Novel Fluorescent Benzimidazole-Hydrazone-Loaded Micellar Carriers for Controlled Release: Impact on Cell Toxicity, Nuclear and Microtubule Alterations in Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Lines
2.3. Methods
2.4. Synthesis
2.4.1. Synthesis of 1H-Benzimidazole-2-yl Hydrazone Derivative
2.4.2. Synthesis of Poly (Acrylic Acid)-Block-Poly (n-Butyl Acrylate) (PAA-b-PnBA) Copolymer
2.4.3. Preparation of Polymeric Micelles
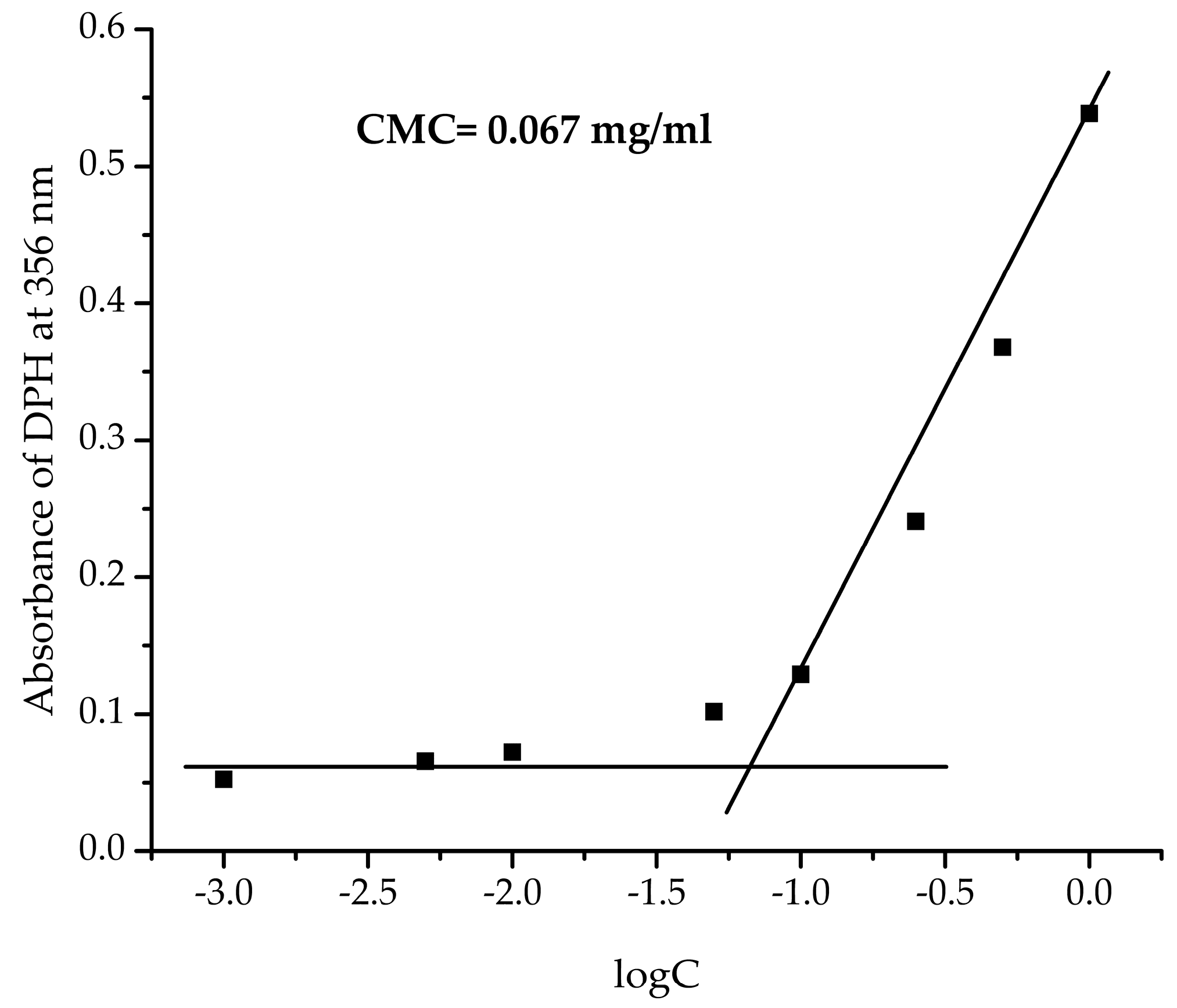
2.4.4. Determination of Critical Micelle Concentration (CMC)
2.4.5. Drug Loading
2.4.6. Drug Release
2.5. Cell Treatment with Micelles and Drug
2.5.1. MTT Test for Cell Viability
2.5.2. Fluorescent Imaging
Tubulin Immunofluorescence
DAPI Staining
Autofluorescence of Micelles with/without Drug
2.6. Data Analysis
3. Results and Discussion
3.1. Synthesis of Benzimidazole-Hydrazone Derivative 5
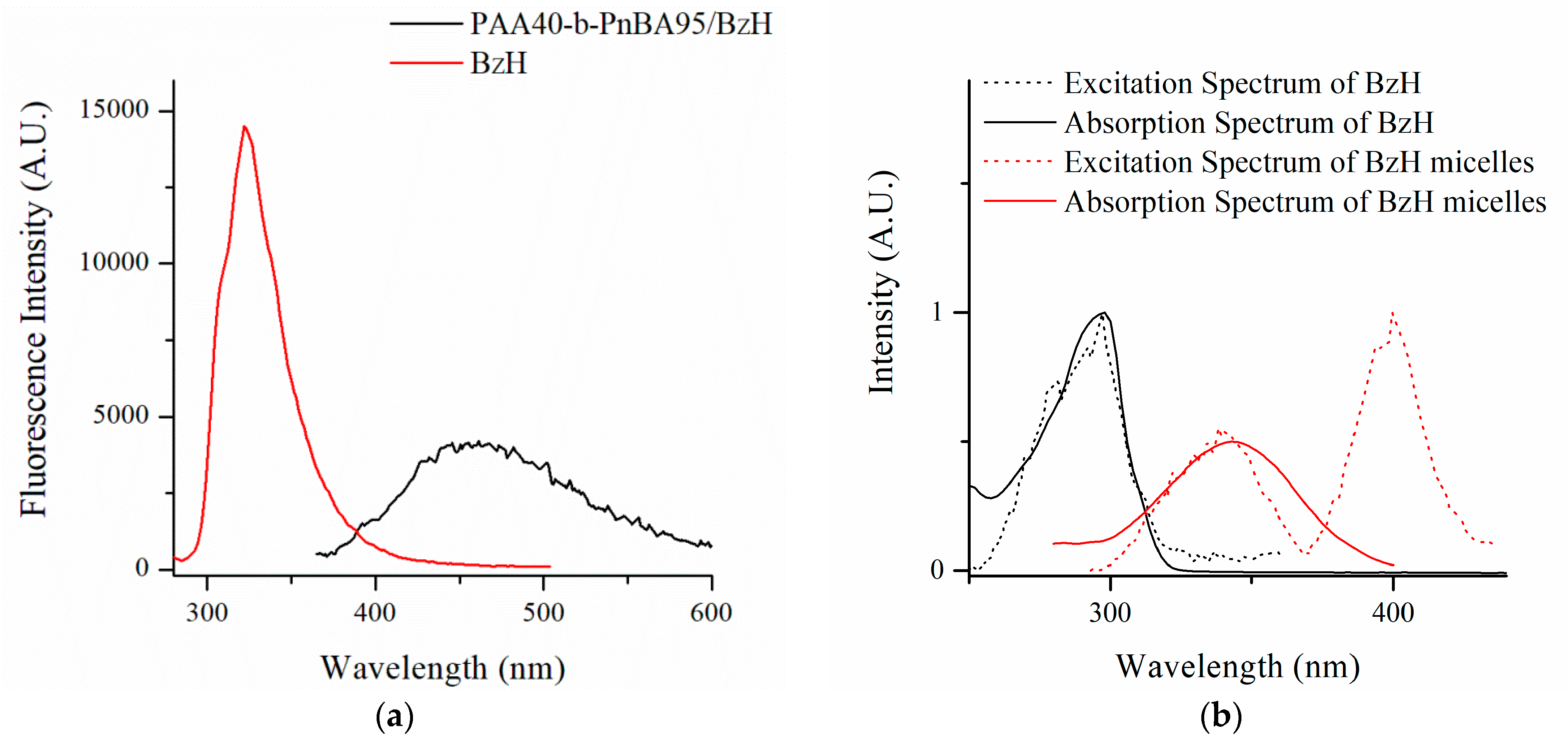
3.2. Preparation of Nanosized Fluorescent Micelles with Embedded Benzimidazole-Hydrazone Derivative 5 (PAA40-b-PnBA95/BzH)
3.3. Cell Treatment with Micelles and Drug
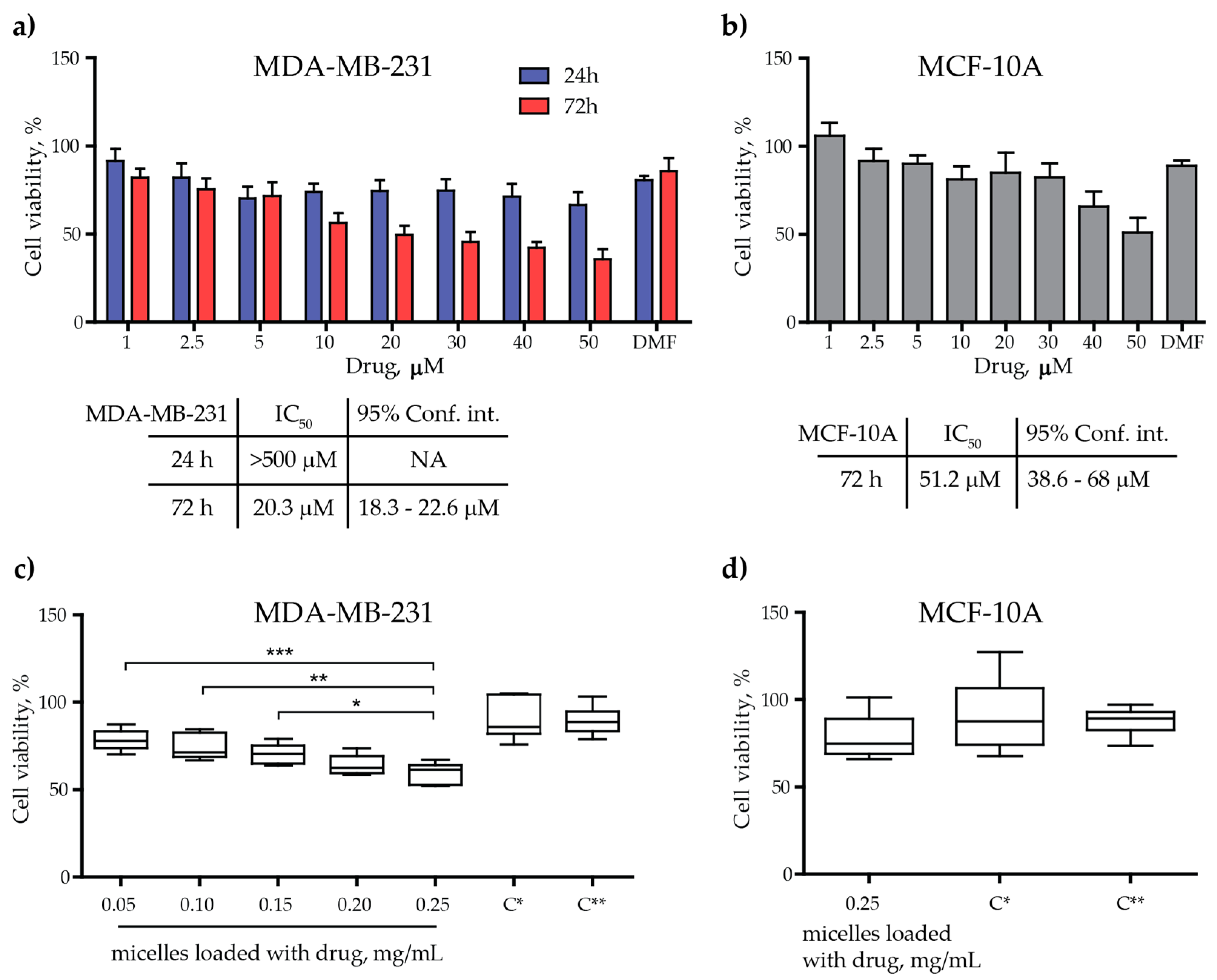
3.3.1. Cytotoxicity Assessment
3.3.2. Fluorescent Imaging
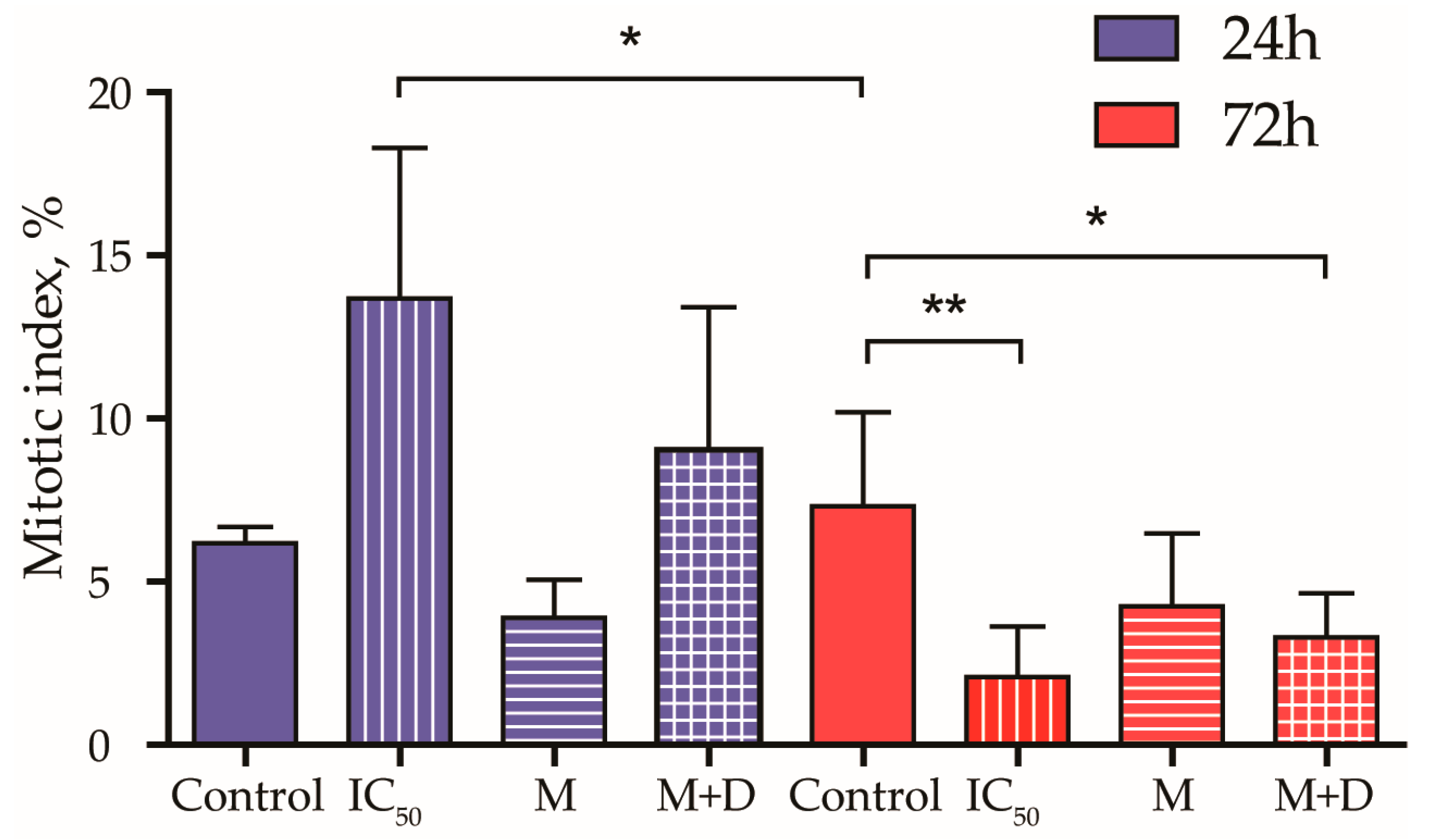
3.3.3. Mitotic Index
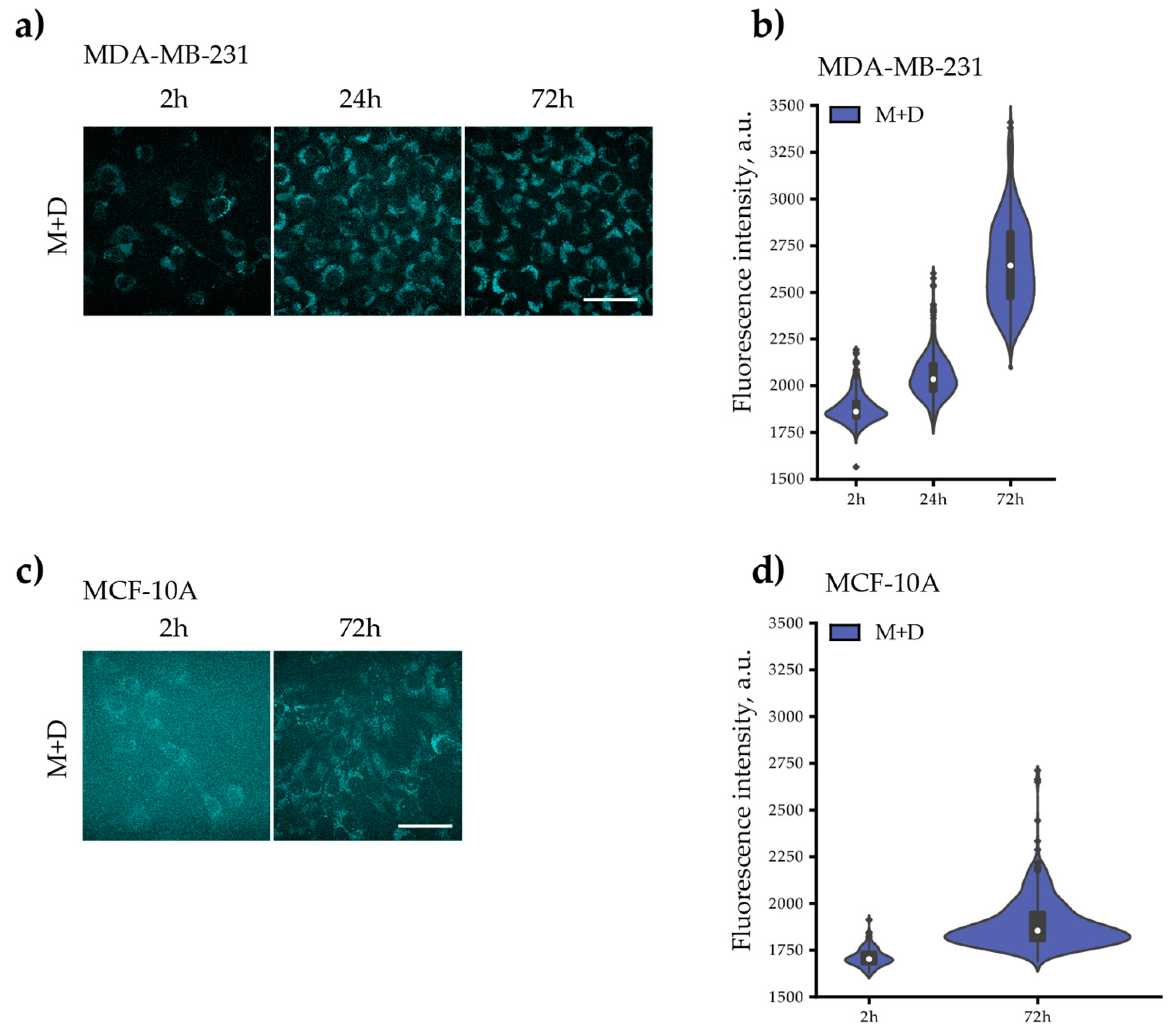
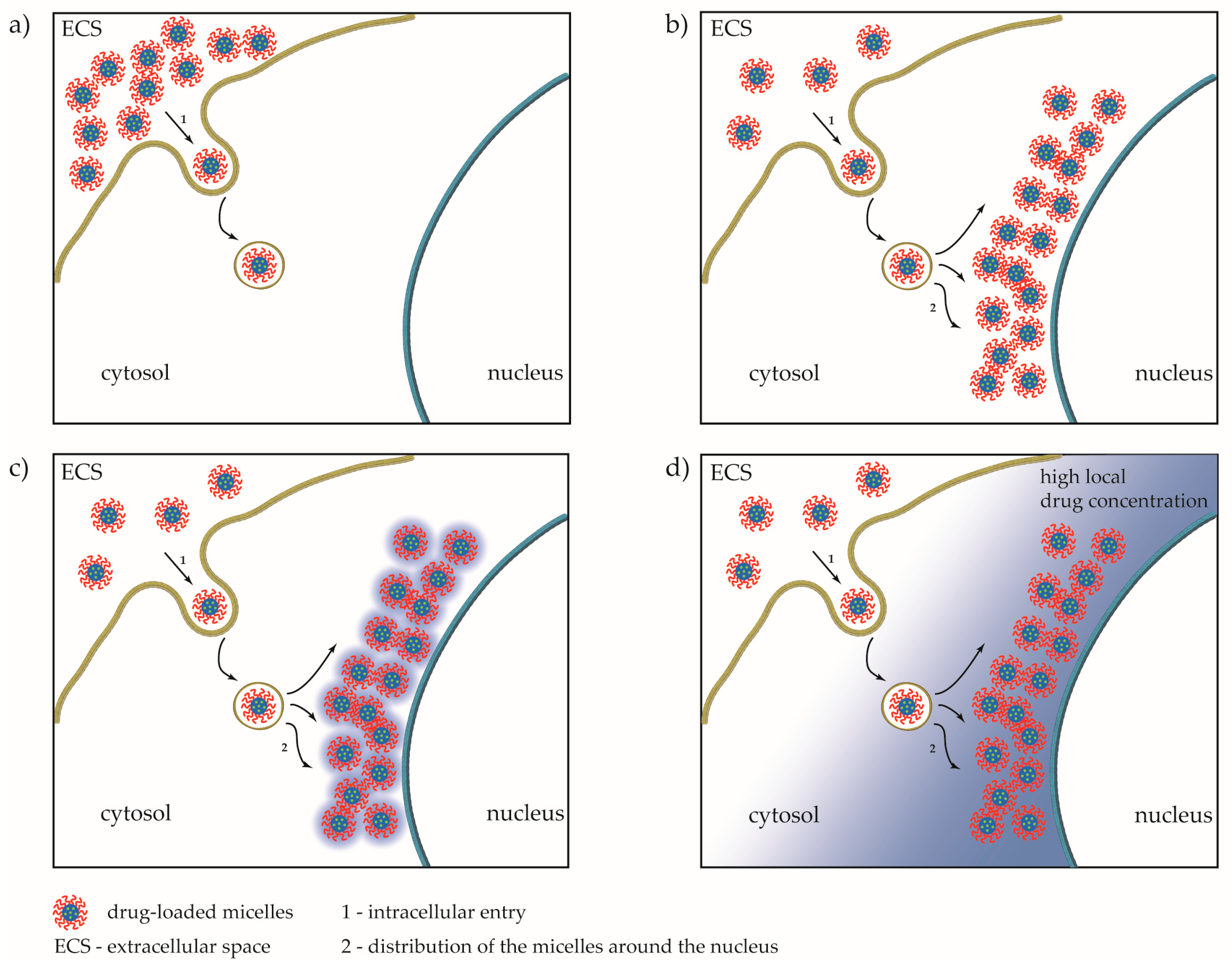
3.3.4. Fluorescent Intensity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.X.; Jin, L.; Qi, R.Q.; Ma, T. pH-responsive polymeric micelles self-assembled from amphiphilic copolymer modified with lipid used as doxorubicin delivery carriers. R. Soc. Open Sci. 2018, 5, 171654. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Yang, Y.Q.; Huang, T.X.; Zhao, B.; Guo, X.D.; Wang, J.F.; Zhang, L.J. Self-assembled pH-responsive MPEG-b-(PLA-co-PAE) block copolymer micelles for anticancer drug delivery. Biomaterials 2012, 33, 6273–6283. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Benzimidazole and its derivatives as cancer therapeutics: The potential role from traditional to precision medicine. Acta Pharm. Sin. B 2022, 13, 478–497. [Google Scholar] [CrossRef] [PubMed]
- Tahlan, S.; Kumar, S.; Kakkar, S.; Narasimhan, B. Benzimidazole scaffolds as promising antiproliferative agents: A review. BMC Chem. 2019, 13, 66. [Google Scholar] [CrossRef]
- Lalic, H.; Aurer, I.; Batinic, D.; Visnjic, D.; Smoljo, T.; Babic, A. Bendamustine: A review of pharmacology, clinical use and immunological effects (Review). Oncol. Rep. 2022, 47, 11. [Google Scholar] [CrossRef]
- Cheng, Y.; Tian, H. Current Development Status of MEK Inhibitors. Molecules 2017, 22, 1551. [Google Scholar] [CrossRef]
- Wagner, L. Profile of veliparib and its potential in the treatment of solid tumors. OncoTargets Ther. 2015, 8, 1931–1939. [Google Scholar] [CrossRef]
- Thomas, X.; Heiblig, M. An evaluation of glasdegib for the treatment of acute myelogenous leukemia. Expert Opin. Pharmacother. 2020, 21, 523–530. [Google Scholar] [CrossRef]
- Lee, C.-K.; Lee, M.E.; Lee, W.S.; Kim, J.M.; Park, K.H.; Kim, T.S.; Lee, K.Y.; Ahn, J.B.; Chung, H.C.; Rha, S.Y. Dovitinib (TKI258), a multi-target angiokinase inhibitor, is effective regardless of KRAS or BRAF mutation status in colorectal cancer. Am. J. Cancer Res. 2015, 5, 72–86. [Google Scholar]
- Chai, J.-Y.; Jung, B.-K.; Hong, S.-J. Albendazole and Mebendazole as Anti-Parasitic and Anti-Cancer Agents: An Update. Korean J. Parasitol. 2021, 59, 189–225. [Google Scholar] [CrossRef]
- Nawrocka, W.; Sztuba, B.; Kowalska, M.W.; Liszkiewicz, H.; Wietrzyk, J.; Nasulewicz, A.; Pełczyńska, M.; Opolski, A. Synthesis and antiproliferative activity in vitro of 2-aminobenzimidazole derivatives. Il Farm. 2004, 59, 83–91. [Google Scholar] [CrossRef]
- Walia, R.; Hedaitullah, M.; Naaz, S.F.; Iqbal, K.; Lamba, H.S. Benzimidazole derivatives-an overview. Int. J. Res. Pharm. Chem. 2011, 1, 565–574. [Google Scholar]
- Argirova, M.; Guncheva, M.; Momekov, G.; Cherneva, E.; Mihaylova, R.; Rangelov, M.; Todorova, N.; Denev, P.; Anichina, K.; Mavrova, A.; et al. Modulation Effect on Tubulin Polymerization, Cytotoxicity and Antioxidant Activity of 1H-Benzimidazole-2-Yl Hydrazones. Molecules 2022, 28, 291. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.E.; Shahmabadi, H.E. Anthelmintics for drug repurposing: Opportunities and challenges. Saudi Pharm. J. 2021, 29, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Zahidah, K.A.; Kakooei, S.; Ismail, M.C.; Mohebbi, H.; Kermanioryani, M.; Raja, P.B. Benzimidazole-loaded Halloysite Nanotube as a Smart Coating Application. Int. J. Eng. Technol. Innov. 2017, 7, 243–254. [Google Scholar]
- Liu, M.; Jia, Z.; Jia, D.; Zhou, C. Recent advance in research on halloysite nanotubes-polymer nanocomposite. Prog. Polym. Sci. 2014, 39, 1498–1525. [Google Scholar] [CrossRef]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Nair, A.B.; Kamal, Y.T. Progress in Polymeric Micelles for Drug Delivery Applications. Pharmaceutics 2022, 14, 1636. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Kedar, U.; Phutane, P.; Shidhaye, S.; Kadam, V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 714–729. [Google Scholar] [CrossRef]
- Eltoukhy, A.A.; Siegwart, D.J.; Alabi, C.A.; Rajan, J.S.; Langer, R.; Anderson, D.G. Effect of molecular weight of amine end-modified poly(b-amino ester)s on gene delivery efficiency and toxicity. Biomaterials 2012, 33, 3594–3603. [Google Scholar] [CrossRef]
- Noorani, L.; Pourgholami, M.H.; Liang, M.; Morris, D.L.; Stenzel, M. Albendazole loaded albumin nanoparticles for ovarian cancer therapy. Eur. J. Nanomed. 2014, 6, 227–236. [Google Scholar] [CrossRef]
- Telsang, M.; Pradeep, D.; Wilson, B.; Tamreen, V.; Sadik, S.; Habeebuddin, M.; Asif, A.H.; Asdaq, M.B.; Nagaraja, S.H.; Noor, S.D. Formulation and Evaluation of Bendamustine Loaded Polymeric Nanoparticle. Indian J. Pharm. Educ. Res. 2022, 56, 415–419. [Google Scholar] [CrossRef]
- Racoviceanu, R.; Trandafirescu, C.; Voicu, M.; Ghiulai, R.; Borcan, F.; Dehelean, C.; Watz, C.; Aigner, Z.; Ambrus, R.; Coricovac, D.E.; et al. Solid Polymeric Nanoparticles of Albendazole: Synthesis, Physico-Chemical Characterization and Biological Activity. Molecules 2020, 25, 5130. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, N.I.; Bakov, V.V.; Anichina, K.K.; Bojinov, V.B. Fluorescent Probes as a Tool in Diagnostic and Drug Delivery Systems. Pharmaceuticals 2023, 16, 381. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Wang, J.; Liang, X.-J. Aggregation-induced emission (AIE) fluorophores as imaging tools to trace the biological fate of nano-based drug delivery systems. Adv. Drug Deliv. Rev. 2019, 143, 161–176. [Google Scholar] [CrossRef]
- Wang, S.; Ren, W.X.; Hou, J.-T.; Won, M.; An, J.; Chen, X.; Shu, J.; Kim, J.S. Fluorescence imaging of pathophysiological microenvironments. Chem. Soc. Rev. 2021, 50, 8887–8902. [Google Scholar] [CrossRef]
- Han, H.-H.; Tian, H., Jr.; Zang, Y.; Sedgwick, A.C.; Li, J.; Sessler, J.L.; He, X.-P.; James, T.D. Small-molecule fluorescence-based probes for interrogating major organ diseases. Chem. Soc. Rev. 2021, 50, 9391–9429. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liang, X.; Yin, J.; Lin, W. Organic fluorescent probes for monitoring autophagy in living cells. Chem. Soc. Rev. 2021, 50, 102–119. [Google Scholar] [CrossRef]
- Sun, W.; Li, M.; Fan, J.; Peng, X. Activity-Based Sensing and Theranostic Probes Based on Photoinduced Electron Transfer. Acc. Chem. Res. 2019, 52, 2818–2831. [Google Scholar] [CrossRef]
- Ding, F.; Feng, J.; Zhang, X.; Sun, J.; Fan, C.; Ge, Z. Responsive optical probes for deep-tissue imaging: Photoacoustics and second near-infrared fluorescence. Adv. Drug Deliv. Rev. 2021, 173, 141–163. [Google Scholar] [CrossRef] [PubMed]
- Anichina, K.; Argirova, M.; Tzoneva, R.; Uzunova, V.; Mavrova, A.; Vuchev, D.; Popova-Daskalova, G.; Fratev, F.; Guncheva, M.; Yancheva, D. 1H-benzimidazole-2-yl hydrazones as tubulin-targeting agents: Synthesis, structural characterization, anthelmintic activity and antiproliferative activity against MCF-7 breast carcinoma cells and molecular docking studies. Chem.-Biol. Interact. 2021, 345, 109540. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.R.; Bryaskova, R.G.; Georgiev, N.I.; Philipova, N.D.; Bakov, V.V.; Uzunova, V.; Tzoneva, R.; Bojinov, V.B. Design and synthesis of fluorescent shell functionalized polymer micelles for biomedical application. Polym. Adv. Technol. 2020, 31, 1365–1376. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Bryaskova, R.G.; Ismail, S.R.; Philipova, N.D.; Uzunova, V.P.; Bakov, V.V.; Tzoneva, R.D.; Bojinov, V.B. Aggregation induced emission in 1,8-naphthalimide embedded nanomicellar architecture as a platform for fluorescent ratiometric pH-probe with biomedical applications. J. Photochem. Photobiol. A Chem. 2021, 418, 113380. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Mutterer, J.; Zinck, E. Quick-and-clean article figures with FigureJ. J. Microsc. 2013, 252, 89–91. [Google Scholar] [CrossRef]
- Argirova, M.A.; Georgieva, M.K.; Hristova-Avakumova, N.G.; Vuchev, D.I.; Popova-Daskalova, G.V.; Anichina, K.K.; Yancheva, D.Y. New 1H-benzimidazole-2-yl hydrazones with combined antiparasitic and antioxidant activity. RSC Adv. 2021, 11, 39848–39868. [Google Scholar] [CrossRef] [PubMed]
- Letchford, K.; Liggins, R.; Burt, H. Pharmaceutics, preformulation and drug delivery: Solubilization of hydrophobic drugs by methoxy poly(ethylene glycol)-block-polycaprolactone diblock copolymer micelles: Theoretical and experimental data and correlations. J. Pharm. Sci. 2008, 97, 1179–1190. [Google Scholar] [CrossRef]
- Soni, M.; Das, S.K.; Sahu, P.K.; Kar, U.P.; Rahaman, A.; Sarkar, M. Synthesis, Photophysics, Live Cell Imaging, and Aggregation Behavior of Some Structurally Similar Alkyl Chain Containing Bromonaphthalimide Systems: Influence of Alkyl Chain Length on the Aggregation Behavior. J. Phys. Chem. C 2013, 117, 14338–14347. [Google Scholar] [CrossRef]
- Kasha, M.; Rawls, H.R.; El-Bayoumi, M.A. The exciton model in molecular spectroscopy. Pure Appl. Chem. 1965, 11, 371–392. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, R.; Cai, S.; Lin, Y.; Sellers, L.; Sakamoto, K.; He, B.; Peterson, B.R. Synthesis and Biological Evaluation of Analogues of AKT (Protein Kinase B) Inhibitor-IV. J. Med. Chem. 2011, 54, 1126–1139. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Deng, X.; Xiong, S.; Xiong, R.; Liu, J.; Zou, L.; Lei, X.; Cao, X.; Xie, Z.; Chen, Y.; et al. Design, synthesis and biological evaluation of chrysin benzimidazole derivatives as potential anticancer agents. Nat. Prod. Res. 2018, 32, 2900–2909. [Google Scholar] [CrossRef] [PubMed]
- Raducka, A.; Świątkowski, M.; Korona-Głowniak, I.; Kaproń, B.; Plech, T.; Szczesio, M.; Gobis, K.; Czylkowska, A. Design, Synthesis, and Characterization of Novel Coordination Compounds of Benzimidazole Derivatives with Cadmium. Pharmaceutics 2022, 14, 1626. [Google Scholar] [CrossRef] [PubMed]
- Miao, T.-T.; Tao, X.-B.; Li, D.-D.; Chen, H.; Jin, X.-Y.; Geng, Y.; Wang, S.-F.; Gu, W. Synthesis and biological evaluation of 2-aryl-benzimidazole derivatives of dehydroabietic acid as novel tubulin polymerization inhibitors. RSC Adv. 2018, 8, 17511–17526. [Google Scholar] [CrossRef]
- Laxmikeshav, K.; Sharma, P.; Palepu, M.; Sharma, P.; Mahale, A.; George, J.; Phanindranath, R.; Dandekar, M.P.; Kulkarni, O.P.; Nagesh, N.; et al. Benzimidazole based bis-carboxamide derivatives as promising cytotoxic agents: Design, synthesis, in silico and tubulin polymerization inhibition. J. Mol. Struct. 2023, 1271, 134078. [Google Scholar] [CrossRef]
- Akhtar, J.; Khan, A.A.; Ali, Z.; Haider, R.; Yar, M.S. Structure-activity relationship (SAR) study and design strategies of nitrogen-containing heterocyclic moieties for their anticancer activities. Eur. J. Med. Chem. 2017, 125, 143–189. [Google Scholar] [CrossRef]
- Pisano, C.; Battistoni, A.; Antoccia, A.; Degrassi, F.; Tanzarella, C. Changes in microtubule organization after exposure to a benzimidazole derivative in Chinese hamster cells. Mutagenesis 2000, 15, 507–515. [Google Scholar] [CrossRef]
- Chang, W.; Chang, C.; Chiang, P.; Ho, Y.; Liu, J.; Chang, K.; Guh, J. 2-Phenyl-5-(pyrrolidin-1-yl)-1-(3,4,5-trimethoxybenzyl)-1H-benzimidazole, a benzimidazole derivative, inhibits growth of human prostate cancer cells by affecting tubulin and c-Jun N-terminal kinase. Br. J. Pharmacol. 2010, 160, 1677–1689. [Google Scholar] [CrossRef]
- Naumenko, V.; Nikitin, A.; Kapitanova, K.; Melnikov, P.; Vodopyanov, S.; Garanina, A.; Valikhov, M.; Ilyasov, A.; Vishnevskiy, D.; Markov, A.; et al. Intravital microscopy reveals a novel mechanism of nanoparticles excretion in kidney. J. Control. Release 2019, 307, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Dogra, P.; Adolphi, N.L.; Wang, Z.; Lin, Y.-S.; Butler, K.S.; Durfee, P.N.; Croissant, J.G.; Noureddine, A.; Coker, E.N.; Bearer, E.L.; et al. Establishing the effects of mesoporous silica nanoparticle properties on in vivo disposition using imaging-based pharmacokinetics. Nat. Commun. 2018, 9, 4551. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bryaskova, R.; Georgiev, N.; Philipova, N.; Bakov, V.; Anichina, K.; Argirova, M.; Apostolova, S.; Georgieva, I.; Tzoneva, R. Novel Fluorescent Benzimidazole-Hydrazone-Loaded Micellar Carriers for Controlled Release: Impact on Cell Toxicity, Nuclear and Microtubule Alterations in Breast Cancer Cells. Pharmaceutics 2023, 15, 1753. https://doi.org/10.3390/pharmaceutics15061753
Bryaskova R, Georgiev N, Philipova N, Bakov V, Anichina K, Argirova M, Apostolova S, Georgieva I, Tzoneva R. Novel Fluorescent Benzimidazole-Hydrazone-Loaded Micellar Carriers for Controlled Release: Impact on Cell Toxicity, Nuclear and Microtubule Alterations in Breast Cancer Cells. Pharmaceutics. 2023; 15(6):1753. https://doi.org/10.3390/pharmaceutics15061753
Chicago/Turabian StyleBryaskova, Rayna, Nikolai Georgiev, Nikoleta Philipova, Ventsislav Bakov, Kameliya Anichina, Maria Argirova, Sonia Apostolova, Irina Georgieva, and Rumiana Tzoneva. 2023. "Novel Fluorescent Benzimidazole-Hydrazone-Loaded Micellar Carriers for Controlled Release: Impact on Cell Toxicity, Nuclear and Microtubule Alterations in Breast Cancer Cells" Pharmaceutics 15, no. 6: 1753. https://doi.org/10.3390/pharmaceutics15061753
APA StyleBryaskova, R., Georgiev, N., Philipova, N., Bakov, V., Anichina, K., Argirova, M., Apostolova, S., Georgieva, I., & Tzoneva, R. (2023). Novel Fluorescent Benzimidazole-Hydrazone-Loaded Micellar Carriers for Controlled Release: Impact on Cell Toxicity, Nuclear and Microtubule Alterations in Breast Cancer Cells. Pharmaceutics, 15(6), 1753. https://doi.org/10.3390/pharmaceutics15061753







