Dendrimer-Mediated Delivery of DNA and RNA Vaccines
Abstract
:1. Introduction
2. The Use of Dendrimers for Biomedical Applications
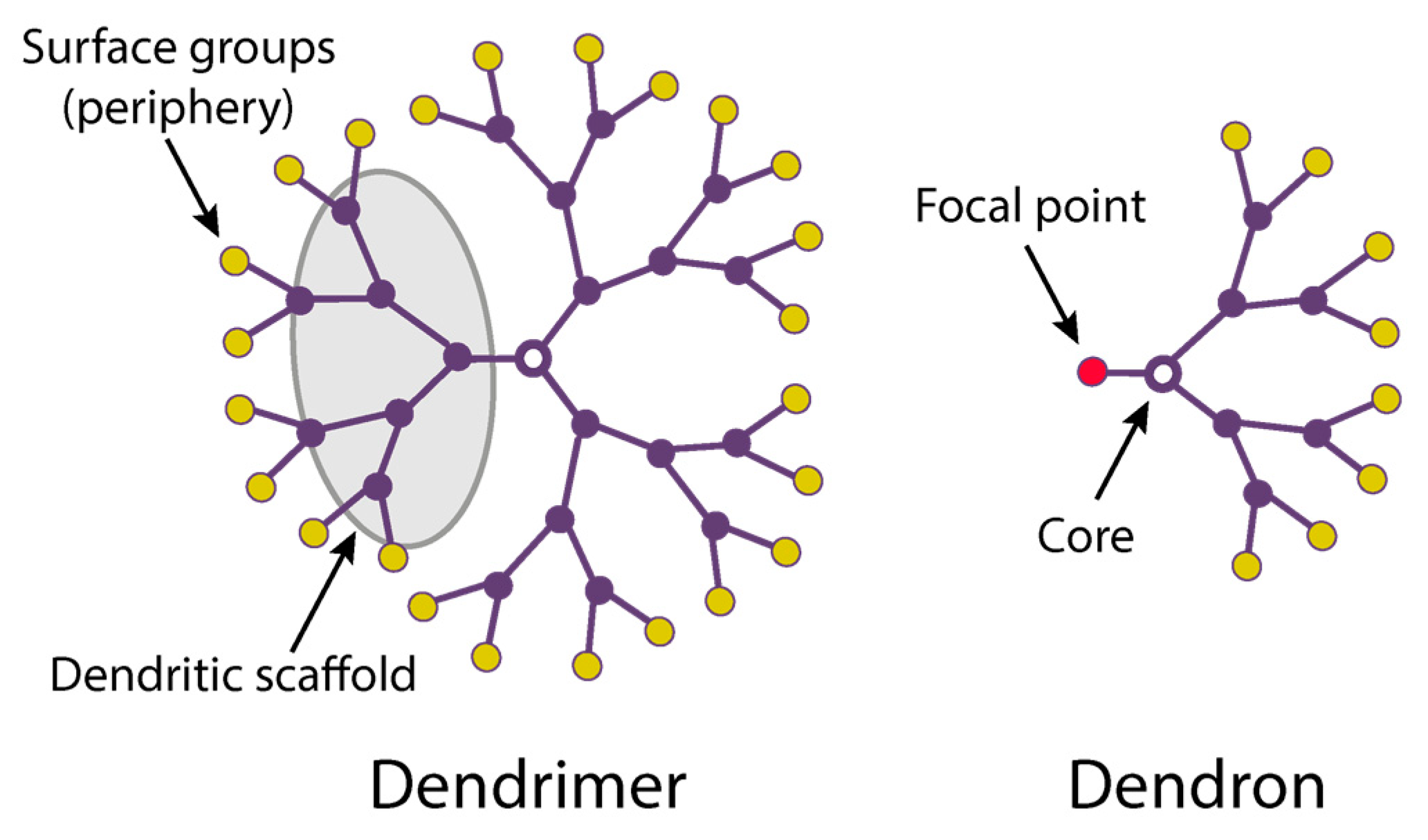
2.1. Dendrimer Structure and Properties
2.2. Validation of Dendrimers in Clinical Trials
3. Delivery of DNA and mRNA Vaccines Using Dendrimers
3.1. Dendrimers for the Delivery of DNA Vaccines against Viral Infections
3.2. Dendrimers for mRNA Delivery of Vaccines against Viral Infections
3.3. Dendrimers for the Delivery of DNA Vaccines against Bacterial Infection
3.4. Dendrimers for the Delivery of DNA Vaccines against Parasitic Infections
3.5. Dendrimers for the Delivery of DNA Vaccines against Cancer
3.6. Dendrimers for the Delivery of RNA Vaccines for Treating Protein Metabolism Disorders
3.7. The Use of Complexes of Dendrimers with Metal Nanoparticles for mRNA Delivery
4. Discussion
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.A. A Comparison of Plasmid DNA and mRNA as Vaccine Technologies. Vaccines 2019, 7, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Momin, T.; Kansagra, K.; Patel, H.; Sharma, S.; Sharma, B.; Patel, J.; Mittal, R.; Sanmukhani, J.; Maithal, K.; Dey, A.; et al. Safety and Immunogenicity of a DNA SARS-CoV-2 vaccine (ZyCoV-D): Results of an open-label, non-randomized phase I part of phase I/II clinical study by intradermal route in healthy subjects in India. EClinicalMedicine 2021, 38, 101020. [Google Scholar] [CrossRef]
- Mallapaty, S. India’s DNA COVID vaccine is a world first—More are coming. Nature 2021, 597, 161–162. [Google Scholar] [CrossRef]
- Li, L.; Petrovsky, N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert Rev. Vaccines 2016, 15, 313–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braathen, R.; Spång, H.C.L.; Hinke, D.M.; Blazevski, J.; Bobic, S.; Fossum, E.; Bogen, B. A DNA Vaccine That Encodes an Antigen-Presenting Cell-Specific Heterodimeric Protein Protects against Cancer and Influenza. Mol. Ther. Methods Clin. Dev. 2020, 17, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Wu, Y.; Kim, Y.B.; Oh, Y.-K. Advances in vaccine delivery systems against viral infectious diseases. Drug Deliv. Transl. Res. 2021, 11, 1401–1419. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Pandya, R.; Apostolopoulos, V. DNA vaccines for SARS-CoV-2: Toward third-generation vaccination era. Expert Rev. Vaccines 2021, 20, 1549–1560. [Google Scholar] [CrossRef]
- Ilyichev, A.A.; Orlova, L.A.; Sharabrin, S.V.; Karpenko, L.I. mRNA technology as one of the promising platforms for the SARS-CoV-2 vaccine development. Vavilov J. Genet. Breed. 2020, 24, 802–807. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Weissman, D. Recent advances in mRNA vaccine technology. Curr. Opin. Immunol. 2020, 65, 14–20. [Google Scholar] [CrossRef]
- Kauffman, K.J.; Webber, M.J.; Anderson, D.G. Materials for non-viral intracellular delivery of messenger RNA therapeutics. J. Control. Release 2016, 240, 227–234. [Google Scholar] [CrossRef]
- Guan, S.; Rosenecker, J. Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Ther. 2017, 24, 133–143. [Google Scholar] [CrossRef]
- Chang, C.; Sun, J.; Hayashi, H.; Suzuki, A.; Sakaguchi, Y.; Miyazaki, H.; Nishikawa, T.; Nakagami, H.; Yamashita, K.; Kaneda, Y. Stable Immune Response Induced by Intradermal DNA Vaccination by a Novel Needleless Pyro-Drive Jet Injector. AAPS PharmSciTech 2019, 21, 19. [Google Scholar] [CrossRef] [Green Version]
- Conforti, A.; Marra, E.; Palombo, F.; Roscilli, G.; Ravà, M.; Fumagalli, V.; Muzi, A.; Maffei, M.; Luberto, L.; Lione, L.; et al. COVID-eVax, an electroporated DNA vaccine candidate encoding the SARS-CoV-2 RBD, elicits protective responses in animal models. Mol. Ther. 2022, 30, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Peletta, A.; Prompetchara, E.; Tharakhet, K.; Kaewpang, P.; Buranapraditkun, S.; Techawiwattanaboon, T.; Jbilou, T.; Krangvichian, P.; Sirivichayakul, S.; Manopwisedjaroen, S.; et al. DNA Vaccine Administered by Cationic Lipoplexes or by In Vivo Electroporation Induces Comparable Antibody Responses against SARS-CoV-2 in Mice. Vaccines 2021, 9, 874. [Google Scholar] [CrossRef] [PubMed]
- Kisakov, D.N.; Kisakova, L.A.; Borgoyakova, M.B.; Starostina, E.V.; Taranov, O.S.; Ivleva, E.K.; Pyankov, O.V.; Zaykovskaya, A.V.; Shcherbakov, D.N.; Rudometov, A.P.; et al. Optimization of In Vivo Electroporation Conditions and Delivery of DNA Vaccine Encoding SARS-CoV-2 RBD Using the Determined Protocol. Pharmaceutics 2022, 14, 2259. [Google Scholar] [CrossRef] [PubMed]
- Aldosari, B.N.; Alfagih, I.M.; Almurshedi, A.S. Lipid Nanoparticles as Delivery Systems for RNA-Based Vaccines. Pharmaceutics 2021, 13, 206. [Google Scholar] [CrossRef]
- Islam, M.A.; Reesor, E.K.G.; Xu, Y.; Zope, H.R.; Zetter, B.R.; Shi, J. Biomaterials for mRNA delivery. Biomater. Sci. 2015, 3, 1519–1533. [Google Scholar] [CrossRef] [Green Version]
- Karpenko, L.I.; Rudometov, A.P.; Sharabrin, S.V.; Shcherbakov, D.N.; Borgoyakova, M.B.; Bazhan, S.I.; Volosnikova, E.A.; Rudometova, N.B.; Orlova, L.A.; Pyshnaya, I.A.; et al. Delivery of mrna vaccine against SARS-CoV-2 using a polyglucin:Spermidine conjugate. Vaccines 2021, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Geetha, K.M. Lipid nanoparticles in the development of mRNA vaccines for COVID-19. J. Drug Deliv. Sci. Technol. 2022, 74, 103553. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zeng, J.; Yan, J. COVID-19 mRNA vaccines. J. Genet. Genomics 2021, 48, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Banerji, A.; Wickner, P.G.; Saff, R.; Stone, C.A.; Robinson, L.B.; Long, A.A.; Wolfson, A.R.; Williams, P.; Khan, D.A.; Phillips, E.; et al. mRNA Vaccines to Prevent COVID-19 Disease and Reported Allergic Reactions: Current Evidence and Suggested Approach. J. Allergy Clin. Immunol. Pract. 2021, 9, 1423–1437. [Google Scholar] [CrossRef]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Eygeris, Y.; Gupta, M.; Kim, J.; Sahay, G. Chemistry of Lipid Nanoparticles for RNA Delivery. Accounts Chem. Res. 2022, 55, 2–12. [Google Scholar] [CrossRef]
- Caminade, A.-M. Dendrimers, an Emerging Opportunity in Personalized Medicine? J. Pers. Med. 2022, 12, 1334. [Google Scholar] [CrossRef]
- Lee, C.C.; MacKay, J.A.; Fréchet, J.M.J.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef]
- Nanjwade, B.K.; Bechra, H.M.; Derkar, G.K.; Manvi, F.V.; Nanjwade, V.K. Dendrimers: Emerging polymers for drug-delivery systems. Eur. J. Pharm. Sci. 2009, 38, 185–196. [Google Scholar] [CrossRef]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers Designed for Functions: From Physical, Photophysical, and Supramolecular Properties to Applications in Sensing, Catalysis, Molecular Electronics, Photonics, and Nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef]
- Caminade, A.-M. Inorganic dendrimers: Recent advances for catalysis, nanomaterials, and nanomedicine. Chem. Soc. Rev. 2016, 45, 5174–5186. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Hameau, A.; Shi, X.; Mignani, S.; Majoral, J.P.; Caminade, A.M. Fluorescent Phosphorus Dendrimers: Towards Material and Biological Applications. ChemPlusChem 2019, 84, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Apartsin, E.; Caminade, A.-M. Single-Component Physical Hydrogels of Dendritic Molecules. J. Compos. Sci. 2023, 7, 26. [Google Scholar] [CrossRef]
- Mignani, S.; Rodrigues, J.; Roy, R.; Shi, X.; Ceña, V.; El Kazzouli, S.; Majoral, J.-P. Exploration of biomedical dendrimer space based on in-vitro physicochemical parameters: Key factor analysis (Part 1). Drug Discov. Today 2019, 24, 1176–1183. [Google Scholar] [CrossRef]
- Mignani, S.; Rodrigues, J.; Roy, R.; Shi, X.; Ceña, V.; El Kazzouli, S.; Majoral, J.-P. Exploration of biomedical dendrimer space based on in-vivo physicochemical parameters: Key factor analysis (Part 2). Drug Discov. Today 2019, 24, 1184–1192. [Google Scholar] [CrossRef]
- Maysinger, D.; Zhang, Q.; Kakkar, A. Dendrimers as Modulators of Brain Cells. Molecules 2020, 25, 4489. [Google Scholar] [CrossRef]
- Mignani, S.; Shi, X.; Ceña, V.; Shcharbin, D.; Bryszewska, M.; Majoral, J.-P. In vivo therapeutic applications of phosphorus dendrimers: State of the art. Drug Discov. Today 2021, 26, 677–689. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Majoral, J.-P. Phosphorus dendrimers functionalised with nitrogen ligands, for catalysis and biology. Dalton Trans. 2019, 48, 7483–7493. [Google Scholar] [CrossRef]
- Dias, A.P.; da Silva Santos, S.; da Silva, J.V.; Parise-Filho, R.; Igne Ferreira, E.; Seoud, O.E.; Giarolla, J. Dendrimers in the context of nanomedicine. Int. J. Pharm. 2020, 573, 118814. [Google Scholar] [CrossRef]
- Dzmitruk, V.; Apartsin, E.; Ihnatsyeu-Kachan, A.; Abashkin, V.; Shcharbin, D.; Bryszewska, M. Dendrimers Show Promise for siRNA and microRNA Therapeutics. Pharmaceutics 2018, 10, 126. [Google Scholar] [CrossRef] [Green Version]
- Knauer, N.; Pashkina, E.; Apartsin, E. Topological Aspects of the Design of Nanocarriers for Therapeutic Peptides and Proteins. Pharmaceutics 2019, 11, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caminade, A.-M.; Ouali, A.; Laurent, R.; Turrin, C.-O.; Majoral, J.-P. The dendritic effect illustrated with phosphorus dendrimers. Chem. Soc. Rev. 2015, 44, 3890–3899. [Google Scholar] [CrossRef] [PubMed]
- Montilla, F.; Galindo, A.; Andrés, R.; Córdoba, M.; de Jesús, E.; Bo, C. Carbosilane Dendrons as Solubilizers of Metal Complexes in Supercritical Carbon Dioxide. Organometallics 2006, 25, 4138–4143. [Google Scholar] [CrossRef]
- Rodríguez, L.-I.; Rossell, O.; Seco, M.; Muller, G. Carbosilane Dendrons Containing a P-Stereogenic Phosphine at the Focal Point. Catalytic Behavior of Their Allylpalladium Complexes in the Asymmetric Hydrovinylation of Styrene. Organometallics 2008, 27, 1328–1333. [Google Scholar] [CrossRef]
- García-Peña, N.G.; Caminade, A.-M.; Ouali, A.; Redón, R.; Turrin, C.-O. Solventless synthesis of Ru(0) composites stabilized with polyphosphorhydrazone (PPH) dendrons and their use in catalysis. RSC Adv. 2016, 6, 64557–64567. [Google Scholar] [CrossRef]
- Michlewska, S.; Ionov, M.; Shcharbin, D.; Maroto-Díaz, M.; Ramirez, R.G.; de la Mata, F.J.; Bryszewska, M. Ruthenium metallodendrimers with anticancer potential in an acute promyelocytic leukemia cell line (HL60). Eur. Polym. J. 2017, 87, 39–47. [Google Scholar] [CrossRef]
- Fernandez, J.; Acosta, G.; Pulido, D.; Malý, M.; Copa-Patiño, J.L.; Soliveri, J.; Royo, M.; Gómez, R.; Albericio, F.; Ortega, P.; et al. Carbosilane Dendron–Peptide Nanoconjugates as Antimicrobial Agents. Mol. Pharm. 2019, 16, 2661–2674. [Google Scholar] [CrossRef]
- Gutierrez-Ulloa, C.E.; Sepúlveda-Crespo, D.; García-Broncano, P.; Malý, M.; Muñoz-Fernández, M.A.; de la Mata, F.J.; Gómez, R. Synthesis of bow-tie carbosilane dendrimers and their HIV antiviral capacity: A comparison of the dendritic topology on the biological process. Eur. Polym. J. 2019, 119, 200–212. [Google Scholar] [CrossRef]
- Pędziwiatr-Werbicka, E.; Gorzkiewicz, M.; Horodecka, K.; Abashkin, V.; Klajnert-Maculewicz, B.; Peña-González, C.E.; Sánchez-Nieves, J.; Gómez, R.; de la Mata, F.J.; Bryszewska, M. Silver Nanoparticles Surface-Modified with Carbosilane Dendrons as Carriers of Anticancer siRNA. Int. J. Mol. Sci. 2020, 21, 4647. [Google Scholar] [CrossRef]
- Apartsin, E.K.; Knauer, N.; Kahlert, U.D.; Caminade, A.-M. Amphiphilic Triazine-Phosphorus Metallodendrons Possessing Anti-Cancer Stem Cell Activity. Pharmaceutics 2022, 14, 393. [Google Scholar] [CrossRef]
- Ramos, E.; Davin, L.; Angurell, I.; Ledesma, C.; Llorca, J. Improved Stability of Pd/Al2O3 Prepared from Palladium Nanoparticles Protected with Carbosilane Dendrons in the Dimethyl Ether Steam Reforming Reaction. ChemCatChem 2015, 7, 2179–2187. [Google Scholar] [CrossRef] [Green Version]
- González-García, E.; Gutiérrez Ulloa, C.E.; de la Mata, F.J.; Marina, M.L.; García, M.C. Sulfonate-terminated carbosilane dendron-coated nanotubes: A greener point of view in protein sample preparation. Anal. Bioanal. Chem. 2017, 409, 5337–5348. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Villanueva, R.; Peña-González, C.E.; Sánchez-Nieves, J.; de la Mata, F.J.; Marina, M.L.; García, M.C. Gold nanoparticles coated with carbosilane dendrons in protein sample preparation. Microchim. Acta 2019, 186, 508. [Google Scholar] [CrossRef]
- Barrios-Gumiel, A.; Sepúlveda-Crespo, D.; Jiménez, J.L.; Gómez, R.; Muñoz-Fernández, M.Á.; de la Mata, F.J. Dendronized magnetic nanoparticles for HIV-1 capture and rapid diagnostic. Colloid Surf. B-Biointerfaces 2019, 181, 360–368. [Google Scholar] [CrossRef]
- Gutierrez-Ulloa, C.E.; Buyanova, M.Y.; Apartsin, E.K.; Venyaminova, A.G.; de la Mata, F.J.; Valiente, M.; Gómez, R. Amphiphilic carbosilane dendrons as a novel synthetic platform toward micelle formation. Org. Biomol. Chem. 2017, 15, 7352–7364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Z.; Cai, C.; Mayeux, A.; Milenkovic, A. The First Organosiloxane Thin Films Derived from SiCl3-Terminated Dendrons. Thickness-Dependent Nano-and Mesoscopic Structures of the Films Deposited on Mica by Spin-Coating. Langmuir 2002, 18, 7728–7739. [Google Scholar] [CrossRef]
- Zhang, Q.; Archer, L.A. Step-Growth Synthesis and Interfacial Friction Properties of Surface Dendron Coatings. Langmuir 2006, 22, 717–722. [Google Scholar] [CrossRef]
- Peterca, M.; Imam, M.R.; Ahn, C.-H.; Balagurusamy, V.S.K.; Wilson, D.A.; Rosen, B.M.; Percec, V. Transfer, Amplification, and Inversion of Helical Chirality Mediated by Concerted Interactions of C3-Supramolecular Dendrimers. J. Am. Chem. Soc. 2011, 133, 2311–2328. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, H.-J.; Hughes, A.D.; Moussodia, R.-O.; Bertin, A.; Chen, Y.; Pochan, D.J.; Heiney, P.A.; Klein, M.L.; Percec, V. Self-assembly of amphiphilic Janus dendrimers into uniform onion-like dendrimersomes with predictable size and number of bilayers. Proc. Natl. Acad. Sci. USA 2014, 111, 9058–9063. [Google Scholar] [CrossRef] [Green Version]
- Apartsin, E.; Caminade, A.M. Supramolecular Self-Associations of Amphiphilic Dendrons and Their Properties. Chem. Eur. J. 2021, 27, 17976–17998. [Google Scholar] [CrossRef]
- Wei, T.; Chen, C.; Liu, J.; Liu, C.; Posocco, P.; Liu, X.; Cheng, Q.; Huo, S.; Liang, Z.; Fermeglia, M.; et al. Anticancer drug nanomicelles formed by self-assembling amphiphilic dendrimer to combat cancer drug resistance. Proc. Natl. Acad. Sci. USA 2015, 112, 2978–2983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apartsin, E.; Knauer, N.; Arkhipova, V.; Pashkina, E.; Aktanova, A.; Poletaeva, J.; Sánchez-Nieves, J.; de la Mata, F.J.; Gómez, R. pH-Sensitive Dendrimersomes of Hybrid Triazine-Carbosilane Dendritic Amphiphiles-Smart Vehicles for Drug Delivery. Nanomaterials 2020, 10, 1899. [Google Scholar] [CrossRef] [PubMed]
- Sztandera, K.; Gorzkiewicz, M.; Bątal, M.; Arkhipova, V.; Knauer, N.; Sánchez-Nieves, J.; de la Mata, F.J.; Gómez, R.; Apartsin, E.; Klajnert-Maculewicz, B. Triazine–Carbosilane Dendrimersomes Enhance Cellular Uptake and Phototoxic Activity of Rose Bengal in Basal Cell Skin Carcinoma Cells. Int. J. Nanomed. 2022, 17, 1139–1154. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Liu, X.; Bolcato-Bellemin, A.L.; Wang, Y.; Liu, C.; Erbacher, P.; Qu, F.; Rocchi, P.; Behr, J.P.; Peng, L. An Amphiphilic Dendrimer for Effective Delivery of Small Interfering RNA and Gene Silencing In Vitro and In Vivo. Angew. Chem. Int. Edit. 2012, 51, 8478–8484. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, J.; Yu, T.; Chen, C.; Cheng, Q.; Sengupta, K.; Huang, Y.; Li, H.; Liu, C.; Wang, Y.; et al. Adaptive Amphiphilic Dendrimer-Based Nanoassemblies as Robust and Versatile siRNA Delivery Systems. Angew. Chem. Int. Edit. 2014, 53, 11822–11827. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Chen, C.; Tintaru, A.; Cao, Y.; Liu, J.; Ziarelli, F.; Tang, J.; Guo, H.; Rosas, R.; et al. A Fluorinated Bola-Amphiphilic Dendrimer for On-Demand Delivery of siRNA, via Specific Response to Reactive Oxygen Species. Adv. Funct. Mater. 2016, 26, 8594–8603. [Google Scholar] [CrossRef]
- Sherman, S.E.; Xiao, Q.; Percec, V. Mimicking Complex Biological Membranes and Their Programmable Glycan Ligands with Dendrimersomes and Glycodendrimersomes. Chem. Rev. 2017, 117, 6538–6631. [Google Scholar] [CrossRef]
- Torre, P.; Xiao, Q.; Buzzacchera, I.; Sherman, S.E.; Rahimi, K.; Kostina, N.Y.; Rodriguez-Emmenegger, C.; Möller, M.; Wilson, C.J.; Klein, M.L.; et al. Encapsulation of hydrophobic components in dendrimersomes and decoration of their surface with proteins and nucleic acids. Proc. Natl. Acad. Sci. USA 2019, 116, 15378–15385. [Google Scholar] [CrossRef] [Green Version]
- Mignani, S.; Rodrigues, J.; Tomas, H.; Zablocka, M.; Shi, X.; Caminade, A.-M.; Majoral, J.-P. Dendrimers in combination with natural products and analogues as anti-cancer agents. Chem. Soc. Rev. 2018, 47, 514–532. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymenrs–starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Newkome, G.R.; Yao, Z.Q.; Baker, G.R.; Gupta, V.K. Micelles. 1 Cascade molecules—A new approach to micelles—A [27]-arborol. J. Org. Chem. 1985, 50, 2003–2004. [Google Scholar] [CrossRef]
- Wooley, K.L.; Hawker, C.J.; Frechet, J.M.J. Hyperbranched macromolecules via a novel double-stage convergent growth approach. J. Am. Chem. Soc. 1991, 113, 4252–4261. [Google Scholar] [CrossRef]
- Zhou, L.L.; Roovers, J. Synthesis of novel carbosilane dendritic macromolecules. Macromolecules 1993, 26, 963–968. [Google Scholar] [CrossRef]
- de Brabander van den Berg, E.M.M.; Meijer, E.W. Poly(Propylene Imine) Dendrimers—Large-Scale Synthesis by Hetereogeneously Catalyzed Hydrogenations. Angew. Chem. Int. Edit. 1993, 32, 1308–1311. [Google Scholar] [CrossRef] [Green Version]
- Percec, V.; Mitchell, C.M.; Cho, W.D.; Uchida, S.; Glodde, M.; Ungar, G.; Zeng, X.B.; Liu, Y.S.; Balagurusamy, V.S.K.; Heiney, P.A. Designing libraries of first generation AB(3) and AB(2) self-assembling dendrons via the primary structure generated from combinations of (AB)(y)-AB(3) and (AB)(y)-AB(2) building blocks. J. Am. Chem. Soc. 2004, 126, 6078–6094. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Intern. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef] [PubMed]
- Newkome, G.R.; Moorefield, C.N.; Chakraborty, S. A Long Pathway to the Quantitative Assembly of Metallodendrimers. J. Inorg. Organomet. Polym. Mater. 2018, 28, 360–368. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Nixon, L.S.; Hedstrand, D.M. The Role of Branch Cell Symmetry and Other Critical Nanoscale Design Parameters in the Determination of Dendrimer Encapsulation Properties. Biomolecules 2020, 10, 642. [Google Scholar] [CrossRef]
- Zhang, D.P.; Atochina-Vasserman, E.N.; Lu, J.C.; Maurya, D.S.; Xiao, Q.; Liu, M.; Adamson, J.; Ona, N.; Reagan, E.K.; Ni, H.P.; et al. The Unexpected Importance of the Primary Structure of theHydrophobic Part of One-Component Ionizable Amphiphilic JanusDendrimers in Targeted mRNA Delivery Activity. J. Am. Chem. Soc. 2022, 144, 4746–4753. [Google Scholar] [CrossRef]
- De la Mata, F.J.; Gómez, R.; Cano, J.; Sánchez-Nieves, J.; Ortega, P.; Gallego, S.G. Carbosilane dendritic nanostructures, highly versatile platforms for pharmaceutical applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, e1871. [Google Scholar] [CrossRef]
- Caminade, A.-M. Phosphorus Dendrimers as Nanotools against Cancers. Molecules 2020, 25, 3333. [Google Scholar] [CrossRef] [PubMed]
- Majoral, J.-P.; Zablocka, M.; Ciepluch, K.; Milowska, K.; Bryszewska, M.; Shcharbin, D.; Katir, N.; El Kadib, A.; Caminade, A.-M.; Mignani, S. Hybrid phosphorus–viologen dendrimers as new soft nanoparticles: Design and properties. Org. Chem. Front. 2021, 8, 4607–4622. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Q.; Chang, H.; Xiao, J.; Cheng, Y. Stimuli-responsive dendrimers in drug delivery. Biomater. Sci. 2016, 4, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Kalomiraki, M.; Thermos, K.; Chaniotakis, N.A. Dendrimers as tunable vectors of drug delivery systems and biomedical and ocular applications. Int. J. Nanomed. 2016, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Hermanson, G.T. (Ed.) Bioconjugate Techniques, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2008; p. 1195. [Google Scholar]
- Mendes, L.P.; Pan, J.; Torchilin, V.P. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef] [Green Version]
- SPL7013 Gel—Male Tolerance Study. Available online: https://clinicaltrials.gov/ct2/show/NCT00370357 (accessed on 29 January 2023).
- VivaGel™ in Healthy Young Women. Available online: https://clinicaltrials.gov/ct2/show/NCT00331032 (accessed on 29 January 2023).
- Safety and Acceptability of SPL7013 Gel (VivaGel™) in Sexually Active Women. Available online: https://clinicaltrials.gov/ct2/show/NCT00442910 (accessed on 29 January 2023).
- Retention and Duration of Activity of SPL7013 (VivaGel®) after Vaginal Dosing. Available online: https://clinicaltrials.gov/ct2/show/NCT00740584 (accessed on 29 January 2023).
- Dose Ranging Study of SPL7013 Gel for Treatment of Bacterial Vaginosis (BV). Available online: https://clinicaltrials.gov/ct2/show/NCT01201057 (accessed on 29 January 2023).
- Dose-ranging Study of SPL7013 Gel for the Prevention of Bacterial Vaginosis (BV). Available online: https://clinicaltrials.gov/ct2/show/NCT01437722 (accessed on 29 January 2023).
- A Phase 3 Study of SPL7013 Gel (VivaGel) for the Treatment of Bacterial Vaginosis. Available online: https://clinicaltrials.gov/ct2/show/NCT01577537 (accessed on 29 January 2023).
- Efficacy and Safety Study of SPL7013 Gel to Prevent the Recurrence of Bacterial Vaginosis (BV). Available online: https://clinicaltrials.gov/ct2/show/NCT02236156 (accessed on 29 January 2023).
- Efficacy and Safety Study of SPL7013 Gel to Prevent the Recurrence of Bacterial Vaginosis (BV). Available online: https://clinicaltrials.gov/ct2/show/NCT02237950 (accessed on 29 January 2023).
- A Study of AZD0466 in Patients With Advanced Hematologic or Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT04214093 (accessed on 17 March 2023).
- A Phase I/II Study of AZD0466 as Monotherapy or in Combination With Anticancer Agents in Advanced Non-Hodgkin Lymphoma. Available online: https://clinicaltrials.gov/ct2/show/NCT05205161 (accessed on 29 January 2023).
- Partnered-DEP® products—AZD0466. Available online: https://starpharma.com/drug_delivery/dep-azd0466 (accessed on 29 January 2023).
- Study of AZD0466 Monotherapy or in Combination in Patients With Advanced Haematological Malignancies. Available online: https://clinicaltrials.gov/ct2/show/NCT04865419 (accessed on 29 January 2023).
- Treatment of Non-responding to Conventional Therapy Inoperable Liver Cancers by In Situ Introduction of ImDendrim (ImDendrim). Available online: https://clinicaltrials.gov/ct2/show/NCT03255343 (accessed on 29 January 2023).
- A Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of OP-101 after Intravenous Administration in Healthy Volunteers. Available online: https://clinicaltrials.gov/ct2/show/NCT03500627 (accessed on 29 January 2023).
- A Clinical Study to Measure the Effect of OP-101 after Being Administered Subcutaneous in Healthy Volunteers. Available online: https://clinicaltrials.gov/ct2/show/NCT04321980 (accessed on 29 January 2023).
- A Study to Evaluate OP-101 (Dendrimer N-acetyl-cysteine) in Severe Coronavirus Disease 2019 (COVID-19) Patients (PRANA). Available online: https://clinicaltrials.gov/ct2/show/NCT04458298 (accessed on 29 January 2023).
- A Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of D-4517.2 after Subcutaneous Administration in Healthy Participants. Available online: https://clinicaltrials.gov/ct2/show/NCT05105607 (accessed on 29 January 2023).
- A Study to Evaluate the Safety, Tolerability and Pharmacokinetics of D-4517.2 after Subcutaneous Administration in Subjects With Neovascular (Wet) Age-Related Macular Degeneration (AMD) or Subjects With Diabetic Macular Edema (DME) (Tejas). Available online: https://clinicaltrials.gov/ct2/show/NCT05387837 (accessed on 29 January 2023).
- The siCoV/KK46 Drug Open-safety Study. Available online: https://clinicaltrials.gov/ct2/show/NCT05208996 (accessed on 29 January 2023).
- Evaluation of Safety & Efficacy of MIR 19 ® Inhalation Solution in Patients With Moderate COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT05184127 (accessed on 29 January 2023).
- Holmes, W.R.; Maher, L.; Rosenthal, S.L. Attitudes of men in an Australian male tolerance study towards microbicide use. Sex Health 2008, 5, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, S.L.; Holmes, W.; Maher, L. Australian men’s experiences during a microbicide male tolerance study. Aids Care Psychol. Socio Med. Asp. Aids/HIV 2009, 21, 125–130. [Google Scholar] [CrossRef]
- Chen, M.Y.; Millwood, I.Y.; Wand, H.; Poynten, M.; Law, M.; Kaldor, J.M.; Wesselingh, S.; Price, C.F.; Clark, L.J.; Paull, J.R.A.; et al. A Randomized Controlled Trial of the Safety of Candidate Microbicide SPL7013 Gel When Applied to the Penis. Jaids 2009, 50, 375–380. [Google Scholar] [CrossRef] [Green Version]
- Cohen, C.R.; Brown, J.; Moscicki, A.B.; Bukusi, E.A.; Paull, J.R.A.; Price, C.F.; Shiboski, S. A Phase I Randomized Placebo Controlled Trial of the Safety of 3% SPL7013 Gel (VivaGel (R)) in Healthy Young Women Administered Twice Daily for 14 Days. PLoS ONE 2011, 6, e16285. [Google Scholar] [CrossRef] [Green Version]
- McGowan, I.; Gomez, K.; Bruder, K.; Febo, I.; Chen, B.A.; Richardson, B.A.; Husnik, M.; Livant, E.; Price, C.; Jacobson, C.; et al. Phase 1 randomized trial of the vaginal safety and acceptability of SPL7013 gel (VivaGel) in sexually active young women (MTN-004). Aids 2011, 25, 1057–1064. [Google Scholar] [CrossRef] [Green Version]
- Price, C.F.; Tyssen, D.; Sonza, S.; Davie, A.; Evans, S.; Lewis, G.R.; Xia, S.; Spelman, T.; Hodsman, P.; Moench, T.R.; et al. SPL7013 Gel (VivaGel (R)) Retains Potent HIV-1 and HSV-2 Inhibitory Activity following Vaginal Administration in Humans. PLoS ONE 2011, 6, e24095. [Google Scholar] [CrossRef] [Green Version]
- Moscicki, A.B.; Kaul, R.; Ma, Y.F.; Scott, M.E.; Scott, M.E.; DAUD, I.I.; Bukusi, E.A.; Shiboski, S.; Rebbapragada, A.; Huibner, S.; et al. Measurement of Mucosal Biomarkers in a Phase 1 Trial of Intravaginal 3% StarPharma LTD 7013 Gel (VivaGel) to Assess Expanded Safety. Jaids 2012, 59, 134–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carballo-Dieguez, A.; Giguere, R.; Dolezal, C.; Chen, B.A.; Kahn, J.; Zimet, G.; Mabragana, M.; Leu, C.S.; McGowan, I. “Tell Juliana”: Acceptability of the Candidate Microbicide VivaGel(A (R)) and Two Placebo Gels Among Ethnically Diverse, Sexually Active Young Women Participating in a Phase 1 Microbicide Study. AIDS Behav. 2012, 16, 1761–1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldbaum, A.S.; Schwebke, J.R.; Paull, J.R.A.; Price, C.F.; Edmondson, S.R.; Castellarnau, A.; McCloud, P.; Kinghorn, G.R. A phase 2, double-blind, multicenter, randomized, placebo-controlled, dose-ranging study of the efficacy and safety of Astodrimer Gel for the treatment of bacterial vaginosis. PLoS ONE 2020, 15, e0232394. [Google Scholar] [CrossRef] [PubMed]
- Chavoustie, S.E.; Carter, B.A.; Waldbaum, A.S.; Donders, G.G.G.; Peters, K.H.; Schwebke, J.R.; Paull, J.R.A.; Price, C.F.; Castellarnau, A.; McCloud, P.; et al. Two phase 3, double-blind, placebo-controlled studies of the efficacy and safety of Astodrimer 1% Gel for the treatment of bacterial vaginosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 245, 13–18. [Google Scholar] [CrossRef]
- Australian New Zealand Clinical Trials Registry. Available online: https://anzctr.org.au/ (accessed on 17 March 2023).
- Patterson, C.M.; Balachander, S.B.; Grant, I.; Pop-Damkov, P.; Kelly, B.; McCoull, W.; Parker, J.; Giannis, M.; Hill, K.J.; Gibbons, F.D.; et al. Design and optimisation of dendrimer-conjugated Bcl-2/x(L) inhibitor, AZD0466, with improved therapeutic index for cancer therapy. Commun. Biol. 2021, 4, 112. [Google Scholar] [CrossRef]
- Arulananda, S.; O’Brien, M.; Evangelista, M.; Jenkins, L.J.; Poh, A.R.; Walkiewicz, M.; Leong, T.; Mariadason, J.M.; Cebon, J.; Balachander, S.B.; et al. A novel BH3-mimetic, AZD0466, targeting BCL-XL and BCL-2 is effective in pre-clinical models of malignant pleural mesothelioma. Cell Death Discov. 2021, 7, 122. [Google Scholar] [CrossRef]
- Feeney, O.M.; Ardipradja, K.; Noi, K.F.; Mehta, D.; De Rose, R.; Yuen, D.; Johnston, A.P.R.; Kingston, L.; Ericsson, C.; Elmore, C.S.; et al. Subcutaneous delivery of a dendrimer-BH3 mimetic improves lymphatic uptake and survival in lymphoma. J. Control. Release 2022, 348, 420–430. [Google Scholar] [CrossRef]
- Akhtar, N.; Ashford, M.B.; Beer, L.; Bowes, A.; Bristow, T.; Broo, A.; Buttar, D.; Coombes, S.; Cross, R.; Eriksson, E.; et al. The Global Characterisation of a Drug-Dendrimer Conjugate—PEGylated poly-lysine Dendrimer. J. Pharm. Sci. 2022, 112, 844–858. [Google Scholar] [CrossRef]
- Yang, G.; Sadeg, N.; Tahar, H.B. New Potential In Situ Anticancer Agent Derived from [188Re]rhenium Nitro-Imidazole Ligand Loaded 5th Generation Poly-L-Lysine Dendrimer for Treatment of Transplanted Human Liver Carcinoma in Nude Mice. Drug Design. 2017, 06, 1–7. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Naylor, A.M.; Goddard, W.A. STARBURST Dendrimers: Molecular Level Control of Size, Shape, Surface Chemistry, Topology and Flexibility from Atoms to Macroscopic Matter. Angew. Chem. Int. Edit. 1990, 29, 138–175. [Google Scholar] [CrossRef]
- Tomalia, D.A. Dendrimer research. Science 1991, 252, 1231–1232. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A. In quest of a systematic framework for unifying and defining nanoscience. J. Nanopart. Res. 2009, 11, 1251–1310. [Google Scholar] [CrossRef] [Green Version]
- Stanwix, H.; Tomalia, D.A. An architectural journey: From trees, dendrons/dendrimers to nanomedicine. Nanomedicine 2012, 7, 953–956. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Christensen, J.B.; Boas, U. Dendrimers, Dendrons, and Dendritic Polymers: Discovery, Applications, and the Future; Cambridge University Press: Cambridge, UK, 2012; pp. 1–412. [Google Scholar]
- Kukowska-Latallo, J.F.; Bielinska, A.U.; Johnson, J.; Spindler, R.; Tomalia, D.A.; Baker, J.R. Efficient transfer of genetic material into mammalian cells using Starburst polyamidoamine dendrimers. Proc. Natl. Acad. Sci. USA 1996, 93, 4897–4902. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, T.D.; Karellas, P.; Henderson, S.A.; Giannis, M.; O’Keefe, D.F.; Heery, G.; Paull, J.R.A.; Matthews, B.R.; Holan, G. Dendrimers as drugs: Discovery and preclinical and clinical development of dendrimer-based microbicides for HIV and STI prevention. Mol. Pharm. 2005, 2, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Gusdon, A.M.; Faraday, N.; Aita, J.S.; Kumar, S.; Mehta, I.; Choi, H.A.; Cleland, J.L.; Robinson, K.; McCullough, L.D.; Ng, D.K.; et al. Dendrimer nanotherapy for severe COVID-19 attenuates inflammation and neurological injury markers and improves outcomes in a phase2a clinical trial. Sci. Transl. Med. 2022, 14, eabo2652. [Google Scholar] [CrossRef]
- Ashvattha Therapeutics. Available online: https://avttx.com/pipeline/ophthalmology/ (accessed on 17 March 2023).
- Khaitov, M.R.; Shilovskii, I.P.; Kozhikhova, K.V.; Kofiadi, I.A.; Smirnov, V.V.; Koloskova, O.O.; Sergeev, I.V.; Trofimov, D.Y.; Trukhin, V.P.; Skvortsova, V.I. Combination Antiviral Formulation against SARS-CoV-2 Comprising SARS-CoV-2 Genome-Targeting siRNAs and Transfection-Enhancing Cationic Peptide Dendrimer. RU2746362 C1, 11 March 2021. [Google Scholar]
- Khaitov, M.; Nikonova, A.; Shilovskiy, I.; Kozhikhova, K.; Kofiadi, I.; Vishnyakova, L.; Nikolskii, A.; Gattinger, P.; Kovchina, V.; Barvinskaia, E.; et al. Silencing of SARS-CoV-2 with modified siRNA-peptide dendrimer formulation. Allergy 2021, 76, 2840–2854. [Google Scholar] [CrossRef]
- Khaitov, M.; Nikonova, A.; Kofiadi, I.; Shilovskiy, I.; Smirnov, V.; Elisytina, O.; Maerle, A.; Shatilov, A.; Shatilova, A.; Andreev, S.; et al. Treatment of COVID-19 patients with a SARS-CoV-2-specific siRNA-peptide dendrimer formulation. Allergy 2023, 1–15. [Google Scholar] [CrossRef]
- Registration Certificate LP-007720. Available online: https://grls.rosminzdrav.ru/Grls_View_v2.aspx?routingGuid=bb62a3b8-7b38-4d71-aa9b-51660813a32a (accessed on 17 March 2023).
- Ullas, P.T.; Madhusudana, S.N.; Desai, A.; Sagar, B.K.C.; Jayamurugan, G.; Rajesh, Y.B.R.D.; Jayaraman, N. Enhancement of immunogenicity and efficacy of a plasmid DNA rabies vaccine by nanoformulation with a fourth-generation amine-terminated poly(ether imine) dendrimer. Int. J. Nanomed. 2014, 9, 627–634. [Google Scholar] [CrossRef] [Green Version]
- Dutta, T.; Garg, M.; Jain, N.K. Poly(propyleneimine) dendrimer and dendrosome mediated genetic immunization against hepatitis B. Vaccine 2008, 26, 3389–3394. [Google Scholar] [CrossRef] [PubMed]
- Karpenko, L.I.; Apartsin, E.K.; Dudko, S.G.; Starostina, E.V.; Kaplina, O.N.; Antonets, D.V.; Volosnikova, E.A.; Zaitsev, B.N.; Bakulina, A.Y.; Venyaminova, A.G.; et al. Cationic Polymers for the Delivery of the Ebola DNA Vaccine Encoding Artificial T-Cell Immunogen. Vaccines 2020, 8, 718. [Google Scholar] [CrossRef]
- Bahadoran, A.; Moeini, H.; Bejo, M.H.; Hussein, M.Z.; Omar, A.R. Development of Tat-Conjugated Dendrimer for Transdermal DNA Vaccine Delivery. J. Pharm. Pharm. Sci. 2016, 19, 325–338. [Google Scholar] [CrossRef] [Green Version]
- Bahadoran, A.; Ebrahimi, M.; Yeap, S.K.; Safi, N.; Moeini, H.; Hair-Bejo, M.; Hussein, M.Z.; Omar, A.R. Induction of a robust immune response against avian influenza virus following transdermal inoculation with H5-DNA vaccine formulated in modified dendrimer-based delivery system in mouse model. Int. J. Nanomed. 2017, 12, 8573–8585. [Google Scholar] [CrossRef] [Green Version]
- Chahal, J.S.; Khan, O.F.; Cooper, C.L.; McPartlan, J.S.; Tsosie, J.K.; Tilley, L.D.; Sidik, S.M.; Lourido, S.; Langer, R.; Bavari, S.; et al. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc. Natl. Acad. Sci. USA 2016, 113, e4133–e4142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chahal, J.S.; Fang, T.; Woodham, A.W.; Khan, O.F.; Ling, J.; Anderson, D.G.; Ploegh, H.L. An RNA nanoparticle vaccine against Zika virus elicits antibody and CD8+ T cell responses in a mouse model. Sci. Rep. 2017, 7, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, S.; Rijpkema, S.G.; Durrani, Z.; Florence, A.T. PLGA-dendron nanoparticles enhance immunogenicity but not lethal antibody production of a DNA vaccine against anthrax in mice. Int. J. Pharm. 2007, 331, 228–232. [Google Scholar] [CrossRef]
- Verminnen, K.; Beeckman, D.S.A.; Sanders, N.N.; De Smedt, S.; Vanrompay, D.C.G. Vaccination of turkeys against Chlamydophila psittaci through optimised DNA formulation and administration. Vaccine 2010, 28, 3095–3105. [Google Scholar] [CrossRef]
- Wang, X.; Dai, Y.; Zhao, S.; Tang, J.; Li, H.; Xing, Y.; Qu, G.; Li, X.; Dai, J.; Zhu, Y.; et al. PAMAM-Lys, a Novel Vaccine Delivery Vector, Enhances the Protective Effects of the SjC23 DNA Vaccine against Schistosoma japonicum Infection. PLoS ONE 2014, 9, e86578. [Google Scholar] [CrossRef] [Green Version]
- Daftarian, P.; Kaifer, A.E.; Li, W.; Blomberg, B.B.; Frasca, D.; Roth, F.; Chowdhury, R.; Berg, E.A.; Fishman, J.B.; Sayegh, H.A.A.; et al. Peptide-Conjugated PAMAM Dendrimer as a Universal DNA Vaccine Platform to Target Antigen-Presenting Cells. Cancer Res. 2011, 71, 7452–7462. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Q.; Wei, T.; Jia, Y.; Farbiak, L.; Zhou, K.; Zhang, S.; Wei, Y.; Zhu, H.; Siegwart, D.J. Dendrimer-Based Lipid Nanoparticles Deliver Therapeutic FAH mRNA to Normalize Liver Function and Extend Survival in a Mouse Model of Hepatorenal Tyrosinemia Type I. Adv. Mater. 2018, 30, e1805308. [Google Scholar] [CrossRef]
- Mbatha, L.S.; Maiyo, F.; Daniels, A.; Singh, M. Dendrimer-Coated Gold Nanoparticles for Efficient Folate-Targeted mRNA Delivery In Vitro. Pharmaceutics 2021, 13, 900. [Google Scholar] [CrossRef] [PubMed]
- Folliero, V.; Zannella, C.; Chianese, A.; Stelitano, D.; Ambrosino, A.; De Filippis, A.; Galdiero, M.; Franci, G.; Galdiero, M. Application of Dendrimers for Treating Parasitic Diseases. Pharmaceutics 2021, 13, 343. [Google Scholar] [CrossRef]
- Singh, M.; Briones, M.; Ott, G.; O’Hagan, D. Cationic microparticles: A potent delivery system for DNA vaccines. Proc. Natl. Acad. Sci. USA 2000, 97, 811–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillay, N.S.; Daniels, A.; Singh, M. Folate-Targeted Transgenic Activity of Dendrimer Functionalized Selenium Nanoparticles In Vitro. Int. J. Mol. Sci. 2020, 21, 7177. [Google Scholar] [CrossRef] [PubMed]
- Mbatha, L.S.; Maiyo, F.C.; Singh, M. Dendrimer functionalized folate-targeted gold nanoparticles for luciferase gene silencing in vitro: A proof of principle study. Acta Pharm. 2018, 69, 49–61. [Google Scholar] [CrossRef] [Green Version]
- Mbatha, L.S.; Singh, M. Starburst Poly(amidoamine) Dendrimer Grafted Gold Nanoparticles as a Scaffold for Folic Acid-Targeted Plasmid DNA Delivery In Vitro. J. Nanosci. Nanotechnol. 2019, 19, 1959–1970. [Google Scholar] [CrossRef]
- Shan, Y.; Luo, T.; Peng, C.; Sheng, R.; Cao, A.; Cao, X.; Shen, M.; Guo, R.; Tomás, H.; Shi, X. Gene delivery using dendrimer-entrapped gold nanoparticles as nonviral vectors. Biomaterials 2012, 33, 3025–3035. [Google Scholar] [CrossRef]
- Yuan, X.; Wen, S.; Shen, M.; Shi, X. Dendrimer-stabilized silver nanoparticles enable efficient colorimetric sensing of mercury ions in aqueous solution. Anal. Methods 2013, 5, 5486–5492. [Google Scholar] [CrossRef]
- Figueroa, E.R.; Lin, A.Y.; Yan, J.; Luo, L.; Foster, A.E.; Drezek, R.A. Optimization of PAMAM-gold nanoparticle conjugation for gene therapy. Biomaterials 2014, 35, 1725–1734. [Google Scholar] [CrossRef] [Green Version]
- Oladimeji, O.; Akinyelu, J.; Singh, M. Co-Polymer Functionalised Gold Nanoparticles Show Efficient Mitochondrial Targeted Drug Delivery in Cervical Carcinoma Cells. J. Biomed. Nanotechnol. 2020, 16, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Akinyelu, J.; Oladimeji, O.; Singh, M. Lactobionic acid-chitosan functionalised gold-coated poly(lactide-co-glycolide) nanoparticles for hepatocyte targeted gene delivery. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 045017. [Google Scholar] [CrossRef]
- Mecke, A.; Majoros, I.J.; Patri, A.K.; Baker, J.R.; Holl, M.M.B.; Orr, B.G. Lipid Bilayer Disruption by Polycationic Polymers: The Roles of Size and Chemical Functional Group. Langmuir 2005, 21, 10348–10354. [Google Scholar] [CrossRef] [PubMed]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatullin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Amano, T.; Yu, H.; Amano, M.; Leyder, E.; Badiola, M.; Ray, P.; Kim, J.; Ko, A.C.; Achour, A.; Weng, N.P.; et al. Controllable self-replicating RNA vaccine delivered intradermally elicits predominantly cellular immunity. bioRxiv 2022. [Google Scholar] [CrossRef]
- Luong, D.; Kesharwani, P.; Deshmukh, R.; Amin, M.; Gupta, U.; Greish, K.; Iyer, A.K. PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016, 43, 14–29. [Google Scholar] [CrossRef]
- Chowdhury, S.; Toth, I.; Stephenson, R.J. Dendrimers in vaccine delivery: Recent progress and advances. Biomaterials 2022, 280, 121303. [Google Scholar] [CrossRef]
- Beg, S.; Samad, A.; Alam, M.I.; Nazish, I. Dendrimers as Novel Systems for Delivery of Neuropharmaceuticals to the Brain. CNS Neurol. Disord. Drug Targets 2011, 10, 576–588. [Google Scholar] [CrossRef]
- Bolhassani, A.; Javanzad, S.; Saleh, T.; Hashemi, M.; Aghasadeghi, M.R.; Sadat, S.M. Polymeric nanoparticles Potent vectors for vaccine delivery targeting cancer and infectious diseases. Human Vaccines Immunother. 2014, 10, 321–332. [Google Scholar] [CrossRef] [Green Version]
- Roberts, J.C.; Bhalgat, M.K.; Zera, R.T. Preliminary biological evaluation of polyamidoamine (PAMAM) StarburstTM dendrimers. J. Biomed. Mater. Res. 1996, 30, 53–65. [Google Scholar] [CrossRef]
- Wolinsky, J.B.; Grinstaff, M.W. Therapeutic and diagnostic applications of dendrimers for cancer treatment. Adv. Drug Deliv. Rev. 2008, 60, 1037–1055. [Google Scholar] [CrossRef]
- Gothwal, A.; Khan, I.; Kumar, P.; Raza, K.; Kaul, A.; Mishra, A.K.; Gupta, U. Bendamustine–PAMAM Conjugates for Improved Apoptosis, Efficacy, and in Vivo Pharmacokinetics: A Sustainable Delivery Tactic. Mol. Pharm. 2018, 15, 2084–2097. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, D.; Bhadra, S.; Jain, S.; Jain, N.K. A PEGylated dendritic nanoparticulate carrier of fluorouracil. Int. J. Pharm. 2003, 257, 111–124. [Google Scholar] [CrossRef]
- Gajbhiye, V.; Kumar, P.V.; Tekade, R.K.; Jain, N.K. PEGylated PPI dendritic architectures for sustained delivery of H2 receptor antagonist. Eur. J. Med. Chem. 2009, 44, 1155–1166. [Google Scholar] [CrossRef]
- Agrawal, P.; Gupta, U.; Jain, N.K. Glycoconjugated peptide dendrimers-based nanoparticulate system for the delivery of chloroquine phosphate. Biomaterials 2007, 28, 3349–3359. [Google Scholar] [CrossRef]
- Bhadra, D.; Yadav, A.K.; Bhadra, S.; Jain, N.K. Glycodendrimeric nanoparticulate carriers of primaquine phosphate for liver targeting. Int. J. Pharm. 2005, 295, 221–233. [Google Scholar] [CrossRef]
- Konda, S.D.; Aref, M.; Wang, S.; Brechbiel, M.; Wiener, E.C. Specific targeting of folate–dendrimer MRI contrast agents to the high affinity folate receptor expressed in ovarian tumor xenografts. Magn. Reson. Mat. Phys. Biol. Med. 2001, 12, 104–113. [Google Scholar] [CrossRef]
- Knauer, N.; Arkhipova, V.; Li, G.; Hewera, M.; Pashkina, E.; Nguyen, P.-H.; Meschaninova, M.; Kozlov, V.; Zhang, W.; Croner, R.S.; et al. In Vitro Validation of the Therapeutic Potential of Dendrimer-Based Nanoformulations against Tumor Stem Cells. Int. J. Mol. Sci. 2022, 23, 5691. [Google Scholar] [CrossRef] [PubMed]
- Knauer, N.; Pashkina, E.; Aktanova, A.; Boeva, O.; Arkhipova, V.; Barkovskaya, M.; Meschaninova, M.; Karpus, A.; Majoral, J.-P.; Kozlov, V.; et al. Effects of Cationic Dendrimers and Their Complexes with microRNAs on Immunocompetent Cells. Pharmaceutics 2023, 15, 148. [Google Scholar] [CrossRef] [PubMed]
- Knauer, N.; Meschaninova, M.; Muhammad, S.; Hänggi, D.; Majoral, J.-P.; Kahlert, U.D.; Kozlov, V.; Apartsin, E.K. Effects of Dendrimer-microRNA Nanoformulations against Glioblastoma Stem Cells. Pharmaceutics 2023, 15, 968. [Google Scholar] [CrossRef] [PubMed]

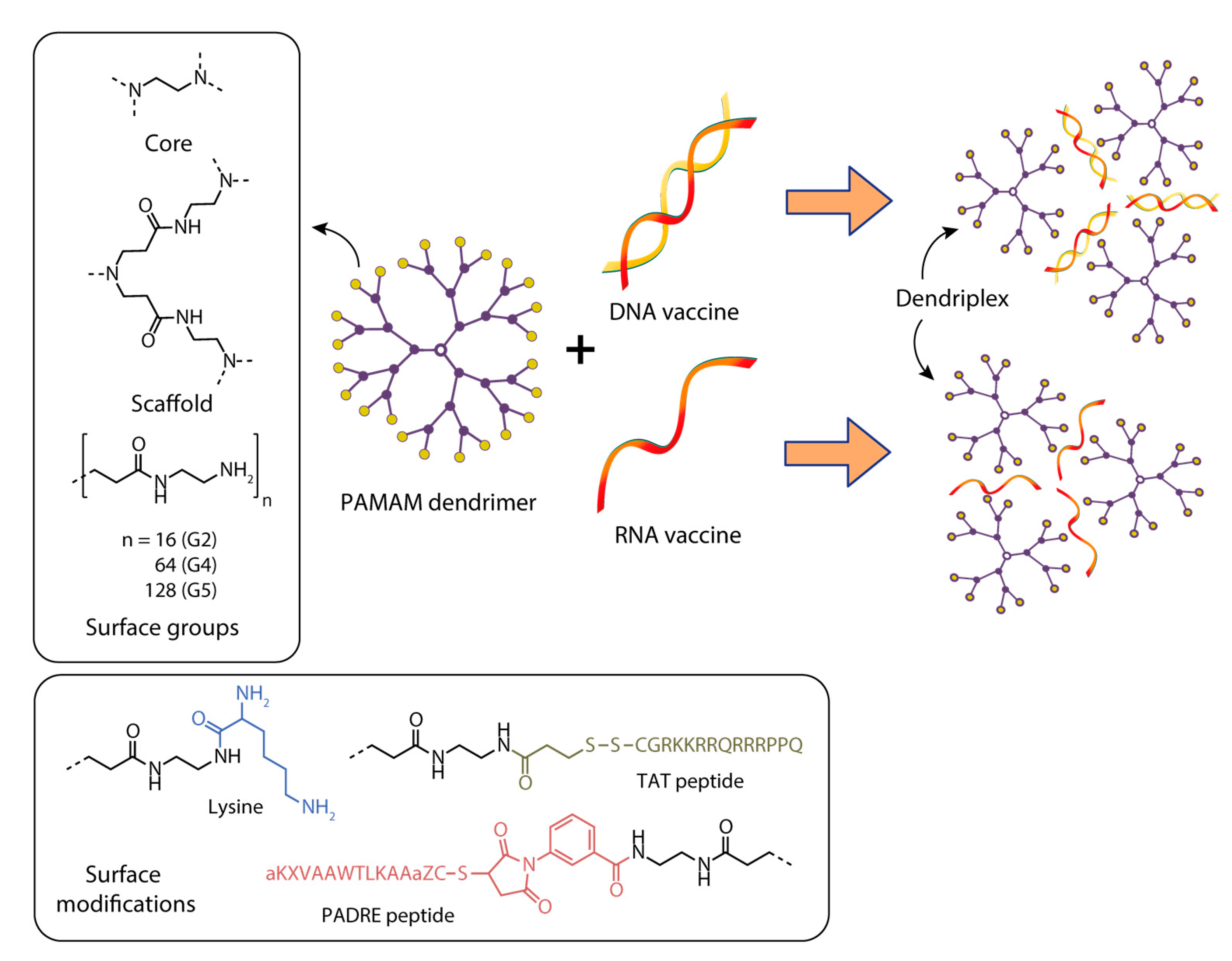


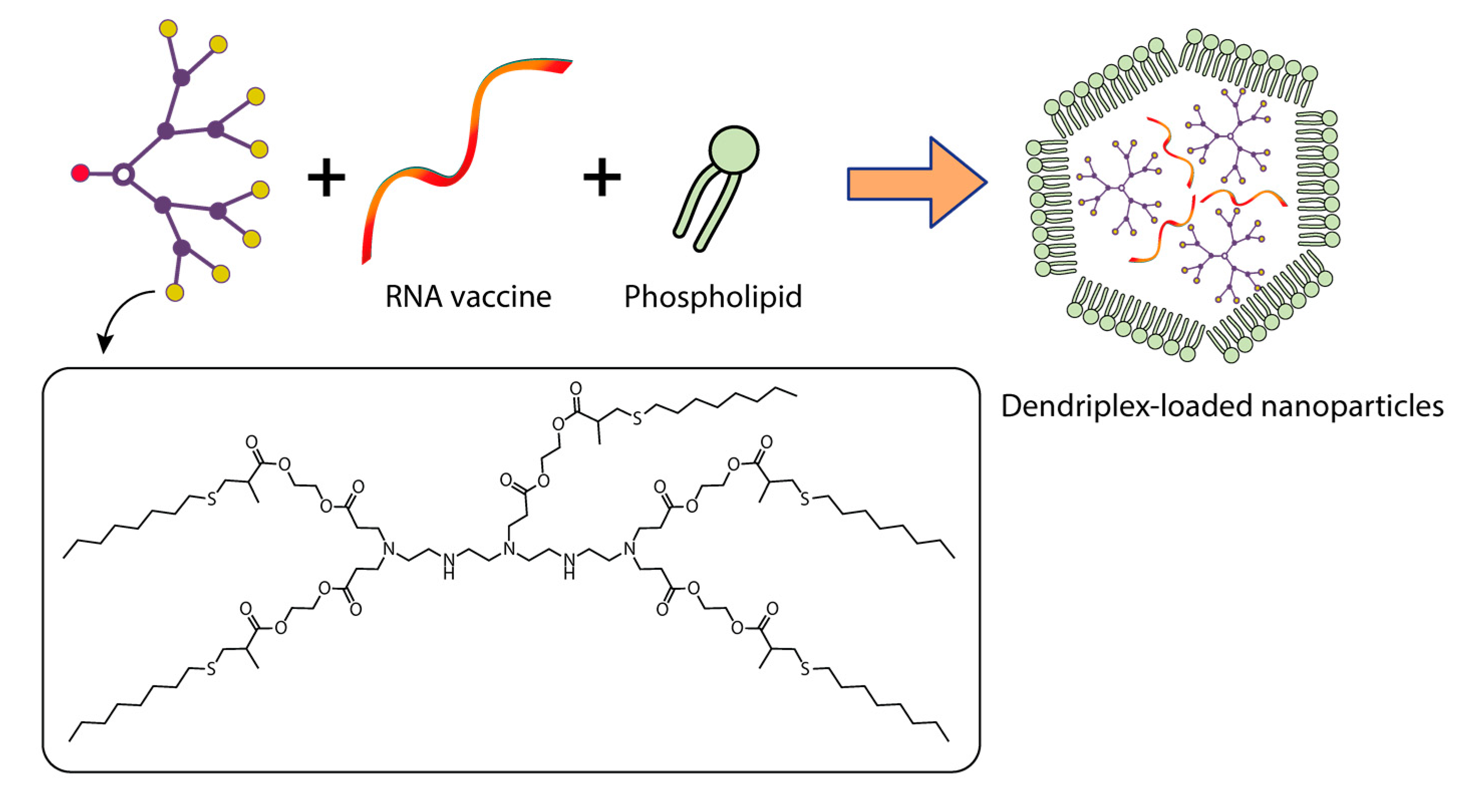
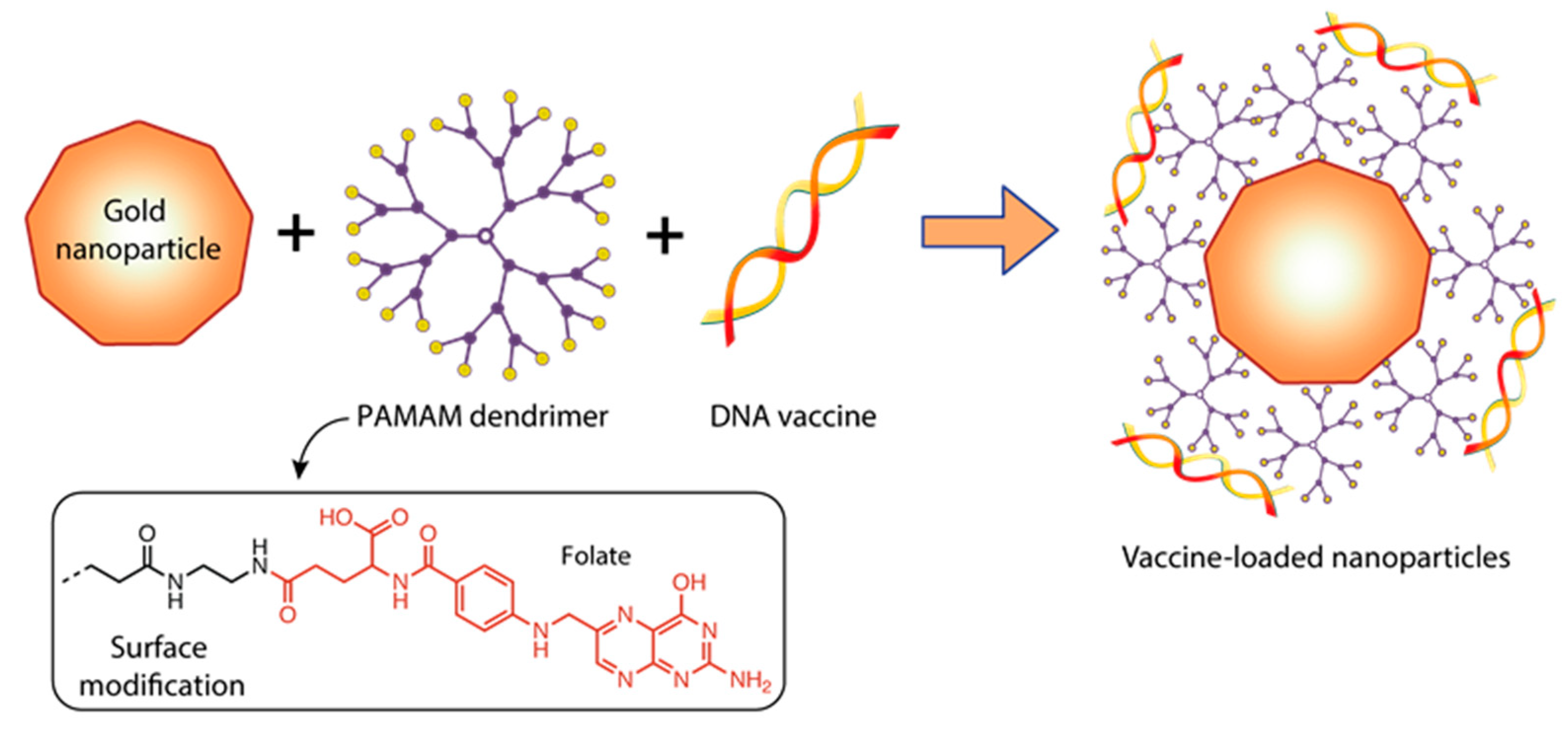

| Drug | Description of the Drug | Study Title | Study Dates | Brief Study Description | Company | Clinicaltrials.gov Identifier | Ref. |
|---|---|---|---|---|---|---|---|
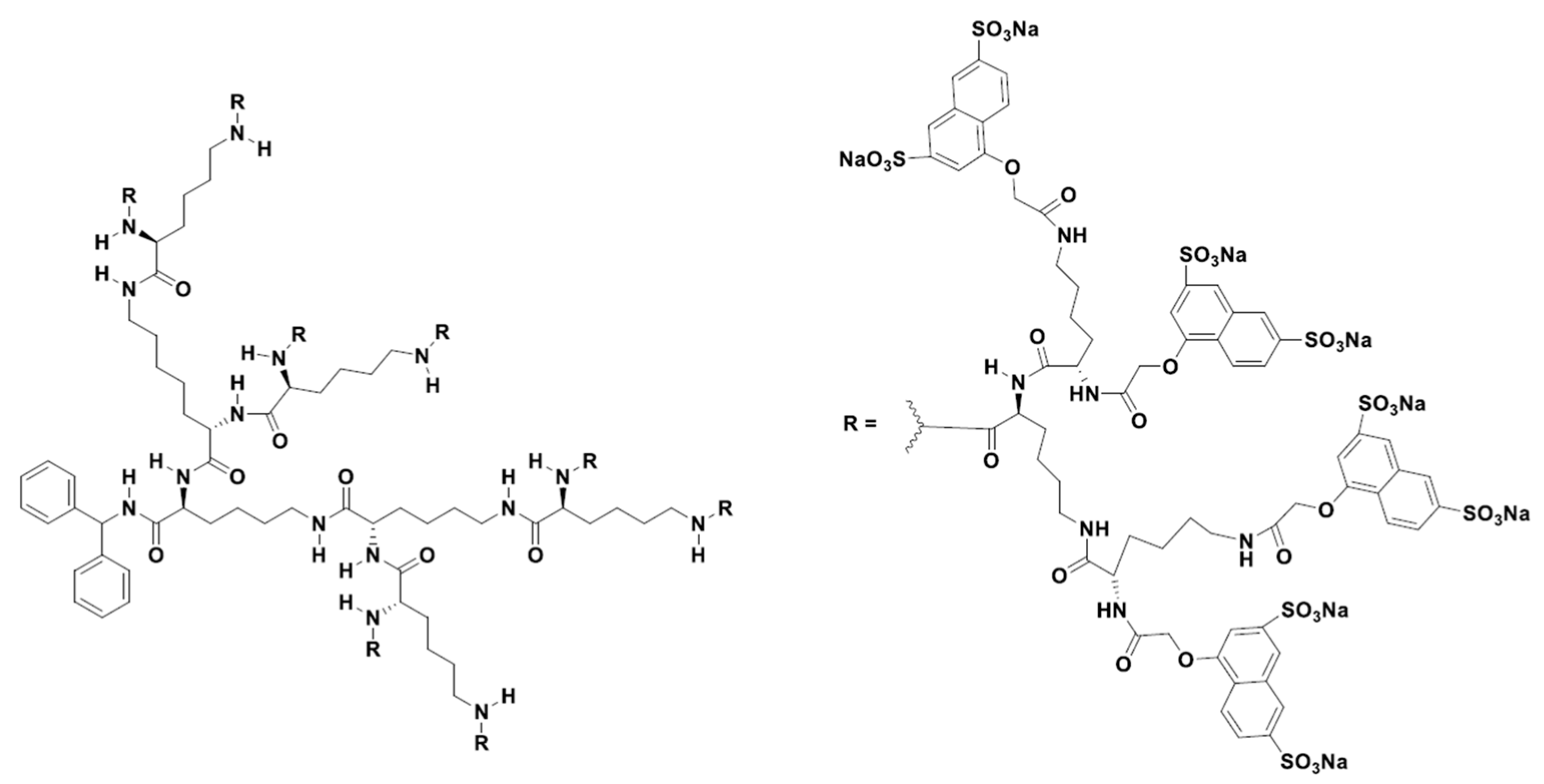
| SPL-7013 Gel (VivaGel™) | G4 poly(L-lysine) dendrimer bearing 32 sodium 1-(carboxymethoxy) naphthalene 3,6-disulfonate on the surface | SPL7013 gel—male tolerance study | August 2006–June 2007 | A phase 1, placebo-controlled study of the safety of a 3% w/w SPL7013 gel, administered to the penis of healthy male volunteers once daily for seven days | Starpharma Pty Ltd., Abbotsford, Australia | NCT00370357 | [87] |
| SPL-7013 Gel (VivaGel™) | “ | VivaGel™ in healthy young women | December 2006–November 2007 | A phase 1, expanded, randomized placebo-controlled trial of the safety and tolerability of a 3% w/w SPL7013 gel in healthy young women when administered twice daily for 14 days | Starpharma Pty Ltd., Abbotsford, Australia | NCT00331032 | [88] |
| SPL-7013 Gel (VivaGel™) | “ | Safety and acceptability of SPL7013 gel (VivaGel™) in sexually active women | July 2007–December 2009 | A phase 1 study of the safety and acceptability of a 3% w/w SPL7013 Gel applied vaginally in sexually active young women | Starpharma Pty Ltd., Abbotsford, Australia | NCT00442910 | [89] |
| SPL-7013 Gel (VivaGel™) | “ | Retention and duration of activity of SPL7013 (VivaGel®) after vaginal dosing | August 2008–March 2009 | Phase 1 and phase 2 assessments of local retention and duration of activity following vaginal application of a 3% VivaGel in healthy volunteers | Starpharma Pty Ltd., Abbotsford, Australia | NCT00740584 | [90] |
| SPL-7013 Gel (VivaGel™) | “ | Dose-ranging study of SPL7013 gel for treatment of bacterial vaginosis (BV) | August 2010–May 2011 | A phase 2, double-blind, multicenter, randomized, placebo-controlled, dose-ranging study to determine the efficacy and safety of the VivaGel administered vaginally in the treatment of bacterial vaginosis | Starpharma Pty Ltd., Abbotsford, Australia | NCT01201057 | [91] |
| SPL-7013 Gel (VivaGel™) | “ | Dose-ranging study of SPL7013 gel for the prevention of bacterial vaginosis (BV) | August 2011–December 2012 | A phase 2, double-blind, multicenter, randomized, placebo-controlled, dose-ranging study to determine the efficacy and safety of the SPL7013 gel administered vaginally to prevent the recurrence of bacterial vaginosis | Starpharma Pty Ltd., Abbotsford, Australia | NCT01437722 | [92] |
| SPL-7013 Gel (VivaGel™) | “ | A phase 3 study of SPL7013 gel (VivaGel) for the treatment of bacterial vaginosis | April 2012–October 2012 | A phase 3, double-blind, multicenter, randomized, placebo-controlled study to assess the efficacy and safety of a 1% SPL7013 gel for the treatment of bacterial vaginosis | Starpharma Pty Ltd., Abbotsford, Australia | NCT01577537 | [93] |
| SPL-7013 Gel (VivaGel™) | “ | A phase 3 study of SPL7013 gel (VivaGel) for the treatment of bacterial vaginosis | March 2012–July 2012 | A phase 3, double-blind, multicenter, randomized, placebo-controlled study to assess the efficacy and safety of a 1% SPL7013 gel for the treatment of bacterial vaginosis | Starpharma Pty Ltd., Abbotsford, Australia | NCT01577238 | [93] |
| SPL-7013 Gel (VivaGel™) | “ | Efficacy and safety study of SPL7013 gel to prevent the recurrence of bacterial vaginosis (BV) | October 2014–October 2016 | A phase 3, double-blind, multicenter, randomized, placebo-controlled study to determine the efficacy and safety of the SPL7013 gel to prevent the recurrence of bacterial vaginosis | Starpharma Pty Ltd., Abbotsford, Australia | NCT02236156 | [94] |
| SPL-7013 Gel (VivaGel™) | “ | Efficacy and safety study of SPL7013 gel to prevent the recurrence of bacterial vaginosis (BV) | October 2014–February 2017 | A phase 3, double-blind, multicenter, randomized, placebo-controlled study to determine the efficacy and safety of the SPL7013 gel to prevent the recurrence of bacterial vaginosis | Starpharma Pty Ltd., Abbotsford, Australia | NCT02237950 | [95] |
| AZD0466 | Astra Zeneca cancer drug AZD4320, chemically conjugated to a PEGylated poly-lysine dendrimer | A Study of AZD0466 in patients with advanced hematologic or solid tumors | December 2019–June 2021 | A phase 1, first-in-human study to determine the safety, tolerability, maximum tolerated dose (MTD), recommended Phase 2 dose (RP2D), and pharmacokinetics (PK) of AZD0466 in patients with solid tumors, lymphoma, and multiple myeloma at low, intermediate, or high risk for tumor lysis syndrome (TLS) with hematologic malignancies for whom no standard therapy exists | Starpharma, Abbotsford, Australia; AstraZeneca, UK, Cambridge | NCT04214093 | [96] |
| AZD0466 | “ | A phase I/II study of AZD0466 as monotherapy or in combination with anticancer agents in advanced non-Hodgkin lymphoma | July 2022–November 2024 [Estimated] | A phase 1/2, modular, open-label, dose escalation and expansion, multicenter study of the safety, tolerability, PK, and preliminary efficacy of AZD0466 as a monotherapy, or in combination with other anticancer agents in patients with advanced NHL | Starpharma, Abbotsford, Australia; AstraZeneca, UK, Cambridge | NCT05205161 | [97,98] |
| AZD0466 | “ | Study of AZD0466 monotherapy or in combination in patients with advanced hematological malignancies | June 2021–June 2024 [Estimated] | A phase 1/2, modular, open-label, multicenter study to assess the safety, tolerability, pharmacokinetics, and preliminary efficacy of AZD0466 as a monotherapy and drug-drug interaction potential between AZD0466 and the azole antifungal voriconazole in participants with advanced hematological malignancies | Starpharma, Abbotsford, Australia; AstraZeneca, UK, Cam-bridge | NCT04865419 | [99] |
| ImDendrim | G5 polylysine dendrimer mixed with nitro-imidazole-methyl-1,2,3-triazol-methyl-di-(2-pycolyl) amine | Treatment of non-responding to conventional therapy inoperable liver cancers by in situ introduction of ImDendrim | March 2017–December 2017 | An open-label and unicenter study in patients with primary hepatocellular cancer or metastatic liver cancer without standard therapeutic options for treatment, including chemotherapy or surgery | National Institute of Allergy and Infectious Diseases (NIAID), North Bethesda, Maryland, USA | NCT03255343 | [100] |
| OP-101 | G4 PAMAM dendrimer N-acetyl-cysteine | A study to evaluate the safety, tolerability, and pharmacokinetics of OP-101 after intravenous administration in healthy volunteers | March 2018–July 2018 | A phase 1, open-label single ascending dose study to evaluate the safety, tolerability, and pharmacokinetics after intravenous administration in healthy volunteers | Orpheris, Inc. Redwood City, California, USA | NCT03500627 | [101] |
| OP-101 | “ | A clinical study to measure the effect of OP-101 after being administered subcutaneous in healthy volunteers | March 2020–May 2020 | A phase 1, open-label single ascending dose study to evaluate the safety, tolerability, and pharmacokinetics after subcutaneous administration in healthy volunteers | Orpheris, Inc. Redwood City, California, USA | NCT04321980 | [102] |
| OP-101 | “ | A study to evaluate OP-101 (dendrimer N-acetyl-cysteine) in severe coronavirus disease 2019 (COVID-19) patients (PRANA) | August 2020–August 2022 [Estimated] | A phase 2, two-stage, double-blind, placebo-controlled study to evaluate the safety, tolerability, pharmacokinetics, and efficacy in patients with severe COVID-19 | Ashvattha Therapeutics, Inc. Redwood City, California, USA | NCT04458298 | [103] |
| D-4517.2 | Hydroxyl dendrimer, VEGFR tyrosine kinase inhibitor | A study to evaluate the safety, tolerability, and pharmacokinetics of D-4517.2 after subcutaneous administration in healthy participants | January 2022–August 2022 | A phase 1, open-label, single-ascending dose study of the safety, tolerability, and pharmacokinetics after subcutaneous administration in healthy volunteers | Ashvattha Therapeutics, Inc. Redwood City, California, USA | NCT05105607 | [104] |
| D-4517.2 | “ | A study to evaluate the safety, tolerability, and pharmacokinetics of D-4517.2 after subcutaneous administration in subjects with neovascular (Wet) age-related macular degeneration (AMD), or subjects with diabetic macular edema (DME) (Tejas) | August 2022–June 2023 [Estimated] | A phase 2, two-stage study: open-label assessment of safety and pharmacodynamic response as well as a visual examiner-masked, randomized active, sham, and placebo controlled study evaluating the efficacy of D-4517.2 administered subcutaneously to subjects with neovascular age-related macular degeneration or subjects with diabetic macular edema | Ashvattha Therapeutics, Inc. Redwood City, California, USA | NCT05387837 | [105] |
| siCoV/KK46 | Anti-SARS-CoV-2 siRNA (targeting RNA-dependent RNA polymerase)/KK-46 (peptide dendrimer) complex | The siCoV/KK46 drug open-safety study | January 2021–March 2021 | A phase 1, open-label, dose-escalation study to assess the safety and tolerability of single and multiple doses in healthy volunteers (inhalation use) | National Research Center —Institute of Immunology FMBA, Saint Petersburg, Russia | NCT05208996 | [106] |
| MIR 19® | “ | Evaluation of safety and efficacy of a MIR 19 ® inhalation solution in patients with moderate COVID-19 | April 2021–September 2021 | A phase 2, multicenter controlled randomized study to assess the efficacy and safety of MIR 19® via 14 days of treatment of participants with symptomatic moderate COVID-19 | National Research Center—Institute of Immunology FMBA, Saint Petersburg, Russia | NCT05184127 | [107] |
| Vaccine Antigen/Type of Tumor | Complex Type | Physicochemical Characteristics of Particles | Immunization | Immune Response | Ref. | ||
|---|---|---|---|---|---|---|---|
| Model | Administration Route/Regimen | Dose | |||||
| Viral infections | |||||||
| DNA vaccine | |||||||
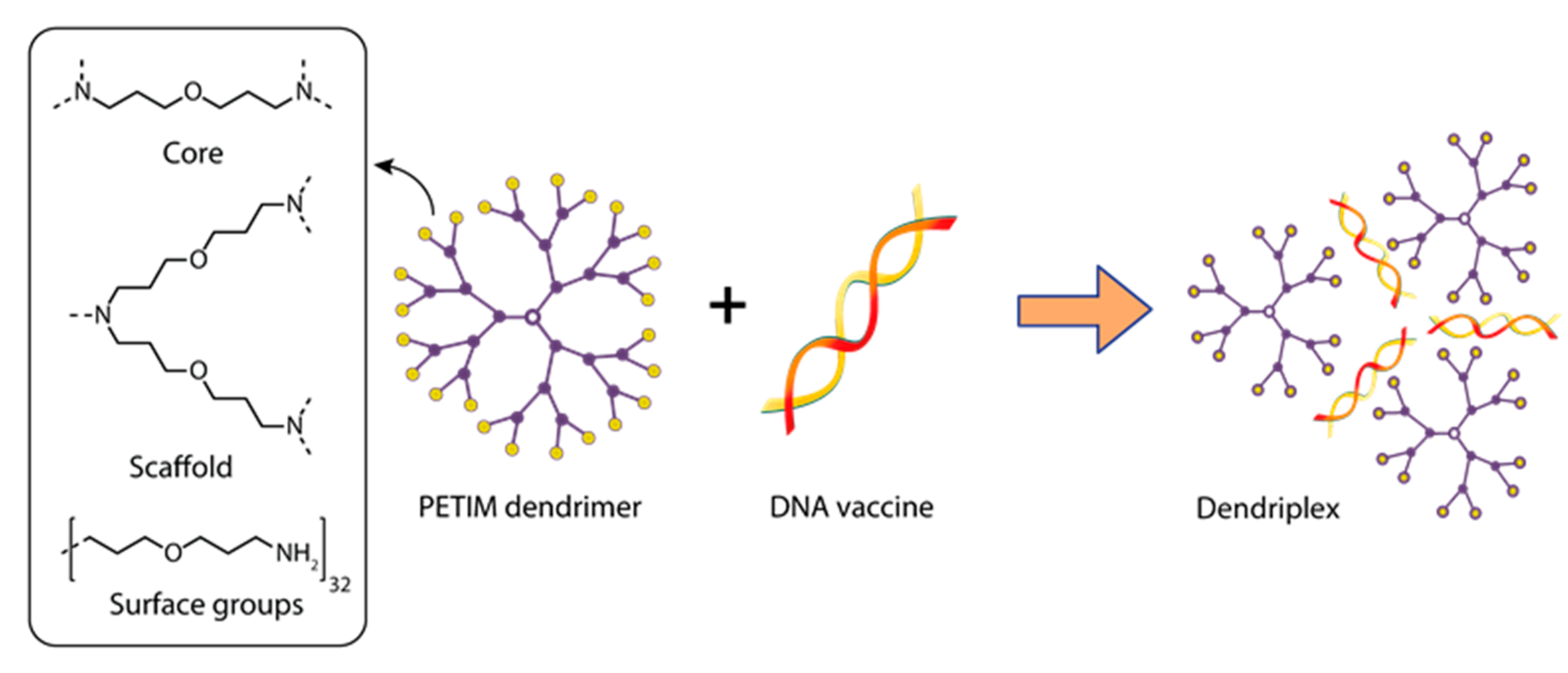
| Rabies surface glycoprotein (Rgp) | Dendriplex PETIM:pIRES-Rgp | PETIM:pIRES-Rgp ratio (w/w)—10:1; Particle size—500 nm | Swiss albino mice | IM/triple injection on days 0, 7, and 21 | 90 μg of pIRES-Rgp 10 μg of PETIM: pIRES-Rgp | Immunization PETIM:pIRES-Rgp provided induction of specific anti-rabies IgG starting from the 14th day and provided 100% protection of animals against virus infection | [137] |
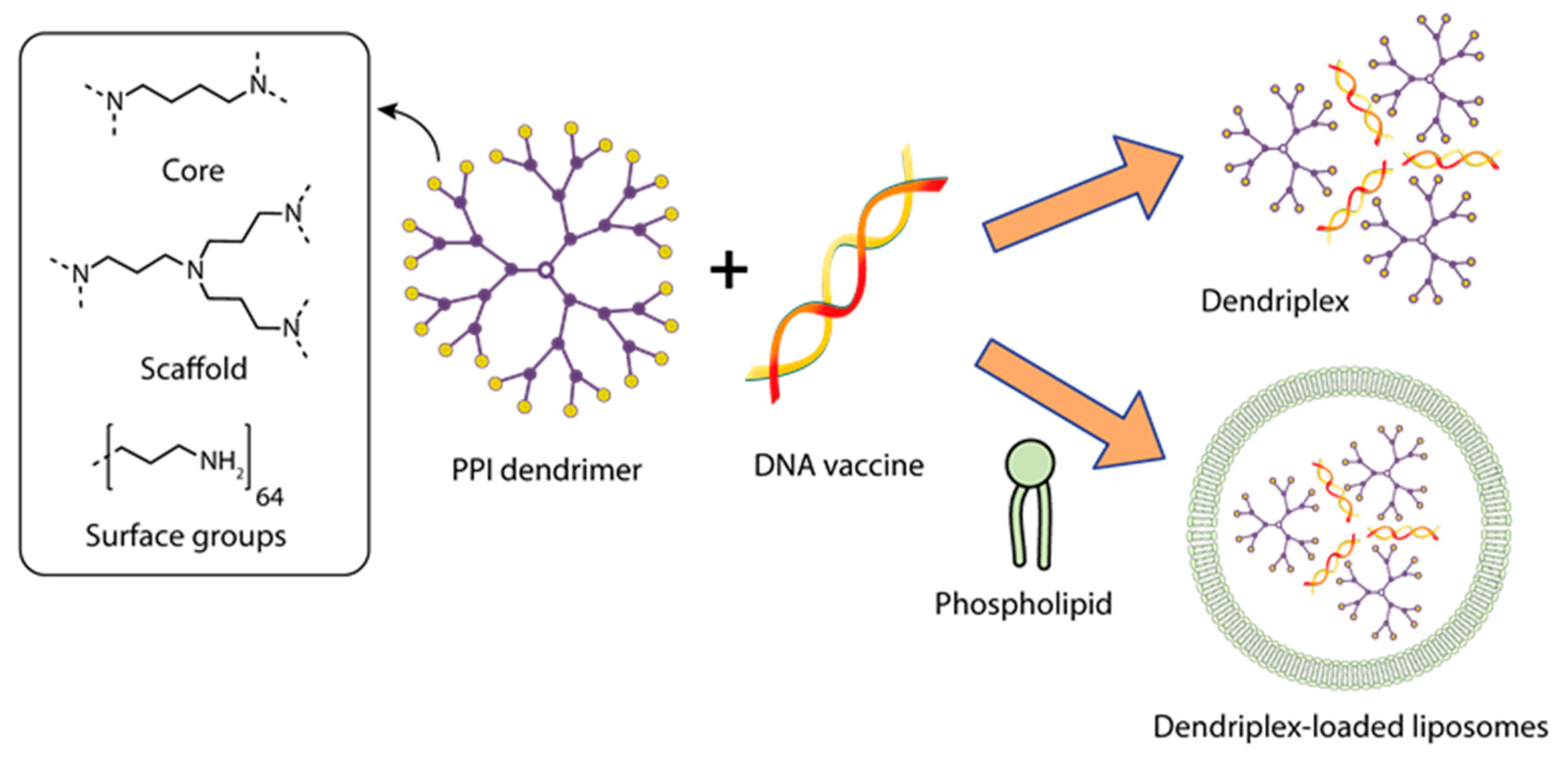
| Hepatitis B surface antigen | Dendriplex: pRc/CMV-HBs/ Poly(propylene imine) dendrimer PPI G5 DF3: Dendriplex-loaded phosphatidylcholine (PC) and cholesterol (C) vesicles | Dendriplex PPI 50: Molar ratio plasmid: PPI—1:50 Zeta potential (mV)—21.3 ± 0.33 DF3: Molar ratio PC:C—7:3 PPI 50 entrapment efficiency (%)—46.79 ± 1.33 Vesicle size (nm)—121 ± 2.9 Zeta potential (mV)—29.33 ± 0.21 | Balb/c mice | IM/single injection on day 1 | 10 µg of plasmid pRc/CMV-HBs in dendrimer or dendrosome form | Complex DF3 provided the induction of specific anti-HBs IgG and Th1 response significantly higher and longer than complex PPI 50. The “naked” pRc/CMV-HBs had weak immunogenicity. | [138] |
| Ebola virus | Dendriplex PAMAM G4 dendrimer + DNA encoding artificial T-cell antigens EBOV EV.CTL and EV.Th | pEV.CTL/pEV.Th + PAMAM N/P ratio—3:1; Particle size (nm)—< 100; Zeta Potential, (mV): pEV.CTL + PAMAM—27.3 ± 6.9; pEV.Th + PAMAM—9.6 ±6.7 | Balb/c mice | IM/triple injection on days 0, 14, and 28 | 100 μg of plasmid pEV.CTL /pEV.Th + PAMAM G4 | The immune response to both naked DNA vaccines and DNA vaccines in combination with PAMAM was the same. | [139] |
| H5N1 avian influenza virus | Dendriplex PAMAM G5 dendrimer + TAT polypeptide + DNA (pBud-H5-GFP) | TAT-PAMAM-DNA polyplexes: Molar ratio—6:1 Average particle size (nm)—105 Zeta potential (mV)—42 | Balb/c mice | IM/ double injection on days 0 and 21 | 50 μL of PAMAM—pDNA polyplexes and TAT-PAMAM-pDNA polyplexes | Immunization with TAT-PAMAM-DNA and PAMAM-DNA polyplexes caused the formation of specific HI-antibodies and induced T-cell activation | [140,141] |
| PAMAM—DNA polyplexes: Molar ratio—6:1 Average particle size (nm)—103 Zeta potential (mV)—32 | |||||||
| mRNA-vaccine | |||||||
| (a) Ebola virus glycoprotein (b) H1N1 influenza hemagglutinin (c) Toxoplasma gondii | Amphiphilic dendrimer + replicating VEEV mRNAs encoding pathogen antigens | Mass ratio of modified dendrimer to 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] to RNA—11.5:1:2.3 Diameter (nm)~ 200–300 | C57BL/6 mice | IM/single injection on day 1 | 40 µg of MDNP-encapsulated VEEV RNAs encoding different pathogen antigens | Immunization with PAMAM G1-mRNAs caused the formation of IgG and induced T-cell activation. Immunization of PAMAM G1-mRNA encoding the Ebola virus antigens provided 60% protection of animals from virus infection. Immunization of PAMAM G1-mRNAs encoding H1N1 and Toxoplasma gondii antigens ensured 100% protection of animals against virus infection. | [142] |
| Zika virus | Amphiphilic dendrimer + RNA, VEEV with RNA encoding ZIKV E protein | Mass ratio of modified dendrimer to 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] to RNA—11.5:1:2.3 Diameter (nm)~ 200–300 | Balb/c mice | IM/single injection on Day 1 | 40 µg of MDNP-encapsulated VEEV RNAs, encoding ZIKV E protein | Immunization with PAMAM G1-mRNA caused the formation of IgG and induced T-cell activation. | [143] |
| Bacterial infections | |||||||
| Protective antigen (PA) gene of Bacillus anthracis | Amphiphilic poly-L-lysine dendron + PA antigen DNA encapsulated in PLGA particles | PLGA-PA DNA: with the same molar charge ratio Mean particle size (nm)—> 800 Zeta Potential, (mV)~ −19 | Balb/c mice | IM/quadruple injection on days 1, 21, 42, and 63 | Prime injection: 20 µg of PLGA-PA DNA or DNA:Dendron Boost injection: 14 µg of PLGA-PA DNA or DNA:Dendron | Immunization with PLGA-PA DNA induced specific IgG1 but was not able to neutralize the toxin | [144] |
| DNA:Dendron: Molar charge ratio—10:1; Mean particle size (nm)~ 400; Zeta Potential, (mV)~ −17 | |||||||
| Chlamydophila (Cp.) psittaci | Poliplex brPEI-pcDNA1/MOMPopt | brPEI polyplexes: N/P ratio—8; Particle size (nm)~ 114;Zeta Potential, mV~ 48 | Turkeys | IM / aerosol/ double injection on days 1 and 21 | 100 µg of plasmid pcDNA1/MOMPopt and brPEI-pcDNA1/MOMP opt (IM); | Immunization with brPEI polyplex induces the formation of specific IgG, identifying a significantly higher average percentage of CD4+ T cells and provides a high level of protection against virus infection for animals | [145] |
| 500 µg of brPEI-pcDNA1/MOMP opt (Aerosol) | |||||||
| Parasitic infections | |||||||
| Schistosoma japonicum | Dendriplex G4 PAMAM-Lys + membrane protein DNA (SjC23) | PAMAM-Lys/DNA complex Charge ratio—4:1; Particle size (nm)–50–100; | Balb/c mice | IM/ Triple injection on days 0, 14, and 28 | 100 μg of plasmid PAMAM-Lys/DNA complex | Immunization with PAMAM-Lys elicited a predominantly humoral IgG2a response and a dramatic increase in IL-2 and IFN-γ production compared to the SjC23 naked DNA vaccine. | [146] |
| Oncological diseases | |||||||
| Melanoma | DNA (pcDNA3-tyrosine-related protein-2 (TRP2) and pcDNA3-gp70) conjugated with G5-PAMAM-PADRE epitope | N/P ratio—10:1; Particle size (nm)–600; | mice C57BL/6 | Subcutaneous electroporation/ double injection on days 0 and 14 | 20 μg of plasmid pcDNA3-TRP2 | Subcutaneous injection of DNA-peptide-dendrimer complexes, followed by dermal electroporation, transfected APC, mostly DCs, in vivo directly in the lymph nodes, induced T cell immunity and humoral response, and reduced tumor growth in a B16F10 melanoma model. | [147] |
| Protein metabolism disorders | |||||||
| mRNA-FAH (fumarylacetoacetate hydrolase) | 5A2-SC8-mRNA-loaded dendrimer lipid nanoparticles (mDLNPs) | 5A2-SC8 + mRNA Mass ratio—20:1; Diameter (nm)—95–101; Zeta Potential, (mV)—3.58 | Balb/c mice | IM/single injection on Day 1 | 0.5 µg of mDLNPs | mDLNPs transfect >44% of all hepatocytes in the liver and produce high levels of FAH protein | [148] |
| The use of complexes of dendrimers with metal particles for mRNA delivery | |||||||
| FLuc-mRNA | Gold nanoparticles modified with folate-conjugated PAMAM G5 complexed with FLuc-mRNA | Au:G5D:FA-mRNA: NP:mRNA(w/w) Ratio–4:1 Mean Diameter(nm) ± SD— 101.8 ± 36.9 Zeta Potential (mV) ± SD—65.7 ± 1.4 Polydispersity Index—0.131 | Cell lines: HEK293, HepG2, MCF-7, KB and Caco-2 | - | 0.05 µg of Au:G5D:FA+ FLuc-mRNA | Folic acid modification of Au:G5D:FA + FLuc-mRNA nanoparticles with grafted gold particles resulted in higher transfection efficiency in all cell lines. The use of G5 dendrimer increased stability of mRNA molecule. | [149] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kisakova, L.A.; Apartsin, E.K.; Nizolenko, L.F.; Karpenko, L.I. Dendrimer-Mediated Delivery of DNA and RNA Vaccines. Pharmaceutics 2023, 15, 1106. https://doi.org/10.3390/pharmaceutics15041106
Kisakova LA, Apartsin EK, Nizolenko LF, Karpenko LI. Dendrimer-Mediated Delivery of DNA and RNA Vaccines. Pharmaceutics. 2023; 15(4):1106. https://doi.org/10.3390/pharmaceutics15041106
Chicago/Turabian StyleKisakova, Lyubov A., Evgeny K. Apartsin, Lily F. Nizolenko, and Larisa I. Karpenko. 2023. "Dendrimer-Mediated Delivery of DNA and RNA Vaccines" Pharmaceutics 15, no. 4: 1106. https://doi.org/10.3390/pharmaceutics15041106
APA StyleKisakova, L. A., Apartsin, E. K., Nizolenko, L. F., & Karpenko, L. I. (2023). Dendrimer-Mediated Delivery of DNA and RNA Vaccines. Pharmaceutics, 15(4), 1106. https://doi.org/10.3390/pharmaceutics15041106







