fluoroquinolone Antimicrobial: Evening versus Morning Dosing. Clin. Pharmacol. Ther. 1994, 56, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Petersen, U. Quinolone Antibiotics: The Development of Moxifloxacin. In Analogue-Based Drug Discovery; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 315–370. ISBN 978-3-527-60800-3. [Google Scholar]
- Jaillon, P.; Morganroth, J.; Brumpt, I.; Talbot, G. Overview of Electrocardiographic and Cardiovascular Safety Data for Sparfloxacin. Sparfloxacin Safety Group. J. Antimicrob. Chemother. 1996, 37, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, E. Safety Profile of Sparfloxacin in the Treatment of Respiratory Tract Infections. J. Antimicrob. Chemother. 1996, 37, 145–160. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Dorr, M.B.; Magner, D.J.; Talbot, G.H. Safety Profile of Sparfloxacin, a New Fluoroquinolone Antibiotic. Clin. Ther. 1999, 21, 148–159. [Google Scholar] [CrossRef] [PubMed]
- King, D.E.; Malone, R.; Lilley, S.H. New Classification and Update on the Quinolone Antibiotics. Am. Fam. Physician 2000, 61, 2741–2748. [Google Scholar] [PubMed]
- Qureshi, Z.P.; Seoane-Vazquez, E.; Rodriguez-Monguio, R.; Stevenson, K.B.; Szeinbach, S.L. Market Withdrawal of New Molecular Entities Approved in the United States from 1980 to 2009. Pharmacoepidemiol. Drug Saf. 2011, 20, 772–777. [Google Scholar] [CrossRef]
- Melvani, S.; Speed, B.R. Alatrofloxacin-Induced Seizures during Slow Intravenous Infusion. Ann. Pharmacother. 2000, 34, 1017–1019. [Google Scholar] [CrossRef] [PubMed]
- Gales, B.J.; Sulak, L.B. Severe Thrombocytopenia Associated with Alatrofloxacin. Ann. Pharmacother. 2000, 34, 330–334. [Google Scholar] [CrossRef]
- File, T.M., Jr.; Schlemmer, B.; Garau, J.; Cupo, M.; Young, C.; The 049 Clinical Study Group. Efficacy and Safety of Gemifloxacin in the Treatment of Community-Acquired Pneumonia: A Randomized, Double-Blind Comparison with Trovafloxacin. J. Antimicrob. Chemother. 2001, 48, 67–74. [Google Scholar] [CrossRef]
- Pannu, H.K.; Gottlieb, L.; Fishman, E.K. Acute Liver Failure Due to Trovafloxacin: CT Findings. Emerg. Radiol. 2001, 8, 108–110. [Google Scholar] [CrossRef]
- Stahlmann, R.; Schwabe, R. Safety Profile of Grepafloxacin Compared with Other Fluoroquinolones. J. Antimicrob. Chemother. 1997, 40, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.E.; Mazur, A.; Yang, T.; Roden, D.M. Potassium Current Antagonist Properties and Proarrhythmic Consequences of Quinolone Antibiotics. J. Pharmacol. Exp. Ther. 2001, 296, 806–810. [Google Scholar]
- Zhanel, G.G.; Walkty, A.; Vercaigne, L.; Karlowsky, J.A.; Embil, J.; Gin, A.S.; Hoban, D.J. The New Fluoroquinolones: A Critical Review. Can. J. Infect. Dis. 1999, 10, 207–238. [Google Scholar] [CrossRef] [Green Version]
- FDA. Determination That TEQUIN (Gatifloxacin) Was Withdrawn From Sale for Reasons of Safety or Effectiveness. Available online: https://www.federalregister.gov/documents/2008/09/09/E8-20938/determination-that-tequin-gatifloxacin-was-withdrawn-from-sale-for-reasons-of-safety-or (accessed on 14 September 2022).
- Mandell, L.; Tillotson, G. Safety of Fluoroquinolones: An Update. Can. J. Infect. Dis. 2002, 13, 54–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EMA. Questions and Answers on the Withdrawal of the Marketing Authorisation Application for Factive Gemifloxacin. Available online: https://www.ema.europa.eu/en/documents/medicine-qa/questions-answers-withdrawal-marketing-authorisation-application-factive-gemifloxacin_en.pdf (accessed on 22 September 2022).
- EMA. Menarini International Operations Luxembourg Withdraws Its Marketing Authorisation Application for Factive (Gemifloxacin). Available online: https://www.ema.europa.eu/en/news/menarini-international-operations-luxembourg-withdraws-its-marketing-authorisation-application (accessed on 22 September 2022).
- Center for Drug Evaluation and Research. CDER Division of Drug Information. Available online: https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/cder-division-drug-information (accessed on 22 September 2022).
- Molnar, D.M.; Kremzner, M.E. Fluoroquinolones: A Hot Topic for Pharmacists and the Food and Drug Administration’s Division of Drug Information. J. Am. Pharm. Assoc. 2019, 59, 13–16. [Google Scholar] [CrossRef]
- Nenoff, P. Acne Vulgaris and Bacterial Skin Infections: Review of the Topical Quinolone Nadifloxacin. Expert Rev. Dermatol. 2006, 1, 643–654. [Google Scholar] [CrossRef]
- EMA. EMA/150639/2017 Nadifloxacin, List of Nationally Authorised Medicinal Products. Available online: https://www.ema.europa.eu/en/documents/psusa/nadifloxacin-list-nationally-authorised-medicinal-products-psusa/00002102/201605_en.pdf (accessed on 11 June 2021).
- Wetzel, C.; Lonneman, M.; Wu, C. Polypharmacological Drug Actions of Recently FDA Approved Antibiotics. Eur. J. Med. Chem. 2021, 209, 112931. [Google Scholar] [CrossRef] [PubMed]
- Center for Drug Evaluation and Research Drug Trial Snapshot: Xepi. FDA 2020. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trial-snapshot-xepi (accessed on 22 September 2022).
- Troy Brown FDA Approves Ozenoxacin Cream for Impetigo. Available online: https://www.medscape.com/viewarticle/890180 (accessed on 22 September 2022).
- Jacobs, M.R.; Appelbaum, P.C. Nadifloxacin: A Quinolone for Topical Treatment of Skin Infections and Potential for Systemic Use of Its Active Isomer, WCK 771. Expert Opin. Pharmacother. 2006, 7, 1957–1966. [Google Scholar] [CrossRef]
- Anderson, D.L. Sitafloxacin Hydrate for Bacterial Infections. Drugs Today 2008, 44, 489–501. [Google Scholar] [CrossRef]
- Chen, C.-K.; Cheng, I.-L.; Chen, Y.-H.; Lai, C.-C. Efficacy and Safety of Sitafloxacin in the Treatment of Acute Bacterial Infection: A Meta-Analysis of Randomized Controlled Trials. Antibiotics 2020, 9, 106. [Google Scholar] [CrossRef] [Green Version]
- NCATS Inxight Drugs—SITAFLOXACIN. Available online: https://drugs.ncats.io/drug/9TD681796G (accessed on 26 September 2022).
- Kawada, Y.; Ishihara, S.; Matsui, T.; Tsugawa, M.; Matsumoto, T. Clinical Study of Sitafloxacin in Febrile Complicated Pyelonephritis. Jpn. J. Chemother. 2008, 56, 103–109. [Google Scholar]
- Kawada, Y.; Matsumoto, T.; Onodera, S.; Kaku, M.; Hori, S. Clinical Study of Sitafloxacin in Male Nongonococcal Urethritis. Jpn. J. Chemother. 2008, 56, 130–138. [Google Scholar]
- Onodera, S.; Hori, S. Clinical Study of Sitafloxacin in the Treatment of Male Gonococcal Urethritis. Jpn. J. Chemother. 2008, 56, 146–153. [Google Scholar]
- Matsuda, S.; Noguchi, M.; Yasuda, J.; Hori, S. Clinical Study of Sitafloxacin in Treatment of Cervicitis with Chlamydia Trachomatis. Jpn. J. Chemother. 2008, 56, 139–145. [Google Scholar]
- Kawada, Y.; Ishihara, S.; Matsui, T.; Tsugawa, M.; Matsumoto, T.; Watanabe, K.; Nakashima, M. Comparative Study on Sitafloxacin and Levofloxacin in Complicated Urinary Tract Infections. Jpn. J. Chemother. 2008, 56, 81–91. [Google Scholar] [CrossRef]
- Kawada, Y.; Yasuda, M.; Tanaka, K.; Monden, K.; Akasaka, S.; Egashira, T.; Kaku, M.; Hori, S. Dose-Comparative Study of Sitafloxacin in Complicated Urinary Tract Infections. Jpn. J. Chemother. 2008, 56, 92–102. [Google Scholar] [CrossRef]
- Saito, A.; Tanigawara, Y.; Watanabe, A.; Aoki, N.; Niki, Y.; Kohno, S.; Kaku, M.; Hori, S.; Totsuka, K. Open Study of Sitafloxacin in Patients with Respiratory Tract Infections. Jpn. J. Chemother. 2008, 56, 63–80. [Google Scholar]
- Sasaki, J.; Hori, S. Oral Tissue Distribution, Efficacy, and Safety of Sitafloxacin in Patients with Dentistry and Oral Surgery Infection. Jpn. J. Chemother. 2008, 56, 121–129. [Google Scholar]
- Saito, A.; Watanabe, A.; Aoki, N.; Niki, Y.; Kohno, S.; Kaku, M.; Hori, S. Phase III Double-Blind Comparative Study of Sitafloxacin versus Tosufloxacin in Patients with Community-Acquired Pneumonia. Jpn. J. Chemother. 2008, 56, 49–62. [Google Scholar]
- Kobayashi, H.; Watanabe, A.; Nakata, K.; Wada, K.; Niki, Y.; Kohno, S. Double-Blind Comparative Study of Sitafloxacin versus Levofloxacin in Patients with Respiratory Tract Infection. Jpn. J. Chemother. 2008, 56, 36–48. [Google Scholar] [CrossRef]
- FDA, N. 22308/S-013 Besifloxacin Label 2009. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/022308s013lbl.pdf (accessed on 5 June 2021).
- Khimdas, S.; Visscher, K.L.; Hutnik, C.M.L. Besifloxacin Ophthalmic Suspension: Emerging Evidence of Its Therapeutic Value in Bacterial Conjunctivitis. Ophthalmol. Eye Dis. 2011, 3, OED.S4102. [Google Scholar] [CrossRef] [Green Version]
- Therapeutic Goods Administration (TGA). AusPAR: Besifloxacin Hydrochloride. Available online: https://www.tga.gov.au/resources/auspar/auspar-besifloxacin-hydrochloride (accessed on 27 September 2022).
- FDA. Drug Approval Package Xtoro (Finafloxacin) Otic Suspension. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/206307Orig1s000TOC.cfm (accessed on 7 June 2021).
- Barnes, K.B.; Zumbrun, S.D.; Halasohoris, S.A.; Desai, P.D.; Miller, L.L.; Richards, M.I.; Russell, P.; Bentley, C.; Harding, S.V. Demonstration of the Broad-Spectrum In Vitro Activity of Finafloxacin against Pathogens of Biodefense Interest. Antimicrob. Agents Chemother. 2019, 63, e01470-19. [Google Scholar] [CrossRef] [Green Version]
- Barnes, K.B.; Richards, M.; Laws, T.R.; Nunez, A.; Thwaite, J.E.; Bentley, C.; Harding, S. Finafloxacin Is an Effective Treatment for Inhalational Tularemia and Plague in Mouse Models of Infection. Antimicrob. Agents Chemother. 2021, 65, e02294-20. [Google Scholar] [CrossRef] [PubMed]
- Product-Taigexyn®-TaiGen Biotechnology—A Pharmaceutical Company Dedicating in Drug Discovery. Available online: https://www.taigenbiotech.com/en/product/detail/Taigexyn (accessed on 26 September 2022).
- Cao, G.; Zhang, J.; Zhang, Y.; Guo, B.; Yu, J.; Wu, X.; Chen, Y.; Wu, J.-F.; Shi, Y. Safety, Tolerability, and Pharmacokinetics of Intravenous Nemonoxacin in Healthy Chinese Volunteers. Antimicrob. Agents Chemother. 2014, 58, 6116–6121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocsis, B.; Szabo, D. Zabofloxacin for Chronic Bronchitis. Drugs Today 2016, 52, 495. [Google Scholar] [CrossRef] [PubMed]
- Dongwha News Dong Wha Pharm’s Quinolone Antibacterial Agent, “Zabolante,” Wins at the 19th KNDA. Available online: https://www.dong-wha.co.kr/english/customer/dnews/content.asp?t_idx=1139 (accessed on 27 September 2022).
- Dong Wha Pharmaceutical CO., LTD Antibiotics. Available online: https://www.dong-wha.co.kr/english/product/content.asp?t_idx=545&t_page=1&d=&b=10&s=11 (accessed on 27 September 2022).
- FDA. Baxdela (Delafloxacin) Tablets and Injection. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208610Orig1s000,208611Orig1s000TOC.cfm (accessed on 5 June 2021).
- Scott, L.J. Delafloxacin: A Review in Acute Bacterial Skin and Skin Structure Infections. Drugs 2020, 80, 1247–1258. [Google Scholar] [CrossRef]
- EMA. Quofenix. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/quofenix (accessed on 27 September 2022).
- Eudaley, S. Delafloxacin (Baxdela) for Skin Infections. Am. Fam. Physician 2018, 98, 246–247. [Google Scholar]
- FDA. XEPITM (Ozenoxacin) Cream, for Topical Use 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208945lbl.pdf (accessed on 27 September 2022).
- Garcia Ron, G.; Villa Arranz, M. New Therapeutic Applications of Ozenoxacin in Superficial Skin Infections. Dermatol. Rep. 2022, 14. [Google Scholar] [CrossRef]
- Torrelo, A.; Grimalt, R.; Masramon, X.; Albareda López, N.; Zsolt, I. Ozenoxacin, a New Effective and Safe Topical Treatment for Impetigo in Children and Adolescents. Dermatology 2020, 236, 199–207. [Google Scholar] [CrossRef]
- Ogihara, S. NHI Drug Price Listing and Release of Oral Quinolone Antibacterial Agent “Lasvic®Tablets 75mg”. 2019. Available online: https://www.kyorin-pharm.co.jp/en/news/a329f0ae64024c1173f40660eede0efb37f1cbb0.pdf (accessed on 25 June 2021).
- Ogihara, S.; Liang, X. KYORIN and Nanjing Neiwa Faith Signed License Agreement for Lascufloxacin in China. 2022, 1. Available online: https://www.kyorin-pharm.co.jp/en/news/KYORIN%20and%20Nanjing%20Neiwa%20Faith%20Signed%20License%20Agreement%20for%20Lascufloxacin%20in%20China.pdf (accessed on 28 September 2022).
- Tateda, K.; Tanioka, S.; Totsuka, K.; Kohno, S. An Overview of Oral Lascufloxacin, a Novel Quinolone Antibiotic. Jpn. J. Chemother. 2020, 68, 1–15. [Google Scholar]
- Bakthavatchalam, Y.D.; Shankar, A.; Muniyasamy, R.; Peter, J.V.; Marcus, Z.; Triplicane Dwarakanathan, H.; Gunasekaran, K.; Iyadurai, R.; Veeraraghavan, B. Levonadifloxacin, a Recently Approved Benzoquinolizine Fluoroquinolone, Exhibits Potent in Vitro Activity against Contemporary Staphylococcus Aureus Isolates and Bengal Bay Clone Isolates Collected from a Large Indian Tertiary Care Hospital. J. Antimicrob. Chemother. 2020, 75, 2156–2159. [Google Scholar] [CrossRef]
- Saxena, D.; Kaul, G.; Dasgupta, A.; Chopra, S. Levonadifloxacin Arginine Salt to Treat MRSA Infection and Acute Bacterial Skin and Skin Structure Infection. Drugs Today 2020, 56, 583. [Google Scholar] [CrossRef]
- Koulenti, D.; Xu, E.; Song, A.; Sum Mok, I.Y.; Karageorgopoulos, D.E.; Armaganidis, A.; Tsiodras, S.; Lipman, J. Emerging Treatment Options for Infections by Multidrug-Resistant Gram-Positive Microorganisms. Microorganisms 2020, 8, 191. [Google Scholar] [CrossRef] [Green Version]
- Richards, G.A.; Brink, A.J.; Feldman, C. Rational Use of the Fluoroquinolones. S. Afr. Med. J. 2019, 109, 378–381. [Google Scholar] [CrossRef] [Green Version]
- Kuula, L.S.M.; Viljemaa, K.M.; Backman, J.T.; Blom, M. Fluoroquinolone-Related Adverse Events Resulting in Health Service Use and Costs: A Systematic Review. PLoS ONE 2019, 14, e0216029. [Google Scholar] [CrossRef]
- Van Bambeke, F.; Michot, J.-M.; Van Eldere, J.; Tulkens, P.M. Quinolones in 2005: An Update. Clin. Microbiol. Infect. 2005, 11, 256–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipsky, B.A.; Baker, C.A. Fluoroquinolone Toxicity Profiles: A Review Focusing on Newer Agents. Clin. Infect. Dis. 1999, 28, 352–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, N.; Nakata, Y.; Yazaki, A. New Findings on the Structure-Phototoxicity Relationship and Photostability of Fluoroquinolones with Various Substituents at Position 1. Antimicrob. Agents Chemother. 2004, 48, 799–803. [Google Scholar] [CrossRef] [Green Version]
- Emami, S.; Shafiee, A.; Foroumadi, A. Quinolones: Recent Structural and Clinical Developments. Iran. J. Pharm. Res. 2005, 4, 123–136. [Google Scholar] [CrossRef]
- Sutter, R.; Rüegg, S.; Tschudin-Sutter, S. Seizures as Adverse Events of Antibiotic Drugs: A Systematic Review. Neurology 2015, 85, 1332–1341. [Google Scholar] [CrossRef]
- Bennett, A.C.; Bennett, C.L.; Witherspoon, B.J.; Knopf, K.B. An Evaluation of Reports of Ciprofloxacin, Levofloxacin, and Moxifloxacin-Association Neuropsychiatric Toxicities, Long-Term Disability, and Aortic Aneurysms/Dissections Disseminated by the Food and Drug Administration and the European Medicines Agency. Expert Opin. Drug Saf. 2019, 18, 1055–1063. [Google Scholar] [CrossRef]
- Lee, C.-C.; Lee, M.G.; Hsieh, R.; Porta, L.; Lee, W.-C.; Lee, S.-H.; Chang, S.-S. Oral Fluoroquinolone and the Risk of Aortic Dissection. J. Am. Coll. Cardiol. 2018, 72, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Londhe, A.A.; Holy, C.E.; Weaver, J.; Fonseca, S.; Villasis, A.; Fife, D. Risk of Aortic Aneurysm and Dissection Following Exposure to Fluoroquinolones, Common Antibiotics, and Febrile Illness Using a Self-Controlled Case Series Study Design: Retrospective Analyses of Three Large Healthcare Databases in the US. PLoS ONE 2021, 16, e0255887. [Google Scholar] [CrossRef]
- Jun, C.; Fang, B. Current Progress of Fluoroquinolones-Increased Risk of Aortic Aneurysm and Dissection. BMC Cardiovasc. Disord. 2021, 21, 470. [Google Scholar] [CrossRef]
- George Sakoulas Adverse Effects of Fluoroquinolones: Where Do We Stand? Available online: https://www.jwatch.org/na48248/2019/02/13/adverse-effects-fluoroquinolones-where-do-we-stand (accessed on 12 August 2021).
- Hoefer, I.E.; den Adel, B.; Daemen, M.J.A.P. Biomechanical Factors as Triggers of Vascular Growth. Cardiovasc. Res. 2013, 99, 276–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orobello, N.C.; Dirain, C.O.; Schultz, G.; Milne-Davies, B.A.; Ng, M.R.A.; Antonelli, P.J. Ciprofloxacin Decreases Collagen in Mouse Tympanic Membrane Fibroblasts. Otolaryngol. Head Neck Surg. 2016, 155, 127–132. [Google Scholar] [CrossRef]
- Sendzik, J.; Shakibaei, M.; Schäfer-Korting, M.; Lode, H.; Stahlmann, R. Synergistic Effects of Dexamethasone and Quinolones on Human-Derived Tendon Cells. Int. J. Antimicrob. Agents 2010, 35, 366–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzzardi, D.; Teng, G.; Svystonyuk, D.; Kang, S.; Park, D.; Belke, D.; Turnbull, J.; Fedak, P. Fluoroquinolone induces human aortic fibroblast-mediated extracellular matrix dysregulation. Can. J. Cardiol. 2017, 33, S38–S39. [Google Scholar] [CrossRef] [Green Version]
- Uivarosi, V. Metal Complexes of Quinolone Antibiotics and Their Applications: An Update. Molecules 2013, 18, 11153–11197. [Google Scholar] [CrossRef] [PubMed]
- Walden, D.M.; Khotimchenko, M.; Hou, H.; Chakravarty, K.; Varshney, J. Effects of Magnesium, Calcium, and Aluminum Chelation on Fluoroquinolone Absorption Rate and Bioavailability: A Computational Study. Pharmaceutics 2021, 13, 594. [Google Scholar] [CrossRef]
- Lecomte, S.; Baron, M.H.; Chenon, M.T.; Coupry, C.; Moreau, N.J. Effect of Magnesium Complexation by Fluoroquinolones on Their Antibacterial Properties. Antimicrob. Agents Chemother. 1994, 38, 2810–2816. [Google Scholar] [CrossRef] [Green Version]
- Akinremi, C.A.; Obaleye, J.A.; Amolegbe, S.A.; Adediji, J.F.; Bamigboye, M.O. Biological Activities of Some Fluoroquinolones-Metal Complexes. Int. J. Med. Biomed. Res. 2012, 1, 24–34. [Google Scholar] [CrossRef]
- Badal, S.; Her, Y.F.; Maher, L.J. Nonantibiotic Effects of Fluoroquinolones in Mammalian Cells. J. Biol. Chem. 2015, 290, 22287–22297. [Google Scholar] [CrossRef] [Green Version]
- Shakibaei, M.; Pfister, K.; Schwabe, R.; Vormann, J.; Stahlmann, R. Ultrastructure of Achilles Tendons of Rats Treated with Ofloxacin and Fed a Normal or Magnesium-Deficient Diet. Antimicrob. Agents Chemother. 2000, 44, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Stahlmann, R.; Kühner, S.; Shakibaei, M.; Flores, J.; Vormann, J.; van Sickle, D.C. Effects of Magnesium Deficiency on Joint Cartilage in Immature Beagle Dogs: Immunohistochemistry, Electron Microscopy, and Mineral Concentrations. Arch. Toxicol. 2000, 73, 573–580. [Google Scholar] [CrossRef]
- Daneman, N.; Lu, H.; Redelmeier, D.A. Fluoroquinolones and Collagen Associated Severe Adverse Events: A Longitudinal Cohort Study. BMJ Open 2015, 5, e010077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, P.-H.; Hu, C.-F.; Liu, J.-W.; Chung, C.-H.; Chen, Y.-C.; Sun, C.-A.; Chien, W.-C. The Incidence of Collagen-Associated Adverse Events in Pediatric Population with the Use of Fluoroquinolones: A Nationwide Cohort Study in Taiwan. BMC Pediatr. 2020, 20, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markham, A. Delafloxacin: First Global Approval. Drugs 2017, 77, 1481–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lodise, T.; Corey, R.; Hooper, D.; Cammarata, S. Safety of Delafloxacin: Focus on Adverse Events of Special Interest. Open Forum. Infect. Dis. 2018, 5, ofy220. [Google Scholar] [CrossRef]
- Office of the Commissioner FDA Updates Warnings for Fluoroquinolone Antibiotics on Risks of Mental Health and Low Blood Sugar Adverse Reactions. Available online: https://www.fda.gov/news-events/press-announcements/fda-updates-warnings-fluoroquinolone-antibiotics-risks-mental-health-and-low-blood-sugar-adverse (accessed on 19 September 2022).
- Hornak, J.P.; Reynoso, D. Early Clinical Experience with Delafloxacin: A Case Series. Am. J. Med. Sci. 2022, 363, 359–363. [Google Scholar] [CrossRef]
- Lee, A.; Lamb, Y.N.; Shirley, M. Delafloxacin: A Review in Community-Acquired Pneumonia. Drugs 2022, 82, 913–923. [Google Scholar] [CrossRef]
- Stahlmann, R.; Lode, H. Toxicity of Quinolones. Drugs 1999, 58, 37–42. [Google Scholar] [CrossRef]
- Kim, G.K. The Risk of Fluoroquinolone-Induced Tendinopathy and Tendon Rupture. J. Clin. Aesthet. Dermatol. 2010, 3, 49–54. [Google Scholar] [PubMed]
- Fernández-Cuadros, M.E.; Casique-Bocanegra, L.O.; Albaladejo-Florín, M.J.; Gómez-Dueñas, S.; Ramos-Gonzalez, C.; Pérez-Moro, O.S. Bilateral Levofloxacin-Induced Achilles Tendon Rupture: An Uncommon Case Report and Review of the Literature. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2019, 12, 1179544119835222. [Google Scholar] [CrossRef]
- Melhus, A.; Apelqvist, J.; Larsson, J.; Eneroth, M. Levofloxacin-Associated Achilles Tendon Rupture and Tendinopathy. Scand. J. Infect. Dis. 2003, 35, 768–770. [Google Scholar] [CrossRef]
- Gold, L.; Igra, H. Levofloxacin-Induced Tendon Rupture: A Case Report and Review of the Literature. J. Am. Board Fam. Pract. 2003, 16, 458–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowatari, K.; Nakashima, K.; Ono, A.; Yoshihara, M.; Amano, M.; Toh, S. Levofloxacin-Induced Bilateral Achilles Tendon Rupture: A Case Report and Review of the Literature. J. Orthop. Sci. 2004, 9, 186–190. [Google Scholar] [CrossRef]
- Baik, S.; Lau, J.; Huser, V.; McDonald, C.J. Association between Tendon Ruptures and Use of Fluoroquinolone, and Other Oral Antibiotics: A 10-Year Retrospective Study of 1 Million US Senior Medicare Beneficiaries. BMJ Open 2020, 10, e034844. [Google Scholar] [CrossRef] [PubMed]
- Childs, S.G. Pathogenesis of Tendon Rupture Secondary to Fluoroquinolone Therapy. Orthop. Nurs. 2007, 26, 175–182. [Google Scholar] [CrossRef]
- Williams, R.J.; Attia, E.; Wickiewicz, T.L.; Hannafin, J.A. The Effect of Ciprofloxacin on Tendon, Paratenon, and Capsular Fibroblast Metabolism. Am. J. Sports Med. 2000, 28, 364–369. [Google Scholar] [CrossRef]
- Shiu, J.; Ting, G.; Kiang, T.K. Clinical Pharmacokinetics and Pharmacodynamics of Delafloxacin. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 305–317. [Google Scholar] [CrossRef]
- Bassetti, M.; Puente, F.D.; Magnasco, L.; Giacobbe, D.R. Innovative Therapies for Acute Bacterial Skin and Skin-Structure Infections (ABSSSI) Caused by Methicillin-Resistant Staphylococcus Aureus: Advances in Phase I and II Trials. Expert Opin. Investig. Drugs 2020, 29, 495–506. [Google Scholar] [CrossRef]
- O’Riordan, W.; McManus, A.; Teras, J.; Poromanski, I.; Cruz-Saldariagga, M.; Quintas, M.; Lawrence, L.; Liang, S.; Cammarata, S. PROCEED Study Group A Comparison of the Efficacy and Safety of Intravenous Followed by Oral Delafloxacin with Vancomycin Plus Aztreonam for the Treatment of Acute Bacterial Skin and Skin Structure Infections: A Phase 3, Multinational, Double-Blind, Randomized Study. Clin. Infect. Dis. 2018, 67, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Pullman, J.; Gardovskis, J.; Farley, B.; Sun, E.; Quintas, M.; Lawrence, L.; Ling, R.; Cammarata, S. PROCEED Study Group Efficacy and Safety of Delafloxacin Compared with Vancomycin plus Aztreonam for Acute Bacterial Skin and Skin Structure Infections: A Phase 3, Double-Blind, Randomized Study. J. Antimicrob. Chemother. 2017, 72, 3471–3480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horcajada, J.P.; Salata, R.A.; Álvarez-Sala, R.; Nitu, F.M.; Lawrence, L.; Quintas, M.; Cheng, C.-Y.; Cammarata, S. DEFINE-CABP Study Group A Phase 3 Study to Compare Delafloxacin With Moxifloxacin for the Treatment of Adults With Community-Acquired Bacterial Pneumonia (DEFINE-CABP). Open Forum. Infect. Dis. 2020, 7, ofz514. [Google Scholar] [CrossRef] [Green Version]
- Blair, K.; Czyz, C.N. Retinal Detachment. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Marchant, J. When Antibiotics Turn Toxic. Nature 2018, 555, 431–433. [Google Scholar] [CrossRef] [Green Version]
- Etminan, M.; Forooghian, F.; Brophy, J.M.; Bird, S.T.; Maberley, D. Oral Fluoroquinolones and the Risk of Retinal Detachment. JAMA 2012, 307, 1414–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasternak, B.; Svanström, H.; Melbye, M.; Hviid, A. Association Between Oral Fluoroquinolone Use and Retinal Detachment. JAMA 2013, 310, 2184–2190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brett, A.S. Oral Fluoroquinolone Use and Retinal Detachment: Reconciling Conflicting Findings in Observational Research. JAMA 2013, 310, 2151–2153. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, K.; Ghodasra, D.H.; Haynes, K.; Chen, J.; Kempen, J.H.; VanderBeek, B.L. Risk of Retinal Tear or Detachment with Oral Fluoroquinolone Use: A Cohort Study. Pharmacoepidemiol. Drug Saf. 2014, 23, 745–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chui, C.S.L.; Wong, I.C.K.; Wong, L.Y.L.; Chan, E.W. Association between Oral Fluoroquinolone Use and the Development of Retinal Detachment: A Systematic Review and Meta-Analysis of Observational Studies. J. Antimicrob. Chemother. 2015, 70, 971–978. [Google Scholar] [CrossRef] [Green Version]
- Raguideau, F.; Dray-Spira, R.; Zureik, M. Oral Fluoroquinolone Use and Retinal Detachment-Reply. JAMA Ophthalmol. 2016, 134, 1448–1449. [Google Scholar] [CrossRef]
- Douglas, I.J.; Root, A.; Krishnan, B. Oral Fluoroquinolone Use and Retinal Detachment. JAMA Ophthalmol. 2016, 134, 1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, Y.-H.; Park, S.J.; Jeong, S.; Oh, I.-S.; Jeong, H.E.; Park, K.H.; Shin, J.-Y. Signal Detection Between Fluoroquinolone Use and the Risk of Rhegmatogenous Retinal Detachment: Sequence Symmetry Analysis Using Nationwide South Korean Healthcare Database Between 2004 and 2015. Clin. Drug Investig. 2018, 38, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-Y.; Jeong, S.; Jeon, H.-L.; Byun, S.; Park, K.H.; Jeong, H.E.; Park, S.J. The Risk Profile of Rhegmatogenous Retinal Detachment before and after Using a Fluoroquinolone: A 12 Year Nationwide Self-Controlled Case Series Study. J. Antimicrob. Chemother. 2018, 73, 3442–3453. [Google Scholar] [CrossRef]
- Taher, M.K.; Habsah, M.; Bjerre, L.M.; Momoli, F.; Mattison, D.; Krewski, D. Systemic Quinolones and Risk of Retinal Detachment II: Systematic Review of Clinical Trials. Clin. Med. Rev. Case Rep. 2021, 8, 369. [Google Scholar] [CrossRef]
- Taher, M.K.; Alami, A.; Gravel, C.A.; Tsui, D.; Bjerre, L.M.; Momoli, F.; Mattison, D.; Krewski, D. Systemic Quinolones and Risk of Retinal Detachment I: Analysis of Data from the US FDA Adverse Event Reporting System. Expert Opin. Drug Saf. 2022, 21, 269–276. [Google Scholar] [CrossRef]
- Taher, M.K.; Crispo, J.A.G.; Fortin, Y.; Moog, R.; McNair, D.; Bjerre, L.M.; Momoli, F.; Mattison, D.; Krewski, D. Systemic Quinolones and Risk of Retinal Detachment III: A Nested Case-Control Study Using a US Electronic Health Records Database. Eur. J. Clin. Pharmacol. 2022, 78, 1019–1028. [Google Scholar] [CrossRef]
- Ponsioen, T.L.; van Luyn, M.J.A.; van der Worp, R.J.; van Meurs, J.C.; Hooymans, J.M.M.; Los, L.I. Collagen Distribution in the Human Vitreoretinal Interface. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4089–4095. [Google Scholar] [CrossRef] [Green Version]
- Reviglio, V.E.; Hakim, M.A.; Song, J.K.; O’Brien, T.P. Effect of Topical Fluoroquinolones on the Expression of Matrix Metalloproteinases in the Cornea. BMC Ophthalmol. 2003, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Sharma, C.; Velpandian, T.; Baskar Singh, S.; Ranjan Biswas, N.; Bihari Vajpayee, R.; Ghose, S. Effect of Fluoroquinolones on the Expression of Matrix Metalloproteinase in Debrided Cornea of Rats. Toxicol. Mech. Methods 2011, 21, 6–12. [Google Scholar] [CrossRef]
- Khaliq, Y.; Zhanel, G.G. Fluoroquinolone-Associated Tendinopathy: A Critical Review of the Literature. Clin. Infect. Dis. 2003, 36, 1404–1410. [Google Scholar] [CrossRef] [Green Version]
- Granowitz, E.V.; Brown, R.B. Antibiotic Adverse Reactions and Drug Interactions. Crit. Care Clin. 2008, 24, 421–442. [Google Scholar] [CrossRef] [PubMed]
- Hedenmalm, K.; Spigset, O. Peripheral Sensory Disturbances Related to Treatment with Fluoroquinolones. J. Antimicrob. Chemother. 1996, 37, 831–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J.S. Peripheral Neuropathy Associated with Fluoroquinolones. Ann. Pharmacother. 2001, 35, 1540–1547. [Google Scholar] [CrossRef]
- Morales, D.; Pacurariu, A.; Slattery, J.; Pinheiro, L.; McGettigan, P.; Kurz, X. Association Between Peripheral Neuropathy and Exposure to Oral Fluoroquinolone or Amoxicillin-Clavulanate Therapy. JAMA Neurol. 2019, 76, 827–833. [Google Scholar] [CrossRef] [Green Version]
- Popescu, C. Severe Acute Axonal Neuropathy Induced by Ciprofloxacin: A Case Report. CRN 2018, 10, 124–129. [Google Scholar] [CrossRef]
- Francis, J.K.; Higgins, E. Permanent Peripheral Neuropathy: A Case Report on a Rare but Serious Debilitating Side-Effect of Fluoroquinolone Administration. J. Investig. Med. High Impact Case Rep. 2014, 2, 2324709614545225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estofan, L.J.F.; Naydin, S.; Gliebus, G. Quinolone-Induced Painful Peripheral Neuropathy: A Case Report and Literature Review. J. Investig. Med. High Impact Case Rep. 2018, 6, 2324709617752736. [Google Scholar] [CrossRef] [Green Version]
- Scavone, C.; Mascolo, A.; Ruggiero, R.; Sportiello, L.; Rafaniello, C.; Berrino, L.; Capuano, A. Quinolones-Induced Musculoskeletal, Neurological, and Psychiatric ADRs: A Pharmacovigilance Study Based on Data from the Italian Spontaneous Reporting System. Front. Pharm. 2020, 11, 428. [Google Scholar] [CrossRef]
- Althaqafi, A.; Ali, M.; Alzahrani, Y.; Ming, L.C.; Hussain, Z. How Safe Are Fluoroquinolones for Diabetic Patients? A Systematic Review of Dysglycemic and Neuropathic Effects of Fluoroquinolones. Ther. Clin. Risk Manag. 2021, 17, 1083–1090. [Google Scholar] [CrossRef]
- Menarini Group A Randomized, Observer-Blinded, Active-Controlled, Phase Illb Study to Compare IV/Oral Delafloxacin Fixed-Dose Monotherapy With Best Available Treatments in a Microbiologically Enriched Population With Surgical Site Infections; clinicaltrials.gov, 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04042077 (accessed on 9 November 2022).
- Miki, M.; Mikasa, K.; Kadota, J.; Mukae, H.; Fujita, J.; Hori, S.; Yanagihara, K.; Tateda, K.; Totsuka, K.; Umemoto, Y.; et al. Phase III Double-Blind Comparative Study of Lascufloxacin versus Levofloxacin in Patients with Community-Acquired Pneumonia|Cochrane Library. Jpn. J. Chemother. 2021, 69, 255–269. [Google Scholar] [CrossRef]
- Chung, D.T.; Tsai, C.-Y.; Chen, S.-J.; Chang, L.-W.; King, C.-H.R.; Hsu, C.-H.; Chiu, K.-M.; Tan, H.-C.; Chang, Y.-T.; Hsu, M.-C. Multiple-Dose Safety, Tolerability, and Pharmacokinetics of Oral Nemonoxacin (TG-873870) in Healthy Volunteers. Antimicrob. Agents Chemother. 2010, 54, 411–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Rensburg, D.J.J.; Perng, R.-P.; Mitha, I.H.; Bester, A.J.; Kasumba, J.; Wu, R.-G.; Ho, M.-L.; Chang, L.-W.; Chung, D.T.; Chang, Y.-T.; et al. Efficacy and Safety of Nemonoxacin versus Levofloxacin for Community-Acquired Pneumonia. Antimicrob. Agents Chemother. 2010, 54, 4098–4106. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, Y.; Wu, J.; Zhu, D.; Sun, S.; Zhao, L.; Wang, X.; Liu, H.; Ren, Z.; Wang, C.; et al. A Randomized, Double-Blind, Multicenter Phase II Study Comparing the Efficacy and Safety of Oral Nemonoxacin with Oral Levofloxacin in the Treatment of Community-Acquired Pneumonia. J. Microbiol. Immunol. Infect. 2017, 50, 811–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, J.; Cash, H.L.; Niki, Y.; Kadota, J.; Yanagihara, K.; Kohno, S.; Kaku, M.; Watanabe, A.; Aoki, N.; Hori, S.; et al. Clinical and Bacteriological Efficacies of Sitafloxacin against Community-Acquired Pneumonia Caused by Streptococcus Pneumoniae: Nested Cohort within a Multicenter Clinical Trial. J. Infect. Chemother. 2013, 19, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Kim, S.E.; Shin, K.-H.; Lim, C.; Lim, K.S.; Yu, K.-S.; Cho, J.-Y. Comparison of Pharmacokinetics between New Quinolone Antibiotics: The Zabofloxacin Hydrochloride Capsule and the Zabofloxacin Aspartate Tablet. Curr. Med. Res. Opin. 2013, 29, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.K.; Chang, J.H.; Choi, E.G.; Kim, H.K.; Kwon, Y.-S.; Kyung, S.Y.; Lee, J.-H.; Park, M.J.; Yoo, K.H.; Oh, Y.M. Zabofloxacin versus Moxifloxacin in Patients with COPD Exacerbation: A Multicenter, Double-Blind, Double-Dummy, Randomized, Controlled, Phase III, Non-Inferiority Trial. Int. J. Chron. Obs. Pulmon. Dis. 2015, 10, 2265–2275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomé, A.M.; Filipe, A. Quinolones: Review of Psychiatric and Neurological Adverse Reactions. Drug Saf. 2011, 34, 465–488. [Google Scholar] [CrossRef]
- Kaur, K.; Fayad, R.; Saxena, A.; Frizzell, N.; Chanda, A.; Das, S.; Chatterjee, S.; Hegde, S.; Baliga, M.S.; Ponemone, V.; et al. Fluoroquinolone-Related Neuropsychiatric and Mitochondrial Toxicity: A Collaborative Investigation by Scientists and Members of a Social Network. J. Community Support Oncol. 2016, 14, 54–65. [Google Scholar] [CrossRef]
- Samyde, J.; Petit, P.; Hillaire-Buys, D.; Faillie, J.-L. Quinolone Antibiotics and Suicidal Behavior: Analysis of the World Health Organization’s Adverse Drug Reactions Database and Discussion of Potential Mechanisms. Psychopharmacology 2016, 233, 2503–2511. [Google Scholar] [CrossRef]
- Sellick, J.; Mergenhagen, K.; Morris, L.; Feuz, L.; Horey, A.; Risbood, V.; Wojciechowski, A.; Ruh, C.; Bednarczyk, E.; Conway, E.; et al. Fluoroquinolone-Related Neuropsychiatric Events in Hospitalized Veterans. Psychosomatics 2018, 59, 259–266. [Google Scholar] [CrossRef]
- Kushner, J.M.; Peckman, H.J.; Snyder, C.R. Seizures Associated with Fluoroquinolones. Ann. Pharmacother. 2001, 35, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.Z.; Cannizzaro, D.N.; Naughton, L.F.; Bove, C. Fluoroquinolones-Associated Disability: It Is Not All in Your Head. NeuroSci 2021, 2, 235–253. [Google Scholar] [CrossRef]
- Owens, R.C.; Ambrose, P.G. Clinical Use of the Fluoroquinolones. Med. Clin. N. Am. 2000, 84, 1447–1469. [Google Scholar] [CrossRef] [PubMed]
- Akahane, K.; Sekiguchi, M.; Une, T.; Osada, Y. Structure-Epileptogenicity Relationship of Quinolones with Special Reference to Their Interaction with Gamma-Aminobutyric Acid Receptor Sites. Antimicrob. Agents Chemother. 1989, 33, 1704–1708. [Google Scholar] [CrossRef] [Green Version]
- O’Riordan, W.; Mehra, P.; Manos, P.; Kingsley, J.; Lawrence, L.; Cammarata, S. A Randomized Phase 2 Study Comparing Two Doses of Delafloxacin with Tigecycline in Adults with Complicated Skin and Skin-Structure Infections. Int. J. Infect. Dis. 2015, 30, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Jorgensen, S.C.J.; Mercuro, N.J.; Davis, S.L.; Rybak, M.J. Delafloxacin: Place in Therapy and Review of Microbiologic, Clinical and Pharmacologic Properties. Infect. Dis. Ther. 2018, 7, 197–217. [Google Scholar] [CrossRef] [Green Version]
- Jjingo, C.J. Clinical Review (Baxdela) 2016. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208610Orig1s000,208611Orig1s000MedR.pdf (accessed on 10 November 2022).
- Maddix, D.S.; Stefani, A. Comment: Myasthenia Gravis and Ciprofloxacin. Ann. Pharmacother. 1992, 26, 265–266. [Google Scholar] [CrossRef]
- Sieb, J.P.; Milone, M.; Engel, A.G. Effects of the Quinoline Derivatives Quinine, Quinidine, and Chloroquine on Neuromuscular Transmission. Brain Res. 1996, 712, 179–189. [Google Scholar] [CrossRef]
- Tintinalli, J.E. Fluoroquinolones Should Be Avoided in Myasthenia Gravis. Ann. Emerg. Med. 2004, 44, 87–88. [Google Scholar] [CrossRef]
- Gunduz, A.; Turedi, S.; Kalkan, A.; Nuhoglu, I. Levofloxacin Induced Myasthenia Crisis. Emerg Med. J. 2006, 23, 662. [Google Scholar] [CrossRef] [Green Version]
- Pham Nguyen, T.P.; Leonard, C.E.; Bird, S.J.; Willis, A.W.; Hamedani, A.G. Pharmacosafety of Fluoroquinolone and Macrolide Antibiotics in the Clinical Care of Patients with Myasthenia Gravis. Muscle Nerve 2021, 64, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.S.; Sheridan, R.E.; Adler, M. Efficacy of Certain Quinolines as Pharmacological Antagonists in Botulinum Neurotoxin Poisoning. Toxicon 1997, 35, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Sieb, J.P. Fluoroquinolone Antibiotics Block Neuromuscular Transmission. Neurology 1998, 50, 804–807. [Google Scholar] [CrossRef]
- van der Linden, P.D.; van der Lei, J.; Vlug, A.E.; Stricker, B.H.C. Skin Reactions to Antibacterial Agents in General Practice. J. Clin. Epidemiol. 1998, 51, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Balakirski, G.; Merk, H.F. Cutaneous Allergic Drug Reactions: Update on Pathophysiology, Diagnostic Procedures and Differential Diagnosic. Cutan. Ocul. Toxicol. 2017, 36, 307–316. [Google Scholar] [CrossRef]
- Sable, D.; Murakawa, G.J. Quinolones in Dermatology. Clin. Dermatol. 2003, 21, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Scherer, K.; Bircher, A.J. Hypersensitivity Reactions to Fluoroquinolones. Curr. Allergy Asthma Rep. 2005, 5, 15–21. [Google Scholar] [CrossRef]
- Tang, W.; Rao, E. Anaphylaxis to Ciprofloxacin Requiring Emergent Surgical Cricothyrotomy. Eur. J. Case Rep. Intern. Med. 2022, 9, 003180. [Google Scholar] [CrossRef]
- EMA. Factive: Withdrawn Application. Available online: https://www.ema.europa.eu/en/medicines/human/withdrawn-applications/factive (accessed on 22 September 2022).
- Menarini International Operations Luxembourg S.A. Withdrawal Letter Factive. Available online: https://www.ema.europa.eu/en/documents/other/withdrawal-letter-factive_en.pdf (accessed on 22 September 2022).
- Yilmaz, İ.; Doğan, S.; Tutar, N.; Kanbay, A.; Büyükoğlan, H.; Demir, R. Biphasic Anaphylaxis to Gemifloxacin. Asia Pac. Allergy 2012, 2, 280–282. [Google Scholar] [CrossRef] [Green Version]
- McGee, E.U.; Samuel, E.; Boronea, B.; Dillard, N.; Milby, M.N.; Lewis, S.J. Quinolone Allergy. Pharmacy 2019, 7, 97. [Google Scholar] [CrossRef] [Green Version]
- Renaudin, J.-M.; Beaudouin, E.; Ponvert, C.; Demoly, P.; Moneret-Vautrin, D.-A. Severe Drug-Induced Anaphylaxis: Analysis of 333 Cases Recorded by the Allergy Vigilance Network from 2002 to 2010. Allergy 2013, 68, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, M.; Severino, M.; Testi, S.; Macchia, D.; Ermini, G.; Pichler, W.J.; Campi, P. Detection of Specific IgE to Quinolones. J. Allergy Clin. Immunol. 2004, 113, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Kulthanan, K.; Chularojanamontri, L.; Manapajon, A.; Dhana, N.; Jongjarearnprasert, K. Cutaneous Adverse Reactions to Fluoroquinolones. Dermatitis 2011, 22, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Devadarshini, S.; Pattnaik, K.P.; Samal, R.; Mohapatra, J.; Sahoo, S.S. Comparative Analysis of Cutaneous Drug Reactions among Different Fluoroquinolones: An Experimental Study. Int. J. Basic Clin. Pharmacol. 2017, 6, 1254–1260. [Google Scholar] [CrossRef] [Green Version]
- Neuman, M.G.; Cohen, L.B.; Nanau, R.M. Quinolones-Induced Hypersensitivity Reactions. Clin. Biochem. 2015, 48, 716–739. [Google Scholar] [CrossRef]
- Li, R.; Bernstein, J.A. Anaphylactic Shock Caused By Moxifloxacin without Cross-Reactivity to Other Fluoroquinolones. J. Allergy Clin. Immunol. 2016, 137, AB40. [Google Scholar] [CrossRef] [Green Version]
- Doña, I.; Moreno, E.; Pérez-Sánchez, N.; Andreu, I.; Hernández Fernandez de Rojas, D.; Torres, M.J. Update on Quinolone Allergy. Curr. Allergy Asthma Rep. 2017, 17, 56. [Google Scholar] [CrossRef]
- Portilho, N.C.; Aun, M.V.; Kalil, J.; Giavina-Bianchi, P. Quinolone-Induced Anaphylaxis. Curr. Treat. Options Allergy 2020, 7, 370–380. [Google Scholar] [CrossRef]
- Fernández, T.D.; Ariza, A.; Palomares, F.; Montañez, M.I.; Salas, M.; Martín-Serrano, A.; Fernández, R.; Ruiz, A.; Blanca, M.; Mayorga, C.; et al. Hypersensitivity to Fluoroquinolones. Medicine 2016, 95, e3679. [Google Scholar] [CrossRef]
- Doña, I.; Pérez-Sánchez, N.; Salas, M.; Barrionuevo, E.; Ruiz-San Francisco, A.; Hernández Fernández de Rojas, D.; Martí-Garrido, J.; Andreu-Ros, I.; López-Salgueiro, R.; Moreno, E.; et al. Clinical Characterization and Diagnostic Approaches for Patients Reporting Hypersensitivity Reactions to Quinolones. J. Allergy Clin. Immunol. Pract. 2020, 8, 2707–2714.e2. [Google Scholar] [CrossRef]
- Doña, I.; Blanca-López, N.; Boteanu, C.; Cueva-Oliver, B.; Fernández-Sánchez, F.; Gajate, P.; García-Avilés, M.; García-Núñez, I.; Lobera, T.; Moreno, E.; et al. Clinical Practice Guidelines for Diagnosis and Management of Hypersensitivity Reactions to Quinolones. J. Investig. Allergol. Clin. Immunol. 2021, 31, 292–307. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hu, S.; Zhang, Y.; Che, D.; Cao, J.; Wang, J.; Zhao, T.; Jia, Q.; Wang, N.; Zhang, T. Mast Cell-Mediated Hypersensitivity to Fluoroquinolone Is MRGPRX2 Dependent. Int. Immunopharmacol. 2019, 70, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Rodvold, K.A.; Gotfried, M.H.; Chugh, R.; Gupta, M.; Yeole, R.; Patel, A.; Bhatia, A. Intrapulmonary Pharmacokinetics of Levonadifloxacin Following Oral Administration of Alalevonadifloxacin to Healthy Adult Subjects. Antimicrob. Agents Chemother. 2018, 62, e02297-17. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Chang, L.-W.; Tsai, C.-Y.; Hsu, C.-H.; Chung, D.T.; Aronstein, W.S.; Ajayi, F.; Kuzmak, B.; Lyon, R.A. Dose Escalation Study of the Safety, Tolerability, and Pharmacokinetics of Nemonoxacin (TG-873870), a Novel Potent Broad-Spectrum Nonfluorinated Quinolone, in Healthy Volunteers. Antimicrob. Agents Chemother. 2010, 54, 405–410. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.; Wu, X.; Zhang, Y.; Shi, Y.; Yu, J.; Cao, G.; Zhang, J. Safety and Clinical Pharmacokinetics of Nemonoxacin, a Novel Non-Fluorinated Quinolone, in Healthy Chinese Volunteers following Single and Multiple Oral Doses. Clin. Drug Investig. 2012, 32, 475–486. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, J.; Guo, B.; Zhang, Y.; Yu, J.; Cao, G.; Chen, Y.; Zhu, D.; Ye, X.; Wu, J.; et al. Pharmacokinetics and Pharmacodynamics of Multiple-Dose Intravenous Nemonoxacin in Healthy Chinese Volunteers. Antimicrob. Agents Chemother. 2015, 59, 1446–1454. [Google Scholar] [CrossRef] [Green Version]
- Sousa, J.; Alves, G.; Fortuna, A.; Falcão, A. Third and Fourth Generation Fluoroquinolone Antibacterials: A Systematic Review of Safety and Toxicity Profiles. Curr. Drug Saf. 2014, 9, 89–105. [Google Scholar] [CrossRef]
- Singh, A.P.; Kumar, V.; Singh, N. Phototoxic Potential Assessment of Enoxacin In Vitro: Phototoxicity; LAP LAMBERT Academic Publishing: Saarbruecken, Germany, 2012; ISBN 978-3-659-19507-5. [Google Scholar]
- Kawada, A.; Hatanaka, K.; Gomi, H.; Matsuo, I. In Vitro Phototoxicity of New Quinolones: Production of Active Oxygen Species and Photosensitized Lipid Peroxidation. Photodermatol. Photoimmunol. Photomed. 1999, 15, 226–230. [Google Scholar] [CrossRef]
- Jałbrzykowska, K.; Chrzanowska, A.; Roszkowski, P.; Struga, M. The New Face of a Well-Known Antibiotic: A Review of the Anticancer Activity of Enoxacin and Its Derivatives. Cancers 2022, 14, 3056. [Google Scholar] [CrossRef]
- Mang, R.; Stege, H.; Krutmann, J. Mechanisms of Phototoxic and Photoallergic Reactions. In Contact Dermatitis; Johansen, J.D., Frosch, P.J., Lepoittevin, J.-P., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 155–163. ISBN 978-3-642-03827-3. [Google Scholar]
- de Guidi, G.; Bracchitta, G.; Catalfo, A. Photosensitization Reactions of Fluoroquinolones and Their Biological Consequences. Photochem. Photobiol. 2011, 87, 1214–1229. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.G.; Epling, G.A.; Kiely, J.S.; Bailey, D.L.; Song, B. Mechanistic Studies of the Phototoxic Potential of PD 117596, a Quinolone Antibacterial Compound. Toxicol. Appl. Pharmacol. 1991, 111, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.E. Mechanisms of Photosensitization by Phototoxic Drugs. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1998, 422, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Chignell, C.F. Photocleavage of DNA by the Fluoroquinolone Antibacterials. J. Photochem. Photobiol. B Biol. 1998, 45, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Kojima, K.; Nagano, H.; Matsubara, S.; Yokota, T. Photostability and Biological Activity of Fluoroquinolones Substituted at the 8 Position after UV Irradiation. Antimicrob. Agents Chemother. 1992, 36, 1715–1719. [Google Scholar] [CrossRef] [Green Version]
- Thompson, A.M. Ocular Toxicity of Fluoroquinolones. Clin. Exp. Ophthalmol. 2007, 35, 566–577. [Google Scholar] [CrossRef]
- Marrot, L.; Agapakis-Causse, C. Differences in the Photogenotoxic Potential of Two Fluoroquinolones as Shown in Diploid Yeast Strain (Saccharomyces Cerevisae) and Supercoiled Plasmid DNA. Mutat. Res. 2000, 468, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fasani, E.; Mella, M.; Caccia, D.; Tassi, S.; Fagnoni, M.; Albini, A. The Photochemistry of Lomefloxacin. An Aromatic Carbene as the Keyintermediate in Photodecomposition. Chem. Commun. 1997, 14, 1329–1330. [Google Scholar] [CrossRef]
- Cuquerella, M.C.; Miranda, M.A.; Bosca, F. Generation of Detectable Singlet Aryl Cations by Photodehalogenation of Fluoroquinolones. J. Phys. Chem. B 2006, 110, 6441–6443. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, J.; Banach, K.; Rok, J.; Beberok, A.; Rzepka, Z.; Wrześniok, D. Molecular and Biochemical Basis of Fluoroquinolones-Induced Phototoxicity—The Study of Antioxidant System in Human Melanocytes Exposed to UV-A Radiation. Int. J. Mol. Sci. 2020, 21, 9714. [Google Scholar] [CrossRef] [PubMed]
- Viola, G.; Facciolo, L.; Canton, M.; Vedaldi, D.; Dall’Acqua, F.; Aloisi, G.G.; Amelia, M.; Barbafina, A.; Elisei, F.; Latterini, L. Photophysical and Phototoxic Properties of the Antibacterial Fluoroquinolones Levofloxacin and Moxifloxacin. Chem. Biodivers. 2004, 1, 782–801. [Google Scholar] [CrossRef] [PubMed]
- Dawe, R.S.; Ferguson, J.; Ibbotson, S.; Lawrence, L.; Paulson, S.; Duffy, E.; Cammarata, S. Lack of Phototoxicity Potential with Delafloxacin in Healthy Male and Female Subjects: Comparison to Lomefloxacin. Photochem. Photobiol. Sci. 2018, 17, 773–780. [Google Scholar] [CrossRef] [Green Version]
- Nandanwar, M.; Kansagara, A.; Gupta, S.; Patel, A.; Patel, M.A.; Yeole, R.; Thorve, D.; Patel, M. Preclinical Safety Evaluation of Levonadifloxacin, a Novel Anti-Methicillin-Resistant Staphyloccocus Aureus Benzoquinolizine Fluoroquinolone by Intravenous and Oral Administration. J. Appl. Toxicol. 2022, 42, 1354–1370. [Google Scholar] [CrossRef]
- Chugh, R.; Lakdavala, F.; Bhatia, A. Safety and Pharmacokinetics of Multiple Ascending Doses of WCK 771 and WCK 2349. In Proceedings of the Abstract P1268; European Society of Clinical Microbiology and Infectious Diseases: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Bhawsar, S.; Kale, R.; Deshpande, P.; Yeole, R.; Bhagwat, S.; Patel, M. Design and Synthesis of an Oral Prodrug Alalevonadifloxacin for the Treatment of MRSA Infection. Bioorganic Med. Chem. Lett. 2021, 54, 128432. [Google Scholar] [CrossRef]
- Dawe, R.S.; Ibbotson, S.H.; Sanderson, J.B.; Thomson, E.M.; Ferguson, J. A Randomized Controlled Trial (Volunteer Study) of Sitafloxacin, Enoxacin, Levofloxacin and Sparfloxacin Phototoxicity. Br. J. Dermatol. 2003, 149, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Bech-Thomsen, N.; Angelo, H.R.; Wulf, H.C. Skin Pigmentation as a Predictor of Minimal Phototoxic Dose after Oral Methoxsalen. Arch. Dermatol. 1994, 130, 464–468. [Google Scholar] [CrossRef]
- Lojanapiwat, B.; Nimitvilai, S.; Bamroongya, M.; Jirajariyavej, S.; Tiradechavat, C.; Malithong, A.; Predanon, C.; Tanphaichitra, D.; Lertsupphakul, B. Oral Sitafloxacin vs Intravenous Ceftriaxone Followed by Oral Cefdinir for Acute Pyelonephritis and Complicated Urinary Tract Infection: A Randomized Controlled Trial. Infect. Drug Resist. 2019, 12, 173–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, R.L.; Suda, K.J.; Evans, C.T. Antibiotic Treatment for Clostridium Difficile-associated Diarrhoea in Adults. Cochrane Database Syst. Rev. 2017, 2017, CD004610. [Google Scholar] [CrossRef]
- Dhalla, I.A.; Mamdani, M.M.; Simor, A.E.; Kopp, A.; Rochon, P.A.; Juurlink, D.N. Are Broad-Spectrum Fluoroquinolones More Likely To Cause Clostridium Difficile-Associated Disease? Antimicrob. Agents Chemother. 2006, 50, 3216–3219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, K. Clostridium Difficile and Fluoroquinolones: Is There a Link? Int. J. Antimicrob. Agents 2009, 33, S29–S32. [Google Scholar] [CrossRef]
- McCusker, M.E.; Harris, A.D.; Perencevich, E.; Roghmann, M.-C. Fluoroquinolone Use and Clostridium Difficile–Associated Diarrhea. Emerg. Infect. Dis. 2003, 9, 730–733. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, A.; Pant, C.; Jain, A.; Fraser, T.G.; Rolston, D.D.K. Do Fluoroquinolones Predispose Patients to Clostridium Difficile Associated Disease? A Review of the Evidence. Curr. Med. Res. Opin. 2008, 24, 329–333. [Google Scholar] [CrossRef]
- Johnson, S. Recurrent Clostridium Difficile Infection: A Review of Risk Factors, Treatments, and Outcomes. J. Infect. 2009, 58, 403–410. [Google Scholar] [CrossRef]
- Eze, P.; Balsells, E.; Kyaw, M.H.; Nair, H. Risk Factors for Clostridium Difficile Infections—An Overview of the Evidence Base and Challenges in Data Synthesis. J. Glob. Health 2017, 7, 010417. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.; Lawrence, J.; Berry, C.; Davis, G.; Yu, H.; Cai, B.; Gonzalez, E.; Prantner, I.; Kurcz, A.; Macovei, I.; et al. Risk Factors for Primary Clostridium Difficile Infection; Results from the Observational Study of Risk Factors for Clostridium Difficile Infection in Hospitalized Patients With Infective Diarrhea (ORCHID). Front. Public Health 2020, 8. [Google Scholar] [CrossRef]
- Zaiss, N.H.; Witte, W.; Nübel, U. Fluoroquinolone Resistance and Clostridium Difficile, Germany. Emerg. Infect. Dis. 2010, 16, 675–677. [Google Scholar] [CrossRef]
- Donskey, C.J. Fluoroquinolone Restriction to Control Fluoroquinolone-Resistant Clostridium Difficile. Lancet Infect. Dis. 2017, 17, 353–354. [Google Scholar] [CrossRef] [Green Version]
- Kyorin Pharmacetical Lasvic Tablets 75mg. Available online: https://www.rad-ar.or.jp/siori/english/search/result?n=42565 (accessed on 14 November 2022).
- Mehta, K.D.; Sharma, J.B.; Anand, A.; Reddy N, P.K.; Kadam, P.; Debnath, K.; Bhapkar, S.; Thampi, B.M. Real-World Evidence of Efficacy and Safety of Levonadifloxacin (Oral and IV) in the Management of Acute Bacterial Skin and Skin Structure Infections (ABSSSI): Findings of a Retrospective, Multi-Center Study. Cureus 2022, 14, e24299. [Google Scholar] [CrossRef] [PubMed]
- Recanatini, M.; Poluzzi, E.; Masetti, M.; Cavalli, A.; De Ponti, F. QT Prolongation through HERG K+ Channel Blockade: Current Knowledge and Strategies for the Early Prediction during Drug Development. Med. Res. Rev. 2005, 25, 133–166. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.; Walter, E.A.; Gaspar, D.K.S.; Obodozie-Ofoegbu, O.O.; Frei, C.R. Torsades de Pointes and QT Prolongation Associations with Antibiotics: A Pharmacovigilance Study of the FDA Adverse Event Reporting System. Int. J. Med. Sci. 2019, 16, 1018–1022. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Wang, L.; Chen, X.-L.; Triggle, D.J.; Rampe, D. Interactions of a Series of Fluoroquinolone Antibacterial Drugs with the Human Cardiac K+ Channel HERG. Mol. Pharmacol. 2001, 59, 122–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, R.C. QT Prolongation with Antimicrobial Agents. Drugs 2004, 64, 1091–1124. [Google Scholar] [CrossRef] [PubMed]
- Briasoulis, A.; Agarwal, V.; Pierce, W.J. QT Prolongation and Torsade de Pointes Induced by Fluoroquinolones: Infrequent Side Effects from Commonly Used Medications. CRD 2011, 120, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, E.; Camm, J. Cardiotoxicity of Fluoroquinolones. J. Antimicrob. Chemother. 2002, 49, 593–596. [Google Scholar] [CrossRef] [Green Version]
- Roden, D.M. Long-QT Syndrome. N. Engl. J. Med. 2008, 358, 169–176. [Google Scholar] [CrossRef]
- Khan, F.; Ismail, M.; Khan, Q.; Ali, Z. Moxifloxacin-Induced QT Interval Prolongation and Torsades de Pointes: A Narrative Review. Expert Opin. Drug Saf. 2018, 17, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Owens, R.C., Jr. Risk Assessment for Antimicrobial Agent-Induced QTc Interval Prolongation and Torsades de Pointes. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2001, 21, 301–319. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, M.; Krahn, A.D. Ciprofloxacin-Induced Acquired Long QT Syndrome. Heart Rhythm. 2004, 1, 624–626. [Google Scholar] [CrossRef] [PubMed]
- Keivanidou, A.; Arnaoutoglou, C.; Krommydas, A.; Papanikolaou, G.; Tsiptses, K.; Chrisopoulos, C.; Kirpizidis, C. Ciprofloxacin Induced Acquired Long QT Syndrome in a Patient under Class III Antiarrhythmic Therapy. Cardiol. J. 2009, 16, 172–174. [Google Scholar] [PubMed]
- Noel, G.J.; Natarajan, J.; Chien, S.; Hunt, T.L.; Goodman, D.B.; Abels, R. Effects of Three Fluoroquinolones on QT Interval in Healthy Adults after Single Doses. Clin. Pharmacol. Ther. 2003, 73, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Tsikouris, J.P.; Peeters, M.J.; Cox, C.D.; Meyerrose, G.E.; Seifert, C.F. Effects of Three Fluoroquinolones on QT Analysis after Standard Treatment Courses. Ann. Noninvasive Electrocardiol. 2006, 11, 52–56. [Google Scholar] [CrossRef]
- Stancampiano, F.F.; Palmer, W.C.; Getz, T.W.; Serra-Valentin, N.A.; Sears, S.P.; Seeger, K.M.; Pagan, R.J.; Racho, R.G.; Ray, J.C.; Snipelisky, D.F.; et al. Rare Incidence of Ventricular Tachycardia and Torsades de Pointes in Hospitalized Patients with Prolonged QT Who Later Received Levofloxacin: A Retrospective Study. Mayo Clin. Proc. 2015, 90, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Badshah, A.; Janjua, M.; Younas, F.; Halabi, A.R.; Cotant, J.F. Moxifloxacin-Induced QT Prolongation and Torsades: An Uncommon Effect of a Common Drug. Am. J. Med. Sci. 2009, 338, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Matsukura, S.; Nakamura, Y.; Hoshiai, K.; Hayashi, T.; Koga, T.; Goto, A.; Chiba, K.; Lubna, N.J.; Hagiwara-Nagasawa, M.; Izumi-Nakaseko, H.; et al. Effects of Moxifloxacin on the Proarrhythmic Surrogate Markers in Healthy Filipino Subjects: Exposure-Response Modeling Using ECG Data of Thorough QT/QTc Study. J. Pharmacol. Sci. 2018, 136, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Täubel, J.; Prasad, K.; Rosano, G.; Ferber, G.; Wibberley, H.; Cole, S.T.; Van Langenhoven, L.; Fernandes, S.; Djumanov, D.; Sugiyama, A. Effects of the Fluoroquinolones Moxifloxacin and Levofloxacin on the QT Subintervals: Sex Differences in Ventricular Repolarization. J. Clin. Pharmacol. 2020, 60, 400–408. [Google Scholar] [CrossRef]
- Taubel, J.; Ferber, G.; Lorch, U.; Batchvarov, V.; Savelieva, I.; Camm, A.J. Thorough QT Study of the Effect of Oral Moxifloxacin on QTc Interval in the Fed and Fasted State in Healthy Japanese and Caucasian Subjects. Br. J. Clin. Pharmacol. 2014, 77, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Litwin, J.S.; Benedict, M.S.; Thorn, M.D.; Lawrence, L.E.; Cammarata, S.K.; Sun, E. A Thorough QT Study to Evaluate the Effects of Therapeutic and Supratherapeutic Doses of Delafloxacin on Cardiac Repolarization. Antimicrob. Agents Chemother. 2015, 59, 3469–3473. [Google Scholar] [CrossRef] [Green Version]
- Mason, J.W.; Chugh, R.; Patel, A.; Gutte, R.; Bhatia, A. Electrocardiographic Effects of a Supratherapeutic Dose of WCK 2349, a Benzoquinolizine Fluoroquinolone. Clin. Transl. Sci. 2019, 12, 47–52. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, X.; Wang, T.; Chen, X.; Liang, L.; Qiao, H.; Tsai, C.; Chang, L.; Huang, P.; Hsu, C.; et al. Effects of an Al3+- and Mg2+-Containing Antacid, Ferrous Sulfate, and Calcium Carbonate on the Absorption of Nemonoxacin (TG-873870) in Healthy Chinese Volunteers. Acta Pharmacol. Sin. 2014, 35, 1586–1592. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Dai, X.; Yang, Y.; Chen, X.; Wang, T.; Tang, Y.; Tsai, C.; Chang, L.; Chang, Y.; Zhong, D. Effects of Probenecid and Cimetidine on the Pharmacokinetics of Nemonoxacin in Healthy Chinese Volunteers. Drug Des. Devel. Ther. 2016, 10, 357–370. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Lv, Y.; Li, X.; Hou, F.; Ma, X.; Wei, M.; Kang, Z.; Cui, L.; Xia, Y.; Liu, Y.; et al. Effects of Nemonoxacin on thorough ECG QT/QTc Interval: A Randomized, Placebo- and Positive-Controlled Crossover Study in Healthy Chinese Adults. Clin. Ther. 2018, 40, 983–992. [Google Scholar] [CrossRef]
- Kang, Y.; Li, Y.; Xu, F.; Zhang, J.; Wang, K.; Chen, Y.; Wu, J.; Guo, B.; Yu, J.; Zhang, Y. Population Pharmacokinetics Study of Nemonoxacin Among Chinese Patients with Moderate Hepatic Impairment. Clin. Ther. 2019, 41, 505–517.e0. [Google Scholar] [CrossRef]
- Kelesidis, T.; Canseco, E. Quinolone-Induced Hypoglycemia: A Life-Threatening but Potentially Reversible Side Effect. Am. J. Med. 2010, 123, e5–e6. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.E.; Hubbard, R.A.; Willis, A.W.; Zuppa, A.F.; Zaoutis, T.E.; Hennessy, S. Comparative Risk of Serious Hypoglycemia among Persons Dispensed a Fluoroquinolone versus a Non-Fluoroquinolone Antibiotic. Diabetes Res. Clin. Pract. 2022, 185, 109225. [Google Scholar] [CrossRef] [PubMed]
- El Ghandour, S.; Azar, S.T. Dysglycemia Associated with Quinolones. Prim. Care Diabetes 2015, 9, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, T.; Canseco, E. Levofloxacin-Induced Hypoglycemia: A Rare but Life-Threatening Side Effect of a Widely Used Antibiotic. Am. J. Med. 2009, 122, e3–e4. [Google Scholar] [CrossRef] [PubMed]
- Park-Wyllie, L.Y.; Juurlink, D.N.; Kopp, A.; Shah, B.R.; Stukel, T.A.; Stumpo, C.; Dresser, L.; Low, D.E.; Mamdani, M.M. Outpatient Gatifloxacin Therapy and Dysglycemia in Older Adults. N. Engl. J. Med. 2006, 354, 1352–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yip, C.; Lee, A.J. Gatifloxacin-Induced Hyperglycemia: A Case Report and Summary of the Current Literature. Clin. Ther. 2006, 28, 1857–1866. [Google Scholar] [CrossRef]
- Bobba, R.K.; Arsura, E.L. Hyperglycemia in an Elderly Diabetic Patient: Drug-Drug or Drug-Disease Interaction? South. Med. J. 2006, 99, 94–95. [Google Scholar] [CrossRef] [Green Version]
- Murad, M.H.; Coto-Yglesias, F.; Wang, A.T.; Sheidaee, N.; Mullan, R.J.; Elamin, M.B.; Erwin, P.J.; Montori, V.M. Drug-Induced Hypoglycemia: A Systematic Review. J. Clin. Endocrinol. Metab. 2009, 94, 741–745. [Google Scholar] [CrossRef] [Green Version]
- Ishiwata, Y.; Sanada, Y.; Yasuhara, M. Effects of Gatifloxacin on Serum Glucose Concentration in Normal and Diabetic Rats. Biol. Pharm. Bull. 2006, 29, 527–531. [Google Scholar] [CrossRef] [Green Version]
- Kingsley, J.; Mehra, P.; Lawrence, L.E.; Henry, E.; Duffy, E.; Cammarata, S.K.; Pullman, J. A Randomized, Double-Blind, Phase 2 Study to Evaluate Subjective and Objective Outcomes in Patients with Acute Bacterial Skin and Skin Structure Infections Treated with Delafloxacin, Linezolid or Vancomycin. J. Antimicrob. Chemother. 2016, 71, 821–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Totsuka, K.; Sesoko, S.; Fukase, H.; Ikushima, I.; Odajima, M.; Niwayama, Y. Pharmacokinetic Study of Lascufloxacin in Non-Elderly Healthy Men and Elderly Men. J. Infect. Chemother. 2020, 26, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Furuie, H.; Tanioka, S.; Shimizu, K.; Manita, S.; Nishimura, M.; Yoshida, H. Intrapulmonary Pharmacokinetics of Lascufloxacin in Healthy Adult Volunteers. Antimicrob. Agents Chemother. 2018, 62, e02169-17. [Google Scholar] [CrossRef] [Green Version]
- NIDDK Fluoroquinolones. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2020.
- Giustarini, G.; Huppelschoten, S.; Barra, M.; Oppelt, A.; Wagenaar, L.; Weaver, R.J.; Bol-Schoenmakers, M.; Smit, J.J.; van de Water, B.; Klingmüller, U.; et al. The Hepatotoxic Fluoroquinolone Trovafloxacin Disturbs TNF- and LPS-Induced P65 Nuclear Translocation in Vivo and in Vitro. Toxicol. Appl. Pharmacol. 2020, 391, 114915. [Google Scholar] [CrossRef] [PubMed]
- Leitner, J.M.; Graninger, W.; Thalhammer, F. Hepatotoxicity of Antibacterials: Pathomechanisms and Clinical Data. Infection 2010, 38, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.J.; Tulkens, P.M. Hepatic Safety of Antibiotics Used in Primary Care. J. Antimicrob. Chemother. 2011, 66, 1431–1446. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, A.C.; Lundquist, L.M. Ciprofloxacin-Induced Hepatotoxicity Resolved with Levofloxacin: A Case Report and a Review of the Literature. Hosp. Pharm. 2009, 44, 978–983. [Google Scholar] [CrossRef]
- Orman, E.S.; Conjeevaram, H.S.; Vuppalanchi, R.; Freston, J.W.; Rochon, J.; Kleiner, D.E.; Hayashi, P.H. Clinical and Histopathologic Features of Fluoroquinolone-Induced Liver Injury. Clin. Gastroenterol. Hepatol. 2011, 9, 517–523.e3. [Google Scholar] [CrossRef] [Green Version]
- Alshammari, T.M.; Larrat, E.P.; Morrill, H.J.; Caffrey, A.R.; Quilliam, B.J.; Laplante, K.L. Risk of Hepatotoxicity Associated with Fluoroquinolones: A National Case–Control Safety Study. Am. J. Health Syst. Pharm. 2014, 71, 37–43. [Google Scholar] [CrossRef]
- Taher, M.K.; Alami, A.; Gravel, C.A.; Tsui, D.; Bjerre, L.M.; Momoli, F.; Mattison, D.R.; Krewski, D. Systemic Quinolones and Risk of Acute Liver Failure I: Analysis of Data from the US FDA Adverse Event Reporting System. JGH Open 2021, 5, 778–784. [Google Scholar] [CrossRef]
- Sun, Q.; Zhu, R.; Foss, F.W.; Macdonald, T.L. Mechanisms of Trovafloxacin Hepatotoxicity: Studies of a Model Cyclopropylamine-Containing System. Bioorg. Med. Chem. Lett. 2007, 17, 6682–6686. [Google Scholar] [CrossRef]
- Hoover, R.; Hunt, T.; Benedict, M.; Paulson, S.K.; Lawrence, L.; Cammarata, S.; Sun, E. Single and Multiple Ascending-Dose Studies of Oral Delafloxacin: Effects of Food, Sex, and Age. Clin. Ther. 2016, 38, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Temple, R. Hy’s Law: Predicting Serious Hepatotoxicity. Pharmacoepidemiol. Drug Saf. 2006, 15, 241–243. [Google Scholar] [CrossRef]
- Yuan, J.; Mo, B.; Ma, Z.; Lv, Y.; Cheng, S.-L.; Yang, Y.; Tong, Z.; Wu, R.; Sun, S.; Cao, Z.; et al. Safety and Efficacy of Oral Nemonoxacin versus Levofloxacin in Treatment of Community-Acquired Pneumonia: A Phase 3, Multicenter, Randomized, Double-Blind, Double-Dummy, Active-Controlled, Non-Inferiority Trial. J. Microbiol. Immunol. Infect. 2019, 52, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Kohno, S.; Niki, Y.; Kadota, J.-I.; Yanagihara, K.; Kaku, M.; Watanabe, A.; Aoki, N.; Hori, S.; Fujita, J.; Tanigawara, Y. Clinical Dose Findings of Sitafloxacin Treatment: Pharmacokinetic-Pharmacodynamic Analysis of Two Clinical Trial Results for Community-Acquired Respiratory Tract Infections. J. Infect. Chemother. 2013, 19, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Alder, A.C.; Koller, T.; Widmer, R.M. Identification of Fluoroquinolone Antibiotics as the Main Source of UmuC Genotoxicity in Native Hospital Wastewater. Environ. Toxicol. Chem. 1998, 17, 377–382. [Google Scholar] [CrossRef]
- Ikbal, M.; Doğan, H.; Odabaş, H.; Pirim, İ. Genotoxic Evaluation of the Antibacterial Drug, Ciprofloxacin, in Cultured Lymphocytes of Patients with Urinary Tract Infection. Turk. J. Med. Sci. 2004, 34, 309–313. [Google Scholar]
- Hu, J.; Wang, W.; Zhu, Z.; Chang, H.; Pan, F.; Lin, B. Quantitative Structure−Activity Relationship Model for Prediction of Genotoxic Potential for Quinolone Antibacterials. Environ. Sci. Technol. 2007, 41, 4806–4812. [Google Scholar] [CrossRef] [PubMed]
- Khadra, A.; Pinelli, E.; Lacroix, M.Z.; Bousquet-Melou, A.; Hamdi, H.; Merlina, G.; Guiresse, M.; Hafidi, M. Assessment of the Genotoxicity of Quinolone and Fluoroquinolones Contaminated Soil with the Vicia Faba Micronucleus Test. Ecotoxicol. Environ. Saf. 2012, 76, 187–192. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, T.; Wei, X.; Li, J.; Wang, W. In Silico and in Vitro Genotoxicity Evaluation of Descarboxyl Levofloxacin, an Impurity in Levofloxacin. Drug Chem. Toxicol. 2014, 37, 311–315. [Google Scholar] [CrossRef]
- Du, M.; Zhang, D.; Hou, Y.; Zhao, X.; Li, Y. Combined 2D-QSAR, Principal Component Analysis and Sensitivity Analysis Studies on Fluoroquinolones’ Genotoxicity. Int. J. Environ. Res. Public Health 2019, 16, 4156. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Wang, X.; Li, Y. Combined HQSAR Method and Molecular Docking Study on Genotoxicity Mechanism of Quinolones with Higher Genotoxicity. Environ. Sci. Pollut. Res. 2019, 26, 34830–34853. [Google Scholar] [CrossRef]
- PubChem Amifloxacin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/55492 (accessed on 17 November 2022).
- Arjona, A. Nemonoxacin Quinolone Antibiotic. Drug Future 2009, 34, 196–203. [Google Scholar] [CrossRef]
- Qin, X.; Huang, H. Review of Nemonoxacin with Special Focus on Clinical Development. Drug Des. Devel. Ther. 2014, 8, 765–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Wei, D.; Zhao, H.; Du, Y. Genotoxicity of Quinolones: Substituents Contribution and Transformation Products QSAR Evaluation Using 2D and 3D Models. Chemosphere 2014, 95, 220–226. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Mukherjee, S.; Mandal, S.M. Fluoroquinolone Antibiotics Show Genotoxic Effect through DNA-Binding and Oxidative Damage. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117634. [Google Scholar] [CrossRef]
- Fengxian, C.; Reti, H. Analysis of Positions and Substituents on Genotoxicity of Fluoroquinolones with Quantitative Structure-Activity Relationship and 3D Pharmacophore Model. Ecotoxicol. Environ. Saf. 2017, 136, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Kishii, R.; Yamaguchi, Y.; Takei, M. In Vitro Activities and Spectrum of the Novel Fluoroquinolone Lascufloxacin (KRP-AM1977). Antimicrob Agents Chemother 2017, 61, e00120-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taigexyn® Nemonoxacin Capsule 250 Mg; TaiGen Biotechnology Co., Ltd.: Taipei, Taiwan, 2020.
- Shiomi, M.; Togawa, M.; Fujita, K.; Murata, R. Effect of Early Oral Fluoroquinolones in Hemorrhagic Colitis Due to Escherichia Coli O157:H7. Pediatr. Int. 1999, 41, 228–232. [Google Scholar] [CrossRef]
- Safdar, N.; Said, A.; Gangnon, R.E.; Maki, D.G. Risk of Hemolytic Uremic Syndrome after Antibiotic Treatment of Escherichia Coli O157:H7 Enteritis: A Meta-Analysis. JAMA 2002, 288, 996–1001. [Google Scholar] [CrossRef]
- Panos, G.Z.; Betsi, G.I.; Falagas, M.E. Systematic Review: Are Antibiotics Detrimental or Beneficial for the Treatment of Patients with Escherichia Coli O157:H7 Infection? Aliment. Pharmacol. Ther. 2006, 24, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, K.; Nakamura-Uchiyama, F. Does Levofloxacin Induce Hemolytic Uremic Syndrome in Patients Infected with Verotoxin-Producing Escherichia Coli O157 Infections? Jpn. J. Infect. Dis. 2012, 65, 442–443. [Google Scholar] [CrossRef] [Green Version]
- Geerdes-Fenge, H.F.; Löbermann, M.; Nürnberg, M.; Fritzsche, C.; Koball, S.; Henschel, J.; Höhn, R.; Schober, H.C.; Mitzner, S.; Podbielski, A.; et al. Ciprofloxacin Reduces the Risk of Hemolytic Uremic Syndrome in Patients with Escherichia Coli O104:H4-Associated Diarrhea. Infection 2013, 41, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Kakoullis, L.; Papachristodoulou, E.; Chra, P.; Panos, G. Shiga Toxin-Induced Haemolytic Uraemic Syndrome and the Role of Antibiotics: A Global Overview. J. Infect. 2019, 79, 75–94. [Google Scholar] [CrossRef]
- Mody, R.K.; Hoekstra, R.M.; Scott, M.K.; Dunn, J.; Smith, K.; Tobin-D’Angelo, M.; Shiferaw, B.; Wymore, K.; Clogher, P.; Palmer, A.; et al. Risk of Hemolytic Uremic Syndrome Related to Treatment of Escherichia Coli O157 Infection with Different Antimicrobial Classes. Microorganisms 2021, 9, 1997. [Google Scholar] [CrossRef] [PubMed]
- Maguire, R.B.; Stroncek, D.F.; Gale, E.; Yearlsey, M. Hemolytic Anemia and Acute Renal Failure Associated with Temafloxacin-Dependent Antibodies. Am. J. Hematol. 1994, 46, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Moise, P.A.; Birmingham, M.C.; Schentag, J.J. Pharmacokinetics and Metabolism of Moxifloxacin. Drugs Today 2000, 36, 229–244. [Google Scholar] [CrossRef]
- Mulgaonkar, A.; Venitz, J.; Sweet, D.H. Fluoroquinolone Disposition: Identification of the Contribution of Renal Secretory and Reabsorptive Drug Transporters. Expert Opin. Drug Metab. Toxicol. 2012, 8, 553–569. [Google Scholar] [CrossRef]
- Lomaestro, B.M. Fluoroquinolone-Induced Renal Failure. Drug Saf. 2000, 22, 479–485. [Google Scholar] [CrossRef]
- Stahlmann, R.; Lode, H. Safety Considerations of Fluoroquinolones in the Elderly. Drugs Aging 2010, 27, 193–209. [Google Scholar] [CrossRef]
- Bird, S.T.; Etminan, M.; Brophy, J.M.; Hartzema, A.G.; Delaney, J.A.C. Risk of Acute Kidney Injury Associated with the Use of Fluoroquinolones. CMAJ 2013, 185, E475–E482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farid, S.; Mahmood, M.; Abu Saleh, O.M.; Hamadah, A.; Nasr, S.H.; Garrigos, Z.E.; Leung, N.; Sohail, M.R. Clinical Manifestations and Outcomes of Fluoroquinolone-Related Acute Interstitial Nephritis. Mayo Clin. Proc. 2018, 93, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Stratta, P.; Lazzarich, E.; Canavese, C.; Bozzola, C.; Monga, G. Ciprofloxacin Crystal Nephropathy. Am. J. Kidney Dis. 2007, 50, 330–335. [Google Scholar] [CrossRef]
- Khan, M.; Ortega, L.M.; Bagwan, N.; Nayer, A. Crystal-Induced Acute Kidney Injury Due to Ciprofloxacin. J. Nephropathol. 2015, 4, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Perazella, M.A.; Shirali, A. 37—Kidney Disease Caused by Therapeutic Agents. In National Kidney Foundation Primer on Kidney Diseases, 6th ed.; Gilbert, S.J., Weiner, D.E., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2014; pp. 326–336. ISBN 978-1-4557-4617-0. [Google Scholar]
- Jaman, M.; Chowdhury, A.A.; Rana, A.A.; Masum, S.M.; Ferdous, T.; Rashid, M.A.; Karim, M.M. In Vitro Evaluation of Ciprofloxacin Hydrochloride. Bangladesh J. Sci. Ind. Res. 2015, 50, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Torniainen, K.; Tammilehto, S.; Ulvi, V. The Effect of PH, Buffer Type and Drug Concentration on the Photodegradation of Ciprofloxacin. Int. J. Pharm. 1996, 132, 53–61. [Google Scholar] [CrossRef]
- Roca Jalil, M.E.; Baschini, M.; Sapag, K. Influence of PH and Antibiotic Solubility on the Removal of Ciprofloxacin from Aqueous Media Using Montmorillonite. Appl. Clay Sci. 2015, 114, 69–76. [Google Scholar] [CrossRef]
- Hoover, R.K.; Alcorn, H.; Lawrence, L.; Paulson, S.K.; Quintas, M.; Cammarata, S.K. Delafloxacin Pharmacokinetics in Subjects With Varying Degrees of Renal Function. J. Clin. Pharmacol. 2018, 58, 514–521. [Google Scholar] [CrossRef] [Green Version]
- Hoover, R.; Hunt, T.; Benedict, M.; Paulson, S.K.; Lawrence, L.; Cammarata, S.; Sun, E. Safety, Tolerability, and Pharmacokinetic Properties of Intravenous Delafloxacin After Single and Multiple Doses in Healthy Volunteers. Clin. Ther. 2016, 38, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Hoover, R.; Alcorn, H.; Lawrence, L.; Paulson, S.K.; Quintas, M.; Cammarata, S.K. Pharmacokinetics of Intravenous Delafloxacin in Patients With End-Stage Renal Disease. J. Clin. Pharmacol. 2018, 58, 913–919. [Google Scholar] [CrossRef]
- Pierfitte, C.; Royer, R.J.; Moore, N.; Bégaud, B. The Link between Sunshine and Phototoxicity of Sparfloxacin. Br. J. Clin. Pharmacol. 2000, 49, 609–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trisciuoglio, D.; Krasnowska, E.; Maggi, A.; Pozzi, R.; Parasassi, T.; Sapora, O. Phototoxic Effect of Fluoroquinolones on Two Human Cell Lines. Toxicol Vitr. 2002, 16, 449–456. [Google Scholar] [CrossRef]
- Fasano, C.J.; O’Malley, G.; Dominici, P.; Aguilera, E.; Latta, D.R. Comparison of Octreotide and Standard Therapy versus Standard Therapy Alone for the Treatment of Sulfonylurea-Induced Hypoglycemia. Ann. Emerg. Med. 2008, 51, 400–406. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, S.A.; Crandall, C.S.; McKinney, P.E. Octreotide: An Antidote for Sulfonylurea-Induced Hypoglycemia. Ann. Emerg. Med. 2000, 36, 133–138. [Google Scholar] [CrossRef]
- Hassan, Z.; Wright, J. Use of Octreotide Acetate to Prevent Rebound Hypoglycaemia in Sulfonylurea Overdose. Emerg. Med. J. 2007, 24, 580–581. [Google Scholar] [CrossRef] [Green Version]
- Muanda, F.T.; Sood, M.M.; Weir, M.A.; Sontrop, J.M.; Ahmadi, F.; Yoo, E.; Kim, R.B.; Silverman, M.S.; Knoll, G.A.; Garg, A.X. Association of Higher-Dose Fluoroquinolone Therapy with Serious Adverse Events in Older Adults With Advanced Chronic Kidney Disease. JAMA Netw. Open 2022, 5, e2224892. [Google Scholar] [CrossRef]
- de With, K.; Allerberger, F.; Amann, S.; Apfalter, P.; Brodt, H.-R.; Eckmanns, T.; Fellhauer, M.; Geiss, H.K.; Janata, O.; Krause, R.; et al. Strategies to Enhance Rational Use of Antibiotics in Hospital: A Guideline by the German Society for Infectious Diseases. Infection 2016, 44, 395–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartelli, M.; Weber, D.G.; Ruppé, E.; Bassetti, M.; Wright, B.J.; Ansaloni, L.; Catena, F.; Coccolini, F.; Abu-Zidan, F.M.; Coimbra, R.; et al. Antimicrobials: A Global Alliance for Optimizing Their Rational Use in Intra-Abdominal Infections (AGORA). World J. Emerg. Surg. 2016, 11, 33. [Google Scholar] [CrossRef] [Green Version]
- Adikwu, E.; Deo, O. Fluoroquinolones Reported Hepatotoxicity. Pharmacol. Pharm. 2012, 3, 20646. [Google Scholar] [CrossRef] [Green Version]
- Yan, K.; Zhu, M.; Jia, Y.; Wang, J.; Cai, Y. Efficacy and Safety of Quinolones vs. Other Antimicrobials for the Treatment of Uncomplicated Urinary Tract Infections in Adults: A Systematic Review and Meta-Analysis. Int. Urogynecol. J. 2022, 33, 1103–1123. [Google Scholar] [CrossRef]
- Haiping, L.; Ziqiang, J.; Qina, Z.; Yuhua, D. Adverse Reactions of Fluoroquinolones to Central Nervous System and Rational Drug Use in Nursing Care. Pak. J. Pharm. Sci. 2019, 32, 427–432. [Google Scholar] [PubMed]
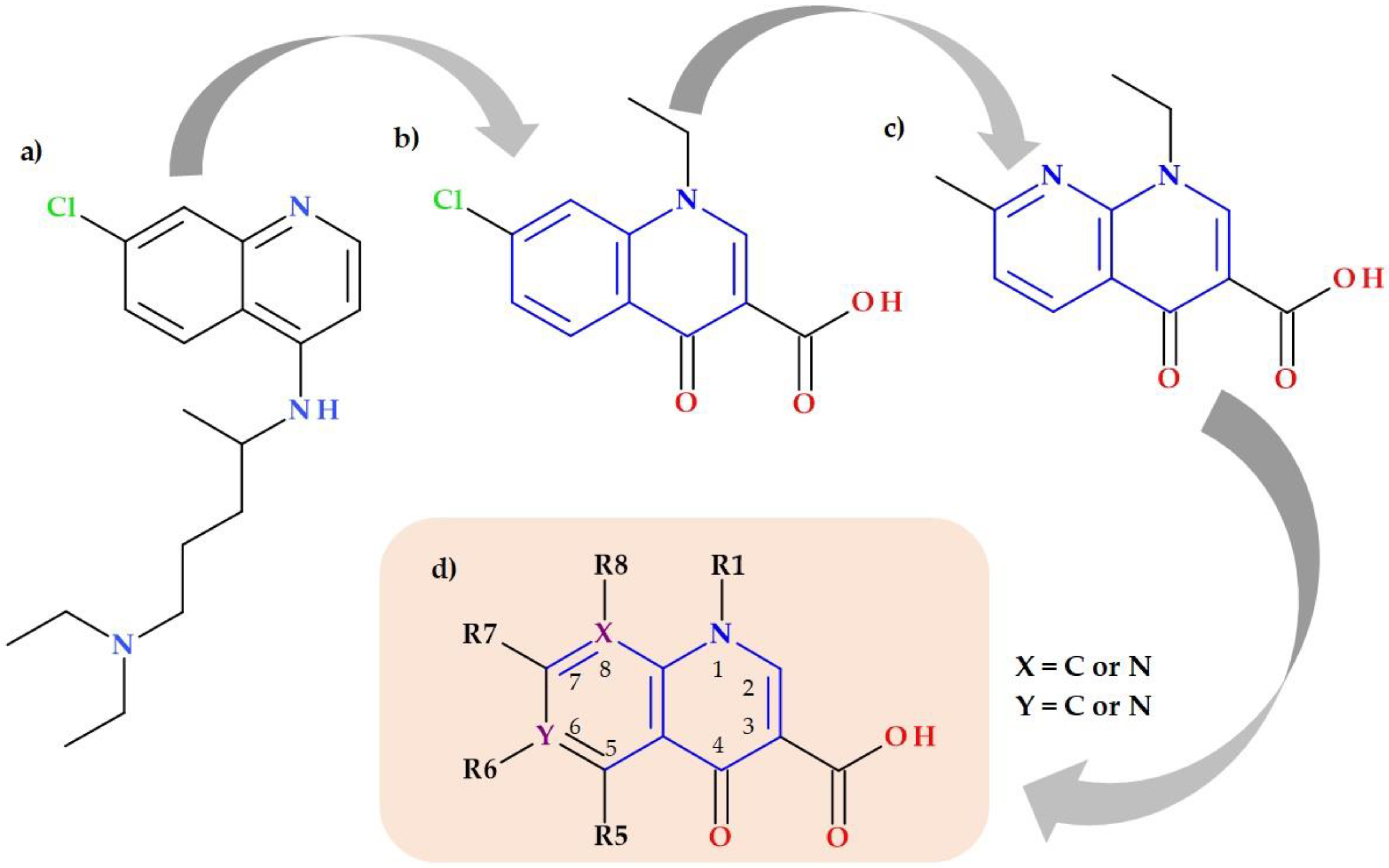


| No. | FQs (Generation) | Manufacturer | Approval Year | Withdrawn Year | Side-Effects | References |
|---|---|---|---|---|---|---|
| 1 | Fleroxacin (2nd) | Kyorin Pharmaceutical | 1981 | 1990 | CNS effects, phototoxicity | [37,93,94] |
| 2 | Tosufloxacin (2nd) | Toyama Chemical | 1990 | 2006 | Thrombocytopenia, nephritis, toxic epidermal necrosis, eosinophilic pneumonitis | [37,95,96,97] |
| 3 | Temafloxacin (2nd) | Abbott Laboratories | 1992 | 1992 | “Temafloxacin syndrome”: hemolytic-uremic syndrome | [2,37,49,98,99,100] |
| 4 | Lomefloxacin (2nd) | Serle | 1992 | 1993 | CNS effects, phototoxicity | [101,102,103] |
| 5 | Sparfloxacin (3rd) | Mylan | 1996 | 2001 | QT prolongation, phototoxicity | [103,104,105,106,107,108] |
| 6 | Alatrofloxacin (3rd) | Pfizer | 1997 | 2006 | Seizures, thrombocytopenia, hepatotoxicity | [108,109,110] |
| 7 | Trovafloxacin (3rd) | Pfizer | 1997 | 2000 | Hepatotoxicity | [99,108,111,112] |
| 8 | Grepafloxacin (3rd) | Glaxo | 1997 | 1999 | QT prolongation, fatal cardiotoxicity, gastrointestinal toxicity | [99,108,113,114] |
| 9 | Clinafloxacin (3rd) | Parke Davis | 1999 | 1999 | Hypoglycemia, phototoxicity | [115] |
| 10 | Gatifloxacin (3rd) | Bristol-Myers Squibb | 1999 | 2006 | Increased risk of dysglycemia | [108,116,117] |
| 11 | Gemifloxacin (3rd) | Vansen Pharma | 1999 | 2009 | Rash erythematous | [117,118,119] |
| Approval Year(s) | QNs/FQs | Antibacterial Spectrum | Indications | Formulations | Administration | Observations | Ref. |
|---|---|---|---|---|---|---|---|
| 1998 2000 | Nadifloxacin | G(+) (including MRSA and coagulase-negative staphylococci), G(−), and anaerobic bacteria | Acne vulgaris Other skin infections | Topical (1% cream) | Twice daily | Approved in Japan Approved by EMA | [42,43,122,123,127] |
| 2008 2012 | Sitafloxacin | Broad-spectrum: G(+) and G(−) bacteria, including anaerobic bacteria, atypical pathogens (particularly against bacteria resistant to other FQs) | Respiratory infections and UTI | Oral formulation (tablets 50 mg) | 50–100 mg twice daily | Approved in Japan Approved in Thailand | [128,129,130,131,132,133,134,135,136,137,138,139,140] |
| 2009 | Besifloxacin | Staphylococcus aureus, Streptococcus pneumoniae, Staphylococcus epidermidis, Moraxella catarrhalis, Hemophilus influenzae, Corynebacterium spp. | Bacterial conjunctivitis | Ophthalmic suspension (0.6%) | One drop in the affected eye(s), three times daily, 4 to 12 h apart (for 7 days) | Approved by the FDA Approved in Canada and later in other countries (Argentina, South Korea, Brazil etc.) | [141,142,143] |
| 2014 | Finafloxacin | Broad-spectrum (particularly against Staphylococcus aureus and Pseudomonas aeruginosa) | Acute otitis externa | Otic suspension (0.3%) | Four drops in the affected ear(s), twice daily (for seven days) | Approved by the FDA | [27,144,145,146] |
| 2014 2016 | Nemonoxacin 1 | Broad spectrum: typical and atypical respiratory pathogens (particularly against resistant G(+) cocci, including penicillin-resistant Streptococcus pneumoniae and MRSA) | CAP (pending for diabetic foot ulcer infections, Skin and soft tissue infections approval) | Oral formulation (capsules 250 mg) Intravenous (i.v.) formulation | 500 mg once a day | Approved in Taiwan Approved in China | [28,29,147,148] |
| 2015 | Zabofloxacin | Broad-spectrum (particularly against major respiratory tract pathogens) | Acute bacterial exacerbation of COPD | Oral (tablets) | 367 mg once daily (for five days) | Approved in South Korea, the Middle East, and North-African countries FDA’s Clinical phase 3 trial approval for CAP patients | [21,149,150,151] |
| 2017 2019 | Delafloxacin | Broad-spectrum: G(+) (including MRSA) and G(−) bacteria | ABSSSI, CAP | Oral (tablets) Parenteral (i.v. infusion) | Oral: 450 mg every 12 h (for 5 to 14 days) Parenteral: 300 mg by i.v. infusion over 60 min every 12 h. | Approved by the FDA Approved by the EMA | [21,152,153,154,155] |
| 2017 2018 2019 | Ozenoxacin | Staphylococcus aureus, Staphylococcus pyogenes, and other G(+) bacteria sensitive and resistant to methicillin, QNs, mupirocin and fusidic acid | Impetigo | Topical (1% cream) | Twice daily (for five days) | Approved by the FDA Approved in Spain Approved in 12 EU countries | [126,156,157,158] |
| 2019 2022 | Lascufloxacin | Major respiratory tract pathogens (e.g., Streptococcus pneumoniae, Moraxella catarrhalis, Hemophilus influenzae, and Mycoplasma pneumoniae) | Respiratory tract and ear, nose, and throat infections, CAP, otorhinolaryngological infections | Oral (tablets) | 75 mg once daily | Approved in Japan Approved in China | [23,159,160,161] |
| 2020 | Levonadifloxacin | Broad-spectrum: G(+) (including MRSA and FQs-resistant Staphylococcus aureus) and G(−) bacteria, atypical bacteria, anaerobic bacteria, bioterror pathogens | ABSSSI with concurrent bacteraemia and diabetic foot infections | Oral (tablets) I.v. injection | 500 mg twice daily 800 mg twice daily | Approved in India | [162,163,164] |
| No. | Substituents on Chemical Structure (X and Y: C or N)  | Associated Side-Effects | Ref. |
|---|---|---|---|
| 1 | N1: Cyclopropyl, Ethyl, 2,4-Diphluorophenyl | Interactions with the cytochrome P450 | [167] |
| N1: Cyclopropyl ≥ Ethyl > 2,4-Diphluorophenyl > Fluorethyl | Theophylline interactions | [168] | |
| N1: Cyclopropyl ≥ Tert-Butyl > 2,4-Diphluorophenyl > Ethyl | Genotoxicity | [168] | |
| N1: 2,4-Diphluorophenyl (combined with halogen at position 8) | Phototoxicity | [169] | |
| N1: Cyclopropyl, Ethyl | Phototoxicity | [169] | |
| N1: Aminodifluorophenyl, 1-Isoxazolyl | Phototoxicity | [169] | |
| 2 | C2: No substitution | No side-effects | [168,170] |
| 3 | C3: Carboxyl | Decrease absorption of biological metals; interactions with antacids, multimineral supplements, and milk products (due to metal binding and chelating) | [168] |
| C3: Carboxyl | Chondrotoxicity (due to specific chelation of Mg2+) | [167,170] | |
| 4 | C4: Oxo | Decrease absorption of biological metals; interactions with antacids, multimineral supplements, and milk products (due to metal binding and chelating) | [168] |
| C4: Oxo | Chondrotoxicity (due to specific chelation of Mg2+) | [167,170] | |
| 5 | C5: Methyl/amino substituents | QT prolongation | [104,168,170] |
| 5 | C5: Methyl >> H > Amino substituents | Phototoxicity | [167,168] |
| 5 | C5: Methyl > Amino substituents > H | Genotoxicity | [167,168] |
| 6 | C6: Fluorine substituent | Genotoxicity | [167] |
| 7 | C7: Pyrrolidine > Piperazine > Alkyl | Genotoxicity | [167] |
| C7: Pyrrolidine (unsubstituted) > Piperazine (unsubstituted) > Pyrrolidine (substituted) > Piperazine (substituted) | Genotoxicity | [168] | |
| C7: Alkyl > Piperazine (unsubstituted) > Pyrrolidine (unsubstituted) > Piperazine (substituted) or Pyrrolidine (substituted) | Neuropsychiatric toxicity, seizures (GABA receptor binding) | [8,44,117,167,168,171] | |
| C7: Piperazine (unsubstituted) | Crystalluria | [167] | |
| C7: Piperazine (unsubstituted) > Pyrrolidine (unsubstituted) > Piperazine (substituted) or Pyrrolidine (substituted) | Some NSAIDs interactions | [167] | |
| C7: Pyrrolidine (unsubstituted) > Piperazine (unsubstituted) > Piperazine (substituted) or Pyrrolidine (substituted) | Theophylline interactions | [168] | |
| 8 | C8: Fluorine/chlorine substituents (F > Cl) | Phototoxicity, Genotoxicity | [167,170] |
| C-F > C-Cl > N > C-H > C-O-Methyl, C-CF3 | Phototoxicity | [168] | |
| C-F > C-Cl ≥ C-O-Methyl > N > C-H | Genotoxicity | [168] | |
| C8: Fluorine/chlorine, methoxy substituents | Crystalluria | [167] | |
| C8: Bulky substituent | Decreased interactions with the cytochrome P450 | [167] | |
| N8: Naphtiridone nucleus | Increased interactions with the cytochrome P450 | [167,170] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusu, A.; Munteanu, A.-C.; Arbănași, E.-M.; Uivarosi, V. Overview of Side-Effects of Antibacterial Fluoroquinolones: New Drugs versus Old Drugs, a Step Forward in the Safety Profile? Pharmaceutics 2023, 15, 804. https://doi.org/10.3390/pharmaceutics15030804
Rusu A, Munteanu A-C, Arbănași E-M, Uivarosi V. Overview of Side-Effects of Antibacterial Fluoroquinolones: New Drugs versus Old Drugs, a Step Forward in the Safety Profile? Pharmaceutics. 2023; 15(3):804. https://doi.org/10.3390/pharmaceutics15030804
Chicago/Turabian StyleRusu, Aura, Alexandra-Cristina Munteanu, Eliza-Mihaela Arbănași, and Valentina Uivarosi. 2023. "Overview of Side-Effects of Antibacterial Fluoroquinolones: New Drugs versus Old Drugs, a Step Forward in the Safety Profile?" Pharmaceutics 15, no. 3: 804. https://doi.org/10.3390/pharmaceutics15030804
APA StyleRusu, A., Munteanu, A.-C., Arbănași, E.-M., & Uivarosi, V. (2023). Overview of Side-Effects of Antibacterial Fluoroquinolones: New Drugs versus Old Drugs, a Step Forward in the Safety Profile? Pharmaceutics, 15(3), 804. https://doi.org/10.3390/pharmaceutics15030804








