Use of Poly Lactic-co-glycolic Acid Nano and Micro Particles in the Delivery of Drugs Modulating Different Phases of Inflammation
Abstract
1. Introduction
2. Oxidative Stress
3. Inflammatory Bowel Disease
4. Cardiovascular Diseases
5. Transplant Rejection

6. Neurologic Diseases
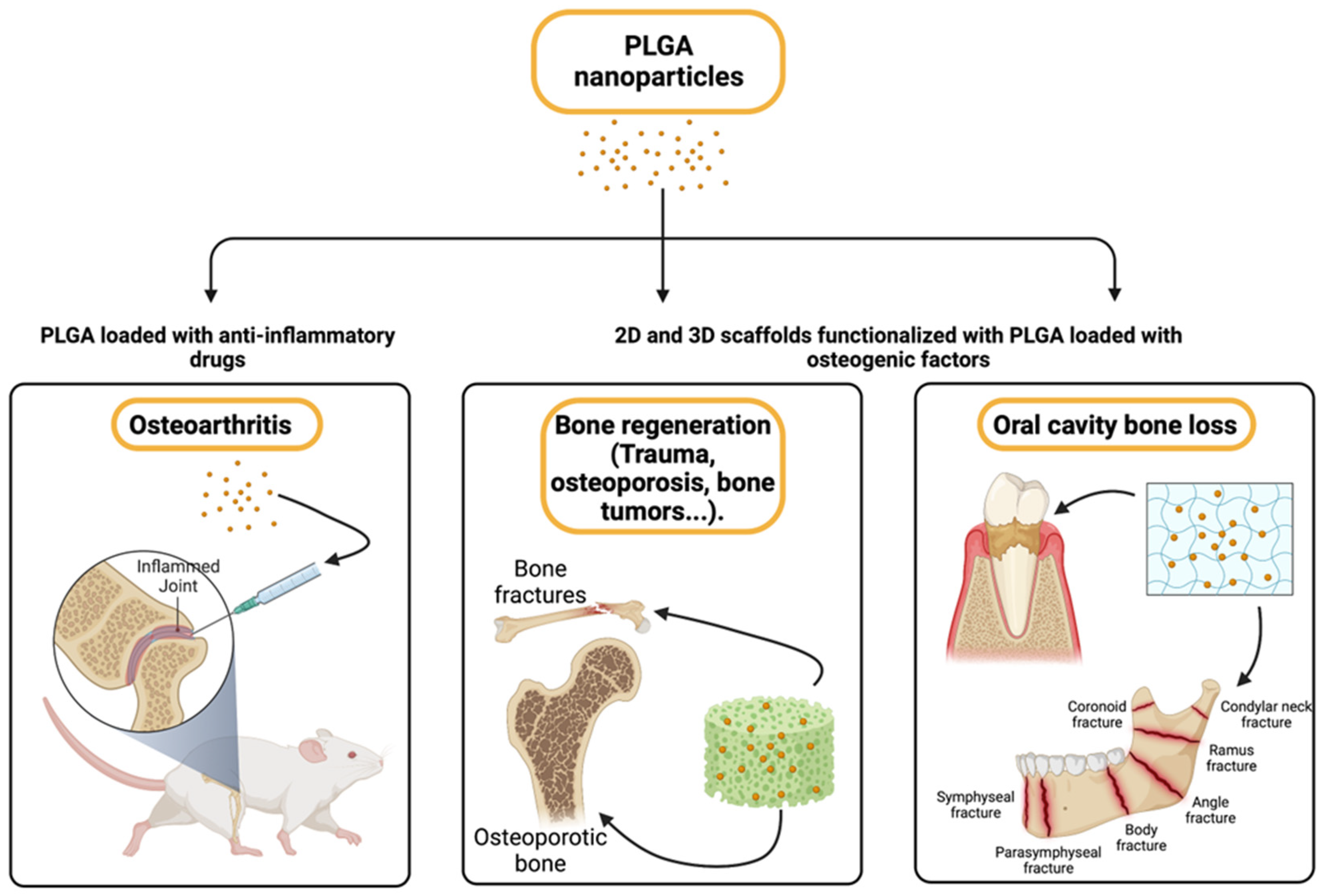
7. Osteoarthritis and Bone Regeneration
8. Skin Wound Healing
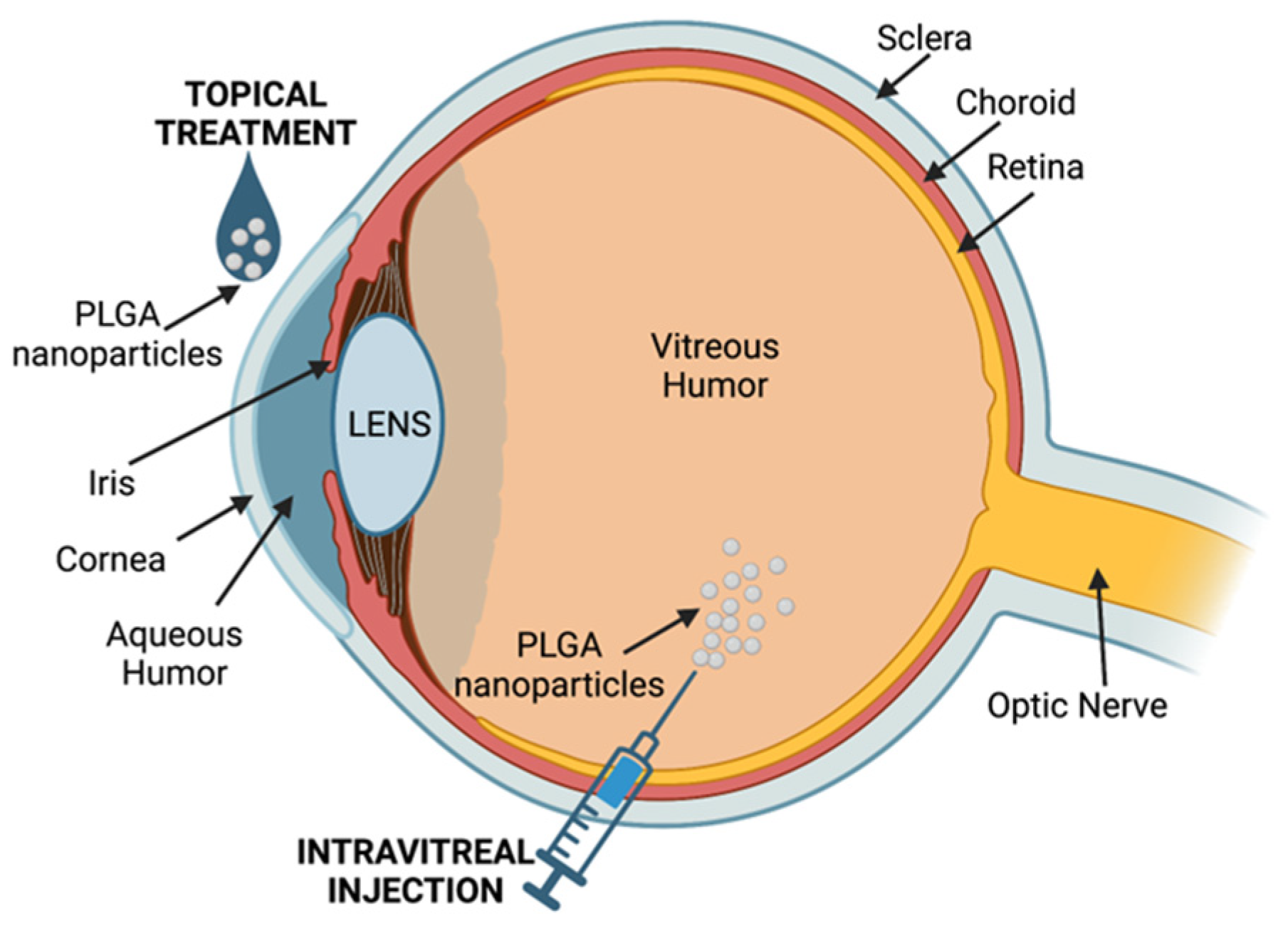
9. Eye Diseases
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D. Chronic Inflammation in the Etiology of Disease across the LifeSpan. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Cannito, S.; Dianzani, U.; Parola, M.; Albano, E.; Sutti, S. Inflammatory Processes Involved in NASH-Related Hepatocellular Carcinoma. Biosci. Rep. 2023, 43, BSR20221271. [Google Scholar] [CrossRef] [PubMed]
- Todoric, J.; Antonucci, L.; Karin, M. Targeting Inflammation in Cancer Prevention and Therapy. Cancer Prev. Res. 2016, 9, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Badri, W.; Miladi, K.; Nazari, Q.A.; Greige-Gerges, H.; Fessi, H.; Elaissari, A. Encapsulation of NSAIDs for Inflammation Management: Overview, Progress, Challenges and Prospects. Int. J. Pharm. 2016, 515, 757–773. [Google Scholar] [CrossRef]
- Haley, R.M.; von Recum, H.A. Localized and Targeted Delivery of NSAIDs for Treatment of Inflammation: A Review. Exp. Bio. Med. 2019, 244, 433–444. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and Organ Damage: A Current Perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Maniar, K.H.; Jones, I.A.; Gopalakrishna, R.; Vangsness, C.T. Lowering Side Effects of NSAID Usage in Osteoarthritis: Recent Attempts at Minimizing Dosage. Expert. Opin. Pharmacother. 2018, 19, 93–102. [Google Scholar] [CrossRef]
- Salice, M.; Rizzello, F.; Calabrese, C.; Calandrini, L.; Gionchetti, P.A. Current Overview of Corticosteroid Use in Active Ulcerative Colitis. Expert. Rev. Gastroenterol. Hepatol. 2019, 13, 557–561. [Google Scholar] [CrossRef]
- Volmer, T.; Effenberger, T.; Trautner, C.; Buhl, R. Consequences of Long-Term Oral Corticosteroid Therapy and Its Side-Effects in Severe Asthma in Adults: A Focused Review of the Impact Data in the Literature. Eur. Respir. J. 2018, 52, 1800703. [Google Scholar] [CrossRef] [PubMed]
- Al-Shalabi, E.; Abusulieh, A.; Hammad, A.M.; Sunoqrot, S. Rhoifolin Loaded in PLGA Nanoparticles Alleviates Oxidative Stress and Inflammation in Vitro and in Vivo. Biomater. Sci. 2022, 10, 5504–5519. [Google Scholar] [CrossRef] [PubMed]
- Pecorini, G.; Ferraro, E.; Puppi, D. Polymeric Systems for the Controlled Release of Flavonoids. Pharmaceutics 2023, 15, 628. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Alharbi, F.D.; Alhibs, A.S.; Alanazi, N.B.; Alshehri, B.Y.; Saleh, M.A.; Alshehri, F.S.; Algarni, M.A.; Almugaiteeb, T.; Uddin, M.N.; et al. PLGA-Based Nanomedicine: History of Advancement and Development in Clinical Applications of Multiple Diseases. Pharmaceutics 2022, 14, 2728. [Google Scholar] [CrossRef]
- Hines, D.J.; Kaplan, D.L. Poly(Lactic-Co-Glycolic) Acid-Controlled-Release Systems: Experimental and Modeling Insights. Crit. Rev. Ther. Drug Carrier Syst. 2013, 30, 257–276. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-Based Nanoparticles: An Overview of Biomedical Applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Conniot, J.; Scomparin, A.; Peres, C.; Yeini, E.; Pozzi, S. Immunization with Mannosylated Nanovaccines and Inhibition of the Immune-Suppressing Microenvironment Sensitizes Melanoma to Immune Checkpoint Modulators. Nat. Nanotechnol. 2019, 14, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Kocbek, P.; Obermajer, N.; Cegnar, M.; Kos, J.; Kristl, J. Targeting Cancer Cells Using PLGA Nanoparticles Surface Modified with Monoclonal Antibody. J. Control. Release 2007, 120, 18–26. [Google Scholar] [CrossRef]
- Zhukova, V.; Osipova, N.; Semyonkin, A.; Malinovskaya, J.; Melnikov, P.; Valikhov, M. Fluorescently Labeled PLGA Nanoparticles for Visualization in Vitro and in Vivo: The Importance of Dye Properties. Pharmaceutics 2021, 13, 1145. [Google Scholar] [CrossRef]
- Ghitman, J.; Biru, E.I.; Stan, R.; Iovu, H. Review of Hybrid PLGA Nanoparticles: Future of Smart Drug Delivery and Theranostics Medicine. Mater. Des. 2020, 193, 108805. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, X.; Zhang, J.; Lu, W.; Lin, X.; Zhang, Y.; Tian, B.; Yang, H.; He, H. PEG-PLGA Copolymers: Their Structure and Structure-Influenced Drug Delivery Applications. J. Control. Release 2014, 183, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Giottonini, K.Y.; Rodríguez-Córdova, R.J.; Gutiérrez-Valenzuela, C.A.; Peñuñuri-Miranda, O. PLGA Nanoparticle Preparations by Emulsification and Nanoprecipitation Techniques: Effects of Formulation Parameters. RSC Adv. 2020, 10, 4218–4231. [Google Scholar] [CrossRef] [PubMed]
- Govender, T. PLGA Nanoparticles Prepared by Nanoprecipitation: Drug Loading and Release Studies of a Water Soluble Drug. J. Control. Release 1999, 57, 171–185. [Google Scholar] [CrossRef]
- Pieper, S.; Langer, K. Doxorubicin-Loaded PLGA Nanoparticles—A Systematic Evaluation of Preparation Techniques and Parameters. Mater. Today Proc. 2017, 4, S188–S192. [Google Scholar] [CrossRef]
- Graham, P.D.; Brodbeck, K.J.; McHugh, A.J. Phase Inversion Dynamics of PLGA Solutions Related to Drug Delivery. J. Control. Release 1999, 58, 233–245. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, C. Tuning the Size of Poly(Lactic-Co-Glycolic Acid) (PLGA) Nanoparticles Fabricated by Nanoprecipitation. Biotechnol. J. 2017, 13, 1700203. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef]
- Clemente, N.; Boggio, E.; Gigliotti, L.C.; Raineri, D.; Ferrara, B.; Miglio, G.; Argenziano, M.; Chiocchetti, A.; Cappellano, G.; Trotta, F.; et al. Immunotherapy of experimental melanoma with ICOS-Fc loaded in biocompatible and biodegradable nanoparticles. J. Control. Release 2020, 320, 112–124. [Google Scholar] [CrossRef]
- Soumya, R.S.; Raghu, K.G. Recent Advances on Nanoparticle-Based Therapies for Cardiovascular Diseases. J. Cardiol. 2022, 81, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Horvath, D.; Basler, M. PLGA Particles in Immunotherapy. Pharmaceutics 2023, 15, 615. [Google Scholar] [CrossRef]
- Triantafyllakou, I.; Clemente, N.; Khetavat, R.K.; Dianzani, U.; Tselios, T. Development of PLGA Nanoparticles with a Glycosylated Myelin Oligodendrocyte Glycoprotein Epitope (MOG35–55) against Experimental Autoimmune Encephalomyelitis (EAE). Mol. Pharm. 2022, 19, 3795–3805. [Google Scholar] [CrossRef] [PubMed]
- Cappellano, G.; Abreu, H.; Casale, C.; Dianzani, U.; Chiocchetti, A. Nano-Microparticle Platforms in Developing Next-Generation Vaccines. Vaccines 2021, 9, 606. [Google Scholar] [CrossRef] [PubMed]
- Cappellano, G.; Comi, C.; Chiocchetti, A.; Dianzani, U. Exploiting PLGA-Based Biocompatible Nanoparticles for Next-Generation Tolerogenic Vaccines against Autoimmune Disease. Int. J. Mol. Sci. 2019, 20, 204. [Google Scholar] [CrossRef]
- Cappellano, G.; Woldetsadik, A.D.; Orilieri, E.; Shivakumar, Y.; Rizzi, M.; Carniato, F.; Gigliotti, C.L.; Boggio, E.; Clemente, N.; Comi, C.; et al. Subcutaneous Inverse Vaccination with PLGA Particles Loaded with a MOG Peptide and IL-10 Decreases the Severity of Experimental Autoimmune Encephalomyelitis. Vaccine 2014, 32, 5681–5689. [Google Scholar] [CrossRef]
- Jin, S.; Xia, X.; Huang, J.; Yuan, C.; Zuo, Y.; Li, Y.; Li, J. Recent Advances in PLGA-Based Biomaterials for Bone Tissue Regeneration. Acta Biomater. 2021, 127, 56–79. [Google Scholar] [CrossRef]
- Brannon, E.R.; Guevara, M.V.; Pacifici, N.J.; Lee, J.K.; Lewis, J.S.; Eniola-Adefeso, O. Polymeric particle-based therapies for acute inflammatory diseases. Nat. Rev. Mater. 2022, 7, 796–813. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.P.; Bolandparvaz, A.; Ma, J.A.; Manickam, V.A.; Lewis, J.S. Latent, Immunosuppressive Nature of Poly(lactic-co-glycolic acid) Microparticles. ACS Biomater. Sci. Eng. 2018, 4, 900–918. [Google Scholar] [CrossRef]
- Ulbrich, W.; Lamprecht, A. Targeted drug-delivery approaches by nanoparticulate carriers in the therapy of inflammatory diseases. J. R. Soc. Interface 2010, 7 (Suppl. S1), S55–S66. [Google Scholar] [CrossRef]
- Silva, A.L.; Peres, C.; Conniot, J.; Matos, A.I.; Moura, L.; Carreira, B.; Sainz, V.; Scomparin, A.; Satchi-Fainaro, R.; Préat, V.; et al. Nanoparticle impact on innate immune cell pattern-recognition receptors and inflammasomes activation. Semin. Immunol. 2017, 34, 3–24. [Google Scholar] [CrossRef]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Khan, M.; Siddique, H. PLGA-based nanoparticles for the treatment of inflammatory diseases. PLGA Nanoparticles Drug Deliv. 2023, 211–233. [Google Scholar]
- Xiao, B.; Xu, Z.; Viennois, E.; Zhang, Y.; Zhang, Z.; Zhang, M.; Han, M.K.; Kang, Y.; Merlin, D. Orally targeted delivery of tripeptide KPV via hyaluronic acid-functionalized nanoparticles efficiently alleviates ulcerative colitis. Mol. Ther. 2017, 25, 1628–1640. [Google Scholar] [CrossRef]
- Gou, S.; Huang, Y.; Wan, Y.; Ma, Y.; Zhou, X.; Tong, X.; Huang, J.; Kang, Y.; Pan, G.; Dai, F.; et al. Multi-bioresponsive silk fibroin-based nanoparticles with on-demand cytoplasmic drug release capacity for CD44-targeted alleviation of ulcerative colitis. Biomaterials 2019, 212, 39–54. [Google Scholar] [CrossRef]
- Truffi, M.; Colombo, M.; Peñaranda-Avila, J.; Sorrentino, L.; Colombo, F.; Monieri, M.; Collico, V.; Zerbi, P.; Longhi, E.; Allevi, R.; et al. Nano-targeting of mucosal addressin cell adhesion molecule-1 identifies bowel inflammation foci in murine model. Nanomedicine 2017, 12, 1547–1560. [Google Scholar] [CrossRef]
- Sun-Mi, L.; Hyung, J.K.; You-Jung, H.; Young, N.P.; Soo-Kon, L.; Yong-Beom, P.; Kyung-Hwa, Y. Targeted Chemo-Photothermal Treatments of Rheumatoid Arthritis Using Gold Half-Shell Multifunctional Nanoparticles. ACS Nano 2013, 7, 50–57. [Google Scholar]
- Wan, S.; Zhang, L.; Quan, Y.; Wei, K. Resveratrol-loaded PLGA nanoparticles: Enhanced stability, solubility and bioactivity of resveratrol for non-alcoholic fatty liver disease therapy. R. Soc. Open. Sci. 2018, 5, 181457. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.V.; Gonçalves, V.; da Silva, M.C.; Bañobre-López, M.; Gallo, J. PLGA-Based Composites for Various Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 2034. [Google Scholar] [CrossRef] [PubMed]
- Operti, M.C.; Bernhardt, A.; Grimm, S.; Engel, A.; Figdor, C.G.; Tagit, O. PLGA-based nanomedicines manufacturing: Technologies overview and challenges in industrial scale-up. Int. J. Pharm. 2021, 605, 120807. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Alpini, G.; Glaser, S.; Merlin, D. Lipid nanoparticles for oral delivery of nucleic acids for treating inflammatory bowel disease. Nanomedicine 2022, 17, 1501–1504. [Google Scholar] [CrossRef] [PubMed]
- Hornos Carneiro, M.F.; Barbosa, F., Jr. Gold nanoparticles: A critical review of therapeutic applications and toxicological aspects. J. Toxicol. Environ. Health B Crit. Rev. 2016, 19, 129–148. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.H.; Joo, H.S.; Hong, E.B.; Lee, H.J.; Lee, J.M. Therapeutic Application of Exosomes in Inflammatory Diseases. Int. J. Mol. Sci. 2021, 22, 1144. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Yun, Y.; Park, K. PLA micro- and nano-particles. Adv. Drug. Deliv. Rev. 2016, 107, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Feelisch, M.; Faber, K.N.; Pasch, A.; Dijkstra, G.; van Goor, H. Oxidative Stress and Redox-Modulating Therapeutics in Inflammatory Bowel Disease. Trends Mol. Med. 2020, 26, 1034–1046. [Google Scholar] [CrossRef]
- Lingappan, K. NF-ΚB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Denat, L.; Kadekaro, A.L.; Marrot, L.; Leachman, S.A.; Abdel-Malek, Z.A. Melanocytes as Instigators and Victims of Oxidative Stress. J. Investig. Dermatol. 2014, 134, 1512–1518. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Cueto, R.; Effi, C.; Zhang, Y.; Tan, H.; Qin, X.; Ji, Y.; Yang, X.; Wang, H. Biochemical Basis and Metabolic Interplay of Redox Regulation. Redox. Biol. 2019, 26, 101284. [Google Scholar] [CrossRef]
- Nathan, C.; Shiloh, M.U. Reactive Oxygen and Nitrogen Intermediates in the Relationship between Mammalian Hosts and Microbial Pathogens. Proc. Natl. Acad. Sci. USA 2000, 97, 8841–8848. [Google Scholar] [CrossRef]
- Barrera, G.; Pizzimenti, S.; Daga, M.; Dianzani, C.; Arcaro, A.; Cetrangolo, G.P.; Giordano, G.; Cucci, M.A.; Graf, M.; Gentile, F. Lipid Peroxidation-Derived Aldehydes, 4-Hydroxynonenal and Malondialdehydein Aging-Related Disorders. Antioxidants 2018, 7, 102. [Google Scholar] [CrossRef]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant Responses and Cellular Adjustments to Oxidative Stress. Redox. Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Jesus, S.; Schmutz, M.; Som, C.; Borchard, G.; Wick, P.; Borges, O. Hazard Assessment of Polymeric Nanobiomaterials for Drug Delivery: What Can We Learn from Literature so Far. Front. Bioeng. Biotechnol. 2019, 7, 261. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. Nitric Oxide Signaling in Health and Disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef]
- Chakraborty, S.; Stalin, S.; Das, N.; Thakur Choudhury, S.; Ghosh, S.; Swarnakar, S. The Use of Nano-Quercetin to Arrest Mitochondrial Damage and MMP-9 Upregulation during Prevention of Gastric Inflammation Induced by Ethanol in Rat. Biomaterials 2012, 33, 2991–3001. [Google Scholar] [CrossRef] [PubMed]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Binmahfouz, L.S.; Bakhaidar, R.B.; Sreeharsha, N.; Nair, A.B.; Ramnarayanan, C. Lung Targeted Lipopolymeric Microspheres of Dexamethasone for the Treatment of ARDS. Pharmaceutics 2021, 13, 1347. [Google Scholar] [CrossRef]
- Li, Y.; Che, J.; Chang, L.; Guo, M.; Bao, X.; Mu, D.; Sun, X.; Zhang, X.; Lu, W.; Xie, J. CD47- and Integrin α 4/ β 1-Comodified-Macrophage-Membrane-Coated Nanoparticles Enable Delivery of Colchicine to Atherosclerotic Plaque. Adv. Healthc. Mater. 2021, 11, 2101788. [Google Scholar] [CrossRef]
- Völs, S.; Kaisar-Iluz, N.; Shaul, M.E.; Ryvkin, A.; Ashkenazy, H. Targeted Nanoparticles Modify Neutrophil Function in Vivo. Front. Immunol. 2022, 13, 1003871. [Google Scholar] [CrossRef] [PubMed]
- Medrano-Bosch, M.; Moreno-Lanceta, A.; Melgar-Lesmes, P. Nanoparticles to Target and Treat Macrophages: The Ockham’s Concept? Pharmaceutics 2021, 13, 1340. [Google Scholar] [CrossRef]
- Boltnarova, B.; Kubackova, J.; Skoda, J.; Stefela, A.; Smékalová, M.; Svačinová, P.; Ivona, P.; Dittrich, M. PLGA Based Nanospheres as a Potent Macrophage-Specific Drug Delivery System. Nanomaterials 2021, 11, 749. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Guo, M.; Xu, J.; Wu, F.; Fan, J.; Huang, Q.; Yang, G.; Lv, Z.; Wang, X.; Jin, Y. Nanoparticles Targeting Macrophages as Potential Clinical Therapeutic Agents against Cancer and Inflammation. Front. Immunol. 2019, 10, 1998. [Google Scholar] [CrossRef] [PubMed]
- Windsor, J.W.; Kaplan, G.G. Evolving Epidemiology of IBD. Curr. Gastroenterol. Rep. 2019, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef]
- McGuckin, M.A.; Eri, R.; Simms, L.A.; Florin, T.H.J.; Radford-Smith, G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm. Bowel. Dis. 2009, 15, 100–113. [Google Scholar] [CrossRef]
- Gomollón, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J. Crohn’s Colitis 2017, 11, 3–25. [Google Scholar] [CrossRef]
- Magro, F.; Doherty, G.; Peyrin-Biroulet, L.; Svrcek, M.; Borralho, P.; Walsh, A.; Carneiro, F.; Rosini, F.; de Hertogh, G.; Biedermann, L. ECCO Position Paper: Harmonization of the Approach to Ulcerative Colitis Histopathology. J. Crohn’s Colitis 2020, 14, 1503–1511. [Google Scholar] [CrossRef]
- De Souza, H.S.P.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef]
- Yilmaz, B.; Juillerat, P.; Øyås, O.; Ramon, C.; Bravo, F.D.; Franc, Y.; Fournier, N.; Michetti, P.; Mueller, C.; Geuking, M.; et al. Microbial network disturbances in relapsing refractory Crohn’s disease. Nat. Med. 2019, 25, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef] [PubMed]
- Zurba, Y.; Gros, B.; Shehab, M. Exploring the Pipeline of Novel Therapies for Inflammatory Bowel Disease; State of the Art Review. Biomedicines 2023, 11, 762. [Google Scholar] [CrossRef]
- De Jong, M.E.; Smits, L.J.T.; van Ruijven, B.; den Broeder, N.; Russel, M.G.V.M.; Römkens, T.E.H.; West, R.L.; Jansen, J.M.; Hoentjen, F. Increased Discontinuation Rates of Anti-TNF Therapy in Elderly Inflammatory Bowel Disease Patients. J. Crohn’s Colitis 2020, 14, 888–895. [Google Scholar] [CrossRef]
- Yarur, A.J.; Jain, A.; Sussman, D.A.; Barkin, J.S.; Quintero, M.A.; Princen, F.; Kirkland, R.; Deshpande, A.R.; Singh, S.; Abreu, M.T. The association of tissue anti-TNF drug levels with serological and endoscopic disease activity in inflammatory bowel disease: The ATLAS study. Gut 2016, 65, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Tahara, K.; Samura, S.; Tsuji, K.; Yamamoto, H.; Tsukada, Y.; Bando, Y.; Tsujimoto, H.; Morishita, R.; Kawashima, Y. Oral nuclear factor-κB decoy oligonucleotides delivery system with chitosan modified poly(d,l-lactide-co-glycolide) nanospheres for inflammatory bowel disease. Biomaterials 2011, 32, 870–878. [Google Scholar] [CrossRef]
- Lamprecht, A.; Ubrich, N.; Yamamoto, H.; Schäfer, U.; Takeuchi, H.; Maincent, P.; Kawashima, Y.; Lehr, C.M. Biodegradable Nanoparticles for Targeted Drug Delivery in Treatment of Inflammatory Bowel Disease. J. Pharmacol. Exp. Ther. 2001, 299, 775–781. [Google Scholar]
- Friend, D.R. New oral delivery systems for treatment of inflammatory bowel disease. Adv. Drug. Deliv. Rev. 2005, 57, 247–265. [Google Scholar] [CrossRef]
- Meissner, Y.; Lamprecht, A. Alternative Drug Delivery Approaches for the Therapy of Inflammatory Bowel Disease. J. Pharm. Sci. 2008, 97, 2878–2891. [Google Scholar] [CrossRef]
- Watts, P.J.; Barrow, L.; Steed, K.P.; Wilson, C.D.; Spiller, R.C.; Melia, C.D.; Davies, M. The Transit Rate of Different-Sized Model Dosage Forms through the Human Colon and the Effects of a Lactulose-Induced Catharsis. Int. J. Pharm. 1992, 87, 215–221. [Google Scholar] [CrossRef]
- Meissner, Y.; Pellequer, Y.; Lamprecht, A. Nanoparticles in inflammatory bowel disease: Particle targeting versus pH-sensitive delivery. Int. J. Pharm. 2006, 316, 138–143. [Google Scholar] [CrossRef]
- Davoudi, Z.; Peroutka-Bigus, N.; Bellaire, B.; Wannemuehler, M.; Barrett, T.A.; Narasimhan, B.; Wang, Q. Intestinal Organoids Containing PLGA Nanoparticles for the Treatment of Inflammatory Bowel Diseases. J. Biomed. Mater. Res. A 2018, 106, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Tabata, Y.; Inoue, Y.; Ikada, Y. Size effect on systemic and mucosal immune responses induced by oral administration of biodegradable microspheres. Vaccine 1996, 14, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Mohan, L.J.; Daly, J.S.; Ryan, B.M.; Ramtoola, Z. Oral infliximab nanomedicines for targeted treatment of inflammatory bowel diseases. Eur. J. Pharm. Sci. 2023, 183, 106379. [Google Scholar] [CrossRef] [PubMed]
- Lautenschläger, C.; Schmidt, C.; Lehr, C.M.; Fischer, D.; Stallmach, A. PEG-functionalized microparticles selectively target inflamed mucosa in inflammatory bowel disease. Eur. J. Pharm. Biopharm. 2013, 85, 578–586. [Google Scholar] [CrossRef]
- Mohan, L.J.; McDonald, L.; Daly, J.S.; Ramtoola, Z. Optimising PLGA-PEG Nanoparticle Size and Distribution for Enhanced Drug Targeting to the Inflamed Intestinal Barrier. Pharmaceutics 2020, 12, 1114. [Google Scholar] [CrossRef]
- Coco, R.; Plapied, L.; Pourcelle, V.; Jérôme, C.; Brayden, D.J.; Schneider, Y.J.; Préat, V. Drug delivery to inflamed colon by nanoparticles: Comparison of different strategies. Int. J. Pharm. 2013, 440, 3–12. [Google Scholar] [CrossRef]
- Pakulska, M.M.; Elliott Donaghue, I.; Obermeyer, J.M.; Tuladhar, A.; McLaughlin, C.K.; Shendruk, T.N.; Shoichet, M.S. Encapsulation-free controlled release: Electrostatic adsorption eliminates the need for protein encapsulation in PLGA nanoparticles. Sci. Adv. 2016, 2, e1600519. [Google Scholar] [CrossRef]
- Ruiz-Pulido, G.; Medina, D.I. An overview of gastrointestinal mucus rheology under different pH conditions and introduction to pH-dependent rheological interactions with PLGA and chitosan nanoparticles. Eur. J. Pharm. Biopharm. 2021, 159, 123–136. [Google Scholar] [CrossRef]
- Bai, X.; Feng, Z.; Peng, S.; Zhu, T.; Jiao, L.; Mao, N.; Gu, P.; Liu, Z.; Yang, Y.; Wang, D. Chitosan-modified Phellinus igniarius polysaccharide PLGA nanoparticles ameliorated inflammatory bowel disease. Biomater. Adv. 2022, 139, 213002. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N. Trimethyl chitosan and its applications in drug delivery. J. Mater. Sci. Mater. Med. 2009, 20, 1057–1079. [Google Scholar] [CrossRef]
- Shrestha, N.; Xu, Y.; Prévost, J.R.C.; McCartney, F.; Brayden, D.; Frédérick, R.; Beloqui, A.; Préat, V. Impact of PEGylation on an Antibody-Loaded Nanoparticle-Based Drug Delivery System for the Treatment of Inflammatory Bowel Disease. Acta Biomater. 2022, 140, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.; Wu, R.; Zhou, M.; Wang, P. Mechanism of the Anti-inflammatory Effect of Curcumin: PPAR-γ Activation. PPAR Res. 2007, 2007, 89369. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, F.; Cofelice, M.; Venditti, F.; Ceglie, A.; Miguel, M.; Lindman, B.; Lopez, F. In-vitro digestion of curcumin loaded chitosan-coated liposomes. Colloids Surf. B 2018, 168, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Hlaing, S.P.; Cao, J.; Lee, J.; Kim, J.; Saparbayeva, A.; Kwak, D.; Kim, H.; Hwang, S.; Yun, H.; Moon, H.R.; et al. Hyaluronic Acid-Conjugated PLGA Nanoparticles Alleviate Ulcerative Colitis via CD44-Mediated Dual Targeting to Inflamed Colitis Tissue and Macrophages. Pharmaceutics 2022, 14, 2118. [Google Scholar] [CrossRef] [PubMed]
- Beloqui, A.; Coco, R.; Memvanga, P.B.; Ucakar, B.; Des Rieux, A.; Préat, V. pH-sensitive nanoparticles for colonic delivery of curcumin in inflammatory bowel disease. Int. J. Pharm. 2014, 473, 203–212. [Google Scholar] [CrossRef]
- Truffi, M.; Sevieri, M.; Morelli, L.; Monieri, M.; Mazzucchelli, S.; Sorrentino, L.; Allevi, R.; Bonizzi, A.; Zerbi, P.; Marchini, B.; et al. Anti-MAdCAM-1-Conjugated Nanocarriers Delivering Quantum Dots Enable Specific Imaging of Inflammatory Bowel Disease. Int. J. Nanomed. 2020, 15, 8537–8552. [Google Scholar] [CrossRef]
- Ross, R. The Pathogenesis of Atherosclerosis: A Perspective for the 1990s. Nature 1993, 362, 801–809. [Google Scholar] [CrossRef]
- Sanz, J.; Fayad, Z.A. Imaging of Atherosclerotic Cardiovascular Disease. Nature 2008, 451, 953–957. [Google Scholar] [CrossRef]
- Napoli, C.; D’Armiento, F.P.; Mancini, F.P.; Postiglione, A.; Witztum, J.L.; Palumbo, G.; Palinski, W. Fatty Streak Formation Occurs in Human Fetal Aortas and Is Greatly Enhanced by Maternal Hypercholesterolemia. Intimal Accumulation of Low Density Lipoprotein and Its Oxidation Precede Monocyte Recruitment into Early Atherosclerotic Lesions. J. Clin. Investig. 1997, 100, 2680–2690. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of Plaque Formation and Rupture. Cir. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Randolph, G.J. Mechanisms That Regulate Macrophage Burden in Atherosclerosis. Circ. Res. 2014, 114, 1757–1771. [Google Scholar] [CrossRef]
- Olie, R.H.; van der Meijden, P.E.J.; ten Cate, H. The Coagulation System in Atherothrombosis: Implications for New Therapeutic Strategies. Res. Pract. Thromb. Haemost. 2018, 2, 188–198. [Google Scholar] [CrossRef]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef]
- Soehnlein, O.; Libby, P. Targeting Inflammation in Atherosclerosis—From Experimental Insights to the Clinic. Nat. Rev. Drug. Discov. 2021, 20, 589–610. [Google Scholar] [CrossRef]
- Cheng, Y.; Rong, J. Macrophage Polarization as a Therapeutic Target in Myocardial Infarction. Curr. Drug. Targ. 2018, 19, 651–662. [Google Scholar] [CrossRef]
- Moskalik, A.; Niderla-Bielińska, J.; Ratajska, A. Multiple Roles of Cardiac Macrophages in Heart Homeostasis and Failure. Heart Fail. Rev. 2022, 27, 1413–1430. [Google Scholar] [CrossRef]
- Hetherington, I.; Totary-Jain, H. Anti-Atherosclerotic Therapies: Milestones, Challenges, and Emerging Innovations. Mol. Ther. 2022, 30, 3106–3117. [Google Scholar] [CrossRef]
- Leal, B.H.; Velasco, B.; Cambón, A.; Pardo, A.; Fernández Vega, J.; Arellano, L.; Al-Modlej, A.; Mosquera, V.; Bouzas, A.; Prieto, G.; et al. Combined Therapeutics for Atherosclerosis Treatment Using Polymeric Nanovectors. Pharmaceutics 2022, 14, 258. [Google Scholar] [CrossRef]
- Cheng, P.; Zeng, W.; Li, L.; Huo, D.; Zeng, L.; Tan, J.; Zhou, J.; Sun, J.; Liu, G.; Li, Y.; et al. PLGA-PNIPAM Microspheres Loaded with the Gastrointestinal Nutrient NaB Ameliorate Cardiac Dysfunction by Activating Sirt3 in Acute Myocardial Infarction. Adv. Sci. 2016, 3, 1600254. [Google Scholar] [CrossRef]
- Liang, X.; Li, H.; Li, X.; Tian, X.; Zhang, A.; Luo, Q. Highly Sensitive H2O2-Scavenging Nano-Bionic System for Precise Treatment of Atherosclerosis. Acta Pharma. Sin. B 2022, 13, 372–389. [Google Scholar] [CrossRef]
- Gasparyan, A.Y.; Ayvazyan, L.; Yessirkepov, M.; Kitas, G.D. Colchicine as an Anti-Inflammatory and Cardioprotective Agent. Exp. Opin. Drug. Met. Toxic. 2015, 11, 1781–1794. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.M.; Navarini, A.; Brandt, O.; Müller, S. Colchicine—Renaissance of an “Ancient” Drug. J. Dtsch. Dermatol. Ges. 2023, 21, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Dasgeb, B.; Kornreich, D.; McGuinn, K.; Okon, L.; Brownell, I.; Sackett, D.L. Colchicine: An Ancient Drug with Novel Applications. Brit. J. Dermatol. 2018, 178, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.J.; Celermajer, D.S.; Patel, S. Corrigendum To: “the NLRP3 Inflammasome and the Emerging Role of Colchicine to Inhibit Atherosclerosis-Associated Inflammation”. Atherosclerosis 2018, 273, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, Y.; Aks, S.E.; Hutson, J.R.; Juurlink, D.N.; Nguyen, P.; Dubnov-Raz, G.; Pollak, U.; Koren, G.; Bentur, Y. Colchicine Poisoning: The Dark Side of an Ancient Drug. Clin. Toxicol. 2010, 48, 407–414. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, Y.; Han, D.; Zhang, X.; Qin, C.; Liu, J.; Tian, L.; Xu, M.; Fang, Y.; Zhang, Y.; et al. Scavenger Receptor-AI Targeted Theranostic Nanoparticles for Regression of Atherosclerotic Plaques via ABCA1 Modulation. Nanomed. Nanotechnol. Biol. Med. 2023, 50, 102672. [Google Scholar] [CrossRef]
- Bei, W.; Jing, L.; Chen, N. Cardio Protective Role of Wogonin Loaded Nanoparticle against Isoproterenol Induced Myocardial Infarction by Moderating Oxidative Stress and Inflammation. Colloids Surf. B Biointerfaces 2020, 185, 110635. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Zheng, L.; Du, J.; Wei, S.; Zhu, X.; Xiong, J.-W. A DUSP6 Inhibitor Suppresses Inflammatory Cardiac Remodeling and Improves Heart Function after Myocardial Infarction. Dis. Models. Mech. 2023, 16, dmm049662. [Google Scholar] [CrossRef]
- Hu, S.; Zhu, D.; Li, Z.; Cheng, K. Detachable Microneedle Patches Deliver Mesenchymal Stromal Cell Factor-Loaded Nanoparticles for Cardiac Repair. ACS Nano 2022, 16, 15935–15945. [Google Scholar] [CrossRef]
- Yokoyama, R.; Ii, M.; Tabata, Y.; Hoshiga, M.; Ishizaka, N.; Asahi, M. Cardiac Regeneration by Statin-Polymer Nanoparticle-Loaded Adipose-Derived Stem Cell Therapy in Myocardial Infarction. Stem Cells Transl. Med. 2019, 8, 1055–1067. [Google Scholar] [CrossRef]
- Ghassemi, A.H.; van Steenbergen, M.J.; Talsma, H.; van Nostrum, C.F.; Jiskoot, W.; Crommelin, D.J.A.; Hennink, W.E. Preparation and Characterization of Protein Loaded Microspheres Based on a Hydroxylated Aliphatic Polyester, Poly(Lactic-Co-Hydroxymethyl Glycolic Acid). J. Control. Release 2009, 138, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kunjachan, S.; Gremse, F.; Theek, B.; Koczera, P.; Pola, R.; Pechar, M.; Etrych, T.; Ulbrich, K.; Storm, G.; Kiessling, F.; et al. Noninvasive Optical Imaging of Nanomedicine Biodistribution. ACS Nano 2013, 7, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Nakanishi, K.; Yoshioka, T.; Tsutsui, Y.; Maeda, A.; Kondo, H.; Sako, K. Oral Tacrolimus Oil Formulations for Enhanced Lymphatic Delivery and Efficient Inhibition of T-Cell’s Interleukin-2 Production. Eur. J. Pharm. Biopharm. 2016, 100, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Staatz, C.E.; Tett, S.E. Clinical Pharmacokinetics and Pharmacodynamics of Tacrolimus in Solid Organ Transplantation. Clin. Pharmacokinet. 2004, 43, 623–653. [Google Scholar] [CrossRef]
- Niu, L.; Chu, L.Y.; Burton, S.A.; Hansen, K.J.; Panyam, J. Intradermal Delivery of Vaccine Nanoparticles Using Hollow Microneedle Array Generates Enhanced and Balanced Immune Response. J. Control. Release 2019, 294, 268–278. [Google Scholar] [CrossRef]
- Deng, C.; Jin, Q.; Wu, Y.; Li, H.; Yi, L.; Chen, Y.-H.; Gao, T. Immunosuppressive Effect of PLGA-FK506-NPs in Treatment of Acute Cardiac Rejection via Topical Subcutaneous Injection. Drug. Deliv. 2021, 28, 1759–1768. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, D.; Zhang, X.-L.; Wang, D.-N.; Meimei, D.; Chen, W.; Lin, D.; Zhu, F.; Chen, W.; Lin, H. Development and Effects of Tacrolimus-Loaded Nanoparticles on the Inhibition of Corneal Allograft Rejection. Drug. Deliv. 2019, 26, 290–299. [Google Scholar] [CrossRef]
- Liu, D.; Wu, Q.; Chen, W.; Lin, H.; Liu, Y.; Liang, H.; Zhu, F. Tacrolimus-Loaded Methoxy Poly(Ethylene Glycol)-Block-Poly(D,L)-Lactic–Co-Glycolic Acid Micelles Self-Assembled in Aqueous Solution for Treating Cornea Immune Rejection after Allogenic Penetrating Keratoplasty in Rats. Eur. Pharm. Sci. 2019, 133, 104–114. [Google Scholar] [CrossRef]
- Shahzad, K.A.; Wan, X.; Zhang, L.; Pei, W.; Zhang, A.; Younis, M.; Wang, W.; Shen, C. On-Target and Direct Modulation of Alloreactive T Cells by a Nanoparticle Carrying MHC Alloantigen, Regulatory Molecules and CD47 in a Murine Model of Alloskin Transplantation. Drug. Deliv. 2018, 25, 703–715. [Google Scholar] [CrossRef]
- Li, Y.; Frei, A.W.; Labrada, I.; Rong, Y.; Liang, J.-P.; Samojlik, M.M.; Sun, C.; Barash, S.D. Immunosuppressive PLGA TGF-β1 Microparticles Induce Polyclonal and Antigen-Specific Regulatory T Cells for Local Immunomodulation of Allogeneic Islet Transplants. Front. Immunol. 2021, 12, 653088. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.R. Optimizing the Long-Term Outcome of Renal Transplants: Opportunities Created by Sirolimus. Transplant. Proc. 2003, 35, S67–S72. [Google Scholar] [CrossRef]
- Shahzad, K.A.; Naeem, M.; Zhang, L.; Wan, X.; Song, S.; Pei, W.; Zhao, C.; Jin, X.; Shen, C. Design and Optimization of PLGA Particles to Deliver Immunomodulatory Drugs for the Prevention of Skin Allograft Rejection. Immunol. Investig. 2020, 49, 840–857. [Google Scholar] [CrossRef]
- Keshavarz Shahbaz, S.; Foroughi, F.; Soltaninezhad, E.; Jamialahmadi, T.; Penson, P.E.; Sahebkar, A. Application of PLGA Nano/Microparticle Delivery Systems for Immunomodulation and Prevention of Allotransplant Rejection. Expert. Opin. Drug. Deliv. 2020, 17, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Li, M.O.; Flavell, R.A. TGF-β: A Master of All T Cell Trades. Cell 2008, 134, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.; Nguyen, T.; Pathak, S.; Regmi, S.; Nguyen, H.V.; Tran, T.; Yong, C.S.; Kim, J.M.; Park, P.; Park, M. Tissue Adhesive FK506–Loaded Polymeric Nanoparticles for Multi–Layered Nano–Shielding of Pancreatic Islets to Enhance Xenograft Survival in a Diabetic Mouse Model. Biomaterials 2018, 154, 182–196. [Google Scholar] [CrossRef]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Xie, A.; Gao, J.; Xu, L.; Meng, D. Shared Mechanisms of Neurodegeneration in Alzheimer’s Disease and Parkinson’s Disease. BioMed. Res. International. 2014, 2014, 648740. [Google Scholar] [CrossRef]
- Mistretta, M.; Farini, A.; Torrente, Y.; Villa, C. Multifaceted Nanoparticles: Emerging Mechanisms and Therapies in Neurodegenerative Diseases. Brain 2023, awad014. [Google Scholar] [CrossRef]
- Teixeira, M.I.; Lopes, C.M.; Amaral, M.H.; Costa, P.C. Current Insights on Lipid Nanocarrier-Assisted Drug Delivery in the Treatment of Neurodegenerative Diseases. Eur. J. Pharm. Biopharm. 2020, 149, 192–217. [Google Scholar] [CrossRef]
- Gilhus, N.E.; Deuschl, G. Neuroinflammation—A Common Thread in Neurological Disorders. Nat. Rev. Neurol. 2019, 15, 429–430. [Google Scholar] [CrossRef]
- Schain, M.; Kreisl, W.C. Neuroinflammation in Neurodegenerative Disorders—A Review. Curr. Neurol. Neurosci. Rep. 2017, 17, 25. [Google Scholar] [CrossRef]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s Disease: Current Evidence and Future Directions. Alzheimer’s Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Phelps, W. Overview on Clinical Data of Dexibuprofen. Clin. Rheumatol. 2001, 20, 15–21. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Ettcheto, M.; Egea, M.A.; Espina, M.; Calpena, A.C.; Folch, J.; Camins, A.; García, M.L. New Potential Strategies for Alzheimer’s Disease Prevention: Pegylated Biodegradable Dexibuprofen Nanospheres Administration to APPswe/PS1dE9. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Ettcheto, M.; Chang, J.-H.; Barroso, E.; Espina, M.; Kühne, B.A.; Barenys, M.; Auladell, C.; Folch, J.; Souto, E.B.; et al. Dual-Drug Loaded Nanoparticles of Epigallocatechin-3-Gallate (EGCG)/Ascorbic Acid Enhance Therapeutic Efficacy of EGCG in an APPswe/PS1dE9 Alzheimer’s Disease Mice Model. J. Control. Release 2019, 301, 62–75. [Google Scholar] [CrossRef]
- Vilella, A.; Belletti, D.; Sauer, A.K.; Hagmeyer, S.; Sarowar, T.; Masoni, M.; Stasiak, N.; Mulvihill, J.J.E.; Ruozi, B.; Forni, F.; et al. Reduced Plaque Size and Inflammation in the APP23 Mouse Model for Alzheimer’s Disease after Chronic Application of Polymeric Nanoparticles for CNS Targeted Zinc Delivery. J. Trace Elements Med. Biol. 2018, 49, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.G.; Cha, M.-Y.; Kim, J.; Hwang, T.W.; Kim, K.A.; Kim, T.H.; Song, K.C.; Kim, J.-J.; Moon, M. Vitamin D-Binding Protein-Loaded PLGA Nanoparticles Suppress Alzheimer’s Disease-Related Pathology in 5XFAD Mice. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 297–307. [Google Scholar] [CrossRef]
- Doggui, S.; Sahni, J.K.; Arseneault, M.; Dao, L.; Ramassamy, C. Neuronal Uptake and Neuroprotective Effect of Curcumin-Loaded PLGA Nanoparticles on the Human SK-N-SH Cell Line. J. Alzheimer’s Dis. 2012, 30, 377–392. [Google Scholar] [CrossRef]
- Huang, N.; Lu, S.; Liu, X.G.; Zhu, J.; Wang, Y.J.; Liu, R.T. PLGA nanoparticles modified with a BBB-penetrating peptide co-delivering Aβ generation inhibitor and curcumin attenuate memory deficits and neuropathology in Alzheimer’s disease mice. Oncotarget 2017, 8, 81001–81013. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Agarwal, S.; Seth, B.; Yadav, A.; Nair, S.; Bhatnagar, P.; Karmakar, M.; Kumari, M.; Chauhan, L.K.S.; Patel, D.K.; et al. Curcumin-Loaded Nanoparticles Potently Induce Adult Neurogenesis and Reverse Cognitive Deficits in Alzheimer’s Disease Model Via Canonical Wnt/β-Catenin Pathway. ACS Nano 2013, 8, 76–103. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Yang, Z.; Xia, X.; Chen, Y.; Yang, X.; Xie, R.; Tong, F.; Wang, X.; Gao, H. A Nanocleaner Specifically Penetrates the Blood–Brain Barrier at Lesions to Clean Toxic Proteins and Regulate Inflammation in Alzheimer’s Disease. Acta Pharma. Sin. B 2021, 11, 4032–4044. [Google Scholar] [CrossRef]
- Brodie, M.J. Antiepileptic Drug Therapy the Story so Far. Seizure 2010, 19, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Ettcheto, M.; Espina, M.; Auladell, C.; Calpena, A.C.; Folch, J.; Barenys, M.; Sánchez-López, E.; Camins, A.; García, M.L. Epigallocatechin-3-Gallate Loaded PEGylated-PLGA Nanoparticles: A New Anti-Seizure Strategy for Temporal Lobe Epilepsy. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1073–1085. [Google Scholar] [CrossRef]
- Dauer, W.; Przedborski, S. Parkinson’s Disease: Mechanisms and Models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [PubMed]
- Delcroix, G.J.R.; Garbayo, E.; Sindji, L.; Thomas, O.; Vanpouille-Box, C.; Schiller, P.C.; Montero-Menei, C.N. The Therapeutic Potential of Human Multipotent Mesenchymal Stromal Cells Combined with Pharmacologically Active Microcarriers Transplanted in Hemi-Parkinsonian Rats. Biomaterials 2011, 32, 1560–1573. [Google Scholar] [CrossRef] [PubMed]
- Herrán, E.; Ruiz-Ortega, J.Á.; Aristieta, A.; Igartua, M.; Requejo, C.; Lafuente, J.V.; Ugedo, L.; Pedraz, J.L.; Hernández, R.M. In Vivo Administration of VEGF- and GDNF-Releasing Biodegradable Polymeric Microspheres in a Severe Lesion Model of Parkinson’s Disease. Eur. J. Pharm. Biopharm. 2013, 85, 1183–1190. [Google Scholar] [CrossRef]
- Bali, N.; Salve, P.S. Impact of Rasagiline Nanoparticles on Brain Targeting Efficiency via Gellan Gum Based Transdermal Patch: A Nanotheranostic Perspective for Parkinsonism. Int. J. Biol. Macromol. 2020, 164, 1006–1024. [Google Scholar] [CrossRef]
- Medina, D.X.; Chung, E.P.; Teague, C.D.; Bowser, R.; Sirianni, R.W. Intravenously Administered, Retinoid Activating Nanoparticles Increase Lifespan and Reduce Neurodegeneration in the SOD1G93A Mouse Model of ALS. Front. Bioeng. Biotechnol. 2020, 8, 224. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Jiang, M.; Fang, J.; Yang, M.-F.; Zhang, S. Enhanced Therapeutic Potential of Nano-Curcumin against Subarachnoid Hemorrhage-Induced Blood-Brain Barrier Disruption through Inhibition of Inflammatory Response and Oxidative Stress. Mol. Neurobiol. 2017, 54, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Maher, R.; Moreno-Borrallo, A.; Jindal, D.; Mai, B.T.; Ruiz-Hernandez, E.; Harkin, A. Intranasal Polymeric and Lipid-Based Nanocarriers for CNS Drug Delivery. Pharmaceutics 2023, 15, 746. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Nigam, K.; Tyagi, A.; Dang, S. A Preliminary Pharmacodynamic Study for the Management of Alzheimer’s Disease Using Memantine-Loaded PLGA Nanoparticles. AAPS PharmSciTech 2022, 23, 298. [Google Scholar] [CrossRef]
- Yan, X.; Xu, L.; Bi, C.; Duan, D.; Chu, L.; Yu, X.; Wu, Z.; Wang, A.; Sun, K. Lactoferrin-modified rotigotine nanoparticles for enhanced nose-to-brain delivery: LESA-MS/MS-based drug biodistribution, pharmacodynamics, and neuroprotective effects. Int. J. Nanomed. 2018, 13, 273–281. [Google Scholar] [CrossRef]
- Arisoy, S.; Sayiner, O.; Comoglu, T.; Onal, D.; Atalay, O.; Pehlivanoglu, B. In Vitro and In Vivo evaluation of levodopa-loaded nanoparticles for nose to brain delivery. Pharm. Dev. Technol. 2020, 25, 735–747. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Ma, C.; Li, T.; Yang, L. Preparation of baicalin-loaded ligand-modified nanoparticles for nose-to-brain delivery for neuroprotection in cerebral ischemia. Drug. Deliv. 2022, 29, 1282–1298. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmad, R.; Alrasheed, R.; Almatar, H.; Al-Ramadan, A.; Amir, M.; Sarafroz, M. Quantification and Evaluations of Catechin Hydrate Polymeric Nanoparticles Used in Brain Targeting for the Treatment of Epilepsy. Pharmaceutics 2020, 12, 203. [Google Scholar] [CrossRef]
- Gregory, H.; Phillips, J.B. Materials for peripheral nerve repair constructs: Natural proteins or synthetic polymers? Neurochem. Int. 2021, 143, 104953. [Google Scholar] [CrossRef]
- Jeon, J.; Lee, M.S.; Lim, J.; Park, S.; Kim, S.M.; Kim, D.; Tae, G.; Yang, H.S. Micro-grooved nerve guidance conduits combined with microfiber for rat sciatic nerve regeneration. J. Ind. Eng. Chem. 2020, 90, 214–223. [Google Scholar] [CrossRef]
- Cheong, H.; Kim, J.; Kim, B.J.; Kim, E.; Park, H.Y.; Choi, B.H.; Joo, K.I.; Cho, M.L.; Rhie, J.W.; Lee, J.I.; et al. Multi-dimensional bioinspired tactics using an engineered mussel protein glue-based nanofiber conduit for accelerated functional nerve regeneration. Acta Biomater. 2019, 90, 87–99. [Google Scholar] [CrossRef]
- Huang, W.C.; Lin, C.C.; Chiu, T.W.; Chen, S.Y. 3D Gradient and Linearly Aligned Magnetic Microcapsules in Nerve Guidance Conduits with Remotely Spatiotemporally Controlled Release to Enhance Peripheral Nerve Repair. ACS Appl. Mater. Interfaces 2022, 14, 46188–46200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Jiang, Y.K.; Li, J.A.; Wei, W.F.; Shi, M.P.; Wang, Y.B.; Jia, G.L. The effect of poly(lactic-co-glycolic acid) conduit loading insulin-like growth factor 1 modified by a collagen-binding domain on peripheral nerve injury in rats. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 2100–2109. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.F.; Chen, J.H.; Li, C.W.; Hsu, H.Y.; Chen, Y.P.; Wang, C.C.; Chiu, I.M. Human IL12p80 Promotes Murine Oligodendrocyte Differentiation to Repair Nerve Injury. Int. J. Mol. Sci. 2022, 23, 7002. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Fu, C.; Qi, Z.-P.; Guo, W.-L.; You, D.; Li, R.; Zhu, Z. Localised Delivery of Quercetin by Thermo-Sensitive PLGA-PEG-PLGA Hydrogels for the Treatment of Brachial Plexus Avulsion. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1010–1021. [Google Scholar] [CrossRef]
- Ham, T.R.; Leipzig, N.D. Biomaterial Strategies for Limiting the Impact of Secondary Events Following Spinal Cord Injury. Biomed. Mater. 2018, 13, 024105. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, P.; Jiang, Y.; Li, L.; Ju, F.; Cheng, Q.; Zhou, Y.L.; Zhou, Y. Sustained-Release Esketamine Based Nanoparticle-Hydrogel Delivery System for Neuropathic Pain Management. Int. J. Nanomed. 2023, 18, 1131–1143. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, X.; Fang, J.; Sun, X. Nanomedicines for the Treatment of Rheumatoid Arthritis: State of Art and Potential Therapeutic Strategies. Acta Pharm. Sin. B 2021, 11, 1158–1174. [Google Scholar] [CrossRef]
- Wenham, C.Y.; Conaghan, P.G. New horizons in osteoarthritis. Age Ageing 2013, 42, 272–278. [Google Scholar] [CrossRef]
- Anita, C.; Munira, M.; Mural, Q.; Shaily, L. Topical nanocarriers for management of Rheumatoid Arthritis: A review. Biomed. Pharmacother. 2021, 141, 111880. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Gao, S.; Chen, C.; Xu, W.; Xiao, P.; Chen, Z.; Du, C.; Chen, B.; Gao, Y.; Wang, C.; et al. The natural product salicin alleviates osteoarthritis progression by binding to IRE1α and inhibiting endoplasmic reticulum stress through the IRE1α-IκBα-p65 signaling pathway. Exp. Mol. Med. 2022, 54, 1927–1939. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Gu, X.; Hao, T.; Liu, J.; Gao, R.; Li, Y.; Yu, B.; Xu, H. Intra-articular injection PLGA blends sustained-release microspheres loaded with meloxicam: Preparation, optimization, evaluation in vitro and in vivo. Drug Deliv. 2022, 29, 3317–3327. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.F.; Li, Z.H.; Li, S.J.; Li, J.A.; Hou, T.T.; Wang, Y. PLGA scaffold carrying icariin to inhibit the progression of osteoarthritis in rabbits. R. Soc. Open. Sci. 2019, 6, 181877. [Google Scholar] [CrossRef] [PubMed]
- Pape, E.; Parent, M.; Pinzano, A.; Sapin-Minet, A.; Henrionnet, C.; Gillet, P.; Scala-Bertola, J.; Gambier, N. Rapamycin-loaded Poly(lactic-co-glycolic) acid nanoparticles: Preparation, characterization, and in vitro toxicity study for potential intra-articular injection. Int. J. Pharm. 2021, 609, 121198. [Google Scholar] [CrossRef]
- Ko, J.Y.; Choi, Y.J.; Jeong, G.J.; Im, G.I. Sulforaphane-PLGA microspheres for the intra-articular treatment of osteoarthritis. Biomaterials 2013, 34, 5359–5368. [Google Scholar] [CrossRef]
- Dhanabalan, K.M.; Dravid, A.A.; Agarwal, S.; Sharath, R.K.; Padmanabhan, A.K.; Agarwal, R. Intra-articular injection of rapamycin microparticles prevent senescence and effectively treat osteoarthritis. Bioeng. Transl. Med. 2022, 8, e10298. [Google Scholar] [CrossRef]
- Park, H.; Lee, H.R.; Shin, H.J.; Park, J.A.; Joo, Y.; Kim, S.M.; Beom, J.; Kang, S.W.; Kim, D.W.; Kim, J. p16INK4a-siRNA nanoparticles attenuate cartilage degeneration in osteoarthritis by inhibiting inflammation in fibroblast-like synoviocytes. Biomater. Sci. 2022, 10, 3223–3235. [Google Scholar] [CrossRef]
- Shin, H.J.; Park, H.; Shin, N.; Kwon, H.H.; Yin, Y.; Hwang, J.A.; Kim, S.I.; Kim, S.R.; Kim, S.; Joo, Y.; et al. p47phox siRNA-Loaded PLGA Nanoparticles Suppress ROS/Oxidative Stress-Induced Chondrocyte Damage in Osteoarthritis. Polymers 2020, 12, 443. [Google Scholar] [CrossRef]
- Moon, S.J.; Woo, Y.J.; Jeong, J.H.; Park, M.K.; Oh, H.J.; Park, J.S.; Kim, E.K.; Cho, M.L.; Park, S.H.; Kim, H.Y.; et al. Rebamipide attenuates pain severity and cartilage degeneration in a rat model of osteoarthritis by downregulating oxidative damage and catabolic activity in chondrocytes. Osteoarthr. Cartil. 2012, 20, 1426–1438. [Google Scholar] [CrossRef]
- Kim, S.E.; Choi, S.J.; Park, K.; Kim, H.J.; Song, G.G.; Jung, J.H. Intra-Articular Injection of Rebamipide-Loaded Nanoparticles Attenuate Disease Progression and Joint Destruction in Osteoarthritis Rat Model: A Pilot Study. Cartilage 2022, 13, 19476035211069250. [Google Scholar] [CrossRef]
- Li, J.; Liu, N.; Huang, Z.; Wang, W.; Hou, D.; Wang, W. Intra-articular injection of loaded sPL sustained-release microspheres inhibits osteoarthritis and promotes cartilaginous repairs. J. Orthop. Surg. Res. 2021, 16, 646. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tu, Q.; Xie, D.; Chen, S.; Gao, K.; Xu, X.; Zhang, Z.; Mei, X. Triamcinolone acetonide-loaded nanoparticles encapsulated by CD90+ MCSs-derived microvesicles drive anti-inflammatory properties and promote cartilage regeneration after osteoarthritis. J. Nanobiotechnol. 2022, 20, 150. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Kim, S.E.; Kim, H.J.; Park, K.; Song, G.G.; Choi, S.J. A comparative pilot study of oral diacerein and locally treated diacerein-loaded nanoparticles in a model of osteoarthritis. Int. J. Pharm. 2020, 581, 119249. [Google Scholar] [CrossRef] [PubMed]
- Babaie, E.; Bhaduri, S.B. Fabrication Aspects of Porous Biomaterials in Orthopedic Applications: A Review. ACS Biomater. Sci. Eng. 2017, 4, 1–39. [Google Scholar] [CrossRef]
- Chen, X.; Fan, H.; Deng, X.; Wu, L.; Yi, T.; Gu, L.; Zhou, C.; Fan, Y.; Zhang, X. Scaffold Structural Microenvironmental Cues to Guide Tissue Regeneration in Bone Tissue Applications. Nanomaterials 2018, 8, 960. [Google Scholar] [CrossRef]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and Cells for Tissue Regeneration: Different Scaffold Pore Sizes—Different Cell Effects. Cytotechnology 2015, 68, 355–369. [Google Scholar] [CrossRef]
- Weisgerber, D.W.; Erning, K.; Flanagan, C.L.; Hollister, S.J.; Harley, B.A.C. Evaluation of Multi-Scale Mineralized Collagen–Polycaprolactone Composites for Bone Tissue Engineering. J. Mech. Behav. Biomed. Mater. 2016, 61, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Zhang, W.; Zhang, R.; Luo, Z.P. Synchronization of Calcium Sulphate Cement Degradation and New Bone Formation Is Improved by External Mechanical Regulation. J. Orthop. Resp. 2015, 33, 685–691. [Google Scholar] [CrossRef]
- Liao, C.Z.; Li, K.; Wong, H.M.; Tong, W.Y.; Yeung, K.W.K.; Tjong, S.C. Novel Polypropylene Biocomposites Reinforced with Carbon Nanotubes and Hydroxyapatite Nanorods for Bone Replacements. Mater. Sci. Eng. C 2013, 33, 1380–1388. [Google Scholar] [CrossRef]
- Paşcu, E.I.; Stokes, J.; McGuinness, G.B. Electrospun Composites of PHBV, Silk Fibroin and Nano-Hydroxyapatite for Bone Tissue Engineering. Mater. Sci. Eng. C 2013, 33, 4905–4916. [Google Scholar] [CrossRef]
- Lobo, S.E.; Livingston Arinzeh, T. Biphasic Calcium Phosphate Ceramics for Bone Regeneration and Tissue Engineering Applications. Materials 2010, 3, 815–826. [Google Scholar] [CrossRef]
- Nihouannen, D.L.; Duval, L.; Lecomte, A.; Julien, M.; Guicheux, J.; Daculsi, G.; Layrolle, P. Interactions of Total Bone Marrow Cells with Increasing Quantities of Macroporous Calcium Phosphate Ceramic Granules. J. Mater. Sci. Mater. Med. 2007, 18, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Ghanaati, S.; Barbeck, M.; Orth, C.; Willershausen, I.; Thimm, B.W.; Hoffmann, C.; Rasic, A.; Sader, R.A.; Unger, R.E.; Peters, F. Influence of β-Tricalcium Phosphate Granule Size and Morphology on Tissue Reaction in Vivo. Acta Biomater. 2010, 6, 4476–4487. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fan, H.; Zhang, Z.-Y.; Lou, A.-J.; Pei, G.-X.; Jiang, S.; Mu, T.-W.; Qin, J.-J.; Chen, S.-Y.; Jin, D. Osteogenesis and Angiogenesis of Tissue-Engineered Bone Constructed by Prevascularized β-Tricalcium Phosphate Scaffold and Mesenchymal Stem Cells. Biomaterials 2010, 31, 9452–9461. [Google Scholar] [CrossRef]
- Liu, Y.; Dang, Z.; Wang, Y.-Y.; Huang, J.; Li, H. Hydroxyapatite/Graphene-Nanosheet Composite Coatings Deposited by Vacuum Cold Spraying for Biomedical Applications: Inherited Nanostructures and Enhanced Properties. Carbon 2014, 67, 250–259. [Google Scholar] [CrossRef]
- Mullick Chowdhury, S.; Lalwani, G.; Zhang, K.; Yang, J.Y.; Neville, K.; Sitharaman, B. Cell Specific Cytotoxicity and Uptake of Graphene Nanoribbons. Biomaterials 2013, 34, 283–293. [Google Scholar] [CrossRef]
- Fan, J.P.; Kalia, P.; Di Silvio, L.; Huang, J. In Vitro Response of Human Osteoblasts to Multi-Step Sol–Gel Derived Bioactive Glass Nanoparticles for Bone Tissue Engineering. Mater. Sci. Eng. C 2014, 36, 206–214. [Google Scholar] [CrossRef]
- Pilia, M.; Guda, T.; Appleford, M. Development of Composite Scaffolds for Load-Bearing Segmental Bone Defects. BioMed Res. Int. 2013, 2013, 1–15. [Google Scholar] [CrossRef]
- Berruto, M.; Delcogliano, M.; de Caro, F.; Carimati, G.; Uboldi, F.; Ferrua, P.; Ziveri, G.; Felice, C. Treatment of Large Knee Osteochondral Lesions with a Biomimetic Scaffold. Am. J. Sport. Med. 2014, 42, 1607–1617. [Google Scholar] [CrossRef]
- Kruger, T.E.; Miller, A.H.; Wang, J. Collagen Scaffolds in Bone Sialoprotein-Mediated Bone Regeneration. Sci. World J. 2013, 2013, 812718. [Google Scholar] [CrossRef]
- Duarte, A.R.C.; Mano, J.F.; Reis, R.L. Novel 3D Scaffolds of Chitosan–PLLA Blends for Tissue Engineering Applications: Preparation and Characterization. J. Supercrit. Fluids 2010, 54, 282–289. [Google Scholar] [CrossRef]
- Zhu, T.; Cui, Y.; Zhang, M.; Zhao, D.; Liu, G.; Ding, J. Engineered Three-Dimensional Scaffolds for Enhanced Bone Regeneration in Osteonecrosis. Bioact. Mater. 2020, 5, 584–601. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable Polymeric Nanoparticles Based Drug Delivery Systems. Colloids Surf. B Biointer. 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ding, J. In Vitro Degradation of Three-Dimensional Porous Poly(D,L-Lactide-Co-Glycolide) Scaffolds for Tissue Engineering. Biomaterials 2004, 25, 5821–5830. [Google Scholar] [CrossRef]
- Yu, Y.; Sun, B.; Yi, C.; Mo, X. Stem Cell Homing-Based Tissue Engineering Using Bioactive Materials. Front. Mater. Sci. 2017, 11, 93–105. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef]
- Liang, X.; Qi, Y.; Pan, Z.; He, Y.; Liu, X.; Cui, S.; Ding, J. Design and Preparation of Quasi-Spherical Salt Particles as Water-Soluble Porogens to Fabricate Hydrophobic Porous Scaffolds for Tissue Engineering and Tissue Regeneration. Mater. Chem. Front. 2018, 2, 1539–1553. [Google Scholar] [CrossRef]
- Lee, H.; Yoo, J.J.; Kang, H.-W.; Cho, D.-W. Investigation of Thermal Degradation with Extrusion-Based Dispensing Modules for 3D Bioprinting Technology. Biofabrication 2016, 8, 015011. [Google Scholar] [CrossRef]
- Wascher, D.C.; Bulthuis, L. Extremity Trauma: Field Management of Sports Injuries. Curr. Rev. Musculoskelet. Med. 2014, 7, 387–393. [Google Scholar] [CrossRef]
- Sozen, T.; Ozisik, L.; Calik Basaran, N. An Overview and Management of Osteoporosis. Eur. J. Rheum. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Lems, W.F.; Raterman, H.G. Critical Issues and Current Challenges in Osteoporosis and Fracture Prevention. An Overview of Unmet Needs. Ther. Adv. Musculoskelet. Dis. 2017, 9, 299–316. [Google Scholar] [CrossRef]
- Guise, T.A. Bone Loss and Fracture Risk Associated with Cancer Therapy. Oncologist 2006, 11, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.; Mercatali, L.; Amadori, D. Bone and Cancer: The Osteoncology. Clinical cases in mineral and bone metabolism. Off. J. Ital. Soc. Osteoporos. Min. Metab. Skelet. Dis. 2013, 10, 121–123. [Google Scholar]
- Saridis, A.; Panagiotopoulos, E.; Tyllianakis, M.; Matzaroglou, C.; Vandoros, N.; Lambiris, E. The Use of the Ilizarov Method as a Salvage Procedure in Infected Nonunion of the Distal Femur with Bone Loss. J. Bone Jt. Surg. 2006, 88-B, 232–237. [Google Scholar] [CrossRef]
- Assouline-Dayan, Y. Pathogenesis and Natural History of Osteonecrosis. Semin. Arthritis. Rheum. 2002, 32, 94–124. [Google Scholar] [CrossRef]
- Alazraki, N.P.; Moitoza, J.; Heaphy, J.H.; Taylor, A.M. The Effect of Iatrogenic Trauma on the Bone Scintigram: An Animal Study: Concise Communication. J. Nucl. Med. 1984, 25, 978–981. [Google Scholar] [PubMed]
- Hansson, S.; Halldin, A. Alveolar ridge resorption after tooth extraction: A consequence of a fundamental principle of bone physiology. J. Dent. Biomech. 2012, 3, 1758736012456543. [Google Scholar] [CrossRef] [PubMed]
- Cohn, S.A. Disuse atrophy of the periodontium in mice, Arch. Oral. Biol. 1965, 10, 909–919. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Xiao, E.; Graves, D.T. Diabetes mellitus related bone metabolism and periodontal disease. Int. J. Oral. Sci. 2015, 7, 63–72. [Google Scholar] [CrossRef]
- Mackie, E.J.; Tatarczuch, L.; Mirams, M. The skeleton: A multi-functional complex organ. The growth plate chondrocyte and endochondral ossification. J. Endocrinol. 2011, 211, 109–121. [Google Scholar] [CrossRef]
- Lim, J.; Lee, J.; Yun, H.-S.; Shin, H.-I.; Park, E.K. Comparison of bone regeneration rate in flat and long bone defects: Calvarial and tibial bone. Tissue Eng. Regener. Med. 2013, 10, 336–340. [Google Scholar] [CrossRef]
- Chang, H.C.; Yang, C.; Feng, F.; Lin, F.H.; Wang, C.H.; Chang, P.C. Bone morphogenetic protein-2 loaded poly(D, L-lactide-co-glycolide) microspheres enhance osteogenic potential of gelatin/ hydroxyapatite/b-tricalcium phosphate cryogel composite for alveolar ridge augmentation. J. Formos. Med. Assoc. 2017, 116, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Green, J.R.; Lyles, K.W.; Reid, D.M.; Trechsel, U.; Hosking, D.J.; Black, D.M.; Cummings, S.R.; Russell, R.G.G.; Eriksen, E.F. Zoledronate. Bone 2020, 137, 115390. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, Q.; Li, J.; She, P.; Kong, F.; Du, Y.; Zhang, F. Implantable zoledronate-PLGA microcapsules ameliorate alveolar bone loss, gingival inflammation and oxidative stress in an experimental periodontitis rat model. J. Biomater. Appl. 2021, 35, 569–578. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef]
- Atala, A.; Kasper, F.K.; Mikos, A.G. Engineering complex tissues. Sci. Transl. Med. 2012, 4, 160rv12. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Hajibandeh, J.; Suzuki, T.; Fan, A.; Shang, P.; Mao, J.J. Three-dimensional printed multiphase scaffolds for regeneration of periodontium complex. Tissue Eng. Part A 2014, 20, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Onizuka, S.; Iwata, T. Application of Periodontal Ligament-Derived Multipotent Mesenchymal Stromal Cell Sheets for Periodontal Regeneration. Int. J. Mol. Sci. 2019, 20, 2796. [Google Scholar] [CrossRef]
- Yang, J.; Yamato, M.; Kohno, C.; Nishimoto, A.; Sekine, H.; Fukai, F.; Okano, T. Cell sheet engineering: Recreating tissues without biodegradable scaffolds. Biomaterials 2005, 26, 6415–6422. [Google Scholar] [CrossRef]
- Akizuki, T.; Oda, S.; Komaki, M.; Tsuchioka, H.; Kawakatsu, N.; Kikuchi, A.; Yamato, M.; Okano, T.; Ishikawa, I. Application of periodontal ligament cell sheet for periodontal regeneration: A pilot study in beagle dogs. J. Periodontal Res. 2005, 40, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Progress in the field of electrospinning for tissue engineering applications. Adv. Mater. 2009, 21, 3343–3351. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chen, J.; Yang, B.; Li, L.; Luo, X.; Zhang, X.; Feng, L.; Jiang, Z.; Yu, M.; Guo, W.; et al. Combination of aligned PLGA/Gelatin electrospun sheets, native dental pulp extracellular matrix and treated dentin matrix as substrates for tooth root regeneration. Biomaterials 2015, 52, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug. Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Tulbah, A.S.; Pisano, E.; Landh, E.; Scalia, S.; Young, P.M.; Traini, D.; Ong, H.X. Simvastatin Nanoparticles Reduce Inflammation in LPS-Stimulated Alveolar Macrophages. J. Pharm. Sci. 2019, 108, 3890–3897. [Google Scholar] [CrossRef]
- Kirmizis, D.; Papagianni, A.; Dogrammatzi, F.; Efstratiadis, G.; Memmos, D. Anti-inflammatory effects of simvastatin in diabetic compared to non-diabetic patients on chronic hemodialysis. J. Diabetes 2013, 5, 492–494. [Google Scholar] [CrossRef]
- Al-Rasheed, N.M.; Al-Rasheed, N.M.; Bassiouni, Y.A.; Hasan, I.H.; Al-Amin, M.A.; Al-Ajmi, H.N.; Mahmoud, A.M. Simvastatin ameliorates diabetic nephropathy by attenuating oxidative stress and apoptosis in a rat model of streptozotocin-induced type 1 diabetes. Biomed. Pharmacother. 2018, 105, 290–298. [Google Scholar] [CrossRef]
- Niu, Y.; Li, Q.; Ding, Y.; Dong, L.; Wang, C. Engineered delivery strategies for enhanced control of growth factor activities in wound healing. Adv. Drug. Deliv. Rev. 2019, 146, 190–208. [Google Scholar] [CrossRef]
- Leader, B.; Baca, Q.J.; Golan, D.E. Protein therapeutics: A summary and pharmacological classification. Nat. Rev. Drug. Discov. 2008, 7, 21–39. [Google Scholar] [CrossRef]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng. B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef]
- Yuan, X.; Yuan, Z.; Wang, Y.; Wan, Z.; Wang, X.; Yu, S.; Han, J.; Huang, J.; Xiong, C.; Ge, L.; et al. Vascularized pulp regeneration via injecting simvastatin functionalized gelMA cryogel microspheres loaded with stem cells from human exfoliated deciduous teeth. Mater. Today Bio 2022, 13, 100209. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Ishida, Y. Molecular pathology of wound healing. Forensic Sci. Int. 2010, 203, 93–98. [Google Scholar] [CrossRef]
- Tiwari, N.; Osorio-Blanco, E.R.; Sonzogni, A.; Esporrín-Ubieto, D.; Wang, H.; Calderón, M. Nanocarriers for Skin Applications: Where Do We Stand? Angew. Chem. Int. Ed. Eng. 2022, 61, e202107960. [Google Scholar]
- Chereddy, K.K.; Coco, R.; Memvanga, P.B.; Ucakar, B.; des Rieux, A.; Vandermeulen, G.; Préat, V. Combined effect of PLGA and curcumin on wound healing activity. J. Control. Release 2013, 171, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, D.H.; Tambuwala, M.M.; Mitchell, C.A.; Osman, M.A.; El-Gizawy, S.A.; Faheem, A.M.; El-Tanani, M.; McCarron, P.A. Enhanced cutaneous wound healing in rats following topical delivery of insulin-loaded nanoparticles embedded in poly(vinyl alcohol)-borate hydrogels. Drug. Deliv. Transl. Res. 2018, 8, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.; Cheng, G.; Guo, J.; Du, S.; Qiu, J.; Wang, C.; Li, C.; Yang, X.; Chen, T.; et al. Tailored Hydrogel Delivering Niobium Carbide Boosts ROS-Scavenging and Antimicrobial Activities for Diabetic Wound Healing. Small 2022, 18, 2201300. [Google Scholar] [CrossRef]
- Liao, H.-T.; Lai, Y.-T.; Kuo, C.-Y.; Chen, J.-P. A Bioactive Multi-Functional Heparin-Grafted Aligned Poly(Lactide-Co-Glycolide)/Curcumin Nanofiber Membrane to Accelerate Diabetic Wound Healing. Mat. Sci. Eng. C 2021, 120, 111689. [Google Scholar] [CrossRef]
- Gao, S.; Chen, T.; Wang, Z.; Ji, P.; Xu, L.; Cui, W.; Wang, Y. Immuno-Activated Mesenchymal Stem Cell Living Electrospun Nanofibers for Promoting Diabetic Wound Repair. J. Nanobiotechnol. 2022, 20, 294. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, L.; Liu, J.; Huang, X.; Wang, X.; Guo, X.; You, X.; Li, W.; Li, L.; Sun, T.; et al. Chitosan/PLGA shell nanoparticles as Tylotoin delivery platform for advanced wound healing. Int. J. Biol. Macromol. 2022, 220, 395–405. [Google Scholar] [CrossRef]
- Xu, K.; Chai, B.; Zhang, K.; Xiong, J.; Zhu, Y.; Xu, J.; An, N.; Xia, W.; Ji, H.; Wu, Y.; et al. Topical Application of Fibroblast Growth Factor 10-PLGA Microsphere Accelerates Wound Healing via Inhibition of ER Stress. Oxid. Med. Cell. Longev. 2020, 2020, 8586314. [Google Scholar] [CrossRef]
- Lee, C.H.; Huang, C.H.; Hung, K.C.; Huang, S.C.; Kuo, C.C.; Liu, S.J. Nanofibrous Vildagliptin/PLGA Membranes Accelerate Diabetic Wound Healing by Angiogenesis. Pharmaceuticals 2022, 15, 1358. [Google Scholar] [CrossRef]
- Ali, I.H.; Khalil, I.A.; El-Sherbiny, I.M. Single-Dose Electrospun Nanoparticles-in-Nanofibers Wound Dressings with Enhanced Epithelialization, Collagen Deposition, and Granulation Properties. ACS Appl. Mater. Interfaces 2016, 8, 14453–14469. [Google Scholar] [CrossRef]
- Mouritzen, M.V.; Abourayale, S.; Ejaz, R.; Ardon, C.B.; Carvalho, E.; Dalgaard, L.T.; Roursgaard, M.; Jenssen, H. Neurotensin, substance P, and insulin enhance cell migration. J. Pept. Sci. 2018, 24, e3093. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, Y.; Huang, W.; Mo, Y.; Lan, Y.; Guo, R.; Cheng, B. Neurotensin-loaded PLGA/CNC composite nanofiber membranes accelerate diabetic wound healing. Artif. Cells Nanomed. Biotechnol. 2018, 46, 493–501. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int. J. Mol. Sci. 2020, 21, 1306. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, J.; Zhang, X.; Fan, C.; Huang, J. VAP-PLGA microspheres (VAP-PLGA) promote adipose-derived stem cells (ADSCs)-induced wound healing in chronic skin ulcers in mice via PI3K/Akt/HIF-1α pathway. Bioengineered 2021, 12, 10264–10284. [Google Scholar] [CrossRef]
- Hsieh, C.F.; Chen, C.H.; Kao, H.H.; Govindaraju, D.T.; Dash, B.S.; Chen, J.P. PLGA/Gelatin/Hyaluronic Acid Fibrous Membrane Scaffold for Therapeutic Delivery of Adipose-Derived Stem Cells to Promote Wound Healing. Biomedicines 2022, 10, 2902. [Google Scholar] [CrossRef] [PubMed]
- David Boothe, W.; Tarbox, J.A.; Tarbox, M.B. Atopic Dermatitis: Pathophysiology. Adv. Exp. Med. Biol. 2017, 1027, 21–37. [Google Scholar] [PubMed]
- Feng, S.; Nie, L.; Zou, P.; Suo, J. Drug-loaded PLGA-mPEG microparticles as treatment for atopic dermatitis-like skin lesions in BALB/c mice model. J. Microencapsul. 2015, 32, 201–209. [Google Scholar] [CrossRef]
- Lin, C.Y.; Hsieh, Y.T.; Chan, L.Y.; Yang, T.Y.; Maeda, T.; Chang, T.M.; Huang, H.C. Dictamnine delivered by PLGA nanocarriers ameliorated inflammation in an oxazolone-induced dermatitis mouse model. J. Control. Release. 2021, 329, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Yoon, G.H.; Lee, H.C.; Jung, M.H.; Yu, S.I.; Yeon, S.J.; Min, S.K.; Kwon, Y.S.; Hwang, J.H.; Shin, H.S. Thermodynamic Insights and Conceptual Design of Skin-Sensitive Chitosan Coated Ceramide/PLGA Nanodrug for Regeneration of Stratum Corneum on Atopic Dermatitis. Sci. Rep. 2015, 5, 1808. [Google Scholar] [CrossRef] [PubMed]
- Balmert, S.C.; Donahue, C.; Vu, J.R.; Erdos, G.; Falo, L.D., Jr.; Little, S.R. In vivo induction of regulatory T cells promotes allergen tolerance and suppresses allergic contact dermatitis. J. Control. Release 2017, 261, 223–233. [Google Scholar] [CrossRef]
- Fox, L.; Csongradi, C.; Aucamp, M.; du Plessis, J.; Gerber, M. Treatment Modalities for Acne. Molecules 2016, 21, 1063. [Google Scholar] [CrossRef] [PubMed]
- Folle, C.; Marqués, A.M.; Díaz-Garrido, N.; Espina, M.; Sánchez-López, E.; Badia, J.; Baldoma, L.; Calpena, A.C.; García, M.L. Thymol-loaded PLGA nanoparticles: An efficient approach for acne treatment. J. Nanobiotechnology 2021, 19, 359. [Google Scholar] [CrossRef] [PubMed]
- Folle, C.; Díaz-Garrido, N.; Sánchez-López, E.; Marqués, A.M.; Badia, J.; Baldomà, L.; Espina, M.; Calpena, A.C.; García, M.L. Surface-Modified Multifunctional Thymol-Loaded Biodegradable Nanoparticles for Topical Acne Treatment. Pharmaceutics 2021, 13, 1501. [Google Scholar] [CrossRef]
- Yang, Y.; Lockwood, A. Topical ocular drug delivery systems: Innovations for an unmet need. Exp. Eye Res. 2022, 218, 109006. [Google Scholar] [CrossRef]
- Bravo-Osuna, I.; Andrés-Guerrero, V.; Arranz-Romera, A.; Esteban-Pérez, S.; Molina-Martínez, I.T.; Herrero-Vanrell, R. Microspheres as intraocular therapeutic tools in chronic diseases of the optic nerve and retina. Adv. Drug. Deliv. Rev. 2018, 126, 127–144. [Google Scholar] [CrossRef]
- Huang, H.; Yang, X.; Li, H.; Lu, H.; Oswald, J.; Liu, Y.; Zeng, J.; Jin, C.; Peng, X.; Liu, J.; et al. iRGD decorated liposomes: A novel actively penetrating topical ocular drug delivery strategy. Nano Res. 2020, 13, 3105–3109. [Google Scholar] [CrossRef]
- Jager, R.D.; Aiello, L.P.; Patel, S.C.; Cunningham, E.T.J. Risks of intravitreous injection: A comprehensive review. Retina 2004, 24, 676–698. [Google Scholar] [CrossRef]
- Singh, R.; Davoudi, S.; Ness, S. Preventive factors, diagnosis, and management of injection-related endophthalmitis: A literature review. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 2399–2416. [Google Scholar] [CrossRef]
- Barcia, E.; Herrero-Vanrell, R.; Díez, A.; Alvarez-Santiago, C.; López, I.; Calonge, M. Downregulation of endotoxin-induced uveitis by intravitreal injection of polylactic-glycolic acid (PLGA) microspheres loaded with dexamethasone. Exp. Eye Res. 2009, 89, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zhu, L.; Zhang, K.; Li, T.; Huang, S. Nanodelivery of triamcinolone acetonide with PLGA-chitosan nanoparticles for the treatment of ocular inflammation. Artif. Cells Nanomed. Biotechnol. 2021, 49, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Ratay, M.L.; Balmert, S.C.; Bassin, E.J.; Little, S.R. Controlled release of an HDAC inhibitor for reduction of inflammation in dry eye disease. Acta Biomater. 2018, 71, 261–270. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Thapa, R.; Hariani, H.N.; Volyanyuk, M.; Ogle, S.D.; Orloff, K.A.; Ankireddy, S.; Lai, K.; Žiniauskaitė, A.; Stubbs, E.B., Jr.; et al. Poly(lactic-co-glycolic acid) Nanoparticles Encapsulating the Prenylated Flavonoid, Xanthohumol, Protect Corneal Epithelial Cells from Dry Eye Disease-Associated Oxidative Stress. Pharmaceutics 2021, 13, 1362. [Google Scholar] [CrossRef]
- Chowdhury, S.; Guha, R.; Trivedi, R.; Kompella, U.B.; Konar, A.; Hazra, S. Pirfenidone nanoparticles improve corneal wound healing and prevent scarring following alkali burn. PLoS ONE 2013, 8, 70528. [Google Scholar] [CrossRef]
- Tsai, I.L.; Tsai, C.Y.; Kuo, L.L.; Woung, L.C.; Ku, R.Y.; Cheng, Y.H. PLGA nanoparticles containing Lingzhi extracts rescue corneal epithelial cells from oxidative damage. Exp. Eye Res. 2021, 206, 108539. [Google Scholar] [CrossRef]
- Ganugula, R.; Arora, M.; Lepiz, M.A.; Niu, Y.; Mallick, B.K.; Pflugfelder, S.C.; Scott, E.M.; Kumar, M.N.V.R. Systemic anti-inflammatory therapy aided by double-headed nanoparticles in a canine model of acute intraocular inflammation. Sci. Adv. 2020, 6, 7878. [Google Scholar] [CrossRef]
- Yang, H.; Leffler, C.T. Hybrid dendrimer hydrogel/poly(lactic-co-glycolic acid) nanoparticle platform: An advanced vehicle for topical delivery of antiglaucoma drugs and a likely solution to improving compliance and adherence in glaucoma management. J. Ocul. Pharmacol. Ther. 2013, 29, 166–172. [Google Scholar] [CrossRef]
- Arranz-Romera, A.; Davis, B.M.; Bravo-Osuna, I.; Esteban-Pérez, S.; Molina-Martínez, I.T.; Shamsher, E.; Ravindran, N.; Guo, L.; Cordeiro, M.F.; Herrero-Vanrell, R. Simultaneous co-delivery of neuroprotective drugs from multi-loaded PLGA microspheres for the treatment of glaucoma. J. Control. Release 2019, 297, 26–38. [Google Scholar] [CrossRef]
- Warsi, M.H.; Anwar, M.; Garg, V.; Jain, G.K.; Talegaonkar, S.; Ahmad, F.J.; Khar, R.K. Dorzolamide-loaded PLGA/vitamin E TPGS nanoparticles for glaucoma therapy: Pharmacoscintigraphy study and evaluation of extended ocular hypotensive effect in rabbits. Colloids Surf. B Biointerfaces 2014, 122, 423–431. [Google Scholar] [CrossRef]
- Park, C.G.; Kim, Y.K.; Kim, S.N.; Lee, S.H.; Huh, B.K.; Park, M.A.; Won, H.; Park, K.H.; Choy, Y.B. Enhanced ocular efficacy of topically-delivered dorzolamide with nanostructured mucoadhesive microparticles. Int. J. Pharm. 2017, 522, 66–73. [Google Scholar] [CrossRef]
- Jiang, C.; Moore, M.J.; Zhang, X.; Klassen, H.; Langer, R.; Young, M. Intravitreal injections of GDNF-loaded biodegradable microspheres are neuroprotective in a rat model of glaucoma. Mol. Vis. 2007, 13, 1783–1792. [Google Scholar] [PubMed]
- Shirley Ding, S.L.; Leow, S.N.; Munisvaradass, R.; Koh, E.H.; Bastion, M.L.; Then, K.Y.; Kumar, S.; Mok, P.L. Revisiting the role of erythropoietin for treatment of ocular disorders. Eye 2016, 30, 1293–1309. [Google Scholar] [CrossRef] [PubMed]
- Naguib, S.; DeJulius, C.R.; Backstrom, J.R.; Haider, A.A.; Ang, J.M.; Boal, A.M.; Calkins, D.J.; Duvall, C.L.; Rex, T.S. Intraocular Sustained Release of EPO-R76E Mitigates Glaucoma Pathogenesis by Activating the NRF2/ARE Pathway. Antioxidants 2023, 12, 556. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Egea, M.A.; Davis, B.M.; Guo, L.; Espina, M.; Silva, A.M.; Calpena, A.C.; Souto, E.M.B.; Ravindran, N.; Ettcheto, M.; et al. Memantine-Loaded PEGylated Biodegradable Nanoparticles for the Treatment of Glaucoma. Small 2018, 14, 1701808. [Google Scholar] [CrossRef]
- Sharma, M.; Bhowmick, R.; Gappa-Fahlenkamp, H. Drug-Loaded Nanoparticles Embedded in a Biomembrane Provide a Dual-Release Mechanism for Drug Delivery to the Eye. J. Ocul. Pharm. Ther. 2016, 32, 565–573. [Google Scholar] [CrossRef]

| Disease | Cargo Molecules | |
|---|---|---|
| Allergic diseases | Allergic Asthma | Antisense oligonucleotides targeting Dnmt3, chrysin, OVA, A20, CpG, Der p2 |
| Allergic conjunctivitis | Recombinant Amb a 1 | |
| Cow’s milk allergy | β-lactoglobulin derived peptides, β-lactoglobulin | |
| Honey bee venom allergy | PLA2, protamine stabilized CpG | |
| Autoimmunity | Multiple sclerosis | LIF, MOG35–55, IL-10, TGF-β, GM-CSF, Glucosamine-MOG35–55, PLP139–151 |
| Guillain-Barrè Syndrome | Cargo-free | |
| Type I diabetes | B9–23, TGF-β, GM-CSF, Vitamin D3, Denatured insulin, CM-GSF, CpG | |
| Rheumatoid arthritis | CII, Altered CII AP268–270, Tacrolimus, Cargo-free | |
| Systemic lupus erythermatosus | miRNA-125a, TGF-β, IL-2 | |
| Infectious diseases | Viral hepatitis | HBV nucleocapsid core antigen, MPLA, HBsAg, HCV E2 envelope glycoprotein |
| Influenza | HA (H5N1) imiquimod, Inactivated H4N6, CpG, Capsomere presenting M2e, M158–66, PA46–54, CpG, polyIC, OVA | |
| HIV | HIV-1 gp120, PEI/plasmid-DNA complex (Gag, Pol, Env) | |
| COVID-19 | Recombinant SARS-CoV-2 S1-E, Trivalent SARS-CoV-2 subunits | |
| Cancer immunotherapy | Immune checkpoint inhibition | Cargo-free |
| PTT + immune checkpoint inhibition | Anti-PD1 mAb, Imiquimod, ICG | |
| Modulation of tumor microenvironment | Oxaliplatin, R848, Paclitaxel, CXCL-1 | |
| Modulation of tumor microenvironment + immune checkpoint inhibition | Metformin, siRNA FGL1 | |
| Cancer vaccine | OVA mRNA, gardiquimod, 4T1 cell lysate, NY-ESO-1 TAA, IMM60 | |
| Cancer vaccine + immune checkpoint inhibition | Imiquimod, OVA, HLA-A*0201 restricted TAA, Riboxxim, HPV-E7, siRNA PD1, siRNA PD-L1 | |
| Disease | Drug | Citation |
|---|---|---|
| Intestinal Bowel Disease | Phellinus igniarius | [86] |
| Myocardial infarction | (E)-2-benzylidene-3-(cyclohexylamino)-2,3-dihydro-1Hinden-1-one (BCI) | [117] |
| Mesenchymal stromal cell-secreted factors (MSCFs) | [118] | |
| Simvastatin (plus adipose-derived stem cells | [119] | |
| Parkinson disease | Neurotrophin 3 (NT3) | [154] |
| Vascular endothelial growth factor (VEGF) plus glial cell line-derived neurotrophic factor (GDNF) | [155] | |
| Peripheral nerve damage | Mussel adhesive peptides and IKVAV peptides | [167] |
| Nerve growth factor | [168] | |
| Insulin-like growth factor | [169] | |
| IL-12 | [170] | |
| Quercetin | [171] | |
| Osteoarthritis | Super-activated platelet lysate (sPL) | [187] |
| Synovial mesenchymal stem cells microvesicles | [188] | |
| Bone loss | Zoledronate | [229] |
| Simvastatin | [241,242] | |
| Wound healing | Curcumin | [253] |
| Niobium carbide (Nb2 C) | [254] | |
| Curcumin plus heparin | [255] | |
| LPS/IFN-γ activated macrophage cell membrane | [256] | |
| Tylotoin | [257] | |
| Fibroblast growth factor 10 (FGF10) | [258] | |
| Vildagliptin | [259] | |
| Phenytoin | [260] | |
| Neurotensin (NT) | [261,262] | |
| IL-8 plus mesenchymal stem cells | [263] | |
| Velvet antler polypeptide plus adipose-derived stem cells | [264] | |
| Hyaluronic acid (HA) plus adipose-derived stem cells | [265] | |
| Ocular wound healing | Pirfenidone | [283] |
| Lingzhi extracts | [284] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puricelli, C.; Gigliotti, C.L.; Stoppa, I.; Sacchetti, S.; Pantham, D.; Scomparin, A.; Rolla, R.; Pizzimenti, S.; Dianzani, U.; Boggio, E.; et al. Use of Poly Lactic-co-glycolic Acid Nano and Micro Particles in the Delivery of Drugs Modulating Different Phases of Inflammation. Pharmaceutics 2023, 15, 1772. https://doi.org/10.3390/pharmaceutics15061772
Puricelli C, Gigliotti CL, Stoppa I, Sacchetti S, Pantham D, Scomparin A, Rolla R, Pizzimenti S, Dianzani U, Boggio E, et al. Use of Poly Lactic-co-glycolic Acid Nano and Micro Particles in the Delivery of Drugs Modulating Different Phases of Inflammation. Pharmaceutics. 2023; 15(6):1772. https://doi.org/10.3390/pharmaceutics15061772
Chicago/Turabian StylePuricelli, Chiara, Casimiro Luca Gigliotti, Ian Stoppa, Sara Sacchetti, Deepika Pantham, Anna Scomparin, Roberta Rolla, Stefania Pizzimenti, Umberto Dianzani, Elena Boggio, and et al. 2023. "Use of Poly Lactic-co-glycolic Acid Nano and Micro Particles in the Delivery of Drugs Modulating Different Phases of Inflammation" Pharmaceutics 15, no. 6: 1772. https://doi.org/10.3390/pharmaceutics15061772
APA StylePuricelli, C., Gigliotti, C. L., Stoppa, I., Sacchetti, S., Pantham, D., Scomparin, A., Rolla, R., Pizzimenti, S., Dianzani, U., Boggio, E., & Sutti, S. (2023). Use of Poly Lactic-co-glycolic Acid Nano and Micro Particles in the Delivery of Drugs Modulating Different Phases of Inflammation. Pharmaceutics, 15(6), 1772. https://doi.org/10.3390/pharmaceutics15061772









