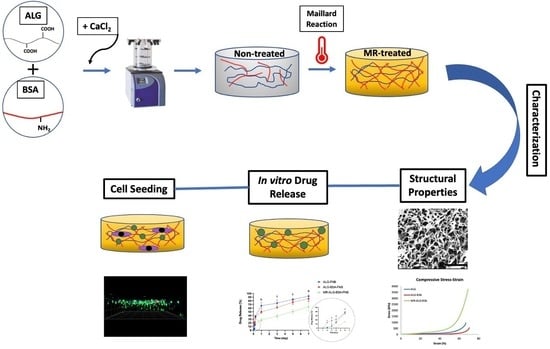
Maillard Reaction Crosslinked Alginate-Albumin Scaffolds for Enhanced Fenofibrate Delivery to the Retina: A Promising Strategy to Treat RPE-Related Dysfunction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of the Scaffolds
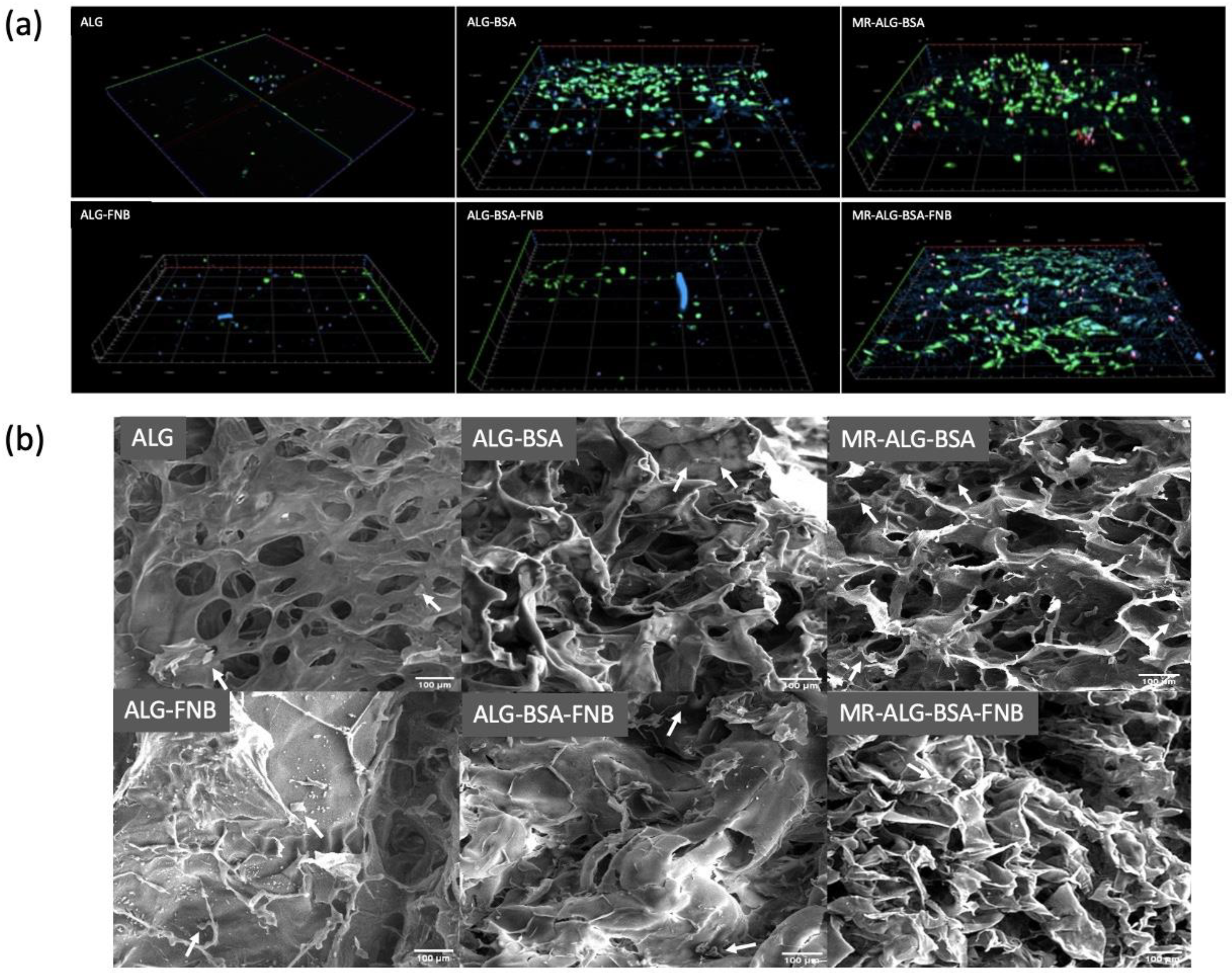
2.3. Morphological Characterisation
2.4. Mechanical Properties Testing
2.5. Swelling and Degradation Studies
2.6. Drug Loading and Release Studies
2.6.1. Loading Capacity Study
2.6.2. Drug Release Study
2.7. Biological Investigations
2.7.1. Cell Culture and Seeding on Scaffolds
2.7.2. MTS-Based Cytotoxicity Assay
2.7.3. Analysis of ARPE-19 Cells-Scaffold Interactions
2.7.4. Distribution of Cells on the Scaffolds
2.8. Statistical Analysis
3. Results & Discussion
3.1. Scaffolds Structural Morphology
3.2. Mechanical Properties of the Scaffolds
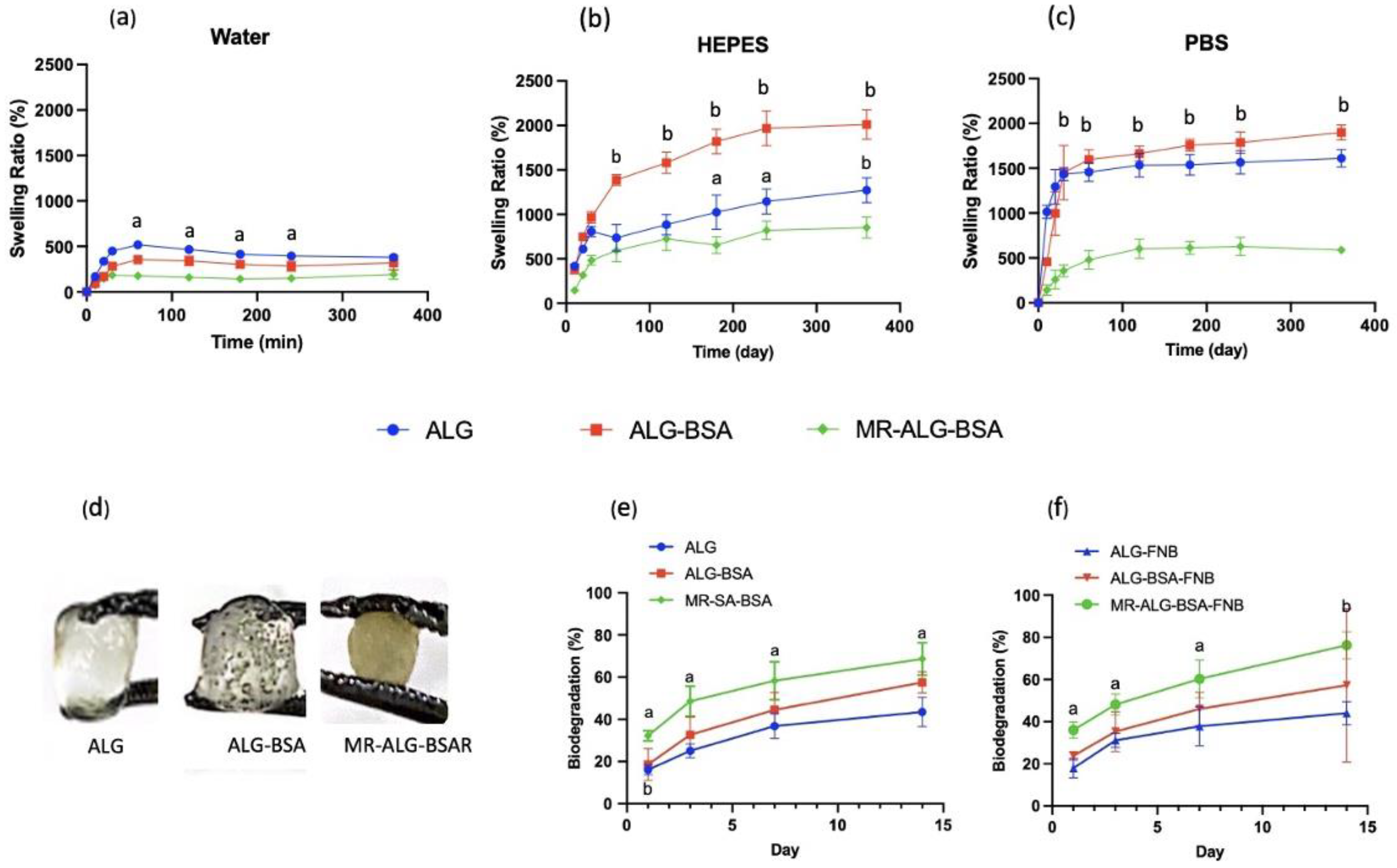
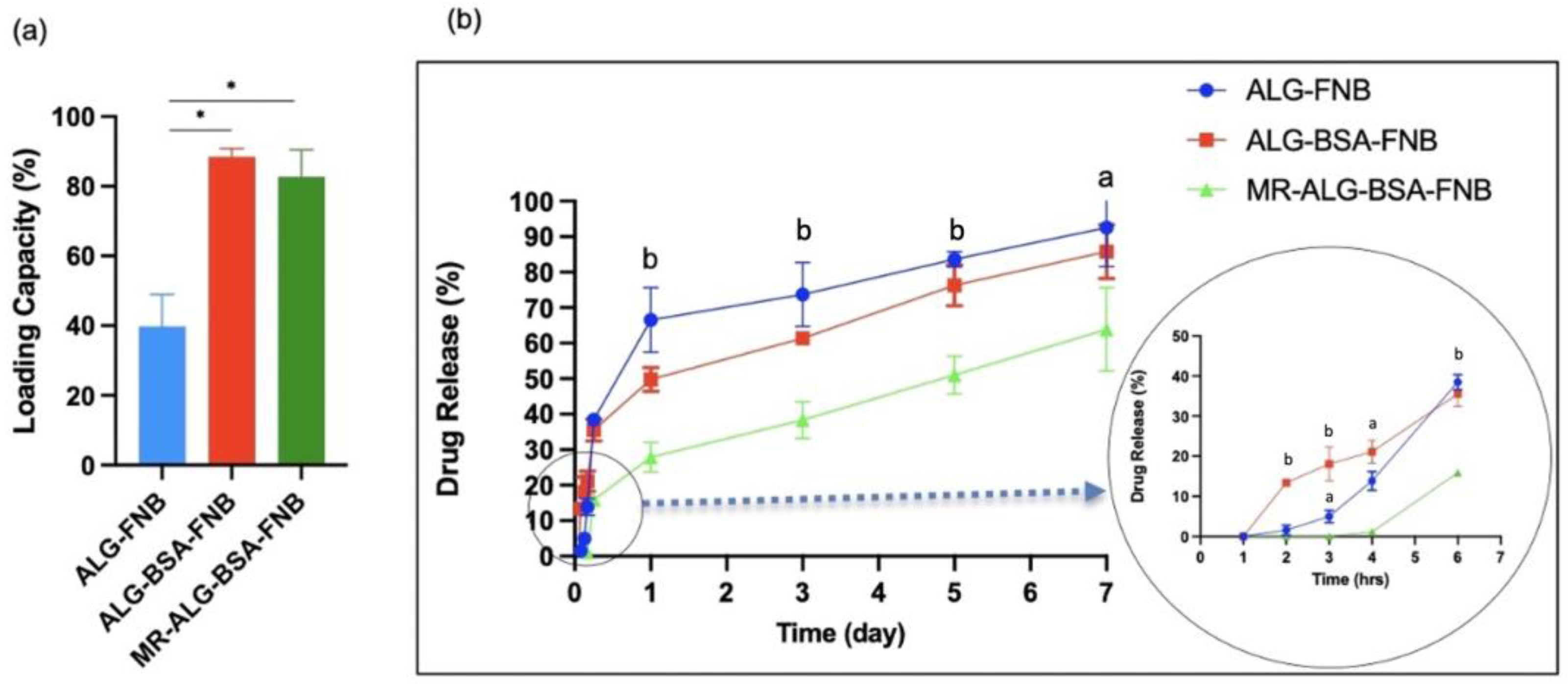
3.3. Scaffolds Swelling, Biodegradation, and Drug Release Studies
- Swelling Studies
- Biodegradation
- Drug Loading and In-Vitro Release Studies:
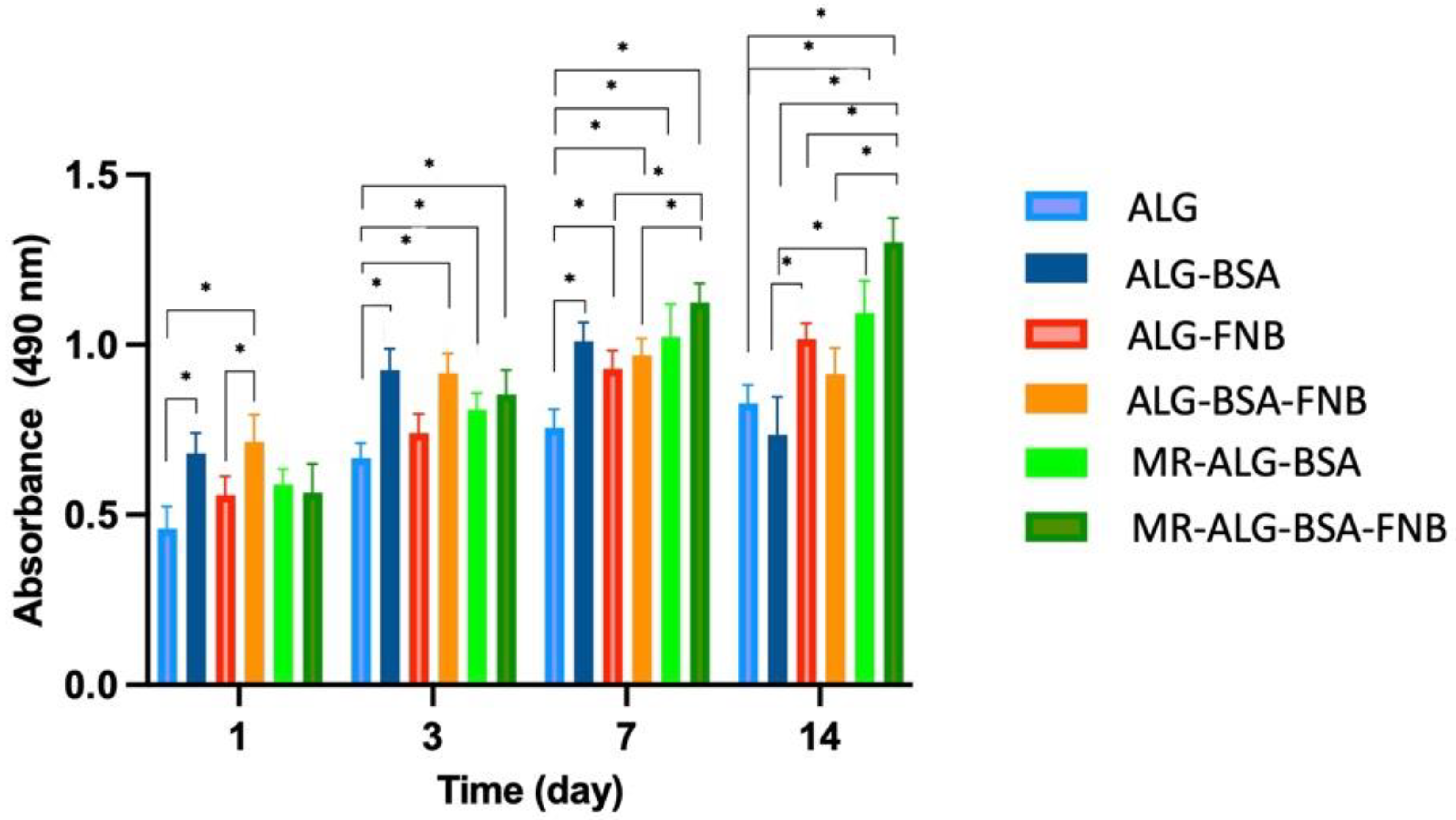
3.4. In Vitro Biological Investigation with ARPE-19 Cells
- Viability of the ARPE-19 cells within scaffolds
- In vitro cell–scaffold interaction studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abedin Zadeh, M.; Khoder, M.; Al-Kinani, A.A.; Younes, H.M.; Alany, R.G. Retinal Cell Regeneration Using Tissue Engineered Polymeric Scaffolds. Drug Discov. Today 2019, 24, 1669–1678. [Google Scholar] [CrossRef]
- Ou, K.; Li, Y.; Liu, L.; Li, H.; Cox, K.; Wu, J.; Liu, J.; Dick, A. Recent Developments of Neuroprotective Agents for Degenerative Retinal Disorders. Neural Regen. Res. 2022, 17, 1919. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global Prevalence of Age-Related Macular Degeneration and Disease Burden Projection for 2020 and 2040: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Wallsh, J.O.; Gallemore, R.P. Anti-VEGF-Resistant Retinal Diseases: A Review of the Latest Treatment Options. Cells 2021, 10, 1049. [Google Scholar] [CrossRef]
- Bisht, R.; Mandal, A.; Jaiswal, J.K.; Rupenthal, I.D. Nanocarrier Mediated Retinal Drug Delivery: Overcoming Ocular Barriers to Treat Posterior Eye Diseases. WIREs Nanomed. Nanobiotechnol. 2018, 10, e1473. [Google Scholar] [CrossRef]
- Guay, D.R.P. Update on Fenofibrate. Cardiovasc. Drug Rev. 2006, 20, 281–302. [Google Scholar] [CrossRef]
- Qiu, F.; Meng, T.; Chen, Q.; Zhou, K.; Shao, Y.; Matlock, G.; Ma, X.; Wu, W.; Du, Y.; Wang, X.; et al. Fenofibrate-Loaded Biodegradable Nanoparticles for the Treatment of Experimental Diabetic Retinopathy and Neovascular Age-Related Macular Degeneration. Mol. Pharm. 2019, 16, 1958–1970. [Google Scholar] [CrossRef]
- Bogdanov, P.; Hernández, C.; Corraliza, L.; Carvalho, A.R.; Simó, R. Effect of Fenofibrate on Retinal Neurodegeneration in an Experimental Model of Type 2 Diabetes. Acta Diabetol. 2015, 52, 113–122. [Google Scholar] [CrossRef]
- Tezel, T.H.; Del Priore, L.V.; Berger, A.S.; Kaplan, H.J. Adult Retinal Pigment Epithelial Transplantation in Exudative Age-Related Macular Degeneration. Am. J. Ophthalmol. 2007, 143, 584–595.e2. [Google Scholar] [CrossRef]
- Kim, H.M.; Woo, S.J. Ocular Drug Delivery to the Retina: Current Innovations and Future Perspectives. Pharmaceutics 2021, 13, 108. [Google Scholar] [CrossRef]
- Garg, T.; Singh, O.; Arora, S.; Murthy, R.S.R. Scaffold: A Novel Carrier for Cell and Drug Delivery. Crit. Rev. Ther. Drug Carr. Syst. 2012, 29, 1–63. [Google Scholar] [CrossRef]
- Chan, B.P.; Leong, K.W. Scaffolding in Tissue Engineering: General Approaches and Tissue-Specific Considerations. Eur. Spine J. 2008, 17 (Suppl. S4), 467–479. [Google Scholar] [CrossRef]
- White, C.E.; Olabisi, R.M. Scaffolds for Retinal Pigment Epithelial Cell Transplantation in Age-Related Macular Degeneration. J. Tissue Eng. 2017, 8, 204173141772084. [Google Scholar] [CrossRef]
- Al Dalaty, A.; Karam, A.; Najlah, M.; Alany, R.G.; Khoder, M. Effect of Non-Cross-Linked Calcium on Characteristics, Swelling Behaviour, Drug Release and Mucoadhesiveness of Calcium Alginate Beads. Carbohydr. Polym. 2016, 140, 163–170. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Nayak, A.K.; Mohanta, B.C.; Hasnain, M.S.; Hoda, M.N.; Tripathi, G. Alginate-Based Scaffolds for Drug Delivery in Tissue Engineering. In Alginates in Drug Delivery; Academic Press: Cambridge, MA, USA, 2020; pp. 359–386. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baharvand, H.; Daemi, H. Chemical Crosslinking of Biopolymeric Scaffolds: Current Knowledge and Future Directions of Crosslinked Engineered Bone Scaffolds. Int. J. Biol. Macromol. 2018, 107, 678–688. [Google Scholar] [CrossRef]
- Niknejad, H.; Mahmoudzadeh, R. Comparison of Different Crosslinking Methods for Preparation of Docetaxel-Loaded Albumin Nanoparticles. Iran. J. Pharm. Res. 2015, 14, 385–394. [Google Scholar]
- Khoder, M.; Gbormoi, H.K.; Ryan, A.; Karam, A.; Alany, R.G. Potential Use of the Maillard Reaction for Pharmaceutical Applications: Gastric and Intestinal Controlled Release Alginate-Albumin Beads. Pharmaceutics 2019, 11, 83. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Tang, T.-K.; Phuah, E.-T.; Alitheen, N.B.M.; Tan, C.-P.; Lai, O.-M. New Functionalities of Maillard Reaction Products as Emulsifiers and Encapsulating Agents, and the Processing Parameters: A Brief Review. J. Sci. Food Agric. 2017, 97, 1379–1385. [Google Scholar] [CrossRef]
- Morales, F.J.; Jiménez-Pérez, S. Free Radical Scavenging Capacity of Maillard Reaction Products as Related to Colour and Fluorescence. Food Chem. 2001, 72, 119–125. [Google Scholar] [CrossRef]
- Chen, X.-M.; Kitts, D.D. Antioxidant and Anti-Inflammatory Activities of Maillard Reaction Products Isolated from Sugar–Amino Acid Model Systems. J. Agric. Food Chem. 2011, 59, 11294–11303. [Google Scholar] [CrossRef] [PubMed]
- Prasopdee, T.; Sinthuvanich, C.; Chollakup, R.; Uttayarat, P.; Smitthipong, W. The Albumin/Starch Scaffold and Its Biocompatibility with Living Cells. Mater. Today Commun. 2021, 27, 102164. [Google Scholar] [CrossRef]
- Yuan, H.; Zheng, X.; Liu, W.; Zhang, H.; Shao, J.; Yao, J.; Mao, C.; Hui, J.; Fan, D. A Novel Bovine Serum Albumin and Sodium Alginate Hydrogel Scaffold Doped with Hydroxyapatite Nanowires for Cartilage Defects Repair. Colloids Surf. B Biointerfaces 2020, 192, 111041. [Google Scholar] [CrossRef] [PubMed]
- Fereshteh, Z. Freeze-Drying Technologies for 3D Scaffold Engineering. In Functional 3D Tissue Engineering Scaffolds: Materials, Technologies, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 151–174. [Google Scholar] [CrossRef]
- Li, C. Electrolyte Quantification in Aqueous and Vitreous Humor of Common Preclinical Species. ARVO Annu. Meet. Abstr. 2019, 60, 3421. [Google Scholar]
- Wan, L.Q.; Jiang, J.; Arnold, D.E.; Guo, X.E.; Lu, H.H.; Mow, V.C. Calcium Concentration Effects on the Mechanical and Biochemical Properties of Chondrocyte-Alginate Constructs. Cell Mol. Bioeng. 2008, 1, 93–102. [Google Scholar] [CrossRef]
- Sangkert, S.; Kamolmatyakul, S.; Gelinsky, M.; Meesane, J. 3D Printed Scaffolds of Alginate/Polyvinylalcohol with Silk Fibroin Based on Mimicked Extracellular Matrix for Bone Tissue Engineering in Maxillofacial Surgery. Mater. Today Commun. 2021, 26, 102140. [Google Scholar] [CrossRef]
- Lacroix, P.M.; Dawson, B.A.; Sears, R.W.; Black, D.B.; Cyr, T.D.; Ethier, J.C. Fenofibrate Raw Materials: HPLC Methods for Assay and Purity and an NMR Method for Purity. J. Pharm. Biomed. Anal. 1998, 18, 383–402. [Google Scholar] [CrossRef]
- Jain, D.; Jain, N.; Raghuwanshi, R. Development and Validation of RP-HPLC Method for Simultaneous Estimation of Atorvastatin Calcium and Fenofibrate in Tablet Dosage Forms. Indian J. Pharm. Sci. 2008, 70, 263. [Google Scholar] [CrossRef]
- Shaqour, B.; Reigada, I.; Górecka, Ż.; Choińska, E.; Verleije, B.; Beyers, K.; Święszkowski, W.; Fallarero, A.; Cos, P. 3D-Printed Drug Delivery Systems: The Effects of Drug Incorporation Methods on Their Release and Antibacterial Efficiency. Materials 2020, 13, 3364. [Google Scholar] [CrossRef]
- Wong, F.S.Y.; Tsang, K.K.; Chu, A.M.W.; Chan, B.P.; Yao, K.M.; Lo, A.C.Y. Injectable Cell-Encapsulating Composite Alginate-Collagen Platform with Inducible Termination Switch for Safer Ocular Drug Delivery. Biomaterials 2019, 201, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Ke, C.-J.; Yen, K.-C.; Hsieh, H.-C.; Sun, J.-S.; Lin, F.-H. 3D Porous Calcium-Alginate Scaffolds Cell Culture System Improved Human Osteoblast Cell Clusters for Cell Therapy. Theranostics 2015, 5, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Ardiles, C.S.; Rodríguez, C.C. Theoretical Study for Determining the Type of Interactions between a GG Block of an Alginate Chain with Metals Cu2+, Mn2+, Ca2+ and Mg2+. Arab. J. Chem. 2021, 14, 103325. [Google Scholar] [CrossRef]
- Liu, F.; Ma, C.; Gao, Y.; McClements, D.J. Food-Grade Covalent Complexes and Their Application as Nutraceutical Delivery Systems: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 76–95. [Google Scholar] [CrossRef]
- Lavik, E.B.; Klassen, H.; Warfvinge, K.; Langer, R.; Young, M.J. Fabrication of Degradable Polymer Scaffolds to Direct the Integration and Differentiation of Retinal Progenitors. Biomaterials 2005, 26, 3187–3196. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakkak, J.; Al-Hakkak, F. Functional Egg White–Pectin Conjugates Prepared by Controlled Maillard Reaction. J. Food Eng. 2010, 100, 152–159. [Google Scholar] [CrossRef]
- Ekemen, Z.; Ahmad, Z.; Stride, E.; Kaplan, D.; Edirisinghe, M. Electrohydrodynamic Bubbling: An Alternative Route to Fabricate Porous Structures of Silk Fibroin Based Materials. Biomacromolecules 2013, 14, 1412–1422. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Stachewicz, U.; Szewczyk, P.K.; Kruk, A.; Barber, A.H.; Czyrska-Filemonowicz, A. Pore Shape and Size Dependence on Cell Growth into Electrospun Fiber Scaffolds for Tissue Engineering: 2D and 3D Analyses Using SEM and FIB-SEM Tomography. Mater. Sci. Eng. C 2019, 95, 397–408. [Google Scholar] [CrossRef]
- Prasadh, S.; Wong, R.C.W. Unraveling the Mechanical Strength of Biomaterials Used as a Bone Scaffold in Oral and Maxillofacial Defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Khoder, M.; Tsapis, N.; Huguet, H.; Besnard, M.; Gueutin, C.; Fattal, E. Removal of Ciprofloxacin in Simulated Digestive Media by Activated Charcoal Entrapped within Zinc-Pectinate Beads. Int. J. Pharm. 2009, 379, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Etxabide, A.; Ribeiro, R.D.C.; Guerrero, P.; Ferreira, A.M.; Stafford, G.P.; Dalgarno, K.; de la Caba, K.; Gentile, P. Lactose-Crosslinked Fish Gelatin-Based Porous Scaffolds Embedded with Tetrahydrocurcumin for Cartilage Regeneration. Int. J. Biol. Macromol. 2018, 117, 199–208. [Google Scholar] [CrossRef]
- Varley, M.C.; Neelakantan, S.; Clyne, T.W.; Dean, J.; Brooks, R.A.; Markaki, A.E. Cell Structure, Stiffness and Permeability of Freeze-Dried Collagen Scaffolds in Dry and Hydrated States. Acta Biomater. 2016, 33, 166–175. [Google Scholar] [CrossRef]
- Wendland, R.J.; Jiao, C.; Russell, S.R.; Han, I.C.; Wiley, L.A.; Tucker, B.A.; Sohn, E.H.; Worthington, K.S. The Effect of Retinal Scaffold Modulus on Performance during Surgical Handling. Exp. Eye Res. 2021, 207, 108566. [Google Scholar] [CrossRef]
- Worthington, K.S.; Wiley, L.A.; Bartlett, A.M.; Stone, E.M.; Mullins, R.F.; Salem, A.K.; Guymon, C.A.; Tucker, B.A. Mechanical Properties of Murine and Porcine Ocular Tissues in Compression. Exp. Eye Res. 2014, 121, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Gentile, P.; Mattioli-Belmonte, M.; Chiono, V.; Ferretti, C.; Baino, F.; Tonda-Turo, C.; Vitale-Brovarone, C.; Pashkuleva, I.; Reis, R.L.; Ciardelli, G. Bioactive Glass/Polymer Composite Scaffolds Mimicking Bone Tissue. J. Biomed Mater. Res. A 2012, 100A, 2654–2667. [Google Scholar] [CrossRef] [PubMed]
- Meydani, B.; Vahedifar, A.; Askari, G.; Madadlou, A. Influence of the Maillard Reaction on the Properties of Cold-Set Whey Protein and Maltodextrin Binary Gels. Int. Dairy J. 2019, 90, 79–87. [Google Scholar] [CrossRef]
- Fatimi, A.; Okoro, O.V.; Podstawczyk, D.; Siminska-Stanny, J.; Shavandi, A. Natural Hydrogel-Based Bio-Inks for 3D Bioprinting in Tissue Engineering: A Review. Gels 2022, 8, 179. [Google Scholar] [CrossRef]
- Shavandi, A.; Hosseini, S.; Okoro, O.V.; Nie, L.; Eghbali Babadi, F.; Melchels, F. 3D Bioprinting of Lignocellulosic Biomaterials. Adv. Healthc. Mater. 2020, 9, 2001472. [Google Scholar] [CrossRef]
- Shah, R.; Saha, N.; Saha, P. Influence of Temperature, PH and Simulated Biological Solutions on Swelling and Structural Properties of Biomineralized (CaCO3) PVP–CMC Hydrogel. Prog. Biomater. 2015, 4, 123–136. [Google Scholar] [CrossRef]
- Will, M.A.; Clark, N.A.; Swain, J.E. Biological PH Buffers in IVF: Help or Hindrance to Success. J. Assist. Reprod. Genet. 2011, 28, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Khoder, M.; Tsapis, N.; Domergue-Dupont, V.; Gueutin, C.; Fattal, E. Removal of Residual Colonic Ciprofloxacin in the Rat by Activated Charcoal Entrapped within Zinc-Pectinate Beads. Eur. J. Pharm. Sci. 2010, 41, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, J.; Mossine, V.V.; Feather, M.S. The Detection of Some Dicarbonyl Intermediates Arising from the Degradation of Amadori Compounds (the Maillard Reaction). Carbohydr. Res. 1995, 273, 171–177. [Google Scholar] [CrossRef]
- Zhang, M.; Li, H.; Lang, B.; O’Donnell, K.; Zhang, H.; Wang, Z.; Dong, Y.; Wu, C.; Williams, R.O. Formulation and Delivery of Improved Amorphous Fenofibrate Solid Dispersions Prepared by Thin Film Freezing. Eur. J. Pharm. Biopharm. 2012, 82, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Bismarck, A.; Steinke, J.H.G. Ion-Responsive Alginate Based Macroporous Injectable Hydrogel Scaffolds Prepared by Emulsion Templating. J. Mater. Chem. B 2013, 1, 4736. [Google Scholar] [CrossRef] [PubMed]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-Polysaccharide Composite Scaffolds for 3D Cell Culture and Tissue Engineering: Towards Natural Therapeutics. Bioeng. Transl. Med. 2019, 4, 96–115. [Google Scholar] [CrossRef]
- Shoichet, M.S.; Li, R.H.; White, M.L.; Winn, S.R. Stability of Hydrogels Used in Cell Encapsulation: An in Vitro Comparison of Alginate and Agarose. Biotechnol. Bioeng. 1996, 50, 374–381. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, X.; Deng, X.; Hao, L.; Wang, W. Modification of Alginate Hydrogel Films for Delivering Hydrophobic Kaempferol. J. Nanomater. 2019, 2019, 9170732. [Google Scholar] [CrossRef]
- Eral, H.B.; López-Mejías, V.; O’Mahony, M.; Trout, B.L.; Myerson, A.S.; Doyle, P.S. Biocompatible Alginate Microgel Particles as Heteronucleants and Encapsulating Vehicles for Hydrophilic and Hydrophobic Drugs. Cryst. Growth Des. 2014, 14, 2073–2082. [Google Scholar] [CrossRef]
- Al-Husseini, J.K.; Stanton, N.J.; Selassie, C.R.D.; Johal, M.S. The Binding of Drug Molecules to Serum Albumin: The Effect of Drug Hydrophobicity on Binding Strength and Protein Desolvation. Langmuir 2019, 35, 17054–17060. [Google Scholar] [CrossRef] [PubMed]
- Hazim, R.A.; Volland, S.; Yen, A.; Burgess, B.L.; Williams, D.S. Rapid Differentiation of the Human RPE Cell Line, ARPE-19, Induced by Nicotinamide. Exp. Eye Res. 2019, 179, 18–24. [Google Scholar] [CrossRef]
- Hsu, Y.-J.; Lin, C.-W.; Cho, S.-L.; Yang, W.-S.; Yang, C.-M.; Yang, C.-H. Protective Effect of Fenofibrate on Oxidative Stress-Induced Apoptosis in Retinal–Choroidal Vascular Endothelial Cells: Implication for Diabetic Retinopathy Treatment. Antioxidants 2020, 9, 712. [Google Scholar] [CrossRef]
- Deng, G.; Moran, E.P.; Cheng, R.; Matlock, G.; Zhou, K.; Moran, D.; Chen, D.; Yu, Q.; Ma, J.-X. Therapeutic Effects of a Novel Agonist of Peroxisome Proliferator-Activated Receptor Alpha for the Treatment of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5030. [Google Scholar] [CrossRef]
- Popescu, L.M.; Gherghiceanu, M.; Suciu, L.C.; Manole, C.G.; Hinescu, M.E. Telocytes and Putative Stem Cells in the Lungs: Electron Microscopy, Electron Tomography and Laser Scanning Microscopy. Cell Tissue Res. 2011, 345, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Uggeri, J.; Gatti, R.; Belletti, S.; Scandroglio, R.; Corradini, R.; Rotoli, B.M.; Orlandini, G. Calcein-AM Is a Detector of Intracellular Oxidative Activity. Histochem. Cell Biol. 2000, 122, 499–505. [Google Scholar] [CrossRef]
- Stiefel, P.; Schmidt-Emrich, S.; Maniura-Weber, K.; Ren, Q. Critical Aspects of Using Bacterial Cell Viability Assays with the Fluorophores SYTO9 and Propidium Iodide. BMC Microbiol. 2015, 15, 36. [Google Scholar] [CrossRef]
- Murphy, C.M.; O’Brien, F.J. Understanding the Effect of Mean Pore Size on Cell Activity in Collagen-Glycosaminoglycan Scaffolds. Cell Adhes. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef]
- Choi, S.-W.; Xie, J.; Xia, Y. Chitosan-Based Inverse Opals: Three-Dimensional Scaffolds with Uniform Pore Structures for Cell Culture. Adv. Mater. 2009, 21, 2997–3001. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abedin Zadeh, M.; Alany, R.G.; Satarian, L.; Shavandi, A.; Abdullah Almousa, M.; Brocchini, S.; Khoder, M. Maillard Reaction Crosslinked Alginate-Albumin Scaffolds for Enhanced Fenofibrate Delivery to the Retina: A Promising Strategy to Treat RPE-Related Dysfunction. Pharmaceutics 2023, 15, 1330. https://doi.org/10.3390/pharmaceutics15051330
Abedin Zadeh M, Alany RG, Satarian L, Shavandi A, Abdullah Almousa M, Brocchini S, Khoder M. Maillard Reaction Crosslinked Alginate-Albumin Scaffolds for Enhanced Fenofibrate Delivery to the Retina: A Promising Strategy to Treat RPE-Related Dysfunction. Pharmaceutics. 2023; 15(5):1330. https://doi.org/10.3390/pharmaceutics15051330
Chicago/Turabian StyleAbedin Zadeh, Maria, Raid G. Alany, Leila Satarian, Amin Shavandi, Mohamed Abdullah Almousa, Steve Brocchini, and Mouhamad Khoder. 2023. "Maillard Reaction Crosslinked Alginate-Albumin Scaffolds for Enhanced Fenofibrate Delivery to the Retina: A Promising Strategy to Treat RPE-Related Dysfunction" Pharmaceutics 15, no. 5: 1330. https://doi.org/10.3390/pharmaceutics15051330
APA StyleAbedin Zadeh, M., Alany, R. G., Satarian, L., Shavandi, A., Abdullah Almousa, M., Brocchini, S., & Khoder, M. (2023). Maillard Reaction Crosslinked Alginate-Albumin Scaffolds for Enhanced Fenofibrate Delivery to the Retina: A Promising Strategy to Treat RPE-Related Dysfunction. Pharmaceutics, 15(5), 1330. https://doi.org/10.3390/pharmaceutics15051330