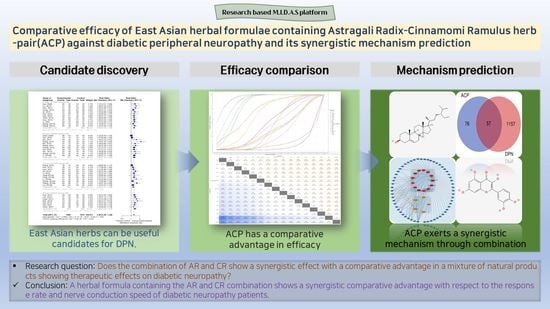
Comparative Efficacy of East Asian Herbal Formulae Containing Astragali Radix–Cinnamomi Ramulus Herb-Pair against Diabetic Peripheral Neuropathy and Mechanism Prediction: A Bayesian Network Meta-Analysis Integrated with Network Pharmacology
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.2.1. Type of Studies
2.2.2. Type of Participants
2.2.3. Type of Interventions
2.2.4. Type of Outcome Measures
2.3. Data Extraction
2.4. Methodological Quality Assessment
2.5. Data Analysis
2.5.1. Pairwise Meta-Analysis
2.5.2. Network Meta-Analysis
2.5.3. Network Pharmacology Analysis of the Synergistic Mechanism of the ACP against DPN
3. Results
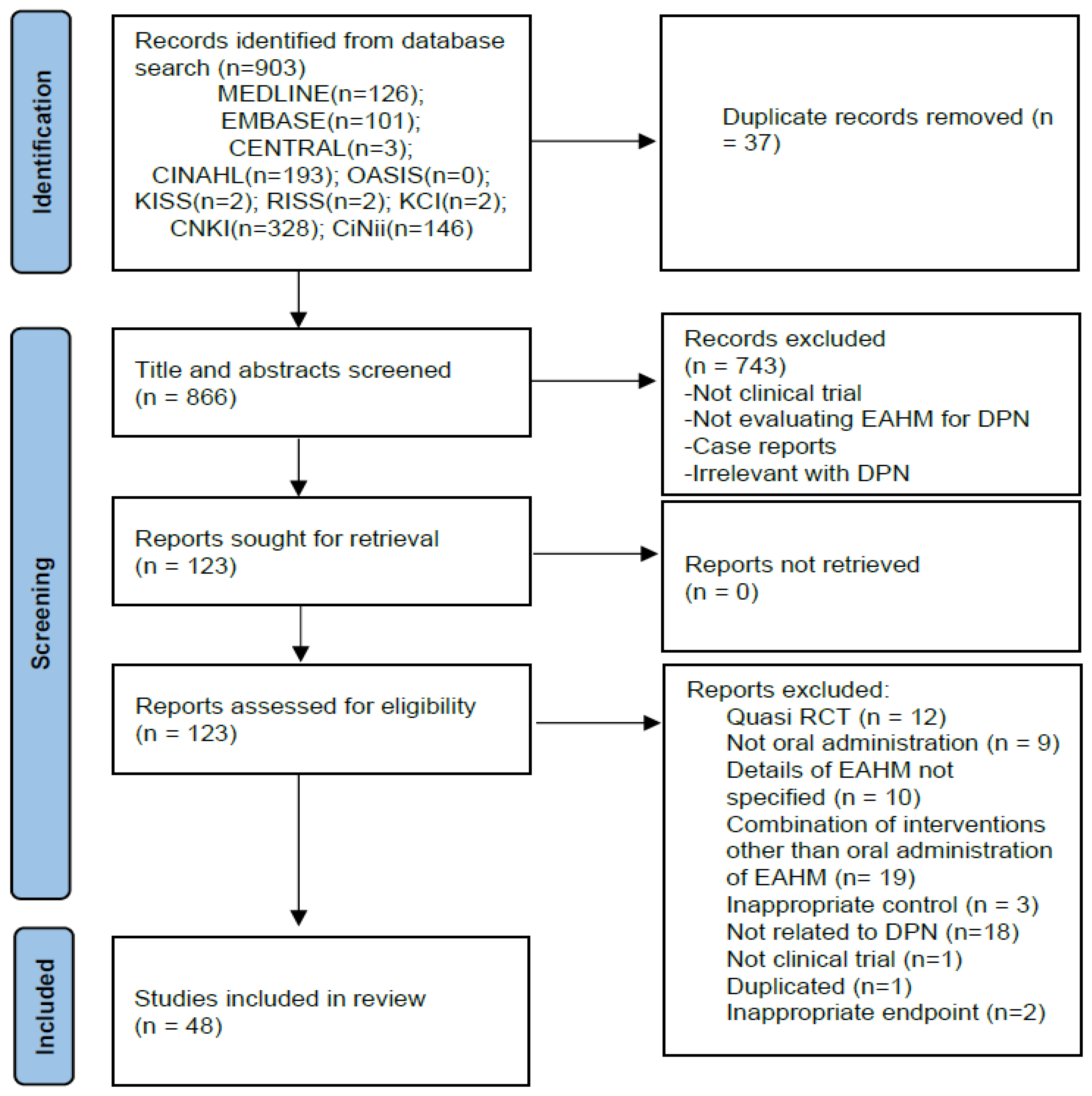
3.1. Study Identification
3.2. Study Characteristics
3.3. Risk of Bias
| Included Study (Reference) | Type of Diabetes/Diagnosis Criteria | Trial Design/Randomization Method | Number of Participants (Male/Female); Age (Mean ± SD) | Interventions | Morbidity Period (Mean ± SD or Range) | Outcome Index (Intergroup Differencies p-Value) | Course of Treatment | Adverse Event (Case/Symptom) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial | Control | Trial | Control | Trial | Control | ||||||
| Jin 2004 [59] | T1DM and T2DM/ WHO criteria, 1999 | Randomized; Single center; Parallel/NR | 103 (54/49) 59.4 ± 5.61 y | 99 (51/48) 58.81 ± 6.01 y | Tangmaitong tablets (0.5 g × 4 t, t.i.d.) | Mecobalamin tablets (500 mg, t.i.d.) | 3.31 ± 1.25 y | 3.82 ± 1.17 y | 1. MMNCV (p > 0.05) 2. MSNCV (p < 0.01) 3. PMNCV (p < 0.05) 4. PSNCV (p < 0.01) | 8 w | Trial: 1 AE/diarrhea Control: 3 AEs/abdomnial pain with diarrhea |
| Sun 2008 [60] | T2DM/WHO criteria, 1999 | Randomized; Single center; Parallel/NR | 30 (18/12) 40~70 y | 30 (16/14) 43~69 y | 1. Ziyinbushenhuoxuetonglou fang decoction (300 mL, b.i.d.) 2. Mecobalamin tablets (500 mg, t.i.d.) | Mecobalamin tablets (500 mg, t.i.d.) | 1~33 m | 1~34 m | 1. Response rate (p < 0.05) | 4 w | NR |
| Shen 2009 [61] | T2DM/WHO criteria, 1999 | Randomized; Single center; Parallel/Block randomization method | 50 (21/29) 60 ± 4.2 y | 50 (27/23) 58.81 ± 6.01 y | Tangmaining capsule (4.5 g × 5 c, b.i.d.) | Mecobalamin tablets (500 mg, t.i.d.) | 8.5 y | 7.9 y | 1. Response rate (p < 0.05) 2. MMNCV (p < 0.05) 3. MSNCV (p < 0.05) 4. UMNCV (p < 0.01) 5. USNCV (p < 0.01) 6. PMNCV (p < 0.05) 7. PSNCV (p > 0.05) 8. TMNCV (p > 0.05) 9. TSNCV (p < 0.01) | 8 w | Trial: No AE Control: No AE |
| Lin 2010 [62] | T2DM/WHO criteria, 1999 | Randomized; Single center; Parallel/NR | 40 (22/18) median 55.6 y | 40 (23/19) median 54.2 y | 1. Tongxinluo capsule (3c, t.i.d.) 2. Mecobalamin tablets (500 mg, t.i.d.) | Mecobalamin tablets (500 mg, t.i.d.) | NR | NR | 1. Response rate (p < 0.05) 2. PMNCV (p < 0.01) 3. PSNCV (p < 0.01) 4. TMNCV (p < 0.01) 5. TSNCV (p < 0.01) | 4 w | NR |
| Wang 2010 [63] | T2DM/WHO criteria, 1999 | Randomized; Single center; Parallel/Simple randomization using random number table | 80 (45/35) 62.68 ± 7.35 y | 79 (43/36) 62.78 ± 7.57 y | 1. Huangqiguizhiwuwu decoction (300 mL, b.i.d.) 2. Mecobalamin injection (0.5 mg, q.d., i.m.) | Mecobalamin injection (0.5 mg, q.d., i.m.) | 7.12 ± 4.25 y | 6.98 ± 4.62 y | 1. Response rate (p < 0.01) 2. MMNCV (p < 0.01) 3. MNSCV (p < 0.01) 4. PMNCV (p < 0.01) 5. PSNCV (p < 0.01) | 12 w | NR |
| Yan 2010 [64] | T2DM/WHO criteria, 1999 | Randomized; Single center; Parallel/NR | 14 (7/7) 57.79 ± 6.73 y | 15 (6/9) 52.53 ± 8.0 y | Shutangluofang granule (b.i.d.) | Methylcobalamine (500 mg, t.i.d.) | 13.14 ± 10.58 m | 10.67 ± 11.14 m | 1. Response rate (p < 0.05) | 12 w | NR |
| Wu 2011 [65] | T1DM and T2DM/Guidelines for the Prevention and Treatment of Diabetes in China, 2004 | Randomized; Single center; Parallel/NR | 30 (16/14) mean 49.9 y | 27 (15/12) mean 48 y | Modified yiqihuoxue decoction (300 mL, b.i.d.) | Vitamin B1 (20 mg, t.i.d.) Vitamin B6 (20 mg, t.i.d.) | mean 12 m | mean 11.4 m | 1. Response rate (p < 0.01) 2. PMNCV (p < 0.01) 3. PSNCV (p < 0.01) | 6 w | NR |
| Gao 2012 [66] | T2DM/WHO criteria, 1999 | Randomized; Single center; Parallel/NR | 30 (16/14) NR | 30 (17/13) NR | 1. Nourishing the liver to stop the wind and tongluo decoction 2. Methylcobalamine (0.5 mg, t.i.d.) | Methylcobalamine (0.5 mg, t.i.d.) | NR | NR | 1. Response rate (p < 0.05) 2. MMNCV (p < 0.01) 3. MSNCV (p < 0.01) 4. PMNCV (p < 0.01) 5. PSNCV (p < 0.01) | 8 w | Trial: 2 AEs/ nausea, upper abdominal discomfort Control: No AE |
| Gong 2013 [67] | T1DM and T2DM/WHO criteria, 1999 | Randomized; Single center; Parallel/NR | 60 (32/28) 56.42 ± 5.28 y | 60 (33/27) 57.16 ± 5.34 y | 1. Modified aconiti decoction (400 mL, b.i.d.) 2. Methylcobalamine (500 mg, t.i.d.) | Methylcobalamine (500 mg t.i.d.) | 7.65 ± 3.84 m | 7.83 ± 3.29 m | 1. Response rate (p < 0.05) 2. PMNCV (p < 0.01) 3. PSNCV (p > 0.05) | 30 d | Trial: No AE Control: No AE |
| Han 2013 [68] | T1DM and T2DM/WHO criteria, 1999 | Randomized; Single center; Parallel/Simple randomization using random number table | 31 (17/14) 54.2 ± 9.6 y | 31 (16/15) 55.3 ± 10.1 y | 1. Modified huangqiguizhiwuwu decoction (400 mL, b.i.d.) 2. Methylcobalamine (0.5 mg, t.i.d.) | Methylcobalamine (0.5 mg, t.i.d.) | NR | NR | 1. Response rate (p < 0.05) 2. PMNCV (p < 0.01) 3. PSNCV (p < 0.01) 4. MMNCV (p < 0.01) 5. MSNCV (p < 0.01) | 8 w | NR |
| Zhang 2013a [69] | T2DM/WHO criteria, 1999 | Randomized; Single center; Parallel/NR | 30 (16/14) 54.32 ± 7.14 y | 30 (15/15) 56.24 ± 7.40 y | 1. Mudan tong luo fang (b.i.d.) 2. α-Lipoic acid injection (600 mg, q.d., i.v. drip) | α-Lipoic acid injection (600 mg, q.d., i.v. drip) | 8.3 ± 1.67 y | 8.5 ± 1.54 y | 1. Response rate (p < 0.05) 2. MMNCV (p < 0.05) 3. MSNCV (p < 0.05) 4. PMNCV (p < 0.05) 5. PSNCV (p < 0.05) | 3 w | NR |
| Zhang 2013b [70] | T1DM and T2DM/Only diagnostic criteria are presented without reference. | Randomized; Single center; Parallel/NR | 30 Total 60 (36/14) 56 ± 8 y | 30 Total 60 (36/14) 56 ± 8 y | Tang bao kang (20 pills, t.i.d.) | 1. Methylcobalamine (500 mg, t.i.d.) 2. Vitamin B1 (30 mg, t.i.d.) 3. Vitamin B6 (30 mg, t.i.d.) | Total 5~10 y | Total 5~10 y | 1. Response rate (p < 0.01) 2. MMNCV (p < 0.01) 3. MSNCV (p < 0.01) 4. UMNCV (p < 0.01) 5. USNCV (p < 0.01) 6. PMNCV (p < 0.01) 7. PSNCV (p < 0.01) | 24 w | Trial: No AE Control: 1 AE/skin rash |
| Guo 2014 [71] | T1DM and T2DM/WHO criteria, 1999 | Randomized; Single center; Parallel/NR | 32 (19/13) 64.78 ± 8.90 y | 32 (15/17) 65.59 ± 8.35 y | 1. Modified huangqiguizhiwuwu decoction (b.i.d.) 2. Mecobalamin tablets (0.5 mg, t.i.d.) 3. Gabapentin (600 mg, t.i.d.) | 1. Mecobalamin tablets (0.5 mg, t.i.d.) 2. Gabapentin (600 mg, t.i.d.) | NR | NR | 1. Response rate (p < 0.01) | 8 w | NR |
| Yang 2014a [73] | T2DM/Diagnostic criteria of Chinese guidelines for the prevention and treatment of type 2 diabetes, 2008 | Randomized; Single center; Parallel/NR | 60 (35/25) 51.30 ± 6.03 y | 60 (37/23) 51.26 ± 5.38 y | 1. Shenqixuebi feng (b.i.d.) 2. α-Lipoic acid injection (0.3 g, q.d., i.v. drip) 3. Mecobalamin injection (0.5 mg, q.d., i.v. drip) | 1. α-Lipoic acid injection (0.3 g, q.d., i.v. drip) 2. Mecobalamin injection (0.5 mg, q.d., i.v. drip) | 3.65 ± 1.12 y | 3.36 ± 1.18 y | 1. Response rate (p < 0.05) | 4 w | NR |
| Yang 2014b [72] | T1DM and T2DM/WHO criteria, 1999 | Randomized; Single center; Parallel/NR | 36 (23/13) 47.8 ± 8.3 y | 36 (20/16) 46.5 ± 8.1 y | 1. Modified huangqiguizhiwuwu decoction (200 mL, q.d.) 2. Methylcobalamine injection (500 mg, q.d., i.m.) | 1. Methylcobalamine injection (500 mg, q.d., i.m.) | 4.1 ± 1.3 m | 3.9 ± 1.4 m | 1. Response rate (p < 0.05) | 4 w | NR |
| Qi 2015 [74] | T1DM and T2DM/WHO criteria, 1999 | Randomized; Single center; Parallel/NR | 32 (17/15) 53.2 ± 7.1 y | 32 (16/16) 52.4 ± 7.0 y | 1. Mudan granule (7 g, t.i.d.) 2. 0.9% Sodium chloride 200 mL + α-Lipoic acid injection (450 mg, q.d., i.v. drip) | 1. 0.9% Sodium chloride 200 mL + α-Lipoic acid injection (450 mg, q.d., i.v. drip) | 2.3 ± 2.1 y | 2.6 ± 1.9 y | 1. Response rate (p < 0.05) 2. PMNCV (p < 0.01) 3. PSNCV (p < 0.01) | 4 w | Trial: No AE Control: No AE |
| Wang 2015 [75] | T2DM/TCM diagnosis and treatment plan for 95 diseases in 22 specialties | Randomized; Single center; Parallel/NR | 40 (20/20) mean 68.5 y | 40 (23/17) mean 71.2 y | 1. Yinxinshu capsule (3c, t.i.d.) 2. Maixuekang capsule (3c, t.i.d.) | 1. Oryzanol (20 mg, t.i.d.) 2. Vitamin B1 (10 mg, t.i.d.) 3. Adenosylcobalamin (1 mg, t.i.d.) | 10~12 y | 10~12 y | 1. Response rate (p < 0.05) | 4 w | Trial: No AE Control: No AE |
| Xue 2015 [76] | T2DM/WHO criteria, 1999 | Randomized; Single center; Parallel/Simple randomization using random number table | 42 (23/19) 36~78 y | 42 (22/20) 35~78 y | 1. Modified liutengshuilushexian decoction (150 mL, q.d.) | 1. Methylcobalamine tablet (0.5 mg, t.i.d.) | 28~73 d | 30~73 d | 1. Response rate (p < 0.01) 2. MSNCV (p < 0.01) 3. TSNCV (p < 0.01) 4. PSNCV (p < 0.01) | 3 w | Trial: No AE Control: No AE |
| Guo 2016 [77] | T1DM and T2DM/Diabetic peripheral neuropathy diagnosis and treatment guidelines of China, 2009 | Randomized; Single center; Parallel/Simple randomization using random number table | 51 (26/25) 69.54 ± 5.06 y | 51 (28/23) 69.78 ± 5.96 y | 1. Qitengtongluo decoction (b.i.d.) 2. Epalrestat (50 mg, 1t, t.i.d.) | 1. Epalrestat (50 mg, 1 t, t.i.d.) | 1.91 ± 2.09 y | 6.59 ± 1.91 y | 1. Response rate (p < 0.05) 2. NCSS (p < 0.05) 3. MSNCV (p < 0.05) 4. TSNCV (p < 0.05) 5. PMNCV (p < 0.05) 6. PSNCV (p < 0.05) | 12 w | NR |
| Han 2016 [78] | TIDM and T2DM/Diagnostic criteria for diabetic peripheral neuropathy formulated by the Chinese Medical Doctor Association | Randomized; Single center; Parallel/NR | 20 (12/8) 54.3 ± 7.2 y | 20 (11/9) 53.7 ± 6.8 y | 1. Zhanjin tongluo Chinese medicine (b.i.d.) 2. Mecobalamin tablets (500 mg, t.i.d.) | 1. Mecobalamin tablets (500 mg, t.i.d.) | 2.4 ± 1.2 y | 2.6 ± 1.3 y | 1. Response rate (p < 0.05) | 4 w | NR |
| Lan 2016 [79] | T2DM/WHO criteria, 1999 | Randomized; Single center; Parallel/NR | 54 Other information NR | 54 Other information NR | Yiqihuoxue tongluo capsule (1.2 g, t.i.d.) | Epalrestat tablets (50 mg, t.i.d.) | NR | NR | 1. Response rate (p < 0.05) 2. PMNCV (p < 0.05) | 12 w | Trial: No AE Control: No AE |
| Mo 2016 [82] | T2DM/Guidelines for the Prevention and Treatment of Type 2 Diabetes in China, 2013 | Randomized; Single center; Parallel/Simple randomization using random number table | 33 (19/14) 65.28 ± 9.098 y | 32 (17/15) 62.34 ± 8.168 y | Yangyinjiedu decoction (300 mL, b.i.d.) | Methylcobalamine (0.5 mg t.i.d.) | 2~23 y | 2~19 y | 1. Response rate (p < 0.01) | 8 w | NR |
| Wang 2016 [83] | T2DM/Guidelines for the Prevention and Treatment of Type 2 Diabetes in China, 2013 | Randomized; Single center; Parallel/Simple randomization using random number table | 124 (72/52) 57.3 ± 6.8 y | 103 (58/45) 58.1 ± 7.2 y | Modified tangbitong feng (150 mL, b.i.d.) | Lifestyle modification | 22.1 ± 5.4 m | 23.5 ± 4.8 m | 1. Response rate (p < 0.01) | 8 w | Trial: No AE Control: No AE |
| Li 2016a [80] | T1DM and T2DM/Diabetic peripheral neuropathy diagnosis and treatment of China, 2009 | Randomized; Single center; Parallel/Simple randomization using random number table | 30 (18/12) 49.6 ± 5.6 y | 30 (17/13) 50.3 ± 5.4 y | 1. Wenyanghuoxuetongbi feng (b.i.d.) 2. Methylcobalamine (0.5 mg, t.i.d.) | 1. Methylcobalamine (0.5 mg, t.i.d.) | 18.21 ± 12.37 m | 17.97 ± 12.54 m | 1. Response rate (p < 0.01) 2. TSNCV (p < 0.01) 3. PSNCV (p < 0.05) | 8 w | Trial: No AE Control: No AE |
| Zhang 2016a [85] | T1DM and T2DM/WHO criteria, 1999 | Randomized; Single center; Parallel/Simple randomization using random number table | 48 (26/22) 54.6 y | 48 (28/20) 55.2 y | 1. Huangichifeng decoction combined Dangguisini decoction (q.d.) 2. Methylcobalamine injection (500 mg, q.d., i.m.) | 1. Methylcobalamine injection (500 mg, q.d., i.v.) | 2.8 y | 3.2 y | 1. Response rate (p < 0.01) 2. MSNCV (p < 0.01) 3. USNCV (p < 0.01) 4. PMNCV (p < 0.01) 5. TMNCV (p < 0.01) | 4 w | NR |
| Li 2016b [81] | T2DM/WHO criteria, 1999 | Randomized; Single center; Parallel/NR | 60 (37/23) 57 y | 60 (35/25) 56 y | Huangzhitongnaoluo capsule (3c, t.i.d.) | Mecobalamin dispersible tablets (500 mg, t.i.d.) | 1~13 y | 1~12 y | 1. Response rate (p < 0.05) 2. MSNCV (p < 0.05) 3. TMNCV (p < 0.05) | 12 w | NR |
| Zhang 2016b [84] | T2DM/WHO criteria, 1999 | Randomized; Single center; Parallel/Simple randomization using random number table | 60 (36/24) 55.3 ± 6.4 y | 60 (35/25) 55.6 ± 5.5 y | 1. Qiming granule (4.5 g, t.i.d.) 2. Nimodipine injection (8 mg, q.d., i.v. drip) | 1. Nimodipine injection (8 mg, q.d., i.v. drip) | 2.0 ± 1.1 y | 2.2 ± 1.0 y | 1. Response rate (p < 0.01) 2. MMNCV (p < 0.01) 3. MSNCV (p < 0.01) 4. UMNCV (p < 0.05) 5. USNCV (p < 0.01) 6. TMNCV (p < 0.05) 7. TSNCV (p < 0.01) | 12 w | Trial: No AE Control: 1 AE/mild dizziness |
| Chen 2017 [86] | T2DM/Guidelines for the Prevention and Treatment of Type 2 Diabetes in China, 2013 | Randomized; Single center; Parallel/Simple randomization using random number table | 30 (14/16) 38.72 ± 20.02 y | 30 (13/17) 39.11 ± 19.57 y | Dagguisini decoction (300 mL, b.i.d.) | Epalrestat capsule (50 mg, t.i.d.) | 4.32 ± 2.05 y | 4.20 ± 2.01 y | 1. Response rate (p < 0.05) | 12 w | Trial: No AE Control: No AE |
| Shi 2017 [87] | T2DM/WHO criteria, 1999 | Randomized; Single center; Parallel/NR | 32 (20/12) 38.7 ± 8.1 y | 32 (22/10) 40.3 ± 10.1 y | 1. Fufang danshen dripping pill (10 pill, t.i.d.) | 1. Methylcobalamine (0.5 mg, t.i.d.) 2. Epalrestat (50 mg, t.i.d.) | 3.87 ± 1.5 y | 3.69 ± 1.3 y | 1. TSNCV (p < 0.01) | 15 w | NR |
| Wang 2017 [88] | T2DM/WHO criteria, 1999 | Randomized; Single center; Parallel/Simple randomization using random number table | 30 (15/15) 58.76 ± 4.32 y | 30 (16/14) 57.21 ± 3.56 y | Dangguisini decoction (200 mL, b.i.d.) | Mecobalamin tablets (500 mg, t.i.d.) | 3.56 ± 1.21 y | 3.84 ± 1.36 y | 1. Response rate (p < 0.05) 2. MMNCV (p > 0.05) 3. MSNCV (p > 0.05) 4. PMNCV (p < 0.05) 5. PSNCV (p < 0.05) 6. TMNCV (p < 0.05) 7. TSNCV (p < 0.05) | 8 w | NR |
| Chen 2018 [89] | T2DM/WHO criteria, 1999 | Randomized; Single center; Parallel/Simple randomization using random number table | 40 (19/21) 55.8 ± 4.7 y | 40 (20/20) 56.2 ± 2.8 y | 1. Dangguisinin decoction (b.i.d.) 2. Mecobalamin tablets (500 mg, t.i.d.) | Mecobalamin tablets (500 mg, t.i.d.) | 3.6 ± 1.8 y | 2.4 ± 2.1 y | 1. Response rate (p < 0.05) | 4 w | Trial: 2 AEs/skin rash, gastrointestinal discomfort Control: 3 AEs/diarrhea (2), skin rash |
| Dai 2018 [90] | T2DM/Guidelines for the Prevention and Treatment of Type 2 Diabetes in China, 2013 | Randomized; Single center; Parallel/NR | 40 45~85 y Other information NR | 40 45~85 y Other information NR | Modified huangqiguizhiwuwu decoction (500 mL, b.i.d.) | Epalrestat capsule (50 mg, t.i.d.) | NR | NR | 1. Response rate (p < 0.05) 2. UMNCV (p < 0.05) 3. USNCV (p < 0.05) 4. PMNCV (p < 0.05) 5. PSNCV (p < 0.05) | 3 w | NR |
| Hu 2018 [92] | T2DM/Guidelines for the Prevention and Treatment of Type 2 Diabetes in China, 2013 | Randomized; Single center; Parallel/NR | 31 (13/18) 55.45 ± 11.52 y | 31 (15/16) 53.76 ± 2.03 y | 1. Modified jiajianhuangqiguizhiwuwu decoction (200 mL, b.i.d.) 2. Methylcobalamine (0.5 mg, t.i.d.) | 1. Methylcobalamine tablet (0.5 mg, t.i.d.) | 7.13 ± 2.01 y | 6.52 ± 1.95 y | 1. Response rate (p < 0.05) 2. PMNCV (p < 0.05) | 8 w | NR |
| Huang 2018 [93] | T1DM and T2DM/Diagnostic and therapeutic effect evaluation criteria of diseases and syndromes in traditional Chinese medicine, 1994 | Randomized; Single center; Parallel/Simple randomization using random number table | 120 (52/68) 51.3 ± 11.4 y | 120 (51/69) 50.9 ± 11.6 y | Matong powder (7 g, t.i.d.) | Methylcobalamine tablet (0.5 mg, t.i.d.) | 8.92 ± 8.6 m | 8.97 ± 8.5 m | 1. Response rate (p < 0.05) 2. PMNCV (p < 0.05) 3. TSNCV (p < 0.05) | 8 w | Trial: 3 AEs/ Abdominal bloating with anorexia (3) Control: 2 AEs/Abdominal bloating with anorexia (2) |
| She 2018 [94] | T2DM/Guidelines for the Prevention and Treatment of Type 2 Diabetes in China, 2010 | Randomized; Single center; Parallel/Simple randomization using random number table | 30 (18/12) 63.35 ± 7.12 y | 30 (17/13) 65.13 ± 6.21 y | 1. Huangqiguizhiwuwu granule (b.i.d.) 2. Mecobalamin tablet (1 mg, t.i.d.) | Mecobalamin tablet (1 mg, t.i.d.) | 3.31 ± 2.06 y | 3.82 ± 1.97 y | 1. Response rate (p < 0.05) | 6 w | NR |
| Xin 2018 [95] | T2DM/Diabetic peripheral neuropathy diagnosis and treatment of China, 2009 | Randomized; Single center; Parallel/NR | 30 Total 60 (36/24) 55.3 y | 30 Total 60 (36/24) 55.3 y | 1. Mongolian medicine garidi-13 weiwan (3 g, q.d.) | Mecobalamin tablet (0.5 mg, t.i.d.) | Total 4.2 y | Total 4.2 y | 1. Response rate (p < 0.05) | 4 w | NR |
| Gao 2019 [91] | T1DM and T2DM/WHO criteria, 1999 | Randomized; Single center; Parallel/NR | 50 (26/24) 60.83 ± 5.26 y | 50 (25/25) 61.17 ± 6.05 y | 1. Modified shegmaisan (300 mL, b.i.d.) 2. Mecobalmin tablet (500 mg, t.i.d.) | Mecobalmin tablet (500 mg, t.i.d.) | 3.82 ± 1.04 y | 3.77 ± 1.12 y | 1. Response rate (p < 0.05) 2. MMNCV (p > 0.05) 3. MSNCV (p > 0.05) 4. PMNCV (p < 0.05) 5. PSNCV (p < 0.05) 6. TMNCV (p < 0.05) 7. TSNCV (p < 0.05) | 8 w | Trial: No AE Control: No AE |
| Wu 2019 [99] | T2DM/Guidelines for the Prevention and Treatment of Type 2 Diabetes in China, 2013 | Randomized; Single center; Parallel/Simple randomization using random number table | 30 (16/14) 57.60 ± 7.20 y | 30 (16/14) 57.03 ± 7.63 y | Taohongsiwu decoction (t.i.d.) | Epalrestat tablet (50 mg, t.i.d.) | 4.3 y | 4.3 y | 1. Response rate (p < 0.05) 2. MSNCV (p < 0.05) 3. PSNCV (p < 0.05) | 4 w | Trial: No AE Control: No AE |
| Yi 2019 [100] | T1DM and T2DM/Diabetic neuropathy diagnosis criteria of American Diabetes Association, 2017 | Randomized; Single center; Parallel/Simple randomization using random number table | 60 (31/29) 61.36 ± 4.37 y | 60 (29/31) 61.53 ± 4.64 y | Mongolian medicine zhenbo pills (0.2 g × 15 p, b.i.d.) | α-Lipoic acid tablet (0.3 g × 2 c, q.d.) | 8.23 ± 3.21 y | 8.23 ± 3.12 y | 1. MMNCV (p < 0.05) 2. MSNCV (p < 0.05) 3. PMNCV (p < 0.05) 4. PSNCV (p < 0.05) | 24 w | Trial: 5 AEs/ nausea (2), anorexia (3) Control: 6 AEs/ nausea (2), gastric pain (2) |
| Ji 2019 [96] | T2DM/Guidelines for the Prevention and Treatment of Type 2 Diabetes in China, 2010 | Randomized; Single center; Parallel/Simple randomization using random number table | 54 (32/22) 54.47 ± 9.81 y | 53 (33/20) 54.81 ± 9.44 y | 1. Yangyinzhuyu decoction (150 mL, b.i.d.) 2. Epalrestat tablet (50 mg, t.i.d.) | Epalrestat tablet (50 mg, t.i.d.) | 10.24 ± 3.08 y | 10.53 ± 2.66 y | 1. Response rate (p < 0.05) | 90 d | Trial: No AE Control: No AE |
| Liu 2019a [97] | T2DM/Guidelines for the Prevention and Treatment of Type 2 Diabetes in China, 2013 | Randomized; Single center; Parallel/Simple randomization using random number table | 40 Other information NR | 40 Other information NR | 1. Shengjinsan combined Taohongyin (200 mL, b.i.d.) 2. Mecobalamin tablet (500 mg, t.i.d.) | Mecobalamin tablet (500 mg, t.i.d.) | NR | NR | 1. MMNCV (p < 0.05) 2. MSNCV (p < 0.05) 3. TMNCV (p < 0.05) 4. TSNCV (p < 0.05) | 4 w | NR |
| Liu 2019b [98] | T2DM/Guidelines for the Prevention and Treatment of Type 2 Diabetes in China, 2013 | Randomized; Single center; Parallel/Simple randomization | 45 (27/18) 58.77 ± 4.26 y | 45 (26/19) 59.46 ± 4.77 y | 1. Huangqiguizhiwuwu decoction (400 mL, b.i.d.) 2. Epalrestat tablets (t.i.d.) 3. Mecobalamin tablet (t.i.d.) | 1. Epalrestat tablets (t.i.d.) 2. Mecobalamin tablet (t.i.d.) | 3.28 ± 1.45 m | 3.31 ± 1.13 m | 1. Response rate (p < 0.05) | 8 w | NR |
| Chen 2021 [101] | T2DM/Guidelines for the Prevention and Treatment of Type 2 Diabetes in China, 2013 | Randomized; Single center; Parallel/NR | 28 (15/13) 57.2 ± 8.1 y | 29 (16/13) 56.5 ± 7.6 y | 1. Zicuijuanbi decoction (150 mL, b.i.d.) 2. Normal saline injection (250 mL, i.v.) | 1. gabapentin capsule (0.3 g, t.i.d.) 2. Normal saline injection (250 mL, i.v.) | 15.57 ± 3.68 y | 14.59 ± 4.35 y | 1. Response rate (p < 0.05) | 10 w | NR |
| Hou 2021 [102] | T2DM/WHO criteria, 1999 | Randomized; Single center; Parallel/NR | 39 (24/15) 56.74 ± 11.79 y | 28 (18/10) 55.83 ± 10.60 y | Jiuchongdan (40 pills, t.i.d.) | Mecobalamin tablet (500 mg, t.i.d.) | 15.28 ± 11.23 m | 16.72 ± 10.96 m | 1. Response rate (p < 0.05) 2. PSNCV (p < 0.05) 3. MSNCV (p < 0.05) 4. USNCV (p < 0.05) | 12 w | NR |
| Li 2021 [103] | T2DM/Guidelines for the Prevention and Treatment of Diabetic Peripheral Neuropathy by Traditional Chinese Medicine, 2011 | Randomized; Single center; Parallel/Simple randomization using random number table | 41 (22/19) 59.81 ± 5.63 y | 41 (23/18) 60.20 ± 5.62 y | 1. Huangqiguizhiwuwu decoction (200 mL, t.i.d.) combined Mudan granule (7 g, t.i.d.) 2. Mecobalmin tablet (500 mg, t.i.d.) | 1. Mecobalamin tablet (500 mg, t.i.d.) | 3.15 ± 0.45 y | 3.12 ± 0.43 y | 1. Response rate (p < 0.05) 2. MMNCV (p < 0.05) 3. MSNCV (p < 0.05) 4. PMNCV (p < 0.05) 5. PSNCV (p < 0.05) | 8 w | Trial: 5 AEs/diarrhea (1), nausea (1), constipation (2), dizziness (1) Control: 1 AE/ nausea (1) |
| Wang 2021a [105] | T1DM and T2DM/diagnostic criteria are presented without reference | Randomized; Single center; Parallel/Simple randomization using random number table | 30 (16/14) 64.63 ± 4.72 y | 30 (17/13) 64.71 ± 4.68 y | 1. Yiqiyangyintongluo decoction (200 mL, b.i.d.) 2. Epalrestat tablets (50 mg, t.i.d.) | 1. Epalrestat tablets (50 mg, t.i.d.) | 6.14 ± 1.24 y | 6.12 ± 1.22 y | 1. Response rate (p < 0.05) | 12 w | NR |
| Wang 2021b [104] | T2DM/diagnostic criteria are presented without reference | Randomized; Single center; Parallel/Simple randomization using random number table | 50 (34/16) 67.13 ± 6.29 y | 50 (32/18) 67.13 ± 6.29 y | 1. Taohongsiwu decoction (b.i.d.) 2. Mecobalmin capsule (0.5 mg, t.i.d.) | 1. Mecobalamin capsule (0.5 mg, t.i.d.) | 1.57 ± 0.51 y | 1.42 ± 0.83 y | 1. MMNCV (p < 0.05) 2. MSNCV (p < 0.05) 3. PMNCV (p < 0.05) 4. PSNCV (p < 0.05) 5. TMNCV (p < 0.05) 6. TSNCV (p < 0.05) | 4 w | NR |
| Zhang 2021 [106] | T2DM/Guidelines for the Prevention and Treatment of Diabetic Peripheral Neuropathy by Traditional Chinese Medicine, 2011 | Randomized; Single center; Parallel/NR | 74 Total 148 (78/70) 59.64 ± 8.94 y | 74 Total 148 (78/70) 59.64 ± 8.94 y | 1. Buqi Huoxue Zhitong decoction (b.i.d.) 2. α-Lipoic acid injection (0.6 g, q.d.) combined 0.9% Sodium chloride injection (250 mL, q.d.) | 1. α-Lipoic acid injection (0.6 g, q.d.) combined 0.9% Sodium chloride injection (250 mL, q.d.) | Total 9.33 ± 1.25 y | Total 9.33 ± 1.25 y | 1. TSNCV (p < 0.05) 2. PSNCV (p < 0.05) | 8 w | NR |
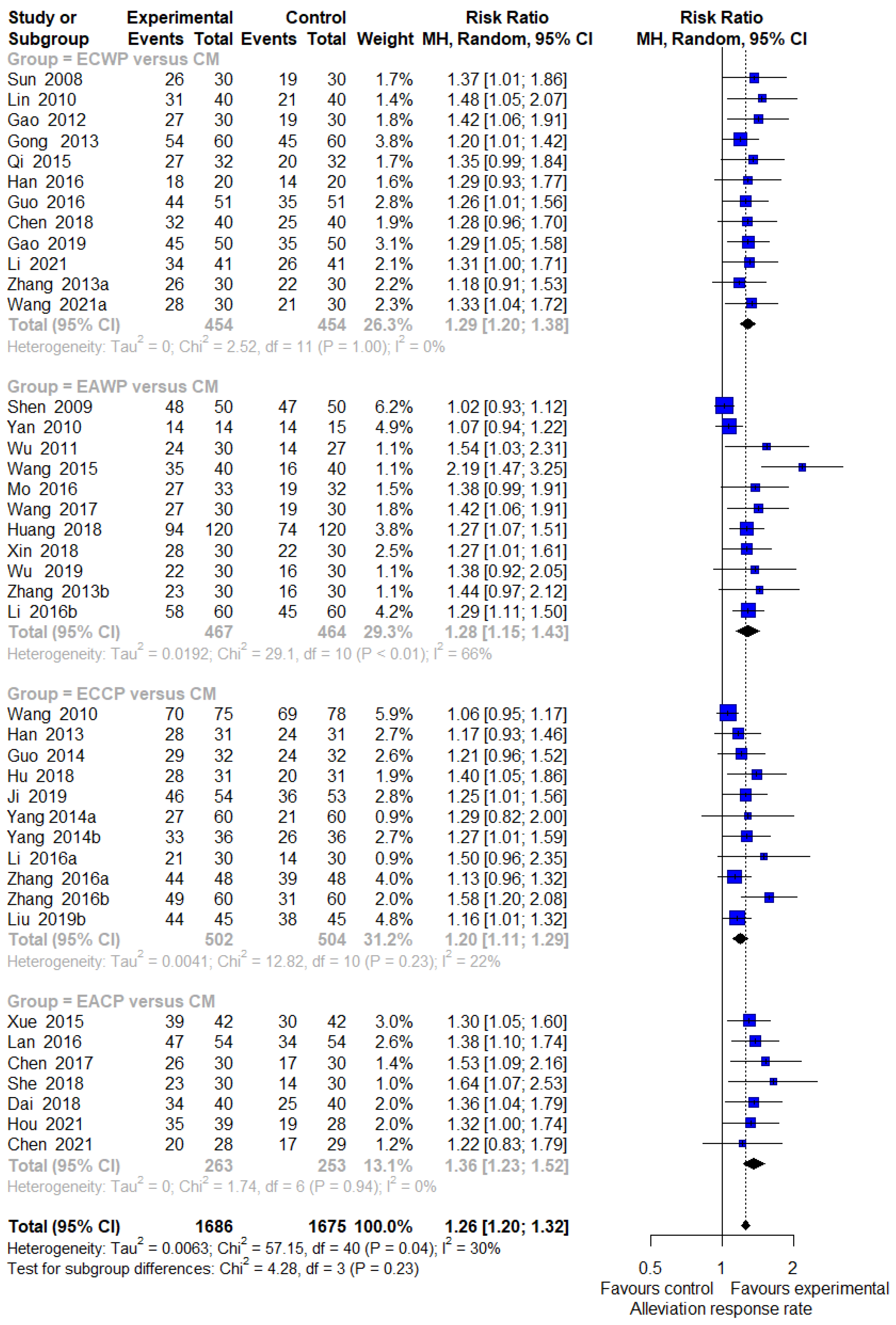
3.4. Pairwise Meta-Analysis
3.4.1. Response Rate
3.4.2. Motor Nerve Outcomes: MMNCV, PMNCV, UMNCV, TMNCV
3.4.3. Sensory Nerve Outcomes: MSNCV, PSNCV, USNCV, TSNCV
3.4.4. Safety Assessment
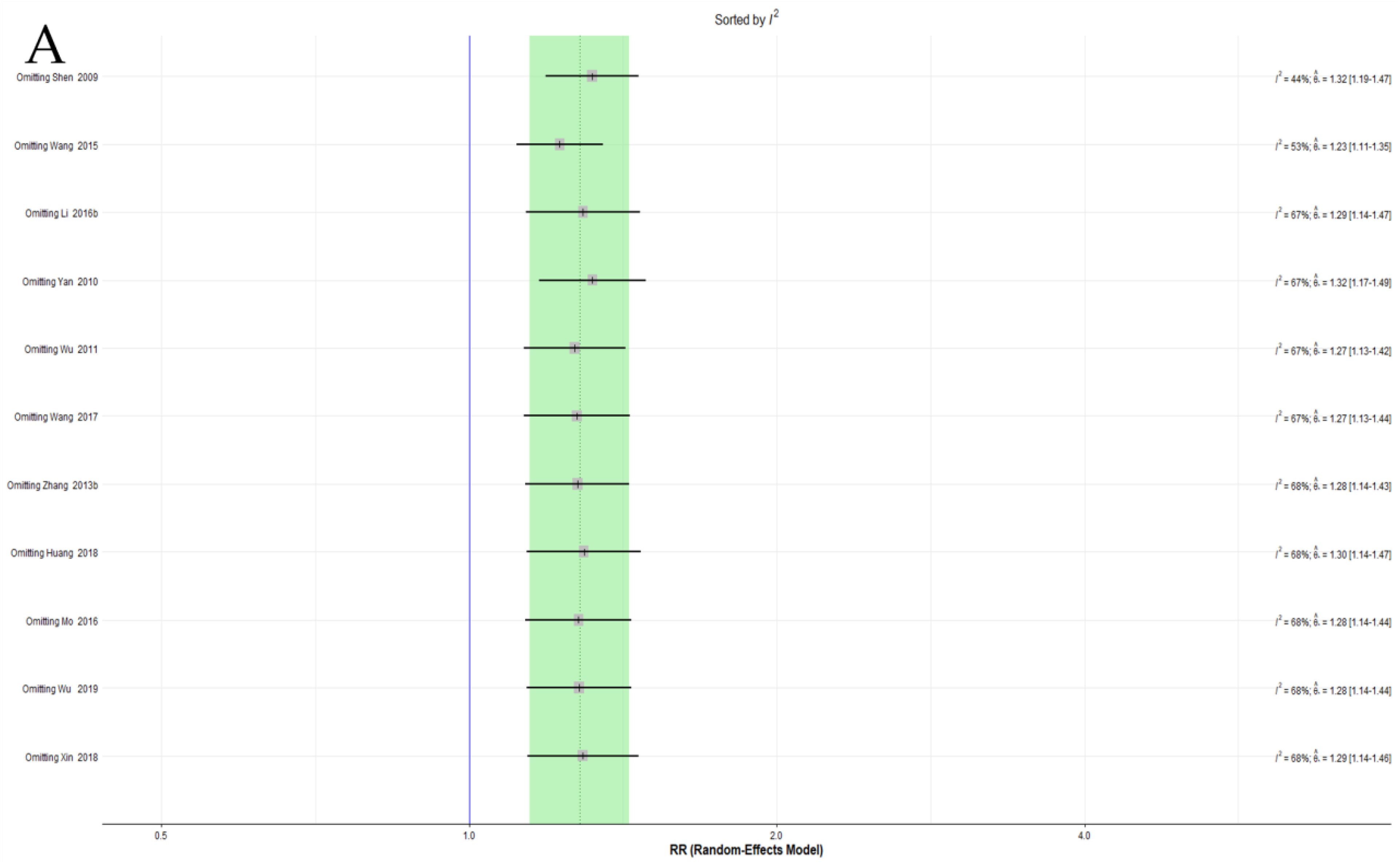
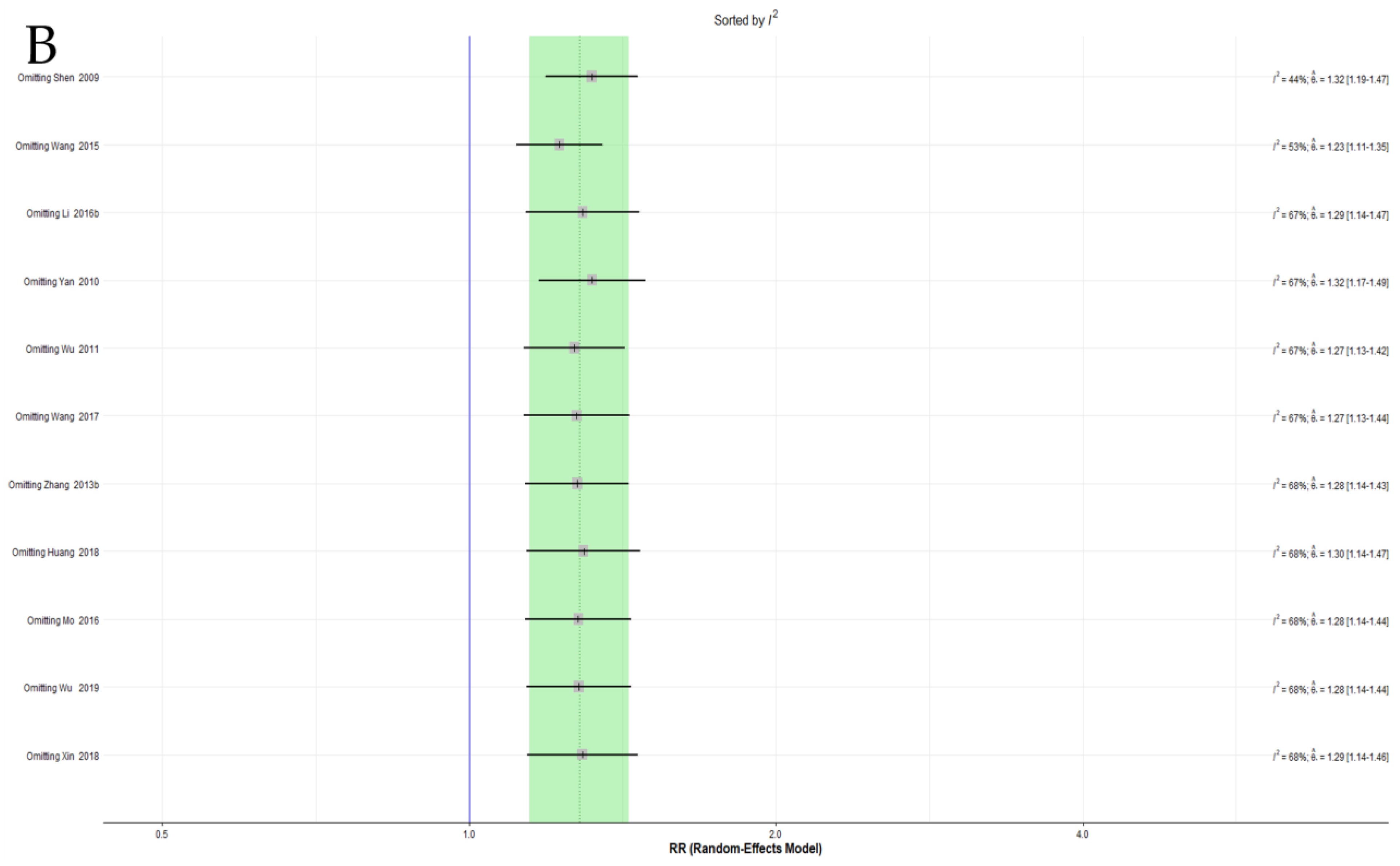
3.4.5. Sensitivity Analysis
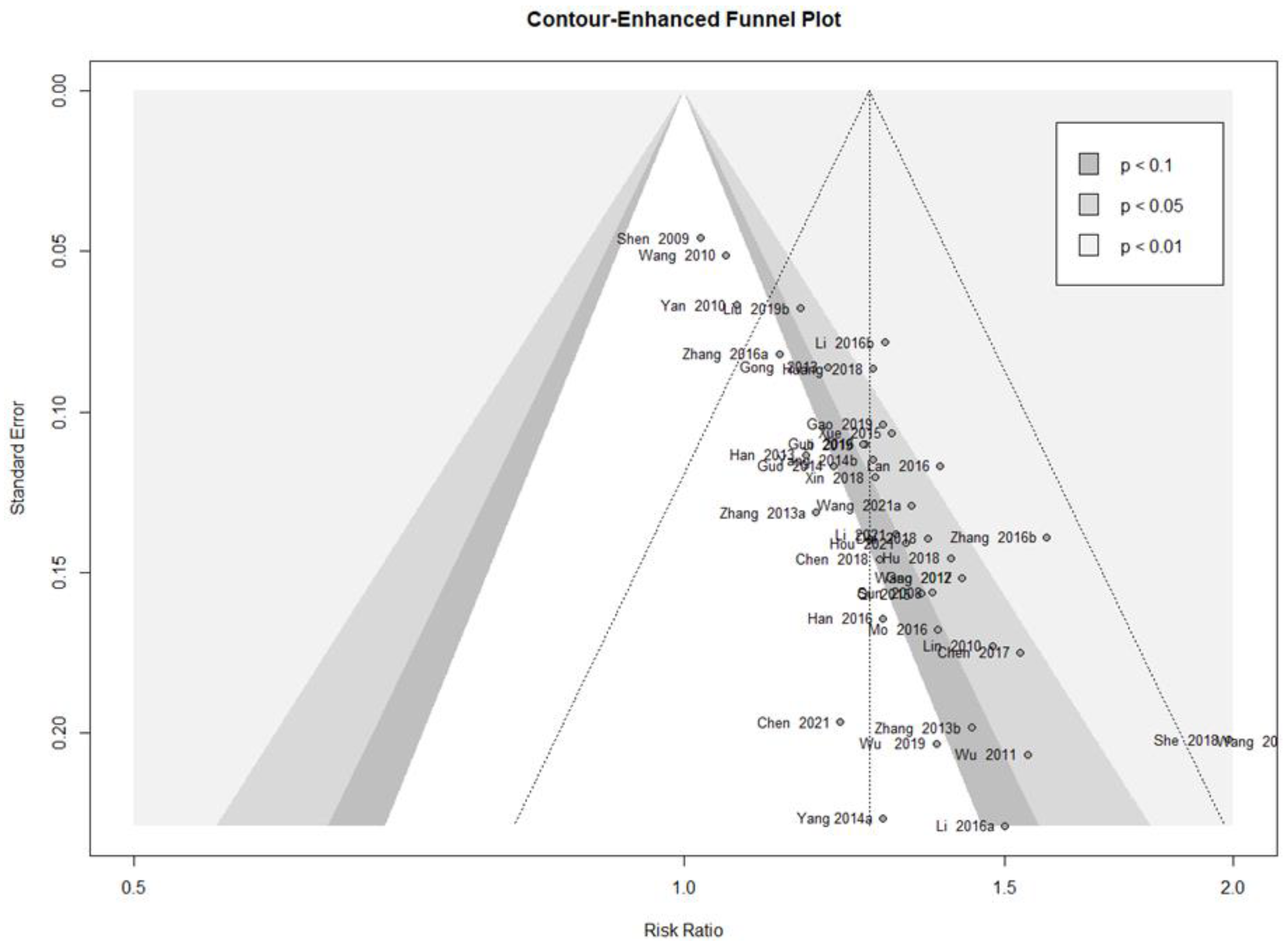
3.4.6. Publication Bias
3.4.7. Quality of Evidence According to Outcome Measures
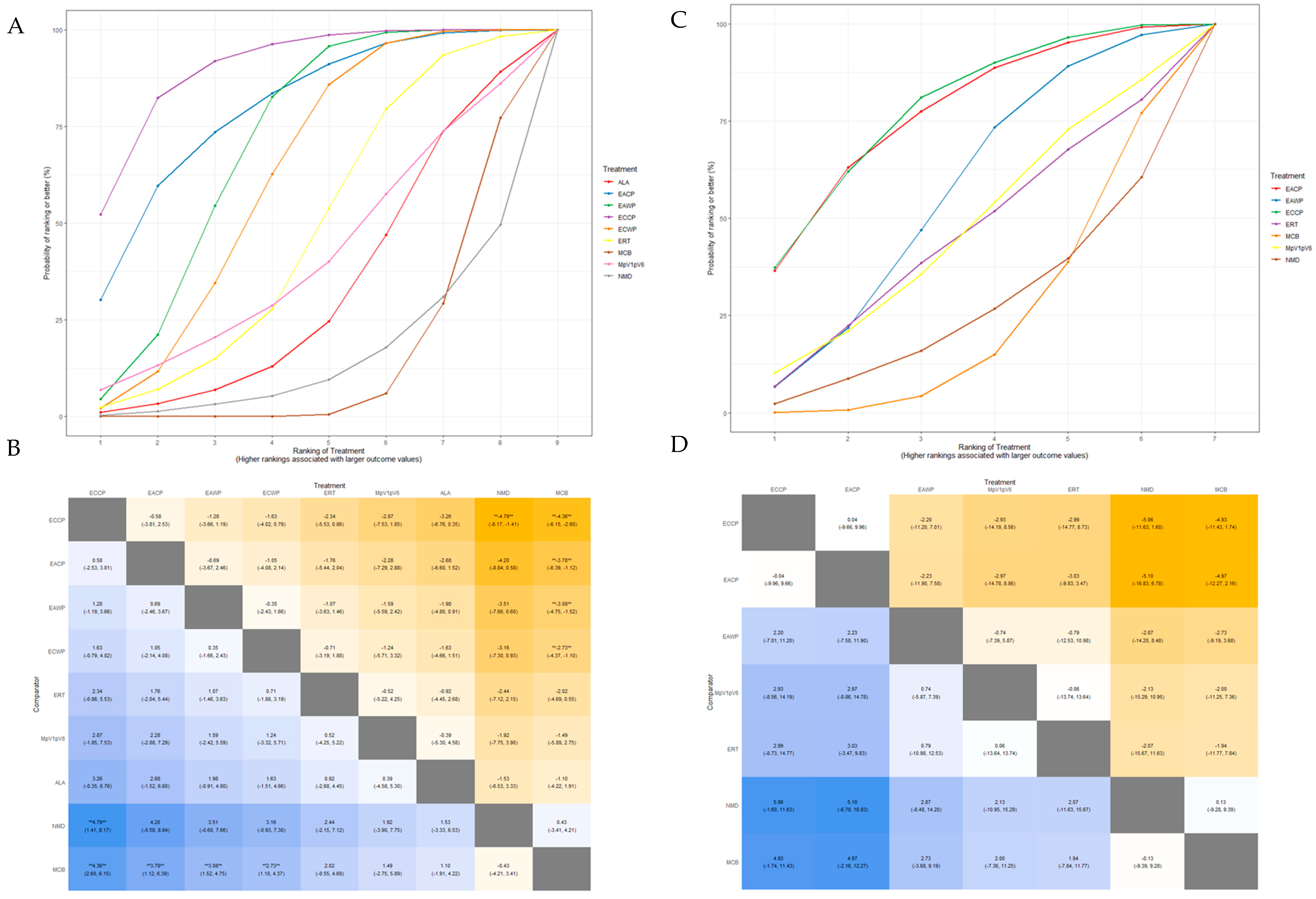
3.5. Network Meta-Analysis
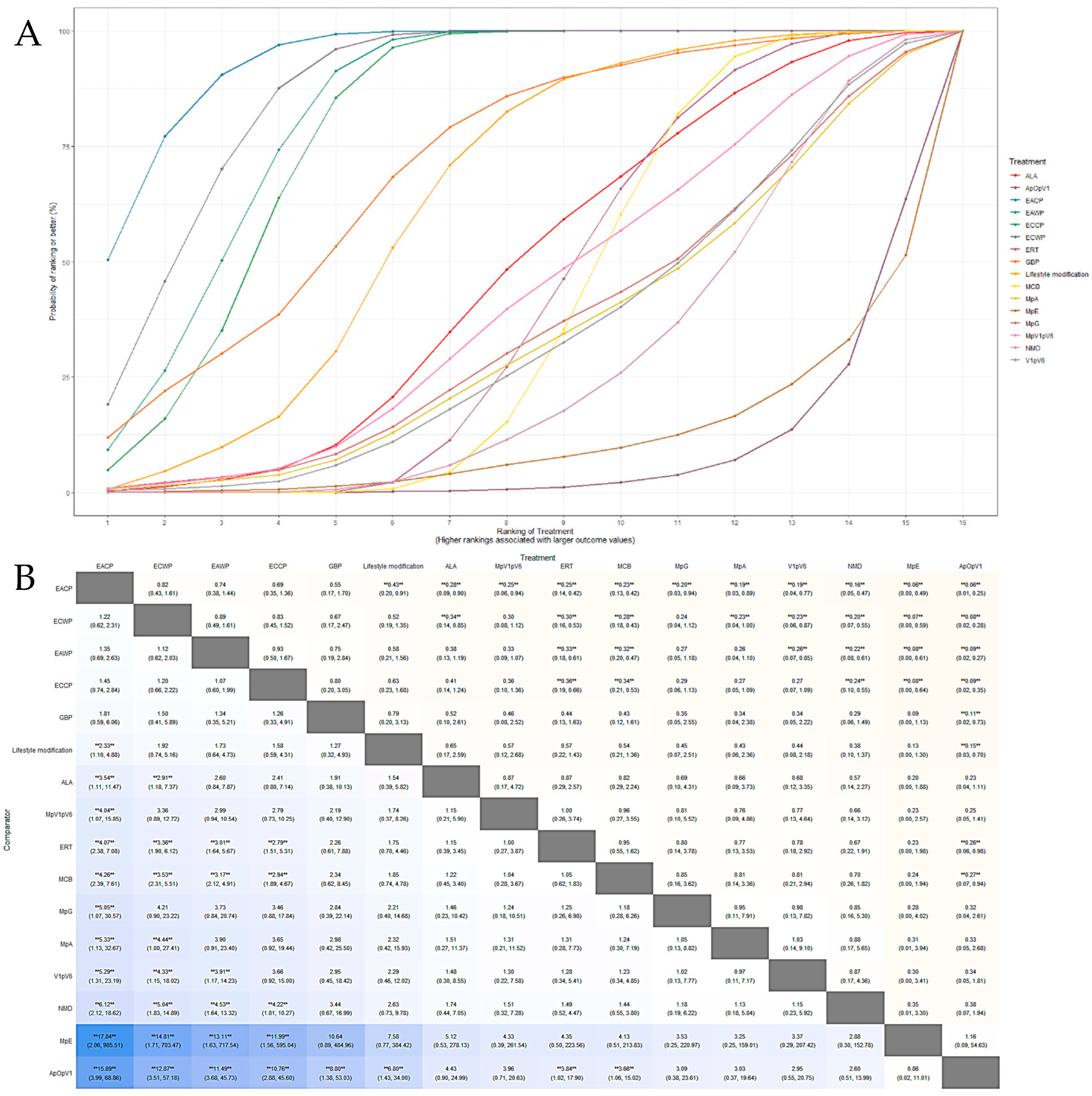
3.5.1. Response Rate
3.5.2. Motor Nerve Outcomes: MMNCV, PMNCV, UMNCV, TMNCV
3.5.3. Sensory Nerve Outcomes: MSNCV, PSNCV, USNCV, TSNCV
3.5.4. Inconsistency Test
3.6. Analysis of the Mechanism of the ACP on DPN through Network Pharmacology
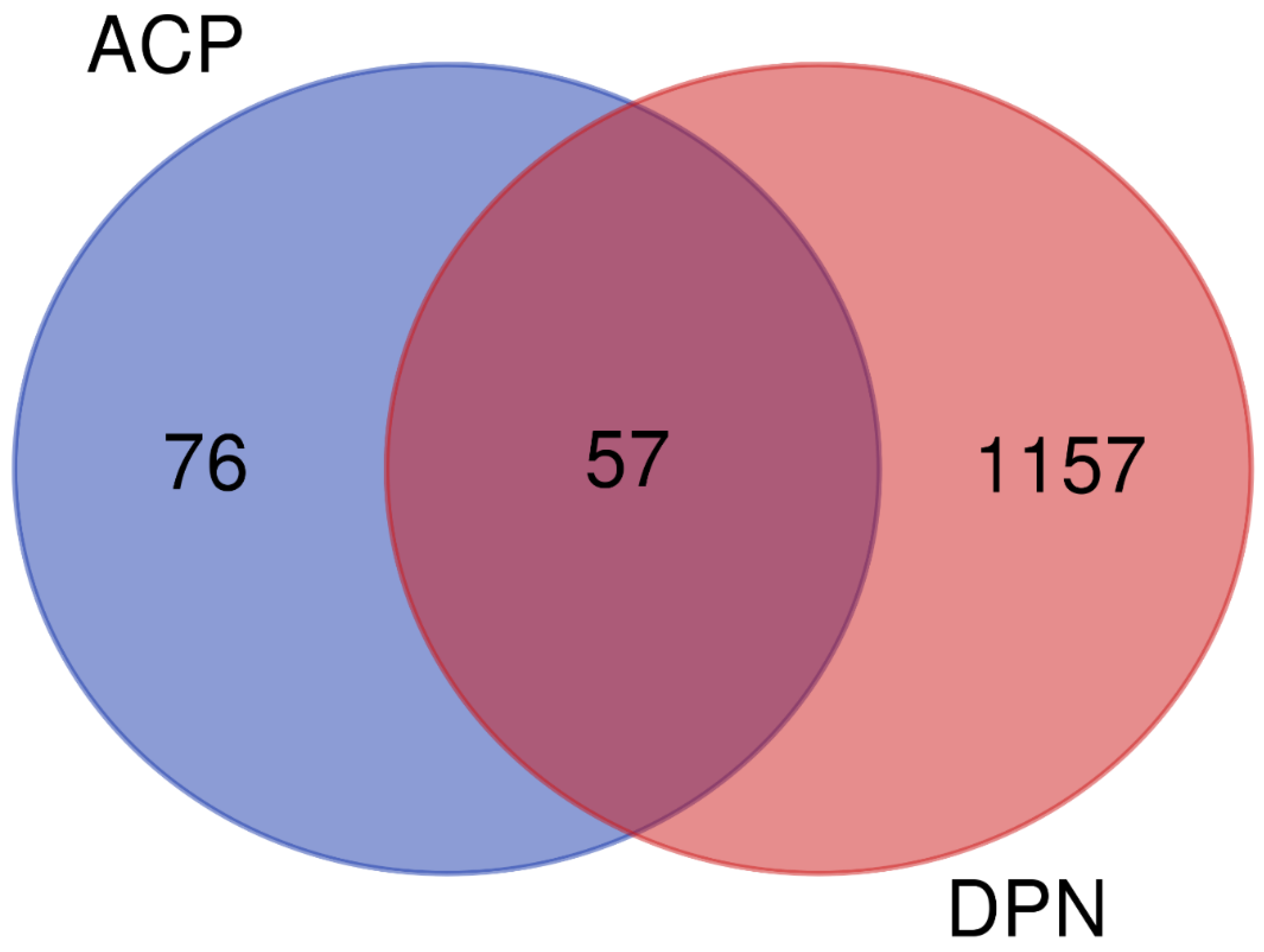
3.6.1. Active Ingredients and Anti-DPN Gene Targets of the ACP
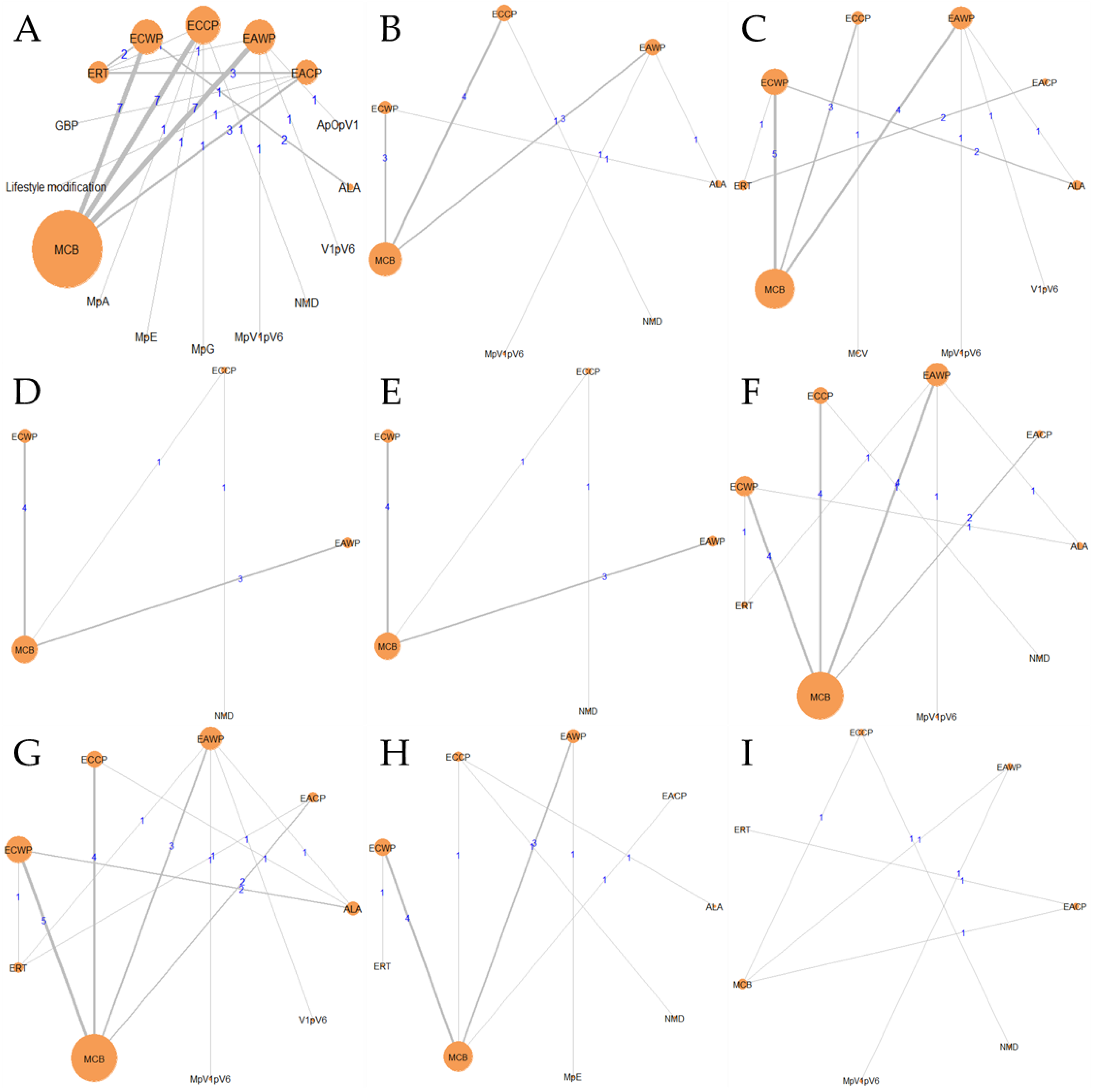
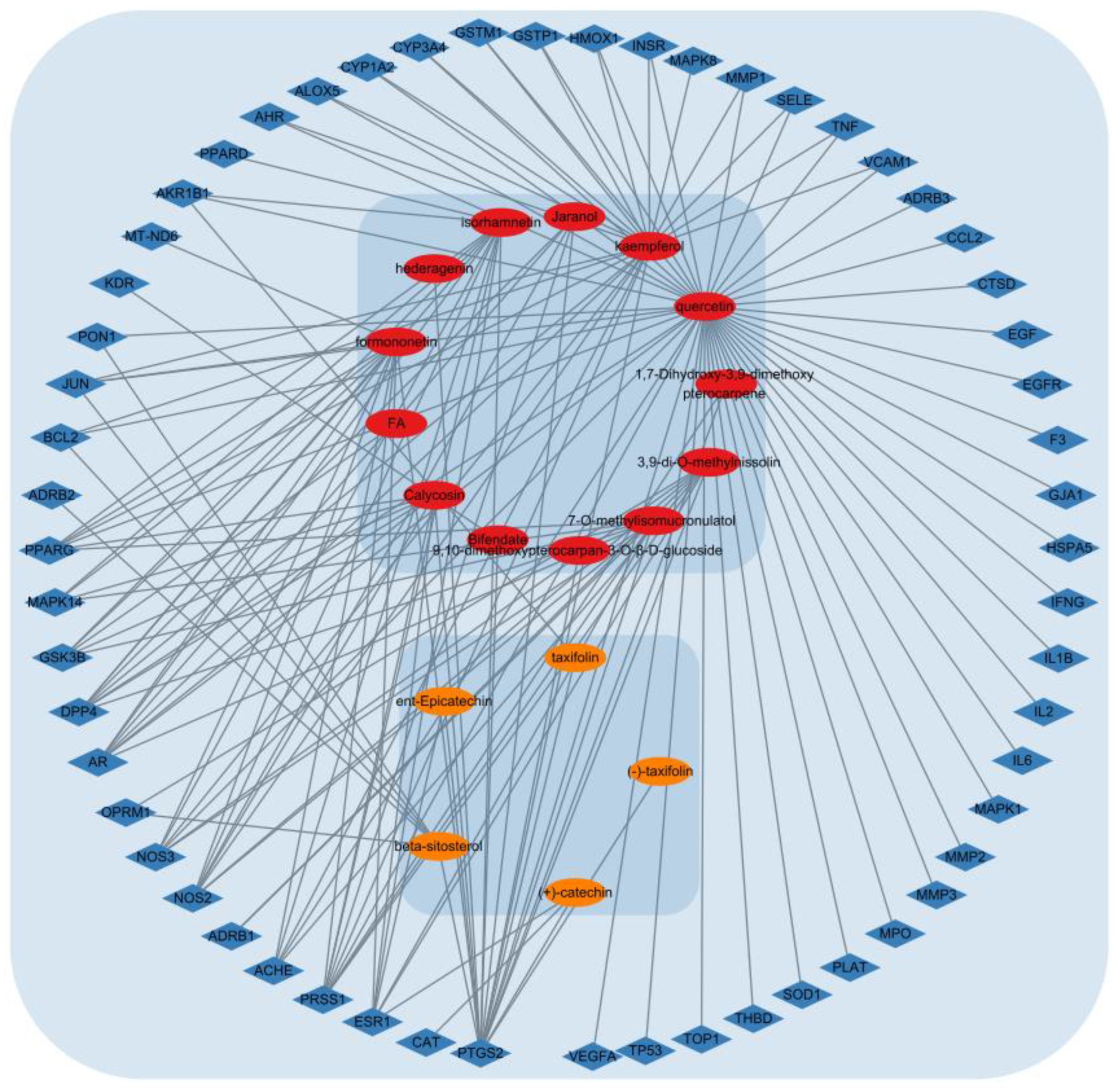
3.6.2. Network Analysis of the ACP and DPN Targets
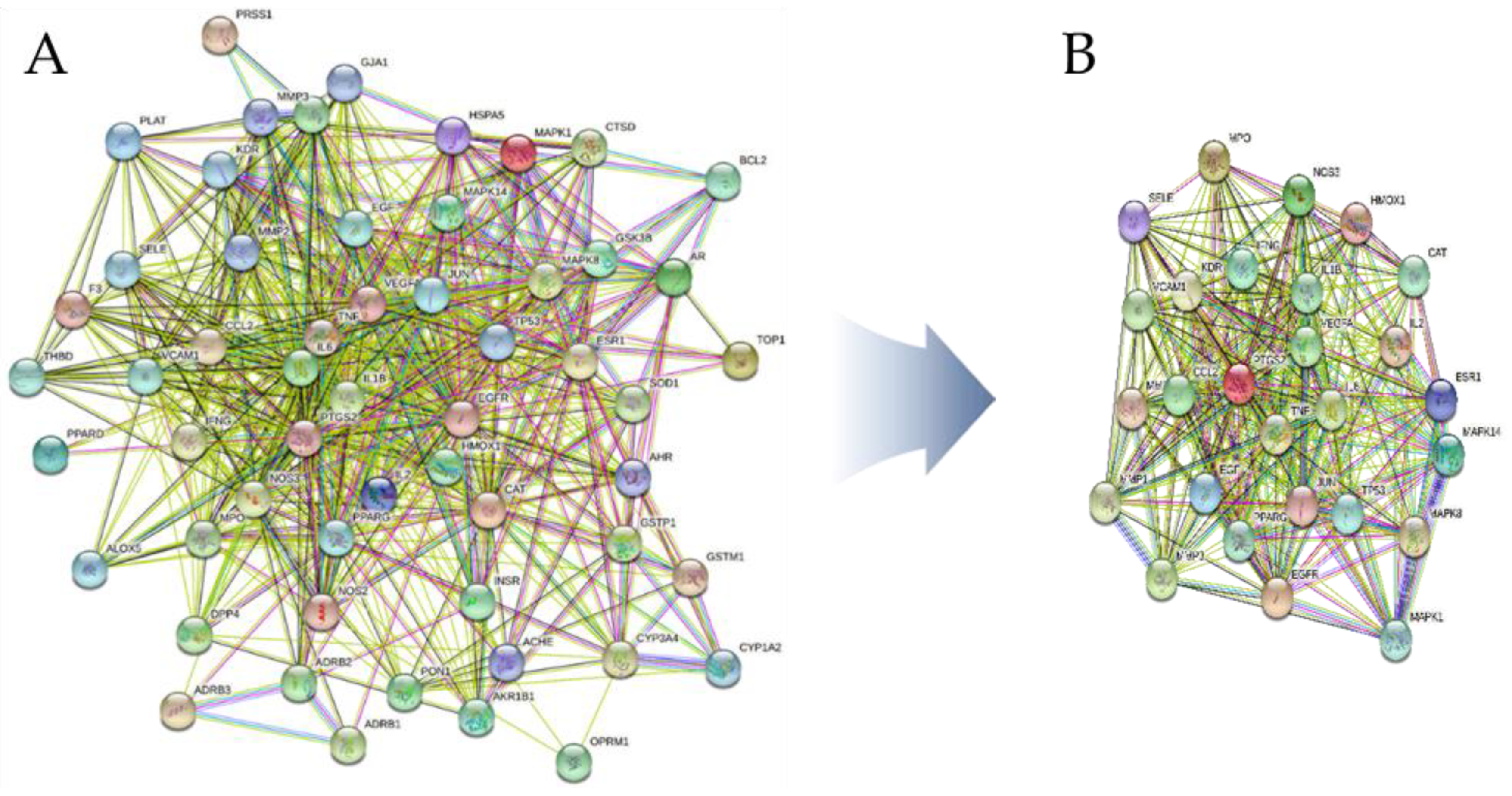
3.6.3. PPI Network Construction
3.6.4. Gene Ontology and KEGG Pathway Enrichment Analysis
4. Discussion
4.1. Summary of the Findings
4.2. Strengths and Limitations
4.3. Implications for Clinical Decision-Making
4.4. Implications for Drug Discovery
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selvarajah, D.; Kar, D.; Khunti, K.; Davies, M.J.; Scott, A.R.; Walker, J.; Tesfaye, S. Diabetic Peripheral Neuropathy: Advances in Diagnosis and Strategies for Screening and Early Intervention. Lancet Diabetes Endocrinol. 2019, 7, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.S.; Karlsson, P.; Gylfadottir, S.S.; Andersen, S.T.; Bennett, D.L.; Tankisi, H.; Finnerup, N.B.; Terkelsen, A.J.; Khan, K.; Themistocleous, A.C.; et al. Painful and Non-Painful Diabetic Neuropathy, Diagnostic Challenges and Implications for Future Management. Brain J. Neurol. 2021, 144, 1632–1645. [Google Scholar] [CrossRef]
- Gonçalves, N.P.; Vægter, C.B.; Andersen, H.; Østergaard, L.; Calcutt, N.A.; Jensen, T.S. Schwann Cell Interactions with Axons and Microvessels in Diabetic Neuropathy. Nat. Rev. Neurol. 2017, 13, 135–147. [Google Scholar] [CrossRef]
- Pang, L.; Lian, X.; Liu, H.; Zhang, Y.; Li, Q.; Cai, Y.; Ma, H.; Yu, X. Understanding Diabetic Neuropathy: Focus on Oxidative Stress. Oxid. Med. Cell Longev. 2020, 2020, e9524635. [Google Scholar] [CrossRef] [PubMed]
- Calcutt, N.A. Diabetic Neuropathy and Neuropathic Pain: A (Con)Fusion of Pathogenic Mechanisms? Pain 2020, 161, S65–S86. [Google Scholar] [CrossRef]
- Kiyani, M.; Yang, Z.; Charalambous, L.T.; Adil, S.M.; Lee, H.-J.; Yang, S.; Pagadala, P.; Parente, B.; Spratt, S.E.; Lad, S.P. Painful Diabetic Peripheral Neuropathy: Health Care Costs and Complications from 2010 to 2015. Neurol. Clin. Pract. 2020, 10, 47–57. [Google Scholar] [CrossRef]
- Waldfogel, J.M.; Nesbit, S.A.; Dy, S.M.; Sharma, R.; Zhang, A.; Wilson, L.M.; Bennett, W.L.; Yeh, H.-C.; Chelladurai, Y.; Feldman, D.; et al. Pharmacotherapy for Diabetic Peripheral Neuropathy Pain and Quality of Life: A Systematic Review. Neurology 2017, 88, 1958–1967. [Google Scholar] [CrossRef]
- Asrar, M.M.; Kumari, S.; Sekhar, B.C.; Bhansali, A.; Bansal, D. Relative Efficacy and Safety of Pharmacotherapeutic Interventions for Diabetic Peripheral Neuropathy: A Systematic Review and Bayesian Network Meta-Analysis. Pain Physician 2021, 24, E1–E14. [Google Scholar]
- Yang, K.; Wang, Y.; Li, Y.-W.; Chen, Y.-G.; Xing, N.; Lin, H.-B.; Zhou, P.; Yu, X.-P. Progress in the Treatment of Diabetic Peripheral Neuropathy. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 148, 112717. [Google Scholar] [CrossRef]
- Huang, D.-D.; Shi, G.; Jiang, Y.; Yao, C.; Zhu, C. A Review on the Potential of Resveratrol in Prevention and Therapy of Diabetes and Diabetic Complications. Biomed. Pharmacother. 2020, 125, 109767. [Google Scholar] [CrossRef] [PubMed]
- Addepalli, V.; Suryavanshi, S.V. Catechin Attenuates Diabetic Autonomic Neuropathy in Streptozotocin Induced Diabetic Rats. Biomed. Pharmacother. 2018, 108, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, F.; Farzaei, M.H.; Bahramsoltani, R.; Heydari, M.; Naderinia, K.; Rahimi, R. Plant-Derived Medicines for Neuropathies: A Comprehensive Review of Clinical Evidence. Rev. Neurosci. 2019, 30, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Arora, K.; Tomar, P.C.; Mohan, V. Diabetic Neuropathy: An Insight on the Transition from Synthetic Drugs to Herbal Therapies. J. Diabetes Metab. Disord. 2021, 20, 1773–1784. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.-Y.; Deng, L.-H.; Huang, Z.-J.; Daror, A.S.; Wang, Z.-H.; Jin, W.-J.; Zhuang, Z.; Tong, Q.; Zheng, G.-Q.; Wang, Y. Buyang Huanwu Decoction Enhances Revascularization via Akt/GSK3β/NRF2 Pathway in Diabetic Hindlimb Ischemia. Oxid. Med. Cell Longev. 2021, 2021, e1470829. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Liu, N.; Tian, M.; Ma, L.; Yang, J.; Lan, X.; Ma, H.; Niu, J.; Yu, J. Effects of Alkaloids on Peripheral Neuropathic Pain: A Review. Chin. Med. 2020, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kaur, N.; Kishore, L.; Gupta, G.K. Management of Diabetic Complications: A Chemical Constituents Based Approach. J. Ethnopharmacol. 2013, 150, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Choi, H.Y.; Park, C.S.; Pyo, M.K.; Yune, T.Y.; Kim, G.W.; Chung, S.H. GS-KG9 Ameliorates Diabetic Neuropathic Pain Induced by Streptozotocin in Rats. J. Ginseng Res. 2019, 43, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.Y.; Liu, K.H.; Kong, T.Y.; Jeong, H.-U.; Choi, S.-Z.; Son, M.; Cho, Y.-Y.; Lee, H.S. Evaluation of DA-9801, a New Herbal Drug for Diabetic Neuropathy, on Metabolism-Mediated Interaction. Arch. Pharm. Res. 2013, 36, 1–5. [Google Scholar] [CrossRef]
- Nam, Y.H.; Moon, H.W.; Lee, Y.R.; Kim, E.Y.; Rodriguez, I.; Jeong, S.Y.; Castañeda, R.; Park, J.-H.; Choung, S.-Y.; Hong, B.N.; et al. Panax Ginseng (Korea Red Ginseng) Repairs Diabetic Sensorineural Damage through Promotion of the Nerve Growth Factor Pathway in Diabetic Zebrafish. J. Ginseng Res. 2019, 43, 272–281. [Google Scholar] [CrossRef]
- Yang, D.; Liang, X.-C. Strategies and Research Progress of Chinese Medicine in Prevention and Treatment of Diabetic Peripheral Neuropathy. Chin. J. Integr. Med. 2018, 24, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.S.; Ko, S.-G. Introduction to the History and Current Status of Evidence-Based Korean Medicine: A Unique Integrated System of Allopathic and Holistic Medicine. Evid.-Based Complement. Altern. Med. ECAM 2014, 2014, 740515. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-M.; Kim, J. Cross-National Differences in the Holistic Use of Traditional East Asian Medicine in East Asia. Health Promot. Int. 2018, 33, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-M.; Lee, Y.-S. The Association between the Use of Biomedical Services and the Holistic Use of Traditional East Asian Medicine: A National Survey of Outpatients in South Korea. BMJ Open 2017, 7, e018414. [Google Scholar] [CrossRef] [PubMed]
- Cha, W.-S.; Oh, J.-H.; Park, H.-J.; Ahn, S.-W.; Hong, S.-Y.; Kim, N.-I. Historical Difference between Traditional Korean Medicine and Traditional Chinese Medicine. Neurol. Res. 2007, 29 (Suppl. 1), S5–S9. [Google Scholar] [CrossRef]
- Shim, J.-M. The Relationship Between the Use of Complementary and Alternative Medicine and the Use of Biomedical Services: Evidence From East Asian Medical Systems. Asia. Pac. J. Public Health 2016, 28, 51–60. [Google Scholar] [CrossRef]
- Kim, H.U.; Ryu, J.Y.; Lee, J.O.; Lee, S.Y. A Systems Approach to Traditional Oriental Medicine. Nat. Biotechnol. 2015, 33, 264–268. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Y.; Tan, W.; Wu, X.; Chen, R.; Cao, J.; Chen, M.; Wang, Y. Compatibility Art of Traditional Chinese Medicine: From the Perspective of Herb Pairs. J. Ethnopharmacol. 2012, 143, 412–423. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Li, S.; Ye, X.; Li, X.; He, K. Synergy Effects of Herb Extracts: Pharmacokinetics and Pharmacodynamic Basis. Fitoterapia 2014, 92, 133–147. [Google Scholar] [CrossRef]
- Zhou, X.; Seto, S.W.; Chang, D.; Kiat, H.; Razmovski-Naumovski, V.; Chan, K.; Bensoussan, A. Synergistic Effects of Chinese Herbal Medicine: A Comprehensive Review of Methodology and Current Research. Front. Pharmacol. 2016, 7, 201. [Google Scholar] [CrossRef]
- Xu, H.-B.; Jiang, R.-H.; Chen, X.-Z.; Li, L. Chinese Herbal Medicine in Treatment of Diabetic Peripheral Neuropathy: A Systematic Review and Meta-Analysis. J. Ethnopharmacol. 2012, 143, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Wu, F.; Lu, L.; Wang, J.; Guo, Y.; Liu, A.; Liao, W.; Zheng, G. Chinese Herbal Medicine for Diabetic Peripheral Neuropathy: An Updated Meta-Analysis of 10 High-Quality Randomized Controlled Studies. PLoS ONE 2013, 8, e76113. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Wang, M.; Fan, Y.-H.; Huang, R.; Rao, Y.; Mai, X.; Liu, M. A Systematic Review of Randomized Controlled Trials of the Wenyang Huoxue Method in Treating Diabetic Peripheral Neuropathy. Medicine 2019, 98, e17618. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wang, J.; Wu, Z.; Huang, C.; Lu, A.; Wang, Y. Systems Pharmacology Exploration of Botanic Drug Pairs Reveals the Mechanism for Treating Different Diseases. Sci. Rep. 2016, 6, 36985. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Y.; Xin, L.; Sun, M.; Yu, C.; Shi, G.; Bao, T.; Liu, J.; Ni, Y.; Lu, R.; et al. Clinical Features and Rules of Chinese Herbal Medicine in Diabetic Peripheral Neuropathy Patients. Evid.-Based Complement. Altern. Med. ECAM 2020, 2020, 5795264. [Google Scholar] [CrossRef]
- Yue, S.-J.; Xin, L.-T.; Fan, Y.-C.; Li, S.-J.; Tang, Y.-P.; Duan, J.-A.; Guan, H.-S.; Wang, C.-Y. Herb Pair Danggui-Honghua: Mechanisms Underlying Blood Stasis Syndrome by System Pharmacology Approach. Sci. Rep. 2017, 7, 40318. [Google Scholar] [CrossRef]
- Jeong, S.J.; Lim, H.-S.; Seo, C.-S.; Kim, J.-H.; Jin, S.-E.; Yoo, S.-R.; Shin, H.-K.; Jeong, S.-J.; Lim, H.-S.; Seo, C.-S.; et al. Traditional Herbal Formula Jakyakgamcho-Tang (Paeonia lactiflora and Glycyrrhiza uralensis) Impairs Inflammatory Chemokine Production by Inhibiting Activation of STAT1 and NF-ΚB in HaCaT Cells. Phytomedicine 2015, 22, 326–332. [Google Scholar] [CrossRef]
- Pang, B.; Zhao, T.-Y.; Zhao, L.-H.; Wan, F.; Ye, R.; Zhou, Q.; Tian, F.; Tong, X.-L. Huangqi Guizhi Wuwu Decoction for Treating Diabetic Peripheral Neuropathy: A Meta-Analysis of 16 Randomized Controlled Trials. Neural Regen. Res. 2016, 11, 1347–1358. [Google Scholar] [CrossRef]
- Liang, L.; Wei, X.; Feng, M.; Zhu, L.; Yu, J.; Yang, G.; Yin, X.; Zhou, S.; Li, K.; Yang, M.; et al. Huangqi Guizhi Wuwu Decoction for Treating Cervical Radiculopathy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine 2020, 99, e19137. [Google Scholar] [CrossRef]
- Jo, H.-G.; Lee, D. Oral Administration of East Asian Herbal Medicine for Peripheral Neuropathy: A Systematic Review and Meta-Analysis with Association Rule Analysis to Identify Core Herb Combinations. Pharmaceuticals 2021, 14, 1202. [Google Scholar] [CrossRef]
- He, Y.; Zheng, H.; Zhong, L.; Zhong, N.; Wen, G.; Wang, L.; Zhang, Y. Identification of Active Ingredients of Huangqi Guizhi Wuwu Decoction for Promoting Nerve Function Recovery After Ischemic Stroke Using HT22 Live-Cell-Based Affinity Chromatography Combined with HPLC-MS/MS. Drug Des. Devel. Ther. 2021, 15, 5165–5178. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Shen, J.; Gao, X.; Ruan, Y.; Ling, J.; Sun, R.; Dai, J.; Fan, H.; Cheng, X.; Cao, P. Herbal Formula Huangqi Guizhi Wuwu Decoction Attenuates Paclitaxel-Related Neurotoxicity via Inhibition of Inflammation and Oxidative Stress. Chin. Med. 2021, 16, 76. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.-Z.; Shen, X.; He, Y.-Y.; Yan, X.-L.; Wang, S.-X.; Yu, A.-M.; Wang, L.-S. Pharmacokinetic Analysis of Huangqi Guizhi Wuwu Decoction on Blood and Brain Tissue in Rats with Normal and Cerebral Ischemia-Reperfusion Injury by Microdialysis with HPLC-MS/MS. Drug Des. Devel. Ther. 2020, 14, 2877–2888. [Google Scholar] [CrossRef]
- Liu, W.; Shi, L.; Wan, Q.; Wu, Y.; Huang, D.; Ou, J.; Liu, Q.; Guan, X.; Yang, Y.; Zhang, X.; et al. Huangqi Guizhi Wuwu Decoction Attenuates Podocyte Cytoskeletal Protein Damage in IgA Nephropathy Rats by Regulating AT1R/Nephrin/c-Abl Pathway. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 142, 111907. [Google Scholar] [CrossRef] [PubMed]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Lortie, C.J.; Filazzola, A. A Contrast of Meta and Metafor Packages for Meta-Analyses in R. Ecol. Evol. 2020, 10, 10916–10921. [Google Scholar] [CrossRef]
- Zhang, Y.; Akl, E.A.; Schünemann, H.J. Using Systematic Reviews in Guideline Development: The GRADE Approach. Res. Synth. Methods 2019, 10, 312–329. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Meng, Z.; Wu, C.; Lin, L. The Effect Direction Should Be Taken into Account When Assessing Small-Study Effects. J. Evid.-Based Dent. Pract. 2023, 23, 101830. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Béliveau, A.; Boyne, D.J.; Slater, J.; Brenner, D.; Arora, P. BUGSnet: An R Package to Facilitate the Conduct and Reporting of Bayesian Network Meta-Analyses. BMC Med. Res. Methodol. 2019, 19, 196. [Google Scholar] [CrossRef]
- Xu, C.; Niu, Y.; Wu, J.; Gu, H.; Zhang, C. Software and Package Applicating for Network Meta-Analysis: A Usage-Based Comparative Study. J. Evid.-Based Med. 2018, 11, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A Database of Systems Pharmacology for Drug Discovery from Herbal Medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinforma. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING Database in 2021: Customizable Protein-Protein Networks, and Functional Characterization of User-Uploaded Gene/Measurement Sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape Provides a Biologist-Oriented Resource for the Analysis of Systems-Level Datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Jin, J.; Chen, H.; Zhao, D.; Feng, Z.; Liu, Q.; Zhang, Z.; Qin, R. Clinical study on tangmaitong tablets in treating 103 cases of diabetic peripheral neuropathy. J. Tradit. Chin. Med. 2004, 45, 429–431. [Google Scholar]
- Sun, L. Clinical Observation on Treatment of 30 Cases of Peripheral Neuroapthy of Type 2 Diabetes with Nourishing Yin Bushen Huoxue Tongluo Decoction. Chin. J. Med. Drug Appl. 2008, 2, 39–40. [Google Scholar]
- Shen, J.; Shu, X. Clinical study of tangmaining capsule in the treatment of diabetic peripheral neuropathy. Chin. J. Exp. Tradit. Med. 2009, 15, 74–76. [Google Scholar]
- Lin, M.; Yu, J.; Deng, Y.; Tan, S. Tongxinluo capsule combined with methyl vitamin B12 in the treatment of multiple diabetes observation of therapeutic effect of neuropathy. Chin. J. Clin. Ration. Drug Use 2010, 3, 31–32. [Google Scholar]
- Wang, Z.; Wang, J. Observation on curative effect of huangqi guizhi wuwu decoction in treating diabetic peripheral neuropathy. Qingdao Med. Health 2010, 42, 275–276. [Google Scholar]
- Yan, Q.; Yu, J. Evaluation of clinical therapeutic effect of the method for nourishing yin and activating blood circulation on diabetic peripheral neuropathy. Chin. Arch. Tradit. Chin. Med. 2010, 28, 2372–2373. [Google Scholar]
- Wu, Y.; Zhang, J.; Qin, F. Modified yiqi huoxue decoction for the treatment of 30 cases of diabetic peripheral neuropathy. China Foreign Med. Treat. 2011, 18, 127–128. [Google Scholar]
- Gao, Z.; Wang, X. Clinical observation of diabetic peripheral neuropathy by the interfere of nourishing the liver to stop the wind and tonglu decoction. Chin. J. Basic Med. Tradit. Chin. Med. 2012, 18, 998–1000. [Google Scholar]
- Gong, Y.; Wang, J.; Tan, Y.; Lu, J. Clinical effects of modified aconiti deccotion for diabetic perpheral neuropathy and its influences on the glucose level. J. Clin. Med. Pract. 2013, 17, 11–13. [Google Scholar]
- Han, J. Modified Huangqi Guizhi Wuwu Decoction Combined with Mecobalamin Tablets in Treating Diabetes Clinical Observation of 62 Cases of Neuropathy. Anhui Med. Pharm. J. 2013, 17, 849. [Google Scholar]
- Zhang, J. Observation of mudan tongluo fang in the treatment of type 2 diabectic peripheral neuropathy. Clin. J. Tradit. Chin. Med. 2013, 25, 753–755. [Google Scholar]
- Zhang, Y. Observation on therapeutic effect of modified “Tangbaokang” on 60 cases of diabetic peripheral neuropathy. J. Med. Pract. 2013, 26, 747–749. [Google Scholar]
- Guo, Y.; Liu, Y.; Sun, Y.; Qin, B.; Cai, D. Clinical observation of modified “huangqi guizi wuwu decoction” for diabetic peripheral neuropathic pain. Shanghai J. Tradit. Chin. Med. 2014, 48, 40–42. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, H. Treatment of 36 Cases of Diabetic Peripheral Neuropathy with Modified Huangqi Guizi Wuwu Decoction. J. Aerosp. Med. 2014, 25, 1009–1010. [Google Scholar]
- Yang, Y. Shenqixuebi Decoction and Western Medicine Treat 60 Cases of Diabetic Peripheral Neuropathy. TCM Res. 2014, 27, 27–29. [Google Scholar]
- Qi, Y.; Yu, S. Effect of Mudan Granules on Oxidative Stress in Painful Diabetic Peripheral Neuropathy. Lishizhen Med. Mater. Med. Res. 2015, 26, 1561–1563. [Google Scholar]
- Wang, L.; Su, J.; Jiao, H.; Zhang, Y.; Liu, T.; Sin, L. 40 Cases of Senile Diabetic Peripheral Neuropathy Treated with Yixinshu Capsule and Maixuekang Capsule. J. Integr. Tradit. Chin. West. Med. Cardiovasc. Dis. 2015, 13, 939–940. [Google Scholar]
- Xue, L.; Chen, H.; Yang, Y.; Zhang, Z. Clinical observation on the treatment of 42 cases of diabetic peripheral neuropathy with modified liuteng shuilu shexian decoction. J. Sichuan Tradit. Chin. Med. 2015, 33, 59–61. [Google Scholar]
- Guo, H. Clinical research of the patients with type 2 diabetic peripheral neuropathy in the elderly treated by compound qiteng tongluo tang combined epalrestat. Acta Chin. Med. 2016, 31, 1874–1879. [Google Scholar]
- Han, L. Therapeutic effect of Zhanjintongluo Chinese medicine combined with mecobalamin in the treatment of patients with diabetic peripheral neuropathy. Diabetes New World 2016, 19, 50–51. [Google Scholar]
- Lan, B.; Wang, X.; Mi, J.; Wang, G. Clinic study of yiqi huoxue tongluo in treating diabetic peripheral neuropathy. Chin. Med. Mod. Distance Educ. China 2016, 14, 58–59. [Google Scholar]
- Li, G.; Huang, D.; Li, M.; Lin, L. Effects of Wenyang Huoxue Tongbi Fang on Nerve Conduction Velocity and Plasma Hcy of Diabetic Peripheral Neurophathy with Yang-Deficiency, Congealing Cold and Blood Stasis Syndrome. J. Tradit. Chin. Med. 2016, 57, 1486–1489. [Google Scholar]
- Li, H.; Zhong, Q. Clinical observatyion of huangzhi tonglnaoluo capsule in treating diabetic periphral neuropathy. Yunnan J. Tradit. Chin. Med. Mater. Med. 2016, 37, 37–38. [Google Scholar]
- Mo, S.; Xiao, T. The observation on the clinical effect of yangyin jiedu decoction in the treatment of diabetic perpheral neuropathy of qi and yin deficiency with blood stasis type. World J. Integr. Tradit. West. Med. 2016, 11, 692–695. [Google Scholar]
- Wang, Z.; An, X.; Chen, L.; Aihua, C.; Wen, H.; Lin, N. Clinical study on modified tangbitong recipe for treatment of type 2 diabetes distal symmetric polyneuropathy. Guangxi Tradit. Chin. Med. 2016, 39, 19–21. [Google Scholar]
- Zhang, H.; Su, H.; Wang, Y.; Zhang, B.; Li, H.; Guo, Y.; Shi, Z.; Cui, S. Observation of the clinical effect of nimodipine combined with qiming granule in the treatment of diabetic peripheral neuropathy. Inf. Tradit. Chin. Med. 2016, 33, 97–100. [Google Scholar]
- Zhang, J.; Zhi, D.; Xie, H. Treatment of 48 cases of diabetic peripheral neuropathy with huangqichifeng decoction combined danggisini decoction. World Latest Med. Inf. 2016, 16, 26–27. [Google Scholar]
- Chen, H.; Wu, J.; Tan, H. Clinical observation of danggui sini tang for diabetic peripheral neuropathy. J. New Chin. Med. 2017, 49, 56–58. [Google Scholar]
- Shi, Z.; Li, L.; Wang, K.; Zhang, J.; Lu, Y.; Zhang, H.; Wang, J.; Zhou, Y. Effect of compound danshen dripping pills on early peripheral neuropathy in patients with type 2 diabetes. New World Diabetes 2017, 4, 174–176. [Google Scholar]
- Wang, P.; Cui, P.; Hong, Y. Effect of danggui sini deccotion on treatment of diabetic peripheral neuropathy with cold congealing and blood stasis. Chin. Arch. Tradit. Chin. Med. 2017, 35, 661–664. [Google Scholar]
- Chen, X. Clinical study on danggui sini tang in treatment of peripheral neuropathy of diabetes mellitus with cold and dampness obstraction spleen syndrome. Acta Chin. Med. 2018, 33, 756–759. [Google Scholar]
- Dai, Q.; Xu, X. Clinical study of huangqi guizhi wuwu decoction combined with yunu decoction in the treatment of diabectic peripheral neuropathy. Shaanxi Tradit. Chin. Med. 2018, 39, 482–484. [Google Scholar]
- Gao, S.; Tian, X.; Jinag, W.; Ma, Y. Clinical Study on the Treatment of 50 Cases of Diabetic Peripheral Neuropathy with Shengmai Powder Combined with Basic Therapy. Jiangsu Tradit. Chin. Med. 2019, 51, 28–30. [Google Scholar]
- Hu, Y.; Liu, H.; Liu, M.; Wang, T. Clinical observation of modified jiajian huangqi guizhi wuwu decoction in treating diabetic peripheral neuropahty patients with Qi deficiency and blood stasis type. Clin. J. Tradit. Chin. Med. 2018, 30, 105–107. [Google Scholar]
- Huang, X.; Lin, X.; Chen, C. Clinical study on matong powder in treating 120 cases of diabetic peripheral neuritis. Mod. Hosp. 2018, 18, 288–290. [Google Scholar]
- She, Y.; Yu, J.; Li, R.; Wang, Y.; Zhang, S.; Zhu, F.; Hu, A.; Wu, J.; Wang, J.; Peng, M. Observations on curative effect of huangqi guizhi wuwu granules combined with acupucnture and moxibustion in treating diabetic peripheral neuropathy. J. Guangxi Univ. Tradit. Chin. Med. 2018, 21, 11–13. [Google Scholar]
- Xin, Y.; Ma, D. Observation on the clinical curative effect of mongolian medicine garidi-13 weiwan in the treatment of diabetic peripheral neuropathy. J. Med. Pharm. Chin. Minor. 2018, 24, 15–16. [Google Scholar] [CrossRef]
- Ji, W.; Hua, W. Efficacy of Yangyin Zhuyu Decoction with Epalrestat in the Treatment of Bi Disease with Yin Deficiency and Blood Stasis Syndrome Caused by Consumptive Thirst. J. Chang. Univ. Chin. Med. 2019, 35, 460–463. [Google Scholar]
- Liu, L.; Bin, J.; Kong, F. Clinical observation on treating diabetic peripheral neuropathy with shengjiang san and taohong yin. Clin. J. Chin. Med. 2019, 11, 61–65. [Google Scholar]
- Liu, M. Huangqi guizhi wuwu decoction combined with western Medicine treat peripheral neuropathy in type 2 diabetes mellitus randomized parallel controlled study. J. Pract. Tradit. Chin. Intern. Med. 2019, 33, 10–12. [Google Scholar]
- Wu, G.; Meng, C.; Zhang, D. A randomized controlled study of acupuncture combined with taohong siwu decocotion in treating diabetic peripheral neuropathy. J. Gansu Univ. Chin. Med. 2019, 36, 64–67. [Google Scholar]
- Yi, W.; Zhang, F.; Wang, Y.; Sun, H.; Hu, Y.; Wu, S.; Liu, T. Clinical observation on 54 cases of diabetic peripheral neuropathy with phlegm and static blood syndrome treated with mongolian medicine zhenbao pills. J. Tradit. Chin. Med. 2019, 60, 42–46. [Google Scholar]
- Chen, J.; Zhang, Y.; Hu, C.; Feng, Z.; Liu, M.; Shi, J.; Shen, Y.; Jiang, J.; Yan, J. Clinical Research on TCM Directional Penetration Combined Zicui Juanbi Decoction in Treating Painful Diabetic Peripheral Neuropathy. Mod. J. Integr. Tradit. Chin. West. Med. 2021, 30, 1844–1848. [Google Scholar]
- Hou, Y.; Guo, L.; Zhang, Y.; Li, X. Clinical study of jiuchongdan in the treatment of diabetic peripheral neuropathy. China Tradit. Chin. Med. Technol. 2021, 19, 9–10. [Google Scholar]
- Li, Q.; Zhang, B. Exploration of the effect of Huanquizhiwuwu decoction combined with mudan granules in 41 cases of diabetic peripheral neuropathy (Qi deficiency and blood stasis syndrome). Anhui Med. Pharm. J. 2021, 25, 1052–1056. [Google Scholar]
- Wang, R. Clinical Effect of Taohong Siwu Decoction Combined with Mecobalamin on Diabetic Peripheral Neuropathy. Diabetes New World 2021, 24, 181–183. [Google Scholar]
- Wang, Y.; Tang, L.; Song, H. Clinical observation of yiqi yangyin tongluo decoction in treating diabetic peripheral neuropathy. China Tradit. Chin. Med. Sci. Technol. 2021, 28, 289–291. [Google Scholar]
- Zhang, R.; Zhong, Y.; Zhao, L. The influence of buqi huoxue zhitong tang and A-lipoic acid in patients with diabetic peripheral neuropathy on inflammatory response and neurological function of lower extremity. Chin. Prim. Health Care 2021, 35, 92–94. [Google Scholar]
- Panthi, S.; Jing, X.; Gao, C.; Gao, T. Yang-Warming Method in the Treatment of Diabetic Peripheral Neuropathy: An Updated Systematic Review and Meta-Analysis. BMC Complement. Altern. Med. 2017, 17, 424. [Google Scholar] [CrossRef]
- Liu, W.; Fan, Y.; Tian, C.; Jin, Y.; Du, S.; Zeng, P.; Wang, A. Deciphering the Molecular Targets and Mechanisms of HGWD in the Treatment of Rheumatoid Arthritis via Network Pharmacology and Molecular Docking. Evid.-Based Complement Altern. Med. ECAM 2020, 2020, 7151634. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, G. Extraction, Structure, and Activity of Polysaccharide from Radix Astragali. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 150, 113015. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Z.; Huang, L.; Zheng, S.; Wang, D.; Chen, S.; Zhang, H.; Yang, S. Review of the Botanical Characteristics, Phytochemistry, and Pharmacology of Astragalus Membranaceus (Huangqi). Phytother. Res. PTR 2014, 28, 1275–1283. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, L.; Gao, C.; Chen, W.; Vong, C.T.; Yao, P.; Yang, Y.; Li, X.; Tang, X.; Wang, S.; et al. Astragali Radix (Huangqi): A Promising Edible Immunomodulatory Herbal Medicine. J. Ethnopharmacol. 2020, 258, 112895. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Q.; Li, R.-L.; Wei, S.-J.; Huang, C.-Y.; Gao, Y.-X.; Pu, X.-F. The Traditional Uses, Phytochemistry, Pharmacology and Toxicology of Cinnamomi Ramulus: A Review. J. Pharm. Pharmacol. 2020, 72, 319–342. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhang, X.; Wang, Z.; Zheng, C.; Li, P.; Huang, C.; Tao, W.; Xiao, W.; Wang, Y.; Huang, L.; et al. Deciphering the Combination Principles of Traditional Chinese Medicine from a Systems Pharmacology Perspective Based on Ma-Huang Decoction. J. Ethnopharmacol. 2013, 150, 619–638. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, J.; Wang, Y.; Zhang, Y.; Bi, K.; Zhang, Z.; Li, Q. Study on the Multitarget Synergistic Effects of Kai-Xin-San against Alzheimer’s Disease Based on Systems Biology. Oxid. Med. Cell Longev. 2019, 2019, e1707218. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Liu, Y.; Chen, Y.; Chen, T.; Li, Y.; Li, Q.; Zhao, M. Identification of the Mechanism of Matrine Combined with Glycyrrhizin for Hepatocellular Carcinoma Treatment through Network Pharmacology and Bioinformatics Analysis. Oxid. Med. Cell Longev. 2022, 2022, e2663758. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and Antagonism in Natural Product Extracts: When 1 + 1 Does Not Equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Cui, H.; Liu, G.; Zhao, X.; Li, W.; Piao, G. How Can Synergism of Traditional Medicines Benefit from Network Pharmacology? Molecules 2017, 22, 1135. [Google Scholar] [CrossRef]
- Gertsch, J. Botanical Drugs, Synergy, and Network Pharmacology: Forth and Back to Intelligent Mixtures. Planta Med. 2011, 77, 1086–1098. [Google Scholar] [CrossRef]
- Zhong, J.; Liu, Z.; Zhou, X.; Xu, J. Synergic Anti-Pruritus Mechanisms of Action for the Radix Sophorae Flavescentis and Fructus Cnidii Herbal Pair. Mol. Basel Switz. 2017, 22, 1465. [Google Scholar] [CrossRef]
- Ma, X.; Hao, C.; Zhang, Z.; Jiang, H.; Zhang, W.; Huang, J.; Chen, X.; Yang, W. Shenjinhuoxue Mixture Attenuates Inflammation, Pain, and Cartilage Degeneration by Inhibiting TLR-4 and NF-κB Activation in Rats with Osteoarthritis: A Synergistic Combination of Multitarget Active Phytochemicals. Oxid. Med. Cell Longev. 2021, 2021, e4190098. [Google Scholar] [CrossRef]
- Ung, C.Y.; Li, H.; Cao, Z.W.; Li, Y.X.; Chen, Y.Z. Are Herb-Pairs of Traditional Chinese Medicine Distinguishable from Others? Pattern Analysis and Artificial Intelligence Classification Study of Traditionally Defined Herbal Properties. J. Ethnopharmacol. 2007, 111, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Qu, C.; Tang, Y.; Pang, H.; Liu, L.; Zhu, Z.; Shang, E.; Huang, S.; Sun, D.; Duan, J.-A. Herb Pairs Containing Angelicae Sinensis Radix (Danggui): A Review of Bio-Active Constituents and Compatibility Effects. J. Ethnopharmacol. 2016, 181, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Bi, W.; Fan, K.; Li, T.; Yan, T.; Xiao, F.; He, B.; Bi, K.; Jia, Y. Ameliorating Effect of Alpinia Oxyphylla—Schisandra Chinensis Herb Pair on Cognitive Impairment in a Mouse Model of Alzheimer’s Disease. Biomed. Pharmacother. 2018, 97, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.L.; Li, H.; Yu, X.J.; Li, Q.N.; Liu, J.Q.; Liu, C.Y.; Han, H. Effect and Mechanism of “Danggui–Kushen” Herb Pair on Ischemic Heart Disease. Biomed. Pharmacother. 2022, 145, 112450. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-P.; Wang, P.-F.; Bai, J.-Q.; Gao, S.; Wang, Y.-H.; Quan, L.-N.; Wang, F.; Wang, X.-T.; Wang, J.; Xie, Y.-D. Investigating the Effects and Possible Mechanisms of Danshen- Honghua Herb Pair on Acute Myocardial Ischemia Induced by Isoproterenol in Rats. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 118, 109268. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Mo, C.; Shi, W.; Meng, L.; Ai, J. Network Pharmacology Combined with Bioinformatics to Investigate the Mechanisms and Molecular Targets of Astragalus Radix-Panax Notoginseng Herb Pair on Treating Diabetic Nephropathy. Evid.-Based Complement. Altern. Med. ECAM 2021, 2021, 9980981. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, C.; Miao, L.; Tan, Y.; Zhou, Y.; Cheong, M.S.; Huang, Y.; Wang, Y.; Yu, H.; Cheang, W.S. Panax Notoginseng Protects against Diabetes-Associated Endothelial Dysfunction: Comparison between Ethanolic Extract and Total Saponin. Oxid. Med. Cell Longev. 2021, 2021, e4722797. [Google Scholar] [CrossRef]
- Bianchi, E.; Rogge, L. The IL-23/IL-17 Pathway in Human Chronic Inflammatory Diseases-New Insight from Genetics and Targeted Therapies. Genes Immun. 2019, 20, 415–425. [Google Scholar] [CrossRef]
- Rajendran, S.; Quesada-Masachs, E.; Zilberman, S.; Graef, M.; Kiosses, W.B.; Chu, T.; Benkahla, M.A.; Lee, J.-H.M.; von Herrath, M. IL-17 Is Expressed on Beta and Alpha Cells of Donors with Type 1 and Type 2 Diabetes. J. Autoimmun. 2021, 123, 102708. [Google Scholar] [CrossRef]
- Zheng, Y.-H.; Ren, C.-Y.; Shen, Y.; Li, J.-B.; Chen, M.-W. A Cross-Sectional Study on the Correlation Between Inflammatory Cytokines, Negative Emotions, and Onset of Peripheral Neuropathy in Type 2 Diabetes. Neuropsychiatr. Dis. Treat. 2020, 16, 2881–2890. [Google Scholar] [CrossRef]
- Sanaye, M.M.; Kavishwar, S.A. Diabetic Neuropathy: Review on Molecular Mechanisms. Curr. Mol. Med. 2023, 23, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Königs, V.; Pierre, S.; Schicht, M.; Welss, J.; Hahnefeld, L.; Rimola, V.; Lütjen-Drecoll, E.; Geisslinger, G.; Scholich, K. GPR40 Activation Abolishes Diabetes-Induced Painful Neuropathy by Suppressing VEGF-A Expression. Diabetes 2022, 71, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Du, X.; Long, M.; Zhang, Z.; Zhou, S.; Zhou, J.; Qian, G. Neuroprotective Effect of Berberine Is Mediated by MAPK Signaling Pathway in Experimental Diabetic Neuropathy in Rats. Eur. J. Pharmacol. 2016, 774, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, H.; Zhang, X.; Lou, W.; Zhang, P.; Qiu, Y.; Zhang, C.; Wang, Y.; Liu, W.J. The Effect of Berberine on Metabolic Profiles in Type 2 Diabetic Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Oxid. Med. Cell Longev. 2021, 2021, e2074610. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Oxid. Med. Cell Longev. 2016, 2016, e2986796. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Lebelo, S.L. Antioxidant Effects and Mechanisms of Medicinal Plants and Their Bioactive Compounds for the Prevention and Treatment of Type 2 Diabetes: An Updated Review. Oxid. Med. Cell Longev. 2020, 2020, e1356893. [Google Scholar] [CrossRef]
- Dhanya, R. Quercetin for Managing Type 2 Diabetes and Its Complications, an Insight into Multitarget Therapy. Biomed. Pharmacother. 2022, 146, 112560. [Google Scholar] [CrossRef]
- Dong, B.; Shi, Z.; Dong, Y.; Chen, J.; Wu, Z.-X.; Wu, W.; Chen, Z.-S.; Han, C. Quercetin Ameliorates Oxidative Stress-induced Cell Apoptosis of Seminal Vesicles via Activating Nrf2 in Type 1 Diabetic Rats. Biomed. Pharmacother. 2022, 151, 113108. [Google Scholar] [CrossRef]
- Sharma, D.; Gondaliya, P.; Tiwari, V.; Kalia, K. Kaempferol Attenuates Diabetic Nephropathy by Inhibiting RhoA/Rho-Kinase Mediated Inflammatory Signalling. Biomed. Pharmacother. 2019, 109, 1610–1619. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A Review of Pharmacological Effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Wei, P.-C.; Lee-Chen, G.-J.; Chen, C.-M.; Chen, Y.; Lo, Y.-S.; Chang, K.-H. Isorhamnetin Attenuated the Release of Interleukin-6 from β-Amyloid-Activated Microglia and Mitigated Interleukin-6-Mediated Neurotoxicity. Oxid. Med. Cell Longev. 2022, 2022, e3652402. [Google Scholar] [CrossRef] [PubMed]
- Matboli, M.; Saad, M.; Hasanin, A.H.; A Saleh, L.; Baher, W.; Bekhet, M.M.; Eissa, S. New Insight into the Role of Isorhamnetin as a Regulator of Insulin Signaling Pathway in Type 2 Diabetes Mellitus Rat Model: Molecular and Computational Approach. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 135, 111176. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yamashita, Y.; Nakamura, A.; Croft, K.; Ashida, H. Quercetin and Its Metabolite Isorhamnetin Promote Glucose Uptake through Different Signalling Pathways in Myotubes. Sci. Rep. 2019, 9, 2690. [Google Scholar] [CrossRef] [PubMed]
- Oza, M.J.; Kulkarni, Y.A. Formononetin Ameliorates Diabetic Neuropathy by Increasing Expression of SIRT1 and NGF. Chem. Biodivers. 2020, 17, e2000162. [Google Scholar] [CrossRef] [PubMed]
- Raafat, K.; Hdaib, F. Neuroprotective Effects of Moringa Oleifera: Bio-Guided GC-MS Identification of Active Compounds in Diabetic Neuropathic Pain Model. Chin. J. Integr. Med. 2017. [Google Scholar] [CrossRef] [PubMed]

| Included Study | D1 | D2 | D3 | D4 | D5 | Overall |
|---|---|---|---|---|---|---|
| Jin 2004 [59] | Sc | H | H | L | Sc | H |
| Sun 2008 [60] | Sc | H | H | Sc | Sc | H |
| Shen 2009 [61] | L | H | H | L | Sc | H |
| Lin 2010 [62] | Sc | H | H | L | Sc | H |
| Wang 2010 [63] | L | H | H | L | Sc | H |
| Yan 2010 [64] | Sc | H | H | Sc | Sc | H |
| Wu 2011 [65] | Sc | H | H | L | Sc | H |
| Gao 2012 [66] | Sc | H | H | L | Sc | H |
| Gong 2013 [67] | Sc | H | H | L | Sc | H |
| Han 2013 [68] | L | H | H | Sc | Sc | H |
| Zhang 2013a [69] | L | H | H | L | Sc | H |
| Zhang 2013b [70] | Sc | H | H | L | Sc | H |
| Guo 2014 [71] | Sc | H | H | Sc | Sc | H |
| Yang 2014a [73] | Sc | H | H | Sc | Sc | H |
| Yang 2014b [72] | L | H | H | Sc | Sc | H |
| Qi 2015 [74] | Sc | H | H | L | Sc | H |
| Wang 2015 [75] | Sc | H | H | Sc | Sc | H |
| Xue 2015 [76] | L | H | H | L | Sc | H |
| Guo 2016 [77] | L | H | H | L | Sc | H |
| Han 2016 [78] | Sc | H | H | L | Sc | H |
| Lan 2016 [79] | H | H | H | L | Sc | H |
| Mo 2016 [82] | L | H | H | Sc | Sc | H |
| Wang 2016 [83] | L | H | H | Sc | Sc | H |
| Li 2016a [80] | L | H | H | L | Sc | H |
| Zhang 2016a [85] | L | H | H | L | Sc | H |
| Li 2016b [81] | Sc | H | H | L | Sc | H |
| Zhang 2016b [84] | L | H | H | L | Sc | H |
| Chen 2017 [86] | L | H | H | L | Sc | H |
| Shi 2017 [87] | Sc | H | H | L | Sc | H |
| Wang 2017 [88] | L | H | H | L | Sc | H |
| Chen 2018 [89] | L | H | H | Sc | Sc | H |
| Dai 2018 [90] | H | H | H | L | Sc | H |
| Hu 2018 [92] | Sc | H | H | L | Sc | H |
| Huang 2018 [93] | L | H | H | L | Sc | H |
| She 2018 [94] | L | H | H | L | Sc | H |
| Xin 2018 [95] | H | H | H | Sc | Sc | H |
| Gao 2019 [91] | Sc | H | H | L | Sc | H |
| Wu 2019 [99] | L | H | H | L | Sc | H |
| Yi 2019 [100] | L | H | H | L | Sc | H |
| Ji 2019 [96] | L | H | H | Sc | Sc | H |
| Liu 2019a [97] | Sc | H | H | L | Sc | H |
| Liu 2019b [98] | Sc | H | H | Sc | Sc | H |
| Chen 2021 [101] | Sc | H | Sc | L | Sc | H |
| Hou 2021 [102] | Sc | H | H | L | Sc | H |
| Li 2021 [103] | L | H | H | L | Sc | H |
| Wang 2021a [105] | L | H | H | L | Sc | H |
| Wang 2021b [104] | L | H | H | L | Sc | H |
| Zhang 2021 [106] | H | H | H | L | Sc | H |
| Intervention and Comparator Intervention | Outcomes | Number of Participants (Studies) | Anticipated Absolute Effects (95% CI) | Quality of the Evidence (GRADE) |
|---|---|---|---|---|
| EACP compared to CM for diabetic peripheral neuropathy | Response rate | 516 (7) | 224 more per 1000 (from 139 more to 318 more) | ⨁⨁⨁◯ MODERATE a |
| ECCP compared to CM for diabetic peripheral neuropathy | Response rate | 1006 (11) | 134 more per 1000 (from 77 more to 196 more) | ⨁⨁◯◯ LOW a,c |
| EAWP compared to CM for diabetic peripheral neuropathy | Response rate | 931 (11) | 184 more per 1000 (from 96 more to 283 more) | ⨁⨁◯◯ LOW a,c |
| ECWP compared to CM for diabetic peripheral neuropathy | Response rate | 908 (12) | 190 more per 1000 (from 130 more to 255 more) | ⨁⨁◯◯ LOW a,c |
| Characteristic | Response Rate | Motor Nerve Outcomes | Sensory Nerve Outcomes | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MMNCV | PMNCV | TMNCV | UMNCV | MSNCV | PSNCV | TSNCV | USNCV | ||
| Number of interventions | 16 | 7 | 10 | 5 | 7 | 9 | 10 | 9 | 7 |
| Number of included trials | 42 | 14 | 21 | 9 | 4 | 20 | 21 | 13 | 6 |
| Total number of patients in network | 3588 | 1365 | 2092 | 866 | 360 | 1894 | 2092 | 1348 | 523 |
| Total possible pairwise comparisons | 120 | 21 | 45 | 10 | 5 | 36 | 45 | 36 | 21 |
| Total number of pairwise comparisons with direct data | 18 | 7 | 10 | 4 | 4 | 10 | 10 | 8 | 6 |
| Is the network connected? | TRUE | TRUE | TRUE | TRUE | FALSE | TRUE | TRUE | TRUE | TRUE |
| Number of two-arm studies | 42 | 14 | 21 | 9 | 4 | 20 | 21 | 13 | 6 |
| Number of multiple arm studies | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Average outcome (for continuous variables) | NA | 49.34 | 42.44 | 43.07 | 46.76 | 44.37 | 42.44 | 39.83 | 42.38 |
| Total number of events in network (for dichotomous variables) | 2777 | NA | NA | NA | NA | NA | NA | NA | NA |
| Number of studies with at least one zero events (for dichotomous variables) | 42 | NA | NA | NA | NA | NA | NA | NA | NA |
| Number of studies with all zero events (for dichotomous variables) | 0 | NA | NA | NA | NA | NA | NA | NA | NA |
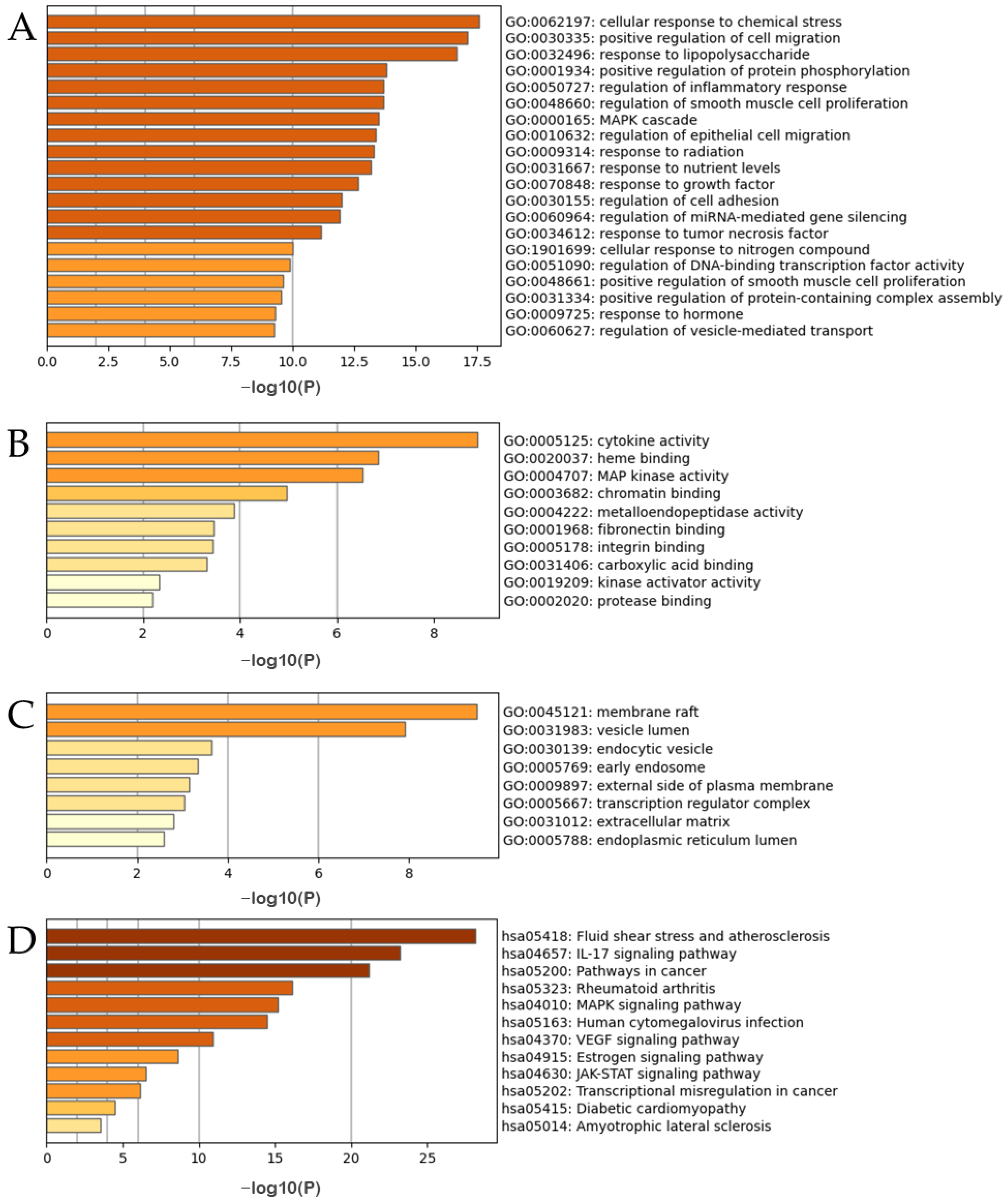
| Mol ID | Mol Name | Structure | OB (%) | DL |
|---|---|---|---|---|
| MOL000359 | sitosterol | 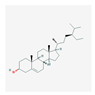 | 36.91 | 0.75 |
| MOL000358 | beta-sitosterol | 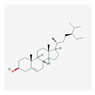 | 36.91 | 0.75 |
| MOL011169 | Peroxyergosterol | 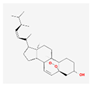 | 44.39 | 0.82 |
| MOL000073 | ent-Epicatechin |  | 48.96 | 0.24 |
| MOL000492 | (+)-catechin | 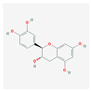 | 54.83 | 0.24 |
| MOL004576 | taxifolin | 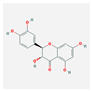 | 57.84 | 0.27 |
| MOL001736 | (−)-taxifolin |  | 60.51 | 0.27 |
| MOL000387 | Bifendate |  | 31.10 | 0.67 |
| MOL000033 | (3S,8S,9S,10R,13R,14S,17R)-10,13-dimethyl-17-[(2R,5S)-5-propan-2-yloctan-2-yl]-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol |  | 36.23 | 0.78 |
| MOL000379 | 9,10-dimethoxypterocarpan-3-O-β-D-glucoside | 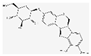 | 36.74 | 0.92 |
| MOL000296 | hederagenin | 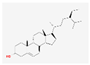 | 36.91 | 0.75 |
| MOL000442 | 1,7-Dihydroxy-3,9-dimethoxy pterocarpene | 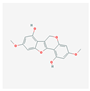 | 39.05 | 0.48 |
| MOL000374 | 5′-hydroxyiso-muronulatol-2′,5′-di-O-glucoside | 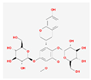 | 41.72 | 0.69 |
| MOL000422 | kaempferol | 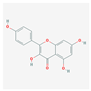 | 41.88 | 0.24 |
| MOL000098 | quercetin |  | 46.43 | 0.28 |
| MOL000417 | Calycosin | 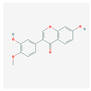 | 47.75 | 0.24 |
| MOL000439 | isomucronulatol-7,2′-di-O-glucosiole | 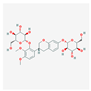 | 49.28 | 0.62 |
| MOL000354 | isorhamnetin | 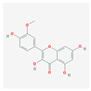 | 49.60 | 0.31 |
| MOL000239 | Jaranol | 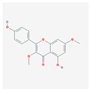 | 50.83 | 0.29 |
| MOL000371 | 3,9-di-O-methylnissolin | 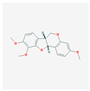 | 53.74 | 0.48 |
| MOL000211 | Mairin | 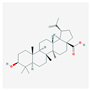 | 55.38 | 0.78 |
| MOL000380 | (6aR,11aR)-9,10-dimethoxy-6a,11a-dihydro-6H-benzofurano [3,2-c]chromen-3-ol | 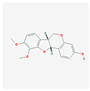 | 64.26 | 0.42 |
| MOL000438 | (3R)-3-(2-hydroxy-3,4-dimethoxyphenyl)chroman-7-ol | 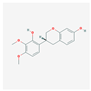 | 67.67 | 0.26 |
| MOL000433 | FA |  | 68.96 | 0.71 |
| MOL000392 | formononetin | 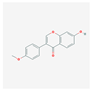 | 69.67 | 0.21 |
| MOL000378 | 7-O-methylisomucronulatol |  | 74.69 | 0.30 |
| MOL000398 | isoflavanone | 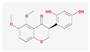 | 109.99 | 0.30 |
| Molecule Name | Degree Centrality | Betweenness Centrality | Closeness Centrality | Included Herb |
|---|---|---|---|---|
| quercetin | 45 | 0.636 | 0.632 | AR |
| kaempferol | 23 | 0.140 | 0.460 | AR |
| isorhamnetin | 13 | 0.067 | 0.413 | AR |
| formononetin | 13 | 0.066 | 0.413 | AR |
| 7-O-methylisomucronulatol | 11 | 0.042 | 0.404 | AR |
| Calycosin | 9 | 0.022 | 0.396 | AR |
| 3,9-di-O-methylnissolin | 8 | 0.034 | 0.387 | AR |
| beta-sitosterol | 6 | 0.044 | 0.379 | CR |
| Jaranol | 5 | 0.003 | 0.376 | AR |
| (+)-catechin | 3 | 0.028 | 0.368 | CR |
| Number | Gene Name | Degree Centrality | Betweenness Centrality | Closeness Centrality |
|---|---|---|---|---|
| 1 | TNF | 45 | 0.054 | 0.846 |
| 2 | IL6 | 44 | 0.047 | 0.833 |
| 3 | VEGFA | 43 | 0.041 | 0.821 |
| 4 | TP53 | 41 | 0.042 | 0.797 |
| 5 | IL1B | 40 | 0.028 | 0.786 |
| 6 | PTGS2 | 38 | 0.025 | 0.764 |
| 7 | CAT | 37 | 0.046 | 0.753 |
| 8 | EGFR | 37 | 0.024 | 0.753 |
| 9 | JUN | 37 | 0.032 | 0.743 |
| 10 | NOS3 | 36 | 0.047 | 0.733 |
| 11 | PPARG | 36 | 0.037 | 0.733 |
| 12 | EGF | 35 | 0.014 | 0.733 |
| 13 | CCL2 | 34 | 0.013 | 0.724 |
| 14 | ESR1 | 33 | 0.033 | 0.714 |
| 15 | HMOX1 | 32 | 0.010 | 0.705 |
| 16 | MMP2 | 32 | 0.014 | 0.705 |
| 17 | VCAM1 | 30 | 0.009 | 0.688 |
| 18 | IFNG | 28 | 0.019 | 0.671 |
| 19 | IL2 | 28 | 0.004 | 0.671 |
| 20 | MPO | 26 | 0.008 | 0.655 |
| 21 | MAPK8 | 25 | 0.008 | 0.647 |
| 22 | SELE | 25 | 0.003 | 0.640 |
| 23 | MAPK14 | 24 | 0.002 | 0.632 |
| 24 | MMP3 | 24 | 0.022 | 0.632 |
| 25 | MAPK1 | 23 | 0.008 | 0.632 |
| 26 | KDR | 22 | 0.018 | 0.611 |
| 27 | MMP1 | 22 | 0.002 | 0.625 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.-G.; Baek, E.; Lee, D. Comparative Efficacy of East Asian Herbal Formulae Containing Astragali Radix–Cinnamomi Ramulus Herb-Pair against Diabetic Peripheral Neuropathy and Mechanism Prediction: A Bayesian Network Meta-Analysis Integrated with Network Pharmacology. Pharmaceutics 2023, 15, 1361. https://doi.org/10.3390/pharmaceutics15051361
Jo H-G, Baek E, Lee D. Comparative Efficacy of East Asian Herbal Formulae Containing Astragali Radix–Cinnamomi Ramulus Herb-Pair against Diabetic Peripheral Neuropathy and Mechanism Prediction: A Bayesian Network Meta-Analysis Integrated with Network Pharmacology. Pharmaceutics. 2023; 15(5):1361. https://doi.org/10.3390/pharmaceutics15051361
Chicago/Turabian StyleJo, Hee-Geun, Eunhye Baek, and Donghun Lee. 2023. "Comparative Efficacy of East Asian Herbal Formulae Containing Astragali Radix–Cinnamomi Ramulus Herb-Pair against Diabetic Peripheral Neuropathy and Mechanism Prediction: A Bayesian Network Meta-Analysis Integrated with Network Pharmacology" Pharmaceutics 15, no. 5: 1361. https://doi.org/10.3390/pharmaceutics15051361
APA StyleJo, H.-G., Baek, E., & Lee, D. (2023). Comparative Efficacy of East Asian Herbal Formulae Containing Astragali Radix–Cinnamomi Ramulus Herb-Pair against Diabetic Peripheral Neuropathy and Mechanism Prediction: A Bayesian Network Meta-Analysis Integrated with Network Pharmacology. Pharmaceutics, 15(5), 1361. https://doi.org/10.3390/pharmaceutics15051361