Development of Dipeptide N–acetyl–L–cysteine Loaded Nanostructured Carriers Based on Inorganic Layered Hydroxides
Abstract
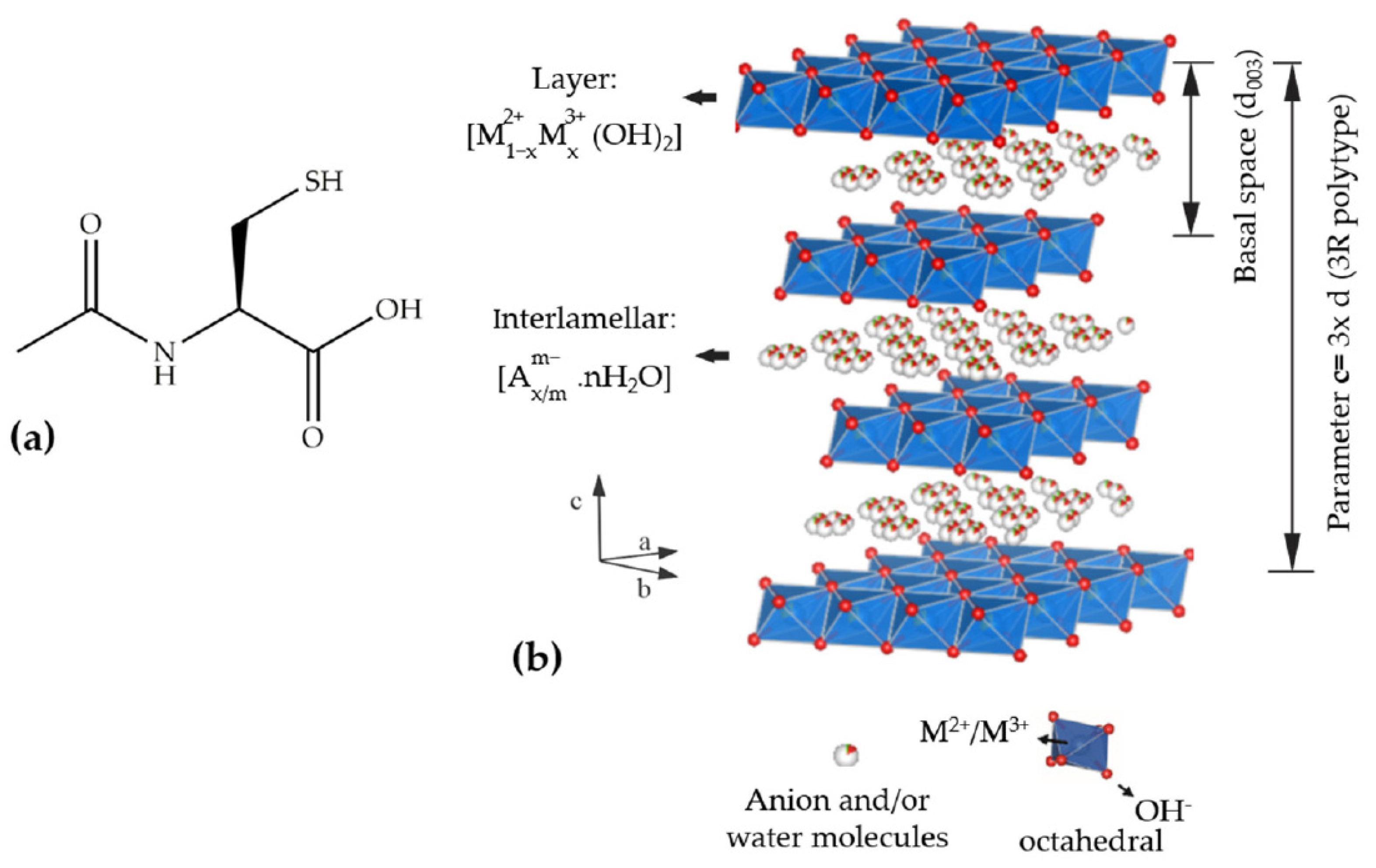
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis of Layered Double Hydroxides
2.3. Preparation of NAC Salts in the Sodium Form
2.4. In Vitro NAC Release Kinetics Experiments
2.5. Equipment
2.6. X-ray Diffraction Data Processing
2.7. Simulation of NAC Vibrational Spectrum
3. Results and Discussion
3.1. XRD, SEM, and Vibrational Spectroscopic Characterization of Zn2Al–NAC Sample
3.2. Vibrational Spectroscopic Characterization of Zn2Al–NAC and NAC Salt Samples
3.3. 13C–NMR Characterization of Zn2Al–NAC55 Sample
3.4. Thermal Analysis Data of Zn2Al-NAC55 Sample
3.5. Composition of Zn2Al–NAC55 Sample
3.6. Characterization of Mg2Al–NAC Sample
3.7. In Vitro NAC Release Kinectics Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rind, L.; Ahmad, M.; Khan, M.I.; Badruddeen; Akhtar, J.; Ahmad, U.; Yadav, C.; Owais, M. An Insight on Safety, Efficacy, and Molecular Docking Study Reports of N-Acetylcysteine and Its Compound Formulations. J. Basic Clin. Physiol. Pharmacol. 2022, 33, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Hurst, G.A.; Shaw, P.B.; LeMaistre, C.A. Laboratory and Clinical Evaluation of the Mucolytic Properties of Acetylcysteine 1, 2. Am. Rev. Respir. Dis. 1967, 96, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Prescott, L.F.; Ballantyne, A.; Proudfoot, A.T.; Park, J.; Adriaenssens, P. Treatment of Paracetamol (Acetaminophen) Poisoning with N-Acetylcysteine. Lancet 1977, 310, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Bateman, D.N. Large Paracetamol Overdose—Higher Dose Acetylcysteine Is Required. Br. J. Clin. Pharmacol. 2023, 89, 34–38. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Hassan, M.H.; Saleem, T.H.; Mohamed, S.A.; El-Zeftawy, M.; Ahmed, E.A.; Mostafa, N.A.M.; Hetta, H.F.; Hasan, A.S.; Abdallah, A.A.M. KIM-1 and GADDI-153 Gene Expression in Paracetamol-Induced Acute Kidney Injury: Effects of N-Acetylcysteine, N-Acetylmethionine, and N-Acetylglucosamine. Turk. J. Biochem. 2022, 47, 409–416. [Google Scholar] [CrossRef]
- Moreira, P.I.; Harris, P.L.R.; Zhu, X.; Santos, M.S.; Oliveira, C.R.; Smith, M.A.; Perry, G. Lipoic Acid and N-Acetyl Cysteine Decrease Mitochondrial-Related Oxidative Stress in Alzheimer Disease Patient Fibroblasts. J. Alzheimer’s Dis. 2007, 12, 195–206. [Google Scholar] [CrossRef]
- Lasram, M.M.; Dhouib, I.B.; Annabi, A.; El Fazaa, S.; Gharbi, N. A Review on the Possible Molecular Mechanism of Action of N-Acetylcysteine against Insulin Resistance and Type-2 Diabetes Development. Clin. Biochem. 2015, 48, 1200–1208. [Google Scholar] [CrossRef]
- Ma, F.; Li, H.; Huo, H.; Han, Q.; Liao, J.; Zhang, H.; Li, Y.; Pan, J.; Hu, L.; Guo, J.; et al. N–Acetyl–L–Cysteine Alleviates FUNDC1-Mediated Mitophagy by Regulating Mitochondrial Dynamics in Type 1 Diabetic Nephropathy Canine. Life Sci. 2023, 313, 121278. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ma, H.; Hou, X.; Song, L.; Feng, M. N–acetyl–L–cysteine Ameliorates Gestational Diabetes Mellitus by Inhibiting Oxidative Stress. Biotechnol. Appl. Biochem. 2022, 13, 938680. [Google Scholar] [CrossRef]
- Dean, O.; Giorlando, F.; Berk, M. N-Acetylcysteine in Psychiatry: Current Therapeutic Evidence and Potential Mechanisms of Action. J. Psychiatry Neurosci. 2011, 36, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Banaclocha, M. N-AcetyL–Cysteine: Modulating the Cysteine Redox Proteome in Neurodegenerative Diseases. Antioxidants 2022, 11, 416. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Wu, T.; Yang, S.; Chang, J.T. Effects of NAC and Gallic Acid on the Proliferation Inhibition and Induced Death of Lung Cancer Cells with Different Antioxidant Capacities. Molecules 2021, 27, 75. [Google Scholar] [CrossRef]
- Safe, I.P.; Lacerda, M.V.G.; Printes, V.S.; Praia Marins, A.F.; Rebelo Rabelo, A.L.; Costa, A.A.; Tavares, M.A.; Jesus, J.S.; Souza, A.B.; Beraldi-Magalhães, F.; et al. Safety and Efficacy of N-Acetylcysteine in Hospitalized Patients with HIV-Associated Tuberculosis: An Open-Label, Randomized, Phase II Trial (RIPENACTB Study). PLoS ONE 2020, 15, e0235381. [Google Scholar] [CrossRef] [PubMed]
- Pontremoli, C.; Boffito, M.; Laurano, R.; Iviglia, G.; Torre, E.; Cassinelli, C.; Morra, M.; Ciardelli, G.; Vitale-Brovarone, C.; Fiorilli, S. Mesoporous Bioactive Glasses Incorporated into an Injectable Thermosensitive Hydrogel for Sustained Co-Release of Sr2+ Ions and N-Acetylcysteine. Pharmaceutics 2022, 14, 1890. [Google Scholar] [CrossRef] [PubMed]
- Bourne, L.E.; Patel, J.J.; Davies, B.K.; Neven, E.; Verhulst, A.; D’Haese, P.C.; Wheeler-Jones, C.P.D.; Orriss, I.R. N-acetylcysteine (NAC) Differentially Affects Arterial Medial Calcification and Bone Formation: The Role of l -cysteine and Hydrogen Sulphide. J. Cell. Physiol. 2022, 237, 1070–1086. [Google Scholar] [CrossRef] [PubMed]
- De Flora, S.; Balansky, R.; La Maestra, S. Rationale for the Use of N-Acetylcysteine in Both Prevention and Adjuvant Therapy of COVID-19. FASEB J. 2020, 34, 13185–13193. [Google Scholar] [CrossRef]
- Andreou, A.; Trantza, S.; Filippou, D.; Filippou, D.; Sipsas, N.; Tsiodras, S. COVID-19: The Potential Role of Copper and N-Acetylcysteine (NAC) in a Combination of Candidate Antiviral Treatments against SARS-CoV-2. Vivo (Brooklyn) 2020, 34, 1567–1588. [Google Scholar] [CrossRef]
- Marco, F.D.I.; Foti, G.; Corsico, A.G. Where Are We with the Use of N-Acetylcysteine as a Preventive and Adjuvant Treatment for COVID-19 ? Eur. Rev. Med. Pharm. Sci. 2022, 26, 715–721. [Google Scholar] [CrossRef]
- Micheletto, C.; Izquierdo, J.L.; Avdeev, S.N.; Escobar, R.A.R. N-Acetylcysteine as a Therapeutic Approach to Post-COVID-19 Pulmonary Fibrosis Adjunctive Treatment. Eur. Rev. Med. Pharm. Sci. 2022, 26, 4872–4880. [Google Scholar] [CrossRef]
- Sadowska, A.M.; Manuel-y-Keenoy, B.; De Backer, W.A. Antioxidant and Anti-Inflammatory Efficacy of NAC in the Treatment of COPD: Discordant in Vitro and in Vivo Dose-Effects: A Review. Pulm. Pharmacol. Ther. 2007, 20, 9–22. [Google Scholar] [CrossRef]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The Chemistry and Biological Activities of N-Acetylcysteine. Biochim. Biophys. Acta 2013, 1830, 4117–4129. [Google Scholar] [CrossRef]
- Rushworth, G.F.; Megson, I.L. Existing and Potential Therapeutic Uses for N-Acetylcysteine: The Need for Conversion to Intracellular Glutathione for Antioxidant Benefits. Pharmacol. Ther. 2014, 141, 150–159. [Google Scholar] [CrossRef]
- Elbini Dhouib, I.; Jallouli, M.; Annabi, A.; Gharbi, N.; Elfazaa, S.; Lasram, M.M. A Minireview on N-Acetylcysteine: An Old Drug with New Approaches. Life Sci. 2016, 151, 359–363. [Google Scholar] [CrossRef]
- Whillier, S.; Raftos, J.E.; Chapman, B.; Kuchel, P.W. Role of N-Acetylcysteine and Cystine in Glutathione Synthesis in Human Erythrocytes. Redox Rep. 2009, 14, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Giustarini, D.; Milzani, A.; Dalle-Donne, I.; Tsikas, D.; Rossi, R. N-Acetylcysteine Ethyl Ester (NACET): A Novel Lipophilic Cell-Permeable Cysteine Derivative with an Unusual Pharmacokinetic Feature and Remarkable Antioxidant Potential. Biochem. Pharmacol. 2012, 84, 1522–1533. [Google Scholar] [CrossRef]
- Holdiness, M.R. Clinical Pharmacokinetics of N-Acetylcysteine. Clin. Pharmacokinet. 1991, 20, 123–134. [Google Scholar] [CrossRef]
- Papi, A.; Di Stefano, A.F.D.; Radicioni, M. Pharmacokinetics and Safety of Single and Multiple Doses of Oral N-Acetylcysteine in Healthy Chinese and Caucasian Volunteers: An Open-Label, Phase I Clinical Study. Adv. Ther. 2021, 38, 468–478. [Google Scholar] [CrossRef]
- Sigma Aldrich, Safety Data Sheet, N–Acetyl–L–Cysteine 2012. Available online: https://www.sigmaaldrich.com/PT/en/product/sial/a7250 (accessed on 15 March 2019).
- Sager, M.; Schneider, F.; Jedamzik, P.; Wiedmann, M.; Schremmer, E.; Koziolek, M.; Weitschies, W. Effect of Coadministered Water on the in Vivo Performance of Oral Formulations Containing N-Acetylcysteine: An in Vitro Approach Using the Dynamic Open Flow-Through Test Apparatus. Mol. Pharm. 2017, 14, 4272–4280. [Google Scholar] [CrossRef]
- Ennadi, A.; Legrouri, A.; De Roy, A.; Besse, J.P. X-ray Diffraction Pattern Simulation for Thermally Treated [Zn–Al–Cl] Layered Double Hydroxide. J. Solid State Chem. 2000, 152, 568–572. [Google Scholar] [CrossRef]
- Ameena Shirin, V.K.; Sankar, R.; Johnson, A.P.; Gangadharappa, H.V.; Pramod, K. Advanced Drug Delivery Applications of Layered Double Hydroxide. J. Control. Release 2021, 330, 398–426. [Google Scholar] [CrossRef]
- Wen, J.; Yang, K.; Huang, J.; Sun, S. Recent Advances in LDH-Based Nanosystems for Cancer Therapy. Mater. Des. 2021, 198, 109298. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered Double Hydroxides: A Brief Review from Fundamentals to Application as Evolving Biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- O’Hare, D. Inorganic Intercalation Compounds. In Inorganic Materials; DW Bruce, D.O., Ed.; John Wiley & Sons, Inc.: Oxford, UK, 1997; pp. 171–254. ISBN 978-0-471-96036-2. [Google Scholar]
- Evans, D.G.; Slade, R.C.T. Structural Aspects of Layered Double Hydroxides. In Layered Double Hydroxides; Structure and Bonding; Duan, X., Evans, D.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 119, pp. 1–87. ISBN 3-540-28279-3. [Google Scholar]
- Crepaldi, E.L.; Valim, J.B. Hidróxidos Duplos Lamelares: Síntese, Estrutura, Propriedades e Aplicações. Quim. Nova 1998, 21, 300–311. [Google Scholar] [CrossRef]
- Cao, Z.; Li, B.; Sun, L.; Li, L.; Xu, Z.P.; Gu, Z. 2D Layered Double Hydroxide Nanoparticles: Recent Progress toward Preclinical/Clinical Nanomedicine. Small Methods 2019, 4, 1900343. [Google Scholar] [CrossRef]
- Tarnawski, A.S.; Tomikawa, M.; Ohta, M.; Sarfeh, I.J. Antacid Talcid Activates in Gastric Mucosa Genes Encoding for EGF and Its Receptor. The Molecular Basis for Its Ulcer Healing Action. J. Physiol. 2000, 94, 93–98. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Hasanur, S.; Saha, S.; Chakraborty, M. Determination of Half Maximal Inhibitory Concentration of CaAl Layered Double Hydroxide on Cancer Cells and Its Role in the Apoptotic Pathway. Appl. Clay Sci. 2019, 168, 31–35. [Google Scholar] [CrossRef]
- Kang, H.; Kim, M.; Feng, Q.; Lin, S.; Wei, K.; Li, R.; Choi, C.J.; Kim, T.-H.; Li, G.; Oh, J.-M.; et al. Nanolayered Hybrid Mediates Synergistic Co-Delivery of Ligand and Ligation Activator for Inducing Stem Cell Differentiation and Tissue Healing. Biomaterials 2017, 149, 12–28. [Google Scholar] [CrossRef]
- Kang, H.R.; da Costa Fernandes, C.J.; da Silva, R.A.; Constantino, V.R.L.; Koh, I.H.J.; Zambuzzi, W.F. Mg–Al and Zn–Al Layered Double Hydroxides Promote Dynamic Expression of Marker Genes in Osteogenic Differentiation by Modulating Mitogen-Activated Protein Kinases. Adv. Healthc. Mater. 2018, 7, 1700693. [Google Scholar] [CrossRef]
- Cunha, V.R.R.; de Souza, R.B.; da Fonseca Martins, A.M.C.R.P.; Koh, I.H.J.; Constantino, V.R.L. Accessing the Biocompatibility of Layered Double Hydroxide by Intramuscular Implantation: Histological and Microcirculation Evaluation. Sci. Rep. 2016, 6, 30547. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, M.P.; Cunha, V.R.R.; Leroux, F.; Taviot-gueho, C.; Nakamae, M.N.; Kang, Y.R.; Souza, R.B.; Martins, A.M.C.R.P.F.; Koh, I.H.J.; Constantino, V.R.L. Iron-Based Layered Double Hydroxide Implants: Potential Drug Delivery Carriers with Tissue Biointegration Promotion and Blood Microcirculation Preservation. ACS Omega 2018, 3, 18263–18274. [Google Scholar] [CrossRef] [Green Version]
- Cunha, V.R.R.; Ferreira, A.M.D.C.; Constantino, V.R.L.; Tronto, J.; Valim, J.B. Layered Double Hydroxides: Inorganic Nanoparticles for Storage and Release of Species of Biological and Therapeutic Interest. Quim. Nova 2010, 33, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Constantino, V.R.L.; Cunha, V.R.; Rocha, M.A.; Figueiredo, M.P.; Magri, V.R.; Eulálio, D.; Perotti, G.F.; Bizeto, M.A.; Zambuzzi, W.F.; Koh, I.H.J. Layered Double Hydroxides: Characterization, Therapeutic Activities and Biocompatibility. In Progress in Layered Double Hydroxides: From Synthesis to New Applications; Nocchetti, M., Costantino, U., Eds.; Series on Chemistry, Energy and the Environment: Volume 8; World Scientific: Singapore, 2022; pp. 413–482. ISBN 978-981-12-4060-7. [Google Scholar]
- Gordijo, C.R.; Barbosa, C.A.S.; Da Costa Ferreira, A.M.; Constantino, V.R.L.; De Oliveira Silva, D. Immobilization of Ibuprofen and Copper-Ibuprofen Drugs on Layered Double Hydroxides. J. Pharm. Sci. 2005, 94, 1135–1148. [Google Scholar] [CrossRef]
- Rocha, M.A.; Petersen, P.A.D.; Teixeira-Neto, E.; Petrilli, H.M.H.M.; Leroux, F.; Taviot-Gueho, C.; Constantino, V.R.L.; La1, M.A.; Rocha, P.A.D.; Petersen, E.; et al. Yered Double Hydroxide and Sulindac Coiled and Scrolled Nanoassemblies for Storage and Drug Rel. RSC Adv. 2016, 6, 16419–16436. [Google Scholar] [CrossRef]
- Cunha, V.R.R.; Guilherme, V.A.; De Paula, E.; De Araujo, D.R.; Silva, R.O.; Medeiros, J.V.R.; Leite, J.R.S.A.; Petersen, P.A.D.; Foldvari, M.; Petrilli, H.M.; et al. Delivery System for Mefenamic Acid Based on the Nanocarrier Layered Double Hydroxide: Physicochemical Characterization and Evaluation of Anti-Inflammatory and Antinociceptive Potential. Mater. Sci. Eng. C 2016, 58, 629–638. [Google Scholar] [CrossRef] [Green Version]
- Cunha, V.R.R.; Petersen, P.A.D.; Souza, R.B.; Martins, A.M.C.R.P.F.; Leroux, F.; Taviot-Gueho, C.; Petrilli, H.M.; Koh, I.H.J.; Constantino, V.R.L. Phytochemical Species Intercalated into Layered Double Hydroxides: Structural Investigation and Biocompatibility Assays. New J. Chem. 2020, 44, 10011–10021. [Google Scholar] [CrossRef]
- Guilherme, V.A.; Cunha, V.R.R.; de Paula, E.; de Araujo, D.R.; Constantino, V.R.L. Anti-Inflammatory and Analgesic Evaluation of a Phytochemical Intercalated into Layered Double Hydroxide. Pharmaceutics 2022, 14, 934. [Google Scholar] [CrossRef]
- Rodrigues, L.A.D.S.; Figueiras, A.; Veiga, F.; de Freitas, R.M.; Nunes, L.C.C.; da Silva Filho, E.C.; da Silva Leite, C.M. The Systems Containing Clays and Clay Minerals from Modified Drug Release: A Review. Colloids Surf. B Biointerfaces 2013, 103, 642–651. [Google Scholar] [CrossRef]
- Perotti, G.F.; Silva, F.F.; de Couto, R.A.A.; Lima, F.C.D.A.; Petrilli, H.M.; Leroux, F.; Ferreira, A.M.C.; Constantino, V.R.L. Intercalation of Apocarotenoids from Annatto (Bixa orellana L.) into Layered Double Hydroxides. J. Braz. Chem. Soc. 2020, 31, 2211–2223. [Google Scholar] [CrossRef]
- Cherif, N.F.; Constantino, V.R.L.; Hamdaoui, O.; Leroux, F.; Taviot-Guého, C. New Insights on Two Intercalated Ciprofloxacin Arrangements into Layered Double Hydroxide Carrier Material. New J. Chem. 2020, 44, 10076–10086. [Google Scholar] [CrossRef]
- Gil, O.M.; Rocha, M.A.; Constantino, V.R.L.; Koh, I.H.J.; de Faria, D.L.A. Modified Drug Release System Based on Sulindac and Layered Double Hydroxide: An in Vivo Raman Investigation. Vib. Spectrosc. 2016, 87, 60–66. [Google Scholar] [CrossRef]
- Figueiredo, M.P.; Layrac, G.; Hébraud, A.; Limousy, L.; Brendle, J.; Schlatter, G.; Constantino, V.R.L. Design of 3D Multi-Layered Electrospun Membranes Embedding Iron-Based Layered Double Hydroxide for Drug Storage and Control of Sustained Release. Eur. Polym. J. 2020, 131, 109675. [Google Scholar] [CrossRef]
- Rojas, R.; Mosconi, G.; Pablo, J.; Gil, G.A. Layered Double Hydroxide Applications in Biomedical Implants. Appl. Clay Sci. 2022, 224, 106514. [Google Scholar] [CrossRef]
- Wei, M.; Shi, Z.; Evans, D.G.; Duan, X. Study on the Intercalation and Interlayer Oxidation Transformation of L–Cysteine in a Confined Region of Layered Double Hydroxides. J. Mater. Chem. 2006, 16, 2102. [Google Scholar] [CrossRef]
- Stimpfling, T.; Vialat, P.; Hintze-Bruening, H.; Keil, P.; Shkirskiy, V.; Volovitch, P.; Ogle, K.; Leroux, F. Amino Acid Interleaved Layered Double Hydroxides as Promising Hybrid Materials for AA2024 Corrosion Inhibition. Eur. J. Inorg. Chem. 2016, 2016, 2006–2016. [Google Scholar] [CrossRef]
- Silva, E.P. Estudo Espectroscópico Da Intercalação de Aminoácidos Em Hidróxidos Duplos Lamelares: Perspectivas Astrobiológicas. Master’s Dissertation, Universidade de São Paulo, São Paulo, Brazil, 2017. [Google Scholar]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- United States Pharmacopeial Convention 〈711〉DISSOLUTION. In The United States Pharmacopeia. 37th Revision: The National Formulary, 32nd ed.; United States Pharmacopeial Convention (Ed.) United States Pharmacopeial Convention: Rockville, MD, USA, 2013; ISBN 3769261208. [Google Scholar]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic Modeling on Drug Release from Controlled Drug Delivery Systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar] [PubMed]
- Bhaskar, R.; Murthy, R.S.R.; Miglani, B.D.; Viswanathan, K. Novel Method to Evaluate Diffusion Controlled Release of Drug from Resinate. Int. J. Pharm. 1986, 28, 59–66. [Google Scholar] [CrossRef]
- Pongjanyakul, T.; Priprem, A.; Chitropas, P.; Puttipipatkhachorn, S. Effect of Polysulfonate Resins and Direct Compression Fillers on Multiple-Unit Sustained-Release Dextromethorphan Resinate Tablets. AAPS PharmSciTech 2005, 6, E190–E197. [Google Scholar] [CrossRef] [Green Version]
- Mourão, S.C.; da Silva, C.; Bresolin, T.M.B.; Serra, C.H.R.; Porta, V. Dissolution Parameters for Sodium Diclofenac-Containing Hypromellose Matrix Tablet. Int. J. Pharm. 2010, 386, 201–207. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. Commission on Powder Diffraction(IUCr). Newsletter 2001, 26, 12–19. [Google Scholar]
- Roisnel, T.; Rodriguez-Carvajal, J. WinPLOTR: A Windows tool for powderdiffraction patterns analysis materials science forum. In Proceedings of the Seventh European Powder Diffraction Conference, Barcelona, Spain, 20–23 March 2000; Delhez, R., Mitemeijer, E.J., Eds.; Trans Tech Publications Inc.: Stafa-Zurich, Switzerland, 2001; pp. 118–123. [Google Scholar]
- Spartan 18, version 1.2.Ø; Wavefunction Inc.: Irvine, CA, USA.
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods II. Applications. J. Comput. Chem. 1989, 10, 221–264. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colic-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troutier-Thuilliez, A.-L.L.; Taviot-Guého, C.; Cellier, J.; Hintze-Bruening, H.; Leroux, F. Layered Particle-Based Polymer Composites for Coatings: Part I. Evaluation of Layered Double Hydroxides. Prog. Org. Coat. 2009, 64, 182–192. [Google Scholar] [CrossRef]
- Figueiredo, M.P.; Duarte, A.; Vendruscolo, V.; Thirouard, R.; Constantino, V.R.L.; Taviot-Guého, C. Investigation about Iron(III) Incorporation into Layered Double Hydroxides: Compositional and Structural Properties of Mg2FeyAl(1−y)(OH)6-Cl and Zn2FeyAl(1−y)(OH)6-Cl. J. Alloys Compd. 2021, 886, 161184. [Google Scholar] [CrossRef]
- Kumar, S.S.; Nangia, A. A New Conformational Polymorph of N–Acetyl–L–Cysteine. The Role of S–H⋯O and C–H⋯O Interactions. CrystEngComm 2013, 15, 6498. [Google Scholar] [CrossRef]
- Poopari, M.R.; Dezhahang, Z.; Yang, G.; Xu, Y. Conformational Distributions of N–Acetyl–L–Cysteine in Aqueous Solutions: A Combined Implicit and Explicit Solvation Treatment of VA and VCD Spectra. ChemPhysChem 2012, 13, 2310–2321. [Google Scholar] [CrossRef]
- Fazary, A.E.; Awwad, N.S.; Ibrahium, H.A.; Shati, A.A.; Alfaifi, M.Y.; Ju, Y.H. Protonation Equilibria of N-Acetylcysteine. ACS Omega 2020, 5, 19598–19605. [Google Scholar] [CrossRef]
- Cunha, V.R.R.; Petersen, P.A.D.; Gonçalves, M.B.; Petrilli, H.M.; Taviot-Gueho, C.; Leroux, F.; Temperini, M.L.A.; Constantino, V.R.L. Structural, Spectroscopic (NMR, IR, and Raman), and DFT Investigation of the Self-Assembled Nanostructure of Pravastatin-LDH (Layered Double Hydroxides) Systems. Chem. Mater. 2012, 24, 1415–1425. [Google Scholar] [CrossRef]
- Corbi, P.P.; Cagnin, F.; Massabni, A.C. Synthesis and Characterization of a Platinum (II) Complex with N–Acetyl–L–Cysteine. J. Coord. Chem. 2009, 62, 2764–2771. [Google Scholar] [CrossRef]
- Corbi, P.P.; Quintão, F.A.; Ferraresi, D.K.D.; Lustri, W.R.; Amaral, A.C.; Massabni, A.C. Chemical, Spectroscopic Characterization, and in Vitro Antibacterial Studies of a New Gold(I) Complex with N–Acetyl–L–Cysteine. J. Coord. Chem. 2010, 63, 1390–1397. [Google Scholar] [CrossRef]
- Abbehausen, C.; Heinrich, T.A.; Abrão, E.P.; Costa-Neto, C.M.; Lustri, W.R.; Formiga, A.L.B.; Corbi, P.P. Chemical, Spectroscopic Characterization, DFT Studies and Initial Pharmacological Assays of a Silver(I) Complex with N–Acetyl–L–Cysteine. Polyhedron 2011, 30, 579–583. [Google Scholar] [CrossRef]
- Adachi, Y.; Yoshikawa, Y.; Sakurai, H. Antidiabetic Zinc(II)-N–Acetyl–L–Cysteine Complex: Evaluations of in Vitro Insulinomimetic and in Vivo Blood Glucose-Lowering Activities. BioFactors 2007, 29, 213–223. [Google Scholar] [CrossRef]
- Benito, P.; Labajos, F.M.; Mafra, L.; Rocha, J.; Rives, V. Carboxylate-Intercalated Layered Double Hydroxides Aged under Microwave—Hydrothermal Treatment. J. Solid State Chem. 2009, 182, 18–26. [Google Scholar] [CrossRef]
- MacKenzie, K.J.D.; Smith, M.E. Multinuclear Solid-State Nuclear Magnetic Resonance of Inorganic Materials; Elsevier: Amsterdam, The Netherlands, 2002; Volume 6, ISBN 9780123744135. [Google Scholar]
- Olszówka, J.E.; Karcz, R.; Bielańska, E.; Kryściak-Czerwenka, J.; Napruszewska, B.D.; Sulikowski, B.; Socha, R.P.; Gaweł, A.; Bahranowski, K.; Olejniczak, Z.; et al. New Insight into the Preferred Valency of Interlayer Anions in Hydrotalcite-like Compounds: The Effect of Mg/Al Ratio. Appl. Clay Sci. 2018, 155, 84–94. [Google Scholar] [CrossRef]
- Pushparaj, S.S.C.; Forano, C.; Prevot, V.; Lipton, A.S.; Rees, G.J.; Hanna, J.V.; Nielsen, U.G. How the Method of Synthesis Governs the Local and Global Structure of Zinc Aluminum Layered Double Hydroxides. J. Phys. Chem. C 2015, 119, 27695–27707. [Google Scholar] [CrossRef]
- Weast, R.C. Handbook of Chemistry and Physics, 61st ed.; CRC Press: Boca Raton, FL, USA, 1973; ISBN 100878194541. [Google Scholar]
- Santoso, S.P.; Chandra, I.K.; Soetaredjo, F.E.; Angkawijaya, A.E. Equilibrium Studies of Complexes between N-Acetylcysteine and Divalent Metal Ions in Aqueous Solutions. J. Chem. Eng. Data 2014, 59, 1661–1666. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, H.; Hirami, S.; Tsuhako, M. Selective Adsorption of Mercury Ion by Mercaptocarboxylic Acid Intercalated Mg-Al Layered Double Hydroxide. J. Colloid Interface Sci. 2007, 315, 177–183. [Google Scholar] [CrossRef]
- Nakayama, H.; Hirami, S.; Tsuhako, M. Intercalation of Mercaptocarboxylic Acid into Layered Double Hydroxide Accompanied with Oxidation of Mercapto Group. Chem. Lett. 2004, 33, 712–713. [Google Scholar] [CrossRef]
- Li, C.; Han, X.; Hong, X.; Li, X.; Zhang, H.; Wang, Z.; Zheng, A. Study on the Complexation and Release Mechanism of Methylphenidate Hydrochloride Ion Exchange Resin Complex. Polymers 2021, 13, 4394. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Benício, L.P.F.; Eulálio, D.; Guimarães, L.D.M.; Pinto, F.G.; Costa, L.M.D.; Tronto, J. Layered Double Hydroxides as Hosting Matrices for Storage and Slow Release of Phosphate Analyzed by Stirred-Flow Method. Mater. Res. 2018, 21, e20171004. [Google Scholar] [CrossRef]
- Frost, R.L.; López, A.; Xi, Y.; Scholz, R. Vibrational Spectroscopic Characterization of the Phosphate Mineral Althausite Mg2(PO4)(OH, F, O)—Implications for the Molecular Structure. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 120, 252–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]












| Model | Mathematical Equation |
|---|---|
| Zero order | |
| First order | |
| Higuchi | |
| Hixson–Crowell | |
| Baskar |
| S1 Method | S2 Method | |||
|---|---|---|---|---|
| R2 | Equation | R2 | Equation | |
| Zero-order | 0.8815 * | y = −0.0028x + 0.8967 | 0.8648 * | y = −0.002x + 0.9601 |
| First-order | 0.9112 * | y = 0.014x − 2.1837 | 0.4522 * | y = 0.0253x − 3.3505 |
| Higuchi | 0.941 * | y = 2.5917x + 7.0461 | 0.9766 * | y = 2.0737x + 0.7088 |
| Hixson–Crowell | 0.9786 * | y = −0.0011x + 0.9629 | 0.8786 * | y = −0.0007x + 0.9866 |
| Baskar | 0.9651 * | y = 0.0164x + 0.0875 | 0.9595 * | y = 0.0112x + 0.0214 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eulálio, D.; Pires Figueiredo, M.; Taviot-Gueho, C.; Leroux, F.; dos Reis Serra, C.H.; Faria, D.L.A.d.; Constantino, V.R.L. Development of Dipeptide N–acetyl–L–cysteine Loaded Nanostructured Carriers Based on Inorganic Layered Hydroxides. Pharmaceutics 2023, 15, 955. https://doi.org/10.3390/pharmaceutics15030955
Eulálio D, Pires Figueiredo M, Taviot-Gueho C, Leroux F, dos Reis Serra CH, Faria DLAd, Constantino VRL. Development of Dipeptide N–acetyl–L–cysteine Loaded Nanostructured Carriers Based on Inorganic Layered Hydroxides. Pharmaceutics. 2023; 15(3):955. https://doi.org/10.3390/pharmaceutics15030955
Chicago/Turabian StyleEulálio, Denise, Mariana Pires Figueiredo, Christine Taviot-Gueho, Fabrice Leroux, Cristina Helena dos Reis Serra, Dalva Lúcia Araújo de Faria, and Vera Regina Leopoldo Constantino. 2023. "Development of Dipeptide N–acetyl–L–cysteine Loaded Nanostructured Carriers Based on Inorganic Layered Hydroxides" Pharmaceutics 15, no. 3: 955. https://doi.org/10.3390/pharmaceutics15030955
APA StyleEulálio, D., Pires Figueiredo, M., Taviot-Gueho, C., Leroux, F., dos Reis Serra, C. H., Faria, D. L. A. d., & Constantino, V. R. L. (2023). Development of Dipeptide N–acetyl–L–cysteine Loaded Nanostructured Carriers Based on Inorganic Layered Hydroxides. Pharmaceutics, 15(3), 955. https://doi.org/10.3390/pharmaceutics15030955








