Nanofibrous Scaffolds for Diabetic Wound Healing
Abstract
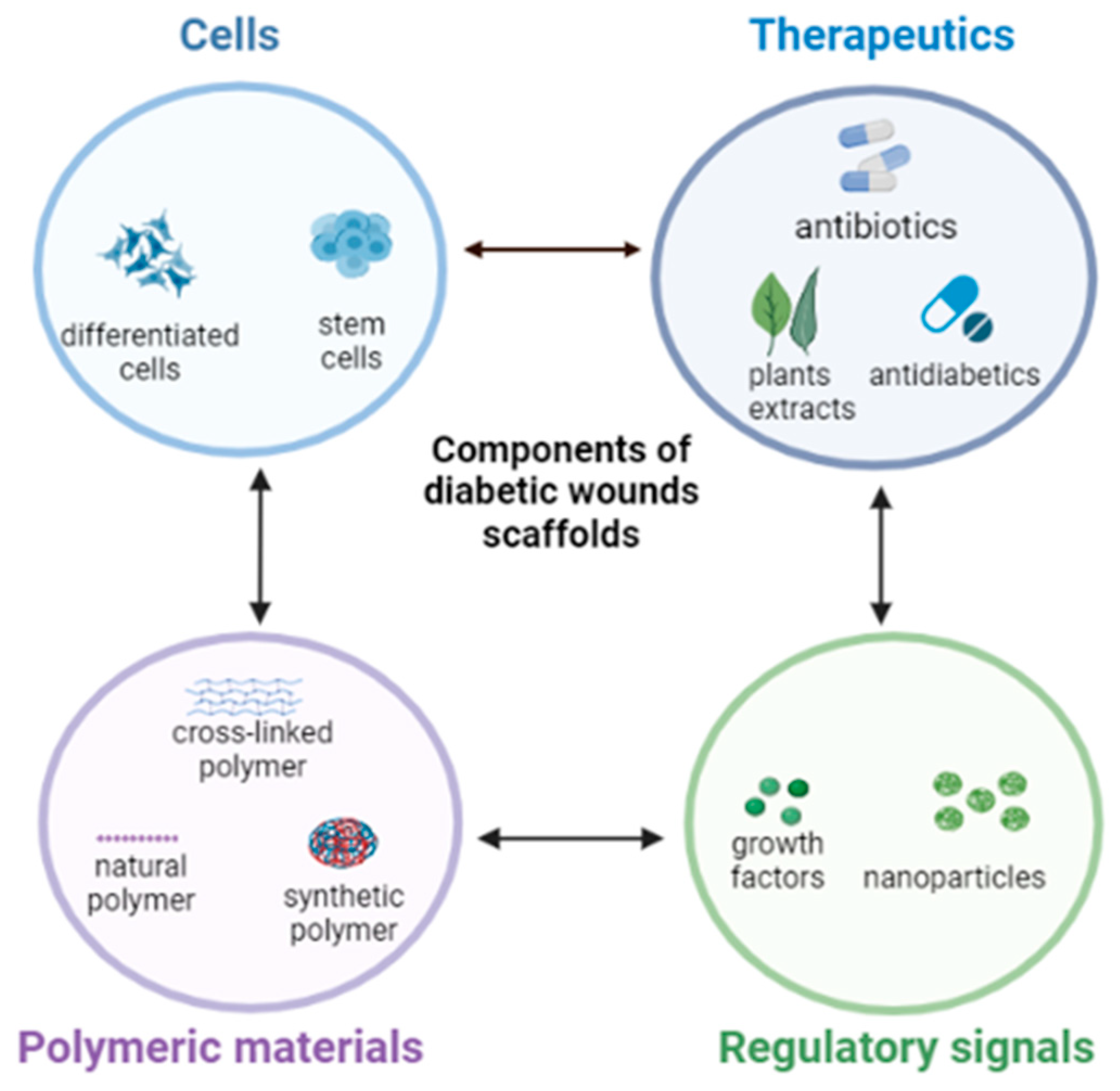
:1. Introduction
2. Conventional Approaches Employed for Treating Diabetic Wounds
3. Polymers Used in the Fabrication of Nanofibrous Scaffolds
3.1. Natural Polymers
3.1.1. Chitosan
3.1.2. Collagen and Gelatin
3.1.3. Hyaluronic Acid
3.1.4. Cellulose
3.1.5. Poly (Amino Acids)
3.1.6. Starch
3.2. Synthetic Polymers
3.2.1. Polycaprolactone
3.2.2. Polylactide Acid
3.2.3. Poly(lactide-co-glycolic) Acid
4. Commonly Employed Manufacturing Techniques
4.1. Electrospinning
4.2. Phase Separation
4.3. Self-Assembly
4.4. Melt Blowing
4.5. Templating System
5. Current Trends in the Development of Nanofibrous Scaffolds for Diabetic Wounds
5.1. Antibiotics
5.2. Herbs and Phytochemicals
5.3. Stem Cells
5.4. Growth Factors
5.5. Anti-Inflammatory and Antioxidants
5.6. Antidiabetic Agents
6. Conclusions and Future Viewpoints
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walicka, M.; Raczyńska, M.; Marcinkowska, K.; Lisicka, I.; Czaicki, A.; Wierzba, W.; Franek, E. Amputations of lower limb in subjects with diabetes mellitus: Reasons and 30-Day mortality. J. Diabetes Res. 2021, 2021, 8866126. [Google Scholar] [CrossRef]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 diabetes and its impact on the immune system. Curr. Diabetes Rev. 2020, 16, 442. [Google Scholar] [PubMed]
- Spampinato, S.F.; Caruso, G.I.; De Pasquale, R.; Sortino, M.A.; Merlo, S. The treatment of impaired wound healing in diabetes: Looking among old drugs. Pharmaceuticals 2020, 13, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, Y.; Gleason, C.E.; Tran, P.O.T.; Harmon, J.S.; Robertson, R.P. Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proc. Natl. Acad. Sci. USA 1999, 96, 10857–10862. [Google Scholar] [CrossRef] [Green Version]
- Rehman, K.; Akash, M.S.H. Mechanism of generation of oxidative stress and pathophysiology of type 2 diabetes mellitus: How are they interlinked? J. Cell. Biochem. 2017, 118, 3577–3585. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Kim, H.J.; Kim, D.; Yoon, H.; Choi, C.S.; Oh, Y.S.; Jun, H.S. Prevention of oxidative stress-induced pancreatic beta cell damage by Broussonetia kazinoki Siebold fruit extract via the ERK-Nox4 pathway. Antioxidants 2020, 9, 406. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108–615. [Google Scholar] [CrossRef]
- Ridiandries, A.; Tan, J.T.; Bursill, C.A. The role of chemokines in wound healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef] [Green Version]
- Luo, R.; Dai, J.; Zhang, J.; Li, Z. Accelerated skin wound healing by electrical stimulation. Adv. Healthc. Mater 2021, 10, e2100557. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hou, Q.; Zhong, L.; Zhao, Y.; Fu, X. Macrophage related chronic inflammation in non-healing wounds. Front. Immunol. 2021, 12, 2289. [Google Scholar] [CrossRef]
- Sheir, M.M.; Nasra, M.M.; Abdallah, O.Y. Phenytoin-loaded bioactive nanoparticles for the treatment of diabetic pressure ulcers: Formulation and in vitro/in vivo evaluation. Drug Deliv. Transl. Res. 2022, 12, 2936–2949. [Google Scholar] [CrossRef]
- Chen, H.; Truckenmüller, R.; Van Blitterswijk, C.; Moroni, L. Fabrication of nanofibrous scaffolds for tissue engineering applications. In Nanomaterials in Tissue Engineering; Woodhead Publishing: Sawston, UK, 2013; pp. 158–183. [Google Scholar]
- Gupta, K.C.; Haider, A.; Choi, Y.R.; Kang, I.K. Nanofibrous scaffolds in biomedical applications. Biomater. Res. 2014, 18, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagaich, U. Nanofibrous scaffold in tissue engineering. J. Adv. Pharm. Technol. Res. 2017, 8, 85. [Google Scholar] [PubMed]
- Liu, Y.; Li, C.; Feng, Z.; Han, B.; Yu, D.G.; Wang, K. Advances in the Preparation of Nanofiber Dressings by Electrospinning for Promoting Diabetic Wound Healing. Biomolecules 2022, 12, 1727. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, H.; Aramideh Khouy, R.; Nosrati, A.; Khodaei, M.; Banitalebi-Dehkordi, M.; Ashrafi-Dehkordi, K.; Sanami, S.; Alizadeh, Z. Nanocomposite scaffolds for accelerating chronic wound healing by enhancing angiogenesis. J. Nanobiotechnol. 2021, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Mavrogenis, A.F.; Megaloikonomos, P.D.; Antoniadou, T.; Igoumenou, V.G.; Panagopoulos, G.N.; Dimopoulos, L.; Moulakakis, K.G.; Sfyroeras, G.S.; Lazaris, A. Current concepts for the evaluation and management of diabetic foot ulcers. EFORT Open Rev. 2018, 3, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Hakkarainen, T.W.; Kopari, N.M.; Pham, T.N.; Evans, H.L. Necrotizing soft tissue infections: Review and current concepts in treatment, systems of care, and outcomes. Curr. Probl. Surg. 2014, 51, 344–362. [Google Scholar] [CrossRef] [Green Version]
- Everett, E.; Mathioudakis, N. Update on management of diabetic foot ulcers. Ann. N. Y. Acad. Sci. 2018, 1411, 153–165. [Google Scholar] [CrossRef]
- Manna, B.; Nahirniak, P.; Morrison, C.A. Wound Debridement—StatPearls—NCBI Bookshelf, Wound Debridement; StatPearls publishing LLC: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Kavitha, K.V. Choice of wound care in diabetic foot ulcer: A practical approach. World J. Diabetes 2014, 5, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.J.; Jang, I. Oxygen-Releasing Composites: A Promising Approach in the Management of Diabetic Foot Ulcers. Polymers 2021, 13, 4131. [Google Scholar] [CrossRef]
- Hilton, J.R.; Williams, D.T.; Beuker, B.; Miller, D.R.; Harding, K.G. Wound dressings in diabetic foot disease. Clin. Infect. Dis. 2004, 39 (Suppl. 2), S100–S103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of appropriate wound dressing for various wounds. Front. Bioeng. Biotechnol. 2020, 8, 182. [Google Scholar] [CrossRef] [Green Version]
- Baker, N.; Osman, I.S. The principles and practicalities of offloading diabetic foot ulcers. Diabet. Foot J. 2016, 19, 172–181. [Google Scholar]
- Namazi, H. Polymers in our daily life. BioImpacts BI 2017, 7, 73–74. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Bacakova, L.; Pajorova, J.; Zikmundova, M.; Filova, E.; Mikes, P.; Jencova, V.; Kostakova, E.K.; Sinica, A. Nanofibrous scaffolds for skin tissue engineering and wound healing based on nature-derived polymers. In Current and Future Aspects of Nanomedicine; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–30. [Google Scholar]
- Ahmed, R.; Tariq, M.; Ali, I.; Asghar, R.; Khanam, P.N.; Augustine, R.; Hasan, A. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int. J. Biol. Macromol. 2018, 120, 385–393. [Google Scholar] [CrossRef]
- Lee, C.H.; Liu, K.S.; Cheng, C.W.; Chan, E.C.; Hung, K.C.; Hsieh, M.J.; Chang, S.H.; Fu, X.; Juang, J.H.; Hsieh, I.C.; et al. Codelivery of sustainable antimicrobial agents and platelet-derived growth factor via biodegradable nanofibers for repair of diabetic infectious wounds. ACS Infect. Dis. 2020, 6, 2688–2697. [Google Scholar] [CrossRef]
- Merrell, J.G.; Mclaughlin, S.W.; Tie, L.; Laurencin, C.T.; Chen, A.F.; Nair, L.S. Curcumin-loaded poly(ε-caprolactone) nanofibres: Diabetic wound dressing with antioxidant and anti-inflammatory properties. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1149–1156. [Google Scholar] [CrossRef] [Green Version]
- Cam, M.E.; Crabbe-Mann, M.; Alenezi, H.; Hazar-Yavuz, A.N.; Ertas, B.; Ekentok, C.; Ozcan, G.S.; Topal, F.; Guler, E.; Yazir, Y.; et al. The comparision of glibenclamide and metformin-loaded bacterial cellulose/gelatin nanofibers produced by a portable electrohydrodynamic gun for diabetic wound healing. Eur. Polym. J. 2020, 134, 109844. [Google Scholar] [CrossRef]
- Alven, S.; Peter, S.; Mbese, Z.; Aderibigbe, B.A. Polymer-based wound dressing materials loaded with bioactive agents: Potential materials for the treatment of diabetic wounds. Polymers 2022, 14, 724. [Google Scholar] [CrossRef]
- Yu, M.; Huang, J.; Zhu, T.; Lu, J.; Liu, J.; Li, X.; Yan, X.; Liu, F. Liraglutide-loaded PLGA/gelatin electrospun nanofibrous mats promote angiogenesis to accelerate diabetic wound healing via the modulation of miR-29b-3p. Biomater. Sci. 2020, 8, 4225–4238. [Google Scholar] [CrossRef]
- Jafari, A.; Amirsadeghi, A.; Hassanajili, S.; Azarpira, N. Bioactive antibacterial bilayer PCL/gelatin nanofibrous scaffold promotes full-thickness wound healing. Int. J. Pharm. 2020, 583, 119413. [Google Scholar] [CrossRef]
- Cai, N.; Li, C.; Han, C.; Luo, X.; Shen, L.; Xue, Y.; Yu, F. Tailoring mechanical and antibacterial properties of chitosan/gelatin nanofiber membranes with Fe3O4 nanoparticles for potential wound dressing application. Appl. Surf. Sci. 2016, 369, 492–500. [Google Scholar] [CrossRef]
- Khandaker, M.; Alkadhem, N.; Progri, H.; Nikfarjam, S.; Jeon, J.; Kotturi, H.; Vaughan, M.B. Glutathione immobilized polycaprolactone nanofiber mesh as a dermal drug delivery mechanism for wound healing in a diabetic patient. Processes 2022, 10, 512. [Google Scholar] [CrossRef]
- Tahami, S.R.; Nemati, N.H.; Keshvari, H.; Khorasani, M.T. In vitro and in vivo evaluation of nanofibre mats containing Calendula officinalis extract as a wound dressing. J. Wound Care 2022, 31, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Fathima, N.N. Fenugreek Incorporated Silk Fibroin Nanofibers A Potential Antioxidant Scaffold for Enhanced Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 5916–5926. [Google Scholar] [CrossRef]
- Selvaraj, S.; Duraipandy, N.; Kiran, M.S.; Fathima, N.N. Anti-oxidant enriched hybrid nanofibers: Effect on mechanical stability and biocompatibility. Int. J. Biol. Macromol. 2018, 117, 209–217. [Google Scholar] [CrossRef]
- Vijayan, A.; Nanditha, C.K.; Kumar, G.V. ECM-mimicking nanofibrous scaffold enriched with dual growth factor carrying nanoparticles for diabetic wound healing. Nanoscale Adv. 2021, 3, 3085–3092. [Google Scholar] [CrossRef]
- Natarajan, J.; Sanapalli, B.K.R.; Bano, M.; Singh, S.K.; Gulati, M.; Karri, V.V.S.R. Nanostructured lipid carriers of pioglitazone loaded collagen/chitosan composite scaffold for diabetic wound healing. Adv. Wound Care 2019, 8, 499–513. [Google Scholar] [CrossRef]
- Jannesari, M.; Varshosaz, J.; Morshed, M.; Zamani, M. Composite poly (vinyl alcohol)/poly (vinyl acetate) electrospun nanofibrous mats as a novel wound dressing matrix for controlled release of drugs. Int. J. Nanomed. 2011, 6, 993. [Google Scholar]
- Guleken, Z.; Depciuch, J.; Ege, H.; İlbay, G.; Kalkandelen, C.; Ozbeyli, D.; Bulut, H.; Sener, G.; Tarhan, N.; Kuruca, S.E. Spectrochemical and biochemical assay comparison study of the healing effect of the Aloe vera and Hypericum perforatum loaded nanofiber dressings on diabetic wound. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 254, 119639. [Google Scholar] [CrossRef]
- Choi, J.S.; Leong, K.W.; Yoo, H.S. In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF). Biomaterials 2008, 29, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Rajinikanth, P.S.; Arya, D.K.; Pandey, P.; Gupta, R.K.; Sankhwar, R.; Chidambaram, K. Multifunctional biomimetic nanofibrous scaffold loaded with asiaticoside for rapid diabetic wound healing. Pharmaceutics 2022, 14, 273. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Su, Y.; John, J.V.; McCarthy, A.; Wong, S.L.; Xie, J. Mesenchymal stem cell-laden, personalized 3D scaffolds with controlled structure and fiber alignment promote diabetic wound healing. Acta Biomater. 2020, 108, 153–167. [Google Scholar] [CrossRef]
- Moura, L.I.F.; Dias, A.M.; Carvalho, E.; de Sousa, H.C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—A review. Acta Biomater. 2013, 9, 7093–7114. [Google Scholar] [CrossRef] [Green Version]
- Sundaramurthi, D.; Krishnan, U.M.; Sethuraman, S. Electrospun nanofibers as scaffolds for skin tissue engineering. Polym. Rev. 2014, 54, 348–376. [Google Scholar] [CrossRef]
- Al-Madhagy, G.; Alghoraibi, I.; Darwich, K.; Hajeer, M.Y. Evaluation of the Chemical, Morphological, Physical, Mechanical, and Biological Properties of Chitosan/Polyvinyl Alcohol Nanofibrous Scaffolds for Potential Use in Oral Tissue Engineering. Cureus 2022, 14, e29850. [Google Scholar] [CrossRef]
- Monroy, D.A.P.; Bravo, J.M.C.; Mercado, I.E.S.; Gómez, L.J.V. Gelatin and collagen nanofiber scaffolds for tissue engineering. In Tissue Regeneration; IntechOpen: London, UK, 2018. [Google Scholar]
- Liu, X.; Ma, P.X. Phase separation, pore structure, and properties of nanofibrous gelatin scaffolds. Biomaterials 2009, 30, 4094–4103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samadian, H.; Zamiri, S.; Ehterami, A.; Farzamfar, S.; Vaez, A.; Khastar, H.; Alam, M.; Ai, A.; Derakhshankhah, H.; Allahyari, Z.; et al. Electrospun cellulose acetate/gelatin nanofibrous wound dressing containing berberine for diabetic foot ulcer healing: In vitro and in vivo studies. Sci. Rep. 2020, 10, 8312. [Google Scholar] [CrossRef]
- Stern, R. Hyaluronan in cancer biology. Semin. Cancer Biol. 2008, 18, 237. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, K.; Madhan, R.; Kumar, G.V. Biodegradable Polymers for Nanofibre Production. Biol. Forum-Int. Jouy 2020, 12, 68–73. [Google Scholar]
- Awasthi, A.; Gulati, M.; Kumar, B.; Kaur, J.; Vishwas, S.; Khursheed, R.; Porwal, O.; Alam, A.; Kr, A.; Corrie, L.; et al. Recent progress in development of dressings used for diabetic wounds with special emphasis on scaffolds. BioMed Res. Int. 2022, 2022, 1–43. [Google Scholar] [CrossRef]
- Naomi, R.; Fauzi, M.B. Cellulose/collagen dressings for diabetic foot ulcer: A review. Pharmaceutics 2020, 12, 881. [Google Scholar] [CrossRef]
- Diaz-Gomez, L.; Gonzalez-Prada, I.; Millan, R.; Da Silva-Candal, A.; Bugallo-Casal, A.; Campos, F.; Concheiro, A.; Alvarez-Lorenzo, C. 3D printed carboxymethyl cellulose scaffolds for autologous growth factors delivery in wound healing. Carbohydr. Polym. 2022, 278, 118924. [Google Scholar] [CrossRef]
- He, M.; Sun, L.; Fu, X.; McDonough, S.P.; Chu, C.C. Biodegradable amino acid-based poly (ester amine) with tunable immunomodulating properties and their in vitro and in vivo wound healing studies in diabetic rats’ wounds. Acta Biomater. 2019, 84, 114–132. [Google Scholar] [CrossRef]
- Ji, Y.; Song, W.; Xu, L.; Yu, D.G.; Annie Bligh, S.W. A Review on Electrospun Poly (amino acid) Nanofibers and Their Applications of Hemostasis and Wound Healing. Biomolecules 2022, 12, 794. [Google Scholar] [CrossRef]
- Ren, S.; Guo, S.; Yang, L.; Wang, C. Effect of composite biodegradable biomaterials on wound healing in diabetes. Front. Bioeng. Biotechnol. 2022, 10, 1060026. [Google Scholar] [CrossRef] [PubMed]
- Mistry, P.; Chhabra, R.; Muke, S.; Narvekar, A.; Sathaye, S.; Jain, R.; Dandekar, P. Fabrication and characterization of starch-TPU based nanofibers for wound healing applications. Mater. Sci. Eng. C 2021, 119, 111316. [Google Scholar] [CrossRef]
- Tan, G.; Wang, L.; Pan, W.; Chen, K. Polysaccharide electrospun nanofibers for wound healing applications. Int. J. Nanomed. 2022, 17, 3913–3931. [Google Scholar] [CrossRef]
- Waghmare, V.S.; Wadke, P.R.; Dyawanapelly, S.; Deshpande, A.; Jain, R.; Dandekar, P. Starch based nanofibrous scaffolds for wound healing applications. Bioact. Mater. 2018, 3, 255–266. [Google Scholar] [CrossRef]
- Afsharian, Y.P.; Rahimnejad, M. Bioactive electrospun scaffolds for wound healing applications: A comprehensive review. Polym. Test. 2021, 93, 106952. [Google Scholar] [CrossRef]
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic polymeric biomaterials for wound healing: A Review. Prog. Biomater. 2018, 7, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadi, S.; Shafiei, S.S.; Sabouni, F. Electrospun Nanofibrous Scaffolds of Polycaprolactone/Gelatin Reinforced with Layered Double Hydroxide Nanoclay for Nerve Tissue Engineering Applications. ACS Omega 2022, 7, 28351–28360. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ruan, L.; Wang, R.; Liu, T.; Song, G.; Gao, X.; Jiang, G.; Liu, X. Electrospun scaffold of collagen and polycaprolactone containing ZnO quantum dots for skin wound regeneration. J. Bionic Eng. 2021, 18, 1378–1390. [Google Scholar] [CrossRef]
- Ilomuanya, M.O.; Okafor, P.S.; Amajuoyi, J.N.; Onyejekwe, J.C.; Okubanjo, O.O.; Adeosun, S.O.; Silva, B.O. Polylactic acid-based electrospun fiber and hyaluronic acid-valsartan hydrogel scaffold for chronic wound healing. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 31. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, S.; Gao, Y.; Zhai, Y. Electrospun nanofibers as a wound dressing for treating diabetic foot ulcer. Asian J. Pharm. Sci. 2019, 14, 130–143. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, Y.; Huang, W.; Mo, Y.; Lan, Y.; Guo, R.; Cheng, B. Neurotensin-loaded PLGA/CNC composite nanofiber membranes accelerate diabetic wound healing. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S2), 493–501. [Google Scholar] [CrossRef] [Green Version]
- Yao, T.; Baker, M.B.; Moroni, L. Strategies to improve nanofibrous scaffolds for vascular tissue engineering. Nanomaterials 2020, 10, 887. [Google Scholar] [CrossRef]
- Al-Hazeem, N.Z. Nanofibers and electrospinning method. In Novel Nanomaterials-Synthesis and Applications; Preprint; IntechOpen: London, UK, 2018. [Google Scholar]
- Sharma, G.K.; James, N.R. Electrospinning: The Technique and Applications. In Recent Developments in Nanofibers Research; IntechOpen: London, UK, 2022. [Google Scholar]
- Ghalia, M.A.; Dahman, Y. Advanced nanobiomaterials in tissue engineering: Synthesis, properties, and applications. In Nanobiomaterials in Soft Tissue Engineering; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 141–172. [Google Scholar]
- Gupta, B.S.; Edwards, J.V. Textile materials and structures for topical management of wounds. In Advanced Textiles for Wound Care; Woodhead Publishing: Sawston, UK, 2019; pp. 55–104. [Google Scholar]
- Qin, W.; Li, J.; Tu, J.; Yang, H.; Chen, Q.; Liu, H. Fabrication of porous chitosan membranes composed of nanofibers by low temperature thermally induced phase separation, and their adsorption behavior for Cu2+. Carbohydr. Polym. 2017, 178, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Li, R.; Liu, G.; Jia, W.; Sun, M.; Liu, Y.; Luo, Y.; Cheng, Z. Phase separation-based electrospun Janus nanofibers loaded with Rana chensinensis skin peptides/silver nanoparticles for wound healing. Mater. Des. 2021, 207, 109864. [Google Scholar] [CrossRef]
- Pochan, D.; Scherman, O. Introduction: Molecular self-assembly. Chem. Rev. 2021, 121, 13699–13700. [Google Scholar] [CrossRef] [PubMed]
- Subramani, K.; Ahmed, W. Self-assembly of proteins and peptides and their applications in bionanotechnology and Dentistry. In Emerging Nanotechnologies in Dentistry; William Andrew Publishing: Norwich, NY, USA, 2012; pp. 209–224. [Google Scholar]
- Kalva, S.N.; Augustine, R.; Al Mamun, A.; Dalvi, Y.B.; Vijay, N.; Hasan, A. Active agents loaded extracellular matrix mimetic electrospun membranes for wound healing applications. J. Drug Deliv. Sci. Technol. 2021, 63, 102500. [Google Scholar] [CrossRef]
- Beachley, V.; Wen, X. Polymer nanofibrous structures: Fabrication, biofunctionalization, and cell interactions. Prog. Polym. Sci. 2010, 35, 868–892. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.; Teixeira, C.; Ferraz, R.; Prudêncio, C.; Gomes, P. Wound-healing peptides for treatment of chronic diabetic foot ulcers and other infected skin injuries. Molecules 2017, 22, 1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, T.L.; Meehan, S.; Pourdeyhimi, B.; Little, D. Meltblown polymer fabrics as candidate scaffolds for rotator cuff tendon tissue engineering. Tissue Eng. Part A 2017, 23, 958–967. [Google Scholar] [CrossRef]
- Dzierzkowska, E.; Scislowska-Czarnecka, A.; Kudzin, M.; Boguń, M.; Szatkowski, P.; Gajek, M.; Kornaus, K.; Chadzinska, M.; Stodolak-Zych, E. Effects of process parameters on structure and properties of melt-blown poly (lactic acid) nonwovens for skin regeneration. J. Funct. Biomater. 2021, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Nadaf, A.; Gupta, A.; Hasan, N.; Ahmed, F.S.; Kesharwani, P.; Ahmad, F.J. Recent update on electrospinning and Electrospun nanofibers: Current trends and their applications. RSC Adv. 2022, 12, 23808–23828. [Google Scholar] [CrossRef] [PubMed]
- Norzain, N.A.; Yu, Z.W.; Lin, W.C.; Su, H.H. Micropatterned fibrous scaffold produced by using template-assisted electrospinning technique for wound healing application. Polymers 2021, 13, 2821. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef]
- Cui, S.; Sun, X.; Li, K.; Gou, D.; Zhou, Y.; Hu, J.; Liu, Y. Polylactide nanofibers delivering doxycycline for chronic wound treatment. Mater. Sci. Eng. C 2019, 104, 109745. [Google Scholar] [CrossRef]
- Ganesh, M.; Aziz, A.S.; Ubaidulla, U.; Hemalatha, P.; Saravanakumar, A.; Ravikumar, R.; Peng, M.M.; Choi, E.Y.; Jang, H.T. Sulfanilamide and silver nanoparticles-loaded polyvinyl alcohol-chitosan composite electrospun nanofibers: Synthesis and evaluation on synergism in wound healing. J. Ind. Eng. Chem. 2016, 39, 127–135. [Google Scholar] [CrossRef]
- Alven, S.; Buyana, B.; Feketshane, Z.; Aderibigbe, B.A. Electrospun nanofibers/nanofibrous scaffolds loaded with silver nanoparticles as effective antibacterial wound dressing materials. Pharmaceutics 2021, 13, 964. [Google Scholar] [CrossRef]
- Sabarees, G.; Velmurugan, V.; Tamilarasi, G.P.; Alagarsamy, V.; Raja Solomon, V. Recent Advances in Silver Nanoparticles Containing Nanofibers for Chronic Wound Management. Polymers 2022, 14, 3994. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, V.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int. J. Biol. Macromol. 2019, 122, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Azlan, A.Y.H.N.; Katas, H.; Busra, M.F.M.; Salleh, N.A.M.; Smandri, A. Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy. Nanotechnol. Rev. 2021, 10, 653–670. [Google Scholar] [CrossRef]
- Adamu, B.F.; Gau, J.; Jhatial, A.K.; Kumelachew, D.M. A review of medicinal plant-based bioactive electrospun nano fibrous wound dressings. Mater. Des. 2021, 209, 109942. [Google Scholar] [CrossRef]
- Tamilarasi, G.P.; Krishnan, M.; Sabarees, G.; Gouthaman, S.; Alagarsamy, V.; Solomon, V.R. Emerging Trends in Curcumin Embedded Electrospun Nanofibers for Impaired Diabetic Wound Healing. Appl. Nano 2022, 3, 202–232. [Google Scholar] [CrossRef]
- Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Correia, I.J. Electrospun polycaprolactone/aloe vera chitosan nanofibrous asymmetric membranes aimed for wound healing applications. Polymers 2017, 9, 183. [Google Scholar] [CrossRef]
- Maleki, H.; Khoshnevisan, K.; Sajjadi-Jazi, S.M.; Baharifar, H.; Doostan, M.; Khoshnevisan, N.; Sharifi, F. Nanofiber-based systems intended for diabetes. J. Nanobiotechnol. 2021, 19, 317. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Gang, X.; Sun, C.; Wang, G. Mesenchymal stem cells improve healing of diabetic foot ulcer. J. Diabetes Res. 2017, 2017, 9328347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, M.-C.W.; Wang, W.T.; Lin, C.Y.; Kuo, Y.R.; Lee, S.S.; Hou, M.F.; Wu, Y.C. Stem cell-based therapeutic strategies in diabetic wound healing. Biomedicines 2022, 10, 2085. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhang, Y.; Chu, J.; Wang, X.; Yan, W.; Zhang, Q.; Liu, H. Reduced graphene oxide incorporated acellular dermal composite scaffold enables efficient local delivery of mesenchymal stem cells for accelerating diabetic wound healing. ACS Biomater. Sci. Eng. 2019, 5, 4054–4066. [Google Scholar] [CrossRef]
- Lu, D.; Chen, B.; Liang, Z.; Deng, W.; Jiang, Y.; Li, S.; Xu, J.; Wu, Q.; Zhang, Z.; Xie, B.; et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: A double-blind, randomized, controlled trial. Diabetes Res. Clin. Pract. 2011, 92, 26–36. [Google Scholar] [CrossRef]
- Amann, B.; Luedemann, C.; Ratei, R.; Schmidt-Lucke, J.A. Autologous bone marrow cell transplantation increases leg perfusion and reduces amputations in patients with advanced critical limb ischemia due to peripheral artery disease. Cell Transplant. 2009, 18, 371–380. [Google Scholar] [CrossRef]
- Vojtaššák, J.; Danišovič, L.; Kubeš, M.; Bakoš, D.; Jarabek, L.; Uličná, M.; Blaško, M. Autologous biograft and mesenchymal stem cells in treatment of the diabetic foot. Neuroendocrinol. Lett. 2006, 27 (Suppl. S2), 134–137. [Google Scholar]
- Dash, N.R.; Dash, S.N.; Routray, P.; Mohapatra, S.; Mohapatra, P.C. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res. 2009, 12, 359–366. [Google Scholar] [CrossRef]
- Procházka, V.; Gumulec, J.; Jalůvka, F.; Šalounová, D.; Jonszta, T.; Czerný, D.; Krajča, J.; Urbanec, R.; Klement, P.; Martinek, J.; et al. Cell therapy, a new standard in management of chronic critical limb ischemia and foot ulcer. Cell Transplant. 2010, 19, 1413–1424. [Google Scholar] [CrossRef] [Green Version]
- Bhansali, A.; Upreti, V.; Khandelwal, N.; Marwaha, N.; Gupta, V.; Sachdeva, N.; Sharma, R.R.; Saluja, K.; Dutta, P.; Walia, R.; et al. Efficacy of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cells Dev. 2009, 18, 1407–1416. [Google Scholar] [CrossRef]
- Ezhilarasu, H.; Vishalli, D.; Dheen, S.T.; Bay, B.H.; Srinivasan, D.K. Nanoparticle-based Therapeutic Approach for diabetic wound healing. Nanomaterials 2020, 10, 1234. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Ren, D.Y.; Feng, Z.X.; Zhang, L.Y.; Zhong, Y.F.; Jin, M.Y.; Xu, F.W.; Feng, C.Y.; Du, Y.Z.; et al. Mussel-inspired collagen-hyaluronic acid composite scaffold with excellent antioxidant properties and sustained release of a growth factor for enhancing diabetic wound healing. Mater. Today Bio 2022, 15, 100320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, L.; Xiong, Y.; Panayi, A.C.; Abududilibaier, A.; Hu, Y.; Yu, C.; Zhou, W.; Sun, Y.; Liu, M.; et al. Antioxidant therapy and antioxidant-related bionanomaterials in diabetic wound healing. Front. Bioeng. Biotechnol. 2021, 9, 707479. [Google Scholar] [CrossRef] [PubMed]
- Cam, M.E.; Ertas, B.; Alenezi, H.; Hazar-Yavuz, A.N.; Cesur, S.; Ozcan, G.S.; Ekentok, C.; Guler, E.; Katsakouli, C.; Demirbas, Z.; et al. Accelerated diabetic wound healing by topical application of combination oral antidiabetic agents-loaded nanofibrous scaffolds: An in vitro and in vivo evaluation study. Mater. Sci. Eng. C 2021, 119, 111586. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Chang, S.H.; Chen, W.J.; Hung, K.C.; Lin, Y.H.; Liu, S.J.; Hsieh, M.J.; Pang, J.H.S.; Juang, J.H. Augmentation of diabetic wound healing and enhancement of collagen content using nanofibrous glucophage-loaded collagen/PLGA scaffold membranes. Colloid Interface Sci. 2015, 439, 88–97. [Google Scholar] [CrossRef]
| Polymers/Blends | Bioactive Agents | Therapeutic Efficacy | References |
|---|---|---|---|
| Chitosan and polyvinyl alcohol | Zinc oxide | Antioxidant and antibacterial effects and accelerated diabetic wound recovery | [31] |
| Poly (lactic-co-glycolic acid | Vancomycin, gentamicin, and platelet-derived growth factor | Improved angiogenesis and healing of infected diabetic wounds | [32] |
| Polycaprolactone and tragacanth gum | Curcumin | Exhibited anti-inflammatory, antioxidant properties, and increased wound closure rate. | [33] |
| Gelatin and cellulose | Metformin and glibenclamide | Lowered risks of cytotoxicity and improved wound healing | [34] |
| Polylactide | Doxycycline | Good antibacterialeffect on diabetic wounds | [35] |
| Poly (lactic-co-glycolic acid and gelatin | Liraglutide | Improved the physical properties of the scaffold template and promoted vascularization on diabetic wound | [36] |
| Polycaprolactone and gelatin | Amoxicillin and Zinc oxide | Sustained drug release and antibacterial activity | [37] |
| Chitosan and gelatin | Ferrous oxide | Boosted the antibacterial properties of the scaffold | [38] |
| Polycaprolactone | Glutathione | Demonstrated anti-inflammatory and antioxidant effects | [39] |
| Polyvinyl alcohol and sodium alginate | Calendula officinalis extract | High wound closure rate and supported cell proliferation | [40] |
| Silk fibroin | Fenugreek extract | Increased collagen deposition and provided antioxidant benefits | [41] |
| Collagen/Silk fibroin composite | Fenugreek extract | Enhanced antioxidant properties, improved viability, and proliferation of fibroblasts, which accelerated wound healing | [42] |
| Poly (lactic-co-glycolic acid), collagen and chitosan | Basic fibroblast growth factor and vascular endothelial growth factor | Promoted angiogenesis, cell proliferation, and prevented scar formation | [43] |
| Collagen and chitosan | Pioglitazone | Elevated cell growth and rapid wound healing | [44] |
| Polyvinyl alcohol and polyvinyl acetate | Ciprofloxacin | Antibacterial activity | [45] |
| Gelatin and polycaprolactone | Aloe Vera extract | Provided anti-inflammatory, antibacterial, and antioxidant effects | [46] |
| Polyethylene glycol and polycaprolactone | Epidermal growth factor | Enhanced mechanical properties and good healing abilities | [47] |
| Cellulose and bacterial cellulose | Metformin and glibenclamide | Sustained drug release and anti-inflammatory properties | [34] |
| Polyvinyl alcohol, sodium alginate, silk fibroin | Asiaticoside | Supplied oxygen to wound and good potential for wound healing | [48] |
| Gelatin, pluronic-F-127 and polycaprolactone | Bone marrow-mesenchymal stem cell | Promoted angiogenesis, formation of granulation tissue and increased collagen deposits and improved wound healing | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusuf Aliyu, A.; Adeleke, O.A. Nanofibrous Scaffolds for Diabetic Wound Healing. Pharmaceutics 2023, 15, 986. https://doi.org/10.3390/pharmaceutics15030986
Yusuf Aliyu A, Adeleke OA. Nanofibrous Scaffolds for Diabetic Wound Healing. Pharmaceutics. 2023; 15(3):986. https://doi.org/10.3390/pharmaceutics15030986
Chicago/Turabian StyleYusuf Aliyu, Anna, and Oluwatoyin A. Adeleke. 2023. "Nanofibrous Scaffolds for Diabetic Wound Healing" Pharmaceutics 15, no. 3: 986. https://doi.org/10.3390/pharmaceutics15030986
APA StyleYusuf Aliyu, A., & Adeleke, O. A. (2023). Nanofibrous Scaffolds for Diabetic Wound Healing. Pharmaceutics, 15(3), 986. https://doi.org/10.3390/pharmaceutics15030986







