Pharmacokinetics of Orally Applied Cannabinoids and Medical Marijuana Extracts in Mouse Nervous Tissue and Plasma: Relevance for Pain Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Medical Marijuana Extracts and Cannabinoid Compounds
2.2. Reference Solutions and Extract Preparation
2.3. LC-ToF-MS Analysis of Medical Marijuana Extracts
2.4. Animals
2.5. Administration of Cannabinoid Formulations
2.6. Spared Nerve Injury (SNI) Model
2.7. Tissue Collection and Sample Preparation
2.8. LC-MS/MS Quantification of Δ9-THC, OH-THC, COOH-THC and CBD in Tissue Samples
2.9. Analysis of Quantitative Mass Spectrometry Data
2.10. Von Frey Test for Mechanical Thresholds
2.11. Data Analysis
3. Results
3.1. Quantification of Bioactive Cannabinoids and Flavonoids in Medical Marijuana Extracts
3.2. Pharmacokinetic Profile of Δ9-THC Bioavailability in Nervous Tissue and Plasma after Application of Medical Marijuana Extracts and Pure Compound
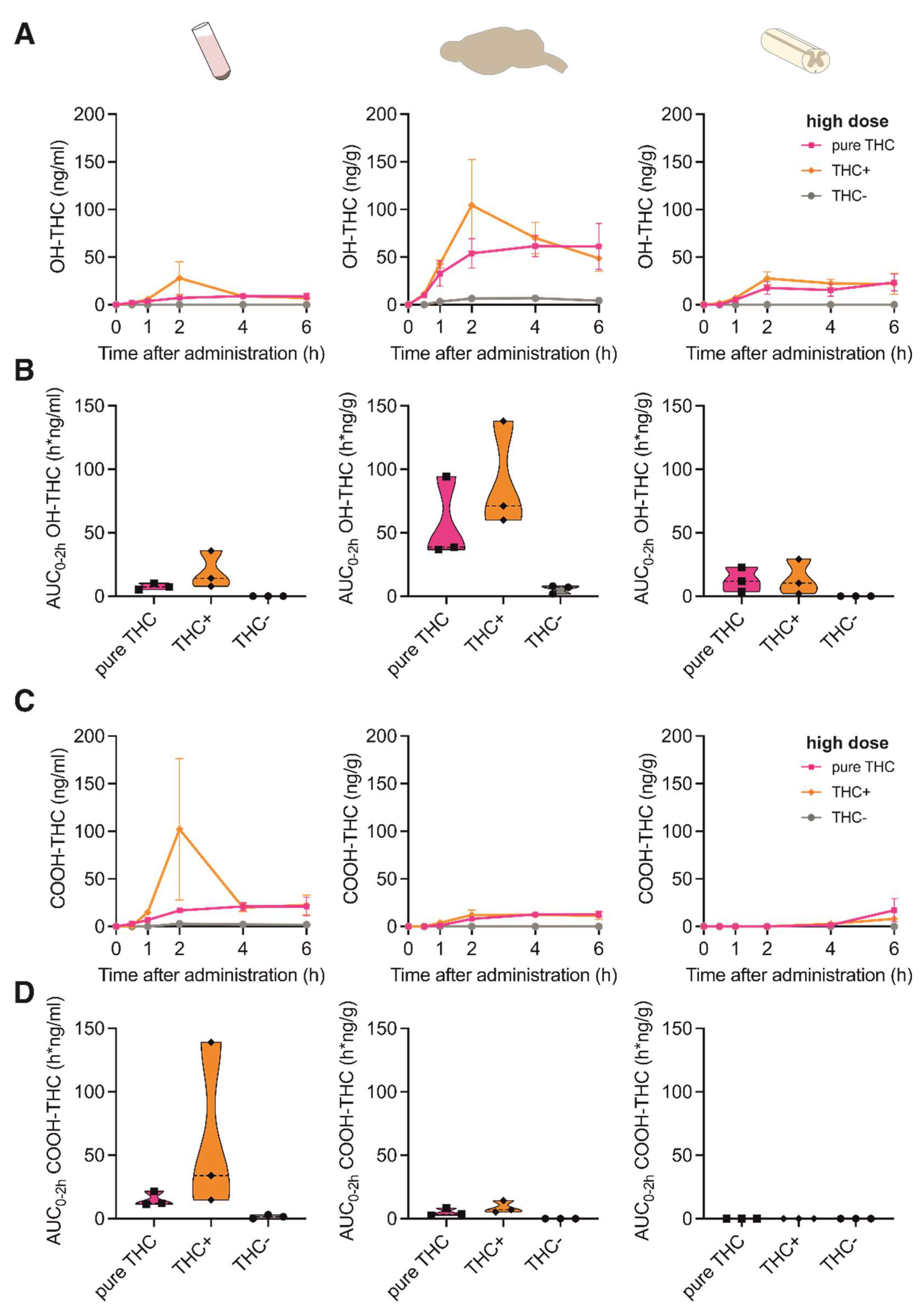
3.3. Pharmacokinetic Profile of Δ9-THC Metabolites in Nervous Tissue and Plasma after Application of Medical Marijuana Extracts and Pure Compound
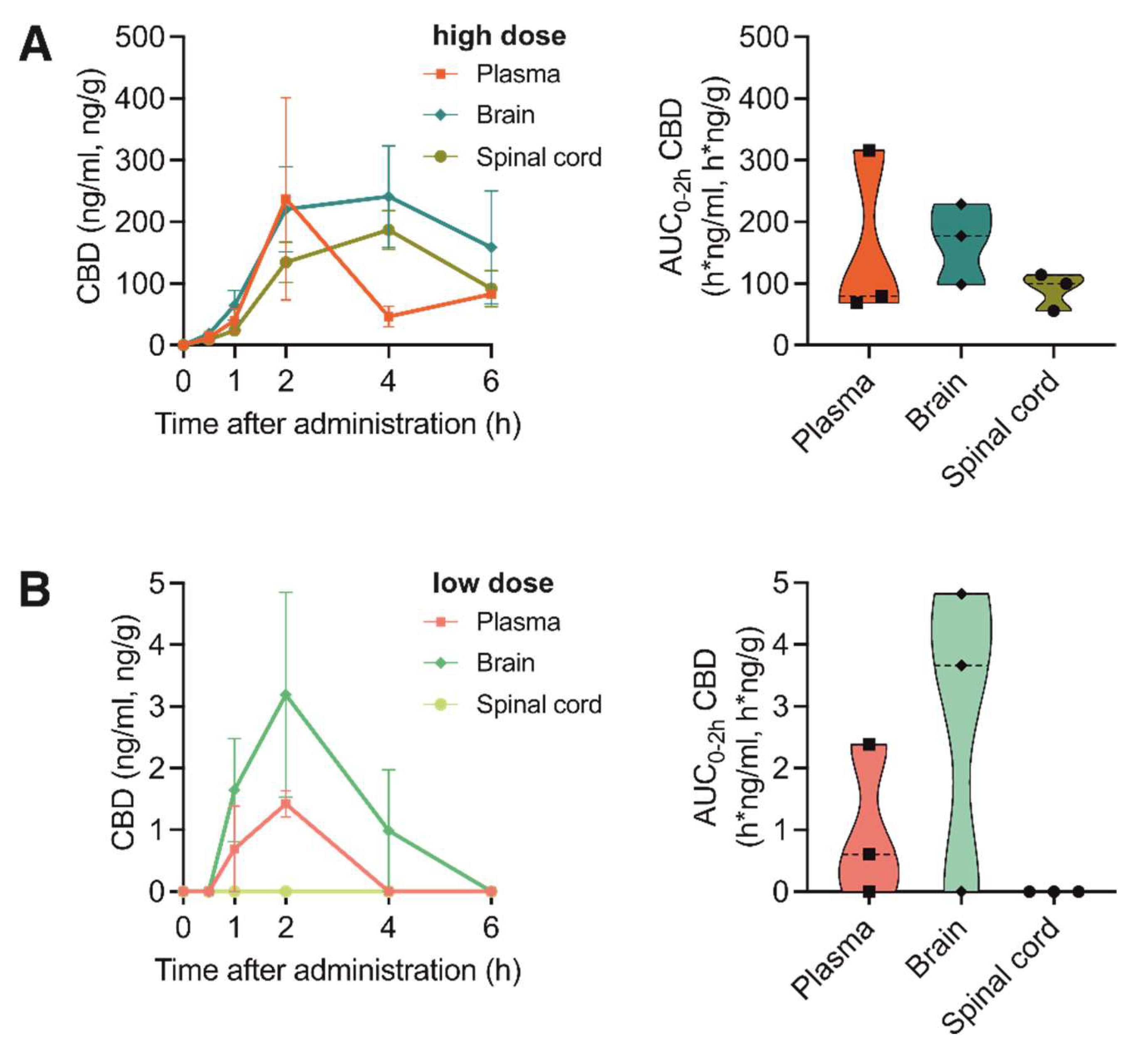
3.4. Pharmacokinetic Profile of CBD Bioavailability in Nervous Tissue and Plasma after Application of Medical Marijuana Extracts and Pure Compound
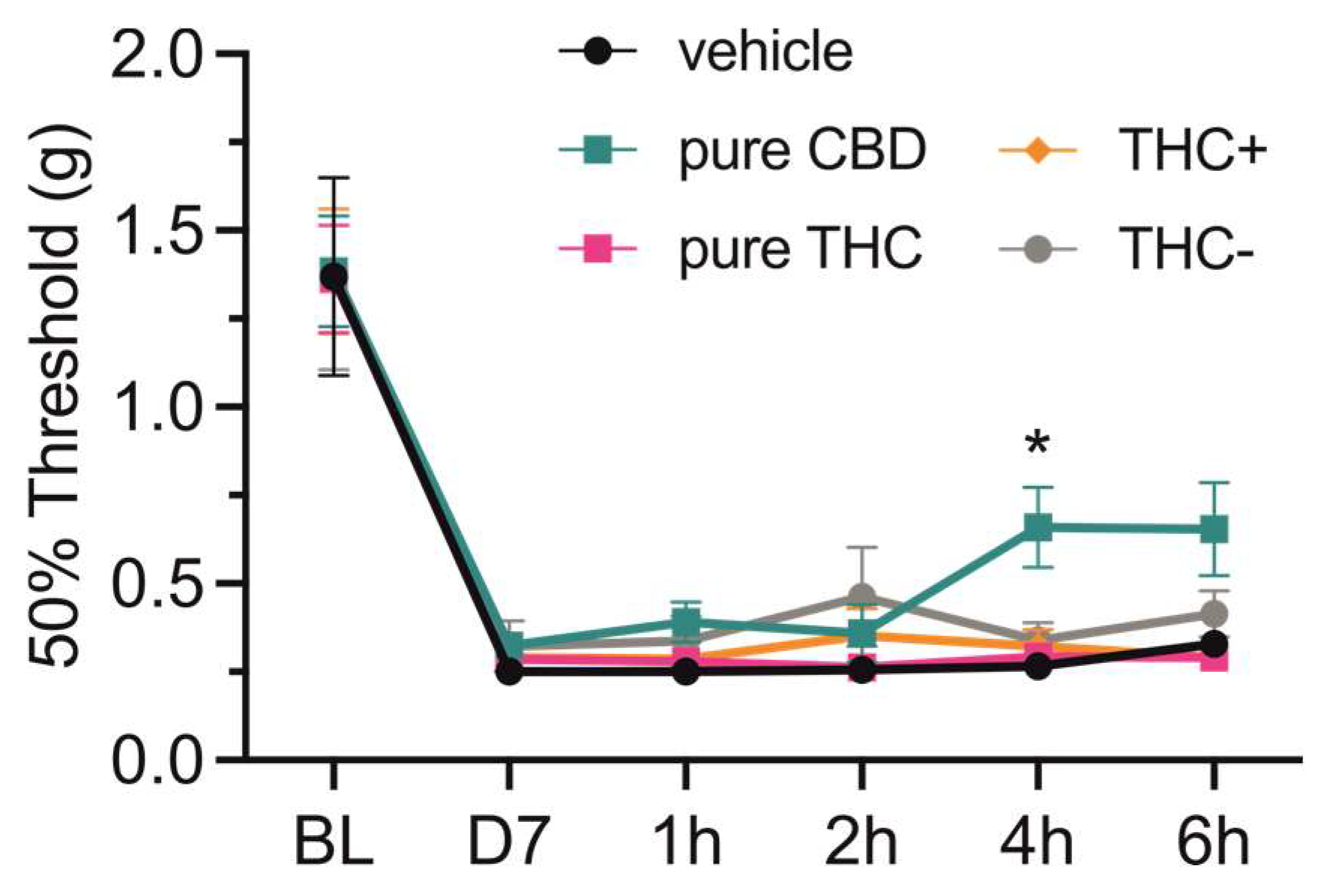
3.5. Analgesic Potency of Medical Marijuana Extracts and Pure Cannabinoid Compounds
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Berman, P.; Futoran, K.; Lewitus, G.M.; Mukha, D.; Benami, M.; Shlomi, T.; Meiri, D. A new ESI-LC/MS ap-proach for comprehensive metabolic profiling of phytocannabinoids in Cannabis. Sci. Rep. 2018, 8, 14280. [Google Scholar] [CrossRef] [Green Version]
- Cicaloni, V.; Salvini, L.; Vitalini, S.; Garzoli, S. Chemical Profiling and Characterization of Different Cultivars of Cannabis sativa L. Inflorescences by SPME-GC-MS and UPLC-MS. Separations 2022, 9, 90. [Google Scholar] [CrossRef]
- Mechoulam, R.; Hanuš, L.O.; Pertwee, R.; Howlett, A.C. Early phytocannabinoid chemistry to endocanna-binoids and beyond. Nat. Rev. Neurosci. 2014, 15, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Svizenska, I.; Dubovy, P.; Sulcova, A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures--a short review. Pharmacol. Biochem. Behav. 2008, 90, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-J.; Gao, M.; Gao, F.-F.; Su, Q.-X.; Wu, J. Brain cannabinoid receptor 2: Expression, function and modulation. Acta Pharmacol. Sin. 2017, 38, 312–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Olives, C.; Rivas-Santisteban, R.; Lillo, J.; Navarro, G.; Franco, R. Recent Advances in the Potential of Cannabinoids for Neuroprotection in Alzheimer’s, Parkinson’s, and Huntington’s Diseases. Adv. Exp. Med. Biol. 2021, 1264, 81–92. [Google Scholar]
- Russo, E.B.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic Properties of Cannabidiol at 5-HT1a Receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef]
- Martínez-Aguirre, C.; Carmona-Cruz, F.; Velasco, A.L.; Velasco, F.; Aguado-Carrillo, G.; Cuéllar-Herrera, M.; Rocha, L. Cannabidiol Acts at 5-HT1A Receptors in the Human Brain: Relevance for Treating Temporal Lobe Epilepsy. Front. Behav. Neurosci. 2020, 14, 611278. [Google Scholar] [CrossRef]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2018, 11, 487. [Google Scholar] [CrossRef] [Green Version]
- Englund, A.; Morrison, P.D.; Nottage, J.; Hague, D.; Kane, F.; Bonaccorso, S.; Stone, J.M.; Reichenberg, A.; Brenneisen, R.; Holt, D.; et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J. Psychopharmacol. 2012, 27, 19–27. [Google Scholar] [CrossRef]
- Hložek, T.; Uttl, L.; Kadeřábek, L.; Balíková, M.; Lhotková, E.; Horsley, R.R.; Nováková, P.; Šíchová, K.; Štefková, K.; Tylš, F.; et al. Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur. Neuropsychopharmacol. 2017, 27, 1223–1237. [Google Scholar] [CrossRef]
- Grotenhermen, F. Pharmacokinetics and Pharmacodynamics of Cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef]
- Huestis, M.A. Human Cannabinoid Pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef] [Green Version]
- Millar, S.A.; Stone, N.L.; Yates, A.S.; O’sullivan, S.E. A Systematic Review on the Pharmacokinetics of Can-nabidiol in Humans. Front. Pharmacol. 2018, 9, 1365. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, T.; Iwawaki, Y.; Watanabe, K.; Yamamoto, I.; Kageyama, T.; Yoshimura, H. Metabolism of delta 9-tetrahydrocannabinol by cytochrome P450 isozymes purified from hepatic microsomes of monkeys. Life Sci. 1995, 56, 2089–2095. [Google Scholar] [CrossRef]
- Abbotts, K.S.S.; Ewell, T.R.; Butterklee, H.M.; Bomar, M.C.; Akagi, N.; Dooley, G.P.; Bell, C. Cannabidiol and Cannabidiol Metabolites: Pharmacokinetics, Interaction with Food, and Influence on Liver Function. Nutrients 2022, 14, 2152. [Google Scholar] [CrossRef] [PubMed]
- Carlini, E.A.; Karniol, I.G.; Renault, P.F.; Schuster, C.R. Effects of marihuana in laboratory animals and in man. Br. J. Pharmacol. 1974, 50, 299–309. [Google Scholar] [CrossRef]
- Ben-Shabat, S.; Fride, E.; Sheskin, T.; Tamiri, T.; Rhee, M.-H.; Vogel, Z.; Bisogno, T.; De Petrocellis, L.; Di Marzo, V.; Mechoulam, R. An entourage effect: Inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur. J. Pharmacol. 1998, 353, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- Benito, S.B.; Seijo-Vila, M.; Caro-Villalobos, M.; Tundidor, I.; Andradas, C.; García-Taboada, E.; Wade, J.; Smith, S.; Guzmán, M.; Pérez-Gómez, E.; et al. Appraising the “entourage effect”: Antitumor action of a pure cannabinoid versus a botanical drug preparation in preclinical models of breast cancer. Biochem. Pharmacol. 2018, 157, 285–293. [Google Scholar] [CrossRef]
- Nallathambi, R.; Mazuz, M.; Ion, A.; Selvaraj, G.; Weininger, S.; Fridlender, M.; Nasser, A.; Sagee, O.; Kumari, P.; Nem-ichenizer, D.; et al. An-ti-Inflammatory Activity in Colon Models Is Derived from Δ9-Tetrahydrocannabinolic Acid That Interacts with Additional Compounds in Cannabis Extracts. Cannabis Cannabinoid Res. 2017, 2, 167–182. [Google Scholar] [CrossRef] [Green Version]
- Wan, B.A.; Diaz, P.L.; Blake, A.; Chan, S.; Wolt, A.; Zaki, P.; Zhang, L.; Slaven, M.; Shaw, E.; Deangelis, C.; et al. Efficacy of different varieties of medical cannabis in relieving symptoms. J. Pain Manag. 2017, 10, 415–422. [Google Scholar]
- Baron, E.P.; Lucas, P.; Eades, J.; Hogue, O. Patterns of medicinal cannabis use, strain analysis, and substitution effect among patients with migraine, headache, arthritis, and chronic pain in a medicinal cannabis cohort. J. Headache Pain 2018, 19, 37. [Google Scholar] [CrossRef] [Green Version]
- Casey, S.L.; Atwal, N.; Vaughan, C.W. Cannabis constituent synergy in a mouse neuropathic pain model. Pain 2017, 158, 2452–2460. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.; Cairns, B.E. Cannabidiol, cannabinol and their combinations act as peripheral analgesics in a rat model of myofascial pain. Arch. Oral Biol. 2019, 104, 33–39. [Google Scholar] [CrossRef]
- Comelli, F.; Giagnoni, G.; Bettoni, I.; Colleoni, M.; Costa, B. Antihyperalgesic effect of a Cannabis sativa extract in a rat model of neuropathic pain: Mechanisms involved. Phytotherapy Res. 2008, 22, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- King, K.M.; Myers, A.M.; Soroka-Monzo, A.J.; Tuma, R.F.; Tallarida, R.J.; Walker, E.A.; Ward, S.J. Single and combined effects of Delta(9)—tetrahydrocannabinol and cannabidiol in a mouse model of chemotherapy-induced neuro-pathic pain. Br. J. Pharmacol. 2017, 174, 2832–2841. [Google Scholar] [CrossRef] [Green Version]
- Anderson, L.L.; Low, I.K.; Banister, S.D.; McGregor, I.S.; Arnold, J.C. Pharmacokinetics of Phytocannabinoid Acids and Anticonvulsant Effect of Cannabidiolic Acid in a Mouse Model of Dravet Syndrome. J. Nat. Prod. 2019, 82, 3047–3055. [Google Scholar] [CrossRef] [Green Version]
- Kalpachidou, T.; Malsch, P.; Qi, Y.; Mair, N.; Geley, S.; Quarta, S.; Kummer, K.K.; Kress, M. Genetic and functional evidence for gp130/IL6ST-induced transient receptor potential ankyrin 1 upregulation in uninjured but not injured neurons in a mouse model of neuropathic pain. Pain 2022, 163, 579–589. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.L.; Hansen, R.B.; Storm, M.A.; Olesen, J.; Hansen, T.F.; Ossipov, M.; Izarzugaza, J.M.G.; Porreca, F.; Kristensen, D.M. Von Frey testing revisited: Provision of an online algorithm for improved accuracy of 50% thresholds. Eur. J. Pain 2019, 24, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, P.; Pichini, S.; Pacifici, R.; Busardò, F.P.; Del Rio, A. Herbal Preparations of Medical Cannabis: A Vademecum for Prescribing Doctors. Medicina 2020, 56, 237. [Google Scholar] [CrossRef] [PubMed]
- Vandrey, R.; Herrmann, E.S.; Mitchell, J.M.; Bigelow, G.E.; Flegel, R.; Lodico, C.; Cone, E.J. Pharmacokinetic Profile of Oral Cannabis in Humans: Blood and Oral Fluid Disposition and Relation to Pharmacodynamic Outcomes. J. Anal. Toxicol. 2017, 41, 83–99. [Google Scholar] [CrossRef] [Green Version]
- Lunn, S.; Diaz, P.; O’hearn, S.; Cahill, S.P.; Blake, A.; Narine, K.; Dyck, J.R.B. Human Pharmacokinetic Pa-rameters of Orally Administered Δ(9)-Tetrahydrocannabinol Capsules Are Altered by Fed Versus Fasted Conditions and Sex Differences. Cannabis Cannabinoid Res. 2019, 4, 255–264. [Google Scholar] [CrossRef]
- Klein, C.; Karanges, E.; Spiro, A.; Wong, A.; Spencer, J.; Huynh, T.; Gunasekaran, N.; Karl, T.; Long, L.E.; Huang, X.-F.; et al. Cannabidiol potentiates Δ9-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology 2011, 218, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Pagano, E.; Capasso, R.; Piscitelli, F.; Romano, B.; Parisi, O.A.; Finizio, S.; Lauritano, A.; Di Marzo, V.; Izzo, A.A.; Borrelli, F. An Orally Active Cannabis Extract with High Content in Cannabidiol attenuates Chemically-induced Intestinal Inflammation and Hypermotility in the Mouse. Front. Pharmacol. 2016, 7, 341. [Google Scholar] [CrossRef] [Green Version]
- Al-Ghezi, Z.Z.; Busbee, P.B.; Alghetaa, H.; Nagarkatti, P.S.; Nagarkatti, M. Combination of cannabinoids, delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), mitigates experimental autoimmune encephalomyelitis (EAE) by altering the gut microbiome. Brain Behav. Immun. 2019, 82, 25–35. [Google Scholar] [CrossRef]
- Anderson, L.L.; Etchart, M.G.; Bahceci, D.; Golembiewski, T.A.; Arnold, J.C. Cannabis constituents interact at the drug efflux pump BCRP to markedly increase plasma cannabidiolic acid concentrations. Sci. Rep. 2021, 11, 14948. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Gurley, B.J.; Markowitz, J.S. The Potential for Pharmacokinetic Interactions Between Cannabis Products and Conventional Medications. J. Clin. Psychopharmacol. 2019, 39, 462–471. [Google Scholar] [CrossRef]
- Nasrin, S.; Watson, C.J.; Perez-Paramo, Y.X.; Lazarus, P. Cannabinoid Metabolites as Inhibitors of Major Hepatic CYP450 Enzymes, with Implications for Cannabis-Drug Interactions. Drug Metab. Dispos. 2021, 49, 1070–1080. [Google Scholar] [CrossRef]
- Wager, T.T.; Chandrasekaran, R.Y.; Hou, X.; Troutman, M.D.; Verhoest, P.R.; Villalobos, A.; Will, Y. Defining Desirable Central Nervous System Drug Space through the Alignment of Molecular Properties, In Vitro ADME, and Safety Attributes. ACS Chem. Neurosci. 2010, 1, 420–434. [Google Scholar] [CrossRef] [Green Version]
- Russo, E.B. Cannabis Therapeutics and the Future of Neurology. Front. Integr. Neurosci. 2018, 12, 51. [Google Scholar] [CrossRef]
- Li, H.; Kong, W.; Chambers, C.R.; Yu, D.; Ganea, D.; Tuma, R.F.; Ward, S.J. The non-psychoactive phyto-cannabinoid cannabidiol (CBD) attenuates pro-inflammatory mediators, T cell infiltration, and thermal sensitivity fol-lowing spinal cord injury in mice. Cell. Immunol. 2018, 329, 1–9. [Google Scholar] [CrossRef]
- Patricio, F.; Morales-Andrade, A.A.; Patricio-Martínez, A.; Limón, I.D. Cannabidiol as a Therapeutic Target: Evidence of its Neuroprotective and Neuromodulatory Function in Parkinson’s Disease. Front. Pharmacol. 2020, 11, 595635. [Google Scholar] [CrossRef] [PubMed]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Arthur, M.; Fleming, S.; Woodcock, H.; Dorward, P.; Pigliacampo, B.; Close, S.; et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ⁹-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacology 2012, 219, 859–873. [Google Scholar] [PubMed]
- Rock, E.M.; Limebeer, C.L.; Parker, L.A. Effect of cannabidiolic acid and ∆(9)-tetrahydrocannabinol on car-rageenan-induced hyperalgesia and edema in a rodent model of inflammatory pain. Psychopharmacology 2018, 235, 3259–3271. [Google Scholar] [CrossRef] [PubMed]
- Britch, S.C.; Goodman, A.G.; Wiley, J.L.; Pondelick, A.M.; Craft, R.M. Antinociceptive and Immune Effects of Delta-9-Tetrahydrocannabinol or Cannabidiol in Male Versus Female Rats with Persistent Inflammatory Pain. Experiment 2020, 373, 416–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, V.A.; Harley, J.; Casey, S.L.; Vaughan, A.C.; Winters, B.L.; Vaughan, C.W. Oral efficacy of Δ(9)-tetrahydrocannabinol and cannabidiol in a mouse neuropathic pain model. Neuropharmacology 2021, 189, 108529. [Google Scholar] [CrossRef]
- Xiong, W.; Cui, T.; Cheng, K.; Yang, F.; Chen, S.-R.; Willenbring, D.; Guan, Y.; Pan, H.-L.; Ren, K.; Xu, Y.; et al. Cannabinoids suppress inflammatory and neuropathic pain by targeting α3 glycine receptors. J. Exp. Med. 2012, 209, 1121–1134. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Shahidi, F. Cannabis and Cannabis Edibles: A Review. J. Agric. Food Chem. 2021, 69, 1751–1774. [Google Scholar] [CrossRef] [PubMed]
- Urits, I.; Gress, K.; Charipova, K.; Habib, K.; Lee, D.; Lee, C.; Jung, J.W.; Kassem, H.; Cornett, E.; Paladini, A.; et al. Use of cannabidiol (CBD) for the treatment of chronic pain. Best Pract. Res. Clin. Anaesthesiol. 2020, 34, 463–477. [Google Scholar] [CrossRef] [PubMed]

| Substance | MRM-Transition 1 | Dwell Time | DP 2 | CE 3 | CXP 4 | EP 5 |
|---|---|---|---|---|---|---|
| Δ9-THC and CBD (Quantifier) | 315.0 → 193.1 | 30 msec | 60 | 30 | 10 | 10 |
| Δ9-THC and CBD (Qualifier) | 315.0 → 259.0 | 10 msec | 60 | 30 | 10 | 10 |
| D3-THC (Quantifier) | 318.2 → 196.1 | 30 msec | 60 | 30 | 10 | 10 |
| D3-THC (Qualifier) | 318.2 → 123.0 | 10 msec | 60 | 30 | 10 | 10 |
| OH-THC (Quantifier) | 331.2 → 313.1 | 30 msec | 60 | 30 | 10 | 10 |
| OH-THC (Qualifier) | 331.2 → 193.1 | 10 msec | 60 | 30 | 10 | 10 |
| COOH-THC (Quantifier) | 345.2 → 299.2 | 30 msec | 60 | 30 | 10 | 10 |
| COOH-THC (Qualifier) | 345.2 → 193.1 | 10 msec | 60 | 30 | 10 | 10 |
| Analyte | C 1 Per Sample [ng/mL] | Mean C [ng/mL] 2 | SEM | C of Analysed Extract [μg/mL] | ng Analyte/ μg Extract | % [m/m] |
|---|---|---|---|---|---|---|
| CBC | 247.5 | 247.40 | 3.44 | 40 | 6.19 | 0.62 |
| 241.4 | ||||||
| 253.3 | ||||||
| CBD | 63.30 | 63.08 | 1.04 | 40 | 1.58 | 0.16 |
| 64.77 | ||||||
| 61.18 | ||||||
| CBDA | <LLOQ | <LLOQ 3 | N/A 4 | N/A | N/A | N/A |
| <LLOQ | ||||||
| <LLOQ | ||||||
| CBDV | 38.35 | 37.46 | 2.49 | 400 | 0.09 | 0.01 |
| 32.77 | ||||||
| 41.26 | ||||||
| CBG | 297.2 | 300.37 | 2.16 | 40 | 7.51 | 0.75 |
| 304.5 | ||||||
| 299.4 | ||||||
| CBN | 2343 | 2677.00 | 175.60 | 40 | 66.93 | 6.69 |
| 2750 | ||||||
| 2938 | ||||||
| THCV | 110.4 | 90.95 | 12.39 | 40 | 2.27 | 0.23 |
| 94.52 | ||||||
| 67.92 | ||||||
| THCA-A | 170 | 161.33 | 6.55 | 400 | 0.4 | 0.04 |
| 165.5 | ||||||
| 148.5 | ||||||
| Δ9-THC | 1890 | 2043.33 | 79.90 | 4 | 510.83 | 51.08 |
| 2081 | ||||||
| 2159 | ||||||
| API | <LLOQ | <LLOQ | N/A | N/A | N/A | N/A |
| <LLOQ | ||||||
| <LLOQ | ||||||
| LUT | <LLOQ | <LLOQ | N/A | N/A | N/A | N/A |
| <LLOQ | ||||||
| <LLOQ |
| Analyte | C 1 Per Sample [ng/mL] | Mean C [ng/mL] 2 | SEM | C of Analysed Extract [μg/mL] | ng Analyte/ μg Extract | % [m/m] |
|---|---|---|---|---|---|---|
| CBC | <LLOQ | <LLOQ 3 | N/A 4 | N/A | N/A | N/A |
| <LLOQ | ||||||
| <LLOQ | ||||||
| CBD | <LLOQ | <LLOQ | N/A | N/A | N/A | N/A |
| <LLOQ | ||||||
| <LLOQ | ||||||
| CBDA | 16.19 | 16.16 | 0.04 | 400 | 0.04 | 0.004 |
| 16.20 | ||||||
| 16.09 | ||||||
| CBDV | <LLOQ | <LLOQ | N/A | N/A | N/A | N/A |
| <LLOQ | ||||||
| <LLOQ | ||||||
| CBG | 62.56 | 57.25 | 2.68 | 400 | 0.14 | 0.014 |
| 55.19 | ||||||
| 54.01 | ||||||
| CBN | 30.61 | 29.66 | 0.55 | 40 | 0.74 | 0.07 |
| 29.66 | ||||||
| 28.72 | ||||||
| THCV | <LLOQ | <LLOQ | N/A | N/A | N/A | N/A |
| <LLOQ | ||||||
| <LLOQ | ||||||
| THCA-A | 216.8 | 213.33 | 2.89 | 40 | 5.33 | 0.53 |
| 215.6 | ||||||
| 207.6 | ||||||
| Δ9-THC | 502.6 | 504.97 | 1.89 | 400 | 1.26 | 0.13 |
| 508.7 | ||||||
| 503.6 | ||||||
| API | <LLOQ | <LLOQ | N/A | N/A | N/A | N/A |
| <LLOQ | ||||||
| <LLOQ | ||||||
| LUT | 17.38 | 18.41 | 0.70 | 400 | 0.05 | 0.005 |
| 19.74 | ||||||
| 18.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumbraveanu, C.; Strommer, K.; Wonnemann, M.; Choconta, J.L.; Neumann, A.; Kress, M.; Kalpachidou, T.; Kummer, K.K. Pharmacokinetics of Orally Applied Cannabinoids and Medical Marijuana Extracts in Mouse Nervous Tissue and Plasma: Relevance for Pain Treatment. Pharmaceutics 2023, 15, 853. https://doi.org/10.3390/pharmaceutics15030853
Dumbraveanu C, Strommer K, Wonnemann M, Choconta JL, Neumann A, Kress M, Kalpachidou T, Kummer KK. Pharmacokinetics of Orally Applied Cannabinoids and Medical Marijuana Extracts in Mouse Nervous Tissue and Plasma: Relevance for Pain Treatment. Pharmaceutics. 2023; 15(3):853. https://doi.org/10.3390/pharmaceutics15030853
Chicago/Turabian StyleDumbraveanu, Cristiana, Katharina Strommer, Meinolf Wonnemann, Jeiny Luna Choconta, Astrid Neumann, Michaela Kress, Theodora Kalpachidou, and Kai K. Kummer. 2023. "Pharmacokinetics of Orally Applied Cannabinoids and Medical Marijuana Extracts in Mouse Nervous Tissue and Plasma: Relevance for Pain Treatment" Pharmaceutics 15, no. 3: 853. https://doi.org/10.3390/pharmaceutics15030853





