Overview of Ethnobotanical–Pharmacological Studies Carried Out on Medicinal Plants from the Serra da Estrela Natural Park: Focus on Their Antidiabetic Potential
Abstract
:1. Introduction
2. Materials and Methods
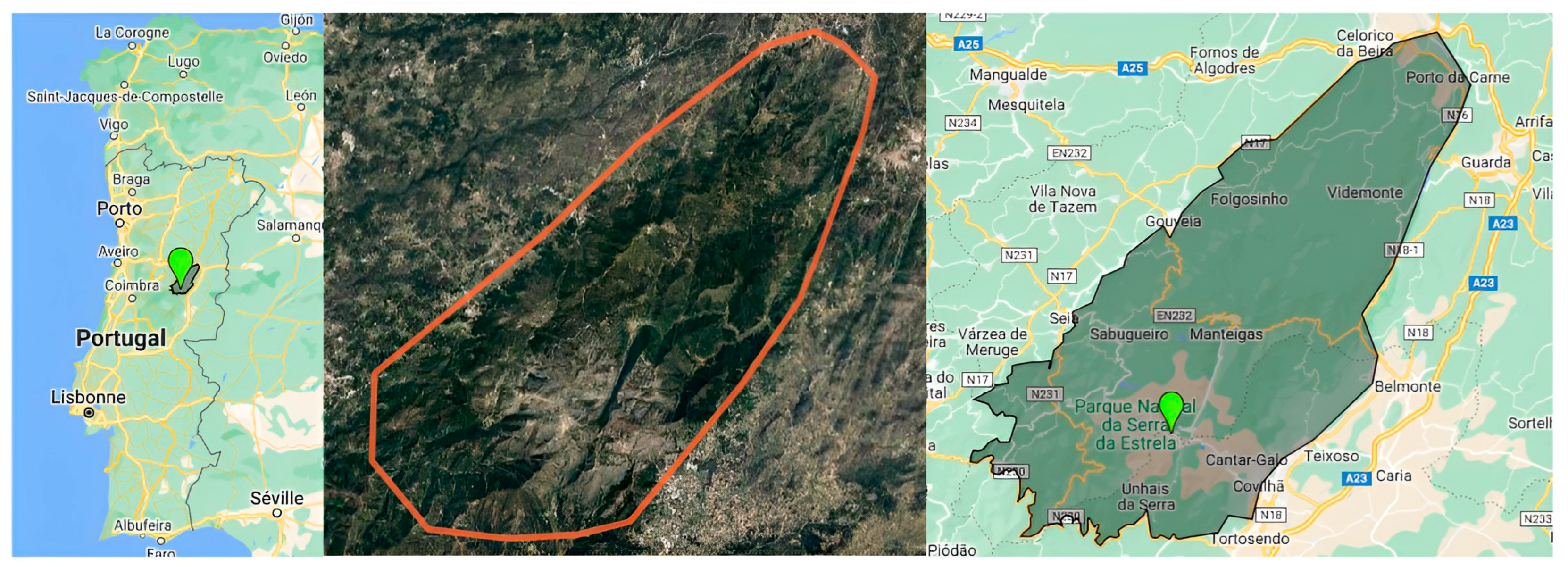
2.1. Geographical and Climate Features of the Serra da Estrela Natural Park
2.2. Ethnobotanical Data Collection and Selection Criteria
3. Results
3.1. Botanical Diversity of NPSE and Ethnopharmacological Uses of Medicinal Plants with Antidiabetic Potential
3.1.1. Asteraceae
3.1.2. Lamiaceae
3.1.3. Fabaceae
3.1.4. Rosaceae
3.1.5. Caryophyllaceae
3.1.6. Polygalaceae
3.1.7. Other families
3.2. Medicinal Plants with Antidiabetic Potential in NPSE
3.2.1. Asteraceae Family
- Arctium minus (Hill) Bernh.
| Pharmacological Uses | Chemical Constituents | References |
|---|---|---|
| Asteraceae | ||
| Arctium minus (Hill) Bernh | ||
| Antimicrobial, antioxidant, anti-inflammatory, antinociceptive, acetylcholinesterase inhibitory activities, anti-cancer. | Phenolic acids: Rosmarinic acid, quinic acid, caffeic acid, chlorogenic acid, cynarin, hydroxy cinnamoyl quinic acid. Flavonoids: Rutin, isoquercetin, luteolin kaempferol-3-O-rhamnoglucoside, quercimeritrin, astragalin, arabinose, rhamnose, mannose, cellulose, inulin. Polysaccharides: Pectic substance, rhamnogalacturonan, hemicellulose (arabinan, arabinogalactan, galactan, xylan, xyloglucan, galacturonic acid, glucose, galactose. | [118,173,183,184,185,188,189] |
| Achillea millefolium | ||
| Anxiolytic, antimicrobial, antioxidant, vasoprotective, vasorelaxant, anti-appetite (orexigenic), anti-tumor, anti-ulcerogenic, hypotensive, analgesic, modulation of mitochondria respiration, anti-inflammatory, anti-neuroinflammatory, anti-proliferative, antiplatelet, skin-rejuvenating, antinociceptive, hepatoprotective, antiplasmodial, anthelmintic, antispasmodic, anti-cancer, antispermatogenic, for haemorrhoids and dysmenorrhea. | Phenolic acids: Cis and trans-3,5-O-dicaffeoylquinic acids, chlorogenic acid, p-coumaric acid, neochlorogenic acid, ferulic acid, stachydrine. Flavonoids: Resveratrol, morin, myricetin, naringin, naringenin, apigenin, quercetin, luteolin O-acetylhexoside, apigenin O-acetylhexoside, centaureidin, casticin, artemetin, luteolin 7-glucoside, luteolin 4′-O-glucosid, apigenin 7-glucoside, apigenin 4′-O-α-glucopyranoside, 5-Hydroxy-3,6,7,4′-tetramethoxyflavone, kaempferol, isorhamnetin glycosides, rutin, cynaroside, cosmosiin, vicenin-2. Sesquiterpenoids: paulitin, isopaulitin, psilostachyin C, desacetylmatricarin, sintenin, achillicin, 8a-(Angeloyloxy), artabsin 1,4-endoperoxide, 8a-(Tigloyloxy)artabsin 1,4-endoperoxide, 7b-Hydroxy-a-longipin-2-en-1-one, a-Longipin-2-en-1-one (longipinanes), Millefoliumins F and G, leucodin, 8α-angeloxy-leucodin, achillin, 8α-angeloxy-achillin, desacetylmatricarin. Organic acids and phenols: oxalic, quinic, citric acids, fatty acids (with linoleic and palmitic acids), tocopherols (γ-tocopherol), ascorbic acid, carboxylic acid, salicylic acid, thymol, carvacrol, pyrocatechol, adenine, mandelic acid, methyl esters of caprylic-linolenic-undecylenic acid. | [172,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216] |
| Anthemis canescens (syn. Matricaria aurea) | ||
| Antioxidant, anti-inflammatory, anti-ulcer, analgesic, antibacterial, anti-cancer. | Phenolic acids: p-coumaric acid, ferulic acid, shikimic acid, protocatechuic acid, p-aminobenzoic acid, digalloyl-shikimic acid, epicatechin, p-hydroxybenzoic acid, rosmarinic acid, 7,8-dihydroxycoumarin, chlorogenic acid, 1-O-b-d-glucopyranosyl sinapate, 5-methoxysalicylic acid. Flavonoids: Apigenin, apigenin-7-O-rhamnoglucoside (Rhoifolin), apigenin 8-C-glucoside, apigenin-7-O-glucoside, 4′-Methoxyapigenin (Acacetin), luteolin, luteolin-6-C-glucoside, quercetin, quercetin-3-D-xyloside, quercetin-7-O-rhamnoside, quercetin-3-arabinoside, quercitrin, kaempferol-3-glucuronide, kaempferol-3-O-alpha-L-rhamnoside, kaempferol-3-O-alpha-L-arabinoside, Kaempferide, eriodictyol-7-O-glucoside, baicalin, isovitexin 7-O-glucoside (saponarin), syringetin-3-O-galactoside, rhamnetin, isorhamnetin, isorhamnetin-3-O-rutinoside, isorhamnetin-3-O-glucoside, myricitrin, daidzein-8-C-glucoside, cyanidin-3-glucoside, myricetin, diosmetin 7-O-rutinoside, hesperetin-7-O-neohesperidoside, maritimetin-6-O-glucoside, acacetin-7-O-neohesperidoside, acacetin-7-O-rutinoside, naringenin, esculetin, formononetin, resveratrol, eriodictyol. Others: Anthocyanins (delphinidin-3-rutinoside), terpenes alkaloids (gibberellin A4), chalcones (Okanin-4′-O-glucoside), coumarins (Scopoletin, 4-methylumbelliferone). | [217,218,219,220,221,222] |
| Arnica montana | ||
| Antiphlogistic, inotropic, antibiotic, anti-inflammatory, immunomodulatory, antiplatelet, uterotonic, anti-rheumatic, anti-osteoarthritic, antimicrobial, improve circulation, increase respiration, ureotonic, antioxidant, hepatoprotective, insecticidal, hypopigmentation, antihair loss, anticough, antihaemorrhagic and analgesic in febrile conditions. | Phenolic acids: Chlorogenic acid, 3,5-dicaffeoylquinic acid, 4,5-dicaffeoylquinic acid Flavonoids: Kaempferol 3-O-glucoside, 6-methoxy-kaempferol 3-O-glucoside, hispidulin, quercetin 3-O-glucoside, quercetin 3-O-glucuronic acid, patuletin 3-O-glucoside, luteolin, apigenin. Sesquiterpene lactones: Helenalin,11a,13-dihydohelenalin. Others: Carotenoids, diterpenes, arnidiol, 2-pyrrolidineacetic acid, pyrrolizidine alkaloids (tussilagine and isotussilagine), polyacetylenes, coumarins (umbelliferone and scopoletin), lignans, dicaffeoyl quinic derivatives (1,3-3,5 and 4,5 dicaffeoyl quinic acids), umbelliferone, scopoletin, oligosaccharides, sesquiterpene lactones (2,3-dihydroaromaticin, chamissonoid, mexicanin 1). | [223,224,225] |
| Bellis perennis | ||
| Wound healing, anxiolytic, anti-tumor, antibacterial, anti-fungal, anti-hyperlipidemic, antioxidant, postpartum anti-hemorrhagic, pancreatic lipase inhibitor, and cytotoxic activities. | Phenolic acids: Chlorogenic acid, neochlorogenic acid, rosmarinic acid, caffeoylquinic acids. Flavonoids: Isorhamnetin 3-O-β-d-galactopyranoside, isorhamnetin 3-O-β-d-(6 ″-acetyl)-galactopyranoside and kaempferol 3-O-β-d-glucopyranoside. Triterpene saponins: Perennisosides I-VII, bellidioside A, asterbatanoside D, bernardioside A/F/B2, bellissaponin BS6/BA1/BA2, Anthocyanins: Cyanidin 3-O-(4″-O-(malonyl)-2″O-(β d-glucuronyl)-β-d-glucopyranoside), cyanidin 3-O-(2″-O-(β-d-glucuronyl)-β-d-glucopyranoside), cyanidin 3-O-(6″-O-(malonyl)-2″-O-(β-d-glucuronyl)-β-d-glucopyranoside). | [226,227,228,229,230,231,232,233,234,235,236,237] |
| Bidens frondose | ||
| Antibacterial, antioxidant, antidiarrheal, anti-malarial, anti-inflammatory, allelopathic. | Phenolic acids and their ethers: Caffeic acid, 4,5-di-O caffeoylquinic acid 1-methyl ether, isoferuloyl ethyl ester, protocatechuic acid. Flavonoids: Okanin-4′-O-(6″-O-acetyl-2”-O-caffeoyl-6″-O-glucopyranoside), okanin-4′-O-(2”-O-caffeoyl-6″-O-p-coumaroyl-β-D-glucopyranoside), 4-O-methylokanin-4′-O-(6″-O-p-coumaroyl-β-D-glucopyranoside), 4-O-methylokanin-4′-O-(6″-O-acetyl-β-D-glucopyranoside), 4-O-methylokanin-4′-O-(6″-O-acetyl-2”-O-caffeoyl-β-D-glucopyranoside), okanin-4′-O-(6″-O-p-coumaroyl β-D-glucopyranoside), okanin-4′-O-(6″-O-acetyl-β-D-glucopyranoside), (Z)-6″-O-p-coumaroyl-maritimein, (Z)-6″-O-acetylmaritimein, apigenin, luteolin, luteolin-7-O- β-D-glucopyranoside, luteolin-7-O-(β-dglucopyranosyl)-2-glucopyranoside, kaempferol-3-O-β-D-glucopyranoside, quercetin-3-O-β-D-glucopyranoside, 8,3′,4′-trihydroxyflavone-7-O-(6′′-O-p-coumaroyl)-β-D-glucopyranoside, 6-hydroxyluteolin-7-O-glucoside, 3′′-(3-hydroxy-3-methylglutaroyl)-ester of 6-hydroxyluteolin-7-O-β-D-glucopyranoside, 8,3′,4′-trihydroxyflavone-7-O-β-D-glucopyranoside, 3′-hydroxyscutellarein-7-O-(6′′-Oprotocatechuoyl)-β-glucopyranoside, (−)-4′-methoxy-7-Oβ-dglucopyranosyl-8,3′-dihydroxyflavanone, (−)-4′-methoxy-7-O-(6′′-acetyl)-βdglucopyranosyl-8,3′-dihydroxyflavanone, hesperetin-7-O-β-D-glucopyranoside. Others: 2′-butoxyethylconiferin, butylconiferin, 2-methoxy-4-(2′-hydroxyethyl)-phenol-1-O-β-D-glucopyranoside, (1′R,2′R)-guaiacyl glycerol 3′-O-β-dglucopyranoside, threo-5-hydroxy3,7-dimethoxyphenylpropane-8,9-diol, 3-(4-hydroxy-3-methoxyphenyl)-3-methoxypropane1,2-diol, 3-(4-Hydroxy-3-methoxyphenyl)propane-1,2-diol, guaiacylglycerol, wilfordiol B, caffeoylcalleryanin, 1-O-(E)-caffeoyl-β-dgentiobiose, dihydrophaseic acid, 1,3,5-trimethoxybenzene, vanillin, galacturonic acid, galactose, glucose, arabinose, xylose, rhamnose. | [238,239,240,241,242] |
| Calendula arvensis | ||
| Antibacterial, anti-fungal, antiparasitic, anti-inflammatory, antioxidant, wound healing, antimutagenic, immunomodulatory, and anti-cancer. | Phenolic acids: Isomeric form hydroxy ferulic acid hexoside, 5-O-caffeoylquinic acid, 4-O-caffeoylquinic acid, caffeic acid, sinapic acid, sinapic acid hexoside, hexoside derivative, caffeoylshikimic acid, 3,4-O-dicaffeoylquinic acid, 5-O-feruloyl quinic acid, protocatechuic acid pentoside, quinic acid with an aldonic residue. Flavonoids: Quercetin hydrate, quercetin dihexoside, quercetin-3-O-rutinoside, quercetin-3-O-neohesperidoside, quercetin-3-omalonylhexoside, quercetin acetyl hexoside, quercetin hexoside I, quercetin 3-O-β-D-glucopyranoside, quercetin 3-O-β-D-galactopyranoside, apigenin-8-C-pentose-6-chexose or apigenin-8-chexose-6-C-pentose, apigenin-O-hexosylpentosyl, isorhamnetin-3-O-hexoside. Saponins: 3-O-(β-D-galactopyranosyl-(1⟶3)-β-D-glucopyranosyl) oleanolic acid-28-O- β-D-glucopyranoside, 3β-O-(β-D-galactopyranosyl-(1⟶3)-β-D-glucopyranosyl) oleanolic acid, 3β-O-(β-D-galactopyranosyl-(1⟶3)-β-D-glucopyranosyluronic acid) oleanolic acid-28-O- β-D-glucopyranoside, 3β-O-(β-D-galactopyranosyl-(1⟶3)-β-D-glucopyranosyluronic acid) oleanolic acid, 4-O-(β-D-fucopyranosyl)-4-alloaromadendrole, arvensoside A, arvensoside B, derivatives of arvensoside B, calenduloside D, calenduloside C, 4-O-(β-D-fucopyranosyl)-4-alloaromadendrole, 4-O-(β -D-fucopyranosyl)-4-alloaromadendrol-2″-methylpropanoyl esters, 4-O-(β -D-fucopyranosyl)-4-alloaromadendrol -2″-methyl-2″-butenoyl esters, Sesquiterpeneglycosides: 3α,7β-dihydroxy-5β,6β-epoxyeudesm-4(15)-ene-11-(O-β-D-fucopyranoside-2′,4′ -diangelate-3′-acetate), 7β-Hydroxy-3β-acetoxy-5β,6β-epoxyeudesm-5(15)-ene-11-(O-β-D-ficopyranoside-2′,4′-diangelate-3′-acetate), 3α,7β-Dihydroxy-5β,6β-epoxyeudesm-4(15)-ene-11-(O-β-D-fucopyranoside-2′,4′-diangelate-3′-isobutyrate), 3α,7β-dihydroxy -5β, 6β-epoxyeudesm-4(15)-ene-11-(O-β-D-fucopyranoside-2′, 4′-diangelate-3′-methylbutyrate), and 3α,7β-dihydroxy-15-acetoxyeudesm-4(5)-ene-11-(O-β-D-fucopyranoside-2′,4′-diangelate-3′-acetate). Carboxylic acids/Fatty acids: Stearic acid, oleic acid, linoleic acid, linolenic acid, palmitic acid, palmitoleic acid, α-linolenic acid, quinic acid, citric acid, and tetracosanoic acid. Polysaccharides: L-threonic acid, D-(−)-tagatofuranose, D-(−)-fructofuranose, D-(−)-fructopyranose, D-(−)-psicopyranose, D-(+)-mannopyranose, D-(+)-galactopyranose, β-D-glucopyranose, D-gluconic acid, galactaric acid, sucrose, cellobiose. Others: Ethyl butyrate, 2-methyl-3-furanthiol, methional, 1-octen-3-one, ethyl hexanoate, 2-6-Dimethyl-3 ethyl pyrazine, (E)-2-nonenal, (E,E)-2,4-octadienal, 5-methyl-2-furanaldehyde, citronellol, phenethylacetate, α-terpineol, lactone-like, and δ-decalactone, Neophytadiene, phytol, α-bisabolol, 8,14-cedranoxide, stigmasterol, stigmast-5-ene, amyrin, lup-20(29)-en-28-al, 3-oxo-ursan-28-oic acid, myo-inositol, 1H-benzocyclohepten-9-ol, 1-hexacosanol, untriacontane, 4-aminobutanoic acid, isomer of platynecine derivative, ligstroside hexoside, calendasaponin A, calenduloside G isomer, β-sitosterol. | [243] |
| Chamaemelum nobile (syn. Anthemis nobilis L. or Chamomilla nobilis) | ||
| Anti-inflammatory, antioxidant, antinociceptive, antimutagenic, sedative, anxiolytic, antispasmodic, anxiety, depression, sleep quality and insomnia, postoperative gastrointestinal dysfunction, diarrhoea, colic, nausea, vomiting, acute, diuretic, chronic pain, antibacterial, anti-fungal, insecticidal, hypotensive, antiplatelet aggregation, antioxidant, effect in asthma and polycystic ovary, nervous endocrine, cytotoxic, bronchodilator, antispasmodic, carminative, anti-emetic, antispasmodic, cytostatic, anti-oedema sedative properties | Phenolic acids: The glucose esters caffeic acid, ferulic acid, anthenobilic acid, trans-caffeic acid-glucose ester, trans- and cis- forms of the caffeic acid, 3-O-caffeoylquinic acid, 5-O-caffeoylquinic acid-hexoside, 3,4-O-dicaffeoylquinic acid, protocatechuic acid, caffeoyl-hexoside-methylglutarate, 5-O-caffeoylquinic acid, p-coumaroyl-hexoside-methylglutarate 1,3,5-O-Tricaffeoylquinic acid. Flavonoids: Apigenin, apigenin 6-C-glucose-8-C-glucose, apigenin O-glucuronide, apigenin O-glucuronylhexoside, luteolin, luteolin O-hexoside, luteolin O-rutinoside, luteolin O-acetylhexoside, luteolin-7-glucoside, luteolin O-pentosylhexoside, luteolin O-glucuronide, luteolin O-rhamnosylhexoside, quercetin, quercetin 3-O-glucuronide, quercetin 7-O-malonylhexoside, quercetin O-acetylhexoside, isorhamnetin O-acetylhexoside, myricetin 3-O-glucoside, rutin, anthemoside (apigenin2,3-dihydorycinnamoyl acid 7-O-β-D-glucose), cosmosioside (apigenin 7-O-β-D-glucose), apiin (apigenin 7-O-β-D-apiosylglucoside), chamaemeloside [apigenin 7-O-β-D-glucose-6″-(3′″-hydroxy-3′″-methyl-glutarate)], luteolin 7-O-β-D-glucose, quercetin 3-O-α-L-rhamnoside, kaempferol, kaempferol O-pentosylhexoside, catechins. Terpenoids and steroids: α-bisabolol, chamazulene, anthesterols, β-amyrin, taraxasterol, pseudotaraxasterol, β-sitosterol. Coumarins: Herniarin, umbelliferone, scopoletin-7-glucoside. Others: Angelic and tiglic acid esters, anthemic acid, choline, phenolic, phytosterols, inositol, oxalic acid, quinic acid, malic acid, citric acid, fumaric acids, octulosonic acid, betahydroperoxyisonobilin, hydroxyisonobiline, germacranolide-type sesquiterpene lactones (nobilin, 3-epinobilin, 1,10-epoxynobilin, 3-dehydronobilin), amyl and isobutyl alcohols, 1β-Hydroperoxyisonobilin, alkyl hydroperoxides, Cis- and trans-spiroether derivatives, cis- and trans-dehydromatricariaester and tiophenesetrs, polyacetylenes. | [244,245,246] |
| Cichorium intybus | ||
| The hepatoprotective, anti-inflammatory, antioxidant, sedative, immunomodulatory effect, cardiovascular, hypolipidemic, gastro-protective, anti-tumor, anti-leukaemic, cytotoxic, antimicrobial, allergenic, antibiotic, anti-cancer, anti hyperuricemia, antiprotozoal, anthelmintic, anti-malarial, sedative. | Phenolic acids: Chlorogenic acid, chicoric acid, p-coumaric acids, protocatechuic acid, p-hydroxybenzoic, iso vanillic, gallic acid, 4-amino-benzoic, p-OH-benzoic, caffeine, ferulic acid, isoferulic acid, vanillic acid, benzoic acid, ellagic acid, alpha-cumaric, 3,4,5-methoxy-cinnamic, salycilic acid, cinnamic acid, 3-O-p-coumaroyl quinic acid. Flavonoids: Quercetin, quercetin glucuronide, luteolin glucuronide, catechin, catechol, epicatechin, cyanidin-3-O-(6″-malonyl-β-glucopyranoside), delphinidin 3,5-di-O-(6-O-malonyl-β-d-glucoside), delphinidin 3-O-(6-O-malonyl-β-d-glucoside)-5-O-β-d-glucoside, delphinidin 3-O-β-d-glucoside-5-O-(6-O-malonyl-β-d-glucoside), delphinidin 3,5-di-O-β-d-glucoside. Fatty acids and derivatives: Lauric acid methyl ester, myristic acid methyl ester, palmitoleic methyl ester, palmitic acid methyl ester, methyl dihydromalvalate, 9,12- linoleic methyl ester, stearic acid methyl ester, methyl linolelaidate, linolenic acid methyl ester, 11-eicosenoic acid methyl ester, eicosanoic acid methyl ester, n-hexadecanyl hexadecanoate, n-pentadecanyl octadec-9-enoate, n-hexadecanyl octadec-9-enoate, n-hexadecanyl octadecenoate, n-octadecanyl octadecenoate, α-linolenic acid, oleic acid, linoleic acid, palmitic acid. Sesquiterpene lactones: Lactucin, 8-deoxylactucin, 11(S),13-dihydro-8-deoxylactucin, lactucopicrin, 11(S),13- dihydrolactucopicrin, jacquinelin, crepidiaside B, lactuside A, 11(S), 13-dihydrolactucin, lactucin, 8-deoxylactucin, 11(S), 13-dihydro-8-deoxylactucin, 11(S),13-dihydrolactucopicrin, lactucopicrin Others: Inulin, coumarin, epigallocatechin gallate. | [247,248] |
| Dittrichia viscosa subsp. Viscosa (Syn. Inula viscosa) | ||
| Antiphlogistic, antiviral, anti-fungal, antibacterial, antiseptic, anti-inflammatory, allelopathic potential, fungicidal, nematicidal, anti-ulcerogenic, antihelmintic, anti-cancer, neuroprotective effects | Phenolic acids and derivatives: Caffeic acid, di-o-caffeoylquinic acid, rosmarinic acid, protocatechuic acid hexoside, caffeoyl hexose, p-coumaroyl hexose, 1-O-caffeoylquinic acid, 3-O-caffeoylquinic acid, 4-O-caffeoylquinic acid, di-O-Caffeoylquinic acid, caffeic acid phenethyl ester, (Epi)-rosmanol methyl ether, rosmanol, epirosmanol, dicaffeoylshikimic acid, N-caffeoyl-tryptophan, dihydroxybenzoic acid. Flavonoids: Dihydroquercetin, 3-O-methylquercetin, quercetin-O-(caffeoyl)-hexoside, quercetin dihexoside, quercetin-3-O-(6″-acetyl) hexoside, quercetin rhamnoside, cirsiliol, 3-O-acetylpadmatin, padmatin, nepetin, spinacetin, diosmetin, rhamnetin, hesperetin, hispidulin, catechin, medioresinol, γ-mangostin, banaxanthone E, epi- granilin, naringenin, isorhamnetin, diosmetin, cirsimaritin derivative, genkwanin, rutin, kaempferol-O-deoxyhexoside, kaempferol-3-O-(6″-acetyl) hexoside, kaempferol-3-O-(caffeoyl)-hexoside, aromadendrin, naringenin-7-O-hexoside, isorhamnetin glycoside, isorhamnetin-O-pentosylhexoside, kaempferol-O-(p-coumaroyl)-hexoside, kaempferol-O-(feruloyl)-hexoside, 3,7-dihydroxycoumarin, nepetin, spinacetin, dihydroxycoumarin, padmatin isomer 1/2, cinchonain. Sesquiterpenes: α- and γ- costic acid isomers, ilicic acid, hydroxyalantolactone, tomentosine/inulviscolide, alantolactone, inulanolide, Others: Galloylquinic acid, (Epi)-gallocatechin-3-gallate, paxanthone, proanthocyanidin dimer, prodelphinidin B3, malic acid I and II, caffeoyl-malic acid, shikimoyl blechnic acid. | [249,250,251,252,253,254,255,256,257,258,259] |
| Galinsoga parviflora | ||
| Antibacterial, antioxidant, anti-arthritic, antiplatelet, anti-inflammatory, anti-fungal. | Kaempferol, gallic acid, 2,4,5-tricaffeolylglucaric acid, 2,3,4,5-tetracaffeolylglucaric acid, 2,3,4-tricaffeolylaltraric acid, 3,4,5-tricaffeolylaltraric acid, beta-sitosterol-3-O-beta-glucoside, quercetine, beta-sitosterol, 3,5,7,3′,4′pentahydroxyflavanone, 4-hydroxybenzoic acid. | [260] |
| Helichrysum stoechas | ||
| Antibacterial, anti-proliferative, neuroprotective, anti-inflammatory, antioxidant treatment for urolithiasis. | Neo-chlorogenic acid, chlorogenic acid and crypto-chlorogenic acid, isomeric dicaffeoyl quinic acids, isomeric naringenin glucosides, quercetin, isoquercitrin, kaempferol, apigenin glucosides, tetrahydroxychalcone-glucoside, Helipyrone A/B/C, Italipyrone, 20-prenylitalipyrone, Bitalin A (R)-form, 6-methyleuparin, helipyrone, 5,7-dihydroxy-3,6,8-trimethoxyflavone, quercetagetin-7-O-glucopyranoside, santinol B. | [261,262,263,264] |
| Hypochaeris radicata | ||
| Treatment of jaundice, rheumatism, and antibacterial, anti-fungal properties with antioxidant and anti-inflammatory, antihemolytic. | Chicoric acid, hypochoeroside C, hypochoeroside D, 5-O-caffeoylshikimic acid, 4-(3,4-dihydroxybenzyl)-2-(3,4-dihydroxyphenyl)tetrahydrofuran-3-carboxy-O-β-D-glucopyranoside, 4-(3,4-dihydroxybenzyl)-2-(3,4-dihydroxyphenyl)tetrahydrofuran-3-carboxy-O-β-D-glucopyranosyl-2′-O-methacrylate, (7S,8R,8′R)-7-(3,4-dihydroxyphenyl)-3′,4′-dihydroxy-7,8,7′,8′-tetrahydronaphtho [8,8′-c]furan-1(3H)-one, (7S,8R,8′R)-7-(3,4-dihydroxyphenyl)-3′,4′-dihydroxy-8′-(hydroxymethyl)-7,8,7′,8′-tetrahydronaphthalen-8-carboxylic acid, confertin, scopoletin. | [265,266,267,268] |
| Lactuca serriola | ||
| Hepatoprotective, antioxidant, antivenom, allelopathic, sedative, anticonvulsant, antiepileptic, anti-inflammatory, anti-carcinogenic activities | Chlorogenic acid, caffeic acid, quercetin, lactutin, 8-deoxylactucin, jacquilenin, 11-β-13-dihydrolactucin, deacetoxymatricarin (=leucodin, leucomisin), loliolide, guaiane ester, the melampolide glucoside, luteolin-7-O-β-D glucoside, protocatechuic acid, 4-hydroxybenzoic acid, lactuside A, kaempferol, lactucone, lactucic acids, lactucopicrin, sesquiterpene esters, vitamins, oxalic acid, β-carotene, iron, lupeol, lupeol acetate, oleanans, α-amyrin, β-amyrin. | [269,270,271,272,273,274,275,276] |
| Onopordum acanthium | ||
| Antihypertensive, bactericide, cardiotonic, hemostatic agent, used against hypotonicity, anti-inflammatory, anti-malarial, anti-inflammatory, anti-tumor, cytotoxicity, antipyretic, analgesic, anti-tumor, regeneration, phytotoxic. | Phenolic acids and derivatives: Isochlorogenic acid, caffeic acid, Flavonoids: Apigenin, luteolin, scutellarein, nepetin, chrysoeriol, hispidulin, pectolinarigenin, scutellarein 4′-methyl ether, quercetin, apigenin-7-O-glucoside, apigenin-7-O-rutinoside, apigenin-7-O-β-D-glucuronide, luteolin-7-O-glucoside, quercetin-3-O-glucoside, isorhamnetin-3-O-glucoside, riodictyol; cyanin, aconiside. Others: Pinoresinol, syringaresinol, medioresinol, nitidanin diisovalerianate; arctiin, aesculin; aesculetin, 4β,15-dihydro-3-dehydrozaluzanin C, zaluzanin C, 4β,15,11β,13-tetrahydrozaluzanin C, onopordopicrin; arctiopicrin, Elemanolide 11(13)-dehydromelitensin β-hydroxyisobutyrate; acanthiolide, α-amyrin; β-amyrin, lupeol; taraxasterol, steroids, heptadecatetraen-(2,8,10,16)-diin-(4, 6)-al-(1), tridecadien-(1,11)-tetrain-(3,5,7,9), heptadecatetraen-(1,7,9,15)-diin-(11,13), heptadecatetraen-(2,8,10,16)-diin-(4,6)-ol-(1)), linoleic acid, oleic acid, palmitic acid, stearic acid, pentadecanoic acid, hentriacontanoic acid, nonacosanoic acid, arachidic acid, margaric acid, myristic acid, behenic acid, palmitoleic acid, gadoleic acid, erucic acid, vaccenic acids, α-tocopherol, α-tocotrienol, β-tocopherol, γ-tocopherol, 1-amino-2-propanol, stachydrine, choline, phytin. | [277,278,279,280,281,282,283,284] |
| Senecio vulgaris | ||
| Antioxidant, cytotoxic, antibacterial, anti-fungal | Phenolic acids and derivatives: Caffeic acid, protocatechuic acid, 3-O-caffeoylquinic acid (chlorogenic acid), dicaffeoylquinic acid, p-hydroxy benzene-acetic acid, vanillic acid, syringic acid, p-hydroxy benzene-acetic acid derivative, p-hydroxycinnamic acid. Flavonoids: Quercitin-3-glucoside (Isoquercitrin), quercetin 3-O-rhamnoside (quercitrin), kaempferol-3-O-di-deoxyhexoside. Pyrrolizidine alkaloid: Retrorsine N-oxide, spartioidine N-oxide, seneciophylline N-oxide, integerrimine N-oxide, senecionine N-oxide, usaramine, neosenkirkine, riddelline, neoplatyphylline, retrorsine, spartioidine, platyphylline, integerrimine, senecionine. | [285,286,287,288] |
| Solidago virgaurea | ||
| Antioxidant, anti-inflammatory, analgesic, spasmolytic, antihypertensive, diuretic effects and benefits in other urinary tract conditions, antibacterial, anti-fungal, antiparasitic, cytotoxic and anti-tumor, antimutagenic, cardioprotective, antisenescence effects. | Phenolic acids and derivatives: Caffeic acid, chlorogenic acid, 5-O-caffeoylquinic (neo chlorogenic) acid, 3,5-di-O-caffeoylquinic acid, 3,4-di-O-caffeoylquinic acid, 4,5-di-O-caffeoylquinic acid, 3,4,5-tri-O-caffeoylquinic acid, methyl 3,5-di-O-caffeoylquinate, 3-hydroxyphenyl acetic acid, 3,4-dihydroxyphenylacetic acid, 5-p-coumaroylquinic acid, homovanilic acid, p-coumaric acid, ferulic acid, sinapic acid, rosmarinic acid benzoic acid, 3-hydroxybenzoic acid, 4-hydroxybenzoic acid, 3,4-dihydroxybenzoic (protocatechuic) acid, salicylic acid, gentisic acid, vanillic acid, gallic acid, leiocarposide, 2-methoxybenzyl-2,6-dimethoxy benzoate. Flavonoids: Quercetin, quercetin-3-O-glucoside (isoquercitrin), quercetin-3-O-galactoside (hyperoside), quercetin-3-O-rhamnoside (quercitrin), quercetin-3-O-rutinoside (rutin), quercetin-3-O-arabinopyranoside (avicularin), kaempferol-3-O-glucoside (astragalin), kaempferol-3-O-rhamnoside (afzelin), kaempferol-3-O-rutinoside (nicotiflorin), kaempferol-3-O-robinobioside (biorobin), myricetin 3-rhamnoside (myricitrin), Isorhamnetin-3-O-rutinoside (narcissin), cyanidin-3-gentiobioside mono-C-glycosylflavones, di-C-glycosyl flavones. Others: Virgaureasaponins 1–6, solidagosaponins X-XXIX, bellisaponin BA2, erythrodiol-3-acetate, α-tocopherol quinone, 2-phyten-1-ol. | [289] |
| Sonchus asper | ||
| Antioxidant, anti-inflammatory, antibacterial, insecticidal, hepatorenal protective, used for treating bronchitis, gastrointestinal infection, cardiac dysfunction, kidney diseases, and cancer. | Phenolic acids and derivatives: Caffeic acid, 3-coumaric acid, chlorogenic acid, gallic acid, luteolin, luteolin-7-o, protocatechuic acid, rosemarinic acid, quinic acid, vanillic acid. Flavonoids: Apigenin, apigenin-7-o, luteolin, pyrocatechol, quercetin, rutin. Others: 11 beta,13-dihydrourospermal A, 15-O-beta-D-glucopyranosyl-11 beta,13-dihydrourospermal A, 15-O-beta-D-glucopyranosylurospermal A, 15-O-[6′-(p-hydroxyphenylacetyl)]-beta-D-glucopyranosylurospermal A and 14-O-methylacetal-15-O-[6′-(p-hydroxyphenylacetyl)]-beta-D-glucopyranosylurospermal A, asperal, emodin, methyl-(3,8-di-hydroxy-6-methyl-9-oxo-9H-xanthene)-1-carboxylate. | [118,290,291,292,293,294,295,296,297,298,299,300] |
| Sonchus oleraceus | ||
| Antioxidant, anti-inflammatory, anti-tumor, antibacterial, anti-fungal, antidepressant, anxiolytic, antinociceptive effects, used for treating cancer, diarrhoea, and enteritis. | Phenolic acids and derivatives: Chicorin, caffeic acid glycoside, 4-cafffeoylquinic acid, 5-caffeoylquinic acid, cis-3′ caffeoylquinic acid, 5-coumaroylquinic acid, caftaric acid, chicoric acid, 3,4 dicaffeoylquinic acid, 3,5 dicaffeoylquinic acid, dicaffeoylquinic acid (isomer), cis-3,5 dicaffeoylquinic acid (isomer), tri-O-caffeyolyquinic acid, cis-3,4 dicaffeoylquinic acid, 4,5 dicaffeoylquinic acid. Flavonoids: Quercetin-glucoronide-glycosyl, quercetin-hexose-hexoside, quercetin glucoside glucoronide, luteolin-glycosyl-glucuronide, luteolin-diglucoside, isorhamnetin diglucoside, luteolin, luteolin glucuronide, luteolin glycoside, quercetin-rutinoside, isorhamnetin rutinoside, luteolin, quercetin acetylglycoside, apigenin glucuronide, apigenin rutinoside, kaempferol acetylglycoside sesquiterpenes, crepidiaside A. Others: 7S,10S-3,9-dioxo-di-nor-eudesma-4-en-11-oic acid, 6 R,7S,10S-3,9-dioxo-7-hydroxyldi-nor-eudesma-4-en-11-oic acid. | [301,302,303,304,305,306] |
| Tanacetum parthenium | ||
| Antioxidant, anxiolytic, antidepressant, anti-migraine agent, anticoagulant, anti-inflammatory, neuroprotective, antiviral, anti-apoptotic, anti-cancer, antiparasitic, pain reliever. | Phenolic acids and derivatives: 4-o-caffeoyl-quinic acid, 3,4-dicaffeoyl-quinic acid, 3,5-dicaffeoyl-quinic acid, 4,5-dicaffeoyl-quinic acid, neochlorogenic acid, ellagic acid, chlorogenic acid. Flavonoids: Kaempferol-3-rutinoside, 6-hydroxykaempferol-3,6,4′-trimethylether (santin), 6-hydroxykaempferol-3,6-dimethylether, quercetagenin-3,6-dimethylether (axillarin), quercetagenin-3,6,3′-trimethylether (jaceidin), quercetagenin-3,6,4′-trimethylether (centaureidin), apigenin, luteolin, santin, chrysoeriol, luteolin-7-glucoronides, methylquercetin, trihydroxy-methoxyflavone, costunolide, dihydro-β-cyclopyrethrosin, sudachitin, aceronin, tanacetol A isomer, nevadensin, parthenolide, casticin, nevadensin, tanaphillin, 3-β-hydroxyanhydroverlotorin, seco-tanapartholide A/B, hispidulin. | [307,308,309,310,311,312,313,314,315,316,317] |
| Tanacetum vulgare | ||
| Antioxidant, anti-inflammatory, anti-tumor, antibacterial, antiparasitic, anthelmintic, repellent, insecticidal, antiviral, anti-fungal. | Phenolic acids and derivatives: Caffeoylgluconic acid, 1-caffeoylquinic acid, protocatechuic acid, p-hydroxyphenylacetic acid 1-O-hexoside, protocatechuic acid-O-hexoside isomer, syringic acid 4-O-hexoside, neochlorogenic (3-caffeoylquinic) acid, O-caffeoyl hexose, vanillic acid 4-O-hexoside, vanillic acid, caffeoylgluconic acid isomer, O-caffeoyl hexose isómer, 4-hydroxybenzoic acid, 4-hydroxybenzoic acid-hexoside, 3-p-coumaroylquinic acid, caffeoylgluconic acid isomer, O-caffeoyl hexose isomer, quinic acid, chlorogenic (5-caffeoylquinic) acid, p-coumaric acid, 3-feruloylquinic acid, caffeic acid-O-hexoside, caffeic acid, gentisic acid, 5-p-coumaroylquinic acid, 3-caffeoyl-5-hydroxy-dihydrocaffeoylquinic acid, p-hydroxyphenylacetic acid, 5-feruloylquinic acid, 1-caffeoyl-3-hydroxy-dihydrocaffeoylquinic acid, vanillic acid-4-O-(6-O-caffeoyl)-hexoside, 3,4-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, 3-dehydrocaffeoyl-5-caffeoylquinic acid, 4,5-dicaffeoylquinic acid, shikimic acid, 4-dehydrocaffeoyl-5-caffeoylquinic acid, salicylic acid, 3-feruloyl-4-caffeoylquinic acid, 3-p-coumaroyl-5-caffeoylquinic acid, caffeic acid-O-(salicyl)-hexoside, 3-caffeoyl-5-p-coumaroylquinic acid, 3-feruloyl-5-caffeoylquinic acid, 4-caffeoyl-5-p-coumaroylquinic acid, 4-caffeoyl-5-feruloylquinic acid, 3,4,5-tricaffeoylquinic acid. Flavonoids: Apigenin, apigenin-6,8-diC-hexoside, apigenin 7-O-glucoside, methoxyeriodictyol-O-hexuronide, apigenin-O-hexuronide, luteolin, luteolin-O-hexuronide, luteolin 7-O-glucoside, 6-hydroxyluteolin-O-hexoside, luteolin 7-O-gentiobioside dihexoside (gentiobioside) 6-glucopyranosyl-glucopyranoside, luteolin-7-O-neohesperidoside, luteolin-O-caffeoylhexoside, luteolin-O-acetylhexosidekaempferol 3-O-glucuronide, rutin, quercetin, quercetin 3-O-acetylhexoside, quercetin 7-O-hexuronide, kaempferol, kaempferol 3-O-glucoside, eriodictyol-O-hexuronide, patuletin-O-hexoside, nepetin-O-hexoside, isorhamnetin 3-O-glucoside, naringenin-O-hexuronide, hesperetin 7-O-rutinoside (hesperidin), nepetin-O-hexuronide, hispidulin-O-hexuronide, isorhamnetin-O-hexuronide, chrysoeriol-O-hexuronide, hesperetin-O-hexuronide, jaceosidin -O-hexuronide, patuletin (6-methoxyquercetin), nepetin (6-methoxyluteolin) 6-methoxykaempferol, naringenin, hispidulin (scutellarein-6-methyl ether), chrysoeriol, hesperetin, Isorhamnetin, jaceosidin (6-hydroxyluteolin-6,3′-dimethyl ether), quercetagetin-3,6,3′(4′)-trimethyl ether, cirsimaritin (6-hydroxyapigenin-6,7-dimethyl ether), eupatilin, casticin, acacetin. Sesquiterpene lactones and derivatives: α/β thujone, hydroxyarbusculin, ludovicin C, tanacetin/hydroxyraynosin/armefolin, parthenolide, camphor, caryophyllene oxide, dehydrosantamarin, caryophyllene/bisabolene, linoleamide, palmitamide, oleamide. | [315,318,319,320,321,322,323,324,325,326,327] |
| Lamiaceae | ||
| Calamintha nepeta subsp. nepeta (Syn. Clinopodium nepeta) | ||
| Stimulant, tonic, antiseptic, antispasmodic, antioxidant, antimicrobial, anti-inflammatory, anti-ulcer, phytotoxic. | Phenolic acids and derivatives: 3-O-Caffeoylquinic acid, 4-O-caffeoylquinic acid, 5-O-caffeoylquinic acid, rosmarinic acid, quercetin-3-O-rutinoside, gallic acid, protocatechuic acid, chlorogenic acid, p-hydroxybenzoic acid, vanillic acid, syringic acid, vanillin, trans-cinnamic acid, coumarin, quinic acid, 12-O-hexosyljasmonate, caffeic acid hexamer, caffeic acid pentamer, rosmarinic acid, 12-O-(6′-caffeoylhexosyl)jasmonate, acacetin 7-O-[hexosyl(1iv → 2″)]deoxyhexosyl(1′″ → 6″) hexoside. Flavonoids: Myricetin, quercetin, luteolin, hesperidin, kaempferol, kaempferol-di-O-hexoside, apigenin, luteolin-8-C-(3-hydroxy-3-methyl-glutaroyl) hexosyl hexoside, 6,8-C-dihexosylapigenin, caffeic acid dimer, quercetin-3-O-hexoside, quercetin-3-O-[6″-O-(3-hydroxy-3-methyl-glutaroyl)]hexoside, kaempferol-3-O-hexoside, salvianolic acid B, acacetin, acacetin 7-O-[6iv-O-acetyl-hexosyl(1iv → 2″)]deoxyhexosyl(1′″ → 6″)hexoside, acacetin 7-O-deoxyhexosyl(1′″ → 6″)hexoside. | [328,329,330,331,332,333,334,335,336] |
| Lavandula pedunculata | ||
| Anti-inflammatory, antioxidant, antimicrobial. | Phenolic acids and derivatives: Salvianolic acid B, rosmarinic acid, caffeic acid, caffeic acid hexoside, p-coumaroyl hexoside, rosmarinic acid, rosmarinic acid hexoside, sangerinic acid, lithospermic acid A, chlorogenic acid, 3-hydroxy-4-methoxybenzaldehyde thiosemicarbazone, ferulic acid, syringic acid, vanilic acid, p-hydroxybenzoic acid, protocatechuic acid, gallic acid. Flavonoids: Luteolin-O-hexosyl-O-glucuronide, eriodictyol-O-glucuronide, luteolin-7-O-glucuronide, methylluteolin-O-glucuronide, eriodictyol-O-glucuronide, herniarin, myricetin. | [337,338,339,340] |
| Lavandula stoechas | ||
| Anti-inflammatory, antioxidant, antispasmodic, sedative, antibacterial, anti-fungal, insecticidal, larvicidal, hepatoprotective, renoprotective, anti-leishmaniasis. | Phenolic acids and derivatives: Protocatechuic acid, chlorogenic acid, caffeic acid, rosmarinic acid, ferulic acid, 7-methoxy coumarin. Flavonoids: Flavone di-O-glycosides, flavone 7-O-monoglycosides, pinobanksin, quercetin, pinocembrin, luteolin, vitexin, acacetin, erythrodiol. Others: Ursolic acid, vergatic acid, oleanolic acid, α-amyrin, α-amyrin acetate, β-sitosterol, lupeol, two longipinane derivatives (longipin-2-ene-7β,9α-diol-1-one and longipin-2-ene-7β,9α-diol-1-one-9-monoacetate), lavanol. | [341,342,343,344,345,346,347] |
| Melissa officinalis | ||
| Anti-proliferative, anti-tumor, antioxidant, antiangiogenic, cardioprotective, anxiolytic antidepressant, antinociceptive, neuroprotective, GABA-T inhibitor, anti-kinetoplastidae, analgesic, hypnotic, anti-Alzheimer, antispasmodic, antiviral, anti-fungal, antibacterial, for premenstrual syndromes. | Phenolic acids: Caffeic acid, caftaric acid, chlorogenic acid, ferulic acid, gentisic acid, p-Coumaric acid, rosmarinic acid. Flavonoids: Apigenin, cynaroside, daidzein, hyperoside, isoquercetin, kaempherol, luteolin, myricetin, quercetin, quercetrol, rutin. Triterpenes: Betulinic acid, oleanolic acid, ursolic acid, 23-sulfate ester of niga-ichigoside F1, 3β,16β,23-trihydroxy-13,28-epoxyurs-11-ene-3-O-β-D-glucopyranoside, 3,23-Disulfate ester of 2α,3β,19α,23-tetrahydroxyurs-12-en-28-oicacid, 3,23-Disulfate ester of 2α,3β,19α,23-tetrahydroxyurs-12-en-28-oicacid 28-O-β-D-glucopyranoside, 3,23-Disulfate ester of2α,3β,23,29-tetrahydroxyolean-12-en-28-oicacid, 3,23-disulfate ester of 3β-23,29-trihydroxyolean-12-en-28-oic acid, 3,23-disulfate ester of 2α,3β-23,29-tetrahydroxyolean-12-ene-28-oicacid, 23-sulfate ester of 2α,3β,19 α,23-tetrahydroxyurs-12-en-28-oic acid, melissioside A, melissioside B, melissioside C. | [348,349,350,351] |
| Mentha aquatica | ||
| Antioxidant, anxiolytic, anti-inflammatory, hepatoprotective, antimicrobial, anti-cancer. | Phenolic acids: Rosmarinic acid, caffeic acid. Flavonoids: Luteolin-7-O-rutinoid, Eriodictyol-O-rutinoside, naringenin-7-O-rutinoside, hesperetin-7-O- rutinoside, luteolin glucoside, luteolin-7-O-β-D-diglucuronide, eriocitrin, apigenin-7-O-β-D-diglucuronide, luteolin-7-O-glucuronide, narirutin, apigenin-7-O-rutinoside, apigenin-7-O-glucuronide, hesperidin, catechin. Others: methyl ester palmitic acid, methyl ester linolenic acid, ethyl ester linolenic acid, neophytadiene, phytol, viridiflorol, rotundifolone, 2,3-seco-triterpene, 3-O-benzoyltormentic acid, tormentic acid, 1-O-benzoylhyptad, ienic acid, 3-epiursolic acid, hyptadienic acid, 3-epi-maslinic acid, 3-epi-tormentic acid, ursolic acid, β-sitosterol, oleanolic acid, pomolic acid, micromeric acid, 21α-hydroxyursolic acid, pomolic acid, hyptadienic acid, 1-O-linoleoyl-2-O-enadecanoyl-3-O-palmitoleoyl-sn-glycerol, 1-O-oleoyl-2-O-enadecanoyl-3-O-palmitoleoyl-sn-glycerol, 1, 3-O-dioleoyl-2-O-eicosanoyl-sn-glycerol, 1-O-linoleoyl-2-O-palmitoleoyl-sn-glycerol, corosolic acid, asiatic acid, choline, acetic acid, formic acid, lactic acid, quinic acid, salicylic acid, succinic acid, fructose, glucose, sucrose, alanine, aspartic acid, glycine, isoleucine, leucine, phenylalanine, threonine, valine. | [352,353,354,355,356,357,358,359,360,361,362,363,364,365,366,367,368,369,370,371] |
| Mentha pulegium | ||
| Insecticidal, nematicidal, allelopathic, antioxidant, antimicrobial, antiviral, antileishmanial, anti-tumor, anti-cancer, anti-hemolytic, antihypertensive, anti-inflammatory, burn wound healing, cardioprotective, stomachic, astringent, emmenagogue, decongestant, antispasmodic, antiseptic, depurative, digestive, anti-rheumatic, anti-arthritic, hepatotoxicity. | Phenolic acids: Gallic acid, chlorogenic acid, caffeic acid, ellagic acid, fumaric acid, protocatechuic acid, p-hydroxybenzoic acid, syringic acid, cinnamic acid, vanillic acid, ferulic acids, p-coumaric acid, chlorogenic acid, rosmarinic acid. Flavonoids: Epicatechin, catechin, apigenin, salvigenin, salvigenin, luteolin, isorhamnetin, quercetagetin-3,6-dimethylether, kaempferol, kaempferol-3-O-rutinoside, hesperidin, thymonin, jaceosidin, pectolinaringenin, ladanein, sorbifolin, pedalitin, diosmin, luteolin, apigenin, naringenin, chrysin, chrysoeriol, vicenin-2, gallocatechin isomer 1. Others: Alterporriol, atropisomer, altersolanol, stemphypyrone, 6-O-methylalater-nin, macrosporin, salvianolic acid, Lithospermic acid, jaceidinisomer 1, Jaceosidin. | [371,372,373,374,375,376,377,378,379] |
| Mentha suaveolens | ||
| Antioxidant, antimicrobial, antimutagenic, analgesic, anti-inflammatory, insecticidal, anti-cancer, antithermal skin-aging effect. | Phenolic acids: Cinnamic acid, chlorogenic acid, rosmarinic acid, caffeic acid, p-methyl coumarate, ferulic acid, p-coumaric acid, gallic acid, hydroxybenzoic acid, hydroxybenzoic acid, 3-dihydroxybenzoic acid, vanillic acid, salicylic acid, salicylic acid 2-O-β-glucoside, trans-cinnamic acid, p-methyl coumarate, p-anisic acid. Flavonoids: Hesperidin, rutin, quercetin, naringenin, luteolin, kaempferol, apigenin. | [352,376,380,381,382,383,384,385,386,387] |
| Origanum vulgare L. | ||
| Antibacterial, anti-fungal, antiviral, antiparasitic, antioxidant, anti-inflammatory, anti-tumoral, beneficial activity on skin disorders, effects on melanin production and on human sperm mobility, anti-Alzheimer, energy producer, stomach booster, nervous system reliever, laxative, reducing the general weakness of the body, anti-cancer, relief of migraine pain, for external use by rubbing in place of fractures and numbness of body parts, toothache, disinfection, antidiarrhoea, anticonvulsant, expectorant, nourishing, menstrual regulator, anti-urinary tract infection, treatment of sexual dysfunction, colic, sinusitis, relaxing, cardiorespiratory booster, nervous system booster, treatment of blockages, hepatoprotective. | Phenolic acids: Rosmarinic acid, chlorogenic acid, cinnamic acid, caffeic acid, syringic acid, benzoic acid, vanillic acid, galo-coumaric acid, gallic acid, protocatechuic acid, 4-hydroxybenzoic acid, p-coumaric acid, ferulic acid, sinapic acid, trans-cinnamic acid, 2,4-dihydroxybenzoic acid, phenyllactic acid. Flavonoids: Quercetin, apigenin, kaempferol, naringenin, eriodictyol, salvianolic acid B, llithospermic acid B, amburosides A, luteolin, luteolin 7-O-glucuronide, apigenin 7-O-glucuronide, (−)-epigallocatechin, (+)-catechin, rutin. Others: Thymoquinone, thymol, carvacrol, demethylbenzolignanoid, chicoric acid, calleryanin 3,4-dihydroxybenzoate, calleryanin 3-hydroxy,4-methoxybenzoate, gastrodin 3,4-dihydroxybenzoate. | [388,389,390,391,392,393,394,395,396] |
| Prunella vulgaris | ||
| Anti-tumor, anti-inflammation, immunoregulation, antiviral, antioxidant, anti-osteoporosis, antidepression, hypotensive, hypolipemic, cardioprotective, anti-dementia, anti-amnesia. | Phenolic acids: p-coumaric acid, caffeic acid, rosmarinic acid. Flavonoids: Kaempferol, luteolin, delphinidin, quercetin, quercetin-3-O-β-D-galactoside, homoorinet, cinaroside, quercetin-3-O-β-D-glucoside, kaempferol-3-O-β-D-glucoside. Steroids and derivatives: Beta-sitosterol, spinasterol, stigmasterol, vulgaxanthin-I, poriferasterol monoglucoside, morin, ducosterol, (22E20S24S)-stigmast-7,22-diene-3-e, Stigmast-7-en-3β-ol. Triterpenes: Oleanolic acid, ursolic acid, vulgarsaponin A/B, methyl oleanolate, methyl ursolate, methyl, maslinate, pravuloside A/B, palmitic acid, ethyl palmitate, tetracosanoic acid, stearic acid, 6,9-octodecadienoic acid, 3,6,7-eicosatrienoic acid, oleic acid, peanut oleic acid, moringoic acid, lauric acid, myristic acid, linolenic acid, palmitic acid, myristic acid, linoleic acid. Coumarins: Umbelliferon, scopoletin, esculetin. | [397] |
| Salvia verbenaca | ||
| Antibacterial, antioxidant, anti-cancer, antiparasitic, insecticidal, antihemolytic. | Phenolic acids: p-Hydroxybenzoic acid, p-coumaric acid, rosmarinic acid, vanillic acid, caffeic acid, ferulic acid, 3-O- and 4-O-caffeoylquinic acids. Flavonoids: Naringenin, cirsiliol, luteolin, apigenin, naringin, hesperidin, genkwanin. Others: Palmitic acid, stearic acid, linolenic acid, arachidic acid, oleic acid, linoleic acid, palmitoleic acid, arachidic acid, verbenacines, salvinines, 6,7-dehydroroyleanone, cryptanol, sitosterol, campesterol, 6-hydroxysalvonolone, microstegiol, stigmasterol, carnosic acids, methyl carnosate contents, carnosol. | [387,398,399,400] |
| Thymus mastichina | ||
| Antibacterial, anti-fungal, antioxidant, anti-cancer, antiviral, insecticidal, insect repellent, anti-Alzheimer, anti-inflammatory. | Phenolic acids: Rosmarinic acid, hydroxycinnamoylquinic acid, 3-methoxysalicylic acid, caffeic acid, chlorogenic acid, salvianolic acid B/E, salvianolic acid A/K isomer. Flavonoids: Quercetin glucoside, 6-hydroxyluteolin-7-O-glucopyranoside, luteolin glucoside, 6- hydroxyapigenin7-Oglucopyranoside, apigenin-7-Oglucoside, naringenin, luteolin, carnosol, apigenin, kaempferol, chrysoeriol-O-hexuronide, sakuranetin, sterubin. Others: Oleanolic acid, ursolic acid, xanthophyll lutein, β-sitosterol. | [401] |
- Achillea millefolium L.
| Target | Part Used/Extraction | Observations | References |
|---|---|---|---|
| Asteraceae | |||
| Arctium minus (Hill) Bernh | |||
| A-GLU/A-AMY | 1 mg/mL of MeOH, CH2Cl2, EtOAc, and BuOH extracts of leaves (L), flowers (F) and roots (R). | AGLU-LMeOHext = 3.32 ± 9.80, AMY-LMeOHext = 12.65 ± 6.40. AGLU-LCH2Cl2-ext = 87.12 ± 8.06, AMY-LCH2Cl2-ext = 28.84 ± 5.57. AGLU-LEtOAc-ext = na, AMY-LEtOAc-ext = na. AGLU- LBuOH-ext = 24.49 ± 15.92, AMY-LBuOH-ext = 30.50 ± 8.35. AGLU-LAqua-ext = 15.51 ± 6.96, AMY-LAqua-ext = 5.74 ± 5.95. AGLU-FMeOHext = na, AMY-FMeOHext = na. AGLU-FCH2Cl2-ext = 21.68 ± 3.12, AMY-FCH2Cl2-ext = na. AGLU-FEtOAc-ext = 40.69 ± 6.90, AMY-FEtOAc-ext = na. AGLU-FBuOH-ext = 6.40 ± 4.45, AMY- FBuOH-ext = na. AGLU-FAqua-ext = 13.32 ± 2.22, AMY-FAqua-ext = na. AGLU-RMeOHext = na, AMY-RMeOHext = na. AGLU-RCH2Cl2-ext = 68.01 ± 7.02, AMY-RCH2Cl2-ext = na. AGLU-REtOAc-ext = 36.11 ± 10.68, AMY-REtOAc-ext = na. AGLU- FBuOH-ext = na, AMY- FBuOH-ext = na. AGLU-RAqua-ext = 30.40 ± 8.50, AMY-RAqua-ext = na. | [173] |
| Achillea millefolium | |||
| A-GLU | Hydromethanolic extract of aerial parts. | AI 55% inhibition at 1.6 mg/mL. | [409] |
| A-GLU | Hydroethanolic extract of aerial parts. | The extract promoted the α-glucosidases inhibition by 55% at 1 mg/mL concerning control. It increased the PPARγ (five times) and GLUT4 (two-fold) relative expression than the control (p < 0.05). Finally, it significantly increased INS secretion and [Ca2+]i compared with the control. | [407] |
| INS secretion and calcium mobilization | |||
| PPARγ and GLUT4 expression analysis. | |||
| Arnica montana | |||
| A-AMY | Methanolic extract fractions (dried cell biomass of seeds germinated). | All fractions inhibited α-amylase activity (almost 12%). | [410] |
| Bellis perennis | |||
| Quantification of GLUT4 translocation. |
| Both extracts had a clear dose-response relationship, with EXT4404 being slightly more effective than EXT4407. However, EXT4407 had no effect at 0.25 mg/L, while EXT4404 at the same concentration only increased by about 4%. Overall, all the extracts are effective inducers of GLUT4 translocation without INS. | [411] |
| Glucose Transport Assay | |||
| A-GLU/A-AMY | Methanol: water (80:20%, v/v) extract of flowers. | IC50A-AMY: 8.48 ± 0.07 mg/mL of dried flowers; IC50A-GLU: 49.62 ± 0.01 mg/mL of dried flowers. | [412] |
| Bidens frondose | |||
| A-GLU/A-AMY | Ethanolic extracts (80%) of aerial parts. | IC50A-GLU = 0.41 mg/mL, the extracts inhibited α-glucosidase enzyme strongly (64.29–75.22% at 2 mg/mL); inactive on α-amylase activity. | [413] |
| Cichorium intybus | |||
| A-AMY | Aqueous extracts of aerial parts. | IC50A-AMY = 136.13 ± 8.09 µg/mL, | |
| Insulinotropic investigations (IC1) | Caffeic, ferulic acids, and Chicoric acid (CAE, extracted from aqueous extract). | Caffeic acid mainly promotes a decrease in hepatic glycogenolysis. Ferulic acid elicits a clear increase in INS release and a reduction in hepatic glycogenolysis. CAE increases INS release and glucose uptake without affecting hepatic glycogenolysis. None of these compounds implicates hepatic glucose 6-phosphatase in contrast to chlorogenic acid, an inhibitor of glucose 6-phosphatase. | [414] |
| Insulin sensitizing investigations (IC2) | |||
| Hepatocyte culture and glycogenolysis test (IC3) | |||
| Evaluation of glucose 6-phosphatase activity (IC4) | |||
| Glucose uptake assay. | Caffeic acid, chlorogenic acid (CGA), and chicoric acid (CAE). | CRA and CGA increased glucose uptake in L6 muscular cells, an effect only observed in the presence of stimulating concentrations of INS. Both CRA and CGA stimulated INS secretion from the INS-1E cells and rat islets of Langerhans. The effect of CRA is only observed in the presence of subnormal glucose levels. | [415] |
| β-cell culture and measurement of INS secretion. | |||
| Rat pancreatic islet experiments. | |||
| Study of G6Pase and PEPCK expression (IC5). | Three di-O-caffeoylquinic acids (CQA) were extracted from chicory roots methanolic extract. | CQA suppressed hepatic glucose production in H4IIE rat hepatoma cells by reducing the expression of G6Pase and PEPCK. Activation of PI3K and MAPK pathways as a method of controlling gene expression. Promoted increased mitochondrial respiration and cellular metabolism by inducing oxidative phosphorylation and proton leak. | [416] |
| Gene expression of PI3K and MAPK pathways | |||
| Cellular bioenergetics (IC6). | |||
| Differentiation induction of embryonal carcinoma stem cells into INS-producing cells (IC7) | Methanolic extracts (100%) of leaves. | The extract efficiently induced the differentiation of P19 EC cells into clusters similar to pancreatic islets with the molecular, cellular, and functional characteristics of mature β cells. | [417] |
| A-GLU/A-AMY | Aqueous methanolic extracts (80% methanol, 19% H2O, 1% HCl; v/v/v) of the plant. | IC50A-AMY: 18.3 ± 0.7 mg/mL; IC50A-GLU: 4.25 ± 0.08 mg/mL | [418] |
| Glucose uptake test. |
| Adding NCRAE increased glucose uptake at 50 mg/mL, which agrees with our previous report. At the same concentration of 50 µg/mL, the SCCAM solution has also increased glucose uptake with a value close to the NCRAE values. | [419] |
| Glucose uptake test and lipid accumulation assays. | Methanolic extract (CME) and CME/DT (detannification). | CME and CME/DT exhibited significant glucose uptake in 3T3-L1 adipocytes with a dose-dependent response. The glucose uptake profile in the presence of PI3K and IRTK inhibitors (Wortmannin and Genistein) substantiates the mechanism used by both extracts. CME inhibited the differentiation of 3T3-L1 preadipocytes but failed to show glucose uptake in inhibitor-treated cells. The activity exhibited by CME/DT is exactly opposite to CME. PTP1B inhibition assay, mRNA, and protein expression analysis revealed the unique behaviour of CME and CME/DT. | [420] |
| PTP1B Inhibition study. | |||
| Glucose uptake assay. | 12, 8-guaianolide sesquiterpene lactones isolated from butan-1-ol and ethyl acetate fractions of roots extract | The compounds significantly facilitated glucose uptake in the hyperglycemic HepG2 cell model at 50 μM. | [259] |
| Dittrichia viscosa subsp. Viscosa (Syn. Inula viscosa) | |||
| A-GLU/A-AMY | Methanol: water (80:20%, v/v) extract of leaves. | IC50A-AMY: 1.381 ± 0.085 mg/mL; IC50A-GLU: 0.118 ± 0.02 mg/mL. | [258] |
| A-GLU/A-AMY | Methanol (MeOH), ethyl acetate (EtOAc), and chloroform (CHL) extracts of leaves. | IC50A-GLU-EtOAc: 29.9 ± 1.04 µg/mL; PI-A-AMY: 22.152 ± 0.387% IC50A-GLU-MeOH: 22.3 ± 2.82 µg/mL; PI-A-AMY: 27.162 ± 1.623% IC50A-GLU-Chlo: 39.8 ± 0.76 µg/mL; PI-A-AMY: 17.157 ± 0.634% | [256] |
| A-GLU/A-AMY | Tomentosin is extracted and purified from dichloromethane and ethanolic extract. | IC50A-GLU-26.61 ± 0.236 μM; IC50A-AMY: 26.89 ± 1.54 μM | [421] |
| Glucose uptake assay (IC8). | 7-O-Methylaromadendrin (MAD) extracted from methanolic extract of the aerial part of the plant. | MAD significantly stimulated INS-induced glucose uptake. MAD increased the P2a and PPARg2 gene expression. MAD stimulated the reactivation of INS-mediated phosphorylation of PI3K-(Akt/PKB) and AMPK in high glucose-induced, INS-resistant HepG2 cells. | [422] |
| Study of aP2 and PPARg2 gene expression. | |||
| Galinsoga parviflora | |||
| A-GLU/A-AMY | Aqueous extracts of leaves. | At 2.5 mg/mL IA% (A-GLU): 40%, A-AMY: no inhibition | [423] |
| A-GLU | Two compounds, Galinsosides A (1) and B (2), flavanone glucosides extracted from methanolic extract of whole plant. | IC50A-GLU (1): 286 ± 0.68 μM; IC50A-GLU (2): 46.7 ± 0.32 μM. | [424] |
| Helichrysum stoechas | |||
| A-GLU/DPP-4 | Methanol extracts of aerial parts. | IC50A-GLU: 481.01 μg/mL, IC50DPP-4: 81.71 μg/mL. | [261] |
| Hypochaeris radicata | |||
| A-GLU/A-AMY | Aqueous extracts of leaves. HR1: Extract fresh plant materials; HR2: Extract plant materials after blanching; HR3: Blanching water extract. | IC50A-GLU-HR1: 79.4 ± 1.7 μg/mL; IC50A-GLU-HR2: 99.1 ± 1.9 μg/mL; IC50A-GLU-HR3: 83.4 ± 1.8 μg/mL IC50A-AMY-HR1: 41.9 ± 1.4 μg/mL; IC50A-AMY-HR2: 84.5 ± 1.8 μg/mL; IC50A-AMY-HR3: 51.9 ± 1.5 μg/mL | [267] |
| Lactuca serriola | |||
| A-GLU | 4-hydroxybenzoic acid (1), protocatechuic acid (2), kaempferol (3), quercetin (4), lactuside A (5), luteolin-7-O-β-D-glucoside (6) are extracted from methanolic extracts of the leaves. | IC50A-GLU-(1): 810.31 ± 1.03 µM; IC50A-GLU-(2): 126.65 ± 1.82 µM; IC50A-GLU-(3): 39.72 ± 0.43 µM; IC50A-GLU-(4): 39.82 ± 1.12 µM; IC50A-GLU-(5): 468.98 ± 0.45 µM; IC50A-GLU-(6): 161.29 ± 0.31 µM. | [270] |
| Senecio vulgaris | |||
| A-AMY | Methanol extract (MeOH = 1 mg/mL), Dichloromethane extract (DCM1 = 100 and DCM2 = 50 μg/mL). | MeOH-IA%: 82.46 ± 0.0041%, DCM1-IA%: 90.95 ± 0.0001%, DCM2-IA%: 59.05 ± 0.0001%. | [286] |
| ALDO | Methanol extracts of aerial parts. | IA%: 42.00% at 1mg/mL. | [425] |
| Solidago virgaurea | |||
| A-GLU/A-AMY | Conc-ASE (concentrated extract obtained after accelerated solvent extraction) Conc-LE (concentrated extract obtained after laser extraction). | Conc-ASE = IC50A-GLU: 9.3 ± 0.9 µg/mL, IC50A-AMY-33.9 ± 2.4 µg/mL. Conc-LE = IC50A-GLU: 8.7 ± 0.6 µg/mL, IC50A-AMY-32.1±1.9 µg/mL. | [426] |
| Sonchus oleraceus | |||
| Glucose uptake assay (IC13) Analysis of p-AMPK/Akt/GSK3-β expression in cells. | Hydroethanolic extract (90%) of the leaves (SOL). | The glucose uptake in HepG2 cells treated with 200 μg/mL SOL was significantly increased to 145%, but the uptake was lower than that treated with insulin (320%). After treatment with SOL extracts for 24 h, the p-AMPK, Akt, and GSK3β expression levels significantly increased by approximately 1.7, 1.0, and 0.8 times, respectively, compared with the control. | [427] |
| Tanacetum parthenium | |||
| ra-ALDO/AGEs | Methanolic extract (70%) (ME) Ferulic acid (FA), Apigenin (API), Luteolin-7-O-glucoside (LUG), Luteolin (LUT), Chrysosplenol (CHR), Kaempferol (KAE), Santin (SAN) were extracted and purified from the methanolic extract. | ME: ra-ALDO-IA% (61.1 ± 0.5%), IC50-ra-ALDO (8.04 ± 0.61 µg/mL), IC50-AGEs (163.71 ± 6.31 µg/mL). FA: IC50-ra-ALDO (3.20 ±0.12 µg/mL), IC50-AGEs (5.59 ± 0.26 µg/mL). API: IC50-ra-ALDO (1.97 ± 0.10 µg/mL), IC50-AGEs (NA). LUG: IC50-ra-ALDO (1.31 ± 0.09 µg/mL), IC50-AGEs (3.43 ± 0.12 µg/mL). LUT: IC50-ra-ALDO (1.76 ± 0.03 µg/mL), IC50-AGEs (6.73 ± 0.43 µg/mL). CHR: IC50-ra-ALDO (1.92 ± 0.08 µg/mL), IC50-AGEs (NA). KAE: IC50-ra-ALDO (1.11 ± 0.03 µg/mL), IC50-AGEs (NA). SAN: IC50-ra-ALDO (NA), IC50-AGEs (NA). | [428] |
| A-GLU/A-AMY | Ethanolic extract of aerial parts. Extraction by accelerated solvent extraction (ASE), microwave-assisted extraction (MAE), maceration (MAC), Soxhlet (SOX), and ultrasound−assisted extraction (UAE). | ASE: IC50A-GLU (1.63 ± 0.02 mmol acarbose equivalent (ACAE)/g extracts), IC50A-AMY (0.51 ± 0.02 ACAE/g extract). MAE: IC50A-GLU (1.64 ± 0.01 mmol ACAE/g extracts), IC50A-AMY (0.53 ± 0.05 mmol ACAE/g extract). MAC: IC50A-GLU (1.65 ± 0.01 mmol ACAE/g extracts), IC50A-AMY (0.52 ± 0.02 mmol ACAE/g extract). SOX: IC50A-GLU (1.67 ± 0.01 mmol ACAE/g extracts), IC50A-AMY (0.51 ± 0.03 mmol ACAE/g extract). UAE: IC50A-GLU (1.64 ± 0.01 mmol ACAE/g extracts), IC50A-AMY (0.56 ± 0.01 mmol ACAE/g extract). | [429] |
| Tanacetum vulgare | |||
| A-GLU/A-AMY | Hexan, hydroethanolic, and infusion of flowers (HEXF, HETF, INFF), stems (HEXS, HETS, INFS), and aerial parts (HEXAP, HETAP, INFAP). | HEXF: IC50A-GLU (10.41 ± 0.06 mmol acarbose equivalent (ACAE)/g extracts), IC50A-AMY (0.53 ± 0.01 mmol ACAE/g extract). HETF: IC50A-GLU (10.77 ± 0.15 mmol ACAE/g extracts), IC50A-AMY (0.33 ± 0.01 mmol ACAE/g extract). INFF: IC50A-GLU (3.57 ± 0.13 mmol ACAE/g extracts), IC50A-AMY (0.07 ± 0.01 mmol ACAE/g extract). HEXS: IC50A-GLU (10.60 ± 0.06 mmol ACAE/g extracts), IC50A-AMY (0.50 ± 0.02 mmol ACAE/g extract). HETS: IC50A-GLU (7.54 ± 0.65 mmol ACAE/g extracts), IC50A-AMY (0.33 ± 0.02 mmol ACAE/g extract). INFS: IC50A-GLU (4.00 ± 0.09 mmol ACAE/g extracts), IC50A-AMY (0.10 ± 0.01 mmol ACAE/g extract). HEXAP: IC50A-GLU (10.56 ± 0.04 mmol ACAE/g extracts), IC50A-AMY (0.48 ± 0.03 mmol ACAE/g extract). HETAP: IC50A-GLU (8.67 ± 1.19 mmol ACAE/g extracts), IC50A-AMY (0.35 ± 0.03 mmol ACAE/g extract). INFAP: IC50A-GLU (4.26 ± 0.12 mmol ACAE/g extracts), IC50A-AMY (0.09 ± 0.01 mmol ACAE/g extract). | [322] |
| Lamiaceae | |||
| Calamintha nepeta subsp. Nepeta (Syn. Clinopodium nepeta) | |||
| A-GLU/A-AMY | Methanolic extract (80%) of leaves. | At 10 mg/mL IA% (A-GLU): 66.62 ± 1.61%, IA% (A-AMY): 16.45 ± 0.94% | [333] |
| A-AMY | Methyl alcohol: water (7:3) extract fractionated with ethyl acetate (AcOEt), dichloromethane (DCM), and n-butanol (BuOH). | IC50A-AMY of DCM, AcOEt, and BuOH >200 µg/mL | [332] |
| A-AMY | Methanolic extract (ME), essential oil (EO), and aqueous extract (AQ). | IC50A-AMY-ME: 24.46 mg/mL, IC50A-AMY-EO: 31.54 mg/mL, IC50A-AMY-AQ: 115.47 mg/ml | [335] |
| Lavandula pedunculata | |||
| A-GLU/A-AMY | Aqueous extract of flowering tops. | IC50A-AMY: 0.44 ± 0.05 mg/mL, IC50A-GLU-131 ± 20 mg/mL. | [430] |
| Intestinal Glucose Absorption in vitro | The extract inhibited the intestinal glucose absorption (IC50 = 81.28 ± 4.01 µg/mL) in a concentration-dependent manner. | ||
| Lavandula stoechas | |||
| A-GLU/A-AMY | Aqueous extract of aerial parts. | IC50A-AMY: 0.485 ± 0.13 mg/mL, IC50A-GLU: 168 ± 40.10 μg/mL | [431] |
| Intestinal Glucose Absorption assay, in situ. | The extract lowered intestinal glucose absorption in situ at 250 mg/kg. | ||
| A-GLU/A-AMY | EO of aerial parts. | IC50A-AMY: 3.00 ± 0.008 mg/mL, IC50A-GLU: 2.58 ± 0.04 mg/mL | [347] |
| Glucose production assay (IC9) | Ethyl acetate (EE) and n-butanol (BE) fractions are prepared from an aqueous extract of aerial parts. | EE and BE at low doses (25–50 µg/mL) significantly decreased hepatic gluconeogenesis in the H4IIE cell line by suppressing the expression of PEPCK and G6Pase. In C2C12 myotubes, both extracts increased the INS-stimulated glucose uptake more effectively than metformin. They also effectively increased the expression of lipoprotein lipase protein levels in INS-resistant myotubes at low doses. EE increased the protein level of PPARγ and stimulated the activation of AKT in INS-resistant H4IIE and C2C12 cell lines. | [432] |
| Glucose uptake assay (IC10) | |||
| Effects on PEPCK and G6Pase gene expression. | |||
| Effects on AKT activation and GLUT4 expression. | |||
| Transcriptome analyses | |||
| A-GLU | Ursolic acid extracted from Methanol (ME), ethanol (ET), methanol-dichloromethane (1: 1, v/v) (MDI), acetone (AC), ethyl acetate (EA), diethyl ether (DEE), and chloroform extracts (CHL). | IC50A-GLU-ME: 49.86 ± 0.36 mg/mL, IC50A-GLU-ET: 17.81 ± 0.55 mg/mL, IC50A-GLU-MDI: 29.57 ± 0.19 mg/mL, IC50A-GLU-AC: 24.63 ± 0.13 mg/mL, IC50A-GLU-EA: 40.31 ± 0.84 mg/mL, IC50A-GLU-DEE: 23.60 ± 1.04 mg/mL, IC50A-GLU-CHL: 26.21 ± 1.00 mg/mL. | [346] |
| A-GLU | EO of flowering leaves. | IC50A-AMY: 106.73 ± 3.27 µg/mL, IC50A-GLU: 98.54 ± 4.84 µg/mL. | [344] |
| Melissa officinalis | |||
| Anti-glycation assay. | Aqueous extract of leaves (AQ). Rosmarinic acid (RA), melitric acid A (MA), salviaic acid A (SA), caffeic acid (CA). | IC50-AQ: 0.24 mg/mL, IC50-RA: 0.34 mM, IC50-MA: 0.38 mM, IC50-SA: 0.16 mM, IC50-CA: 0.48 mM. | [433] |
| A-GLU/A-AMY | Aqueous extract of leaves | IA%: 83.9%, A-AMY: No activity. | [434] |
| A-AMY | Lemon balm-based extract with 50% RA | IA%: 50% | [435] |
| Uptake inhibition of glucose (UIG) and fructose (UIF) (IC12) | Methanolic and aqueous extract of leaves. | UIG%: <25%, UIF: No activity for both extracts. | [436] |
| Glucose consumption (IC8) | EO (A, B, and C compagnies) | EO-A: 63.64 ± 11.46%, EO-B: 59.96 ± 3.65%, EO-C: 65.63 ± 9.76%. The Western blot data suggest that the key factors of the adenosine monophosphate-activated protein kinase/acetyl-CoA carboxylase pathway can be mediated by the EOs. | [437] |
| Gene expressions analysis of p-AMPK, AMPK, p-ACC, ACC, PPAR, CEBPα, and SREBP1 proteins. | |||
| Mentha aquatica | |||
| A-AMY | Hydroethanolic extract (70%) of the leaves. | IC50A-AMY: 229.50 ± 4.1 µg/mL. | [438] |
| A-AMY | Methanolic (ME) and aqueous extracts (AQ) of the leaves. | IA%-ME: 61.7 ± 5.5%, IA%-AQ: 14.0 ± 3.0% | [439] |
| Uptake inhibition of glucose (UIG) and fructose (UIF) (IC12) | Methanolic and aqueous extract of leaves. | UIG%: <25%, UIF: No activity for both extracts. | [436] |
| Mentha pulegium | |||
| A-GLU/A-AMY | Methanolic and aqueous extract of leaves. | IC50A-GLU-ME: 20.38 µg/mL, IC50A-GLU-AQ: 21.65 µg/mL, IC50A-AMY-ME: 23.11µg/mL, IC50A-GLU-AQ: 36.47 µg/mL | [372] |
| A-GLU/A-AMY | Ethyl acetate fraction of aerial part. | IC50A-GLU: 61.85 ± 1.69 µg/mL, IC50A-AMY: 16.37 ± 0.11 µg/mL | [440] |
| Mentha suaveolens | |||
| A-GLU/A-AMY | EO of the aerial part. | IC50A-GLU: 141.16 ± 0.2 µg/mL, IC50A-AMY: 94.30 ± 0.06 µg/mL | [384] |
| Origanum vulgare | |||
| A-AMY | Clonal oregano shoots ethanolic extracts (50%) (O-1, O-9, O-11Y, O-11M, O-12, O-17, OK-17, O-23, O-24, O-26, GO-19-1). | The strongest anti-amylase activity was observed for extract O-24, which had an AI index value of 2.32 ± 0.28 and corresponded to 57% inhibition of enzyme activity. Eight of the eleven clonal oregano extracts tested had AI index values greater than or equal to 1.5. For these experiments, an AI index value of 1.5 corresponded to approximately 33% α-amylase enzyme inhibition. | [441] |
| ALDO | Caffeic acid (CA), rosmarinic acid (RO), lithospermic acid B (LTO), 12-hydroxy jasmonic acid 12-O-β-glucopyranoside (HDG), p-menth-3-ene-1,2-diol 1-O-β-glucopyranoside (MDG) isolated from the polar extracts of aerial parts. | ALDO-CA: 8 ± 4.6%, ALDO-RO: 95 ± 0.0%, ALDO-LTO: 96 ± 1.7, ALDO-HDG: 77 ± 1.4%, ALDO-MDG: 41 ± 0.6%. EB-CA: −7.68 kcal/mol, EB-RO: 15.71 kcal/mol, EB-LTO: −16.08 kcal/mol, EB-HDG: −14.58 kcal/mol, EB-MDG: −10.57 kcal/mol. | [442] |
| Docking studies of ALDO inhibitory activity (EB). | |||
| A-GLU/A-AMY | Aqueous and ethanolic (12%) extract of plant clonal lines. | At 1000 µg/mL: A-GLU (93.7%), A-AMY (95%). | [434] |
| Analysis of PPARγ- and δ-mediated transactivation, a test of adipogenic potential, INS-stimulated glucose uptake, neutral red assay. | Origanum vulgare ssp. vulgare (1): hexane (Hex), dichloromethane (DCM), and ethyl acetate (EtOAc) extracts of the aerial part. Origanum vulgare ssp. hirtum (2): dichloromethane (DCM), methanol (MeOH) extracts of the aerial part. | (1): Hex ext = Activation of the γ, δ PPARs, adipocyte differentiation (NA), INS-stimulated GLU-uptake (+), Viability of endothelial cells (NA), Viability of macrophages (NA), DCM ext = Activation of the γ PPARs, adipocyte differentiation (NA), INS-stimulated GLU-uptake (+), Viability of endothelial cells (-), Viability of macrophages (66.1 ± 5.3%). EtOAc ext = Activation of the γ PPARs, adipocyte differentiation (NA), INS-stimulated GLU-uptake (+), Viability of endothelial cells (NA), Viability of macrophages (2.7 ± 1.4%). (2): DCM ext = Activation of the γ PPARs, adipocyte differentiation (NA), INS-stimulated GLU-uptake (+), Viability of endothelial cells (NA), Viability of macrophages (NA), MeOH ext = Activation of the γ, δ PPARs, adipocyte differentiation (NA), INS-stimulated GLU-uptake (+), Viability of endothelial cells (NA), Viability of macrophages (NA). | [443] |
| DPP-IV/PTP1B | Methanolic extracts of leaves: commercial oregano extract (E1) and greenhouse-grown oregano extract (E2). Chemical fractionation by flash chromatography system (fractions FA to FI). | DPP-IV-IC50: (E1) = 28.4 ± 6.3 μM GAE, (E2) = 86.2 ± 18.8 μM GAE, FA > 500 μM GAE, FB = 206.3 ± 47.2 μM GAE, FC > 500 μM GAE, FD = 317.4 ± 60.7 μM GAE, FE = 20.3 ± 3.9 μM GAE, FF = 23.3 ± 1.9 μM GAE, FG = NA, FH = NA, FI = NA. PTP1B-IA: (E1)/(E2) = NA, FA = 7.0 ± 3.5%, FB = 13.3 ± 4.2%, FC = 1.3 ± 1.0%, FD = NA, FE = 32.1 ± 3.3%, FF = 77.4 ± 18.4%, FG = NA, FH = NA, FI = NA. | [444] |
| A-GLU/A-AMY | (1): EO of O. vulgare subsp. Hirtum. (2): EO of O. vulgare subsp. Vulgare. | IC50A-AMY (1): 0.14 ± 0.008 mmol ACEs/g; IC50A-GLU (1): 0.88 ± 0.03 mmol ACEs/g. IC50A-AMY (2): 0.13 ± 0.004 mmol ACEs/g, IC50A-GLU (2): 6.04 ± 0.91 mmol ACEs/g. | [390] |
| A-GLU/A-AMY | Methanolic extract (80%) of leaves. | A-GLU-IA = 58.41 ± 1.97%, A-AMY-IA = 6.79 ± 0.57%. | [333] |
| A-GLU | Aqueous acetonitrile (50%) of powder leaves. | IC50A-GLU = 0.35 ± 0.03 μg/mL, AGEs-IC50 = 0.55 ± 0.07 mg/mL. Cells treated with extract leaf extract at a 100 μg/mL concentration showed significantly enhanced 2-NBDG uptake compared with INS-treated cells. The extract decreased the promoter activity and the mRNA and protein expression of PEPCK and SREBP-1c. I. The extract inhibited the expression of CPY2E1 and enhanced the expression of GLUT2. | [393] |
| AGEs assay | |||
| Glucose uptake assay (IC13). | |||
| The mRNA and protein expression of PEPCK, SREBP-1c, GLUT2, CYP2E1 (IC14). | |||
| A-GLU/A-AMY/LIPA | Ethanolic extracts (80% v/v). | IC50A-AMY = 44.71 ± 0.86 µg/mL, IC50A-GLU = 7.11 ± 1.37 µg/mL, IC50-LIPA = 922.35 ± 30.99 µg/mL. | [395] |
| Prunella vulgaris | |||
| Study of Glucose Production (IC15). | Methanolic extract of arial part (PVA). Rosmarinic and caffeic acids were extracted from solid residue PVA by organic solvent, representing about 26% and 0.3% w/w of total extract. | The PVA lowered glucose production from glycogen (glycogenolysis), dihydroxyacetone, and alanine (gluconeogenesis). None of the phenolic acids influenced PEPCK mRNA expression, which INS downregulated. G6Pase mRNA was decreased by INS, increased by PVA, and remained unaffected by other treatments. Both compounds significantly increased GK mRNA expression; PVA did not affect this gene expression. | [445] |
| The mRNA expression analysis of G6Pase, GLUK, CALM, PEPCK, and GK (IC16). | |||
| ra-ALDO/hu-ALDO/AGEs assay | Aqueous extract (AQE) partitioned sequentially with n-hexane (HEX), methylene chloride (CH2Cl2), ethyl acetate (EtOAc), n-butanol (BuOH), and water (H2O). Compounds (C1 to C6) isolated from AQE fractionation. | ra-ALDO: AQE-IA = 36.18 ± 1.13%, HEX-IA = 33.94 ± 0.49%, CH2Cl2-IA= 32.49 ± 0.54%, EtOAc-IA = 87.33 ± 2.39% (IC50 = 2.99 ± 0.10 μg/mL), BuOH-IA = 59.56 ± 2.34%, H2O-IA = NA, C1 = NA, C2 = NA, C3-IC50 = 8.35 ± 0.51 μM, C4-IC50 = 2.77 ± 0.48 μM, C5-IC50 = 3.20 ± 0.55 μM, C6 = NA. hu-ALDO: C1 = NA, C2 = NA, C3 = NA, C4-IC50 = 18.62 ± 0.40 μM, C5-IC50 = 12.58 ± 0.32 μM, C6 = NA. AGEs assay: AQE-IA = 29.26 ± 0.94%, HEX-IA = 33.94 ± 0.41%, CH2Cl2-IA = 54.03 ± 1.00% (IC50 = 186.72 ± 2.05 µg/mL), EtOAc-IA = 68.31 ± 1.06% (IC50 = 141.34 ± 1.27 µg/mL), BuOH-IA = 40.47 ± 0.68%, H2O-IA = 30.24 ± 1.01%, C1-IA = 9.33 ± 0.27%, C2= NA, C3 = NA, C4 = 20.67 ± 0.37%, C5-IA = 74.81 ± 1.41% (IC50 = 33.16 ± 0.54 µg/mL), C6-IA = 88.69 ± 0.56% (IC50 = 304.36 ± 3.41 µg/mL). | [446] |
| Cas-3 activity and activation of the apoptotic signaling pathway (IC16) analysis (Bax/Bcl-2, Fas/FasL, phospho-JNK, phospho-ERK, phospho-p38, NF-κB binding activity, phosphorylated-IκB, TNF-α, IL-6). | Aqueous extract (AQE). | AQE administration significantly prevented IL-1β-increased INS-1 cell death and LDH activity and attenuated IL-1β-increased cas-3 activity. | [447] |
| A-GLU/A-AMY | Hydroethanolic extract of inflorescence (PV) contained RA (4.5%), CA (9.8%), and pCA (11.6%). | IC50A-GLU-PV: 90.9 μg/mL, IC50A-AMY-PV: 47.2 μg/mL IC50A-GLU-CA: 4.7 μg/mL, IC50A-AMY-CA: 5.1 μg/mL IC50A-GLU-RA: 11.6 μg/mL, IC50A-AMY-RA: 21.7 μg/ml | [448] |
| NPMDA | Active compounds tested (Kaempferol, luteolin, delphinidin, quercetin, beta-sitosterol, spinasterol, stigmasterol, vulgaxanthin-I, poriferasterol monoglucoside, stigmast-7-enol, morin) | The sterols and flavonoids play an active role by affecting the TNF signalling pathway, AGE-RAGE signalling pathway, MAPK pathway, and PI3K-Akt pathway-related targets such as IL-6 and INS. | [158] |
| A-GLU | Hydroethanolic extract (75%) (HE) partitioned sequentially with Petroleum ether (PE), Ethyl acetate (EtOAc), n-butanol (BuOH), and water (H2O) fractions. Compounds Caffeic acid (C1), Isoquercitrin (C2), and Rosmarinic acid (C3) isolated from AQE fractionation. | HE-IC50 = 130.46 ± 4.33 µg/mL, PE-IC50 = 194.61 ± 4.69 µg/mL, EtOAc-IC50 = 69.13 ± 2.86 µg/mL, BuOH-IC50 = 124.97 ± 2.56 µg/mL, H2O-IC50 = 191.88 ± 3.34 µg/mL, C1-IC50 = 3.91 ± 0.07 µg/mL, C2-IC50 = 85.52 ± 2.94 µg/mL, C3-IC50 = 0.65 ± 0.04 µg/mL. | [449] |
| Salvia verbenaca | |||
| A-GLU/A-AMY | Methanolic extract (85%) (ME) and decoction (DE) of the aerial part. | IC50A-GLU-ME: 50.5 ± 1.40 µg/mL, IC50A-AMY-ME = 101.30 ± 0.08 µg/mL. IC50A-GLU-DE: 313.7 ± 1.36 µg/mL, IC50A-AMY-DE = NA. | [399] |
| Thymus mastichina | |||
| A-GLU/A-AMY | Essential oil. | IC50A-GLU = 100 ± 0.0 µg/mL, IC50A-AMY = 4600 ± 0.0 µg/mL. | [450] |
- Anthemis canescens var. aurea
- Bellis perennis L. and Bidens frondose
- Chamaemelum nobile
- Cichorium intybus L.
| Part Used/Extract Tested | Model/Parameters Studies | Intervention and Duration | Observations | References |
|---|---|---|---|---|
| Asteraceae | ||||
| Arctium minus (Hill) Bernh | ||||
| R/DEC | Male diabetic GK (Goto-kakizak) rats. | 125 g/L DR ad libitum/4 weeks. | The DEC led to a GK rats’ occasional glycaemia decrease. It did not significantly affect glycemic control; long-lasting treatments induced toxic effects. The DEC decreased several parameters of GK liver mitochondria respiratory activity. | [182] |
| R/L/AQ | ALLO-induced diabetic rats. | RAQ (500 mg/kg) and LAQ (200 mg/kg) OG/21 days. | RAQ was reduced by 34.6 ± 5.8%, and LAQ was reduced by 6.2 ± 22.9%. | [174] |
| Measurement of biochemical parameters (B1) | ||||
| Achillea millefolium | ||||
| NI/HET | STZ-induced diabetic rats. | 25 mg/kg/day and 100 mg/kg/day OG/28 days. | Compared with Metformin, the HET reduces lipid abnormality, HYG and hepatic enzymes with a dose-dependent effect in diabetic rats. | [402] |
| Measurement of biochemical parameters (B2) | ||||
| NI/AQ/MET | OGTT | 250 and 500 mg·kg−1 BW DR/18h. | The AQ/MET at dose levels of 250 and 500 mg/kg BW showed a significant decrease in BG level, TGL, VLDL, cholesterol, SGOT, SGPT, and ALP in diabetic rats. | [404] |
| ALLO-induced diabetic rats. | 250 and 500 mg/kg BW DR/14 days. | |||
| Measurement of biochemical parameters (B3) | ||||
| AP/HET (70%) | OGTT | 100 mg/kg 0, 0.5, 1, 1.5, 2, and 3 h | The HET showed significant glucose diminution on oral glucose tolerance tests and in acute experimental T2DM assay. It reduced the BG levels in a dose-dependent manner. | [407] |
| STZ-induced diabetic mice. | 33, 100 and 330 mg/kg 1, 3, 5, and 7 h | |||
| AP/HET | STZ-induced diabetic rats. | 100 mg/kg/day i.p./14 days | The HET significantly reduced the expression of both IL-1β and iNOS genes. The serum INS levels in the HET group animals increased while the BG levels decreased significantly. The HET enhanced overexpression of IL-1β and iNOS genes, which may have a protective effect on β-cells. | [408] |
| Measurement of biochemical parameters (B4) and IL-1β/iNOS gene expression. | ||||
| NI/HET | STZ-induced diabetic rats. | 250 mg/kg NI/21 days | The results indicated that the HET improves renal function due to antioxidant activity and modulates some biochemical factors in diabetic rats. | [406] |
| Measurement of biochemical parameters (B5) Analysis of oxidative stress-related factors (O1). | ||||
| Anthemis canescens (syn. Matricaria aurea) | ||||
| ET/EA/DCM/HEX | STZ-induced diabetic rats. | ETH1 = 100 mg/kg, ETH2 = 200 mg/kg EA1 = 100 mg/kg, EA2 = 200 mg/kg DCM1 = 100 mg/kg, DCM2 = 200 mg/kg HEX1 = 100 mg/kg, HEX2 = 200 mg/kg OG/4 weeks. | Treatment with either ETH1/2 extracts or pioglitazone successfully ameliorated INS resistance, hyperlipidemia, and fatty liver without significantly affecting fasting INS levels or pancreatic secretory capacity. It increased liver protection from injury associated with T2DM, as evidenced by a significant decrease in ALT and AST. | [217] |
| Measurement of biochemical parameters (B6). | ||||
| Oxidative stress and antioxidant markers in the liver (O2) | ||||
| Bellis perennis | ||||
| L/F/ EXT4404/EXT4407/HMET | Avian embryos in the first two-thirds of embryonic development (lasting 21 days) Hens egg test-chorioallantoic membrane (HET-CAM) assay. | EXT4404 (300 mg/L), EXT4407 (300 mg/L), HMET (300 mg/L). | All three extracts resulted in a comparable decrease in BG levels (~20% after 1 h and 30% after 2 h) and were statistically significant after 2 h incubation. The three extracts significantly reduced BG levels at both time points with comparable efficacy (~12% after 1 and 2 h). | [411] |
| Bidens frondosa | ||||
| AP/ET (80%) | OGTT | 250 and 500 mg/kg BW OG/7 days | ET exhibited weak to moderate hypoglycaemic effects on normoglycemic rats at the tested doses. In OGTT, higher doses of the extract indicated significant inhibitory activities. The ET lowered BG levels in varying ratios. The body weight of the animals was not changed significantly during this experiment. | [413] |
| Healthy and STZ-induced diabetic rats. | ||||
| Measurement of biochemical parameters (B1) | ||||
| Chamaemelum nobile (syn. Anthemis nobilis L. or Chamomilla nobilis) | ||||
| AP/AQ | STZ-induced diabetic rats. | 20 mg/kg BW OG/15 days. | Single oral administration of AQ reduced BG levels in normal and STZ diabetic rats. BG levels were decreased in normal and STZ diabetic rats, respectively, after 15 days of treatment. | [451] |
| Measurement of biochemical parameters (B7) | ||||
| Cichorium intybus | ||||
| R/AQ | ALLO-induced diabetic rat | 2.5, 5, 10, and 15 g DPM/kg OG/2 weeks | Treatment with 10 g/kg of the herbal mixture significantly lowered glycaemic values compared with the diabetic controls. The treatment with the highest tested concentration (15 g/kg) completely restored BG level to normoglycemic values in experimental groups. The lipid status of treated animals and serum AST, ALT, ALP, CRE, URE, and MDA were completely normalised. The polyherbal mixture completely restored the histopathological changes in the liver, kidneys, and hippocampus. | [458] |
| Measurement of physiological and biochemical parameters (P1) | ||||
| WP/ET (80%) | STZ-induced diabetic rats (male Sprague–Dawley rats) | 125 mg of plant extract/kg BW OG/14 days | Daily administration of ET (125 mg/kg) for 14 days to diabetic rats attenuated BG by 20%, TG by 91%, and tTC by 16%. | [455] |
| OGTT | ||||
| Measurement of biochemical parameters (B8) | ||||
| R/AQ | STZ- niacinamide (NIA/STZ) induced diabetic rats | 125 mg/kg BW i.p. injections/28 days. | The extract prevented body-weight loss and decreased BG level. ALT activities and TG, TC, and HbA1c levels decreased, and NO concentration increased in the chicory-treated groups. Unlike late-stage diabetes, fasting serum INS concentrations were higher, and the OGTT pattern was approximated to normal in chicory-treated early-stage diabetic rats. | [456] |
| Measurement of biochemical parameters (B9) | ||||
| OGTT | ||||
| AP/CAE | Healthy rats | 3, 15 or 30 mg/kg i.p. injections/4 days. | The CAE can decrease BG without any effect hepatic effect. Daily i.p. administrations of CAE improve i.p. glucose tolerance in a dose-dependent manner, mainly via an INS sensitising effect. | [414] |
| OGTT | ||||
| Measurement of biochemical parameters (B10) | ||||
| S/CQA-ET | Healthy rats | Diet with CQA-ET FEE/28 days | The CQA-ET was found to decrease the atherogenic index to the level observed in the control rats’ group and to increase blood antioxidant status. Both dietary supplements reduced the content of thiobarbituric acid-reactive substances in kidney and heart tissue compared with the experimental group. | [457] |
| High-fructose diets | ||||
| Measurement of physiological and biochemical parameters (P2) | ||||
| Antioxidant status of rats (O3) | ||||
| NCRAE, SCCAM | STZ-induced diabetic rats | 15 mg/kg i.p. injections/7 days. | Both NCRAE and SCCAM can improve glucose tolerance in STZ diabetic rats after a subchronic administration of seven days. Alone, NCRAE significantly decreases the basal HYG after six days of treatment. | [419] |
| OGTT | ||||
| Measurement of biochemical parameters (B11). | ||||
| Dittrichia viscosa subsp. iscosa (Syn. Inula viscosa) | ||||
| L/AuNPs | High-fat diet (HFD)/STZ-induced diabetes in rats | 2.5 mg/kg i.p. injections/21 days. | Treatment with AuNP significantly lowered the BG level, the gene expression, and the activity of hepatic PEPCK in comparison with the untreated diabetic group. The AuNPs synthesised can alleviate HYG in HFD/STZ-induced diabetes in rats by reducing hepatic gluconeogenesis by inhibiting the expression and activity of the hepatic PEPCK gene. | [472] |
| Measurement of biochemical parameters (B12) | ||||
| AP/AQ | Normal and STZ-induced diabetes rats | 20 mg/kg OG/for 2 weeks. | A significant reduction in BG levels was observed in normal rats 2 h after a single oral administration. Repeated daily oral administration significantly reduced BG levels after 4 days of treatment. In diabetic rats, a significant reduction in BG levels was observed 1 h after a single oral administration. Repeated oral administration reduced BG levels on the 4th day. No change in TC and TG levels was observed after a single and repeated oral administration in both normal and diabetic rats. Plasma INS levels and body weight remained unchanged after 15 days of repeated oral administration in normal and diabetic rats. | [473] |
| Measurement of biochemical parameters (B13) | ||||
| Galinsoga parviflora | ||||
| WP/HET 80% | STZ-induced diabetic rats. | 400 mg/kg BW NI | The extract reduced the BG level equivalent to GLIB (5 mg/kg BW) in diabetic rats. | [474] |
| Measurement of biochemical parameters (B13) | ||||
| Lactuca serriola | ||||
| L/AQ | STZ-induced diabetic rats. | 200 and 500 mg/kg BW OG/NI | Both doses of extracts restored β -cell function and INS secretion. | [475] |
| OGTT | ||||
| Measurement of biochemical parameters (B14) | ||||
| Onopordum acanthium | ||||
| L/MET | STZ-induced diabetic rats | 200 and 400 mg/kg OG/8 days | Administration of extracts significantly increased INS content in β-cells with a marked enhancement of pancreatic islet structure, significantly reducing BG level and BW loss. Extract treatment suppressed the increase in inflammatory cell score in myocardial tissue with an M2–like macrophage elevation. | [476] |
| Measurement of biochemical parameters (B15) | ||||
| Solidago virgaurea | ||||
| AP/HE | ALLO-induced diabetic rat. | 250 mg/kg BW OG 15 days | Extract significantly reduced BG level, serum AMY activity, TNF-α level, and pancreatic MDA level, as well as increasing the serum INS, liver GLY level, pancreatic SOD, and CAT activities in comparison with their corresponding diabetic rats. | [477] |
| Measurement of physiological and biochemical parameters (P3) | ||||
| Sonchus asper | ||||
| NI/ME | STZ-induced diabetic rats. | 200 mg/kg 21 days | The ME improve the activity of the antioxidant enzymes, TBARS contents, and cholesterol profile of the diabetic rats. DTR’s BG and INS levels were significantly lower in treatment than the diabetic rats on day 21. | [478] |
| Measurement of physiological and biochemical parameters (P4) | ||||
| Sonchus oleraceus | ||||
| WP/ME | STZ-induced diabetic rats. | 75, 150, 300 mg/kg 14 days | The Me (150 mg/kg) treatment exhibited 39.40% glycaemia reduction. The measurement of stress markers in plasma, liver, and kidney after ME administration showed a significant reduction in MDA and hydrogen peroxide levels, coupled with a substantial increase in CAT activity. | [479] |
| OGTT | ||||
| Antioxidant status of rats (O4) | ||||
| L/HET (90%) | STZ-induced diabetes in rats | 100, 200, 400 mg/kg/day BW 6 weeks | HET significantly increased both SOD activity and GSH levels while causing a reduction of MDA levels in the liver. Moreover, HET ameliorates STZ-induced liver function and pathological damage. DTR fed with HET daily for 6 weeks showed significantly decreased levels of TNF-α and IL-1β in the liver. HET decreased MyD88, TGF-β, and TLR4 expression levels in the liver of DTR. | [480] |
| Measurement of physiological and biochemical parameters (P5) | ||||
| L/HET (90%) | HFD/STZ-induced diabetes in rats | 100, 200, 400 mg/kg/day BW 6 weeks | In DTR treated by HET (400 mg/kg/day for 6 weeks), TG, TC, and LDL-c were reduced by 43%, 22%, and 16%, respectively. Meanwhile, it was also found that daily feeding of DTR decreased plasma glucose levels by approximately 23%. DTR with HET at 400 mg/kg/day for 6 weeks show portal tract and mild fibrous expansion without sep inflammation formed of lymphocytes. The administration of HET exhibited a protective effect against the hepatic damage induced by STZ, which was also corroborated by the apparent condition and colour observed in HET-administered rats. | [427] |
| OGTT | ||||
| Measurement of physiological and biochemical parameters (P6) | ||||
| L/ET (80%) | ALLO-induced diabetic rat | 100, 200, and 300 mg/kg BW 56 days | The treatment of SOE 200 and 300 mg/kg in diabetic rats for two months dramatically decreased BG, total lipid, TC, TG, and LDLc, while HDLc levels improved liver and kidney functions. The histological assay revealed that the treatment of SOE 300 mg/kg significantly improved the pancreas tissues. | [481] |
| Measurement of physiological and biochemical parameters (P7) | ||||
| Lamiaceae | ||||
| Lavandula pedunculata | ||||
| FTO/AQ | Healthy rats | 1 g/kg BW NI | Acute and chronic oral administration of extract reduced the peak of the BG (30 min) and the area under the curve. The effect was at the same amplitude as the positive control Metformin. | [430] |
| Acute OGTT and chronic | ||||
| OGTT for plant mixtures | ||||
| Measurement of biochemical parameters (B13) | ||||
| Lavandula stoechas | ||||
| AP/EO | ALLO-induced diabetic rat | 50 mg/kg BW i.p. injections/15 days. | Subacute EO administration prevented BW gain decline and protected against alloxan-induced increase in hepatic and renal relative weights. EO treatment corrected the BG level significantly, protected against lipoperoxidation and decreased (−SH) group levels, and reversed antioxidant enzyme depletion. Induced by alloxan treatment. Treatment with EO significantly protected against hepatic and renal dysfunctions and the disturbance of lipid metabolic parameters induced by alloxan treatment. | [482] |
| Measurement of biochemical parameters (B16) | ||||
| AP/EO | ALLO-induced diabetic rat | 50 mg and 160 mg/kg BW i.p. injections/15 days | EO treatment protects against decreased BW gain, relative reproductive organ weights, testosterone level, and sperm quality decline. EO treatment protects against oxidative damage to DTR’s male reproductive organ systems. | [483] |
| Measurement of physiological and biochemical parameters (P8) | ||||
| R/ET (70%) | ALLO-induced diabetic rat | 50, 100, and 150 mg/kg BW i.p. injections/NI | The extract significantly reduced BG levels of DTR in a dose-dependent manner. | [484] |
| Measurement of biochemical parameters (B13) | ||||
| NI/EO | STZ-induced diabetic rats | 0.05 mL DDR/21 days | The percentage of healing was highest in the EO group on Days 7, 14, and 21. Microscopic examination of the biopsies supported accelerated wound healing on Days 7 and 14. Reduced mononuclear cell density and increased hair follicle and adipose tissue development were observed in the T2DM-EO group on Day 7. On Day 14, the T2DM-EO group increased collagen levels and hair follicles. | [485] |
| Wound healing test | ||||
| Measurement of physiological and biochemical parameters (P9) | ||||
| AP/AQ | ALLO-induced diabetic rat | 150 mg/kg OG/NI | Oral extract administration reduced HYG induced by sucrose and starch in normal and diabetic rats. | [431] |
| OGTT | ||||
| Measurement of biochemical parameters (B13) | ||||
| Melissa officinalis | ||||
| EO | db/db mice | 0.0125 mg EO/d FEE/6 weeks | Mice administered EO for 6 weeks showed significantly reduced BG (65%; p < 0.05) and TG concentrations, improved glucose tolerance, as assessed by an OGTT, and significantly higher serum INS levels than the CGr. All the genes were significantly upregulated, whereas G6Pase and PEPCK expression was downregulated in the livers of the EO group. | [486] |
| Measurement of biochemical parameters (B17) | ||||
| OGTT | ||||
| L/ET | HFD C57BL/6 mice | 200 mg/kg/day FEE/6 weeks | The DTR revealed significantly reduced fasting BG concentrations (14% decrease versus vehicle). The extract showed no significant effects on FPIL. It significantly decreased the HFD-induced INS resistance by 35%. It reduced the HFD-provoked rise in fasting plasma concentrations of nonesterified FAs by 59% and plasma TAG gain by 66%. The extract-fed mice showed reduced plasma levels of LDL/VLDL-c (32% decrease) and a slight decrease in TC (8% decrease). The extract treatment led to an increase in the HDL/LDL ratio of 56%. | [487] |
| Measurement of biochemical parameters (B18) | ||||
| NI/EO | STZ-induced diabetic rats | 0.01, 0.02 and 0.04 mg/day FEE/4 weeks | EO at both high doses restored glycemia and reduced the BW of DTR compared with untreated diabetic animals. | [488] |
| Measurement of biochemical parameters (B1) | ||||
| L/HAE | ALLO-induced diabetic rat | 20, 100 or 500 mg/kg BW OG/4 weeks | There was a significant decrease in blood sugar levels, TC, TG, and LDL in DTR with HAE. An increase in HDL levels was observed in HAE-DTR. | [489] |
| Measurement of biochemical parameters (B19) | ||||
| L/HE-EA (ALS-L1023) | HFD C57BL/6 mice | HFD supplemented with 0.4% (w/w) ALS-L1023 (HFD-ALS) FEE/12 weeks | Administration of ALS-L1023 to high-fat-diet-induced OMI caused reductions in BW gain, VFM, and VAS without changes in FC profiles. ALS-L1023 improved HYG, HYIN, BG, and INIT and normalised INS-positive β-cell area in OMI. ALS-L1023 decreased hepatic LIA and concomitantly increased the expression of PPARα target genes responsible for fatty acid β-oxidation in livers. | [490] |
| Measurement of physiological and biochemical parameters (P10) | ||||
| OGTT and IPITT | ||||
| L/HE/EA | Otsuka Long-Evans Tokushima fatty (OLETF) rats | HFD with 0.4% or 0.8% (w/w) of extract FEE/4 weeks | The EAE administration resulted in a BW reduction without changes in FI. It also markedly inhibited HYG and HYIN, restoring the β-cell mass severely impaired in OLETF rats. There was a decrease in LIA in the liver and skeletal muscle of the ORAT after treatment with EAE. After EAE treatment, the liver and skeletal muscle increases the expression levels of FAs-oxidizing enzymes (AMPKα2, ACOX, MCAD, and VLCAD). The EAE attenuated the pancreatic inflammation, including the infiltration of CD68-positive macrophages and mast cells, and attenuated the expression of inflammatory factors (IL-6 and CD68). | [491] |
| Measurement of physiological and biochemical parameters (P111) | ||||
| Mentha aquatica | ||||
| L/AQ | STZ-induced diabetic rats | 50 mg, 100 mg and 150 mg OG/90 days. | FBG and HbA1c levels decreased in DTR. The BW and INS levels of DTR were significantly increased. The levels of TC TG were reduced, and the levels of HDL were significantly increased. The ALB of DTR were significantly increased. However, the levels of UR and CREA were decreased in DTR. TBARS/MDA level formation significantly decreased in DTR. The activities of CAT, SOD, GPx, and GST were increased in DTR. DTR at a dose of 100 mg/kg bw/day showed normal glomeruli, normal intertubular vessels, and tubular epithelial cells, indicating degenerative changes in the kidney. | [492] |
| Measurement of physiological and biochemical parameters (P12) | ||||
| Mentha pulegium | ||||
| AP/AQ | STZ-induced diabetic rats | 20 mg/kg BW OG/15 days | The AQE caused a significant reduction in BG levels in DTR. The morphometric analysis and histological sections realised in the pancreas and liver have shown the beneficial effect of AQE in the cellular population. According to OGTT, the AQE has improved glucose tolerance in normal rats. | [493] |
| OGTT | ||||
| Measurement of physiological and biochemical parameters (P13) | ||||
| AP/AQ | STZ-induced diabetic rats | 20 mg/kg BW OG/15 days | The AQE alleviated hyperlipidemia in diabetic rats by lowering significantly the TC levels without affecting the TG levels significantly. It exerted some increasing activity on plasma HDL-c level. | [494] |
| Measurement of biochemical parameters (B20) | ||||
| Mentha suaveolens | ||||
| AP/AQ | STZ-induced diabetic rats. | 20 mg/kg BW OG/15 days | The AQE decrease the BG, TC, and TG levels in both normal and diabetic rats. The AQE treatment was demonstrated to act positively on the liver and pancreas histopathological tissues. | [495] |
| OGTT | ||||
| Measurement of physiological and biochemical parameters (P14) | ||||
| Origanum vulgare L. | ||||
| L/AQ | STZ-induced diabetic rats | 20 mg/kg OG/2 weeks | The AQE produced a significant decrease in BG levels in DTR. The BG levels were normalised from the fourth day after daily repeated oral administration of AQE. No changes in basal plasma INS concentrations were observed after treatment in either normal or DTR. | [496] |
| Measurement of biochemical parameters (B4) | ||||
| L/AQ | STZ-induced diabetic rats | 20 mg/kg OG/6 weeks | Administration of AQE significantly decreased BG levels, HbA1c, and AMY in DTR. | [497] |
| Measurement of biochemical parameters (B21). | ||||
| L/ME/AQ | STZ-induced diabetic rats | 5 mg/kg per day i.p. injections/10 days | ME reduced diabetes incidence and preserved normal insulin secretion. ME scavenged reactive oxygen and nitrogen species and alleviated the need to upregulate antioxidant enzymes. ME treatment attenuated the pro-inflammatory response mediated by T helper 17 cells. It enhanced anti-inflammatory T helper 2 and T regulatory cells by impacting specific signalling pathways and transcription factors. | [394] |
| Measurement of physiological and biochemical parameters (P15) | ||||
| L/EtOAc | STZ-induced diabetic rats | 2 mg/mouse OG/10 days | EtOAc treatment significantly preserved pancreatic islets and reduced diabetes incidence in DTR. Besides the down-modulatory effect on macrophages, EtOAc reduced the number of total CD4+ and activated CD4+ CD25+ T cells. EtOAc affected the number of T helper 1 (Th1) and T helper 17 (Th17) cells by downregulating their key transcription factors T-bet and RORγT. | [498] |
| Measurement of physiological and biochemical parameters (P16) | ||||
| L/AQ | ALLO-induced diabetic rat | 150 mg/kg, 300 mg/kg BW 300 mg/kg Equal mixture (150 mg chamomile + 150 mg oregano) OG/6 weeks | Treatment with higher or lower doses or a mixture of extracts had significant weight gain, hypoglycemic effect, decreased AMY activity, and increased INS levels. Restoration of the renal profile and lipid profile with an increase in HDL-C and the reversal of Bax and Bcl-2 were well observed, with a 300 mg/kg mixture showing synergistic activity of the extracts compared with individual low doses of 150 mg/kg. | [499] |
| Measurement of biochemical parameters (B22) | ||||
| L/HE | Glucose-induced-diabetic zebrafish | 10 μg/L FEE/24H | The BG level, TC, and TG were significantly reduced in diabetic zebrafish treated. | [500] |
| Measurement of biochemical parameters (B23) | ||||
| L/INF | ALLO-induced diabetic rat | 55 mL OG/40 days | The INF reduced BG levels after the first day of treatment, compared with the diabetic CGr. The INF appears to stimulate INS secretion. | [501] |
| Measurement of biochemical parameters (B10) | ||||
| Prunella vulgaris | ||||
| WP/AQ | db/db mice HCF/HFD | 100 mg, 200 mg/kg/day DR/8 weeks | AQE treatment markedly lowered BG and SBP. The CRE clearance was restored by treatment with AQE. The AQE markedly decreased TC, TG, LDL-c, MDA, and TGFβ1 and increased HDL-c and NO levels. AQE ameliorated vascular relaxation of aortic rings by acetylcholine or SNP-inducement in a dose-dependent manner. AQE treatment significantly reduced the aortic expressions of ICAM-1, VCAM-1, ET-1, and nitrotyrosine. The expression of eNOS in aortic was increased by AQE treatment. | [502] |
| Measurement of physiological and biochemical parameters (P17) | ||||
| Fr/HE/TAP | STZ-induced diabetic rats | 50 mg/kg, 100 mg/kg, 200 mg/kg of TAP OG/6 weeks | The BW and the levels of BG, FMN, MDA, NO, and the activity of NOS in serum decreased significantly compared with the STZ group in a dose-dependent manner. The activity of SOD in serum and BW increased significantly compared with the STZ group in a dose-dependent manner. The DTR showed a significant increase in SOD mRNA expression in pancreatic β cells. Histopathological examination also showed the protective effect of TAP on pancreatic β cells. | [503] |
| Measurement of physiological and biochemical parameters (P18) | ||||
| HFOR/HE/AQ | Male CD-1 (ICR) mice/FFF | 8.02 g/kg OG/10 weeks | HEE could improve glucose intolerance and normalise the lipid profile. HEE provokes an increase in peripheral and hepatic INS sensitivity, a decrease in FAs level, enhanced GLUK activity and GLY content, and improved serum antioxidant activity. Hepatic histopathological examination showed that HEE administration markedly decreased fatty deposits in the liver of mice. | [504] |
| OGTT and IPITT | ||||
| Measurement of physiological and biochemical parameters (P19) | ||||
| IF/PV(HE)/CARF/CA/RA | ALLO-induced diabetic rat model | 50, 100, 150 mg/kg i.p. injections/8 weeks | CARF reduced BG levels and improved in vivo oxidative stress. CARF reduced HbA1c levels more significantly than PV and equivalent amounts of CA or RA. CARF had significantly increased serum-INS, and ameliorated thermal hyperalgesia and tactile allodynia more significantly than the effects of PV and equivalent amounts of CA or RA. The tested compounds showed potential restoration of the lipid peroxide levels. | [448] |
| Measurement of physiological and biochemical parameters (P20) | ||||
| WP/AQ | Male Sprague-Dawley (SD) STZ-induced diabetic rats | 100 mg, 300 mg/kg/day DR/8 weeks | In DTR, AQE significantly decreased BG and BUN and ameliorated plasma CRE. AQE reduced the PAS positivity staining intensity and basement membrane thickening in the glomeruli of DTR. | [505] |
| Measurement of physiological and biochemical parameters (P21) | ||||
3.2.2. Lamiaceae Family
- Lavandula stoechas
- Melissa officinalis
- Mentha sp.
- Origanum vulgare L.
- Prunella vulgaris
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Maleki, V.; Jafari-Vayghan, H.; Saleh-Ghadimi, S.; Adibian, M.; Kheirouri, S.; Alizadeh, M. Effects of Royal Jelly on Metabolic Variables in Diabetes Mellitus: A Systematic Review. Complement. Ther. Med. 2019, 43, 20–27. [Google Scholar] [CrossRef]
- ADA 15. Diabetes Advocacy: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, S152–S153. [Google Scholar] [CrossRef]
- Bełtowski, J.; Wójcicka, G.; Jamroz-Wiśniewska, A. Hydrogen Sulfide in the Regulation of Insulin Secretion and Insulin Sensitivity: Implications for the Pathogenesis and Treatment of Diabetes Mellitus. Biochem. Pharmacol. 2018, 149, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Gaglia, J.L.; Hilliard, M.E.; Isaacs, D.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Am. Diabetes Assoc. 2022, 46, S19–S40. [Google Scholar] [CrossRef] [PubMed]
- Halim, M.; Halim, A. The Effects of Inflammation, Aging and Oxidative Stress on the Pathogenesis of Diabetes Mellitus (Type 2 Diabetes). Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M. Molecular Pathways Associated with Oxidative Stress in Diabetes Mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes Mellitus and Oxidative Stress—A Concise Review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef]
- LeRoith, D. β-Cell Dysfunction and Insulin Resistance in Type 2 Diabetes: Role of Metabolic and Genetic Abnormalities. Am. J. Med. 2002, 113, 3–11. [Google Scholar] [CrossRef]
- Mooradian, A.D. Diabetes-Related Perturbations in the Integrity of Physiologic Barriers. J. Diabetes Complicat. 2023, 37, 108552. [Google Scholar] [CrossRef]
- Fu, J.; Yu, M.G.; Li, Q.; Park, K.; King, G.L. Insulin’s Actions on Vascular Tissues: Physiological Effects and Pathophysiological Contributions to Vascular Complications of Diabetes. Mol. Metab. 2021, 52, 101236. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.A.; Whaley-Connell, A.; Habibi, J.; Wei, Y.; Lastra, G.; Manrique, C.; Stas, S.; Sowers, J.R. Renin-Angiotensin-Aldosterone System and Oxidative Stress in Cardiovascular Insulin Resistance. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H2009–H2023. [Google Scholar] [CrossRef]
- Rawshani, A.; Rawshani, A.; Franzén, S.; Eliasson, B.; Svensson, A.-M.; Miftaraj, M.; McGuire, D.K.; Sattar, N.; Rosengren, A.; Gudbjörnsdottir, S. Mortality and Cardiovascular Disease in Type 1 and Type 2 Diabetes. N. Engl. J. Med. 2017, 376, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Nabrdalik, K.; Kwiendacz, H.; Moos, J.; Moos, Ł.; Kulpa, J.; Brzoza, Z.; Stompór, T.; Gumprecht, J.; Lip, G.Y.H. Diabetic Peripheral Neuropathy Is Associated with Diabetic Kidney Disease and Cardiovascular Disease: The Silesia Diabetes-Heart Project. Curr. Probl. Cardiol. 2023, 48, 101726. [Google Scholar] [CrossRef]
- Koulis, C.; Watson, A.M.D.; Gray, S.P.; Jandeleit-Dahm, K.A. Linking RAGE and Nox in Diabetic Micro- and Macrovascular Complications. Diabetes Metab. 2015, 41, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, W.; Ji, Q.; Ran, X.; Kuang, H.; Yu, X.; Fang, H.; Yang, J.; Liu, J.; Xue, Y.; et al. Prevalence of Painful Diabetic Peripheral Neuropathy in Type 2 Diabetes Mellitus and Diabetic Peripheral Neuropathy: A Nationwide Cross-Sectional Study in Mainland China. Diabetes Res. Clin. Pract. 2023, 198, 110602. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimpour, S.; Zakeri, M.; Esmaeili, A. Crosstalk between Obesity, Diabetes, and Alzheimer’s Disease: Introducing Quercetin as an Effective Triple Herbal Medicine. Ageing Res. Rev. 2020, 62, 101095. [Google Scholar] [CrossRef] [PubMed]
- The International Diabetes Federation. IDF Diabetes Atlas; The International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- World Health Organisation Diabetes. Available online: https://www.who.int/health-topics/diabetes#tab=tab_1 (accessed on 23 July 2023).
- SNS Servico National de Saude-Diabetes. Available online: https://www.sns.gov.pt/noticias/2018/05/14/diabetes/ (accessed on 23 July 2023).
- Soares, A.R.; Coelho, M.; Tracey, M.; Carvalho, D.; Silva-Nunes, J. Epidemiological, Social and Economic Burden of Severe Hypoglycaemia in Patients with Diabetes Mellitus in Portugal: A Structured Literature Review. Diabetes Ther. 2023, 14, 265. [Google Scholar] [CrossRef] [PubMed]
- João Filipe, R. Diabetes: Factos e Números 2016, 2017 e 2018. Rev. Port. Diabetes 2020, 15, 19–27. [Google Scholar]
- Kyrou, I.; Tsigos, C.; Mavrogianni, C.; Cardon, G.; Van Stappen, V.; Latomme, J.; Kivelä, J.; Wikström, K.; Tsochev, K.; Nanasi, A.; et al. Sociodemographic and Lifestyle-Related Risk Factors for Identifying Vulnerable Groups for Type 2 Diabetes: A Narrative Review with Emphasis on Data from Europe. BMC Endocr. Disord. 2020, 20, 134. [Google Scholar] [CrossRef]
- Ojo, O.A.; Ibrahim, H.S.; Rotimi, D.E.; Ogunlakin, A.D.; Ojo, A.B. Diabetes Mellitus: From Molecular Mechanism to Pathophysiology and Pharmacology. Med. Nov. Technol. Devices 2023, 19, 100247. [Google Scholar] [CrossRef]
- Wołos-Kłosowicz, K.; Bandurska-Stankiewicz, E. Effects of Common Weight Loss Plans on Diabetes Mellitus and Cardiovascular Risk Factors. Prim. Care Diabetes 2022, 16, 252–256. [Google Scholar] [CrossRef]
- Wei, X.; Meng, E.; Yu, S. A Meta-Analysis of Passive Smoking and Risk of Developing Type 2 Diabetes Mellitus. Diabetes Res. Clin. Pract. 2015, 107, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.J.; Hu, H.Y.; Lee, Y.L.; Ko, M.C.; Ku, P.W.; Yen, Y.F.; Chu, D. Frequency of Alcohol Consumption and Risk of Type 2 Diabetes Mellitus: A Nationwide Cohort Study. Clin. Nutr. 2019, 38, 1368–1372. [Google Scholar] [CrossRef] [PubMed]
- The Lancet Type 2 Diabetes: The Urgent Need to Protect Young People. Lancet 2018, 392, 2325. [CrossRef]
- Ortiz, M.S.; Cabieses, B.; Repetto, P. Type 2 Diabetes in Young People: Adding Socioeconomic Inequality to the Discussion. Diabetes Res. Clin. Pract. 2019, 156, 107795. [Google Scholar] [CrossRef]
- Wong, N.D.; Zhao, Y.; Patel, R.; Patao, C.; Malik, S.; Bertoni, A.G.; Correa, A.; Folsom, A.R.; Kachroo, S.; Mukherjee, J.; et al. Cardiovascular Risk Factor Targets and Cardiovascular Disease Event Risk in Diabetes: A Pooling Project of the Atherosclerosis Risk in Communities Study, Multi-Ethnic Study of Atherosclerosis, and Jackson Heart Study. Diabetes Care 2016, 39, 668–676. [Google Scholar] [CrossRef]
- Meeks, K.A.C.; Freitas-Da-Silva, D.; Adeyemo, A.; Beune, E.J.A.J.; Modesti, P.A.; Stronks, K.; Zafarmand, M.H.; Agyemang, C. Disparities in Type 2 Diabetes Prevalence among Ethnic Minority Groups Resident in Europe: A Systematic Review and Meta-Analysis. Intern. Emerg. Med. 2016, 11, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2020.
- Ciarambino, T.; Crispino, P.; Leto, G.; Mastrolorenzo, E.; Para, O.; Giordano, M. Influence of Gender in Diabetes Mellitus and Its Complication. Int. J. Mol. Sci. 2022, 23, 8850. [Google Scholar] [CrossRef] [PubMed]
- Kautzky-Willer, A.; Leutner, M.; Harreiter, J. Sex Differences in Type 2 Diabetes. Diabetologia 2023, 66, 986–1002. [Google Scholar] [CrossRef]
- Gerdts, E.; Regitz-Zagrosek, V. Sex Differences in Cardiometabolic Disorders. Nat. Med. 2019, 25, 1657–1666. [Google Scholar] [CrossRef]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.F.; Montagner, A.; Gourdy, P. Sex Differences in Metabolic Regulation and Diabetes Susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef]
- Geer, E.B.; Shen, W. Gender Differences in Insulin Resistance, Body Composition, and Energy Balance. Gend. Med. 2009, 6, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Christen, T.; Trompet, S.; Noordam, R.; van Klinken, J.B.; van Dijk, K.W.; Lamb, H.J.; Cobbaert, C.M.; den Heijer, M.; Jazet, I.M.; Jukema, J.W.; et al. Sex Differences in Body Fat Distribution Are Related to Sex Differences in Serum Leptin and Adiponectin. Peptides 2018, 107, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Senthil Kumar, S.P.D. Sex Differences in Obesity-Related Glucose Intolerance and Insulin Resistance. In Glucose Tolerance; InTech: London, UK, 2012. [Google Scholar] [CrossRef]
- Palmisano, B.T.; Zhu, L.; Eckel, R.H.; Stafford, J.M. Sex Differences in Lipid and Lipoprotein Metabolism. Mol. Metab. 2018, 15, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Colditz, G.A.; Willett, W.C.; Rotnitzky, A.; Manson, J.E. Weight Gain as a Risk Factor for Clinical Diabetes Mellitus in Women. Ann. Intern. Med. 1995, 122, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Gastaldelli, A.; Yki-Järvinen, H.; Scherer, P.E. Why Does Obesity Cause Diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, X.; Li, M.; Xie, J.; Yang, X.-L. Prevalence of Type 2 Diabetes and Pre-diabetes among Overweight or Obese Children in Tianjin, China. Diabet. Med. 2013, 30, 1457–1465. [Google Scholar] [CrossRef]
- He, Q.X.; Zhao, L.; Tong, J.S.; Liang, X.Y.; Li, R.N.; Zhang, P.; Liang, X.H. The Impact of Obesity Epidemic on Type 2 Diabetes in Children and Adolescents: A Systematic Review and Meta-Analysis. Prim. Care Diabetes 2022, 16, 736–744. [Google Scholar] [CrossRef]
- Hossain, M.F.; Rashid, M.; Burniston, T.; Wu, M.A.W.; Kataye, K.A.; Sidhu, R.; Justice, M.; Abdelfattah, S. Evaluation of Fucoxanthin Content in Popular Weight Loss Supplements: The Case for Stricter Regulation of Dietary Supplements. J. Obes. Weight Loss Medicat. 2019, 5, 31. [Google Scholar] [CrossRef]
- North Dakota Legislative. North Dakota 2022 Diabetes Report. North Dakota Century Code 23-01-40-North Dakota Health, US Statutes, Codes, and Regulations. 2022. Available online: https://ndlegis.gov/files/committees/67-2021/23_5151_03000appendixd.pdf (accessed on 23 July 2023).
- Word Health Organization Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 23 July 2023).
- Aras, M.; Tchang, B.G.; Pape, J. Obesity and Diabetes. Nurs. Clin. N. Am. 2021, 56, 527–541. [Google Scholar] [CrossRef]
- North Dakota Legislative. Title 23. Health and Safety. Chapter 23-01, Section 23-01-40—Diabetes Goals and Plans—Report to Legislative Management. US Statutes, Codes, and Regulations. 2022. Available online: https://ndlegis.gov/cencode/t23c01.pdf (accessed on 23 July 2023).
- The, N.S.; Richardson, A.; Gordon-Larsen, P. Timing and Duration of Obesity in Relation to Diabetes: Findings from an Ethnically Diverse, Nationally Representative Sample. Diabetes Care 2001, 36, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, M.; Bentham, J.; Stevens, G.A.; Zhou, B.; Danaei, G.; Lu, Y.; Bixby, H.; Cowan, M.J.; Riley, L.M.; Hajifathalian, K.; et al. Trends in Adult Body-Mass Index in 200 Countries from 1975 to 2014: A Pooled Analysis of 1698 Population-Based Measurement Studies with 19·2 Million Participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- Power, C.; Thomas, C. Changes in BMI, Duration of Overweight and Obesity, and Glucose Metabolism: 45 Years of Follow-up of a Birth Cohort. Diabetes Care 2011, 34, 1986–1991. [Google Scholar] [CrossRef]
- Dandona, P.; Aljada, A. A Rational Approach to Pathogenesis and Treatment of Type 2 Diabetes Mellitus, Insulin Resistance, Inflammation, and Atherosclerosis. Am. J. Cardiol. 2002, 90, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative Stress and Reactive Oxygen Species in Endothelial Dysfunction Associated with Cardiovascular and Metabolic Diseases. Vascul. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hopps, E.; Noto, D.; Caimi, G.; Averna, M.R. A Novel Component of the Metabolic Syndrome: The Oxidative Stress. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 72–77. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Martinez-González, M.Á.; Bulló, M.; Ros, E. The Role of Diet in the Prevention of Type 2 Diabetes. Nutr. Metab. Cardiovasc. Dis. 2011, 21, B32–B48. [Google Scholar] [CrossRef]
- Testa, R.; Bonfigli, A.R.; Prattichizzo, F.; La Sala, L.; De Nigris, V.; Ceriello, A. The “Metabolic Memory” Theory and the Early Treatment of Hyperglycemia in Prevention of Diabetic Complications. Nutrients 2017, 9, 437. [Google Scholar] [CrossRef]
- Dong, K.; Ni, H.; Wu, M.; Tang, Z.; Halim, M.; Shi, D. ROS-Mediated Glucose Metabolic Reprogram Induces Insulin Resistance in Type 2 Diabetes. Biochem. Biophys. Res. Commun. 2016, 476, 204–211. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxidative Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef]
- Rolo, A.P.; Palmeira, C.M. Diabetes and Mitochondrial Function: Role of Hyperglycemia and Oxidative Stress. Toxicol. Appl. Pharmacol. 2006, 212, 167–178. [Google Scholar] [CrossRef]
- Luca, P. Pros and Cons of Selective Inhibition of Cyclooxygenase-2 versus Dual/Cyclooxygenase Inhibition: Is Two Better than One? J. Rheumatol. 2001, 28, 2375–2382. [Google Scholar]
- Hakim, J. Reactive Oxygen Species and Inflammation. Comptes Rendus Seances Soc. Biol. Fil. 1993, 187, 286–295. [Google Scholar] [CrossRef]
- Pickup, J.C. Inflammation and Activated Innate Immunity in the Pathogenesis of Type 2 Diabetes. Diabetes Care 2004, 27, 813–823. [Google Scholar] [CrossRef]
- American Diabetes Association. 5. Prevention or Delay of Type 2 Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, S51–S54. [Google Scholar] [CrossRef]
- Raptis, S.A.; Dimitriadis, G.D. Oral Hypoglycemic Agents: Insulin Secretagogues, α-Glucosidase Inhibitors and Insulin Sensitizers. Exp. Clin. Endocrinol. Diabetes 2001, 109. [Google Scholar] [CrossRef]
- Sudhir, R.; Mohan, V. Postprandial Hyperglycemia in Patients with Type 2 Diabetes Mellitus. Treat. Endocrinol. 2002, 1, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Chehade, J.M.; Mooradian, A.D. A Rational Approach to Drug Therapy of Type 2 Diabetes Mellitus. Drugs 2000, 60, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, M.T. Current Therapeutic Options in Type 2 Diabetes Mellitus: A Practical Approach. Clin. Med. Res. 2003, 1, 189–200. [Google Scholar] [CrossRef]
- Feingold, K.R. Oral and Injectable (Non-Insulin) Pharmacological Agents for the Treatment of Type 2 Diabetes. In Endotext; MDText.com, Inc.: South Dartmouth, MA, USA, 2022. [Google Scholar]
- McFarlane, S.I. Insulin Therapy and Type 2 Diabetes: Management of Weight Gain. J. Clin. Hypertens. 2009, 11, 601. [Google Scholar] [CrossRef]
- Chentli, F.; Azzoug, S.; Mahgoun, S. Diabetes Mellitus in Elderly. Indian J. Endocrinol. Metab. 2015, 19, 744–752. [Google Scholar] [CrossRef]
- Bastaki, S. Diabetes Mellitus and Its Treatment. Int. J. Diabetes Metab. 2005, 13, 111–134. [Google Scholar] [CrossRef]
- Pandey, A.; Tripathi, P.; Pandey, R.; Srivatava, R.; Goswami, S. Alternative Therapies Useful in the Management of Diabetes: A Systematic Review. J. Pharm. Bioallied Sci. 2011, 3, 504–512. [Google Scholar] [CrossRef]
- Gurib-Fakim, A. Medicinal Plants: Traditions of Yesterday and Drugs of Tomorrow. Mol. Aspects Med. 2006, 27, 1–93. [Google Scholar] [CrossRef]
- Kouretas, D.; Skaperda, Z.; Wu, P.; Wang, X. Natural Drugs: A New Direction for the Prevention and Treatment of Diabetes. Molecules 2023, 28, 5525. [Google Scholar] [CrossRef]
- Tripathy, B.; Sahoo, N.; Sahoo, S.K. Trends in Diabetes Care with Special Emphasis to Medicinal Plants: Advancement and Treatment. Biocatal. Agric. Biotechnol. 2021, 33, 102014. [Google Scholar] [CrossRef]
- Shabab, S.; Gholamnezhad, Z.; Mahmoudabady, M. Protective Effects of Medicinal Plant against Diabetes Induced Cardiac Disorder: A Review. J. Ethnopharmacol. 2021, 265, 113328. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Sinniah, U.R. Patchouli (Pogostemon Cablin Benth.): Botany, Agrotechnology and Biotechnological Aspects. Ind. Crops Prod. 2016, 87, 161–176. [Google Scholar] [CrossRef]
- Mohanty, S.; Swamy, M.; Sinniah, U.; Anuradha, M. Leptadenia Reticulata (Retz.) Wight & Arn. (Jivanti): Botanical, Agronomical, Phytochemical, Pharmacological, and Biotechnological Aspects. Molecules 2017, 22, 1019. [Google Scholar] [CrossRef]
- Singh, S.; Bansal, A.; Singh, V.; Chopra, T.; Poddar, J. Flavonoids, Alkaloids and Terpenoids: A New Hope for the Treatment of Diabetes Mellitus. J. Diabetes Metab. Disord. 2022, 21, 941. [Google Scholar] [CrossRef]
- Choudhury, H.; Pandey, M.; Hua, C.K.; Mun, C.S.; Jing, J.K.; Kong, L.; Ern, L.Y.; Ashraf, N.A.; Kit, S.W.; Yee, T.S.; et al. An Update on Natural Compounds in the Remedy of Diabetes Mellitus: A Systematic Review. J. Tradit. Complement. Med. 2018, 8, 361. [Google Scholar] [CrossRef]
- Chen, L.; Gnanaraj, C.; Arulselvan, P.; El-Seedi, H.; Teng, H. A Review on Advanced Microencapsulation Technology to Enhance Bioavailability of Phenolic Compounds: Based on Its Activity in the Treatment of Type 2 Diabetes. Trends Food Sci. Technol. 2019, 85, 149–162. [Google Scholar] [CrossRef]
- Akhlaghipour, I.; Nasimi Shad, A.; Askari, V.R.; Maharati, A.; Baradaran Rahimi, V. How Caffeic Acid and Its Derivatives Combat Diabetes and Its Complications: A Systematic Review. J. Funct. Foods 2023, 110, 105862. [Google Scholar] [CrossRef]
- Salehi, B.; Ata, A.; Kumar, N.V.A.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Ayatollahi, S.A.; Fokou, P.V.T.; Kobarfard, F.; Zakaria, Z.A.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef]
- Khan, V.; Najmi, A.K.; Akhtar, M.; Aqil, M.; Mujeeb, M.; Pillai, K.K. A Pharmacological Appraisal of Medicinal Plants with Antidiabetic Potential. J. Pharm. Bioallied Sci. 2012, 4, 27. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Heinrich, M.; Atanasov, A.G. Ethnopharmacology-A Bibliometric Analysis of a Field of Research Meandering between Medicine and Food Science? Front. Pharmacol. 2018, 9, 347011. [Google Scholar] [CrossRef]
- Weckerle, C.S.; de Boer, H.J.; Puri, R.K.; van Andel, T.; Bussmann, R.W.; Leonti, M. Recommended Standards for Conducting and Reporting Ethnopharmacological Field Studies. J. Ethnopharmacol. 2018, 210, 125–132. [Google Scholar] [CrossRef]
- Jansen, J. Geobotanical Guide of the Serra Da Estrela; Instituto da Conservaçao da Natureza: Lisbon, Portugal, 2002; 276p. [Google Scholar]
- Vieira, G.; Jansen, J. Environmental Setting of the Serra Da Estrela, Portugal: A Short-Note. In Landscape Ecology and Management of Atlantic Mountains; Correia, T.P., Bunce, R.G.H., Howard, D.C., Eds.; IALE: Logan, UT, USA, 2005; ISBN 9780954713003. [Google Scholar]
- Marques, P.P. Serra Da Estrela: Management and Conservation of Priority Habitats; Associação; Gráfica do Tortosendo, Lda: Vila Real, Portugal, 2006. [Google Scholar]
- Alexandre, S.; Catarina, M.; Claudia, D.; Fatima, S.J.C.L.S.T.B. Plantas Aromáticas e Medicinais Do Parque Natural Da Serra Da Estrela: Guia Etnobotânico; CISE-Município de Seia: Seia, Portugal, 2011; pp. 1–225. [Google Scholar]
- Da Cunha, A.P.; Ribeiro, J.A.; Roque, O.R. Plantas Aromáticas Em Portugal: Caracterização e Utilizações; Fundação Calouste Gulbenkian: Lisbon, Portugal, 2007. [Google Scholar]
- Fernandes, R.B. As Explorações Botânicas Do Instituto Botânico Da Universidade de Coimbra No Parque Natural Da Serra Da Estrela Nos Últimos 50 Anos. Anu. Soc. Brot. 1992, 58, 1–12. [Google Scholar]
- Jansen, J. Stands of Cytisus Oromediterraneus in the Serra Da Estrela, with Some Remarks on the Habitats of the Bluethroat (Luscinia Svecica Cyanecula). In 11 Seminário Técnico Conservaçao da Natureza na Serra da Estrela, Parque Natural da Serra da Estrela, Manteigas; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Boa, V.; Coelho, H.; Amaro, C.; Castanheira, I.; Delgado, F.; Jacinto, P.; Oliveira, M.R.; Caldeira, R.E. Inventariação e Propagação de Thymus mastichina Na Beira Interior. In Proceedings of the II Congresso Nacional de Plnatas Aromáticas e Medicinais, Gerês, Portugal, 28–29 September 2007; pp. 46–51. [Google Scholar]
- Gil, C.; Duarte, A.P. Antioxidant Activity of Extracts of Portuguese Shrubs: Pterospartum Tridentatum, Cytisus Scoparius and Erica Spp. J. Med. Plants Res. 2009, 3, 886–893. [Google Scholar]
- Carocho, M.; Barros, L.; Barreira, J.C.M.; Calhelha, R.C.; Soković, M.; Fernández-Ruiz, V.; Buelga, C.S.; Morales, P.; Ferreira, I.C.F.R. Basil as Functional and Preserving Ingredient in “Serra Da Estrela” Cheese. Food Chem. 2016, 207, 51–59. [Google Scholar] [CrossRef]
- Carocho, M.; Barreira, J.C.M.; Antonio, A.L.; Bento, A.; Morales, P.; Ferreira, I.C.F.R. The Incorporation of Plant Materials in “Serra Da Estrela” Cheese Improves Antioxidant Activity without Changing the Fatty Acid Profile and Visual Appearance. Eur. J. Lipid Sci. Technol. 2015, 117, 1607–1614. [Google Scholar] [CrossRef]
- Mora, C. A Synthetic Map of the Climatopes of the Serra Da Estrela (Portugal). J. Maps 2010, 6, 591–608. [Google Scholar] [CrossRef]
- Presidência do Conselho de Ministros. Resolução Do Conselho de Ministros 76/2000 Proposto Para. Integrar o Sítio “Serra Da Estrela” a Rede Natura 2000; Presidência do Conselho de Ministros: Lisbon, Portugal, 1976. [Google Scholar]
- Ministério do Ambiente Decreto Regulamentar 50/97, Reclassifica o Parque Natural Da Serra Da Estrela. Available online: https://dre.tretas.org/dre/88089/decreto-regulamentar-50-97-de-20-de-novembro (accessed on 20 June 2023).
- CISE Centro de Interpretação Da Serra Da Estrela. Available online: http://www.cise.pt/pt/index.php/cise/missao (accessed on 20 June 2023).
- Jansen, J.; Sequeire, M.P.S.M. The Vegetation of Shallow Waters and Seasonally Inundated Habitats (Littorelletea and Isoeto-Nanojuncetea) in the Higher Parts of the Serra Da Estrela. Mitteilungen Badischen Landesver. Naturkunde 1999, 17, 449–462. [Google Scholar]
- Garcia, C.; Sérgio, C.; Jansen, J. The Bryophyte Flora of the Natural Park of Serra Da Estrela (Portugal): Conservation and Biogeographical Approaches. Cryptogam. Bryol. 2008, 29, 49–73. [Google Scholar]
- Daveau, S.; Coelho, C.; Costa, V.G.E.; Carvalho, L. Repartition et Rythme Des Precipitations Au Portugal; Daveau, D., Ed.; Mem. CEG.: Lisbon, Portugal, 1977; Volume 3. [Google Scholar]
- Google Maps Serra Da Estrela. Available online: https://www.google.com/maps/place/Serra+da+Estrela/@40.32303,-7.5960533,15z/data=!3m1!4b1!4m6!3m5!1s0xd3d28a0408be839:0x1d00ebbed2102ce0!8m2!3d40.3230306!4d-7.5960533!16zL20vMDNqdHNw?entry=ttu (accessed on 12 December 2023).
- WCVP The World Checklist of Vascular Plants. Available online: https://powo.science.kew.org/about-wcvp (accessed on 22 June 2023).
- Sociedade Portuguesa de Botânica Flora-On. Available online: https://flora-on.pt/ (accessed on 22 June 2023).
- INaturalist INaturalist. Available online: https://www.inaturalist.org/pages/what+is+it (accessed on 22 June 2023).
- IPCC Intergovernmental Panel on Climate Change. Available online: https://www.ipcc.ch/ (accessed on 29 November 2023).
- Adler, C.; Huggel, C.; Orlove, B.; Nolin, A. Climate Change in the Mountain Cryosphere: Impacts and Responses. Reg. Environ. Chang. 2019, 19, 1225–1228. [Google Scholar] [CrossRef]
- Abram, N.J.; Henley, B.J.; Gupta, A.S.; Lippmann, T.J.R.; Clarke, H.; Dowdy, A.J.; Sharples, J.J.; Nolan, R.H.; Zhang, T.; Wooster, M.J.; et al. Connections of Climate Change and Variability to Large and Extreme Forest Fires in Southeast Australia. Commun. Earth Environ. 2021, 2, 8. [Google Scholar] [CrossRef]
- Albrich, K.; Rammer, W.; Seidl, R. Climate Change Causes Critical Transitions and Irreversible Alterations of Mountain Forests. Glob. Chang. Biol. 2020, 26, 4013–4027. [Google Scholar] [CrossRef]
- Körner, C. Mountain Ecosystems in a Changing Environment. Eco Mont 2014, 6, 71–77. [Google Scholar] [CrossRef]
- Xu, J.; Badola, R.; Chettri, N.; Chaudhary, R.P.; Zomer, R.; Pokhrel, B.; Hussain, S.A.; Pradhan, S.; Pradhan, R. Sustaining Biodiversity and Ecosystem Services in the Hindu Kush Himalaya. In The Hindu Kush Himalaya Assessment: Mountains, Climate Change, Sustainability and People; Springer: Berlin/Heidelberg, Germany, 2019; pp. 127–165. [Google Scholar]
- The Royal Botanic Gardens Kew. Plants of the World Online. Available online: https://powo.science.kew.org/results?q=Prunella%20vulgaris (accessed on 7 December 2023).
- Cabral, C.; Cavaleiro, C.; Gonçalves, M.J.; Cruz, M.T.; Lopes, M.C.; Salgueiro, L. Otanthus maritimus (L.) Hoffmanns. & Link as a Source of a Bioactive and Fragrant Oil. Ind. Crops Prod. 2013, 43, 484–489. [Google Scholar] [CrossRef]
- Kenny, O.; Smyth, T.J.; Walsh, D.; Kelleher, C.T.; Hewage, C.M.; Brunton, N.P. Investigating the Potential of Under-Utilised Plants from the Asteraceae Family as a Source of Natural Antimicrobial and Antioxidant Extracts. Food Chem. 2014, 161, 79–86. [Google Scholar] [CrossRef]
- Simpson, M.G. Diversity and Classification of Flowering Plants: Eudicots. In Plant Systematics; Academic Press: Cambridge, MA, USA, 2010; pp. 275–448. [Google Scholar] [CrossRef]
- Broholm, S.K.; Teeri, T.H.; Elomaa, P. Molecular Control of Inflorescence Development in Asteraceae. Adv. Bot. Res. 2014, 72, 297–333. [Google Scholar] [CrossRef]
- Harris, E.M. Inflorescence and Floral Ontogeny in Asteraceae: A Syn (Doctoral Dissertation, Thesis of Historical and Current Concepts). Bot. Rev. 1995, 61, 93–278. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Mishra, A.K.; Nongbet, A.; Chakrabartty, I.; Mahanta, S.; Sarma, B.; Panda, J.; Panda, S.K. Potential Use of the Asteraceae Family as a Cure for Diabetes: A Review of Ethnopharmacology to Modern Day Drug and Nutraceuticals Developments. Front. Pharmacol. 2023, 14, 1153600. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Oliveira, P.; Barral, M.; Carpena, M.; Gullón, P.; Fraga-Corral, M.; Otero, P.; Prieto, M.A.; Simal-Gandara, J. Traditional Plants from Asteraceae Family as Potential Candidates for Functional Food Industry. Food Funct. 2021, 12, 2850–2873. [Google Scholar] [CrossRef] [PubMed]
- Woldeamanuel, M.M.; Geda, M.K.; Mohapatra, S.; Bastia, T.K.; Rath, P.; Panda, A.K. Ethnobotanical Study of Endemic and Non-Endemic Medicinal Plants Used by Indigenous People in Environs of Gullele Botanical Garden Addis Ababa, Central Ethiopia: A Major Focus on Asteraceae Family. Front. Pharmacol. 2022, 13, 1020097. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.; Abd Rani, N.Z.; Husain, K. A Review on the Potential Use of Medicinal Plants From Asteraceae and Lamiaceae Plant Family in Cardiovascular Diseases. Front. Pharmacol. 2020, 11, 484919. [Google Scholar] [CrossRef]
- Panda, S.K.; Da Silva, L.C.N.; Sahal, D.; Leonti, M. Editorial: Ethnopharmacological Studies for the Development of Drugs with Special Reference to Asteraceae. Front. Pharmacol. 2019, 10, 483288. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Barreira, J.C.M.; Oliveira, M.B.P.P. Asteraceae Species with Most Prominent Bioactivity and Their Potential Applications: A Review. Ind. Crops Prod. 2015, 76, 604–615. [Google Scholar] [CrossRef]
- Panda, S.K.; Luyten, W. Antiparasitic Activity in Asteraceae with Special Attention to Ethnobotanical Use by the Tribes of Odisha, India. Parasite 2018, 25, 10. [Google Scholar] [CrossRef]
- Devkota, H.P.; Aftab, T. Medicinal Plants of the Asteraceae Family: Traditional Uses, Phytochemistry and Pharmacological Activities; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–230. [Google Scholar]
- Mouhid, L.; Gómez De Cedrón, M.; Vargas, T.; García-Carrascosa, E.; Herranz, N.; García-Risco, M.; Reglero, G.; Fornari, T.; De Molina, A.R. Identification of Antitumoral Agents against Human Pancreatic Cancer Cells from Asteraceae and Lamiaceae Plant Extracts. BMC Complement. Altern. Med. 2018, 18, 254. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, H.; Matos, F.J.A. Plantas Medicinais No Brasil: Nativas e Exoticas; Instituto Plantarum, Ed.; Instituto Plantarum de Estudos da Flora: Nova Odessa, Brazil, 2002. [Google Scholar]
- Petlevski, R.; Hadžija, M.; Slijepčevič, M.; Juretič, D. Effect of “antidiabetis” Herbal Preparation on Serum Glucose and Fructosamine in NOD Mice. J. Ethnopharmacol. 2001, 75, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Paradise, L. Homeopathic Pharmaceutical Compositions. U.S. Patent 5795573A, 18 August 1998. [Google Scholar]
- Chaachouay, N.; Benkhnigue, O.; Fadli, M.; Ibaoui, H.E.; Zidane, L. Ethnobotanical and Ethnopharmacological Studies of Medicinal and Aromatic Plants Used in the Treatment of Metabolic Diseases in the Moroccan Rif. Heliyon 2019, 5, e02191. [Google Scholar] [CrossRef] [PubMed]
- Wazaify, M.; Afifi, F.; El-Khateeb, M.; Ajlouni, K. Complementary and Alternative Medicine Use among Jordanian Patients with Diabetes. Complement. Ther. Clin. Pract. 2011, 17, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, L.; Pugni, F.; Fico, G. Tradition of Use on Medicinal Species in Valfurva (Sondrio, Italy). J. Ethnopharmacol. 2015, 163, 113–134. [Google Scholar] [CrossRef] [PubMed]
- Errajraji, A.; Ouhdouch, F.; El-Anssari, N. Usage Des Plantes Médicinales Dans Le Traitement Du Diabète de Type 2 Au Maroc: Use of Medicinal Plants for Type 2 Diabetes Treatment, in Morocco. Médecine Des Mal. Métaboliques 2010, 4, 301–304. [Google Scholar] [CrossRef]
- Trojan-Rodrigues, M.; Alves, T.L.S.; Soares, G.L.G.; Ritter, M.R. Plants Used as Antidiabetics in Popular Medicine in Rio Grande Do Sul, Southern Brazil. J. Ethnopharmacol. 2012, 139, 155–163. [Google Scholar] [CrossRef]
- Pullaiah, T. Encyclopedia of World Medicinal Plants; Regency Publications: Delhi, India, 2006. [Google Scholar]
- Móricz, Á.M.; Ott, P.G.; Häbe, T.T.; Darcsi, A.; Böszörményi, A.; Alberti, Á.; Krüzselyi, D.; Csontos, P.; Béni, S.; Morlock, G.E. Effect-Directed Discovery of Bioactive Compounds Followed by Highly Targeted Characterization, Isolation and Identification, Exemplarily Shown for Solidago virgaurea. Anal. Chem. 2016, 88, 8202–8209. [Google Scholar] [CrossRef]
- Kamau, L.N.; Mbaabu, M.P.; Mbaria, J.M.; Karuri, G.P.; Kiama, S.G. Knowledge and Demand for Medicinal Plants Used in the Treatment and Management of Diabetes in Nyeri County, Kenya. J. Ethnopharmacol. 2016, 189, 218–229. [Google Scholar] [CrossRef]
- Cornara, L.; La Rocca, A.; Marsili, S.; Mariotti, M.G. Traditional Uses of Plants in the Eastern Riviera (Liguria, Italy). J. Ethnopharmacol. 2009, 125, 16–30. [Google Scholar] [CrossRef]
- Benkhnigue, O.; Akka, B.; Salhi, S.; Fadli, M.; Douira, A.; Zidane, L. Catalogue Des Plantes Médicinales Utilisées Dans Le Traitement Du Diabète Dans La Région d’Al Haouz-Rhamna (Maroc). J. Anim. Plant Sci. 2014, 23, 3539–3568. [Google Scholar]
- Tamokou, J.D.D.; Mbaveng, A.T.; Kuete, V. Antimicrobial Activities of African Medicinal Spices and Vegetables. In Medicinal Spices and Vegetables from Africa: Therapeutic Potential. Against Metabolic, Inflammatory, Infectious and Systemic Diseases; Academic Press: Cambridge, MA, USA, 2017; pp. 207–237. [Google Scholar] [CrossRef]
- Kokkini, S.; Karousou, R.; Hanlidou, E. HERBS|Herbs of the Labiatae. In Encyclopedia of Food Sciences and Nutrition; Academic Press: Cambridge, MA, USA, 2003; pp. 3082–3090. [Google Scholar] [CrossRef]
- Etsassala, N.G.E.R.; Hussein, A.A.; Nchu, F. Potential Application of Some Lamiaceae Species in the Management of Diabetes. Plants 2021, 10, 279. [Google Scholar] [CrossRef] [PubMed]
- Ghourri, M.; Zidane, L.; Douira, A. Catalogue Des Plantes Médicinales Utilisées Dans Le Traitement de La Lithiase Rénale Dans La Province de Tan-Tan (Maroc Saharien). Int. J. Biol. Chem. Sci. 2014, 7, 1688. [Google Scholar] [CrossRef]
- Skalli, S.; Hassikou, R.; Arahou, M. An Ethnobotanical Survey of Medicinal Plants Used for Diabetes Treatment in Rabat, Morocco. Heliyon 2019, 5, e01421. [Google Scholar] [CrossRef] [PubMed]
- Idm’hand, E.; Msanda, F.; Cherifi, K. Ethnopharmacological Review of Medicinal Plants Used to Manage Diabetes in Morocco. Clin. Phytosci. 2020, 6, 18. [Google Scholar] [CrossRef]
- Arifah, F.H.; Nugroho, A.E.; Rohman, A.; Sujarwo, W. A Review of Medicinal Plants for the Treatment of Diabetes Mellitus: The Case of Indonesia. S. Afr. J. Bot. 2022, 149, 537–558. [Google Scholar] [CrossRef]
- Tahraoui, A.; El-Hilaly, J.; Israili, Z.H.; Lyoussi, B. Ethnopharmacological Survey of Plants Used in the Traditional Treatment of Hypertension and Diabetes in South-Eastern Morocco (Errachidia Province). J. Ethnopharmacol. 2007, 110, 105–117. [Google Scholar] [CrossRef]
- Eddouks, M.; Ajebli, M.; Hebi, M. Ethnopharmacological Survey of Medicinal Plants Used in Daraa-Tafilalet Region (Province of Errachidia), Morocco. J. Ethnopharmacol. 2017, 198, 516–530. [Google Scholar] [CrossRef]
- Ziyyat, A.; Legssyer, A.; Mekhfi, H.; Dassouli, A.; Serhrouchni, M.; Benjelloun, W. Phytotherapy of Hypertension and Diabetes in Oriental Morocco. J. Ethnopharmacol. 1997, 58, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Cetto, A.; Heinrich, M. Mexican Plants with Hypoglycaemic Effect Used in the Treatment of Diabetes. J. Ethnopharmacol. 2005, 99, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F.; Mootoosamy, A.; Wambugu, S. Traditional Therapies Used to Manage Diabetes and Related Complications in Mauritius: A Comparative Ethnoreligious Study. Evid. Based Complement. Altern. Med. 2016, 2016, 4523828. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.Y.; Zhang, Q.F. Enhanced Analysis of Triterpenes, Flavonoids and Phenolic Compounds in Prunella vulgaris L. by Capillary Zone Electrophoresis with the Addition of Running Buffer Modifiers. J. Chromatogr. A 2008, 1213, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Orch, H.; Douira, A.; Zidane, L. Étude Ethnobotanique Des Plantes Médicinales Utilisées Dans Le Traitement Du Diabète, et Des Maladies Cardiaques Dans La Région d’Izarène (Nord Du Maroc). J. Appl. Biosci. 2015, 86, 7940. [Google Scholar] [CrossRef]
- Jiao, X.; Liu, H.; Lu, Q.; Wang, Y.; Zhao, Y.; Liu, X.; Liu, F.; Zuo, Y.; Wang, W.; Li, Y. Study on the Mechanism of Prunella vulgaris L on Diabetes Mellitus Complicated with Hypertension Based on Network Pharmacology and Molecular Docking Analyses. J. Diabetes Res. 2021, 2021, 9949302. [Google Scholar] [CrossRef] [PubMed]
- Sokovic, M.; Marin, P.D.; Brkic, D.; van Griensven, L.J. Chemical Composition and Antibacterial Activity of Essential Oils against Human Pathogenic Bacteria. Food 2008, 1, 220–226. [Google Scholar]
- Nadon, B.; Jackson, S. The Polyploid Origins of Crop Genomes and Their Implications: A Case Study in Legumes. Adv. Agron. 2020, 159, 275–313. [Google Scholar] [CrossRef]
- Harris, S. TROPICAL FORESTS|Woody Legumes (Excluding Acacias). In Encyclopedia of Forest Sciences; Academic Press: Cambridge, MA, USA, 2004; pp. 1793–1797. [Google Scholar] [CrossRef]
- Tekdal, D. Plant Genes for Abiotic Stress in Legumes. In Abiotic Stress and Legumes: Tolerance and Management; Elsevier: Amsterdam, The Netherlands, 2021; pp. 291–301. [Google Scholar] [CrossRef]
- Kumar, S.; Bamboriya, S.D.; Rani, K.; Meena, R.S.; Sheoran, S.; Loyal, A.; Kumawat, A.; Jhariya, M.K. Grain Legumes: A Diversified Diet for Sustainable Livelihood, Food, and Nutritional Security. In Advances in Legumes for Sustainable Intensification; Elsevier: Amsterdam, The Netherlands, 2022; pp. 157–178. [Google Scholar] [CrossRef]
- Serrano-Sandoval, S.N.; Guardado-Felix, D.; Gutiérrez-Uribe, J.A. Legumes in Human Health and Nutrition. In Encyclopedia of Human Nutrition, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 1–4, pp. 430–437. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Composition and Antioxidant Potential of Grain Legume Seeds: A Review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef]
- Hummer, K.E.; Janick, J. Rosaceae: Taxonomy, Economic Importance, Genomics. In Genetics and Genomics of Rosaceae; Springer: New York, NY, USA, 2009; pp. 1–17. [Google Scholar] [CrossRef]
- George, J.P.; Konrad, H.; Collin, E.; Thevenet, J.; Ballian, D.; Idzojtic, M.; Kamm, U.; Zhelev, P.; Geburek, T. High Molecular Diversity in the True Service Tree (Sorbus domestica) despite Rareness: Data from Europe with Special Reference to the Austrian Occurrence. Ann. Bot. 2015, 115, 1105–1115. [Google Scholar] [CrossRef]
- Cachi, A.; Wünsch, A.; Vilanova, A.; Guàrdia, M.; Ciordia, M.; Aletà, N. S-locus Diversity and Cross-compatibility of Wild Prunus Avium for Timber Breeding. Plant Breed. 2017, 136, 126–131. [Google Scholar] [CrossRef]
- Chandra, S.; Rawat, D.S. Medicinal Plants of the Family Caryophyllaceae: A Review of Ethno-Medicinal Uses and Pharmacological Properties. Integr. Med. Res. 2015, 4, 123–131. [Google Scholar] [CrossRef]
- Böttger, S.; Melzig, M.F. Triterpenoid Saponins of the Caryophyllaceae and Illecebraceae Family. Phytochem. Lett. 2011, 4, 59–68. [Google Scholar] [CrossRef]
- Forest, F.; Chase, M.W.; Persson, C.; Crane, P.R.; Hawkins, J.A. The role of biotic and abiotic factors in evolution of ant dispersal in the milkwort family (Polygalaceae). Evolution 2007, 61, 1675–1694. [Google Scholar] [CrossRef]
- Wang, D.; Bădărau, A.S.; Swamy, M.K.; Shaw, S.; Maggi, F.; da Silva, L.E.; López, V.; Yeung, A.W.K.; Mocan, A.; Atanasov, A.G. Arctium Species Secondary Metabolites Chemodiversity and Bioactivities. Front. Plant Sci. 2019, 10, 439246. [Google Scholar] [CrossRef]
- İlgün, S.; Karatoprak, G.Ş.; Polat, D.Ç.; Şafak, E.K.; Yıldız, G.; Küpeli Akkol, E.; Sobarzo-Sánchez, E. Phytochemical Composition and Biological Activities of Arctium minus (Hill) Bernh.: A Potential Candidate as Antioxidant, Enzyme Inhibitor, and Cytotoxic Agent. Antioxidants 2022, 11, 1852. [Google Scholar] [CrossRef]
- De Liz Oliveira Cavalli, V.L.; Sordi, C.; Tonini, K.; Grando, A.; Muneron, T.; Guigi, A.; Júnior, W.A.R. Avaliação In Vivo Do Efeito Hipoglicemiante de Extratos Obtidos Da Raiz e Folha de Bardana Arctium minus (Hill.) Bernh. Rev. Bras. Farmacogn. 2007, 17, 64–70. [Google Scholar] [CrossRef]
- Tawfick, M.M.; Xie, H.; Zhao, C.; Shao, P.; Farag, M.A. Inulin Fructans in Diet: Role in Gut Homeostasis, Immunity, Health Outcomes and Potential Therapeutics. Int. J. Biol. Macromol. 2022, 208, 948–961. [Google Scholar] [CrossRef]
- Akram, W.; Garud, N.; Joshi, R. Role of Inulin as Prebiotics on Inflammatory Bowel Disease. Drug Discov. Ther. 2019, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.B. Prebiotics: Preferential Substrates for Specific Germs? Am. J. Clin. Nutr. 2001, 73, 406s–409s. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Wang, Q.; Wang, X.; Fan, C.; Luan, T.; Yan, L.; Zhang, Y.; Zeng, X.; Dai, Y.; Li, P. The Protective Effects of Inulin-Type Fructans Against High-Fat/Sucrose Diet-Induced Gestational Diabetes Mice in Association with Gut Microbiota Regulation. Front. Microbiol. 2022, 13, 832151. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Luo, J.; Cai, Y.; Fu, M.; Li, W.; Shi, L.; Liu, J.; Dong, R.; Xu, X.; Tu, L.; et al. Inulin-Type Fructans Change the Gut Microbiota and Prevent the Development of Diabetic Nephropathy. Pharmacol. Res. 2022, 183, 106367. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, L.; Xue, J.; Yang, X.; Dong, X.; Sha, L.; Lei, H.; Zhang, X.; Zhu, L.; Wang, Z.; et al. Dietary Inulin Alleviates Diverse Stages of Type 2 Diabetes Mellitus via Anti-Inflammation and Modulating Gut Microbiota in Db/Db Mice. Food Funct. 2019, 10, 1915–1927. [Google Scholar] [CrossRef]
- Fernanda, M.F.; Peixoto, F.P.; Seiçad, R.; Santos, M.S. Diabetes and Medicinal Plants in Portugal. In Natural Products: Research Reviews; Gupta, V.K., Ed.; Daya Publisher: Delhi, India, 2012; Volume 1, pp. 1–41. [Google Scholar]
- Ferreira, F.M.; Peixoto, F.P.; Nunes, E.; Sena, C.; Seiça, R.; Santos, S.M. Inhibitory Effect of Arctium minus on Mitochondrial Bioenergetics in Diabetic Goto-Kakizaki Rats. Sci. Res. Essay 2010, 5, 2136–2142. [Google Scholar]
- Fischer, S.P.M.; Brusco, I.; Camponogara, C.; Piana, M.; Faccin, H.; Gobo, L.A.; de Carvalho, L.M.; Oliveira, S.M. Arctium minus Crude Extract Presents Antinociceptive Effect in a Mice Acute Gout Attack Model. Inflammopharmacology 2018, 26, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Erdemoglu, N.; Turan, N.N.; Akkol, E.K.; Sener, B.; Abacioglu, N. Estimation of Anti-Inflammatory, Antinociceptive and Antioxidant Activities on Arctium minus (Hill) Bernh. Ssp. Minus. J. Ethnopharmacol. 2009, 121, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Guettaf, S.; Benmerzoug, A.; Chawki, B.; Çakmak, Y.S.; Dahamna, S.; Baghiani, A.; Harzallah, D. Contribution to Pharmacological Valorisation of Algerian Arctium minus (Hill) Bernh. Subsp. Atlanticum (Pomel) Maire; Antioxidant an d Acetylcholinesterase Inhibitory Activities. Curr. Enzym. Inhib. 2022, 18, 135–144. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, C.; Guven, E.C.; Paoli, P.; Simal-Gandara, J.; Ramkumar, K.M.; Wang, S.; Buleu, F.; Pah, A.; Turi, V.; et al. Dietary Polyphenols as Antidiabetic Agents: Advances and Opportunities. Food Front. 2020, 1, 18–44. [Google Scholar] [CrossRef]
- Alam, S.; Sarker, M.M.R.; Sultana, T.N.; Chowdhury, M.N.R.; Rashid, M.A.; Chaity, N.I.; Zhao, C.; Xiao, J.; Hafez, E.E.; Khan, S.A.; et al. Antidiabetic Phytochemicals From Medicinal Plants: Prospective Candidates for New Drug Discovery and Development. Front Endocrinol 2022, 13, 800714. [Google Scholar] [CrossRef]
- Saleh, N.A.M.; Bohm, B.A. Flavonoids of Arctium minus (Compositae). Experientia 1971, 27, 1494. [Google Scholar] [CrossRef]
- Watkins, F.; Pendry, B.; Sanchez-Medina, A.; Corcoran, O. Antimicrobial Assays of Three Native British Plants Used in Anglo-Saxon Medicine for Wound Healing Formulations in 10th Century England. J. Ethnopharmacol. 2012, 144, 408–415. [Google Scholar] [CrossRef]
- Vitalini, S.; Beretta, G.; Iriti, M.; Orsenigo, S.; Basilico, N.; Dall’Acqua, S.; Iorizzi, M.; Fico, G. Phenolic Compounds from Achillea millefolium L. and Their Bioactivity. Acta Biochim. Pol. 2011, 58, 203–209. [Google Scholar] [CrossRef]
- Trumbeckaite, S.; Benetis, R.; Bumblauskiene, L.; Burdulis, D.; Janulis, V.; Toleikis, A.; Viškelis, P.; Jakštas, V. Achillea millefolium L. s.l. Herb Extract: Antioxidant Activity and Effect on the Rat Heart Mitochondrial Functions. Food Chem. 2011, 127, 1540–1548. [Google Scholar] [CrossRef]
- De Souza, P.; Gasparotto, A.; Crestani, S.; Stefanello, M.É.A.; Marques, M.C.A.; Da Silva-Santos, J.E.; Kassuya, C.A.L. Hypotensive Mechanism of the Extracts and Artemetin Isolated from Achillea millefolium L. (Asteraceae) in Rats. Phytomedicine 2011, 18, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Potrich, F.B.; Allemand, A.; da Silva, L.M.; dos Santos, A.C.; Baggio, C.H.; Freitas, C.S.; Mendes, D.A.G.B.; Andre, E.; de Paula Werner, M.F.; Marques, M.C.A. Antiulcerogenic Activity of Hydroalcoholic Extract of Achillea millefolium L.: Involvement of the Antioxidant System. J. Ethnopharmacol. 2010, 130, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Goli, S.A.H.; Talebi, M. Total Phenolic Content and Antioxidant Activity of Three Iranian Endemic Achillea Species. Ind. Crops Prod. 2013, 50, 154–158. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Dueñas, M.; Pereira, E.; Carvalho, A.M.; Alves, R.C.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical Composition of Wild and Commercial Achillea millefolium L. and Bioactivity of the Methanolic Extract, Infusion and Decoction. Food Chem. 2013, 141, 4152–4160. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Bolego, C.; Cignarella, A.; Gaion, R.M.; Innocenti, G. Vasoprotective Activity of Standardized Achillea millefolium Extract. Phytomedicine 2011, 18, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Candan, F.; Unlu, M.; Tepe, B.; Daferera, D.; Polissiou, M.; Sökmen, A.; Akpulat, H.A. Antioxidant and Antimicrobial Activity of the Essential Oil and Methanol Extracts of Achillea millefolium Subsp. Millefolium Afan. (Asteraceae). J. Ethnopharmacol. 2003, 87, 215–220. [Google Scholar] [CrossRef]
- Baretta, I.P.; Felizardo, R.A.; Bimbato, V.F.; Dos Santos, M.G.J.; Kassuya, C.A.L.; Gasparotto Junior, A.; Da Silva, C.R.; De Oliveira, S.M.; Ferreira, J.; Andreatini, R. Anxiolytic-like Effects of Acute and Chronic Treatment with Achillea millefolium L. Extract. J. Ethnopharmacol. 2012, 140, 46–54. [Google Scholar] [CrossRef]
- Jonsdottir, G.; Omarsdottir, S.; Vikingsson, A.; Hardardottir, I.; Freysdottir, J. Aqueous Extracts from Menyanthes Trifoliate and Achillea millefolium Affect Maturation of Human Dendritic Cells and Their Activation of Allogeneic CD4 + T Cells In Vitro. J. Ethnopharmacol. 2011, 136, 88–93. [Google Scholar] [CrossRef]
- Cavalcanti, A.M.; Baggio, C.H.; Freitas, C.S.; Rieck, L.; de Sousa, R.S.; Da Silva-Santos, J.E.; Mesia-Vela, S.; Marques, M.C.A. Safety and Antiulcer Efficacy Studies of Achillea millefolium L. after Chronic Treatment in Wistar Rats. J. Ethnopharmacol. 2006, 107, 277–284. [Google Scholar] [CrossRef]
- Csupor-Löffler, B.; Hajdú, Z.; Zupkó, I.; Réthy, B.; Falkay, G.; Forgo, P.; Hohmann, J. Antiproliferative Effect of Flavonoids and Sesquiterpenoids from Achillea millefolium s.l. on Cultured Human Tumour Cell Lines. Phytother. Res. 2009, 23, 672–676. [Google Scholar] [CrossRef]
- Chanishvili, S.; Badridze, G.; Rapava, L.; Janukashvili, N. Effect of Altitude on the Contents of Antioxidants in Leaves of Some Herbaceous Plants. Russ. J. Ecol. 2007, 38, 367–373. [Google Scholar] [CrossRef]
- Si, X.T.; Zhang, M.L.; Shi, Q.W.; Kiyota, H. Chemical Constituents of the Plants in the Genus Achillea. Chem. Biodivers. 2006, 3, 1163–1180. [Google Scholar] [CrossRef]
- Jenabi, E.; Fereidoony, B. Effect of Achillea millefolium on Relief of Primary Dysmenorrhea: A Double-Blind Randomized Clinical Trial. J. Pediatr. Adolesc. Gynecol. 2015, 28, 402–404. [Google Scholar] [CrossRef]
- Arias-Durán, L.; Estrada-Soto, S.; Hernández-Morales, M.; Chávez-Silva, F.; Navarrete-Vázquez, G.; León-Rivera, I.; Perea-Arango, I.; Villalobos-Molina, R.; Ibarra-Barajas, M. Tracheal Relaxation through Calcium Channel Blockade of Achillea millefolium Hexanic Extract and Its Main Bioactive Compounds. J. Ethnopharmacol. 2020, 253, 112643. [Google Scholar] [CrossRef]
- Farhadi, N.; Babaei, K.; Farsaraei, S.; Moghaddam, M.; Ghasemi Pirbalouti, A. Changes in Essential Oil Compositions, Total Phenol, Flavonoids and Antioxidant Capacity of Achillea millefolium at Different Growth Stages. Ind. Crops Prod. 2020, 152, 112570. [Google Scholar] [CrossRef]
- Arias-Durán, L.; Estrada-Soto, S.; Hernández-Morales, M.; Millán-Pacheco, C.; Navarrete-Vázquez, G.; Villalobos-Molina, R.; Ibarra-Barajas, M.; Almanza-Pérez, J.C. Antihypertensive and Vasorelaxant Effect of Leucodin and Achillin Isolated from Achillea millefolium through Calcium Channel Blockade and NO Production: In Vivo, Functional Ex Vivo and in Silico Studies. J. Ethnopharmacol. 2021, 273, 113948. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, L.; Liu, G.; Li, J.; Aisa, H.A. Chlorine-Containing Guaianolide Sesquiterpenoids from Achillea millefolium L. with Inhibitory Effects against LPS-Induced NO Release in BV-2 Microglial Cells. Phytochemistry 2023, 207, 113567. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, A.; Seyedsadeghi, M.; Miran, M.; Ahari, S.S.; Layegh, H.; Mostafalou, S. Therapeutic Effect of Achillea millefolium on the Hemorrhoids; A Randomized Double-Blind Placebo-Controlled Clinical Trial. J. Herb. Med. 2023, 39, 100657. [Google Scholar] [CrossRef]
- Mohammadhosseini, M.; Sarker, S.D.; Akbarzadeh, A. Chemical Composition of the Essential oils and Extracts of Achillea Species and Their Biological Activities: A Review. J. Ethnopharmacol. 2017, 199, 257–315. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Naderi, G.; Ghannadi, A.; Gharipour, M.; Golbon, S. Protective Effect of Achillea millefolium, Crataegus Curvisepala and Matricaria chamomilla on Oxidative Hemolysis of Human Erythrocytes and-SH Capacity. J. Med. Plants 2003, 2, 41–48. [Google Scholar]
- Nematy, M.; Mazidi, M.; Jafari, A.; Baghban, S.; Rakhshandeh, H.; Norouzy, A.; Esmaily, H.; Etemad, L.; Patterson, M.; Mohammadpour, A.H. The Effect of Hydro-Alcoholic Extract of Achillea millefolium on Appetite Hormone in Rats. Avicenna J. Phytomed. 2017, 7, 10–15. [Google Scholar] [PubMed]
- Ali, S.I.; Gopalakrishnan, B.; Venkatesalu, V. Pharmacognosy, Phytochemistry and Pharmacological Properties of Achillea millefolium L.: A Review. Phytother. Res. 2017, 31, 1140–1161. [Google Scholar] [CrossRef] [PubMed]
- Düsman, E.; Almeida, I.V.D.; Coelho, A.C.; Balbi, T.J.; Düsman Tonin, L.T.; Vicentini, V.E.P. Antimutagenic Effect of Medicinal Plants Achillea millefolium and Bauhinia Forficata in Vivo. Evid.-Based Complement. Altern. Med. 2013, 2013, 893050. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M. Chemical Composition and Antimicrobial, Antioxidant Activities and Anti-Inflammatory Potential of Achillea millefolium L., Anethum graveolens L., and Carum copticum L. Essential oils. J. Herb. Med. 2015, 5, 217–222. [Google Scholar] [CrossRef]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Goli, S.A.H. Effect of Drought Stress on Total Phenolic, Lipid Peroxidation, and Antioxidant Activity of Achillea Species. Appl. Biochem. Biotechnol. 2016, 178, 796–809. [Google Scholar] [CrossRef] [PubMed]
- Ismail, Y.; Fahmy, D.M.; Ghattas, M.H.; Ahmed, M.M.; Zehry, W.; Saleh, S.M.; Abo-elmatty, D.M. Integrating Experimental Model, LC-MS/MS Chemical Analysis, and Systems Biology Approach to Investigate the Possible Antidiabetic Effect and Mechanisms of Matricaria aurea (Golden Chamomile) in Type 2 Diabetes Mellitus. Front. Pharmacol. 2022, 13, 924478. [Google Scholar] [CrossRef]
- Yousefbeyk, F.; Hemmati, G.; Gholipour, Z.; Ghasemi, S.; Evazalipour, M.; Schubert, C.; Koohi, D.E.; Böhm, V. Phytochemical Analysis, Antioxidant, Cytotoxic, and Antimicrobial Activities of Golden Chamomile (Matricaria aurea (Loefl.) Schultz Bip). Z. Fur Naturforschung C 2022, 77, 331–342. [Google Scholar] [CrossRef]
- Rizwana, H.; Soliman Alwahibi, M.; Soliman, D. Antimicrobial Activity and Chemical Composition of Flowers of Matricaria aurea a Native Herb of Saudi Arabia Article In. Int. J. Pharmacol. 2016, 12, 576–586. [Google Scholar] [CrossRef]
- Kheder, F.B.H.; Mahjoub, M.A.; Zaghrouni, F.; Kwaja, S.; Helal, A.N.; Mighri, Z. Chemical Composition Antioxidant and Antimicrobial Activities of the Essential Oils of Matricaria aurea Loefl. Growing in Tunisia. J. Essent. Oil Bear. Plants 2014, 17, 493–505. [Google Scholar] [CrossRef]
- Khan, M.; Abdullah, M.M.S.; Mahmood, A.; Al-Mayouf, A.M.; Alkhathlan, H.Z. Evaluation of Matricaria aurea Extracts as Effective Anti-Corrosive Agent for Mild Steel in 1.0 M HCl and Isolation of Their Active Ingredients. Sustainability 2019, 11, 7174. [Google Scholar] [CrossRef]
- Bohm, B.A.; Stuessy, T.F. Flavonoids of the Sunflower Family (Asteraceae). In Flavonoids of the Sunflower Family (Asteraceae); Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar] [CrossRef]
- Kriplani, P.; Guarve, K.; Baghael, U.S. Arnica montana L.—A Plant of Healing: Review. J. Pharm. Pharmacol. 2017, 69, 925–945. [Google Scholar] [CrossRef] [PubMed]
- Duthen, S.; Gadéa, A.; Trempat, P.; Boujedaini, N.; Fabre, N. Comparison of the Phytochemical Variation of Non-Volatile Metabolites within Mother Tinctures of Arnica montana Prepared from Fresh and Dried Whole Plant Using UHPLC-HRMS Fingerprinting and Chemometric Analysis. Molecules 2022, 27, 2737. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bai, R.; Liu, Y.; Zhang, X.; Kan, J.; Jin, C. Isolation, Structural Characterization and Bioactivities of Naturally Occurring Polysaccharide–Polyphenolic Conjugates from Medicinal Plants—A Reivew. Int. J. Biol. Macromol. 2018, 107, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- Schöpke, T.; Wray, V.; Rzazewska, B.; Hiller, K. Bellissaponins BA1 and BA2, Acylated Saponins from Bellis perennis. Phytochemistry 1991, 30, 627–631. [Google Scholar] [CrossRef]
- Scognamiglio, M.; Esposito, A.; D’Abrosca, B.; Pacifico, S.; Fiumano, V.; Tsafantakis, N.; Monaco, P.; Fiorentino, A. Isolation, Distribution and Allelopathic Effect of Caffeic Acid Derivatives from Bellis perennis L. Biochem. Syst. Ecol. 2012, 43, 108–113. [Google Scholar] [CrossRef]
- Avato, P.; Tava, A. Acetylenes and Terpenoids of Bellis perennis. Phytochemistry 1995, 40, 141–147. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Li, X.; Nishida, E.; Nakamura, S.; Matsuda, H.; Muraoka, O.; Morikawa, T. Medicinal Flowers. XXI. Structures of Perennisaponins A, B, C, D, E, and F, Acylated Oleanane-Type Triterpene Oligoglycosides, from the Flowers of Bellis perennis. Chem. Pharm. Bull. 2008, 56, 559–568. [Google Scholar] [CrossRef]
- Toki, K.; Saito, N.; Honda, T. Three Cyanidin 3-Glucuronylglucosides from Red Flowers of Bellis perennis. Phytochemistry 1991, 30, 3769–3771. [Google Scholar] [CrossRef]
- Morikawa, T.; Li, X.; Nishida, E.; Ito, Y.; Matsuda, H.; Nakamura, S.; Muraoka, O.; Yoshikawa, M. Perennisosides I-VII, Acylated Triterpene Saponins with Antihyperlipidemic Activities from the Flowers of Bellis perennis. J. Nat. Prod. 2008, 71, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Gudej, J.; Nazaruk, J. Flavonol Glycosides from the Flowers of Bellis perennis. Fitoterapia 2001, 72, 839–840. [Google Scholar] [CrossRef]
- Karakas, F.; Karakas, A.; CoÅŸkun, H.; Turker, U. Effects of Common Daisy (Bellis perennis L.) Aqueous Extracts on Anxiety-like Behaviour and Spatial Memory Performance in Wistar Albino Rats. Afr. J. Pharm. Pharmacol. 2011, 5, 1378–1388. [Google Scholar] [CrossRef]
- Pehlivan Karakaş, F.; Karakaş, A.; Boran, Ç.; Uçar Türker, A.; Nuray Yalçin, F.; Bilensoy, E. The Evaluation of Topical Administration of Bellis perennis Fraction on Circular Excision Wound Healing in Wistar Albino Rats. Pharm. Biol. 2012, 50, 1031–1037. [Google Scholar] [CrossRef]
- Karakas, F.P.; Turker, A.U. An Efficient in Vitro Regeneration System for Bellis perennis L. and Comparison of Phenolic Contents of Field-Grown and in Vitro-Grown Leaves by LC-MS/MS. Ind. Crops Prod. 2013, 48, 162–170. [Google Scholar] [CrossRef]
- Karakas, F.P.; Turker, A.U.; Karakas, A.; Mshvildadze, V.; Pichette, A.; Legault, J. In Vitro Cytotoxic, Antibacterial, Anti-Inflammatory and Antioxidant Activities and Phenolic Content in Wild-Grown Flowers of Common Daisy—A Medicinal Plant. J. Herb. Med. 2017, 8, 31–39. [Google Scholar] [CrossRef]
- Li, W.; Asada, Y.; Koike, K.; Nikaido, T.; Furuya, T.; Yoshikawa, T. Bellisosides A–F, Six Novel Acylated Triterpenoid Saponins from Bellis perennis (Compositae). Tetrahedron 2005, 61, 2921–2929. [Google Scholar] [CrossRef]
- Lapava, N. Bidens Frondosa: Component Composition and Pharmacological Profile. In Proceedings of the International Scientific Conference of Young Scientists “Current Development of Health Care Technology”, Vilar, Moscow, 1–4 June 2021; pp. 475–484. [Google Scholar]
- Brandão, M.G.L.; Krettli, A.U.; Soares, L.S.R.; Nery, C.G.C.; Marinuzzi, H.C. Antimalarial Activity of Extracts and Fractions from Bidens Pilosa and Other Bidens Species (Asteraceae) Correlated with the Presence of Acetylene and Flavonoid Compounds. J. Ethnopharmacol. 1997, 57, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Le, J.; Lu, W.; Xiong, X.; Wu, Z.; Chen, W. Anti-Inflammatory Constituents from Bidens Frondosa. Molecules 2015, 20, 18496–18510. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Bajpai, V.K.; Dung, N.T.; Kang, S.C. Antibacterial and Antioxidant Activities of the Essential Oil and Methanol Extracts of Bidens Frondosa Linn. Int. J. Food Sci. Technol. 2011, 46, 1238–1244. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Panchagnula, G.K.; Subbaraju, G.V. Synthesis and Antioxidative Activity of 3′,4′,6,7-Tetrahydroxyaurone, a Metabolite of Bidens Frondosa. Biosci. Biotechnol. Biochem. 2004, 68, 2183–2185. [Google Scholar] [CrossRef]
- Khouchlaa, A.; El Baaboua, A.; El Moudden, H.; Lakhdar, F.; Bakrim, S.; El Menyiy, N.; Belmehdi, O.; Harhar, H.; El Omari, N.; Balahbib, A.; et al. Traditional Uses, Bioactive Compounds, and Pharmacological Investigations of Calendula Arvensis L.: A Comprehensive Review. Adv. Pharmacol. Pharm. Sci. 2023, 2023, 2482544. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.; Barros, L.; Dueñas, M.; Calhelha, R.C.; Carvalho, A.M.; Santos-Buelga, C.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Nutrients, Phytochemicals and Bioactivity of Wild Roman Chamomile: A Comparison between the Herb and Its Preparations. Food Chem. 2013, 136, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Committee on Herbal Medicinal Products (HMPC). Assessment Report on Chamaemelum nobile (L.) All., Flos; Committee on Herbal Medicinal Products (HMPC): London, UK, 2012. [Google Scholar]
- Al-Snafi, A.E. Medical Importance of Anthemis Nobilis (Chamaemelum nobile)—A Review. Some of the Authors of This Publication Are Also Working on These Related Projects: Pharmacology of Medicinal Plants View Project Immunological Effects of Medicinal Plants: A Review (Part 2). View Project. Asian J. Pharm. Sci. Technol. 2016, 6, 89–95. [Google Scholar]
- Aisa, H.A.; Xin, X.; Tang, D. Chemical Constituents and Their Pharmacological Activities of Plants from Cichorium Genus. Chin. Herb. Med. 2020, 12, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Puhlmann, M.L.; de Vos, W.M. Back to the Roots: Revisiting the Use of the Fiber-Rich Cichorium intybus L. Taproots. Adv. Nutr. 2020, 11, 878–890. [Google Scholar] [CrossRef] [PubMed]
- Brahmi-Chendouh, N.; Piccolella, S.; Crescente, G.; Pacifico, F.; Boulekbache, L.; Hamri-Zeghichi, S.; Akkal, S.; Madani, K.; Pacifico, S. A Nutraceutical Extract from Inula viscosa Leaves: UHPLC-HR-MS/MS Based Polyphenol Profile, and Antioxidant and Cytotoxic Activities. J. Food Drug Anal. 2019, 27, 692. [Google Scholar] [CrossRef] [PubMed]
- Vuko, E.; Dunkić, V.; Maravić, A.; Ruščić, M.; Nazlić, M.; Radan, M.; Ljubenkov, I.; Soldo, B.; Fredotović, Ž. Not Only a Weed Plant—Biological Activities of Essential Oil and Hydrosol of Dittrichia viscosa (L.) Greuter. Plants 2021, 10, 1837. [Google Scholar] [CrossRef] [PubMed]
- Kheyar-Kraouche, N.; da Silva, A.B.; Serra, A.T.; Bedjou, F.; Bronze, M.R. Characterization by Liquid Chromatography–Mass Spectrometry and Antioxidant Activity of an Ethanolic Extract of Inula viscosa Leaves. J. Pharm. Biomed. Anal. 2018, 156, 297–306. [Google Scholar] [CrossRef]
- Parolin, P.; Ion Scotta, M.; Bresch, C. Biología de Dittrichia viscosa, Una Planta Ruderal Del Mediterráneo: Revisión. Phyton 2014, 83, 251–262. [Google Scholar]
- Ozkan, E.; Karakas, F.P.; Yildirim, A.B.; Tas, I.; Eker, I.; Yavuz, M.Z.; Turker, A.U. Promising Medicinal Plant Inula viscosa L.: Antiproliferative, Antioxidant, Antibacterial and Phenolic Profiles. Prog. Nutr. 2019, 21, 652–661. [Google Scholar] [CrossRef]
- Rozenblat, S.; Grossman, S.; Bergman, M.; Gottlieb, H.; Cohen, Y.; Dovrat, S. Induction of G2/M Arrest and Apoptosis by Sesquiterpene Lactones in Human Melanoma Cell Lines. Biochem. Pharmacol. 2008, 75, 369–382. [Google Scholar] [CrossRef]
- Kheyar-Kraouche, N.; Boucheffa, S.; Bellik, Y.; Farida, K.; Brahmi-Chendouh, N. Exploring the Potential of Inula viscosa Extracts for Antioxidant, Antiproliferative and Apoptotic Effects on Human Liver Cancer Cells and a Molecular Docking Study. BioTechnologia 2023, 104, 183. [Google Scholar] [CrossRef]
- Asraoui, F.; Kounnoun, A.; Cacciola, F.; Mansouri, F.E.; Kabach, I.; Majdoub, Y.O.E.; Alibrando, F.; Arena, K.; Trovato, E.; Mondello, L.; et al. Phytochemical Profile, Antioxidant Capacity, α-Amylase and α-Glucosidase Inhibitory Potential of Wild Moroccan Inula viscosa (L.) Aiton Leaves. Molecules 2021, 26, 3134. [Google Scholar] [CrossRef] [PubMed]
- Rotundo, G.; Paventi, G.; Barberio, A.; De Cristofaro, A.; Notardonato, I.; Russo, M.V.; Germinara, G.S. Biological Activity of Dittrichia viscosa (L.) Greuter Extracts against Adult Sitophilus granarius (L.) (Coleoptera, Curculionidae) and Identification of Active Compounds. Sci. Rep. 2019, 9, 6429. [Google Scholar] [CrossRef]
- Mrid, R.B.; Bouchmaa, N.; Kabach, I.; Zouaoui, Z.; Chtibi, H.; El Maadoudi, M.; Kounnoun, A.; Cacciola, F.; El Majdoub, Y.O.; Mondello, L.; et al. Dittrichia viscosa L. Leaves: A Valuable Source of Bioactive Compounds with Multiple Pharmacological Effects. Molecules 2022, 27, 2108. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-H.; Pan, Y.-A.; Lv, H.; Ding, X.-Q.; Yin, D.-Q.; Gai, Y.-N.; Niu, G.-T.; Ren, B.-R.; Qian, X.-G.; Chen, J.; et al. One New 12, 8-Guaianolide Sesquiterpene Lactone with Antihyperglycemic Activity from the Roots of Cichorium intybus. Nat. Prod. Res. 2023. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ripanda, A.; Luanda, A.; Sule, K.S.; Mtabazi, G.S.; Makangara, J.J. Galinsoga parviflora (Cav.): A Comprehensive Review on Ethnomedicinal, Phytochemical and Pharmacological Studies. Heliyon 2023, 9, e13517. [Google Scholar] [CrossRef] [PubMed]
- Les, F.; Venditti, A.; Cásedas, G.; Frezza, C.; Guiso, M.; Sciubba, F.; Serafini, M.; Bianco, A.; Valero, M.S.; López, V. Everlasting Flower (Helichrysum Stoechas Moench) as a Potential Source of Bioactive Molecules with Antiproliferative, Antioxidant, Antidiabetic and Neuroprotective Properties. Ind. Crops Prod. 2017, 108, 295–302. [Google Scholar] [CrossRef]
- Albayrak, S.; Aksoy, A.; Sagdic, O.; Hamzaoglu, E. Compositions, Antioxidant and Antimicrobial Activities of Helichrysum (Asteraceae) Species Collected from Turkey. Food Chem. 2010, 119, 114–122. [Google Scholar] [CrossRef]
- Onaran, M.; Orhan, N.; Farahvash, A.; Ekin, H.N.I.; Kocabiyik, M.; Gönül, I.I.; Şen, I.; Aslan, M. Successful Treatment of Sodium Oxalate Induced Urolithiasis with Helichrysum Flowers. J. Ethnopharmacol. 2016, 186, 322–328. [Google Scholar] [CrossRef]
- Bremner, P.; Rivera, D.; Calzado, M.A.; Obón, C.; Inocencio, C.; Beckwith, C.; Fiebich, B.L.; Muñoz, E.; Heinrich, M. Assessing Medicinal Plants from South-Eastern Spain for Potential Anti-Inflammatory Effects Targeting Nuclear Factor-Kappa B and Other pro-Inflammatory Mediators. J. Ethnopharmacol. 2009, 124, 295–305. [Google Scholar] [CrossRef]
- Jamuna, S.; Karthika, K.; Paulsamy, S.; Thenmozhi, K.; Kathiravan, S.; Venkatesh, R. Confertin and Scopoletin from Leaf and Root Extracts of Hypochaeris radicata Have Anti-Inflammatory and Antioxidant Activities. Ind. Crops Prod. 2015, 70, 221–230. [Google Scholar] [CrossRef]
- Shulha, O.; Çiçek, S.S.; Wangensteen, H.; Kroes, J.; Mäder, M.; Girreser, U.; Sendker, J.; Jöhrer, K.; Greil, R.; Schühly, W.; et al. Lignans and Sesquiterpene Lactones from Hypochaeris radicata Subsp. Neapolitana (Asteraceae, Cichorieae). Phytochemistry 2019, 165, 112047. [Google Scholar] [CrossRef]
- Sicari, V.; Loizzo, M.R.; Silva, A.S.; Romeo, R.; Spampinato, G.; Tundis, R.; Leporini, M.; Musarella, C.M. The Effect of Blanching on Phytochemical Content and Bioactivity of Hypochaeris and Hyoseris Species (Asteraceae), Vegetables Traditionally Used in Southern Italy. Foods 2020, 10, 32. [Google Scholar] [CrossRef]
- Senguttuvan, J.; Paulsamy, S.; Karthika, K. Phytochemical Analysis and Evaluation of Leaf and Root Parts of the Medicinal Herb, Hypochaeris radicata L. for In Vitro Antioxidant Activities. Asian Pac. J. Trop. Biomed. 2014, 4, S359. [Google Scholar] [CrossRef] [PubMed]
- Shoaib Akhtar, M.; Naveed Mushtaq, M.; Ali, I.; Pharm Sci, P.J.; Farooq Awan, A.; Anjum, I.; Fatima, A.; Mannan, A. Anti-Oxidant and Hepatoprotective Effects of Lactuca serriola and Its Phytochemical Screening by HPLC and FTIR Analysis. Pak. J. Pharm. Sci. 2020, 33, 2823–2830. [Google Scholar] [CrossRef]
- Fatah, N.H.A.; Amen, Y.; Abdel Bar, F.M.; Halim, A.F.; Saad, H.-E.A. Supporting Information Antioxidants and α-Glucosidase Inhibitors from Lactuca serriola L. Nat. Prod. 2020, 14, 410–415. [Google Scholar]
- Abd-ElGawad, A.M.; Elshamy, A.I.; El-Nasser El Gendy, A.; Al-Rowaily, S.L.; Assaeed, A.M. Preponderance of Oxygenated Sesquiterpenes and Diterpenes in the Volatile Oil Constituents of Lactuca serriola L. Revealed Antioxidant and Allelopathic Activity. Chem. Biodivers. 2019, 16, e1900278. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Nabavi, S.M.; Latifi, A.M.; Mirzaei, M.; Habtemariam, S.; Moghaddam, A.H. Mitigating Role of Quercetin against Sodium Fluoride-Induced Oxidative Stress in the Rat Brain. Pharm. Biol. 2012, 50, 1380–1383. [Google Scholar] [CrossRef]
- Elsharkawy, E.; Aljohar, H. Anticancer Screening of Medicinal Plants Growing in the Northern Region of Saudi Arabia. Natl. J. Physiol. Pharm. Pharmacol. 2016, 6, 241–246. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Elkelish, A.; Elansary, H.O.; Ali, H.M.; Elshikh, M.; Witczak, J.; Ahmad, M. Genetic Transformation and Hairy Root Induction Enhance the Antioxidant Potential of Lactuca serriola L. Oxidative Med. Cell. Longev. 2017, 2017, 5604746. [Google Scholar] [CrossRef]
- Mohammad, A. Traditional Use of Kahu (Lactuca scariola L.)—A Review. Glob. J. Res. Med. Plants Indig. Med. 2013, 2, 465–474. [Google Scholar]
- Ullah, M.I.; Anwar, R.; Kamran, S.; Gul, B.; Elhady, S.S.; Youssef, F.S. Evaluation of the Anxiolytic and Anti-Epileptogenic Potential of Lactuca serriola Seed Using Pentylenetetrazol-Induced Kindling in Mice and Metabolic Profiling of Its Bioactive Extract. Antioxidants 2022, 11, 2232. [Google Scholar] [CrossRef]
- Ghods, R.; Gharouni, M.; Amanlou, M.; Sharifi, N.; Ghobadi, A.; Amin, G. Effect of Onopordon acanthium L. as Add on Antihypertensive Therapy in Patients with Primary Hypertension Taking Losartan: A Pilot Study. Adv. Pharm. Bull. 2018, 8, 69. [Google Scholar] [CrossRef]
- Bande-De León, C.; Buendía-Moreno, L.; Abellán, A.; Manzi, P.; Al Mohandes Dridi, B.; Essaidi, I.; Aquilanti, L.; Tejada, L. Clotting and Proteolytic Activity of Freeze-Dried Crude Extracts Obtained from Wild Thistles Cynara humilis L. and Onopordum platylepis Murb. Foods 2023, 12, 2325. [Google Scholar] [CrossRef]
- Molnár, J.; Szebeni, G.J.; Csupor-Löffler, B.; Hajdú, Z.; Szekeres, T.; Saiko, P.; Ocsovszki, I.; Puskás, L.G.; Hohmann, J.; Zupkó, I. Investigation of the Antiproliferative Properties of Natural Sesquiterpenes from Artemisia Asiatica and Onopordum acanthium on HL-60 Cells In Vitro. Int. J. Mol. Sci. 2016, 17, 83. [Google Scholar] [CrossRef]
- Wei, C.; Zhou, S.; Shi, K.; Zhang, C.; Shao, H. Chemical Profile and Phytotoxic Action of Onopordum acanthium Essential oil. Sci. Rep. 2020, 10, 13568. [Google Scholar] [CrossRef] [PubMed]
- Garsiya, E.R.; Konovalov, D.A.; Shamilov, A.A.; Glushko, M.P.; Orynbasarova, K.K. Traditional Medicine Plant, Onopordum acanthium L. (Asteraceae): Chemical Composition and Pharmacological Research. Plants 2019, 8, 40. [Google Scholar] [CrossRef]
- Esmaeili, A.; Saremnia, B. Preparation of Extract-Loaded Nanocapsules from Onopordon leptolepis DC. Ind. Crops Prod. 2012, 37, 259–263. [Google Scholar] [CrossRef]
- Khalilov, L.M.; Khalilova, A.Z.; Shakurova, E.R.; Nuriev, I.F.; Kachala, V.V.; Shashkov, A.S.; Dzhemilev, U.M. PMR and 13C NMR Spectra of Biologically Active Compounds. XII. Taraxasterol and Its Acetate from the Aerial Part of Onopordum acanthium. Chem. Nat. Compd. 2003, 39, 285–288. [Google Scholar] [CrossRef]
- Tyumkina, T.V.; Nuriev, I.F.; Khalilov, L.M.; Akhmetova, V.R.; Dzhemilev, U.M. PMR and 13C NMR Spectra of Biologically Active Compounds. XIII. Structure and Stereochemistry of a New Phenylpropanoid Glycoside Isolated from Onopordum acanthium Seeds. Chem. Nat. Compd. 2009, 45, 61–65. [Google Scholar] [CrossRef]
- Yang, X.; Yang, L.; Xiong, A.; Li, D.; Wang, Z. Authentication of Senecio Scandens and S. Vulgaris Based on the Comprehensive Secondary Metabolic Patterns Gained by UPLC–DAD/ESI-MS. J. Pharm. Biomed. Anal. 2011, 56, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Loizzo, M.R.; Statti, G.; Houghton, P.; Menichini, F. Biological Properties of Different Extracts of Two Senecio Species. Int. J. Food Sci. Nutr. 2006, 57, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Acito, M.; Russo, C.; Fatigoni, C.; Mercanti, F.; Moretti, M.; Villarini, M. Cytotoxicity and Genotoxicity of Senecio vulgaris L. Extracts: An In Vitro Assessment in HepG2 Liver Cells. Int. J. Environ. Res. Public Health 2022, 19, 14824. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Statti, G.A.; Tundis, R.; Conforti, F.; Bonesi, M.; Autelitano, G.; Houghton, P.J.; Miljkovic-Brake, A.; Menichini, F. Antibacterial and Antifungal Activity of Senecio inaequidens DC. and Senecio vulgaris L. Phytother. Res. 2004, 18, 777–779. [Google Scholar] [CrossRef]
- Fursenco, C.; Calalb, T.; Uncu, L.; Dinu, M.; Ancuceanu, R. Solidago virgaurea L.: A Review of Its Ethnomedicinal Uses, Phytochemistry, and Pharmacological Activities. Biomolecules 2020, 10, 1619. [Google Scholar] [CrossRef]
- Khan, R.A.; Khan, M.R.; Sahreen, S. Brain Antioxidant Markers, Cognitive Performance and Acetylcholinesterase Activity of Rats: Efficiency of Sonchus asper. Behav. Brain Funct. 2012, 8, 21. [Google Scholar] [CrossRef]
- Khan, R.A.; Khan, M.R.; Sahreen, S. Protective Effect of Sonchus asper Extracts against Experimentally Induced Lung Injuries in Rats: A Novel Study. Exp. Toxicol. Pathol. 2012, 64, 725–731. [Google Scholar] [CrossRef]
- Khan, R.A.; Khan, M.R.; Sahreen, S.; Bokhari, J. Prevention of CCl4-Induced Nephrotoxicity with Sonchus asper in Rat. Food Chem. Toxicol. 2010, 48, 2469–2476. [Google Scholar] [CrossRef]
- Khan, R.A. Protective Effects of Sonchus asper (L.) Hill, (Asteraceae) against CCl4-Induced Oxidative Stress in the Thyroid Tissue of Rats. BMC Complement. Altern. Med. 2012, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Badar, I.; Siddiquah, A. Prevention of Hepatorenal Toxicity with Sonchus asper in Gentamicin Treated Rats. BMC Complement. Altern. Med. 2011, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.N.; Sati, S.C.; Kumar, P. An Invasive Plant Sonchus asper (L.) Hill: A Review of Its Ethnopharmacology, Phytochemistry, and Pharmacological Properties. Indian J. Nat. Prod. Resour. 2022, 13, 468–473. [Google Scholar] [CrossRef]
- Khan, I.U.; Khan, F.U.; Hussain, J.; Badshah, S.; Muhammad, N.; Khan, R.A.; Kait, C.F.; Ali, M.A.; Khan, H.; Aslam, M.W.; et al. Asperal: A New Clerodane Diterpene from Sonchus asper. Asian J. Chem. 2014, 26, 2699–2701. [Google Scholar] [CrossRef]
- Helal, A.M.; Nakamura, N.; El-Askary, H.; Hattori, M. Sesquiterpene Lactone Glucosides from Sonchus asper. Phytochemistry 2000, 53, 473–477. [Google Scholar] [CrossRef]
- Xia, D.Z.; Yu, X.F.; Zhu, Z.Y.; Zou, Z.D. Antioxidant and Antibacterial Activity of Six Edible Wild Plants (Sonchus spp.) in China. Nat. Prod. Res. 2011, 25, 1893–1901. [Google Scholar] [CrossRef]
- Li, X.M.; Yang, P.L. Research Progress of Sonchus Species. Int. J. Food Prop. 2018, 21, 147–157. [Google Scholar] [CrossRef]
- Wang, L.; Xu, M.L.; Liu, J.; Wang, Y.; Hu, J.H.; Wang, M.H. Sonchus asper Extract Inhibits LPS-Induced Oxidative Stress and pro-Inflammatory Cytokine Production in RAW264.7 Macrophages. Nutr. Res. Pract. 2015, 9, 579–585. [Google Scholar] [CrossRef]
- Chen, L.; Lin, X.; Xiao, J.; Tian, Y.; Zheng, B.; Teng, H. Sonchus oleraceus Linn Protects against LPS-Induced Sepsis and Inhibits Inflammatory Responses in RAW264.7 Cells. J. Ethnopharmacol. 2019, 236, 63–69. [Google Scholar] [CrossRef]
- Lei, M.; Wang, Q.; Liu, B.; Che, Y. Two New Sesquiterpenes from Sonchus oleraceus and Inhibitory Mechanism on Murine Haemangioendothelioma (EOMA) Cell Lines. Nat. Prod. Res. 2021, 36, 2814–2820. [Google Scholar] [CrossRef]
- Nobela, O.; Ndhlala, A.R.; Tugizimana, F.; Njobeh, P.; Raphasha, D.G.; Ncube, B.; Madala, N.E. Tapping into the Realm of Underutilised Green Leafy Vegetables: Using LC-IT-Tof-MS Based Methods to Explore Phytochemical Richness of Sonchus oleraceus (L.) L. S. Afr. J. Bot. 2022, 145, 207–212. [Google Scholar] [CrossRef]
- Huyan, T.; Li, Q.; Wang, Y.L.; Li, J.; Zhang, J.Y.; Liu, Y.X.; Shahid, M.R.; Yang, H.; Li, H.Q. Anti-Tumor Effect of Hot Aqueous Extracts from Sonchus oleraceus (L.) L. and Juniperus sabina L—Two Traditional Medicinal Plants in China. J. Ethnopharmacol. 2016, 185, 289–299. [Google Scholar] [CrossRef]
- El-Desouky, T.A. Evaluation of Effectiveness Aqueous Extract for Some Leaves of Wild Edible Plants in Egypt as Anti-Fungal and Anti-Toxigenic. Heliyon 2021, 7, e06209. [Google Scholar] [CrossRef]
- Vilela, F.C.; De Mesquita Padilha, M.; Alves-Da-Silva, G.; Soncini, R.; Giusti-Paiva, A. Antidepressant-Like Activity of Sonchus oleraceus in Mouse Models of Immobility Tests. J. Med. Food 2010, 13, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Végh, K.; Riethmüller, E.; Hosszú, L.; Darcsi, A.; Müller, J.; Alberti, Á.; Tóth, A.; Béni, S.; Könczöl, Á.; Balogh, G.T.; et al. Three Newly Identified Lipophilic Flavonoids in Tanacetum parthenium Supercritical Fluid Extract Penetrating the Blood-Brain Barrier. J. Pharm. Biomed. Anal. 2018, 149, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Hordiei, K.; Gontova, T.; Trumbeckaite, S.; Yaremenko, M.; Raudone, L. Phenolic Composition and Antioxidant Activity of Tanacetum parthenium Cultivated in Different Regions of Ukraine: Insights into the Flavonoids and Hydroxycinnamic Acids Profile. Plants 2023, 12, 2940. [Google Scholar] [CrossRef]
- Cárdenas, J.; Reyes-Pérez, V.; Hernández-Navarro, M.D.; Dorantes-Barrón, A.M.; Almazán, S.; Estrada-Reyes, R. Anxiolytic- and Antidepressant-like Effects of an Aqueous Extract of Tanacetum parthenium L. Schultz-Bip (Asteraceae) in Mice. J. Ethnopharmacol. 2017, 200, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Benassi-Zanqueta, É.; Marques, C.F.; Valone, L.M.; Pellegrini, B.L.; Bauermeister, A.; Ferreira, I.C.P.; Lopes, N.P.; Nakamura, C.V.; Dias Filho, B.P.; Natali, M.R.M.; et al. Evaluation of Anti-HSV-1 Activity and Toxicity of Hydroethanolic Extract of Tanacetum parthenium (L.) Sch.Bip. (Asteraceae). Phytomedicine 2019, 55, 249–254. [Google Scholar] [CrossRef] [PubMed]
- di Giacomo, V.; Ferrante, C.; Ronci, M.; Cataldi, A.; Di Valerio, V.; Rapino, M.; Recinella, L.; Chiavaroli, A.; Leone, S.; Vladimir-Knežević, S.; et al. Multiple Pharmacological and Toxicological Investigations on Tanacetum parthenium and Salix Alba Extracts: Focus on Potential Application as Anti-Migraine Agents. Food Chem. Toxicol. 2019, 133, 110783. [Google Scholar] [CrossRef] [PubMed]
- Ataollahi, M.; Akrami, E.; Kalani, M.; Zarei, M.; Chijan, M.R.; Sedigh-Rahimabadi, M.; Alipanah, H. Evaluation of Anticoagulant and Inflammatory Effects of Tanacetum parthenium (L.) in a Randomized Controlled Clinical Trial. J. Herb. Med. 2022, 36, 100613. [Google Scholar] [CrossRef]
- Wu, C.; Chen, F.; Wang, X.; Wu, Y.; Dong, M.; He, G.; Galyean, R.D.; He, L.; Huang, G. Identification of Antioxidant Phenolic Compounds in Feverfew (Tanacetum parthenium) by HPLC-ESI-MS/MS and NMR. Phytochem. Anal. 2007, 18, 401–410. [Google Scholar] [CrossRef]
- Rabito, M.F.; Britta, E.A.; Pelegrini, B.L.; Scariot, D.B.; Almeida, M.B.; Nixdorf, S.L.; Nakamura, C.V.; Ferreira, I.C.P. In Vitro and in Vivo Antileishmania Activity of Sesquiterpene Lactone-Rich Dichloromethane Fraction Obtained from Tanacetum parthenium (L.) Schultz-Bip. Exp. Parasitol. 2014, 143, 18–23. [Google Scholar] [CrossRef]
- Liu, J.; Cui, M.; Wang, Y.; Wang, J. Trends in Parthenolide Research over the Past Two Decades: A Bibliometric Analysis. Heliyon 2023, 9, e17843. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, X. Recent Advances on the Structural Modification of Parthenolide and Its Derivatives as Anticancer Agents. Chin. J. Nat. Med. 2022, 20, 814–829. [Google Scholar] [CrossRef] [PubMed]
- Toraman, E.; Budak, B.; Bayram, C.; Sezen, S.; Mokhtare, B.; Hacımüftüoğlu, A. Role of Parthenolide in Paclitaxel-Induced Oxidative Stress Injury and Impaired Reproductive Function in Rat Testicular Tissue. Chem. Biol. Interact. 2024, 387, 110793. [Google Scholar] [CrossRef] [PubMed]
- Coté, H.; Boucher, M.-A.; Pichette, A.; Legault, J. Anti-Inflammatory, Antioxidant, Antibiotic, and Cytotoxic Activities of Tanacetum vulgare L. Essential Oil and Its Constituents. Medicines 2017, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Bączek, K.B.; Kosakowska, O.; Przybył, J.L.; Pióro-Jabrucka, E.; Costa, R.; Mondello, L.; Gniewosz, M.; Synowiec, A.; Węglarz, Z. Antibacterial and Antioxidant Activity of Essential Oils and Extracts from Costmary (Tanacetum balsamita L.) and Tansy (Tanacetum vulgare L.). Ind. Crops Prod. 2017, 102, 154–163. [Google Scholar] [CrossRef]
- Schinella, G.R.; Giner, R.M.; Del Carmen Recio, M.; De Buschiazzo, P.M.; Ríos, J.L.; Máñez, S. Anti-Inflammatory Effects of South American Tanacetum vulgare. J. Pharm. Pharmacol. 2011, 50, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Kavallieratos, N.G.; Skourti, A.; Nika, E.P.; Mártonfi, P.; Spinozzi, E.; Maggi, F. Tanacetum vulgare Essential Oil as Grain Protectant against Adults and Larvae of Four Major Stored-Product Insect Pests. J. Stored Prod. Res. 2021, 94, 101882. [Google Scholar] [CrossRef]
- Ak, G.; Gevrenova, R.; Sinan, K.I.; Zengin, G.; Zheleva, D.; Mahomoodally, M.F.; Senkardes, I.; Brunetti, L.; Leone, S.; Di Simone, S.C.; et al. Tanacetum vulgare L. (Tansy) as an Effective Bioresource with Promising Pharmacological Effects from Natural Arsenal. Food Chem. Toxicol. 2021, 153, 112268. [Google Scholar] [CrossRef]
- Babich, O.; Larina, V.; Krol, O.; Ulrikh, E.; Sukhikh, S.; Gureev, M.A.; Prosekov, A.; Ivanova, S. In Vitro Study of Biological Activity of Tanacetum vulgare Extracts. Pharmaceutics 2023, 15, 616. [Google Scholar] [CrossRef]
- Vilhelmova, N.; Simeonova, L.; Nikolova, N.; Pavlova, E.; Gospodinova, Z.; Antov, G.; Galabov, A.; Nikolova, I. Antiviral, Cytotoxic and Antioxidant Effects of Tanacetum vulgare L. Crude Extract In Vitro. Folia Med. 2020, 62, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, Á.L.; Habtemariam, S.; Juan-Badaturuge, M.; Jackson, C.; Parra, F. In Vitro Anti HSV-1 and HSV-2 Activity of Tanacetum vulgare Extracts and Isolated Compounds: An Approach to Their Mechanisms of Action. Phytother. Res. 2011, 25, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, J.; Kostic, I.; Milanovic, S.; Šešlija Jovanović, D.; Krnjajic, S.; C¨alic, D.; Stankovic, S.; Kostic, M. Repellent Activity of Tanacetum parthenium (L.) and Tanacetum vulgare (L.) Essential oils against Leptinotarsa Decemlineata (Say). Bull. Entomol. Res. 2021, 111, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Kļaviņa, A.; Keidāne, D.; Ganola, K.; Lūsis, I.; Šukele, R.; Bandere, D.; Kovalcuka, L. Anthelmintic Activity of Tanacetum vulgare L. (Leaf and Flower) Extracts against Trichostrongylidae Nematodes in Sheep In Vitro. Animals 2023, 13, 2176. [Google Scholar] [CrossRef] [PubMed]
- Arantes, S.M.; Piçarra, A.; Guerreiro, M.; Salvador, C.; Candeias, F.; Caldeira, A.T.; Martins, M.R. Toxicological and Pharmacological Properties of Essential oils of Calamintha nepeta, Origanum virens and Thymus mastichina of Alentejo (Portugal). Food Chem. Toxicol. 2019, 133, 110747. [Google Scholar] [CrossRef] [PubMed]
- Marongiu, B.; Piras, A.; Porcedda, S.; Falconieri, D.; Maxia, A.; Goncalves, M.J.; Cavaleiro, C.; Salgueiro, L. Chemical Composition and Biological Assays of Essential Oils of Calamintha nepeta (L.) Savi Subsp. Nepeta (Lamiaceae). Nat. Prod. Res. 2010, 24, 1734–1742. [Google Scholar] [CrossRef]
- Božović, M.; Ragno, R.; Tzakou, O. Calamintha nepeta (L.) Savi and Its Main Essential Oil Constituent Pulegone: Biological Activities and Chemistry. Molecules 2017, 22, 290. [Google Scholar] [CrossRef]
- Rodenak-Kladniew, B.; Castro, M.A.; Gambaro, R.C.; Girotti, J.; Cisneros, J.S.; Viña, S.; Padula, G.; Crespo, R.; Castro, G.R.; Gehring, S.; et al. Cytotoxic Screening and Enhanced Anticancer Activity of Lippia alba and Clinopodium nepeta Essential Oils-Loaded Biocompatible Lipid Nanoparticles against Lung and Colon Cancer Cells. Pharmaceutics 2023, 15, 2045. [Google Scholar] [CrossRef]
- Beddiar, H.; Boudiba, S.; Benahmed, M.; Tamfu, A.N.; Ceylan, Ö.; Hanini, K.; Kucukaydin, S.; Elomari, A.; Bensouici, C.; Laouer, H.; et al. Chemical Composition, Anti-Quorum Sensing, Enzyme Inhibitory, and Antioxidant Properties of Phenolic Extracts of Clinopodium nepeta L. Kuntze. Plants 2021, 10, 1955. [Google Scholar] [CrossRef]
- Gonçalves, S.; Moreira, E.; Grosso, C.; Andrade, P.B.; Valentão, P.; Romano, A. Phenolic Profile, Antioxidant Activity and Enzyme Inhibitory Activities of Extracts from Aromatic Plants Used in Mediterranean Diet. J. Food Sci. Technol. 2017, 54, 219. [Google Scholar] [CrossRef]
- Pacifico, S.; Galasso, S.; Piccolella, S.; Kretschmer, N.; Pan, S.P.; Marciano, S.; Bauer, R.; Monaco, P. Seasonal Variation in Phenolic Composition and Antioxidant and Anti-Inflammatory Activities of Calamintha nepeta (L.) Savi. Food Res. Int. 2015, 69, 121–132. [Google Scholar] [CrossRef]
- Benabed, K.H.; Boussoussa, H.; Khacheba, I.; bekhaoua, A.; Douadji, F.Z.; Daïdi, S.; Djaâfour, S.; Yousfi, M. Alpha-Amylase Inhibitory Activity of Extracts from Algerian Calamintha nepeta (L.). Curr. Enzym. Inhib. 2023, 19, 136–141. [Google Scholar] [CrossRef]
- Araniti, F.; Lupini, A.; Mercati, F.; Statti, G.A.; Abenavoli, M.R. Calamintha nepeta L. (Savi) as Source of Phytotoxic Compounds: Bio-Guided Fractionation in Identifying Biological Active Molecules. Acta Physiol. Plant 2013, 35, 1979–1988. [Google Scholar] [CrossRef]
- Xiong, L.; Yang, J.; Jiang, Y.; Lu, B.; Hu, Y.; Zhou, F.; Mao, S.; Shen, C. Phenolic Compounds and Antioxidant Capacities of 10 Common Edible Flowers from China. J. Food Sci. 2014, 79, C517–C525. [Google Scholar] [CrossRef]
- Lopes, C.L.; Pereira, E.; Soković, M.; Carvalho, A.M.; Barata, A.M.; Lopes, V.; Rocha, F.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Phenolic Composition and Bioactivity of Lavandula pedunculata (Mill.) Cav. Samples from Different Geographical Origin. Molecules 2018, 23, 1037. [Google Scholar] [CrossRef] [PubMed]
- Boutahiri, S.; Eto, B.; Bouhrim, M.; Mechchate, H.; Saleh, A.; Al Kamaly, O.; Drioiche, A.; Remok, F.; Samaillie, J.; Neut, C.; et al. Lavandula pedunculata (Mill.) Cav. Aqueous Extract Antibacterial Activity Improved by the Addition of Salvia rosmarinus Spenn., Salvia lavandulifolia Vahl and Origanum compactum Benth. Life 2022, 12, 328. [Google Scholar] [CrossRef] [PubMed]
- Dobros, N.; Zawada, K.D.; Paradowska, K. Phytochemical Profiling, Antioxidant and Anti-Inflammatory Activity of Plants Belonging to the Lavandula Genus. Molecules 2023, 28, 256. [Google Scholar] [CrossRef]
- Ez zoubi, Y.; Bousta, D.; Farah, A. A Phytopharmacological Review of a Mediterranean Plant: Lavandula stoechas L. Clin. Phytosci. 2020, 6, 1–9. [Google Scholar] [CrossRef]
- Mushtaq, A.; Anwar, R.; Gohar, U.F.; Ahmad, M.; Marc, R.A.; Mureşan, C.C.; Irimie, M.; Bobescu, E. Biomolecular Evaluation of Lavandula stoechas L. For Nootropic Activity. Plants 2021, 10, 1259. [Google Scholar] [CrossRef]
- Selmi, S.; Jallouli, M.; Gharbi, N.; Marzouki, L. Hepatoprotective and Renoprotective Effects of Lavender (Lavandula stoechas L.) Essential Oils Against Malathion-Induced Oxidative Stress in Young Male Mice. J. Med. Food 2015, 18, 1103–1111. [Google Scholar] [CrossRef]
- Balahbib, A.; El Omari, N.; Bakrim, S.; Benali, T.; Ullah, R.; Alotaibi, A.; El Mrabti, H.N.; Goh, B.H.; Ardianto, C.; Ming, L.C.; et al. Evaluation of Antioxidant, Antidiabetic, and Dermatoprotective Properties of Lavandula stoechas Essential Oils and Their Main Chemotypes. Heliyon 2023, 1–20. [Google Scholar] [CrossRef]
- Benali, T.; Lemhadri, A.; Harboul, K.; Chtibi, H.; Khabbach, A.; Jadouali, S.M.; Quesada-Romero, L.; Louahlia, S.; Hammani, K.; Ghaleb, A.; et al. Chemical Profiling and Biological Properties of Essential Oils of Lavandula stoechas L. Collected from Three Moroccan Sites: In Vitro and In Silico Investigations. Plants 2023, 12, 1413. [Google Scholar] [CrossRef]
- Aydin, T.; Saglamtas, R.; Gumustas, M.; Genisel, M.; Kazaz, C.; Cakir, A. Lavandula stoechas L. Subsp. Stoechas, a New Herbal Source for Ursolic Acid: Quantitative Analysis, Purification and Bioactivity Studies. Chem. Biodivers. 2023, 20, e202300414. [Google Scholar] [CrossRef]
- El Hachlafi, N.; Benkhaira, N.; Al-Mijalli, S.H.; Mrabti, H.N.; Abdnim, R.; Abdallah, E.M.; Jeddi, M.; Bnouham, M.; Lee, L.H.; Ardianto, C.; et al. Phytochemical Analysis and Evaluation of Antimicrobial, Antioxidant, and Antidiabetic Activities of Essential Oils from Moroccan Medicinal Plants: Mentha suaveolens, Lavandula stoechas, and Ammi visnaga. Biomed. Pharmacother. 2023, 164, 114937. [Google Scholar] [CrossRef]
- Shakeri, A.; Sahebkar, A.; Javadi, B. Melissa officinalis L.—A Review of Its Traditional Uses, Phytochemistry and Pharmacology. J. Ethnopharmacol. 2016, 188, 204–228. [Google Scholar] [CrossRef]
- Petrisor, G.; Motelica, L.; Craciun, L.N.; Oprea, O.C.; Ficai, D.; Ficai, A. Melissa officinalis: Composition, Pharmacological Effects and Derived Release Systems—A Review. Int. J. Mol. Sci. 2022, 23, 3591. [Google Scholar] [CrossRef] [PubMed]
- Alimoradi, Z.; Jafari, E.; Abdi, F.; Griffiths, M.D. Therapeutic Applications of Lemon Balm (Melissa officinalis) for Obstetrics and Gynecological Health Issues: A Systematic Review. J. Herb. Med. 2023, 42, 100751. [Google Scholar] [CrossRef]
- Carvalho, F.; Duarte, A.P.; Ferreira, S. Antimicrobial Activity of Melissa officinalis and Its Potential Use in Food Preservation. Food Biosci. 2021, 44, 101437. [Google Scholar] [CrossRef]
- Zeljković, S.Ć.; Šišková, J.; Komzáková, K.; De Diego, N.; Kaffková, K.; Tarkowski, P. Phenolic Compounds and Biological Activity of Selected Mentha Species. Plants 2021, 10, 550. [Google Scholar] [CrossRef] [PubMed]
- Olsen, H.T.; Stafford, G.I.; van Staden, J.; Christensen, S.B.; Jäger, A.K. Isolation of the MAO-Inhibitor Naringenin from Mentha aquatica L. J. Ethnopharmacol. 2008, 117, 500–502. [Google Scholar] [CrossRef]
- Ferreira, F.M.; Pereira, O.R.; Cardoso, S.M.; Oliveira, P.J.; Moreno, A.J.M. Mentha aquatica L. In Extract Effects on Mitochondrial Bioenergetics. In Proceedings of the 18th European Bioenergetics Conference, Lisbon, Portugal, 12–17 July 2014. [Google Scholar]
- Hanafy, D.M.; Prenzler, P.D.; Burrows, G.E.; Ryan, D.; Nielsen, S.; El Sawi, S.A.; El Alfy, T.S.; Abdelrahman, E.H.; Obied, H.K. Biophenols of Mints: Antioxidant, Acetylcholinesterase, Butyrylcholinesterase and Histone Deacetylase Inhibition Activities Targeting Alzheimer’s Disease Treatment. J. Funct. Foods 2017, 33, 345–362. [Google Scholar] [CrossRef]
- Benabdallah, A.; Boumendjel, M.; Aissi, O.; Rahmoune, C.; Boussaid, M.; Messaoud, C. Chemical Composition, Antioxidant Activity and Acetylcholinesterase Inhibitory of Wild Mentha Species from Northeastern Algeria. S. Afr. J. Bot. 2018, 116, 131–139. [Google Scholar] [CrossRef]
- Pereira, O.R.; Macias, R.I.R.; Domingues, M.R.M.; Marin, J.J.G.; Cardoso, S.M. Hepatoprotection of Mentha aquatica L., Lavandula dentata L. and Leonurus cardiaca L. Antioxidants 2019, 8, 267. [Google Scholar] [CrossRef]
- Jäger, A.K.; Almqvist, J.P.; Vangsøe, S.A.K.; Stafford, G.I.; Adsersen, A.; Van Staden, J. Compounds from Mentha aquatica with Affinity to the GABA-Benzodiazepine Receptor. S. Afr. J. Bot. 2007, 73, 518–521. [Google Scholar] [CrossRef]
- Chang, C.T.; Soo, W.N.; Chen, Y.H.; Shyur, L.F. Essential Oil of Mentha aquatica Var. Kenting Water Mint Suppresses Two-Stage Skin Carcinogenesis Accelerated by BRAF Inhibitor Vemurafenib. Molecules 2019, 24, 2344. [Google Scholar] [CrossRef]
- Thi, N.Q.N.; Duc, L.T.; Minh, L.V.; Tien, L.X. Phytochemicals and Antioxidant Activity of Aqueous and Ethanolic Extracts of Mentha aquatica L. IOP Conf. Ser. Mater. Sci. Eng. 2020, 991, 012027. [Google Scholar] [CrossRef]
- Ferhat, M.; Erol, E.; Beladjila, K.A.; Çetintaş, Y.; Duru, M.E.; Öztürk, M.; Kabouche, A.; Kabouche, Z. Antioxidant, Anticholinesterase and Antibacterial Activities of Stachys guyoniana and Mentha aquatica. Pharm. Biol. 2017, 55, 324–329. [Google Scholar] [CrossRef]
- Dhifi, W.; Litaiem, M.; Jelali, N.; Hamdi, N.; Mnif, W. Identification of A New Chemotye of the Plant Mentha aquatica Grown in Tunisia: Chemical Composition, Antioxidant and Biological Activities of Its Essential Oil. J. Essent. Oil Bear. Plants 2013, 14, 320–328. [Google Scholar] [CrossRef]
- De Oliveira Braga, L.E.; da Silva, G.G.; de Oliveira Sousa, I.M.; de Oliveira, E.C.S.; Jorge, M.P.; Monteiro, K.M.; Sedano, T.C.; Foglio, M.A.; Ruiz, A.L.T.G. Gastrointestinal Effects of Mentha aquatica L. Essential Oil. Inflammopharmacology 2022, 30, 2127–2137. [Google Scholar] [CrossRef]
- de Souza, L.B.; Tinti, S.V.; Sousa, I.M.D.O.; Montanari, I.; da Costa, J.L.; de Carvalho, J.E.; Foglio, M.A.; Ruiz, A.L.T.G. Mentha aquatica L. Aerial Parts: In Vitro Anti-Proliferative Evaluation on Human Tumour and Non-Tumour Cell Lines. Nat. Prod. Res. 2022, 36, 3117–3123. [Google Scholar] [CrossRef] [PubMed]
- Rahimifard, N.; Haji, M.; Hedayati, M.; Bagheri, O. Cytotoxic Effects of Essential Oils and Extracts of Some Mentha Species on Vero, Hela and Hep2 Cell Lines. J. Med. Plants 2010, 9, 88–92. [Google Scholar]
- Anderson, W.; Barrows, M.; Lopez, F.; Rogers, S.; Ortiz-Coffie, A.; Norman, D.; Hodges, J.; McDonald, K.; Barnes, D.; McCall, S.; et al. Investigation of the Anxiolytic Effects of Naringenin, a Component of Mentha aquatica, in the Male Sprague-Dawley Rat. Holist. Nurs. Pract. 2012, 26, 52–57. [Google Scholar] [CrossRef]
- Safaiee, P.; Taghipour, A.; Vahdatkhoram, F.; Movagharnejad, K. Extraction of Phenolic Compounds from Mentha aquatica: The Effects of Sonication Time, Temperature and Drying Method. Chem. Pap. 2019, 73, 3067–3073. [Google Scholar] [CrossRef]
- Venditti, A.; Frezza, C.; Celona, D.; Sciubba, F.; Foddai, S.; Delfini, M.; Serafini, M.; Bianco, A.; Venditti, A.; Frezza, C.; et al. Phytochemical Comparison with Quantitative Analysis between Two Flower Phenotypes of Mentha aquatica L.: Pink-Violet and White. AIMS Mol. Sci. 2017, 4, 288–300. [Google Scholar] [CrossRef]
- Mimica-Dukić, N.; Božin, B.; Soković, M.; Mihajlović, B.; Matavulj, M. Antimicrobial and Antioxidant Activities of Three Mentha Species Essential Oils. Planta Med. 2003, 69, 413–419. [Google Scholar]
- Conforti, F.; Ioele, G.; Statti, G.A.; Marrelli, M.; Ragno, G.; Menichini, F. Antiproliferative Activity against Human Tumor Cell Lines and Toxicity Test on Mediterranean Dietary Plants. Food Chem. Toxicol. 2008, 46, 3325–3332. [Google Scholar] [CrossRef] [PubMed]
- Saqib, S.; Ullah, F.; Naeem, M.; Younas, M.; Ayaz, A.; Ali, S.; Zaman, W. Mentha: Nutritional and Health Attributes to Treat Various Ailments Including Cardiovascular Diseases. Molecules 2022, 27, 6728. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ.; Gören, A.C.; Taslimi, P.; Alwasel, S.H.; Kılıc, O.; Bursal, E. Anticholinergic, Antidiabetic and Antioxidant Activities of Anatolian Pennyroyal (Mentha pulegium)-Analysis of Its Polyphenol Contents by LC-MS/MS. Biocatal. Agric. Biotechnol. 2020, 23, 101441. [Google Scholar] [CrossRef]
- Bouyahya, A.; Et-Touys, A.; Bakri, Y.; Talbaui, A.; Fellah, H.; Abrini, J.; Dakka, N. Chemical Composition of Mentha pulegium and Rosmarinus officinalis Essential Oils and Their Antileishmanial, Antibacterial and Antioxidant Activities. Microb. Pathog. 2017, 111, 41–49. [Google Scholar] [CrossRef]
- Cherrat, L.; Espina, L.; Bakkali, M.; Pagán, R.; Laglaoui, A. Chemical Composition, Antioxidant and Antimicrobial Properties of Mentha pulegium, Lavandula stoechas and Satureja calamintha Scheele Essential Oils and an Evaluation of Their Bactericidal Effect in Combined Processes. Innov. Food Sci. Emerg. Technol. 2014, 22, 221–229. [Google Scholar] [CrossRef]
- Chraibi, M.; Farah, A.; Lebrazi, S.; El Amine, O.; Iraqui Houssaini, M.; Fikri-Benbrahim, K. Antimycobacterial Natural Products from Moroccan Medicinal Plants: Chemical Composition, Bacteriostatic and Bactericidal Profile of Thymus Satureioides and Mentha pulegium Essential Oils. Asian Pac. J. Trop. Biomed. 2016, 6, 836–840. [Google Scholar] [CrossRef]
- Eftekhari, A.; Khusro, A.; Ahmadian, E.; Dizaj, S.M.; Dinparast, L.; Bahadori, M.B.; Hasanzadeh, A.; Cucchiarini, M. Phytochemical and Nutra-Pharmaceutical Attributes of Mentha Spp.: A Comprehensive Review. Arab. J. Chem. 2021, 14, 103106. [Google Scholar] [CrossRef]
- Al-Rajhi, A.M.H.; Qanash, H.; Almuhayawi, M.S.; Al Jaouni, S.K.; Bakri, M.M.; Ganash, M.; Salama, H.M.; Selim, S.; Abdelghany, T.M. Molecular Interaction Studies and Phytochemical Characterization of Mentha pulegium L. Constituents with Multiple Biological Utilities as Antioxidant, Antimicrobial, Anticancer and Anti-Hemolytic Agents. Molecules 2022, 27, 4824. [Google Scholar] [CrossRef]
- Amtaghri, S.; Slaoui, M.; Eddouks, M. Mentha pulegium: A Plant with Several Medicinal Properties. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 302–320. [Google Scholar] [CrossRef]
- Hadi, M.Y.; Hameed, I.H.; Ibraheam, I.A. Mentha pulegium: Medicinal Uses, Anti-Hepatic, Antibacterial, Antioxidant Effect and Analysis of Bioactive Natural Compounds: A Review. Res. J. Pharm. Technol. 2017, 10, 3580–3584. [Google Scholar] [CrossRef]
- Pietrella, D.; Angiolella, L.; Vavala, E.; Rachini, A.; Mondello, F.; Ragno, R.; Bistoni, F.; Vecchiarelli, A. Beneficial Effect of Mentha suaveolens Essential Oil in the Treatment of Vaginal Candidiasis Assessed by Real-Time Monitoring of Infection. BMC Complement. Altern. Med. 2011, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Oumzil, H.; Ghoulami, S.; Rhajaoui, M.; Ilidrissi, A.; Fkih-Tetouani, S.; Faid, M.; Benjouad, A. Antibacterial and Antifungal Activity of Essential Oils of Mentha suaveolens. Phytother. Res. Int. J. Devoted 2002, 16, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Aldogman, B.; Bilel, H.; Moustafa, S.M.N.; Elmassary, K.F.; Ali, H.M.; Alotaibi, F.Q.; Hamza, M.; Abdelgawad, M.A.; El-Ghorab, A.H. Investigation of Chemical Compositions and Biological Activities of Mentha suaveolens L. from Saudi Arabia. Molecules 2022, 27, 2949. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Hemmerich, I.; Zivcak, M.; Rauh, C.; Brestic, M. Comparative Analysis of Bioactive Phenolic Compounds Composition from 26 Medicinal Plants. Saudi J. Biol. Sci. 2018, 25, 631–641. [Google Scholar] [CrossRef]
- Al-Mijalli, S.H.; Assaggaf, H.; Qasem, A.; El-Shemi, A.G.; Abdallah, E.M.; Mrabti, H.N.; Bouyahya, A. Antioxidant, Antidiabetic, and Antibacterial Potentials and Chemical Composition of Salvia officinalis and Mentha suaveolens Grown Wild in Morocco. Adv. Pharmacol. Pharm. Sci. 2022, 2022, 2844880. [Google Scholar] [CrossRef]
- Lee, H.; Yeom, M.; Shin, S.; Jeon, K.; Park, D.; Jung, E. Protective Effects of Aqueous Extract of Mentha suaveolens against Oxidative Stress-Induced Damages in Human Keratinocyte HaCaT Cells. Evid.-Based Complement. Altern. Med. 2019, 2019, 5045491. [Google Scholar] [CrossRef] [PubMed]
- Moreno, L.; Bello, R.; Primo-Yúfera, E.; Esplugues, J. Pharmacological Properties of the Methanol Extract from Mentha suaveolens Ehrh. Phytother. Res. 2002, 16, 10–13. [Google Scholar] [CrossRef]
- El-Akhal, J.; Oliveira, A.P.; Bencheikh, R.; Valentão, P.; Andrade, P.B.; Morato, M. Vasorelaxant Mechanism of Herbal Extracts from Mentha suaveolens, Conyza canadensis, Teucrium polium and Salvia verbenaca in the Aorta of Wistar Rats. Molecules 2022, 27, 8752. [Google Scholar] [CrossRef] [PubMed]
- Oniga, I.; Pus, C.; Silaghi-Dumitrescu, R.; Olah, N.K.; Sevastre, B.; Marica, R.; Marcus, I.; Sevastre-Berghian, A.C.; Benedec, D.; Pop, C.E.; et al. Origanum vulgare ssp. Vulgare: Chemical Composition and Biological Studies. Molecules 2018, 23, 2077. [Google Scholar] [CrossRef] [PubMed]
- Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.M.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A Recent Insight Regarding the Phytochemistry and Bioactivity of Origanum vulgare L. Essential Oil. Int. J. Mol. Sci. 2020, 21, 9653. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Zengin, G.; Oskay, M.; Uysal, S.; Ceylan, R.; Aktumsek, A. Composition, Antioxidant, Antimicrobial and Enzyme Inhibition Activities of Two Origanum vulgare Subspecies (Subsp. Vulgare and Subsp. Hirtum) Essential Oils. Ind. Crops Prod. 2015, 70, 178–184. [Google Scholar] [CrossRef]
- Milos, M.; Mastelic, J.; Jerkovic, I. Chemical Composition and Antioxidant Effect of Glycosidically Bound Volatile Compounds from Oregano (Origanum vulgare L. Ssp. Hirtum). Food Chem. 2000, 71, 79–83. [Google Scholar] [CrossRef]
- Jafari Khorsand, G.; Morshedloo, M.R.; Mumivand, H.; Emami Bistgani, Z.; Maggi, F.; Khademi, A. Natural Diversity in Phenolic Components and Antioxidant Properties of Oregano (Origanum vulgare L.) Accessions, Grown under the Same Conditions. Sci. Rep. 2022, 12, 5813. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, P.; Liu, H.; Sun, X.; Liang, J.; Sun, L.; Chen, Y. Hypoglycemic Activity of Origanum vulgare L. and Its Main Chemical Constituents Identified with HPLC-ESI-QTOF-MS. Food Funct. 2021, 12, 2580–2590. [Google Scholar] [CrossRef]
- Vujicic, M.; Nikolic, I.; Kontogianni, V.G.; Saksida, T.; Charisiadis, P.; Orescanin-Dusic, Z.; Blagojevic, D.; Stosic-Grujicic, S.; Tzakos, A.G.; Stojanovic, I. Methanolic Extract of Origanum vulgare Ameliorates Type 1 Diabetes through Antioxidant, Anti-Inflammatory and Anti-Apoptotic Activity. Br. J. Nutr. 2015, 113, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Parra, C.; Muñoz, P.; Bustos, L.; Parra, F.; Simirgiotis, M.J.; Escobar, H. UHPLC-DAD Characterization of Origanum vulgare L. from Atacama Desert Andean Region and Antioxidant, Antibacterial and Enzyme Inhibition Activities. Molecules 2021, 26, 2100. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Berkay Yılmaz, Y.; Antika, G.; Salehi, B.; Tumer, T.B.; Kulandaisamy Venil, C.; Das, G.; Patra, J.K.; Karazhan, N.; Akram, M.; et al. Phytochemical Constituents, Biological Activities, and Health-Promoting Effects of the Genus Origanum. Phytother. Res. 2021, 35, 95–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-J.; Wang, X.-H.; Dai, Y.-Y.; Ma, M.-H.; Rahman, K.; Nian, H.; Zhang, H. Prunella vulgaris: A Comprehensive Review of Chemical Constituents, Pharmacological Effects and Clinical Applications. Curr. Pharm. Des. 2019, 25, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Mrabti, H.N.; El Menyiy, N.; Charfi, S.; Saber, M.; Bakrim, S.; Alyamani, R.A.; Rauf, A.; Ali, A.M.H.; Abdallah, E.M.; El Omari, N.; et al. Phytochemistry and Biological Properties of Salvia verbenaca L.: A Comprehensive Review. Biomed. Res. Int. 2022, 2022, 3787818. [Google Scholar] [CrossRef]
- Mamache, W.; Amira, S.; Ben Souici, C.; Laouer, H.; Benchikh, F. In Vitro Antioxidant, Anticholinesterases, Anti-α-Amylase, and Anti-α-Glucosidase Effects of Algerian Salvia aegyptiaca and Salvia verbenaca. J. Food Biochem. 2020, 44, e13472. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.; Zadri, F.; Slimani, S. Assessment of Protective Effects of Methanolic Extract of Salvia verbenaca Roots Against Oxidative Damage Induced by Hydrogen Peroxide. Turk. J. Pharm. Sci. 2021, 18, 360. [Google Scholar] [CrossRef]
- Rodrigues, M.; Lopes, A.C.; Vaz, F.; Filipe, M.; Alves, G.; Ribeiro, M.P.; Coutinho, P.; Araujo, A.R.T.S. Thymus mastichina: Composition and Biological Properties with a Focus on Antimicrobial Activity. Pharmaceuticals 2020, 13, 479. [Google Scholar] [CrossRef]
- Rezaei, S.; Ashkar, F.; Koohpeyma, F.; Mahmoodi, M.; Gholamalizadeh, M.; Mazloom, Z.; Doaei, S. Hydroalcoholic Extract of Achillea millefolium Improved Blood Glucose, Liver Enzymes and Lipid Profile Compared to Metformin in Streptozotocin-Induced Diabetic Rats. Lipids Health Dis. 2020, 19, 81. [Google Scholar] [CrossRef]
- Coskun, O.; Kanter, M.; Korkmaz, A.; Oter, S. Quercetin, a Flavonoid Antioxidant, Prevents and Protects Streptozotocin-Induced Oxidative Stress and β-Cell Damage in Rat Pancreas. Pharmacol. Res. 2005, 51, 117–123. [Google Scholar] [CrossRef]
- Mustafa, K.G.; Ganai, B.A.; Akbar, S.; Dar, M.Y.; Masood, A. β-Cell Protective Efficacy, Hypoglycemic and Hypolipidemic Effects of Extracts of Achillea millifolium in Diabetic Rats. Chin. J. Nat. Med. 2012, 10, 185–189. [Google Scholar] [CrossRef]
- Asakawa, A.; Inui, A.; Kaga, T.; Yuzuriha, H.; Nagata, T.; Ueno, N.; Makino, S.; Fujimiya, M.; Niijima, A.; Fujino, M.A.; et al. Ghrelin Is an Appetite-Stimulatory Signal from Stomach with Structural Resemblance to Motilin. Gastroenterology 2001, 120, 337–345. [Google Scholar] [CrossRef]
- Karimi, A.; Niazkar, H.R.; Sefidmooye Azar, P.; Tutunchi, H.; Karimi, M.; Asghariazar, V.; Kooshki, F. Protective Effect of Hydro-Alcoholic Extract of Achillea millefolium on Renal Injury and Biochemical Factors in Streptozotocin-Induced Diabetic Rats. Nutr. Food Sci. 2021, 51, 1068–1083. [Google Scholar] [CrossRef]
- Chávez-Silva, F.; Cerón-Romero, L.; Arias-Durán, L.; Navarrete-Vázquez, G.; Almanza-Pérez, J.; Román-Ramos, R.; Ramírez-Ávila, G.; Perea-Arango, I.; Villalobos-Molina, R.; Estrada-Soto, S. Antidiabetic Effect of Achillea millefollium through Multitarget Interactions: α-Glucosidases Inhibition, Insulin Sensitization and Insulin Secretagogue Activities. J. Ethnopharmacol. 2018, 212, 1–7. [Google Scholar] [CrossRef]
- Zolghadri, Y.; Fazeli, M.; Kooshki, M.; Shomali, T.; Karimaghayee, N.; Dehghani, M. Achillea millefolium L. Hydro- Alcoholic Extract Protects Pancreatic Cells by Down Regulating IL- 1β and INOS Gene Expression in Diabetic Rats. Int. J. Mol. Cell Med. 2014, 3, 255. [Google Scholar]
- Noda, K.; Kato, E.; Kawabata, J. Intestinal α-Glucosidase Inhibitors in Achillea millefolium. Nat. Prod. Commun. 2017, 12, 1259–1261. [Google Scholar] [CrossRef]
- Nieto-Trujillo, A.; Cruz-Sosa, F.; Luria-Pérez, R.; Gutiérrez-Rebolledo, G.A.; Román-Guerrero, A.; Burrola-Aguilar, C.; Zepeda-Gómez, C.; Estrada-Zúñiga, M.E. Arnica montana Cell Culture Establishment, and Assessment of Its Cytotoxic, Antibacterial, α-Amylase Inhibitor, and Antioxidant In Vitro Bioactivities. Plants 2021, 10, 2300. [Google Scholar] [CrossRef]
- Haselgrübler, R.; Stadlbauer, V.; Stübl, F.; Schwarzinger, B.; Rudzionyte, I.; Himmelsbach, M.; Iken, M.; Weghuber, J. Insulin Mimetic Properties of Extracts Prepared from Bellis perennis. Molecules 2018, 23, 2605. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, P.; Wojdyło, A. Anti-Hyperglycemic and Anticholinergic Effects of Natural Antioxidant Contents in Edible Flowers. Antioxidants 2019, 8, 308. [Google Scholar] [CrossRef]
- Icoz, U.G.; Orhan, N.; Altun, L.; Aslan, M. In Vitro and in Vivo Antioxidant and Antidiabetic Activity Studies on Standardized Extracts of Two Bidens Species. J. Food Biochem. 2017, 41, e12429. [Google Scholar] [CrossRef]
- Azay-Milhau, J.; Ferrare, K.; Leroy, J.; Aubaterre, J.; Tournier, M.; Lajoix, A.D.; Tousch, D. Antihyperglycemic Effect of a Natural Chicoric Acid Extract of Chicory (Cichorium intybus L.): A Comparative in Vitro Study with the Effects of Caffeic and Ferulic Acids. J. Ethnopharmacol. 2013, 150, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Tousch, D.; Lajoix, A.D.; Hosy, E.; Azay-Milhau, J.; Ferrare, K.; Jahannault, C.; Cros, G.; Petit, P. Chicoric Acid, a New Compound Able to Enhance Insulin Release and Glucose Uptake. Biochem. Biophys. Res. Commun. 2008, 377, 131–135. [Google Scholar] [CrossRef]
- Jackson, K.M.P.; Rathinasabapathy, T.; Esposito, D.; Komarnytsky, S. Structural Constraints and Importance of Caffeic Acid Moiety for Anti-Hyperglycemic Effects of Caffeoylquinic Acids from Chicory. Mol. Nutr. Food Res. 2017, 61, 1601118. [Google Scholar] [CrossRef]
- Ebrahiminia, M.; Esmaeili, F.; Shabani, L. In Vitro Differentiation Induction of Embryonal Carcinoma Stem Cells into Insulin-Producing Cells by Cichorium intybus L. Leaf Extract. J. Ethnopharmacol. 2020, 246, 112214. [Google Scholar] [CrossRef]
- Dalar, A.; Konczak, I. Cichorium intybus from Eastern Anatolia: Phenolic Composition, Antioxidant and Enzyme Inhibitory Activities. Ind. Crops Prod. 2014, 60, 79–85. [Google Scholar] [CrossRef]
- Ferrare, K.; Bidel, L.P.R.; Awwad, A.; Poucheret, P.; Cazals, G.; Lazennec, F.; Azay-Milhau, J.; Tournier, M.; Lajoix, A.D.; Tousch, D. Increase in Insulin Sensitivity by the Association of Chicoric Acid and Chlorogenic Acid Contained in a Natural Chicoric Acid Extract (NCRAE) of Chicory (Cichorium intybus L.) for an Antidiabetic Effect. J. Ethnopharmacol. 2018, 215, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, V.S.; Anand, S.; Sangeetha, K.N.; Sujatha, S.; Arun, B.; Lakshmi, B.S. Tannins Present in Cichorium intybus Enhance Glucose Uptake and Inhibit Adipogenesis in 3T3-L1 Adipocytes through PTP1B Inhibition. Chem. Biol. Interact. 2008, 174, 69–78. [Google Scholar] [CrossRef]
- Aydin, T.; Saglamtas, R.; Dogan, B.; Kostekci, E.; Durmus, R.; Cakir, A. A New Specific Method for Isolation of Tomentosin with a High Yield from Inula viscosa (L.) and Determination of Its Bioactivities. Phytochem. Anal. 2022, 33, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Lee, J.-J.; Kim, I.-S.; Kim, Y.; Park, J.-S.; Myung, C.-S. 7-O-Methylaromadendrin Stimulates Glucose Uptake and Improves Insulin Resistance In Vitro. Article Biol. Pharm. Bull. 2010, 33, 1494–1499. [Google Scholar] [CrossRef]
- Ranilla, L.G.; Kwon, Y.I.; Apostolidis, E.; Shetty, K. Phenolic Compounds, Antioxidant Activity and in Vitro Inhibitory Potential against Key Enzymes Relevant for Hyperglycemia and Hypertension of Commonly Used Medicinal Plants, Herbs and Spices in Latin America. Bioresour. Technol. 2010, 101, 4676–4689. [Google Scholar] [CrossRef]
- Ferheen, S.; Ur-Rehman, A.; Afza, N.; Malik, A.; Iqbal, L.; Azam Rasool, M.; Irfan Ali, M.; Bakhsh Tareen, R. Galinsosides A and B, Bioactive Flavanone Glucosides from Galinsoga parviflora. J. Enzyme Inhib. Med. Chem. 2009, 24, 1128–1132. [Google Scholar] [CrossRef]
- Kim, H.-M.; Lee, J.-M.; Choi, K.; Ku, J.-J.; Park, K.-W. Inhibition of Aldose Reductase from Rat Lenses by Methanol Extracts from Korean Folk Plants. Nat. Prod. Sci. 2010, 16, 285–290. [Google Scholar]
- Paun, G.; Neagu, E.; Alecu, A.; Albu, C.; Seciu-Grama, A.-M.; Radu, G.L. Evaluation of the Antioxidant, and Antidiabetic Properties of Flavonoids and Isoflavonoids-Rich Extracts of Medicago sativa and Solidago virgaurea. Complement. Altern. Med. Prepr. 2023, 2023, 101307. [Google Scholar] [CrossRef]
- Chen, L.; Lin, X.; Fan, X.; Qian, Y.; Lv, Q.; Teng, H. Sonchus oleraceus Linn Extract Enhanced Glucose Homeostasis through the AMPK/Akt/ GSK-3β Signaling Pathway in Diabetic Liver and HepG2 Cell Culture. Food Chem. Toxicol. 2020, 136, 111072. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kim, H.Y.; Quispe, Y.N.G.; Wang, Z.; Zuo, G.; Lim, S.S. Aldose Reductase, Protein Glycation Inhibitory and Antioxidant of Peruvian Medicinal Plants: The Case of Tanacetum parthenium L. and Its Constituents. Molecules 2019, 24, 2010. [Google Scholar] [CrossRef]
- Zengin, G.; Cvetanović, A.; Gašić, U.; Stupar, A.; Bulut, G.; Şenkardes, I.; Dogan, A.; Ibrahime Sinan, K.; Uysal, S.; Aumeeruddy-Elalfi, Z.; et al. Modern and Traditional Extraction Techniques Affect Chemical Composition and Bioactivity of Tanacetum parthenium (L.) Sch.Bip. Ind. Crops Prod. 2020, 146, 112202. [Google Scholar] [CrossRef]
- Boutahiri, S.; Bouhrim, M.; Abidi, C.; Mechchate, H.; Alqahtani, A.S.; Noman, O.M.; Elombo, F.K.; Gressier, B.; Sahpaz, S.; Bnouham, M.; et al. Antihyperglycemic Effect of Lavandula Pedunculata: In Vivo, In Vitro and Ex Vivo Approaches. Pharmaceutics 2021, 13, 2019. [Google Scholar] [CrossRef] [PubMed]
- Elrherabi, A.; Bouhrim, M.; Abdnim, R.; Berraaouan, A.; Ziyyat, A.; Mekhfi, H.; Legssyer, A.; Bnouham, M. Antihyperglycemic Potential of the Lavandula stoechas Aqueous Extract via Inhibition of Digestive Enzymes and Reduction of Intestinal Glucose Absorption. J. Ayurveda Integr. Med. 2023, 14, 100795. [Google Scholar] [CrossRef]
- Kulabas, S.S.; Ipek, H.; Tufekci, A.R.; Arslan, S.; Demirtas, I.; Ekren, R.; Sezerman, U.; Tumer, T.B. Ameliorative Potential of Lavandula stoechas in Metabolic Syndrome via Multitarget Interactions. J. Ethnopharmacol. 2018, 223, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Yui, S.; Fujiwara, S.; Harada, K.; Motoike-Hamura, M.; Sakai, M.; Matsubara, S.; Miyazaki, K. Beneficial Effects of Lemon Balm Leaf Extract on In Vitro Glycation of Proteins, Arterial Stiffness, and Skin Elasticity in Healthy Adults. J. Nutr. Sci. Vitaminol. 2017, 63, 59–68. [Google Scholar] [CrossRef]
- Kwon, Y.-I.I.; Vattem, D.A.; Shetty, K. Evaluation of Clonal Herbs of Lamiaceae Species for Management of Diabetes and Hypertension. Asia Pac. J. Clin. Nutr. 2006, 15, 107–118. [Google Scholar]
- Mccue, P.P.; Shetty, K. Inhibitory Effects of Rosmarinic Acid Extracts on Porcine Pancreatic Amylase in Vitro. Asia Pacific J. Clin. Nutr. 2004, 13, 101–106. [Google Scholar]
- Schreck, K.; Melzig, M.F. Traditionally Used Plants in the Treatment of Diabetes Mellitus: Screening for Uptake Inhibition of Glucose and Fructose in the Caco2-Cell Model. Front. Pharmacol. 2021, 12, 692566. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.F.; Hsieh, C.T.; Hsieh, T.J.; Chang, F.R.; Wang, C.K. In Vitro Anti-Diabetic Effect and Chemical Component Analysis of 29 Essential Oils Products. J. Food Drug Anal. 2015, 23, 124. [Google Scholar] [CrossRef]
- Marrelli, M.; Loizzo, M.R.; Nicoletti, M.; Menichini, F.; Conforti, F. In Vitro Investigation of the Potential Health Benefits of Wild Mediterranean Dietary Plants as Anti-Obesity Agents with α-Amylase and Pancreatic Lipase Inhibitory Activities. J. Sci. Food Agric. 2014, 94, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, T.; Melzig, M.F. Medicinal Plants Traditionally Used for Treatment of Obesity and Diabetes Mellitus—Screening for Pancreatic Lipase and α-Amylase Inhibition. Phytother. Res. 2016, 30, 260–266. [Google Scholar] [CrossRef]
- Abbou, F.; Azzi, R.; Ouffai, K.; El Haci, I.A.; Belyagoubi-Benhammou, N.; Bensouici, C.; Benamar, H. Phenolic Profile, Antioxidant and Enzyme Inhibitory Properties of Phenolic-Rich Fractions from the Aerial Parts of Mentha pulegium L. South Afr. J. Bot. 2022, 146, 196–204. [Google Scholar] [CrossRef]
- Mccue, P.; Vattem, D.; Shetty, K. Inhibitory Effect of Clonal Oregano Extracts against Porcine Pancreatic Amylase in Vitro. Asia Pac. J. Clin. Nutr. 2004, 13, 401–408. [Google Scholar]
- Koukoulitsa, C.; Zika, C.; Geromichalos, G.D.; Demopoulos, V.J.; Skaltsa, H. Evaluation of Aldose Reductase Inhibition and Docking Studies of Some Secondary Metabolites, Isolated from Origanum vulgare L. Ssp. Hirtum. Bioorg. Med. Chem. 2006, 14, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.B.; Minet, A.; Svenstrup, H.; Grevsen, K.; Zhang, H.; Schrader, E.; Rimbach, G.; Wein, S.; Wolffram, S.; Kristiansen, K.; et al. Identification of Plant Extracts with Potential Antidiabetic Properties: Effect on Human Peroxisome Proliferator-Activated Receptor (PPAR), Adipocyte Differentiation and Insulin-Stimulated Glucose Uptake. Phytother. Res. 2009, 23, 1316–1325. [Google Scholar] [CrossRef]
- Bower, A.M.; Real Hernandez, L.M.; Berhow, M.A.; De Mejia, E.G. Bioactive Compounds from Culinary Herbs Inhibit a Molecular Target for Type 2 Diabetes Management, Dipeptidyl Peptidase IV. J. Agric. Food Chem. 2014, 62, 6147–6158. [Google Scholar] [CrossRef]
- Valentová, K.; Nhu, T.T.; Moncion, A.; De Waziers, I.; Ulrichová, J. Induction of Glucokinase MRNA by Dietary Phenolic Compounds in Rat Liver Cells in Vitro. J. Agric. Food Chem. 2007, 55, 7726–7731. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Kim, J.K.; Jang, J.M.; Kwon, S.O.; Cui, C.B.; Lim, S.S. The Inhibitory Effect of Prunella vulgaris L. on Aldose Reductase and Protein Glycation. J. Biomed. Biotechnol. 2012, 2012, 928159. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Gao, M.; Ha, T.; Kelley, J.; Young, A.; Breuel, K. Prunella vulgaris Aqueous Extract Attenuates IL-1β-Induced Apoptosis and NF-κB Activation in INS-1 Cells. Exp. Ther. Med. 2012, 3, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Raafat, K.; Wurglics, M.; Schubert-Zsilavecz, M. Prunella vulgaris L. Active Components and Their Hypoglycemic and Antinociceptive Effects in Alloxan-Induced Diabetic Mice. Biomed. Pharmacother. 2016, 84, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, F.; Cao, Y.; Cai, G.; Wei, Q.; Shi, S.; Guo, Y. Screening of Potent α-Glucosidase Inhibitory and Antioxidant Polyphenols in Prunella vulgaris L. by Bioreaction–HPLC–Quadrupole-Time-of-Flight-MS/MS and in Silico Analysis. J. Sep. Sci. 2022, 45, 3393–3403. [Google Scholar] [CrossRef]
- Aazza, S.; El-Guendouz, S.; Graça Miguel, M.; Antunes, M.D.; Faleiro, M.L.; Correia, A.I.; Figueiredo, A.C. Antioxidant, Anti-Inflammatory and Anti-Hyperglycaemic Activities of Essential Oils from Thymbra capitata, Thymus albicans, Thymus caespititius, Thymus carnosus, Thymus lotocephalus and Thymus mastichina from Portugal. Nat. Prod. Commun. 2016, 11, 1029–1038. [Google Scholar] [CrossRef]
- Eddouks, M.; Lemhadri, A.; Zeggwagh, N.A.; Michel, J.B. Potent Hypoglycaemic Activity of the Aqueous Extract of Chamaemelum Nobile in Normal and Streptozotocin-Induced Diabetic Rats. Diabetes Res. Clin. Pract. 2005, 67, 189–195. [Google Scholar] [CrossRef]
- König, G.M.; Wright, A.D.; Keller, W.J.; Judd, R.L.; Bates, S.; Day, C. Hypoglycaemic Activity of an HMG-Containing Flavonoid Glucoside, Chamaemeloside, from Chamaemelum Nobile. Planta Med. 1998, 64, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Witherup, K.M.; McLaughlin, J.L.; Judd, R.L.; Ziegler, M.H.; Medon, P.J.; Keller, W.J. Identification of 3-Hydroxy-3-Methylglutaric Acid (HMG) as a Hypoglycemic Principle of Spanish Moss (Tillandsia usneoides). J. Nat. Prod. 1995, 58, 1285–1290. [Google Scholar] [CrossRef]
- Yonei, Y.; Miyazaki, R.; Takahashi, Y.; Takahashi, H.; Nomoto, K.; Yagi, M.; Kawai, H.; Kubo, M.; Matsuura, N. Anti-Glycation Effect of Mixed Herbal Extract in Individuals with Pre-Diabetes Mellitus A Double-Blind, Placebo-Controlled, Parallel Group Study. Anti-Aging Med. 2010, 7, 26–35. [Google Scholar] [CrossRef]
- Pushparaj, P.N.; Low, H.K.; Manikandan, J.; Tan, B.K.H.; Tan, C.H. Anti-Diabetic Effects of Cichorium intybus in Streptozotocin-Induced Diabetic Rats. J. Ethnopharmacol. 2007, 111, 430–434. [Google Scholar] [CrossRef]
- Ghamarian, A.; Abdollahi, M.; Su, X.; Amiri, A.; Ahadi, A.; Nowrouzi, A. Effect of Chicory Seed Extract on Glucose Tolerance Test (GTT) and Metabolic Profile in Early and Late Stage Diabetic Rats. DARU J. Pharm. Sci. 2012, 20, 56. [Google Scholar] [CrossRef]
- Jurgoński, A.; Juśkiewicz, J.; Zduńczyk, Z.; Król, B. Caffeoylquinic Acid-Rich Extract from Chicory Seeds Improves Glycemia, Atherogenic Index, and Antioxidant Status in Rats. Nutrition 2012, 28, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Petrović, A.; Madić, V.; Stojanović, G.; Zlatanović, I.; Zlatković, B.; Vasiljević, P.; Đorđević, L. Antidiabetic Effects of Polyherbal Mixture Made of Centaurium erythraea, Cichorium intybus and Potentilla erecta. J. Ethnopharmacol. 2024, 319, 117032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Sirovina, D.; Odeh, D.; Gajski, G.; Balta, V.; Šver, L.; Jembrek, M.J. Efficacy of Caffeic Acid on Diabetes and Its Complications in the Mouse. Molecules 2021, 26, 3262. [Google Scholar] [CrossRef]
- Schaalan, M.; El-Abhar, H.S.; Barakat, M.; El-Denshary, E.S. Westernized-like-Diet-Fed Rats: Effect on Glucose Homeostasis, Lipid Profile, and Adipocyte Hormones and Their Modulation by Rosiglitazone and Glimepiride. J. Diabetes Complicat. 2009, 23, 199–208. [Google Scholar] [CrossRef]
- Saleh Aldayel, T.; Alshammari, G.M.; Mohammed Omar, U.; Grace, M.H.; Ann Lila, M.; Yahya, M.A. Hypoglycaemic, Insulin Releasing, and Hepatoprotective Effect of the Aqueous Extract of Aloe perryi Baker Resin (Socotran Aloe) in Streptozotocin-Induced Diabetic Rats. J. Taibah Univ. Sci. 2020, 14, 1671–1685. [Google Scholar] [CrossRef]
- Peungvicha, P.; Temsiririrkkul, R.; Prasain, J.K.; Tezuka, Y.; Kadota, S.; Thirawarapan, S.S.; Watanabe, H. 4-Hydroxybenzoic Acid: A Hypoglycemic Constituent of Aqueous Extract of Pandanus odorus Root. J. Ethnopharmacol. 1998, 62, 79–84. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Cheung, F.; Hong, M.; Feng, Y. The Potential and Action Mechanism of Polyphenols in the Treatment of Liver Diseases. Oxidative Med. Cell. Longev. 2018, 2018, 8394818. [Google Scholar] [CrossRef]
- Ganesan, D.; Albert, A.; Paul, E.; Ananthapadmanabhan, K.; Andiappan, R.; Sadasivam, S.G. Rutin Ameliorates Metabolic Acidosis and Fibrosis in Alloxan Induced Diabetic Nephropathy and Cardiomyopathy in Experimental Rats. Mol. Cell. Biochem. 2020, 471, 41–50. [Google Scholar] [CrossRef]
- Quine, S.; Raghu, P.S. Effects of (-)-Epicatechin, a Flavonoid on Lipid Peroxidation and Antioxidants in Streptozotocin-Induced Diabetic Liver, Kidney and Heart. Pharmacol. Rep. 2005, 57, 610–615. [Google Scholar]
- Zhang, L.; He, S.; Yang, F.; Yu, H.; Xie, W.; Dai, Q.; Zhang, D.; Liu, X.; Zhou, S.; Zhang, K. Hyperoside Ameliorates Glomerulosclerosis in Diabetic Nephropathy by Downregulating MiR-21. Can. J. Physiol. Pharmacol. 2016, 94, 1249–1256. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, S.; Sun, X.; Lou, Y.; Bao, J.; Yu, J. Hyperoside Ameliorates Diabetic Nephropathy Induced by STZ via Targeting the MiR-499–5p/APC Axis. J. Pharmacol. Sci. 2021, 146, 10–20. [Google Scholar] [CrossRef]
- Zhang, R.; Yao, Y.; Wang, Y.; Ren, G. Antidiabetic Activity of Isoquercetin in Diabetic KK -A y Mice. Nutr. Metab. 2011, 8, 85. [Google Scholar] [CrossRef]
- Jayachandran, M.; Zhang, T.; Ganesan, K.; Xu, B.; Chung, S.S.M. Isoquercetin Ameliorates Hyperglycemia and Regulates Key Enzymes of Glucose Metabolism via Insulin Signaling Pathway in Streptozotocin-Induced Diabetic Rats. Eur. J. Pharmacol. 2018, 829, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Ajala-Lawal, R.A.; Adeyiga, A.B. Caffeic Acid Abrogates 1,3-Dichloro-2-Propanol-Induced Hepatotoxicity by Upregulating Nuclear Erythroid-Related Factor 2 and Downregulating Nuclear Factor-Kappa B. Hum. Exp. Toxicol. 2019, 38, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Ayyoub, S.; Al-Trad, B.; Aljabali, A.A.A.; Alshaer, W.; Al Zoubi, M.; Omari, S.; Fayyad, D.; Tambuwala, M.M. Biosynthesis of Gold Nanoparticles Using Leaf Extract of Dittrichia viscosa and In Vivo Assessment of Its Anti-Diabetic Efficacy. Drug Deliv. Transl. Res. 2022, 12, 2993. [Google Scholar] [CrossRef] [PubMed]
- Zeggwagh, N.A.; Ouahidi, M.L.; Lemhadri, A.; Eddouks, M. Study of Hypoglycaemic and Hypolipidemic Effects of Inula viscosa L. Aqueous Extract in Normal and Diabetic Rats. J. Ethnopharmacol. 2006, 108, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, I.; El-Aziz, E.A.; Hafez, S.; El-Shazly, A. Chemical Constituents and Biological Activities of Galinsoga parviflora Cav. (Asteraceae) from Egypt. Z. Fur Naturforschung C 2013, 68, 285–292. [Google Scholar] [CrossRef]
- Salih, B.A. Effect of Lactuca serriola on β-Cell Dysfunction and Glucose Tolerance Induced by High Sucrose Fed in Albino Rats. J. Phys. Conf. Ser. 2019, 1294, 062092. [Google Scholar] [CrossRef]
- Sharef, A.Y.; Hamdi, B.A.; Alrawi, R.A.; Ahmad, H.O. Onopordum acanthium L. Extract Attenuates Pancreatic β-Cells and Cardiac Inflammation in Streptozocin-Induced Diabetic Rats. PLoS ONE 2023, 18, e0280464. [Google Scholar] [CrossRef]
- Sanad, F.A.A.; Ahmed, S.F.; El-Tantawy, W.H. Antidiabetic and Hypolipidemic Potentials of Solidago virgaurea Extract in Alloxan-Induced Diabetes Type 1. Arch. Physiol. Biochem. 2022, 128, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A.; Khan, M.R.; Shah, N.A.; Sahreen, S.; Siddiq, P. Modulation of Carbon Tetrachloride-Induced Nephrotoxicity in Rats by n-Hexane Extract of Sonchus asper. Toxicol. Ind. Health 2015, 31, 955–959. [Google Scholar] [CrossRef]
- Teugwa, C.M.; Mejiato, P.C.; Zofou, D.; Tchinda, B.T.; Boyom, F.F. Antioxidant and Antidiabetic Profiles of Two African Medicinal Plants: Picralima Nitida (Apocynaceae) and Sonchus oleraceus (Asteraceae). BMC Complement. Altern. Med. 2013, 13, 175. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fan, X.; Lin, X.; Qian, L.; Zengin, G.; Delmas, D.; Paoli, P.; Teng, H.; Xiao, J. Phenolic Extract from Sonchus oleraceus L. Protects Diabetes-Related Liver Injury in Rats through TLR4/NF-κB Signaling Pathway. eFood 2020, 1, 77–84. [Google Scholar] [CrossRef]
- Salim, N.S.; Abdel-Alim, M.; Said, H.E.M.; Foda, M.F. Phenolic Profiles, Antihyperglycemic, Anti-Diabetic, and Antioxidant Properties of Egyptian Sonchus oleraceus Leaves Extract: An In Vivo Study. Molecules 2023, 28, 6389. [Google Scholar] [CrossRef]
- Sebai, H.; Selmi, S.; Rtibi, K.; Souli, A.; Gharbi, N.; Sakly, M. Lavender (Lavandula stoechas L.) Essential Oils Attenuate Hyperglycemia and Protect against Oxidative Stress in Alloxan-Induced Diabetic Rats. Lipids Health Dis. 2013, 12, 189. [Google Scholar] [CrossRef]
- Sebai, H.; Selmi, S.; Rtibi, K.; Gharbi, N.; Sakly, M. Protective Effect of Lavandula stoechas and Rosmarinus officinalis Essential Oils Against Reproductive Damage and Oxidative Stress in Alloxan-Induced Diabetic Rats. J. Med. Food 2015, 18, 241–249. [Google Scholar] [CrossRef]
- Mustafa, S.B.; Akram, M.; Muhammad Asif, H.; Qayyum, I.; Hashmi, A.M.; Munir, N.; Khan, F.S.; Riaz, M.; Ahmad, S. Antihyperglycemic Activity of Hydroalcoholic Extracts of Selective Medicinal Plants Curcuma longa, Lavandula stoechas, Aegle marmelos, and Glycyrrhiza glabra and Their Polyherbal Preparation in Alloxan-Induced Diabetic Mice. Dose-Response 2019, 17, 1559325819852503. [Google Scholar] [CrossRef]
- Demir, D.; Toygar, I.; Soylu, E.; Aksu, A.T.; Türeyen, A.; Yıldırım, I.; Çetinkalp, Ş. The Effect of Lavandula stoechas on Wound Healing in an Experimental Diabetes Model. Cureus 2023, 15, e45001. [Google Scholar] [CrossRef]
- Chung, M.J.; Cho, S.Y.; Bhuiyan, M.J.H.; Kim, K.H.; Lee, S.J. Anti-Diabetic Effects of Lemon Balm (Melissa officinalis) Essential Oil on Glucose- and Lipid-Regulating Enzymes in Type 2 Diabetic Mice. Br. J. Nutr. 2010, 104, 180–188. [Google Scholar] [CrossRef]
- Weidner, C.; Wowro, S.J.; Freiwald, A.; Kodelja, V.; Abdel-Aziz, H.; Kelber, O.; Sauer, S. Lemon Balm Extract Causes Potent Antihyperglycemic and Antihyperlipidemic Effects in Insulin-Resistant Obese Mice. Mol. Nutr. Food Res. 2014, 58, 903–907. [Google Scholar] [CrossRef]
- Hasanein, P.; Riahi, H. Antinociceptive and Antihyperglycemic Effects of Melissa officinalis Essential Oil in an Experimental Model of Diabetes. Med. Princ. Pract. 2015, 24, 47–52. [Google Scholar] [CrossRef]
- Moshtaghian, J.; Khodsooz, S.; Moshtaghian, J.; Eivani, M. Antihyperglycemic and Antihyperlipidemic Effects of Hydroalcoholic Extract of Melissa officinalis (Lemon Balm) in Alloxan-Induced Diabetic Rats. Physiol. Pharmacol. 2016, 20, 24–30. [Google Scholar]
- Lee, D.; Shin, Y.; Roh, J.S.; Ahn, J.; Jeoong, S.; Shin, S.S.; Yoon, M. Lemon Balm Extract ALS-L1023 Regulates Obesity and Improves Insulin Sensitivity via Activation of Hepatic PPARα in High-Fat Diet-Fed Obese C57BL/6J Mice. Int. J. Mol. Sci. 2020, 21, 4256. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Lee, D.; Ahn, J.; Lee, M.; Shin, S.S.; Yoon, M. The Herbal Extract ALS-L1023 from Melissa officinalis Reduces Weight Gain, Elevated Glucose Levels and β-Cell Loss in Otsuka Long-Evans Tokushima Fatty Rats. J. Ethnopharmacol. 2021, 264, 113360. [Google Scholar] [CrossRef] [PubMed]
- Yellanur Konda, P.; Egi, J.Y.; Dasari, S.; Katepogu, R.; Jaiswal, K.K.; Nagarajan, P. Ameliorative Effects of Mentha aquatica on Diabetic and Nephroprotective Potential Activities in STZ-Induced Renal Injury. Comp. Clin. Pathol. 2020, 29, 189–199. [Google Scholar] [CrossRef]
- Farid, O.; Zeggwagh, N.A.; Ouadi, F.E.L.; Eddouks, M. Mentha pulegium Aqueous Extract Exhibits Antidiabetic and Hepatoprotective Effects in Streptozotocin-Induced Diabetic Rats. Endocr. Metab. Immune Disord. Drug Targets 2018, 19, 292–301. [Google Scholar] [CrossRef]
- Farid, O.; Eddouks, M. Evaluation of the Anti-Hypercholesterolemic and Antioxidant Activity of Mentha pulegium (L.) Aqueous Extract in Normal and Streptozotocin-Induced Diabetic Rats. Nat. Prod. J. 2019, 10, 236–243. [Google Scholar] [CrossRef]
- Ajebli, M.; Eddouks, M. Pharmacological and Phytochemical Study of Mentha suaveolens Ehrh in Normal and Streptozotocin-Induced Diabetic Rats. Nat. Prod. J. 2018, 8, 213–227. [Google Scholar] [CrossRef]
- Lemhadri, A.; Zeggwagh, N.A.; Maghrani, M.; Jouad, H.; Eddouks, M. Anti-Hyperglycaemic Activity of the Aqueous Extract of Origanum vulgare Growing Wild in Tafilalet Region. J. Ethnopharmacol. 2004, 92, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Nassier, O.A. The Antihyperglycaemic Effect of the Aqueous Extract of Origanium vulgare Leaves in Streptozotocin-Induced Diabetic Rats. Jordan J. Biol. Sci. 2013, 6, 31–38. [Google Scholar] [CrossRef]
- Vujicic, M.; Nikolic, I.; Kontogianni, V.G.; Saksida, T.; Charisiadis, P.; Vasic, B.; Stosic-Grujicic, S.; Gerothanassis, I.P.; Tzakos, A.G.; Stojanovic, I. Ethyl Acetate Extract of Origanum vulgare L. Ssp. Hirtum Prevents Streptozotocin-Induced Diabetes in C57BL/6 Mice. J. Food Sci. 2016, 81, H1846–H1853. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, R.; Ashraf, E.A.; Essam, M.A. Chamomile and Oregano Extracts Synergistically Exhibit Antihyperglycemic, Antihyperlipidemic, and Renal Protective Effects in Alloxan-Induced Diabetic Rats. Can. J. Physiol. Pharmacol. 2017, 95, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Martha erez Guti errez, R.P.; Fernando Martínez Jer onimo, F.; Guadalupe Contreras Soto, J.; Mu, A.; Ramírez, N.; Fernanda Estrella Mendoza, M. Optimization of Ultrasonic-Assisted Extraction of Polyphenols from the Polyherbal Formulation of Cinnamomum verum, Origanum majorana, and Origanum vulgare and Their Anti-Diabetic Capacity in Zebrafish (Danio rerio). Heliyon 2017, 8, e08682. [Google Scholar] [CrossRef]
- Silva, M.L.A.E.; Lucarini, R.; dos Santos, F.F.; Martins, C.H.G.; Pauletti, P.M.; Januario, A.H.; Santos, M.F.C.; Cunha, W.R. Hypoglycemic Effect of Rosmarinic Acid-Rich Infusion (RosCE) from Origanum vulgare in Alloxan-Induced Diabetic Rats. Nat. Prod. Res. 2022, 36, 4525–4531. [Google Scholar] [CrossRef]
- Hwang, S.M.; Kim, J.S.; Lee, Y.J.; Yoon, J.J.; Lee, S.M.; Kang, D.G.; Lee, H.S. Anti-Diabetic Atherosclerosis Effect of Prunella vulgaris in Db/Db Mice with Type 2 Diabetes. Am. J. Chin. Med. 2012, 40, 937–951. [Google Scholar] [CrossRef]
- Zhou, Q.X.; Liu, F.; Zhang, J.S.; Lu, J.G.; Gu, Z.L.; Gu, G.X. Effects of Triterpenic Acid from Prunella vulgaris L. On Glycemia and Pancreas in Rat Model of Streptozotozin Diabetes. Chin. Med. J. 2013, 126, 1647–1653. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhang, X.; Wang, O.; Liu, J.; Cai, S.; Wang, R.; Zhou, F.; Ji, B. Anti-Diabetic Effects of the Ethanol Extract of a Functional Formula Diet in Mice Fed with a Fructose/Fat-Rich Combination Diet. J. Sci. Food Agric. 2015, 95, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Namgung, S.; Yoon, J.J.; Yoon, C.S.; Han, B.H.; Choi, E.S.; Oh, H.; Kim, Y.C.; Lee, Y.J.; Kang, D.G.; Lee, H.S. Prunella vulgaris Attenuates Diabetic Renal Injury by Suppressing Glomerular Fibrosis and Inflammation. Am. J. Chin. Med. 2017, 45, 475–495. [Google Scholar] [CrossRef]
- Upson, T.; Andrews, S.; Royal Botanic Gardens, K. The Genus Lavandula; Timber Pr: Springfield, OR, USA, 2004; p. 442. [Google Scholar]
- Sahranavard, S.; Ghafari, S.; Mosaddegh, M. Medicinal Plants Used in Iranian Traditional Medicine to Treat Epilepsy. Seizure 2014, 23, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Cocco, E.; Maccioni, D.; Sanjust, E.; Falconieri, D.; Farris, E.; Maxia, A. Ethnopharmacobotany and Diversity of Mediterranean Endemic Plants in Marmilla Subregion, Sardinia, Italy. Plants 2022, 11, 3165. [Google Scholar] [CrossRef]
- Nayebi, N.; Esteghamati, A.; Meysamie, A.; Khalili, N.; Kamalinejad, M.; Emtiazy, M.; Hashempur, M.H. The Effects of a Melissa officinalis L. Based Product on Metabolic Parameters in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blinded Controlled Clinical Trial. J. Complement. Integr. Med. 2019, 16, 20180088. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Shidfar, F.; Safari, M.; Hosseini, A.F.; Fallah Huseini, H.; Heidari, I.; Rajab, A. Efficacy of Melissa officinalis L. (Lemon Balm) Extract on Glycemic Control and Cardiovascular Risk Factors in Individuals with Type 2 Diabetes: A Randomized, Double-Blind, Clinical Trial. Phytother. Res. 2019, 33, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M. The Role of PPARα in Lipid Metabolism and Obesity: Focusing on the Effects of Estrogen on PPARα Actions. Pharmacol. Res. 2009, 60, 151–159. [Google Scholar] [CrossRef]
- Yoon, M. PPAR in Obesity: Sex Difference and Estrogen Involvement. PPAR Res. 2010, 2010, 584296. [Google Scholar] [CrossRef]
- Kumar, P.; Mishra, S.; Malik, A.; Satya, S. Insecticidal Properties of Mentha Species: A Review. Ind. Crops Prod. 2011, 34, 802–817. [Google Scholar] [CrossRef]
- Peter, K. Handbook of Herbs and Spices; Woodhead Publishing: Sawston, UK, 2006; Volume 3. [Google Scholar]
- Rahman, M.M.; Dhar, P.S.; Sumaia; Anika, F.; Ahmed, L.; Islam, M.R.; Sultana, N.A.; Cavalu, S.; Pop, O.; Rauf, A. Exploring the Plant-Derived Bioactive Substances as Antidiabetic Agent: An Extensive Review. Biomed. Pharmacother. 2022, 152, 113217. [Google Scholar] [CrossRef]
- Pereira, A.S.P.; Banegas-Luna, A.J.; Peña-García, J.; Pérez-Sánchez, H.; Apostolides, Z. Evaluation of the Anti-Diabetic Activity of Some Common Herbs and Spices: Providing New Insights with Inverse Virtual Screening. Molecules 2019, 24, 4030. [Google Scholar] [CrossRef]
- Singh, A.-K.; Yadav, D.; Sharma, N.; Jin, J.-O.; Dipeptidyl, J. Dipeptidyl Peptidase (DPP)-IV Inhibitors with Antioxidant Potential Isolated from Natural Sources: A Novel Approach for the Management of Diabetes. Pharmaceuticals 2021, 14, 586. [Google Scholar] [CrossRef]
- Liao, H.J.; Tzen, J.T.C. The Potential Role of Cyclopeptides from Pseudostellaria heterophylla, Linum usitatissimum and Drymaria diandra, and Peptides Derived from Heterophyllin B as Dipeptidyl Peptidase IV Inhibitors for the Treatment of Type 2 Diabetes: An In Silico Study. Metabolites 2022, 12, 387. [Google Scholar] [CrossRef]
- Scott, L.J. Sitagliptin: A Review in Type 2 Diabetes. Drugs 2017, 77, 209–224. [Google Scholar] [CrossRef]
- Godinho, R.; Mega, C.; Teixeira-De-Lemos, E.; Carvalho, E.; Teixeira, F.; Fernandes, R.; Reis, F. The Place of Dipeptidyl Peptidase-4 Inhibitors in Type 2 Diabetes Therapeutics: A “Me Too” or “the Special One” Antidiabetic Class? J. Diabetes Res. 2015, 2015, 806979. [Google Scholar] [CrossRef]
- Klemann, C.; Wagner, L.; Stephan, M.; von Hörsten, S. Cut to the Chase: A Review of CD26/Dipeptidyl Peptidase-4′s (DPP4) Entanglement in the Immune System. Clin. Exp. Immunol. 2016, 185, 1–21. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Shamilov, A.A.; Kashchenko, N.I. New Glycoside of Quercetin from the Genus Prunella. Chem. Nat. Compd. 2023, 59, 647–650. [Google Scholar] [CrossRef]
- Bai, Y.; Xia, B.; Xie, W.; Zhou, Y.; Xie, J.; Li, H.; Liao, D.; Lin, L.; Li, C. Phytochemistry and Pharmacological Activities of the Genus Prunella. Food Chem. 2016, 204, 483–496. [Google Scholar] [CrossRef]
- Pan, J.; Wang, H.; Chen, Y. Prunella vulgaris L.—A Review of Its Ethnopharmacology, Phytochemistry, Quality Control and Pharmacological Effects. Front. Pharmacol. 2022, 13, 23. [Google Scholar] [CrossRef]
- National Health Commission. List of New Food Materials and Common Foods; National Health Commission: Beijing, China, 2016.
- Chinese Pharmacopoeia Commission. The 2020 Edition of Pharmacopoeia of the People’s Republic of China; Chinese Pharmacopoeia Commission: Beijing, China, 2020. [Google Scholar]
- Li, K.; Hui-Xia, Y. Value of Fructosamine Measurement in Pregnant Women with Abnormal Glucose Tolerance. Chin. Med. J. 2006, 119, 1861–1865. [Google Scholar] [CrossRef]
- Katakami, N. Mechanism of Development of Atherosclerosis and Cardiovascular Disease in Diabetes Mellitus. J. Atheroscler. Thromb. 2018, 25, 27–39. [Google Scholar] [CrossRef]
- La Sala, L.; Prattichizz, F.; Ceriello, A. The Link between Diabetes and Atherosclerosis. Eur. J. Prev. Cardiol. 2019, 26, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus–Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef] [PubMed]
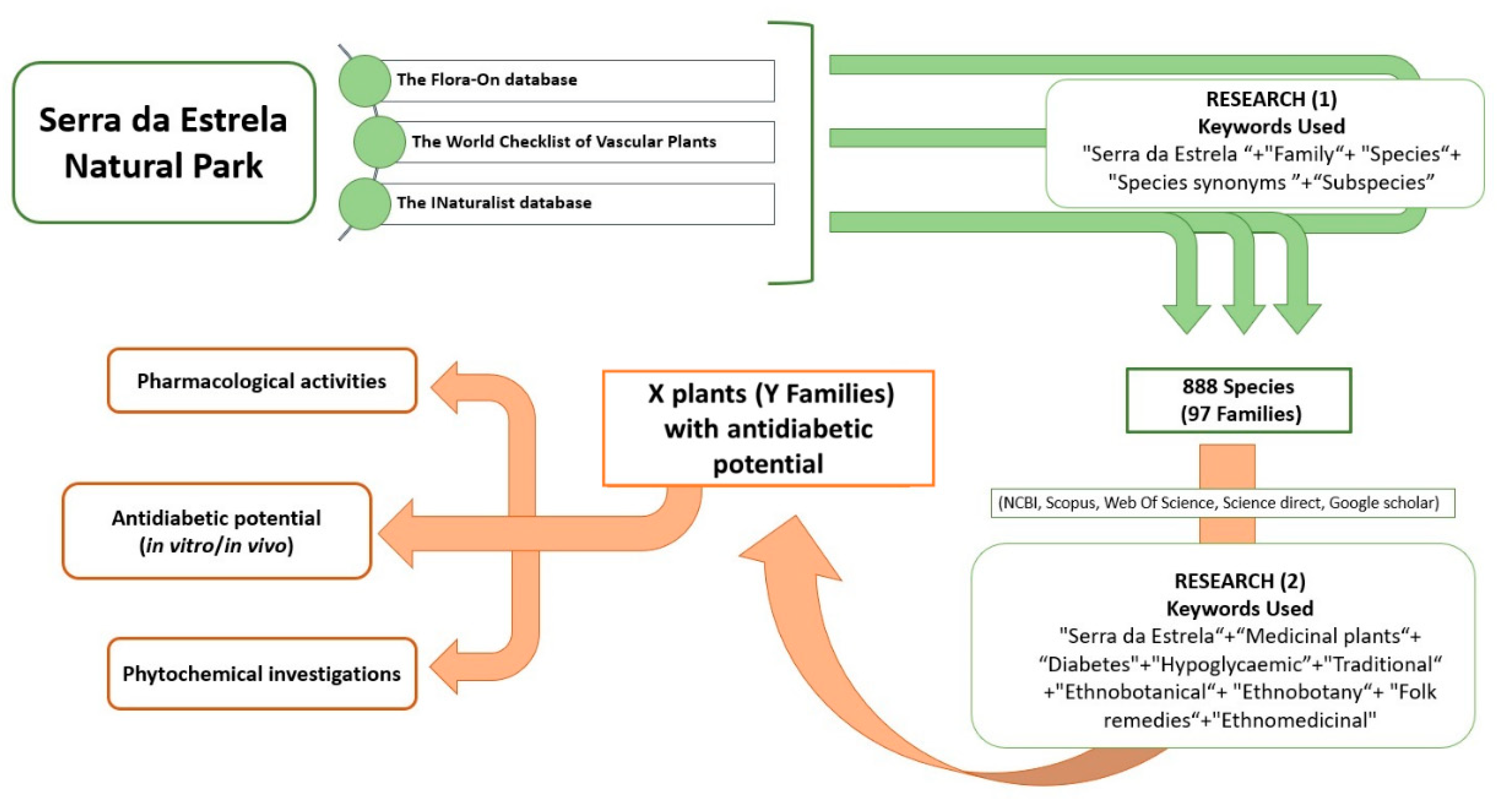
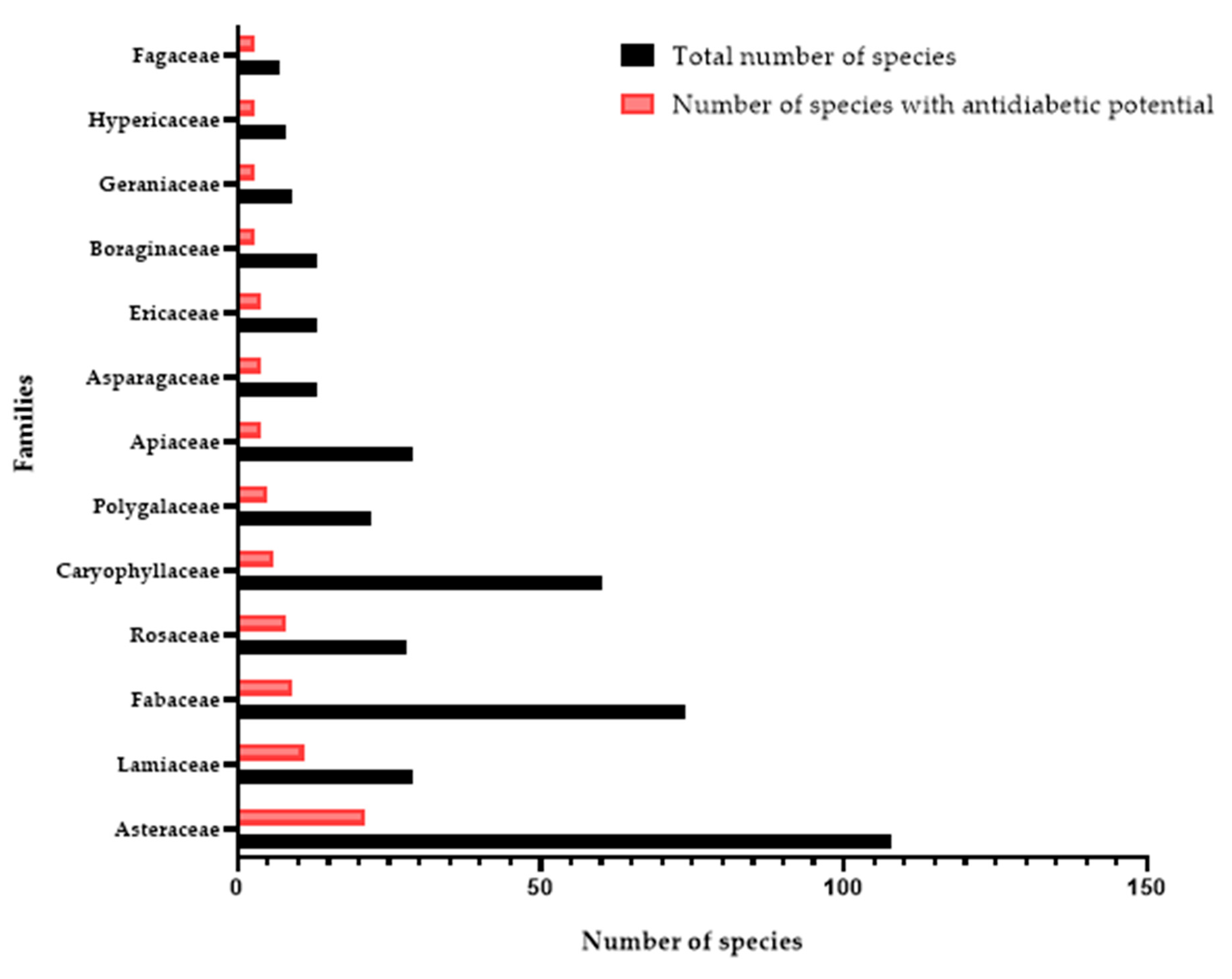
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lahlou, R.A.; Carvalho, F.; Pereira, M.J.; Lopes, J.; Silva, L.R. Overview of Ethnobotanical–Pharmacological Studies Carried Out on Medicinal Plants from the Serra da Estrela Natural Park: Focus on Their Antidiabetic Potential. Pharmaceutics 2024, 16, 454. https://doi.org/10.3390/pharmaceutics16040454
Lahlou RA, Carvalho F, Pereira MJ, Lopes J, Silva LR. Overview of Ethnobotanical–Pharmacological Studies Carried Out on Medicinal Plants from the Serra da Estrela Natural Park: Focus on Their Antidiabetic Potential. Pharmaceutics. 2024; 16(4):454. https://doi.org/10.3390/pharmaceutics16040454
Chicago/Turabian StyleLahlou, Radhia Aitfella, Filomena Carvalho, Maria João Pereira, João Lopes, and Luís R. Silva. 2024. "Overview of Ethnobotanical–Pharmacological Studies Carried Out on Medicinal Plants from the Serra da Estrela Natural Park: Focus on Their Antidiabetic Potential" Pharmaceutics 16, no. 4: 454. https://doi.org/10.3390/pharmaceutics16040454
APA StyleLahlou, R. A., Carvalho, F., Pereira, M. J., Lopes, J., & Silva, L. R. (2024). Overview of Ethnobotanical–Pharmacological Studies Carried Out on Medicinal Plants from the Serra da Estrela Natural Park: Focus on Their Antidiabetic Potential. Pharmaceutics, 16(4), 454. https://doi.org/10.3390/pharmaceutics16040454








