Comprehensive Insights into Medicinal Research on Imidazole-Based Supramolecular Complexes
Abstract
1. Introduction
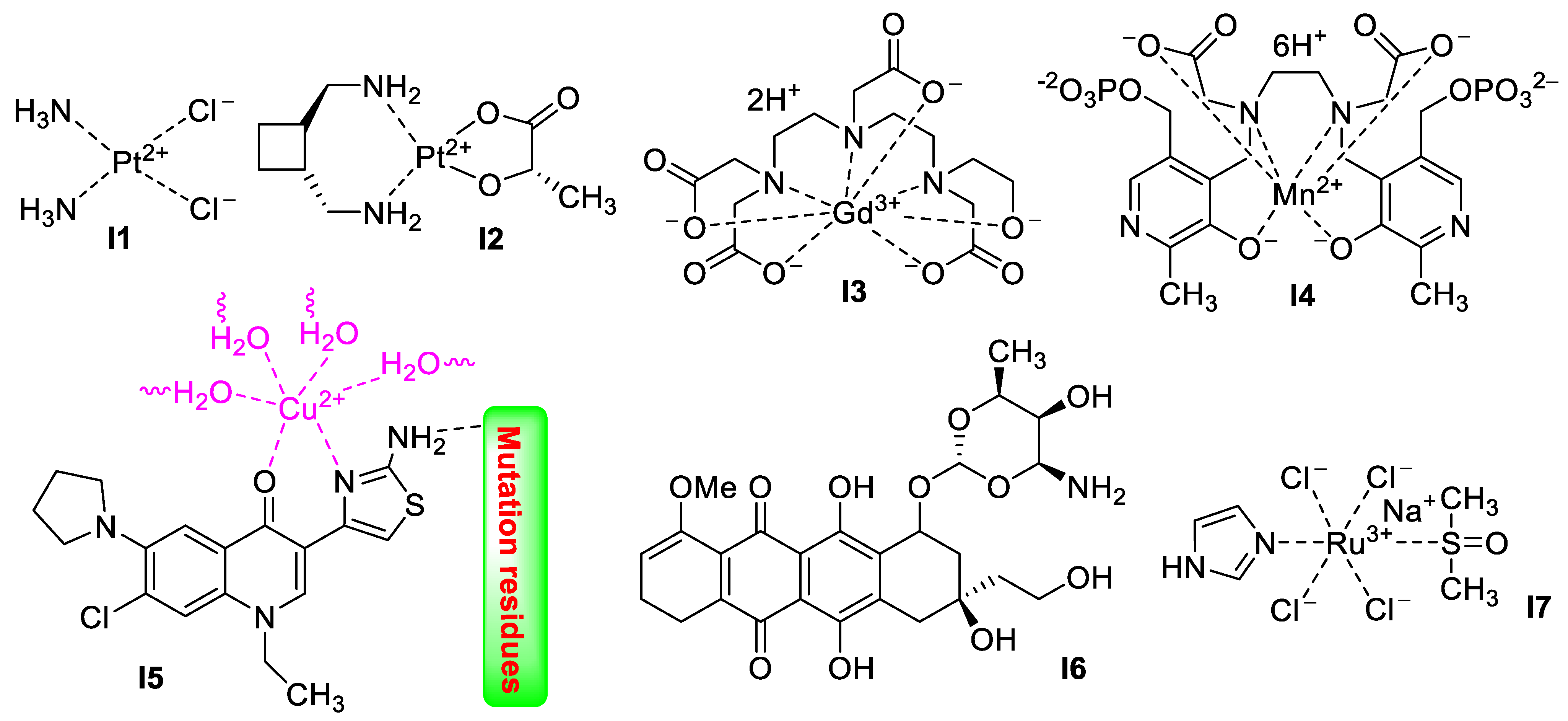
2. Imidazole-Based Supermolecules as Medicinal Agents
2.1. Imidazole-Based Supermolecules as Anticancer Agents
2.1.1. Noble Metal-Based Imidazole Supermolecules as Anticancer Agents
Platinum-Based Imidazole Supermolecules as Anticancer Agents

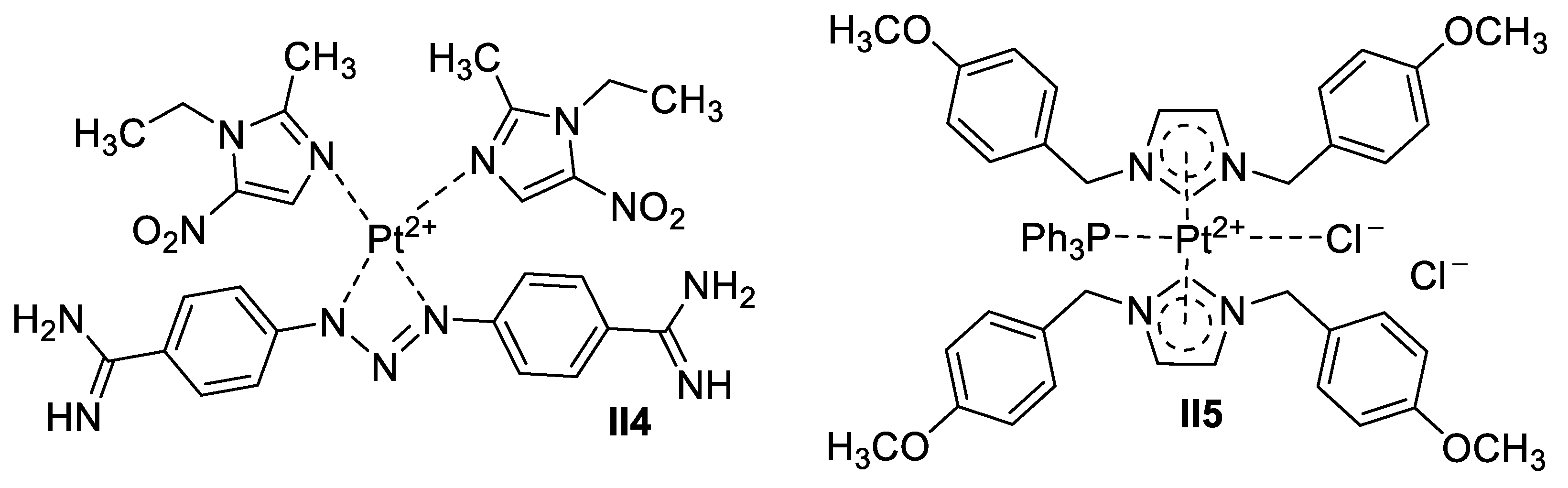

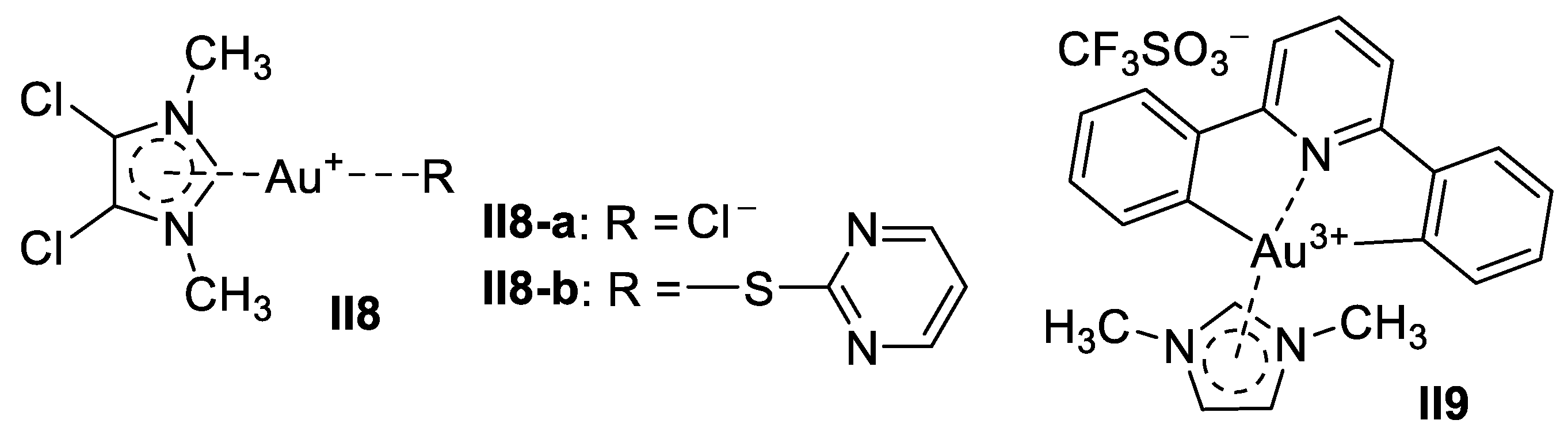
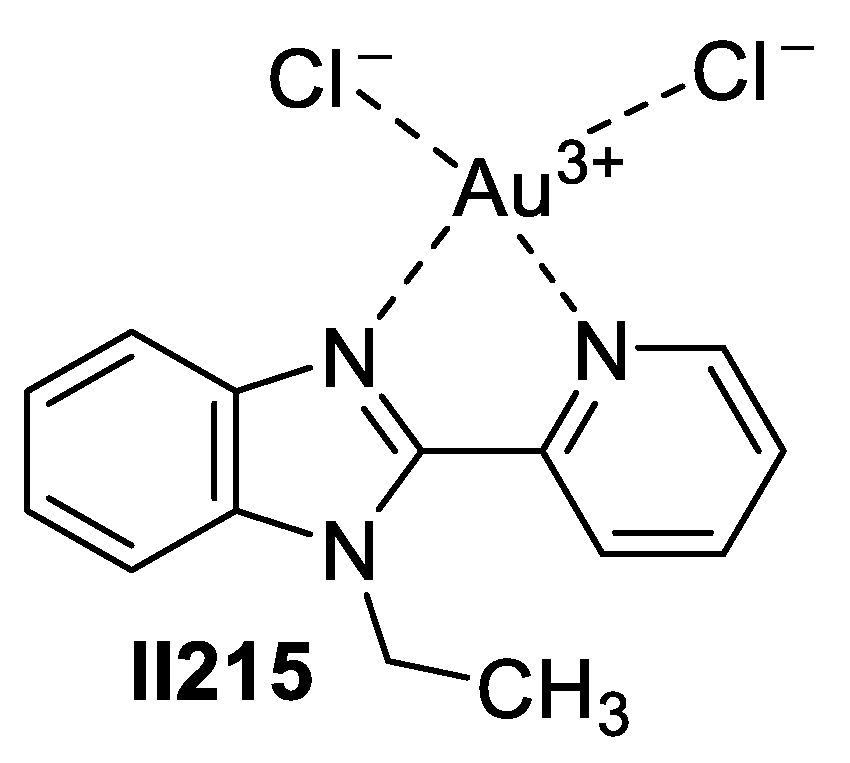
Gold-Based Imidazole Supermolecules as Anticancer Agents




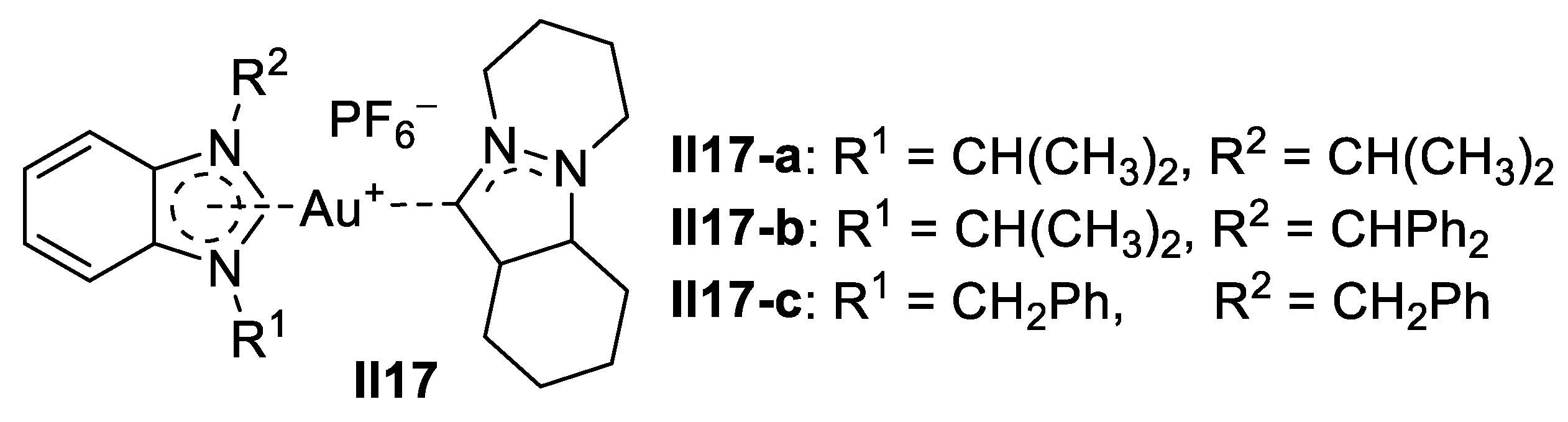
Silver-Based Imidazole Supermolecules as Anticancer Agents
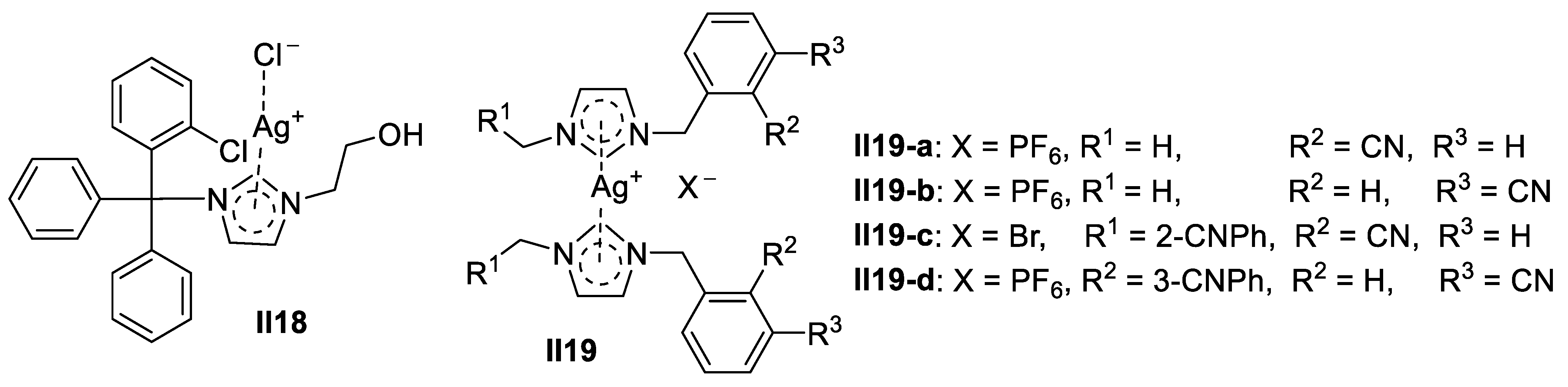
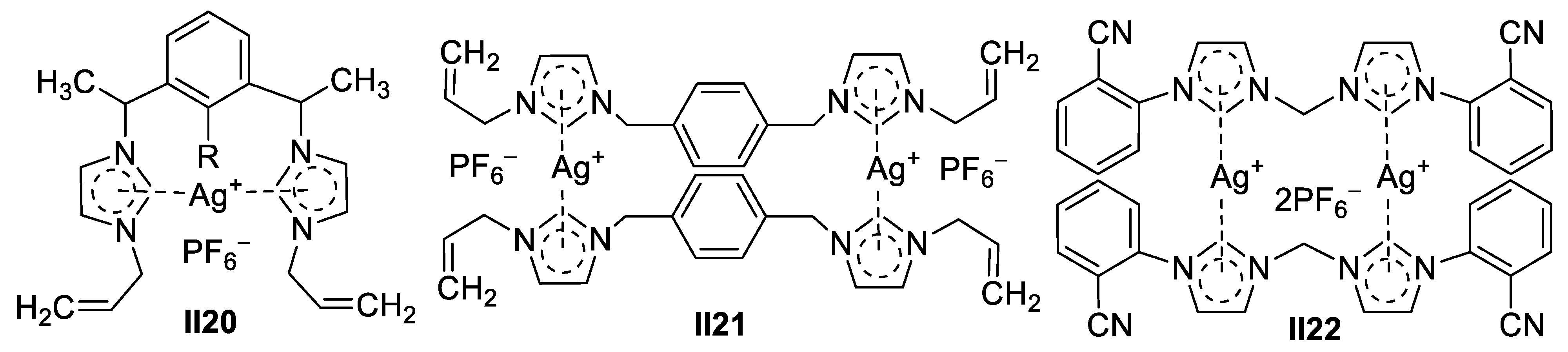
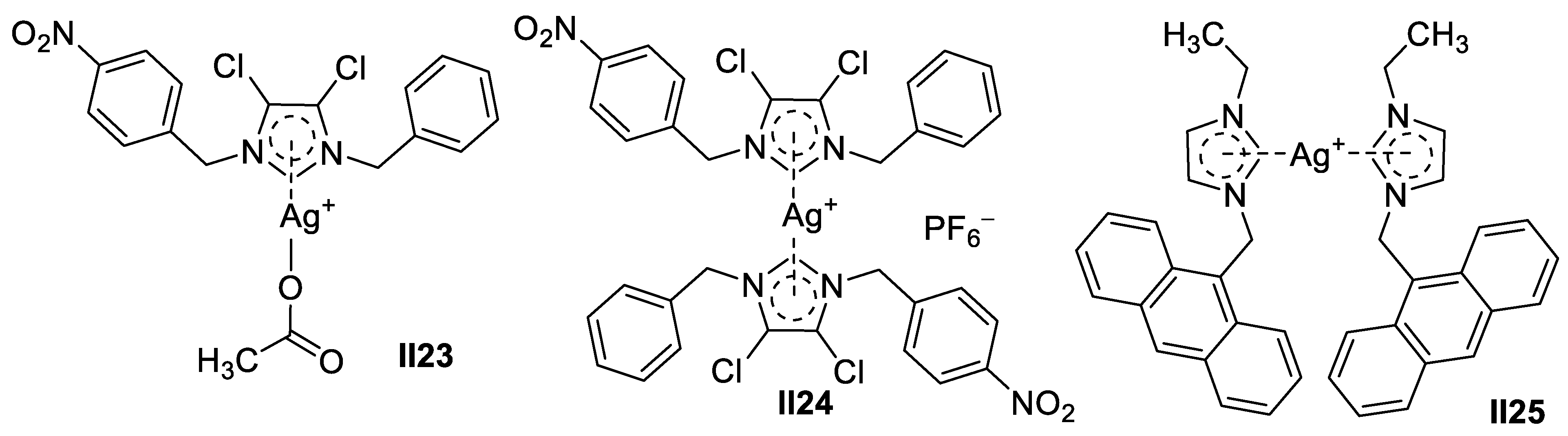



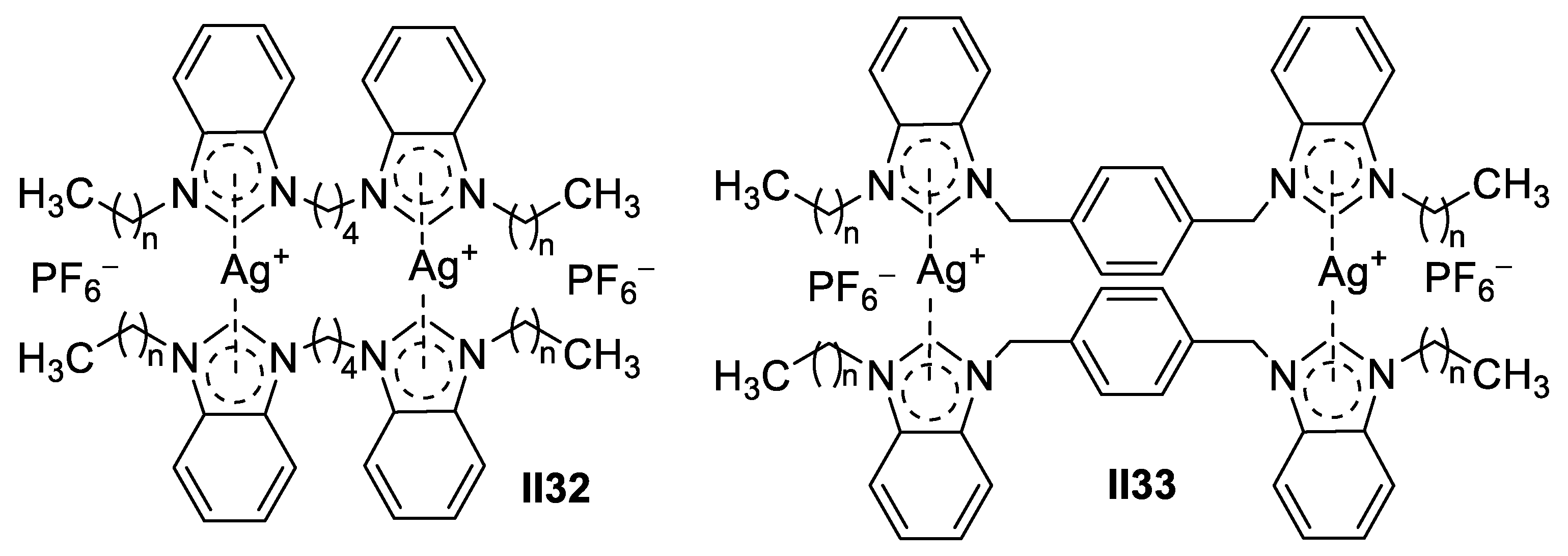
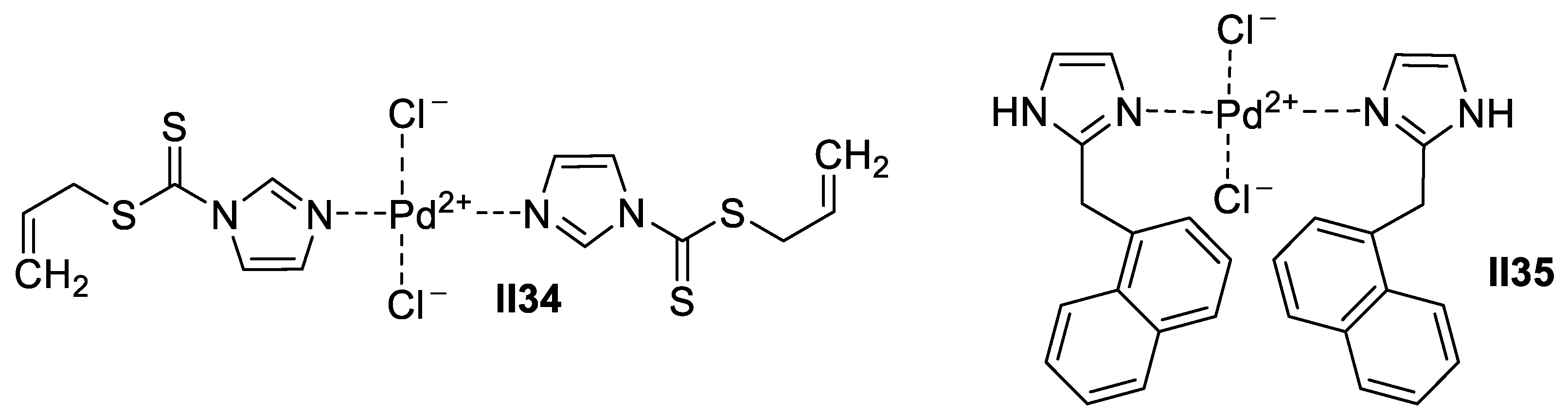
Palladium-Based Imidazole Supermolecules as Anticancer Agents

2.1.2. Transitional Metal-Based Imidazole Supermolecules as Anticancer Agents
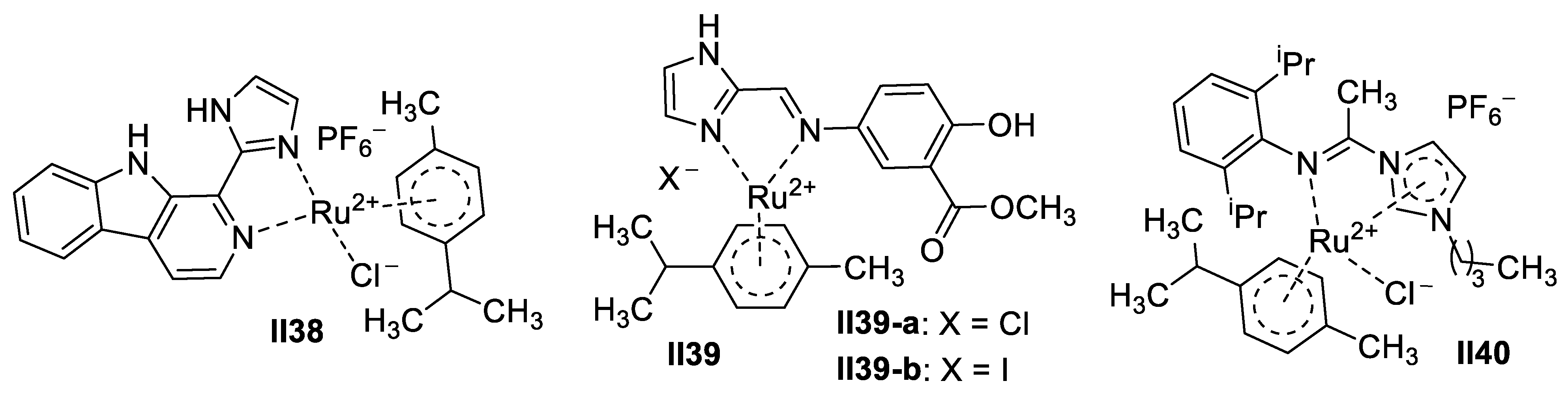
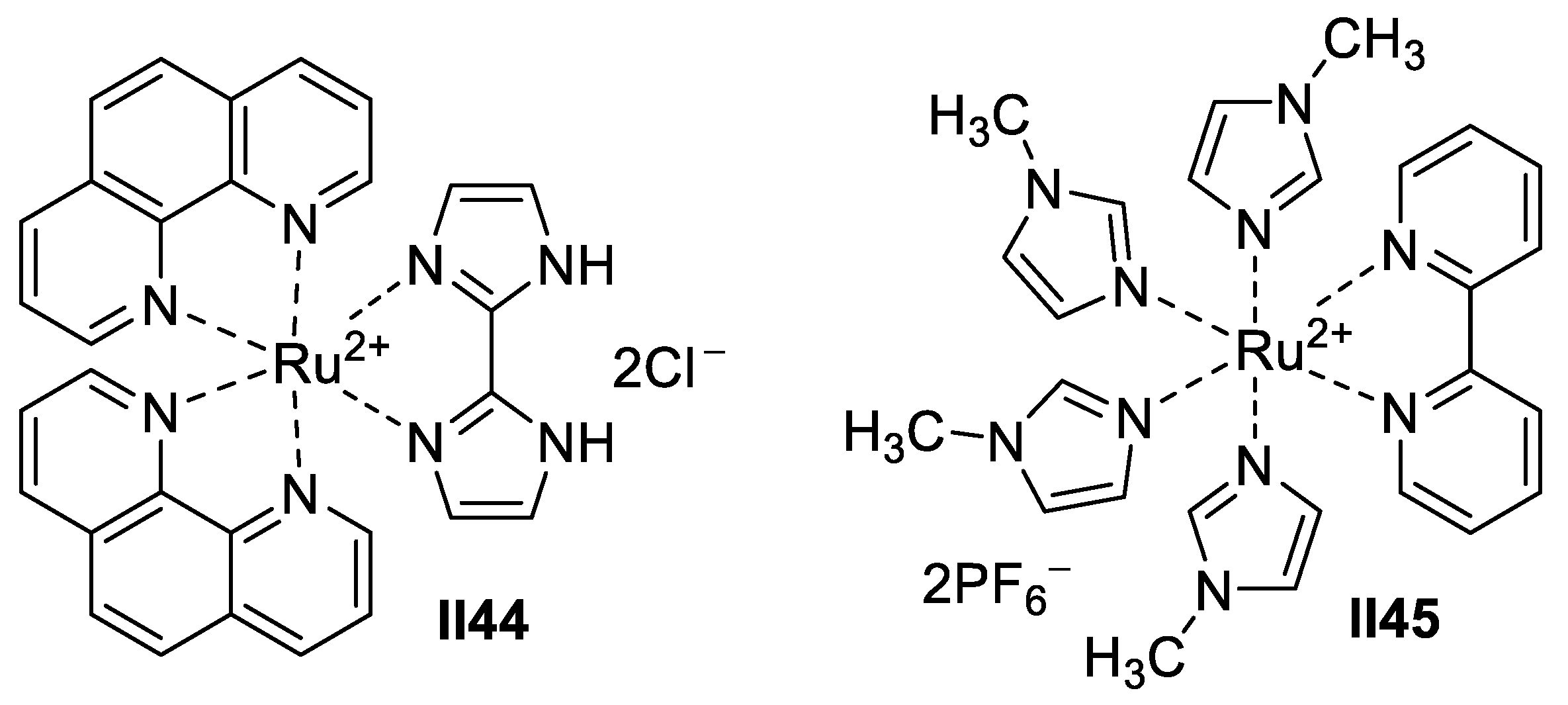
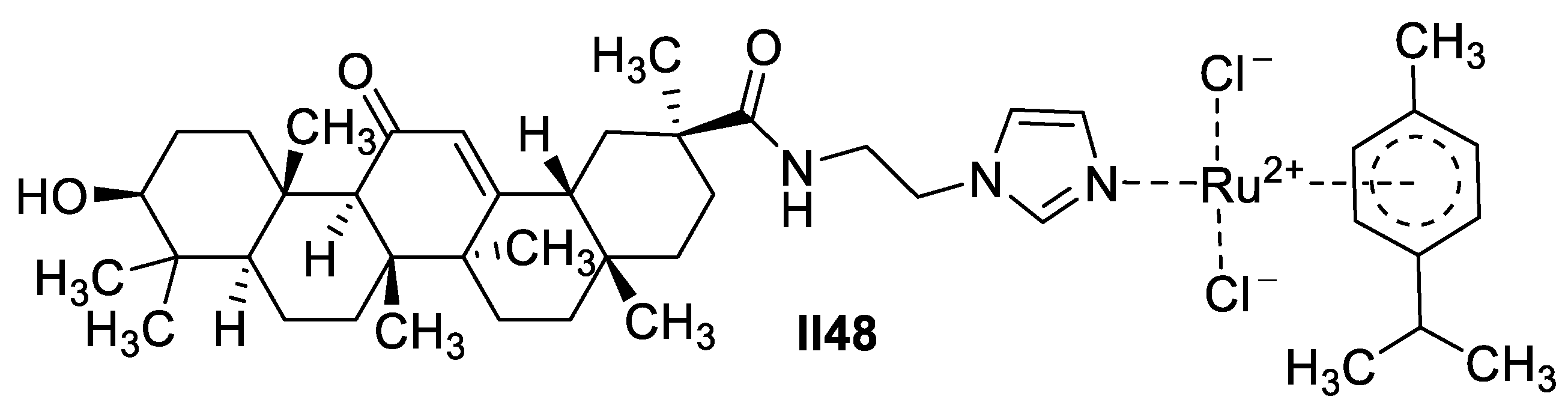
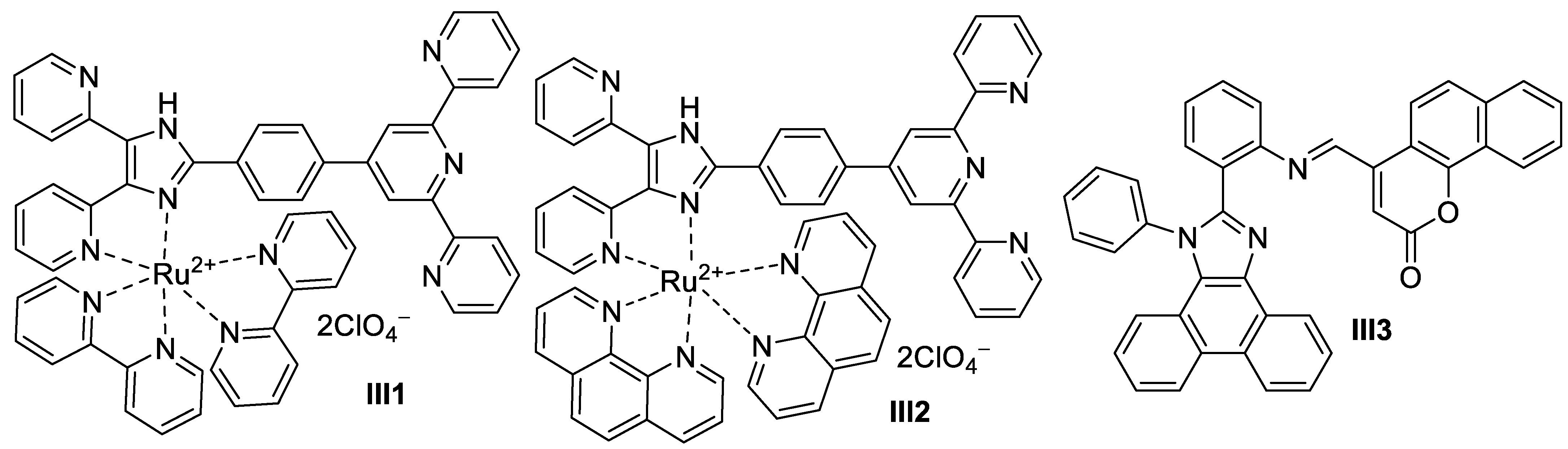
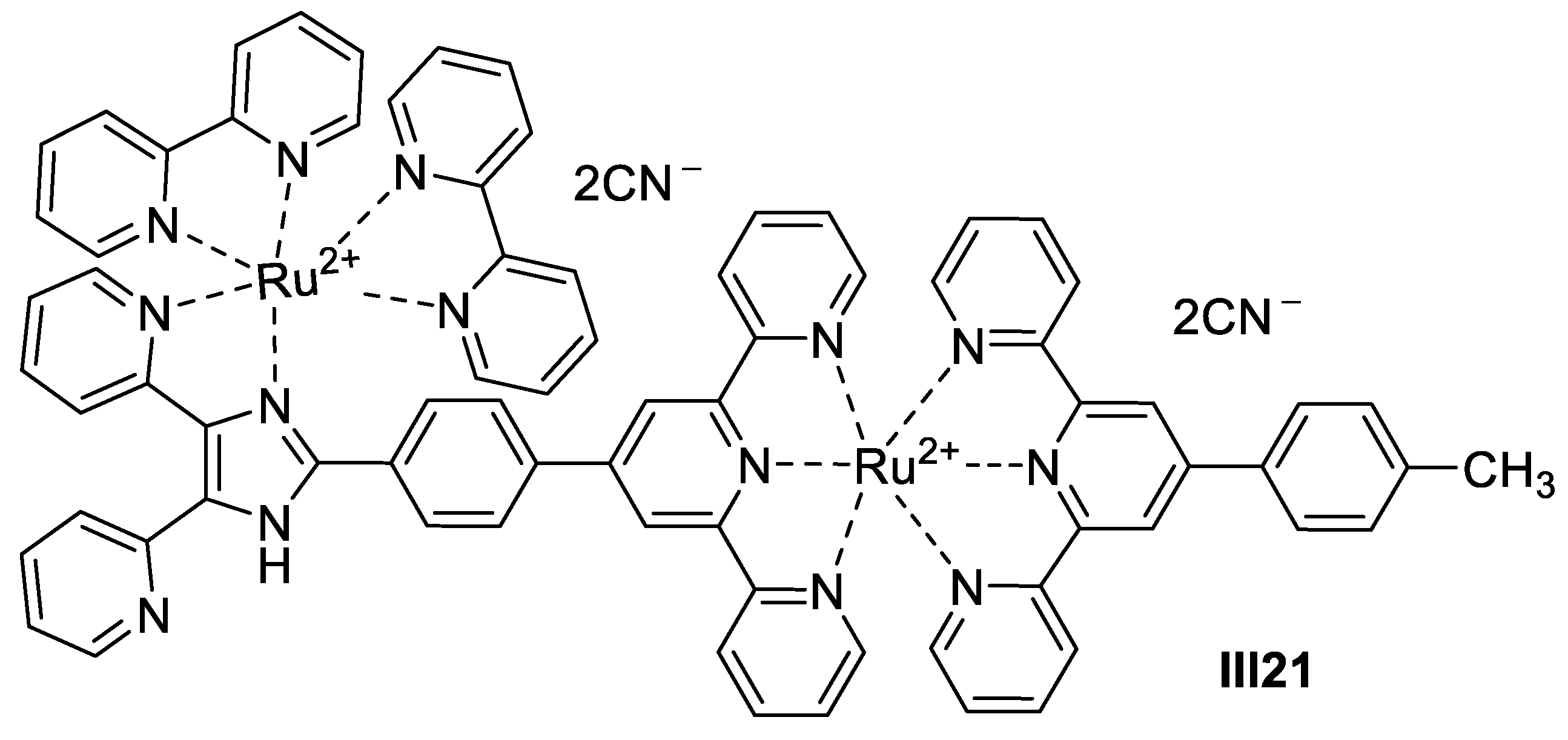
Ruthenium-Based Imidazole Supermolecules as Anticancer Agents



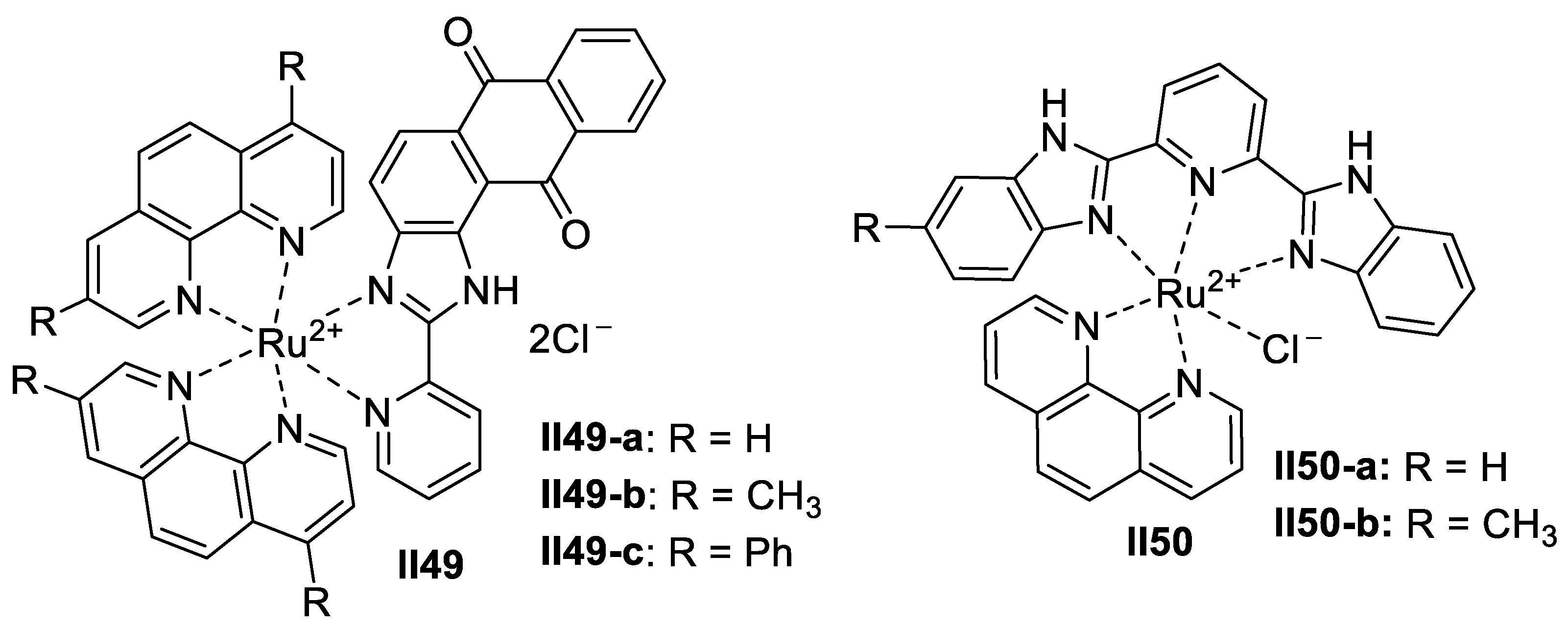


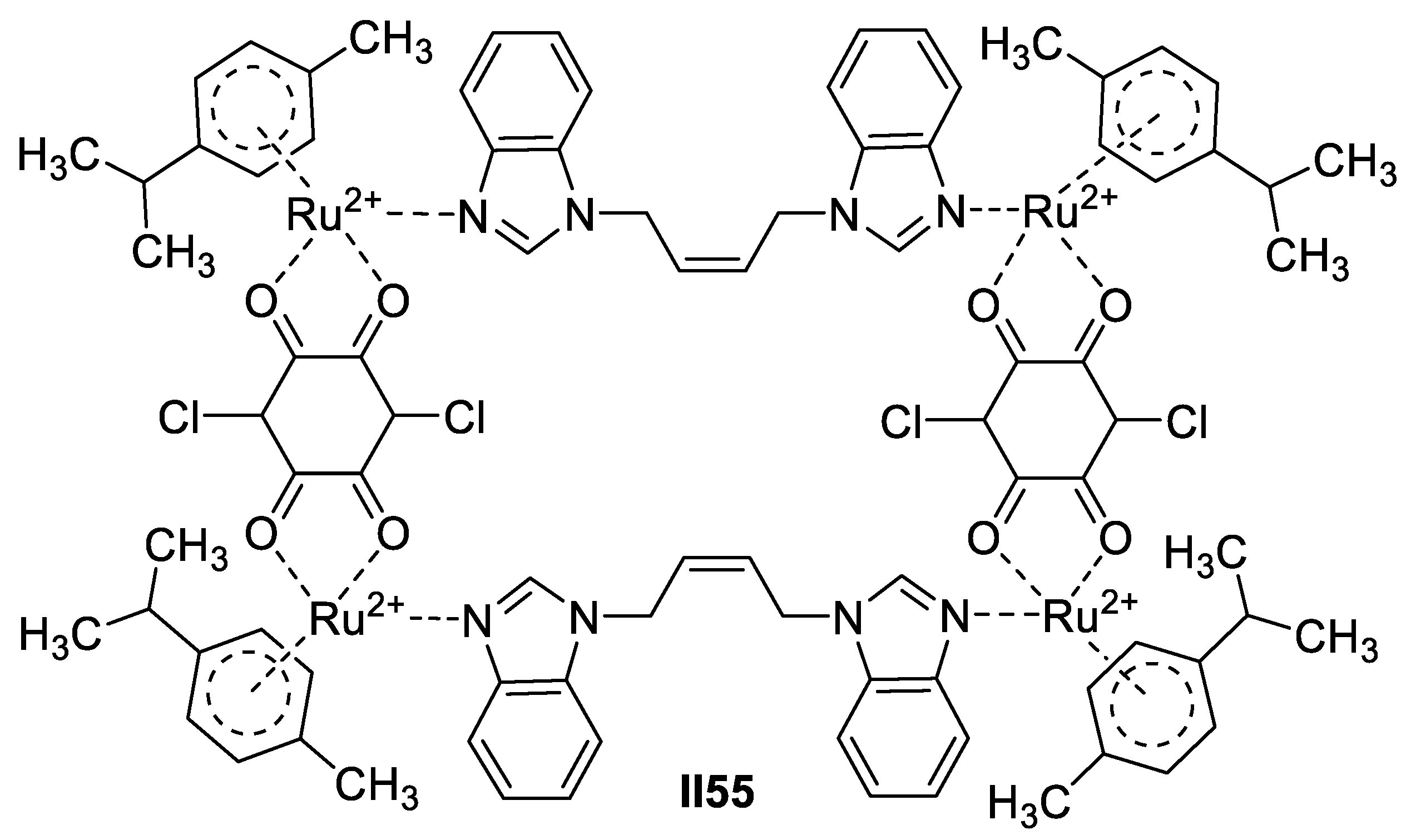
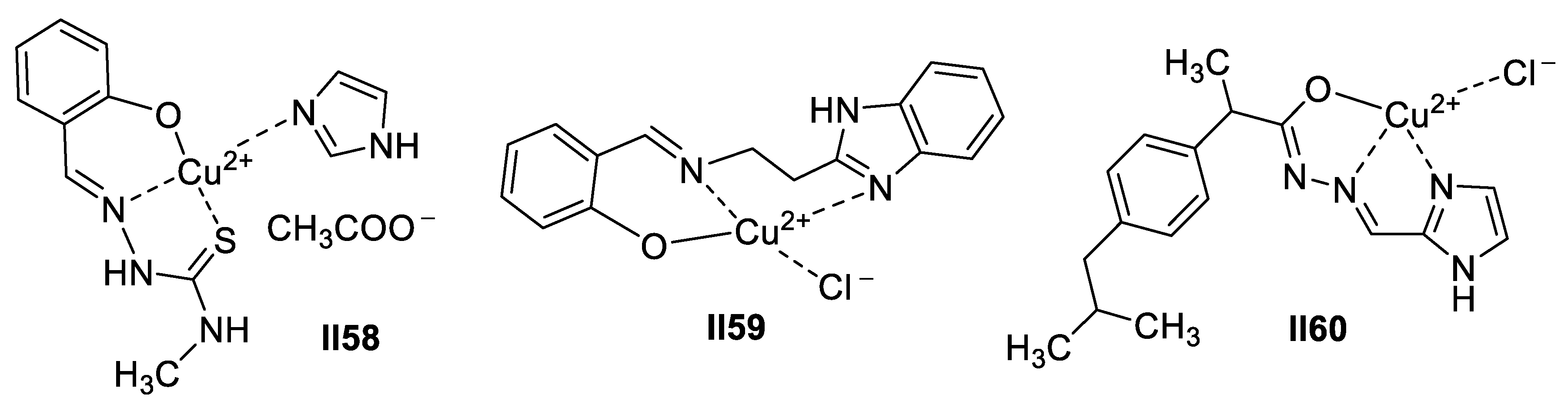
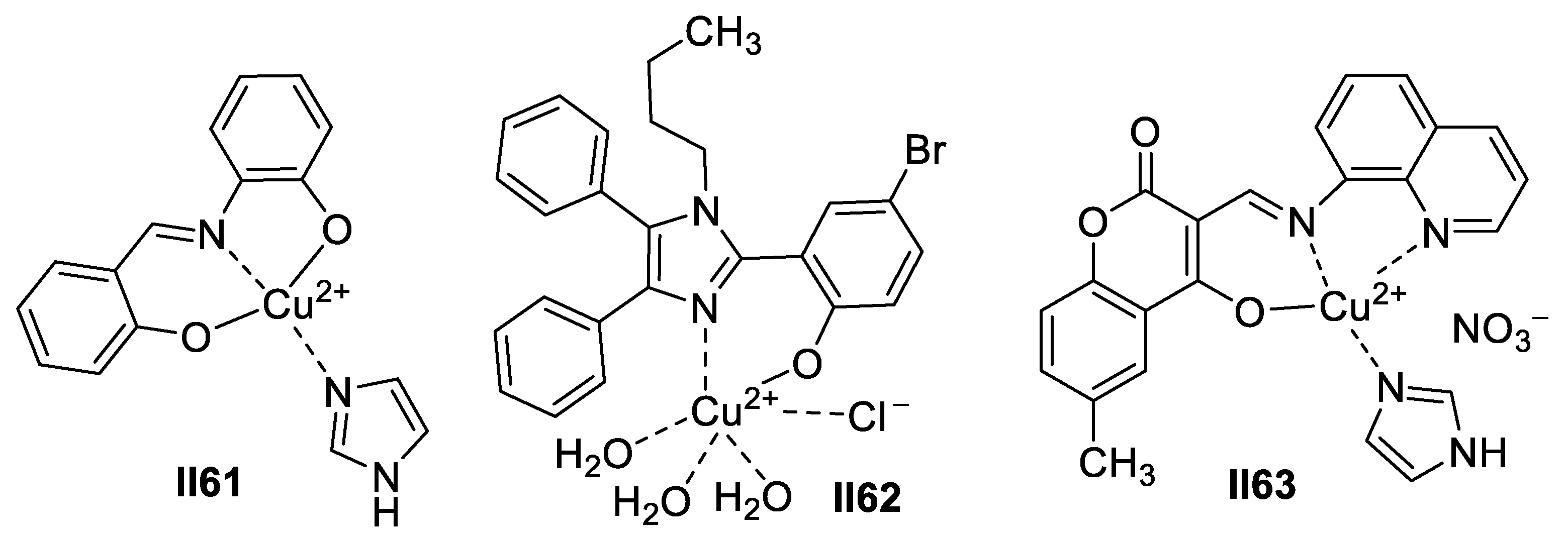
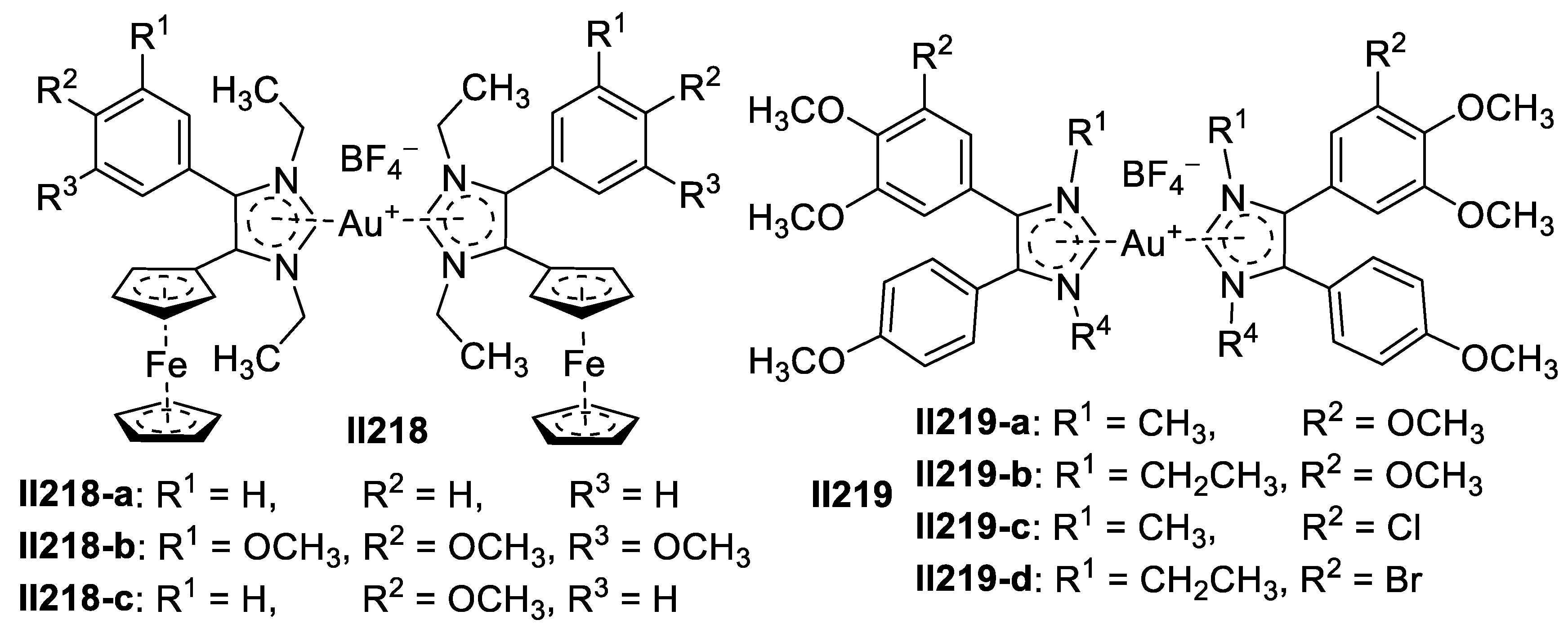
Copper-Based Imidazole Supermolecules as Anticancer Agents




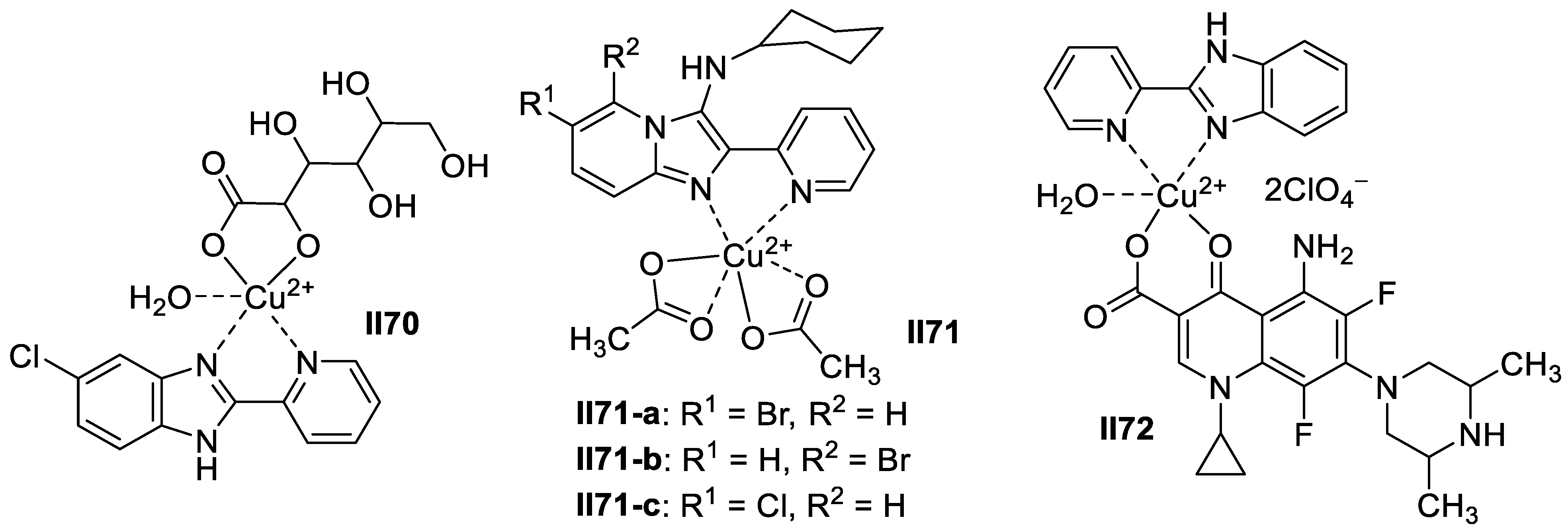
Iridium-Based Imidazole Supermolecules as Anticancer Agents

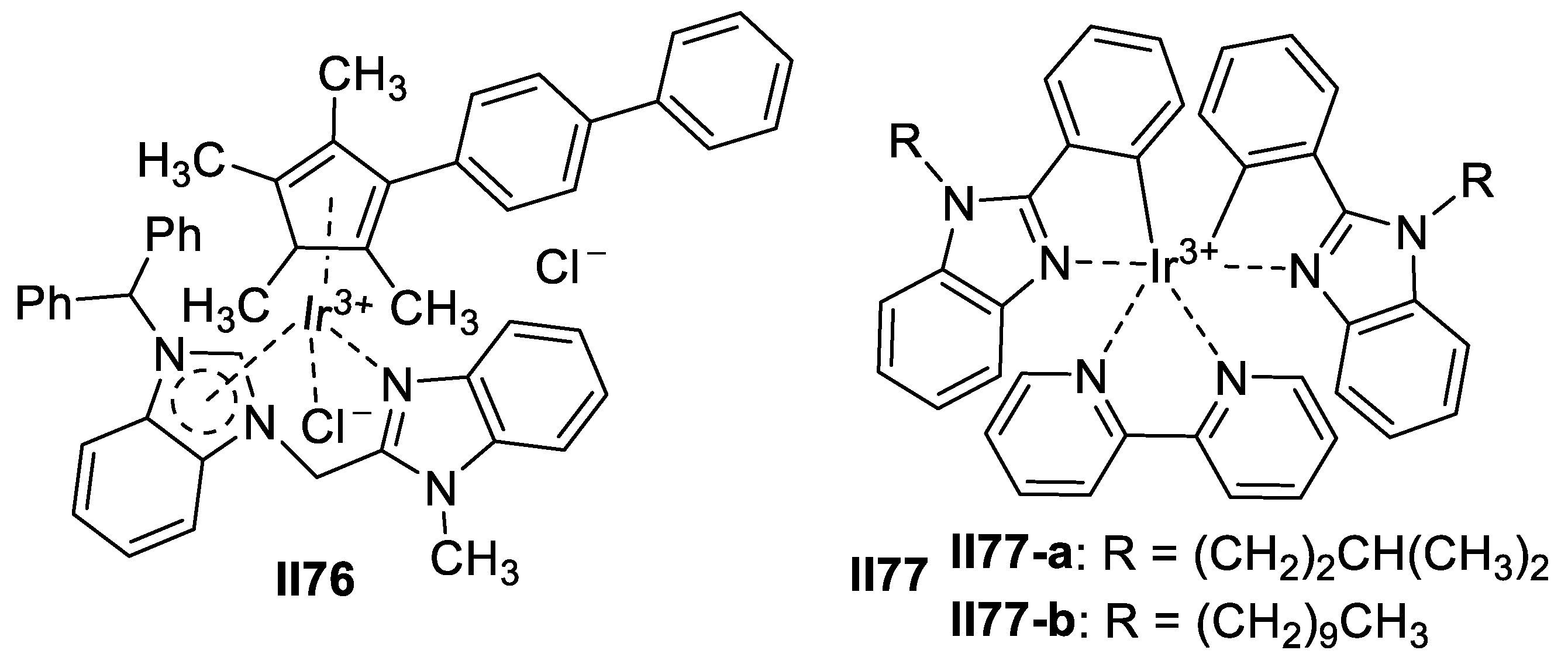
Iron-Based Imidazole Supermolecules as Anticancer Agents

Rhenium-Based Imidazole Supermolecules as Anticancer Agents

Vanadium-Based Imidazole Supermolecules as Anticancer Agents

Other Transitional Metal-Based Imidazole Supermolecules as Anticancer Agents

2.1.3. Other Metal-Based Imidazole Supermolecules as Anticancer Agents
2.2. Imidazole-Based Supermolecules as Antibacterial Agents
2.2.1. Imidazole-Based Supermolecules as Antibacterial Agents

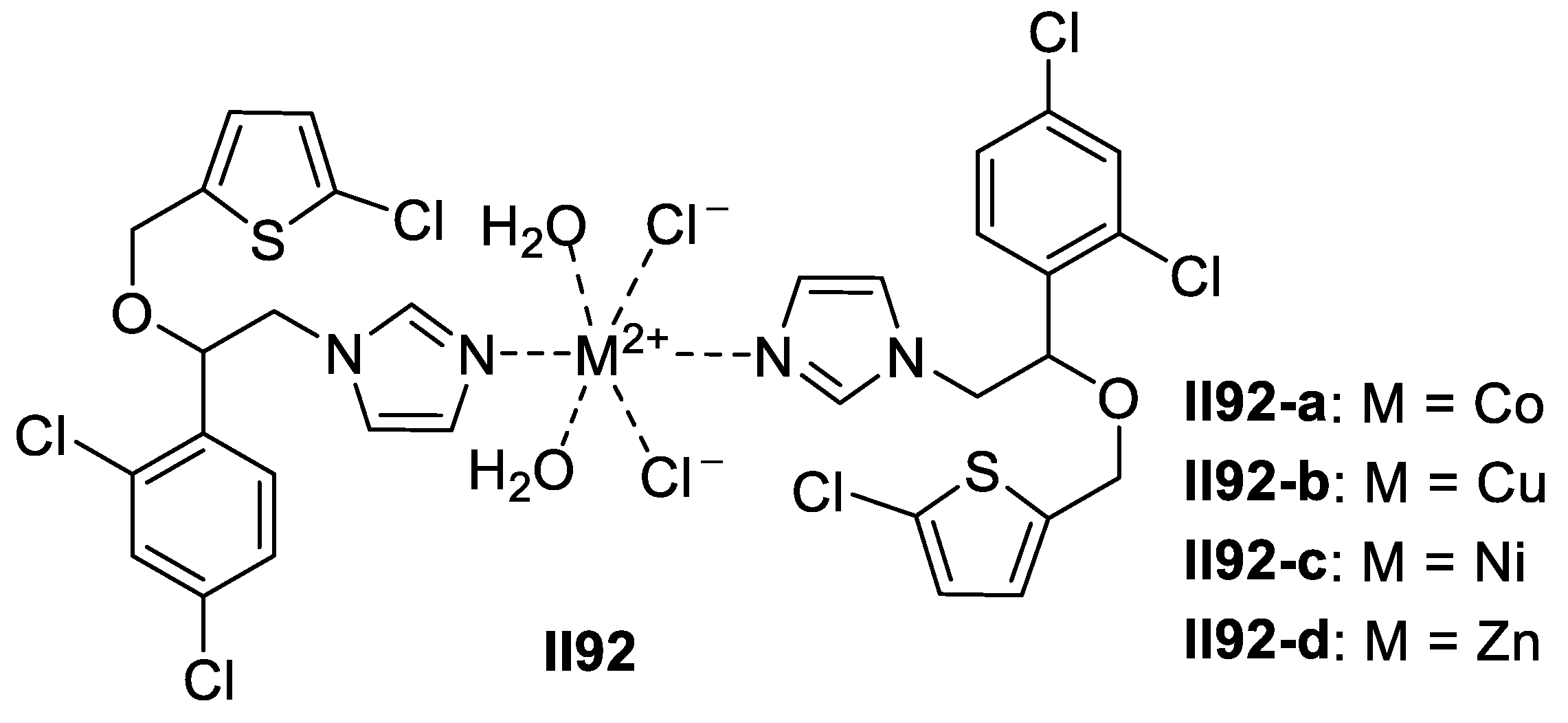
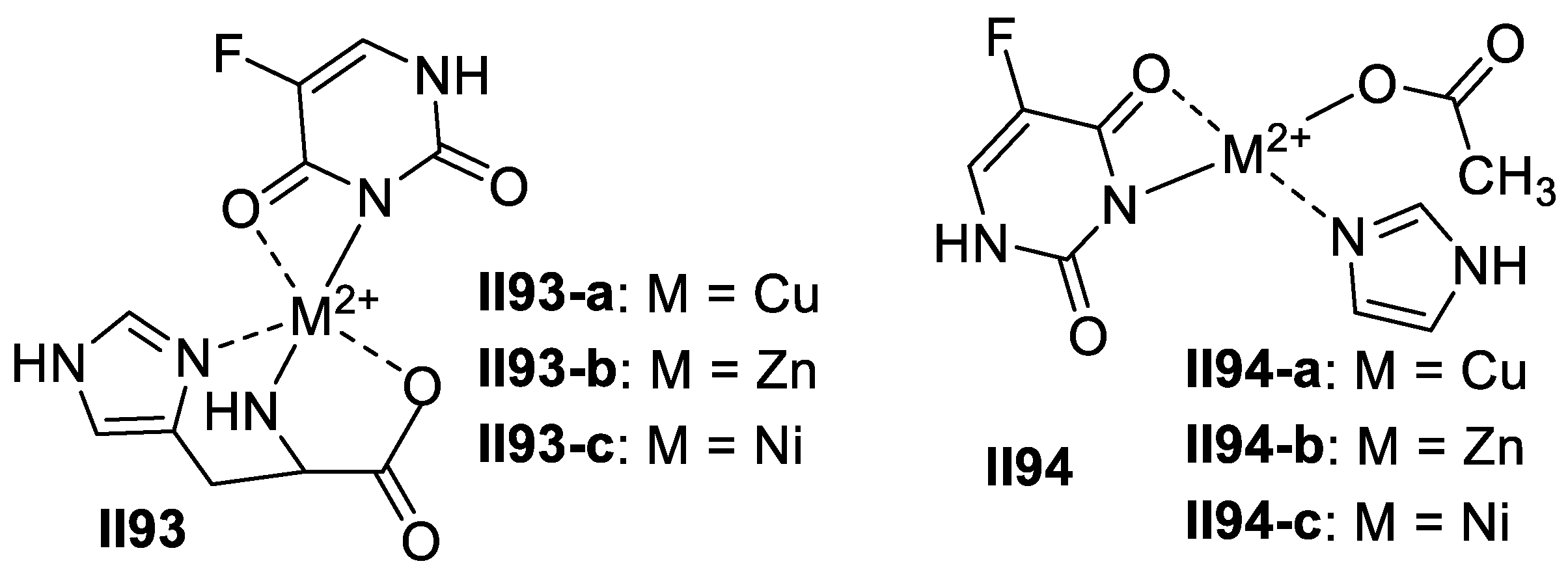

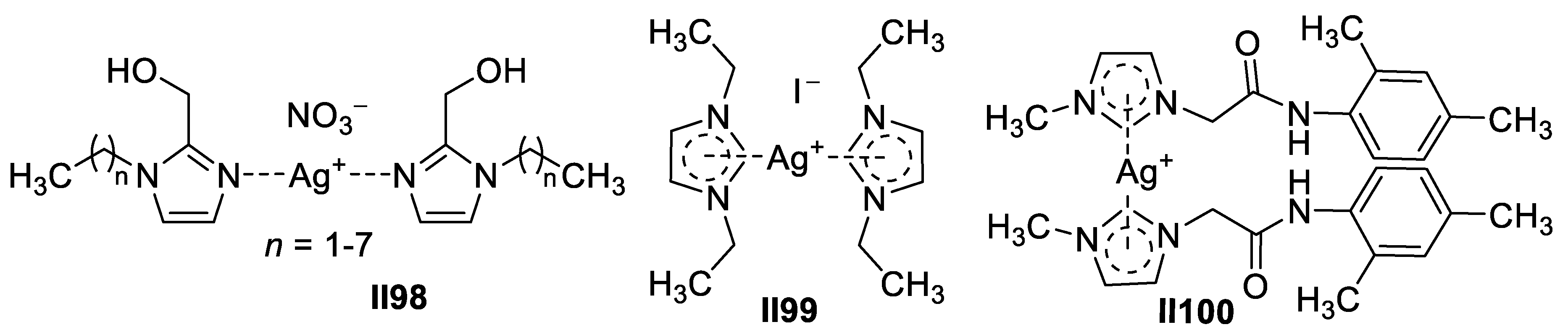
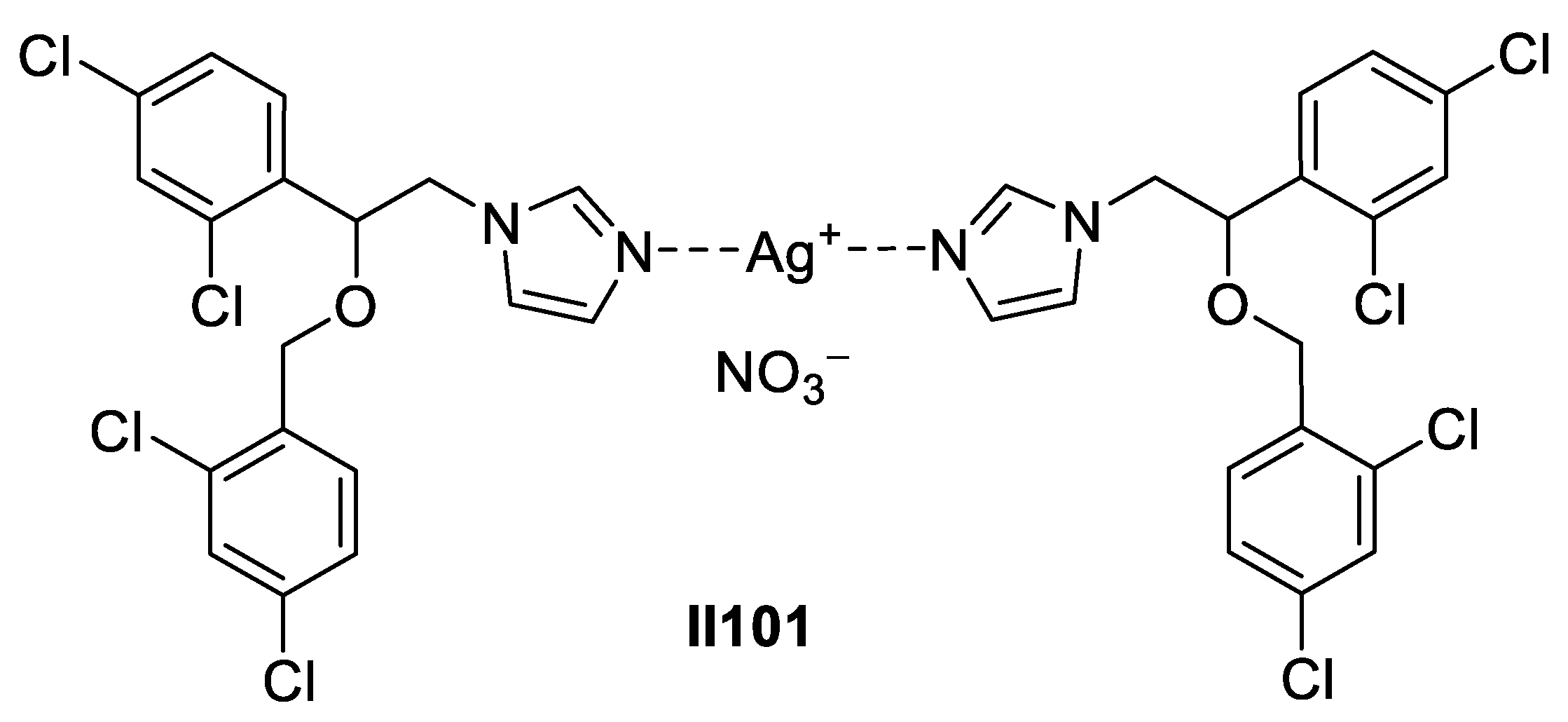




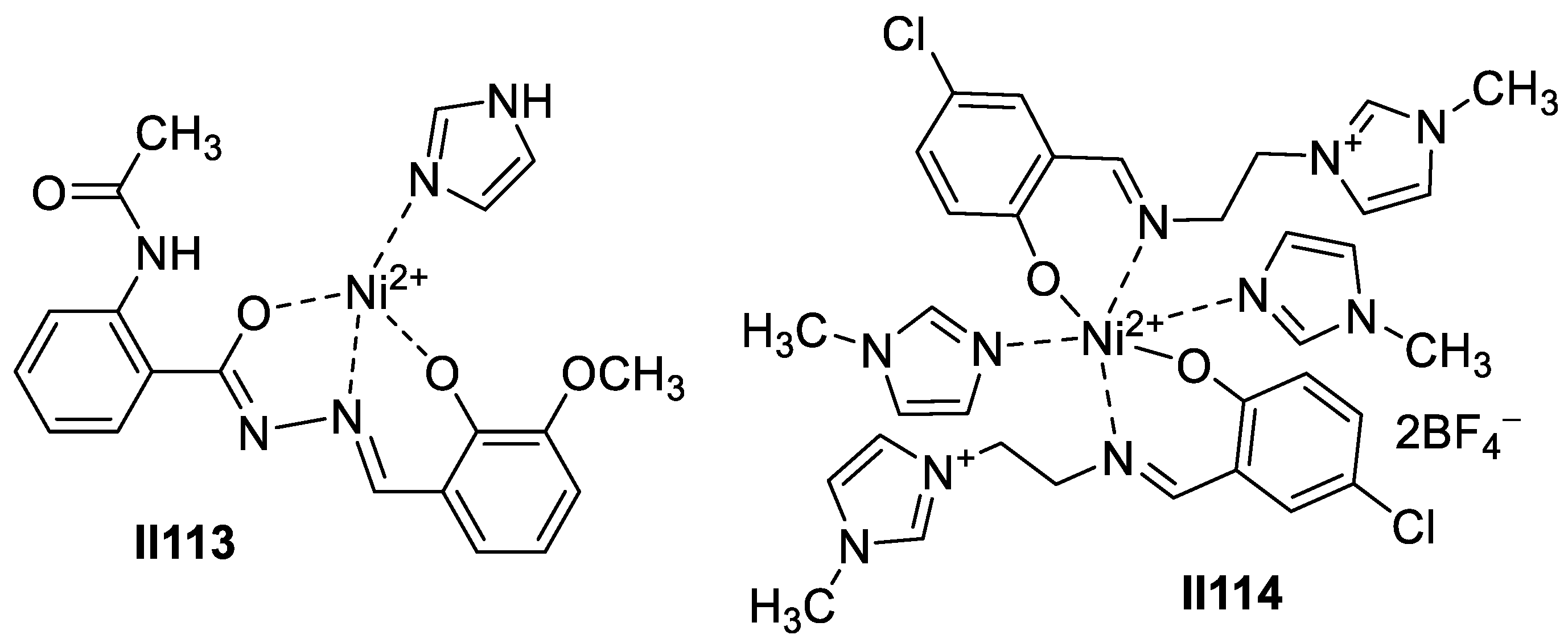




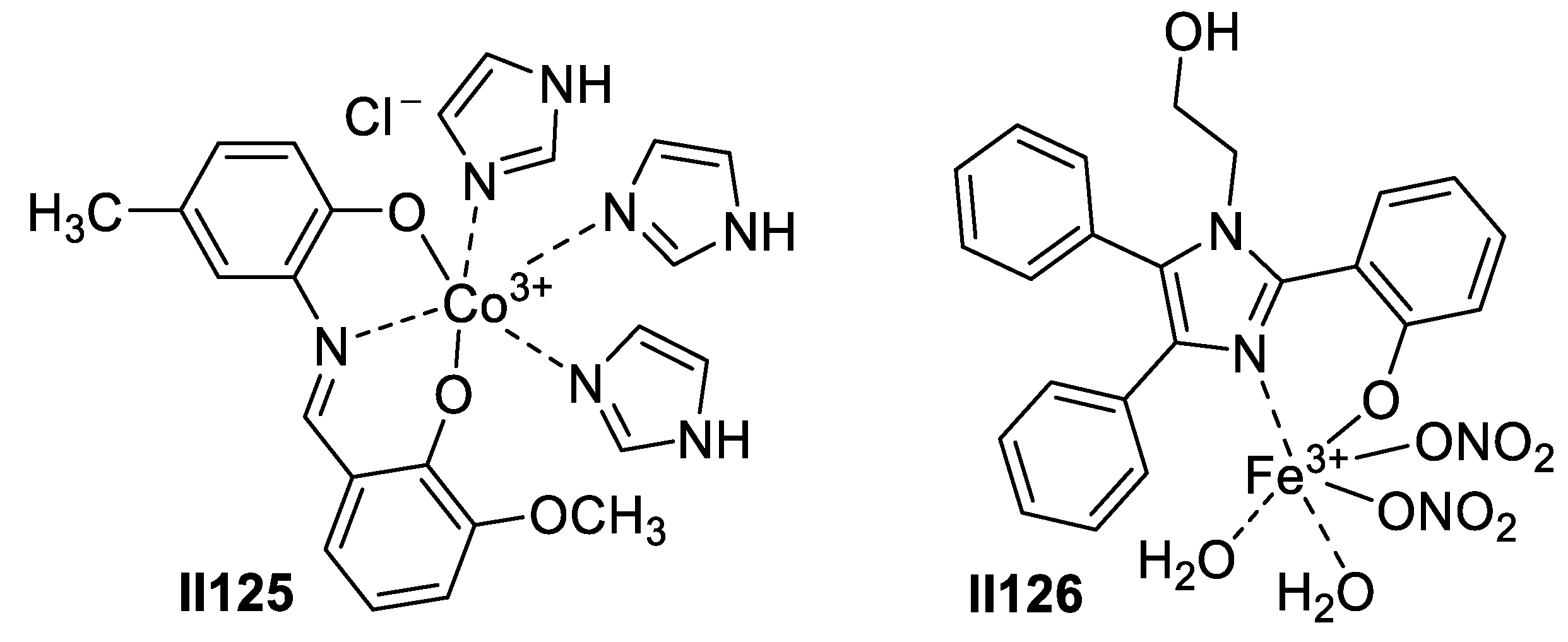




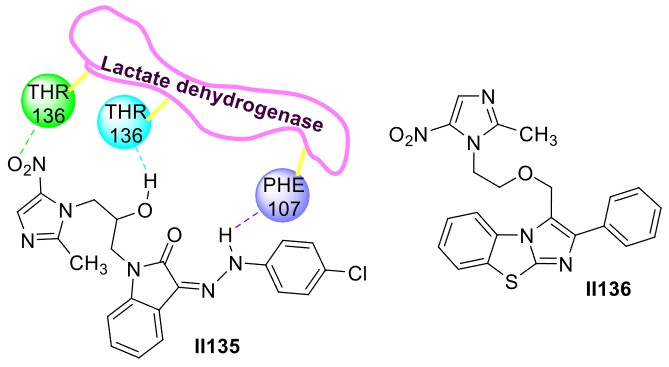
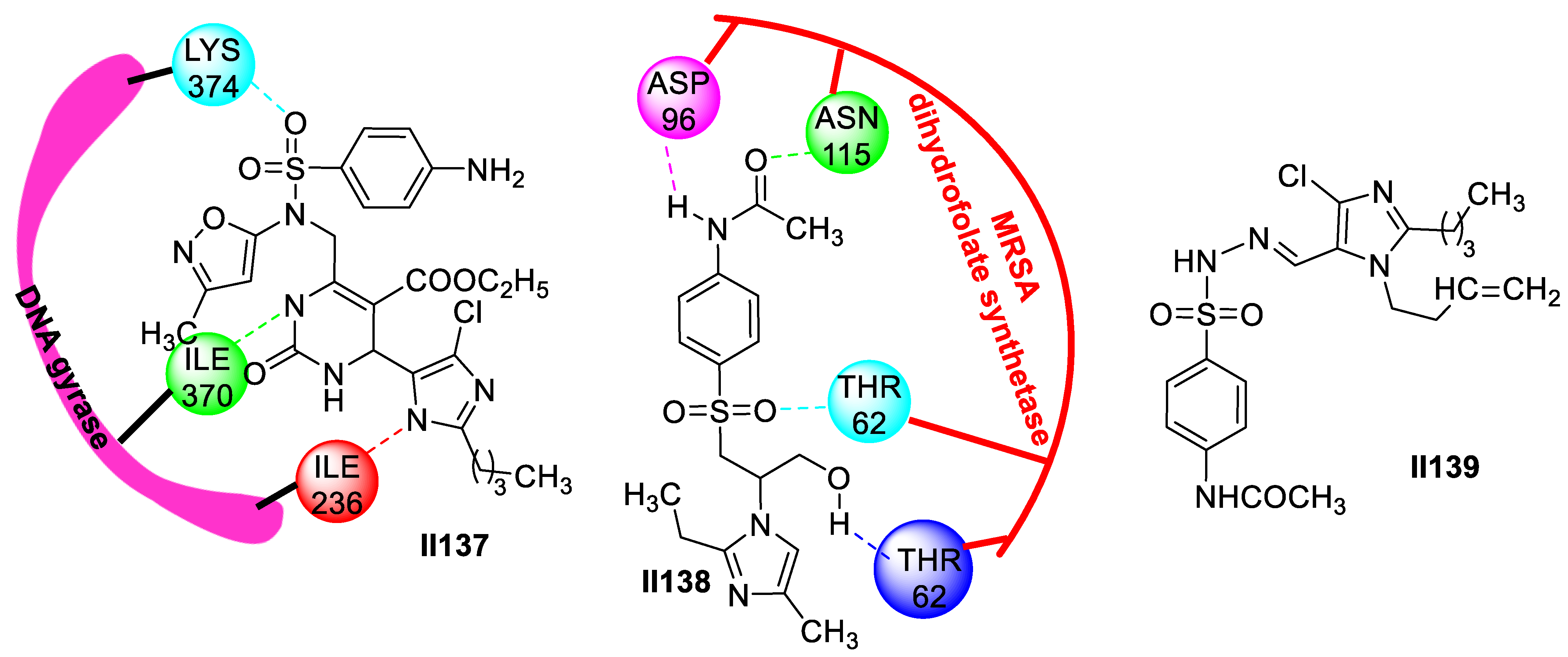
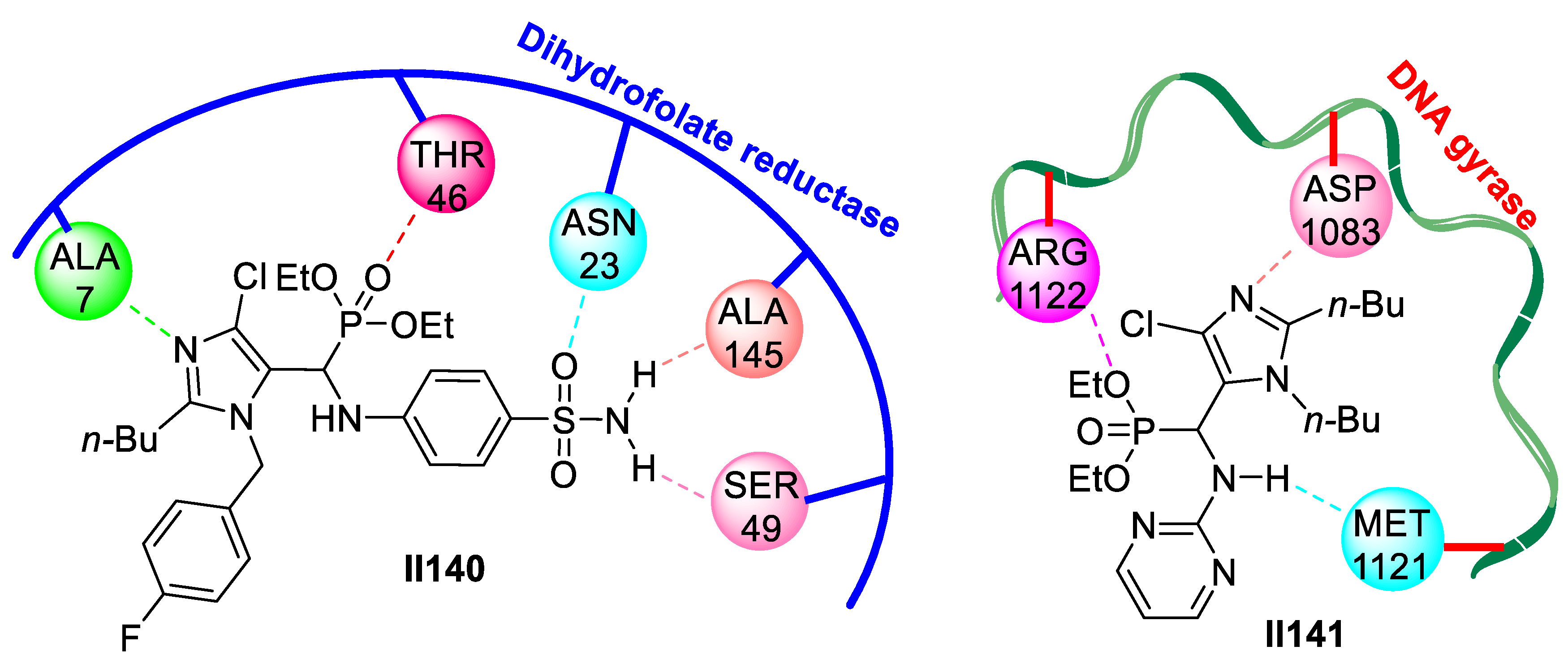


2.2.2. Benzimidazole-Based Supermolecules as Antibacterial Agents









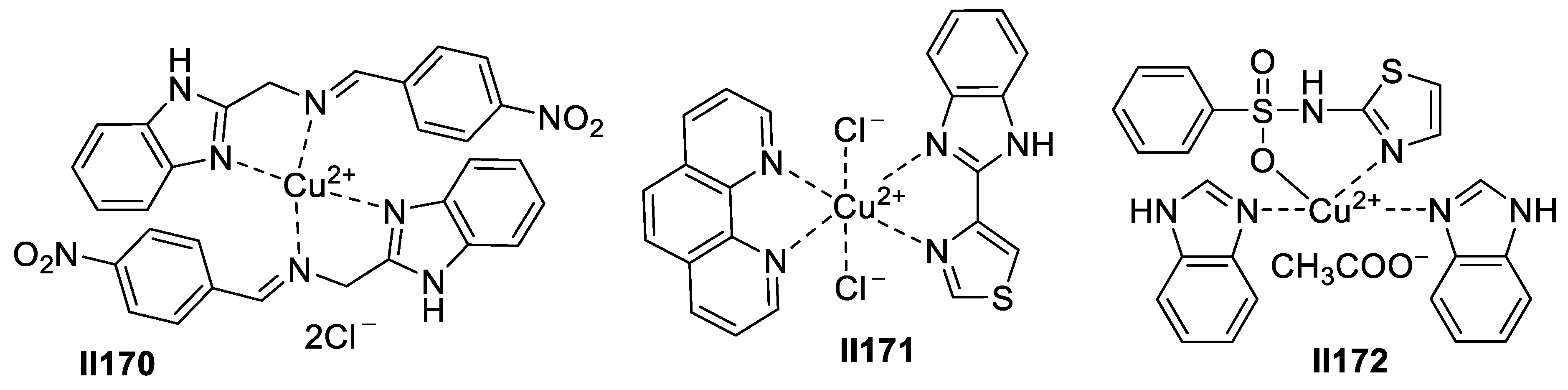

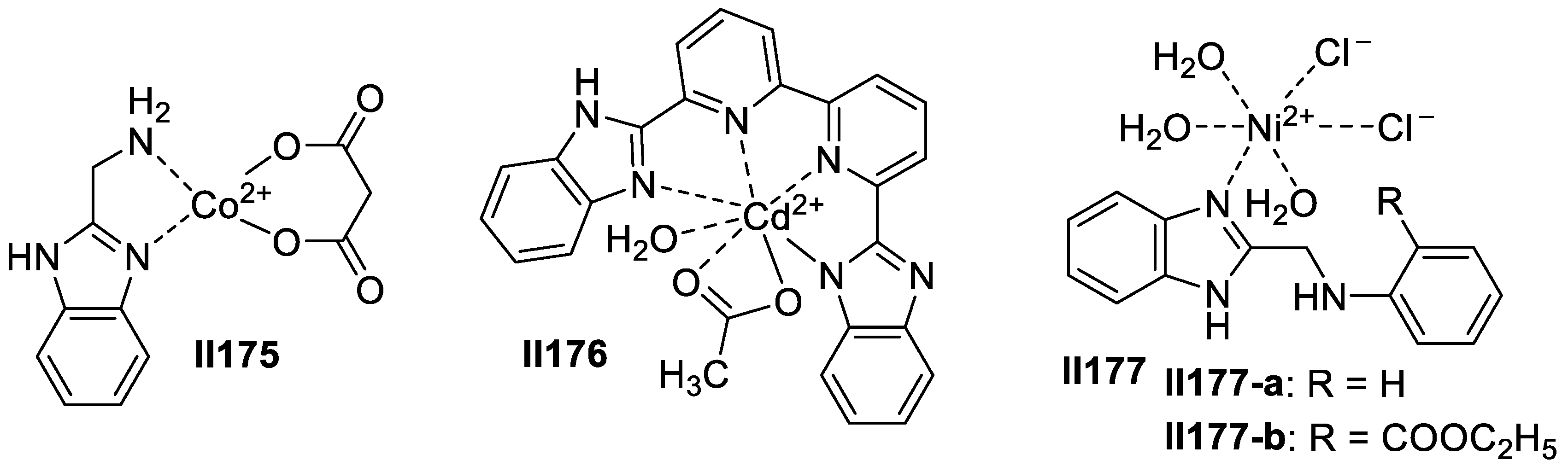







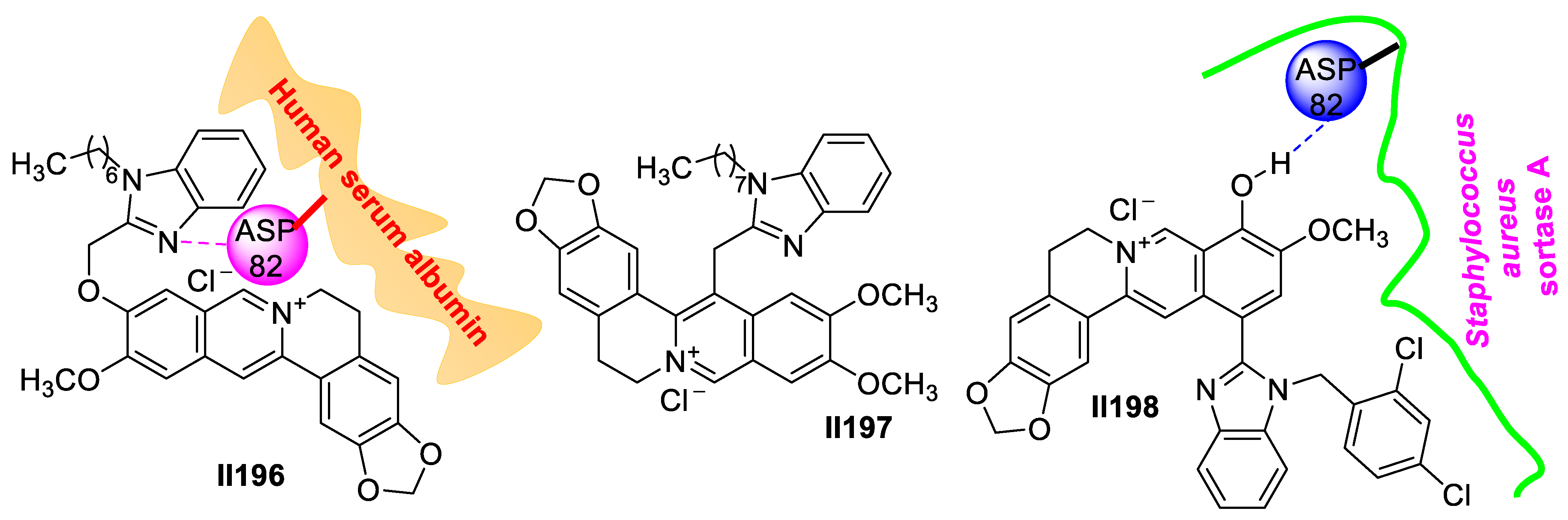

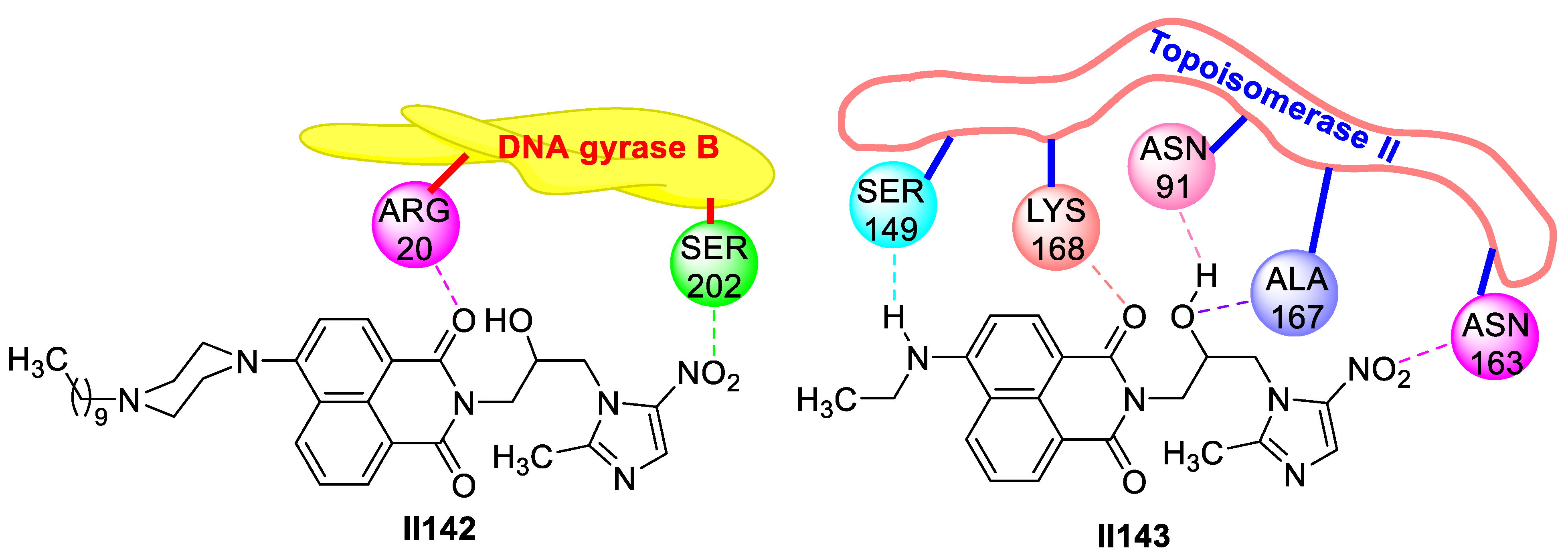
2.3. Imidazole-Based Supermolecules as Antifungal Agents



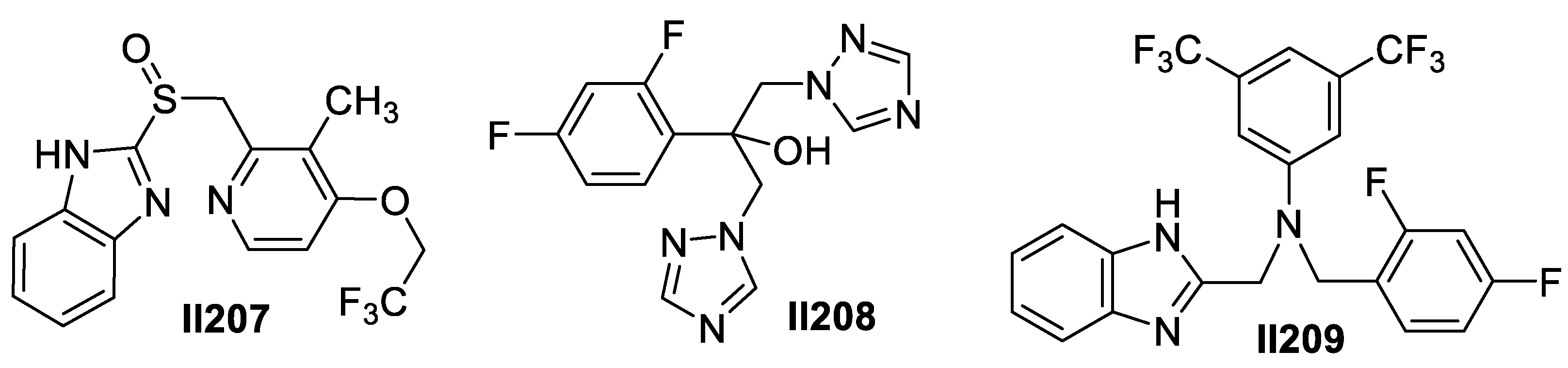


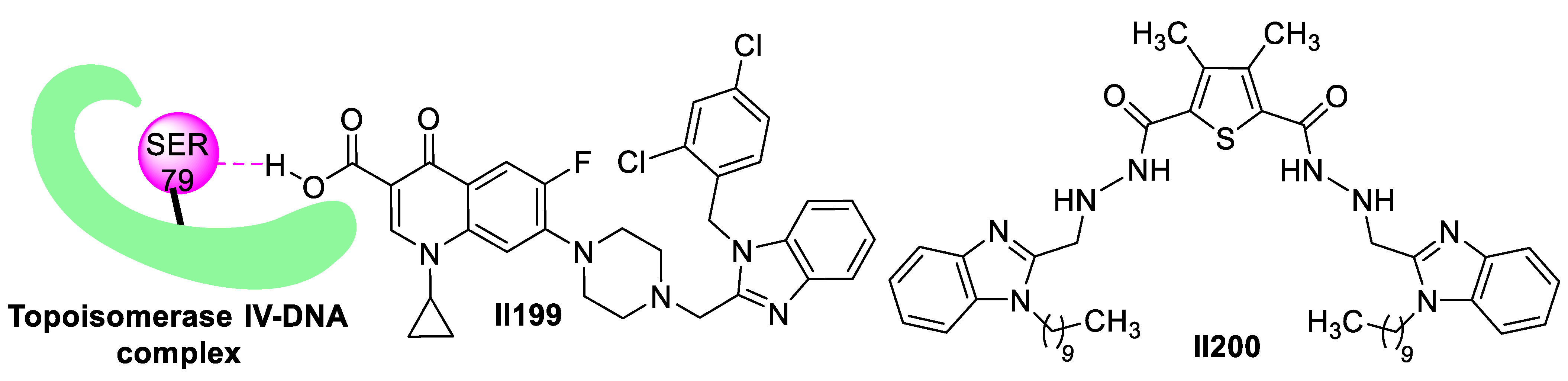
2.4. Imidazole-Based Supermolecules as Antiparasitic Agents


2.5. Imidazole-Based Supermolecules as Antidiabetic Agents

2.6. Imidazole-Based Supermolecules as Antihypertensive Agents
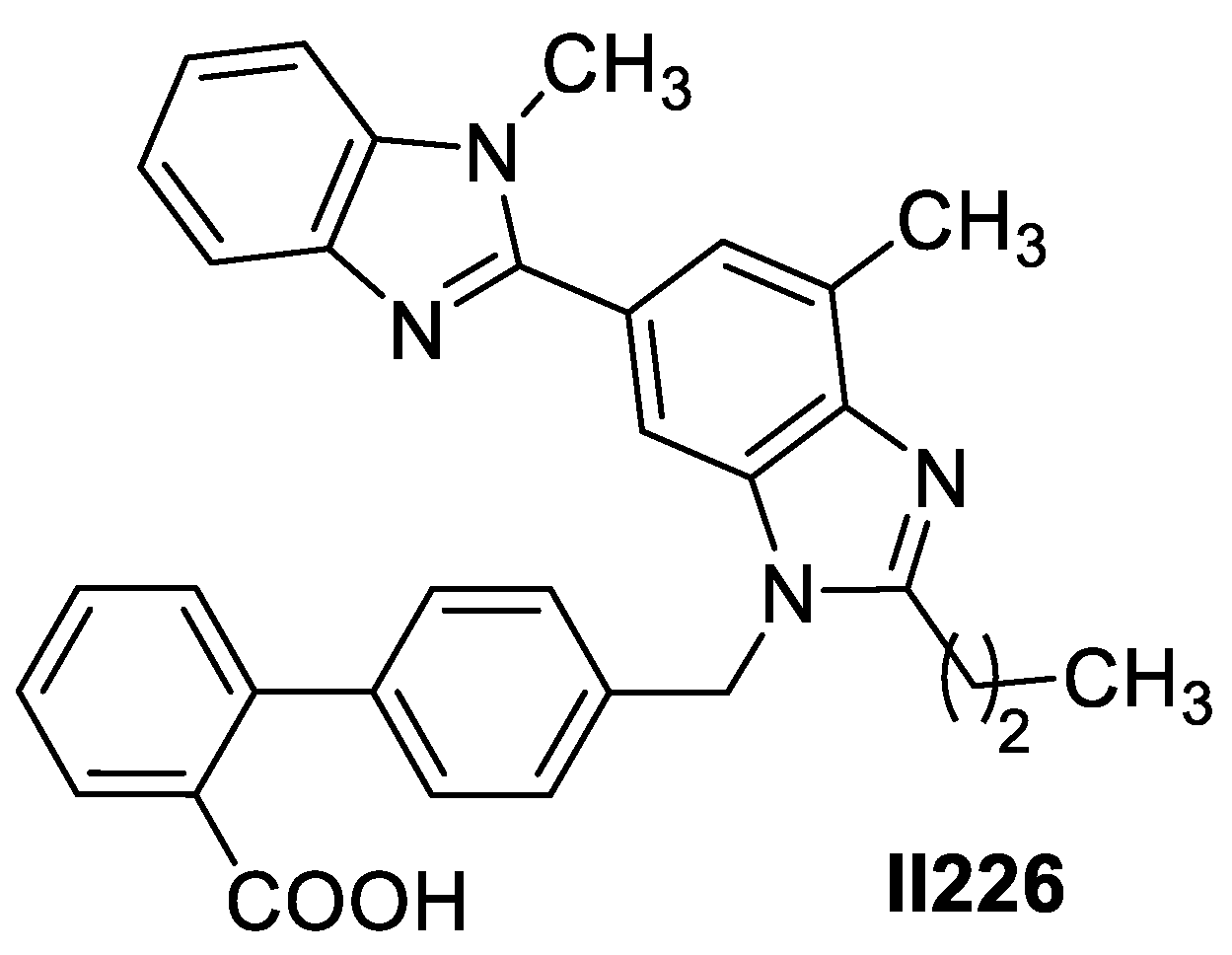
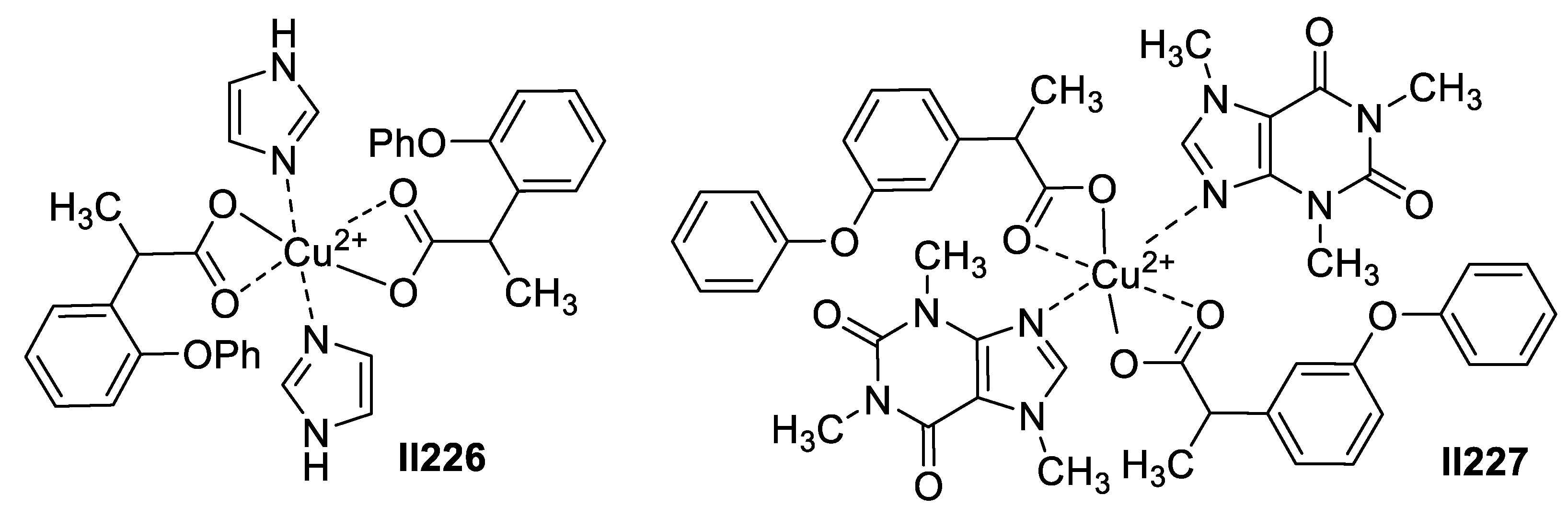
2.7. Imidazole-Based Supermolecules as Anti-Inflammatory Agents

2.8. Imidazole-Based Supermolecules as Other Medicinal Agents
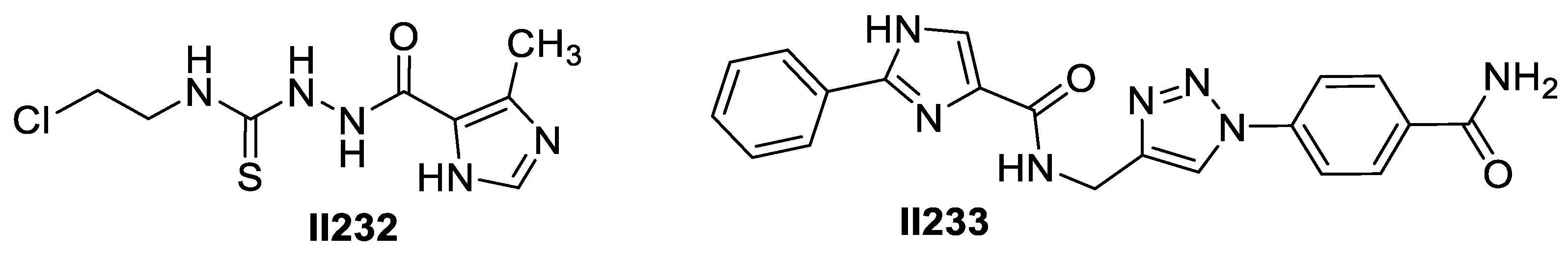
3. Imidazole-Based Supermolecules as Ion Receptors
3.1. Imidazole-Based Supermolecules as Cation Receptors
3.1.1. Imidazole-Based Supermolecules as Cation Receptors for Iron Ions
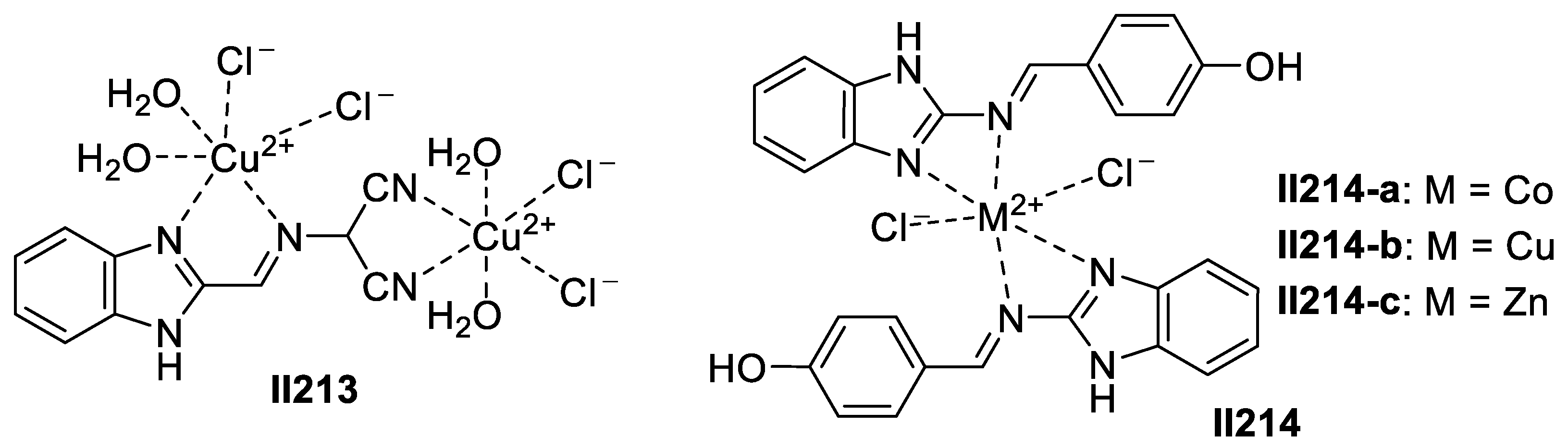
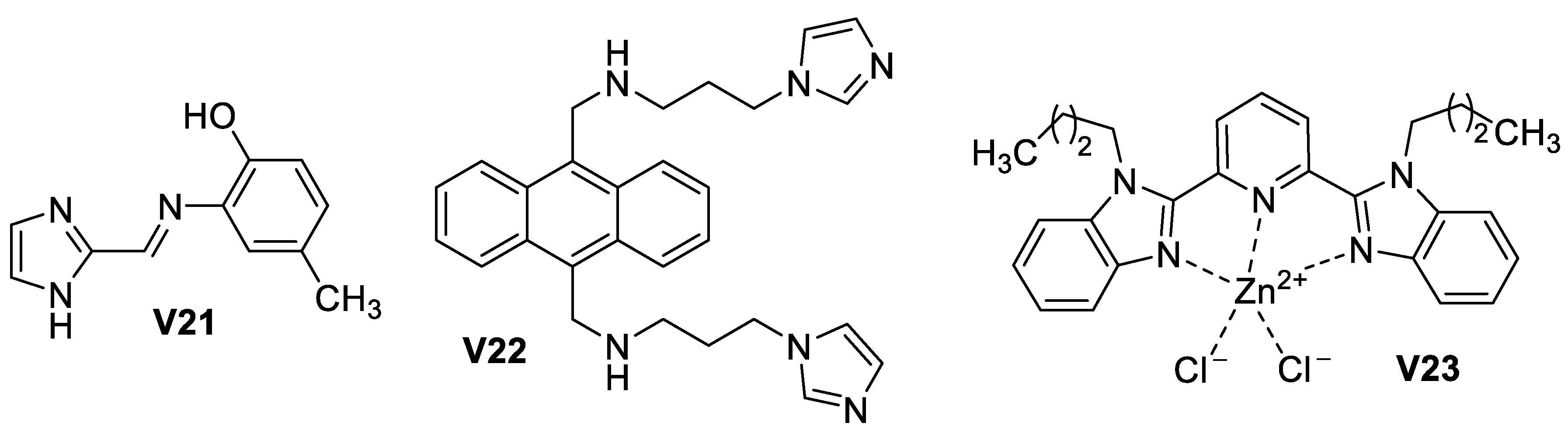
3.1.2. Imidazole-Based Supermolecules as Cation Receptors for Copper Ions


3.1.3. Imidazole-Based Supermolecules as Cation Receptors for Zinc Ions

3.1.4. Imidazole-Based Supermolecules as Cation Receptors for Mercury Ions

3.2. Imidazole-Based Supermolecules as Anion Receptors




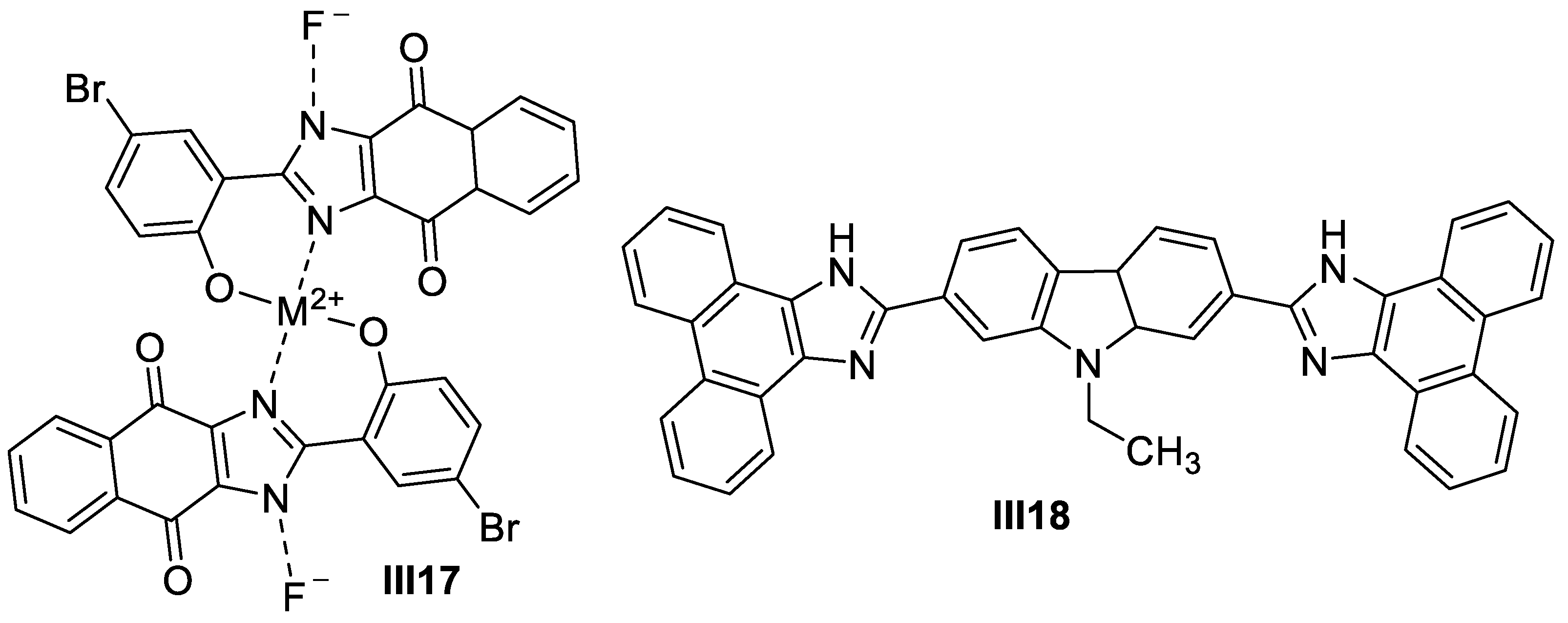

4. Imidazole-Based Supermolecules as Imaging Agents
4.1. Imidazole-Based Supermolecules as Imaging Agents for Cells


4.2. Imidazole-Based Supermolecules as Imaging Agents for Intracellular Materials

5. Imidazole-Based Supermolecules as Pathological Probes
5.1. Imidazole-Based Supermolecules as Pathological Probes toward Organelles


5.2. Imidazole-Based Supermolecules as Pathological Probes toward the Detection of Biological Active Substances
5.2.1. Imidazole-Based Supermolecules as Probes to Detect Biological Mercaptans
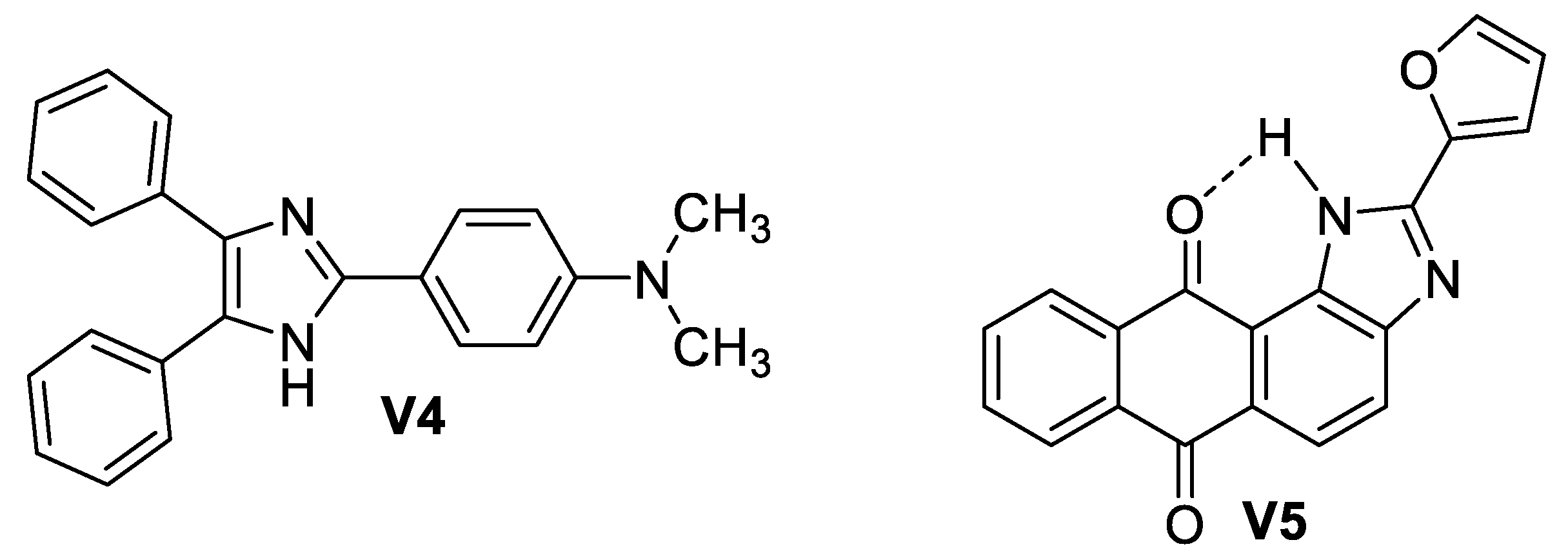
5.2.2. Imidazole-Based Supermolecules as Probes to Detect Adenine

5.2.3. Imidazole-Based Supermolecules as Probes to Detect Metal Ions In Vivo
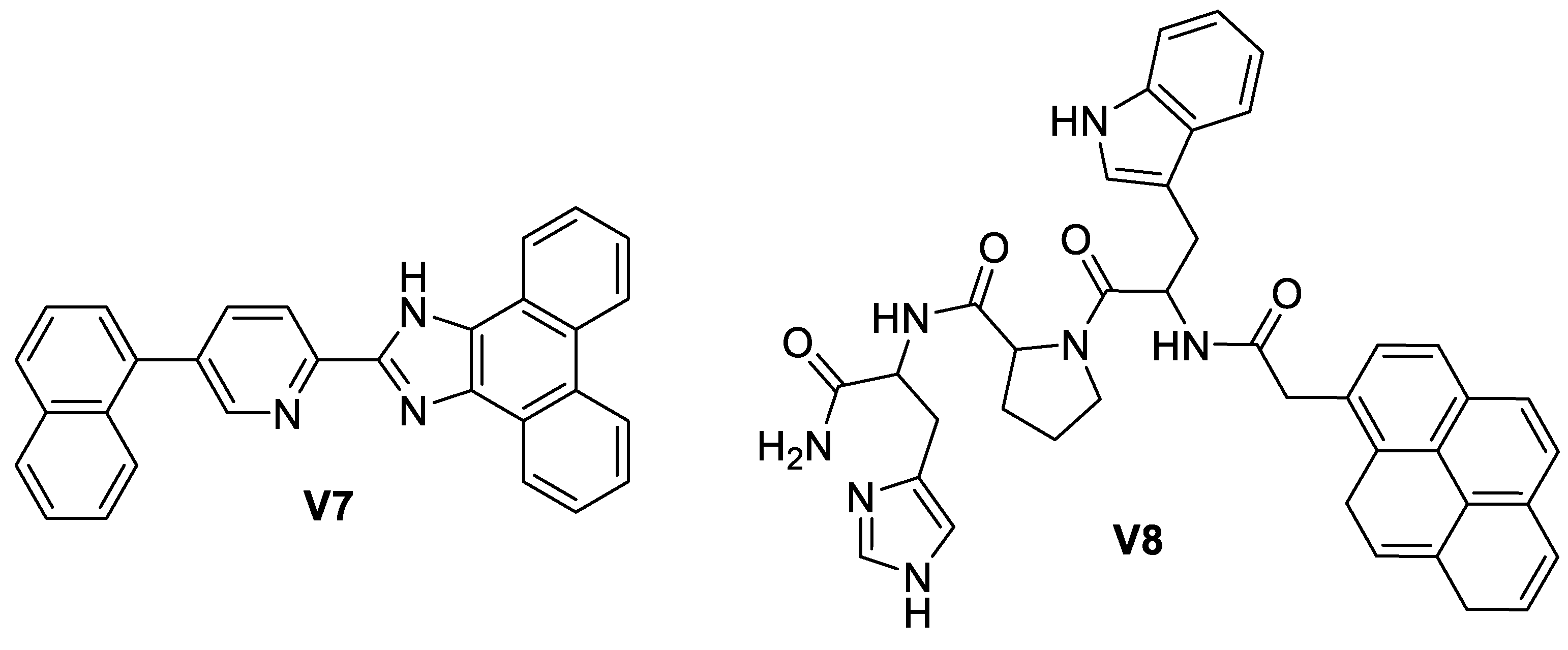

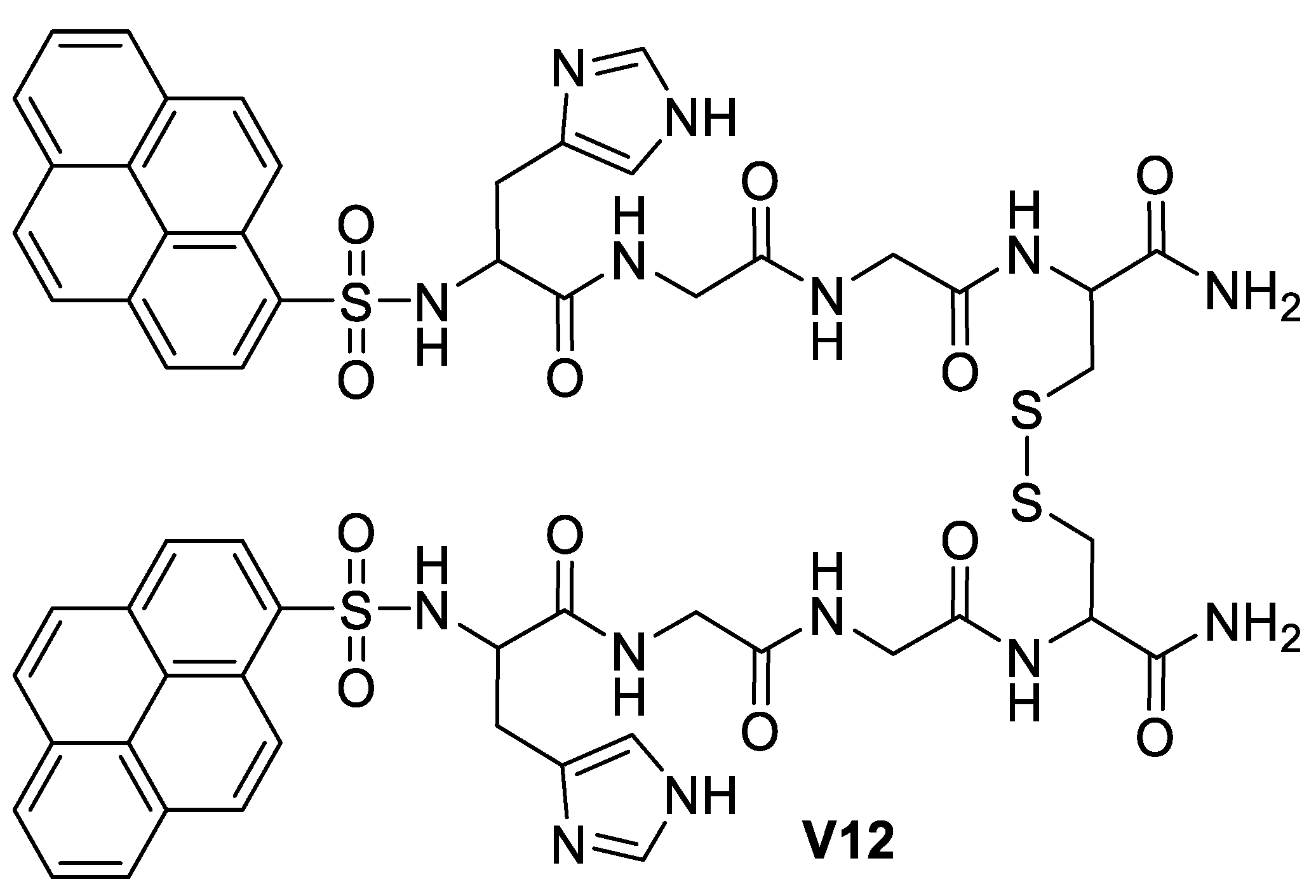
5.3. Imidazole-Based Supermolecules as Other Pathological Probes
5.3.1. Imidazole-Based Supermolecules as Probes to Detect the Change in pH
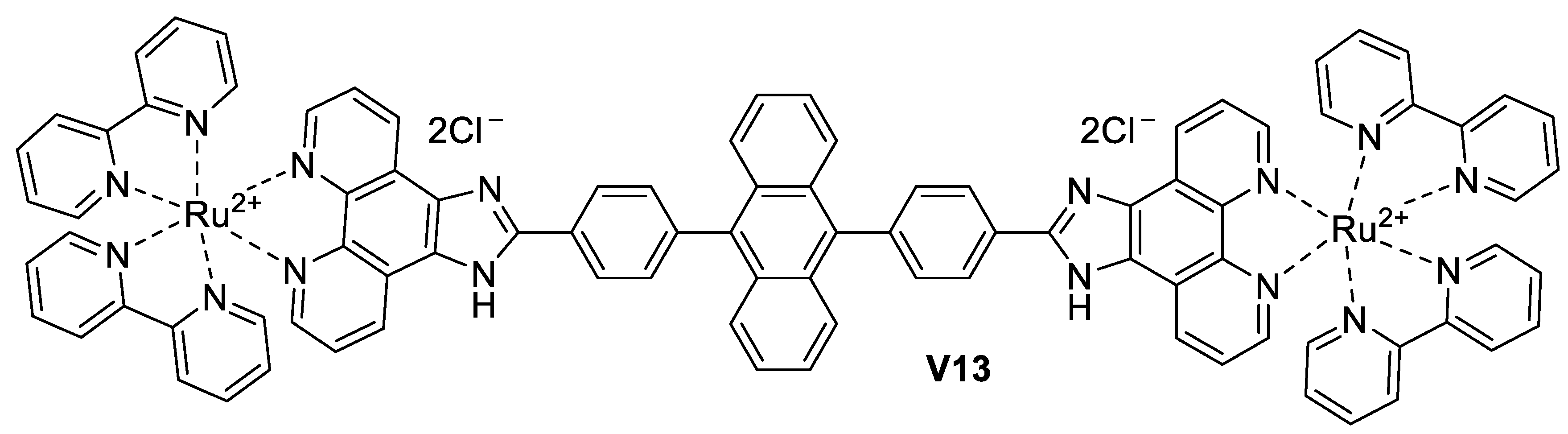
5.3.2. Imidazole-Based Supermolecules as Probes to Detect Fluoride Ions

5.3.3. Imidazole-Based Supermolecules as Probes to Detect Hydrogen Sulfide (H2S)

5.3.4. Imidazole-Based Supermolecules as Probes to Detect Pyrophosphate Ions
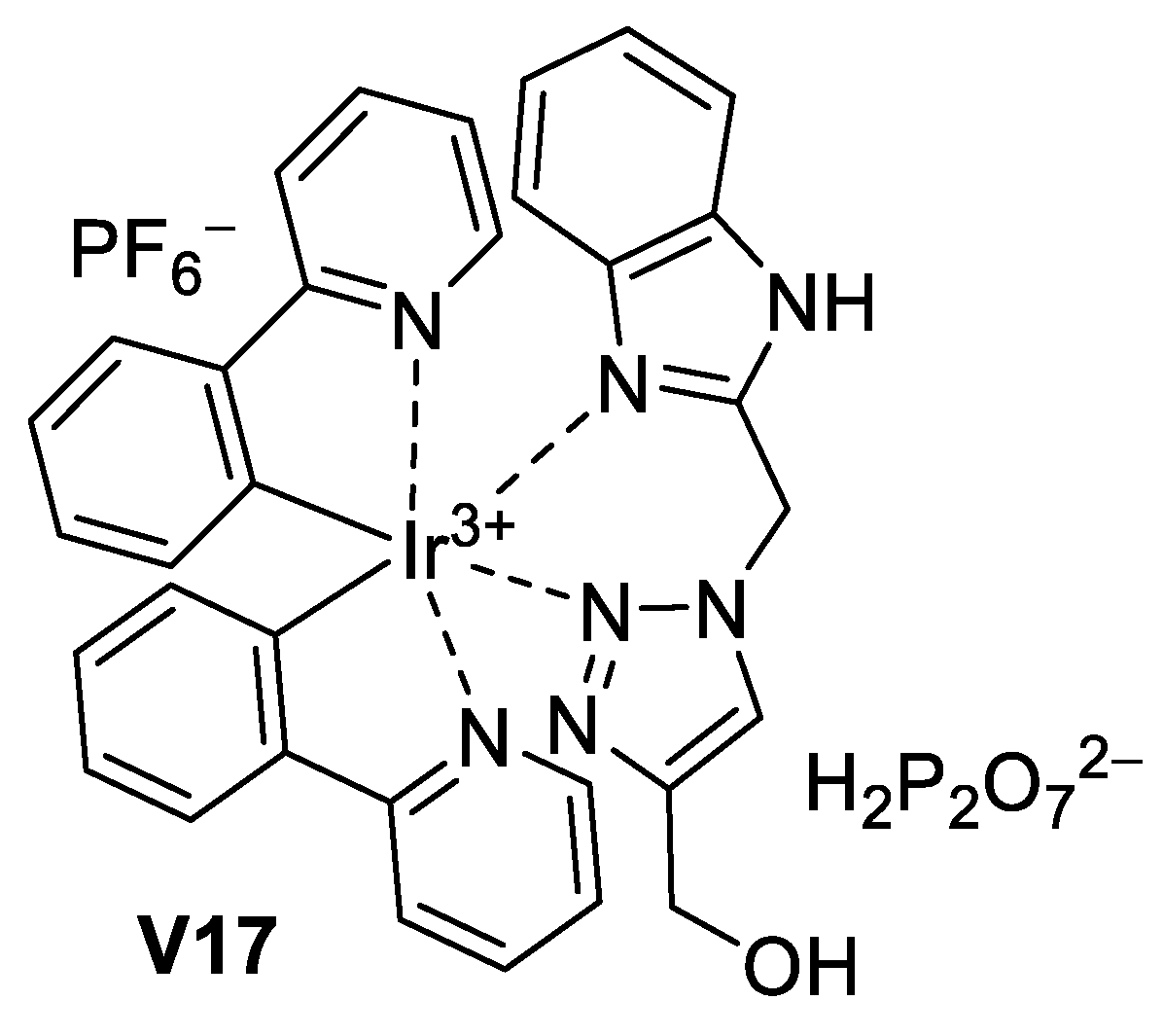
5.3.5. Imidazole-Based Supermolecules as Probes to Detect Silver Ions

5.3.6. Imidazole-Based Supermolecules as Probes to Detect Mercury Ions

5.3.7. Imidazole-Based Supermolecules as Probes to Detect Zinc Ions
5.3.8. Imidazole-Based Supermolecules as Probes to Detect Copper Ions
5.3.9. Imidazole-Based Supermolecules as Probes to Detect Iron Ions

6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, C.H.; Sui, Y.F. Supermolecules as Medicinal Drugs. In Handbook of Macrocyclic Supramolecular Assembly, 2nd ed.; Liu, Y., Chen, Y., Zhang, H.Y., Eds.; Springer: Singapore, 2020; Volume 2, pp. 1587–1633. [Google Scholar]
- Joyce, L.A.; Shabbir, S.H.; Anslyn, E.V. The uses of supramolecular chemistry in synthetic methodology development: Examples of anion and neutral molecular recognition. Chem. Soc. Rev. 2019, 39, 3621–3632. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Wang, F.; Dong, S.Y.; Huang, F.H. Supramolecular polymers constructed by crown ether-based molecular recog-nition. Chem. Soc. Rev. 2012, 41, 1621–1636. [Google Scholar] [CrossRef]
- Zhang, J.; Meng, X.G.; Zeng, X.C.; Yu, X.Q. Metallomicellar supramolecular systems and their applications in catalytic reac-tions. Coord. Chem. Rev. 2009, 253, 2166–2177. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.-Y. Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef]
- Zhou, W.L.; Lin, W.J.; Chen, Y.; Liu, Y. Supramolecular assembly confined purely organic room temperature phosphores-cence and its biological imaging. Chem. Sci. 2022, 13, 7976–7989. [Google Scholar] [CrossRef]
- Cheng, H.B.; Li, Y.Y.; Tang, B.Z.; Yoon, J. Assembly strategies of organic-based imaging agents for fluorescence and photoa-coustic bioimaging applications. Chem. Soc. Rev. 2020, 49, 21–31. [Google Scholar] [CrossRef]
- Accardo, A.; Tesauro, D.; Aloj, L.; Pedone, C.; Morelli, G. Supramolecular aggregates containing lipophilic Gd(III) complex-es as contrast agents in MRI. Coord. Chem. Rev. 2009, 253, 2193–2213. [Google Scholar] [CrossRef]
- Zhou, C.H.; Zhang, F.F.; Gan, L.L.; Zhang, Y.Y.; Geng, R.X. Research in supramolecular chemical drugs. Sci. China Ser. B Chem. 2009, 39, 208–252. [Google Scholar]
- Zhou, C.; Gan, L.; Zhang, Y.; Zhang, F.; Wang, G.; Jin, L.; Geng, R. Review on supermolecules as chemical drugs. Sci. China Chem. 2009, 52, 415–458. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, H.; Addla, D.; Zhou, C.H. Current researches and applications of azole-based supermolecules as medici-nal agents. Chin. J. Org. Chem. 2016, 36, 1–42. [Google Scholar] [CrossRef]
- Cui, S.F.; Addla, D.; Zhou, C.H. Novel 3-aminothiazolquinolones: Design, synthesis, bioactive evaluation, SARs, and pre-liminary antibacterial mechanism. J. Med. Chem. 2016, 59, 4488–4510. [Google Scholar] [CrossRef]
- Zhou, C.H.; Zhang, H.Z.; Cui, S.F.; Lv, J.S.; Yan, C.Y.; Wan, K.; Zhang, Y.Y.; Zhang, S.L.; Cai, G.X.; Geng, R.X.; et al. Recent developments in organometallic supramolecular complexes as anticancer drugs. Adv. Anticancer. Agents Med. Chem. 2013, 2, 46–129. [Google Scholar]
- Peng, X.-M.; Cai, G.-X.; Zhou, C.-H. Recent Developments in Azole Compounds as Antibacterial and Antifungal Agents. Curr. Top. Med. Chem. 2013, 13, 1963–2010. [Google Scholar] [CrossRef]
- Zhang, H.-Z.; Gan, L.-L.; Wang, H.; Zhou, C.-H. New Progress in Azole Compounds as Antimicrobial Agents. Mini-Rev. Med. Chem. 2016, 17, 122–166. [Google Scholar] [CrossRef]
- Peng, X.M.; Peng, L.P.; Avula, S.R.; Kannekanti, V.K.; Li, S.; Zhou, C.H. Quinazolinone azolyl ethanols: Potential lead anti-bacterial agents with dual action modes targeting MRSA DNA. Future Med. Chem. 2016, 8, 1927–1940. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, X.-M.; Damu, G.L.V.; Geng, R.-X.; Zhou, C.-H. Comprehensive Review in Current Developments of Imidazole-Based Medicinal Chemistry. Med. Res. Rev. 2014, 34, 340–437. [Google Scholar] [CrossRef]
- Li, Z.Z.; Kameswari, M.S.; Reddy, T.V.K.; Yadav, B.R.R.; Lin, J.M.; Yang, R.G.; Cai, G.X.; Zhou, C.H. Researches and applica-tions of nitroimidazole heterocycles in medicinal chemistry. Sci. Sin. Chim. 2019, 49, 230–255. [Google Scholar] [CrossRef]
- Wu, J.; Mi, J.L.; Zhou, C.H. Progress in research of histamine H3 receptor ligands. Chin. Pharm. J. 2007, 42, 404–409. [Google Scholar]
- Zhou, C.H.; Wang, Y. Recent researches in triazole compounds as medicinal drugs. Curr. Med. Chem. 2012, 19, 239–280. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C.H. Recent advances in the researches of triazole compounds as medicinal drugs. Sci. Sin. Chim. 2011, 41, 1429–1456. [Google Scholar] [CrossRef]
- Wei, J.J.; Zhou, C.H.; Wang, Y.; Wang, X.L.; Ji, Q.G. Research progress of 1,2,3-triazole medicinal compounds. Chin. Pharm. J. 2011, 46, 481–485. [Google Scholar]
- Chang, J.J.; Wang, Y.; Zhang, H.Z.; Zhou, C.H.; Geng, R.X.; Ji, Q.G. Recent advances in supramolecular chemistry and drugs of triazole. Chem. J. Chin. Univ. 2011, 32, 1970–1985. [Google Scholar]
- Dai, L.L.; Cui, S.F.; Guri, L.V.D.; Zhou, C.H. Recent Advances in the Synthesis and Application of Tetrazoles. Chin. J. Org. Chem. 2013, 33, 224. [Google Scholar] [CrossRef]
- Dai, L.-L.; Zhang, H.-Z.; Nagarajan, S.; Rasheed, S.; Zhou, C.-H. Synthesis of tetrazole compounds as a novel type of potential antimicrobial agents and their synergistic effects with clinical drugs and interactions with calf thymus DNA. Med. Chem. Comm. 2015, 6, 147–154. [Google Scholar] [CrossRef]
- Cui, S.F.; Wang, Y.; Lv, J.S.; Damu, G.L.V.; Zhou, C.H. Recent advances in application researches of thiazole compounds. Sci. Sin. Chim. 2012, 42, 1105–1131. [Google Scholar]
- Cui, S.F.; Zhou, C.H.; Geng, R.X.; Ji, Q.G. Recent advances in the researches on thiazole compounds as enzyme and acceptor inhibitors. Chin. J. Biochem. Pharm. 2012, 33, 311–315. [Google Scholar]
- Chen, J.-P.; Battini, N.; Ansari, M.F.; Zhou, C.-H. Membrane active 7-thiazoxime quinolones as novel DNA binding agents to decrease the genes expression and exert potent anti-methicillin-resistant Staphylococcus aureus activity. Eur. J. Med. Chem. 2021, 217, 113340. [Google Scholar] [CrossRef]
- Zhang, H.-Z.; Zhao, Z.-L.; Zhou, C.-H. Recent advance in oxazole-based medicinal chemistry. Eur. J. Med. Chem. 2018, 144, 444–492. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Zhou, C.H.; Geng, R.X.; Ji, Q.G. Recent advances in syntheses of oxazole compounds. Chin. J. Org. Chem. 2011, 31, 1963–1976. [Google Scholar]
- Meng, J.P.; Geng, R.X.; Zhou, C.H.; Gan, L.L. Advances in the research of benzimidazole drugs. Chin. J. New Drugs 2009, 18, 1505–1514. [Google Scholar]
- Meng, J.P.; Lu, Y.H.; Halqam, I.; Zhou, C.H. Advances in the research of benzimidazole compounds as enzyme inhibitors. Chin. J. Biochem. Pharm. 2008, 29, 418–421. [Google Scholar]
- Ren, Y.; Zhang, L.; Zhou, C.-H.; Geng, R.-X. Recent Development of Benzotriazole-based Medicinal Drugs. Med. Chem. 2014, 4, 640–662. [Google Scholar] [CrossRef]
- Liu, H.; Gopala, L.; Avula, S.R.; Jeyakkumar, P.; Peng, X.; Zhou, C.; Geng, R. Chalcone-Benzotriazole Conjugates as New Potential Antimicrobial Agents: Design, Synthesis, Biological Evaluation and Synergism with Clinical Drugs. Chin. J. Chem. 2017, 35, 483–496. [Google Scholar] [CrossRef]
- Zhang, F.F.; Zhou, C.H.; Yan, J.P. New progress of researches in carbazole compounds. Chin. J. Org. Chem. 2010, 30, 783–796. [Google Scholar]
- Zhang, Y.; Tangadanchu, V.K.R.; Bheemanaboina, R.R.Y.; Cheng, Y.; Zhou, C.-H. Novel carbazole-triazole conjugates as DNA-targeting membrane active potentiators against clinical isolated fungi. Eur. J. Med. Chem. 2018, 155, 579–589. [Google Scholar] [CrossRef]
- Xie, Y.-P.; Sangaraiah, N.; Meng, J.-P.; Zhou, C.-H. Unique Carbazole-Oxadiazole Derivatives as New Potential Antibiotics for Combating Gram-Positive and -Negative Bacteria. J. Med. Chem. 2022, 65, 6171–6190. [Google Scholar] [CrossRef]
- Zhang, Y.; Tangadanchu, V.K.R.; Cheng, Y.; Yang, R.-G.; Lin, J.-M.; Zhou, C.-H. Potential Antimicrobial Isopropanol-Conjugated Carbazole Azoles as Dual Targeting Inhibitors of Enterococcus faecalis. ACS Med. Chem. Lett. 2018, 9, 244–249. [Google Scholar] [CrossRef]
- He, S.-C.; Ponmani, J.; Avula, S.; Zhang, H.-Z.; Wang, X.-L.; Zhou, C.-H. Recent advance in sulfonamide-based medicinal chemistry. Sci. Sin. Chim. 2016, 46, 823–847. [Google Scholar] [CrossRef]
- Chinthakindi, P.K.; Naicker, T.; Thota, N.; Govender, T.; Kruger, H.G.; Arvidsson, P.I. Sulfonimidamides in Medicinal and Agricultural Chemistry. Angew. Chem. Int. Ed. 2017, 56, 4100–4109. [Google Scholar] [CrossRef]
- Wan, Y.; Fang, G.; Chen, H.; Deng, X.; Tang, Z. Sulfonamide derivatives as potential anti-cancer agents and their SARs elucidation. Eur. J. Med. Chem. 2021, 226, 113837. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.H.; Addla, D.; Lv, J.S.; Zhou, C.H. Heterocyclic naphthalimides as new skeleton structure of compounds with in-creasingly expanding relational medicinal applications. Curr. Top. Med. Chem. 2016, 16, 3303–3364. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Gopala, L.; Bheemanaboina, R.R.Y.; Liu, H.B.; Cheng, Y.; Geng, R.X.; Zhou, C.H. Novel naphthalimide amino-thiazoles as potential multitargeting antimicrobial agents. ACS Med. Chem. Lett. 2017, 8, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Ingrassia, L.; Lefranc, F.; Kiss, R.; Mijatovic, T. Naphthalimides and azonafides as promising anti-cancer agents. Curr. Med. Chem. 2009, 16, 1192–1213. [Google Scholar] [CrossRef]
- Peng, X.M.; Damu, G.L.V.; Zhou, C.H. Current Developments of Coumarin Compounds in Medicinal Chemistry. Curr. Pharm. Des. 2013, 19, 3884–3930. [Google Scholar] [CrossRef]
- Yang, X.-C.; Zeng, C.-M.; Avula, S.R.; Peng, X.-M.; Geng, R.-X.; Zhou, C.-H. Novel coumarin aminophosphonates as potential multitargeting antibacterial agents against Staphylococcus aureus. Eur. J. Med. Chem. 2023, 245, 114891. [Google Scholar] [CrossRef]
- Patil, S.A.; Kandathil, V.; Sobha, A.; Somappa, S.B.; Feldman, M.R.; Bugarin, A.; Patil, S.A. Comprehensive review on medic-inal applications of coumarin-derived imine-metal complexes. Molecules 2022, 27, 5220. [Google Scholar] [CrossRef]
- Qin, H.-L.; Zhang, Z.-W.; Ravindar, L.; Rakesh, K. Antibacterial activities with the structure-activity relationship of coumarin derivatives. Eur. J. Med. Chem. 2020, 207, 112832. [Google Scholar] [CrossRef]
- Gao, W.W.; Zhou, C.H. Antimicrobial 2-aminothiazolyl quinolones: What is their potential in the clinic? Future Med. Chem. 2017, 9, 1461–1464. [Google Scholar] [CrossRef]
- Cui, S.F.; Ren, Y.; Zhang, S.L.; Peng, X.M.; Damu, G.L.V.; Geng, R.X.; Zhou, C.H. Synthesis and biological evaluation of a novel class of quinolone triazoles as potential antimicrobial agents and their interactions with calf thymus DNA. Bioorg. Med. Chem. Lett. 2013, 23, 3267–3272. [Google Scholar] [CrossRef]
- Zhang, J.; Battini, N.; Ou, J.M.; Zhang, S.L.; Zhang, L.; Zhou, C.H. New efforts toward aminothiazolylquinolones with multi-targeting antibacterial potential. J. Agric. Food Chem. 2023, 71, 2322–2332. [Google Scholar] [CrossRef]
- Wang, J.; Battini, N.; Ansari, M.F.; Zhou, C. Synthesis and Biological Evaluation of Quinazolonethiazoles as New Potential Conquerors towards Pseudomonas aeruginosa. Chin. J. Chem. 2020, 39, 1093–1103. [Google Scholar] [CrossRef]
- Wang, J.; Ansari, M.F.; Zhou, C.-H. Identification of Unique Quinazolone Thiazoles as Novel Structural Scaffolds for Potential Gram-Negative Bacterial Conquerors. J. Med. Chem. 2021, 64, 7630–7645. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.C.; Wang, H.; Tangadanchu, V.K.R.; Gopala, L.; Cai, G.X.; Zhou, C.H. Design and synthesis of quinazolinone imid-azoles and their antibacterial and DNA-targeting investigation. Sci. Sin. Chim. 2017, 47, 844–858. [Google Scholar]
- Sun, H.; Huang, S.-Y.; Jeyakkumar, P.; Cai, G.-X.; Fang, B.; Zhou, C.-H. Natural Berberine-derived Azolyl Ethanols as New Structural Antibacterial Agents against Drug-Resistant Escherichia coli. J. Med. Chem. 2022, 65, 436–459. [Google Scholar] [CrossRef]
- Zhang, G.B.; Maddili, S.K.; Tangadanchu, V.K.R.; Gopala, L.; Gao, W.W.; Cai, G.X.; Zhou, C.H. Discovery of natural berberine-derived nitroimidazoles as potentially multi-targeting agents against drug-resistant Escherichia coli. Sci. China Chem. 2018, 61, 557–568. [Google Scholar] [CrossRef]
- Gao, W.-W.; Gopala, L.; Bheemanaboina, R.R.Y.; Zhang, G.-B.; Li, S.; Zhou, C.-H. Discovery of 2-aminothiazolyl berberine derivatives as effectively antibacterial agents toward clinically drug-resistant Gram-negative Acinetobacter baumanii. Eur. J. Med. Chem. 2018, 146, 15–37. [Google Scholar] [CrossRef]
- Sun, H.; Ansari, M.F.; Battini, N.; Bheemanaboina, R.R.Y.; Zhou, C.-H. Novel potential artificial MRSA DNA intercalators: Synthesis and biological evaluation of berberine-derived thiazolidinediones. Org. Chem. Front. 2019, 6, 319–334. [Google Scholar] [CrossRef]
- Li, F.-F.; Zhao, W.-H.; Tangadanchu, V.K.R.; Meng, J.-P.; Zhou, C.-H. Discovery of novel phenylhydrazone-based oxindole-thiolazoles as potent antibacterial agents toward Pseudomonas aeruginosa. Eur. J. Med. Chem. 2022, 239, 114521. [Google Scholar] [CrossRef]
- Tangadanchu, V.K.R.; Sui, Y.-F.; Zhou, C.-H. Isatin-derived azoles as new potential antimicrobial agents: Design, synthesis and biological evaluation. Bioorganic Med. Chem. Lett. 2021, 41, 128030. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C.H.; Verma, A.K.; Choi, E.H. Biomedical Importance of Indoles. Molecules 2013, 18, 6620–6662. [Google Scholar] [CrossRef]
- Sui, Y.-F.; Ansari, M.F.; Fang, B.; Zhang, S.-L.; Zhou, C.-H. Discovery of novel purinylthiazolylethanone derivatives as anti-Candida albicans agents through possible multifaceted mechanisms. Eur. J. Med. Chem. 2021, 221, 113557. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, V.; Kumaran, M.D.B.; Saravanan, R.K.; Kalaichelvan, P.T.; Verma, S. Luminescent Silver-Purine Double Helicate: Synthesis, Self-Assembly and Antibacterial Action. Chempluschem 2016, 81, 1266–1271. [Google Scholar] [CrossRef]
- Deng, Z.; Bheemanaboina, R.R.Y.; Luo, Y.; Zhou, C.H. Aloe emodin-conjugated sulfonyl hydrazones as novel type of anti-bacterial modulators against S. aureus 25923 through multifaceted synergistic effects. Bioorg. Chem. 2022, 127, 106035. [Google Scholar] [CrossRef]
- Liang, X.-Y.; Battini, N.; Sui, Y.-F.; Ansari, M.F.; Gan, L.-L.; Zhou, C.-H. Aloe-emodin derived azoles as a new structural type of potential antibacterial agents: Design, synthesis, and evaluation of the action on membrane, DNA, and MRSA DNA isomerase. RSC Med. Chem. 2021, 12, 602–608. [Google Scholar] [CrossRef]
- Deng, Z.; Sun, H.; Bheemanaboina, R.R.Y.; Luo, Y.; Zhou, C.H. Natural aloe emodin-hybridized sulfonamide aminophos-phates as novel potential membrane-perturbing and DNA-intercalating agents against Enterococcus faecalis. Bioorg. Med. Chem. Lett. 2022, 64, 128695. [Google Scholar] [CrossRef]
- Sui, Y.-F.; Li, D.; Wang, J.; Bheemanaboina, R.R.Y.; Ansari, M.F.; Gan, L.-L.; Zhou, C.-H. Design and biological evaluation of a novel type of potential multi-targeting antimicrobial sulfanilamide hybrids in combination of pyrimidine and azoles. Bioorganic Med. Chem. Lett. 2020, 30, 126982. [Google Scholar] [CrossRef]
- Tan, Y.-M.; Li, D.; Li, F.-F.; Ansari, M.F.; Fang, B.; Zhou, C.-H. Pyrimidine-conjugated fluoroquinolones as new potential broad-spectrum antibacterial agents. Bioorganic Med. Chem. Lett. 2022, 73, 128885. [Google Scholar] [CrossRef]
- Tolomeu, H.V.; Fraga, C.A.M. Imidazole: Synthesis, functionalization and physicochemical properties of a privileged struc-ture in medicinal chemistry. Molecules 2023, 28, 838. [Google Scholar] [CrossRef]
- Serli, B.; Zangrando, E.; Iengo, E.; Mestroni, G.; Yellowlees, L.; Alessio, E. Synthesis and structural, spectroscopic, and elec-trochemical characterization of new ruthenium dimethyl sulfoxide nitrosyls. Inorg. Chem. 2002, 41, 4033–4043. [Google Scholar] [CrossRef]
- Bansal, R.; Acharya, P.C. Man-Made Cytotoxic Steroids: Exemplary Agents for Cancer Therapy. Chem. Rev. 2014, 114, 6986–7005. [Google Scholar] [CrossRef]
- Zhuang, Y.Y.; Zhou, C.H.; Wang, Y.F.; Li, D.H. Research progress in antitumor drugs of nitrogen mustard. Chin. Pharm. J. 2008, 44, 1281–1287. [Google Scholar]
- Ralhan, R.; Kaur, J. Alkylating agents and cancer therapy. Expert Opin. Ther. Patents 2007, 17, 1061–1075. [Google Scholar] [CrossRef]
- Zhou, C.H.; Zhang, Y.Y.; Yan, C.Y.; Wan, K.; Gan, L.L.; Shi, Y. Recent researches in metal supramolecular complexes as anti-cancer agents. Anti Cancer Agents Med. Chem. 2010, 10, 371–395. [Google Scholar] [CrossRef]
- Frezza, M.; Hindo, S.; Chen, D.; Davenport, A.; Schmitt, S.; Tomco, D.; Dou, Q.P. Novel metals and metal complexes as plat-forms for cancer therapy. Curr. Pharm. Des. 2010, 16, 1813–1825. [Google Scholar] [CrossRef]
- Yu, K.G.; Zhou, C.H.; Li, D.H. Advances in macrocyclic drugs. Chin. Pharm. J. 2008, 43, 481–510. [Google Scholar]
- Barsoum, I.B.; Koti, M.; Siemens, D.R.; Graham, C.H. Mechanisms of Hypoxia-Mediated Immune Escape in Cancer. Cancer Res 2014, 74, 7185–7190. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef]
- Kaur, G.; Cholia, R.P.; Mantha, A.K.; Kumar, R. DNA repair and redox activities and inhibitors of apurinic/apyrimidinic endonuclease 1/redox effector factor 1 (ape1/ref-1): A comparative analysis and their scope and limitations toward anti-cancer drug development. J. Med. Chem. 2014, 57, 10241–10256. [Google Scholar] [CrossRef]
- Liu, P.; Jia, J.; Zhao, Y.; Wang, K.Z. Recent advances on dark and light-activated cytotoxity of imidazole-containing rutheni-um complexes. Mini Rev. Med. Chem. 2016, 16, 272–289. [Google Scholar] [CrossRef]
- Liu, W.-K.; Gust, R. Metal N-heterocyclic carbene complexes as potential antitumor metallodrugs. Chem. Soc. Rev. 2013, 42, 755–773. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Li, H.; Liu, C.; Xia, J.; Ma, L.; Chu, W.; Zhang, Z.; Chen, C.; Li, S.; et al. Recent Progress and Future Potential for Metal Complexes as Anticancer Drugs Targeting G-quadruplex DNA. Curr. Med. Chem. 2012, 19, 2957–2975. [Google Scholar] [CrossRef]
- Ceresa, C.; Bravin, A.; Cavaletti, G.; Pellei, M.; Santini, C. The combined therapeutical effect of metal-based drugs and radia-tion therapy: The present status of research. Curr. Med. Chem. 2014, 21, 2237–2265. [Google Scholar] [CrossRef]
- Shaili, E. Platinum Anticancer Drugs and Photochemotherapeutic Agents: Recent Advances and Future Developments. Sci. Prog. 2014, 97, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Guo, Z.J. Targeting and delivery of platinum-based anticancer drugs. Chem. Soc. Rev. 2013, 42, 202–224. [Google Scholar] [CrossRef]
- Rimoldi, I.; Facchetti, G.; Lucchini, G.; Castiglioni, E.; Marchianò, S.; Ferri, N. In Vitro anticancer activity evaluation of new cationic platinum(II) complexes based on imidazole moiety. Bioorganic Med. Chem. 2017, 25, 1907–1913. [Google Scholar] [CrossRef]
- Sen, C.; Patra, C.; Mondol, S.; Datta, A.; Mallick, D.; Mondal, T.K.; Askun, T.; Celikboyun, P.; Canturk, Z.; Sinha, C. Plati-num(II)-azoimidazole drugs against TB and cancer: Structural studies, cytotoxicity and anti-mycobacterial activity. Polyhedron 2018, 152, 1–10. [Google Scholar] [CrossRef]
- Bian, M.; Fan, R.; Yang, Z.; Chen, Y.; Xu, Z.; Lu, Y.; Liu, W. Pt(II)-NHC Complex Induces ROS-ERS-Related DAMP Balance to Harness Immunogenic Cell Death in Hepatocellular Carcinoma. J. Med. Chem. 2022, 65, 1848–1866. [Google Scholar] [CrossRef]
- Czarnomysy, R.; Muszynska, A.; Rok, J.; Rzepka, Z.; Bielawski, K. Mechanism of anticancer action of novel imidazole plati-num(ii) complex conjugated with G2 PAMAM-OH dendrimer in breast cancer cells. Int. J. Mol. Sci. 2021, 22, 5581. [Google Scholar] [CrossRef]
- Rehm, T.; Rothemund, M.; Muenzner, J.K.; Noor, A.; Kempe, R.; Schobert, R. Novel cis-[(NHC)1(NHC)2(L)Cl]platinum(ii) complexes synthesis, structures, and anticancer activities. Dalton Trans. 2016, 45, 15390–15398. [Google Scholar] [CrossRef]
- Ghani, N.T.A.; Mansour, A.M. Molecular structure of 2-chloromethyl-1H-benzimidazole hydrochloride: Single crystal, spectral, biological studies, and DFT calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 86, 605–613. [Google Scholar] [CrossRef]
- Utku, S.; Gumus, F.; Tezcan, S.; Serin, M.S.; Ozkul, A. Synthesis, characterization, cytotoxicity, and DNA binding of some new platinum(II) and platinum(IV) complexes with benzimidazole ligands. J. Enzym. Inhib. Med. Chem. 2010, 25, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Özçelik, A.B.; Utku, S.; Gümüs, F.; Keskin, A.; Açık, L.; Yılmaz, S.; Özgüngör, A. Cytotoxicity and DNA interactions of some platinum(II) complexes with substituted benzimidazole ligands. J. Enzym. Inhib. Med. Chem. 2012, 27, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Nardon, C.; Boscutti, G.; Fregona, D. Beyond platinums: Gold complexes as anticancer agents. Anticancer. Res. 2014, 34, 487–492. [Google Scholar]
- Aher, S.B.; Muskawar, P.N.; Thenmozhi, K.; Bhagat, P.R. Recent developments of metal N-heterocyclic carbenes as anti-cancer agents. Eur. J. Med. Chem. 2014, 81, 408–419. [Google Scholar] [CrossRef]
- Skander, M.; Retailleau, P.; Bourrié, B.; Schio, L.; Mailliet, P.; Marinetti, A. N-Heterocyclic Carbene-Amine Pt(II) Complexes, a New Chemical Space for the Development of Platinum-Based Anticancer Drugs. J. Med. Chem. 2010, 53, 2146–2154. [Google Scholar] [CrossRef]
- Schuh, E.; Pflüger, C.; Citta, A.; Folda, A.; Rigobello, M.P.; Bindoli, A.; Casini, A.; Mohr, F. Gold(I) carbene complexes caus-ing thioredoxin 1 and thioredoxin 2 oxidation as potential anticancer agents. J. Med. Chem. 2012, 55, 5518–5528. [Google Scholar] [CrossRef]
- Fung, S.K.; Zou, T.T.; Cao, B.; Lee, P.Y.; Fung, Y.M.E.; Hu, D.; Lok, C.N.; Che, C.M. Cyclometalated gold(III) complexes con-taining N-heterocyclic carbene ligands engage multiple anti-cancer molecular targets. Angew. Chem. Int. Edit. 2017, 56, 3892–3896. [Google Scholar] [CrossRef]
- Gambini, V.; Tilio, M.; Maina, E.W.; Andreani, C.; Bartolacci, C.; Wang, J.; Iezzi, M.; Ferraro, S.; Ramadori, A.T.; Simon, O.C.; et al. In Vitro and In Vivo studies of gold(I) azolate/phosphane complexes for the treatment of basal like breast cancer. Eur. J. Med. Chem. 2018, 155, 418–427. [Google Scholar] [CrossRef]
- Dada, O.; Sánchez-Sanz, G.; Tacke, M.; Zhu, X. Synthesis and anticancer activity of novel NHC-gold(I)-sugar complexes. Tetrahedron Lett. 2018, 59, 2904–2908. [Google Scholar] [CrossRef]
- Trommenschlager, A.; Chotard, F.; Bertrand, B.; Amor, S.; Dondaine, L.; Picquet, M.; Richard, P.; Bettaieb, A.; Gendre, P.L.; Paul, C.; et al. Gold(I)-BODIPY-imidazole bimetallic complexes as new potential anti-inflammatory and anti-cancer trackable agents. Dalton Trans. 2017, 46, 8051–8056. [Google Scholar] [CrossRef]
- Messori, L.; Marchetti, L.; Massai, L.; Scaletti, F.; Guerri, A.; Landini, I.; Nobili, S.; Perrone, G.; Mini, E.; Leoni, P.; et al. Chemistry and Biology of Two Novel Gold(I) Carbene Complexes as Prospective Anticancer Agents. Inorg. Chem. 2014, 53, 2396–2403. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.M.; Liu, Y.H.; Zhang, Z.B. Heteroleptic Gold(I)-bisNHC complex with excellent activity In Vitro, ex vivo and In Vivo against endometrial cancer. Eur. J. Med. Chem. 2022, 236, 114302. [Google Scholar] [CrossRef] [PubMed]
- Bian, M.L.; Fan, R.; Jiang, G.Z.; Wang, Y.X.; Lu, Y.L.; Liu, W.K. Halo and pseudohalo gold(I)-NHC complexes derived from 4,5-diarylimidazoles with excellent In Vitro and In Vivo anticancer activities against HCC. J. Med. Chem. 2020, 63, 9197–9211. [Google Scholar] [CrossRef]
- Baron, M.; Bellemin-Laponnaz, S.; Tubaro, C.; Basato, M.; Bogialli, S.; Dolmella, A. Synthesis and biological assays on cancer cells of dinuclear gold complexes with novel functionalised di(N-heterocyclic carbene) ligands. J. Inorg. Biochem. 2014, 141, 94–102. [Google Scholar] [CrossRef]
- Sivaram, H.; Tan, J.; Huynh, H.V. Cationic gold(I) heteroleptic complexes bearing a pyrazole-derived N-heterocyclic car-bene: Syntheses, characterizations, and cytotoxic activities. Dalton Trans. 2013, 42, 12421–12428. [Google Scholar] [CrossRef]
- Hu, C.; Li, X.; Wang, W.; Zhang, R.; Deng, L. Metal-N-heterocyclic carbene complexes as anti-tumor agents. Curr. Med. Chem. 2014, 21, 1220–1230. [Google Scholar] [CrossRef]
- Mohamed, H.A.; Shepherd, S.; William, N.; Blundell, H.A.; Das, M.; Pask, C.M.; Lake, B.R.M.; Phillips, R.M.; Nelson, A.; Willans, C.E. Silver(I) N-Heterocyclic Carbene Complexes Derived from Clotrimazole: Antiproliferative Activity and Interaction with an Artificial Membrane-Based Biosensor. Organometallics 2020, 39, 1318–1331. [Google Scholar] [CrossRef]
- Haque, R.A.; Budagumpi, S.; Zulikha, H.Z.; Hasanudin, N.; Ahamed, M.B.K.; Majid, A.M.S.A. Silver(I)-N-heterocyclic carbene complexes of nitrile-functionalized imidazol-2-ylidene ligands as anticancer agents. Inorg. Chem. Commun. 2014, 44, 128–133. [Google Scholar] [CrossRef]
- Gandin, V.; Pellei, M.; Marinelli, M.; Marzano, C.; Dolmella, A.; Giorgetti, M.; Santini, C. Synthesis and In Vitro antitumor activity of water-soluble sulfonate—And ester-functionalized silver(I) N-heterocyclic carbene complexes. J. Inorg. Biochem. 2013, 129, 135–144. [Google Scholar] [CrossRef]
- Pellei, M.; Gandin, V.; Marinelli, M.; Marzano, C.; Yousufuddin, M.; Dias, H.V.R.; Santini, C. Synthesis and biological activity of ester—And amide-functionalized imidazolium salts and related water-soluble coinage metal N-heterocyclic carbene complexes. Inorg. Chem. 2012, 51, 9873–9882. [Google Scholar] [CrossRef]
- Zulikha, H.Z.; Haque, R.A.; Budagumpi, S.; Majid, A.M.A. Topology control in nitrile-functionalized silver(I)-N-heterocyclic carbene complexes: Synthesis, molecular structures, and In Vitro anticancer studies. Inorg. Chim. Acta 2014, 411, 40–47. [Google Scholar] [CrossRef]
- Haque, R.A.; Ghdhayeb, M.Z.; Salman, A.W.; Budagumpi, S.; Ahamed, M.B.K.; Majid, A.M.A. Ag(I)-N-heterocyclic carbene complexes of N-allyl substituted imidazol-2-ylidenes with ortho-, meta- and para-xylyl spacers: Synthesis, crystal structures and In Vitro anticancer studies. Inorg. Chem. Commun. 2012, 22, 113–119. [Google Scholar] [CrossRef]
- Haque, R.A.; Hasanudin, N.; Iqbal, M.A.; Ahmad, A.; Hashim, S.; Majid, A.A.; Ahamed, M.B.K. Synthesis, crystal structures, In Vitro anticancer, and In Vivo acute oral toxicity studies of bis-imidazolium/benzimidazolium salts and respective dinu-clear Ag(I)-N-heterocyclic carbene complexes. J. Coord. Chem. 2013, 66, 3211–3228. [Google Scholar] [CrossRef]
- Shahini, C.R.; Achar, G.; Budagumpi, S.; Tacke, M.; Patil, S.A. Non-symmetrically p -nitrobenzyl-substituted N -heterocyclic carbene-silver(I) complexes as metallopharmaceutical agents. Appl. Organomet. Chem. 2017, 31, e3819. [Google Scholar] [CrossRef]
- Fabbrini, M.G.; Cirri, D.; Pratesi, A.; Ciofi, L.; Marzo, T.; Guerri, A.; Nistri, S.; Dell’Accio, A.; Gamberi, T.; Severi, M.; et al. A Fluorescent Silver(I) Carbene Complex with Anticancer Properties: Synthesis, Characterization, and Biological Studies. Chemmedchem 2019, 14, 182–188. [Google Scholar] [CrossRef]
- Carrasco, C.J.; Montilla, F.; Álvarez, E.; Calderón-Montaño, J.M.; López-Lázaro, M.; Galindo, A. Chirality influence on the cytotoxic properties of anionic chiral bis(N-heterocyclic carbene)silver complexes. J. Inorg. Biochem. 2022, 235, 111924. [Google Scholar] [CrossRef]
- Stryjska, K.; Radko, L.; Chęcińska, L.; Kusz, J.; Posyniak, A.; Ochocki, J. Synthesis, Spectroscopy, Light Stability, Single-Crystal Analysis, and In Vitro Cytotoxic Activity on HepG2 Liver Cancer of Two Novel Silver(I) Complexes of Miconazole. Int. J. Mol. Sci. 2020, 21, 3629. [Google Scholar] [CrossRef]
- Kutlu, T.; Yildirim, I.; Karabiyik, H.; Kilincli, A.; Tekedereli, I.; Gok, Y.; Dikmen, M.; Aktas, A. Cytotoxic activity and apop-tosis induction by a series Ag(I)-NHC complexes on human breast cancer cells and non-tumorigenic epithelial cell line. J. Mol. Struct. 2021, 1228, 129462. [Google Scholar] [CrossRef]
- Çevik-Yıldız, E.; Şahin, N.; Şahin-Bölükbaşı, S. Synthesis, characterization, and investigation of antiproliferative activity of novel Ag (I)-N-Heterocyclic Carbene (NHC) compounds. J. Mol. Struct. 2019, 1199, 126987. [Google Scholar] [CrossRef]
- Sahin, N.; Sahin-Bolukbasi, S.; Marsan, H. Synthesis and antitumor activity of new silver(I)-N-heterocyclic carbene com-plexes. J. Coord. Chem. 2019, 72, 3602–3613. [Google Scholar] [CrossRef]
- Habib, A.; Nazari, V.M.; Iqbal, M.A.; Bhatti, H.N.; Ahmed, M.B.K.; Majid, A.M.S.A. Unsymmetrically substituted benzimid-azolium based Silver(I)-N-heterocyclic carbene complexes: Synthesis, characterization and In Vitro anticancer study against human breast cancer and colon cancer. J. Saudi Chem. Soc. 2019, 23, 795–808. [Google Scholar] [CrossRef]
- Fatima, T.; Haque, R.A.; Razali, M.R.; Ahmad, A.; Iqbal, M.A.; Asif, M.; Ahamed, M.B.K.; Majid, A.M.S.A. Synthesis, crystal structure, In Vitro anticancer and In Vivo acute oral toxicity studies of tetramethylene linked bis-benzimidazolium salts and their respective dinuclear Ag(I)–NHC complexes. J. Coord. Chem. 2016, 69, 3367–3383. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Haque, R.A.; Ahamed, M.B.K.; Majid, A.M.S.A.; Al-Rawi, S.S. Synthesis and anticancer activity of para-xylyl linked bis-benzimidazolium salts and respective Ag(I) N-heterocyclic carbene complexes. Med. Chem. Res. 2013, 22, 2455–2466. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Bernès, S.; Hernández, G.; Portillo, O.; Moreno, G.E.; Sharma, M.; Sharma, P.; Gutiérrez, R. New chiral α-ketoimine-Pd(II) complexes and their anticancer activity. J. Coord. Chem. 2015, 68, 3805–3813. [Google Scholar] [CrossRef]
- Hou, X.L.; Li, X.B.; Hemit, H.; Aisa, H.A. Synthesis, characterization, and antitumor activities of new palladium(II) complex-es with 1-(alkyldithiocarbonyl)imidazoles. J. Coord. Chem. 2014, 67, 461–469. [Google Scholar] [CrossRef]
- Ramezanpour, A.; Karami, K.; Kharaziha, M.; Zakariazadeh, M.; Lipkowski, J.; Shahpiri, A.; Azizi, N.; Namazian, M. A mononuclear PdII complex with Naphcon; crystal structure, experimental and computational studies of the interaction with DNA/BSA and evaluation of anticancer activity. Polyhedron 2021, 206, 115333. [Google Scholar] [CrossRef]
- El-Sherif, A.A. Synthesis and characterization of some potential antitumor palladium(II) complexes of 2-aminomethylbenzimidazole and amino acids. J. Coord. Chem. 2011, 64, 2035–2055. [Google Scholar] [CrossRef]
- Bernd, M.A.; Bauer, E.B.; Oberkofler, J.; Bauer, A.; Reich, R.M.; Kühn, F.E. Macrocyclic NHC complexes of group 10 elements with enlarged aromaticity for biological studies. Dalton Trans. 2020, 49, 14106–14114. [Google Scholar] [CrossRef]
- Muhammad, N.; Guo, Z. Metal-based anticancer chemotherapeutic agents. Curr. Opin. Chem. Biol. 2014, 19, 144–153. [Google Scholar] [CrossRef]
- Xu, G.; Cui, Y.B.; Cui, K.; Gou, S.H. Progress on the study of non-platinum metallic drugs in antitumor. Prog. Chem. 2006, 18, 107–113. [Google Scholar]
- van Rijt, S.H.; Sadler, P.J. Current applications and future potential for bioinorganic chemistry in the development of anticancer drugs. Drug Discov. Today 2009, 14, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Hambley, T.W. Developing new metal-based therapeutics: Challenges and opportunities. Dalton Trans. 2007, 43, 4929–4937. [Google Scholar] [CrossRef] [PubMed]
- Rademaker-Lakhai, J.M.; van den Bongard, D.; Pluim, D.; Beijnen, J.H.; Schellens, J.H.M. A phase I and pharmacological study with imidazolium-trans-DMSO-imidazole-tetrachlororuthenate, a novel ruthenium anticancer agent. Clin. Cancer Res. 2004, 10, 3717–3727. [Google Scholar] [CrossRef]
- He, L.; Liao, S.Y.; Tan, C.P.; Ye, R.R.; Xu, Y.W.; Zhao, M.; Ji, L.N.; Mao, Z.W. Ruthenium-arene-carboline complexes as potent inhibitors of cyclin-dependent kinase1: Synthesis, characterization and anticancer mechanism studies. Chem. Eur. J. 2013, 19, 12152–12160. [Google Scholar] [CrossRef]
- Acharya, S.; Ghosh, S.; Maji, M.; Parambil, A.R.U.; Singh, S.; Mukherjee, A. Inhibition of 3D colon cancer stem cell spheroids by cytotoxic RuII-p-cymene complexes of mesalazine derivatives. Chem. Commun. 2020, 56, 5421–5424. [Google Scholar] [CrossRef]
- Yang, Y.L.; Guo, L.H.; Tian, Z.Z.; Liu, X.C.; Gong, Y.T.; Zheng, H.M.; Ge, X.X.; Liu, Z. Imine-N-heterocyclic carbenes as versa-tile ligands in ruthenium(II) p-cymene anticancer complexes: A structure-activity relationship study. Chem. Asian J. 2018, 13, 2923–2933. [Google Scholar] [CrossRef]
- Huber, W.; Bröhler, P.; Wätjen, W.; Frank, W.; Spingler, B.; Kunz, P.C. Cytotoxicity of ruthenium(II) piano-stool complexes with imidazole-based PN ligands. J. Organomet. Chem. 2012, 717, 187–194. [Google Scholar] [CrossRef]
- Morais, T.S.; Marques, F.; Madeira, P.J.A.; Robalo, M.P.; Garcia, M.H. Design and Anticancer Properties of New Water-Soluble Ruthenium–Cyclopentadienyl Complexes. Pharmaceuticals 2022, 15, 862. [Google Scholar] [CrossRef]
- Lv, J.-S.; Peng, X.-M.; Kishore, B.; Zhou, C.-H. 1,2,3-Triazole-derived naphthalimides as a novel type of potential antimicrobial agents: Synthesis, antimicrobial activity, interaction with calf thymus DNA and human serum albumin. Bioorganic Med. Chem. Lett. 2014, 24, 308–313. [Google Scholar] [CrossRef]
- Kilpin, K.J.; Clavel, C.M.; Edafe, F.; Dyson, P.J. Naphthalimide-Tagged Ruthenium–Arene Anticancer Complexes: Combining Coordination with Intercalation. Organometallics 2012, 31, 7031–7039. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, Q.C.; Qin, X.Y.; Sun, D.D.; Zhang, J.N.; Liu, J. Studies of ruthenium(II)-2,2′-bisimidazole complexes on bind-ing to G-quadruplex DNA and inducing apoptosis in HeLa cells. New J. Chem. 2013, 37, 3706–3715. [Google Scholar] [CrossRef]
- de Souza, A.E.C.; Pires, A.D.R.; Cardoso, C.R.; Carlos, R.M.; Cadena, S.M.S.C.; Acco, A. Antineoplastic activity of a novel ruthenium complex against human hepatocellular carcinoma (HepG2) and human cervical adeno-carcinoma (HeLa) cells. Heliyon 2020, 5, e03862. [Google Scholar]
- Chen, J.; Zhang, Y.; Li, B.; Li, G.; Jie, X.; Cui, Y.; Zou, Z.; Huang, X.; Qu, J.; Chen, L. A comparative study on In Vitro cytotoxicity, cellular uptake, localization and apoptosis-inducing mechanism of two ruthenium(II) complexes. Transit. Met. Chem. 2018, 43, 149–159. [Google Scholar] [CrossRef]
- Ma, L.; Lin, X.; Li, C.; Xu, Z.; Chan, C.-Y.; Tse, M.-K.; Shi, P.; Zhu, G. A Cancer Cell-Selective and Low-Toxic Bifunctional Heterodinuclear Pt(IV)–Ru(II) Anticancer Prodrug. Inorg. Chem. 2018, 57, 2917–2924. [Google Scholar] [CrossRef]
- Wang, H.Y.; Qian, Y.; Wang, F.X.; Habtemariam, A.; Mao, Z.W.; Sadler, P.J.; Liu, H.K. Ruthenium(II)-arene metallacycles: Crystal structures, interaction with DNA, and cytotoxicity. Eur. J. Inorg. Chem. 2017, 12, 1792–1799. [Google Scholar] [CrossRef]
- Kong, Y.Q.; Chen, F.; Su, Z.; Qian, Y.; Wang, F.X.; Wang, X.X.; Zhao, J.; Mao, Z.W.; Liu, H.K. Bioactive ruthenium(II)-arene complexes containing modified 18β-glycyrrhetinic acid ligands. J. Inorg. Biochem. 2018, 182, 194–199. [Google Scholar] [CrossRef]
- Li, L.; Wong, Y.-S.; Chen, T.; Fan, C.; Zheng, W. Ruthenium complexes containing bis-benzimidazole derivatives as a new class of apoptosis inducers. Dalton Trans. 2011, 41, 1138–1141. [Google Scholar] [CrossRef]
- Xicheng, W.; Wu, Q.; Wang, X.; Xie, Q.; Tang, Y.; Lan, Y.; Zhang, S.; Mei, W. Microwave-Assisted Synthesis of Arene Ru(II) Complexes Induce Tumor Cell Apoptosis Through Selectively Binding and Stabilizing bcl-2 G-Quadruplex DNA. Materials 2016, 9, 386. [Google Scholar]
- Mondal, A.; Sen, U.; Roy, N.; Muthukumar, V.; Sahoo, S.K.; Bose, B.; Paira, P. DNA targeting half sandwich Ru(ii)-p-cymene-N^N complexes as cancer cell imaging and terminating agents: Influence of regioisomers in cytotoxicity. Dalton Trans. 2021, 50, 979–997. [Google Scholar] [CrossRef]
- Slimani, I.; Chakchouk-Mtibaa, A.; Mansour, L.; Mellouli, L.; Ozdemir, I.; Gurbuzd, N.; Hamdi, N. Synthesis, characteriza-tion, biological determination and catalytic evaluation of ruthenium(II) complexes bearing benzimidazole-based NHC lig-ands in transfer hydrogenation catalysis. New J. Chem. 2020, 44, 5309–5323. [Google Scholar] [CrossRef]
- Tu, L.; Li, C.; Liu, C.; Bai, S.; Yang, J.; Zhang, X.; Xu, L.; Xiong, X.; Sun, Y. Rationally designed Ru(ii) metallacycles with tunable imidazole ligands for synergistical chemo-phototherapy of cancer. Chem. Commun. 2022, 58, 9068–9071. [Google Scholar] [CrossRef] [PubMed]
- Orhan, E.; Dülger, G.; Alpay, M.; Öksüz, N.; Dülger, B. Synthesis, antimicrobial and antiproliferative activities of new self-assembly benzimidazole-bridged aren ruthenium rectangles in human breast cancer cells. J. Incl. Phenom. Macrocycl. Chem. 2021, 102, 45–54. [Google Scholar] [CrossRef]
- Tisato, F.; Marzano, C.; Porchia, M.; Pellei, M.; Santini, C. Copper in diseases and treatments, and copper-based anticancer strategies. Med. Res. Rev. 2010, 30, 708–749. [Google Scholar] [CrossRef]
- Tardito, S.; Bassanetti, I.; Bignardi, C.; Elviri, L.; Tegoni, M.; Mucchino, C.; Bussolati, O.; Franchi-Gazzola, R.; Marchiò, L.J. Copper binding agents acting as copper lonophores lead to caspase inhibition and paraptotic cell death in human cancer cells. Am. Chem. Soc. 2011, 133, 6235–6242. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Z.-Z.; Liu, H.; Liu, J.-C. Copper (II) complexes modified with water-soluble porphyrin and various small molecules ligand for DNA binding and potential selectivity antitumor agents. Dye. Pigment. 2020, 173, 107923. [Google Scholar] [CrossRef]
- Wang, X.-D.; Zhou, M.; Liu, Y.; Si, Z.-Z. Cope with copper: From copper linked mechanisms to copper-based clinical cancer therapies. Cancer Lett. 2023, 561, 216157. [Google Scholar] [CrossRef]
- Manikandamathavan, V.M.; Nair, B.U. DNA binding and cytotoxicity of copper (II) imidazole terpyridine complexes: Role of oxyanion, hydrogen bonding and π–π interaction. Eur. J. Med. Chem. 2013, 68, 244–252. [Google Scholar] [CrossRef]
- Tabassum, S.; Zaki, M.; Afzal, M.; Arjmand, F. Synthesis and characterization of Cu(II)-based anticancer chemotherapeutic agent targeting topoisomerase I alpha: In Vitro DNA binding, pBR322 cleavage, molecular docking studies and cytotoxicity against human cancer cell lines. Eur. J. Med. Chem. 2014, 74, 509–523. [Google Scholar] [CrossRef]
- Mazlan, N.A.; Ravoof, T.B.S.A.; Tiekink, E.R.T.; Tahir, M.I.M.; Veerakumarasivam, A.; Crouse, K.A. Mixed-ligand metal complexes containing an ONS Schiff base and imidazole/benzimidazole ligands: Synthesis, characterization, crystallography and biological activity. Transit. Met. Chem. 2014, 39, 633–639. [Google Scholar] [CrossRef]
- Rajarajeswari, C.; Loganathan, R.; Palaniandavar, M.; Suresh, E.; Riyasdeen, A.; Akbarsha, M.A. Copper(ii) complexes with 2NO and 3N donor ligands: Synthesis, structures and chemical nuclease and anticancer activities. Dalton Trans. 2013, 42, 8347–8363. [Google Scholar] [CrossRef]
- Kaur, J.; Chikate, T.; Bandyopadhyay, P.; Basu, S.; Chikate, R. Cu(II) complexes of hydrazones-NSAID conjugates: Synthesis, characterization and anticancer activity. J. Coord. Chem. 2020, 73, 3186–3202. [Google Scholar] [CrossRef]
- Koley, M.K.; Parsekar, S.U.; Duraipandy, N.; Kiran, M.S.; Varghese, B.; Manoharan, P.T.; Koley, A.P. DNA binding and cyto-toxicity of two Cu(II) complexes containing a Schiff base ligand along with 1,10-phenanthroline or imidazole as a coligand. Inorg. Chim. Acta 2018, 478, 211–221. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abdelhamid, A.A.; Abu-Dief, A.M.; Shehata, M.R.; Bakheet, M.A. Facile synthesis, X-Ray structure of new multi-substituted aryl imidazole ligand, biological screening and DNA binding of its Cr(III), Fe(III) and Cu(II) coordi-nation compounds as potential antibiotic and anticancer drugs. J. Mol. Struct. 2020, 1200, 127034. [Google Scholar] [CrossRef]
- Usman, M.; Zaki, M.; Khan, R.A.; Alsalme, A.; Ahmad, M.; Tabassum, S. Coumarin centered copper(II) complex with ap-pended-imidazole as cancer chemotherapeutic agents against lung cancer: Molecular insight via DFT-based vibrational analysis. RSC Adv. 2017, 7, 36056–36071. [Google Scholar] [CrossRef]
- Al-Asbahy, W.M.; Usman, M.; Arjmand, F.; Shamsi, M.; Tabassum, S. A dinuclear copper(II) complex with piperazine bridge ligand as a potential anticancer agent: DFT computation and biological evaluation. Inorganica Chim. Acta 2016, 445, 167–178. [Google Scholar] [CrossRef]
- Ünver, H.; Dıkmen, G.; Kiyan, H.T. Synthesis, X-ray characterization and evaluation of potent anti-angiogenic activity of a novel copper(II)-imidazole-bipyridyl complex. Inorg. Nano Metal Chem. 2022, 521, 1153–1160. [Google Scholar] [CrossRef]
- Mirzaahmadi, A.; Hosseini-Yazdi, S.A.; Safarzadeh, E.; Baradaran, B.; Samolova, E.; Dusek, M. New series of water-soluble thiosemicarbazones and their copper(II) complexes as potentially promising anticancer compounds. J. Mol. Liq. 2019, 293, 111412. [Google Scholar] [CrossRef]
- Prosser, K.E.; Chang, S.W.; Saraci, F.; Le, P.H.; Walsby, C.J. Anticancer copper pyridine benzimidazole complexes: ROS gen-eration, biomolecule interactions, and cytotoxicity. J. Inorg. Biochem. 2017, 167, 89–99. [Google Scholar] [CrossRef]
- Fu, X.B.; Zhang, J.J.; Liu, D.D.; Gan, Q.; Gao, H.W.; Mao, Z.W.; Le, X.Y. Cu(II)-dipeptide complexes of 2-(4′-thiazolyl)benzimidazole: Synthesis, DNA oxidative damage, antioxidant and In Vitro antitumor activity. J. Inorg. Biochem. 2015, 143, 77–87. [Google Scholar] [CrossRef]
- Zhao, J.A.; Yu, H.B.; Wang, X.X. Synthesis, chemical nuclease activity, and In Vitro cytotoxicity of benzimidazole-based Cu(II)/Co(II) complexes. Chin. Chem. Lett. 2017, 28, 1539–1546. [Google Scholar] [CrossRef]
- Cai, D.-H.; Zhang, C.-L.; Liu, Q.-Y.; He, L.; Liu, Y.-J.; Xiong, Y.-H.; Le, X.-Y. Synthesis, DNA binding, antibacterial and anticancer properties of two novel water-soluble copper(II) complexes containing gluconate. Eur. J. Med. Chem. 2021, 213, 113182. [Google Scholar] [CrossRef] [PubMed]
- Ismail, Z.; Dam, J.; Penny, C.; de Koning, C.B.; Harmse, L. Copper-imidazo[1,2-a]pyridines differentially modulate pro- and anti-apoptotic protein and gene expression in HL-60 and K562 leukaemic cells to cause apoptotic cell death. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2022, 1869, 119160. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Y.; Qi, Y.Y.; Cai, D.H.; Liu, Y.J.; He, L.; Le, X.Y. Sparfloxacin-Cu(II)-aromatic heterocyclic complexes: Synthesis, charac-terization and In Vitro anticancer evaluation. Dalton Trans. 2022, 51, 9878–9887. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.A.; Sudhindra, P.; Roy, N.; Paira, P. Advances in novel iridium (III) based complexes for anticancer applications: A review. Inorg. Chim. Acta 2020, 513, 119925. [Google Scholar] [CrossRef]
- Li, Y.; Tan, C.-P.; Zhang, W.; He, L.; Ji, L.-N.; Mao, Z.-W. Phosphorescent iridium(III)-bis-N-heterocyclic carbene complexes as mitochondria-targeted theranostic and photodynamic anticancer agents. Biomaterials 2015, 39, 95–104. [Google Scholar] [CrossRef]
- Ouyang, M.; Zeng, L.; Qiu, K.Q.; Chen, Y.; Ji, L.N.; Chao, H. Cyclometalated IrIII complexes as mitochondria-targeted photo-dynamic anticancer agents. Eur. J. Inorg. Chem. 2017, 12, 1764–1771. [Google Scholar] [CrossRef]
- Thamilarasan, V.; Sethuraman, V.; Karunakaran, P.; Sethupathi, M.; Manisankar, P.; Selvaraju, C.; Sengottuvelan, N. Synthe-sis, physicochemical properties, thermal analysis and biological application of phosphorescent cationic iridium(III) com-plexes. Inorg. Chim. Acta 2017, 467, 264–275. [Google Scholar]
- Liu, X.C.; Han, Y.L.; Ge, X.X.; Liu, Z. Imidazole and benzimidazole modified half-sandwich iridium(III) N-heterocyclic car-bene complexes: Synthesis, anticancer application, and organelle targeting. Front. Chem. 2020, 8, 182. [Google Scholar] [CrossRef]
- Laha, P.; De, U.; Chandra, F.; Dehury, N.; Khullar, S.; Kim, H.S.; Patra, S. Alkyl chain-modified cyclometalated iridium com-plexes as tunable anticancer and imaging agents. Dalton Trans. 2018, 47, 15873–15881. [Google Scholar] [CrossRef]
- Shen, Y.; Li, X.; Dong, D.; Zhang, B.; Xue, Y.; Shang, P. Transferrin receptor 1 in cancer: A new sight for cancer therapy. Am. J. Cancer Res. 2018, 8, 916–931. [Google Scholar]
- Gras, M.; Therrien, B.; Süss-Fink, G.; Zava, O.; Dyson, P.J. Thiophenolato-bridged dinuclear arene ruthenium complexes: A new family of highly cytotoxic anticancer agents. Dalton Trans. 2010, 39, 10305–10313. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Morais, T.S.; Robalo, M.P.; Marques, F.; Avecilla, F.; Matos, C.P.; Santos, I.; Tomaz, A.I.; Garcia, M.H. Important cytotoxicity of novel iron(II) cyclopentadienyl complexes with imidazole based ligands. J. Inorg. Biochem. 2013, 129, 1–8. [Google Scholar] [CrossRef]
- Guo, J.L.; Liu, G.Y.; Wang, R.Y.; Sun, S.X. Synthesis and structure elucidation of two essential metal complexes: In-vitro stud-ies of their BSA/HSA-binding properties, docking simulations, and anticancer activities. Molecules 2022, 27, 1886. [Google Scholar] [CrossRef]
- Al-Hakimi, A.N.; Alminderej, F.; Aroua, L.; Alhag, S.K.; Alfaifi, M.Y.; Samir, O.M.; Mahyoub, J.A.; Elbehairi, S.E.I.; Alnafisah, A.S. Design, synthesis, characterization of zirconium (IV), cadmium (II) and iron (III) complexes derived from Schiff base 2-aminomethylbenzimidazole, 2-hydroxynaphtadehyde and evaluation of their biological activity. Arab. J. Chem. 2020, 13, 7378–7389. [Google Scholar] [CrossRef]
- Weissleder, R.; Pittet, M.J. Imaging in the era of molecular oncology. Nature 2008, 452, 580–589. [Google Scholar] [CrossRef]
- Simpson, P.V.; Casari, I.; Paternoster, S.; Skelton, B.W.; Falasca, M.; Massi, M. Defining the anticancer activity of tricarbonyl rhenium complexes: Induction of G2/M cell cycle arrest and blockade of aurora-a kinase phosphorylation. Chem. Eur. J. 2017, 23, 6518–6521. [Google Scholar] [CrossRef]
- Wähler, K.; Ludewig, A.; Szabo, P.; Harms, K.; Meggers, E. Rhenium complexes with red-light-induced anticancer activity. Eur. J. Inorg. Chem. 2014, 2014, 807–811. [Google Scholar] [CrossRef]
- Kostova, I. Titanium and vanadium complexes as anticancer agents. Anti Cancer Agents Med. Chem. 2009, 9, 827–842. [Google Scholar] [CrossRef]
- Biswal, D.; Pramanik, N.R.; Chakrabarti, S.; Drew, M.G.B.; Acharya, K.; Chandra, S. Syntheses, crystal structures, DFT calculations, protein interaction and anticancer activities of water soluble dipicolinic acid-imidazole based oxidovanadium(iv) complexes. Dalton Trans. 2017, 46, 16682–16702. [Google Scholar] [CrossRef]
- Ghosh, N.; Chatterjee, S.; Biswal, D.; Pramanik, N.R.; Chakrabarti, S.; Sil, P.C. Oxidative stress imposed In Vivo anticancer therapeutic efficacy of novel imidazole-based oxidovanadium (IV) complex in solid tumor. Life Sci. 2022, 301, 120606. [Google Scholar] [CrossRef]
- Prasad, P.; Pant, I.; Khan, I.; Kondaiah, P.; Chakravarty, A.R. Mitochondria-targeted photoinduced anticancer activity of oxi-dovanadium(IV) complexes of curcumin in visible light. Eur. J. Inorg. Chem. 2014, 2014, 2420–2431. [Google Scholar] [CrossRef]
- Ustun, E.; Ozgur, A.; Coskun, K.A.; Demir, S.; Ozdemir, I.; Tutar, Y. CO-releasing properties and anticancer activities of manganese complexes with imidazole/benzimidazole ligands. J. Coord. Chem. 2016, 69, 3384–3394. [Google Scholar] [CrossRef]
- Mahdy, A.R.E.; Abu Ali, O.A.; Serag, W.M.; Fayad, E.; Elshaarawy, R.F.M.; Gad, E.M. Synthesis, characterization, and biolog-ical activity of Co(II) and Zn(II) complexes of imidazoles-based azo-functionalized Schiff bases. J. Mol. Struct. 2022, 1259, 132726. [Google Scholar] [CrossRef]
- Tabassum, S.; Asim, A.; Khan, R.A.; Hussain, Z.; Srivastav, S.; Srikrishna, S.; Arjmand, F. Chiral heterobimetallic complexes targeting human DNA-topoisomerase Iα. Dalton Trans. 2013, 42, 16749–16761. [Google Scholar] [CrossRef]
- Sayed, F.N.; Mohamed, G.G. Ruthenium(II)-mercapto complexes with anticancer activity interact with topoisomerase IB. J. Organomet. Chem. 2022, 977, 122450. [Google Scholar]
- Padilha, D.S.; Santos, Y.F.; Giacomin, L.C.; Castro, F.A.V.; Pereira, M.D.; Rocha, A.B.; Resende, J.A.L.C.; Scarpellini, M. Syn-thesis, characterization and biological activity of gallium(III) complexes with non-symmetrical N2O-donor Schiff bases. Polyhedron 2017, 123, 480–489. [Google Scholar] [CrossRef]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef]
- Harada, S.; Ishii, Y.; Yamaguchi, K. Extended-spectrum beta-lactamases: Implications for the clinical laboratory and therapy. Korean J. Lab. Med. 2008, 28, 401–412. [Google Scholar]
- LaPlante, K.L.; Dhand, A.; Wright, K.; Lauterio, M. Re-establishing the utility of tetracycline-class antibiotics for current challenges with antibiotic resistance. Ann. Med. 2022, 54, 1686–1700. [Google Scholar] [CrossRef]
- Jadhav, R.W.; Al Kobaisi, M.; Jones, L.A.; Vinu, A.; Bhosale, S.V. The supramolecular self-assembly of aminoglycoside anti-biotics and their applications. Chem. Open 2019, 8, 1154–1166. [Google Scholar]
- Chu, D.T. Recent developments in macrolides and ketolides. Curr. Opin. Microbiol. 1999, 2, 467–474. [Google Scholar] [CrossRef]
- Vázquez-Laslop, N.; Mankin, A.S. How Macrolide Antibiotics Work. Trends Biochem. Sci. 2018, 43, 668–684. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Zhou, C.-H. Synthesis and activities of naphthalimide azoles as a new type of antibacterial and antifungal agents. Bioorganic Med. Chem. Lett. 2011, 21, 4349–4352. [Google Scholar] [CrossRef]
- Zhang, P.L.; Gopala, L.; Zhang, S.L.; Cai, G.X.; Zhou, C.H. An unanticipated discovery towards novel naphthalimide cor-belled aminothiazoximes as potential anti-MRSA agents and allosteric modulators for PBP2a. Eur. J. Med. Chem. 2022, 229, 114050. [Google Scholar] [CrossRef]
- Zhang, P.L.; Laiche, M.H.; Li, Y.L.; Gao, W.W.; Lin, J.M.; Zhou, C.H. An unanticipated discovery of novel naph-thalimidopropanediols as potential broad-spectrum antibacterial members. Eur. J. Med. Chem. 2022, 241, 114657. [Google Scholar] [CrossRef]
- Gong, H.-H.; Baathulaa, K.; Lv, J.-S.; Cai, G.-X.; Zhou, C.-H. Synthesis and biological evaluation of Schiff base-linked imidazolyl naphthalimides as novel potential anti-MRSA agents. Med. Chem. Comm. 2016, 7, 924–931. [Google Scholar] [CrossRef]
- Zhou, X.-M.; Hu, Y.-Y.; Fang, B.; Zhou, C.-H. Benzenesulfonyl thiazoloimines as unique multitargeting antibacterial agents towards Enterococcus faecalis. Eur. J. Med. Chem. 2023, 248, 115088. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Jeyakkumar, P.; Kumar, K.V.; Zhou, C.H. Synthesis of novel sulfonamide azoles via C–N cleavage of sulfona-mides by azole ring and relational antimicrobial study. New J. Chem. 2015, 39, 5776–5796. [Google Scholar] [CrossRef]
- Zhang, L.; Kumar, K.V.; Rasheed, S.; Zhang, S.-L.; Geng, R.-X.; Zhou, C.-H. Design, synthesis, and antibacterial evaluation of novel azolylthioether quinolones as MRSA DNA intercalators. Med. Chem. Comm. 2015, 6, 1303–1310. [Google Scholar] [CrossRef]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of Quinolone Action and Resistance. Biochemistry 2014, 53, 1565–1574. [Google Scholar] [CrossRef]
- Peng, X.M.; Kumar, K.V.; Damu, G.L.; Zhou, C.H. Coumarin-derived azolyl ethanols: Synthesis, antimicrobial evaluation and preliminary action mechanism study. Sci. China Chem. 2016, 59, 878–894. [Google Scholar] [CrossRef]
- Damu, G.L.; Cui, S.-F.; Peng, X.-M.; Wen, Q.-M.; Cai, G.-X.; Zhou, C.-H. Synthesis and bioactive evaluation of a novel series of coumarinazoles. Bioorganic Med. Chem. Lett. 2014, 24, 3605–3608. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhou, C.-H. Synthesis and evaluation of a class of new coumarin triazole derivatives as potential antimicrobial agents. Bioorganic Med. Chem. Lett. 2011, 21, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.-W.; Rasheed, S.; Tangadanchu, V.K.R.; Sun, Y.; Peng, X.-M.; Cheng, Y.; Zhang, F.-X.; Lin, J.-M.; Zhou, C.-H. Design, synthesis and biological evaluation of amino organophosphorus imidazoles as a new type of potential antimicrobial agents. Sci. China Chem. 2017, 60, 769–785. [Google Scholar] [CrossRef]
- Maddili, S.K.; Katla, R.; Kannekanti, V.K.; Bejjanki, N.K.; Tuniki, B.; Zhou, C.H.; Gandham, H. Molecular interaction of nov-el benzothiazolyl triazolium analogues with calf thymus DNA and HSA-their biological investigation as potent antimicro-bial agents. Eur. J. Med. Chem. 2018, 150, 228–247. [Google Scholar] [CrossRef]
- Xie, Y.-P.; Ansari, M.F.; Zhang, S.-L.; Zhou, C.-H. Novel carbazole-oxadiazoles as potential Staphylococcus aureus germicides. Pestic. Biochem. Physiol. 2021, 175, 104849. [Google Scholar] [CrossRef]
- Wang, J.; Ansari, M.F.; Zhou, C.-H. Unique para-aminobenzenesulfonyl oxadiazoles as novel structural potential membrane active antibacterial agents towards drug-resistant methicillin resistant Staphylococcus aureus. Bioorganic Med. Chem. Lett. 2021, 41, 127995. [Google Scholar] [CrossRef]
- Krishnamoorthy, M.; Hakobyan, S.; Ramstedt, M.; Gautrot, J.E. Surface-initiated polymer brushes in the biomedical field: Ap-plications in membrane science, biosensing, cell culture, regenerative medicine and antibacterial coatings. Chem. Rev. 2014, 114, 10976–11026. [Google Scholar] [CrossRef]
- Wang, L.-L.; Battini, N.; Bheemanaboina, R.R.; Zhang, S.-L.; Zhou, C.-H. Design and synthesis of aminothiazolyl norfloxacin analogues as potential antimicrobial agents and their biological evaluation. Eur. J. Med. Chem. 2019, 167, 105–123. [Google Scholar] [CrossRef]
- Cheng, Y.; Avula, S.R.; Gao, W.W.; Addla, D.; Tangadanchu, V.K.R.; Zhang, L.; Lin, J.M.; Zhou, C.H. Multi-targeting explora-tion of new 2-aminothiazolyl quinolones: Synthesis, antimicrobial evaluation, interaction with DNA, combination with topoisomerase IV and penetrability into cells. Eur. J. Med. Chem. 2016, 124, 935–945. [Google Scholar] [CrossRef]
- Bem, A.E.; Velikova, N.; Pellicer, M.T.; van Baarlen, P.; Marina, A.; Wells, J.M. Bacterial Histidine Kinases as Novel Antibacterial Drug Targets. ACS Chem. Biol. 2014, 10, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, J.R.; Etzerodt, T.P.; Gjetting, T.; Andresen, T.L. Side Chain Hydrophobicity Modulates Therapeutic Activity and Membrane Selectivity of Antimicrobial Peptide Mastoparan-X. PLoS ONE 2014, 9, e91007. [Google Scholar] [CrossRef]
- He, S.C.; Zhang, H.Z.; Zhang, H.J.; Sun, Q.; Zhou, C.H. Design and synthesis novel sulfonamide-derived triazoles and bioac-tivity exploration. Med. Chem. 2020, 16, 104–118. [Google Scholar] [CrossRef]
- Uivarosi, V. Metal Complexes of Quinolone Antibiotics and Their Applications: An Update. Molecules 2013, 18, 11153–11197. [Google Scholar] [CrossRef]
- Ahamed, M.A.R.; Azarudeen, R.S.; Kani, N.M. Antimicrobial Applications of Transition Metal Complexes of Benzothiazole Based Terpolymer: Synthesis, Characterization, and Effect on Bacterial and Fungal Strains. Bioinorg. Chem. Appl. 2014, 2014, 764085. [Google Scholar]
- Siwach, A.; Verma, P.K. Synthesis and therapeutic potential of imidazole containing compounds. BMC Chem. 2021, 15, 12. [Google Scholar] [CrossRef]
- Wen, J.; Luo, Y.-L.; Zhang, H.-Z.; Zhao, H.-H.; Zhou, C.-H.; Cai, G.-X. A green and convenient approach toward benzimidazole derivatives and their antimicrobial activity. Chin. Chem. Lett. 2016, 27, 391–394. [Google Scholar] [CrossRef]
- Rani, N.; Sharma, A.; Singh, R. Imidazoles as promising scaffolds for antibacterial activity: A review. Mini Reviews Med. Chem. 2013, 13, 1812–1835. [Google Scholar] [CrossRef]
- Zhang, L.; Kumar, K.V.; Rasheed, S.; Geng, R.X.; Zhou, C.H. Design, synthesis, and antimicrobial evaluation of novel quino-lone imidazoles and interactions with MRSA DNA. Chem. Biol. Drug Des. 2015, 86, 648–655. [Google Scholar] [CrossRef]
- Zhang, H.-Z.; Damu, G.L.; Cai, G.-X.; Zhou, C.-H. Design, synthesis and antimicrobial evaluation of novel benzimidazole type of Fluconazole analogues and their synergistic effects with Chloromycin, Norfloxacin and Fluconazole. Eur. J. Med. Chem. 2013, 64, 329–344. [Google Scholar] [CrossRef]
- Zhang, S.-L.; Chang, J.-J.; Damu, G.L.; Fang, B.; Zhou, X.-D.; Geng, R.-X.; Zhou, C.-H. Novel berberine triazoles: Synthesis, antimicrobial evaluation and competitive interactions with metal ions to Human Serum Albumin. Bioorganic Med. Chem. Lett. 2013, 23, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.-T.; Wang, Z.-C.; Sang, Y.-L.; Tao, X.-X.; Zhu, H.-L. Exploration of structure-based on imidazole core as antibacterial agents. Curr. Top. Med. Chem. 2013, 13, 3118–3130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ge, Y.; Song, H.-M.; Wang, Q.-M.; Zhou, C.-H. Design, synthesis of novel azolyl flavonoids and their protein tyrosine Phosphatase-1B inhibitory activities. Bioorg. Chem. 2018, 80, 195–203. [Google Scholar] [CrossRef]
- Andrei, G.S.; Andrei, B.F.; Roxana, P.R. Imidazole derivatives and their antibacterial activity—A Mini-Review. Mini Rev. Med. Chem. 2021, 21, 1380–1392. [Google Scholar] [CrossRef]
- Samanta, T.; Roymahapatra, G.; Porto, W.F.; Seth, S.; Ghorai, S.; Saha, S.; Sengupta, J.; Franco, O.L.; Dinda, J.; Mandal, S.M. N,N′-Olefin Functionalized Bis-Imidazolium Gold(I) Salt Is an Efficient Candidate to Control Keratitis-Associated Eye Infection. PLoS ONE 2013, 8, e58346. [Google Scholar] [CrossRef]
- El-Halim, H.F.A.; El-Dien, F.A.N.; Mohamed, G.G.; Mohamed, N.A. Chelating behavior, thermal studies and biocidal efficiency of tioconazole and its complexes with some transition metal ions. J. Therm. Anal. Calorim. 2013, 111, 173–181. [Google Scholar] [CrossRef]
- Fang, X.-F.; Li, D.; Tangadanchu, V.K.R.; Gopala, L.; Gao, W.-W.; Zhou, C.-H. Novel potentially antifungal hybrids of 5-flucytosine and fluconazole: Design, synthesis and bioactive evaluation. Bioorganic Med. Chem. Lett. 2017, 27, 4964–4969. [Google Scholar] [CrossRef]
- Shobana, S.; Dharmaraja, J.; Selvaraj, S. Mixed ligand complexation of some transition metal ions in solution and solid state: Spectral characterization, antimicrobial, antioxidant, DNA cleavage activities and molecular modeling. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 107, 117–132. [Google Scholar] [CrossRef]
- Shobana, S.; Subramaniam, P.; Dharmaraja, J.; Narayan, A. Morphological and pharmacological investigation on some biopo-tent materials derived from substituted pyrimidine and imidazole enzyme constituents. Spectrochim. Acta. Part A 2014, 126, 242–253. [Google Scholar] [CrossRef]
- Hackenberg, F.; Lally, G.; Muller-Bunz, H.; Paradisi, F.; Quaglia, D.; Streciwilk, W.; Tacke, M. Novel symmetrically p-benzyl-substituted 4,5-diaryl-imidazole N-heterocyclic carbene-silver(I) acetate complexes—Synthesis and biological evaluation. J. Organomet. Chem. 2012, 717, 123–134. [Google Scholar] [CrossRef]
- Azócar, M.I.; Gómez, G.; Levín, P.; Paez, M.; Muñoz, H.; Dinamarca, N. Review: Antibacterial behavior of carboxylate silver(I) complexes. J. Coord. Chem. 2014, 67, 3840–3853. [Google Scholar] [CrossRef]
- Li, Y.G.; Lu, X.J.; Jing, H.R.; Wang, Q.; Cai, Y.J. Synthesis, structures and antimicrobial activities of silver(I) complexes de-rived from 2-propyl-1H-imidazole-4,5-dicarboxylic acid. Inorg. Chim. Acta 2017, 467, 117–122. [Google Scholar] [CrossRef]
- Kalinowska-Lis, U.; Felczak, A.; Chęcińska, L.; Małecka, M.; Lisowska, K.; Ochocki, J. Influence of selected inorganic counter-ions on the structure and antimicrobial properties of silver(i) complexes with imidazole-containing ligands. N. J. Chem. 2015, 40, 694–704. [Google Scholar] [CrossRef]
- Kleyi, P.; Walmsley, R.S.; Fernandes, M.A.; Torto, N.; Tshentu, Z.R. Syntheses, characterization and antimicrobial activity of silver(I) complexes containing 2-hydroxymethyl-N-alkylimidazole ligands. Polyhedron 2012, 41, 25–29. [Google Scholar] [CrossRef]
- Mather, J.C.; Wyllie, J.A.; Hamilton, A.; da Costa, T.P.S.; Barnard, P.J. Antibacterial silver and gold complexes of imidazole and 1,2,4-triazole derived N-heterocyclic carbenes. Dalton Trans. 2022, 51, 12056–12070. [Google Scholar] [CrossRef]
- Ghdhayeb, M.Z.; Sabah, K.J.; Salman, A.W.; Kadhim, M.M. New Ag(I) and Pd(II) complexes derived from symmetrical and asymmetrical NHC precursors: Synthesis, Characterization, Antibacterial activity, and Theoretical calculations. J. Mol. Struct. 2021, 1245, 131254. [Google Scholar] [CrossRef]
- Stryjska, K.; Korona-Glowniak, I.; Checinska, L.; Kusz, J.; Ochocki, J. Synthesis, spectroscopy, single-crystal structure analy-sis and antibacterial activity of two novel complexes of silver(I) with miconazole drug. Int. J. Mol. Sci. 2021, 22, 1510. [Google Scholar] [CrossRef]
- Haque, R.A.; Asekunowo, P.O.; Razali, M.R. Synthesis and crystal structures of sterically tuned ether functionalized NHC-silver(I) complexes: Antibacterial and nucleic acid interaction studies. J. Coord. Chem. 2014, 67, 2131–2147. [Google Scholar] [CrossRef]
- Haque, R.A.; Asekunowo, P.O.; Razali, M.R.; Mohamad, F. NHC-Silver(I) Complexes as Chemical Nucleases; Synthesis, Crystal Structures, and Antibacterial Studies. Heteroat. Chem. 2014, 25, 194–204. [Google Scholar] [CrossRef]
- Napoli, M.; Saturnino, C.; Cianciulli, E.I.; Varcamonti, M.; Zanfardino, A.; Tommonaro, G.; Longo, P. Silver(I) N-heterocyclic carbene complexes: Synthesis, characterization and antibacterial activity. J. Organomet. Chem. 2013, 725, 46–53. [Google Scholar] [CrossRef]
- Schmidt, C.; Karge, B.; Misgeld, R.; Prokop, A.; Franke, R.; Brönstrup, M.; Ott, I. Gold(I) NHC Complexes: Antiproliferative Activity, Cellular Uptake, Inhibition of Mammalian and Bacterial Thioredoxin Reductases, and Gram-Positive Directed Antibacterial Effects. Chem. A Eur. J. 2017, 23, 1869–1880. [Google Scholar] [CrossRef]
- Azarkish, M.; Akbari, A.; Sedaghat, T.; Simpson, J. Heteroleptic complexes of Zn(II) based on 1-(5-bromo-2-hydroxybenzylidene)-4-phenylthiosemicarbazide: Synthesis, structural characterization, theoretical studies and antibacterial activity. J. Mol. Struct. 2017, 1134, 126–134. [Google Scholar] [CrossRef]
- Amiri, N.; Ben Taheur, F.; Chevreux, S.; Wenger, E.; Lemercier, G.; Nasri, H. Synthesis, crystal structure and spectroscopic characterizations of porphyrin-based Mg(II) complexes—Potential application as antibacterial agent. Tetrahedron 2017, 73, 7011–7016. [Google Scholar] [CrossRef]
- Tabrizi, L.; McArdle, P.; Ektefan, M.; Chiniforoshan, H. Synthesis, crystal structure, spectroscopic and biological properties of mixed ligand complexes of cadmium(II), cobalt(II) and manganese(II) valproate with 1,10-phenanthroline and imidazole. Inorganica Chim. Acta 2016, 439, 138–144. [Google Scholar] [CrossRef]
- Giacomazzo, G.E.; Conti, L.; Guerri, A.; Pagliai, M.; Fagorzi, C.; Sfragano, P.S.; Palchetti, I.; Pietraperzia, G.; Mengoni, A.; Valtancoli, B.; et al. Nitroimidazole-based ruthenium(II) complexes: Playing with structural parameters to design pho-tostable and light-responsive antibacterial agents. Inorg. Chem. 2022, 61, 6689–6694. [Google Scholar] [CrossRef]
- Paramanik, K.; Bandopadhyay, N.; Debnath, R.; Roy, S.; Kotakonda, M.; Adak, M.K.; Biswas, B.; Das, H.S. A hemilabile 2-(2′-pyridyl)-imidazole based nickel(II) complex: Proton-coupled-electron-transfer, bactericidal and cytotoxicity studies. New J. Chem. 2022, 46, 17517–17526. [Google Scholar] [CrossRef]
- Salehi, M.; Rahimifar, F.; Kubicki, M.; Asadi, A. Structural, spectroscopic, electrochemical and antibacterial studies of some new nickel(II) Schiff base complexes. Inorganica Chim. Acta 2016, 443, 28–35. [Google Scholar] [CrossRef]
- Nejad, F.K.; Khosravan, M.; Ebrahimipour, S.Y.; Bisceglie, F. A mixed-ligand quinazoline-based Ni(II) Schiff base complex: Synthesis, characterization, crystal structure, antimicrobial investigation and catalytic activity for the synthesis of 2H-indazolo[2,1-b]phthalazine-triones. Appl. Organomet. Chem. 2018, 32, e3907. [Google Scholar] [CrossRef]
- Sinha, B.; Bhattacharya, M.; Saha, S.; Saha, S. Spectroscopic studies and antimicrobial evaluation of new mixed ligand Mn(II), Ni(II), Cu(II) complexes synthesized from an ionic liquid-supported Schiff base and 1-methyl imidazole. Polycycl. Aromat. Compd. 2022, 42, 5962–5974. [Google Scholar] [CrossRef]
- Nakahata, D.H.; Ribeiro, M.A.; Corbi, P.P.; Machado, D.; Lancellotti, M.; Lustri, W.R.; Ferreira, A.M.D.C.; Formiga, A.L. Synthesis, characterization and preliminary antimicrobial assays of copper(II) complexes with 2-(imidazole-2-yl)heteroaryl ligands. Inorganica Chim. Acta 2017, 458, 224–232. [Google Scholar] [CrossRef]
- Gulya, A.P.; Lozan-Tyrshu, K.S.; Tsapkov, V.I.; Chumakov, Y.M.; Zhanno, E.; Rudik, V.F. Synthesis, structure, and antimicro-bial activity of copper(II) chelates containing imidazole and condensation products of alpha-amino acids with salicylaldehyde and its derivatives. Russ. J. Gen. Chem. 2013, 83, 530–537. [Google Scholar] [CrossRef]
- Ebrahimipour, S.Y.; Mohamadi, M.; Castro, J.; Mollania, N.; Rudbari, H.A.; Saccá, A. Syntheses, characterizations, crystal struc-tures, and biological activities of two new mixed ligand Ni(II) and Cu(II) Schiff base complexes. J. Coord. Chem. 2015, 68, 632–649. [Google Scholar] [CrossRef]
- Arish, D.; Nair, M.S. Synthesis, characterization and biological studies of Co(II), Ni(II),Cu(II) and Zn(II) complexes with pyr-ral-l-histidinate. Arab. J. Chem. 2012, 5, 179–186. [Google Scholar] [CrossRef]
- Mendoza, Á.; Mendoza-Díaz, G.; Pedraza-Reyes, M.; Bernès, S.Z. Copper(II) complex with the tridentate ligand N,N-bis(2-ethyl-4-methyl-imidazol-5-ylmethyl)phenylethylamine (biaq). X-ray crystal structure and biological activity on bacillus subtilis of [Cu(biaq)Cl-2]. Anorg. Allg. Chem. 2013, 639, 1455–1460. [Google Scholar] [CrossRef]
- Kalanithi, M.; Rajarajan, M.; Tharmaraj, P.; Sheela, C.D. Spectral, biological screening of metal chelates of chalcone based Schiff bases of N-(3-aminopropyl) imidazole. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 87, 155–162. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; Ismail, N.M.; Ismael, M. Synthesis, characterization, and biological activity of new mixed ligand transition metal complexes of glutamine, glutaric, and glutamic acid with nitrogen based ligands. Inorg. Nano-Metal Chem. 2017, 47, 467–480. [Google Scholar] [CrossRef]
- Ali, B.; Tahir, S.; Akhtar, M.N.; Yameen, M.; Ashraf, R.; Hussain, T.; Ghaffar, A.; Abbas, M.; Bokhari, T.H.; Iqbal, M. Cytotoxi-city and antimicrobial activity of pivalic and benzoic acid-complexed cu and mn complexes. Pol. J. Environ. Stud. 2017, 26, 2861–2867. [Google Scholar] [CrossRef]
- Chai, L.-Q.; Zhou, L.; Zhang, K.-Y.; Zhang, H.-S. Structural characterizations, spectroscopic, electrochemical properties, and antibacterial activities of copper (II) and cobalt (II) complexes containing imidazole ring. Appl. Organomet. Chem. 2018, 32, e4576. [Google Scholar] [CrossRef]
- Ebrahimipour, S.Y.; Machura, B.; Mohamadi, M.; Khaleghi, M. A novel cationic cobalt(III) Schiff base complex: Preparation, crystal structure, Hirshfeld surface analysis, antimicrobial activities and molecular docking. Microb. Pathog. 2017, 113, 160–167. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Abdel-Rahman, L.H.; Abdelhamid, A.A.; Marzouk, A.A.; Shehata, M.R.; Bakheet, M.A.; Almaghrabi, O.A.; Nafady, A. Synthesis and characterization of new Cr(III), Fe(III) and Cu(II) complexes incorporating multi-substituted aryl imidazole ligand: Structural, DFT, DNA binding, and biological implications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 228, 117700. [Google Scholar] [CrossRef]
- Stevanović, N.L.; Kljun, J.; Aleksic, I.; Bogojevic, S.S.; Milivojevic, D.; Veselinović, A.; Turel, I.; Djuran, M.I.; Nikodinovic-Runic, J.; Glišić, B.D. Clinically used antifungal azoles as ligands for gold(iii) complexes: The influence of the Au(iii) ion on the antimicrobial activity of the complex. Dalton Trans. 2022, 51, 5322–5334. [Google Scholar] [CrossRef]
- Pasayat, S.; Dash, S.P.; Saswati; Majhi, P.K.; Patil, Y.P.; Nethaji, M.; Dash, H.R.; Das, S.; Dinda, R. Mixed-ligand aroylhydrazone complexes of molybdenum: Synthesis, structure and biological activity. Polyhedron 2012, 38, 198–204. [Google Scholar] [CrossRef]
- Cui, S.F.; Peng, L.P.; Zhang, H.Z.; Rasheed, S.; Kumar, K.V.; Zhou, C.H. Novel hybrids of metronidazole and quinolones: Synthesis, bioactive evaluation, cytotoxicity, preliminary antimicrobial mechanism and effect of metal ions on their transpor-tation by human serum albumin. Eur. J. Med. Chem. 2014, 86, 318–334. [Google Scholar] [CrossRef]
- Zhang, L.; Kumar, K.V.; Geng, R.-X.; Zhou, C.-H. Design and biological evaluation of novel quinolone-based metronidazole derivatives as potent Cu2+ mediated DNA-targeting antibacterial agents. Bioorganic Med. Chem. Lett. 2015, 25, 3699–3705. [Google Scholar] [CrossRef]
- Peng, L.P.; Nagarajan, S.; Rasheed, S.; Zhou, C.H. Synthesis and biological evaluation of a new class of quinazolinone azoles as potential antimicrobial agents and their interactions with calf thymus DNA and human serum albumin. Med. Chem. Comm. 2015, 6, 222–229. [Google Scholar] [CrossRef]
- Li, Z.-Z.; Gopala, L.; Tangadanchu, V.K.R.; Gao, W.-W.; Zhou, C.-H. Discovery of novel nitroimidazole enols as Pseudomonas aeruginosa DNA cleavage agents. Bioorganic Med. Chem. 2017, 25, 6511–6522. [Google Scholar] [CrossRef]
- Li, Z.-Z.; Tangadanchu, V.K.R.; Battini, N.; Bheemanaboina, R.R.Y.; Zang, Z.-L.; Zhang, S.-L.; Zhou, C.-H. Indole-nitroimidazole conjugates as efficient manipulators to decrease the genes expression of methicillin-resistant Staphylococcus aureus. Eur. J. Med. Chem. 2019, 179, 723–735. [Google Scholar] [CrossRef]
- Li, F.-F.; Zhang, P.-L.; Tangadanchu, V.K.R.; Li, S.; Zhou, C.-H. Novel metronidazole-derived three-component hybrids as promising broad-spectrum agents to combat oppressive bacterial resistance. Bioorganic Chem. 2022, 122, 105718. [Google Scholar] [CrossRef]
- Maddili, S.K.; Li, Z.-Z.; Kannekanti, V.K.; Bheemanaboina, R.R.Y.; Tuniki, B.; Tangadanchu, V.K.R.; Zhou, C.-H. Azoalkyl ether imidazo[2,1-b]benzothiazoles as potentially antimicrobial agents with novel structural skeleton. Bioorganic Med. Chem. Lett. 2018, 28, 2426–2431. [Google Scholar] [CrossRef]
- Yang, X.; Sun, H.; Maddili, S.K.; Li, S.; Yang, R.G.; Zhou, C.H. Dihydropyrimidinone imidazoles as unique structural anti-bacterial agents for drug-resistant gram-negative pathogens. Eur. J. Med. Chem. 2022, 232, 114188. [Google Scholar] [CrossRef]
- Bheemanaboina, R.R.Y.; Wang, J.; Hu, Y.Y.; Meng, J.P.; Guan, Z.; Zhou, C.H. A facile reaction to access novel structural sul-fonyl-hybridized imidazolyl ethanols as potential DNA-targeting antibacterial agents. Bioorg. Med. Chem. Lett. 2021, 47, 128198. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Pan, G.; Yang, Z.; Li, T.; Wang, J.; Ansari, M.F.; Hu, C.; Bheemanaboina, R.R.Y.; Cheng, Y.; Zhou, C.; et al. Novel Schiff base-bridged multi-component sulfonamide imidazole hybrids as potentially highly selective DNA-targeting membrane active repressors against methicillin-resistant Staphylococcus aureus. Bioorganic Chem. 2021, 107, 104575. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ansari, M.F.; Lin, J.M.; Zhou, C.H. Design and synthesis of sulfanilamide aminophosphonates as novel antibacte-rial agents towards Escherichia coli. Chin. J. Chem. 2021, 39, 2251–2263. [Google Scholar] [CrossRef]
- Li, D.; Bheemanaboina, R.R.Y.; Battini, N.; Tangadanchu, V.K.R.; Fang, X.F.; Zhou, C.H. Novel organophosphorus aminopy-rimidines as unique structural DNA-targeting membrane active inhibitors towards drug-resistant MRSA. Med. Chem. Comm. 2018, 9, 1529–1537. [Google Scholar] [CrossRef]
- Kang, J.; Gopala, L.; Tangadanchu, V.K.R.; Gao, W.W.; Zhou, C.H. Novel naphthalimide nitroimidazoles as multi-targeting antibacterial agents against resistant Acinetobacter baumanii. Future Med. Chem. 2018, 10, 711–724. [Google Scholar] [CrossRef]
- Kang, J.; Tangadanchu, V.K.R.; Gopala, L.; Gao, W.-W.; Cheng, Y.; Liu, H.-B.; Geng, R.-X.; Li, S.; Zhou, C.-H. Novel potentially antibacterial naphthalimide-derived metronidazoles: Design, synthesis, biological evaluation and supramolecular interactions with DNA, human serum albumin and topoisomerase II. Chin. Chem. Lett. 2017, 28, 1369–1374. [Google Scholar] [CrossRef]
- Zhang, L.; Chang, J.J.; Zhang, S.L.; Damu, G.L.V.; Geng, R.X.; Zhou, C.H. Synthesis and bioactive evaluation of novel hy-brids of metronidazole and berberine as new type of antimicrobial agents and their transportation behavior by human se-rum albumin. Bioorg. Med. Chem. 2013, 21, 4158–4169. [Google Scholar] [CrossRef]
- Wen, S.-Q.; Jeyakkumar, P.; Avula, S.R.; Zhang, L.; Zhou, C.-H. Discovery of novel berberine imidazoles as safe antimicrobial agents by down regulating ROS generation. Bioorganic Med. Chem. Lett. 2016, 26, 2768–2773. [Google Scholar] [CrossRef]
- Ansari, M.F.; Tan, Y.-M.; Sun, H.; Li, S.; Zhou, C.-H. Unique iminotetrahydroberberine-corbelled metronidazoles as potential membrane active broad-spectrum antibacterial agents. Bioorganic Med. Chem. Lett. 2022, 76, 129012. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Bheemanaboina, R.R.Y.; Battini, N.; Zhou, C.H. Sulfonamide-derived four-component molecular hybrids as novel DNA-targeting membrane active potentiators against clinical Escherichia coil. Mol. Pharmaceutics 2019, 16, 1036–1052. [Google Scholar] [CrossRef]
- Li, S.; Xiang, Q.X.; Chen, J.X.; Zhou, C.H. Catalytic hydrolysis and supramolecular recognition by benzimidazoly macrocyclic polyamine Zn(II) complex. Chem. Res. Appl. 2009, 26, 1375–1380. [Google Scholar]
- Liu, H.-B.; Gao, W.-W.; Tangadanchu, V.K.R.; Zhou, C.-H.; Geng, R.-X. Novel aminopyrimidinyl benzimidazoles as potentially antimicrobial agents: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2018, 143, 66–84. [Google Scholar] [CrossRef]
- Meng, J.; Xu, Q.; Song, Z.-R.; Ling, L.-X.; Zhou, C.-H. Recent advance in research of benzimidazole containing compounds as antimicrobial drugs. Chin. J. Antibiot. 2012, 36, 81–89. [Google Scholar]
- Zhang, H.-Z.; Cui, S.-F.; Nagarajan, S.; Rasheed, S.; Cai, G.-X.; Zhou, C.-H. A unique one-pot reaction via C–C cleavage from aminomethylene benzimidazoles to access benzimidazolones with wide potentiality. Tetrahedron Lett. 2014, 55, 4105–4109. [Google Scholar] [CrossRef]
- Gan, L.-L.; Fang, B.; Zhou, C.-H. Synthesis of azole-containing piperazine derivatives and evaluation of their antibacterial, antifungal and cytotoxic activities. Bull. Korean Chem. Soc. 2010, 31, 3684–3692. [Google Scholar] [CrossRef]
- Wang, X.-L.; Wang, X.L.; Geng, R.-X.; Zhou, C.-H. Advance in the research of antimicrobial drugs with sulfamide group. Chin. J. New Drugs 2010, 19, 2050–2059. [Google Scholar]
- Jeyakkumar, P.; Liu, H.-B.; Gopala, L.; Cheng, Y.; Peng, X.-M.; Geng, R.-X.; Zhou, C.-H. Novel benzimidazolyl tetrahydroprotoberberines: Design, synthesis, antimicrobial evaluation and multi-targeting exploration. Bioorganic Med. Chem. Lett. 2017, 27, 1737–1743. [Google Scholar] [CrossRef]
- Kalinowska-Lis, U.; Szewczyk, E.M.; Chęcińska, L.; Wojciechowski, J.M.; Wolf, W.M.; Ochocki, J. Synthesis, Characterization, and Antimicrobial Activity of Silver(I) and Copper(II) Complexes of Phosphate Derivatives of Pyridine And Benzimidazole. Chemmedchem 2014, 9, 169–176. [Google Scholar] [CrossRef]
- Kalinowska-Lis, U.; Felczak, A.; Chęcińska, L.; Szabłowska-Gadomska, I.; Patyna, E.; Małecki, M.; Lisowska, K.; Ochocki, J. Antibacterial Activity and Cytotoxicity of Silver(I) Complexes of Pyridine and (Benz)Imidazole Derivatives. X-ray Crystal Structure of [Ag(2,6-di(CH2OH)py)2]NO3. Molecules 2016, 21, 87. [Google Scholar] [CrossRef]
- Prasad, T.V.S.; Shahini, C.R.; Patil, S.A.; Huang, X.J.; Bugarin, A.; Patil, S.A. Non-symmetrically pnitrobenzyl—And pcyanobenzyl-substituted N-heterocyclic carbene-silver(I) complexes: Synthesis, characterization and antibacterial studies. J. Coord. Chem. 2016, 70, 600–614. [Google Scholar] [CrossRef]
- Shahini, C.R.; Achar, G.; Budagumpi, S.; Tacke, M.; Patil, S.A. Synthesis, structural investigation and antibacterial studies of non–symmetrically p–nitrobenzyl substituted benzimidazole N–heterocyclic carbene–silver(I) complexes. Inorg. Chim. Acta 2017, 466, 432–441. [Google Scholar] [CrossRef]
- Achar, G.; Ramya, V.C.; Upendranath, K.; Budagumpi, S. Coumarin-tethered (benz)imidazolium salts and their silver(I) N-heterocyclic carbene complexes: Synthesis, characterization, crystal structure and antibacterial studies. Appl. Organomet. Chem. 2017, 31, e3770. [Google Scholar] [CrossRef]
- Achar, G.; Uppendranath, K.; Ramya, V.; Biffis, A.; Keri, R.S.; Budagumpi, S. Synthesis, characterization, crystal structure and biological studies of silver(I) complexes derived from coumarin-tethered N-heterocyclic carbene ligands. Polyhedron 2017, 123, 470–479. [Google Scholar] [CrossRef]
- Achar, G.; Shahini, C.R.; Patil, S.A.; Malecki, J.G.; Pan, S.H.; Lan, A.; Chen, X.R.; Budagumpi, S. Sterically modulated silver(I) complexes of coumarin substituted benzimidazol-2-ylidenes: Synthesis, crystal structures and evaluation of their antimi-crobial and antilung cancer potentials. J. Inorg. Biochem. 2018, 183, 43–57. [Google Scholar] [CrossRef]
- Achar, G.; Shahini, C.R.; Patil, S.A.; Budagumpi, S. Synthesis, structural characterization, crystal structures and antibacterial potentials of coumarin-tethered N-heterocyclic carbene silver(I) complexes. J. Organomet. Chem. 2017, 833, 28–42. [Google Scholar] [CrossRef]
- Yang, X.C.; Zhang, P.L.; Kumar, K.V.; Li, S.; Geng, R.X.; Zhou, C.H. Discovery of unique thiazolidinone-conjugated couma-rins as novel broad spectrum antibacterial agents. Eur. J. Med. Chem. 2022, 232, 114192. [Google Scholar] [CrossRef]
- Zeng, C.; Avula, S.R.; Meng, J.; Zhou, C. Synthesis and Biological Evaluation of Piperazine Hybridized Coumarin Indolylcyanoenones with Antibacterial Potential. Molecules 2023, 28, 2511. [Google Scholar] [CrossRef]
- Yang, X.-C.; Hu, C.-F.; Zhang, P.-L.; Li, S.; Geng, R.-X.; Zhou, C.-H. Coumarin thiazoles as unique structural skeleton of potential antimicrobial agents. Bioorganic Chem. 2022, 124, 105855. [Google Scholar] [CrossRef]
- Hu, C.-F.; Zhang, P.-L.; Sui, Y.-F.; Lv, J.-S.; Ansari, M.F.; Battini, N.; Li, S.; Zhou, C.-H.; Geng, R.-X. Ethylenic conjugated coumarin thiazolidinediones as new efficient antimicrobial modulators against clinical methicillin-resistant Staphylococcus aureus. Bioorganic Chem. 2020, 94, 103434. [Google Scholar] [CrossRef]
- Achar, G.; Agarwal, P.; Brinda, K.N.; Malecki, J.G.; Keri, R.S.; Budagumpi, S. Ether and coumarinefunctionalized (benz) im-idazolium salts and their silver(I)-N-heterocyclic carbene complexes: Synthesis, characterization, crystal structures and an-timicrobial studies. J. Organomet. Chem. 2018, 854, 64–75. [Google Scholar] [CrossRef]
- Achar, G.; Hokrani, P.P.; Brinda, K.N.; Malecki, J.G.; Budagumpi, S. Synthesis, characterization, crystal structure and antibac-terial properties of N- and O-functionalized (benz)imidazolium salts and their N-heterocyclic carbene silver(I) complexes. J. Mol. Struct. 2019, 1196, 627–636. [Google Scholar] [CrossRef]
- Turker, D.; Ustun, E.; Gunal, S.; Yildiz, H.; Dusunceli, S.D.; Ozdemir, I. Cyanopropyl functionalized benzimidazolium salts and their silver N-heterocyclic carbene complexes: Synthesis, antimicrobial activity, and theoretical analysis. Arch. Pharm. 2022, 355, e2200041. [Google Scholar] [CrossRef]
- Celik, C.; Ustun, E.; Sahin, N.; Tutar, U. Antimicrobial and antibiofilm activities and bovine serum albumin binding prop-erties of benzimidazolium derivative NHC salts and their Ag(I)-NHC complexes. Appl. Organomet. Chem. 2022, 36, e6891. [Google Scholar] [CrossRef]
- Yığıt, B.; Gök, Y.; Özdemır, I.; Günal, S. Synthesis and antimicrobial studies of 1-methyl-2-dimethylaminoethyl-substituted benzimidazolium salts and N-heterocyclic carbene–silver complexes. J. Coord. Chem. 2012, 65, 371–379. [Google Scholar] [CrossRef]
- Karataş, M.O.; Özdemir, N.; Sarıman, M.; Günal, S.; Ulukaya, E.; Özdemir, I. Water-soluble silver(i) complexes with N-donor benzimidazole ligands containing an imidazolium core: Stability and preliminary biological studies. Dalton Trans. 2021, 50, 11596–11603. [Google Scholar] [CrossRef]
- Ashraf, R.; Javed, M.; Taskin-Tok, T.; Nadeem, R.; Javaid, M.K.; El-Naggar, M.; Alzahrani, O.M.; Mahmoud, S.F. Design, DFT studies, antimicrobial and antioxidant potential of Binuclear N-heterocyclic Carbene (NHCs) complexes, Probing the aspect of DNA interaction through In-vitro and In-silico approach. Comput. Biol. Chem. 2021, 95, 107591. [Google Scholar] [CrossRef]
- Ozdemir, I.; Temelli, N.; Gunal, S.; Demir, S. Gold(I) Complexes of N-Heterocyclic Carbene Ligands Containing Benzimidazole: Synthesis and Antimicrobial Activity. Molecules 2010, 15, 2203–2210. [Google Scholar] [CrossRef]
- Bouchouit, M.; Said, M.E.; Ali, M.K.; Bouacida, S.; Merazig, H.; Chaouche, N.K.; Chibani, A.; Zouchoune, B.; Belfaitah, A.; Bouraiou, A. Synthesis, X-ray structure, theoretical investigation, corrosion inhibition and antimicrobial activity of ben-zimidazole thioether and their metal complexes. Polyhedron 2016, 119, 248–259. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.X.; Xiang, Q.X.; Zhang, L.Q.; Zhou, C.H.; Xie, J.Q.; Yu, L.; Li, F.Z. The synthesis and activities of novel mono-nuclear or dinuclear cyclen complexes bearing azole pendants as antibacterial and antifungal agents. Eur. J. Med. Chem. 2014, 84, 677–686. [Google Scholar] [CrossRef]
- Meier, S.M.; Novak, M.; Kandioller, W.; Jakupec, M.A.; Arion, V.B.; Metzler-Nolte, N.; Keppler, B.K.; Hartinger, C.G. Identifi-cation of the structural determinants for anticancer activity of a ruthenium arene peptide conjugate. Chem. Eur. J. 2013, 19, 9297–9307. [Google Scholar] [CrossRef]
- Patel, M.N.; Dosi, P.A.; Bhatt, B.S. Nucleic acid interaction and antibacterial behaviours of a ternary palladium(II) complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 86, 508–514. [Google Scholar] [CrossRef]
- Lu, Y.; Ou, Z.; Hu, W.; Le, X.-Y. Structure, Antibacterial Activities and DNA Cleavage of a Copper(II) Complex with 2-(2′-Pyridyl)benzimidazole and L-Alaninate. Acta Chim. Sin. 2012, 70, 973–979. [Google Scholar] [CrossRef]
- Ou, Z.B.; Lu, Y.H.; Lu, Y.M.; Chen, S.; Xiong, Y.H.; Zhou, X.H.; Mao, Z.W.; Le, X.Y. A copper(II) complex with 2-(2′-pyridyl)benzimidazole and L-arginine: Synthesis, structure, antibacterial activities, and DNA interaction. J. Coord. Chem. 2013, 66, 2152–2165. [Google Scholar] [CrossRef]
- Neelakantan, M.; Sundaram, M.; Thalamuthu, S.; Nair, M.S. Synthesis, characterization, thermal and redox behavior, and biological activity of Ni(II), Cu(II), and Zn(II) complexes containing pyridoxine and imidazole moieties. J. Coord. Chem. 2010, 63, 1969–1985. [Google Scholar] [CrossRef]
- Pramanik, H.A.R.; Das, D.; Paul, P.C.; Mondal, P.; Bhattacharjee, C.R. Newer mixed ligand Schiff base complexes from aquo-N-(2′-hydroxy acetophenone) glycinatocopper(II) as synthon: DFT, antimicrobial activity and molecular docking study. J. Mol. Struct. 2014, 1059, 309–319. [Google Scholar] [CrossRef]
- Qi, Y.Y.; Liu, Y.X.; Gan, Q.; Xiong, Y.H.; Mao, Z.W.; Le, X.Y. Three new mixed-ligand copper(II) complexes containing glycyl-L-valine and N,N-aromatic heterocyclic compounds: Synthesis, characterization, DNA interaction, cytotoxicity and antimicrobial activity. Appl. Organomet. Chem. 2017, 32, e4126. [Google Scholar] [CrossRef]
- Kumaravel, G.; Raman, N. A treatise on benzimidazole based Schiff base metal(II) complexes accentuating their biological efficacy: Spectroscopic evaluation of DNA interactions, DNA cleavage and antimicrobial screening. Mater. Sci. Eng. C 2016, 70, 184–194. [Google Scholar] [CrossRef]
- Wang, L.-L.; Battini, N.; Bheemanaboina, R.R.Y.; Ansari, M.F.; Chen, J.-P.; Xie, Y.-P.; Cai, G.-X.; Zhang, S.-L.; Zhou, C.-H. A new exploration towards aminothiazolquinolone oximes as potentially multi-targeting antibacterial agents: Design, synthesis and evaluation acting on microbes, DNA, HSA and topoisomerase IV. Eur. J. Med. Chem. 2019, 179, 166–181. [Google Scholar] [CrossRef]
- Ren, X.X.; Chen, J.Y.; Le, X.Y. Antibacterial activities and nuclease properties of two new ternary copper(II) complexes with 2-(4′-Thiazolyl)-benzimidazole and 2,2′-bipyridine/1,10-phenanthroline. Chin. J. Chem. 2011, 29, 1380–1388. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.-L.; Ansari, M.F.; Li, S.; Zhou, C.-H. Molecular design and preparation of 2-aminothiazole sulfanilamide oximes as membrane active antibacterial agents for drug resistant Acinetobacter baumannii. Bioorganic Chem. 2021, 113, 105039. [Google Scholar] [CrossRef]
- Azevedo-Barbosa, H.; Dias, D.F.; Franco, L.L.; Hawkes, J.A.; Carvalho, D.T. From Antibacterial to Antitumour Agents: A Brief Review on The Chemical and Medicinal Aspects of Sulfonamides. Mini-Rev. Med. Chem. 2021, 20, 2052–2066. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.S.; Regupathy, S. Studies on Cu(II)-mixed ligand complexes containing a sulfa drug and some enzyme constituents. J. Coord. Chem. 2010, 63, 361–372. [Google Scholar] [CrossRef]
- Malik, S.; Das, S.; Singh, A.; Mitu, L. 3D-Metal Complexes Derived from Proton Pump Inhibitors-Synthesis, Characterization and Biological Studies. E J. Chem. 2012, 9, 1919–1928. [Google Scholar] [CrossRef]
- Raman, N.; Selvan, A. Investigation of DNA binding mechanism, photoinduced cleavage activity, electrochemical proper-ties and biological functions of mixed ligand copper(II) complexes with benzimidazole derivatives: Synthesis and spectral characterization. J. Enzym. Inhib. Med. Chem. 2012, 27, 380–389. [Google Scholar] [CrossRef]
- El-Sherif, A.A. Synthesis, Solution Equilibria and Antibacterial Activity of Co(II) with 2-(Aminomethyl)-Benzimidazole and Dicarboxylic Acids. J. Solut. Chem. 2010, 39, 1562–1581. [Google Scholar] [CrossRef]
- Liang, L.L.; Miao, M.M.; Liu, C.S.; Zong, Z.H.; Zhang, J.; Fang, Q. Antibacterial and aqueous dual-responsive sensing activi-ties of monomeric complexes with uncoordinated imidazole sites. New J. Chem. 2019, 43, 16691–16698. [Google Scholar] [CrossRef]
- Abdel-Ghani, N.T.; El-Ghar, M.F.A.; Mansour, A.M. Novel Ni(II) and Zn(II) complexes coordinated by 2-arylaminomethyl-1H-benzimidazole: Molecular structures, spectral, DFT studies and evaluation of biological activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 104, 134–142. [Google Scholar] [CrossRef]
- Sharma, K.Y.; Prasad, M. Synthesis, Spectral, Cyclic Voltammetric and Antimicrobial Studies of Iron (III) Complexes with Tetradentate Bis-benzimidazole Based Diamide Ligand. Orient. J. Chem. 2012, 28, 1419–1424. [Google Scholar] [CrossRef]
- Dubey, R.K.; Dubey, U.K.; Mishra, S.K. Synthesis, spectroscopic (IR, electronic, FAB-mass, and PXRD), magnetic, and antimi-crobial studies of new iron(III) complexes containing Schiff bases and substituted benzoxazole ligands. J. Coord. Chem. 2011, 64, 2292–2301. [Google Scholar] [CrossRef]
- Siddiqi, Z.A.; Shahid, A.M.; Khalid, M.; Sharma, P.K.; Siddique, A. Spectroscopic, luminescence, electrochemical and antimi-crobial studies of lanthanide complexes of bis-benzimidazole derived ligands. J. Mol. Struct. 2013, 1037, 402–411. [Google Scholar] [CrossRef]
- Baartzes, N.; Jordaan, A.; Warner, D.F.; Combrinck, J.; Taylor, D.; Chibale, K.; Smith, G.S. Antimicrobial evaluation of neu-tral and cationic iridium(III) and rhodium(III) aminoquinoline-benzimidazole hybrid complexes. Eur. J. Med. Chem. 2020, 206, 112694. [Google Scholar] [CrossRef]
- Roopashree, B.; Gayathri, V.; Mukund, H. Synthesis, characterization, and biological activities of zinc, cadmium, copper, and nickel complexes containing meta-aminophenyl benzimidazole. J. Coord. Chem. 2012, 65, 1354–1370. [Google Scholar] [CrossRef]
- Roopashree, B.; Gayathri, V.; Gopi, A.; Devaraju, K. Syntheses, characterizations, and antimicrobial activities of binuclear ruthenium(III) complexes containing 2-substituted benzimidazole derivatives. J. Coord. Chem. 2012, 65, 4023–4040. [Google Scholar] [CrossRef]
- Elaaraj, I.; Raouan, S.E.; Nakkabi, A.; Es-Sounni, B.; Koraichi, I.; El Moualij, N.; Fahim, M. Synthesis, characterization and antioxidant, antibacterial activity Zn2+, Cu2+, Ni2+ and Co2+, complexes of ligand [2-(thiophen-2-yl)-1-(thiophen-2-ylmethyl)-1H-benzo[d]imidazole]. J. Indian Chem. Soc. 2022, 99, 100404. [Google Scholar] [CrossRef]
- Arjmand, F.; Parveen, S.; Afzal, M.; Shahid, M. Synthesis, characterization, biological studies (DNA binding, cleavage, antibac-terial and topoisomerase I) and molecular docking of copper(II) benzimidazole complexes. J. Photochem. Photobiol. B 2012, 114, 15–26. [Google Scholar] [CrossRef]
- Regupathy, S.; Nair, M.S. Studies on Cu(II) ternary complexes involving an aminopenicillin drug and imidazole containing ligands. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 656–663. [Google Scholar] [CrossRef]
- Sunitha, M.; Jogi, P.; Ushaiah, B.; Kumari, C.G. Synthesis, characterization and antimicrobial activity of transition metal com-plexes of Schiff base ligand derived from 3-ethoxy salicylaldehyde and 2-(2-aminophenyl) 1-H-benzimidazole. E-J. Chem. 2012, 9, 2516–2523. [Google Scholar] [CrossRef]
- Tang, Y.-D.; Zhang, J.; Zhang, S.; Geng, R.; Zhou, C.-H. Synthesis and Characterization of Thiophene-derived Amido Bis-nitrogen Mustard and Its Antimicrobial and Anticancer Activities. Chin. J. Chem. 2012, 30, 1831–1840. [Google Scholar] [CrossRef]
- Khalifa, M.E. Synthetic strategies and functional reactivity of versatile thiophene synthons. Synth. Commun. 2020, 50, 2590–2616. [Google Scholar] [CrossRef]
- Sperry, J.B.; Wright, D.L. Furans, thiophenes and related heterocycles in drug discovery. Curr. Opin. drug Discov. Dev. 2005, 8, 723–740. [Google Scholar] [CrossRef]
- Jogi, P.; Mounika, K.; Padmaja, M.; Lakshmi, M.; Gyanakumari, C. Synthesis, Characterization and Antibacterial Studies of Some Transition Metal Complexes of a Schiff Base Derived from 2-(Aminomethyl)- benzimidazole and Thiophene-2-carbaxaldehyde. E-J. Chem. 2011, 8, 1662–1669. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Lin, J.M.; Rasheed, S.; Zhou, C.H. Design, synthesis, and biological evaluation of novel benzimidazole deriva-tives and their interaction with calf thymus DNA and synergistic effects with clinical drugs. Sci. China Chem. 2014, 57, 807–822. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Bheemanaboina, R.R.Y.; Gao, W.-W.; Kang, J.; Cai, G.-X.; Zhou, C.-H. Discovery of Benzimidazole-Quinolone Hybrids as New Cleaving Agents toward Drug-Resistant Pseudomonas aeruginosa DNA. Chemmedchem 2018, 13, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Bheemanaboina, R.R.Y.; Cai, G.X.; Zhou, C.H. Novel purine benzimidazoles as antimicrobial agents by regulating ROS generation and targeting clinically resistant Staphylococcus aureus DNA groove. Bioorg. Med. Chem. Lett. 2018, 28, 1621–1628. [Google Scholar] [CrossRef]
- Fang, X.-J.; Jeyakkumar, P.; Avula, S.R.; Zhou, Q.; Zhou, C.-H. Design, synthesis and biological evaluation of 5-fluorouracil-derived benzimidazoles as novel type of potential antimicrobial agents. Bioorganic Med. Chem. Lett. 2016, 26, 2584–2588. [Google Scholar] [CrossRef]
- Luo, Y.-L.; Baathulaa, K.; Kannekanti, V.K.; Zhou, C.-H.; Cai, G.-X. Novel benzimidazole derived naphthalimide triazoles: Synthesis, antimicrobial activity and interactions with calf thymus DNA. Sci. China Chem. 2015, 58, 483–494. [Google Scholar] [CrossRef]
- Yang, X.; Syed, R.; Fang, B.; Zhou, C.H. A new discovery towards novel skeleton of benzimidazole conjugated pyrimidi-nones as unique effective antibacterial agents. Chin. J. Chem. 2022, 40, 2642–2654. [Google Scholar] [CrossRef]
- Zhang, H.-Z.; He, S.-C.; Peng, Y.-J.; Zhang, H.-J.; Gopala, L.; Tangadanchu, V.K.R.; Gan, L.-L.; Zhou, C.-H. Design, synthesis and antimicrobial evaluation of novel benzimidazole-incorporated sulfonamide analogues. Eur. J. Med. Chem. 2017, 136, 165–183. [Google Scholar] [CrossRef]
- Jeyakkumar, P.; Zhang, L.; Avula, S.R.; Zhou, C.-H. Design, synthesis and biological evaluation of berberine-benzimidazole hybrids as new type of potentially DNA-targeting antimicrobial agents. Eur. J. Med. Chem. 2016, 122, 205–215. [Google Scholar] [CrossRef]
- Sun, H.; Li, Z.-Z.; Jeyakkumar, P.; Zang, Z.-L.; Fang, B.; Zhou, C.-H. A New Discovery of Unique 13-(Benzimidazolylmethyl)berberines as Promising Broad-Spectrum Antibacterial Agents. J. Agric. Food Chem. 2022, 70, 12320–12329. [Google Scholar] [CrossRef]
- Sun, H.; Ansari, M.F.; Fang, B.; Zhou, C.-H. Natural Berberine-Hybridized Benzimidazoles as Novel Unique Bactericides against Staphylococcus aureus. J. Agric. Food Chem. 2021, 69, 7831–7840. [Google Scholar] [CrossRef]
- Zhang, L.; Addla, D.; Ponmani, J.; Wang, A.; Xie, D.; Wang, Y.N.; Zhang, S.L.; Geng, R.X.; Cai, G.X.; Li, S.; et al. Discov-ery of membrane active benzimidazole quinolones-based topoisomerase inhibitors as potential DNA-binding antimicrobi-al agents. Eur. J. Med. Chem. 2016, 111, 160–182. [Google Scholar] [CrossRef]
- Zhang, S.L.; Damu, G.L.V.; Zhang, L.; Geng, R.X.; Zhou, C.H. Synthesis and biological evaluation of novel benzimidazole de-rivatives and their binding behavior with bovine serum albumin. Eur. J. Med. Chem. 2012, 55, 164–175. [Google Scholar] [CrossRef]
- Tan, Y.-M.; Wang, Y.; Li, S.; Zhang, S.-L.; Zhou, C.-H. Azolylpyrimidinediols as Novel Structural Scaffolds of DNA-Groove Binders against Intractable Acinetobacter baumannii. J. Med. Chem. 2023, 66, 4910–4931. [Google Scholar] [CrossRef]
- Zhang, Y.; Damu, G.L.V.; Cui, S.-F.; Mi, J.-L.; Tangadanchu, V.K.R.; Zhou, C.-H. Discovery of potential antifungal triazoles: Design, synthesis, biological evaluation, and preliminary antifungal mechanism exploration. Med. Chem. Comm. 2017, 8, 1631–1639. [Google Scholar] [CrossRef]
- Zhang, H.-Z.; Wei, J.-J.; Kumar, K.V.; Rasheed, S.; Zhou, C.-H. Synthesis and biological evaluation of novel d-glucose-derived 1,2,3-triazoles as potential antibacterial and antifungal agents. Med. Chem. Res. 2015, 24, 182–196. [Google Scholar] [CrossRef]
- Wang, X.L.; Wan, K.; Zhou, C.H. Synthesis of novel sulfanilamide-derived 1,2,3-triazoles and their evaluation for antibacte-rial and antifungal activities. Eur. J. Med. Chem. 2010, 45, 4631–4639. [Google Scholar] [CrossRef]
- Jampilek, J. Recent Advances in Design of Potential Quinoxaline Anti-Infectives. Curr. Med. Chem. 2014, 21, 4347–4373. [Google Scholar] [CrossRef]
- Felton, T.; Troke, P.F.; Hope, W.W.; Simonsen, K.A.; Anderson-Berry, A.L.; Delair, S.F.; Dele Davies, H. Tissue Penetration of Antifungal Agents. Clin. Microbiol. Rev. 2014, 27, 68–88. [Google Scholar] [CrossRef]
- Vos, M.G.J.; Bollenbach, T. Suppressive drug interactions between antifungals. Chem. Biol. 2014, 21, 439–440. [Google Scholar]
- Zhang, P.L.; Lavanya, G.; Yu, Y.; Fang, B.; Zhou, C.H. Identification of a novel antifungal backbone of naphthalimide thia-zoles with synergistic potential for chemical and dynamic treatment. Future Med. Chem. 2021, 13, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Galczynska, K.; Kurdziel, K.; Ciepluch, K.; Rachuna, J.; Kowalska, M.; Madej, L.; Wegierek-Ciuk, A.; Lankoff, A.; Arabski, M. Synthesis, physicochemical and biological characterization of Ni(II) complex with imidazole-4-acetate anion as new anti-fungal agent. J. Chem. Sci. 2018, 130, 169. [Google Scholar] [CrossRef]
- Rodriguez-Arguelles, M.C.; Lopez-Silva, E.C.; Sanmartin, J.; Pelagatti, P.; Zani, F. Copper complexes of imidazole-2-, pyr-role-2- and indol-3-carbaldehyde thiosemicarbazones: Inhibitory activity against fungi and bacteria. J. Inorg. Biochem. 2005, 99, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.F.; Ansari, M.F.; Zhou, C.H. Pyrimidinetrione-imidazoles as a unique structural type of potential agents towards can-dida albicans: Design, synthesis and biological evaluation. Chem. Asian J. 2021, 16, 1417–1429. [Google Scholar] [CrossRef]
- Zhao, L.; Tian, L.; Sun, N.; Sun, Y.; Chen, Y.; Wang, X.; Zhao, S.; Su, X.; Zhao, D.; Cheng, M. Design, synthesis, and structure-activity relationship studies of l-amino alcohol derivatives as broad-spectrum antifungal agents. Eur. J. Med. Chem. 2019, 177, 374–385. [Google Scholar] [CrossRef]
- Jana, A.; Nguyen, K.T.; Li, X.; Zhu, P.C.; Tan, N.S.; Agren, H.; Zhao, Y.L. Perylene-derived single-component organic nano-particles with tunable emission: Efficient anticancer drug carriers with real-time monitoring of drug release. ACS Nano 2014, 8, 5939–5952. [Google Scholar] [CrossRef]
- Xiong, L.; Zheng, L.; Han, K.; Liu, Q.; Li, Y.; Liu, W.; Xia, J.; Wang, W. Drug carriers based on cyclodextrin inclusion complexes for the controlled release of hydrophobic drugs. J. Control. Release 2012, 152, e121–e123. [Google Scholar] [CrossRef]
- Demirel, M.; Yurtdas, G.; Genc, L. Inclusion complexes of ketoconazole with beta-cyclodextrin: Physicochemical characteri-zation and In Vitro dissolution behaviour of its vaginal suppositories. J. Incl. Phenom. Macrocycl. Chem. 2011, 70, 437–445. [Google Scholar] [CrossRef]
- McGinley, J.; McCann, M.; Ni, K.; Tallon, T.; Kavanagh, K.; Devereux, M.; Ma, X.; McKee, V. Imidazole Schiff base ligands: Synthesis, coordination complexes and biological activities. Polyhedron 2013, 55, 169–178. [Google Scholar] [CrossRef]
- McCann, M.; Curran, R.; Ben-Shoshan, M.; McKee, V.; Devereux, M.; Kavanagh, K.; Kellett, A. Synthesis, structure and bio-logical activity of silver(I) complexes of substituted imidazoles. Polyhedron 2013, 56, 180–188. [Google Scholar] [CrossRef]
- McCann, M.; Curran, R.; Ben-Shoshan, M.; McKee, V.; Tahir, A.A.; Devereux, M.; Kavanagh, K.; Creaven, B.S.; Kellett, A. Silver(i) complexes of 9-anthracenecarboxylic acid and imidazoles: Synthesis, structure and antimicrobial activity. Dalton Trans. 2012, 41, 6516–6527. [Google Scholar] [CrossRef]
- Verma, S.; Shrivastva, S.; Shrivastva, R. Synthesis, Characterization, and Physicochemical Studies of Mixed Ligand Complexes of Inner Transition Metals with Lansoprazole and Cytosine. J. Chem. 2012, 2013, 309179. [Google Scholar] [CrossRef]
- Wang, Y.; Damu, G.L.V.; Lv, J.S.; Geng, R.X.; Yang, D.C.; Zhou, C.H. Design, synthesis and evaluation of clinafloxacin tria-zole hybrids as a new type of antibacterial and antifungal agents. Bioorg. Med. Chem. Lett. 2012, 22, 5363–5366. [Google Scholar] [CrossRef]
- Wang, Q.P.; Zhang, J.Q.; Damu, G.L.V.; Wan, K.; Zhang, H.Z.; Zhou, C.H. Synthesis and biological activities of thio-triazole derivatives as new potential antibacterial and antifungal agents. Sci. China Chem. 2012, 55, 2134–2153. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Mi, J.-L.; Zhou, C.-H.; Zhou, X.-D. Synthesis of novel fluconazoliums and their evaluation for antibacterial and antifungal activities. Eur. J. Med. Chem. 2011, 46, 4391–4402. [Google Scholar] [CrossRef]
- Zhang, P.; Tangadanchu, V.K.R.; Zhou, C. Identification of Novel Antifungal Skeleton of Hydroxyethyl Naphthalimides with Synergistic Potential for Chemical and Dynamic Treatments. Molecules 2022, 27, 8453. [Google Scholar] [CrossRef]
- Zala, A.R.; Rajani, D.P.; Ahmad, I.; Patel, H.; Kumari, P. Synthesis, characterization, molecular dynamic simulation, and biological assessment of cinnamates linked to imidazole/benzimidazole as a CYP51 inhibitor. J. Biomol. Struct. Dyn. 2023, 1–17. [Google Scholar] [CrossRef]
- Düşünceli, S.D.; Ayaz, D.; Üstün, E.; Günal, S.; Özdemir, N.; Dinçer, M.; Özdemir, I. Synthesis, antimicrobial properties, and theoretical analysis of benzimidazole-2-ylidene silver(I) complexes. J. Coord. Chem. 2020, 73, 1967–1986. [Google Scholar] [CrossRef]
- Gök, Y.; Akkoç, S.; Erdoğan, H.; Albayrak, S. In Vitro antimicrobial studies of new benzimidazolium salts and silver N-heterocyclic carbene complexes. J. Enzym. Inhib. Med. Chem. 2016, 31, 1322–1327. [Google Scholar] [CrossRef]
- Murthy, Y.L.N.; Durga, G.; Jha, A. Synthesis, characterization, and antimicrobial activity of some new 2-diazo-benzimidazole derivatives and their Ni(II), Cu(II), and Ag(I) complexes. Med. Chem. Res. 2013, 22, 2266–2272. [Google Scholar] [CrossRef]
- Kalarani, R.; Sankarganesh, M.; Kumar, G.G.V.; Kalanithi, M. Synthesis, spectral, DFT calculation, sensor, antimicrobial and DNA binding studies of Co(II), Cu(II) and Zn(II) metal complexes with 2-amino benzimidazole Schiff base. J. Mol. Struct. 2020, 1206, 127725. [Google Scholar] [CrossRef]
- Mansour, A.M.; Shehab, O.R. Pyridylbenzimidazole-based gold(III) complexes: Lysozyme metalation, DNA binding studies, and biological activity. Eur. J. Inorg. Chem. 2019, 23, 2830–2838. [Google Scholar] [CrossRef]
- Wang, J.-L.; Li, T.-T.; Huang, S.-Y.; Cong, W.; Zhu, X.-Q. Major parasitic diseases of poverty in mainland China: Perspectives for better control. Infect. Dis. Poverty 2016, 5, 67. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Farhoudi, R. Leishmaniasis in humans: Drug or vaccine therapy? Drug Des. Dev. Ther. 2018, 12, 25–40. [Google Scholar] [CrossRef]
- Murkin, A.S.; Moynihan, M.M. Transition-state-guided drug design for treatment of parasitic neglected tropical diseases. Curr. Med. Chem. 2014, 21, 1781–1793. [Google Scholar] [CrossRef]
- Horn, D.; Duraisingh, M.T. Antiparasitic Chemotherapy: From Genomes to Mechanisms. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 71–94. [Google Scholar] [CrossRef]
- Butera, J.A. Phenotypic Screening as a Strategic Component of Drug Discovery Programs Targeting Novel Antiparasitic and Antimycobacterial Agents: An Editorial. J. Med. Chem. 2013, 56, 7715–7718. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Eseola, A.O.; Plass, W.; Atolani, O.; Sugi, T.; Han, Y.; Batiha, G.E.-S.; Kato, K.; Awakan, O.J.; Olaolu, T.D.; et al. Imidazole derivatives as antiparasitic agents and use of molecular modeling to investigate the structure-activity relationship. Parasitol. Res. 2020, 119, 1925–1941. [Google Scholar] [CrossRef]
- Sanz, A.M.; Gómez-Contreras, F.; Navarro, P.; Sánchez-Moreno, M.; Boutaleb-Charki, S.; Campuzano, J.; Pardo, M.; Osuna, A.; Cano, C.; Yunta, M.J.R.; et al. Efficient Inhibition of Iron Superoxide Dismutase and of Trypanosoma cruzi Growth by Benzo[g]phthalazine Derivatives Functionalized with One or Two Imidazole Rings. J. Med. Chem. 2008, 51, 1962–1966. [Google Scholar] [CrossRef]
- Beltran-Hortelano, I.; Alcolea, V.; Font, M.; Perez-Silanes, S. The role of imidazole and benzimidazole heterocycles in Cha-gas disease: A review. Eur. J. Med. Chem. 2020, 206, 112692. [Google Scholar] [CrossRef]
- Turner, T.L.; Nguyen, V.H.; McLauchlan, C.C.; Dymon, Z.; Dorsey, B.M.; Hooker, J.D.; Jones, M.A. Inhibitory effects of decavanadate on several enzymes and Leishmania tarentolae In Vitro. J. Inorg. Biochem. 2012, 108, 96–104. [Google Scholar] [CrossRef]
- Rosa, L.B.; Galuppo, C.; Lima, R.L.A.; Fontes, J.V.; Siqueira, F.S.; Judice, W.A.S.; Abbehausen, C.; Miguel, D.C. Antileishma-nial activity and insights into the mechanisms of action of symmetric Au(I) benzyl and aryl-N-heterocyclic carbenes. J. Inorg. Biochem. 2022, 229, 111726. [Google Scholar] [CrossRef]
- Koko, W.S.; Jentzsch, J.; Kalie, H.; Schobert, R.; Ersfeld, K.; Al Nasr, I.S.; Khan, T.A.; Biersack, B. Evaluation of the antipara-sitic activities of imidazol-2-ylidene-gold(I) complexes. Arch. Pharm. 2020, 353, e1900363. [Google Scholar] [CrossRef]
- Huang, Q.; Li, B.P.; Yang, S.; Ma, P.P.; Wang, Z.Z. Preparation and cyclodextrin solubilization of the antibacterial agent ben-zoyl metronidazole. Sci. World J. 2013, 306476. [Google Scholar]
- Celebioglu, A.; Uyar, T. Metronidazole/Hydroxypropyl-beta-Cyclodextrin inclusion complex nanofibrous webs as fast-dissolving oral drug delivery system. Int. J. Pharm. 2019, 572, 118828. [Google Scholar] [CrossRef]
- Rojas-Aguirre, Y.; Yépez-Mulia, L.; Castillo, I.; López-Vallejo, F.; Soria-Arteche, O.; Hernández-Campos, A.; Castillo, R.; Hernández-Luis, F. Studies on 6-chloro-5-(1-naphthyloxy)-2-(trifluoromethyl)-1H-benzimidazole/2-hydroxypropyl-β-cyclodextrin association: Characterization, molecular modeling studies, and In Vivo anthelminthic activity. Bioorg. Med. Chem. 2011, 19, 789–797. [Google Scholar] [CrossRef]
- Mota, V.Z.; de Carvalho, G.S.G.; da Silva, A.D.; Costa, L.A.S.; Machado, P.d.A.; Coimbra, E.S.; Ferreira, C.V.; Shishido, S.M.; Cuin, A. Gold complexes with benzimidazole derivatives: Synthesis, characterization and biological studies. Biometals 2014, 27, 183–194. [Google Scholar] [CrossRef]
- Mushtaq, A.; Azam, U.; Mehreen, S.; Naseer, M.M. Synthetic α-glucosidase inhibitors as promising anti-diabetic agents: Re-cent developments and future challenges. Eur. J. Med. Chem. 2023, 249, 115119. [Google Scholar] [CrossRef]
- Alcantara, A.; Pace, V.; Hoyos, P.; Sandoval, M.; Holzer, W.; Hernaiz, M. Chemoenzymatic synthesis of carbohydrates as antidiabetic and anticancer drugs. Curr. Top. Med. Chem. 2014, 14, 2694–2711. [Google Scholar] [CrossRef]
- Kushwaha, R.; Haq, W.; Katti, S. Sixteen-years of clinically relevant dipeptidyl peptidase-IV (DPP-IV) inhibitors for treatment of type-2 diabetes: A perspective. Curr. Med. Chem. 2014, 21, 4013–4045. [Google Scholar] [CrossRef]
- Xu, G.; Lv, B.; Roberge, J.Y.; Xu, B.; Du, J.; Dong, J.; Chen, Y.; Peng, K.; Zhang, L.; Tang, X.; et al. Design, Synthesis, and Biological Evaluation of DeuteratedC-Aryl Glycoside as a Potent and Long-Acting Renal Sodium-Dependent Glucose Cotransporter 2 Inhibitor for the Treatment of Type 2 Diabetes. J. Med. Chem. 2014, 57, 1236–1251. [Google Scholar] [CrossRef] [PubMed]
- Shaik, A.; Kondaparthy, V.; Aveli, R.; Das Manwal, D. Studies on the serum glucose reducing effect of vanadium metal complexes on Wistar rats. J. Mol. Struct. 2022, 1261, 132825. [Google Scholar] [CrossRef]
- Patel, N.; Prajapati, A.K.; Jadeja, R.N.; Patel, R.N.; Patel, S.K.; Tripathi, I.P.; Dwivedi, N.; Gupta, V.K.; Butcher, R.J. Dioxi-dovanadium(V) complexes of a tridentate ONO Schiff base ligand: Structural characterization, quantum chemical calcula-tions and In Vitro antidiabetic activity. Polyhedron 2020, 180, 114434. [Google Scholar] [CrossRef]
- Sadaf, H.; Imtiaz-ud-Din; Zahra, S.S.; Ihsan-ul-Haq; Nadeem, S.; Tahir, M.N.; Ahmad, S.; Andleeb, S. Synthesis, X-ray struc-tures and biological properties of palladium(II) complexes of 1,2-dimethylimidazole and benzimidazole. Polyhedron 2019, 160, 101–107. [Google Scholar] [CrossRef]
- Sadaf, H.; Imtiaz-ud-Din; Fettouhi, M.; Fazal, A.; Ahmad, S.; Farooqi, B.A.; Nadeem, S.; Ihsan-ul-Haq; Ahmad, W. Synthesis, crystal structures and biological activities of palladium(II) complexes of benzimidazole and 2-methylbenzimidazole. Polyhedron 2019, 170, 537–543. [Google Scholar] [CrossRef]
- Tamargo, J.; Duarte, J.; Ruilope, L.M. New Antihypertensive Drugs Under Development. Curr. Med. Chem. 2015, 22, 305–342. [Google Scholar] [CrossRef]
- Bassareo, P.P.; Bassareo, V.; Iacovidou, N.; Mercuro, G. Antihypertensive therapy in children: Differences in medical ap-proach between the unitedstates and europe. Curr. Med. Chem. 2014, 21, 3121–3131. [Google Scholar] [CrossRef]
- Islas, M.S.; Medina, J.M.; Tévez, L.L.L.; Rojo, T.; Lezama, L.; Merino, M.G.; Calleros, L.; Cortes, M.A.; Puyol, M.R.; Echeverría, G.A.; et al. Antitumoral, Antihypertensive, Antimicrobial, and Antioxidant Effects of an Octanuclear Copper(II)-Telmisartan Complex with an Hydrophobic Nanometer Hole. Inorg. Chem. 2014, 53, 5724–5737. [Google Scholar] [CrossRef]
- Alghamdi, S.S.; Suliman, R.S.; Almutairi, K.; Kahtani, K.; Aljatli, D. Imidazole as a Promising Medicinal Scaffold: Current Status and Future Direction. Drug Des. Dev. Ther. 2021, 15, 3289–3312. [Google Scholar] [CrossRef]
- Chaudhary, D.; Robinson, S.; Romero, D.L. Recent Advances in the Discovery of Small Molecule Inhibitors of Interleukin-1 Receptor-Associated Kinase 4 (IRAK4) as a Therapeutic Target for Inflammation and Oncology Disorders. J. Med. Chem. 2015, 58, 96–110. [Google Scholar] [CrossRef]
- Maccari, R.; Ottanà, R. Targeting Aldose Reductase for the Treatment of Diabetes Complications and Inflammatory Diseases: New Insights and Future Directions. J. Med. Chem. 2015, 58, 2047–2067. [Google Scholar] [CrossRef]
- Kong, T.T.; Zhang, C.M.; Liu, Z.P. Recent developments of p38 alpha MAP kinase inhibitors as anti-inflammatory agents based on the imidazole scaffolds. Curr. Med. Chem. 2013, 20, 1997–2016. [Google Scholar] [CrossRef]
- Agotegaray, M.A.; Dennehy, M.; Boeris, M.A.; Grela, M.A.; Burrow, R.A.; Quinzani, O.V. Therapeutic properties, SOD and catecholase mimetic activities of novel ternary copper(II) complexes of the anti-inflammatory drug Fenoprofen with imid-azole and caffeine. Polyhedron 2012, 34, 74–83. [Google Scholar] [CrossRef]
- Sasahara, G.L.; Gouveia, F.S.; Rodrigues, R.D.; Zampieri, D.S.; Fonseca, S.G.C.; Goncalves, R.D.R.; Athaydes, B.R.; Kitagawa, R.R.; Santos, F.A.; Sousa, E.H.S.; et al. Nitro-imidazole-based ruthenium complexes with antioxi-dant and anti-inflammatory activities. J. Inorg. Biochem. 2020, 206, 111048. [Google Scholar] [CrossRef]
- AlAjmi, M.F.; Hussain, A.; Alsalme, A.; Khan, R.A. In Vivo assessment of newly synthesized achiral copper(ii) and zinc(ii) complexes of a benzimidazole derived scaffold as a potential analgesic, antipyretic and anti-inflammatory. RSC Adv. 2016, 6, 19475–19481. [Google Scholar] [CrossRef]
- Nath, M.; Saini, P.K.; Kumar, A. New di- and triorganotin(IV) complexes of tripodal Schiff base ligand containing three im-idazole arms: Synthesis, structural characterization, anti-inflammatory activity and thermal studies. J. Organomet. Chem. 2010, 695, 1353–1362. [Google Scholar] [CrossRef]
- Zhou, F.W.; Lei, H.S.; Fan, L.; Jiang, L.; Liu, J.; Peng, X.M.; Xu, X.R.; Chen, L.; Zhou, C.H.; Zou, Y.Y.; et al. Design, synthesis, and biological evaluation of dihydroartemisinin–fluoroquinolone conjugates as a novel type of poten-tial antitubercular agents. Bioorg. Med. Chem. Lett. 2014, 24, 1912–1917. [Google Scholar] [CrossRef]
- Bekier, A.; Kawka, M.; Lach, J.; Dziadek, J.; Paneth, A.; Gatkowska, J.; Dzitko, K.; Dziadek, B. Imidazole-Thiosemicarbazide Derivatives as Potent Anti-Mycobacterium tuberculosis Compounds with Antibiofilm Activity. Cells 2021, 10, 3476. [Google Scholar] [CrossRef]
- Zhang, F.-F.; Gan, L.-L.; Zhou, C.-H. Synthesis, antibacterial and antifungal activities of some carbazole derivatives. Bioorganic Med. Chem. Lett. 2010, 20, 1881–1884. [Google Scholar] [CrossRef]
- Kashyap, S.; Singh, R.; Singh, U.P. Inorganic and organic anion sensing by azole family members. Coord. Chem. Rev. 2020, 417, 213369. [Google Scholar] [CrossRef]
- Alfonso, M.; Tárraga, A.; Molina, P. Pyrrole, imidazole, and triazole derivatives as ion-pair recognition receptors. Tetrahedron Lett. 2016, 57, 3053–3059. [Google Scholar] [CrossRef]
- McConnell, A.J.; Docker, A.; Beer, P.D. From Heteroditopic to Multitopic Receptors for Ion-Pair Recognition: Advances in Receptor Design and Applications. Chempluschem 2020, 85, 1824–1841. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-H.; Xue, L.; Qian, Y.-Y.; Jiang, H. Novel Ratiometric Fluorescent Sensor for Silver Ions. Org. Lett. 2010, 12, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, R.; Ma, J.; Yan, L.; Zhao, Y.; Wang, Y.; Zhang, W.; Fan, J.; Chen, X. Graphitic carbon nitride solid nanofilms for selective and recyclable sensing of Cu2+ and Ag+ in water and serum. Chem. Commun. 2014, 50, 15415–15418. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Santra, M.; Won, N.; Kim, S.; Kim, J.K.; Bin Kim, S.; Ahn, K.H. Selective Fluorogenic and Chromogenic Probe for Detection of Silver Ions and Silver Nanoparticles in Aqueous Media. J. Am. Chem. Soc. 2009, 131, 2040–2041. [Google Scholar] [CrossRef]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent Sensors for Measuring Metal Ions in Living Systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef]
- Culzoni, M.J.; Munoz de la Pena, A.; Machuca, A.; Goicoechea, H.C.; Babiano, R. Rhodamine and BODIPY chemodosimeters and chemosensors for the detection of Hg2+, based on fluorescence enhancement effects. Anal. Methods 2013, 5, 30–49. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, T.; Fang, Y.; Wang, L.Y.; Song, B.; Deng, Q.G. Two ‘turn-off’ Schiff base fluorescence sensors based on phenan-thro [9,10-d]imidazole-coumarin derivatives for Fe3+ in aqueous solution. Tetrahedron Lett. 2016, 57, 4417–4423. [Google Scholar] [CrossRef]
- Das, S.; Karmakar, S.; Mardanya, S.; Baitalik, S. Synthesis, structural characterization, and multichannel anion and cation sensing studies of a bifunctional Ru(II) polypyridyl-imidazole based receptor. Dalton Trans. 2014, 43, 3767–3782. [Google Scholar] [CrossRef]
- Karmakar, S.; Nandi, M.; Mukherjee, S.; Baitalik, S. Polypyridyl-imidazole based smart Ru(II) complex mimicking advanced Boolean and Fuzzy logic functions. Inorg. Chim. Acta 2017, 454, 76–88. [Google Scholar] [CrossRef]
- Dey, N.; Kulhanek, J.; Bures, F.; Bhattacharya, S. Simultaneous detection of Cu2+ and Hg2+ via two mutually independent sensing pathways of biimidazole push–pull dye. J. Org. Chem. 2019, 84, 1787–1796. [Google Scholar] [CrossRef]
- Sun, J.L.; Suo, Q.L.; Hou, J.N.; Ma, T.; Gao, X.C.; Lv, L.; Gao, Y.Y.; Jia, H.J.; Wang, Y.Q. 2-Ferrocenylimidazole-based multire-sponsive receptors for Al3+, Cu2+, and H2PO4− ions: Effect of structural modification on the ion sensing performance. Tetrahedron 2021, 99, 132434. [Google Scholar] [CrossRef]
- Joel, C.; Livingston, D.J.; Bennie, R.B.; Jeyanthi, D.; Solomon, R.V. Designing bifunctional phenanthroimidazole chromo-phores for highly selective ratiometric chemosensing of Cu2+/F- and Co2+/F—Ions in organic solvents. J. Photochem. Photobiol. A Chem. 2021, 423, 113612. [Google Scholar]
- Saha, B.; Saha, P.; Mandal, A.; Naskar, J.P.; Maiti, D.; Chowdhury, S. Sequential detection of Cu2+ and cysteine using an imid-azole-based chemosensor in aqueous solution. J. Chin. Chem. Soc. 2019, 66, 506–514. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, S. Copper ion luminescence on/off sensing by a quinoline-appended ruthenium(II)-polypyridyl complex in aqueous media. J. Mol. Struct. 2019, 1202, 127242. [Google Scholar] [CrossRef]
- Honnappa, N.; Anil, A.G.; Shekar, S.; Behera, S.K.; Ramamurthy, P.C. Design of a highly selective benzimidazole-based de-rivative for optical and solid-state detection of zinc ion. Inorg. Chem. 2022, 61, 15085–15097. [Google Scholar] [CrossRef]
- Parthiban, C.; Elango, K.P. Selective and sensitive colorimetric detection of Hg(II) in aqueous solution by quinone-diimidazole ensemble with mimicking YES-OR-INHIBIT logic gate operation. Sens. Actuators B Chem. 2016, 237, 284–290. [Google Scholar] [CrossRef]
- Beer, P.D.; Gale, P.A. Anion recognition and sensing: The state of the art and future perspectives. Angew. Chem. Int. Ed. 2001, 40, 486–516. [Google Scholar] [CrossRef]
- Hu, Y.; Long, S.; Fu, H.; She, Y.; Xu, Z.; Yoon, J. Revisiting imidazolium receptors for the recognition of anions: Highlighted research during 2010–2019. Chem. Soc. Rev. 2021, 50, 589–618. [Google Scholar] [CrossRef]
- Parthiban, C.; Ciattini, S.; Chelazzi, L.; Elango, K.P. Colorimetric sensing of anions by Cu(II), Co(II), Ni(II) and Zn(II) com-plexes of naphthoquinone-imidazole hybrid—Influence of complex formation on selectivity and sensing medium. Sens. Actuators B Chem. 2016, 231, 768–778. [Google Scholar] [CrossRef]
- Lo, K.K.W.; Li, S.P.Y.; Zhang, K.Y. Development of luminescent iridium(III) polypyridine complexes as chemical and bio-logical probes. New J. Chem. 2011, 35, 265–287. [Google Scholar] [CrossRef]
- Monti, F.; Baschieri, A.; Gualandi, I.; Serrano-Perez, J.J.; Junquera-Hernandez, J.M.; Tonelli, D.; Mazzanti, A.; Muzzioli, S.; Stagni, S.; Roldan-Carmona, C.; et al. Iridium(III) complexes with phe-nyl-tetrazoles as cyclometalating ligands. Inorg. Chem. 2014, 53, 7709–7721. [Google Scholar] [CrossRef] [PubMed]
- Howarth, A.J.; Davies, D.L.; Lelj, F.; Wolf, M.O.; Patrick, B.O. Tuning the Emission Lifetime in Bis-cyclometalated Iridium(III) Complexes Bearing Iminopyrene Ligands. Inorg. Chem. 2014, 53, 11882–11889. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.B.; Zhang, Y.X. A water–soluble cationic Ir(III) complex for turn–on sensing of ClO4-, based on aggregation–induced emission. Sens. Actuators B Chem. 2017, 245, 599–604. [Google Scholar] [CrossRef]
- Donato, L.; McCusker, C.E.; Castellano, F.N.; Zysman-Colman, E. Mono- and dinuclear cationic Iridium(III) complexes bear-ing a 2,5-dipyridylpyrazine (2,5-dpp) ligand. Inorg. Chem. 2013, 52, 8495–8504. [Google Scholar] [CrossRef]
- Yin, H.-J.; Liu, Y.-J.; Gao, J.; Wang, K.-Z. A highly sensitive and selective visible-light excitable luminescent probe for singlet oxygen based on a dinuclear ruthenium complex. Dalton Trans. 2017, 46, 3325–3331. [Google Scholar] [CrossRef]
- Paul, A.; Bar, M.; Ahmed, T.; Baitalik, S. Anion-sensitive photophysics of luminescent trimetallic complexes of Fe(II), Ru(II), and Os(II) with polarized NH motifs. Polyhedron 2020, 190, 114772. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, P.; Xie, Q.; Ma, Q.-Q.; Meng, Y.-H.; Wang, Z.-W.; Zhang, S.; Zhao, X.-J.; Chen, J.; Wang, Z.-L. Cadmium(II)-Triazole Framework as a Luminescent Probe for Ca2+ and Cyano Complexes. Chem. A Eur. J. 2016, 22, 10459–10474. [Google Scholar] [CrossRef]
- Mardanya, S.; Mondal, D.; Karmakar, S.; Baitalik, S. Smart ruthenium and osmium complexes mimic the complicated func-tions of traffic signal and memory device. Sens. Actuators B Chem. 2017, 239, 635–641. [Google Scholar] [CrossRef]
- Parthiban, C.; Elango, K.P. Selective colorimetric sensing of fluoride ion via H-bonding in 80% aqueous solution by transi-tion metal chelates. Sens. Actuators B Chem. 2017, 245, 321–333. [Google Scholar] [CrossRef]
- Beneto, A.J.; Siva, A. A phenanthroimidazole based effective colorimetric chemosensor for copper(II) and fluoride ions. Sens. Actuators B Chem. 2017, 247, 526–531. [Google Scholar] [CrossRef]
- Alreja, P.; Kaur, N. Establishing the anion recognition correlation of the 2-(2-methoxyphenyl)-1H-imidazo [4, 5-f][1,10] phenanthroline and its Ru(bipy) 2 2+ complex via fluorimetry. J. Lumin 2016, 179, 372–377. [Google Scholar] [CrossRef]
- Mondal, D.; Pal, P.; Baitalik, S. Anthraquinone-biimidazole based ruthenium(II) complexes as selective multichannel anion sensors and multi-readout molecular logic gates and memory devices: Combined experimental and DFT/TD-DFT study. Sens. Actuators B Chem. 2017, 242, 746–759. [Google Scholar] [CrossRef]
- Bar, M.; Maity, D.; Das, K.; Baitalik, S. Asymmetric bimetallic ruthenium(II) complexes selectively sense cyanide in water through significant modulation of their ground and excited state properties. Sens. Actuators B Chem. 2017, 251, 208–223. [Google Scholar] [CrossRef]
- Yazdani, A.; Janzen, N.; Czorny, S.; Valliant, J.F. Technetium(I) Complexes of Bathophenanthrolinedisulfonic Acid. Inorg. Chem. 2017, 56, 2958–2965. [Google Scholar] [CrossRef]
- Yang, C.; Mehmood, F.; Lam, T.L.; Chan, S.L.-F.; Wu, Y.; Yeung, C.-S.; Guan, X.; Li, K.; Chung, C.Y.-S.; Zhou, C.-Y.; et al. Stable luminescent iridium(iii) complexes with bis(N-heterocyclic carbene) ligands: Photo-stability, excited state properties, visible-light-driven radical cyclization and CO2 reduction, and cellular imaging. Chem. Sci. 2016, 7, 3123–3136. [Google Scholar] [CrossRef]
- Qiu, K.; Ouyang, M.; Liu, Y.; Huang, H.; Liu, C.; Chen, Y.; Jia, L.; Chao, H. Two-photon photodynamic ablation of tumor cells by mitochondria-targeted iridium(iii) complexes in aggregate states. J. Mater. Chem. B 2017, 5, 5488–5498. [Google Scholar] [CrossRef]
- Rizvi, S.F.A.; Zhang, H.X.; Mehmood, S.; Sanad, M. Synthesis of 99mTc-labeled 2-Mercaptobenzimidazole as a novel radio-tracer to diagnose tumor hypoxia. Transl. Oncol. 2020, 13, 100854. [Google Scholar] [CrossRef]
- Kydonaki, T.E.; Tsoukas, E.; Mendes, F.; Hatzidimitriou, A.G.; Paulo, A.; Papadopoulou, L.C.; Papagiannopoulou, D.; Pso-mas, G. Synthesis, characterization and biological evaluation of 99mTc/Re-tricarbonyl quinolone complexes. J. Inorg. Biochem. 2016, 160, 94–105. [Google Scholar] [CrossRef]
- Liu, H.Y.; Zhang, B.B.; Tan, C.Y.; Liu, F.; Cao, J.K.; Tan, Y.; Jiang, Y.Y. Simultaneous bioimaging recognition of Al3+ and Cu2+ in living-cell, and further detection of F− and S2− by a simple fluorogenic benzimidazole-based chemosensor. Talanta 2016, 161, 309–319. [Google Scholar] [CrossRef]
- Ganesan, J.S.; Sepperumal, M.; Ayyanar, S. A novel pyrazole bearing imidazole frame as ratiometric fluorescent chemosen-sor for Al3+/Fe3+ ions and its application in HeLa cell imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 230, 117993. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Liu, T.; Fang, Y.; Wang, L.Y.; Kan, W.; Deng, Q.G.; Song, B. A new selective chemosensor based on phenan-thro[9,10-d]imidazole-coumarin with sequential “on-off-on” fluorescence response to Fe3+ and phosphate anions and its ap-plication in live cell. Sens. Actuator B Chem. 2017, 246, 370–379. [Google Scholar] [CrossRef]
- Liu, C.L.; Yang, W.; Du, J.Y.; Shen, P.; Yang, C.Y. A boron 2-(2′-pyridyl) imidazole fluorescence probe for bovine serum al-bumin: Discrimination over other proteins and identification of its denaturation. Photochem. Photobiol. 2017, 93, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Chen, Z.W.; Hu, B.H.; Cai, P.Q.; Wang, S.; Xiao, S.Z.; Wu, Y.L.; Chen, X.D. Synergistic lysosomal activatable pol-ymeric nanoprobe encapsulating ph sensitive imidazole derivative for tumor diagnosis. Small 2018, 14, 1703164. [Google Scholar]
- Liu, H.Y.; Zhang, S.Q.; Cui, M.C.; Gao, L.H.; Zhao, H.; Wang, K.Z. pH-Sensitive Near-IR emitting dinuclear ruthenium com-plex for recognition, two-photon luminescent imaging, and subcellular localization of cancer cells. ACS Appl. Bio Mater. 2021, 3, 5420–5427. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, C.; Wang, Y.; Chen, Y.; Ding, X.; Yu, B. Luminescent detection of the lipopolysaccharide endotoxin and rapid discrimination of bacterial pathogens using cationic platinum(ii) complexes. RSC Adv. 2017, 7, 32632–32636. [Google Scholar] [CrossRef]
- Lee, S.; Li, J.; Zhou, X.; Yin, J.; Yoon, J. Recent progress on the development of glutathione (GSH) selective fluorescent and colorimetric probes. Coord. Chem. Rev. 2018, 366, 29–68. [Google Scholar] [CrossRef]
- Okda, H.E.; Sayed, S.E.; Ferreira, R.C.M.; Costa, S.P.G.; Raposo, M.M.M.; Martínez-Máñez, R.; Sancenón, F. 4-(4,5-Diphenyl-1H-imidazole-2-yl)-N,N-dimethylaniline-Cu(II) complex, a highly selective probe for glutathione sensing in water-acetonitrile mixtures. Dye. Pigment. 2018, 159, 45–48. [Google Scholar] [CrossRef]
- Zhao, C.; Kong, X.; Shuang, S.; Wang, Y.; Dong, C. An anthraquinone-imidazole-based colorimetric and fluorescent sensor for the sequential detection of Ag+ and biothiols in living cells. Analyst 2020, 145, 3029–3037. [Google Scholar] [CrossRef]
- Tian, F.; Jiang, X.; Dou, X.; Wu, Q.; Wang, J.; Song, Y. Design and synthesis of novel adenine fluorescence probe based on Eu(III) complexes with dtpa-bis(guanine) ligand. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 179, 194–200. [Google Scholar] [CrossRef]
- DU, Z.-H.; Li, X.-Y.; Tian, J.-J.; Zhang, Y.-Z.; Tian, H.-T.; Xu, W.-T. Progress on Detection of Metals Ions by Functional Nucleic Acids Biosensor. Chin. J. Anal. Chem. 2018, 46, 995–1004. [Google Scholar] [CrossRef]
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. A review on detection of heavy metal ions in water—An electrochemical approach. Sens. Actuators B Chem. 2015, 213, 515–533. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Sun, Q.; Shi, J.W.; Xia, H.Y.; Wang, J.L.; Chen, H.Y.; He, H.F.; Shen, L.; Zhao, F.; Zhong, J. A novel triple substituted imidazole fluorescent sensor for Ag plus and its imaging in living cell and zebrafish. J. Photochem. Photobiol. A Chem. 2020, 389, 112244. [Google Scholar] [CrossRef]
- Mehta, P.K.; Oh, E.-T.; Park, H.J.; Lee, K.-H. Ratiometric detection of Cu+ in aqueous buffered solutions and in live cells using fluorescent peptidyl probe to mimic the binding site of the metalloprotein for Cu+. Sens. Actuators B Chem. 2018, 256, 393–401. [Google Scholar] [CrossRef]
- Suresh, S.; Bhuvanesh, N.; Raman, A.; Sugumar, P.; Padmanabhan, D.; Easwaramoorthi, S.; Ponnuswamy, M.N.; Kavitha, S.; Nandhakumar, R. Experimental and theoretical studies of imidazole based chemosensor for Palladium and their biological applications. J. Photochem. Photobiol. A Chem. 2019, 385, 112092. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Mahata, S.; Bandyopadhyay, A.; Mandal, B.B.; Manivannan, V. Application of 2,4,5-tris(2-pyridyl)imidazole as ‘turn-off’ fluorescence sensor for Cu(II) and Hg(II) ions and In Vitro cell imaging. Luminescence 2022, 37, 883–891. [Google Scholar] [CrossRef]
- Ansari, S.N.; Saini, A.K.; Kumari, P.; Mobin, S.M. An imidazole derivative-based chemodosimeter for Zn2+ and Cu2+ ions through “ON–OFF–ON” switching with intracellular Zn2+ detection. Inorg. Chem. Front. 2019, 6, 736–745. [Google Scholar] [CrossRef]
- Mehta, P.K.; Oh, E.-T.; Park, H.J.; Lee, K.-H. Ratiometric fluorescent probe based on symmetric peptidyl receptor with picomolar affinity for Zn2+ in aqueous solution. Sens. Actuators B Chem. 2017, 245, 996–1003. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Q.; Jiang, Z.; Shen, J.; Wu, W.; Liu, X.; Fan, Q.; Huang, W. Recent Progress of SERS Nanoprobe for pH Detecting and Its Application in Biological Imaging. Biosensors 2021, 11, 282. [Google Scholar] [CrossRef]
- Yin, H.J.; Cheng, F.X.; Liu, Z.N.; He, C.X.; Yang, Y.T.; Wang, K.Z. Preparation, characterization, pH titration, and electro-chemical properties of an anthracene-bridged binuclear ruthenium complex containing imidazole. J. Coord. Chem. 2019, 72, 2957–2967. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhu, B.; Chen, J.; Duan, X. Fluorescent Sensing of Fluoride in Cellular System. Theranostics 2015, 5, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.C.; Liu, J.; Gao, S.Y.; Gao, J.J.; Zhou, Y.; Liu, S.Z.; Ma, C.; Zhao, Y.Z. Fluoride ions detection in aqueous media by un-precedented ring opening of fluorescein dye: A novel multimodal sensor for fluoride ions and its utilization in live cell imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 287, 122001. [Google Scholar] [CrossRef] [PubMed]
- El-Maghrabey, M.H.; Watanabe, R.; Kishikawa, N.; Kuroda, N. Detection of hydrogen sulfide in water samples with 2-(4-hydroxyphenyl)-4,5-di(2-pyridyl)imidazole-copper(II) complex using environmentally green microplate fluorescence assay method. Anal. Chim. Acta 2019, 1057, 123–131. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, Y.; Shao, B.; Cheng, J.; Li, X. Recent advances of small-molecule fluorescent probes for detecting biological hydrogen sulfide. Front. Chem. Sci. Eng. 2021, 16, 34–63. [Google Scholar] [CrossRef]
- Strianese, M.; Brenna, S.; Ardizzoia, G.A.; Guarnieri, D.; Lamberti, M.; D’Auria, I.; Pellecchia, C. Imidazo-pyridine-based zinc(ii) complexes as fluorescent hydrogen sulfide probes. Dalton Trans. 2021, 50, 17075–17085. [Google Scholar] [CrossRef]
- Lee, S.; Yuen, K.K.Y.; Jolliffe, K.A.; Yoon, J. Fluorescent and colorimetric chemosensors for pyrophosphate. Chem. Soc. Rev. 2015, 44, 1749–1762. [Google Scholar] [CrossRef]
- Rabha, M.; Sen, B.; Sheet, S.K.; Aguan, K.; Khatua, S. Cyclometalated iridium(iii) complex of a 1,2,3-triazole-based ligand for highly selective sensing of pyrophosphate ion. Dalton Trans. 2022, 51, 11372–11380. [Google Scholar] [CrossRef]
- Ratte, H.T. Bioaccumulation and toxicity of silver compounds: A review. Environ. Toxicol. Chem. 1999, 18, 89–108. [Google Scholar] [CrossRef]
- Asaithambi, G.; Periasamy, V. Phenanthrene-imidazole-based fluorescent sensor for selective detection of Ag+ and F− ions: Real sample application and live cell imaging. Res. Chem. Intermed. 2019, 45, 1295–1308. [Google Scholar] [CrossRef]
- Lai, Q.; Liu, Q.; He, Y.; Zhao, K.; Wei, C.Y.; Wojtas, L.; Shi, X.D.; Song, Z.G. Triazole-imidazole (TA-IM) derivatives as ultra-fast fluorescent probes for selective Ag+ detection. Org. Biomol. Chem. 2018, 16, 7801–7805. [Google Scholar] [CrossRef]
- Li, L.Y.; Wen, Y.L.; Xu, L.; Xu, Q.; Song, S.P.; Zuo, X.L.; Yan, J.; Zhang, W.J.; Liu, G. Development of mercury (II) ion biosen-sors based on mercury-specific oligonucleotide probes. Biosens. Bioelectron. 2015, 75, 433–445. [Google Scholar] [CrossRef]
- Li, G.; Gao, G.; Cheng, J.; Chen, X.; Zhao, Y.; Ye, Y. Two new reversible naphthalimide-based fluorescent chemosensors for Hg2+. Luminescence 2015, 31, 992–996. [Google Scholar] [CrossRef]
- Wang, F.; Wang, K.J.; Kong, Q.; Wang, J.; Xi, D.Z.; Gu, B.W.; Lu, S.; Wei, T.W.; Chen, X.Q. Recent studies focusing on the de-velopment of fluorescence probes for zinc ion. Coord. Chem. Rev. 2021, 429, 213636. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.K.; Angamaly, S.A.; Pradeep, S.D.; Madhusoodhanan, D.T.; Manoharan, D.K.; Mohanan, P.V. A Novel Imidazole Bound Schiff Base as Highly Selective “Turn-on” Fluorescence Sensor for Zn2+ and Colorimetric Kit for Co2+. J. Fluoresc. 2022, 32, 189–202. [Google Scholar] [CrossRef]
- Pandith, A.; Uddin, N.; Choi, C.H.; Kim, H.S. Highly selective imidazole-appended 9,10-N,N′-diaminomethylanthracene fluorescent probe for switch-on Zn2+ detection and switch-off H2PO4− and CN− detection in 80% aqueous DMSO, and applica-tions to sequential logic gate operations. Sens. Actuators B Chem. 2017, 247, 840–849. [Google Scholar] [CrossRef]
- Kırpık, H.; Kose, M.; Ballı, N.J. Tridentate benzimidazole ligand and its metal complexes: Synthesis, characterization, photo physical and sensor properties. Appl. Organomet. Chem. 2020, 34, 5992. [Google Scholar] [CrossRef]
- Mahnashi, M.H.; Mahmoud, A.M.; Alkahtani, S.A.; Ali, R.; El-Wekil, M.M. A novel imidazole derived colorimetric and fluo-rometric chemosensor for bifunctional detection of copper (II) and sulphide ions in environmental water samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117846. [Google Scholar] [CrossRef]
- Pan, J.; Yu, J.; Qiu, S.; Zhu, A.; Liu, Y.; Ban, X.; Li, W.; Yu, H.; Li, L. A novel dibenzimidazole-based fluorescent probe with high sensitivity and selectivity for copper ions. J. Photochem. Photobiol. A Chem. 2020, 406, 113018. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, S.; He, X.; Xu, X.; Zhang, G.; Yang, L.; Kong, L.; Yang, J. A novel tetraphenylethylene-functionalized arylimidazole AIEgen for detections of picric acid and Cu2+. Chem. Pap. 2021, 75, 6297–6306. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Sharma, D.; Bera, R.K.; Crisponi, G.; Callan, J.F. Iron(iii) selective molecular and supramolecular fluorescent probes. Chem. Soc. Rev. 2012, 41, 7195–7227. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, X.; Mi, Z.; Zheng, R.; Fan, J.; Gu, Q.; Zhang, Y. A New Tetrasubstituted Imidazole Based Difunctional Probe for UV-spectrophotometric and Fluorometric Detecting of Fe3+ Ion in Aqueous Solution. Chem. Res. Chin. Univ. 2019, 35, 200–208. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.-R.; Tan, Y.-M.; Zhang, L.; Zhou, C.-H. Comprehensive Insights into Medicinal Research on Imidazole-Based Supramolecular Complexes. Pharmaceutics 2023, 15, 1348. https://doi.org/10.3390/pharmaceutics15051348
Li S-R, Tan Y-M, Zhang L, Zhou C-H. Comprehensive Insights into Medicinal Research on Imidazole-Based Supramolecular Complexes. Pharmaceutics. 2023; 15(5):1348. https://doi.org/10.3390/pharmaceutics15051348
Chicago/Turabian StyleLi, Shu-Rui, Yi-Min Tan, Ling Zhang, and Cheng-He Zhou. 2023. "Comprehensive Insights into Medicinal Research on Imidazole-Based Supramolecular Complexes" Pharmaceutics 15, no. 5: 1348. https://doi.org/10.3390/pharmaceutics15051348
APA StyleLi, S.-R., Tan, Y.-M., Zhang, L., & Zhou, C.-H. (2023). Comprehensive Insights into Medicinal Research on Imidazole-Based Supramolecular Complexes. Pharmaceutics, 15(5), 1348. https://doi.org/10.3390/pharmaceutics15051348






