Cryogels: Advancing Biomaterials for Transformative Biomedical Applications
Abstract
:1. Introduction
2. Natural Polysaccharides
2.1. Alginate
2.2. Chitosan
2.3. Gelatin
2.4. Cyclodextrine
2.5. Agarose, Carrageenan, Locust Bean Gum, Natural Polysaccharides Blends
3. Synthetic and Other Polymers
3.1. Peg Derivatives
3.2. Polyvinyl Alcohol (PVOH)
3.3. PVOH Hybrids
3.4. Acrylate- and Methacrylate-Based Cryogels
3.5. Acrylamide-Based Cryogels
3.6. Other Synthetic Cryogels
3.7. Other Cryogel Materials
3.8. Developments
| Alginate-Based Cryogel | ||
|---|---|---|
| Material Used | Main Outcomes | Ref. |
| Alginate cryogel loaded with chemoimmunotherapy drug-loaded nanoparticles Sp-AcDEX NPs and Nutlin-3a | Chemoimmunotherapy drug delivery system for cancer treatment, enhancing accumulation of Sp-AcDEX NPs in tumor tissue and inducing immunogenic cell death with Nutlin-3a | [26] |
| Macroporous alginate cryogel incorporated with gold nanorods (GNRs) | Controlled drug delivery system for Mitoxantrone with on-demand release through near-infrared irradiation, suppressing tumor growth in vivo | [27,28] |
| Nanocomposite hydrogel formed by bio-orthogonal crosslinking of alginate using tetrazine-norbornene coupling and pre-adsorbed charged Laponite nanoparticles | Sustained, bioactive release of therapeutic proteins with precise tuning of release kinetics, simplifying the design of hydrogel drug delivery systems | [27,28] |
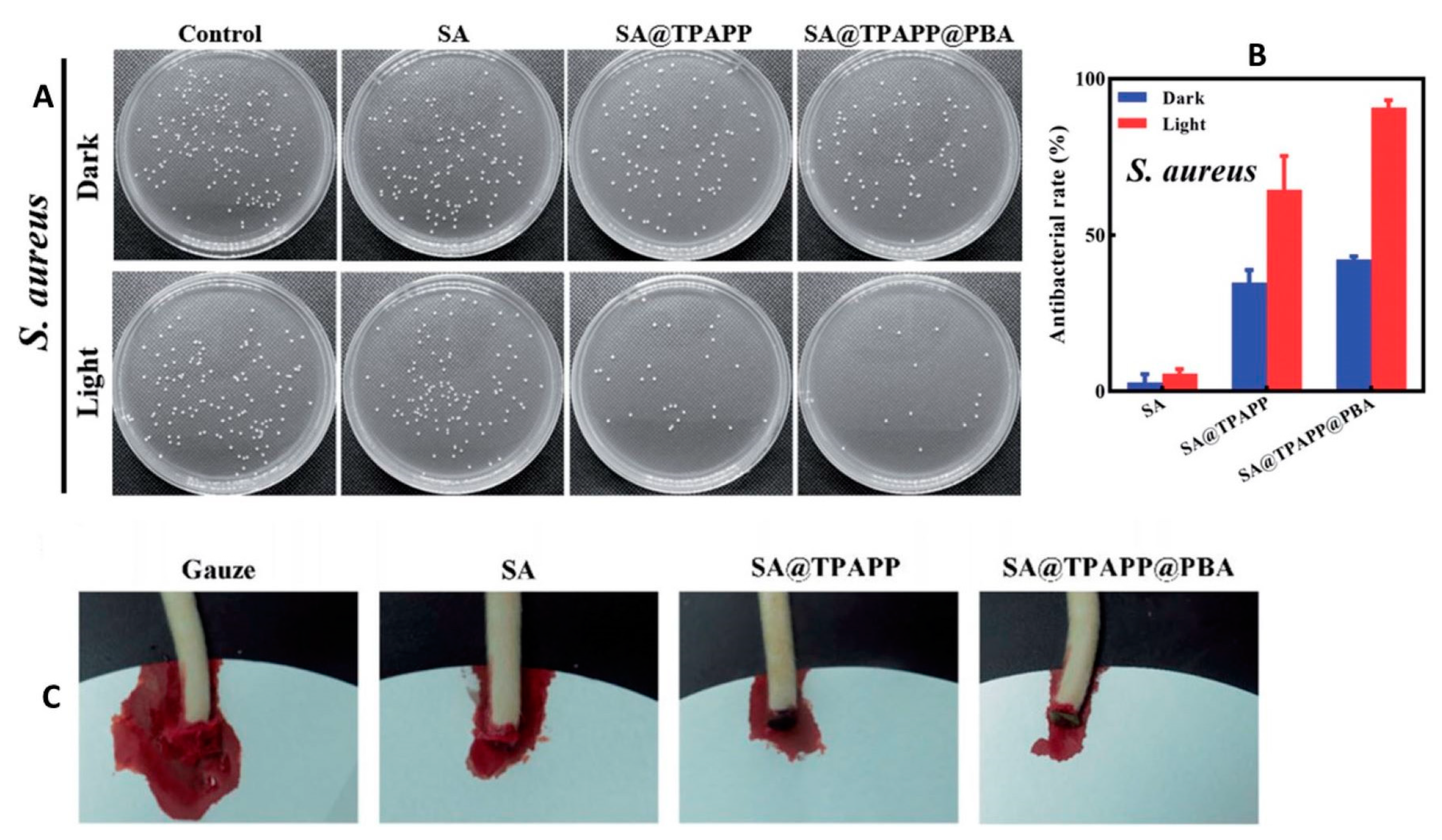
| Sodium alginate-based aerogel with photosensitizers and phenylboronic acid | Antibacterial photodynamic wound dressing with improved solubility, hemostasis capacity, and antibacterial activity against Staphylococcus aureus | [29] |
| Alginate and carboxymethyl-cellulose-based cryogel neural scaffold | Injectable surgical scaffold for minimally invasive delivery of an extended living neuronal network, with high local Young’s modulus for neuronal network protection and soft macroscopic scale for easy injection | [30,31,32] |
| 3D porous scaffold made from carrageenan and alginate with EDC/NHS cross-linker | Scaffold with a porous and interconnected structure, good physical and mechanical stability, higher cell attachment, better cellular response, and higher metabolic activity than matrices with other cross-linkers | [31,32] |
| Autoclavable cryogels made from several naturally derived polymeric precursors (alginate, hyaluronic acid, and gelatin) | Maintain their structural and physical properties, including their syringe injectability signature, after autoclave sterilization | [31,32] |
| Chitosan-Based Cryogel | ||
| Material Used | Main Outcomes | Ref. |
| Hydrophobically modified chitosan cryogel | Potential material for controlled drug delivery applications | [33] |
| Chitosan aerogel microparticles | Suitable for pulmonary drug delivery systems; effects of chitosan molecular weight, polymer concentration, and tripolyphosphate concentration on drug release were investigated | [34] |
| Chitosan sponges Cross-linked with glutaraldehyde | It possesses antibacterial, antioxidant, and controlled delivery properties for plant-derived polyphenols, showed remarkable shape recovery, good radical scavenging activity, and strong antibacterial properties against both Gram-positive and Gram-negative strains | [35] |
| Cryogel–microparticle composite with polymeric network and microstructured biocompatible pore surface | Incorporating microparticles (MPs) into the polymeric network of cryogels to deliver bioactive molecules, demonstrated good biocompatibility with the growth of various cell lines, potential for delivering bioactive/drug molecules to cells growing in 3D conditions | [36] |
| Double cryogel system, gelatin/chitosan cryogel (GC) surrounded by Gelatin/heparin cryogel (GH) | The double cryogel system (DC) is used for dual drug delivery with different release kinetics to induce bone regeneration. The GH layer loaded with VEGF induces initial release, while the GC layer loaded with BMP-4 induces sustained release. | [37] |
| Chitosan and nanofibrillated cellulose hydrogel | Investigated as a biomaterial membrane for peripheral nerve regeneration due to its mechanical strength, thermal resistance, slow rate of swelling, and non-toxicity to Schwann cells. Used in vivo with autologous implant to promote functional recovery within 15 days. | [38] |
| Chitosan-clinoptilolite 3D biocomposites | Investigated for their potential as drug carriers. Biocomposites with higher content of clinoptilolite were found to have a more ordered porous structure and lower water uptake. Effective drug release was observed in phosphate buffer saline, suggesting potential for drug delivery systems. | [39] |
| Pectin/Chitosan Composite Cryogel | Potential candidate for biomedical applications due to improved mechanical strength, surface morphology, degradation time, adhesion to biological tissues, and biocompatibility. | [40] |
| Poly(N-isopropylacrylamide) scaffold loaded with chitosan/bemiparin nanoparticles | Used for tissue engineering and controlled release of heparin. Cryogel exhibits a highly porous structure, is non-cytotoxic, and exhibits excellent properties for application in tissue engineering. | [41] |
| Chitosan/Hydroxyapatite/Heparin/PVA Composite Cryogel Scaffold | Potential for bone regeneration and tissue engineering applications, as it efficiently immobilizes BMP-2 and supports differentiation of bone marrow mesenchymal stem cells into the osteogenic lineage. | [42,43] |
| Thiolated Chitosan/Oxidized Dextran/Locust Bean Gum Semi-IPN Cryogel | Promising candidate for use as a hemostatic dressing due to its improved mechanical strength, decreased hydrophobicity, increased swelling ratio, and enhanced hemostatic properties. Cryogel also exhibits good cytocompatibility, blood compatibility, and hemostatic potential. | [42,43] |
| Chitosan-graft-poly(N-isopropyl acrylamide)/PVA | Development of a smart polymeric vehicle for antifungal drug delivery for mucosal applications and various parameters of cryogels were also characterized. | [44] |
| Chitosan/2-hydroxyethylcellulose cryogel | Development of a pH-sensitive cryogel for drug delivery applications. The cryogels were characterized for physico-mechanical properties and bioadhesive properties. | [45] |
| Methacrylated chitosan/chondroitin sulfate cryogel microparticle (CMP) system | Development of an injectable CMP system for growth factor delivery in tissue regeneration applications. The cryogel microparticles were characterized for in vitro and in vivo neovascularization using rhVEGF. | [46] |
| Chitosan-based polyelectrolyte complex cryogels | Development of a sustainable strategy to fabricate drug delivery systems using polyelectrolyte complex cryogels physically stabilized by spontaneous interactions. The cryogels were characterized for their structure, morphology, and drug release properties. | [47] |
| Glutaraldehyde-crosslinked chitosan cryogels | Fabricated with superamphiphilicity and high separation efficiency for various surfactant-stabilized oil-in-water emulsions under continuous flow mode, make them promising for applications in separation processes driven by gravity or a peristaltic pump. | [151] |
| Colloidal chitosan/k-carrageenan/carboxymethylcellulose sodium salt cryogel | Encapsulation of curcumin in a controlled release system. | [152] |
| Chitosan/xanthan gum polyelectrolyte complex (PEC) aerogel | Development of bone-like structured aerogels suitable for biomedical and environmental applications. | [48] |
| Chitosan/citric acid/silver nanoparticle cryogel | Development of a shape memory cryogel dressing for skin wounds, promoting hemostasis, blood cell adhesion, and wound healing. | [49,50] |
| Chitosan and gluconic acid conjugate cryogels | Development of physically crosslinked chitosan cryogels as practical wound dressings, which retained the same biological properties as the pre-autoclaved ones and showed enhanced resistance to enzymatic degradation. | [49,50] |
| Methacrylic acid/acrylamide or 2-hydroxyethyl methacrylate cryogel 1st network crosslinked with chitosan/poly(ethyleneglycol) diglycidyl ether 2nd network | Used for designing macroporous hydrogels for controlled release of macromolecular drugs | [51] |
| Chitosan/silk fibroin/tannic acid/ferric ion cryogel | Developed as a wound dressing with stimuli-responsive photothermal therapy and good antibacterial and cell-proliferative properties | [49,50,52] |
| Chitosan-gelatin-chondroitin sulfate/nano-hydroxyapatite-gelatin cryogel scaffold | Used for tissue-engineering repair of articular cartilage injuries with potential therapeutic approaches for osteochondral repair | [21] |
| Chitosan-gelatin-polypyrrole 3D scaffolds with chitosan and gelatin microspheres | Evaluated for delivering alpha-ketoglutarate (alpha-KG) to cells in tissue engineering applications | [53] |
| Gelatin-Based Cryogel | ||
| Material Used | Main Outcomes | Ref. |
| Gelatin-based cryogel system embedded with CaCO3 microspheres and ciprofloxacin hydrochloride | Address the condition of osteomyelitis and osteoporosis, sustained drug release for up to 21 days, significant increase in cell viability and alkaline phosphatase levels in rat osteoblasts | [54] |
| Injectable 3D microscale cellular niches using biodegradable gelatin microcryogels | Optimize cell therapy for damaged tissues or organs, facilitate cell protection during injection and in vivo cell retention, survival, and therapeutic functions, superior therapeutic efficacy for treating critical limb ischemia in mouse models | [59] |
| Gelatin microcryogels loaded with hASCs | Injectable gelatin microcryogels were loaded and primed with human adipose-derived stem cells to create 3D cellular micro-niches that accelerate wound healing. | [60] |
| Injectable cryogels made of gelatin methacryloyl and poly(ethylene)glycol | Tunable degradability and porosity suitable for cell and drug delivery applications. | [56] |
| Cryogel scaffold composed of gelatin | Evaluation of polyelectrolyte multilayer microcapsules for controlled release of transforming growth factor-beta 1 (TGF-beta 1) in gelatin-based hydrogels and cryogel scaffolds for tissue engineering applications. | [57] |
| Gelatin-based cryogel with cellulose nanocrystal (CNC) and poly-amidoamine (PAMAM) dendrimer | For sustained drug release and improved mechanical properties. | [58] |
| Cryogenic Gelatin-Hyaluronic Acid Composite | Elastic scaffold for 3D bioprinting with continuous interconnected macroporous structure, allowing for cell attachment, viability, and proliferation | [61] |
| Nanocellulose and Gelatin Composite Cryogel | Controlled drug release carrier with controllable and sustained release of drug 5-FU | [62] |
| Nanosilicate and gelatin methacrylate Cryogel | Tough, macroporous hydrogel for drug delivery with sustained release under physiological conditions, reducing endothelial cell injury caused by nutrient deprivation | [63] |
| Gelatin/ascorbic acid (AA) cryogels | Carriers for corneal keratocyte growth in corneal stromal tissue engineering | [64] |
| Gelatin/heparin cryogel and BMP-2-loaded cryoelectrospun poly(epsilon-caprolactone) hybrid scaffold | Bone regeneration scaffold with enhanced mechanical support and sustained release of BMP-2 | [66] |
| Gelatin and heparin-based injectable cryogel | Carrier for in vivo cell and growth factor delivery in hindlimb ischemic disease | [67] |
| Amino acid-based functional monomer with HEMA and gelatin | Development of degradable molecularly imprinted cryogel for pH-responsive delivery of doxorubicin | [65] |
| Usnic acid encapsulated in Rhamnolipid biosurfactant nanoparticles enriched cryogel nanocomposite scaffold | Design of cryogel nanocomposite scaffold with dual properties of bone regeneration and antibacterial effect for the treatment of osteomyelitis | [55] |
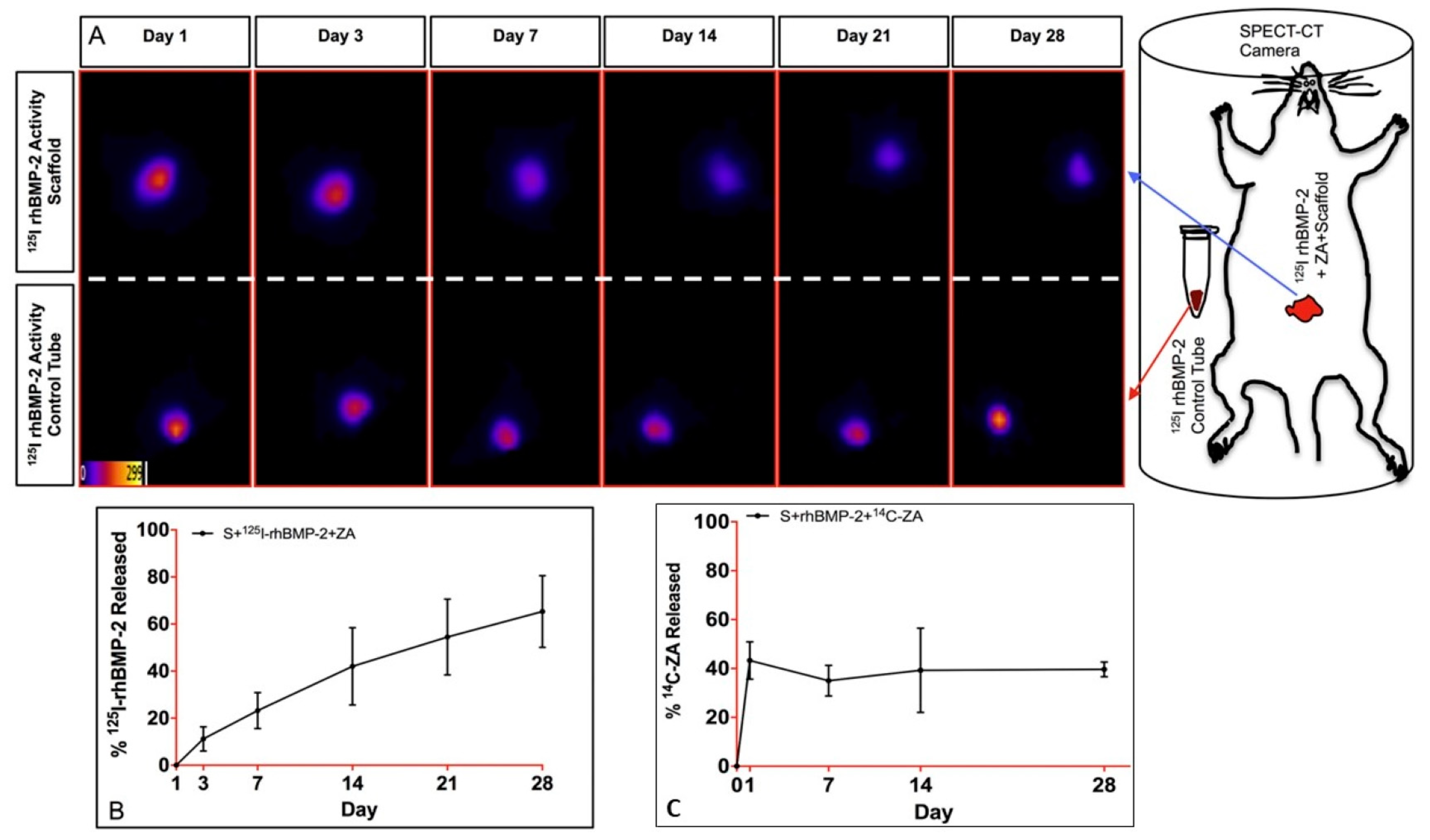
| Macroporous composite biomaterial of gelatin-hydroxyapatite-calcium sulphate | Development of biomaterial with spatio-temporal delivery of rhBMP-2 and ZA leading to increased bone formation compared to commercially available carriers | [68] |
| Gelatin-based cryogel | Use of gene delivery and tissue-engineering approaches for regenerating cartilage tissue lost due to trauma, tumor surgery, or congenital defects | [69] |
| Polysaccharides and Their Hybrids-Based Cryogel | ||
| Material Used | Main Outcomes | Ref. |
| Hydrophobically modified agarose cryogels (HMA cryogels) | Drug delivery systems, low cytotoxicity, adsorb more hydrophobic dye, and controlled release of dye | [75] |
| Mesophyll-inspired agarose cryogel (MAC) | Component of leaf-inspired micropump (LIM) for smart microfluidic applications, adjusts delivery rate of therapeutic liquid in response to temperature changes | [76] |
| Agarose-based cryogels, including cellulose fibers and microparticles | Water absorption and retention ability with high structural stability, potential for drug delivery devices | [77,78] |
| Carrageenan cryogels loaded with alpha-aminophosphonates | Improved mechanical properties and sustainable release of antimicrobial compounds for potential use against S. aureus | [79] |
| Kappa-Carrageenan (kappaC) matrix | Transdermal controlled delivery patch for Metformin, with enhanced drug release-permeation and diffusion coefficients when an electrical potential is applied | [80] |
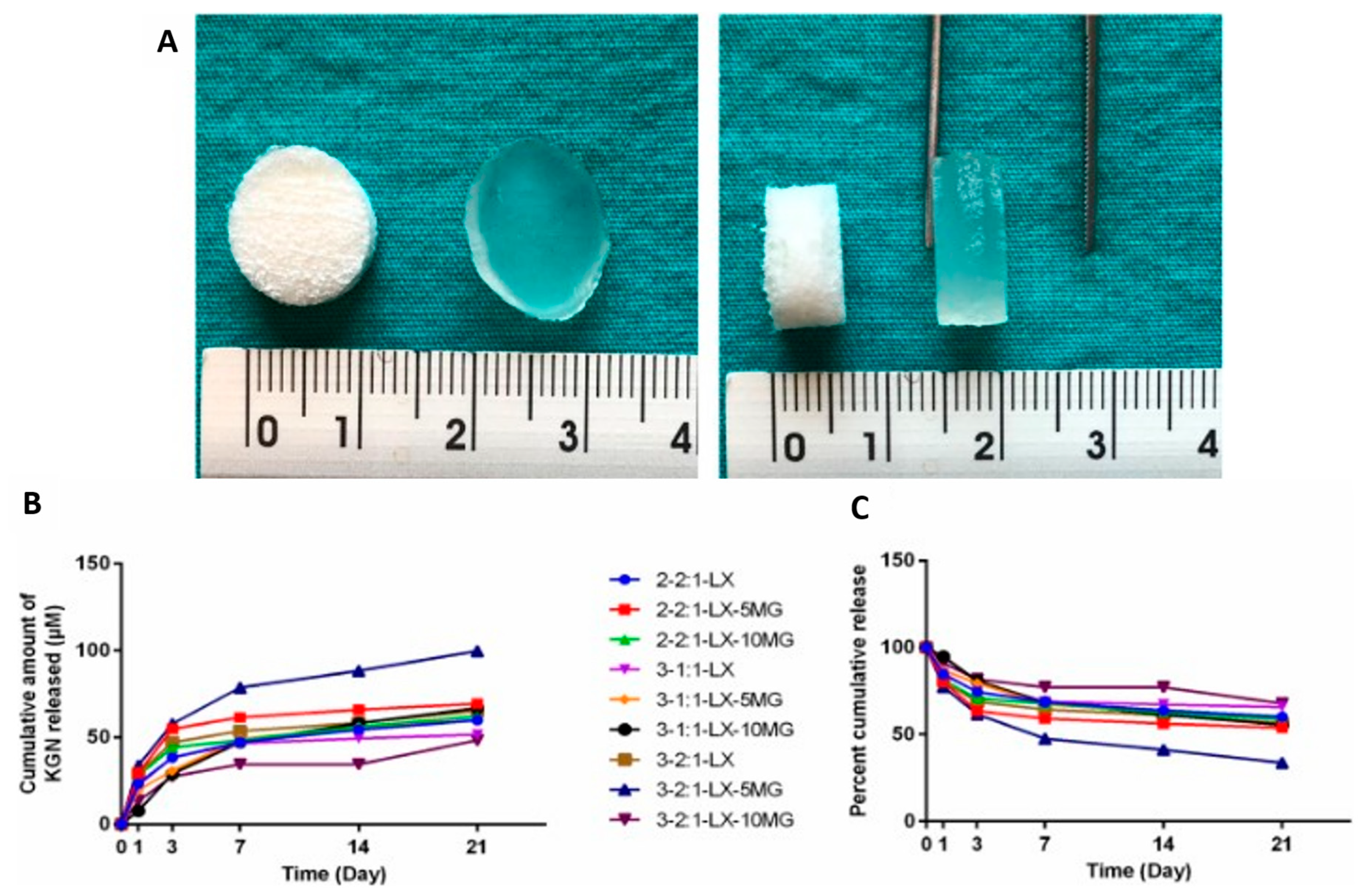
| LBG, XG, and MG-based cryogel scaffolds | Promising properties for cartilage and soft tissue engineering and drug delivery, sustained release of Kartogenin achieved | [81] |
| Cellulose cryogels microsphere | For wound healing, tissue regeneration, and drug delivery | [22,82] |
| CNF-PNIPAm hybrid | Synthesized temperature-sensitive polymer-modified cellulose nanofibril (CNF) cryogel microspheres with potential application in controlled drug release. | [83] |
| Biopolymer cryogels | Developed as an effective delivery system for curcumin in the form of Curcumin-nanostructured lipid carrier-loaded oleogels (Cur-NLC-OGs). Biopolymer cryogels were used to stabilize and self-stand the Cur-OGs. | [84] |
| Super-macroporous hydroxypropyl cellulose (HPC) cryogels embedded with stabilized core-shell micelles (SPM) | For sustained delivery of poorly water-soluble drugs | [153] |
| Hydroxyethyl cellulose (HEC) cryogels with polymeric micelles | Sustained topical delivery of hydrophobic natural substances such as cannabidiol | [85] |
| Composite cryogels (PVP/NaCMC) microspheres loaded with Mupirocin (MP) | Controlled release of antibiotic Mupirocin | [86] |
| Enzymatically modified starch cryogels | Carriers of active molecules for drug delivery systems and potential applications in biomedical and food packaging scenarios | [154] |
| Composite cryogels (OPS or OWS with DMAEM) fabrication of semi-IPN cryogels using N,N-dimethylaminoethyl methacrylate (DMAEM) and oxidized starches oxidized potato starch (OPS) or oxidized wheat starch (OWS) | For controlled drug release in simulated gastric fluid at pH 1.3 | [155] |
| PEG Derivative-Based Cryogel | ||
| Material Used | Main Outcomes | Ref. |
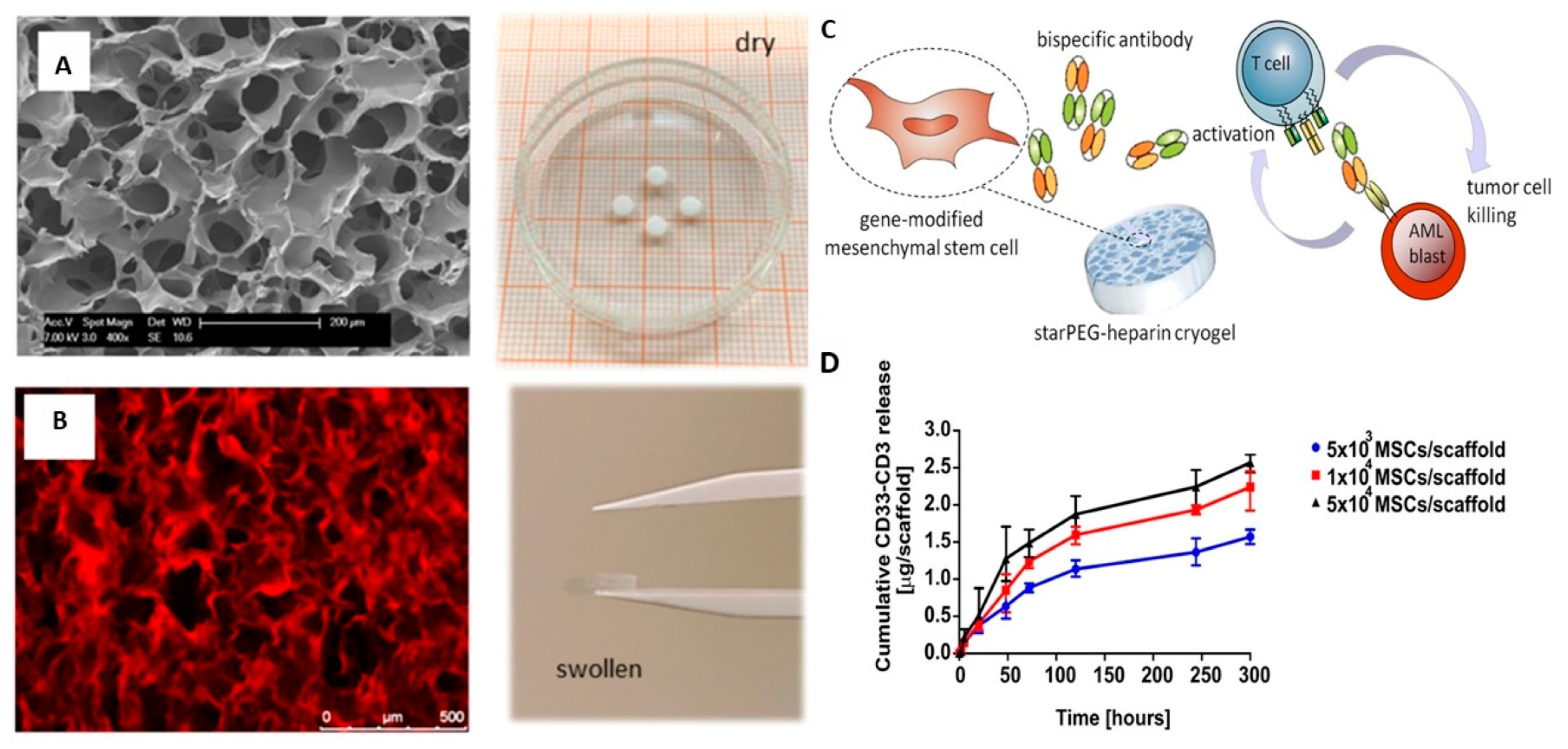
| Poly(ethylene glycol)-heparin cryogel scaffold | Promotes proliferation and survival of bsAb-releasing-MSCs, constant release of sustained and detectable levels of bsAb, and effective in triggering T-cell-mediated anti-tumor responses for treatment of acute myeloid leukemia (AML) | [87] |
| Activated carbonate group- with PEG functionalized cryogels | Slow-releasing drug reservoirs for anticancer drug delivery, with 7-fold higher drug release compared to hydrogels | [23] |
| Poly(ethylene glycol) diacrylate and maleimide-functionalized heparin cryogels | Sustained delivery of growth factors for tissue engineering and regenerative medicine applications, with the ability to load and release nerve growth factor over a period of 2 weeks and induce neurite outgrowth | [88] |
| Dextran methacrylate and polyethylene glycol dimethacrylate cryogel | Spongy scaffold for promoting the delivery of biomolecules in drug delivery and tissue engineering applications, with improved swelling, increased interconnected porosity, and higher mechanical resistance than conventional hydrogels | [142] |
| StarPEG-heparin cryogel | Allows for tunable, long-term delivery of different signaling proteins for tissue engineering applications, inducing local differences in protein concentration, and inducing neuronal differentiation of cells | [24] |
| Dendrimer cryogel made of hyperbranched amine-terminated polyamidoamine (PAMAM) dendrimer G4.0 and linear polyethylene glycol (PEG) diacrylate | Superelastic network with high compression elasticity, super resilience, and stability at acidic pH, suitable for biomedical applications due to its self-triggered degradation at physiological pH | [156] |
| Poly(ethylene glycol) diacrylate microcryogels | Utilized in a syringe-based 3D culture system for the mechanical preconditioning of mesenchymal stromal/stem cells toward nucleus pulposus (NP)-like cells, with potential for NP regeneration | [89] |
| PVOH-Based Cryogel | ||
| Material Used | Main Outcomes | Ref. |
| PVA/ZnO/FA nanocomposite | Drug delivery application with controlled release of fulvic acid | [90] |
| PVA cryogel with thermochromic ink | Thermochromic material for temperature estimation in ultrasound therapy | [91] |
| PVA cryogel | Enhancing solubility of Simvastatin for use as a prolonged-release cryogel matrix for hydrophobic drugs and potential biomaterial for tissue engineering | [92,143] |
| Poly(vinyl) alcohol and propylene glycol cryogel | Used as a hydrophilic active wound dressing loaded with trans-resveratrol for controlled release and reduced irritation | [144] |
| Composite cryogels containing PVA particle and porous adsorbent particles | Used for capturing glycoproteins and evaluated for repeated use in batch and chromatographic experiments | [145] |
| Polyvinyl alcohol cryogel with high-methoxylated pectin | Used for the controlled release of the antibiotic enrofloxacin, and a two-layer film system was designed to modulate the release rate of the drug | [146] |
| Polyvinyl alcohol cryogel with urea additives | Used as a polymeric carrier in drug delivery systems with widened macropores, and the release rate of the drug depends on the urea content in the initial PVA solution | [147] |
| Highly porous composite PVA cryogels loaded with drug molecule | Highly porous composite cryogels loaded with PHB microbeads containing simvastatin for controlled drug delivery | [93] |
| PVA/iron oxide NPs | Cryogels for thermally triggered drug release based on shape-selective heat transfer using magnetic nanoparticles coated with acetaminophen | [148] |
| Polyvinyl alcohol (PVA) | Investigation of PVA cryogel for iontophoretic transdermal drug delivery | [94,149] |
| PVA and its nanocomposites | Used for biomedical and medical device applications due to its unique mechanical properties that can be tailored to match soft tissues | [96] |
| PVA-NLC, nanostructured lipid carriers loaded with drug molecules | Serves as an adhesive film that contains nanostructured lipid carriers loaded with olanzapine and simvastatin for transdermal treatment of psychiatric disorders | [95] |
| PVA-DNA gel matrices | Examines factors that affect the release rate of deoxyribonucleic acid (DNA) from PVA hydrogels and its blend with DNA. Investigates the potential of designing controlled DNA release PVA-based devices | [97,98] |
| PVOH Derivatives-Based Cryogel | ||
| Material Used | Main Outcomes | Ref. |
| Molecularly imprinted cryogel | Used for solid-phase extraction of propranolol from aqueous solution and complex plasma sample due to its high selectivity and stability | [99] |
| PVA and gelatin cryogels | Evaluated for their water-uptake potential, influence of various factors on water sorption, and biocompatibility for potential use in biomedical applications | [100] |
| PVA and egg albumin cryogels | Investigated for their water sorption capacity, swelling behavior, in vitro biocompatibility, thermal and morphological characterization for potential use in the biomedical field | [101] |
| pH-sensitive hydrogel made from a blend of polyvinyl-alcohol, polyacrylic acid, and synthetic hydroxyapatite | Explored for specific drug delivery applications due to their smart properties. Investigated the effects of hydroxyapatite on mechanical strength, bioactivity, and drug release profiles, with promising results seen in terms of swelling, gel fraction, and drug delivery | [102] |
| Gum tragacanth-polyvinyl alcohol (GT-PVA) cryo- and xerogels | Prepared and evaluated for their physical, mechanical, and release properties, including the effect of GT ratio and inclusion of silymarin. Found to have potential applications due to their porosity, microstructure, and mucoadhesive properties | [157] |
| poly(vinyl alcohol),pullulan and zeolite Composite cryogel | Controlled release of Enalapril Maleate drug used to treat hypertension and heart failure | [104] |
| Dual network hydrogel of poly(vinyl alcohol) cryogel and sodium alginate | Synthesized for potential biomedical applications. Can absorb and retain fluids, incorporate biological molecules/drugs | [105] |
| PVA and polyacrylic acid Cryogel carrier system | Controls the release rate of extracted propolis for enhanced efficacy. Can be used as bactericidal dressing | [106] |
| Two-phase hydrogel prepared by physically imbedding a xerogel in the core of a cryogel that was freeze thawed with PVA and PAA | Temperature-sensitive drug delivery systems, drug release at a slower rate from hydrogels containing acrylic acid | [158] |
| PVA/HA pH-responsive cryogel | Drug delivery system for the treatment of psoriasis, significant decrease in toxicity, swelling and drug release at pH 5.5 | [103] |
| Acrylate and Methacrylate Base Cryogel | ||
| Material Used | Main Outcomes | Ref. |
| NIPA and HEMA-lactate-Dextran-based biodegradable and thermoresponsive cryogels | Potential use in bone tissue engineering with controlled drug release capabilities | [107] |
| Poly 2-hydroxyethyl methacrylate (pHEMA) and halloysite nanotubes (HNTs) embedded with thymol (Thy) | Sustained release drug delivery system for wound healing in space | [25] |
| Poly(2-hydroxyethyl methacrylate) | Purification of plasmid expressing Influenza hemagglutinin gene | [108] |
| Metal-chelate monomer N-methacryloyl-L-histidine, hydroxyethyl methacrylate and Cu(2+) ion | Imprinted cryogel discs for delivering chemotherapy drug 5-fluorouracil (5-FU) | [109] |
| Cryogel-based molecularly imprinted membranes | Implantable drug delivery system for controlled release of antineoplastic agent Mitomycin C for cancer treatment | [110] |
| HEMA-based cryogels | Potential candidates for controlled drug delivery systems in biomedical applications | [150] |
| Polymeric cryogels | Efficient removal of emerging contaminants from water and suitable for drug delivery applications | [111] |
| Poly(hydroxyethyl acrylate-co-phenyl vinyl sulfide) cryogel | Suitable anticancer drug delivery carrier | [112] |
| PETEGA cryogels | Controlled drug release for verapamil hydrochloride | [113] |
| Terpolymer hydrogels and cryogels | Potential to improve loading capacity of polymers used in anticancer drug delivery systems | [114] |
| Other Cryogels | ||
| Material Used | Main Outcomes | Ref. |
| Poly(N-isopropylacrylamide) cryogels with conducting polyaniline or polypyrrole nanoparticles | Potential application in electrical devices, tissue engineering scaffolds, drug delivery vehicle, and electronic skin | [115] |
| Zwitterionic cryogels | For drug delivery, chemoimmunotherapy, and long-term release of proteins | [119,120,121] |
| Calcium phosphate-based cryogel encapsulating alkaline phosphatase (ALP) | Preserves activity of ALP and has chemical and structural properties determined using X-ray diffraction, helium pycnometry and mercury porosimetry | [129] |
| Furan-based cryogel (CG) scaffold covalently conjugated with maleimide-modified antimicrobial peptides | Rapid loading and release of therapeutic peptides with high water uptake; “on-demand” photothermal heating upon NIR irradiation | [131] |
| Hybrid and composite biomaterials | To enhance mechanical strength and porosity | [126,130,135] |
4. Challenges and Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Babanejad, N.; Mfoafo, K.; Thumma, A.; Omidi, Y.; Omidian, H. Advances in cryostructures and their applications in biomedical and pharmaceutical products. Polym. Bull. 2023, 48. [Google Scholar] [CrossRef]
- Babanejad, N.; Mfoafo, K.; Zhang, E.; Omidi, Y.; Razeghifard, R.; Omidian, H. Applications of cryostructures in the chromatographic separation of biomacromolecules. J. Chromatogr. A 2022, 1683, 463546. [Google Scholar] [CrossRef]
- Ambreen, J.; Haleem, A.; Shah, A.A.; Mushtaq, F.; Siddiq, M.; Bhatti, M.A.; Shah Bukhari, S.N.U.; Chandio, A.D.; Mahdi, W.A.; Alshehri, S. Facile Synthesis and Fabrication of NIPAM-Based Cryogels for Environmental Remediation. Gels 2023, 9, 64. [Google Scholar] [CrossRef]
- Omidian, H.; Muppalaneni, S.; Joshi, Y.; Mastropietro, D.J. Compositions for Deterring Abuse of Pharmaceutical Products and Alcohol. U.S. Patent WO2017070462A1, 21 October 2016. [Google Scholar]
- Omidian, H.; Qiu, Y.; Kim, D.J.; Yang, S.C.; Park, H.; Park, K. Hydrogels Having Enhanced Elasticity and Mechanical Strength Properties. U.S. Patent No. 6,960,617, 1 November 2005. [Google Scholar]
- Muppalaneni, S.; Omidian, H. Polyvinyl Alcohol in Medicine and Pharmacy: A Perspective. J. Dev. Drugs 2013, 2, 1000112. [Google Scholar] [CrossRef] [Green Version]
- Haleem, A.; Pan, J.-M.; Shah, A.; Hussain, H.; He, W.-d. A systematic review on new advancement and assessment of emerging polymeric cryogels for environmental sustainability and energy production. Sep. Purif. Technol. 2023, 316, 123678. [Google Scholar] [CrossRef]
- Çimen, D.; Özbek, M.A.; Bereli, N.; Mattiasson, B.; Denizli, A. Injectable Cryogels in Biomedicine. Gels 2021, 7, 38. [Google Scholar] [CrossRef]
- Eigel, D.; Werner, C.; Newland, B. Cryogel biomaterials for neuroscience applications. Neurochem. Int. 2021, 147, 105012. [Google Scholar] [CrossRef]
- Raschip, I.E.; Darie-Nita, R.N.; Fifere, N.; Hitruc, G.E.; Dinu, M.V. Correlation between Mechanical and Morphological Properties of Polyphenol-Laden Xanthan Gum/Poly(vinyl alcohol) Composite Cryogels. Gels 2023, 9, 281. [Google Scholar] [CrossRef]
- Qi, X.; Hu, X.; Wei, W.; Yu, H.; Li, J.; Zhang, J.; Dong, W. Investigation of Salecan/poly(vinyl alcohol) hydrogels prepared by freeze/thaw method. Carbohydr. Polym. 2015, 118, 60–69. [Google Scholar] [CrossRef]
- Bakhshpour, M.; Idil, N.; Percin, I.; Denizli, A. Biomedical Applications of Polymeric Cryogels. Appl. Sci. 2019, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Eggermont, L.J.; Rogers, Z.J.; Colombani, T.; Memic, A.; Bencherif, S.A. Injectable Cryogels for Biomedical Applications. Trends Biotechnol. 2020, 38, 418–431. [Google Scholar] [CrossRef]
- Idumah, C.I. Recently emerging advancements in polymeric cryogel nanostructures and biomedical applications. Int. J. Polym. Mater. Polym. Biomater. 2022, 1–21. [Google Scholar] [CrossRef]
- Joukhdar, H.; Seifert, A.; Jungst, T.; Groll, J.; Lord, M.S.; Rnjak-Kovacina, J. Ice Templating Soft Matter: Fundamental Principles and Fabrication Approaches to Tailor Pore Structure and Morphology and Their Biomedical Applications. Adv. Mater. 2021, 33, e2100091. [Google Scholar] [CrossRef]
- Klivenko, A.N.; Mussabayeva, B.K.; Gaisina, B.S.; Sabitova, A.N. Biocompatible cryogels: Preparation and application. Bull. Univ. Karaganda Chem. 2021, 103, 4–20. [Google Scholar] [CrossRef]
- Leach, D.G.; Young, S.; Hartgerink, J.D. Advances in immunotherapy delivery from implantable and injectable biomaterials. Acta Biomater. 2019, 88, 15–31. [Google Scholar] [CrossRef]
- Rojo, L. Combination of Polymeric Supports and Drug Delivery Systems for Osteochondral Regeneration. Adv. Exp. Med. Biol. 2018, 1059, 301–313. [Google Scholar] [CrossRef]
- Savina, I.N.; Zoughaib, M.; Yergeshov, A.A. Design and Assessment of Biodegradable Macroporous Cryogels as Advanced Tissue Engineering and Drug Carrying Materials. Gels 2021, 7, 79. [Google Scholar] [CrossRef]
- Haleem, A.; Qi Chen, S.; Ullah, M.; Siddiq, M.; He, W.D. Highly porous cryogels loaded with bimetallic nanoparticles as an efficient antimicrobial agent and catalyst for rapid reduction of water-soluble organic contaminants. J. Environ. Chem. Eng. 2021, 9, 106510. [Google Scholar] [CrossRef]
- Nikhil, A.; Kumar, A. Evaluating potential of tissue-engineered cryogels and chondrocyte derived exosomes in articular cartilage repair. Biotechnol. Bioeng. 2022, 119, 605–625. [Google Scholar] [CrossRef]
- Tyshkunova, I.V.; Poshina, D.N.; Skorik, Y.A. Cellulose Cryogels as Promising Materials for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 2037. [Google Scholar] [CrossRef]
- Aydin, D.; Arslan, M.; Sanyal, A.; Sanyal, R. Hooked on Cryogels: A Carbamate Linker Based Depot for Slow Drug Release. Bioconjugate Chem. 2017, 28, 1443–1451. [Google Scholar] [CrossRef]
- Sievers, J.; Zimmermann, R.; Friedrichs, J.; Pette, D.; Limasale, Y.D.P.; Werner, C.; Welzel, P.B. Customizing biohybrid cryogels to serve as ready-to-use delivery systems of signaling proteins. Biomaterials 2021, 278, 15. [Google Scholar] [CrossRef]
- Zagni, C.; Scamporrino, A.A.; Riccobene, P.M.; Floresta, G.; Patamia, V.; Rescifina, A.; Carroccio, S.C. Portable Nanocomposite System for Wound Healing in Space. Nanomaterials 2023, 13, 741. [Google Scholar] [CrossRef]
- Bauleth-Ramos, T.; Shih, T.Y.; Shahbazi, M.A.; Najibi, A.J.; Mao, A.S.; Liu, D.F.; Granja, P.; Santos, H.A.; Sarmento, B.; Mooney, D.J. Acetalated Dextran Nanoparticles Loaded into an Injectable Alginate Cryogel for Combined Chemotherapy and Cancer Vaccination. Adv. Funct. Mater. 2019, 29, 10. [Google Scholar] [CrossRef]
- Im, P.; Kim, J. On-Demand Macroscale Delivery System Based on a Macroporous Cryogel with a High Drug Loading Capacity for Enhanced Cancer Therapy. ACS Biomater. Sci. Eng. 2018, 4, 3498–3505. [Google Scholar] [CrossRef]
- Koshy, S.T.; Zhang, D.K.Y.; Grolman, J.M.; Stafford, A.G.; Mooney, D.J. Injectable nanocomposite cryogels for versatile protein drug delivery. Acta Biomater. 2018, 65, 36–43. [Google Scholar] [CrossRef]
- Guo, N.; Xia, Y.; Zeng, W.S.; Chen, J.; Wu, Q.X.; Shi, Y.X.; Li, G.Y.; Huang, Z.Y.; Wang, G.H.; Liu, Y. Alginate-based aerogels as wound dressings for efficient bacterial capture and enhanced antibacterial photodynamic therapy. Drug Deliv. 2022, 29, 1086–1099. [Google Scholar] [CrossRef]
- Beduer, A.; Braschler, T.; Peric, O.; Fantner, G.E.; Mosser, S.; Fraering, P.C.; Bencherif, S.; Mooney, D.J.; Renaud, P. A compressible scaffold for minimally invasive delivery of large intact neuronal networks. Adv. Healthc. Mater. 2015, 4, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Ki, S.B.; Singh, D.; Kim, S.C.; Son, T.W.; Han, S.S. Effect of cross-linkers in fabrication of carrageenan-alginate matrices for tissue engineering application. Biotechnol. Appl. Biochem. 2013, 60, 589–595. [Google Scholar] [CrossRef]
- Memic, A.; Rezaeeyazdi, M.; Villard, P.; Rogers, Z.J.; Abudula, T.; Colombani, T.; Bencherif, S.A. Effect of Polymer Concentration on Autoclaved Cryogel Properties. Macromol. Mater. Eng. 2020, 305, 11. [Google Scholar] [CrossRef]
- Evans, C.; Morimitsu, Y.; Hisadome, T.; Inomoto, F.; Yoshida, M.; Takei, T. Optimized hydrophobically modified chitosan cryogels for strength and drug delivery systems. J. Biosci. Bioeng. 2021, 132, 81–87. [Google Scholar] [CrossRef]
- Obaidat, R.M.; Tashtoush, B.M.; Bayan, M.F.; Al Bustami, R.T.; Alnaief, M. Drying Using Supercritical Fluid Technology as a Potential Method for Preparation of Chitosan Aerogel Microparticles. AAPS PharmSciTech 2015, 16, 1235–1244. [Google Scholar] [CrossRef] [Green Version]
- Platon, I.V.; Ghiorghita, C.A.; Lazar, M.M.; Raschip, I.E.; Dinu, M.V. Chitosan Sponges with Instantaneous Shape Recovery and Multistrain Antibacterial Activity for Controlled Release of Plant-Derived Polyphenols. Int. J. Mol. Sci. 2023, 24, 4452. [Google Scholar] [CrossRef]
- Sami, H.; Kumar, A. Tunable hybrid cryogels functionalized with microparticles as supermacroporous multifunctional biomaterial scaffolds. J. Biomater. Sci. Polym. Ed. 2013, 24, 1165–1184. [Google Scholar] [CrossRef]
- Lee, S.S.; Kim, J.H.; Jeong, J.; Kim, S.H.L.; Koh, R.H.; Kim, I.; Bae, S.; Lee, H.; Hwang, N.S. Sequential growth factor releasing double cryogel system for enhanced bone regeneration. Biomaterials 2020, 257, 120223. [Google Scholar] [CrossRef]
- de Lima, G.G.; Junior, E.L.S.; Aggio, B.B.; Shee, B.S.; Filho, E.M.M.; Segundo, F.A.S.; Fournet, M.B.; Devine, D.M.; Magalhaes, W.L.E.; de Sa, M.J.C. Nanocellulose for peripheral nerve regeneration in rabbits using citric acid as crosslinker with chitosan and freeze/thawed PVA. Biomed. Mater. 2021, 16, 055011. [Google Scholar] [CrossRef]
- Dinu, M.V.; Cocarta, A.I.; Dragan, E.S. Synthesis, characterization and drug release properties of 3D chitosan/clinoptilolite biocomposite cryogels. Carbohydr. Polym. 2016, 153, 203–211. [Google Scholar] [CrossRef]
- Konovalova, M.V.; Markov, P.A.; Durnev, E.A.; Kurek, D.V.; Popov, S.V.; Varlamov, V.P. Preparation and biocompatibility evaluation of pectin and chitosan cryogels for biomedical application. J. Biomed. Mater. Res. Part A 2017, 105, 547–556. [Google Scholar] [CrossRef]
- Peniche, H.; Reyes-Ortega, F.; Aguilar, M.R.; Rodriguez, G.; Abradelo, C.; Garcia-Fernandez, L.; Peniche, C.; San Roman, J. Thermosensitive Macroporous Cryogels Functionalized with Bioactive Chitosan/Bemiparin Nanoparticles. Macromol. Biosci. 2013, 13, 1556–1567. [Google Scholar] [CrossRef]
- Sultankulov, B.; Berillo, D.; Kauanova, S.; Mikhalovsky, S.; Mikhalovska, L.; Saparov, A. Composite Cryogel with Polyelectrolyte Complexes for Growth Factor Delivery. Pharmaceutics 2019, 11, 650. [Google Scholar] [CrossRef] [Green Version]
- Meena, L.K.; Raval, P.; Kedaria, D.; Vasita, R. Study of locust bean gum reinforced cyst-chitosan and oxidized dextran based semi-IPN cryogel dressing for hemostatic application. Bioact. Mater. 2018, 3, 370–384. [Google Scholar] [CrossRef]
- Cheaburu-Yilmaz, C.N.; Yilmaz, O.; Kose, F.A.; Bibire, N. Chitosan-Graft-Poly(N-Isopropylacrylamide)/PVA Cryogels as Carriers for Mucosal Delivery of Voriconazole. Polymers 2019, 11, 1432. [Google Scholar] [CrossRef] [Green Version]
- Stoyneva, V.; Momekova, D.; Kostova, B.; Petrov, P. Stimuli sensitive super-macroporous cryogels based on photo-crosslinked 2-hydroxyethylcellulose and chitosan. Carbohydr. Polym. 2014, 99, 825–830. [Google Scholar] [CrossRef]
- Park, M.J.; An, Y.H.; Choi, Y.H.; Kim, H.D.; Hwang, N.S. Enhanced Neovascularization Using Injectable and rhVEGF-Releasing Cryogel Microparticles. Macromol. Biosci. 2021, 21, e2100234. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V.; Ghiorghita, C.A. Chitosan-Based Polyelectrolyte Complex Cryogels with Elasticity, Toughness and Delivery of Curcumin Engineered by Polyions Pair and Cryostructuration Steps. Gels 2022, 8, 240. [Google Scholar] [CrossRef]
- Tome, L.I.N.; Reis, M.S.; de Sousa, H.C.; Braga, M.E.M. Chitosan-xanthan gum PEC-based aerogels: A chemically stable PEC in scCO(2). Mater. Chem. Phys. 2022, 287, 10. [Google Scholar] [CrossRef]
- Cao, S.; Bi, Z.; Li, Q.; Zhang, S.; Singh, M.; Chen, J. Shape memory and antibacterial chitosan-based cryogel with hemostasis and skin wound repair. Carbohydr. Polym. 2023, 305, 120545. [Google Scholar] [CrossRef]
- Takei, T.; Danjo, S.; Sakoguchi, S.; Tanaka, S.; Yoshinaga, T.; Nishimata, H.; Yoshida, M. Autoclavable physically-crosslinked chitosan cryogel as a wound dressing. J. Biosci. Bioeng. 2018, 125, 490–495. [Google Scholar] [CrossRef]
- Dragan, E.S.; Cocarta, A.I.; Gierszewska, M. Designing novel macroporous composite hydrogels based on methacrylic acid copolymers and chitosan and in vitro assessment of lysozyme controlled delivery. Colloids Surf. B Biointerfaces 2016, 139, 33–41. [Google Scholar] [CrossRef]
- Yu, Y.L.; Li, P.F.; Zhu, C.L.; Ning, N.; Zhang, S.Y.; Vancso, G.J. Multifunctional and Recyclable Photothermally Responsive Cryogels as Efficient Platforms for Wound Healing. Adv. Funct. Mater. 2019, 29, 11. [Google Scholar] [CrossRef]
- Vishnoi, T.; Kumar, A. Comparative study of various delivery methods for the supply of alpha-ketoglutarate to the neural cells for tissue engineering. Biomed. Res. Int. 2013, 2013, 294679. [Google Scholar] [CrossRef]
- Pandey, G.; Mittapelly, N.; Pant, A.; Sharma, S.; Singh, P.; Banala, V.T.; Trivedi, R.; Shukla, P.K.; Mishra, P.R. Dual functioning microspheres embedded crosslinked gelatin cryogels for therapeutic intervention in osteomyelitis and associated bone loss. Eur. J. Pharm. Sci. 2016, 91, 105–113. [Google Scholar] [CrossRef]
- Falakaflaki, M.; Varshosaz, J.; Mirian, M. Local delivery of usnic acid loaded Rhamnolipid vesicles by gelatin/tragacanth gum /montmorillonite/vanillin cryogel scaffold for expression of osteogenic biomarkers and antimicrobial activity. J. Drug Deliv. Sci. Technol. 2022, 69, 12. [Google Scholar] [CrossRef]
- Dalal, N.; Challa, R.; Thimukonda, J.J.; Tayalia, P. Gelatin Methacryloyl Based Injectable Cryogels with Tunable Degradability for Cell Delivery. Macromol. Biosci. 2023, 2023, e2200562. [Google Scholar] [CrossRef]
- De Cock, L.J.; De Wever, O.; Van Vlierberghe, S.; Vanderleyden, E.; Dubruel, P.; De Vos, F.; Vervaet, C.; Remon, J.P.; De Geest, B.G. Engineered (hep/pARG)(2) polyelectrolyte capsules for sustained release of bioactive TGF-beta 1. Soft Matter. 2012, 8, 1146–1154. [Google Scholar] [CrossRef]
- Goodarzi, K.; Shariatzadeh, F.J.; Solouk, A.; Akbari, S.; Mirzadeh, H. Injectable drug loaded gelatin based scaffolds as minimally invasive approach for drug delivery system: CNC/PAMAM nanoparticles. Eur. Polym. J. 2020, 139, 10. [Google Scholar] [CrossRef]
- Li, Y.; Liu, W.; Liu, F.; Zeng, Y.; Zuo, S.; Feng, S.; Qi, C.; Wang, B.; Yan, X.; Khademhosseini, A.; et al. Primed 3D injectable microniches enabling low-dosage cell therapy for critical limb ischemia. Proc. Natl. Acad. Sci. USA 2014, 111, 13511–13516. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Zhu, L.; Han, Q.; Liu, W.; Mao, X.; Li, Y.; Yu, N.; Feng, S.; Fu, Q.; Wang, X.; et al. Preformed gelatin microcryogels as injectable cell carriers for enhanced skin wound healing. Acta Biomater. 2015, 25, 291–303. [Google Scholar] [CrossRef]
- Unluer, O.B.; Diltemiz, S.E.; Say, M.G.; Hur, D.; Say, R.; Ersoz, A. A powerful combination in designing polymeric scaffolds: 3D bioprinting and cryogelation. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 278–290. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.J.; Zhang, L.; Xu, Z.Y.; Dai, H.Q.; Wu, W.B. Nanocellulose/Gelatin Composite Cryogels for Controlled Drug Release. ACS Sustain. Chem. Eng. 2019, 7, 6381–6389. [Google Scholar] [CrossRef]
- Chakraborty, A.; Pacelli, S.; Alexander, S.; Huayamares, S.; Rosenkrans, Z.; Vergel, F.E.; Wu, Y.; Chakravorty, A.; Paul, A. Nanoparticle-Reinforced Tough Hydrogel as a Versatile Platform for Pharmaceutical Drug Delivery: Preparation and in Vitro Characterization. Mol. Pharm. 2023, 20, 767–774. [Google Scholar] [CrossRef]
- Luo, L.J.; Lai, J.Y.; Chou, S.F.; Hsueh, Y.J.; Ma, D.H.K. Development of gelatin/ascorbic acid cryogels for potential use in corneal stromal tissue engineering. Acta Biomater. 2018, 65, 123–136. [Google Scholar] [CrossRef]
- Cetin, K.; Alkan, H.; Bereli, N.; Denizli, A. Molecularly imprinted cryogel as a pH-responsive delivery system for doxorubicin. J. Macromol. Sci. Part A 2017, 54, 502–508. [Google Scholar] [CrossRef]
- Lee, S.S.; Santschi, M.; Ferguson, S.J. A Biomimetic Macroporous Hybrid Scaffold with Sustained Drug Delivery for Enhanced Bone Regeneration. Biomacromolecules 2021, 22, 2460–2471. [Google Scholar] [CrossRef]
- Kim, I.; Lee, S.S.; Bae, S.; Lee, H.; Hwang, N.S. Heparin Functionalized Injectable Cryogel with Rapid Shape-Recovery Property for Neovascularization. Biomacromolecules 2018, 19, 2257–2269. [Google Scholar] [CrossRef]
- Raina, D.B.; Larsson, D.; Mrkonjic, F.; Isaksson, H.; Kumar, A.; Lidgren, L.; Tagil, M. Gelatin- hydroxyapatite- calcium sulphate based biomaterial for long term sustained delivery of bone morphogenic protein-2 and zoledronic acid for increased bone formation: In-vitro and in-vivo carrier properties. J. Control. Release 2018, 272, 83–96. [Google Scholar] [CrossRef]
- Odabas, S.; Feichtinger, G.A.; Korkusuz, P.; Inci, I.; Bilgic, E.; Yar, A.S.; Cavusoglu, T.; Menevse, S.; Vargel, I.; Piskin, E. Auricular cartilage repair using cryogel scaffolds loaded with BMP-7-expressing primary chondrocytes. J. Tissue Eng. Regen. Med. 2013, 7, 831–840. [Google Scholar] [CrossRef]
- Ari, B.; Demirci, S.; Ayyala, R.S.; Salih, B.; Sahiner, N. Superporous poly(?-Cyclodextrin) cryogels as promising materials for simultaneous delivery of both hydrophilic and hydrophobic drugs. Eur. Polym. J. 2022, 176, 12. [Google Scholar] [CrossRef]
- Michel, B.; Bras, J.; Dufresne, A.; Heggset, E.B.; Syverud, K. Production and Mechanical Characterisation of TEMPO-Oxidised Cellulose Nanofibrils/beta-Cyclodextrin Films and Cryogels. Molecules 2020, 25, 2381. [Google Scholar] [CrossRef]
- Danov, Y.; Georgieva, D.; Mihaylova, R.; Kostova, B.; Petrov, P.D. Cryogel Carriers Comprising beta-Cyclodextrin Moieties for Improved Solubilization and Delivery of Aripiprazole. Macromol. Chem. Phys. 2021, 222, 8. [Google Scholar] [CrossRef]
- Momekova, D.; Danov, Y.; Momekov, G.; Ivanov, E.; Petrov, P. Polysaccharide Cryogels Containing beta-Cyclodextrin for the Delivery of Cannabidiol. Pharmaceutics 2021, 13, 1774. [Google Scholar] [CrossRef]
- Hajidariyor, T.; Nuntawad, N.; Somsaen, P.; Prukdamrongchai, R.; Cherdchoo, H.; Posoknistakul, P.; Khemthong, P.; Wanmolee, W.; Arjfuk, P.; Pongchaikul, P.; et al. Cryo-Induced Cellulose-Based Nanogel from Elaeis guineensis for Antibiotic Delivery Platform. Int. J. Mol. Sci. 2023, 24, 1230. [Google Scholar] [CrossRef]
- Evans, C.; Morimitsu, Y.; Nishi, R.; Yoshida, M.; Takei, T. Novel hydrophobically modified agarose cryogels fabricated using dimethyl sulfoxide. J. Biosci. Bioeng. 2022, 133, 390–395. [Google Scholar] [CrossRef]
- Kim, H.; Kim, K.; Lee, S.J. Compact and Thermosensitive Nature-inspired Micropump. Sci. Rep. 2016, 6, 36085. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Kim, J.; Lee, S.J. Fast and Efficient Water Absorption Material Inspired by Cactus Root. ACS Macro Lett. 2018, 7, 387–394. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef]
- Elsherbiny, D.A.; Abdelgawad, A.M.; El-Naggar, M.E.; El-Sherbiny, R.A.; El-Rafie, M.H.; El-Sayed, I.E.T. Synthesis, antimicrobial activity, and sustainable release of novel alpha-aminophosphonate derivatives loaded carrageenan cryogel. Int. J. Biol. Macromol. 2020, 163, 96–107. [Google Scholar] [CrossRef]
- Saramas, T.; Sakunpongpitiporn, P.; Rotjanasuworapong, K.; Morarad, R.; Niamlang, S.; Sirivat, A. Metformin delivery via iontophoresis based on kappa-carrageenan cryogels. Int. J. Biol. Macromol. 2022, 223, 702–712. [Google Scholar] [CrossRef]
- Bektas, E.I.; Pekozer, G.G.; Kok, F.N.; Kose, G.T. Evaluation of natural gum-based cryogels for soft tissue engineering. Carbohydr. Polym. 2021, 271, 15. [Google Scholar] [CrossRef]
- De Wever, P.; Janssens, J.; Fardim, P. Fabrication of cellulose cryogel beads via room temperature dissolution in onium hydroxides. Carbohydr. Polym. Technol. Appl. 2022, 3, 9. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, W.B.; Zhang, X.D.; Meng, X.Z.; Tong, G.L.; Deng, Y.L. Temperature-sensitive poly-NIPAm modified cellulose nanofibril cryogel microspheres for controlled drug release. Cellulose 2016, 23, 415–425. [Google Scholar] [CrossRef]
- Zhang, J.; Chuesiang, P.; Kim, J.T.; Shin, G.H. The role of nanostructured lipid carriers and type of biopolymers on the lipid digestion and release rate of curcumin from curcumin-loaded oleogels. Food Chem. 2022, 392, 133306. [Google Scholar] [CrossRef]
- Momekova, D.; Ivanov, E.; Konstantinov, S.; Ublekov, F.; Petrov, P.D. Nanocomposite Cryogel Carriers from 2-Hydroxyethyl Cellulose Network and Cannabidiol-Loaded Polymeric Micelles for Sustained Topical Delivery. Polymers 2020, 12, 1172. [Google Scholar] [CrossRef]
- Yavuz, B.; Solak, E.K.; Oktar, C. Preparation of biocompatible microsphere-cryogel composite system and controlled release of mupirocin. Int. J. Polym. Mater. Polym. Biomater. 2023, 1–8. [Google Scholar] [CrossRef]
- Aliperta, R.; Welzel, P.B.; Bergmann, R.; Freudenberg, U.; Berndt, N.; Feldmann, A.; Arndt, C.; Koristka, S.; Stanzione, M.; Cartellieri, M.; et al. Cryogel-supported stem cell factory for customized sustained release of bispecific antibodies for cancer immunotherapy. Sci. Rep. 2017, 7, 42855. [Google Scholar] [CrossRef] [Green Version]
- Newland, B.; Newland, H.; Lorenzi, F.; Eigel, D.; Welzel, P.B.; Fischer, D.; Wang, W.X.; Freudenberg, U.; Rosser, A.; Werner, C. Injectable Glycosaminoglycan-Based Cryogels from Well-Defined Microscale Templates for Local Growth Factor Delivery. ACS Chem. Neurosci. 2021, 12, 1178–1188. [Google Scholar] [CrossRef]
- Zeng, Y.; Feng, S.; Liu, W.; Fu, Q.; Li, Y.; Li, X.; Chen, C.; Huang, C.; Ge, Z.; Du, Y. Preconditioning of mesenchymal stromal cells toward nucleus pulposus-like cells by microcryogels-based 3D cell culture and syringe-based pressure loading system. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 507–520. [Google Scholar] [CrossRef]
- Akbari, A.; Jafari, H.; Gohari, G.; Kheiri, G.; Mahdavinia, G.R. Fulvic acid-embedded poly (vinyl alcohol)-zinc oxide hydrogel nanocomposite: Synthesis, characterization, swelling and release kinetic. Int. Nano Lett. 2021, 11, 347–354. [Google Scholar] [CrossRef]
- Ambrogio, S.; Baesso, R.D.; Gomis, A.; Rivens, I.; ter Haar, G.; Zeqiri, B.; Ramnarine, K.V.; Fedele, F.; Miloro, P. A Polyvinyl Alcohol-Based Thermochromic Material for Ultrasound Therapy Phantoms. Ultrasound Med. Biol. 2020, 46, 3135–3144. [Google Scholar] [CrossRef]
- Baptista, J.G.C.; Rodrigues, S.P.J.; Matsushita, A.F.Y.; Vitorino, C.; Maria, T.M.R.; Burrows, H.D.; Pais, A.; Valente, A.J.M. Does poly(vinyl alcohol) act as an amphiphilic polymer? An interaction study with simvastatin. J. Mol. Liq. 2016, 222, 287–294. [Google Scholar] [CrossRef]
- Michurov, D.A.; Makhina, T.K.; Siracusa, V.; Bonartsev, A.P.; Lozinsky, V.I.; Iordanskii, A.L. Cryo-Structuring of Polymeric Systems. Poly(Vinyl Alcohol)-Based Cryogels Loaded with the Poly(3-hydroxybutyrate) Microbeads and the Evaluation of Such Composites as the Delivery Vehicles for Simvastatin. Polymers 2022, 14, 2196. [Google Scholar] [CrossRef]
- Rac, V.; Levic, S.; Balanc, B.; Olalde Graells, B.; Bijelic, G. PVA Cryogel as model hydrogel for iontophoretic transdermal drug delivery investigations. Comparison with PAA/PVA and PAA/PVP interpenetrating networks. Colloids Surf. B Biointerfaces 2019, 180, 441–448. [Google Scholar] [CrossRef]
- Solis, A.C.; Bento, D.; Nunes, S.; Valente, A.; Pais, A.; Vitorino, C. Rethinking transdermal drug delivery using PVA-NLC based films. Polymer 2021, 230, 13. [Google Scholar] [CrossRef]
- Wan, W.K.; Bannerman, A.D.; Yang, L.F.; Mak, H. Poly(Vinyl Alcohol) Cryogels for Biomedical Applications. In Polymeric Cryogels: Macroporous Gels with Remarkable Properties; Okay, O., Ed.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2014; Volume 263, pp. 283–321. [Google Scholar]
- Valente, A.J.M.; Cruz, S.M.A.; Moran, M.C.; Murtinho, D.B.; Muniz, E.C.; Miguel, M.G. Release of DNA from cryogel PVA-DNA membranes. Express. Polym. Lett. 2010, 4, 480–487. [Google Scholar] [CrossRef]
- Valente, A.J.M.; Cruz, S.M.A.; Murtinho, D.M.B.; Miguel, M.G.; Muniz, E.C. DNA-poly(vinyl alcohol) gel matrices: Release properties are strongly dependent on electrolytes and cationic surfactants. Colloids Surf. B Biointerfaces 2013, 101, 111–117. [Google Scholar] [CrossRef]
- Hajizadeh, S.; Xu, C.G.; Kirsebom, H.; Ye, L.; Mattiasson, B. Cryogelation of molecularly imprinted nanoparticles: A macroporous structure as affinity chromatography column for removal of beta-blockers from complex samples. J. Chromatogr. A 2013, 1274, 6–12. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Saini, R. Preparation and characterization of biocompatible spongy cryogels of poly(vinyl alcohol)-gelatin and study of water sorption behaviour. Polym. Int. 2005, 54, 1233–1242. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Saini, R. Preparation and characterization of novel biocompatible cryogels of poly (vinyl alcohol) and egg-albumin and their water sorption study. J. Mater. Sci. Mater. Med. 2006, 17, 49–61. [Google Scholar] [CrossRef]
- de Lima, G.G.; Campos, L.; Junqueira, A.; Devine, D.M.; Nugent, M.J.D. A novel pH-sensitive ceramic-hydrogel for biomedical applications. Polym. Adv. Technol. 2015, 26, 1439–1446. [Google Scholar] [CrossRef]
- Yilmaz, C.N.C.; Pamfil, D.; Vasile, C.; Bibire, N.; Lupusoru, R.V.; Zamfir, C.L.; Lupusoru, C.E. Toxicity, Biocompatibility, pH-Responsiveness and Methotrexate Release from PVA/Hyaluronic Acid Cryogels for Psoriasis Therapy. Polymers 2017, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Ipate, A.M.; Hamciuc, C.; Kalvachev, Y.; Gherman, S.; Ochiuz, L. New cryogels based on polymers and zeolite L for controlled Enalapril maleate release. J. Drug Deliv. Sci. Technol. 2018, 44, 505–512. [Google Scholar] [CrossRef]
- Nkhwa, S.; Kemal, E.; Gurav, N.; Deb, S. Dual polymer networks: A new strategy in expanding the repertoire of hydrogels for biomedical applications. J. Mater. Sci. Mater. Med. 2019, 30, 114. [Google Scholar] [CrossRef] [Green Version]
- de Lima, G.G.; de Souza, R.O.; Bozzi, A.D.; Poplawska, M.A.; Devine, D.M.; Nugent, M.J.D. Extraction Method Plays Critical Role in Antibacterial Activity of Propolis-Loaded Hydrogels. J. Pharm. Sci. 2016, 105, 1248–1257. [Google Scholar] [CrossRef]
- Bolgen, N.; Aguilar, M.R.; Fernandez, M.D.; Gonzalo-Flores, S.; Villar-Rodil, S.; Roman, J.S.; Piskin, E. Thermoresponsive biodegradable HEMA-Lactate-Dextran-co-NIPA cryogels for controlled release of simvastatin. Artif. Cells Nanomed. Biotechnol. 2015, 43, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Santos, T.; Brito, A.; Boto, R.; Sousa, P.; Almeida, P.; Cruz, C.; Tomaz, C. Influenza DNA vaccine purification using pHEMA cryogel support. Sep. Purif. Technol. 2018, 206, 192–198. [Google Scholar] [CrossRef]
- Cetin, K.; Denizli, A. 5-Fluorouracil delivery from metal-ion mediated molecularly imprinted cryogel discs. Colloids Surf. B Biointerfaces 2015, 126, 401–406. [Google Scholar] [CrossRef]
- Oncel, P.; Cetin, K.; Topcu, A.A.; Yavuz, H.; Denizli, A. Molecularly imprinted cryogel membranes for mitomycin C delivery. J. Biomater. Sci. Polym. Ed. 2017, 28, 519–531. [Google Scholar] [CrossRef]
- Zagni, C.; Dattilo, S.; Mecca, T.; Gugliuzzo, C.; Scamporrino, A.A.; Privitera, V.; Puglisi, R.; Carroccio, S.C. Single and dual polymeric sponges for emerging pollutants removal. Eur. Polym. J. 2022, 179, 12. [Google Scholar] [CrossRef]
- Park, S.C.; Sharma, G.; Kim, J.C. Temperature- and oxidation-dependent doxorubicin release from poly(hydroxyethyl acrylate-co-phenyl vinyl sulfide) cryogel. Colloid Polym. Sci. 2023, 301, 331–345. [Google Scholar] [CrossRef]
- Kostova, B.; Momekova, D.; Petrov, P.; Momekov, G.; Toncheva-Moncheva, N.; Tsvetanov, C.B.; Lambov, N. Poly(ethoxytriethyleneglycol acrylate) cryogels as novel sustained drug release systems for oral application. Polymer 2011, 52, 1217–1222. [Google Scholar] [CrossRef]
- Okten Besli, N.S.; Orakdogen, N. Charge-balanced terpolymer poly(diethylaminoethyl methacrylate-hydroxyethyl methacrylate-2-acrylamido-2-methyl-propanesulfonic acid) hydrogels and cryogels: Scaling parameters and correlation with composition. Soft Matter. 2020, 16, 10470–10487. [Google Scholar] [CrossRef]
- Deng, Z.X.; Guo, Y.; Ma, P.X.; Guo, B.L. Rapid thermal responsive conductive hybrid cryogels with shape memory properties, photothermal properties and pressure dependent conductivity. J. Colloid Interface Sci. 2018, 526, 281–294. [Google Scholar] [CrossRef]
- Hepokur, A.I.; Hepokur, C.; Kariper, I.A. Synthesis and Characterization of Macroporous Poly(Acrylamide-Methacrylic Acid) Cryogel and its Interaction with Clarithromycin. Lat. Am. J. Pharm. 2015, 34, 1842–1848. [Google Scholar]
- Hernandez, C.; Gawlik, N.; Goss, M.; Zhou, H.Y.; Jeganathan, S.; Gilbert, D.; Exner, A.A. Macroporous acrylamide phantoms improve prediction of in vivo performance of in situ forming implants. J. Control. Release 2016, 243, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Golunova, A.; Jaros, J.; Jurtikova, V.; Kotelnikov, I.; Kotek, J.; Hlidkova, H.; Streit, L.; Hampl, A.; Rypacek, F.; Proks, V. N-(2-Hydroxypropyl) Methacrylamide Based Cryogels—Synthesis and Biomimetic Modification for Stem Cell Applications. Physiol. Res. 2015, 64, S19–S27. [Google Scholar] [CrossRef]
- Jing, Z.; Jie, L.; Sunxiang, Q.; Haifeng, N.; Jie, F. Injectable zwitterionic cryogels for accurate and sustained chemoimmunotherapy. J. Mater. Chem. B 2023, 11, 2733–2744. [Google Scholar] [CrossRef]
- Sener, G.; Krebs, M.D. Zwitterionic cryogels for sustained release of proteins. RSC Adv. 2016, 6, 29608–29611. [Google Scholar] [CrossRef]
- Sener, G.; Hilton, S.A.; Osmond, M.J.; Zgheib, C.; Newsom, J.P.; Dewberry, L.; Singh, S.; Sakthivel, T.S.; Seal, S.; Liechty, K.W.; et al. Injectable, self-healable zwitterionic cryogels with sustained microRNA-cerium oxide nanoparticle release promote accelerated wound healing. Acta Biomater. 2020, 101, 262–272. [Google Scholar] [CrossRef]
- Ghatee, R.; Tolouei, A.; Fijalkowski, J.; Alsasa, A.; Hayes, J.; Besio, W.; Kennedy, S. Cryogel-Based Electronic-Tissue Interfaces with Soft, Highly Compressible, and Tunable Mechanics. Macromol. Mater. Eng. 2019, 304, 10. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V.; Ghiorghita, C.A.; Lazar, M.M.; Doroftei, F. Preparation and Characterization of Semi-IPN Cryogels Based on Polyacrylamide and Poly(N,N-dimethylaminoethyl methacrylate); Functionalization of Carrier with Monochlorotriazinyl-beta-cyclodextrin and Release Kinetics of Curcumin. Molecules 2021, 26, 21. [Google Scholar] [CrossRef]
- Reddy, R.M.; Srivastava, A.; Kumar, A. Monosaccharide-responsive phenylboronate-polyol cell scaffolds for cell sheet and tissue engineering applications. PLoS ONE 2013, 8, e77861. [Google Scholar] [CrossRef]
- Sengel, S.B.; Sahiner, N. Poly(vinyl phosphonic acid) nanogels with tailored properties and their use for biomedical and environmental applications. Eur. Polym. J. 2016, 75, 264–275. [Google Scholar] [CrossRef]
- Dragan, E.S.; Lazar, M.M.; Dinu, M.V.; Doroftei, F. Macroporous composite IPN hydrogels based on poly(acrylamide) and chitosan with tuned swelling and sorption of cationic dyes. Chem. Eng. J. 2012, 204, 198–209. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V. Interpenetrating Polymer Network Composite Cryogels with Tailored Porous Morphology and Sorption Properties. In Affinity Chromatography: Methods and Protocols, 3rd ed.; Reichelt, S., Ed.; Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2015; Volume 1286, pp. 239–252. [Google Scholar]
- Dragan, E.S.; Cocarta, A.I. Smart Macroporous IPN Hydrogels Responsive to pH, Temperature, and Ionic Strength: Synthesis, Characterization, and Evaluation of Controlled Release of Drugs. ACS Appl. Mater. Interfaces 2016, 8, 12018–12030. [Google Scholar] [CrossRef]
- Jiang, P.J.; Wynn-Jones, G.; Grover, L.M. A calcium phosphate cryogel for alkaline phosphatase encapsulation. J. Mater. Sci. 2010, 45, 5257–5263. [Google Scholar] [CrossRef]
- Abdelmonem, A.M.; Zambo, D.; Rusch, P.; Schlosser, A.; Klepzig, L.F.; Bigall, N.C. Versatile Route for Multifunctional Aerogels Including Flaxseed Mucilage and Nanocrystals. Macromol. Rapid Commun. 2022, 43, e2100794. [Google Scholar] [CrossRef]
- Chambre, L.; Rosselle, L.; Barras, A.; Aydin, D.; Loczechin, A.; Gunbay, S.; Sanyal, R.; Skandrani, N.; Metzler-Nolte, N.; Bandow, J.E.; et al. Photothermally Active Cryogel Devices for Effective Release of Antimicrobial Peptides: On-Demand Treatment of Infections. ACS Appl. Mater. Interfaces 2020, 12, 56805–56814. [Google Scholar] [CrossRef]
- Dolak, I.; Kecili, R.; Yilmaz, F.; Ersoz, A.; Say, R. Selective Recognition and Separation of Ubiquitin by Nanoparticle Embedded Cryogel Traps with Ubiquitin Memories Based on Photosensitive Covalent Imprinting. J. Anal. Chem. 2021, 76, 165–171. [Google Scholar] [CrossRef]
- Radhouani, H.; Bicho, D.; Goncalves, C.; Maia, F.R.; Reis, R.L.; Oliveira, J.M. Kefiran cryogels as potential scaffolds for drug delivery and tissue engineering applications. Mater. Today Commun. 2019, 20, 6. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Feng, S.; Ning, J.; Wang, J.; Gou, M.; Chen, H.; Xu, F.; Du, Y. Magnetically controllable 3D microtissues based on magnetic microcryogels. Lab Chip 2014, 14, 2614–2625. [Google Scholar] [CrossRef]
- Qayoom, I.; Srivastava, E.; Kumar, A. Anti-infective composite cryogel scaffold treats osteomyelitis and augments bone healing in rat femoral condyle. Biomater. Adv. 2022, 142, 213133. [Google Scholar] [CrossRef]
- Abdullah, T.; Su, E.S.; Memic, A. Designing Silk-Based Cryogels for Biomedical Applications. Biomimetics 2023, 8, 16. [Google Scholar] [CrossRef]
- Georgiev, G.L.; Trzebicka, B.; Kostova, B.; Petrov, P.D. Super-macroporous dextran cryogels via UV-induced crosslinking: Synthesis and characterization. Polym. Int. 2017, 66, 1306–1311. [Google Scholar] [CrossRef]
- Slavkova, M.I.; Momekova, D.B.; Kostova, B.D.; Momekov, G.T.; Petrov, P.D. Novel dextran/beta-cyclodextrin and dextran macroporous cryogels for topical delivery of curcumin in the treatment of cutaneous T-cell lymphoma. Bulg. Chem. Commun. 2017, 49, 792–799. [Google Scholar]
- He, T.F.; Li, B.T.; Colombani, T.; Joshi-Navare, K.; Mehta, S.; Kisiday, J.; Bencherif, S.A.; Bajpayee, A.G. Hyaluronic Acid-Based Shape-Memory Cryogel Scaffolds for Focal Cartilage Defect Repair. Tissue Eng. Part A 2021, 27, 748–760. [Google Scholar] [CrossRef]
- How, K.N.; Yap, W.H.; Lim, C.L.H.; Goh, B.H.; Lai, Z.W. Hyaluronic Acid-Mediated Drug Delivery System Targeting for Inflammatory Skin Diseases: A Mini Review. Front. Pharmacol. 2020, 11, 1105. [Google Scholar] [CrossRef]
- Sa’adon, S.; Ansari, M.N.M.; Razak, S.I.A.; Anand, J.S.; Nayan, N.H.M.; Ismail, A.E.; Khan, M.U.A.; Haider, A. Preparation and Physicochemical Characterization of a Diclofenac Sodium-Dual Layer Polyvinyl Alcohol Patch. Polymers 2021, 13, 2459. [Google Scholar] [CrossRef]
- Pacelli, S.; Di Muzio, L.; Paolicelli, P.; Fortunati, V.; Petralito, S.; Trilli, J.; Casadei, M.A. Dextran-polyethylene glycol cryogels as spongy scaffolds for drug delivery. Int. J. Biol. Macromol. 2021, 166, 1292–1300. [Google Scholar] [CrossRef]
- Getangama, N.N.; de Bruyn, J.R.; Hutter, J.L. Dielectric properties of PVA cryogels prepared by freeze-thaw cycling. J. Chem. Phys. 2020, 153, 044901. [Google Scholar] [CrossRef]
- Gorska, A.; Krupa, A.; Majda, D.; Kulinowski, P.; Kurek, M.; Weglarz, W.P.; Jachowicz, R. Poly(Vinyl Alcohol) Cryogel Membranes Loaded with Resveratrol as Potential Active Wound Dressings. AAPS PharmSciTech 2021, 22, 14. [Google Scholar] [CrossRef]
- Hajizadeh, S.; Kirsebom, H.; Leistner, A.; Mattiasson, B. Composite cryogel with immobilized concanavalin A for affinity chromatography of glycoproteins. J. Sep. Sci. 2012, 35, 2978–2985. [Google Scholar] [CrossRef]
- Martinez, Y.N.; Pinuel, L.; Castro, G.R.; Breccia, J.D. Polyvinyl alcohol-pectin cryogel films for controlled release of enrofloxacin. Appl. Biochem. Biotechnol. 2012, 167, 1421–1429. [Google Scholar] [CrossRef]
- Michurov, D.A.; Kolosova, O.Y.; Lozinsky, V.I. Cryostructuring of Polymeric Systems. 61. Physicochemical Properties of Poly(vinyl alcohol) Cryogels Prepared on the Basis of Urea-Containing DMSO-Solutions of the Polymer and Evaluation of the Resultant Gel Materials as Potential Drug Carriers. Bull. Univ. Karaganda Chem. 2022, 107, 75–86. [Google Scholar] [CrossRef]
- Perera, A.S.; Jackson, R.J.; Bristow, R.M.D.; White, C.A. Magnetic cryogels as a shape-selective and customizable platform for hyperthermia-mediated drug delivery. Sci. Rep. 2022, 12, 9654. [Google Scholar] [CrossRef]
- Sa’adon, S.; Ansari, M.N.M.; Razak, S.I.A.; Yusof, A.H.M.; Faudzi, A.A.M.; Sagadevan, S.; Nayan, N.H.M.; Anand, J.S.; Amin, K.A.M. Electrospun Nanofiber and Cryogel of Polyvinyl Alcohol Transdermal Patch Containing Diclofenac Sodium: Preparation, Characterization and In Vitro Release Studies. Pharmaceutics 2021, 13, 1900. [Google Scholar] [CrossRef]
- Sarkaya, K.; Alli, A. Synthesis and characterization of cryogels of p(HEMA-N-vinylformamide) and p(HEMA-N-Vinylpyrrolidone) for chemical release behaviour. J. Porous Mater. 2021, 28, 853–865. [Google Scholar] [CrossRef]
- Gao, C.P.; Wang, Y.A.; Shi, J.S.; Wang, Y.Y.; Huang, X.L.; Chen, X.L.; Chen, Z.Y.; Xie, Y.F.; Yang, Y.Z. Superamphiphilic Chitosan Cryogels for Continuous Flow Separation of Oil-In-Water Emulsions. ACS Omega 2022, 7, 5937–5945. [Google Scholar] [CrossRef]
- Nakagawa, K.; Sowasod, N.; Tanthapanichakoon, W.; Charinpanitkul, T. Hydrogel based oil encapsulation for controlled release of curcumin by using a ternary system of chitosan, kappa-carrageenan, and carboxymethylcellulose sodium salt. LWT Food Sci. Technol. 2013, 54, 600–605. [Google Scholar] [CrossRef]
- Pencheva, V.; Margaritova, E.; Borinarova, M.; Slavkova, M.; Momekova, D.; Petrov, P.D. A novel approach for fabricating nanocomposite materials by embedding stabilized core-shell micelles into polysaccharide cryogel matrix. Carbohydr. Polym. 2018, 183, 165–172. [Google Scholar] [CrossRef]
- Boccia, A.C.; Scavia, G.; Schizzi, I.; Conzatti, L. Biobased Cryogels from Enzymatically Oxidized Starch: Functionalized Materials as Carriers of Active Molecules. Molecules 2020, 25, 2557. [Google Scholar] [CrossRef]
- Loghin, D.F.A.; Biliuta, G.; Coseri, S.; Dragan, E.S. Preparation and characterization of oxidized starch/poly(N,N-dimethylaminoethyl methacrylate) semi-IPN cryogels and in vitro controlled release evaluation of indomethacin. Int. J. Biol. Macromol. 2017, 96, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, H. Superelastic and pH-Responsive Degradable Dendrimer Cryogels Prepared by Cryo-aza-Michael Addition Reaction. Sci. Rep. 2018, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Niknia, N.; Kadkhodaee, R. Gum tragacanth-polyvinyl alcohol cryogel and xerogel blends for oral delivery of silymarin: Structural characterization and mucoadhesive property. Carbohydr. Polym. 2017, 177, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Kennedy, J.E.; Higginbotham, C.L. Characterisation of a Two-Phase Hydrogel System for a Potential Wound Healing Application; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2010; pp. 151–170. [Google Scholar]
- Chang, P.C.; Chang, H.C.; Lin, T.C.; Tai, W.C. Preclinical alveolar ridge preservation using small-sized particles of bone replacement graft in combination with a gelatin cryogel scaffold. J. Periodontol. 2018, 89, 1221–1229. [Google Scholar] [CrossRef]
- Bioactive Smart Dressings for Diabetic Foot Ulcers: Randomized Controlled Trial; Clinical Trials.gov ID: NCT05671250. 2023. Available online: https://beta.clinicaltrials.gov/study/NCT05671250 (accessed on 20 June 2023).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omidian, H.; Dey Chowdhury, S.; Babanejad, N. Cryogels: Advancing Biomaterials for Transformative Biomedical Applications. Pharmaceutics 2023, 15, 1836. https://doi.org/10.3390/pharmaceutics15071836
Omidian H, Dey Chowdhury S, Babanejad N. Cryogels: Advancing Biomaterials for Transformative Biomedical Applications. Pharmaceutics. 2023; 15(7):1836. https://doi.org/10.3390/pharmaceutics15071836
Chicago/Turabian StyleOmidian, Hossein, Sumana Dey Chowdhury, and Niloofar Babanejad. 2023. "Cryogels: Advancing Biomaterials for Transformative Biomedical Applications" Pharmaceutics 15, no. 7: 1836. https://doi.org/10.3390/pharmaceutics15071836
APA StyleOmidian, H., Dey Chowdhury, S., & Babanejad, N. (2023). Cryogels: Advancing Biomaterials for Transformative Biomedical Applications. Pharmaceutics, 15(7), 1836. https://doi.org/10.3390/pharmaceutics15071836







