Prosthetic Joint Infections: Biofilm Formation, Management, and the Potential of Mesoporous Bioactive Glass as a New Treatment Option
Abstract
1. Introduction
1.1. Background on Prosthetic Joint Infection
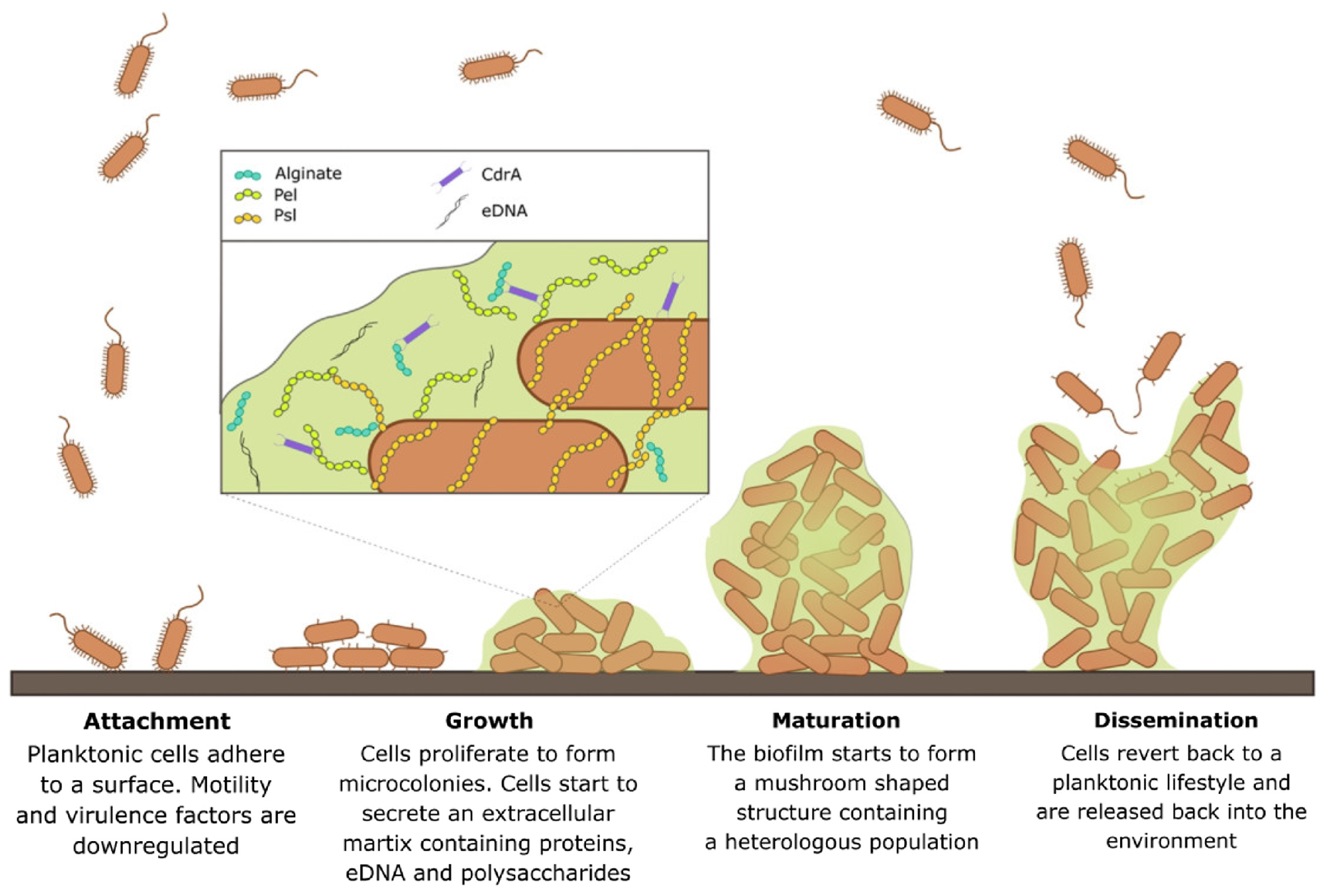
1.2. Biofilm Formation and Challenges in Treating Biofilm Infections
1.3. Challenges of Biofilm Infection Treatment
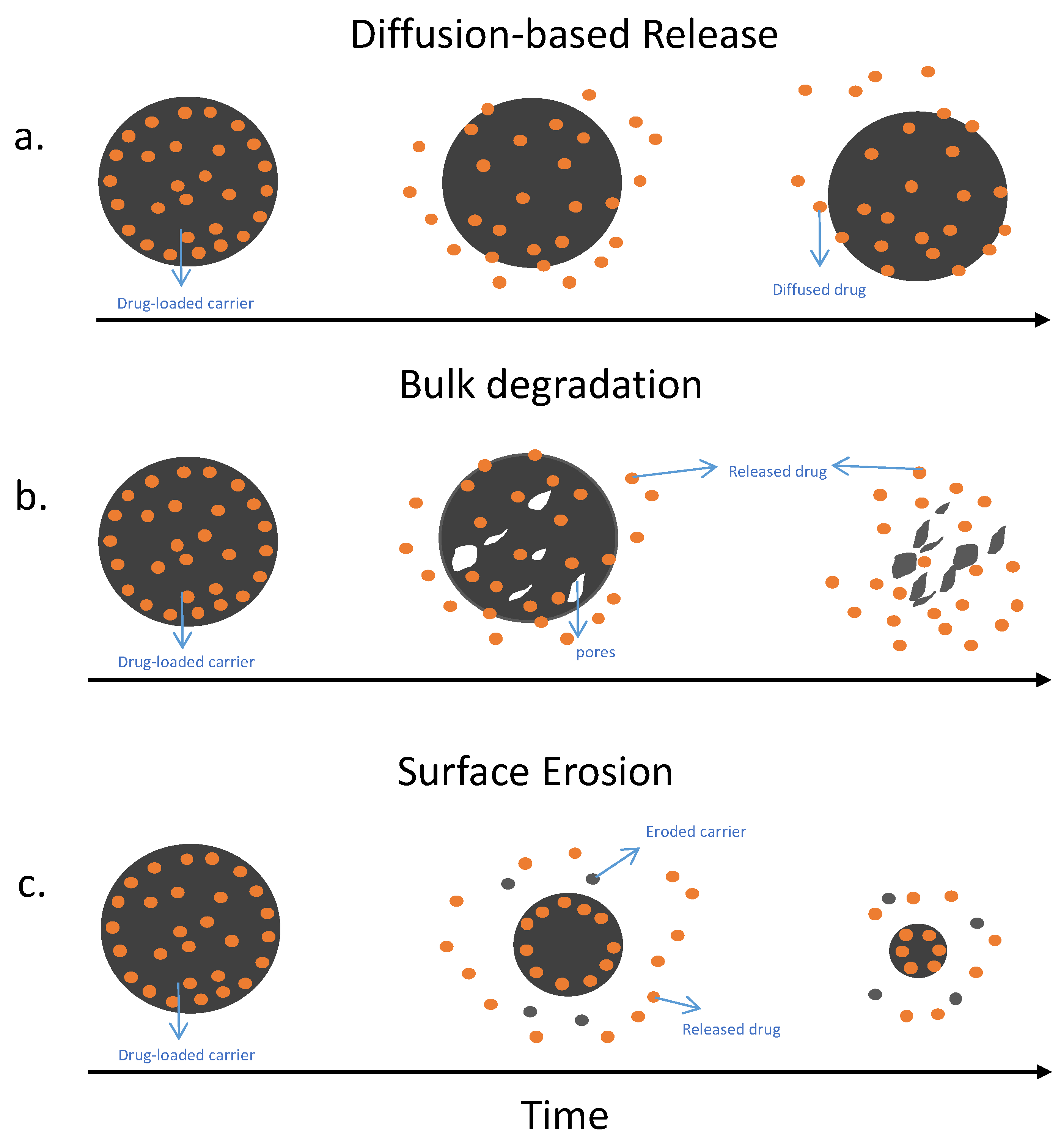
1.4. The Optimal Drug Release Profile for Treating Prosthetic Joint Infection
2. Diagnosis
3. Managing PJI
4. Local Antibiotics Therapy
4.1. Bone Cements
4.2. Degradable Antibiotics Carriers
4.3. Challenges Faced by Current PJI Treatment Methods
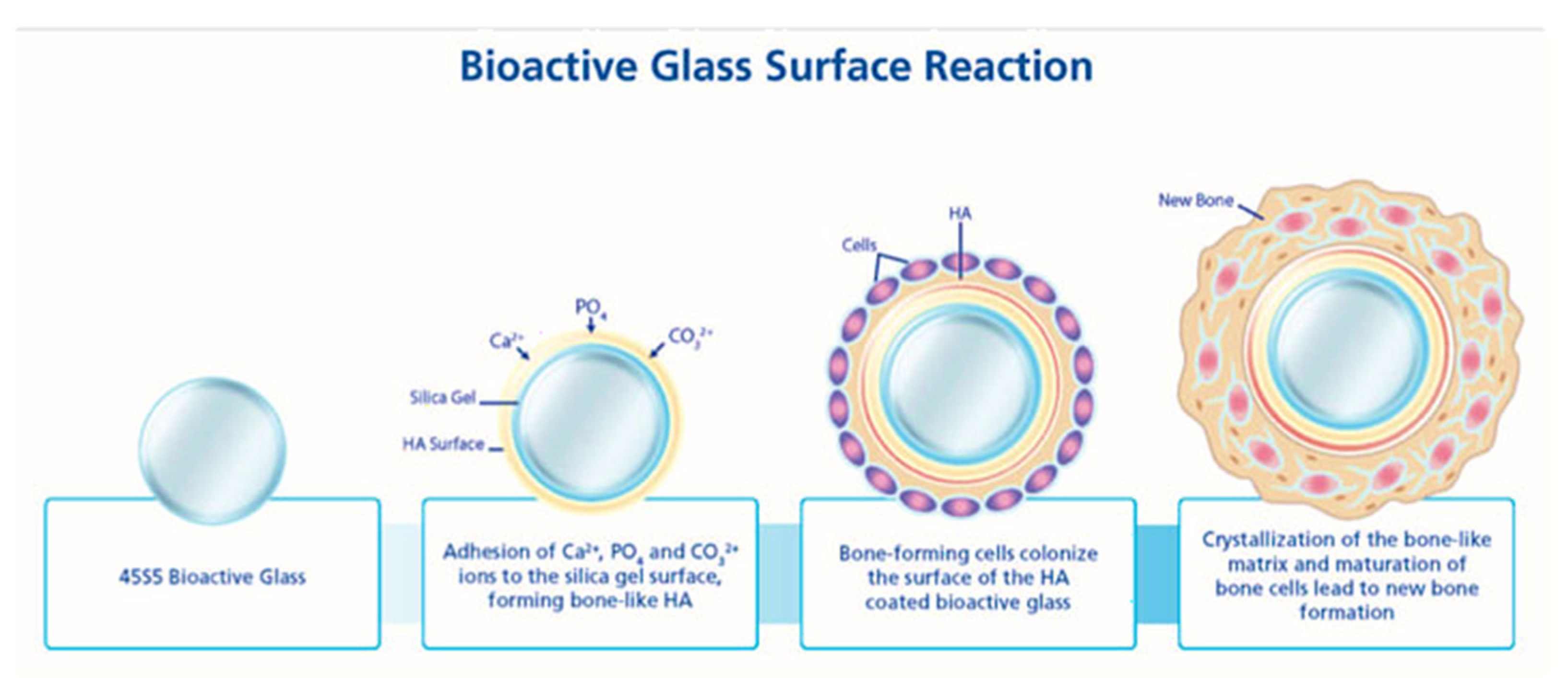
5. Bioactive Glass for Drug Delivery Applications
Review of the Literature on Bioactive Glass as Drug Delivery System
6. Mesoporous Bioactive Glass
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Izakovicova, P.; Borens, O.; Trampuz, A. Periprosthetic joint infection: Current concepts and outlook. EFORT Open Rev. 2019, 4, 482–494. [Google Scholar] [CrossRef]
- Corvec, S.; Portillo, M.E.; Pasticci, B.M.; Borens, O.; Trampuz, A. Epidemiology and new developments in the diagnosis of prosthetic joint infection. Int. J. Artif. Organs 2012, 35, 923–934. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, D.-C. Facing a new challenge. Chin. Med. J. 2019, 132, 1135–1138. [Google Scholar] [CrossRef]
- Francino, M.P. Antibiotics and the human gut microbiome: Dysbioses and accumulation of resistances. Front. Microbiol. 2016, 6, 1543. [Google Scholar] [CrossRef]
- Tande, A.J.; Patel, R. Prosthetic Joint infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef]
- Haddad, F.S.; Ngu, A.; Negus, J.J. Prosthetic Joint infections and cost analysis? In A Modern Approach to Biofilm-Related Orthopaedic Implant Infections. Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 93–100. [Google Scholar] [CrossRef]
- Sousa, A.; Carvalho, A.; Pereira, C.; Reis, E.; Santos, A.C.; Abreu, M.; Soares, D.; Fragoso, R.; Ferreira, S.; Reis, M.; et al. Economic impact of prosthetic joint infection—An evaluation within the Portuguese National Health System. J. Bone Jt. Infect. 2018, 3, 197–202. [Google Scholar] [CrossRef]
- Dapunt, U.; Hänsch, G.; Arciola, C. Innate immune response in implant-associated infections: Neutrophils against biofilms. Materials 2016, 9, 387. [Google Scholar] [CrossRef]
- Kates, S.L.; Borens, O. Principles of Orthopedic Infection Management; Georg Thieme Verlag: Stuttgart, Germany, 2016. [Google Scholar]
- Lamagni, T. Epidemiology and burden of prosthetic joint infections. J. Antimicrob. Chemother. 2014, 69 (Suppl. 1), i5–i10. [Google Scholar] [CrossRef]
- McConoughey, S.J.; Howlin, R.; Granger, J.F.; Manring, M.M.; Calhoun, J.H.; Shirtliff, M.; Kathju, S.; Stoodley, P. Biofilms in periprosthetic orthopedic infections. Future Microbiol. 2014, 9, 987–1007. [Google Scholar] [CrossRef]
- Materials Used in Implants. Available online: https://www.mdbonedocs.com/materials-used-in-implants/ (accessed on 18 July 2020).
- Linke, S.; Thürmer, A.; Bienger, K.; Kleber, C.; Bellova, P.; Lützner, J.; Stiehler, M. Microbiological pathogen analysis in native versus periprosthetic joint infections: A retrospective study. J. Orthop. Surg. Res. 2022, 17, 1–7. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Maunders, E.; Welch, M. Matrix exopolysaccharides; the sticky side of Biofilm Formation. FEMS Microbiol. Lett. 2017, 364, fnx120. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Shi, L. Controlled drug delivery systems in eradicating bacterial biofilm-associated infections. J. Control. Release 2021, 329, 1102–1116. [Google Scholar] [CrossRef]
- Lichstein, P.; Gehrke, T.; Lombardi, A.; Romano, C.; Stockley, I.; Babis, G.; Bialecki, J.; Bucsi, L.; Cai, X.; Cao, L.; et al. One-stage vs two-stage exchange. J. Arthroplast. 2014, 29, 108–111. [Google Scholar] [CrossRef]
- Stone, G.; Wood, P.; Dixon, L.; Keyhan, M.; Matin, A. Tetracycline rapidly reaches all the constituent cells of uropathogenic escherichia coli biofilms. Antimicrob. Agents Chemother. 2002, 46, 2458–2461. [Google Scholar] [CrossRef]
- Vrany, J.D.; Stewart, P.S.; Suci, P.A. Comparison of recalcitrance to ciprofloxacin and levofloxacin exhibited by pseudomonas aeruginosa bofilms displaying rapid-transport characteristics. Antimicrob. Agents Chemother. 1997, 41, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Siala, W.; Mingeot-Leclercq, M.-P.; Tulkens, P.M.; Hallin, M.; Denis, O.; Van Bambeke, F. Comparison of the antibiotic activities of daptomycin, vancomycin, and the investigational fluoroquinolone delafloxacin against biofilms from Staphylococcus aureus clinical isolates. Antimicrob. Agents Chemother. 2014, 58, 6385–6397. [Google Scholar] [CrossRef]
- Ciofu, O.; Rojo-Molinero, E.; Macià, M.D.; Oliver, A. Antibiotic treatment of biofilm infections. APMIS 2017, 125, 304–319. [Google Scholar] [CrossRef]
- Badha, V.; Moore, R.; Heffernan, J.; Castaneda, P.; McLaren, A.; Overstreet, D. Determination of tobramycin and vancomycin exposure required to eradicate biofilms on muscle and bone tissue in vitro. J. Bone Jt. Infect. 2019, 4, 1–9. [Google Scholar] [CrossRef]
- Jacqueline, C.; Caillon, J. Impact of bacterial biofilm on the treatment of prosthetic joint infections. J. Antimicrob. Chemother. 2014, 69 (Suppl. 1), i37–i40. [Google Scholar] [CrossRef]
- Vergidis, P.; Rouse, M.S.; Euba, G.; Karau, M.J.; Schmidt, S.M.; Mandrekar, J.N.; Steckelberg, J.M.; Patel, R. Treatment with linezolid or vancomycin in combination with rifampin is effective in an animal model of methicillin-resistant staphylococcus aureus foreign body osteomyelitis. Antimicrob. Agents Chemother. 2011, 55, 1182–1186. [Google Scholar] [CrossRef]
- Schwarz, E.M.; McLaren, A.C.; Sculco, T.P.; Brause, B.; Bostrom, M.; Kates, S.L.; Parvizi, J.; Alt, V.; Arnold, W.V.; Carli, A.; et al. Adjuvant antibiotic-loaded bone cement: Concerns with current use and research to make it work. J. Orthop. Res. 2020, 39, 227–239. [Google Scholar] [CrossRef]
- Muñoz-Egea, M.-C.; García-Pedrazuela, M.; Mahillo-Fernandez, I.; Esteban, J. Effect of antibiotics and antibiofilm agents in the ultrastructure and development of biofilms developed by nonpigmented rapidly growing mycobacteria. Microb. Drug Resist. 2016, 22, 1–6. [Google Scholar] [CrossRef]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R. Diagnosis and management of Prosthetic Joint Infection: Clinical Practice Guidelines by the Infectious Diseases Society of AMERICAA. Clin. Infect. Dis. 2012, 56, e1–e25. [Google Scholar] [CrossRef]
- van Vugt, T.A.; Arts, J.J.; Geurts, J.A. Antibiotic-loaded polymethylmethacrylate beads and spacers in treatment of orthopedic infections and the role of Biofilm Formation. Front. Microbiol. 2019, 10, 1626. [Google Scholar] [CrossRef]
- Li, C.; Renz, N.; Trampuz, A. Management of periprosthetic joint infection. Hip Pelvis 2018, 30, 138. [Google Scholar] [CrossRef]
- Rowan, F.E.; Donaldson, M.J.; Pietrzak, J.R.; Haddad, F.S. The role of one-stage exchange for prosthetic joint infection. Curr. Rev. Musculoskelet. Med. 2018, 11, 370–379. [Google Scholar] [CrossRef]
- Javad Mortazavi, S.M.; Vegari, D.; Ho, A.; Zmistowski, B.; Parvizi, J. Two-stage exchange arthroplasty for infected total knee arthroplasty: Predictors of failure. Clin. Orthop. Relat. Res. 2011, 469, 3049–3054. [Google Scholar] [CrossRef]
- Wolf, C.F.; Gu, N.Y.; Doctor, J.N.; Manner, P.A.; Leopold, S.S. Comparison of one and two-stage revision of total hip arthroplasty complicated by infection. J. Bone Jt. Surg. 2011, 93, 631–639. [Google Scholar] [CrossRef]
- Weiser, J.R.; Saltzman, W.M. Controlled release for local delivery of drugs: Barriers and Models. J. Control. Release 2014, 190, 664–673. [Google Scholar] [CrossRef]
- Zambanini, T.; Borges, R.; Marchi, J. Bioactive glass/polymer composites for drug delivery. In Clinical Applications of Biomaterials; Springer: Berlin/Heidelberg, Germany, 2017; pp. 287–311. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Schüth, F.; Lozano, D.; Colilla, M.; Manzano, M. Engineering mesoporous silica nanoparticles for drug delivery: Where are we after two decades? Chem. Soc. Rev. 2022, 51, 5365–5451. [Google Scholar] [CrossRef]
- Kluin, O.S.; van der Mei, H.C.; Busscher, H.J.; Neut, D. Biodegradable vs non-biodegradable antibiotic delivery devices in the treatment of osteomyelitis. Expert Opin. Drug Deliv. 2013, 10, 341–351. [Google Scholar] [CrossRef]
- Geraili, A.; Xing, M.; Mequanint, K. Design and fabrication of drug-delivery systems toward adjustable release profiles for personalized treatment. VIEW 2021, 2, 20200126. [Google Scholar] [CrossRef]
- Alt, V.; Franke, J.; Schnettler, R. Local delivery of antibiotics in the surgical treatment of bone infections. Tech. Orthop. 2015, 30, 230–235. [Google Scholar] [CrossRef]
- Hake, M.E.; Young, H.; Hak, D.J.; Stahel, P.F.; Hammerberg, E.M.; Mauffrey, C. Local antibiotic therapy strategies in orthopaedic trauma: Practical tips and tricks and review of the literature. Injury 2015, 46, 1447–1456. [Google Scholar] [CrossRef]
- Bistolfi, A.; Ferracini, R.; Albanese, C.; Vernè, E.; Miola, M. PMMA-based bone cements and the problem of joint arthroplasty infections: Status and new perspectives. Materials 2019, 12, 4002. [Google Scholar] [CrossRef]
- Kallala, R.; Harris, W.E.; Ibrahim, M.; Dipane, M.; McPherson, E. Use of stimulan absorbable calcium sulphate beads in revision lower limb arthroplasty. Bone Jt. Res. 2018, 7, 570–579. [Google Scholar] [CrossRef]
- Taha, M.; Abdelbary, H.; Ross, F.P.; Carli, A.V. New Innovations in the treatment of PJI and biofilms—Clinical and preclinical topics. Curr. Rev. Musculoskelet. Med. 2018, 11, 380–388. [Google Scholar] [CrossRef]
- El-Husseiny, M.; Patel, S.; MacFarlane, R.J.; Haddad, F.S. Biodegradable antibiotic delivery systems. J. Bone Jt. Surgery. Br. Vol. 2011, 93-B, 151–157. [Google Scholar] [CrossRef]
- Inzana, J.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. Biomaterials approaches to treating implant-associated osteomyelitis. Biomaterials 2016, 81, 58–71. [Google Scholar] [CrossRef]
- Liu, S.-J.; Wen-Neng Ueng, S.; Lin, S.-S.; Chan, E.-C. In vivo release of vancomycin from biodegradable beads. J. Biomed. Mater. Res. 2002, 63, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Levack, A.E.; Cyphert, E.L.; Bostrom, M.P.; Hernandez, C.J.; von Recum, H.A.; Carli, A.V. Current options and emerging biomaterials for periprosthetic joint infection. Curr. Rheumatol. Rep. 2018, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sanicola, S.M.; Albert, S.F. The in vitro elution characteristics of vancomycin and tobramycin from calcium sulfate beads. J. Foot Ankle Surg. 2005, 44, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Liu, X.; Jia, W.; Zhang, C.; Huang, W.; Wang, J. Treatment of osteomyelitis and repair of bone defect by degradable Bioactive Borate Glass Releasing Vancomycin. J. Control. Release 2009, 139, 118–126. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Bal, B.S.; Huang, W. Review: Emerging developments in the use of bioactive glasses for treating infected prosthetic joints. Mater. Sci. Eng. C 2014, 41, 224–231. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef]
- Gogia, J.; Meehan, J.; Di Cesare, P.; Jamali, A. Local antibiotic therapy in osteomyelitis. Semin. Plast. Surg. 2009, 23, 100–107. [Google Scholar] [CrossRef]
- Drago, L.; Toscano, M.; Bottagisio, M. Recent evidence on bioactive glass antimicrobial and antibiofilm activity: A mini-review. Materials 2018, 11, 326. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Day, D.E.; Sonny Bal, B.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef]
- Hamadouche, M.; Meunier, A.; Greenspan, D.C.; Blanchat, C.; Zhong, J.P.; La Torre, G.P.; Sedel, L. Long-termin vivo bioactivity and degradability of bulk sol-gel bioactive glasses. J. Biomed. Mater. Res. 2000, 54, 560–566. [Google Scholar] [CrossRef]
- Domingues, Z.R.; Cortés, M.E.; Gomes, T.A.; Diniz, H.F.; Freitas, C.S.; Gomes, J.B.; Faria, A.M.C.; Sinisterra, R.D. Bioactive glass as a drug delivery system of tetracycline and tetracycline associated with β-cyclodextrin. Biomaterials 2004, 25, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-P.; Lü, J.-y.; Ke, Q.-F. Mesoporous bioactive glasses: Fabrication, structure, drug delivery property, and therapeutic potential. In Biomedical, Therapeutic and Clinical Applications of Bioactive Glasses; Elsevier: Amsterdam, The Netherlands, 2019; pp. 127–151. [Google Scholar] [CrossRef]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive glasses: Where are we and where are we going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.; Cresswell, M.; Boccaccini, A.R. Mesoporous silica-based bioactive glasses for antibiotic-free antibacterial applications. Mater. Sci. Eng. C 2018, 83, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Salètes, M.; Vartin, M.; Mocquot, C.; Chevalier, C.; Grosgogeat, B.; Colon, P.; Attik, N. Mesoporous bioactive glasses cytocompatibility assessment: A review of in vitro studies. Biomimetics 2021, 6, 9. [Google Scholar] [CrossRef]
- Yang, F.-S.; Lu, Y.-D.; Wu, C.-T.; Blevins, K.; Lee, M.S.; Kuo, F.-C. Mechanical failure of articulating polymethylmethacrylate (PMMA) spacers in two-stage revision hip arthroplasty: The risk factors and the impact on interim function. BMC Musculoskelet. Disord. 2019, 20, 1–10. [Google Scholar] [CrossRef]
- Thwaites, J.H.; Thwaites, J.F.; Ted, K.L.Y.; Chuang, T. Symptomatic hypercalcaemia following the use of calcium sulfate beads in periprosthetic joint infections. N. Z. Med. J. 2022, 135, 124–126. [Google Scholar]
- Abosala, A.; Ali, M. The use of calcium sulphate beads in periprosthetic joint infection, a systematic review. J. Bone Jt. Infect. 2020, 5, 43–49. [Google Scholar] [CrossRef]
- Tarity, T.D.; Xiang, W.; Jones, C.W.; Gkiatas, I.; Nocon, A.; Selemon, N.A.; Carli, A.; Sculco, P.K. Do antibiotic-loaded calcium sulfate beads improve outcomes after debridement, antibiotics, and implant retention? A matched cohort study. Arthroplast. Today 2022, 14, 90–95. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef]
- Fiume, E.; Migneco, C.; Verné, E.; Baino, F. Comparison between bioactive sol-gel and melt-derived glasses/glass-ceramics based on the multicomponent SiO2–P2O5–CaO–MgO–Na2O–K2O system. Materials 2020, 13, 540. [Google Scholar] [CrossRef]
- Gupta, S.; Majumdar, S.; Krishnamurthy, S. Bioactive Glass: A multifunctional delivery system. J. Control. Release 2021, 335, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chang, J. Mesoporous bioactive glasses: Structure characteristics, drug/growth factor delivery and bone regeneration application. Interface Focus 2012, 2, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, P.; Jones, J.R.; Hench, L.L. Characterization of melt-derived 45S5 and sol-gel-derived 58s bioactive glasses. J. Biomed. Mater. Res. 2001, 58, 734–740. [Google Scholar] [CrossRef]
- Rivadeneira, J.; Gorustovich, A. Bioactive glasses as delivery systems for antimicrobial agents. J. Appl. Microbiol. 2017, 122, 1424–1437. [Google Scholar] [CrossRef]
- Oosthuysen, W.; Venter, R.; Tanwar, Y.; Ferreira, N. Bioactive glass as dead space management following debridement of type 3 chronic osteomyelitis. Int. Orthop. 2019, 44, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Obata, A.; Lee, S.; Kasuga, T. Bioactive glass materials for tissue regeneration. J. Ceram. Soc. Jpn. 2022, 130, 595–604. [Google Scholar] [CrossRef]
- Black, J. Part I. In Biological Performance of Materials: Fundamentals of Biocompatibility; CRC Press: Boca Raton, FL, USA, 2006; pp. 3–14. [Google Scholar]
- Hench, L.L. Bioactive Glass: Chronology, characterization, and genetic control of tissue regeneration. In Advances in Calcium Phosphate Biomaterials; Springer Series in Biomaterials Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2014; pp. 51–70. [Google Scholar] [CrossRef]
- Romanò, C.L.; Logoluso, N.; Meani, E.; Romanò, D.; De Vecchi, E.; Vassena, C.; Drago, L. A comparative study of the use of bioactive glass S53P4 and antibiotic-loaded calcium-based bone substitutes in the treatment of chronic osteomyelitis. Bone Jt. J. 2014, 96-B, 845–850. [Google Scholar] [CrossRef]
- Maximov, M.; Maximov, O.-C.; Craciun, L.; Ficai, D.; Ficai, A.; Andronescu, E. Bioactive glass—An extensive study of the preparation and coating methods. Coatings 2021, 11, 1386. [Google Scholar] [CrossRef]
- Sonatkar, J.; Kandasubramanian, B. Bioactive glass with biocompatible polymers for Bone Applications. Eur. Polym. J. 2021, 160, 110801. [Google Scholar] [CrossRef]
- Lindfors, N.C.; Hyvönen, P.; Nyyssönen, M.; Kirjavainen, M.; Kankare, J.; Gullichsen, E.; Salo, J. Bioactive Glass S53P4 as bone graft substitute in treatment of osteomyelitis. Bone 2010, 47, 212–218. [Google Scholar] [CrossRef]
- Cannio, M.; Bellucci, D.; Roether, J.A.; Boccaccini, D.N.; Cannillo, V. Bioactive glass applications: A literature review of Human Clinical Trials. Materials 2021, 14, 5440. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R.; Brauer, D.S.; Hupa, L.; Greenspan, D.C. Bioglass and bioactive glasses and their impact on Healthcare. Int. J. Appl. Glass Sci. 2016, 7, 423–434. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, D.; Li, N.; Wen, J.; Jiang, X.; Liu, C.; Li, Y. Functionalized mesoporous bioactive glass scaffolds for enhanced bone tissue regeneration. Sci. Rep. 2016, 6, 19361. [Google Scholar] [CrossRef] [PubMed]
- Soundrapandian, C.; Datta, S.; Kundu, B.; Basu, D.; Sa, B. Porous bioactive glass scaffolds for local drug delivery in osteomyelitis: Development and in vitro characterization. AAPS PharmSciTech 2010, 11, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.K.; Kundu, B.; Mukherjee, P.; Mandal, T.K.; Datta, S.; De, D.K.; Basu, D. In vitro and in vivo release of cefuroxime axetil from bioactive glass as an implantable delivery system in experimental osteomyelitis. Ceram. Int. 2009, 35, 3207–3216. [Google Scholar] [CrossRef]
- Araújo, M.; Viveiros, R.; Philippart, A.; Miola, M.; Doumett, S.; Baldi, G.; Perez, J.; Boccaccini, A.R.; Aguiar-Ricardo, A.; Verné, E. Bioactivity, mechanical properties and drug delivery ability of bioactive glass-ceramic scaffolds coated with a natural-derived polymer. Mater. Sci. Eng. C 2017, 77, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yu, C.; Zhou, X.; Tang, J.; Zhao, D. Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew. Chem. Int. Ed. 2004, 43, 5980–5984. [Google Scholar] [CrossRef]
- Yan, X.; Huang, X.; Yu, C.; Deng, H.; Wang, Y.; Zhang, Z.; Qiao, S.; Lu, G.; Zhao, D. The in-vitro bioactivity of mesoporous bioactive glasses. Biomaterials 2006, 27, 3396–3403. [Google Scholar] [CrossRef]
- Letaïef, N.; Lucas-Girot, A.; Oudadesse, H.; Dorbez-Sridi, R.; Boullay, P. Investigation of the surfactant type effect on characteristics and bioactivity of new mesoporous bioactive glass in the ternary system SiO2–CAO–P2O5: Structural, textural and reactivity studies. Microporous Mesoporous Mater. 2014, 195, 102–111. [Google Scholar] [CrossRef]
- Shih, S.-J.; Lin, Y.-C.; Valentino Posma Panjaitan, L.; Rahayu Meyla Sari, D. The correlation of surfactant concentrations on the properties of mesoporous bioactive glass. Materials 2016, 9, 58. [Google Scholar] [CrossRef]
- Hardy, D.; Desuzinges Mandon, E.; Rothnie, A.J.; Jawhari, A. The Yin and Yang of solubilization and stabilization for wild-type and full-length membrane protein. Methods 2018, 147, 118–125. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J.; Xiao, Y. Mesoporous bioactive glasses as drug delivery and bone tissue regeneration platforms. Ther. Deliv. 2011, 2, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yan, X.; Zhou, X.; Zhou, L.; Wang, H.; Tang, J.; Yu, C. Mesoporous bioactive glasses for controlled drug release. Microporous Mesoporous Mater. 2008, 109, 210–215. [Google Scholar] [CrossRef]
- Hum, J.; Boccaccini, A.R. Bioactive glasses as carriers for bioactive molecules and therapeutic drugs: A Review. J. Mater. Sci. Mater. Med. 2012, 23, 2317–2333. [Google Scholar] [CrossRef]
- Xia, W.; Chang, J. Well-ordered mesoporous bioactive glasses (MBG): A promising bioactive drug delivery system. J. Control. Release 2006, 110, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.-Z.; Long, T.; Yu, X.-B.; Tang, T.T.; Dai, K.-R.; Tian, B.; Guo, Y.-P.; Zhu, Z.-A. Mesoporous bioactive glass as a drug delivery system: Fabrication, bactericidal properties and biocompatibility. J. Mater. Sci. Mater. Med. 2013, 24, 1951–1961. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, D.; Weng, W.; Huang, Q.; Zhang, X.; Wen, J.; Wu, J.; Jiang, X. Alendronate delivery on amino modified mesoporous bioactive glass scaffolds to enhance bone regeneration in osteoporosis rats. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 2), 171–181. [Google Scholar] [CrossRef]
- Anand, A.; Das, P.; Nandi, S.K.; Kundu, B. Development of antibiotic loaded mesoporous bioactive glass and its drug release kinetics. Ceram. Int. 2020, 46, 5477–5483. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, H.; Chen, X. The preparation of hollow mesoporous bioglass nanoparticles with excellent drug delivery capacity for bone tissue regeneration. Front. Chem. 2019, 7, 283. [Google Scholar] [CrossRef]
- Berkmann, J.C.; Herrera Martin, A.X.; Pontremoli, C.; Zheng, K.; Bucher, C.H.; Ellinghaus, A.; Boccaccini, A.R.; Fiorilli, S.; Vitale Brovarone, C.; Duda, G.N.; et al. In vivo validation of spray-dried mesoporous bioactive glass microspheres acting as prolonged local release systems for BMP-2 to support bone regeneration. Pharmaceutics 2020, 12, 823. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, F.Y.; Lin, H.M.; Wu, X.; Jiang, J.J. Mesoporous bioactive glass: Ideal material for higher uptake and well sustained release of Ibuprofen. Mater. Res. Innov. 2012, 16, 230–235. [Google Scholar] [CrossRef]
- Xia, W.; Chang, J. Preparation, in vitro bioactivity and drug release property of well-ordered mesoporous 58s bioactive glass. J. Non-Cryst. Solids 2008, 354, 1338–1341. [Google Scholar] [CrossRef]
- López-Noriega, A.; Arcos, D.; Vallet-Regí, M. Functionalizing mesoporous bioglasses for long-term anti-osteoporotic drug delivery. Chem. A Eur. J. 2010, 16, 10879–10886. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, Y.; Shu, Y.; Wu, Z.; Cao, W.; Huang, W. Amino-functionalized mesoporous bioactive glass for drug delivery. Biomed. Mater. 2017, 12, 025017. [Google Scholar] [CrossRef] [PubMed]
- El Baakili, S.; El Mabrouk, K.; Bricha, M. Acellular bioactivity and drug delivery of new strontium doped bioactive glasses prepared through a hydrothermal process. RSC Adv. 2022, 12, 15361–15372. [Google Scholar] [CrossRef]
- El-Kady, A.M.; Ahmed, M.M.; Abd El-Hady, B.M.; Ali, A.F.; Ibrahim, A.M. Optimization of ciprofloxacin release kinetics of novel nano-bioactive glasses: Effect of glass modifier content on drug loading and release mechanism. J. Non-Cryst. Solids 2019, 521, 119471. [Google Scholar] [CrossRef]
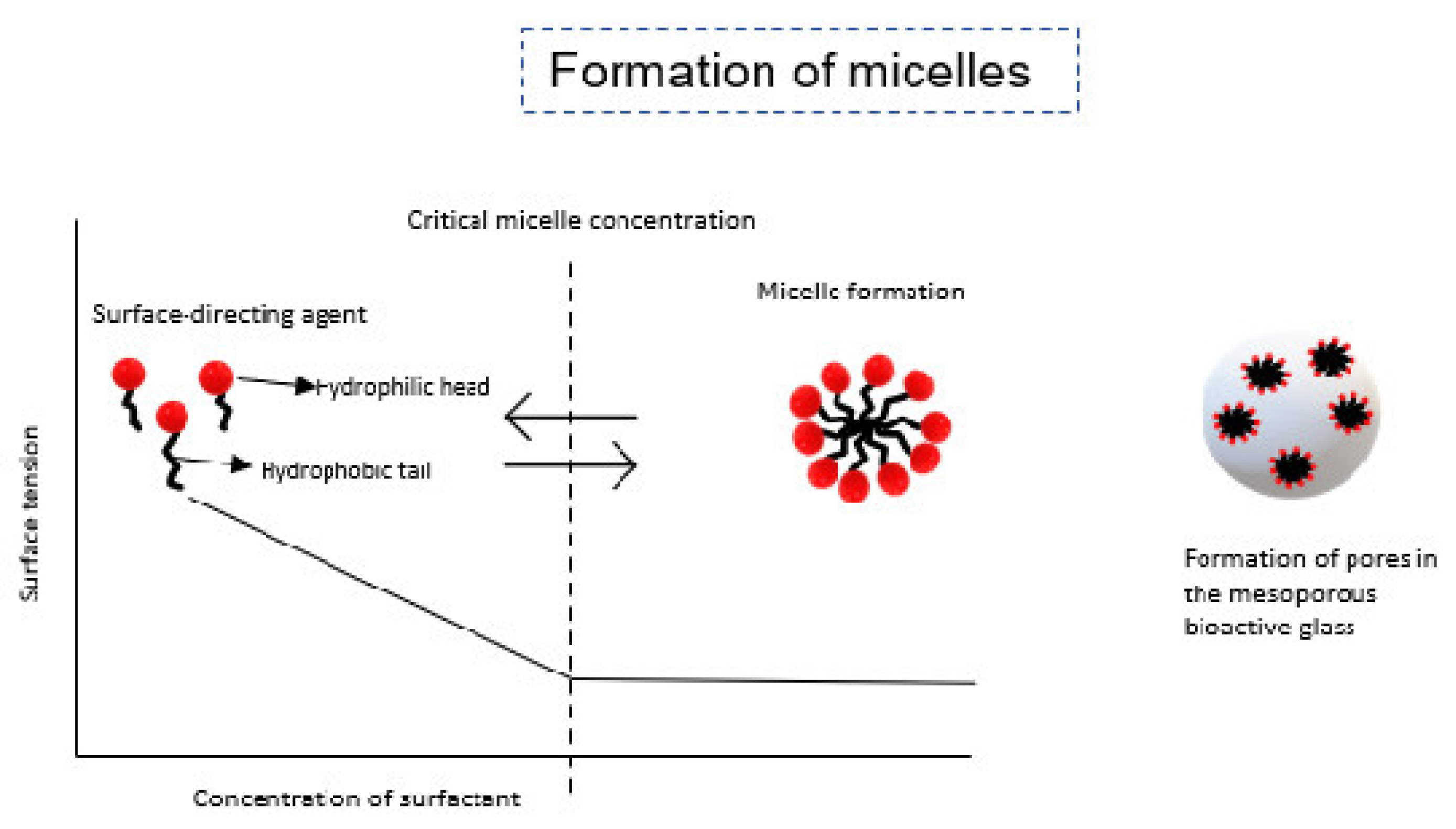

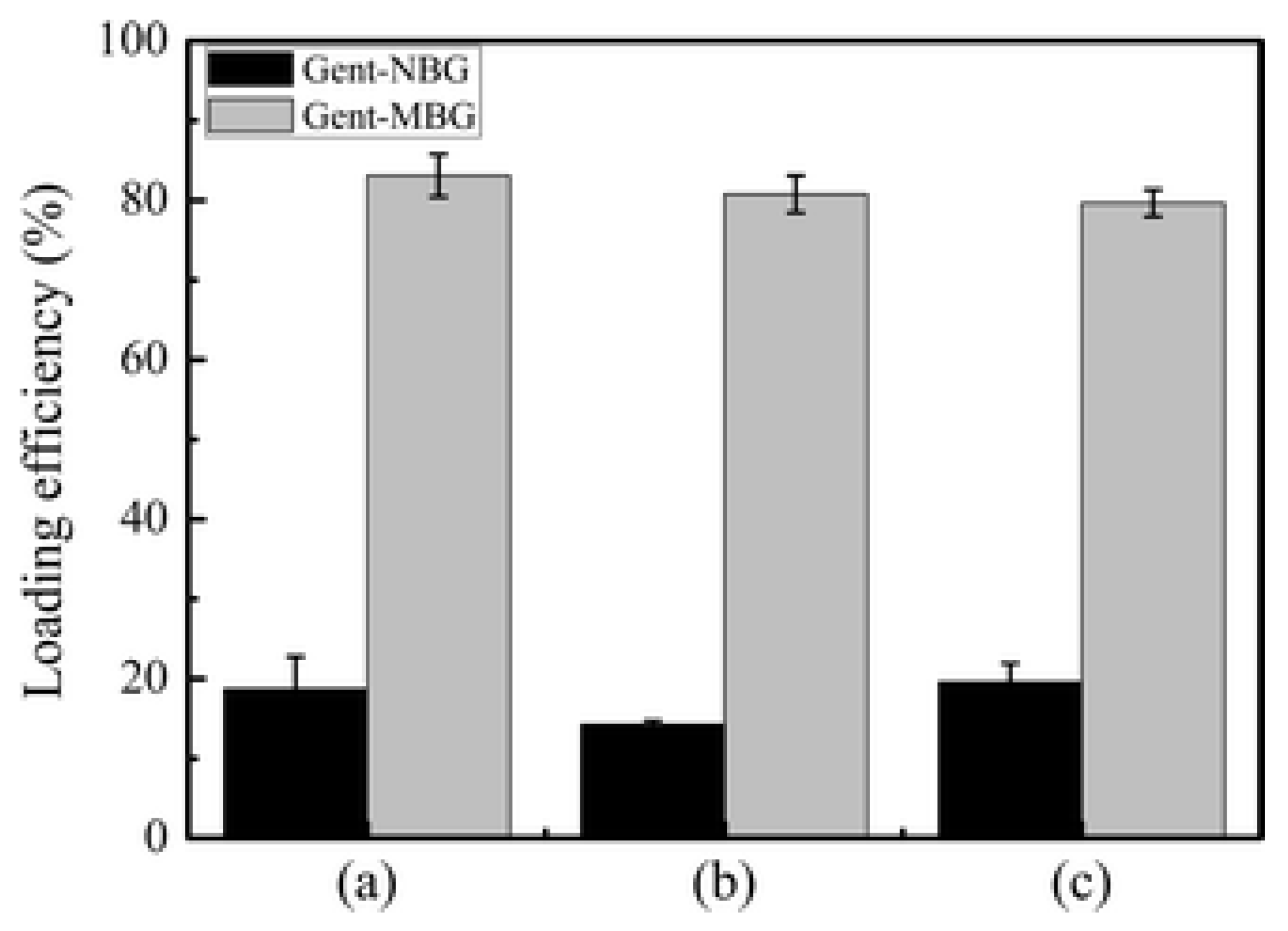

| Infection Type | Success | Type of Joint | Cost | |
|---|---|---|---|---|
| One-stage | Preferred when the infected pathogen is known | Higher eradication rates and functional outcome [30] | Hip and shoulder joints | Lower costs and shorter time at the hospital |
| Two-stage | Preferred when the pathogen is unknown or hard to treat | High success rate for patients with poorer bone stock | Knee joints | Higher cost related to multiple surgeries and longer hospital stay |
| Drug Carrier | Properties | Degradability | Elution Characteristics | Limitations |
|---|---|---|---|---|
| PMMA | Pre-loaded beads or by direct mixing of antibiotics into material before implantation. Can load multiple antibiotics | Non-degradable | Less than 50% of antibiotics eluted after 4 weeks [45] | Nondegradable Limited antibiotics can be used Burst release followed by subtherapeutic antibiotics release. |
| Calcium Sulfate | Can be 100% synthetic to remove any impurities. A wide range of antibiotics can be used. Degradable and osteoconductive. Reabsorbs within 30–60 days [46] | Degrades completely within 4–6 weeks [47] | Releases most loaded antibiotics within 72 h. Burst release 68–74% (w/w) by the second day [48] | Burst release followed by slow release of antibiotics and limited capacity to form new bones [46,49]. Can lead to wound drainage, heterotopic ossification, and hypercalcemia Hypersensitivity reaction [46] |
| Collagen Fleece | Biodegradable Biocompatible and non-toxic | Degrades completely within 8 weeks [43] | Burst release, releases all content within 2 h of implantation | Burst release; releases most antibiotics within 2 h of implantation Mild immunogenicity [50] |
| Polyesters (PLGA) | Biodegradable Highly tunable, can be tweaked to load antibiotics and deliver them efficiently by changing the ratios of the polymers | Depends on composition, can take 8 weeks to few months [44] | Burst release but maintain seroma levels above sensitivity for 55 days [51] | Can cause inflammatory response Lower pH caused by degradation can lead to really fast release of loaded antibiotics [44] |
| Bioactive glass | Synthetic silica-based material. Excellent mechanical and bone-bonding properties [52] and capacity to degrade at controllable rate [53] Osteostimulation and angiogenic potential Has antibacterial properties Many formulations approved for use by FDA | Depending on the composition, sol–gel glasses can take 12–52 weeks [54] | 12% w burst release followed by sustained release for 3 months [55] | Limited porosity [56] Degradation and hydroxyapatite formation depend highly on compositions and production method [57] |
| Mesoporous bioactive glass | Use of surface-directing agents and templating methods Enhanced surface area and higher pore volume Excellent cytocompatibility [58] and better apatite formation than non-mesoporous bioactive glass | Depends on composition, has better apatite formation capacity | These have not been tested in vivo. Brittleness [59] |
| Temperature | Surface Area | Bioactivity | Limitations | |
|---|---|---|---|---|
| Melt quench | Really high temperatures (>1500 °C) | 0.15 to 2.7 m2/g [68] | Limited composition range of SiO2, biologically inert at SiO2 > 60% | Defects such as cracks and bubbles that may affect properties of the glass |
| Sol–gel | Aging 70 °C Drying 120 °C Calcination 600 °C [66] | 126.5–164.7 m2/g [68] | Wider composition range, bioactive at SiO2 of 90% | Shrinkage during drying, microcracks, long hydrolysis stage Carbon traces [69] |
| Materials | Drug | Composition | Loading | Release | Study |
|---|---|---|---|---|---|
| Mesoporous bioglass (MBG) | Tetracycline | Multiple by varying CaO content at 5C, 10C, 25C, and 35C | MBG 100S = 10.5% MBG 90S5C = 15.5% MBG 80S15C = 15.8% MBG 70S25C = 17.4% MBG 60S35C = 18.3% | 100S—high initial release and release 50% at 1.9 h 90S5C, 80S15C, 70S25C, 60S35C equilibrium at 72 h TC total release MBG 100S = 98% MBG 90S5C = 56% MBG 80S15C = 25% MBG 70S25C = 25%, MBG 60S35C =38% | Zhao, 2007 [90] |
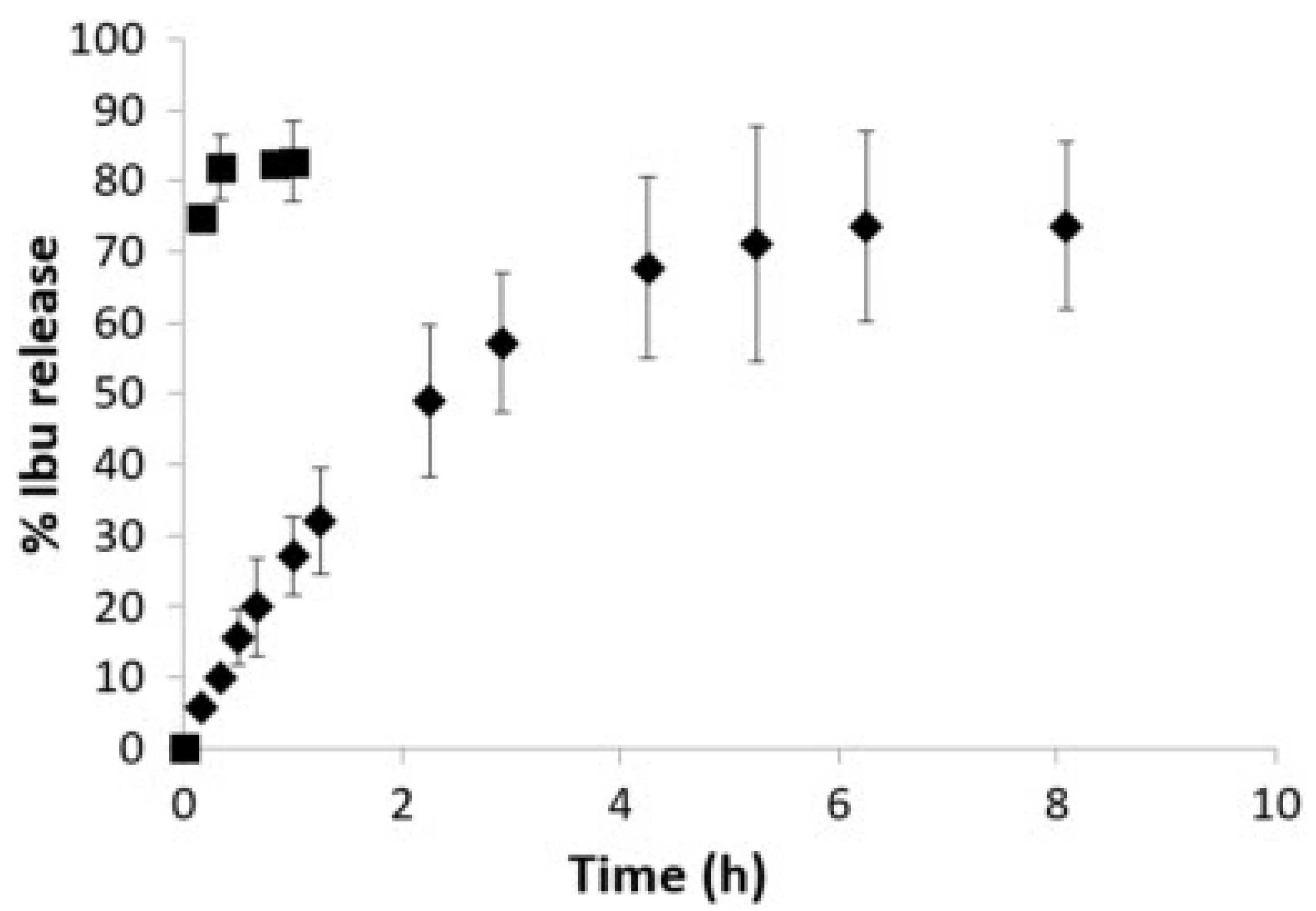
| MBG | Ibuprofen | 80Si2O-15CaO-5P2O5 | 46 wt% of ibuprofens in solution | Release under static conditions 60% of ibuprofen | Zhang, 2012 [98] |
| Borate bioglass (sol–gel) | Vancomycin | 6Na2O-8K2O-8MgO-22CaO-54B2O3-2P2O5 | 80 mg/g of vancomycin per gram of borate glass powder | Cumulative release of vancomycin was 85.99–94.43% and lasted for 18 days | Xie, 2009 [48] |
| MBG | Gentamicin | 58Si2O-23CaO-9P2O5 | 35.2 wt% of total gentamicin in solution | 30 wt% released after 20 days M58S had lower initial burst release in the first 24 h than normal BG (58S) | Xia, 2008 [99] |
| MBG With different surface modifications | Ipriflavone | 85Si2O-10CaO-5P2O5 | Phenyl 85 MBG = 11.7% OH-propyl 85 MBG = 6.08% NH2-propyl 85 MBG = 6.14% SH-propyl 85 MBG = 4.05% Cl-propyl 85 MBG = 1.68% Butyl 85 MBG = 1.06% | Phenyl 85 MBG = 3% OH-propyl 85 MBG = 6.02% NH2-propyl 85 MBG = 7.27% SH-propyl 85 MBG = 13% Cl-propyl 85 MBG = N/A Butyl 85 MBG = N/A | López-Noriea, 2010 [100] |
| MBG | Gentamicin | 79.6–83.1% four times higher than non-MBG used as control | 80% of gentamicin was released in 6 days while maintaining slow and sustained release | Li, 2013 [93] | |
| MBG | CFS a combination of sodium ceftriaxone (CFT) and sodium sulbactam (SUL) in the ratio of 2:1. | 78Si2O-16CaO-6P2O5 | 40.4% of CFS | In different pH conditions, a burst release of >50% occurred in the first 24 h followed by a slow and sustained release for the duration of the study (168 h) | Anand, 2020 [95] |
| Amino-functionalized MBG (N-MBG) | Gentamicin sulfate | 80Si2O-15CaO-5P2O5 | MBG = 48.9 wt% N-MBG = 62.92 wt% | MBG 7-day release 60.7% of the total loaded drug N-MBG 7-day release 45.1% with sustained release after N-MBG and MBG had initial burst release (12 h) = 30–40% of total drug. | Jiang, 2017 [101] |
| MBG nanospheres | Doxorubicin hydrochloride | 80Si2O-15CaO-5P2O5 | High drug loading capacity of 90% | Decreased burst release and sustained release of DOX for 180 h (duration of study) with cumulative release at 15–30% with varying pH of SBF solutions | Wu, 2013 [67] |
| Strontium doped bioactive glass | Ibuprofen | 63Si2O-(37 − x)CaO-xSrO x = 0–1 mol% | High drug loading of 78.5–88.8% depending on the mol% if strontium | Burst release in the first few hours followed by steady release for 28 days | El Baakili, 2022 [102] |
| Nano-bioactive e glass | Ciprofloxacin | 97SiO-3CaO 87SiO-13CaO 77SiO-23CaO 67SiO-33CaO | Loaded drug decreased with increasing mol% of CaO Loading efficiency of 92.5–98.63% | Cumulative release of the total loaded amount of drug for the different compositions ranges from 24.76–52.22% over 28 days | El-Kady, 2019 [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almasri, D.; Dahman, Y. Prosthetic Joint Infections: Biofilm Formation, Management, and the Potential of Mesoporous Bioactive Glass as a New Treatment Option. Pharmaceutics 2023, 15, 1401. https://doi.org/10.3390/pharmaceutics15051401
Almasri D, Dahman Y. Prosthetic Joint Infections: Biofilm Formation, Management, and the Potential of Mesoporous Bioactive Glass as a New Treatment Option. Pharmaceutics. 2023; 15(5):1401. https://doi.org/10.3390/pharmaceutics15051401
Chicago/Turabian StyleAlmasri, Dana, and Yaser Dahman. 2023. "Prosthetic Joint Infections: Biofilm Formation, Management, and the Potential of Mesoporous Bioactive Glass as a New Treatment Option" Pharmaceutics 15, no. 5: 1401. https://doi.org/10.3390/pharmaceutics15051401
APA StyleAlmasri, D., & Dahman, Y. (2023). Prosthetic Joint Infections: Biofilm Formation, Management, and the Potential of Mesoporous Bioactive Glass as a New Treatment Option. Pharmaceutics, 15(5), 1401. https://doi.org/10.3390/pharmaceutics15051401








