Responsive Microneedles as a New Platform for Precision Immunotherapy
Abstract
1. Introduction
2. Microneedles Triggered by Internal Stimuli
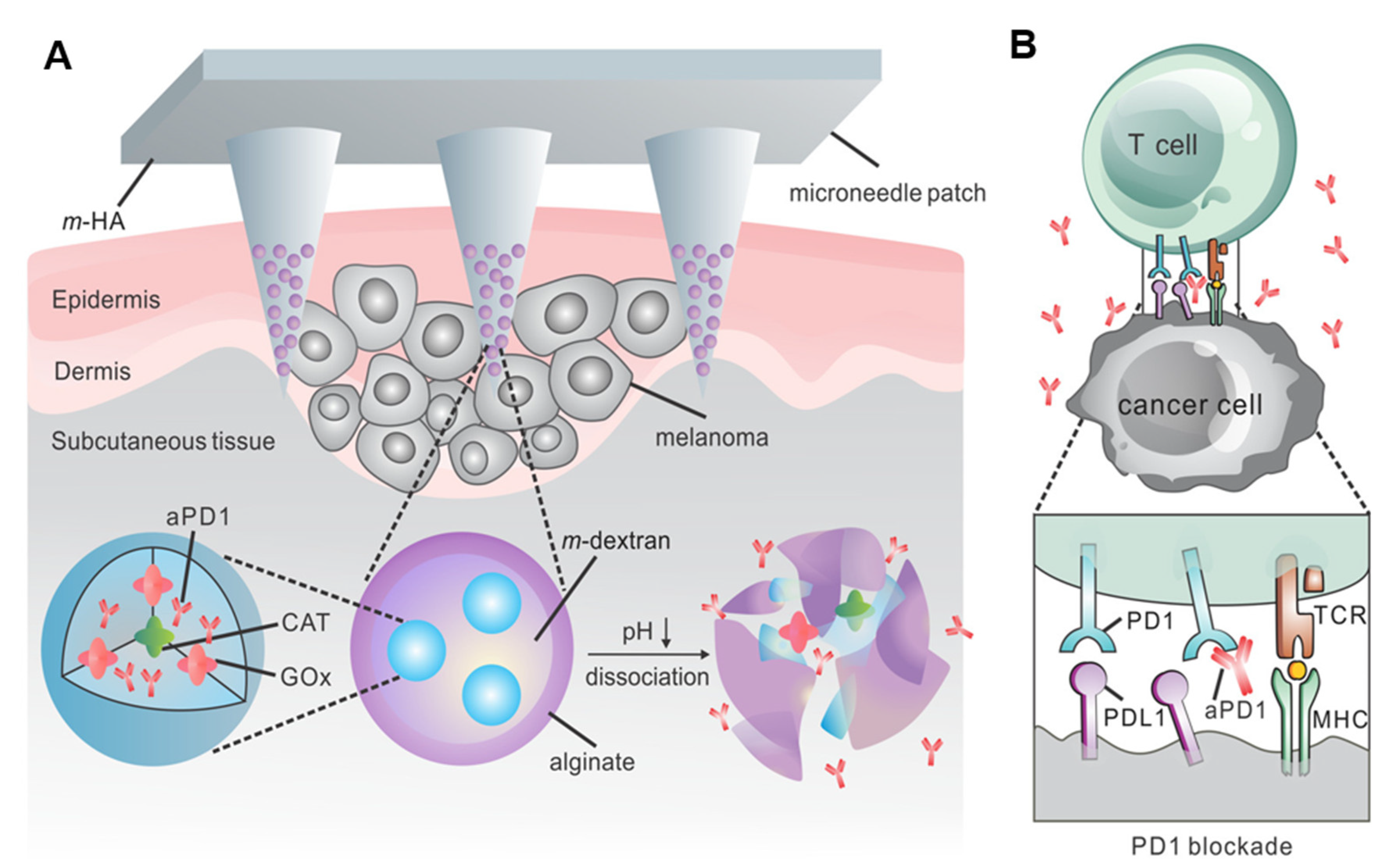
2.1. pH-Responsive Microneedles
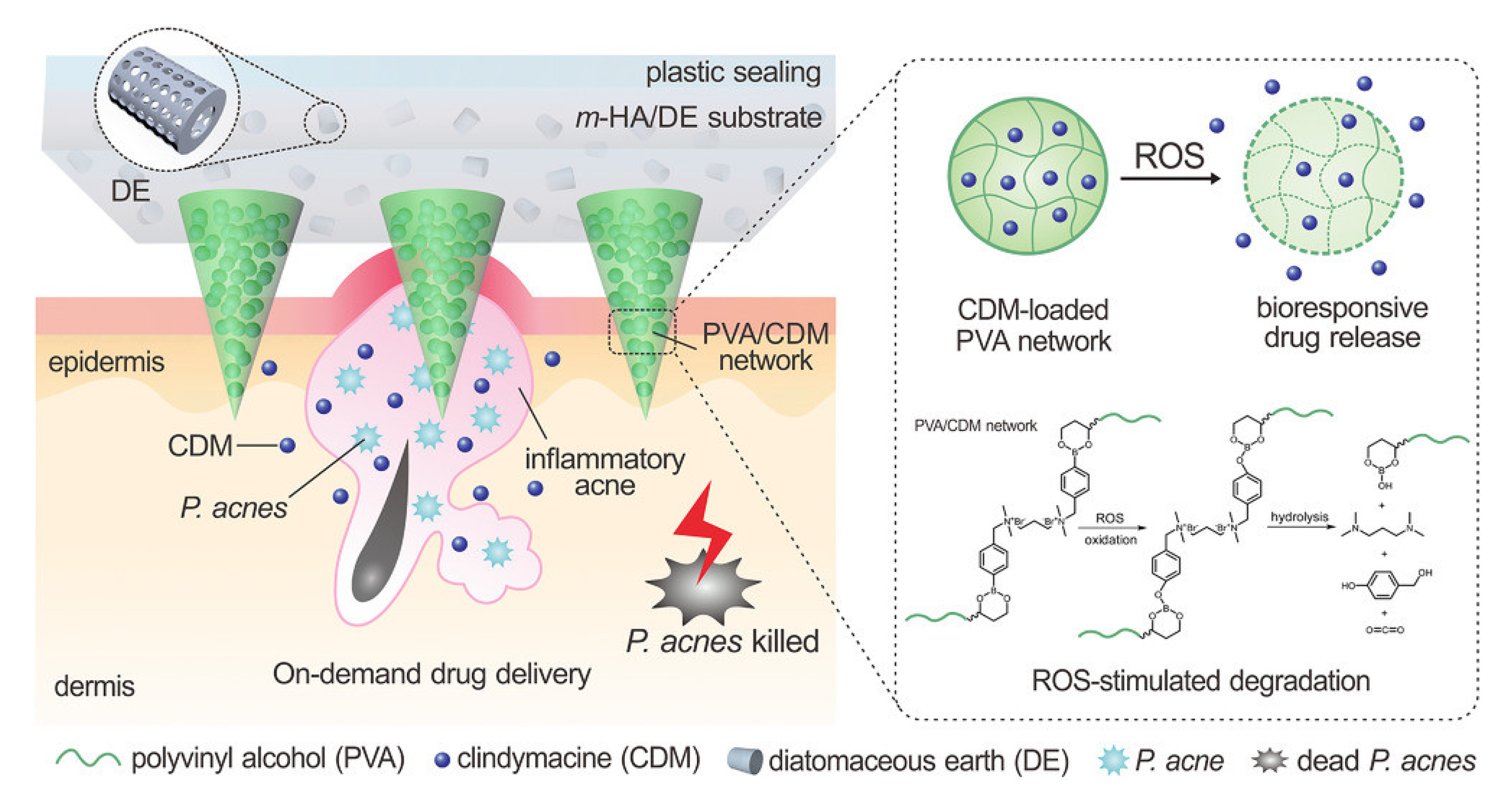
2.2. Reactive Oxygen Species-Responsive Microneedles
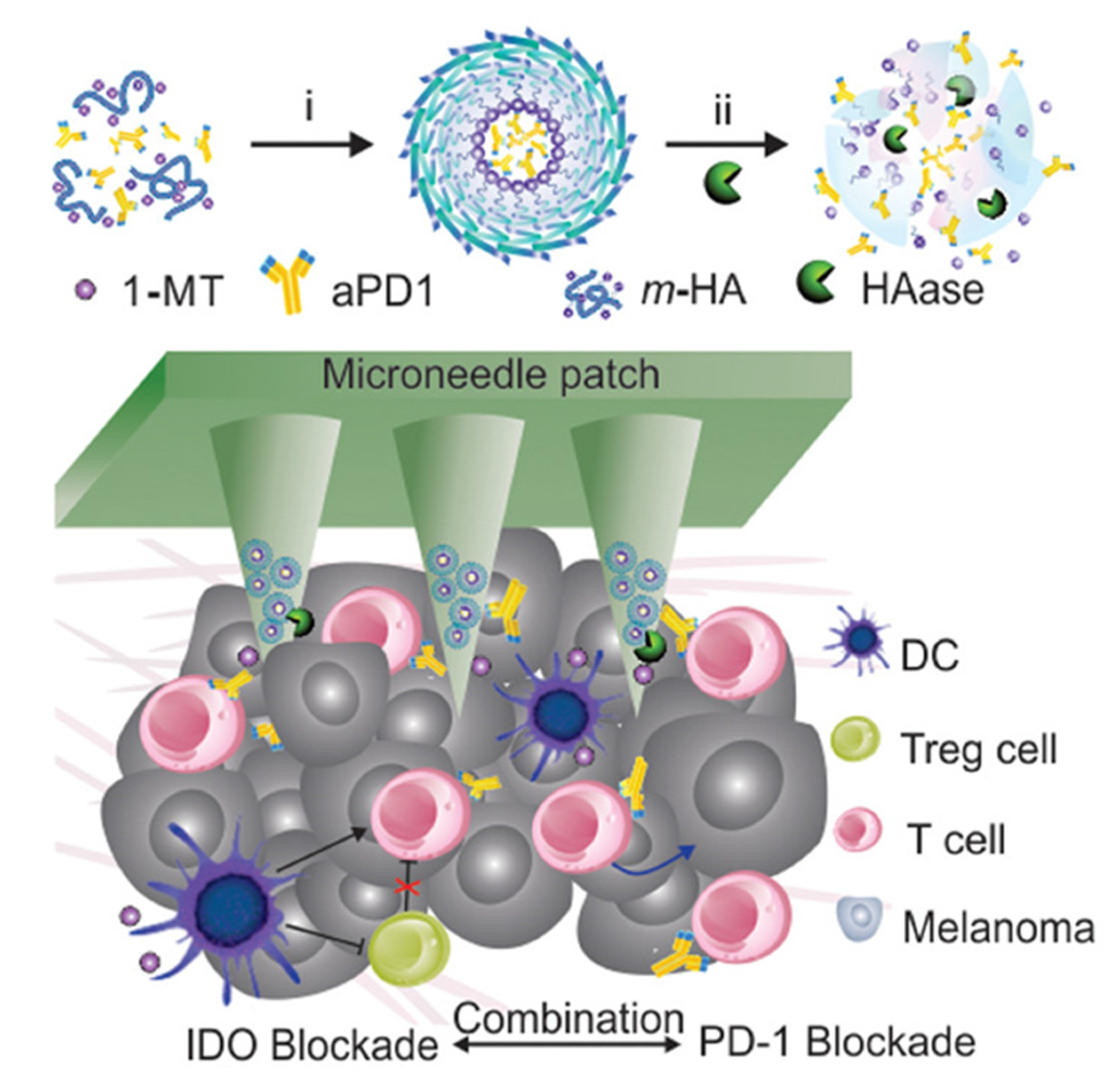
2.3. Enzyme-Responsive Microneedles
2.4. Temperature-Responsive Microneedles
3. Microneedles Triggered by External Stimuli
3.1. Optical-Activated Microneedles
3.2. Mechanical Force-Responsive Microneedles
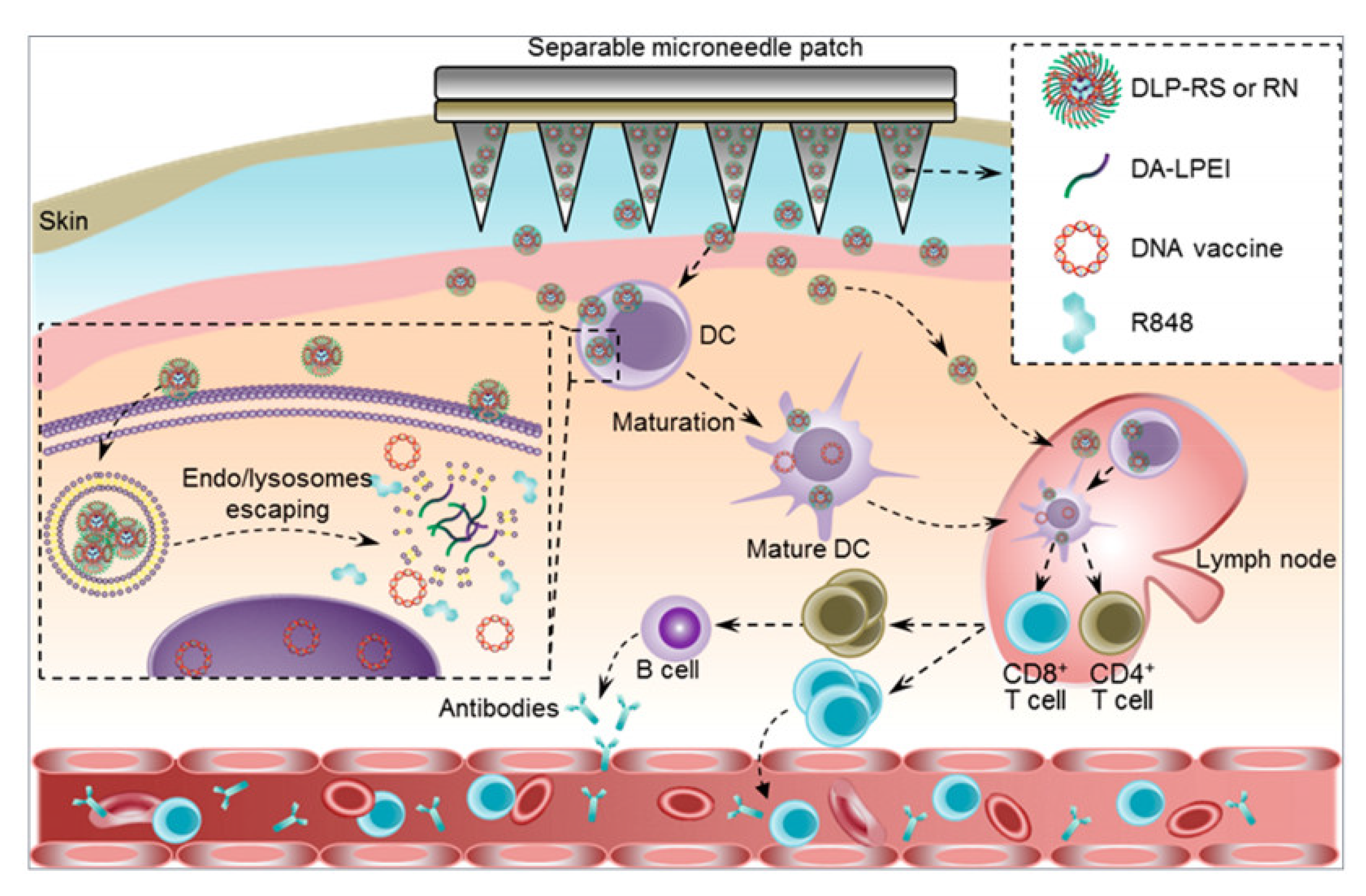
4. Responsive Microneedle for Cancer Immunotherapy
5. The Limitations of the Microneedle-Based Drug Delivery System
6. Future Perspectives and Clinical Translation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- You, Y.; Tian, Y.; Yang, Z.; Shi, J.; Kwak, K.J.; Tong, Y.; Estania, A.P.; Cao, J.; Hsu, W.-H.; Liu, Y.; et al. Intradermally delivered mRNA-encapsulating extracellular vesicles for collagen-replacement therapy. Nat. Biomed. Eng. 2023. [Google Scholar] [CrossRef]
- Yang, T.; Huang, D.; Li, C.; Zhao, D.; Li, J.; Zhang, M.; Chen, Y.; Wang, Q.; Liang, Z.; Liang, X.-J.; et al. Rolling microneedle electrode array (RoMEA) empowered nucleic acid delivery and cancer immunotherapy. Nano Today 2021, 36, 101017. [Google Scholar] [CrossRef]
- Kearney, M.-C.; Caffarel-Salvador, E.; Fallows, S.J.; McCarthy, H.O.; Donnelly, R.F. Microneedle-mediated delivery of donepezil: Potential for improved treatment options in Alzheimer’s disease. Eur. J. Pharm. Biopharm. 2016, 103, 43–50. [Google Scholar] [CrossRef] [PubMed]
- McCrudden, M.T.C.; Alkilani, A.Z.; McCrudden, C.M.; McAlister, E.; McCarthy, H.O.; Woolfson, A.D.; Donnelly, R.F. Design and physicochemical characterisation of novel dissolving polymeric microneedle arrays for transdermal delivery of high dose, low molecular weight drugs. J. Control. Release 2014, 180, 71–80. [Google Scholar] [CrossRef]
- Iachina, I.; Eriksson, A.H.; Bertelsen, M.; Petersson, K.; Jansson, J.; Kemp, P.; Engell, K.M.; Brewer, J.R.; Nielsen, K.T. Dissolvable microneedles for transdermal drug delivery showing skin pentation and modified drug release. Eur. J. Pharm. Sci. 2023, 182, 106371. [Google Scholar] [CrossRef]
- Wang, R.; Zhong, T.; Bian, Q.; Zhang, S.; Ma, X.; Li, L.; Xu, Y.; Gu, Y.; Yuan, A.; Hu, W.; et al. PROTAC Degraders of Androgen Receptor-Integrated Dissolving Microneedles for Androgenetic Alopecia and Recrudescence Treatment via Single Topical Administration. Small Methods 2023, 7, e2201293. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, L.; Yu, H.; Li, C.; Feng, J.; Haq, F.; Khan, A.; Khan, R.U. Preparation, properties and challenges of the microneedles-based insulin delivery system. J. Control. Release 2018, 288, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Jamaledin, R.; Di Natale, C.; Onesto, V.; Taraghdari, Z.B.; Zare, E.N.; Makvandi, P.; Vecchione, R.; Netti, P.A. Progress in microneedle-mediated protein delivery. J. Clin. Med. 2020, 9, 542. [Google Scholar] [CrossRef]
- Liu, T.; Chen, M.; Fu, J.; Sun, Y.; Lu, C.; Quan, G.; Pan, X.; Wu, C. Recent advances in microneedles-mediated transdermal delivery of protein and peptide drugs. Acta Pharm. Sin. B 2021, 11, 2326–2343. [Google Scholar] [CrossRef]
- Chen, Q.; Xiao, Z.; Wang, C.; Chen, G.; Zhang, Y.; Zhang, X.; Han, X.; Wang, J.; Ye, X.; Prausnitz, M.R.; et al. Microneedle Patches Loaded with Nanovesicles for Glucose Transporter-Mediated Insulin Delivery. ACS Nano 2022, 16, 18223–18231. [Google Scholar] [CrossRef]
- Hu, S.; Zhu, D.; Li, Z.; Cheng, K. Detachable Microneedle Patches Deliver Mesenchymal Stromal Cell Factor-Loaded Nanoparticles for Cardiac Repair. ACS Nano 2022, 16, 15935–15945. [Google Scholar] [CrossRef] [PubMed]
- Bui, V.D.; Son, S.; Xavier, W.; Nguyen, V.Q.; Jung, J.M.; Lee, J.; Shin, S.; Um, W.; An, J.Y.; Kim, C.H.; et al. Dissolving microneedles for long-term storage and transdermal delivery of extracellular vesicles. Biomaterials 2022, 287, 121644. [Google Scholar] [CrossRef]
- Liu, S.; Yang, G.; Li, M.; Sun, F.; Li, Y.; Wang, X.; Gao, Y.; Yang, P. Transcutaneous immunization via dissolving microneedles protects mice from lethal influenza H7N9 virus challenge. Vaccine 2022, 40, 6767–6775. [Google Scholar] [CrossRef] [PubMed]
- Mikszta, J.A.; Alarcon, J.B.; Brittingham, J.M.; Sutter, D.E.; Pettis, R.J.; Harvey, N.G. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat. Med. 2002, 8, 415–419. [Google Scholar] [CrossRef]
- Mikszta, J.A.; Sullivan, V.J.; Dean, C.; Waterston, A.M.; Alarcon, J.B.; Dekker, J.P.; Brittingham, J.M.; Huang, J.; Hwang, C.R.; Ferriter, M.; et al. Protective immunization against inhalational anthrax: A comparison of minimally invasive delivery platforms. J. Infect. Dis. 2005, 191, 278–288. [Google Scholar] [CrossRef]
- Rappuoli, R. Vaccines: Science, health, longevity, and wealth. Proc. Natl. Acad. Sci. USA 2014, 111, 12282. [Google Scholar] [CrossRef]
- Chen, Z.; Lv, Y.; Qi, J.; Zhu, Q.; Lu, Y.; Wu, W. Overcoming or circumventing the stratum corneum barrier for efficient transcutaneous immunization. Drug Discov. Today 2018, 23, 181–186. [Google Scholar] [CrossRef]
- Hammond, S.A.; Guebre-Xabier, M.; Yu, J.; Glenn, G.M. Transcutaneous immunization: An emerging route of immunization and potent immunostimulation strategy. Crit. Rev. Ther. Drug Carr. Syst. 2001, 18, 503–526. [Google Scholar] [CrossRef]
- Engelke, L.; Winter, G.; Hook, S.; Engert, J. Recent insights into cutaneous immunization: How to vaccinate via the skin. Vaccine 2015, 33, 4663–4674. [Google Scholar] [CrossRef]
- Sallam, M.A.; Prakash, S.; Kumbhojkar, N.; Shields, C.W.; Mitragotri, S. Formulation-based approaches for dermal delivery of vaccines and therapeutic nucleic acids: Recent advances and future perspectives. Bioeng. Transl. Med. 2021, 6, e10215. [Google Scholar] [CrossRef]
- Amani, H.; Shahbazi, M.A.; D’Amico, C.; Fontana, F.; Abbaszadeh, S.; Santos, H.A. Microneedles for painless transdermal immunotherapeutic applications. J. Control. Release 2021, 330, 185–217. [Google Scholar] [CrossRef]
- Mistilis, M.J.; Joyce, J.C.; Esser, E.S.; Skountzou, I.; Compans, R.W.; Bommarius, A.S.; Prausnitz, M.R. Long-term stability of influenza vaccine in a dissolving microneedle patch. Drug Deliv. Transl. Res. 2017, 7, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.C.; Collins, M.L.; Rota, P.A.; Prausnitz, M.R. Thermostability of Measles and Rubella Vaccines in a Microneedle Patch. Adv. Ther. 2021, 4, 2100095. [Google Scholar] [CrossRef]
- Arya, J.M.; Dewitt, K.; Scott-Garrard, M.; Chiang, Y.W.; Prausnitz, M.R. Rabies vaccination in dogs using a dissolving microneedle patch. J. Control. Release 2016, 239, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, I.; Eassa, H.A.; Mohammed, K.H.A.; Abd El-Fattah, M.A.; Abdo, M.H.; Rashad, E.; Eassa, H.A.; Saleh, A.; Amin, O.M.; Nounou, M.I.; et al. Microneedle-Based Vaccine Delivery: Review of an Emerging Technology. AAPS PharmSciTech 2022, 23, 103. [Google Scholar] [CrossRef]
- Ma, G.; Wu, C. Microneedle, bio-microneedle and bio-inspired microneedle: A review. J. Control. Release 2017, 251, 11–23. [Google Scholar] [CrossRef]
- Yu, J.; Kuwentrai, C.; Gong, H.R.; Li, R.; Zhang, B.Z.; Lin, X.; Wang, X.; Huang, J.D.; Xu, C. Intradermal delivery of mRNA using cryomicroneedles. Acta Biomater. 2022, 148, 133–141. [Google Scholar] [CrossRef]
- Hirobe, S.; Azukizawa, H.; Hanafusa, T.; Matsuo, K.; Quan, Y.-S.; Kamiyama, F.; Katayama, I.; Okada, N.; Nakagawa, S. Clinical study and stability assessment of a novel transcutaneous influenza vaccination using a dissolving microneedle patch. Biomaterials 2015, 57, 50–58. [Google Scholar] [CrossRef]
- Rouphael, N.G.; Paine, M.; Mosley, R.; Henry, S.; McAllister, D.V.; Kalluri, H.; Pewin, W.; Frew, P.M.; Yu, T.; Thornburg, N.J.; et al. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): A randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet 2017, 390, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Ono, A.; Ito, S.; Sakagami, S.; Asada, H.; Saito, M.; Quan, Y.-S.; Kamiyama, F.; Hirobe, S.; Okada, N. Development of Novel Faster-Dissolving Microneedle Patches for Transcutaneous Vaccine Delivery. Pharmaceutics 2017, 9, 27. [Google Scholar] [CrossRef]
- Robertson, C.A.; Tsang, P.; Landolfi, V.A.; Greenberg, D.P. Fluzone(R) Intradermal Quadrivalent Influenza Vaccine. Expert Rev. Vaccines 2016, 15, 1245–1253. [Google Scholar] [CrossRef]
- Ita, K. Ceramic microneedles and hollow microneedles for transdermal drug delivery: Two decades of research. J. Drug Deliv. Sci. Technol. 2018, 44, 314–322. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, L.; Lin, F.; Gomaa, Y.; Flyer, D.; Carrion, R., Jr.; Patterson, J.L.; Prausnitz, M.R.; Smith, G.; Glenn, G.; et al. Intradermal Vaccination With Adjuvanted Ebola Virus Soluble Glycoprotein Subunit Vaccine by Microneedle Patches Protects Mice Against Lethal Ebola Virus Challenge. J. Infect. Dis. 2018, 218 (Suppl. S5), S545–S552. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Wirth, D.M.; Ortega-Rivera, O.A.; Steinmetz, N.F.; Pokorski, J.K. Dissolving Microneedle Delivery of a Prophylactic HPV Vaccine. Biomacromolecules 2022, 23, 903–912. [Google Scholar] [CrossRef]
- Frew, P.M.; Paine, M.B.; Rouphael, N.; Schamel, J.; Chung, Y.; Mulligan, M.J.; Prausnitz, M.R. Acceptability of an inactivated influenza vaccine delivered by microneedle patch: Results from a phase I clinical trial of safety, reactogenicity, and immunogenicity. Vaccine 2020, 38, 7175–7181. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-L.; Zhan, Y.; Villadangos, J.A.; Lew, A.M. The cell biology of cross-presentation and the role of dendritic cell subsets. Immunol. Cell Biol. 2008, 86, 353–362. [Google Scholar] [CrossRef]
- Qin, M.; Du, G.; Sun, X. Recent Advances in the Noninvasive Delivery of mRNA. Acc. Chem. Res. 2021, 54, 4262–4271. [Google Scholar] [CrossRef]
- Zhu, H.; Mah Jian Qiang, J.; Wang, C.G.; Chan, C.Y.; Zhu, Q.; Ye, E.; Li, Z.; Loh, X.J. Flexible polymeric patch based nanotherapeutics against non-cancer therapy. Bioact. Mater. 2022, 18, 471–491. [Google Scholar] [CrossRef]
- Xu, G.; Mao, Y.; Jiang, T.; Gao, B.; He, B. Structural design strategies of microneedle-based vaccines for transdermal immunity augmentation. J. Control. Release 2022, 351, 907–922. [Google Scholar] [CrossRef]
- Ramirez-Garcia, P.D.; Retamal, J.S.; Shenoy, P.; Imlach, W.; Sykes, M.; Truong, N.; Constandil, L.; Pelissier, T.; Nowell, C.J.; Khor, S.Y.; et al. A pH-responsive nanoparticle targets the neurokinin 1 receptor in endosomes to prevent chronic pain. Nat. Nanotechnol. 2019, 14, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.T.T.; Yin, Y.; Thambi, T.; Nguyen, T.L.; Giang Phan, V.H.; Lee, M.S.; Lee, J.E.; Kim, J.; Jeong, J.H.; Lee, D.S. Smart vaccine delivery based on microneedle arrays decorated with ultra-pH-responsive copolymers for cancer immunotherapy. Biomaterials 2018, 185, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef]
- Jager, E.; Murthy, S.; Schmidt, C.; Hahn, M.; Strobel, S.; Peters, A.; Staubert, C.; Sungur, P.; Venus, T.; Geisler, M.; et al. Calcium-sensing receptor-mediated NLRP3 inflammasome response to calciprotein particles drives inflammation in rheumatoid arthritis. Nat. Commun. 2020, 11, 4243. [Google Scholar] [CrossRef]
- Cui, W.; Chen, S.; Chi, Z.; Guo, X.; Zhang, X.; Zhong, Y.; Han, H.; Yao, K. Screening-based identification of xanthone as a novel NLRP3 inflammasome inhibitor via metabolic reprogramming. Clin. Transl. Med. 2021, 11, e496. [Google Scholar] [CrossRef]
- Permana, A.D.; Anjani, Q.K.; Sartini; Utomo, E.; Volpe-Zanutto, F.; Paredes, A.J.; Evary, Y.M.; Mardikasari, S.A.; Pratama, M.R.; Tuany, I.N.; et al. Selective delivery of silver nanoparticles for improved treatment of biofilm skin infection using bacteria-responsive microparticles loaded into dissolving microneedles. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111786. [Google Scholar] [CrossRef]
- Chi, J.; Zhang, X.; Chen, C.; Shao, C.; Zhao, Y.; Wang, Y. Antibacterial and angiogenic chitosan microneedle array patch for promoting wound healing. Bioact. Mater. 2020, 5, 253–259. [Google Scholar] [CrossRef]
- Rainu, S.; Parameswaran, S.; Krishnakumar, S.; Singh, N. Dual-sensitive fluorescent nanoprobes for detection of matrix metalloproteinases and low pH in a 3D tumor microenvironment. J. Mater. Chem. B 2022, 10, 5388–5401. [Google Scholar] [CrossRef]
- Dai, L.; Li, X.; Zheng, X.; Fu, Z.; Yao, M.; Meng, S.; Zhang, J.; Han, B.; Gao, Q.; Chang, J.; et al. TGF-beta blockade-improved chemo-immunotherapy with pH/ROS cascade-responsive micelle via tumor microenvironment remodeling. Biomaterials 2021, 276, 121010. [Google Scholar] [CrossRef]
- Mathesh, M.; Sun, J.; van der Sandt, F.; Wilson, D.A. Supramolecular nanomotors with “pH taxis” for active drug delivery in the tumor microenvironment. Nanoscale 2020, 12, 22495–22501. [Google Scholar] [CrossRef] [PubMed]
- Kuchuk, O.; Tuccitto, A.; Citterio, D.; Huber, V.; Camisaschi, C.; Milione, M.; Vergani, B.; Villa, A.; Alison, M.R.; Carradori, S.; et al. pH regulators to target the tumor immune microenvironment in human hepatocellular carcinoma. Oncoimmunology 2018, 7, e1445452. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luo, Z.; Zhang, J.; Luo, T.; Zhou, J.; Zhao, X.; Cai, K. Hollow mesoporous silica nanoparticles facilitated drug delivery via cascade pH stimuli in tumor microenvironment for tumor therapy. Biomaterials 2016, 83, 51–65. [Google Scholar] [CrossRef]
- Lala, A.; Johnson, K.W.; Januzzi, J.L.; Russak, A.J.; Paranjpe, I.; Richter, F.; Zhao, S.; Somani, S.; Van Vleck, T.; Vaid, A.; et al. Prevalence and Impact of Myocardial Injury in Patients Hospitalized With COVID-19 Infection. J. Am. Coll. Cardiol. 2020, 76, 533–546. [Google Scholar] [CrossRef]
- Ke, C.-J.; Lin, Y.-J.; Hu, Y.-C.; Chiang, W.-L.; Chen, K.-J.; Yang, W.-C.; Liu, H.-L.; Fu, C.-C.; Sung, H.-W. Multidrug release based on microneedle arrays filled with pH-responsive PLGA hollow microspheres. Biomaterials 2012, 33, 5156–5165. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ye, Y.; Hochu, G.M.; Sadeghifar, H.; Gu, Z. Enhanced Cancer Immunotherapy by Microneedle Patch-Assisted Delivery of Anti-PD1 Antibody. Nano Lett. 2016, 16, 2334–2340. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Liu, H.; Shi, Z.; Lin, L.; Li, Y.; Wang, M.; Pan, G.; Lei, Y.; Xue, L. Responsive hydrogel-based microneedle dressing for diabetic wound healing. J. Mater. Chem. B 2022, 10, 3501–3511. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, R.; Wang, Y.; Zhang, Y.; Yu, J.; Gu, Z. Recent Advances in Oral and Transdermal Protein Delivery Systems. Angew. Chem. Int. Ed. Engl. 2022, 62, e202214795. [Google Scholar] [CrossRef]
- Hirama, H.; Ishikura, Y.; Kano, S.; Hayase, M.; Mekaru, H. Monodispersed sodium hyaluronate microcapsules for transdermal drug delivery systems. Mater. Adv. 2021, 2, 7007–7016. [Google Scholar] [CrossRef]
- Rawlings, J.S.; Rosler, K.M.; Harrison, D.A. The JAK/STAT signaling pathway. J. Cell Sci. 2004, 117 Pt 8, 1281–1283. [Google Scholar] [CrossRef]
- Ou, B.S.; Saouaf, O.M.; Baillet, J.; Appel, E.A. Sustained delivery approaches to improving adaptive immune responses. Adv. Drug Deliv. Rev. 2022, 187, 114401. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Nam, G.; Choi, Y.; Woo, S.; Yoon, S.H. Bioinspired microneedle insertion for deep and precise skin penetration with low force: Why the application of mechanophysical stimuli should be considered. J. Mech. Behav. Biomed. Mater. 2018, 78, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Shi, X.; Tian, Y.; Gao, F. pH-Responsive Polymer Nanomaterials for Tumor Therapy. Front. Oncol. 2022, 12, 855019. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Z.; Zhang, M.; Ling, G.; Zhang, P. Research progress of microneedles in the treatment of melanoma. J. Control. Release 2022, 348, 631–647. [Google Scholar] [CrossRef] [PubMed]
- Mu, M.; Liang, X.; Chuan, D.; Zhao, S.; Yu, W.; Fan, R.; Tong, A.; Zhao, N.; Han, B.; Guo, G. Chitosan coated pH-responsive metal-polyphenol delivery platform for melanoma chemotherapy. Carbohydr. Polym. 2021, 264, 118000. [Google Scholar] [CrossRef]
- Wei, S.; Quan, G.; Lu, C.; Pan, X.; Wu, C. Dissolving microneedles integrated with pH-responsive micelles containing AIEgen with ultra-photostability for enhancing melanoma photothermal therapy. Biomater. Sci. 2020, 8, 5739–5750. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, Q.; Zhang, P.; Zhao, X.; Wang, Y. Cutaneous microenvironment responsive microneedle patch for rapid gene release to treat subdermal tumor. J. Control. Release 2019, 314, 72–80. [Google Scholar] [CrossRef]
- Ullah, A.; Jang, M.; Khan, H.; Choi, H.J.; An, S.; Kim, D.; Kim, Y.-R.; Kim, U.-K.; Kim, G.M. Microneedle array with a pH-responsive polymer coating and its application in smart drug delivery for wound healing. Sens. Actuators B Chem. 2021, 345, 130441. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Chio, I.I.C.; Tuveson, D.A. ROS in Cancer: The Burning Question. Trends Mol. Med. 2017, 23, 411–429. [Google Scholar] [CrossRef]
- Balzano-Nogueira, L.; Ramirez, R.; Zamkovaya, T.; Dailey, J.; Ardissone, A.N.; Chamala, S.; Serrano-Quilez, J.; Rubio, T.; Haller, M.J.; Concannon, P.; et al. Integrative analyses of TEDDY Omics data reveal lipid metabolism abnormalities, increased intracellular ROS and heightened inflammation prior to autoimmunity for type 1 diabetes. Genome Biol. 2021, 22, 39. [Google Scholar] [CrossRef]
- De Maranon, A.M.; Iannantuoni, F.; Abad-Jimenez, Z.; Canet, F.; Diaz-Pozo, P.; Lopez-Domenech, S.; Jover, A.; Morillas, C.; Marino, G.; Apostolova, N.; et al. Relationship between PMN-endothelium interactions, ROS production and Beclin-1 in type 2 diabetes. Redox Biol. 2020, 34, 101563. [Google Scholar] [CrossRef]
- Idelchik, M.; Begley, U.; Begley, T.J.; Melendez, J.A. Mitochondrial ROS control of cancer. Semin. Cancer Biol. 2017, 47, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Ijaz, B.; Shabbiri, K.; Ahmed, F.; Rehman, S. Oxidative toxicity in diabetes and Alzheimer’s disease: Mechanisms behind ROS/RNS generation. J. Biomed. Sci. 2017, 24, 76. [Google Scholar] [CrossRef]
- Lv, J.; Yang, Z.; Wang, C.; Duan, J.; Ren, L.; Rong, G.; Feng, Q.; Li, Y.; Cheng, Y. Efficient intracellular and in vivo delivery of toxin proteins by a ROS-responsive polymer for cancer therapy. J. Control. Release 2023, 355, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, W.; Zhu, J.; Zhai, Z.; Xie, J.; Zhou, J.; Feng, X.; Feng, B.; Pan, Q.; Li, S.; Venkatesan, R.; et al. ROS-responsive polymer nanoparticles with enhanced loading of dexamethasone effectively modulate the lung injury microenvironment. Acta Biomater. 2022, 148, 258–270. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, Y.; Wang, Y.; Zhang, D.; Zou, Y.; Ruan, W.; Yin, J.; Tao, W.; Park, J.B.; Shi, B. ROS-Responsive Polymeric siRNA Nanomedicine Stabilized by Triple Interactions for the Robust Glioblastoma Combinational RNAi Therapy. Adv. Mater. 2019, 31, e1903277. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Ru, Z.; Song, W.; Chen, L.; Ma, H.; Sun, L. A ROS-responsive polymeric prodrug nanosystem with self-amplified drug release for PSMA (-) prostate cancer specific therapy. J. Nanobiotechnol. 2019, 17, 91. [Google Scholar] [CrossRef]
- Mohammed, F.; Ke, W.; Mukerabigwi, J.F.; AAM, M.J.; Ibrahim, A.; Wang, Y.; Zha, Z.; Lu, N.; Zhou, M.; Ge, Z. ROS-Responsive Polymeric Nanocarriers with Photoinduced Exposure of Cell-Penetrating Moieties for Specific Intracellular Drug Delivery. ACS Appl. Mater. Interfaces 2019, 11, 31681–31692. [Google Scholar] [CrossRef]
- Cao, Z.; Li, D.; Wang, J.; Yang, X. Reactive oxygen species-sensitive polymeric nanocarriers for synergistic cancer therapy. Acta Biomater. 2021, 130, 17–31. [Google Scholar] [CrossRef]
- Ye, H.; Zhou, Y.; Liu, X.; Chen, Y.; Duan, S.; Zhu, R.; Liu, Y.; Yin, L. Recent Advances on Reactive Oxygen Species-Responsive Delivery and Diagnosis System. Biomacromolecules 2019, 20, 2441–2463. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, P.; Yu, J.; Yang, J.; Zhao, J.; Wang, J.; Shen, Q.; Gu, Z. ROS-Responsive Microneedle Patch for Acne Vulgaris Treatment. Adv. Ther. 2018, 1, 1800035. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, H.; Gao, N.; Yang, C.; Zhang, R.; Zhang, X. An efficient FRET based theranostic nanoprobe for hyaluronidase detection and cancer therapy in vitro. Sens. Actuators B Chem. 2021, 344, 130201. [Google Scholar] [CrossRef]
- Fatima, K.; Masood, N.; Ahmad Wani, Z.; Meena, A.; Luqman, S. Neomenthol prevents the proliferation of skin cancer cells by restraining tubulin polymerization and hyaluronidase activity. J. Adv. Res 2021, 34, 93–107. [Google Scholar] [CrossRef]
- Guan, X.; Chen, J.; Hu, Y.; Lin, L.; Sun, P.; Tian, H.; Chen, X. Highly enhanced cancer immunotherapy by combining nanovaccine with hyaluronidase. Biomaterials 2018, 171, 198–206. [Google Scholar] [CrossRef]
- Stern, R. Hyaluronidases in cancer biology. Semin. Cancer Biol. 2008, 18, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xia, R.; Huang, Y.; Zhao, W.; Li, J.; Zhang, X.; Wang, P.; Venkataramanan, R.; Fan, J.; Xie, W.; et al. An immunostimulatory dual-functional nanocarrier that improves cancer immunochemotherapy. Nat. Commun. 2016, 7, 13443. [Google Scholar] [CrossRef] [PubMed]
- Nayak-Kapoor, A.; Hao, Z.; Sadek, R.; Dobbins, R.; Marshall, L.; Vahanian, N.N.; Jay Ramsey, W.; Kennedy, E.; Mautino, M.R.; Link, C.J.; et al. Phase Ia study of the indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor navoximod (GDC-0919) in patients with recurrent advanced solid tumors. J. Immunother. Cancer 2018, 6, 61. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, J.; Hu, Q.; Hochu, G.M.; Xin, H.; Wang, C.; Gu, Z. Synergistic Transcutaneous Immunotherapy Enhances Antitumor Immune Responses through Delivery of Checkpoint Inhibitors. ACS Nano 2016, 10, 8956–8963. [Google Scholar] [CrossRef]
- Power, G.; Moore, Z.; O’Connor, T. Measurement of pH, exudate composition and temperature in wound healing: A systematic review. J. Wound Care 2017, 26, 381–397. [Google Scholar] [CrossRef]
- Villalba-Rodríguez, A.M.; Martínez-González, S.; Sosa-Hernández, J.E.; Parra-Saldívar, R.; Bilal, M.; Iqbal, H.M.N. Nanoclay/Polymer-Based Hydrogels and Enzyme-Loaded Nanostructures for Wound Healing Applications. Gels 2021, 7, 59. [Google Scholar] [CrossRef]
- Lei, X.; Li, M.; Wang, C.; Cui, P.; Qiu, L.; Zhou, S.; Jiang, P.; Li, H.; Zhao, D.; Ni, X.; et al. Degradable microneedle patches loaded with antibacterial gelatin nanoparticles to treat staphylococcal infection-induced chronic wounds. Int. J. Biol. Macromol. 2022, 217, 55–65. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Singh, T.R.R.; Garland, M.J.; Migalska, K.; Majithiya, R.; McCrudden, C.M.; Kole, P.L.; Mahmood, T.M.T.; McCarthy, H.O.; Woolfson, A.D. Hydrogel-Forming Microneedle Arrays for Enhanced Transdermal Drug Delivery. Adv. Funct. Mater. 2012, 22, 4879–4890. [Google Scholar] [CrossRef]
- Kim, M.; Jung, B.; Park, J.-H. Hydrogel swelling as a trigger to release biodegradable polymer microneedles in skin. Biomaterials 2012, 33, 668–678. [Google Scholar] [CrossRef]
- Thakur, R.R.S.; Fallows, S.J.; McMillan, H.L.; Donnelly, R.F.; Jones, D.S. Microneedle-mediated intrascleral delivery of in situ forming thermoresponsive implants for sustained ocular drug delivery. J. Pharm. Pharmacol. 2014, 66, 584–595. [Google Scholar] [CrossRef]
- Dini, V.; Salvo, P.; Janowska, A.; Di Francesco, F.; Barbini, A.; Romanelli, M. Correlation Between Wound Temperature Obtained With an Infrared Camera and Clinical Wound Bed Score in Venous Leg Ulcers. Wounds 2015, 27, 274–278. [Google Scholar]
- Roh, H.; Yoon, Y.J.; Park, J.S.; Kang, D.-H.; Kwak, S.M.; Lee, B.C.; Im, M. Fabrication of High-Density Out-of-Plane Microneedle Arrays with Various Heights and Diverse Cross-Sectional Shapes. Nano-Micro Lett. 2021, 14, 24. [Google Scholar] [CrossRef]
- Seong, K.-Y.; Seo, M.-S.; Hwang, D.Y.; O’Cearbhaill, E.D.; Sreenan, S.; Karp, J.M.; Yang, S.Y. A self-adherent, bullet-shaped microneedle patch for controlled transdermal delivery of insulin. J. Control. Release 2017, 265, 48–56. [Google Scholar] [CrossRef]
- Li, W.; Terry, R.N.; Tang, J.; Feng, M.R.; Schwendeman, S.P.; Prausnitz, M.R. Rapidly separable microneedle patch for the sustained release of a contraceptive. Nat. Biomed. Eng. 2019, 3, 220–229. [Google Scholar] [CrossRef]
- Yin, Y.; Su, W.; Zhang, J.; Huang, W.; Li, X.; Ma, H.; Tan, M.; Song, H.; Cao, G.; Yu, S.; et al. Separable Microneedle Patch to Protect and Deliver DNA Nanovaccines Against COVID-19. ACS Nano 2021, 15, 14347–14359. [Google Scholar] [CrossRef]
- Li, J.Y.; Feng, Y.H.; He, Y.T.; Hu, L.F.; Liang, L.; Zhao, Z.Q.; Chen, B.Z.; Guo, X.D. Thermosensitive hydrogel microneedles for controlled transdermal drug delivery. Acta Biomater. 2022, 153, 308–319. [Google Scholar] [CrossRef]
- Cui, M.; Zheng, M.; Wiraja, C.; Chew SW, T.; Mishra, A.; Mayandi, V.; Lakshminarayanan, R.; Xu, C. Ocular delivery of predatory bacteria with cryomicroneedles against eye infection. Adv. Sci. 2021, 8, 2102327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Yu, J.; Wen, D.; Kahkoska, A.R.; Lu, Y.; Zhang, X.; Buse, J.B.; Gu, Z. Bioresponsive Microneedles with a Sheath Structure for H(2) O(2) and pH Cascade-Triggered Insulin Delivery. Small 2018, 14, e1704181. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Lu, C.; Qin, W.; Chen, M.; Quan, G.; Liu, H.; Wang, L.; Bai, X.; Pan, X.; Wu, C. Construction of a core-shell microneedle system to achieve targeted co-delivery of checkpoint inhibitors for melanoma immunotherapy. Acta Biomater. 2020, 104, 147–157. [Google Scholar] [CrossRef] [PubMed]
- GhavamiNejad, A.; Li, J.; Lu, B.; Zhou, L.; Lam, L.; Giacca, A.; Wu, X.Y. Glucose-Responsive Composite Microneedle Patch for Hypoglycemia-Triggered Delivery of Native Glucagon. Adv. Mater. 2019, 31, e1901051. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Chen, Y.; He, X.; Yang, F.; Han, R.; Yang, C.; Li, W.; Qian, Z. Near-infrared responsive 5-fluorouracil and indocyanine green loaded MPEG-PCL nanoparticle integrated with dissolvable microneedle for skin cancer therapy. Bioact. Mater. 2020, 5, 542–552. [Google Scholar] [CrossRef]
- Chen, M.C.; Ling, M.H.; Wang, K.W.; Lin, Z.W.; Lai, B.H.; Chen, D.H. Near-infrared light-responsive composite microneedles for on-demand transdermal drug delivery. Biomacromolecules 2015, 16, 1598–1607. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, Z.; Wang, Y.; Lin, S.; Yang, S. Photo-responsive degradable hollow mesoporous organosilica nanoplatforms for drug delivery. J. Nanobiotechnol. 2020, 18, 91. [Google Scholar] [CrossRef]
- Sun, L.; Li, Z.; Su, R.; Wang, Y.; Li, Z.; Du, B.; Sun, Y.; Guan, P.; Besenbacher, F.; Yu, M. Phase-Transition Induced Conversion into a Photothermal Material: Quasi-Metallic WO(2.9) Nanorods for Solar Water Evaporation and Anticancer Photothermal Therapy. Angew. Chem. Int. Ed. Engl. 2018, 57, 10666–10671. [Google Scholar] [CrossRef]
- Liang, R.; Tian, R.; Ma, L.; Zhang, L.; Hu, Y.; Wang, J.; Wei, M.; Yan, D.; Evans, D.G.; Duan, X. A Supermolecular Photosensitizer with Excellent Anticancer Performance in Photodynamic Therapy. Adv. Funct. Mater. 2014, 24, 3144–3151. [Google Scholar] [CrossRef]
- Zha, Z.; Yue, X.; Ren, Q.; Dai, Z. Uniform polypyrrole nanoparticles with high photothermal conversion efficiency for photothermal ablation of cancer cells. Adv. Mater. 2013, 25, 777–782. [Google Scholar] [CrossRef]
- Alamdari, S.G.; Amini, M.; Jalilzadeh, N.; Baradaran, B.; Mohammadzadeh, R.; Mokhtarzadeh, A.; Oroojalian, F. Recent advances in nanoparticle-based photothermal therapy for breast cancer. J. Control. Release 2022, 349, 269–303. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, Q.; Sun, B.; Chu, X.; Zhang, M.; She, Z.; Li, Z.; Zhou, N.; Wang, J.; Li, A. MoO(3-x) nanosheets-based platform for single NIR laser induced efficient PDT/PTT of cancer. J. Control. Release 2021, 338, 46–55. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Chen, Z.; Zhang, F.; Wang, Q.; Guo, W.; Wang, K.; Lin, H.; Qu, F. Construct of MoSe(2)/Bi(2)Se(3) nanoheterostructure: Multimodal CT/PT imaging-guided PTT/PDT/chemotherapy for cancer treating. Biomaterials 2019, 217, 119282. [Google Scholar] [CrossRef]
- Hao, Y.; Dong, M.; Zhang, T.; Peng, J.; Jia, Y.; Cao, Y.; Qian, Z. Novel Approach of Using Near-Infrared Responsive PEGylated Gold Nanorod Coated Poly(l-lactide) Microneedles to Enhance the Antitumor Efficiency of Docetaxel-Loaded MPEG-PDLLA Micelles for Treating an A431 Tumor. ACS Appl. Mater. Interfaces 2017, 9, 15317–15327. [Google Scholar] [CrossRef]
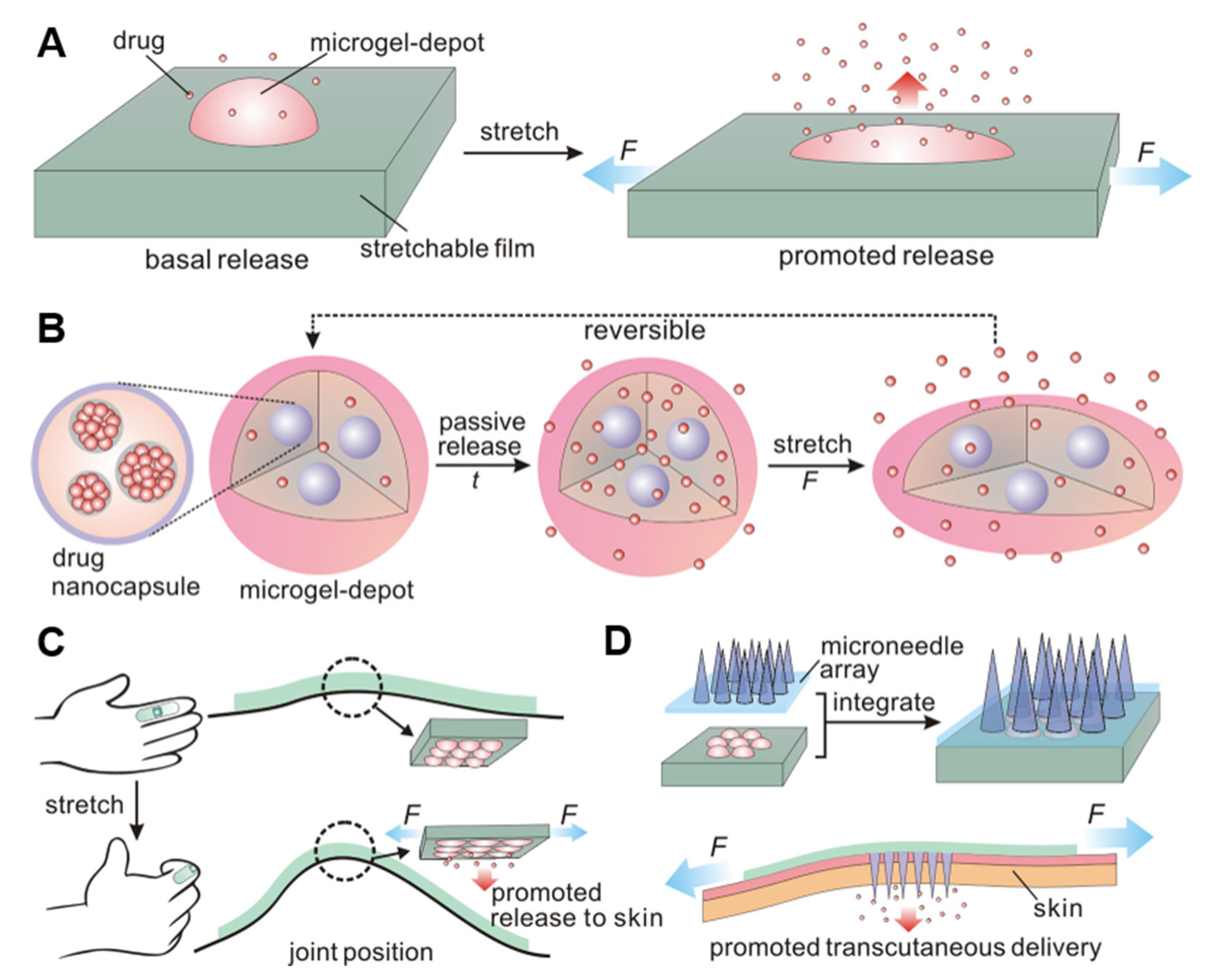
- Di, J.; Yao, S.; Ye, Y.; Cui, Z.; Yu, J.; Ghosh, T.K.; Zhu, Y.; Gu, Z. Stretch-Triggered Drug Delivery from Wearable Elastomer Films Containing Therapeutic Depots. ACS Nano 2015, 9, 9407–9415. [Google Scholar] [CrossRef]
- Jun, H.; Ahn, M.-H.; Choi, I.-J.; Baek, S.-K.; Park, J.-H.; Choi, S.-O. Immediate separation of microneedle tips from base array during skin insertion for instantaneous drug delivery. RSC Adv. 2018, 8, 17786–17796. [Google Scholar] [CrossRef]
- Choi, I.J.; Kang, A.; Ahn, M.H.; Jun, H.; Baek, S.K.; Park, J.H.; Na, W.; Choi, S.O. Insertion-responsive microneedles for rapid intradermal delivery of canine influenza vaccine. J. Control. Release 2018, 286, 460–466. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, X.; Nie, M.; Xu, Y.; Wang, Y.; Shang, L.; Zhao, Y.; Zhao, Y. Photothermal Responsive Microspheres-Triggered Separable Microneedles for Versatile Drug Delivery. Adv. Funct. Mater. 2021, 32, 2110746. [Google Scholar] [CrossRef]
- Semple, S.C.; Akinc, A.; Chen, J.; Sandhu, A.P.; Mui, B.L.; Cho, C.K.; Sah, D.W.Y.; Stebbing, D.; Crosley, E.J.; Yaworski, E.; et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010, 28, 172–176. [Google Scholar] [CrossRef]
- Huang, H.; Hu, D.; Chen, Z.; Xu, J.; Xu, R.; Gong, Y.; Fang, Z.; Wang, T.; Chen, W. Immunotherapy for type 1 diabetes mellitus by adjuvant-free Schistosoma japonicum-egg tip-loaded asymmetric microneedle patch (STAMP). J. Nanobiotechnol. 2022, 20, 377. [Google Scholar] [CrossRef]
- Zheng, Y.; Ye, R.; Gong, X.; Yang, J.; Liu, B.; Xu, Y.; Nie, G.; Xie, X.; Jiang, L. Iontophoresis-driven microneedle patch for the active transdermal delivery of vaccine macromolecules. Microsyst. Nanoeng. 2023, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Niu, B.; Zhao, Y.; Fu, J.; Wen, T.; Liao, K.; Quan, G.; Pan, X.; Wu, C. Multifunctional nanoreactors-integrated microneedles for cascade reaction-enhanced cancer therapy. J. Control. Release 2021, 339, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cao, Y.; Lin, S.; Niu, H.; Zhang, H.; Guan, L.; Shu, C.; Wu, A.; Bian, Y.; Zhu, Y. A responsive microneedle system for efficient anti-melanoma by combining self-enhanced chemodynamic therapy with photothermal therapy. Chem. Eng. J. 2022, 431, 133466. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, G.; Liu, Y.; Sun, L.; Sun, L.; Zhao, Y. Black Phosphorus-Loaded Separable Microneedles as Responsive Oxygen Delivery Carriers for Wound Healing. ACS Nano 2020, 14, 5901–5908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chai, D.; Gao, M.; Xu, B.; Jiang, G. Thermal ablation of separable microneedles for transdermal delivery of metformin on diabetic rats. Int. J. Polym. Mater. Polym. Biomater. 2018, 68, 850–858. [Google Scholar] [CrossRef]
- Hardy, J.G.; Larraneta, E.; Donnelly, R.F.; McGoldrick, N.; Migalska, K.; McCrudden, M.T.; Irwin, N.J.; Donnelly, L.; McCoy, C.P. Hydrogel-Forming Microneedle Arrays Made from Light-Responsive Materials for On-Demand Transdermal Drug Delivery. Mol. Pharm. 2016, 13, 907–914. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, G.; Fu, X.; Wang, Y.; Zhao, Y. Magneto-Responsive Microneedle Robots for Intestinal Macromolecule Delivery. Adv. Mater. 2021, 33, e2104932. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Ye, Y.; DiSanto, R.; Sun, W.; Ranson, D.; Ligler, F.S.; Buse, J.B.; Gu, Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc. Natl. Acad. Sci. USA 2015, 112, 8260–8265. [Google Scholar] [CrossRef]
- Makvandi, P.; Jamaledin, R.; Chen, G.; Baghbantaraghdari, Z.; Zare, E.N.; Di Natale, C.; Onesto, V.; Vecchione, R.; Lee, J.; Tay, F.R.; et al. Stimuli-responsive transdermal microneedle patches. Mater. Today 2021, 47, 206–222. [Google Scholar] [CrossRef]
- Jin, Z.; Zhao, B.; Wan, W.; Li, Y.; Bai, X.; Hu, C.; Wang, J.; Li, Y.; Xin, W.; Kang, L.; et al. Application of microneedle-based vaccines in biosecurity. J. Biosaf. Biosecur. 2022, 4, 75–83. [Google Scholar] [CrossRef]
- Long, L.; Liu, W.; Hu, C.; Yang, L.; Wang, Y. Construction of multifunctional wound dressings with their application in chronic wound treatment. Biomater. Sci. 2022, 10, 4058–4076. [Google Scholar] [CrossRef]
- Higano, C.S.; Small, E.J.; Schellhammer, P.; Yasothan, U.; Gubernick, S.; Kirkpatrick, P.; Kantoff, P.W. Sipuleucel-T. Nat. Rev. Drug Discov. 2010, 9, 513–514. [Google Scholar] [CrossRef]
- Kon, M.; Kiffin, R.; Koga, H.; Chapochnick, J.; Macian, F.; Varticovski, L.; Cuervo, A.M. Chaperone-mediated autophagy is required for tumor growth. Sci. Transl. Med. 2011, 3, 109ra117. [Google Scholar] [CrossRef]
- Tombal, B. Continuous improvement versus innovation: The case for sipuleucel-T. Eur. Urol. 2012, 61, 648–649; discussion 650–641. [Google Scholar] [CrossRef]
- Su, W.; Tan, M.; Wang, Z.; Zhang, J.; Huang, W.; Song, H.; Wang, X.; Ran, H.; Gao, Y.; Nie, G.; et al. Targeted Degradation of PD-L1 and Activation of the STING Pathway by Carbon-Dot-Based PROTACs for Cancer Immunotherapy. Angew. Chem. Int. Ed. Engl. 2023, 62, e202218128. [Google Scholar] [CrossRef]
- Han, X.; Cheng, K.; Xu, Y.; Wang, Y.; Min, H.; Zhang, Y.; Zhao, X.; Zhao, R.; Anderson, G.J.; Ren, L.; et al. Modularly Designed Peptide Nanoprodrug Augments Antitumor Immunity of PD-L1 Checkpoint Blockade by Targeting Indoleamine 2,3-Dioxygenase. J. Am. Chem. Soc. 2020, 142, 2490–2496. [Google Scholar] [CrossRef]
- Moral, J.A.; Leung, J.; Rojas, L.A.; Ruan, J.; Zhao, J.; Sethna, Z.; Ramnarain, A.; Gasmi, B.; Gururajan, M.; Redmond, D.; et al. ILC2s amplify PD-1 blockade by activating tissue-specific cancer immunity. Nature 2020, 579, 130–135. [Google Scholar] [CrossRef]
- Lee, J.; Le, Q.V.; Yang, G.; Oh, Y.K. Cas9-edited immune checkpoint blockade PD-1 DNA polyaptamer hydrogel for cancer immunotherapy. Biomaterials 2019, 218, 119359. [Google Scholar] [CrossRef]
- Gao, M.; Lin, M.; Moffitt, R.A.; Salazar, M.A.; Park, J.; Vacirca, J.; Huang, C.; Shroyer, K.R.; Choi, M.; Georgakis, G.V.; et al. Direct therapeutic targeting of immune checkpoint PD-1 in pancreatic cancer. Br. J. Cancer 2019, 120, 88–96. [Google Scholar] [CrossRef]
- Ye, Y.; Yu, J.; Wen, D.; Kahkoska, A.R.; Gu, Z. Polymeric microneedles for transdermal protein delivery. Adv. Drug Deliv. Rev. 2018, 127, 106–118. [Google Scholar] [CrossRef]
- Raphael, A.P.; Prow, T.W.; Crichton, M.L.; Chen, X.; Fernando, G.J.; Kendall, M.A. Targeted, needle-free vaccinations in skin using multilayered, densely-packed dissolving microprojection arrays. Small 2010, 6, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Gill, H.S.; Andrews, S.N.; Prausnitz, M.R. Kinetics of skin resealing after insertion of microneedles in human subjects. J. Control. Release 2011, 154, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Huang, W.; Yin, Y.; Jiang, Y.; Yang, Q.; Huang, H.; Nie, G.; Wang, H. Stabilizing RNA Nanovaccines with Transformable Hyaluronan Dynamic Hydrogel for Durable Cancer Immunotherapy. Adv. Funct. Mater. 2022, 33, 2204636. [Google Scholar] [CrossRef]
- Martin, J.R.; Howard, M.T.; Wang, S.; Berger, A.G.; Hammond, P.T. Oxidation-Responsive, Tunable Growth Factor Delivery from Polyelectrolyte-Coated Implants. Adv. Healthc. Mater. 2021, 10, e2001941. [Google Scholar] [CrossRef] [PubMed]
- Barberio, A.E.; Smith, S.G.; Correa, S.; Nguyen, C.; Nhan, B.; Melo, M.; Tokatlian, T.; Suh, H.; Irvine, D.J.; Hammond, P.T. Cancer Cell Coating Nanoparticles for Optimal Tumor-Specific Cytokine Delivery. ACS Nano 2020, 14, 11238–11253. [Google Scholar] [CrossRef]
- Zhang, Q.; Dehaini, D.; Zhang, Y.; Zhou, J.; Chen, X.; Zhang, L.; Fang, R.H.; Gao, W.; Zhang, L. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat. Nanotechnol. 2018, 13, 1182–1190. [Google Scholar] [CrossRef]
- Tan, H.; Song, Y.; Chen, J.; Zhang, N.; Wang, Q.; Li, Q.; Gao, J.; Yang, H.; Dong, Z.; Weng, X.; et al. Platelet-Like Fusogenic Liposome-Mediated Targeting Delivery of miR-21 Improves Myocardial Remodeling by Reprogramming Macrophages Post Myocardial Ischemia-Reperfusion Injury. Adv. Sci. 2021, 8, e2100787. [Google Scholar] [CrossRef]
- Sun, J.J.; Chen, Y.C.; Huang, Y.X.; Zhao, W.C.; Liu, Y.H.; Venkataramanan, R.; Lu, B.F.; Li, S. Programmable co-delivery of the immune checkpoint inhibitor NLG919 and chemotherapeutic doxorubicin via a redox-responsive immunostimulatory polymeric prodrug carrier. Acta Pharmacol. Sin. 2017, 38, 823–834. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Ling, Z.; Li, J.; Hu, J.; He, F.; Chen, Q. The role of dendritic cells in the immunomodulation to implanted biomaterials. Int. J. Oral Sci. 2022, 14, 52. [Google Scholar] [CrossRef]
- Bookstaver, M.L.; Zeng, Q.; Oakes, R.S.; Kapnick, S.M.; Saxena, V.; Edwards, C.; Venkataraman, N.; Black, S.K.; Zeng, X.; Froimchuk, E.; et al. Self-Assembly of Immune Signals to Program Innate Immunity through Rational Adjuvant Design. Adv. Sci. 2022, 10, e2202393. [Google Scholar] [CrossRef]
- Leach, D.G.; Young, S.; Hartgerink, J.D. Advances in immunotherapy delivery from implantable and injectable biomaterials. Acta Biomater. 2019, 88, 15–31. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, Y.; Lee, W.; Ren, L.; Liu, B.; Liang, L.; Wang, Z.; Jiang, L. Additive Manufacturing of Honeybee-Inspired Microneedle for Easy Skin Insertion and Difficult Removal. ACS Appl. Mater. Interfaces 2018, 10, 29338–29346. [Google Scholar] [CrossRef]
- Kost, J.; Mathiowitz, E.; Azagury, A. Advances in Drug Delivery and Theranostics. Adv. Funct. Mater. 2021, 31, 2108838. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, G.; Sun, L.; Ye, F.; Shen, X.; Zhao, Y. Claw-inspired microneedle patches with liquid metal encapsulation for accelerating incisional wound healing. Chem. Eng. J. 2021, 406, 126741. [Google Scholar] [CrossRef]
- Abramson, A.; Caffarel-Salvador, E.; Soares, V.; Minahan, D.; Tian, R.Y.; Lu, X.; Dellal, D.; Gao, Y.; Kim, S.; Wainer, J.; et al. A luminal unfolding microneedle injector for oral delivery of macromolecules. Nat. Med. 2019, 25, 1512–1518. [Google Scholar] [CrossRef]
- Gniadecka, M.; Jemec, G.B. Quantitative evaluation of chronological ageing and photoageing in vivo: Studies on skin echogenicity and thickness. Br. J. Dermatol. 1998, 139, 815–821. [Google Scholar] [CrossRef]
- Guo, M.; Wang, Y.; Gao, B.; He, B. Shark Tooth-Inspired Microneedle Dressing for Intelligent Wound Management. ACS Nano 2021, 15, 15316–15327. [Google Scholar] [CrossRef]
- van der Heijden, M.; Vermeulen, L. Stem cells in homeostasis and cancer of the gut. Mol. Cancer 2019, 18, 66. [Google Scholar] [CrossRef]
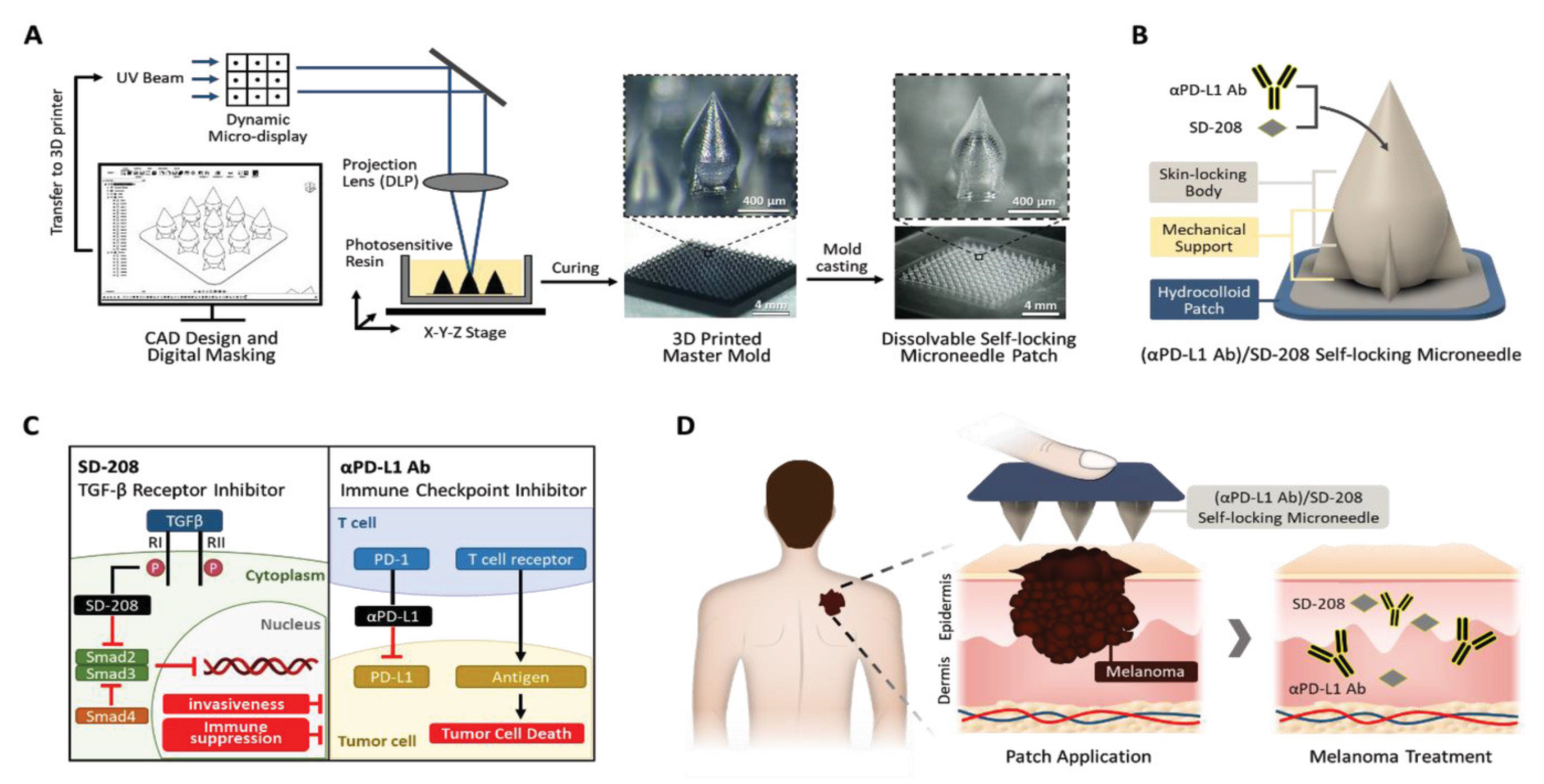
- Joo, S.H.; Kim, J.; Hong, J.; Fakhraei Lahiji, S.; Kim, Y.H. Dissolvable Self-Locking Microneedle Patches Integrated with Immunomodulators for Cancer Immunotherapy. Adv. Mater. 2022, 35, 2209966. [Google Scholar] [CrossRef]
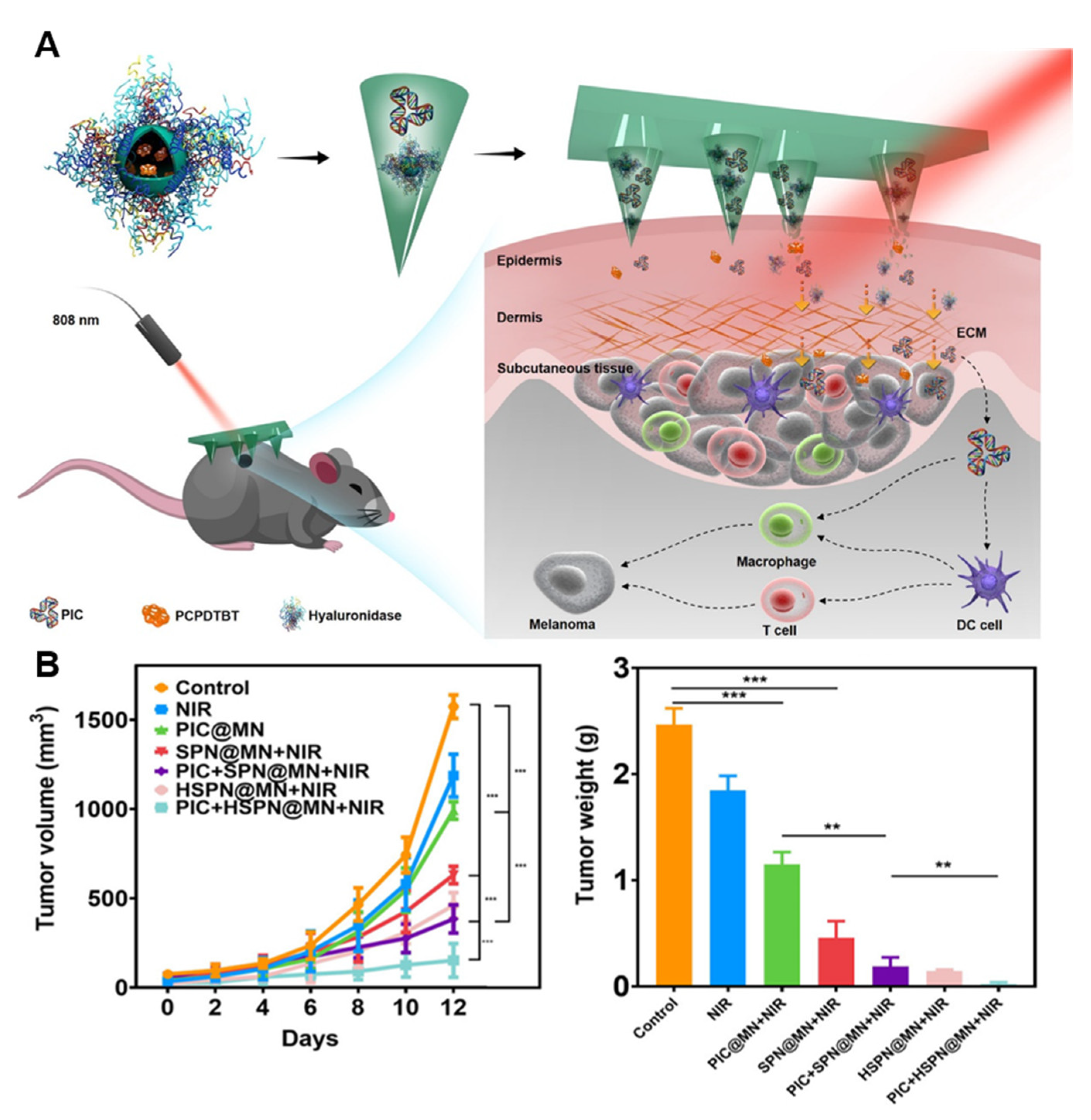
- He, T.; Luo, Y.; Zhang, Q.; Men, Z.; Su, T.; Fan, L.; Chen, H.; Shen, T. Hyalase-Mediated Cascade Degradation of a Matrix Barrier and Immune Cell Penetration by a Photothermal Microneedle for Efficient Anticancer Therapy. ACS Appl. Mater. Interfaces 2021, 13, 26790–26799. [Google Scholar] [CrossRef]
- Thong, T.; Forte, C.A.; Hill, E.M.; Colacino, J.A. Environmental exposures, stem cells, and cancer. Pharmacol. Ther. 2019, 204, 107398. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Tian, N.; Liu, F.; Zhang, Y.; Su, J.; Gao, Y.; Deng, M.; Wei, L.; Ye, J.; Li, H.; et al. A novel dephosphorylation targeting chimera selectively promoting tau removal in tauopathies. Signal Transduct. Target. Ther. 2021, 6, 269. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Cheng, Q.; Zheng, H.; Liu, J.; Liu, L.; Chen, Q. Targeting stemness of cancer stem cells to fight colorectal cancers. Semin. Cancer Biol. 2022, 82, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.; Behrens, A. Are There Specific Cancer Stem Cell Markers? Cancer Res. 2023, 83, 170–172. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Jia, L.; Kim, J.K.; Li, J.; Deng, P.; Zhang, W.; Krebsbach, P.H.; Wang, C.Y. CD276 expression enables squamous cell carcinoma stem cells to evade immune surveillance. Cell Stem Cell 2021, 28, 1597–1613.e7. [Google Scholar] [CrossRef]
- Lee, J.; Jang, E.H.; Kim, J.H.; Park, S.; Kang, Y.; Park, S.; Lee, K.; Kim, J.H.; Youn, Y.N.; Ryu, W. Highly flexible and porous silk fibroin microneedle wraps for perivascular drug delivery. J. Control. Release 2021, 340, 125–135. [Google Scholar] [CrossRef]
- Yang, J.; Li, G.; Yuan, J.; Jing, S.; Wang, X.; Yang, F.; Liu, Y.; Ding, Y.; Li, G.; Xie, G.; et al. A Smart Silk-Based Microneedle for Cancer Stem Cell Synergistic Immunity/Hydrogen Therapy. Adv. Funct. Mater. 2022, 32, 2206406. [Google Scholar] [CrossRef]
- Békés, M.; Langley, D.R.; Crews, C.M. PROTAC targeted protein degraders: The past is prologue. Nat. Rev. Drug Discov. 2022, 21, 181–200. [Google Scholar] [CrossRef]
- Cole, G.; Ali, A.A.; McErlean, E.; Mulholland, E.J.; Short, A.; McCrudden, C.M.; McCaffrey, J.; Robson, T.; Kett, V.L.; Coulter, J.A.; et al. DNA vaccination via RALA nanoparticles in a microneedle delivery system induces a potent immune response against the endogenous prostate cancer stem cell antigen. Acta Biomater. 2019, 96, 480–490. [Google Scholar] [CrossRef]
- Chang, H.; Wen, X.; Li, Z.; Ling, Z.; Zheng, Y.; Xu, C. Co-delivery of dendritic cell vaccine and anti-PD-1 antibody with cryomicroneedles for combinational immunotherapy. Bioeng. Transl. Med. 2022, e10457. [Google Scholar] [CrossRef]
- Xu, J.; Xu, B.; Tao, J.; Yang, Y.; Hu, Y.; Huang, Y. Microneedle-Assisted, DC-Targeted Codelivery of pTRP-2 and Adjuvant of Paclitaxel for Transcutaneous Immunotherapy. Small 2017, 13, 1700666. [Google Scholar] [CrossRef]
- Chen, M.; Yang, D.; Sun, Y.; Liu, T.; Wang, W.; Fu, J.; Wang, Q.; Bai, X.; Quan, G.; Pan, X.; et al. In Situ Self-Assembly Nanomicelle Microneedles for Enhanced Photoimmunotherapy via Autophagy Regulation Strategy. ACS Nano 2021, 15, 3387–3401. [Google Scholar] [CrossRef]
- Chen, S.X.; Ma, M.; Xue, F.; Shen, S.; Chen, Q.; Kuang, Y.; Liang, K.; Wang, X.; Chen, H. Construction of microneedle-assisted co-delivery platform and its combining photodynamic/immunotherapy. J. Control. Release 2020, 324, 218–227. [Google Scholar] [CrossRef]
- Dalvi, M.; Kharat, P.; Thakor, P.; Bhavana, V.; Singh, S.B.; Mehra, N.K. Panorama of dissolving microneedles for transdermal drug delivery. Life Sci. 2021, 284, 119877. [Google Scholar] [CrossRef]
- Dangol, M.; Yang, H.; Li, C.G.; Lahiji, S.F.; Kim, S.; Ma, Y.; Jung, H. Innovative polymeric system (IPS) for solvent-free lipophilic drug transdermal delivery via dissolving microneedles. J. Control. Release 2016, 223, 118–125. [Google Scholar] [CrossRef]
- Alkilani, A.Z.; Nasereddin, J.; Hamed, R.; Nimrawi, S.; Hussein, G.; Abo-Zour, H.; Donnelly, R.F. Beneath the Skin: A Review of Current Trends and Future Prospects of Transdermal Drug Delivery Systems. Pharmaceutics 2022, 14, 1152. [Google Scholar] [CrossRef]




| Design of Microneedle-Based Immunotherapy | Stimulus | Payload | Application | Ref. |
|---|---|---|---|---|
| H2O2-labile and positively charged amphiphilic diblock copolymer | Glucose/H2O2/pH | Insulin | Diabetes | [102] |
| Dimethylmaleic anhydride-modified polylysine | pH | p53 DNA | Cancer therapy | [66] |
| Hyaluronic acid (HA) = integrated dextran nanoparticles | pH | aPD1 | Cancer immunotherapy | [56] |
| Gelatin methacrylate (GelMa)/4-(2-acrylamidoethylcarbamoyl)-3-fluorophenylboronic acid (AFPBA) | Glucose | Gluconic insulin | Diabetic wound | [55] |
| 1-methyl-DL-tryptophan-conjugated HA | Hyaluronidase (HAase) | Anti-PD1 antibody (aPD1) | Cancer immunotherapy | [103] |
| Methacrylated hyaluronic acid (MeHA) | Low glucose | Glucagon | Hypoglycemia | [104] |
| Biomass chitosan microneedle array (CSMNA) patch | Temperature | Vascular endothelial growth factor | Wound healing | [47] |
| Polyvinyl alcohol as the tip and hyaluronic acid/diatomaceous earth as the base | ROS | Clindamycin | Acne vulgaris | [81] |
| Gelatin methacrylate (GelMa)/4-(2-acrylamidoethylcarbamoyl)-3-fluorophenylboronic acid (AFPBA) | Glucose | Gluconic insulin | Diabetic wound | [55] |
| Design of Microneedle-Based Immunotherapy | Stimulus | Payload | Application | Ref. |
|---|---|---|---|---|
| Dissolvable hyaluronic acid (HA) tips and biocompatible polycaprolactone (PCL) bases | Mechanical stress | Antigens derived from canine influenza virus | Influenza vaccination | [117] |
| Photothermal black-phosphorus (BP) and phase-changing gelatin | Near-infrared | Interferon γ (IFN-γ) and Dexa-methasone | Lupus erythematosus treatment | [118] |
| Hyaluronic acid-based microneedles encapsulating tumor lysates, melanin, and adjuvants | Near-infrared | B16F10 whole tumor lysate | Cancer immunotherapy | [119] |
| Asymmetric microneedles loaded with S. japonicum egg tips on CA-CMC | Mechanical stress | Schistosoma japonicum egg | Type 1 diabetes mellitus | [120] |
| A double-sided adhesive impermeable gasket, MN arrays, and Ag/AgCl electrodes | Electric | Ovalbumin | COVID-19 | [121] |
| Rolling structures and a microneedle electrode array | Electric | siRNA(PD-L1) alone or combined with aPD-1 or immunoadjuvant of CPG2395 | c\Cancer immunotherapy | [2] |
| Monomethoxy-poly (ethylene glycol)-polycaprolactone-loaded HA | Near-infrared | 5-fluorouracil (5-Fu) | Cancer therapy | [105] |
| Homologous zeolitic imidazolate framework-8 (ZIF-8) | Near-infrared | Glucose oxidase/catalase (CAT) | Cancer therapy | [122] |
| Poly(vinylpyrrolidone) (PVP) as a substrate | Near-infrared | CuO2 nanoparticles | Cancer therapy | [123] |
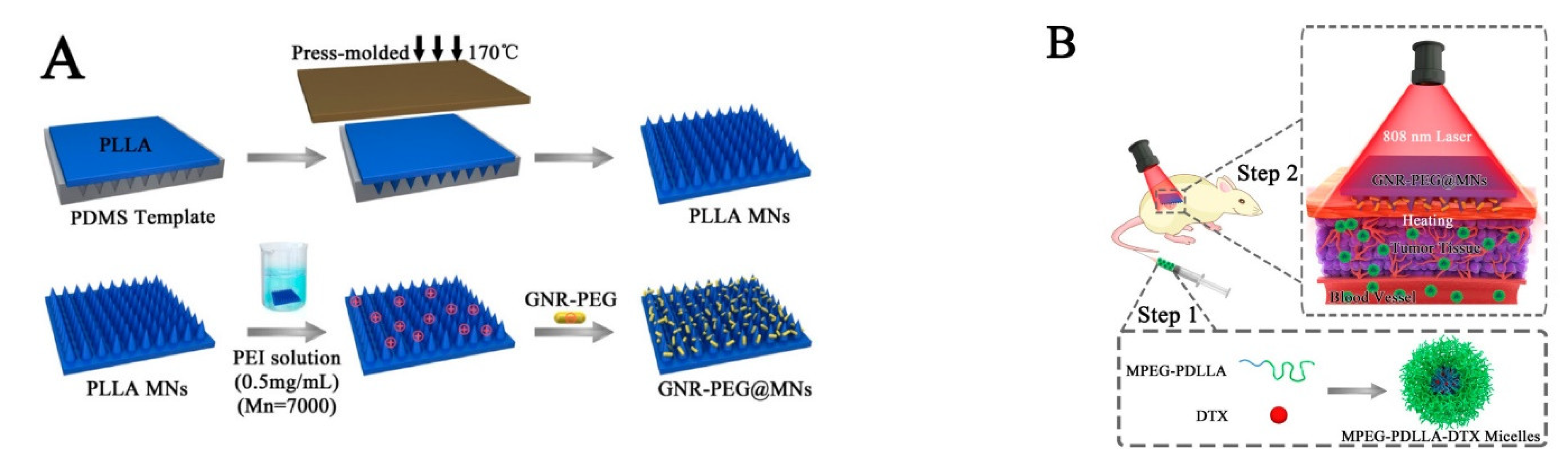
| PEGylated gold nanorod (GNR-PEG) coated poly (L-lactide) | Near-infrared | Docetaxel | Cancer therapy | [114] |
| Polyvinyl acetate (PVA) as base/gelatin methacryloyl (GelMA) as tip | Near-infrared | Black phosphorus/quantum dots | Wound healing | [124] |
| Polycaprolactone | Thermal stimulus | Metformin | Diabetes | [125] |
| 2-hydroxyethyl methacrylate and ethylene glycol Di methacrylate (EGDMA) | UV radiation | Ibuprofen | Pain relief | [126] |
| A magnetically responsive microneedle robot manufactured with 3D printing and encapsulation in commercial enteric capsules. | Magnetic fields | Insulin | Diabetes | [127] |
| Design of Microneedle-Based Immunotherapy | Stimulus | Payload | Application | Ref. |
|---|---|---|---|---|
| Ammonia borane-loaded mesoporous silica nanoparticle (AB-MSN)-encapsulated polycaprolactone | Thermal | aPD-1-loaded silk fibroin (SF) | Melanoma immunotherapy | [167] |
| Hyaluronic acid | Near-infrared | Tumor lysate containing melanin | Melanoma immunotherapy | [168] |
| Polyvinyl acetate (PVA) | Skin environment soluble | RALA/pDNA nanoparticles | TRAMP-C1 tumors | [169] |
| Cryomicroneedles (cryoMNs) | Skin temperature soluble | Dendritic cell (DC)/aPD1 | Melanoma immunotherapy | [170] |
| Positively charged ultra-pH-responsive oligo sulfamethazine conjugated poly (β-amino ester urethane) (OSM-(PEG-PAEU)) and negatively charged immunostimulatory adjuvant poly(I:C) | pH | Immunostimulatory adjuvant poly(I:C)/DNA polyplex | Melanoma immunotherapy | [42] |
| Mannosylated N,N,N-trimethyl chitosan (mTMC) as the gene vector and paclitaxel as the adjuvant | Skin environment soluble | Melanoma antigen tyrosinase-related protein-2 (Trp-2) | Melanoma immunotherapy | [171] |
| Hyaluronic acid | NIR irradiation | Immunogenic cell death-inducer (IR780) and autophagy inhibitor (chloroquine, CQ) coencapsulated micelles (C/I-Mil) | Photoimmunotherapy | [172] |
| Biocompatible hyaluronic acid integrated with the pH-sensitive dextran nanoparticles | pH/UV light | Checkpoint inhibitor anti-CTLA4 antibody (aCTLA4) | 4T1 models photodynamic/immunotherapy | [173] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Song, H.; Sun, T.; Wang, H. Responsive Microneedles as a New Platform for Precision Immunotherapy. Pharmaceutics 2023, 15, 1407. https://doi.org/10.3390/pharmaceutics15051407
Liu X, Song H, Sun T, Wang H. Responsive Microneedles as a New Platform for Precision Immunotherapy. Pharmaceutics. 2023; 15(5):1407. https://doi.org/10.3390/pharmaceutics15051407
Chicago/Turabian StyleLiu, Xinyang, Haohao Song, Tairan Sun, and Hai Wang. 2023. "Responsive Microneedles as a New Platform for Precision Immunotherapy" Pharmaceutics 15, no. 5: 1407. https://doi.org/10.3390/pharmaceutics15051407
APA StyleLiu, X., Song, H., Sun, T., & Wang, H. (2023). Responsive Microneedles as a New Platform for Precision Immunotherapy. Pharmaceutics, 15(5), 1407. https://doi.org/10.3390/pharmaceutics15051407






