Mesenchymal Stem Cell-Derived Exosomes in Ophthalmology: A Comprehensive Review
Abstract
:1. Introduction
2. Overview of MSC-Derived Exosomes
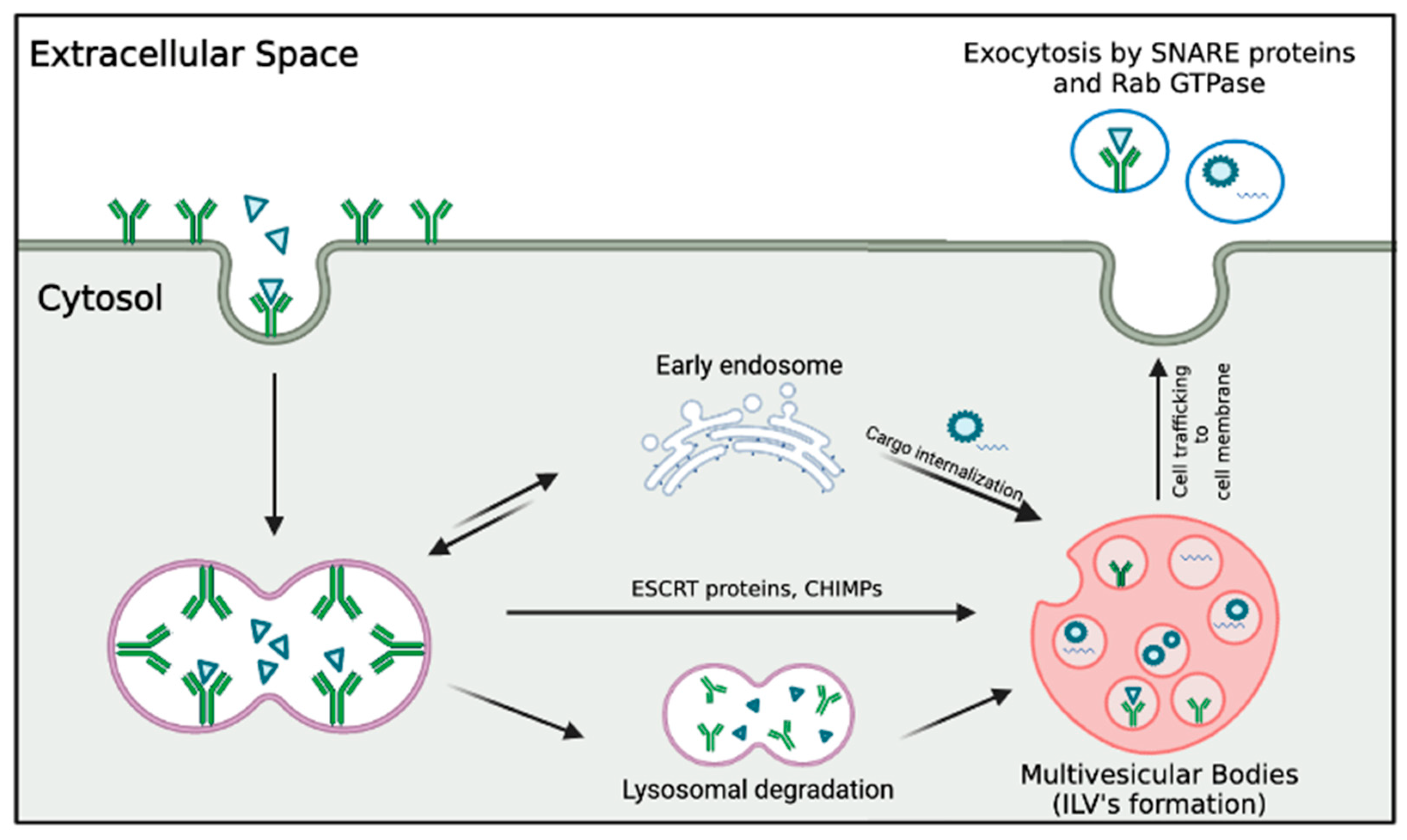
2.1. Exosomes: Characteristics and Biogenesis
2.2. Role of Exosomes in Cellular Communication
2.2.1. The Transfer of Biomolecules by Exosomes and Its Role in Intercellular Communication
2.2.2. The Immunomodulatory Potential of MSC-Exosomes in Immune-Mediated Ocular Diseases
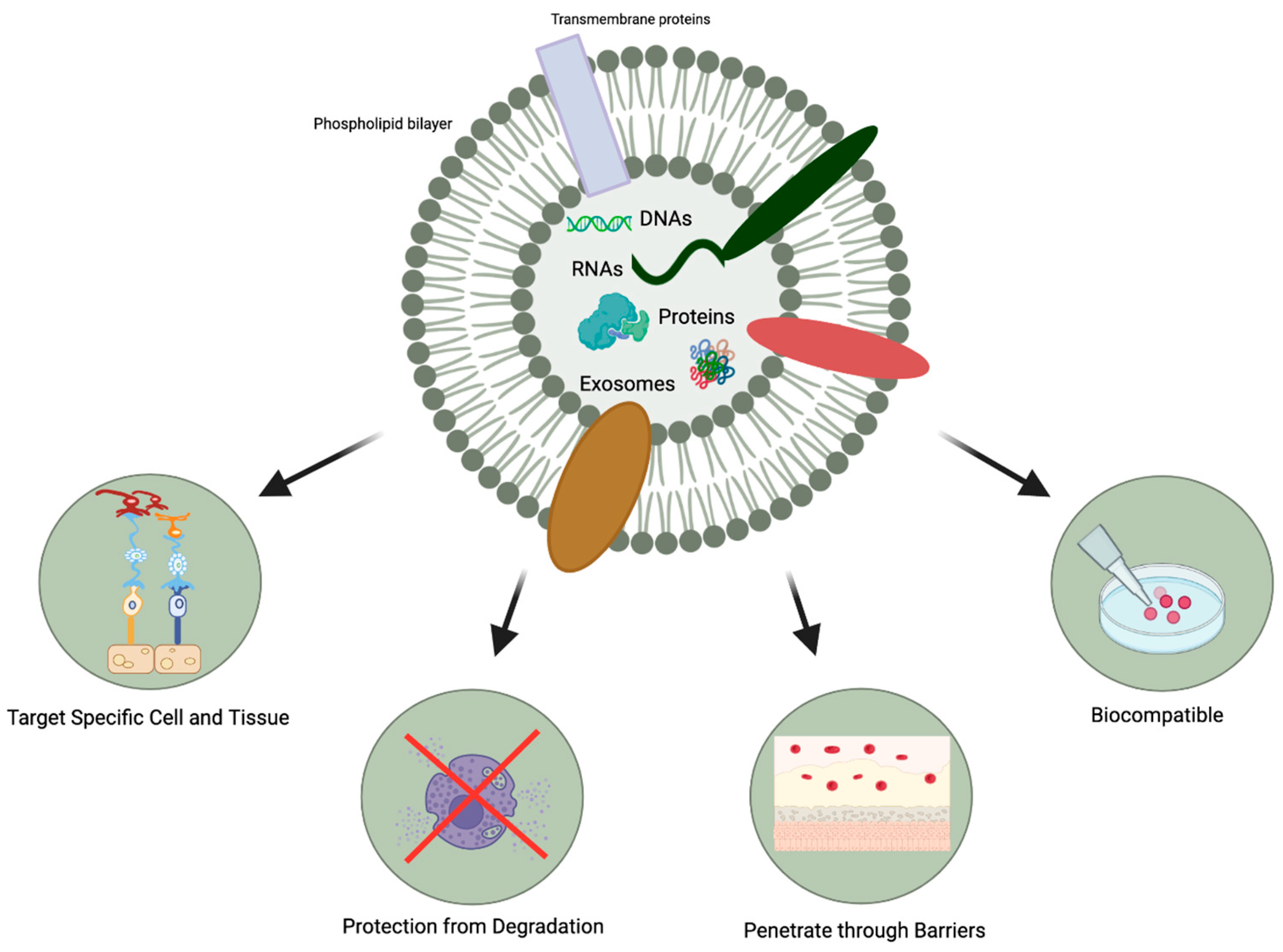
2.3. Advantages of MSC-Exosomes in Ophthalmology
- The protection afforded by the lipid bilayer means that MSC-exosomes can persist in the ocular structure for a long time. Specifically, the bilayer provides stability and structural rigidity and protects the enclosed cargo from premature enzymatic degradation [13].
- They are highly biocompatible due to their bilipid membrane acquired from the parent cells and their natural presence in the body fluids [19,20]. For instance, in comparison to MSC therapy, exosome-based therapies have a lower risk of teratoma formation, embolization, and graft versus host rejection [29,30].
- There is a possibility that they might be able to penetrate through biological barriers of the eye (i.e., blood-retinal and blood-aqueous barriers, tear film, corneal stromal, and vitreous) due to their small size and their bilipid membrane. (Figure 1) However, it is important to note that currently, no articles specifically address the ability of exosomes to penetrate the tear film, corneal, or other ocular barriers. Nevertheless, some research implies that exosomes can successfully traverse the blood-brain barrier (BBB) [31,32,33]. Furthermore, studies in other parts of the body have demonstrated exosomes’ capacity to overcome challenging barriers [34]. Based on this evidence, one might hypothesize that exosomes hold the potential to serve as a delivery platform for penetrating ocular barriers such as the tear film and corneal barriers. However, additional research is necessary to verify this hypothesis. This would offer greater versatility in terms of routes of administration and the ability to deliver a larger quantity of bioactive molecules to the target site.
2.4. MSC-Exosome Isolation and Preservation
2.5. Route of Administration-MSC-Exosome in Ophthalmology
2.6. Bioengineering MSC-Exosomes for Enhanced Drug Delivery
3. The Use of MSC-Derived Exosomes in Anterior Segment Diseases
3.1. MSC-Derived Exosomes for Corneal Regeneration
3.2. MSC-Derived Exosomes for Dry Eye Disease (DED)
3.2.1. GVHD-Associated DED
3.2.2. Sjogren’s Syndrome Dry Eye (SSDE)
3.3. MSC-Derived Exosomes for Corneal Clouding in Mucopolysaccharidosis
3.4. MSC-Derived Exosomes for Glaucoma
3.4.1. MSC-Derived Exosomes for Glaucomatous Optic Neuropathy
3.4.2. MSC-Derived Exosomes for Intraocular Pressure (IOP) Lowering Effect
4. The Use of MSC-Derived Exosomes in Posterior Segment Diseases and Uveitis
4.1. Retinitis Pigmentosa
4.2. Diabetic Retinopathy
4.3. Age-Related Macular Degeneration
4.4. Retinal Ischemia
4.5. Idiopathic Macular Hole
4.6. Uveitis
5. Overcoming Challenges in the Clinical Translation of MSC-Exosomes
5.1. Overcoming the Hurdles of MSC-Exosome Heterogeneity
5.2. Assessment of Parental MSCs as Proxy Indicator of MSC-Exosome Quality
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niamprem, P.; Srinivas, S.P.; Tiyaboonchai, W. Penetration of Nile Red-Loaded Nanostructured Lipid Carriers (NLCs) across the Porcine Cornea. Colloids Surf. B Biointerfaces 2019, 176, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Blass, S.; Teubl, B.; Fröhlich, E.; Meindl, C.; Rabensteiner, D.F.; Trummer, G.; Schmut, O.; Zimmer, A.; Roblegg, E. Permeability Studies on the Ocular Absorbance of Nanostructured Materials Across the Cornea. Sci. Pharm. 2010, 78, 678. [Google Scholar] [CrossRef] [Green Version]
- Mohammadpour, M.; Hashemi, H.; Jabbarvand, M.; Delrish, E. Penetration of Silicate Nanoparticles into the Corneal Stroma and Intraocular Fluids. Cornea 2014, 33, 738. [Google Scholar] [CrossRef]
- Yu, B.; Shao, H.; Su, C.; Jiang, Y.; Chen, X.; Bai, L.; Zhang, Y.; Li, Q.; Zhang, X.; Li, X. Exosomes Derived from MSCs Ameliorate Retinal Laser Injury Partially by Inhibition of MCP-1. Sci. Rep. 2016, 6, 34562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, B.; Li, X.-R.; Zhang, X.-M. Mesenchymal Stem Cell-Derived Extracellular Vesicles as a New Therapeutic Strategy for Ocular Diseases. World J. Stem Cells 2020, 12, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Liu, C.; Huang, L.; Chen, J.; Xu, N. Protective Effects of Intravitreal Administration of Mesenchymal Stem Cell-Derived Exosomes in an Experimental Model of Optic Nerve Injury. Exp. Cell Res. 2021, 407, 112792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, Y.; Kong, Y. Exosomes Derived from Mesenchymal Stem Cells Modulate MiR-126 to Ameliorate Hyperglycemia-Induced Retinal Inflammation Via Targeting HMGB1. Investig. Opthalmol. Vis. Sci. 2019, 60, 294. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.-K.; Chen, L.-J.; Zhou, S.-N.; Li, Y.-F.; Xiang, C. Multifunctional Role of MicroRNAs in Mesenchymal Stem Cell-Derived Exosomes in Treatment of Diseases. World J. Stem Cells 2020, 12, 1276–1294. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of Secretion and Uptake of Exosomes and Other Extracellular Vesicles for Cell-to-Cell Communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Xu, M.; Ji, J.; Jin, D.; Wu, Y.; Wu, T.; Lin, R.; Zhu, S.; Jiang, F.; Ji, Y.; Bao, B.; et al. The Biogenesis and Secretion of Exosomes and Multivesicular Bodies (MVBs): Intercellular Shuttles and Implications in Human Diseases. Genes Dis. 2022, S2352304222000976. [Google Scholar] [CrossRef]
- Wu, H.; Turner, C.; Gardner, J.; Temple, B.; Brennwald, P. The Exo70 Subunit of the Exocyst Is an Effector for Both Cdc42 and Rho3 Function in Polarized Exocytosis. Mol. Biol. Cell 2010, 21, 430–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, M.E.; Leonard, J.N. Stabilization of Exosome-Targeting Peptides via Engineered Glycosylation. J. Biol. Chem. 2015, 290, 8166–8172. [Google Scholar] [CrossRef] [Green Version]
- McKelvey, K.J.; Powell, K.L.; Ashton, A.W.; Morris, J.M.; McCracken, S.A. Exosomes: Mechanisms of Uptake. J. Circ. Biomark. 2015, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Bian, B.; Zhao, C.; He, X.; Gong, Y.; Ren, C.; Ge, L.; Zeng, Y.; Li, Q.; Chen, M.; Weng, C.; et al. Exosomes Derived from Neural Progenitor Cells Preserve Photoreceptors during Retinal Degeneration by Inactivating Microglia. J. Extracell. Vesicles 2020, 9, 1748931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, R.W.Y.; Lai, R.C.; Zhang, B.; Tan, S.S.; Yin, Y.; Teh, B.J.; Lim, S.K. Mesenchymal Stem Cell: An Efficient Mass Producer of Exosomes for Drug Delivery. Adv. Drug Deliv. Rev. 2013, 65, 336–341. [Google Scholar] [CrossRef]
- Liu, X.; Hu, L.; Liu, F. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Cell-Free Therapy of Ocular Diseases. Extracell. Vesicles Circ. Nucleic Acids 2022, 3, 102–117. [Google Scholar] [CrossRef]
- Samaeekia, R.; Rabiee, B.; Putra, I.; Shen, X.; Park, Y.J.; Hematti, P.; Eslani, M.; Djalilian, A.R. Effect of Human Corneal Mesenchymal Stromal Cell-Derived Exosomes on Corneal Epithelial Wound Healing. Investig. Opthalmol. Vis. Sci. 2018, 59, 5194. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Mugisha, A.; Fransisca, S.; Liu, Q.; Xie, P.; Hu, Z. Emerging Role of Exosomes in Retinal Diseases. Front. Cell Dev. Biol. 2021, 9, 643680. [Google Scholar] [CrossRef]
- Boukouris, S.; Mathivanan, S. Exosomes in Bodily Fluids Are a Highly Stable Resource of Disease Biomarkers. PROTEOMICS Clin. Appl. 2015, 9, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.S.; Lai, R.C.; Lee, M.M.; Choo, A.B.H.; Lee, C.N.; Lim, S.K. Mesenchymal Stem Cell Secretes Microparticles Enriched in Pre-MicroRNAs. Nucleic Acids Res. 2010, 38, 215–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, R.C.; Tan, S.S.; Teh, B.J.; Sze, S.K.; Arslan, F.; de Kleijn, D.P.; Choo, A.; Lim, S.K. Proteolytic Potential of the MSC Exosome Proteome: Implications for an Exosome-Mediated Delivery of Therapeutic Proteasome. Int. J. Proteomics 2012, 2012, 971907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glover, K.; Mishra, D.; Singh, T.R.R. Epidemiology of Ocular Manifestations in Autoimmune Disease. Front. Immunol. 2021, 12, 744396. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Kim, H.-S.; Hong, I.-S. Stem Cell-Derived Extracellular Vesicles as Immunomodulatory Therapeutics. Available online: https://www.hindawi.com/journals/sci/2019/5126156/ (accessed on 14 February 2023).
- Kuriyan, A.E.; Albini, T.A.; Townsend, J.H.; Rodriguez, M.; Pandya, H.K.; Leonard, R.E.; Parrott, M.B.; Rosenfeld, P.J.; Flynn, H.W.; Goldberg, J.L. Vision Loss after Intravitreal Injection of Autologous “Stem Cells” for AMD. N. Engl. J. Med. 2017, 376, 1047–1053. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Pratt, R.E.; Hodgkinson, C.P.; Dzau, V.J. Sequential Paracrine Mechanisms Are Necessary for the Therapeutic Benefits of Stem Cell Therapy. Am. J. Physiol. Cell Physiol. 2020, 319, C1141–C1150. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, Biologic Function and Clinical Potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering Exosomes for Targeted Drug Delivery. Theranostics 2021, 11, 3183–3195. [Google Scholar] [CrossRef]
- Seyedrazizadeh, S.-Z.; Poosti, S.; Nazari, A.; Alikhani, M.; Shekari, F.; Pakdel, F.; Shahpasand, K.; Satarian, L.; Baharvand, H. Extracellular Vesicles Derived from Human ES-MSCs Protect Retinal Ganglion Cells and Preserve Retinal Function in a Rodent Model of Optic Nerve Injury. Stem Cell Res. Ther. 2020, 11, 203. [Google Scholar] [CrossRef]
- Pan, D.; Chang, X.; Xu, M.; Zhang, M.; Zhang, S.; Wang, Y.; Luo, X.; Xu, J.; Yang, X.; Sun, X. UMSC-Derived Exosomes Promote Retinal Ganglion Cells Survival in a Rat Model of Optic Nerve Crush. J. Chem. Neuroanat. 2019, 96, 134–139. [Google Scholar] [CrossRef]
- Chen, C.C.; Liu, L.; Ma, F.; Wong, C.W.; Guo, X.E.; Chacko, J.V.; Farhoodi, H.P.; Zhang, S.X.; Zimak, J.; Ségaliny, A.; et al. Elucidation of Exosome Migration across the Blood-Brain Barrier Model In Vitro. Cell. Mol. Bioeng. 2016, 9, 509–529. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Qin, S.; Wen, Y.; Zhao, W.; Huang, Y.; Liu, J. Overcoming the Blood-Brain Barrier: Exosomes as Theranostic Nanocarriers for Precision Neuroimaging. J. Control. Release Off. J. Control. Release Soc. 2022, 349, 902–916. [Google Scholar] [CrossRef] [PubMed]
- Heidarzadeh, M.; Gürsoy-Özdemir, Y.; Kaya, M.; Eslami Abriz, A.; Zarebkohan, A.; Rahbarghazi, R.; Sokullu, E. Exosomal Delivery of Therapeutic Modulators through the Blood–Brain Barrier; Promise and Pitfalls. Cell Biosci. 2021, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.O.; He, M. Unlocking the Power of Exosomes for Crossing Biological Barriers in Drug Delivery. Pharmaceutics 2021, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhou, Y.; Li, H.-J. Advances in Mesenchymal Stem Cell Exosomes: A Review. Stem Cell Res. Ther. 2021, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Ni, Z.; Sun, H.; Wang, C. Microfluidic Approaches Toward the Isolation and Detection of Exosome Nanovesicles. IEEE Access 2019, 7, 45080–45098. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef]
- Moisseiev, E.; Anderson, J.D.; Oltjen, S.; Goswami, M.; Zawadzki, R.J.; Nolta, J.A.; Park, S.S. Protective Effect of Intravitreal Administration of Exosomes Derived from Mesenchymal Stem Cells on Retinal Ischemia. Curr. Eye Res. 2017, 42, 1358–1367. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; He, C.; Lai, P.; Yang, Z.; Liu, Y.; Xu, H.; Lin, X.; Ni, B.; Ju, R.; Yi, W.; et al. MiR-204–Containing Exosomes Ameliorate GVHD-Associated Dry Eye Disease. Sci. Adv. 2022, 8, eabj9617. [Google Scholar] [CrossRef]
- Wang, J.; Chen, D.; Ho, E.A. Challenges in the Development and Establishment of Exosome-Based Drug Delivery Systems. J. Control. Release 2021, 329, 894–906. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, G.; Zhang, K.; Cao, Q.; Liu, T.; Li, J. Mesenchymal Stem Cells-Derived Exosomes for Drug Delivery. Stem Cell Res. Ther. 2021, 12, 561. [Google Scholar] [CrossRef]
- Yu, M.; Liu, W.; Li, J.; Lu, J.; Lu, H.; Jia, W.; Liu, F. Exosomes Derived from Atorvastatin-Pretreated MSC Accelerate Diabetic Wound Repair by Enhancing Angiogenesis via AKT/ENOS Pathway. Stem Cell Res. Ther. 2020, 11, 350. [Google Scholar] [CrossRef]
- Wilson, S.E. Corneal Wound Healing. Exp. Eye Res. 2020, 197, 108089. [Google Scholar] [CrossRef]
- Du, Y.; SundarRaj, N.; Funderburgh, M.L.; Harvey, S.A.; Birk, D.E.; Funderburgh, J.L. Secretion and Organization of a Cornea-like Tissue In Vitro by Stem Cells from Human Corneal Stroma. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5038–5045. [Google Scholar] [CrossRef] [PubMed]
- Sharif, Z.; Sharif, W. Corneal Neovascularization: Updates on Pathophysiology, Investigations & Management. Rom. J. Ophthalmol. 2019, 63, 15–22. [Google Scholar] [PubMed]
- Yu, B.; Zhang, X.; Li, X. Exosomes Derived from Mesenchymal Stem Cells. Int. J. Mol. Sci. 2014, 15, 4142–4157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [Green Version]
- Tao, H.; Chen, X.; Cao, H.; Zheng, L.; Li, Q.; Zhang, K.; Han, Z.; Han, Z.-C.; Guo, Z.; Li, Z.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Corneal Wound Repair. Stem Cells Int. 2019, 2019, 5738510. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Hao, R.; Du, J.; Wu, X.; Chen, X.; Zhang, Y.; Li, W.; Gu, Z.; Yang, H. A Human Cornea-on-a-Chip for the Study of Epithelial Wound Healing by Extracellular Vesicles. iScience 2022, 25, 104200. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Wu, G.; Qi, P.; Zhang, Y.; Liu, Z.; Li, X.; Yu, Y.; Ye, X.; Li, Y.; et al. Umbilical Cord Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Deliver MiR-21 to Promote Corneal Epithelial Wound Healing through PTEN/PI3K/Akt Pathway. Stem Cells Int. 2022, 2022, 1252557. [Google Scholar] [CrossRef]
- Ma, S.; Yin, J.; Hao, L.; Liu, X.; Shi, Q.; Diao, Y.; Yu, G.; Liu, L.; Chen, J.; Zhong, J. Exosomes from Human Umbilical Cord Mesenchymal Stem Cells Treat Corneal Injury via Autophagy Activation. Front. Bioeng. Biotechnol. 2022, 10, 879192. [Google Scholar] [CrossRef]
- Shen, T.; Zheng, Q.-Q.; Shen, J.; Li, Q.-S.; Song, X.-H.; Luo, H.-B.; Hong, C.-Y.; Yao, K. Effects of Adipose-Derived Mesenchymal Stem Cell Exosomes on Corneal Stromal Fibroblast Viability and Extracellular Matrix Synthesis. Chin. Med. J. 2018. Available online: https://mednexus.org/doi/full/10.4103/0366-6999.226889 (accessed on 12 February 2023). [CrossRef] [PubMed]
- Du, Y.; Funderburgh, M.L.; Mann, M.M.; SundarRaj, N.; Funderburgh, J.L. Multipotent Stem Cells in Human Corneal Stroma. Stem Cells 2005, 23, 1266–1275. Available online: https://academic.oup.com/stmcls/article/23/9/1266/6399870 (accessed on 12 February 2023). [CrossRef] [PubMed] [Green Version]
- Du, Y.; Carlson, E.C.; Funderburgh, M.L.; Birk, D.E.; Pearlman, E.; Guo, N.; Kao, W.W.-Y.; Funderburgh, J.L. Stem Cell Therapy Restores Transparency to Defective Murine Corneas. Stem Cells 2009, 27, 1635–1642. Available online: https://academic.oup.com/stmcls/article/27/7/1635/6402401 (accessed on 12 February 2023). [CrossRef] [Green Version]
- Wang, Y.; Gao, G.; Wu, Y.; Wang, Y.; Wu, X.; Zhou, Q. S100A4 Silencing Facilitates Corneal Wound Healing After Alkali Burns by Promoting Autophagy via Blocking the PI3K/Akt/MTOR Signaling Pathway. Investig. Ophthalmol. Vis. Sci. 2020, 61, 19. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, R.; Li, L.; Choi, J.S.; Kim, J.; Yoon, H.J.; Park, J.H.; Yoon, K.C. Blue Light Induces Impaired Autophagy through Nucleotide-Binding Oligomerization Domain 2 Activation on the Mouse Ocular Surface. Int. J. Mol. Sci. 2021, 22, 2015. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Lu, B.; He, J.; Chen, X.; Fu, Q.; Han, H.; Luo, C.; Yin, H.; Qin, Z.; Lyu, D.; et al. Exosomes-Loaded Thermosensitive Hydrogels for Corneal Epithelium and Stroma Regeneration. Biomaterials 2022, 280, 121320. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Song, W.; Teng, L.; Huang, Y.; Liu, J.; Peng, Y.; Lu, X.; Yuan, J.; Zhao, X.; Zhao, Q.; et al. MiRNA 24-3p-Rich Exosomes Functionalized DEGMA-Modified Hyaluronic Acid Hydrogels for Corneal Epithelial Healing. Biocative Mater. 2023, 25, 640–656. Available online: https://www.sciencedirect.com/science/article/pii/S2452199X22003097?via%3Dihub (accessed on 12 February 2023). [CrossRef]
- Lin, H.; Yiu, S.C. Dry Eye Disease: A Review of Diagnostic Approaches and Treatments. Saudi J. Ophthalmol. Off. J. Saudi Ophthalmol. Soc. 2014, 28, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.Y.; Chen, W.T.; Chu-Bédard, Y.-K.; Patel, G.; Tran, S.D. Management of Sjogren’s Dry Eye Disease—Advances in Ocular Drug Delivery Offering a New Hope. Pharmaceutics 2023, 15, 147. [Google Scholar] [CrossRef]
- Lai, P.; Chen, X.; Guo, L.; Wang, Y.; Liu, X.; Liu, Y.; Zhou, T.; Huang, T.; Geng, S.; Luo, C.; et al. A Potent Immunomodulatory Role of Exosomes Derived from Mesenchymal Stromal Cells in Preventing CGVHD. J. Hematol. Oncol. 2018, 11, 135. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Yeo, R.W.Y.; Lai, R.C.; Sim, E.W.K.; Chin, K.C.; Lim, S.K. Mesenchymal Stromal Cell Exosome–Enhanced Regulatory T-Cell Production through an Antigen-Presenting Cell–Mediated Pathway. Cytotherapy 2018, 20, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Liang, Q.; He, Y.; Wang, C.; Jiang, J.; Chen, T.; Zhang, D.; Hu, K. Mesenchymal Stromal Cells-Derived Extracellular Vesicles Regulate Dendritic Cell Functions in Dry Eye Disease. Cells 2023, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, H.; Long, H.; Gong, X.; Hu, S.; Gong, C. Exosomes Derived from Mouse Adipose-Derived Mesenchymal Stem Cells Alleviate Benzalkonium Chloride-Induced Mouse Dry Eye Model via Inhibiting NLRP3 Inflammasome. Ophthalmic Res. 2022, 65, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Chen, P.; Xu, J.; Liu, Y.; Li, H.; Wang, L.; Di, G. HADSCs Derived Extracellular Vesicles Inhibit NLRP3 inflammasome Activation and Dry Eye. Sci. Rep. 2020, 10, 14521. [Google Scholar] [CrossRef]
- Ma, F.; Feng, J.; Liu, X.; Tian, Y.; Wang, W.-J.; Luan, F.-X.; Wang, Y.-J.; Yang, W.-Q.; Bai, J.-Y.; Zhang, Y.-Q.; et al. Ascorbic Acid-Coupled Mesenchymal Stem Cell-Derived Exosomes Ameliorate Dry Eye Disease. Preprints 2020, 2020060316. [Google Scholar] [CrossRef]
- Study Record|Beta ClinicalTrials.Gov. Available online: https://beta.clinicaltrials.gov/study/NCT04213248?tab=results (accessed on 13 February 2023).
- Zhao, J.; An, Q.; Zhu, X.; Yang, B.; Gao, X.; Niu, Y.; Zhang, L.; Xu, K.; Ma, D. Research Status and Future Prospects of Extracellular Vesicles in Primary Sjögren’s Syndrome. Stem Cell Res. Ther. 2022, 13, 230. [Google Scholar] [CrossRef]
- Gong, B.; Zheng, L.; Lu, Z.; Huang, J.; Pu, J.; Pan, S.; Zhang, M.; Liu, J.; Tang, J. Mesenchymal Stem Cells Negatively Regulate CD4+ T Cell Activation in Patients with Primary Sjögren Syndrome through the MiRNA-125b and MiRNA-155 TCR Pathway. Mol. Med. Rep. 2020, 23, 43. [Google Scholar] [CrossRef]
- Li, B.; Xing, Y.; Gan, Y.; He, J.; Hua, H. Labial Gland-Derived Mesenchymal Stem Cells and Their Exosomes Ameliorate Murine Sjögren’s Syndrome by Modulating the Balance of Treg and Th17 Cells. Stem Cell Res. Ther. 2021, 12, 478. Available online: https://stemcellres.biomedcentral.com/articles/10.1186/s13287-021-02541-0 (accessed on 13 February 2023). [CrossRef]
- Lind, E.F.; Ohashi, P.S. Mir-155, a Central Modulator of T-Cell Responses: Highlights. Eur. J. Immunol. 2014, 44, 11–15. [Google Scholar] [CrossRef]
- Rui, K.; Hong, Y.; Zhu, Q.; Shi, X.; Xiao, F.; Fu, H.; Yin, Q.; Xing, Y.; Wu, X.; Kong, X.; et al. Olfactory Ecto-Mesenchymal Stem Cell-Derived Exosomes Ameliorate Murine Sjögren’s Syndrome by Modulating the Function of Myeloid-Derived Suppressor Cells. Cell. Mol. Immunol. 2021, 18, 440–451. [Google Scholar] [CrossRef]
- Tomatsu, S.; Pitz, S.; Hampel, U. Ophthalmological Findings in Mucopolysaccharidoses. J. Clin. Med. 2019, 8, 1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coulson-Thomas, V.J.; Caterson, B.; Kao, W.W.-Y. Transplantation of Human Umbilical Mesenchymal Stem Cells Cures the Corneal Defects of Mucopolysaccharidosis VII Mice. Stem Cells 2013, 31, 2116–2126. Available online: https://academic.oup.com/stmcls/article/31/10/2116/6408126 (accessed on 13 February 2023). [CrossRef] [PubMed] [Green Version]
- Flanagan, M.; Pathak, I.; Gan, Q.; Winter, L.; Emnet, R.; Akel, S.; Montaño, A.M. Umbilical Mesenchymal Stem Cell-Derived Extracellular Vesicles as Enzyme Delivery Vehicle to Treat Morquio a Fibroblasts. Stem Cell Res. Ther. 2021, 12, 276. [Google Scholar] [CrossRef] [PubMed]
- Doozandeh, A.; Yazdani, S. Neuroprotection in Glaucoma. J. Ophthalmic Vis. Res. 2016, 11, 209–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mead, B.; Tomarev, S. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through MiRNA-Dependent Mechanisms. Stem Cells Transl. Med. 2017, 6, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Ahmed, Z.; Tomarev, S. Mesenchymal Stem Cell–Derived Small Extracellular Vesicles Promote Neuroprotection in a Genetic DBA/2J Mouse Model of Glaucoma. Investig. Opthalmol. Vis. Sci. 2018, 59, 5473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mead, B.; Amaral, J.; Tomarev, S. Mesenchymal Stem Cell–Derived Small Extracellular Vesicles Promote Neuroprotection in Rodent Models of Glaucoma. Investig. Opthalmol. Vis. Sci. 2018, 59, 702. [Google Scholar] [CrossRef]
- Mead, B.; Chamling, X.; Zack, D.J.; Ahmed, Z.; Tomarev, S. TNFα-Mediated Priming of Mesenchymal Stem Cells Enhances Their Neuroprotective Effect on Retinal Ganglion Cells. Investig. Opthalmol. Vis. Sci. 2020, 61, 6. [Google Scholar] [CrossRef] [Green Version]
- Park, M.; Shin, H.A.; Duong, V.-A.; Lee, H.; Lew, H. The Role of Extracellular Vesicles in Optic Nerve Injury: Neuroprotection and Mitochondrial Homeostasis. Cells 2022, 11, 3720. [Google Scholar] [CrossRef]
- Berry, M.; Ahmed, Z.; Morgan-Warren, P.; Fulton, D.; Logan, A. Prospects for MTOR-Mediated Functional Repair after Central Nervous System Trauma. Neurobiol. Dis. 2016, 85, 99–110. [Google Scholar] [CrossRef] [Green Version]
- Park, K.K.; Liu, K.; Hu, Y.; Smith, P.D.; Wang, C.; Cai, B.; Xu, B.; Connolly, L.; Kramvis, I.; Sahin, M.; et al. Promoting Axon Regeneration in the Adult CNS by Modulation of the PTEN/MTOR Pathway. Science 2008, 322, 963–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katakowski, M.; Buller, B.; Zheng, X.; Lu, Y.; Rogers, T.; Osobamiro, O.; Shu, W.; Jiang, F.; Chopp, M. Exosomes from Marrow Stromal Cells Expressing MiR-146b Inhibit Glioma Growth. Cancer Lett. 2013, 335, 201–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglas, M.R.; Morrison, K.C.; Jacques, S.J.; Leadbeater, W.E.; Gonzalez, A.M.; Berry, M.; Logan, A.; Ahmed, Z. Off-Target Effects of Epidermal Growth Factor Receptor Antagonists Mediate Retinal Ganglion Cell Disinhibited Axon Growth. Brain 2009, 132, 3102–3121. [Google Scholar] [CrossRef] [Green Version]
- Koprivica, V.; Cho, K.-S.; Park, J.B.; Yiu, G.; Atwal, J.; Gore, B.; Kim, J.A.; Lin, E.; Tessier-Lavigne, M.; Chen, D.F.; et al. EGFR Activation Mediates Inhibition of Axon Regeneration by Myelin and Chondroitin Sulfate Proteoglycans. Science 2005, 310, 106–110. [Google Scholar] [CrossRef]
- Li, H.-J.; Pan, Y.-B.; Sun, Z.-L.; Sun, Y.-Y.; Yang, X.-T.; Feng, D.-F. Inhibition of MiR-21 Ameliorates Excessive Astrocyte Activation and Promotes Axon Regeneration Following Optic Nerve Crush. Neuropharmacology 2018, 137, 33–49. [Google Scholar] [CrossRef]
- Meng, F.; Henson, R.; Wehbe–Janek, H.; Ghoshal, K.; Jacob, S.T.; Patel, T. MicroRNA-21 Regulates Expression of the PTEN Tumor Suppressor Gene in Human Hepatocellular Cancer. Gastroenterology 2007, 133, 647–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, Y.H.; Fingert, J.H.; Kuehn, M.H.; Alward, W.L.M. Primary Open-Angle Glaucoma. N. Engl. J. Med. 2009, 360, 1113–1124. Available online: https://www.nejm.org/doi/full/10.1056/NEJMra0804630 (accessed on 13 February 2023). [CrossRef] [PubMed] [Green Version]
- Tabak, S.; Schreiber-Avissar, S.; Beit-Yannai, E. Crosstalk between MicroRNA and Oxidative Stress in Primary Open-Angle Glaucoma. Int. J. Mol. Sci. 2021, 22, 2421. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, J.; Wang, X.; Wang, X.; Liu, W.; Gao, J. Mesenchymal Stem Cell-Derived Exosomes Protect Trabecular Meshwork from Oxidative Stress. Sci. Rep. 2021, 11, 14863. [Google Scholar] [CrossRef]
- Bradley, J.; Vranka, J.; Colvis, C.; Conger, D.; Alexander, J.; Fisk, A.; Samples, J.; Acott, T. Effect of Matrix Metalloproteinases Activity on Outflow in Perfused Human Organ Culture. Investig. Ophthalmol. Vis. Sci. 1999, 39, 2649–2658. [Google Scholar]
- Tamkovich, S.; Grigor’eva, A.; Eremina, A.; Tupikin, A.; Kabilov, M.; Chernykh, V.; Vlassov, V.; Ryabchikova, E. What Information Can Be Obtained from the Tears of a Patient with Primary Open Angle Glaucoma? Clin. Chim. Acta 2019, 495, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Pantalon, A.; Obadă, O.; Constantinescu, D.; Feraru, C.; Chiseliţă, D. Inflammatory Model in Patients with Primary Open Angle Glaucoma and Diabetes. Int. J. Ophthalmol. 2019, 12, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, Y.; Long, Q. Effects of Mesenchymal Stem Cells Derived Exosomes on Ultrastructure of Corneal Epithelium and Function of the Tear Film in Dry Eye BALB/c Mice. Investing. Opthalmol. Vis. Sci. 2019, 60, 4187. Available online: https://iovs.arvojournals.org/article.aspx?articleid=2743824 (accessed on 13 February 2023).
- Cuenca, N.; Fernández-Sánchez, L.; Campello, L.; Maneu, V.; De la Villa, P.; Lax, P.; Pinilla, I. Cellular Responses Following Retinal Injuries and Therapeutic Approaches for Neurodegenerative Diseases. Prog. Retin. Eye Res. 2014, 43, 17–75. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.-L.; Hu, C.-B.; Ling, S.-T.; Zhao, N.; Bao, L.-H.; Zhou, F.; Xiong, Y.-C.; Chen, T.; Sui, B.-D.; Yu, X.-R.; et al. Photoreceptor Protection by Mesenchymal Stem Cell Transplantation Identifies Exosomal MiR-21 as a Therapeutic for Retinal Degeneration. Cell Death Differ. 2021, 28, 1041–1061. [Google Scholar] [CrossRef]
- Zhang, J.; Li, P.; Zhao, G.; He, S.; Xu, D.; Jiang, W.; Peng, Q.; Li, Z.; Xie, Z.; Zhang, H.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Protect Retina in a Mouse Model of Retinitis Pigmentosa by Anti-Inflammation through MiR-146a-Nr4a3 Axis. Stem Cell Res. Ther. 2022, 13, 394. [Google Scholar] [CrossRef]
- Safwat, A.; Sabry, D.; Ragiae, A.; Amer, E.; Mahmoud, R.H.; Shamardan, R.M. Adipose mesenchymal stem cells–derived exosomes attenuate retina degeneration of streptozotocin-induced diabetes in rabbits. J. Circ. Biomark. 2018, 7, 1849454418807827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Jin, L.; Cui, Y.; Nie, A.; Xie, N.; Liang, G. Bone Marrow Mesenchymal Stem Cells-Induced Exosomal MicroRNA-486-3p Protects against Diabetic Retinopathy through TLR4/NF-ΚB Axis Repression. J. Endocrinol. Investig. 2021, 44, 1193–1207. [Google Scholar] [CrossRef]
- Li, W.; Jin, L.; Cui, Y.; Xie, N. Human Umbilical Cord Mesenchymal Stem Cells-Derived Exosomal MicroRNA-17-3p Ameliorates Inflammatory Reaction and Antioxidant Injury of Mice with Diabetic Retinopathy via Targeting STAT1. Int. Immunopharmacol. 2021, 90, 107010. [Google Scholar] [CrossRef]
- Gu, C.; Zhang, H.; Zhao, S.; He, D.; Gao, Y. Mesenchymal Stem Cell Exosomal MiR-146a Mediates the Regulation of the TLR4/MyD88/NF-ΚB Signaling Pathway in Inflammation Due to Diabetic Retinopathy. Comput. Math. Methods Med. 2022, 2022, 3864863. [Google Scholar] [CrossRef]
- Ebrahim, N.; El-Halim, H.E.A.; Helal, O.K.; El-Azab, N.E.-E.; Badr, O.A.M.; Hassouna, A.; Saihati, H.A.A.; Aborayah, N.H.; Emam, H.T.; El-wakeel, H.S.; et al. Effect of Bone Marrow Mesenchymal Stem Cells-Derived Exosomes on Diabetes-Induced Retinal Injury: Implication of Wnt/b-Catenin Signaling Pathway. Biomed. Pharmacother. 2022, 154, 113554. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Xue, L.-D.; Di, Y.; Li, T.; Tian, Y.-J.; Song, Y. MSC-Derived Exosomal LncRNA SNHG7 Suppresses Endothelial-Mesenchymal Transition and Tube Formation in Diabetic Retinopathy via MiR-34a-5p/XBP1 Axis. Life Sci. 2021, 272, 119232. [Google Scholar] [CrossRef] [PubMed]
- Hajrasouliha, A.R.; Jiang, G.; Lu, Q.; Lu, H.; Kaplan, H.J.; Zhang, H.-G.; Shao, H. Exosomes from Retinal Astrocytes Contain Antiangiogenic Components That Inhibit Laser-Induced Choroidal Neovascularization. J. Biol. Chem. 2013, 288, 28058–28067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, G.-H.; Zhang, W.; Ma, Y.-X.; Yang, J.; Chen, L.; Song, J.; Chen, S. Mesenchymal Stem Cells-Derived Exosomes Ameliorate Blue Light Stimulation in Retinal Pigment Epithelium Cells and Retinal Laser Injury by VEGF-Dependent Mechanism. Int. J. Ophthalmol. 2018, 11, 559–566. [Google Scholar] [CrossRef]
- Mathew, B.; Ravindran, S.; Liu, X.; Torres, L.; Chennakesavalu, M.; Huang, C.-C.; Feng, L.; Zelka, R.; Lopez, J.; Sharma, M.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles and Retinal Ischemia-Reperfusion. Biomaterials 2019, 197, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wen, Y.; Jiang, N.; Li, Z.; Guan, J.; Zhang, Y.; Deng, C.; Zhao, L.; Zheng, S.G.; Zhu, Y.; et al. TNF-α Stimulation Enhances the Neuroprotective Effects of Gingival MSCs Derived Exosomes in Retinal Ischemia-Reperfusion Injury via the MEG3/MiR-21a-5p Axis. Biomaterials 2022, 284, 121484. [Google Scholar] [CrossRef]
- Ma, M.; Li, B.; Zhang, M.; Zhou, L.; Yang, F.; Ma, F.; Shao, H.; Li, Q.; Li, X.; Zhang, X. Therapeutic Effects of Mesenchymal Stem Cell-Derived Exosomes on Retinal Detachment. Exp. Eye Res. 2020, 191, 107899. [Google Scholar] [CrossRef]
- Dervenis, N.; Dervenis, P.; Sandinha, T.; Murphy, D.C.; Steel, D.H. Intraocular Tamponade Choice with Vitrectomy and Internal Limiting Membrane Peeling for Idiopathic Macular Hole: A Systematic Review and Meta-Analysis. Ophthalmol. Retina 2022, 6, 457–468. [Google Scholar] [CrossRef]
- Muqit, M.M.K.; Hamilton, R.; Ho, J.; Tucker, S.; Buck, H. Intravitreal Ocriplasmin for the Treatment of Vitreomacular Traction and Macular Hole- A Study of Efficacy and Safety Based on NICE Guidance. PLoS ONE 2018, 13, e0197072. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Liu, J.; Yu, B.; Ma, F.; Ren, X.; Li, X. Effects of Mesenchymal Stem Cells and Their Exosomes on the Healing of Large and Refractory Macular Holes. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 2041–2052. [Google Scholar] [CrossRef]
- Valdes, L.M.; Sobrin, L. Uveitis Therapy: The Corticosteroid Options. Drugs 2020, 80, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Duplechain, A.; Conrady, C.D.; Patel, B.C.; Baker, S. Uveitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Shigemoto-Kuroda, T.; Oh, J.Y.; Kim, D.; Jeong, H.J.; Park, S.Y.; Lee, H.J.; Park, J.W.; Kim, T.W.; An, S.Y.; Prockop, D.J.; et al. MSC-Derived Extracellular Vesicles Attenuate Immune Responses in Two Autoimmune Murine Models: Type 1 Diabetes and Uveoretinitis. Stem Cell Rep. 2017, 8, 1214–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, L.; Shao, H.; Wang, H.; Zhang, Z.; Su, C.; Dong, L.; Yu, B.; Chen, X.; Li, X.; Zhang, X. Effects of Mesenchymal Stem Cell-Derived Exosomes on Experimental Autoimmune Uveitis. Sci. Rep. 2017, 7, 4323. [Google Scholar] [CrossRef] [Green Version]
- Xie, R.; Bai, L.; Yang, J.; Li, Y.; Dong, L.; Ma, F.; Li, X.; Zhang, X. Effects of rat mesenchymal stem cell-derived exosomes on rat experimental autoimmune uveitis. Chin. J. Ocul. Fundus Dis. 2018, 34, 562–567. [Google Scholar]
- Li, Y.; Ren, X.; Zhang, Z.; Duan, Y.; Li, H.; Chen, S.; Shao, H.; Li, X.; Zhang, X. Effect of Small Extracellular Vesicles Derived from IL-10-Overexpressing Mesenchymal Stem Cells on Experimental Autoimmune Uveitis. Stem Cell Res. Ther. 2022, 13, 100. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, T.; Yang, Z.; Sun, X.; Huang, Z.; Deng, X.; He, C.; Liu, X. Bone Marrow Mesenchymal Stem Cells-Derived Exosomes Attenuate Neuroinflammation and Promote Survival of Photoreceptor in Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3108. [Google Scholar]
- Li, D.; Zhang, J.; Liu, Z.; Gong, Y.; Zheng, Z. Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomal MiR-27b Attenuates Subretinal Fibrosis via Suppressing Epithelial–Mesenchymal Transition by Targeting HOXC6. Stem Cell Res. Ther. 2021, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Kim, T.W.; Jeong, H.J.; Lee, H.J.; Ryu, J.S.; Wee, W.R.; Heo, J.W.; Kim, M.K. Intraperitoneal Infusion of Mesenchymal Stem/Stromal Cells Prevents Experimental Autoimmune Uveitis in Mice. Mediat. Inflamm. 2014, 2014, 624640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Zhang, Z.; Li, Y.; Su, L.; Duan, Y.; Zhang, H.; An, J.; Ni, T.; Li, X.; Zhang, X. Therapeutic Effect of Rapamycin-Loaded Small Extracellular Vesicles Derived from Mesenchymal Stem Cells on Experimental Autoimmune Uveitis. Front. Immunol. 2022, 13, 864956. [Google Scholar] [CrossRef]
- Wei, W.; Ao, Q.; Wang, X.; Cao, Y.; Liu, Y.; Zheng, S.G.; Tian, X. Mesenchymal Stem Cell–Derived Exosomes: A Promising Biological Tool in Nanomedicine. Front. Pharmacol. 2021, 11, 590470. [Google Scholar] [CrossRef]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem Cell-Based Therapy for Human Diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef] [PubMed]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Immunomodulation and Regeneration: A next Generation Therapeutic Tool? Cell Death Dis. 2022, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Varderidou-Minasian, S.; Lorenowicz, M.J. Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles in Tissue Repair: Challenges and Opportunities. Theranostics 2020, 10, 5979–5997. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sun, F.; Qian, H.; Xu, W.; Jiang, J. Preconditioning and Engineering Strategies for Improving the Efficacy of Mesenchymal Stem Cell-Derived Exosomes in Cell-Free Therapy. Stem Cells Int. 2022, 2022, 1779346. [Google Scholar] [CrossRef]
- Qazi, T.H.; Mooney, D.J.; Duda, G.N.; Geissler, S. Biomaterials That Promote Cell-Cell Interactions Enhance the Paracrine Function of MSCs. Biomaterials 2017, 140, 103–114. [Google Scholar] [CrossRef]
- Su, N.; Gao, P.-L.; Wang, K.; Wang, J.-Y.; Zhong, Y.; Luo, Y. Fibrous Scaffolds Potentiate the Paracrine Function of Mesenchymal Stem Cells: A New Dimension in Cell-Material Interaction. Biomaterials 2017, 141, 74–85. [Google Scholar] [CrossRef]
- Samsonraj, R.M.; Rai, B.; Sathiyanathan, P.; Puan, K.J.; Rötzschke, O.; Hui, J.H.; Raghunath, M.; Stanton, L.W.; Nurcombe, V.; Cool, S.M. Establishing Criteria for Human Mesenchymal Stem Cell Potency. Stem Cells 2015, 33, 1878–1891. [Google Scholar] [CrossRef]
- Sathiyanathan, P.; Samsonraj, R.M.; Tan, C.L.L.; Ling, L.; Lezhava, A.; Nurcombe, V.; Stanton, L.W.; Cool, S.M. A Genomic Biomarker That Identifies Human Bone Marrow-Derived Mesenchymal Stem Cells with High Scalability. Stem Cells Dayt. Ohio 2020, 38, 1124–1136. [Google Scholar] [CrossRef]
- Boulestreau, J.; Maumus, M.; Rozier, P.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cell Derived Extracellular Vesicles in Aging. Front. Cell Dev. Biol. 2020, 8, 107. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wu, Q.; Wang, Y.; Li, L.; Bu, H.; Bao, J. Senescence of Mesenchymal Stem Cells (Review). Int. J. Mol. Med. 2017, 39, 775–782. [Google Scholar] [CrossRef] [Green Version]
- Kouroupis, D.; Churchman, S.M.; McGonagle, D.; Jones, E.A. The Assessment of CD146-Based Cell Sorting and Telomere Length Analysis for Establishing the Identity of Mesenchymal Stem Cells in Human Umbilical Cord. F1000Research 2014, 3, 126. [Google Scholar] [CrossRef] [PubMed]
- Laschober, G.T.; Brunauer, R.; Jamnig, A.; Fehrer, C.; Greiderer, B.; Lepperdinger, G. Leptin Receptor/CD295 Is Upregulated on Primary Human Mesenchymal Stem Cells of Advancing Biological Age and Distinctly Marks the Subpopulation of Dying Cells. Exp. Gerontol. 2009, 44, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.M.; Kwon, O.; Kwon, K.-S.; Cho, Y.S.; Rhee, S.K.; Min, J.-K.; Oh, D.-B. Evidences for Correlation between the Reduced VCAM-1 Expression and Hyaluronan Synthesis during Cellular Senescence of Human Mesenchymal Stem Cells. Biochem. Biophys. Res. Commun. 2011, 404, 463–469. [Google Scholar] [CrossRef]
- Simmons, P.J.; Torok-Storb, B. Identification of Stromal Cell Precursors in Human Bone Marrow by a Novel Monoclonal Antibody, STRO-1. Blood 1991, 78, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Yun, S.P.; Han, Y.-S.; Lee, J.H.; Kim, S.M.; Lee, S.H. Melatonin Rescues Mesenchymal Stem Cells from Senescence Induced by the Uremic Toxin p-Cresol via Inhibiting MTOR-Dependent Autophagy. Biomol. Ther. 2018, 26, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Chaker, D.; Mouawad, C.; Azar, A.; Quilliot, D.; Achkar, I.; Fajloun, Z.; Makdissy, N. Inhibition of the RhoGTPase Cdc42 by ML141 Enhances Hepatocyte Differentiation from Human Adipose-Derived Mesenchymal Stem Cells via the Wnt5a/PI3K/MiR-122 Pathway: Impact of the Age of the Donor. Stem Cell Res. Ther. 2018, 9, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, G.; Kluba, T.; Hermanutz-Klein, U.; Bieback, K.; Northoff, H.; Schäfer, R. Phenotype, Donor Age and Gender Affect Function of Human Bone Marrow-Derived Mesenchymal Stromal Cells. BMC Med. 2013, 11, 146. [Google Scholar] [CrossRef] [Green Version]
- Ulum, B.; Teker, H.T.; Sarikaya, A.; Balta, G.; Kuskonmaz, B.; Uckan-Cetinkaya, D.; Aerts-Kaya, F. Bone Marrow Mesenchymal Stem Cell Donors with a High Body Mass Index Display Elevated Endoplasmic Reticulum Stress and Are Functionally Impaired. J. Cell. Physiol. 2018, 233, 8429–8436. [Google Scholar] [CrossRef]
- Li, C.; Zhao, H.; Cheng, L.; Wang, B. Allogeneic vs. Autologous Mesenchymal Stem/Stromal Cells in Their Medication Practice. Cell Biosci. 2021, 11, 187. [Google Scholar] [CrossRef]

| Disease | Origin | Route of Administration | Results | Stage | Reference |
|---|---|---|---|---|---|
| Corneal wound | Human corneal MSC-derived exosomes | Topical |
| Preclinical trial (in vitro and in vivo mouse study) | [18] |
| Corneal alkali injury | Human placenta-derived MSC EVs | Topical |
| Preclinical trial (in vitro and in vivo mouse study) | [48] |
| Corneal wound | Adipocyte-derived MSC-derived exosomes | Topical |
| Preclinical trial (in vitro) | [52] |
| Corneal wound | Bone marrow derives MSC-exosomes | Topical |
| Preclinical trial (in vitro, “cornea on a chip”: combination of human corneal cells and microfluidics to mimic ocular surface in vitro) | [49] |
| Corneal wound | Human umbilical cord MSC-derived exosomes combined with autophagy activator (AA) Rapamycin | Topical |
| Preclinical trial (mouse model) | [51] |
| Corneal wound | Adipose-derived MSC-derived exosomes loaded with miRNA 24-3p in thermosensitive hyaluronic acid hydrogel (THH) | Topical(miRNA 24-3p was tested alone by subconjunctival injection) |
| Preclinical trial (rabbit study) | [58] |
| Corneal wound | Induced pluripotent stem cell-derived MSC (iPSC-MSC) exosomes containing miR-432-5p (in a thermosensitive hydrogel for part of the study) | Topical |
| Preclinical trial (in vitro and in vivo rat study) | [57] |
| Corneal wound | Human umbilical MSC-derived exosomes with miR-21 | Topical |
| Preclinical trial (in vitro and in vivo rat model) | [50] |
| GVHD-associated dry eye disease | Mouse bone marrow-derived MSC-derived exosomes (mouse study), human umbilical cord MSC-derived exosomes (clinical study) | Topical |
| Preclinical trial (2 dry eye mouse models) and clinical study (14 patients/28 eyes with GVHD) | [4] |
| GVHD-associated DED | Human umbilical MSC-derived exosomes | Topical |
| Clinical trial (phase II) with 27 study subjects affected by dry eye symptoms with cGVHD | [67] |
| DED | Human adipose-derived MSC-EVs | Topical |
| Preclinical trial (mouse model) | [65] |
| DED | MSC-derived EVs | Topical |
| Preclinical trial (in vitro and in vivo mouse study) | [63] |
| DED | Mouse adipose-derived MSC-derived exosomes | Topical |
| Preclinical trial (mouse model) | [64] |
| DED | Mouse MSC-derived exosomes coupled with ascorbic acid | Topical |
| Preclinical trial (mouse model) | [66] |
| DED | MSC-exosomes | Topical |
| Preclinical trial (mouse model) | [95] |
| cGVHD | Human bone marrow-derived MSC-exosomes | Tail vein injection |
| Preclinical study | [61] |
| SSDE | Human umbilical-derived MSC-exosomes | In vitro peripheral blood mononuclear cells (PBMCs) |
| Preclinical study (in vitro) | [69] |
| SSDE | Labial gland-derived MSC-exosomes | Tail vein injections |
| Preclinical study (mouse model) | [70] |
| SSDE | Olfactory ecto-MSC-exosomes | Intravenous injections |
| [72] | |
| Mucopolysaccharidosis IVA | Human umbilical MSC-derived EVs |
| Preclinical trial (in vitro) | [75] | |
| Glaucoma/optic nerve crush | Bone marrow-derived MSC-exosomes | Intravitreal injection, just posterior to the limbus |
| Preclinical study (rat study) | [77] |
| Glaucoma/optic nerve crush | Human umbilical MSC-exosomes | Intravitreal injection |
| Preclinical trial (rat study) | [30] |
| Glaucoma/ONC | miR-21 | Intravitreal injection |
| Preclinical trial (rat study) | [87] |
| Glaucoma (POAG) | Bone marrow-derived MSC-exosomes |
| Preclinical trial (in vitro) | [91] | |
| Glaucoma/ONC | Human embryonic stem cell-MSC-EVs | Tail vein injections |
| Preclinical trial (mouse model) | [29] |
| Glaucoma/ONC | Human bone marrow-derived MSC-exosomes |
| Preclinical trial (in vitro) | [80] | |
| Glaucoma | Human bone marrow MSC-derived exosomes | Intravitreal injection |
| Preclinical study (mouse model) | [78] |
| Glaucoma | Human bone marrow MSC-derived exosomes | Intravitreal injection |
| Preclinical study (rat model) | [79] |
| Optic nerve injury | Human placenta-derived mesenchymal stem cells (hPSCs) | In vitro immortalized R28 retinal precursor cells exposure |
| Preclinical study (in vitro) | [81] |
| Disease | Origin | Route of Administration | Results | Stage | Reference |
|---|---|---|---|---|---|
| Idiopathic Macular Holes | Human umbilical cord-derived mesenchymal stem cells (hUCMSCs) | Intravitreal injection | MSC-Exo therapy promotes functional and anatomic recovery from MH and can be used as a. safe method for improving the visual outcomes after MHs surgery. | Clinical Trial | [112] |
| Retinitis Pigmentosa | Mouse bone marrow MSC | Intravitreal Injection | Intravitreal MSCT counteracted photoreceptor apoptosis and alleviated retinal morphological and functional degeneration in a mouse model of photoreceptor loss. | Preclinical Study | [97] |
| Retinitis Pigmentosa | Mouse bone marrow MSC | Intravitreal Injection | MSC-exosomes might suppress the degeneration of photoreceptors with increased thickness of the retinal outer nuclear layer, and mechanically, MSC-exosome treatment inhibits the expression of pro-inflammatory cytokines, indicating the relief of neuroinflammation. | Preclinical Study | [119] |
| Retinitis Pigmentosa | Human umbilical cord mesenchymal stem cells-derived exosome | Intravitreal Injection | MSC-Exosomes increased the survival of photoreceptors and preserved their structure. Visual function, as reflected by optomotor and electroretinogram responses, was significantly enhanced in MSC-EVs-treated rd10 mice. | Preclinical Study | [98] |
| Diabetic Retinopathy | Mice Bone marrow-derived Mesenchymal stem cell (BMSC) | In Vitro culturing of Mouse retinal cells (Muller cells) with MSCs-exosome was performed | BMSC-derived exosomes inhibited oxidative stress, inflammation, and apoptosis and promoted the proliferation of HG-treated Muller cells. | Preclinical Study | [100] |
| Diabetic Retinopathy | Human umbilical cord mesenchymal stem cells-derived exosome | Intravitreal Injection | hucMSCs-derived exosomes shuffle miR-17-3p to ameliorate inflammatory reaction and oxidative injury of DR mice via targeting STAT1. | Preclinical Study | [101] |
| Diabetic Retinopathy | Bone marrow mesenchymal stem cell (BMSC) | Injection into the Retinal Ganglion Cell Culture of DR model mice was conducted ex vivo | BMSC exosomal miR-146a can regulate the inflammatory response of DR by mediating the TLR4/MyD88/NF-κB pathway, providing an experimental basis for the prevention and treatment of DR. | Preclinical Study | [102] |
| Diabetic Retinopathy | Human umbilical cord-derived mesenchymal stem cells (hUCMSCs) | Intravitreal Injection | miR-126 expression in MSC-Exos reduces hyperglycemia-induced retinal inflammation by downregulating the HMGB1 signaling pathway. | Preclinical Study | [7] |
| Diabetic Retinopathy | Animal (Rabbit) adipose-derived mesenchymal stem cells | Intravenous Subconjunctival and Intraocular | Increased expression of micRNA-222 with regenerative changes in the retina following administration of MSCs-derived exosomes. | Preclinical Study | [99] |
| Diabetes Retinopathy | Bone marrow-derived mesenchymal stem cell-derived exosomes (BM-MSCs-Ex) | Intravitreal Injection | By blocking the wnt/b-catenin pathway in the diabetic retina, exosomes demonstrated a significant reduction in features of DR. | Preclinical Study | [103] |
| Retinal Ischemia | Human Bone marrow mesenchymal stem cell (HuBMSC) | Intravitreal | hMSCs were well tolerated without immunosuppression and decreased the severity of retinal ischemia in this murine model. | Preclinical Study | [38] |
| Retinal ischemia-reperfusion injury | Gingival MSC (GMSC)-exosomes | Intraocular | GMSC exosomes significantly reduced inflammation and retinal cell loss caused by glaucoma without any autoimmunity. | Preclinical Study | [108] |
| Retinal Injury/ Ischemia | Mouse adipose and human umbilical cord mesenchymal stem cell | Intravitreal In vivo and in vitro Retinal cell | MSCs and their exosomes reduced damage inhibited apoptosis, and suppressed inflammatory responses to obtain a better visual function to nearly the same extent in vivo. | Preclinical Study | [4] |
| Retinal Detachment | Sprague-Dawley rat bone marrow-derived mesenchymal stem cell exosomes | Subretinal Injection | Suppression of photoreceptor cell apoptosis and maintenance of normal retinal structure observed when treated with MSC-Exosomes. | Preclinical Study | [109] |
| Age-related macular degeneration | Retinal astroglial cell-derived exosomes | Intravitreal | Retinal Astroglial cell-derived exosomes inhibited laser-induced choroidal neo-vascularization. | Preclinical Study | [105] |
| Age-related macular degeneration | Human umbilical cord-derived mesenchymal stem cells (hUCMSCs) | Intravitreal injection in vivo and Retinal pigment epithelial cell model in vitro | In vivo, MSCs-derived exosomes reduced damage, distinctly downregulated VEGF-A, and gradually improved the histological structures of CNV for better visual function. In vitro, MSCs-derived exosomes downregulated the mRNA and protein expression of VEGF-A in RPE cells after blue light stimulation. | Preclinical Study | [106] |
| Subretinal fibrosis resulting from neovascular age-related macular degeneration | Human umbilical cord-derived mesenchymal stem cells (hUCMSCs) | Intravitreal injection | Intravitreal injection of hucMSC-Exo effectively ameliorated laser-induced CNV and subretinal fibrosis via the suppression of epithelial–mesenchymal transition (EMT) process. | Preclinical Study | [120] |
| Experimental Autoimmune Uveitis | Human bone marrow mesenchymal stem/stromal cells (huBMSCs) | Intraperitoneal | hMSCs attenuate EAU by suppressing Th1/Th17 cells and induce IL-10-expressing B220+CD19+ cells. | Preclinical Study | [121] |
| Type 1 Diabetes and Experimental Autoimmune Uveitis | Human Bone marrow mesenchymal stem cells (HuBMSCs) | Tail vein intravenous injection | huBMSC-exosomes effectively prevent the onset of disease in both T1D and EAU. | Preclinical Study | [115] |
| Experimental Autoimmune Uveitis | Human umbilical cord-derived mesenchymal stem cells (hUCMSCs | Periocular Injection | MSC-exosomes effectively ameliorate EAU by inhibiting the migration of inflammatory cells, indicating a potential novel therapy of MSC-Exo for uveitis. | Preclinical Study | [116] |
| Experimental Autoimmune Uveitis | Rat mesenchymal stem cell | Periocular Injection | MSC-exosomes can reduce the clinical and pathological manifestations of EAU, protect retinal function, reduce ocular macrophage infiltration, down-regulate the proportion of inflammatory cells in the eye, and inhibit T cell proliferation. | Preclinical Study | [117] |
| Experimental Autoimmune Uveitis | Human umbilical cord-derived mesenchymal stem cells (hUCMSCs) | Intravenous Injection | Intravenous injection of human umbilical cord MSCs-derived sEVs can reduce inflammation in EAU mice. | Preclinical Study | [118] |
| Experimental Autoimmune Uveitis | Human umbilical cord-derived mesenchymal stem cells (hUCMSCs) | Intravenous Injection (tail vein) | IL-10 overexpressed MSC-exosomes effectively ameliorate EAU by regulating the proliferation and differentiation of T-cells. | Preclinical Study | [118] |
| Experimental Autoimmune Uveitis | Human umbilical cord-derived mesenchymal stem cells (hUCMSCs) | Subconjunctival injection | Compared to sEVs and rapamycin alone, Rapa-sEVs can produce a more marked therapeutic effect and reduce ocular inflammatory cell infiltration. | Preclinical Study | [122] |
| Diabetes Retinopathy | Human Bone marrow mesenchymal stem cell (HuBMSC) | Cellular exposure ex vivo on Human retinal microvascular endothelial cells | MSC-derived exosomal lncRNA SNHG7 suppresses endothelial–mesenchymal transition and tube formation in Human retinal microvascular endothelial cells. | In Vitro Preclinical Study | [104] |
| Retinal Ischemia | Human Bone marrow mesenchymal stem cell (HuBMSC) | Cellular exposure ex vivo on Human retinal microvascular endothelial cells | Retinal cells exposed to MSC-derived EVs significantly reduced cell death and attenuated loss of cell proliferation. | In Vitro Preclinical Study | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.Y.; Ahmad, H.; Lin, G.; Carbonneau, M.; Tran, S.D. Mesenchymal Stem Cell-Derived Exosomes in Ophthalmology: A Comprehensive Review. Pharmaceutics 2023, 15, 1167. https://doi.org/10.3390/pharmaceutics15041167
Wu KY, Ahmad H, Lin G, Carbonneau M, Tran SD. Mesenchymal Stem Cell-Derived Exosomes in Ophthalmology: A Comprehensive Review. Pharmaceutics. 2023; 15(4):1167. https://doi.org/10.3390/pharmaceutics15041167
Chicago/Turabian StyleWu, Kevin Y., Hamza Ahmad, Grace Lin, Marjorie Carbonneau, and Simon D. Tran. 2023. "Mesenchymal Stem Cell-Derived Exosomes in Ophthalmology: A Comprehensive Review" Pharmaceutics 15, no. 4: 1167. https://doi.org/10.3390/pharmaceutics15041167







