Digital Technologies Applied to Control the One-Step Process of Cannabis Olive Oil Preparations
Abstract
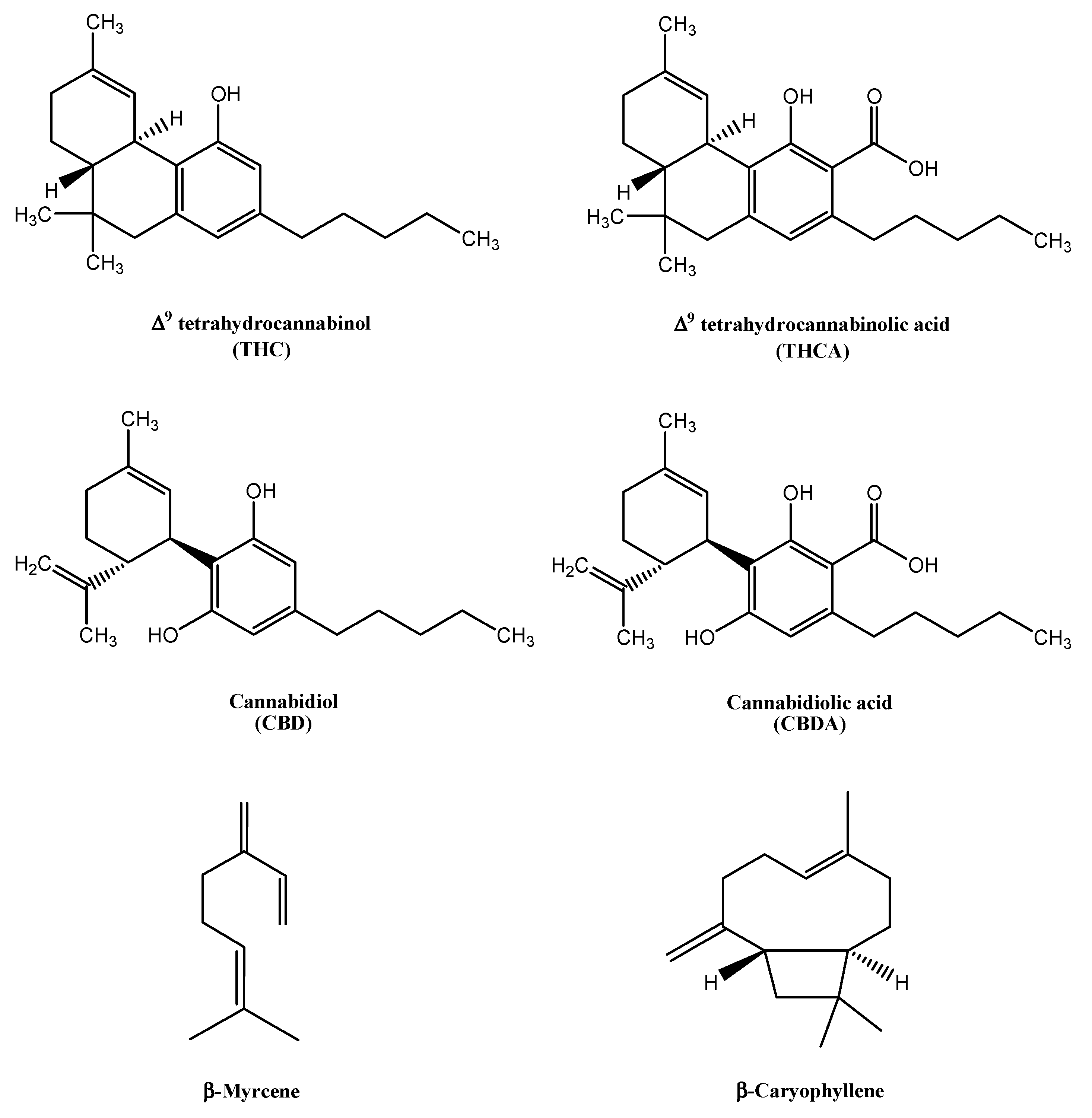
:1. Introduction
2. Materials and Methods
2.1. Chemical and Solvent
2.2. Pharmagear® Apparatus and Instruments
2.3. Preparation of the Cannabis Extracts in Olive Oil
2.4. Cannabis Extract in Olive Oil with the SIFAP Procedure (Maceration Process)
2.5. HPLC Instrument and Method
Calibration Curve
2.6. Sample Preparation and Analysis of Cannabinoids and Terpenes
2.7. Statistical Analysis
3. Results and Discussions
4. Conclusions
5. Patent
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartsel, J.A.; Eades, J.; Hickory, B.; Makriyannis, A. Cannabis Sativa and Hemp, Cannabis Sativa and Hemp; Gupta, R.C., Ed.; Nutraceuticals: Efficacy, Safety and Toxicity; Elsevier Inc.: London, UK, 2016; pp. 735–754. [Google Scholar]
- Ternelli, M.; Brighenti, V.; Anceschi, L.; Poto, M.; Bertelli, D.; Licata, M.; Pellati, F. Innovative methods for the preparation of medical Cannabis oils with a high content of both cannabinoids and terpenes. J. Pharm. Biomed. Anal. 2020, 186, 113296. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecule. Front. Plant Sci. 2016, 7, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appendino, G.; Chianese, G.; Taglialatela-Scafati, O. Cannabinoids: Occurence and medicinal chemistry. Curr. Med. Chem. 2011, 18, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.F.; ElSohly, M.A. The Analytical Chemistry of Cannabis: Quality Assessment, Assurance and Regulation of Medicinal Marijuana and Cannabinoid Preparations, 1st ed.; Elsevier: Amsterdam, The Netherlands; Oxford, UK; Waltham, MA, USA, 2015. [Google Scholar]
- Protti, M.; Brighenti, V.; Battaglia, M.R.; Anceschi, L.; Pellati, F.; Mercolini, L. Cannabinoids from Cannabis sativa L.: A new tool based on HPLC-DAD-MS/MS for a rational use in medicinal chemistry. ACS Med. Chem. Lett. 2019, 10, 539–544. [Google Scholar] [CrossRef]
- Pellati, F.; Brighenti, V.; Sperlea, J.; Marchetti, L.; Bertelli, D.; Benvenuti, S. New methods for the comprehensive analysis of bioactive compounds in Cannabis sativa L. (hemp). Molecules 2018, 23, 2639. [Google Scholar] [CrossRef] [Green Version]
- Brighenti, V.; Pellati, F.; Steinbach, M.; Maran, D.; Benvenuti, S. Development of a new extraction technique and HPLC method for the analysis of non-psychoactive cannabinoids in fibre-type Cannabis sativa L. (hemp). J. Pharm. Biomed. Anal. 2017, 143, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis sativa L. and non-psychoactive cannabinoids: Their chemistry and role against oxidative stress, inflammation, and cancer. Biomed. Res. Int. 2018, 2018, 1691428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iseppi, r.; Brighenti, V.; Licata, M.; Lambertini, A.; Sabia, C.; Messi, P.; Pellati, F.; Benvenuti, S. Chemical characterization and evaluation of the antibacterial activity of essential oils from Fibre-type Cannabis sativa L. (Hemp). Molecules 2019, 24, 2302. [Google Scholar] [CrossRef] [Green Version]
- Mechoulam, R.; Hanuš, L.O.; Pertwee, R.; Howlett, A.C. Early phytocannabinoid chemistry to endo-cannabinoids and beyond. Nat. Rev. Neurosci. 2014, 15, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Corsi, L.; Pellati, F.; Brighenti, V.; Plessi, N.; Benvenuti, S. Chemical composition and in vitro neuroprotective activity of fibre-type Cannabis sativa L. (hemp). Curr. Bioact. Compd. 2019, 15, 201–210. [Google Scholar] [CrossRef]
- Russo, E.; Guy, G.W. A tale of two cannabinoids: The therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med. Hypotheses 2006, 66, 234–246. [Google Scholar] [CrossRef]
- FDA Approves Liquid Marijuana for AIDS and Cancer Patients. Available online: https://hightimes.com/news/fda-approves-liquid-marijuana-for-aids-and-cancer-patients/ (accessed on 28 July 2016).
- Casiraghi, A.; Roda, G.; Casagni, E.; Cristina, C.; Musazzi, U.M.; Franzè, S.; Rocco, P.; Giuliani, C.; Fico, G.; Minghetti, P.; et al. Extraction Method and Analysis of Cannabinoids in Cannabis Olive Oil Preparations. Planta Med. 2018, 84, 242–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, E.B.; Marcu, J. Cannabis pharmacology: The usual suspects and a few promising leads. Adv. Pharmacol. 2017, 80, 67–134. [Google Scholar]
- Russo, E.B. Taming THC: Potential Cannabis Synergy and Phytocannabinoid-Terpenoid Entourage Effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- Navari, R.M. Management of chemotherapy-induced nausea and vomiting: Focus on newer agents and new uses for older agents. Drugs 2013, 73, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; Mcnicol, E.; Baron, R.; Dworkin, R.H.; Gilron, L.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef] [Green Version]
- Leussink, V.I.; Husseini, L.; Warnke, C.; Broussalis, E.; Hartung, H.P.; Kieseier, B.C. Symptomatic therapy in multiple sclerosis: The role of cannabinoids in treating spasticity. Adv. Neurol. Disord. 2012, 5, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Haney, M.; Gunderson, E.W.; Rabkin, J.; Hart, C.L.; Vosburg, S.K.; Comer, S.D.; Foltin, R.W. Dronabinol and marijuana in HIV-positive marijuana smokers. Caloric intake, mood, and sleep. J. Acquir. Immune Defic. Syndr. 2007, 5, 545–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramella, A.; Roda, G.; Pavlovic, R.; Cas, M.D.; Casagni, E.; Mosconi, G.; Cecati, F.; Minghetti, P.; Grizzetti, C. Impact of Lipid Sources on Quality Traits of Medical Cannabis-Based Oil Preparations. Molecules 2020, 25, 2986. [Google Scholar] [CrossRef]
- Palmieri, B.; Laurino, C.; Vadalà, M. Spontaneous, anecdotal, retrospective, open-label study on the efficacy, safety and tolerability of Cannabis galenical preparations (Bedrocan). Int. J. Pharm. Pract. 2019, 27, 264–270. [Google Scholar] [CrossRef] [Green Version]
- Calvi, L.; Pavlovic, R.; Panseri, S.; Giupponi, L.; Leoni, V.; Giorgi, A. Quality Traits of Medical Cannabis sativa L. Inflorescences and Derived Products Based on Comprehensive Mass-Spectrometry Analytical Investigation. In Recent Advances in Cannabinoid Research; Costain, W., Laprairie, R., Eds.; IntechOpen: London, UK, 2018; Available online: https://www.intechopen.com/chapters/63068 (accessed on 5 November 2018).
- Pavlovic, R.; Nenna, G.; Calvi, L.; Panseri, S.; Borgonovo, G.; Giupponi, L.; Cannazza, G.; Giorgi, A. Quality traits of “cannabidiol oils”: Cannabinoids content, terpene fingerprint and oxidation stability of European commercially available preparations. Molecules 2018, 23, 1230. [Google Scholar] [CrossRef] [Green Version]
- Decreto 9 Novembre 2015: Funzioni di Organismo Statale per la Cannabis Previsto Dagli Articoli 23 e 28 Della Convenzione Unica Sugli Stupefacenti del 1961, Come Modificata nel 1972. Available online: http://www.gazzettaufficiale.it/eli/id/2015/11/30/15A08888/sg;jsessionid=p1rnwNujUKlqQ5azhA%20Q95A__.ntc-as3-guri2a (accessed on 1 May 2020).
- Carcieri, C.; Tomasello, C.; Simiele, M.; De Nicolò, A.; Avataneo, V.; Canzoneri, L.; Cusato, J.; Di Perri, G.; D’Avolio, A. Cannabinoids concentration variability in cannabis olive oil galenic preparations. J. Pharm. Pharmacol. 2018, 70, 143–149. [Google Scholar] [CrossRef]
- Pacifici, R.; Marchei, E.; Salvatore, F.; Guandalini, L.; Busardò, F.P.; Pichini, S. Evaluation of cannabinoids concentration and stability in standardized preparations of cannabis tea and cannabis oil by ultra-high performance liquid chromatography tandem mass spectrometry. Clin. Chem. Lab. Med. 2017, 55, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, R.; Marchei, E.; Salvatore, F.; Guandalini, L.; Busardò, F.P.; Pichini, S. Evaluation of long-term stability of cannabinoids in standardized preparations of cannabis flowering tops and cannabis oil by ultra-high-performance liquid chromatography tandem mass spectrometry. Clin. Chem. Lab. Med. 2018, 28, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Citti, C.; Ciccarella, G.; Braghiroli, D.; Parenti, C.; Vandelli, M.A.; Cannazza, G. Medicinal cannabis: Principal cannabinoids concentration and their stability evaluated by a high performance liquid chromatography coupled to diode array and quadrupole time of flight mass spectrometry method. J. Pharm. Biomed. Anal. 2016, 128, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Calvi, L.; Pentimalli, D.; Panseri, S.; Giupponi, L.; Gelmini, F.; Beretta, G.; Vitali, D.; Bruno, M.; Zilio, E.; Pavlovic, R.; et al. Comprehensive quality evaluation of medical Cannabis sativa L. inflorescence and macerated oils based on HS-SPME coupled to GC–MS and LC-HRMS (q-exactive orbitrap®) approach. J. Pharm. Biomed. Anal. 2018, 150, 208–219. [Google Scholar] [CrossRef]
- Romano, L.; Hazekamp, A. An overview of galenic preparation methods for medicinal cannabis. Curr. Bioact. Compd. 2019, 15, 174–195. [Google Scholar] [CrossRef]
- Romano, L.; Hazekamp, A. Cannabis oil: Chemical evaluation of an upcoming Cannabis-based medicine. Cannabinoids 2013, 1, 1–11. [Google Scholar]
- Estratto Oleoso di Infiorescenze Femminili di Cannabis. Available online: https://www.sifap.org/procedure/estrazione-oleosa-di-infiorescenze-femminili-di-cannabi (accessed on 19 September 2021).
- Storm, C.; Zumwalt, M.; Macherone, A. Agilent Note Cannabis and Hemp Testing. Dedicated Cannabinoid Potency Testing in Cannabis or Hemp Products Using the Agilent 1220 Infinity II LC System. Available online: https://www.agilent.com/cs/library/applications/application-dedicated-cannabinoid-potency-testing-5991-9285-en-us-agilent.pdf (accessed on 22 May 2020).
- Aiello, A.; Pizzolongo, F.; Scognamiglio, G.; Romano, A.; Masi, P.; Romano, R. Effects of supercritical and liquid carbon dioxide extraction on hemp (Cannabis sativa L.) seed oil. Int. J. Food Sci. Technol. 2020, 55, 2472–2480. [Google Scholar] [CrossRef]
- Weston-Green, K.; Clunas, H.; Jimenez Naranjo, C. A Review of the Potential Use of Pinene and Linalool as Terpene-Based Medicines for Brain Health: Discovering Novel Therapeutics in the Flavours and Fragrances of Cannabis. Front. Psychiatry 2021, 12, 583211. [Google Scholar] [CrossRef]
- Pamplona, F.A.; da Silva, L.R.; Coan, A.C. Potential Clinical Benefits of CBD-Rich Extracts Over Purified CBD in Treatment-Resistant Epilepsy: Observational Data Meta-Analysis. Front. Neurol. 2018, 9, 759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilbrey, J.A.; Ortiz, Y.T.; Felix, J.S.; McMahon, L.R.; Wilkerson, J.L. Evaluation of the Terpenes-Caryophyllene, -Terpineol, and -Terpinene in the Mouse Chronic Constriction Injury Model of Neuropathic Pain: Possible Cannabinoid Receptor Involvement. Psychopharmacology 2021, 239, 1475–1486. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Takada, T.; Yamamura, Y.; Adachi, I.; Suzuki, H.; Kawakami, J. Inhibitory Effects of Terpenoids on Multidrug Resistance-Associated Protein 2- and Breast Cancer Resistance Protein-Mediated Transport. Drug Metab. Dispos. 2008, 36, 1206–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, S.; Schaefer, U.F.; Doebler, L.; Reichling, J. Cooperative Interaction of Monoterpenes and Phenylpropanoids on the in Vitro Human Skin Permeation of Complex Composed Essential Oils. Planta Med. 2009, 75, 1381–1385. [Google Scholar] [CrossRef]
- Yamane, M.A.; Williams, A.C.; Barry, B.W. Terpene Penetration Enhancers in Propylene Glycol/water Co-Solvent Systems: Effectiveness and Mechanism of Action. J. Pharm. Pharmacol. 1995, 47, 978–989. [Google Scholar] [CrossRef]
- Şenyiğit, T.; Padula, C.; Özer, Ö.; Santi, P. Different Approaches for Improving Skin Accumulation of Topical Corticosteroids. Int. J. Pharm. 2009, 380, 155–160. [Google Scholar] [CrossRef]
- Furuishi, T.; Kato, Y.; Fukami, T.; Suzuki, T.; Endo, T.; Nagase, H.; Ueda, H.; Tomono, K. Effect of Terpenes on the Skin Permeation of Lomerizine Dihydrochloride. J. Pharm. Pharm. Sci. 2013, 16, 551–563. [Google Scholar] [CrossRef] [Green Version]
- Femenía-Font, A.; Balaguer-Fernández, C.; Merino, V.; Rodilla, V.; López-Castellano, A. Effect of Chemical Enhancers on the in Vitro Percutaneous Absorption of Sumatriptan Succinate. Eur. J. Pharm. Biopharm. 2005, 61, 50–55. [Google Scholar] [CrossRef]
- Guo, X.; Rong, Y.; Zhang, L.; Ye, J.C. Enhancing Effect of Chiral Enhancer Linalool on Skin Permeation of Naproxen. Acta Acad. Med. Sin. 2016, 38, 55–61. [Google Scholar]
- Sexton, M.; Shelton, K.; Haley, P.; West, M. Evaluation of Cannabinoid and Terpenoid Content: Cannabis Flower Compared to Supercritical CO2 Concentrate. Planta Med. 2018, 84, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Pang, M.H.; Kim, Y.; Jung, K.W.; Cho, S.; Lee, D.H. A Series of Case Studies: Practical Methodology for Identifying Antinociceptive Multi-Target Drugs. Drug Discov. 2012, 17, 425–434. [Google Scholar] [CrossRef] [PubMed]



| Times (min) | 0.1% (v/v) Formic Acid Aqueous Phase | 0.05% (v/v) Formic Acid in Methanol |
|---|---|---|
| 0 | 40 | 60 |
| 1.0 | 40 | 60 |
| 7.0 | 23 | 77 |
| 8.2 | 5 | 95 |
| 9.5 | 40 | 60 |
| 11.0 | 40 | 60 |
| Compound | Plant Material (g) in Solvent Volume (mL) * | Bedrocan (mg/mL) (Mean ± S.D.) | FM2 (mg/mL) (Mean ± S.D.) | Pedanios (mg/mL) (Mean ± S.D.) | ||
|---|---|---|---|---|---|---|
| TGE | TGE-PE | TGE | TGE-PE | TGE | ||
| THC | 5:50 | 21.084 ± 0.066 | 23.940 ± 2.310 | 7.811 ± 1.640 | 6.686 ± 0.810 | 20.784 ± 0.701 |
| 10:100 | 21.704 ± 2.170 | 23.215 ± 0.380 | 7.107 ± 0.535 | 6.894 ± 0.960 | 19.584 ± 1.420 | |
| 15:150 | 22.259 ± 1.420 | 23.986 ± 0.900 | 7.223 ± 0.880 | 7.758 ± 0.590 | 19.590 ± 0.481 | |
| THCA | 5:50 | 1.000 ± 0.416 | 1.126 ± 0.470 | 0.172 ± 0.170 | N.Q. | 0.548 ± 0.434 |
| 10:100 | 1.526 ± 1.130 | 1.555 ± 0.120 | 0.218 ± 0.380 | N.Q. | 2.521 ± 0.130 | |
| 15:150 | 1.140 ± 0.323 | 0.254 ± 0.260 | N.Q. | N.Q. | 2.012 ± 1.460 | |
| CBD | 5:50 | N.Q. | N.Q. | 8.093 ± 1.480 | 12.126 ± 0.430 | N.Q. |
| 10:100 | N.Q. | N.Q. | 10.712 ± 0.740 | 11.847 ± 0.830 | N.Q. | |
| 15:150 | N.Q. | N.Q. | 12.424 ± 0.280 | 13.217 ± 0.830 | N.Q. | |
| CBDA | 5:50 | N.Q. | N.Q. | 1.386 ± 1.220 | N.Q. | N.Q. |
| 10:100 | N.Q. | N.Q. | 0.768 ± 1.350 | 0.685 ± 1.190 | N.Q. | |
| 15:150 | N.Q. | N.Q. | 0.396 ± 0.690 | N.Q. | N.Q. | |
| CBN | 5:50 | 0.150 ± 0.136 | 0.214 ± 0.370 | N.Q. | 0.264 ± 0.460 | 0.390 ± 0.265 |
| 10:100 | 0.169 ± 0.140 | 0.299 ± 0.520 | N.Q. | 0.485 ± 0.420 | 0.301 ± 0.065 | |
| 15:150 | 0.260 ± 0.133 | N.Q. | N.Q. | N.Q. | 0.298 ± 0.084 | |
| Compound | Bedrocan/Pedanios (mg/mL) (Mean ± S.D.) | FM2 (mg/mL) (Mean ± S.D.) |
|---|---|---|
| THC | 12.236 ± 3.31 | 5.06 ± 1.010 |
| THCA | <1.840 | N.Q. |
| CBD | N.Q. | 7.268 ± 1.840 |
| Compound | TGE (mg/mL) (Mean ± S.D.) | SIFAP (mg/mL) (Mean ± S.D.) |
|---|---|---|
| THC | 23.037 ± 1.956 | 16.719 ± 1.330 |
| THCA | 1.263 ± 0.871 | 1.711 ± 0.504 |
| CBD | N.Q. | N.Q. |
| CBDA | N.Q. | N.Q. |
| CBN | 0.376 ± 0.342 | 0.320 ± 0.231 |
| Terpenes | TGE (mg/Kg) | TGE-PE (mg/Kg) |
|---|---|---|
| α-copaene | 9.14 | 18.82 |
| nerolidol | 17.90 | 25.64 |
| cis-geraniol | 20.96 | 37.83 |
| ylangene | 25.63 | 45.67 |
| α-bergamotene | 29.26 | 42.60 |
| τ-gurjunene | 30.95 | 48.00 |
| borneol | 38.54 | 48.16 |
| iso-caryophyllene | 40.09 | 59.06 |
| α-gurjunene | 41.08 | 48.0 |
| α-selinene | 47.23 | 72.70 |
| eremophilene | 49.04 | 104.72 |
| trans-3-caren-2-ol | 49.53 | 83.68 |
| α-farnesene | 55.27 | 47.43 |
| β-farnesene | 66.83 | 728.77 |
| β-selinene | 69.90 | 139.93 |
| τ-selinene | 71.88 | 72.70 |
| fenchol | 85.03 | 108.87 |
| β-phellandrene | 85.86 | 112.17 |
| 2-pinanol | 105.75 | 193.08 |
| p-mentha-1,3,8-triene | 106.46 | 199.73 |
| β-trans-ocimene | 112.94 | 111.49 |
| aromadendrene | 118.34 | 249.64 |
| α-thujene | 129.13 | 129.14 |
| δ-guaiene | 245.52 | 374.41 |
| τ-terpinene | 271.95 | 299.21 |
| α-pinene | 307.47 | 371.09 |
| 3-carene | 308.99 | 313.79 |
| selina-3,7-diene | 350.59 | 532.26 |
| α-humulene | 354.81 | 677.37 |
| β-pinene | 373.57 | 439.82 |
| guaia-3,7-diene | 377.59 | 560.34 |
| cis-carveol | 393.69 | 557.70 |
| α-terpinene | 402.40 | 371.84 |
| myrtenol | 418.47 | 460.76 |
| α-phellandrene | 443.89 | 482.53 |
| p-cymene | 501.84 | 493.83 |
| linalol | 595.96 | 689.23 |
| p-cymen-8-ol | 738.33 | 1421.52 |
| α-terpineol | 1109.98 | 1507.06 |
| caryophillene | 1224.36 | 1762.26 |
| limonene | 1358.72 | 1431.70 |
| β-cis-ocimene | 3650.65 | 3821.75 |
| β-myrcene | 5144.46 | 5457.31 |
| α-terpinolene | 8544.46 | 9038.39 |
| cis-p-menth-2,8-dienol | N.Q. | 71.71 |
| α-limonene dieposside | N.Q. | 82.25 |
| α-guaiene | N.Q. | 109.64 |
| 5-caranol | N.Q. | 117.39 |
| Total Concentration | 28,524.44 | 33,945.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bongiorno, P.; Lopalco, A.; Casiraghi, A.; Spennacchio, A.; Pitruzzella, A.; Lopedota, A.A.; Minghetti, P.; Denora, N. Digital Technologies Applied to Control the One-Step Process of Cannabis Olive Oil Preparations. Pharmaceutics 2023, 15, 870. https://doi.org/10.3390/pharmaceutics15030870
Bongiorno P, Lopalco A, Casiraghi A, Spennacchio A, Pitruzzella A, Lopedota AA, Minghetti P, Denora N. Digital Technologies Applied to Control the One-Step Process of Cannabis Olive Oil Preparations. Pharmaceutics. 2023; 15(3):870. https://doi.org/10.3390/pharmaceutics15030870
Chicago/Turabian StyleBongiorno, Paolo, Antonio Lopalco, Antonella Casiraghi, Antonio Spennacchio, Alessandro Pitruzzella, Angela Assunta Lopedota, Paola Minghetti, and Nunzio Denora. 2023. "Digital Technologies Applied to Control the One-Step Process of Cannabis Olive Oil Preparations" Pharmaceutics 15, no. 3: 870. https://doi.org/10.3390/pharmaceutics15030870
APA StyleBongiorno, P., Lopalco, A., Casiraghi, A., Spennacchio, A., Pitruzzella, A., Lopedota, A. A., Minghetti, P., & Denora, N. (2023). Digital Technologies Applied to Control the One-Step Process of Cannabis Olive Oil Preparations. Pharmaceutics, 15(3), 870. https://doi.org/10.3390/pharmaceutics15030870










