Kinetics and Mechanism of Camptothecin Release from Transferrin-Gated Mesoporous Silica Nanoparticles through a pH-Responsive Surface Linker
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Mesoporous Silica Nanoparticles (MSN) Preparation
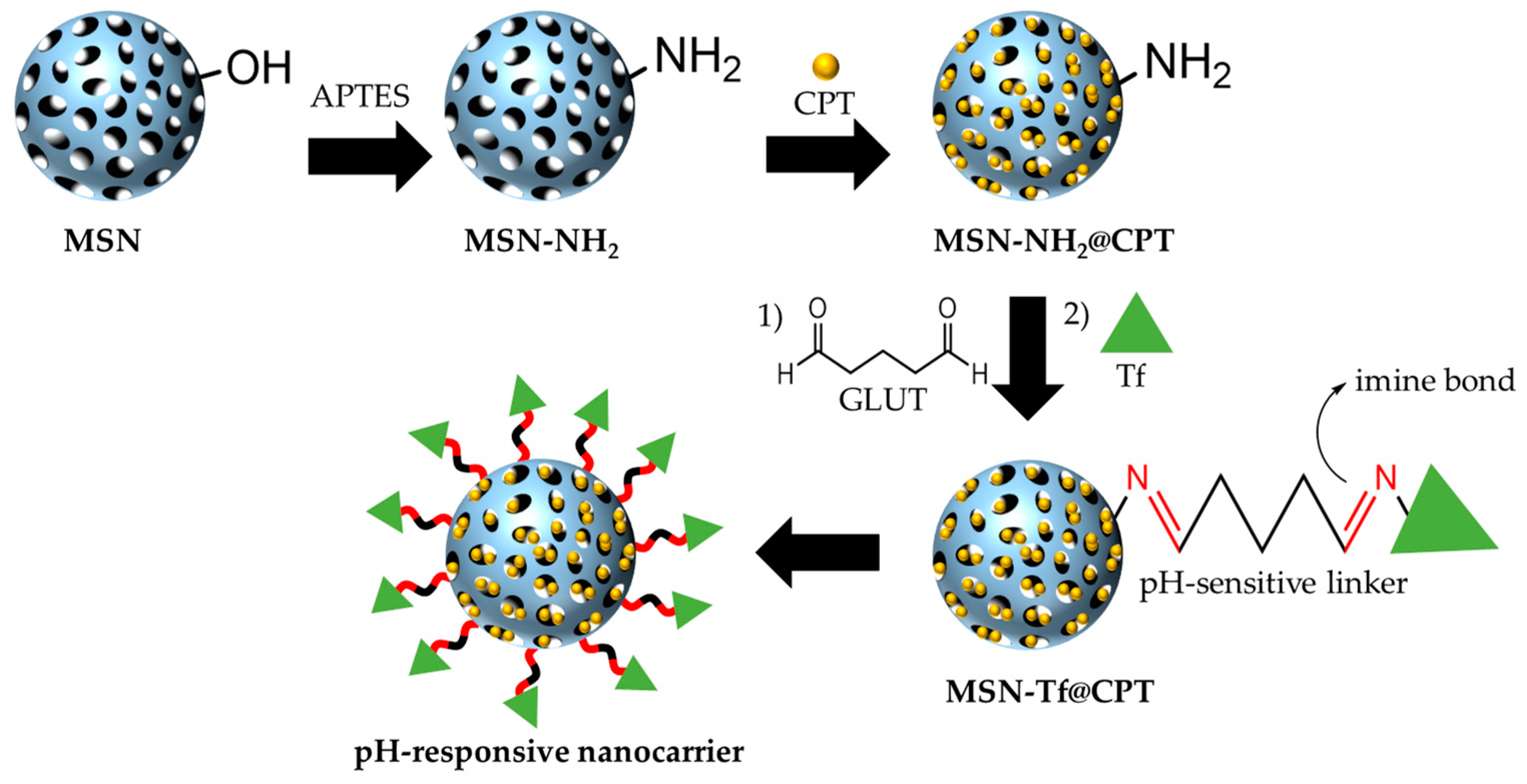
2.4. Amino-Functionalization and Aldehyde-Modified Surface
2.5. Transferrin Conjugation on the Surface of the Nanoparticles
2.6. Drug Loading
2.7. In Vitro Release Profile
2.8. Kinetics Analysis
2.9. Statistical Analysis
3. Results and Discussion
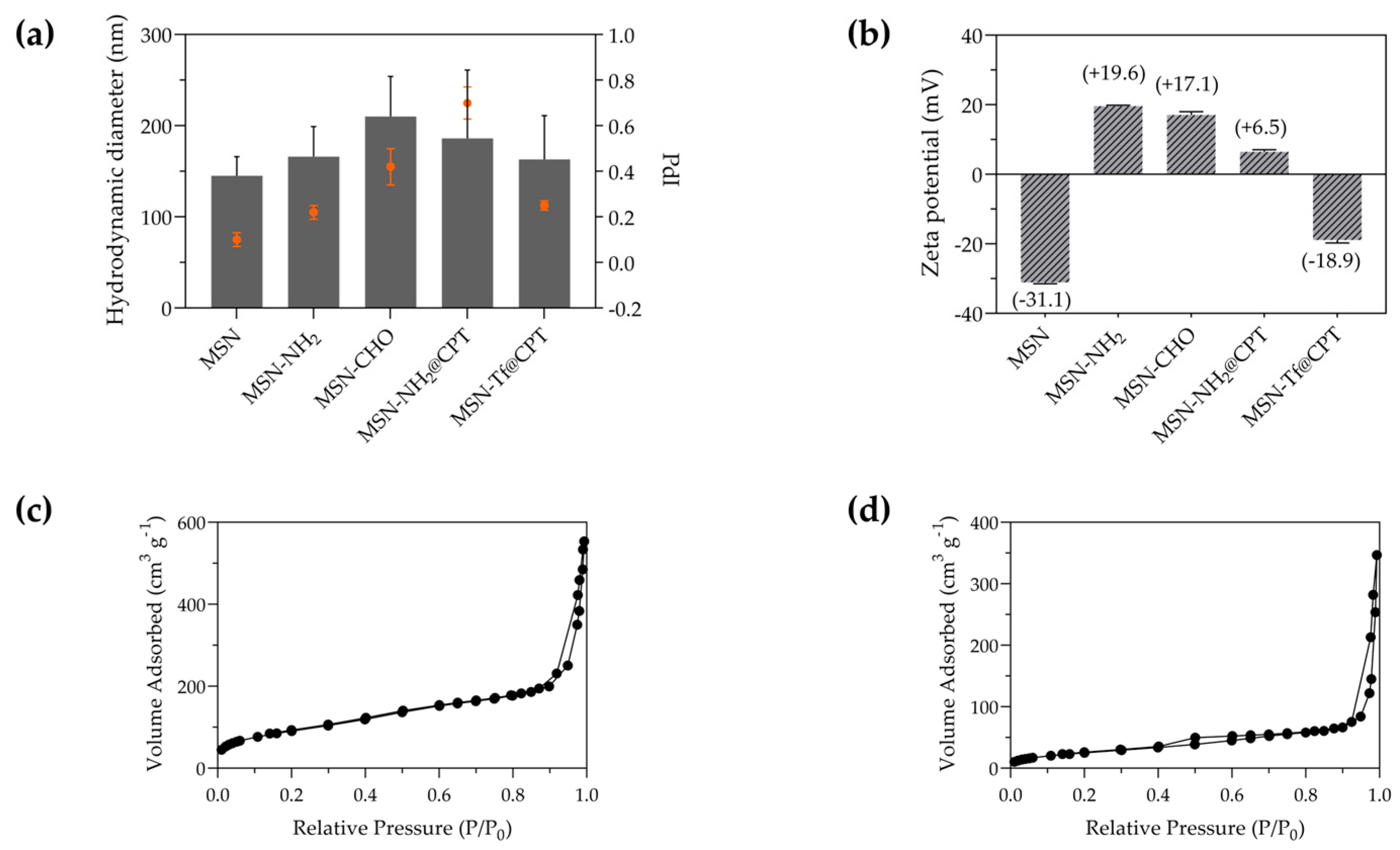
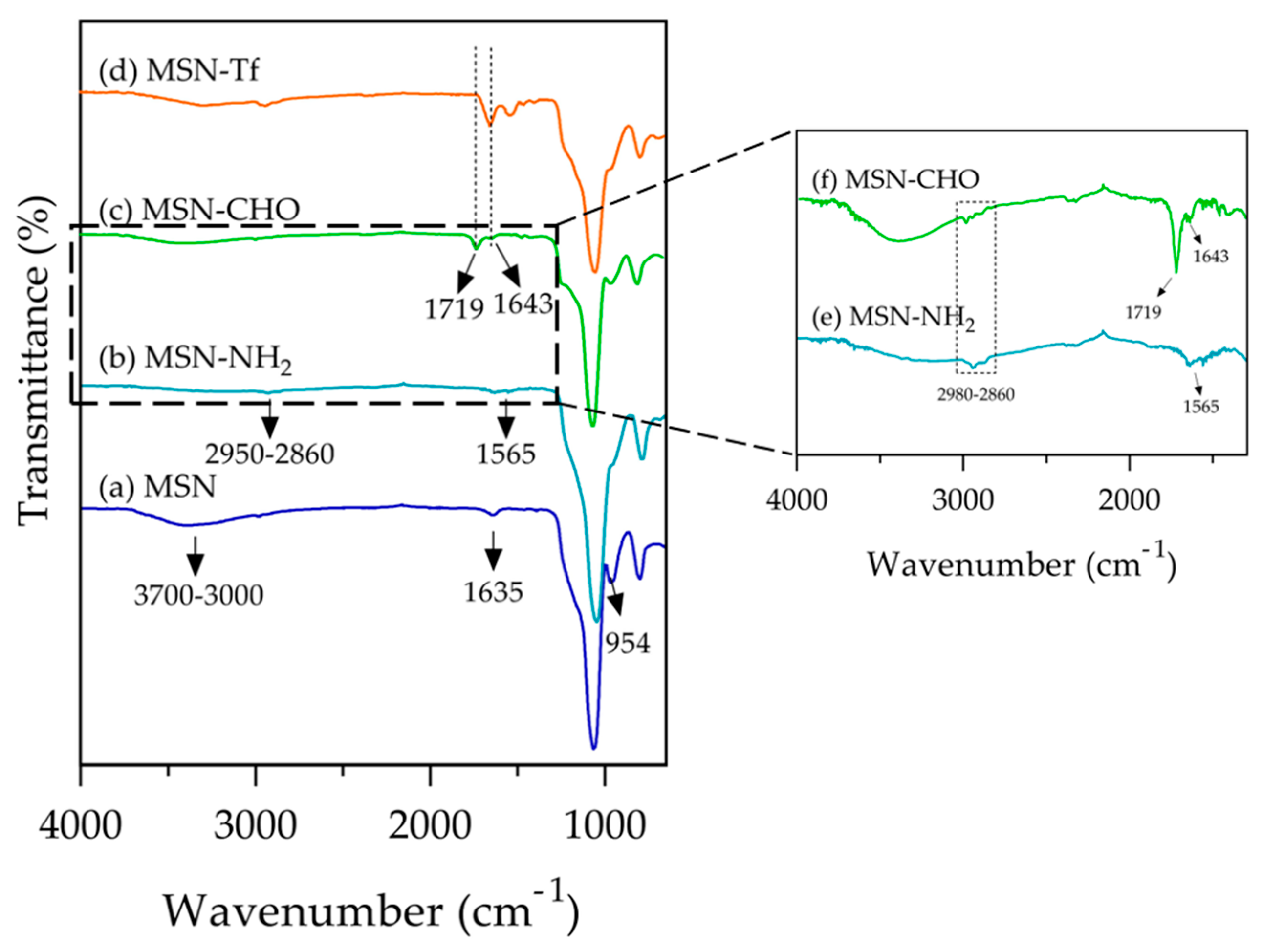
3.1. Fabrication and Characterization of Tf Gated Mesoporous Silica Nanomaterials
3.2. Drug Loading
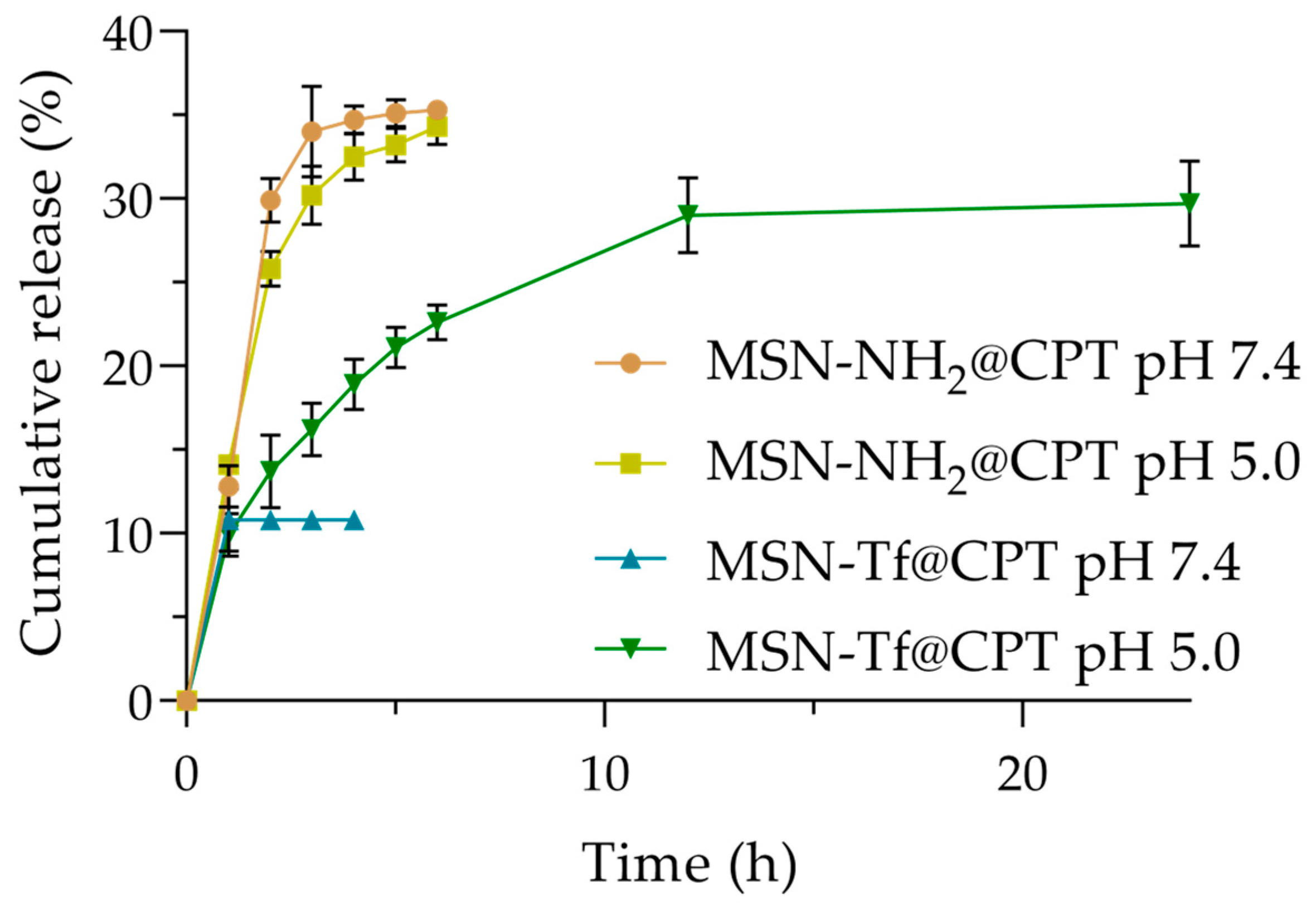
3.3. In Vitro Release Profile
3.4. Kinetics Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Jain, R.K.; Stylianopoulos, T. Delivering Nanomedicine to Solid Tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef]
- Moodley, T.; Singh, M. Current Stimuli-Responsive Mesoporous Silica Nanoparticles for Cancer Therapy. Pharmaceutics 2021, 13, 71. [Google Scholar] [CrossRef]
- Fu, Q.; Hargrove, D.; Lu, X. Improving Paclitaxel Pharmacokinetics by Using Tumor-Specific Mesoporous Silica Nanoparticles with Intraperitoneal Delivery. Nanomedicine 2016, 12, 1951–1959. [Google Scholar] [CrossRef]
- Qiu, Y.; Wu, C.; Jiang, J.; Hao, Y.; Zhao, Y.; Xu, J.; Yu, T.; Ji, P. Lipid-Coated Hollow Mesoporous Silica Nanospheres for Co-Delivery of Doxorubicin and Paclitaxel: Preparation, Sustained Release, Cellular Uptake and Pharmacokinetics. Mater. Sci. Eng. C 2017, 71, 835–843. [Google Scholar] [CrossRef]
- Shakeel, F.; Iqbal, M.; Ezzeldin, E. Bioavailability Enhancement and Pharmacokinetic Profile of an Anticancer Drug Ibrutinib by Self-Nanoemulsifying Drug Delivery System. J. Pharm. Pharmacol. 2016, 68, 772–780. [Google Scholar] [CrossRef]
- Zou, Y.; Mei, D.; Yuan, J.; Han, J.; Xu, J.; Sun, N.; He, H.; Yang, C.; Zhao, L. Preparation, Characterization, Pharmacokinetic, and Therapeutic Potential of Novel 6-Mercaptopurine-Loaded Oral Nanomedicines for Acute Lymphoblastic Leukemia. Int. J. Nanomed. 2021, 16, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Gomte, S.S.; Prathyusha, E.; Prabakaran, A.; Agrawal, M.; Alexander, A. Biomedical Applications of Mesoporous Silica Nanoparticles as a Drug Delivery Carrier. J. Drug Deliv. Sci. Technol. 2022, 76, 103729. [Google Scholar] [CrossRef]
- Kumarage, S.; Munaweera, I.; Kottegoda, N. Contemporary, Multidisciplinary Roles of Mesoporous Silica Nanohybrids/Nanocomposites. ChemistrySelect 2022, 7, e202200574. [Google Scholar] [CrossRef]
- Shah, S.; Famta, P.; Bagasariya, D.; Charankumar, K.; Sikder, A.; Kashikar, R.; Kotha, A.K.; Chougule, M.B.; Khatri, D.K.; Asthana, A.; et al. Tuning Mesoporous Silica Nanoparticles in Novel Avenues of Cancer Therapy. Mol. Pharm. 2022, 19, 4428–4452. [Google Scholar] [CrossRef]
- Rahoui, N.; Jiang, B.; Taloub, N.; Huang, Y.D. Spatio-Temporal Control Strategy of Drug Delivery Systems Based Nano Structures. J. Control. Release 2017, 255, 176–201. [Google Scholar] [CrossRef]
- Palanikumar, L.; Jeena, M.T.; Kim, K.; Yong Oh, J.; Kim, C.; Park, M.-H.; Ryu, J.-H. Spatiotemporally and Sequentially-Controlled Drug Release from Polymer Gatekeeper–Hollow Silica Nanoparticles. Sci. Rep. 2017, 7, 46540. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated PH: A Perfect Storm for Cancer Progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]
- Corbet, C.; Feron, O. Tumour Acidosis: From the Passenger to the Driver’s Seat. Nat. Rev. Cancer 2017, 17, 577–593. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Jin, S.; Muhammad, N.; Guo, Z. Stimuli-Responsive Therapeutic Metallodrugs. Chem. Rev. 2019, 119, 1138–1192. [Google Scholar] [CrossRef]
- Daniels, T.R.; Delgado, T.; Helguera, G.; Penichet, M.L. The Transferrin Receptor Part II: Targeted Delivery of Therapeutic Agents into Cancer Cells. Clin. Immunol. 2006, 121, 159–176. [Google Scholar] [CrossRef]
- Yhee, J.Y.; Lee, S.J.; Lee, S.; Song, S.; Min, H.S.; Kang, S.-W.; Son, S.; Jeong, S.Y.; Kwon, I.C.; Kim, S.H.; et al. Tumor-Targeting Transferrin Nanoparticles for Systemic Polymerized SiRNA Delivery in Tumor-Bearing Mice. Bioconjug. Chem. 2013, 24, 1850–1860. [Google Scholar] [CrossRef]
- Pallares, R.M.; Agbo, P.; Liu, X.; An, D.D.; Gauny, S.S.; Zeltmann, S.E.; Minor, A.M.; Abergel, R.J. Engineering Mesoporous Silica Nanoparticles for Targeted Alpha Therapy against Breast Cancer. ACS Appl. Mater. Interfaces 2020, 12, 40078–40084. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Y.; Zhao, X.; Agarwal, A.; Mueller, L.J.; Feng, P. PH-Responsive Nanogated Ensemble Based on Gold-Capped Mesoporous Silica through an Acid-Labile Acetal Linker. J. Am. Chem. Soc. 2010, 132, 1500–1501. [Google Scholar] [CrossRef]
- Choy, C.J.; Geruntho, J.J.; Davis, A.L.; Berkman, C.E. Tunable PH-Sensitive Linker for Controlled Release. Bioconjug. Chem. 2016, 27, 824–830. [Google Scholar] [CrossRef]
- Tian, Z.; Xu, Y.; Zhu, Y. Aldehyde-Functionalized Dendritic Mesoporous Silica Nanoparticles as Potential Nanocarriers for PH-Responsive Protein Drug Delivery. Mater. Sci. Eng. C 2017, 71, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, F.; Cini, E.; Dreassi, E.; Finetti, F.; Ievoli, G.; Macrì, G.; Petricci, E.; Rango, E.; Trabalzini, L.; Taddei, M. A PH-Responsive Crosslinker Platform for Antibody-Drug Conjugate (ADC) Targeting Delivery. Chem. Comm. 2022, 58, 10532–10535. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Jang, M.-S.; Li, Y.; Du, J.M.; Liu, C.; Lee, J.H.; Fu, Y. PH-Responsive Dynamically Cross-Linked Nanogels with Effective Endo-Lysosomal Escape for Synergetic Cancer Therapy Based on Intracellular Co-Delivery of Photosensitizers and Proteins. Colloids Surf. B 2022, 217, 112638. [Google Scholar] [CrossRef]
- Mao, J.; Li, Y.; Wu, T.; Yuan, C.; Zeng, B.; Xu, Y.; Dai, L. A Simple Dual-PH Responsive Prodrug-Based Polymeric Micelles for Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 17109–17117. [Google Scholar] [CrossRef]
- Travaglini, L.; Picchetti, P.; Totovao, R.; Prasetyanto, E.A.; Cola, L.D. Highly Degradable Imine-Doped Mesoporous Silica Particles. Mater. Chem. Front. 2018, 3, 111–119. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, S.; Zhang, Y.; Chi, Z.; Xu, J. A PH-Responsive Polymer Based on Dynamic Imine Bonds as a Drug Delivery Material with Pseudo Target Release Behavior. Polym. Chem. 2018, 9, 878–884. [Google Scholar] [CrossRef]
- Peng, X.; Liu, P.; Pang, B.; Yao, Y.; Wang, J.; Zhang, K. Facile Fabrication of PH-Responsive Nanoparticles from Cellulose Derivatives via Schiff Base Formation for Controlled Release. Carbohydr. Polym. 2019, 216, 113–118. [Google Scholar] [CrossRef]
- Yan, R.; Liu, X.; Xiong, J.; Feng, Q.; Xu, J.; Wang, H.; Xiao, K. PH-Responsive Hyperbranched Polypeptides Based on Schiff Bases as Drug Carriers for Reducing Toxicity of Chemotherapy. RSC Adv. 2020, 10, 13889–13899. [Google Scholar] [CrossRef]
- Saini, K.; Bandyopadhyaya, R. Transferrin-Conjugated Polymer-Coated Mesoporous Silica Nanoparticles Loaded with Gemcitabine for Killing Pancreatic Cancer Cells. ACS Appl. Nano Mater. 2020, 3, 229–240. [Google Scholar] [CrossRef]
- Llinàs, M.C.; Martínez-Edo, G.; Cascante, A.; Porcar, I.; Borrós, S.; Sánchez-García, D. Preparation of a Mesoporous Silica-Based Nano-Vehicle for Dual DOX/CPT PH-Triggered Delivery. Drug Deliv. 2018, 25, 1137–1146. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, Z.; He, R.; Li, Y.; Xiong, B.; Yi, M.; Chen, Y.; Liu, J.; Lu, B. Bovine Serum Albumin-Based and Dual-Responsive Targeted Hollow Mesoporous Silica Nanoparticles for Breast Cancer Therapy. Colloids Surf. B 2023, 224, 113201. [Google Scholar] [CrossRef] [PubMed]
- Peñaloza, J.P.; Márquez-Miranda, V.; Cabaña-Brunod, M.; Reyes-Ramírez, R.; Llancalahuen, F.M.; Vilos, C.; Maldonado-Biermann, F.; Velásquez, L.A.; Fuentes, J.A.; González-Nilo, F.D.; et al. Intracellular Trafficking and Cellular Uptake Mechanism of PHBV Nanoparticles for Targeted Delivery in Epithelial Cell Lines. J. Nanobiotechnol. 2017, 15, 1. [Google Scholar] [CrossRef]
- Sola, F.; Canonico, B.; Montanari, M.; Volpe, A.; Barattini, C.; Pellegrino, C.; Cesarini, E.; Guescini, M.; Battistelli, M.; Ortolani, C.; et al. Uptake and Intracellular Trafficking Studies of Multiple Dye-Doped Core-Shell Silica Nanoparticles in Lymphoid and Myeloid Cells. Nanotechnol. Sci. Appl. 2021, 14, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Jung, I.; Odom, T.W. Delivery Order of Nanoconstructs Affects Intracellular Trafficking by Endosomes. J. Am. Chem. Soc. 2022, 144, 5274–5279. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Wang, S.; Santos, H.A. Acid-Labile Chemical Bonds-Based Nanoparticles for Endosome Escape and Intracellular Delivery. Biomed. Technol. 2023, 3, 52–58. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular Uptake of Nanoparticles: Journey inside the Cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Vtyurina, N.; Åberg, C.; Salvati, A. Imaging of Nanoparticle Uptake and Kinetics of Intracellular Trafficking in Individual Cells. Nanoscale 2021, 13, 10436–10446. [Google Scholar] [CrossRef]
- Thomas, C.J.; Rahier, N.J.; Hecht, S.M. Camptothecin: Current Perspectives. Bioorg. Med. Chem. 2004, 12, 1585–1604. [Google Scholar] [CrossRef]
- Liu, L.F.; Desai, S.D.; Li, T.-K.; Mao, Y.; Sun, M.; Sim, S.-P. Mechanism of Action of Camptothecin. Ann. N. Y. Acad. Sci. 2000, 922, 1–10. [Google Scholar] [CrossRef]
- Dancey, J.; Eisenhauer, E.A. Current Perspectives on Camptothecins in Cancer Treatment. Br. J. Cancer 1996, 74, 327–338. [Google Scholar] [CrossRef]
- Adams, D.J.; Dewhirst, M.W.; Flowers, J.L.; Gamcsik, M.P.; Colvin, O.M.; Manikumar, G.; Wani, M.C.; Wall, M.E. Camptothecin Analogues with Enhanced Antitumor Activity at Acidic PH. Cancer Chemother. Pharmacol. 2000, 46, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.; Trewyn, B.G.; Lin, V.S.-Y. Effect of Surface Functionalization of MCM-41-Type Mesoporous Silica Nanoparticles on the Endocytosis by Human Cancer Cells. J. Am. Chem. Soc. 2006, 128, 14792–14793. [Google Scholar] [CrossRef] [PubMed]
- Planas, O.; Bresolí-Obach, R.; Nos, J.; Gallavardin, T.; Ruiz-González, R.; Agut, M.; Nonell, S. Synthesis, Photophysical Characterization, and Photoinduced Antibacterial Activity of Methylene Blue-Loaded Amino- and Mannose-Targeted Mesoporous Silica Nanoparticles. Molecules 2015, 20, 6284–6298. [Google Scholar] [CrossRef]
- Arriagada, F.; Günther, G.; Morales, J. Nanoantioxidant–Based Silica Particles as Flavonoid Carrier for Drug Delivery Applications. Pharmaceutics 2020, 12, 302. [Google Scholar] [CrossRef] [PubMed]
- Narayan, S.; Rajagopalan, A.; Reddy, J.S.; Chadha, A. BSA Binding to Silica Capped Gold Nanostructures: Effect of Surface Cap and Conjugation Design on Nanostructure–BSA Interface. RSC Adv. 2013, 4, 1412–1420. [Google Scholar] [CrossRef]
- Yang, Y.; Achazi, K.; Jia, Y.; Wei, Q.; Haag, R.; Li, J. Complex Assembly of Polymer Conjugated Mesoporous Silica Nanoparticles for Intracellular PH-Responsive Drug Delivery. Langmuir 2016, 32, 12453–12460. [Google Scholar] [CrossRef]
- Delpiano, G.R.; Casula, M.F.; Piludu, M.; Corpino, R.; Ricci, P.C.; Vallet-Regí, M.; Sanjust, E.; Monduzzi, M.; Salis, A. Assembly of Multicomponent Nano-Bioconjugates Composed of Mesoporous Silica Nanoparticles, Proteins, and Gold Nanoparticles. ACS Omega 2019, 4, 11044–11052. [Google Scholar] [CrossRef]
- Shakeran, Z.; Keyhanfar, M.; Varshosaz, J.; Sutherland, D.S. Biodegradable Nanocarriers Based on Chitosan-Modified Mesoporous Silica Nanoparticles for Delivery of Methotrexate for Application in Breast Cancer Treatment. Mater. Sci. Eng. C 2021, 118, 111526. [Google Scholar] [CrossRef]
- Kim, C.; Kim, S.; Oh, W.-K.; Choi, M.; Jang, J. Efficient Intracellular Delivery of Camptothecin by Silica/Titania Hollow Nanoparticles. Chem. Eur. J. 2012, 18, 4902–4908. [Google Scholar] [CrossRef]
- di Nunzio, M.R.; Cohen, B.; Douhal, A. Structural Photodynamics of Camptothecin, an Anticancer Drug in Aqueous Solutions. J. Phys. Chem. A 2011, 115, 5094–5104. [Google Scholar] [CrossRef]
- Babaei, M.; Abnous, K.; Taghdisi, S.M.; Taghavi, S.; Saljooghi, A.S.; Ramezani, M.; Alibolandi, M. Targeted Rod-Shaped Mesoporous Silica Nanoparticles for the Co-Delivery of Camptothecin and Survivin ShRNA in to Colon Adenocarcinoma in Vitro and in Vivo. Eur. J. Pharm. Biopharm. 2020, 156, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Sousa Lobo, J.M. Modeling and Comparison of Dissolution Profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Narasimhan, B. Mathematical Models in Drug Delivery: How Modeling Has Shaped the Way We Design New Drug Delivery Systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Prakash Upputuri, R.T.; Azad Mandal, A.K. Sustained Release of Green Tea Polyphenols from Liposomal Nanoparticles; Release Kinetics and Mathematical Modelling. Iran. J. Biotechnol. 2017, 15, 277–283. [Google Scholar] [CrossRef]
- Yu, M.; Yuan, W.; Li, D.; Schwendeman, A.; Schwendeman, S.P. Predicting Drug Release Kinetics from Nanocarriers inside Dialysis Bags. J. Control. Release 2019, 315, 23–30. [Google Scholar] [CrossRef]
- Pourtalebi Jahromi, L.; Ghazali, M.; Ashrafi, H.; Azadi, A. A Comparison of Models for the Analysis of the Kinetics of Drug Release from PLGA-Based Nanoparticles. Heliyon 2020, 6, e03451. [Google Scholar] [CrossRef]
- Huang, S.; Kong, X.; Xiong, Y.; Zhang, X.; Chen, H.; Jiang, W.; Niu, Y.; Xu, W.; Ren, C. An Overview of Dynamic Covalent Bonds in Polymer Material and Their Applications. Eur. Polym. J. 2020, 141, 110094. [Google Scholar] [CrossRef]
- Qiao, Z.-A.; Zhang, L.; Guo, M.; Liu, Y.; Huo, Q. Synthesis of Mesoporous Silica Nanoparticles via Controlled Hydrolysis and Condensation of Silicon Alkoxide. Chem. Mater. 2009, 21, 3823–3829. [Google Scholar] [CrossRef]
- Kachbouri, S.; Mnasri, N.; Elaloui, E.; Moussaoui, Y. Tuning Particle Morphology of Mesoporous Silica Nanoparticles for Adsorption of Dyes from Aqueous Solution. J. Saudi Chem. Soc. 2018, 22, 405–415. [Google Scholar] [CrossRef]
- Ferris, D.P.; Lu, J.; Gothard, C.; Yanes, R.; Thomas, C.R.; Olsen, J.-C.; Stoddart, J.F.; Tamanoi, F.; Zink, J.I. Synthesis of Biomolecule-Modified Mesoporous Silica Nanoparticles for Targeted Hydrophobic Drug Delivery to Cancer Cells. Small 2011, 7, 1816–1826. [Google Scholar] [CrossRef]
- Nairi, V.; Medda, S.; Piludu, M.; Casula, M.F.; Vallet-Regì, M.; Monduzzi, M.; Salis, A. Interactions between Bovine Serum Albumin and Mesoporous Silica Nanoparticles Functionalized with Biopolymers. Chem. Eng. J. 2018, 340, 42–50. [Google Scholar] [CrossRef]
- Beganskienė, A.; Sirutkaitis, V.; Kurtinaitienė, M.; Juškėnas, R.; Kareiva, A. FTIR, TEM and NMR Investigations of Stöber Silica Nanoparticles. Mater. Sci. 2004, 10, 287–290. [Google Scholar]
- Nhavene, E.P.F.; Andrade, G.F.; Faria, J.A.Q.A.; Gomes, D.A.; Sousa, E.M.B.D. Biodegradable Polymers Grafted onto Multifunctional Mesoporous Silica Nanoparticles for Gene Delivery. ChemEngineering 2018, 2, 24. [Google Scholar] [CrossRef]
- Muhammad, F.; Guo, M.; Wang, A.; Zhao, J.; Qi, W.; Guo, Y.; Zhu, G. Responsive Delivery of Drug Cocktail via Mesoporous Silica Nanolamps. J. Colloid Interface Sci. 2014, 434, 1–8. [Google Scholar] [CrossRef]
- Landgraf, M.; Lahr, C.A.; Kaur, I.; Shafiee, A.; Sanchez-Herrero, A.; Janowicz, P.W.; Ravichandran, A.; Howard, C.B.; Cifuentes-Rius, A.; McGovern, J.A.; et al. Targeted Camptothecin Delivery via Silicon Nanoparticles Reduces Breast Cancer Metastasis. Biomaterials 2020, 240, 119791. [Google Scholar] [CrossRef]
- Min Jung, J.; Lip Jung, Y.; Han Kim, S.; Sung Lee, D.; Thambi, T. Injectable Hydrogel Imbibed with Camptothecin-Loaded Mesoporous Silica Nanoparticles as an Implantable Sustained Delivery Depot for Cancer Therapy. J. Colloid Interface Sci. 2023, 636, 328–340. [Google Scholar] [CrossRef]
- Mal, A.; Prabhuraj, R.S.; Malhotra, R.; Valvi, S.K.; Ingle, A.; Srivastava, R.; De, A.; Bandyopadhyaya, R. PH-Responsive Sustained Delivery of Doxorubicin Using Aminated and PEGylated Mesoporous Silica Nanoparticles Leads to Enhanced Antitumor Efficacy in Pre-Clinical Orthotopic Breast Cancer Model. J. Drug Deliv. Sci. Technol. 2022, 77, 103800. [Google Scholar] [CrossRef]
- Gao, J.; Ma, X.; Zhang, L.; Yan, J.; Cui, H.; Zhang, Y.; Wang, D.; Zhang, H. Self-Assembled Disulfide Bond Bearing Paclitaxel—Camptothecin Prodrug Nanoparticle for Lung Cancer Therapy. Pharmaceutics 2020, 12, 1169. [Google Scholar] [CrossRef]
- Yan, Y.; Fu, J.; Wang, T.; Lu, X. Controlled Release of Silyl Ether Camptothecin from Thiol-Ene Click Chemistry-Functionalized Mesoporous Silica Nanoparticles. Acta Biomater. 2017, 51, 471–478. [Google Scholar] [CrossRef]
- Yang, S.C.; Zhu, J.B. Preparation and Characterization of Camptothecin Solid Lipid Nanoparticles. Drug Dev. Ind. Pharm. 2002, 28, 265–274. [Google Scholar] [CrossRef]
- Thakral, N.K.; Ray, A.R.; Bar-Shalom, D.; Eriksson, A.H.; Majumdar, D.K. Soluplus-Solubilized Citrated Camptothecin—A Potential Drug Delivery Strategy in Colon Cancer. AAPS Pharm. Sci. Tech. 2012, 13, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, L.C.; de Sousa, M.; Maia, D.L.S.; de Luna, L.V.; Alves, O.L. Understanding the Driving Forces of Camptothecin Interactions on the Surface of Nanocomposites Based on Graphene Oxide Decorated with Silica Nanoparticles. Nanoscale Adv. 2020, 2, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Solanki, P.; Patel, S.; Devkar, R.; Patel, A. Camptothecin Encapsulated into Functionalized MCM-41: In Vitro Release Study, Cytotoxicity and Kinetics. Mater. Sci. Eng. C 2019, 98, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.R. Elaborations on the Higuchi Model for Drug Delivery. Int. J. Pharm. 2011, 418, 13–17. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A Simple Equation for Description of Solute Release I. Fickian and Non-Fickian Release from Non-Swellable Devices in the Form of Slabs, Spheres, Cylinders or Discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A Simple Equation for the Description of Solute Release. III. Coupling of Diffusion and Relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Zeng, L.; Wu, X. Modeling the Sustained Release of Lipophilic Drugs from Liposomes. Appl. Phys. Lett. 2010, 97, 073701. [Google Scholar] [CrossRef]
- Zeng, L.; An, L.; Wu, X. Modeling Drug-Carrier Interaction in the Drug Release from Nanocarriers. J. Drug Deliv. 2011, 2011, e370308. [Google Scholar] [CrossRef]
- Sebastián Manzano, J.; Singappuli-Arachchige, D.; Parikh, B.L.; Slowing, I.I. Fine-Tuning the Release of Molecular Guests from Mesoporous Silicas by Controlling the Orientation and Mobility of Surface Phenyl Substituents. Chem. Eng. J. 2018, 340, 73–80. [Google Scholar] [CrossRef]
- Mitran, R.-A.; Matei, C.; Berger, D. Correlation of Mesoporous Silica Structural and Morphological Features with Theoretical Three-Parameter Model for Drug Release Kinetics. J. Phys. Chem. C 2016, 120, 29202–29209. [Google Scholar] [CrossRef]


| Nanoparticle | SBET (m2·g−1) | Pore Volume (cm3·g−1) | %LC | %LE | %CE a |
|---|---|---|---|---|---|
| MSN-NH2 | 333 | 0.83 | - | - | - |
| MSN-NH2@CPT | - | - | 13.5 ± 1.8 | 15.7 ± 0.9 | - |
| MSN-Tf@CPT | 95 | 0.51 | 13.4 ± 0.6 | 15.5 ± 0.2 | 9.6 |
| Nanosystem | Medium | Kinetics Model | |||||
|---|---|---|---|---|---|---|---|
| Zero-Order | First-Order | ||||||
| k0 | R2 | AIC | k1 | R2 | AIC | ||
| MSN-Tf@CPT | pH 5.0 | 10.8 | 0.439 | 67.2 | 0.25 | 0.971 | 43.4 |
| Nanosystem | Medium | Kinetics Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Higuchi | Korsmeyer-Peppas | Peppas-Sahlin | |||||||||
| KH | R2 | AIC | n | R2 | AIC | K1 | K2 | R2 | AIC | ||
| MSN-Tf@CPT | pH 5.0 | 30.4 | 0.983 | 38.9 | 0.46 | 0.999 | 3.4 | 32.5 | 0.73 | 0.999 | 5.0 |
| Nanosystem | Medium | Kinetics Model | |||
|---|---|---|---|---|---|
| Three Parameters | |||||
| ks | koff | ΔG (10−21) | R2 | ||
| MSN-Tf@CPT | pH 5.0 | 0.34 | 0.0021 | −5.93 | 0.984 |
| MSN-NH2@CPT | pH 7.4 | 0.67 | 0.0022 | −4.39 | 0.971 |
| pH 5.0 | 0.65 | 0.0017 | −4.68 | 0.992 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, N.; Ortiz, A.C.; Jerez, A.; Morales, J.; Arriagada, F. Kinetics and Mechanism of Camptothecin Release from Transferrin-Gated Mesoporous Silica Nanoparticles through a pH-Responsive Surface Linker. Pharmaceutics 2023, 15, 1590. https://doi.org/10.3390/pharmaceutics15061590
Jackson N, Ortiz AC, Jerez A, Morales J, Arriagada F. Kinetics and Mechanism of Camptothecin Release from Transferrin-Gated Mesoporous Silica Nanoparticles through a pH-Responsive Surface Linker. Pharmaceutics. 2023; 15(6):1590. https://doi.org/10.3390/pharmaceutics15061590
Chicago/Turabian StyleJackson, Nicolás, Andrea C. Ortiz, Alejandro Jerez, Javier Morales, and Francisco Arriagada. 2023. "Kinetics and Mechanism of Camptothecin Release from Transferrin-Gated Mesoporous Silica Nanoparticles through a pH-Responsive Surface Linker" Pharmaceutics 15, no. 6: 1590. https://doi.org/10.3390/pharmaceutics15061590
APA StyleJackson, N., Ortiz, A. C., Jerez, A., Morales, J., & Arriagada, F. (2023). Kinetics and Mechanism of Camptothecin Release from Transferrin-Gated Mesoporous Silica Nanoparticles through a pH-Responsive Surface Linker. Pharmaceutics, 15(6), 1590. https://doi.org/10.3390/pharmaceutics15061590







