Double-Loaded Doxorubicin/Resveratrol Polymeric Micelles Providing Low Toxicity on Cardiac Cells and Enhanced Cytotoxicity on Lymphoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Compatibility between Both Drugs and Hydrophobic Block of the Copolymers
2.3. Preparation of Double-Loaded Micelles
2.4. Characterisation of the Micelles
2.5. In Vitro Release of Doxorubicin and Resveratrol from the Micelles
2.6. Cell Culturing and In Vitro Cell Viability Studies
2.7. Statistical Analysis
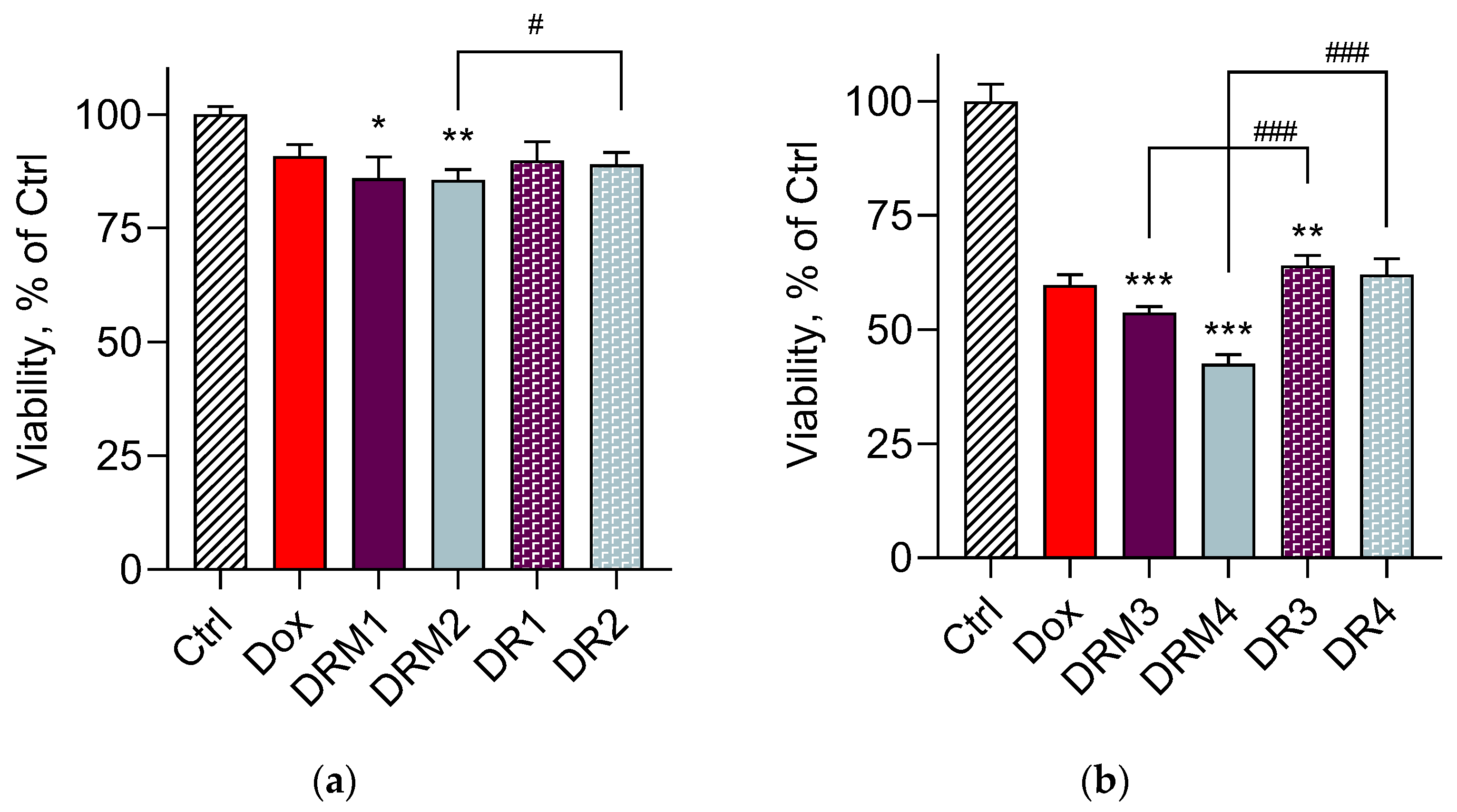
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor Vascular Permeability and the EPR Effect in Macromolecular Therapeutics: A Review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, H.M.; Lavasanifar, A. Polymeric Micelles for Drug Delivery. Expert Opin. Drug Deliv. 2006, 3, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block Copolymer Micelles for Drug Delivery: Design, Characterization and Biological Significance. Adv. Drug Deliv. Rev. 2012, 64, 37–48. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.-X.; Liu, Y.-M.; Chen, K.-L.; Chen, G. A Comparative Study of Anti-Aging Properties and Mechanism: Resveratrol and Caloric Restriction. Oncotarget 2017, 8, 65717–65729. [Google Scholar] [CrossRef] [PubMed]
- Afsharzadeh, M.; Hashemi, M.; Mokhtarzadeh, A.; Abnous, K.; Ramezani, M. Recent Advances in Co-Delivery Systems Based on Polymeric Nanoparticle for Cancer Treatment. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1095–1110. [Google Scholar] [CrossRef] [PubMed]
- Cagel, M.; Tesan, F.C.; Bernabeu, E.; Salgueiro, M.J.; Zubillaga, M.B.; Moretton, M.A.; Chiappetta, D.A. Polymeric Mixed Micelles as Nanomedicines: Achievements and Perspectives. Eur. J. Pharm. Biopharm. 2017, 113, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Scarano, W.; De Souza, P.; Stenzel, M.H. Dual-Drug Delivery of Curcumin and Platinum Drugs in Polymeric Micelles Enhances the Synergistic Effects: A Double Act for the Treatment of Multidrug-Resistant Cancer. Biomater. Sci. 2015, 3, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.H.P.; Yung, L.-Y.L. Synergistic Co-Delivery of Doxorubicin and Paclitaxel Using Multi-Functional Micelles for Cancer Treatment. Int. J. Pharm. 2013, 454, 486–495. [Google Scholar] [CrossRef]
- Riedel, J.; Calienni, M.N.; Bernabeu, E.; Calabro, V.; Lázaro-Martinez, J.M.; Prieto, M.J.; Gonzalez, L.; Martinez, C.S.; del Valle Alonso, S.; Montanari, J.; et al. Paclitaxel and Curcumin Co-Loaded Mixed Micelles: Improving in Vitro Efficacy and Reducing Toxicity against Abraxane®. J. Drug Deliv. Sci. Technol. 2021, 62, 102343. [Google Scholar] [CrossRef]
- Bae, K.H.; Tan, S.; Yamashita, A.; Ang, W.X.; Gao, S.J.; Wang, S.; Chung, J.E.; Kurisawa, M. Hyaluronic Acid-Green Tea Catechin Micellar Nanocomplexes: Fail-Safe Cisplatin Nanomedicine for the Treatment of Ovarian Cancer without off-Target Toxicity. Biomaterials 2017, 148, 41–53. [Google Scholar] [CrossRef]
- Octavia, Y.; Tocchetti, C.G.; Gabrielson, K.L.; Janssens, S.; Crijns, H.J.; Moens, A.L. Doxorubicin-Induced Cardiomyopathy: From Molecular Mechanisms to Therapeutic Strategies. J. Mol. Cell. Cardiol. 2012, 52, 1213–1225. [Google Scholar] [CrossRef]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-Induced Cardiotoxicity: An Update on the Molecular Mechanism and Novel Therapeutic Strategies for Effective Management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Guo, Q.; Li, Y.; Wang, X.; Wang, J.; Tu, P. Co-Assembly of Doxorubicin and Curcumin Targeted Micelles for Synergistic Delivery and Improving Anti-Tumor Efficacy. Eur. J. Pharm. Biopharm. 2017, 112, 209–223. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, Q.; Wang, N.; Yang, Y.; Liu, J.; Yu, G.; Yang, X.; Xu, H.; Wang, H. A Complex Micellar System Co-Delivering Curcumin with Doxorubicin against Cardiotoxicity and Tumor Growth. Int. J. Nanomed. 2018, 13, 4549–4561. [Google Scholar] [CrossRef]
- Qureshi, W.A.; Zhao, R.; Wang, H.; Ji, T.; Ding, Y.; Ihsan, A.; Mujeeb, A.; Nie, G.; Zhao, Y. Co-Delivery of Doxorubicin and Quercetin via MPEG–PLGA Copolymer Assembly for Synergistic Anti-Tumor Efficacy and Reducing Cardio-Toxicity. Sci. Bull. 2016, 61, 1689–1698. [Google Scholar] [CrossRef]
- Bastianetto, S.; Ménard, C.; Quirion, R. Neuroprotective Action of Resveratrol. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2015, 1852, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.; Ingmer, H. Antibacterial and Antifungal Properties of Resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Kasiotis, K.M.; Pratsinis, H.; Kletsas, D.; Haroutounian, S.A. Resveratrol and Related Stilbenes: Their Anti-Aging and Anti-Angiogenic Properties. Food Chem. Toxicol. 2013, 61, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Frémont, L. Biological Effects of Resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic Potential of Resveratrol: The in Vivo Evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Z.-R.; Hsieh, T.-C.; Bruder, J.; Zou, J.-G.; Huang, Y.-Z. Mechanism of Cardioprotection by Resveratrol, a Phenolic Antioxidant Present in Red Wine (Review). Int. J. Mol. Med. 2001, 8, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Hu, W.; Zhang, D. Resveratrol, a Polyphenol Phytoalexin, Protects against Doxorubicin-induced Cardiotoxicity. J. Cell. Mol. Med. 2015, 19, 2324–2328. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Elshaer, M.; Chen, Y.; Wang, X.J.; Tang, X. Resveratrol: An Overview of Its Anti-Cancer Mechanisms. Life Sci. 2018, 207, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Rai, G.; Mishra, S.; Suman, S.; Shukla, Y. Resveratrol Improves the Anticancer Effects of Doxorubicin in Vitro and in Vivo Models: A Mechanistic Insight. Phytomedicine 2016, 23, 233–242. [Google Scholar] [CrossRef]
- Osman, A.-M.M.; Al-Harthi, S.E.; AlArabi, O.M.; Elshal, M.F.; Ramadan, W.S.; Alaama, M.N.; Al-Kreathy, H.M.; Damanhouri, Z.A.; Osman, O.H. Chemosensetizing and Cardioprotective Effects of Resveratrol in Doxorubicin-Treated Animals. Cancer Cell Int. 2013, 13, 52. [Google Scholar] [CrossRef]
- Robinson, K.; Mock, C.; Liang, D. Pre-Formulation Studies of Resveratrol. Drug Dev. Ind. Pharm. 2015, 41, 1464–1469. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of Resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Carlson, L.J.; Cote, B.; Alani, A.W.G.; Rao, D.A. Polymeric Micellar Co-Delivery of Resveratrol and Curcumin to Mitigate in Vitro Doxorubicin-Induced Cardiotoxicity. J. Pharm. Sci. 2014, 103, 2315–2322. [Google Scholar] [CrossRef]
- Fatease, A.A.; Shah, V.; Nguyen, D.X.; Cote, B.; LeBlanc, N.; Rao, D.A.; Alani, A.W.G. Chemosensitization and Mitigation of Adriamycin-Induced Cardiotoxicity Using Combinational Polymeric Micelles for Co-Delivery of Quercetin/Resveratrol and Resveratrol/Curcumin in Ovarian Cancer. Nanomed. Nanotechnol. Biol. Med. 2019, 19, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yuan, S.; Hao, J.; Fang, X. Mechanism of Inhibition of P-Glycoprotein Mediated Efflux by Pluronic P123/F127 Block Copolymers: Relationship between Copolymer Concentration and Inhibitory Activity. Eur. J. Pharm. Biopharm. 2013, 83, 266–274. [Google Scholar] [CrossRef]
- Wei, Z.; Hao, J.; Yuan, S.; Li, Y.; Juan, W.; Sha, X.; Fang, X. Paclitaxel-Loaded Pluronic P123/F127 Mixed Polymeric Micelles: Formulation, Optimization and in Vitro Characterization. Int. J. Pharm. 2009, 376, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shi, Y.; Chen, Y.; Ye, J.; Sha, X.; Fang, X. Multifunctional Pluronic P123/F127 Mixed Polymeric Micelles Loaded with Paclitaxel for the Treatment of Multidrug Resistant Tumors. Biomaterials 2011, 32, 2894–2906. [Google Scholar] [CrossRef]
- Fares, A.R.; ElMeshad, A.N.; Kassem, M.A.A. Enhancement of Dissolution and Oral Bioavailability of Lacidipine via Pluronic P123/F127 Mixed Polymeric Micelles: Formulation, Optimization Using Central Composite Design and in Vivo Bioavailability Study. Drug Deliv. 2018, 25, 132–142. [Google Scholar] [CrossRef]
- Xie, Y.-J.; Wang, Q.-L.; Adu-Frimpong, M.; Liu, J.; Zhang, K.-Y.; Xu, X.-M.; Yu, J.-N. Preparation and Evaluation of Isoliquiritigenin-Loaded F127/P123 Polymeric Micelles. Drug Dev. Ind. Pharm. 2019, 45, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Pellosi, D.S.; Pagliara, V.; Milone, M.R.; Pucci, B.; Caetano, W.; Hioka, N.; Budillon, A.; Ungaro, F.; Russo, G.; et al. Biotin-Targeted Pluronic® P123/F127 Mixed Micelles Delivering Niclosamide: A Repositioning Strategy to Treat Drug-Resistant Lung Cancer Cells. Int. J. Pharm. 2016, 511, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Pellosi, D.S.; Moret, F.; Fraix, A.; Marino, N.; Maiolino, S.; Gaio, E.; Hioka, N.; Reddi, E.; Sortino, S.; Quaglia, F. Pluronic® P123/F127 Mixed Micelles Delivering Sorafenib and Its Combination with Verteporfin in Cancer Cells. Int. J. Nanomed. 2016, 11, 4479–4494. [Google Scholar] [CrossRef]
- Zaharieva, M.M.; Dimitrov Kroumov, A.; Dimitrova, L.; Tsvetkova, I.; Trochopoulos, A.; Mihaylov Konstantinov, S.; Reinhold Berger, M.; Momchilova, M.; Yoncheva, K.; Najdenski, H.M. Micellar Curcumin Improves the Antibacterial Activity of the Alkylphosphocholines Erufosine and Miltefosine against Pathogenic Staphyloccocus Aureus Strains. Biotechnol. Biotechnol. Equip. 2019, 33, 38–53. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Varghese, S.; Manjusha, V. Hyaluronic Acid Coated Pluronic F127/Pluronic P123 Mixed Micelle for Targeted Delivery of Paclitaxel and Curcumin. Int. J. Biol. Macromol. 2021, 192, 950–957. [Google Scholar] [CrossRef]
- Yordanov, Y.; Stefanova, D.; Spassova, I.; Kovacheva, D.; Tzankova, V.; Konstantinov, S.; Yoncheva, K. Formulation of Nanomicelles Loaded with Cannabidiol as a Platform for Neuroprotective Therapy. Pharmaceutics 2022, 14, 2625. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953; ISBN 978-0-8014-0134-3. [Google Scholar]
- Fedors, R.F. A Method for Estimating Both the Solubility Parameters and Molar Volumes of Liquids. Polym. Eng. Sci. 1974, 14, 147–154. [Google Scholar] [CrossRef]
- DIFFRAC.TOPAS. Available online: https://www.bruker.com/en/products-and-solutions/diffractometers-and-scattering-systems/x-ray-diffractometers/diffrac-suite-software/diffrac-topas.html (accessed on 17 February 2023).
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of Solute Release from Porous Hydrophilic Polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral Red Uptake Assay for the Estimation of Cell Viability/Cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (Resazurin) Fluorescent Dye for the Assessment of Mammalian Cell Cytotoxicity: Resazurin as a Cytotoxicity Assay. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Ben-Shachar, M.S.; Lüdecke, D.; Makowski, D. Effectsize: Estimation of Effect Size Indices and Standardized Parameters. J. Open Source Softw. 2020, 5, 2815. [Google Scholar] [CrossRef]
- Wallemacq, P.E.; Capron, A.; Vanbinst, R.; Boeckmans, E.; Gillard, J.; Favier, B. Permeability of 13 Different Gloves to 13 Cytotoxic Agents under Controlled Dynamic Conditions. Am. J. Health. Syst. Pharm. 2006, 63, 547–556. [Google Scholar] [CrossRef]
- Dou, J.; Zhang, H.; Liu, X.; Zhang, M.; Zhai, G. Preparation and Evaluation in Vitro and in Vivo of Docetaxel Loaded Mixed Micelles for Oral Administration. Colloids Surf. B Biointerfaces 2014, 114, 20–27. [Google Scholar] [CrossRef]
- Innocenzi, P.; Malfatti, L.; Piccinini, M.; Marcelli, A. Evaporation-Induced Crystallization of Pluronic F127 Studied in Situ by Time-Resolved Infrared Spectroscopy. J. Phys. Chem. A 2010, 114, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Yoncheva, K.; Tzankov, B.; Yordanov, Y.; Spassova, I.; Kovacheva, D.; Frosini, M.; Valoti, M.; Tzankova, V. Encapsulation of Doxorubicin in Chitosan-Alginate Nanoparticles Improves Its Stability and Cytotoxicity in Resistant Lymphoma L5178 MDR Cells. J. Drug Deliv. Sci. Technol. 2020, 59, 101870. [Google Scholar] [CrossRef]
- Porto, I.C.C.M.; Nascimento, T.G.; Oliveira, J.M.S.; Freitas, P.H.; Haimeur, A.; França, R. Use of Polyphenols as a Strategy to Prevent Bond Degradation in the Dentin-Resin Interface. Eur. J. Oral Sci. 2018, 126, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Billes, F.; Mohammed-Ziegler, I.; Mikosch, H.; Tyihák, E. Vibrational Spectroscopy of Resveratrol. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2007, 68, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Tanski, J.; Villegas-Estrada, A.; Rossi, M. Structural Basis for Antioxidant Activity of Trans-Resveratrol: Ab Initio Calculations and Crystal and Molecular Structure. J. Agric. Food Chem. 2004, 52, 7279–7285. [Google Scholar] [CrossRef]
- Shatalova, O.V.; Krivandin, A.V.; Aksenova, N.A.; Solov’eva, A.B. Structure of Pluronic F-127 and Its Tetraphenylporphyrin Complexes: X-Ray Diffraction Study. Polym. Sci. Ser. A 2008, 50, 417–421. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Alam, M.A.; Ahmad, F.J. Enhancement of Oral Bioavailability of Doxorubicin through Surface Modified Biodegradable Polymeric Nanoparticles. Chem. Cent. J. 2018, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, L.; Zhang, L.; Xing, S.; Wang, T.; Wang, Y.A.; Wang, C.; Su, Z. Designed Fabrication of Unique Eccentric Mesoporous Silica Nanocluster-Based Core–Shell Nanostructures for PH-Responsive Drug Delivery. ACS Appl. Mater. Interfaces 2013, 5, 7282–7290. [Google Scholar] [CrossRef]
- He, L.; Liang, H.; Lin, L.; Shah, B.R.; Li, Y.; Chen, Y.; Li, B. Green-Step Assembly of Low Density Lipoprotein/Sodium Carboxymethyl Cellulose Nanogels for Facile Loading and PH-Dependent Release of Doxorubicin. Colloids Surf. B Biointerfaces 2015, 126, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Asghar, S.; Tian, C.; Hu, Z.; Ping, Q.; Chen, Z.; Shao, F.; Xiao, Y. Lactoferrin/Phenylboronic Acid-Functionalized Hyaluronic Acid Nanogels Loading Doxorubicin Hydrochloride for Targeting Glioma. Carbohydr. Polym. 2021, 253, 117194. [Google Scholar] [CrossRef]
- Monahan, D.S.; Flaherty, E.; Hameed, A.; Duffy, G.P. Resveratrol Significantly Improves Cell Survival in Comparison to Dexrazoxane and Carvedilol in a H9c2 Model of Doxorubicin Induced Cardiotoxicity. Biomed. Pharmacother. 2021, 140, 111702. [Google Scholar] [CrossRef]
- Zhou, W.; Ouyang, J.; Hu, N.; Li, G.; Wang, H. Protective Effect of Two Alkaloids from Hippophae Rhamnoides Linn. against Doxorubicin-Induced Toxicity in H9c2 Cardiomyoblasts. Molecules 2021, 26, 1946. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.H.; Rehman, K. Recent Progress in Biomedical Applications of Pluronic (PF127): Pharmaceutical Perspectives. J. Controlled Release 2015, 209, 120–138. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Garg, T.; Tanmay, M.; Arora, S. Polymeric Drug-Delivery Systems: Role in P-Gp Efflux System Inhibition. Crit. Rev. Ther. Drug Carrier Syst. 2015, 32, 247–275. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K. Alternatives to P Value: Confidence Interval and Effect Size. Korean J. Anesthesiol. 2016, 69, 555–562. [Google Scholar] [CrossRef] [PubMed]
- de la Lastra, C.A.; Villegas, I. Resveratrol as an Antioxidant and Pro-Oxidant Agent: Mechanisms and Clinical Implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
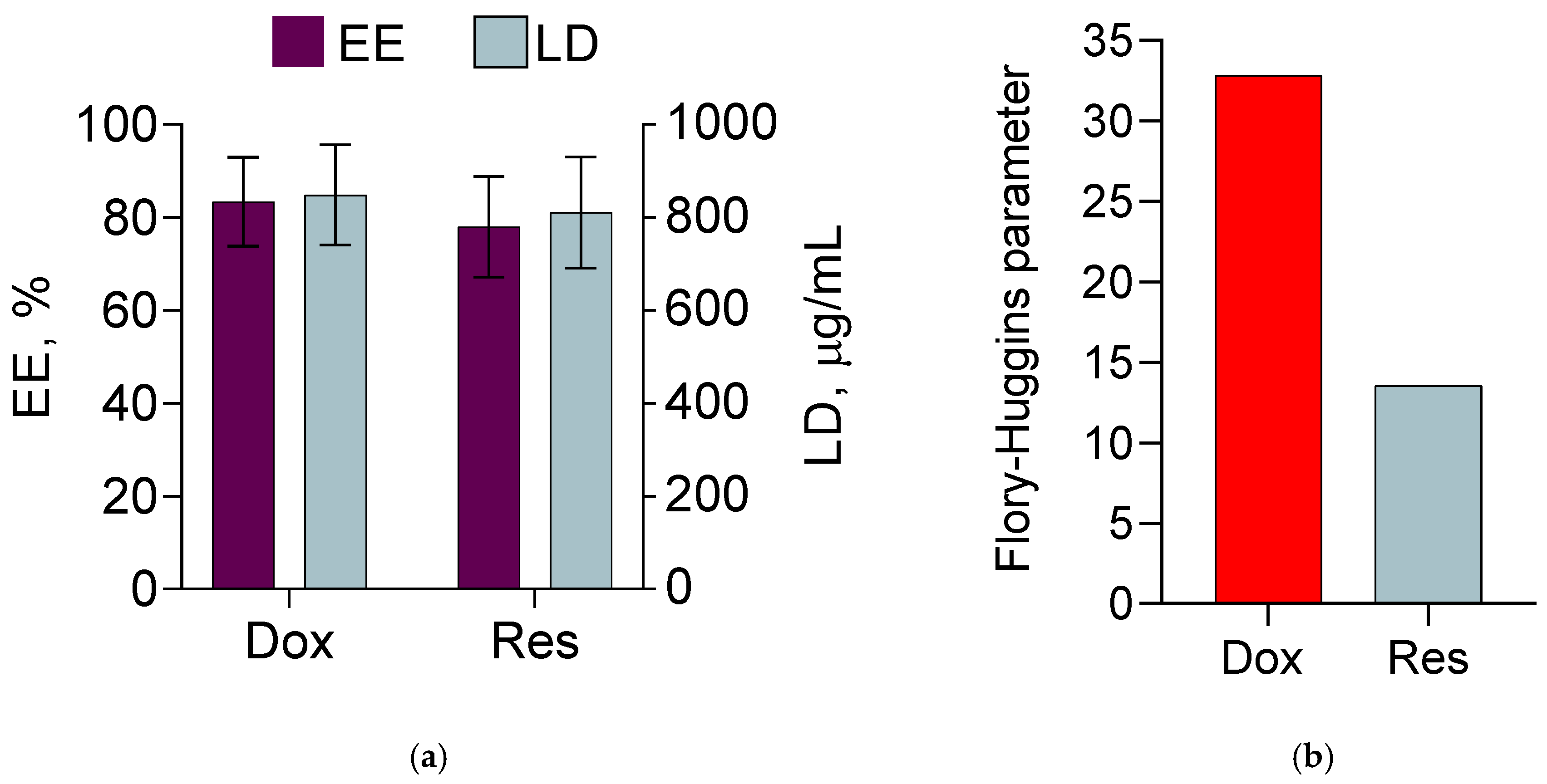
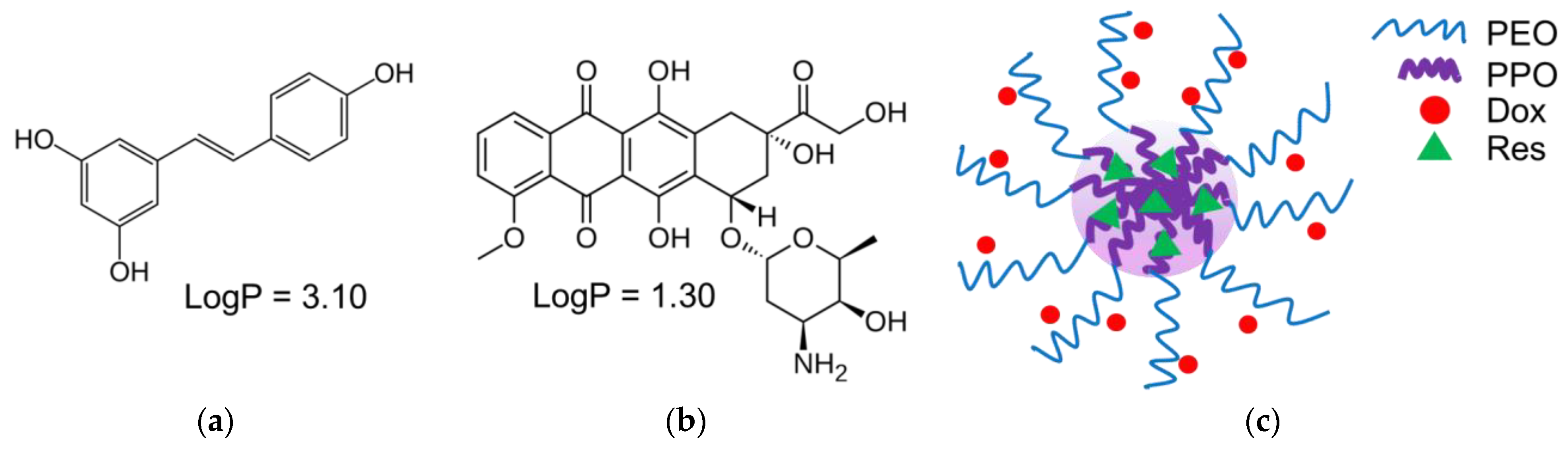

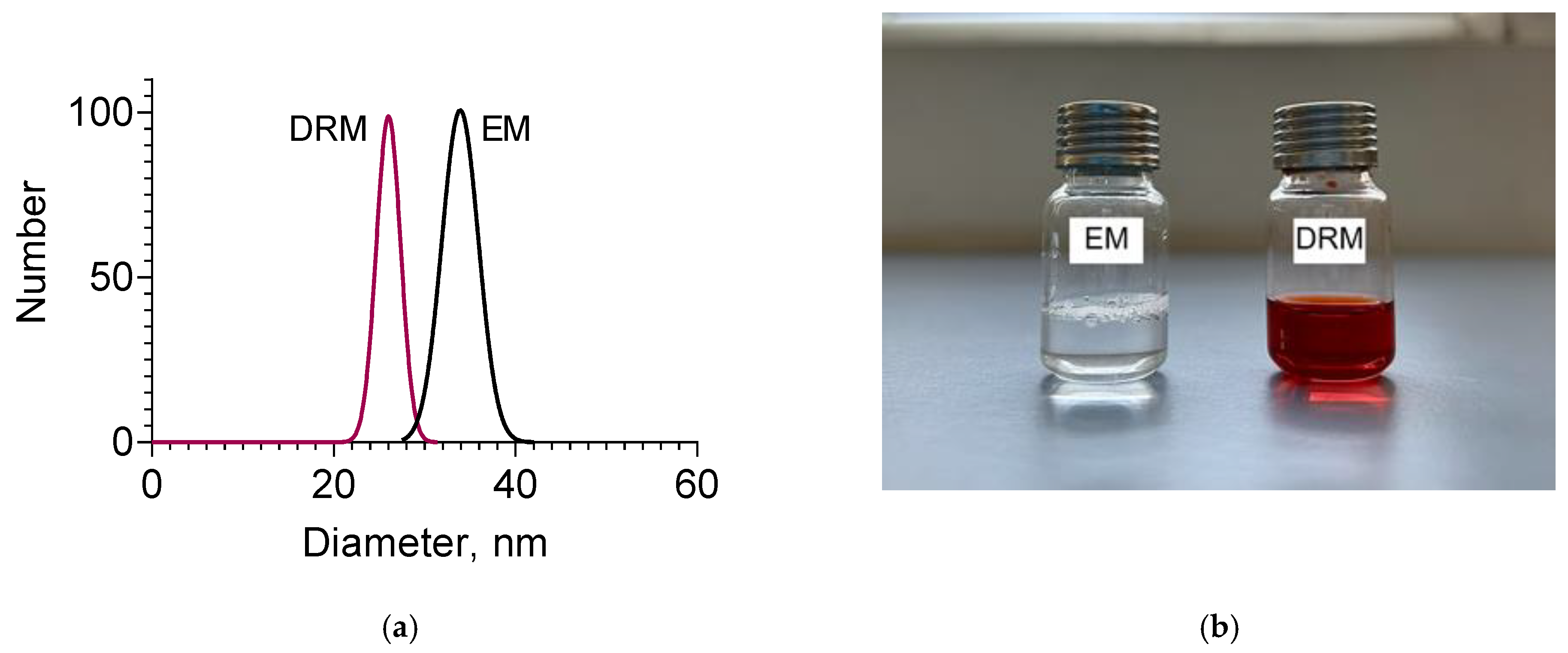

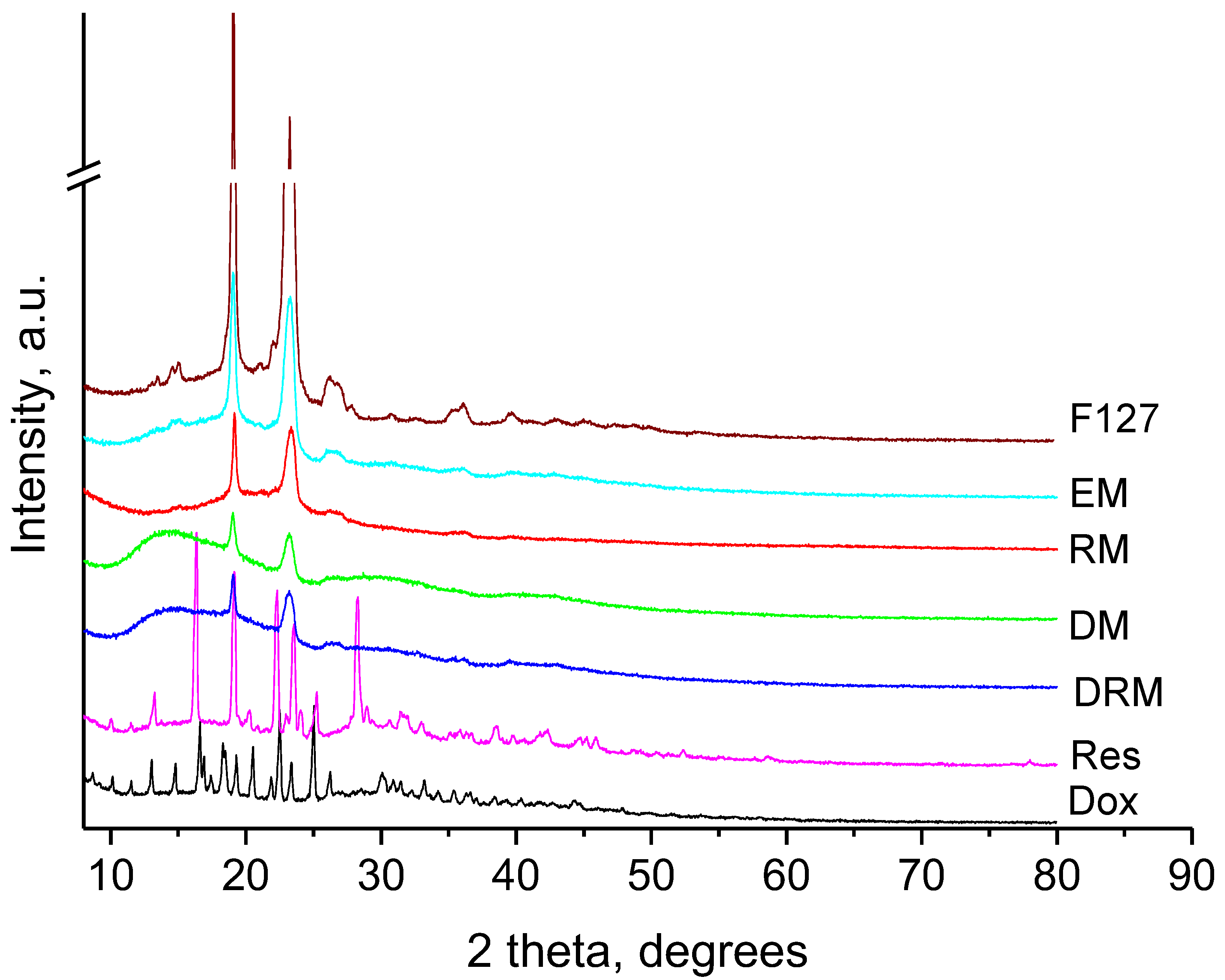



| Sample | Mean Diameter, nm | PDI | Zeta Potential, mV |
|---|---|---|---|
| Empty micelles (EM) | 33 ± 2 | 0.291 | −5.14 |
| Double-loaded micelles (DRM) | 26 ± 3 | 0.283 | +4.15 |
| Release Medium | Doxorubicin | Resveratrol |
|---|---|---|
| pH = 5.0 | r2 = 0.9989 n = 0.497 | r2 = 0.9396 n = 0.443 |
| pH = 7.4 | r2 = 0.9909 n = 0.530 | r2 = 0.9749 n = 0.432 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radeva, L.; Yordanov, Y.; Spassova, I.; Kovacheva, D.; Tzankova, V.; Yoncheva, K. Double-Loaded Doxorubicin/Resveratrol Polymeric Micelles Providing Low Toxicity on Cardiac Cells and Enhanced Cytotoxicity on Lymphoma Cells. Pharmaceutics 2023, 15, 1287. https://doi.org/10.3390/pharmaceutics15041287
Radeva L, Yordanov Y, Spassova I, Kovacheva D, Tzankova V, Yoncheva K. Double-Loaded Doxorubicin/Resveratrol Polymeric Micelles Providing Low Toxicity on Cardiac Cells and Enhanced Cytotoxicity on Lymphoma Cells. Pharmaceutics. 2023; 15(4):1287. https://doi.org/10.3390/pharmaceutics15041287
Chicago/Turabian StyleRadeva, Lyubomira, Yordan Yordanov, Ivanka Spassova, Daniela Kovacheva, Virginia Tzankova, and Krassimira Yoncheva. 2023. "Double-Loaded Doxorubicin/Resveratrol Polymeric Micelles Providing Low Toxicity on Cardiac Cells and Enhanced Cytotoxicity on Lymphoma Cells" Pharmaceutics 15, no. 4: 1287. https://doi.org/10.3390/pharmaceutics15041287





