Development of Nanofibers with Embedded Liposomes Containing an Immunomodulatory Drug Using Green Electrospinning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Liposome Preparation and Characterization
2.3. UPLC Analysis
2.4. Nanofiber Mat Preparation by Green Electrospinning
2.5. Electron Microscopy Analysis
2.6. Determination of the Drug Content in the Nanofibers
2.7. SIM Stability Study
2.8. Release Studies
2.9. Investigation of Liposome Formation after Nanofiber Dissolution
2.10. Evaluation of the Safety and Efficacy of SIM in Nanofibers In Vitro
2.10.1. Formulation Safety Assay
2.10.2. Formulation Efficacy Assay
2.11. Statistical Analysis
3. Results
3.1. Development of Liposomes Containing SIM
3.2. Development of Composite Liposome Nanofibers
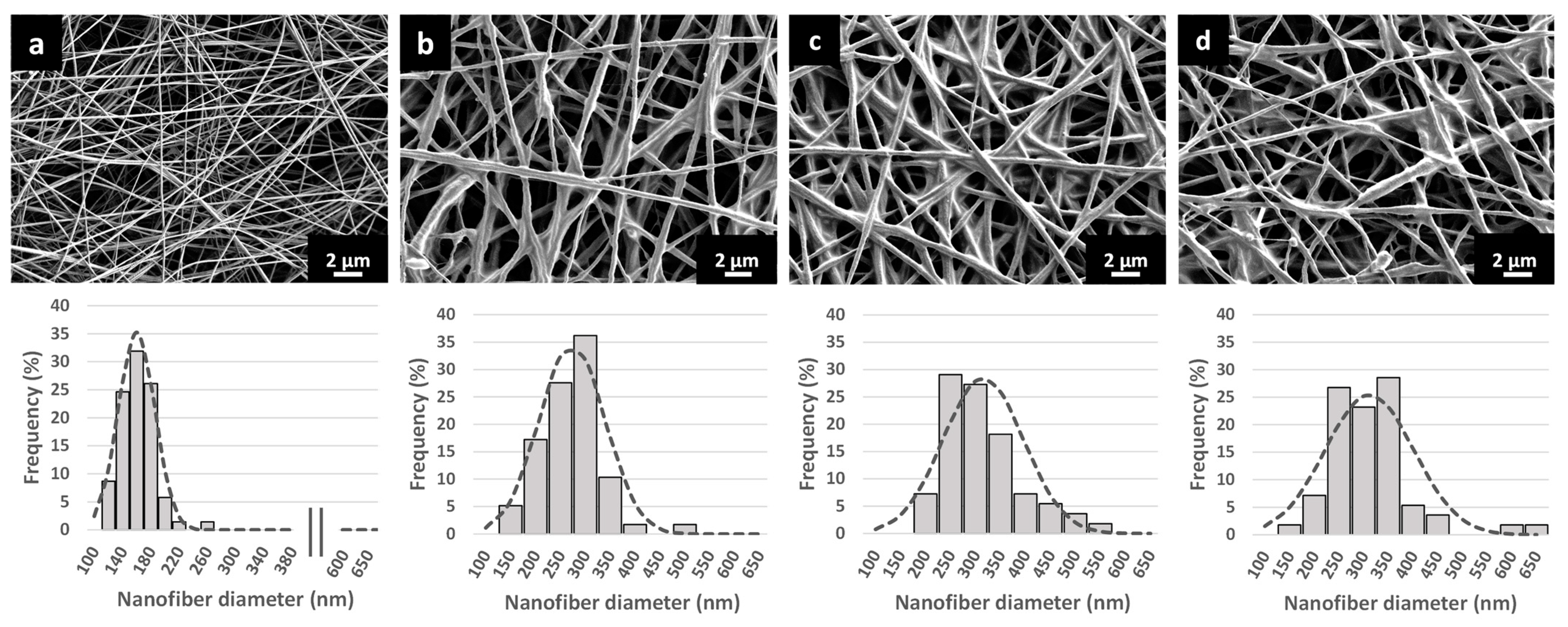
3.3. Characterization of the Nanofibrous Scaffolds
3.4. In Situ Liposomes Formation after Nanofiber Dissolution
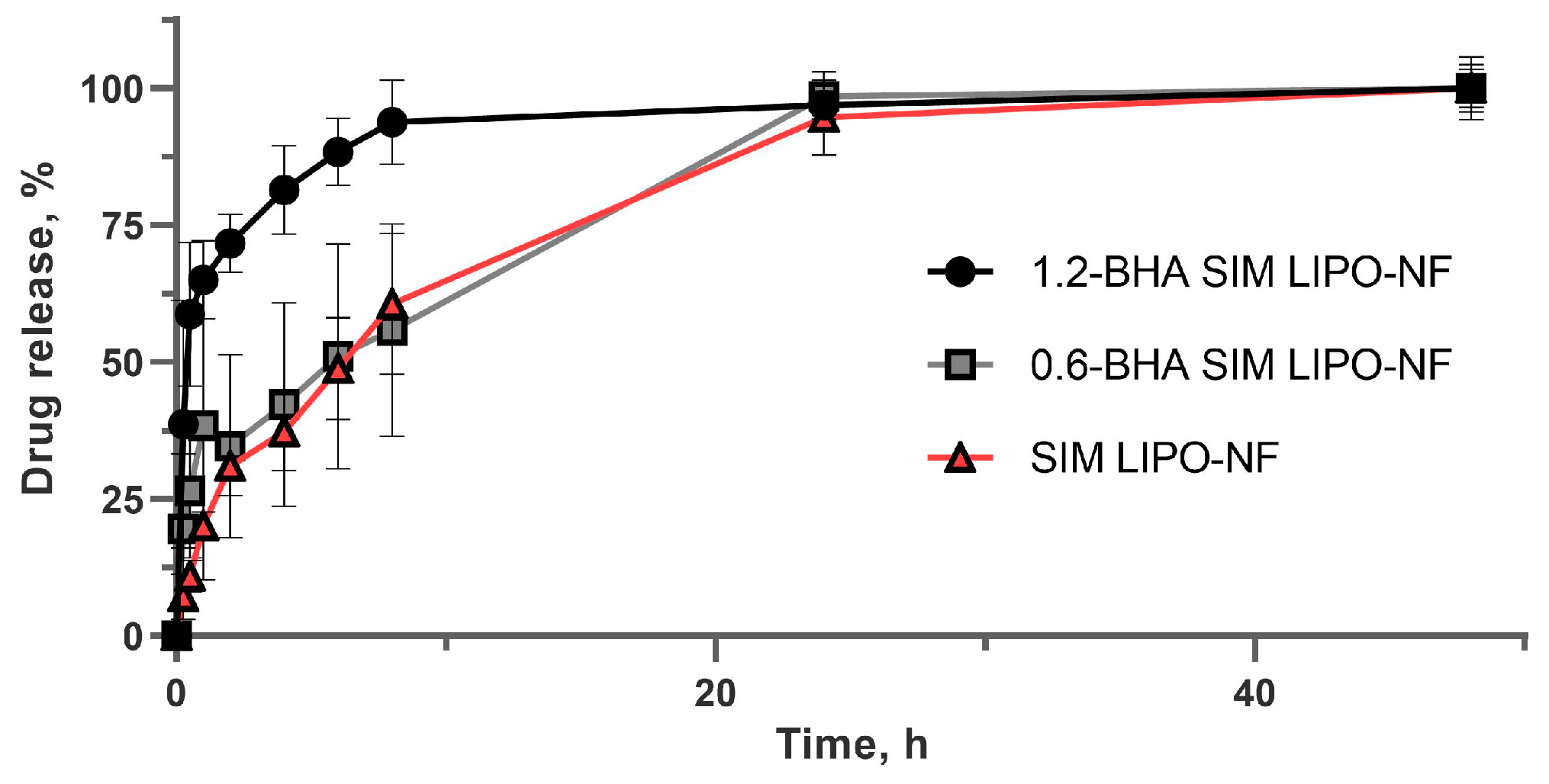
3.5. SIM Release from Nanofibers
3.6. Chemical Stability of SIM in the Nanofibers
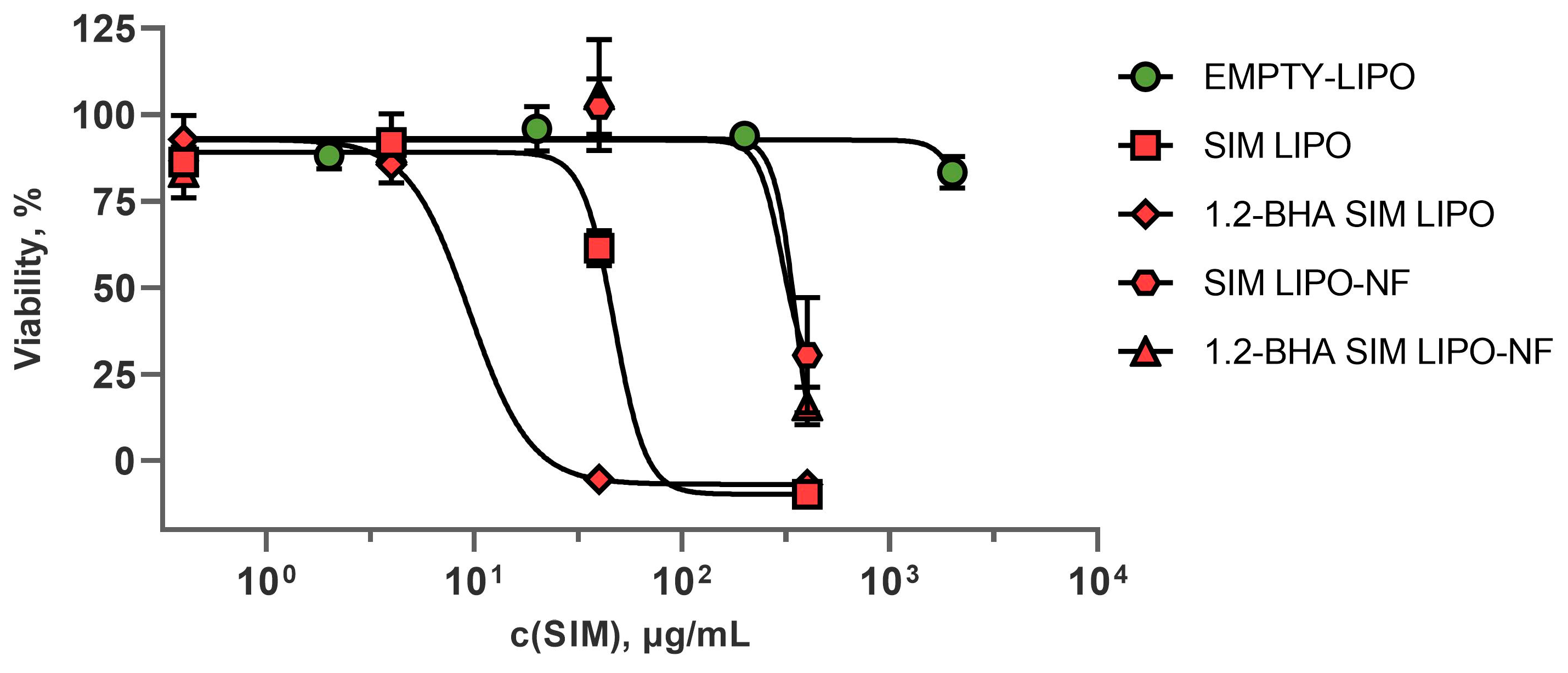
3.7. In Vitro Safety and Efficacy Assessment of SIM Formulations
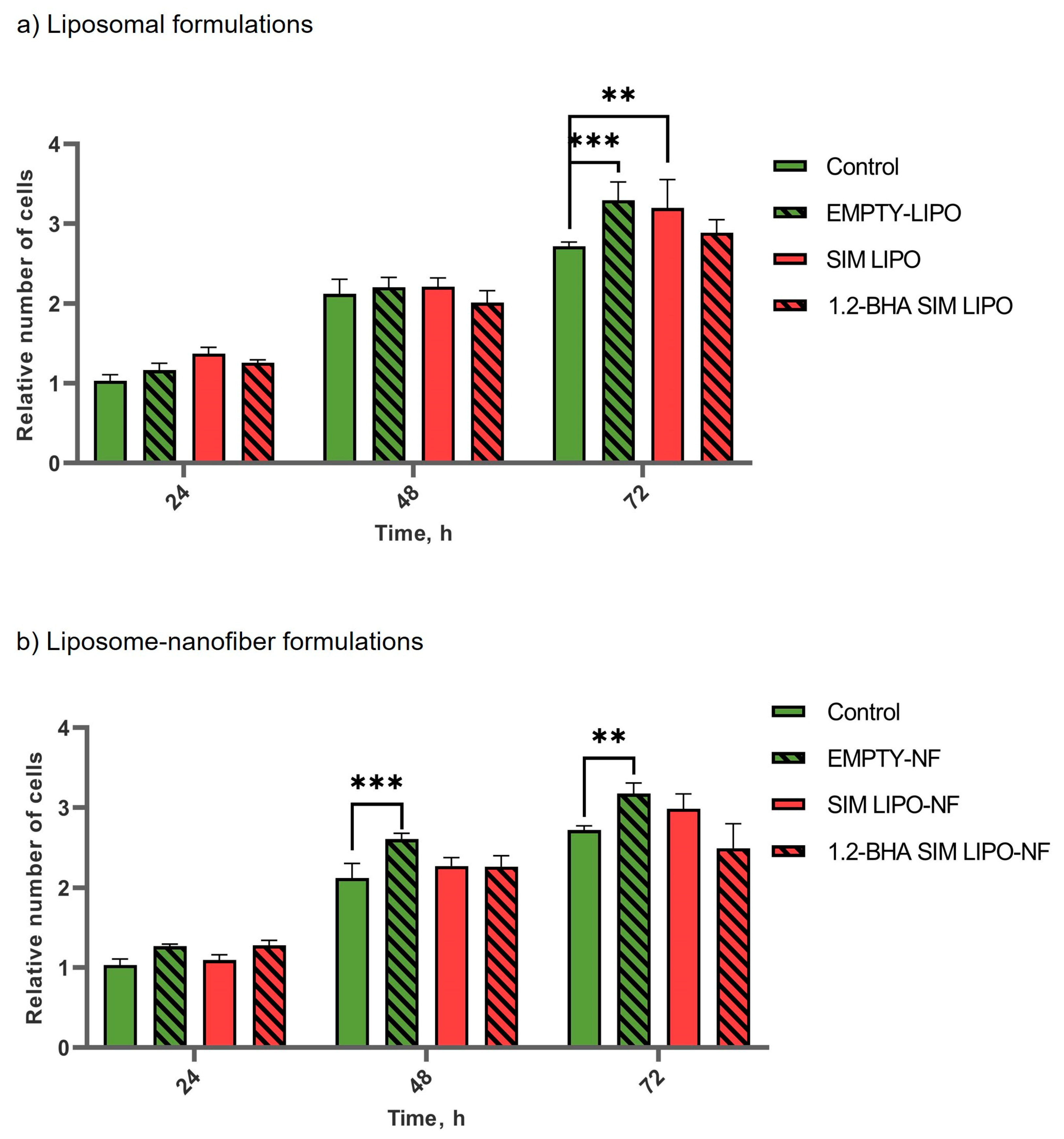
3.7.1. In Vitro Safety Profiles of SIM Formulations
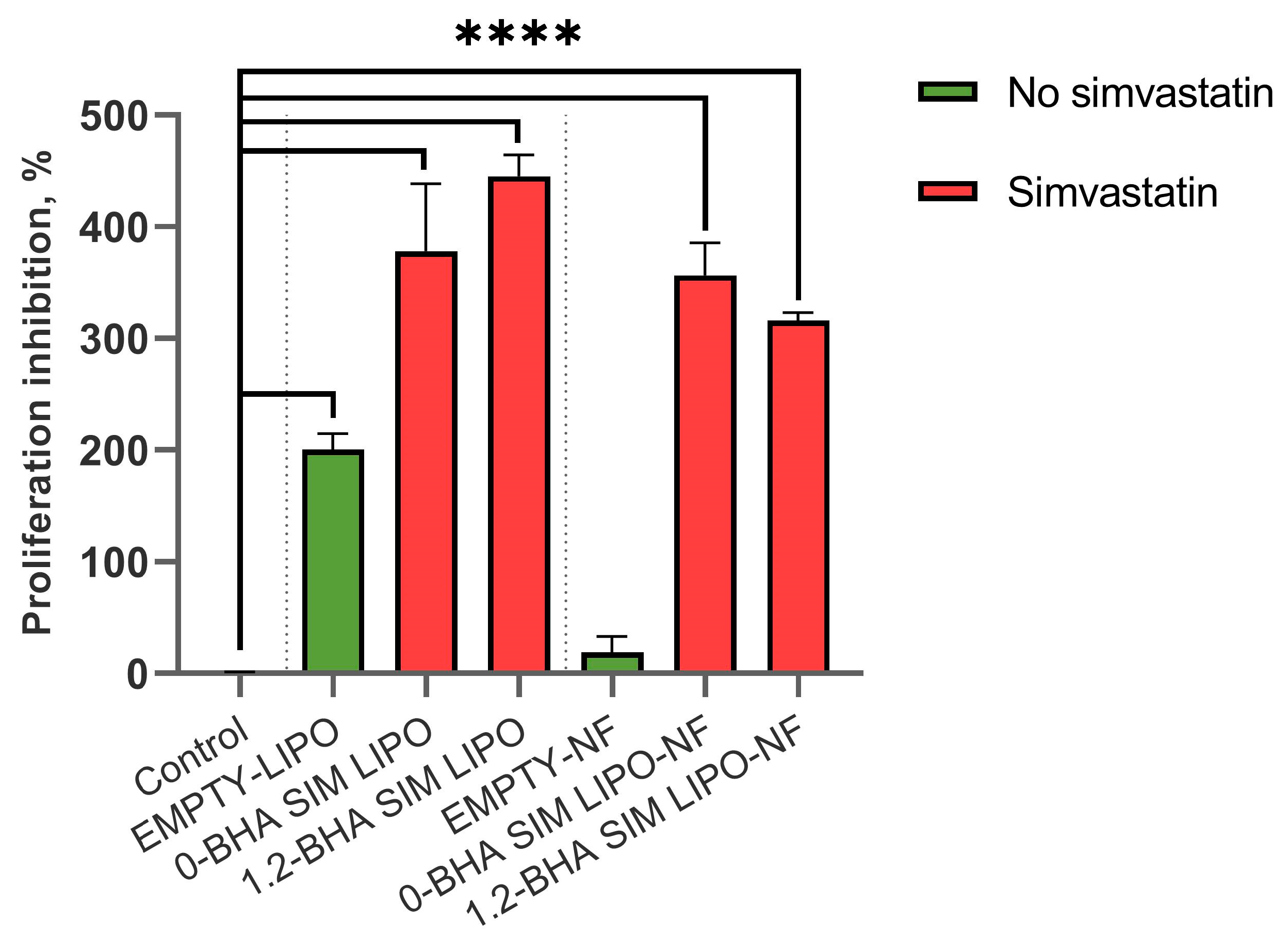
3.7.2. In Vitro Efficacy of SIM Formulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werdin, F.; Tenenhaus, M.; Rennekampff, H.O. Chronic wound care. Lancet 2008, 372, 1860–1862. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.C.; Barbul, A. Guidelines for the best care of chronic wounds. Wound Repair Regen. 2006, 14, 647–648. [Google Scholar] [CrossRef]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef]
- Blanco-Colio, L.M.; Tuñón, J.; Martín-Ventura, J.L.; Egido, J. Anti-inflammatory and immunomodulatory effects of statins. Kidney Int. 2003, 63, 12–23. [Google Scholar] [CrossRef] [Green Version]
- Masadeh, M.; Mhaidat, N.; Alzoubi, K.; Al-azzam, S.; Alnasser, Z. Antibacterial activity of statins: A comparative study of Atorvastatin, Simvastatin, and Rosuvastatin. Ann. Clin. Microbiol. Antimicrob. 2012, 11, 13. [Google Scholar] [CrossRef] [Green Version]
- Uhljar, L.É.; Ambrus, R. Electrospinning of Potential Medical Devices (Wound Dressings, Tissue Engineering Scaffolds, Face Masks) and Their Regulatory Approach. Pharmaceutics 2023, 15, 417. [Google Scholar] [CrossRef]
- Rosic, R.; Pelipenko, J.; Kristl, J.; Kocbeck, P.; Baumgartner, S. Properties, Engineering and Applications of PolymericNanofibers: Current Research and Future Advances. Chem. Biochem. Eng. Q. 2012, 26, 417–425. [Google Scholar]
- Janković, B.; Pelipenko, J.; Škarabot, M.; Muševič, I.; Kristl, J. The design trend in tissue-engineering scaffolds based on nanomechanical properties of individual electrospun nanofibers. Int. J. Pharm. 2013, 455, 338–347. [Google Scholar] [CrossRef]
- Miguel, S.P.; Figueira, D.R.; Simões, D.; Ribeiro, M.P.; Coutinho, P.; Ferreira, P.; Correia, I.J. Electrospun polymeric nanofibres as wound dressings: A review. Colloids Surf. B Biointerfaces 2018, 169, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Godakanda, V.U.; Li, H.; Alquezar, L.; Zhao, L.; Zhu, L.M.; de Silva, R.; de Silva, K.M.N.; Williams, G.R. Tunable drug release from blend poly(vinyl pyrrolidone)-ethyl cellulose nanofibers. Int. J. Pharm. 2019, 562, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Maver, T.; Kurečič, M.; Smrke, D.M.; Kleinschek, K.S.; Maver, U. Electrospun nanofibrous CMC/PEO as a part of an effective pain-relieving wound dressing. J. Sol-Gel Sci. Technol. 2016, 79, 475–486. [Google Scholar] [CrossRef]
- Zupančič, Š.; Casula, L.; Rijavec, T.; Lapanje, A.; Luštrik, M.; Fadda, A.M.; Kocbek, P.; Kristl, J. Sustained release of antimicrobials from double-layer nanofiber mats for local treatment of periodontal disease, evaluated using a new micro flow-through apparatus. J. Control. Release 2019, 316, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Prabhakaran, M.P.; Kai, D.; Ramakrishna, S. Controlled release of multiple epidermal induction factors through core-shell nanofibers for skin regeneration. Eur. J. Pharm. Biopharm. 2013, 85, 689–698. [Google Scholar] [CrossRef]
- Ellison, D.K.; Moore, W.D.; Petts, C.R. Simvastatin. In Analytical Profiles of Drug Substances and Excipients; Academic Press: San Diego, CA, USA, 1993; Volume 22, pp. 359–388. [Google Scholar]
- Contreras-Cáceres, R.; Cabeza, L.; Perazzoli, G.; Díaz, A.; López-Romero, J.M.; Melguizo, C.; Prados, J. Electrospun nanofibers: Recent applications in drug delivery and cancer therapy. Nanomaterials 2019, 9, 656. [Google Scholar] [CrossRef] [Green Version]
- Lai, F.; Caddeo, C.; Manca, M.L.; Manconi, M.; Sinico, C.; Fadda, A.M. What’s new in the field of phospholipid vesicular nanocarriers for skin drug delivery. Int. J. Pharm. 2020, 583, 119398. [Google Scholar] [CrossRef]
- De Freitas Zômpero, R.H.; López-Rubio, A.; de Pinho, S.C.; Lagaron, J.M.; de la Torre, L.G. Hybrid encapsulation structures based on β-carotene-loaded nanoliposomes within electrospun fibers. Colloids Surf. B Biointerfaces 2015, 134, 475–482. [Google Scholar] [CrossRef]
- Ge, Y.; Tang, J.; Fu, H.; Fu, Y. Terpinen-4-ol liposomes-incorporated chitosan/polyethylene oxide electrospun nanofibrous film ameliorates the external microenvironment of healing cutaneous wounds. J. Appl. Polym. Sci. 2021, 138, 49670. [Google Scholar] [CrossRef]
- Li, C.; Bai, M.; Chen, X.; Hu, W.; Cui, H.; Lin, L. Controlled release and antibacterial activity of nanofibers loaded with basil essential oil-encapsulated cationic liposomes against Listeria monocytogenes. Food Biosci. 2022, 46, 101578. [Google Scholar] [CrossRef]
- Chen, J.; Pan, H.; Yang, Y.; Xiong, S.; Duan, H.; Yang, X.; Pan, W. Self-assembled liposome from multi-layered fibrous mucoadhesive membrane for buccal delivery of drugs having high first-pass metabolism. Int. J. Pharm. 2018, 547, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.G.; Branford-White, C.; Williams, G.R.; Bligh, S.W.A.; White, K.; Zhu, L.M.; Chatterton, N.P. Self-assembled liposomes from amphiphilic electrospun nanofibers. Soft Matter 2011, 7, 8239–8247. [Google Scholar] [CrossRef]
- Dickinson, A.M.; Godden, J.M.; Lanovyk, K.; Ahmed, S.S. Assessing the safety of nanomedicines: A mini review. Appl. Vitr. Toxicol. 2019, 5, 114–122. [Google Scholar] [CrossRef]
- Pohlen, M.; Pirker, L.; Luštrik, M.; Dreu, R. A redispersible dry emulsion system with simvastatin prepared via fluid bed layering as a means of dissolution enhancement of a lipophilic drug. Int. J. Pharm. 2018, 549, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Sterle Zorec, B.; Zupančič, Š.; Lavrič, Z.; Dreu, R. Particle properties and drug metastable solubility of simvastatin containing PVP matrix particles prepared by electrospraying technique. Eur. J. Pharm. Sci. 2021, 158, 105649. [Google Scholar] [CrossRef] [PubMed]
- Malvern Panalytical Technical Note: Particle Concentration Measurements on the Zetasizer Ultra—How It Works. 2019. Available online: https://www.malvernpanalytical.com/en/learn/knowledge-center/technical-notes/tn180720particleconczetasizerhowto (accessed on 6 January 2022).
- Malvern Panalytical Technical Note: Best Practice for Making Particle Concentration Measurements on the Zetasizer. 2019. Available online: https://www.malvernpanalytical.com/en/learn/knowledge-center/technical-notes/tn181211guidetoparticleconc (accessed on 6 January 2022).
- FDA/CDER. Dissolution Testing of Immediate Release Solid Oral Dosage Forms. 1997; pp. 15–22. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/dissolution-testing-immediate-release-solid-oral-dosage-forms (accessed on 1 December 2022).
- EMA. CPMP/EWP/QWP/1401/98 Rev. 1. Guideline on the Investigation of BIOEQUIVALENCE. 2010, pp. 1–27. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjq_rn-26H-AhXx-TgGHYYBD2QQFnoECBgQAQ&url=https%3A%2F%2Fwww.ema.europa.eu%2Fen%2Fdocuments%2Fscientific-guideline%2Fguideline-investigation-bioequivalence-rev1_en.pdf&usg=AOvVaw2lPMVvjTJ-LgHASIfLM_8a (accessed on 1 December 2022).
- Kajdič, S.; Zupančič, Š.; Roškar, R.; Kocbek, P. The potential of nanofibers to increase solubility and dissolution rate of the poorly soluble and chemically unstable drug lovastatin. Int. J. Pharm. 2020, 573, 118809. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Buyana, B. Alginate in wound dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Bacakova, L.; Pajorova, J.; Zikmundova, M.; Filova, E.; Mikes, P.; Jencova, V.; Kuzelova Kostakova, E.; Sinica, A. Nanofibrous Scaffolds for Skin Tissue Engineering and Wound Healing Based on Nature-Derived Polymers. In Current and Future Aspects of Nanomedicine; IntechOpen: London, UK, 2020. [Google Scholar]
- Mirtič, J.; Balažic, H.; Zupančič, Š.; Kristl, J. Effect of solution composition variables on electrospun alginate nanofibers: Response surface analysis. Polymers 2019, 11, 692. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, R.; Sundarrajan, S.; Ramakrishna, S. Green processing of nanofibers for regenerative medicine. Macromol. Mater. Eng. 2013, 298, 1034–1058. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Li, C.; Liu, R.; Lin, L. Fabrication of chitosan nanofibers containing tea tree oil liposomes against Salmonella spp. in chicken. LWT 2018, 96, 671–678. [Google Scholar] [CrossRef]
- Mitropoulou, A.; Markatos, D.; Antimisiaris, S.; Mavrillas, D. A Novel Design of a PVA Electrospun Nanofibrous Scaffold Incorporating Liposomes as Drug Delivery Carriers for Tissue Engineering. Ann. Biomed. Technol. Eng. 2018, 1, 1003. [Google Scholar]
- Mickova, A.; Buzgo, M.; Benada, O.; Rampichova, M.; Fisar, Z.; Filova, E.; Tesarova, M.; Lukas, D.; Amler, E. Core/shell nanofibers with embedded liposomes as a drug delivery system. Biomacromolecules 2012, 13, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Hasanbegloo, K.; Banihashem, S.; Faraji, B.; Bybordi, S.; Farrokheslamlou, N.; Abadi, P.G.; Jazi, F.S.; Irani, M. Paclitaxel-loaded liposome-incorporated chitosan (core)/poly(ε-caprolactone)/chitosan (shell) nanofibers for the treatment of breast cancer. Int. J. Biol. Macromol. 2023, 230, 123380. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kang, H.; Che, N.; Liu, Z.; Li, P.; Li, W.; Zhang, C.; Cao, C.; Liu, R.; Huang, Y. Controlled release of liposome-encapsulated Naproxen from core-sheath electrospun nanofibers. Carbohydr. Polym. 2014, 111, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kang, H.; Li, Q.; Che, N.; Liu, Z.; Li, P.; Zhang, C.; Liu, R.; Huang, Y. Ultrathin core-sheath fibers for liposome stabilization. Colloids Surf. B Biointerfaces 2014, 122, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Laidmäe, I.; Meos, A.; Kjærvik, I.A.; Ingebrigtsen, S.G.; Škalko-Basnet, N.; Kirsimäe, K.; Romann, T.; Joost, U.; Kisand, V.; Kogermann, K. Electrospun amphiphilic nanofibers as templates for in situ preparation of chloramphenicol-loaded liposomes. Pharmaceutics 2021, 13, 1742. [Google Scholar] [CrossRef]
- Malvern Panalytical Technical Note: Multi-Angle Dynamic Light Scattering (MADLS) on the Zetasizer Ultra—How It Works. 2018. Available online: https://www.malvernpanalytical.com/en/learn/knowledge-center/technical-notes/tn180719howitworksmadls (accessed on 6 January 2022).
- Austin, J.; Minelli, C.; Hamilton, D.; Wywijas, M.; Jones, H.J. Nanoparticle number concentration measurements by multi-angle dynamic light scattering. J. Nanopart. Res. 2020, 22, 108. [Google Scholar] [CrossRef]
- Śliwa, P.; Śliwa, K.; Sikora, E.; Ogonowski, J.; Oszmiański, J.; Nowicka, P. Incorporation of bioflavonoids from Bidens tripartite into micelles of non-ionic surfactants—Experimental and theoretical studies. Colloids Surf. B Biointerfaces 2019, 184, 110553. [Google Scholar] [CrossRef]
- Leitão, J.H.; Markova, N.; Cairns, S.; Jankevics-Jones, H.; Kaszuba, M.; Caputo, F.; Parot, J. Biophysical Characterization of Viral and Lipid-Based Vectors for Vaccines and Therapeutics with Light Scattering and Calorimetric Techniques. Vaccines 2021, 10, 49. [Google Scholar] [CrossRef]
- Soldi, M.; Sergi Sergi, L.; Unali, G.; Kerzel, T.; Cuccovillo, I.; Capasso, P.; Annoni, A.; Biffi, M.; Rancoita, P.M.V.; Cantore, A.; et al. Laboratory-Scale Lentiviral Vector Production and Purification for Enhanced Ex Vivo and In Vivo Genetic Engineering. Mol. Ther. Methods Clin. Dev. 2020, 19, 411–425. [Google Scholar] [CrossRef]
- Council of Europe. 5.17.1. Reccomendations on Dissolution Testing. European Pharmacopoeia 8.0. 2013, pp. 727–729. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiRl9qv3KH-AhWyjGMGHRKDAOUQFnoECB0QAQ&url=https%3A%2F%2Ffile.wuxuwang.com%2Fyaopinbz%2FEP8.0_1_00338.pdf&usg=AOvVaw3gU5VfqVryBNsdLjEjbHfV (accessed on 1 December 2022).
- Kaufman, M.J. Applications of Oxygen Polarography to Drug Stability Testing and Formulation Development: Solution-Phase Oxidation of Hydroxymethylglutaryl Coenzyme A (HMG-CoA) Reductase Inhibitors. Pharm. Res. 1990, 7, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Aliabadi, H.; Minaiyan, M.; Dabestan, A. Cytotoxic evaluation of doxorubicin in combination with simvastatin against human cancer cells. Res. Pharm. Sci. 2010, 5, 127. [Google Scholar] [PubMed]
- Mozafari, M.R.; Reed, C.J.; Rostron, C. Cytotoxicity evaluation of anionic nanoliposomes and nanolipoplexes prepared by the heating method without employing volatile solvents and detergents. Pharmazie 2007, 62, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Bonnekoh, B.; Röding, J.; Krueger, G.R.F.; Ghyczy, M.; Mahrle, G. Increase of lipid fluidity and suppression of proliferation resulting from liposome uptake by human keratinocytes in vitro. Br. J. Dermatol. 1991, 124, 333–340. [Google Scholar] [CrossRef]
- Zidar, A.; Kristl, J.; Kocbek, P.; Zupančič, Š. Treatment challenges and delivery systems in immunomodulation and probiotic therapies for periodontitis. Expert Opin. Drug Deliv. 2021, 18, 1229–1244. [Google Scholar] [CrossRef] [PubMed]
- Ghittoni, R.; Patrussi, L.; Pirozzi, K.; Pellegrini, M.; Lazzerini, P.E.; Capecchi, P.L.; Pasini, F.L.; Baldari, C.T. Simvastatin inhibits T-cell activation by selectively impairing the function of Ras superfamily GTPases. FASEB J. 2005, 19, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, S.; Su, L.; Amano, M.; Timmerman, L.A.; Kaneshima, H.; Nolan, G.P. The T Cell Activation Factor NF-ATc Positively Regulates HIV-1 Replication and Gene Expression in T Cells. Immunity 1997, 6, 235–244. [Google Scholar] [CrossRef] [Green Version]




| Formulations | Composition | Characterization | |||||
|---|---|---|---|---|---|---|---|
| P90G (mg/mL) | SIM (mg/mL) | BHA (mg/mL) | Average Diameter (nm) | PI | ZP (mV) | EE (%) | |
| SIM LIPO | 100 | 20 | 0 | 65.1 ± 0.8 abc | 0.19 ± 0.01 ab | −14 ± 3 a | 96 ± 5 ab |
| 0.3-BHA SIM LIPO | 100 | 20 | 0.3 | 73.6 ± 4.0 de | 0.16 ± 0.01 acd | −16 ± 1 | 88 ± 3 |
| 0.6-BHA SIM LIPO | 100 | 20 | 0.6 | 77.9 ± 1.2 af | 0.16 ± 0.01 b | −18 ± 1 | 88 ± 5 |
| 0.9-BHA SIM LIPO | 100 | 20 | 0.9 | 86.4 ± 4.6 bdg | 0.18 ± 0.01 c | −21 ± 2 a | 82 ± 5 a |
| 1.2-BHA SIM LIPO | 100 | 20 | 1.2 | 105.9 ± 4.4 cefg | 0.17 ± 0.01 d | −18 ± 3 | 80 ± 4 b |
| Formulations | Nanofiber Diameter (nm) | DL (% (w/w)) | DLE (%) |
|---|---|---|---|
| EMPTY-NF | 159.8 ± 25.2 | - | - |
| SIM LIPO-NF | 273.3 ± 64.6 ab | 10.61 ± 0.28 | 83.6 ± 2.4 |
| 0.6-BHA SIM LIPO-NF | 315.4 ±79.0 a | 10.38 ± 0.45 | 80.9 ± 3.5 |
| 1.2-BHA SIM LIPO-NF | 311.6 ±88.5 b | 10.41 ± 0.27 | 81.3 ± 2.0 |
| Formulations | Peak 1 | Peak 2 | ||||
|---|---|---|---|---|---|---|
| Size (nm) | Intensity (%) | Particle Concentration (Particle/mL) | Size (nm) | Intensity (%) | Particle Concentration (Particle/mL) | |
| SIM LIPO-NF | 135.6 | 44.5 | 1.24 × 1012 | 432.2 | 39.0 | 2.46 × 1010 |
| 0.6-BHA-LIPO-NF | 154.2 | 44.6 | 3.17 × 1010 | 438.0 | 45.7 | 1.31 × 1011 |
| 1.2-BHA-LIPO-NF | 141.2 | 33.2 | 1.11 × 109 | 436.3 | 48.8 | 2.12 × 1012 |
| 0-BHA SIM LIPO-NF vs 0.6-BHA SIM LIPO-NF | 0-BHA SIM LIPO-NF vs 1.2-BHA SIM LIPO-NF | 0.6-BHA SIM LIPO-NF vs 1.2-BHA SIM LIPO-NF | |
|---|---|---|---|
| f2 | 50 | 19 | 24 |
| Formulation | IC50 (μg/mL) |
|---|---|
| EMPTY-LIPO | ~1998 |
| SIM LIPO | ~47 |
| 1.2- BHA SIM LIPO | ~9 |
| SIM LIPO-NF | ~323 |
| 1.2- BHA SIM LIPO-NF | ~392 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casula, L.; Zidar, A.; Kristl, J.; Jeras, M.; Kralj, S.; Fadda, A.M.; Zupančič, Š. Development of Nanofibers with Embedded Liposomes Containing an Immunomodulatory Drug Using Green Electrospinning. Pharmaceutics 2023, 15, 1245. https://doi.org/10.3390/pharmaceutics15041245
Casula L, Zidar A, Kristl J, Jeras M, Kralj S, Fadda AM, Zupančič Š. Development of Nanofibers with Embedded Liposomes Containing an Immunomodulatory Drug Using Green Electrospinning. Pharmaceutics. 2023; 15(4):1245. https://doi.org/10.3390/pharmaceutics15041245
Chicago/Turabian StyleCasula, Luca, Anže Zidar, Julijana Kristl, Matjaž Jeras, Slavko Kralj, Anna Maria Fadda, and Špela Zupančič. 2023. "Development of Nanofibers with Embedded Liposomes Containing an Immunomodulatory Drug Using Green Electrospinning" Pharmaceutics 15, no. 4: 1245. https://doi.org/10.3390/pharmaceutics15041245