Tracers for Cardiac Imaging: Targeting the Future of Viable Myocardium
Abstract
1. Introduction
2. Pathophysiological Bases of Current Tracers
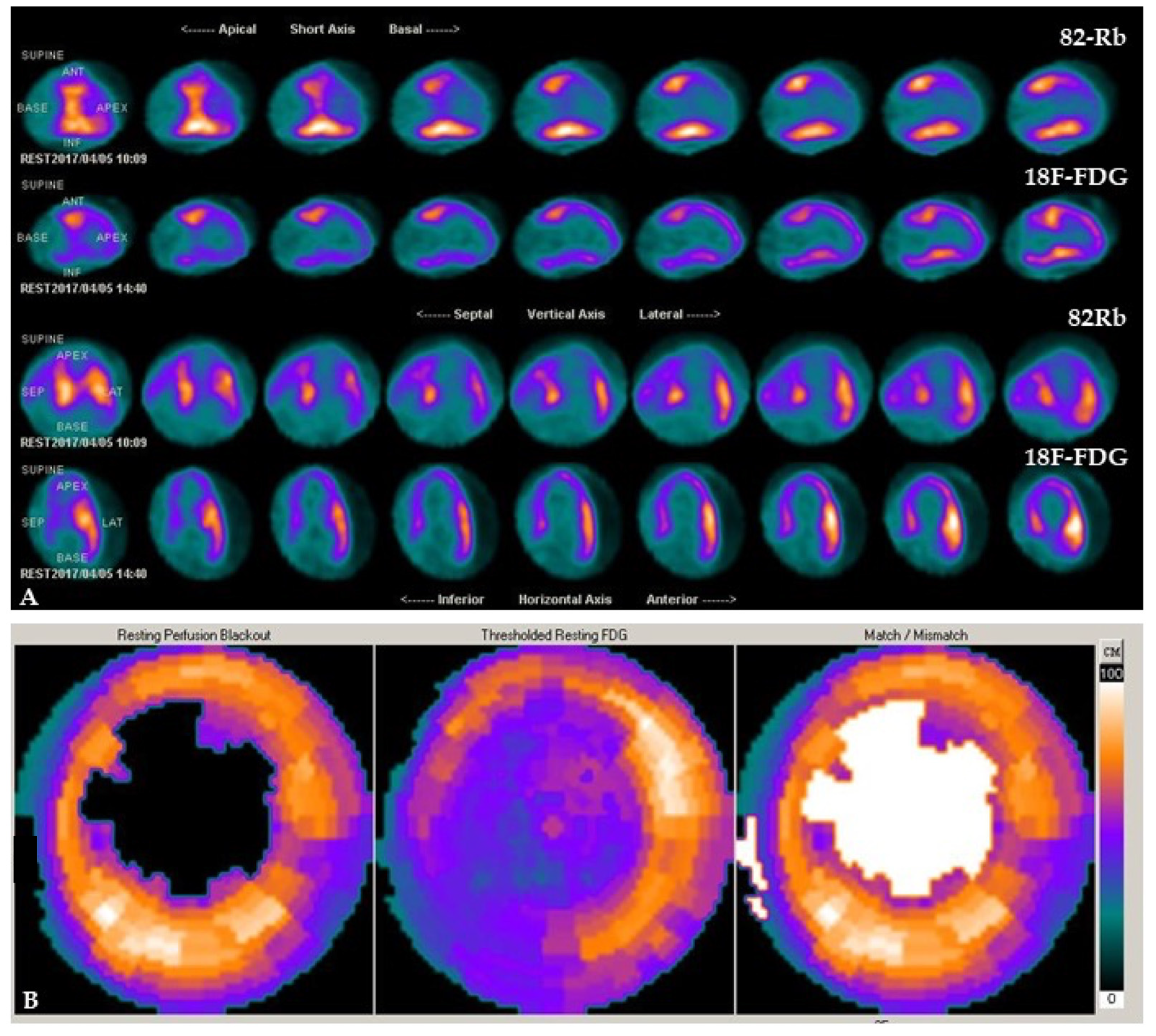
2.1. PET Tracers
2.2. SPECT Tracers
3. The Way to Future Imaging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATP | adenosine triphosphate |
| BMIPP | beta-methyl-iodophenyl-pentadecanoic acid |
| CCR | C-C chemokine receptor type |
| CT | computed tomography |
| FAP | fibroblast activation protein |
| FDG | 2-deoxy-2-fluoro-D-glucose |
| FBBG | flubrobenguane |
| HED | hydroxyephedrine |
| MFBG | meta-fluorobenzylguanidine |
| MIBG | metaiodobenzylguanidine |
| MR | magnetic resonance |
| PET | positron emission tomography |
| SPECT | single-photon emission computed tomography |
References
- Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 9 December 2022).
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, E.J.; Lee, K.L.; Jones, R.H.; Al-Khalidi, H.R.; Hill, J.A.; Panza, J.A.; Michler, R.E.; Bonow, R.O.; Doenst, T.; Petrie, M.C.; et al. Coronary-Artery Bypass Surgery in Patients with Ischemic Cardiomyopathy. N. Engl. J. Med. 2016, 374, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Diamond, G.A.; Forrester, J.S.; deLuz, P.L.; Wyatt, H.L.; Swan, H.J. Post-extrasystolic potentiation of ischemic myocardium by atrial stimulation. Am. Heart J. 1978, 95, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E.; Rutherford, J.D. Reversible ischemic left ventricular dysfunction: Evidence for the “hibernating myocardium”. J. Am. Coll. Cardiol. 1986, 8, 1467–1470. [Google Scholar] [CrossRef][Green Version]
- Alderman, E.L.; Fisher, L.D.; Litwin, P.; Kaiser, G.C.; Myers, W.O.; Maynard, C.; Levine, F.; Schloss, M. Results of coronary artery surgery in patients with poor left ventricular function (CASS). Circulation 1983, 68, 785–795. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pegg, T.J.; Selvanayagam, J.B.; Jennifer, J.; Francis, J.M.; Karamitsos, T.D.; Dall’Armellina, E.; Smith, K.L.; Taggart, D.P.; Neubauer, S. Prediction of global left ventricular functional recovery in patients with heart failure undergoing surgical revascularisation, based on late gadolinium enhancement cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2010, 12, 56. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Panza, J.A.; Ellis, A.M.; Al-Khalidi, H.R.; Holly, T.A.; Berman, D.S.; Oh, J.K.; Pohost, G.M.; Sopko, G.; Chrzanowski, L.; Mark, D.B.; et al. Myocardial viability and long-term outcomes in ischemic cardiomyopathy. N. Engl. J. Med. 2019, 381, 739–748. [Google Scholar] [CrossRef]
- Perera, D.; Clayton, T.; O’Kane, P.D.; Greenwood, J.P.; Weerackody, R.; Ryan, M.; Morgan, H.P.; Dodd, M.; Evans, R.; Canter, R.; et al. Percutaneous Revascularization for Ischemic Left Ventricular Dysfunction. N. Engl. J. Med. 2022, 387, 1351–1360. [Google Scholar] [CrossRef]
- Cleland, J.G.; Pennell, D.J.; Ray, S.G.; Coats, A.J.; Macfarlane, P.W.; Murray, G.D.; Mule, J.D.; Vered, Z.; Lahri, A.; Carvedilol hibernating reversible ischaemia trial: Marker of success investigators (2003). Myocardial viability as a determinant of the ejection fraction response to carvedilol in patients with heart failure (CHRISTMAS trial): Randomised controlled trial. Lancet 2003, 362, 14–21. [Google Scholar] [CrossRef]
- Ypenburg, C.; Schalij, M.J.; Bleeker, G.B.; Steendijk, P.; Boersma, E.; Dibbets-Schneider, P.; Stokkel, M.P.; van der Wall, E.E.; Bax, J.J. Impact of viability and scar tissue on response to cardiac resynchronization therapy in ischaemic heart failure patients. Eur. Heart J. 2007, 28, 33–41. [Google Scholar] [CrossRef]
- Garcia, M.J.; Kwong, R.Y.; Scherrer-Crosbie, M.; Taub, C.C.; Blankstein, R.; Lima, J.; Bonow, R.O.; Eshtehardi, P.; Bois, J.P.; American Heart Association Council on Cardiovascular Radiology and Intervention and Council on Clinical Cardiology. State of the Art: Imaging for Myocardial Viability: A Scientific Statement From the American Heart Association. Circ. Cardiovasc. Imaging 2020, 13, e000053. [Google Scholar] [CrossRef] [PubMed]
- Vanoverschelde, J.L.; Wijns, W.; Borgers, M.; Heyndrickx, G.; Depré, C.; Flameng, W.; Melin, J.A. Chronic myocardial hibernation in humans. From bedside to bench. Circulation 1997, 95, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Wijns, W.; Vatner, S.F.; Camici, P.G. Hibernating myocardium. N. Engl. J. Med. 1998, 339, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Gewirtz, H.; Dilsizian, V. Myocardial viability: Survival mechanisms and molecular imaging targets in acute and chronic ischemia. Circ. Res. 2017, 120, 1197–1212. [Google Scholar] [CrossRef]
- Cheirif, J.; Murgo, J.P. Assessment of myocardial viability by dobutamine echocardiography. Coron. Artery Dis. 1995, 6, 600–605. [Google Scholar] [CrossRef]
- Hoffmann, R.; Lethen, H.; Marwick, T.; Arnese, M.; Fioretti, P.; Pingitore, A.; Picano, E.; Buck, T.; Erbel, R.; Flachskampf, F.A.; et al. Analysis of interinstitutional observer agreement in interpretation of dobutamine stress echocardiograms. J. Am. Coll. Cardiol. 1996, 27, 330–336. [Google Scholar] [CrossRef][Green Version]
- Amzulescu, M.S.; De Craene, M.; Langet, H.; Pasquet, A.; Vancraeynest, D.; Pouleur, A.C.; Vanoverschelde, J.L.; Gerber, B.L. Myocardial strain imaging: Review of general principles, validation, and sources of discrepancies. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 605–619. [Google Scholar] [CrossRef][Green Version]
- Shah, D.V.; Kalekar, D.T.; Gupta, D.A.; Lamghare, D.P. Role of late gadolinium enhancement in the assessment of myocardial viability. Cureus 2022, 14, e22844. [Google Scholar] [CrossRef]
- Parikh, K.; Choy-Shan, A.; Ghesani, M.; Donnino, R. Multimodality imaging of myocardial viability. Curr. Cardiol. Rep. 2021, 23, 5. [Google Scholar] [CrossRef]
- Minamimoto, R.; Nakajima, K.; Okazaki, O. Proliferation PET tracer 11C-4DST PET/CT depicts hibernating myocardium. J. Nucl. Cardiol. 2021, 28, 2379–2383. [Google Scholar] [CrossRef]
- Dell’Aversana, S.; Ascione, R.; De Giorgi, M.; De Lucia, D.R.; Cuocolo, R.; Boccalatte, M.; Sibilio, G.; Napolitano, G.; Muscogiuri, G.; Sironi, S.; et al. Dual-energy CT of the heart: A review. J. Imaging 2022, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Wang, Y.; Zhao, Y.; Chen, Q.; Gao, Y.; Zhou, M.; Liu, B.; Han, R.; Sun, K. Evaluation of myocardial viability in patients with myocardial ischemia reperfusion injury using the dual-energy CT myocardial blood pool imaging. Eur. Radiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Acampa, W.; Petretta, M.; Cuocolo, A. Nuclear medicine procedures in cardiovascular diseases: An evidence-based approach. Q. J. Nucl. Med. 2002, 46, 323–330. [Google Scholar] [PubMed]
- Klocke, F.J.; Baird, M.G.; Lorell, B.H.; Bateman, T.M.; Messer, J.V.; Berman, D.S.; O’Gara, P.T.; Carabello, B.A.; Russell, R.O.; Cerqueira, M.D., Jr.; et al. American Society for Nuclear Cardiology. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging—Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). J. Am. Coll. Cardiol. 2003, 42, 1318–1333. [Google Scholar]
- Bax, J.J.; Poldermans, D.; Elhendy, A.; Boersma, E.; Rahimtoola, S.H. Sensitivity, specificity, and predictive accuracies of various noninvasive techniques for detecting hibernating myocardium. Curr. Probl. Cardiol. 2001, 26, 147–186. [Google Scholar] [CrossRef]
- Dilsizian, V.; Bacharach, S.L.; Beanlands, R.S.; Bergmann, S.R.; Delbeke, D.; Dorbala, S.; Gropler, R.J.; Knuuti, J.; Schelbert, H.R.; Travin, M.I. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J. Nucl. Cardiol. 2016, 23, 1187–1226. [Google Scholar] [CrossRef][Green Version]
- Slart, R.H.; Bax, J.J.; van der Wall, E.E.; van Veldhuisen, D.J.; Jager, P.L.; Dierckx, R.A. Nuclear cardiac imaging for the assessment of myocardial viability. Neth. Heart J. 2005, 13, 408–415. [Google Scholar]
- Thompson, K.; Saab, G.; Birnie, D.; Chow, B.J.; Ukkonen, H.; Ananthasubramaniam, K.; Dekemp, R.A.; Garrard, L.; Ruddy, T.D.; Dasilva, J.N.; et al. Is septal glucose metabolism altered in patients with left bundle branch block and ischemic cardiomyopathy? J. Nucl. Med. 2006, 47, 1763–1768. [Google Scholar]
- Kobylecka, M.; Mączewska, J.; Fronczewska-Wieniawska, K.; Mazurek, T.; Płazińska, M.T.; Królicki, L. Myocardial viability assessment in 18FDG PET/CT study (18FDG PET myocardial viability assessment). Nucl. Med. Rev. Cent. East. Eur. 2012, 15, 52–60. [Google Scholar] [CrossRef]
- Di Carli, M.F.; Asgarzadie, F.; Schelbert, H.R.; Brunken, R.C.; Laks, H.; Phelps, M.E.; Maddahi, J. Quantitative relation between myocardial viability and improvement in heart failure symptoms after revascularization in patients with ischemic cardiomyopathy. Circulation 1995, 92, 3436–3444. [Google Scholar] [CrossRef]
- Beanlands, R.S.; Ruddy, T.D.; deKemp, R.A.; Iwanochko, R.M.; Coates, G.; Freeman, M.; Nahmias, C.; Hendry, P.; Burns, R.J.; Lamy, A.; et al. Positron emission tomography and recovery following revascularization (PARR-1): The importance of scar and the development of a prediction rule for the degree of recovery of left ventricular function. J. Am. Coll. Cardiol. 2002, 40, 1735–1743. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Medical Advisory Secretariat. Positron emission tomography for the assessment of myocardial viability: An evidence-based analysis. Ont. Health Technol. Assess. Ser. 2005, 5, 1–167. [Google Scholar]
- Saha, G.B.; MacIntyre, W.J.; Brunken, R.C.; Go, R.T.; Raja, S.; Wong, C.O.; Chen, E.Q. Present assessment of myocardial viability by nuclear imaging. Semin. Nucl. Med. 1996, 26, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Henes, C.G.; Bergmann, S.R.; Walsh, M.N.; Sobel, B.E.; Geltman, E.M. Assessment of myocardial oxidative metabolic reserve with positron emission tomography and carbon-11 acetate. J. Nucl. Med. 1989, 30, 1489–1499. [Google Scholar]
- Grönman, M.; Tarkia, M.; Stark, C.; Vähäsilta, T.; Kiviniemi, T.; Lubberink, M.; Halonen, P.; Kuivanen, A.; Saunavaara, V.; Tolvanen, T.; et al. Assessment of myocardial viability with [15O]water PET: A validation study in experimental myocardial infarction. J. Nucl. Cardiol. 2021, 28, 1271–1280. [Google Scholar] [CrossRef][Green Version]
- Cuocolo, A.; Nicolai, E.; Petretta, M.; Morisco, C.; De Luca, N.; Salvatore, M.; Trimarco, B. One-year effect of myocardial revascularization on resting left ventricular function and regional thallium uptake in chronic CAD. J. Nucl. Med. 1997, 38, 1684–1692. [Google Scholar]
- Cuocolo, A.; Petretta, M.; Nicolai, E.; Pace, L.; Bonaduce, D.; Salvatore, M.; Trimarco, B. Successful coronary revascularization improves prognosis in patients with previous myocardial infarction and evidence of viable myocardium at thallium-201 imaging. Eur. J. Nucl. Med. 1998, 25, 60–68. [Google Scholar] [CrossRef]
- Berger, B.C.; Petretta, M.; Cuocolo, A.; Bonaduce, D.; Nicolai, E.; Cardei, S.; Berardino, S.; Ianniciello, A.; Apicella, C.; Bianchi, V.; et al. Incremental prognostic value of thallium reinjection after stress-redistribution imaging in patients with previous myocardial infarction and left ventricular dysfunction. J. Nucl. Med. 1997, 38, 195–200. [Google Scholar]
- Dilsizian, V.; Rocco, T.P.; Freedman, N.M.; Leon, M.B.; Bonow, R.O. Enhanced detection of ischemic but viable myocardium by the reinjection of thallium after stress-redistribution imaging. N. Engl. J. Med. 1990, 323, 141–146. [Google Scholar] [CrossRef]
- Schinkel, A.F.; Bax, J.J.; Poldermans, D.; Elhendy, A.; Ferrari, R.; Rahimtoola, S.H. Hibernating myocardium: Diagnosis and patient outcomes. Curr. Probl. Cardiol. 2007, 32, 375–410. [Google Scholar] [CrossRef]
- Udelson, J.E.; Coleman, P.S.; Metherall, J.; Pandian, N.G.; Gomez, A.R.; Griffith, J.L.; Shea, N.L.; Oates, E.; Konstam, M.A. Predicting recovery of severe regional ventricular dysfunction. Comparison of resting scintigraphy with 201Tl and 99mTc-sestamibi. Circulation 1994, 89, 2552–2561. [Google Scholar] [CrossRef] [PubMed]
- Bisi, G.; Sciagrà, R.; Santoro, G.M.; Fazzini, P.F. Rest technetium-99m sestamibi tomography in combination with short-term administration of nitrates: Feasibility and reliability for prediction of postrevascularization outcome of asynergic territories. J. Am. Coll. Cardiol. 1994, 24, 1282–1289. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boschi, A.; Uccelli, L.; Marvelli, L.; Cittanti, C.; Giganti, M.; Martini, P. Technetium-99m Radiopharmaceuticals for Ideal Myocardial Perfusion Imaging: Lost and Found Opportunities. Molecules 2022, 10, 1188. [Google Scholar] [CrossRef] [PubMed]
- Sciagrà, R.; Bisi, G.; Santoro, G.M.; Zerauschek, F.; Sestini, S.; Pedenovi, P.; Pappagallo, R.; Fazzini, P.F. Comparison of baseline-nitrate technetium-99m sestamibi with rest-redistribution thallium-201 tomography in detecting viable hibernating myocardium and predicting postrevascularization recovery. J. Am. Coll. Cardiol. 1997, 30, 384–391. [Google Scholar] [CrossRef][Green Version]
- Cuocolo, A.; Acampa, W.; Nicolai, E.; Pace, L.; Petretta, M.; Salvatore, M. Quantitative thallium-201 and technetium 99m sestamibi tomography at rest in detection of myocardial viability in patients with chronic ischemic left ventricular dysfunction. J. Nucl. Cardiol. 2000, 7, 8–15. [Google Scholar] [CrossRef]
- Acampa, W.; Cuocolo, A.; Petretta, M.; Bruno, A.; Castellani, M.; Finzi, A.; Gerundini, P. Tetrofosmin imaging in the detection of myocardial viability in patients with previous myocardial infarction: Comparison with sestamibi and Tl-201 scintigraphy. J. Nucl. Cardiol. 2002, 9, 33–40. [Google Scholar] [CrossRef]
- Nishimura, T.; Uehara, T.; Shimonagata, T.; Nagata, S.; Haze, K. Clinical experience of 1231-BMIPP myocardial imaging for myocardial infarction and hypertrophic cardiomyopathy. Ann. Nucl. Med. 1993, 7, 35–40. [Google Scholar]
- Kurata, C.; Tawahara, K.; Okayama, K.; Kenichi, W.; Yasushi, K.; Akira, Y.; Noboru, K.M. Myocardial imaging with radioiodinated beta-methyl-branched fatty acid in cardiomyopathy. Ann. Nucl. Med. 1993, 7 (Suppl. 2), 27–33. [Google Scholar]
- Biswas, S.K.; Sarai, M.; Hishida, H.; Ozaki, Y. 123I-BMIPP fatty acid analogue imaging is a novel diagnostic and prognostic approach following acute myocardial infarction. Singap. Med. J. 2009, 50, 943–948. [Google Scholar]
- Nakata, T.; Hashimoto, A.; Eguchi, M. Cardiac BMIPP imaging in acute myocardial infarction. Int. J. Card. Imaging 1999, 15, 21–26. [Google Scholar] [CrossRef]
- Seki, H.; Toyama, T.; Higuchi, K.; Kasama, S.; Ueda, T.; Seki, R.; Hatori, T.; Endo, K.; Kurabayashi, M. Prediction of functional improvement of ischemic myocardium with (123I-BMIPP SPECT and 99mTc-tetrofosmin SPECT imaging: A study of patients with large acute myocardial infarction and receiving revascularization therapy. Circ. J. 2005, 69, 311–319. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Underwood, S.R.; Bax, J.J.; vom Dahl, J.; Henein, M.Y.; Knuuti, J.; van Rossum, A.C.; Schwarz, E.R.; Vanoverschelde, J.L.; van der Wall, E.E.; Wijns, W.; et al. Imaging techniques for the assessment of myocardial hibernation. Report of a Study Group. of the European Society of Cardiology. Eur. Heart J. 2004, 25, 815–836. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Glasenapp, A.; Hess, A.; Thackeray, J.T. Molecular imaging in nuclear cardiology: Pathways to individual precision medicine. J. Nucl. Cardiol. 2020, 27, 2195–2201. [Google Scholar] [CrossRef]
- Borchert, T.; Beitar, L.; Langer, L.B.N.; Polyak, A.; Wester, H.J.; Ross, T.L.; Hilfiker-Kleiner, D.; Bengel, F.M.; Thackeray, J.T. Dissecting the target leukocyte subpopulations of clinically relevant inflammation radiopharmaceuticals. J. Nucl. Cardiol. 2021, 28, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Wargocka-Matuszewska, W.; Uhrynowski, W.; Rozwadowska, N.; Rogulski, Z. Recent advances in cardiovascular diseases research using animal models and PET radioisotope tracers. Int. J. Mol. Sci. 2022, 24, 353. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, J.T.; Bengel, F.M. Molecular imaging of myocardial inflammation with positron emission tomography post-ischemia: A determinant of subsequent remodeling or recovery. JACC Cardiovasc. Imaging 2018, 11, 1340–1355. [Google Scholar] [CrossRef]
- Heo, G.S.; Kopecky, B.; Sultan, D.; Ou, M.; Feng, G.; Bajpai, G.; Zhang, X.; Luehmann, H.; Detering, L.; Su, Y.; et al. Molecular imaging visualizes recruitment of inflammatory monocytes and macrophages to the injured heart. Circ. Res. 2019, 124, 881–890. [Google Scholar] [CrossRef]
- Liu, Y.; Li, W.; Luehmann, H.P.; Zhao, Y.; Detering, L.; Sultan, D.H.; Hsiao, H.M.; Krupnick, A.S.; Gelman, A.E.; Combadiere, C.; et al. Noninvasive imaging of CCR2+ cells in ischemia-reperfusion injury after lung transplantation. Am. J. Transplant. 2016, 16, 3016–3023. [Google Scholar] [CrossRef][Green Version]
- Hess, A.; Thackeray, J.T.; Wollert, K.C.; Bengel, F.M. Radionuclide image-guided repair of the heart. JACC Cardiovasc. Imaging 2020, 13, 2415–2429. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef][Green Version]
- Segura, A.M.; Frazier, O.H.; Buja, L.M. Fibrosis and heart failure. Heart Fail. Rev. 2014, 19, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Ponsiglione, A.; Ascione, R.; Nappi, C.; Imbriaco, M.; Klain, M.; Cuocolo, R.; Cuocolo, A.; Petretta, M. Cardiac hybrid imaging: Novel tracers for novel targets. J. Geriatr. Cardiol. 2021, 18, 748–758. [Google Scholar] [PubMed]
- Garin-Chesa, P.; Old, L.J.; Rettig, W.J. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc. Natl. Acad. Sci. USA 1990, 87, 7235–7239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rettig, W.J.; Garin-Chesa, P.; Healey, J.H.; Su, S.L.; Jaffe, E.A.; Old, L.J. Identification of endosialin, a cell surface glycoprotein of vascular endothelial cells in human cancer. Proc. Natl. Acad. Sci. USA 1992, 89, 10832–10836. [Google Scholar] [CrossRef][Green Version]
- Varasteh, Z.; Mohanta, S.; Robu, S.; Braeuer, M.; Li, Y.; Omidvari, N.; Topping, G.; Sun, T.; Nekolla, S.G.; Richter, A.; et al. Molecular imaging of fibroblast activity after myocardial infarction using a 68Ga-labeled fibroblast activation protein inhibitor, FAPI-04. J. Nucl. Med. 2019, 60, 1743–1749. [Google Scholar] [CrossRef]
- Creemers, E.E.; Pinto, Y.M. Molecular mechanisms that control interstitial fibrosis in the pressure-overloaded heart. Cardiovasc. Res. 2011, 89, 265–272. [Google Scholar] [CrossRef][Green Version]
- Rich, P. Chemiosmotic coupling: The cost of living. Nature 2003, 421, 583. [Google Scholar] [CrossRef]
- Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K. and Walter, P. Molecular biology of the cell. 4th edn. Ann. Bot. 2003, 91, 401. [CrossRef][Green Version]
- Hüttemann, M.; Lee, I.; Pecinova, A.; Pecina, P.; Przyklenk, K.; Doan, J.W. Regulation of oxidative phosphorylation, the mitochondrial membrane potential, and their role in human disease. J. Bioenerg. Biomembr. 2008, 40, 445–456. [Google Scholar] [CrossRef]
- Alpert, N.M.; Guehl, N.; Ptaszek, L.; Pelletier-Galarneau, M.; Ruskin, J.; Mansour, M.C.; Wooten, D.; Ma, C.; Takahashi, K.; Zhou, Y.; et al. Quantitative in vivo mapping of myocardial mitochondrial membrane potential. PLoS ONE 2018, 13, e0190968. [Google Scholar] [CrossRef][Green Version]
- Pelletier-Galarneau, M.; Petibon, Y.; Ma, C.; Han, P.; Kim, S.J.W.; Detmer, F.J.; Yokell, D.; Guehl, N.; Normandin, M.; El Fakhri, G.; et al. In vivo quantitative mapping of human mitochondrial cardiac membrane potential: A feasibility study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Shan, K.; Bick, R.J.; Poindexter, B.J.; Nagueh, S.F.; Shimoni, S.; Verani, M.S.; Keng, F.; Reardon, M.J.; Letsou, G.V.; Howell, J.F.; et al. Altered adrenergic receptor density in myocardial hibernation in humans: A possible mechanism of depressed myocardial function. Circulation 2000, 102, 2599–2606. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Travin, M.I.; Henzlova, M.J.; van Eck-Smit, B.L.F.; Jain, D.; Carrió, I.; Folks, R.D.; Garcia, E.V.; Jacobson, A.F.; Verberne, H.J. Assessment of 123I-mIBG and 99mTc-tetrofosmin single-photon emission computed tomographic images for the prediction of arrhythmic events in patients with ischemic heart failure: Intermediate severity innervation defects are associated with higher arrhythmic risk. J. Nucl. Cardiol. 2017, 24, 377–391. [Google Scholar] [PubMed]
- Travin, M.I. Can the promise of radionuclide cardiac innervation imaging be fulfilled? J. Nucl. Cardiol. 2022, 29, 3189–3193. [Google Scholar] [CrossRef]
- Kobayashi, R.; Chen, X.; Werner, R.A.; Lapa, C.; Javadi, M.S.; Higuchi, T. New horizons in cardiac innervation imaging: Introduction of novel 18F-labeled PET tracers. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2302–2309. [Google Scholar] [CrossRef] [PubMed]
- Das, J.P.; Yeh, R. Prostate cancer cardiac metastasis detected on serial imaging with [68Ga] PSMA-11 PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3952–3953. [Google Scholar] [CrossRef]
- Van de Wiele, C.; Sathekge, M.; de Spiegeleer, B.; De Jonghe, P.J.; Debruyne, P.R.; Borms, M.; Beels, L.; Maes, A. PSMA expression on neovasculature of solid tumors. Histol. Histopathol. 2020, 35, 919–927. [Google Scholar]



| Function | Perfusion | Metabolism | Inotropic Response | Functional Recovery | |
|---|---|---|---|---|---|
| Stunned (viable) | Reduced | Normal | Yes | Yes | Yes |
| Hibernating (viable) | Reduced | Reduced | Yes | Yes | Yes |
| Necrotic (non-viable) | Reduced | Reduced | No | No | No |
| Measure | Viability Marker | |
|---|---|---|
| SPECT | 201Tl or 99mTc uptake | Myocyte membrane integrity |
| PET | 18F-FDG uptake | Glucose metabolism |
| Stress echocardiography | Inotropic stimulation | Contractile reserve |
| Cardiac MR | Late gadolinium enhancement | Extracellular volume |
| Tracer | Half-Life | Pathway | Emission | Energy (MeV) |
|---|---|---|---|---|
| 18F-FDG | 110 min | Hexokinase/glucose metabolism | β+ | 0.633 |
| 11C-acetate | 20 min | Krebs cycle/free fatty acid metabolism | β+, | 0.961 |
| 15O-water | 2 min | Passive diffusion/blood flow | β+ | 0.019 |
| 68Ga-DOTA-ECL1i | 68 min | CCR type 2/inflammatory response to injury | β+ | 1.899 |
| 68Ga-FAPI | 68 min | FAP/inflammatory response to injury | β+ | 1.899 |
| 18F-tetraphenylphosphonium | 110 min | Mitochondria/mitochondrial membrane integrity | β+ | 0.633 |
| 123I-MIBG | 13.2 h | Distribution and integrity of adrenergic nerve endings | γ | 0.159 |
| 18F-FBBG (or 18F-LMI-1195) | 110 min | Distribution and integrity of adrenergic nerve endings | β+ | 0.633 |
| 18F-MFBG | 110 min | Distribution and integrity of adrenergic nerve endings | β+ | 0.633 |
| 11C-HED | 20 min | Distribution and integrity of adrenergic nerve endings | β+ | 0.961 |
| 201Tl | 72.9 h | Na+/K+ pump/blood flow | γ | 0.135, 0.167 |
| 123I-BMIPP | 13.2 h | Krebs cycle/free fatty acid metabolism | γ | 0.159 |
| 99mTc-Sestamibi | 6 h | Mitochondria and cytosol proteins in myocytes | γ | 0.140 |
| 99mTc-Tetrofosmin | 6 h | Mitochondria and cytosol proteins in myocytes | γ | 0.140 |
| Project Title | Sponsors | Study Type | Aim | Status |
|---|---|---|---|---|
| Development and Translation of Generator-Produced PET Tracer for Myocardial Perfusion Imaging-Dosimetry Group (GALMYDAR) | Washington University School of Medicine, USA | Interventional | To evaluate dosimetry, biodistribution, safety and imaging characteristics following a single 68Ga-Galmydar injection in normal healthy volunteers | Recruiting completed |
| 68 Ga-NODAGA-E[c(RGDγK)]2: Positron Emission Tomography Tracer for Imaging of Myocardial Angiogenesis | Rigshospitalet, Denmark | Interventional | To examine the expression of αvβ3 integrin using a novel radiotracer in patients with myocardial infarction and investigate if it is a suitable tool for predicting myocardial recovery and prognosis | Recruiting completed |
| Cardiac FDG PET Viability Registry (CADRE) | Ottawa Heart Institute Research Corporation, Canada | Observational | To evaluate the utility of FDG PET imaging in the decision-making process for patients with poor left ventricular function who may be candidates for revascularization and to study the downstream effect of the clinical management decisions | Recruiting |
| Open-Label, Exploratory, Phase 1/2 Scintigraphy Study Evaluating 18F-mFBG for Imaging Myocardial Sympathetic Innervation in Subjects Without and With Heart Disease | Innervate Radiopharmaceuticals LLC, USA | Interventional | To observe the positron-emitting radiopharmaceutical 18F-mFBG as an imaging agent for quantification of myocardial sympathetic innervation | Recruiting |
| Phase 3, Multicenter, Open Label Study to Confirm the Diagnostic Potential of Intravenously Administered 15O-H2O to Identify Coronary Artery Disease During Pharmacological Stress and Resting Conditions Using PET Imaging (RAPID-WATER-FLOW) | MedTrace Pharma A/S, Denmark | Interventional | To evaluate the sensitivity and specificity of the 15O-H2O PET study using the truth-standard of ICA with FFR or CCTA | Recruiting |
| Tracer | Advantages | Limitations |
|---|---|---|
| 18F-FDG | Long radionuclide half-life allowing delivery; high temporal and spatial resolution of equipment; robust evidence | Metabolic compensation needed; perfusion study required for a combined evaluation |
| 11C-acetate | Single-tracer technique; minimal metabolic dependence | On-site cyclotron required |
| 15O-water | High and linear tracer; uptake rate into myocardium; high temporal and spatial resolution of equipment | On-site cyclotron required; technical demanding protocols |
| 68Ga-DOTA-ECL1i | Commercially available 68Ge/68Ga generator for multiple daily studies; rapid clearance; low liver retention | Very limited data available; low specificity |
| 68Ga-FAPI | Commercially available 68Ge/68Ga generator for multiple daily studies; high abnormal/normal uptake ratio | Limited data available; low specificity |
| 18F-tetraphenylphosphonium | Long radionuclide half-life allowing delivery; High temporal and spatial resolution of equipment; first voltage non-invasive probe | Limited data available; no gold standard method as reference; high distribution heterogeneity |
| 123I-MIBG | Robust evidence; optimal storage in neuronal vesicles; highly specific tracer; high heart-to-background ratios with clear cardiac images | Low resolution of equipment; standardization protocols still required |
| 18F-FBBG (or 18F-LMI-1195) | Simple radiolabeling; procedure for commercial use; high heart-to-background ratios with clear cardiac images; high temporal and spatial resolution of equipment | Limited data available |
| 18F-MFBG | Optimal storage in neuronal vesicles; highly specific tracer; high heart-to-background ratios with clear cardiac images; high temporal and spatial resolution of equipment | Limited data available |
| 11C-HED | Robust evidence; highly specific tracer; high heart-to-background ratios with clear cardiac images high temporal and spatial resolution of equipment | On-site cyclotron required; delayed scans for turnover assessment not feasible due to low radionuclide half-life; high lipophilicity with potential tracer loss across lipid membranes |
| 201Tl | Tissue concentration proportional to flow; potential evaluation of perfusion and viability | Low resolution of equipment; dosimetric issues |
| 123I-BMIPP | Primary energy cardiac source tracer; high specificity | Low resolution of equipment; low sensitivity |
| 99mTc-Sestamibi and 99mTc-Tetrofosmin | Short radionuclide half-life with a feasible dosimetric profile; myocardial uptake proportional to the integrity of membrane with high accuracy | Low resolution of equipment; low first-pass extraction fraction and high liver absorption |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nappi, C.; Panico, M.; Falzarano, M.; Vallone, C.; Ponsiglione, A.; Cutillo, P.; Zampella, E.; Petretta, M.; Cuocolo, A. Tracers for Cardiac Imaging: Targeting the Future of Viable Myocardium. Pharmaceutics 2023, 15, 1532. https://doi.org/10.3390/pharmaceutics15051532
Nappi C, Panico M, Falzarano M, Vallone C, Ponsiglione A, Cutillo P, Zampella E, Petretta M, Cuocolo A. Tracers for Cardiac Imaging: Targeting the Future of Viable Myocardium. Pharmaceutics. 2023; 15(5):1532. https://doi.org/10.3390/pharmaceutics15051532
Chicago/Turabian StyleNappi, Carmela, Mariarosaria Panico, Maria Falzarano, Carlo Vallone, Andrea Ponsiglione, Paolo Cutillo, Emilia Zampella, Mario Petretta, and Alberto Cuocolo. 2023. "Tracers for Cardiac Imaging: Targeting the Future of Viable Myocardium" Pharmaceutics 15, no. 5: 1532. https://doi.org/10.3390/pharmaceutics15051532
APA StyleNappi, C., Panico, M., Falzarano, M., Vallone, C., Ponsiglione, A., Cutillo, P., Zampella, E., Petretta, M., & Cuocolo, A. (2023). Tracers for Cardiac Imaging: Targeting the Future of Viable Myocardium. Pharmaceutics, 15(5), 1532. https://doi.org/10.3390/pharmaceutics15051532








