pH-Responsive Biomaterials for the Treatment of Dental Caries—A Focussed and Critical Review
Abstract
1. Introduction
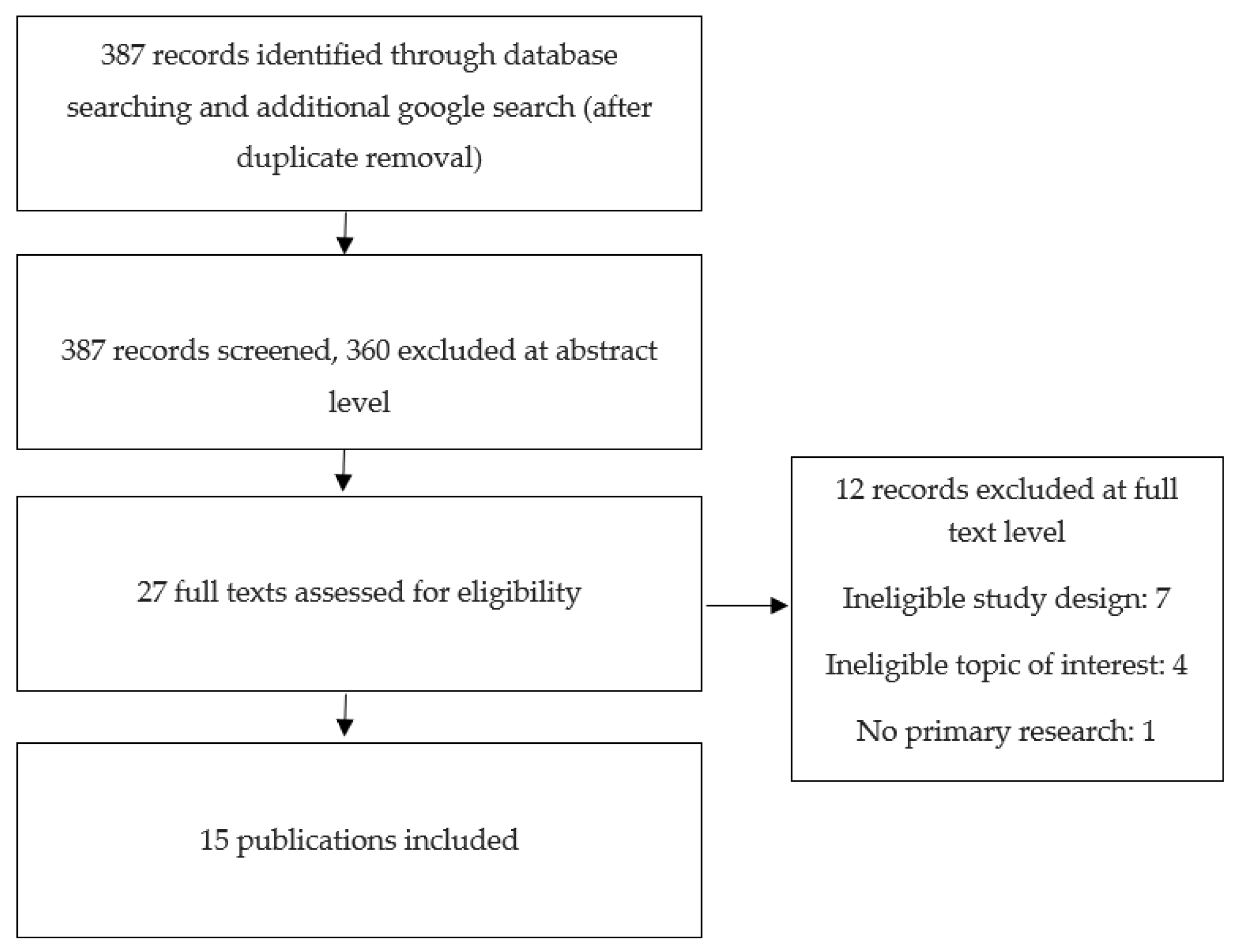
2. Search Strategy
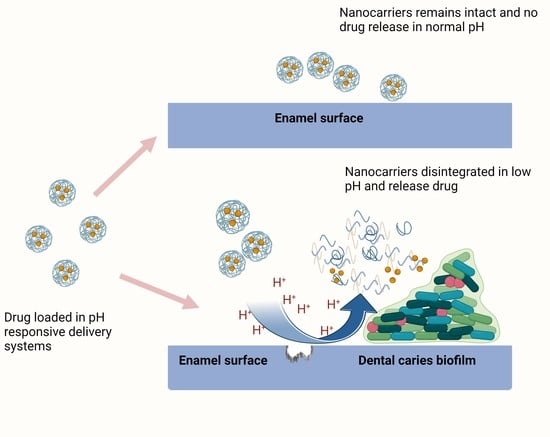
3. Nanoparticle Formulation and pH-Responsive Release Mechanism
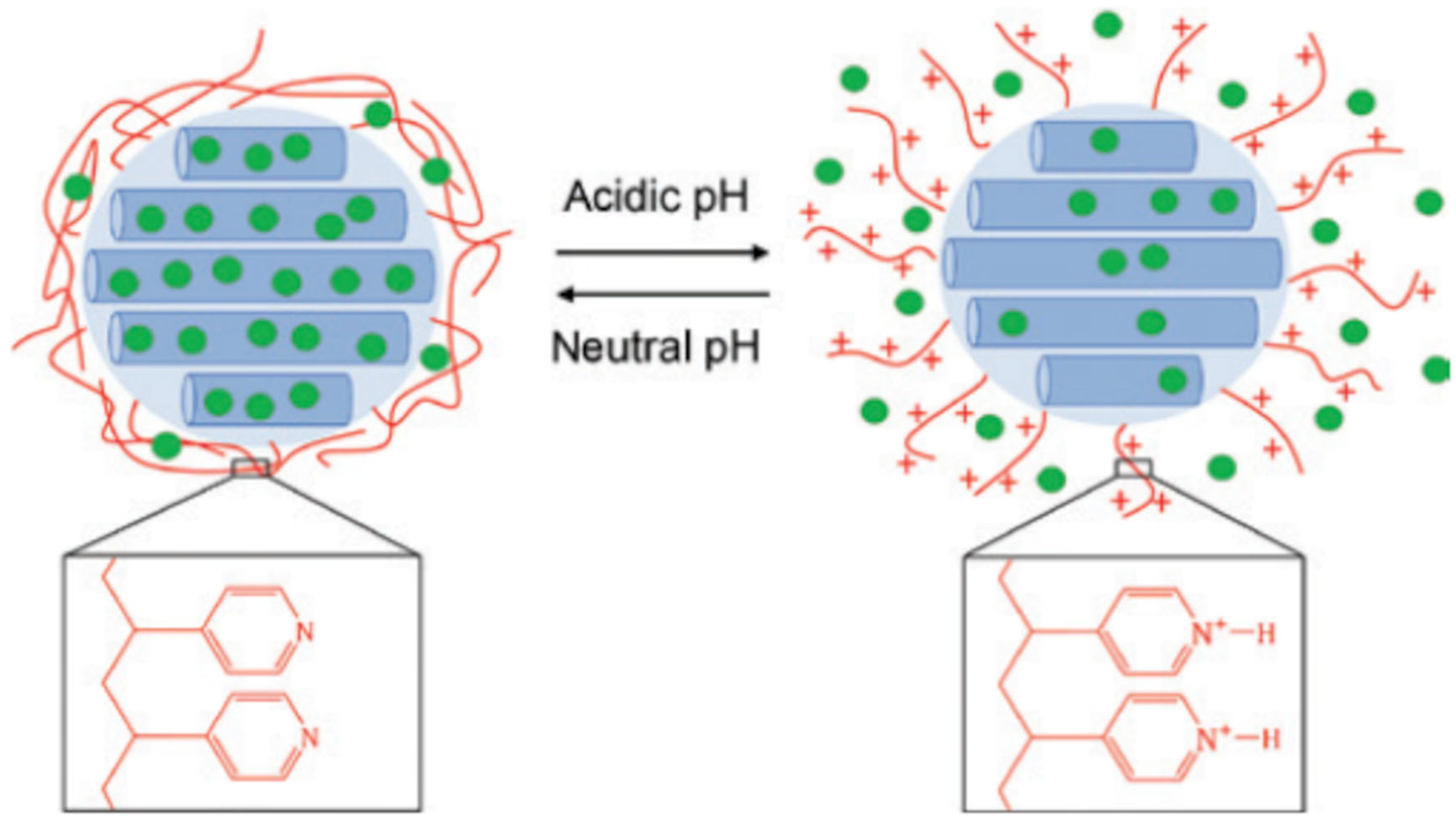
3.1. DMAEMA
3.2. Polyethylene Glycol (PEG)
3.3. Chitosan
3.4. Mesoporous silica Nanoparticles
3.5. Tertiary Amine Modified Restorative Resin
4. Innovation in Binding to Tooth Surfaces and Extracellular Matrix
5. Lessons from Other Fields That Could Be Adopted in Dentistry
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schwendicke, F.; Dörfer, C.; Schlattmann, P.; Page, L.F.; Thomson, W.; Paris, S. Socioeconomic inequality and caries: A systematic review and meta-analysis. J. Dent. Res. 2015, 94, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.E.; Bourgeois, D.; Ogawa, H.; Estupinan-Day, S.; Ndiaye, C. The global burden of oral diseases and risks to oral health. Bull. World Health Organ. 2005, 83, 661–669. [Google Scholar]
- Bagramian, R.A.; Garcia-Godoy, F.; Volpe, A.R. The global increase in dental caries. A pending public health crisis. Am. J. Dent. 2009, 22, 3–8. [Google Scholar]
- Frencken, J.E.; Sharma, P.; Stenhouse, L.; Green, D.; Laverty, D.; Dietrich, T. Global epidemiology of dental caries and severe periodontitis–a comprehensive review. J. Clin. Periodontol. 2017, 44, S94–S105. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [PubMed]
- Dashper, S.; Reynolds, E. pH regulation by Streptococcus mutans. J. Dent. Res. 1992, 71, 1159–1165. [Google Scholar] [CrossRef]
- Jacobson, G.R.; Lodge, J.; Poy, F. Carbohydrate uptake in the oral pathogen Streptococcus mutans: Mechanisms and regulation by protein phosphorylation. Biochimie 1989, 71, 997–1004. [Google Scholar] [CrossRef]
- Aqawi, M.; Sionov, R.V.; Gallily, R.; Friedman, M.; Steinberg, D. Anti-bacterial properties of cannabigerol toward Streptococcus mutans. Front. Microbiol. 2021, 12, 922. [Google Scholar] [CrossRef]
- Jakubovics, N.S. Intermicrobial interactions as a driver for community composition and stratification of oral biofilms. J. Mol. Biol. 2015, 427, 3662–3675. [Google Scholar] [CrossRef]
- Simón-Soro, A.; Belda-Ferre, P.; Cabrera-Rubio, R.; Alcaraz, L.; Mira, A. A tissue-dependent hypothesis of dental caries. Caries Res. 2013, 47, 591–600. [Google Scholar] [CrossRef]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G. Dental caries. Nat. Rev. Dis. Prim. 2017, 3, 17030. [Google Scholar] [CrossRef]
- Nicolae, V.; Neamtu, B.; Picu, O.; Stefanache, M.A.M.; Cioranu, V.S.I. The comparative evaluation of salivary biomarkers (Calcium, Phosphate, Salivary pH) in cario-resistance versus cario-activity. Rev. Chim. 2016, 67, 821–824. [Google Scholar]
- Gilbert, P.; Moore, L. Cationic antiseptics: Diversity of action under a common epithet. J. Appl. Microbiol. 2005, 99, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Zhao, I.S.; Duffin, S.; Duangthip, D.; Lo, E.C.M.; Chu, C.H. Revitalising silver nitrate for caries management. Int. J. Environ. Res. Public. Health 2018, 15, 80. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.H.; Cunha-Cruz, J.; Rajendra, A.; Niederman, R. Controlling caries in exposed root surfaces with silver diamine fluoride: A systematic review with meta-analysis. J. Am. Dent. Assoc. 2018, 149, 671–679. [Google Scholar] [CrossRef]
- Banerjee, A.; Frencken, J.; Schwendicke, F.; Innes, N. Contemporary operative caries management: Consensus recommendations on minimally invasive caries removal. Br. Dent. J. 2017, 223, 215–222. [Google Scholar] [CrossRef]
- Yu, O.Y.; Lam, W.Y.-H.; Wong, A.W.-Y.; Duangthip, D.; Chu, C.-H. Nonrestorative management of dental caries. Dent. J. 2021, 9, 121. [Google Scholar] [CrossRef]
- Weintraub, J.A.; Professor, L.H. Fluoride varnish for caries prevention: Comparisons with other preventive agents and recommendations for a community-based protocol. Spec. Care Dentist. 2003, 23, 180–186. [Google Scholar] [CrossRef]
- Bader, J.D.; Shugars, D.A.; Bonito, A.J. Systematic reviews of selected dental caries diagnostic and management methods. J. Dent. Educ. 2001, 65, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Surendranath, P.; Krishnappa, S.; Srinath, S. Silver Diamine Fluoride in Preventing Caries: A Review of Current Trends. Int. J. Clin. Pediatr. Dent. 2022, 15, S247. [Google Scholar] [CrossRef]
- Walsh, T.; Oliveira-Neto, J.M.; Moore, D. Chlorhexidine treatment for the prevention of dental caries in children and adolescents. Cochrane Database Syst. Rev. 2015, 4, CD008457. [Google Scholar] [CrossRef] [PubMed]
- Cabalén, M.B.; Molina, G.F.; Bono, A.; Burrow, M.F. Nonrestorative Caries Treatment: A Systematic Review Update. Int. Dent. J. 2022, 72, 746–764. [Google Scholar] [CrossRef] [PubMed]
- Balamurali, V.; Pramodkuma, T.; Srujana, N.; Venkatesh, M.; Gupta, N.V.; Krishna, K.; Gangadhara, H. pH sensitive drug delivery systems: A review. Am. J. Drug. Discov. Dev. 2011, 1, 24–48. [Google Scholar] [CrossRef]
- Li, X.; Zheng, B.-Y.; Ke, M.-R.; Zhang, Y.; Huang, J.-D.; Yoon, J. A tumor-pH-responsive supramolecular photosensitizer for activatable photodynamic therapy with minimal in vivo skin phototoxicity. Theranostics 2017, 7, 2746. [Google Scholar] [CrossRef] [PubMed]
- Baliga, S.; Muglikar, S.; Kale, R. Salivary pH: A diagnostic biomarker. J. Indian. Soc. Periodontol. 2013, 17, 461. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Wang, Z.; Deng, X.; Ding, J.; Zhou, W.; Zheng, X.; Tang, G. Mechanisms of drug release in pH-sensitive micelles for tumour targeted drug delivery system: A review. Int. J. Pharm. 2018, 535, 253–260. [Google Scholar] [CrossRef]
- van de Wetering, P.; Moret, E.E.; Schuurmans-Nieuwenbroek, N.M.E.; van Steenbergen, M.J.; Hennink, W.E. Structure− activity relationships of water-soluble cationic methacrylate/methacrylamide polymers for nonviral gene delivery. Bioconjug Chem. 1999, 10, 589–597. [Google Scholar] [CrossRef]
- Brahim, S.; Narinesingh, D.; Guiseppi-Elie, A. Synthesis and hydration properties of pH-sensitive p (HEMA)-based hydrogels containing 3-(trimethoxysilyl) propyl methacrylate. Biomacromolecules 2003, 4, 497–503. [Google Scholar] [CrossRef]
- Horev, B.; Klein, M.I.; Hwang, G.; Li, Y.; Kim, D.; Koo, H.; Benoit, D.S. pH-activated nanoparticles for controlled topical delivery of farnesol to disrupt oral biofilm virulence. ACS Nano. 2015, 9, 2390–2404. [Google Scholar] [CrossRef]
- Zhou, J.; Horev, B.; Hwang, G.; Klein, M.I.; Koo, H.; Benoit, D.S. Characterization and optimization of pH-responsive polymer nanoparticles for drug delivery to oral biofilms. J. Mater. Chem. B 2016, 4, 3075–3085. [Google Scholar] [CrossRef]
- Jones, C.G. Chlorhexidine: Is it still the gold standard? Periodontology 2000 1997, 15, 55–62. [Google Scholar] [CrossRef]
- Pucher, J.J.; Daniel, C. The effects of chlorhexidine digluconate on human fibroblasts in vitro. J. Periodontol. 1992, 63, 526–532. [Google Scholar] [CrossRef]
- Babich, H.; Wurzburger, B.; Rubin, Y.; Sinensky, M.; Blau, L. An in vitro study on the cytotoxicity of chlorhexidine digluconate to human gingival cells. Cell. Biol. Toxicol. 1995, 11, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Flötra, L.; Gjermo, P.; Rölla, G.; Waerhaug, J. Side effects of chlorhexidine mouth washes. Eur. J. Oral. Sci. 1971, 79, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Han, Q.; Zhou, X.; Chen, Y.; Huang, X.; Guo, X.; Peng, R.; Wang, H.; Peng, X.; Cheng, L. Effect of pH-sensitive nanoparticles on inhibiting oral biofilms. Drug Deliv. 2022, 29, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly (ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Verhoef, J.J.; Anchordoquy, T.J. Questioning the use of PEGylation for drug delivery. Drug Deliv. Transl. Res. 2013, 3, 499–503. [Google Scholar] [CrossRef]
- Harris, J.M.; Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug. Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Osada, K.; Yamasaki, Y.; Katayose, S.; Kataoka, K. A synthetic block copolymer regulates S1 nuclease fragmentation of supercoiled plasmid DNA. Angew. Chem. Int. Ed. 2005, 44, 3544–3548. [Google Scholar] [CrossRef]
- Gref, R.; Domb, A.; Quellec, P.; Blunk, T.; Müller, R.H.; Verbavatz, J.M.; Langer, R. The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Adv. Drug. Deliv. Rev. 1995, 16, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ding, C.; Wang, Y.; Tan, H.; Li, J. pH-Responsive polymeric nanocarriers for efficient killing of cariogenic bacteria in biofilms. Biomater. Sci. 2019, 7, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Deirram, N.; Zhang, C.; Kermaniyan, S.S.; Johnston, A.P.; Such, G.K. pH-responsive polymer nanoparticles for drug delivery. Macromol. Rapid Commun. 2019, 40, 1800917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yu, Z.; Lo, E.C.M. A new pH-responsive nano micelle for enhancing the effect of a hydrophobic bactericidal agent on mature Streptococcus mutans biofilm. Front. Microbiol. 2021, 12, 761583. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; You, Y.; Yi, L.; Wu, X.; Zhao, Y.; Yu, J.; Liu, H.; Shen, Y.; Guo, J.; Huang, C. Dental plaque-inspired versatile nanosystem for caries prevention and tooth restoration. Bioact. Mater. 2023, 20, 418–433. [Google Scholar] [CrossRef]
- Min, K.H.; Jang, E.-Y.; Lee, H.J.; Hwang, Y.-S.; Ryu, J.-I.; Moon, J.-H.; Lee, S.C. pH-Responsive mineralized nanoparticles for bacteria-triggered topical release of antibiotics. J. Ind. Eng. Chem. 2019, 71, 210–219. [Google Scholar] [CrossRef]
- Yi, Y.; Wang, L.; Chen, L.; Lin, Y.; Luo, Z.; Chen, Z.; Li, T.; Wu, J.; Zhong, Z. Farnesal-loaded pH-sensitive polymeric micelles provided effective prevention and treatment on dental caries. J. Nanobiotechnol. 2020, 18, 89. [Google Scholar] [CrossRef]
- Koo, H.; Schobel, B.; Scott-Anne, K.; Watson, G.; Bowen, W.H.; Cury, J.A.; Rosalen, P.L.; Park, Y.K. Apigenin and tt-farnesol with fluoride effects on S. mutans biofilms and dental caries. J. Dent. Res. 2005, 84, 1016–1020. [Google Scholar] [CrossRef]
- Jeon, J.G.; Pandit, S.; Xiao, J.; Gregoire, S.; Falsetta, M.L.; Klein, M.I.; Koo, H. Influences of trans-trans farnesol, a membrane-targeting sesquiterpenoid, on Streptococcus mutans physiology and survival within mixed-species oral biofilms. Int. J. Oral Sci. 2011, 3, 98–106. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H.G. Chitosan and its antimicrobial potential–a critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef]
- Souza, M.P.; Vaz, A.F.; Correia, M.T.; Cerqueira, M.A.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Quercetin-loaded lecithin/chitosan nanoparticles for functional food applications. Food Bioprocess Technol. 2014, 7, 1149–1159. [Google Scholar] [CrossRef]
- Bugnicourt, L.; Ladavière, C. Interests of chitosan nanoparticles ionically cross-linked with tripolyphosphate for biomedical applications. Prog. Polym. Sci. 2016, 60, 1–17. [Google Scholar] [CrossRef]
- Olivera, S.; Muralidhara, H.B.; Venkatesh, K.; Guna, V.K.; Gopalakrishna, K.; Kumar, Y. Potential applications of cellulose and chitosan nanoparticles/composites in wastewater treatment: A review. Carbohydr. Polym. 2016, 153, 600–618. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, M.; Rodríguez-Berna, G.; Gonzalez-Alvarez, I.; Hernández, M.J.; Corma, A.; Bermejo, M.; Merino, V.; Gonzalez-Alvarez, M. Ionic hydrogel based on chitosan cross-linked with 6-phosphogluconic trisodium salt as a drug delivery system. Biomacromolecules 2018, 19, 1294–1304. [Google Scholar] [CrossRef]
- Yan, Q.; Chen, X.; Gong, H.; Qiu, P.; Xiao, X.; Dang, S.; Hong, A.; Ma, Y. Delivery of a TNF-α–derived peptide by nanoparticles enhances its antitumor activity by inducing cell-cycle arrest and caspase-dependent apoptosis. FASEB J. 2018, 32, 6948–6964. [Google Scholar] [CrossRef]
- Xiao, B.; Chen, Q.; Zhang, Z.; Wang, L.; Kang, Y.; Denning, T.; Merlin, D. TNFα gene silencing mediated by orally targeted nanoparticles combined with interleukin-22 for synergistic combination therapy of ulcerative colitis. J. Control. Release 2018, 287, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Escudero, C.; Sediqi, N.; Smistad, G.; Hiorth, M. Fluoride loaded polymeric nanoparticles for dental delivery. Eur. J. Pharm. Sci. 2017, 104, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Marin, L.M.; Xiao, Y.; Gillies, E.R.; Siqueira, W.L. Ph-sensitive chitosan nanoparticles for salivary protein delivery. Nanomaterials 2021, 11, 1028. [Google Scholar] [CrossRef]
- Tao, Z.; Toms, B.; Goodisman, J.; Asefa, T. Mesoporous silica microparticles enhance the cytotoxicity of anticancer platinum drugs. ACS Nano. 2010, 4, 789–794. [Google Scholar] [CrossRef]
- Kwon, S.; Singh, R.K.; Perez, R.A.; Abou Neel, E.A.; Kim, H.-W.; Chrzanowski, W. Silica-based mesoporous nanoparticles for controlled drug delivery. J. Tissue Eng. 2013, 4, 2041731413503357. [Google Scholar] [CrossRef]
- Tao, Z. Mesoporous silica-based nanodevices for biological applications. RSC Adv. 2014, 4, 18961–18980. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous materials for drug delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef]
- Natarajan, S.K.; Selvaraj, S. Mesoporous silica nanoparticles: Importance of surface modifications and its role in drug delivery. RSC Adv. 2014, 4, 14328–14334. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124. [Google Scholar] [CrossRef] [PubMed]
- Kamarudin, N.; Jalil, A.; Triwahyono, S.; Salleh, N.; Karim, A.; Mukti, R.; Hameed, B.; Ahmad, A. Role of 3-aminopropyltriethoxysilane in the preparation of mesoporous silica nanoparticles for ibuprofen delivery: Effect on physicochemical properties. Microporous Mesoporous Mater. 2013, 180, 235–241. [Google Scholar] [CrossRef]
- Howarter, J.A.; Youngblood, J.P. Optimization of silica silanization by 3-aminopropyltriethoxysilane. Langmuir 2006, 22, 11142–11147. [Google Scholar] [CrossRef]
- Wang, M.; Wang, T.; Wang, D.; Jiang, W.; Fu, J. Acid and light stimuli-responsive mesoporous silica nanoparticles for controlled release. J. Mater. Sci. 2019, 54, 6199–6211. [Google Scholar] [CrossRef]
- Jin, X.; Wang, Q.; Sun, J.; Panezai, H.; Bai, S.; Wu, X. Dual (pH-and temperature-) stimuli responsive nanocarrier with bimodal mesoporous silica nanoparticles core and copolymer shell for controlled ibuprofen-releasing: Fractal feature and diffusion mechanism. Microporous Mesoporous Mater. 2017, 254, 77–85. [Google Scholar] [CrossRef]
- Anirudhan, T.; Nair, A.S. Temperature and ultrasound sensitive gatekeepers for the controlled release of chemotherapeutic drugs from mesoporous silica nanoparticles. J. Mater. Chem. B 2018, 6, 428–439. [Google Scholar] [CrossRef]
- Wang, P.; Chen, S.; Cao, Z.; Wang, G. NIR light-, temperature-, pH-, and redox-responsive polymer-modified reduced graphene oxide/mesoporous silica sandwich-like nanocomposites for controlled release. ACS Appl. Mater. Interfaces 2017, 9, 29055–29062. [Google Scholar] [CrossRef]
- Akram, Z.; Aati, S.; Ngo, H.; Fawzy, A. pH-dependent delivery of chlorhexidine from PGA grafted mesoporous silica nanoparticles at resin-dentin interface. J. Nanobiotechnology 2021, 19, 43. [Google Scholar] [CrossRef] [PubMed]
- Ayyanaar, S.; Kesavan, M.P.; Sivaraman, G.; Maddiboyina, B.; Annaraj, J.; Rajesh, J.; Rajagopal, G. A novel curcumin-loaded PLGA micromagnetic composite system for controlled and pH-responsive drug delivery. Colloids Surf. A Physicochem. Eng. Asp. 2019, 573, 188–195. [Google Scholar] [CrossRef]
- Fullriede, H.; Abendroth, P.; Ehlert, N.; Doll, K.; Schäske, J.; Winkel, A.; Stumpp, S.N.; Stiesch, M.; Behrens, P. pH-responsive release of chlorhexidine from modified nanoporous silica nanoparticles for dental applications. BioNanoMaterials 2016, 17, 59–72. [Google Scholar] [CrossRef]
- Lu, M.M.; Ge, Y.; Qiu, J.; Shao, D.; Zhang, Y.; Bai, J.; Zheng, X.; Chang, Z.M.; Wang, Z.; Dong, W.F.; et al. Redox/pH dual-controlled release of chlorhexidine and silver ions from biodegradable mesoporous silica nanoparticles against oral biofilms. Int. J. Nanomed. 2018, 13, 7697. [Google Scholar] [CrossRef]
- Bernardo, M.; Luis, H.; Martin, M.D.; Leroux, B.G.; Rue, T.; Leitão, J.; DeRouen, T.A. Survival and reasons for failure of amalgam versus composite posterior restorations placed in a randomized clinical trial. J. Am. Dent. Assoc. 2007, 138, 775–783. [Google Scholar] [CrossRef]
- Soncini, J.A.; Maserejian, N.N.; Trachtenberg, F.; Tavares, M.; Hayes, C. The longevity of amalgam versus compomer/composite restorations in posterior primary and permanent teeth: Findings from the New England Children’s Amalgam Trial. J. Am. Dent. Assoc. 2007, 138, 763–772. [Google Scholar] [CrossRef]
- Heintze, S.; Forjanic, M.; Ohmiti, K.; Rousson, V. Surface deterioration of dental materials after simulated toothbrushing in relation to brushing time and load. Dent. Mater. 2010, 26, 306–319. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Barbosa, R.P.; Pereira-Cenci, T.; da Silva, W.M.; Coelho-de-Souza, F.H.; Demarco, F.F.; Cenci, M.S. Effect of cariogenic biofilm challenge on the surface hardness of direct restorative materials in situ. J. Dent. 2012, 40, 359–363. [Google Scholar] [CrossRef]
- Khalichi, P.; Singh, J.; Cvitkovitch, D.G.; Santerre, J.P. The influence of triethylene glycol derived from dental composite resins on the regulation of Streptococcus mutans gene expression. Biomaterials 2009, 30, 452–459. [Google Scholar] [CrossRef]
- Tărăboanță, I.; Stoleriu, S.; Nica, I.; Georgescu, A.; Gamen, A.C.; Maftei, G.A.; Andrian, S. Roughness Variation of a Nanohybrid Composite Resin Submitted to Acid and Abrasive Challenges. Int. J. Med. Dent. 2020, 24, 182–187. [Google Scholar]
- Fielding, L.A.; Edmondson, S.; Armes, S.P. Synthesis of pH-responsive tertiary amine methacrylate polymer brushes and their response to acidic vapour. J. Mater. Chem. 2011, 21, 11773–11780. [Google Scholar] [CrossRef]
- Jessop, P.G.; Kozycz, L.; Rahami, Z.G.; Schoenmakers, D.; Boyd, A.R.; Wechsler, D.; Holland, A.M. Tertiary amine solvents having switchable hydrophilicity. Green Chem. 2011, 13, 619–623. [Google Scholar] [CrossRef]
- Liang, J.; Liu, F.; Zou, J.; Xu, H.H.K.; Han, Q.; Wang, Z.; Li, B.; Yang, B.; Ren, B.; Li, M.; et al. pH-responsive antibacterial resin adhesives for secondary caries inhibition. J. Dent. Res. 2020, 99, 1368–1376. [Google Scholar] [CrossRef]
- Bowen, W.H. Do we need to be concerned about dental caries in the coming millennium? Crit. Rev. Oral Biol. Med. 2002, 13, 126–131. [Google Scholar] [CrossRef]
- Marsh, P.D. Are dental diseases examples of ecological catastrophes? Microbiology 2003, 149, 279–294. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Fux, C.A.; Costerton, J.W.; Stewart, P.S.; Stoodley, P. Survival strategies of infectious biofilms. Trends Microbiol. 2005, 13, 34–40. [Google Scholar] [CrossRef]
- McCarty, S.M.; Cochrane, C.A.; Clegg, P.D.; Percival, S.L. The role of endogenous and exogenous enzymes in chronic wounds: A focus on the implications of aberrant levels of both host and bacterial proteases in wound healing. Wound Repair. Regen. 2012, 20, 125–136. [Google Scholar] [CrossRef]
- Mah, T.-F. Biofilm-specific antibiotic resistance. Future Microbiol. 2012, 7, 1061–1072. [Google Scholar] [CrossRef]
- Römling, U.; Balsalobre, C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef] [PubMed]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug. Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, J.; Patel, R. The challenge of treating biofilm-associated bacterial infections. Clin. Pharmacol. Ther. 2007, 82, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Schlafer, S.; Raarup, M.K.; Meyer, R.L.; Sutherland, D.S.; Dige, I.; Nyengaard, J.R.; Nyvad, B. pH landscapes in a novel five-species model of early dental biofilm. PLoS ONE 2011, 6, e25299. [Google Scholar] [CrossRef]
- Xiao, J.; Klein, M.I.; Falsetta, M.L.; Lu, B.; Delahunty, C.M.; Yates, J.R., 3rd; Heydorn, A.; Koo, H. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog. 2012, 8, e1002623. [Google Scholar] [CrossRef]
- Vroom, J.M.; De Grauw, K.J.; Gerritsen, H.C.; Bradshaw, D.J.; Marsh, P.D.; Watson, G.K.; Birmingham, J.J.; Allison, C. Depth penetration and detection of pH gradients in biofilms by two-photon excitation microscopy. Appl. Environ. Microbiol. 1999, 65, 3502–3511. [Google Scholar] [CrossRef]
- Bhat, R.R.; Tomlinson, M.R.; Genzer, J. Assembly of Nanoparticles using Surface-Grafted Orthogonal Polymer Gradients. Macromol. Rapid Commun. 2004, 25, 270–274. [Google Scholar] [CrossRef]
- Narayanan, A.; Menefee, J.R.; Liu, Q.; Dhinojwala, A.; Joy, A. Lower critical solution temperature-driven self-coacervation of nonionic polyester underwater adhesives. ACS Nano. 2020, 14, 8359–8367. [Google Scholar] [CrossRef]
- Li, P.; Liu, T.-J. The size effect in adhesive contact on a gradient nanostructured coating. Nanotechnology 2021, 32, 235704. [Google Scholar] [CrossRef]
- Lendenmann, U.; Grogan, J.; Oppenheim, F. Saliva and dental pellicle—A review. Adv. Dent. Res. 2000, 14, 22–28. [Google Scholar] [CrossRef]
- Nobbs, A.H.; Lamont, R.J.; Jenkinson, H.F. Streptococcus adherence and colonization. Microbiol. Mol. Biol. Rev. 2009, 73, 407–450. [Google Scholar] [CrossRef] [PubMed]
- Schilling, K.M.; Bowen, W.H. Glucans synthesized in situ in experimental salivary pellicle function as specific binding sites for Streptococcus mutans. Infect. Immun. 1992, 60, 284–295. [Google Scholar] [CrossRef]
- Bowen, W.; Koo, H. Biology of Streptococcus mutans-derived glucosyltransferases: Role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef]
- Sims, K.R.; Liu, Y.; Hwang, G.; Jung, H.I.; Koo, H.; Benoit, D.S. Enhanced design and formulation of nanoparticles for anti-biofilm drug delivery. Nanoscale 2019, 11, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Miyake, N.; Sato, T.; Maki, Y. Effect of zeta potentials on bovine serum albumin adsorption to hydroxyapatite surfaces. Bull. Tokyo Dent. Coll. 2013, 54, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.C.; Wong, A. Effect of adsorbed protein on hydroxyapatite zeta potential and Streptococcus mutans adherence. Infect. Immun. 1983, 39, 1285–1290. [Google Scholar] [CrossRef]
- Weerkamp, A.; Uyen, H.; Busscher, H. Effect of zeta potential and surface energy on bacterial adhesion to uncoated and saliva-coated human enamel and dentin. J. Dent. Res. 1988, 67, 1483–1487. [Google Scholar] [CrossRef]
- Wilson, W.W.; Wade, M.M.; Holman, S.C.; Champlin, F.R. Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J. Microbiol. Methods 2001, 43, 153–164. [Google Scholar] [CrossRef]
- Young, A.; Smistad, G.; Karlsen, J.; Rölla, G.; Rykke, M. Zeta Potentials of Human Enamel and Hydroxyapatite as Measured by the Coulter® DELSA 440. Adv. Dent. Res. 1997, 11, 560–565. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; London, J. Adhere today, here tomorrow: Oral bacterial adherence. J. Bacteriol. 1993, 175, 3247–3252. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E.; Palmer, R.J., Jr.; Periasamy, S.; Jakubovics, N.S. Oral multispecies biofilm development and the key role of cell–cell distance. Nat. Rev. Microbiol. 2010, 8, 471–480. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, C.; He, L.; Yang, X.; Gou, Y.; Xu, X.; Liu, Y.; Zhao, C.; Li, J.; Li, J. Bioinspired heptapeptides as functionalized mineralization inducers with enhanced hydroxyapatite affinity. J. Mater. Chem. B 2018, 6, 1984–1994. [Google Scholar] [CrossRef]
- Yang, X.; Yang, B.; He, L.; Li, R.; Liao, Y.; Zhang, S.; Yang, Y.; Xu, X.; Zhang, D.; Tan, H.; et al. Bioinspired peptide-decorated tannic acid for in situ remineralization of tooth enamel: In vitro and in vivo evaluation. ACS Biomater. Sci. Eng. 2017, 3, 3553–3562. [Google Scholar] [CrossRef] [PubMed]
- Long, J.R.; Shaw, W.J.; Stayton, P.S.; Drobny, G.P. Structure and dynamics of hydrated statherin on hydroxyapatite as determined by solid-state NMR. Biochemistry 2001, 40, 15451–15455. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Jia, Z.; Rice, K.C.; Reinhardt, R.A.; Bayles, K.W.; Wang, D. The development of drug-free therapy for prevention of dental caries. Pharm. Res. 2014, 31, 3031–3037. [Google Scholar] [CrossRef]
- Chen, F.; Jia, Z.; Rice, K.C.; Reinhardt, R.A.; Bayles, K.W.; Wang, D. The development of dentotropic micelles with biodegradable tooth-binding moieties. Pharm. Res. 2013, 30, 2808–2817. [Google Scholar] [CrossRef]
- Shellis, R.; Addy, M.; Rees, G. In vitro studies on the effect of sodium tripolyphosphate on the interactions of stain and salivary protein with hydroxyapatite. J. Dent. 2005, 33, 313–324. [Google Scholar] [CrossRef]
- Hefferren, J. Historical view of dentifrice functionality methods. J. Clin. Dent. 1998, 9, 53–56. [Google Scholar]
- Zhu, Y.J.; Chen, F. pH-responsive drug-delivery systems. Chem. Asian J. 2015, 10, 284–305. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo-and pH-responsive polymers in drug delivery. Adv. Drug. Del. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Gong, L.; Peng, T.; Yao, J.; Lin, Z. Advances in pH-responsive drug delivery systems. OpenNano 2021, 5, 100031. [Google Scholar] [CrossRef]
- Quek, J.; Uroro, E.; Goswami, N.; Vasilev, K. Design principles for bacteria-responsive antimicrobial nanomaterials. Mater. Today Chem. 2022, 23, 100606. [Google Scholar] [CrossRef]
- Haidari, H.; Kopecki, Z.; Sutton, A.T.; Garg, S.; Cowin, A.J.; Vasilev, K. pH-responsive “smart” hydrogel for controlled delivery of silver nanoparticles to infected wounds. Antibiotics 2021, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Haidari, H.; Vasilev, K.; Cowin, A.J.; Kopecki, Z. Bacteria-Activated Dual pH-and Temperature-Responsive Hydrogel for Targeted Elimination of Infection and Improved Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 51744–51762. [Google Scholar] [CrossRef]
- AlSawaftah, N.M.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. pH-responsive nanocarriers in cancer therapy. Polymers 2022, 14, 936. [Google Scholar] [CrossRef]
- Anirudhan, T.; Mohan, A.M. Novel pH switchable gelatin based hydrogel for the controlled delivery of the anti cancer drug 5-fluorouracil. RSC Adv. 2014, 4, 12109–12118. [Google Scholar] [CrossRef]
- Chu, S.; Shi, X.; Tian, Y.; Gao, F. pH-responsive polymer nanomaterials for tumor therapy. Front. Oncol. 2022, 12, 855019. [Google Scholar] [CrossRef]
- Power, G.; Moore, Z.; O’Connor, T. Measurement of pH, exudate composition and temperature in wound healing: A systematic review. J. Wound Care 2017, 26, 381–397. [Google Scholar] [CrossRef]
- Bennison, L.; Miller, C.; Summers, R.; Minnis, A.; Sussman, G.; McGuiness, W. The pH of wounds during healing and infection: A descriptive literature review. Wound Pract. Res. J. Aust. Wound Manag. Assoc. 2017, 25, 63–69. [Google Scholar]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity Generated by the Tumor Microenvironment Drives Local InvasionAcid-Mediated Invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef]
- Manchun, S.; Dass, C.R.; Sriamornsak, P. Targeted therapy for cancer using pH-responsive nanocarrier systems. Life Sci. 2012, 90, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.; Jones, A.T.; Stephens, D.J. Intracellular trafficking pathways and drug delivery: Fluorescence imaging of living and fixed cells. Adv. Drug. Del. Rev. 2005, 57, 43–61. [Google Scholar] [CrossRef]
- Grabe, M.; Oster, G. Regulation of organelle acidity. J. Gen. Physiol. 2001, 117, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhu, Y.; Liang, Z. Alendronate functionalized mesoporous hydroxyapatite nanoparticles for drug delivery. Mater. Res. Bull. 2013, 48, 2201–2204. [Google Scholar] [CrossRef]
- Yuba, E. Development of functional liposomes by modification of stimuli-responsive materials and their biomedical applications. J. Mater. Chem. B 2020, 8, 1093–1107. [Google Scholar] [CrossRef] [PubMed]
- Zangabad, P.S.; Mirkiani, S.; Shahsavari, S.; Masoudi, B.; Masroor, M.; Hamed, H.; Jafari, Z.; Taghipour, Y.D.; Hashemi, H.; Karimi, M.; et al. Stimulus-responsive liposomes as smart nanoplatforms for drug delivery applications. Nanotechnol. Rev. 2018, 7, 95–122. [Google Scholar] [CrossRef]
- Zhai, L.; Luo, C.; Gao, H.; Du, S.; Shi, J.; Wang, F. A dual pH-responsive DOX-encapsulated liposome combined with glucose administration enhanced therapeutic efficacy of chemotherapy for cancer. Int. J. Nanomed. 2021, 16, 3185. [Google Scholar] [CrossRef]
- Wang, D.Y.; Yang, G.; van Der Mei, H.C.; Ren, Y.; Busscher, H.J.; Shi, L. Liposomes with Water as a pH-Responsive Functionality for Targeting of Acidic Tumor and Infection Sites. Angew. Chem. 2021, 133, 17855–17860. [Google Scholar] [CrossRef]
- Wang, D.; Li, X.; Li, X.; Kang, A.; Sun, L.; Sun, M.; Yang, F.; Xu, C. Magnetic and pH dual-responsive nanoparticles for synergistic drug-resistant breast cancer chemo/photodynamic therapy. Int. J. Nanomed. 2019, 14, 7665–7679. [Google Scholar] [CrossRef]
- Tang, X.L.; Jing, F.; Lin, B.L.; Cui, S.; Yu, R.T.; Shen, X.D.; Wang, T.W. pH-responsive magnetic mesoporous silica-based nanoplatform for synergistic photodynamic therapy/chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 15001–15011. [Google Scholar] [CrossRef]
- Jadhao, A.G.; Bhosale, A.G.; Sitaphale, G.; Rajguru, J.R.; Sonali, S.A.; Deshmane, S.S. Magnetic and pH Sensitive Nanoparticles for Cancer Drug Delivery. Asian J. Pharm. Res. Dev. 2019, 7, 60–71. [Google Scholar] [CrossRef]
- Domiński, A.; Krawczyk, M.; Konieczny, T.; Kasprów, M.; Foryś, A.; Pastuch-Gawołek, G.; Kurcok, P. Biodegradable pH-responsive micelles loaded with 8-hydroxyquinoline glycoconjugates for Warburg effect based tumor targeting. Eur. J. Pharm. Biopharm. 2020, 154, 317–329. [Google Scholar] [CrossRef]
- Hsu, C.-W.; Hsieh, M.-H.; Xiao, M.-C.; Chou, Y.-H.; Wang, T.-H.; Chiang, W.-H. pH-responsive polymeric micelles self-assembled from benzoic-imine-containing alkyl-modified PEGylated chitosan for delivery of amphiphilic drugs. Int. J. Biol. Macromol. 2020, 163, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Son, I.; Lee, Y.; Baek, J.; Park, M.; Han, D.; Min, S.K.; Lee, D.; Kim, B.-S. pH-Responsive amphiphilic polyether micelles with superior stability for smart drug delivery. Biomacromolecules 2021, 22, 2043–2056. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, Y.; Sun, S.; Li, Z.; Wu, A.; Zeng, L. pH-Responsive metal–organic framework encapsulated gold nanoclusters with modulated release to enhance photodynamic therapy/chemotherapy in breast cancer. J. Mater. Chem. B 2020, 8, 1739–1747. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, B.; Sahu, R.K.; Kumari, S.; Jha, C.B.; Singh, N.; Mathur, R.; Hedau, S.T. Development of a pH-sensitive functionalized metal organic framework: In vitro study for simultaneous delivery of doxorubicin and cyclophosphamide in breast cancer. RSC Adv. 2021, 11, 33723–33733. [Google Scholar] [CrossRef]
- Guillen, S.G.; Parres-Gold, J.; Ruiz, A.; Lucsik, E.; Dao, B.; Hang, T.K.L.; Chang, M.; Garcia, A.O.; Wang, Y.; Tian, F. pH-Responsive Metal–Organic Framework Thin Film for Drug Delivery. Langmuir 2022, 38, 16014–16023. [Google Scholar] [CrossRef]
- Karimi, S.; Namazi, H. Simple preparation of maltose-functionalized dendrimer/graphene quantum dots as a pH-sensitive biocompatible carrier for targeted delivery of doxorubicin. Int. J. Biol. Macromol. 2020, 156, 648–659. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Zhao, X.; Chen, L.-J.; Yang, C.-X.; Yan, X.-P. Dendrimer grafted persistent luminescent nanoplatform for aptamer guided tumor imaging and acid-responsive drug delivery. Talanta 2020, 219, 121209. [Google Scholar] [CrossRef] [PubMed]
- Neoh, K.H.; Cheng, S.K.S.; Wu, H.; Chen, A.; Sun, Y.; Li, B.; Cao, A.; Han, R.P.S. pH-Responsive Carbon Nanotube Film-Based Microfluidic Chip for Efficient Capture and Release of Cancer Cells. ACS Appl. Nano Mater. 2022, 5, 6911–6924. [Google Scholar] [CrossRef]
- Seyfoori, A.; Sarfarazijami, S.; Seyyed Ebrahimi, S. pH-responsive carbon nanotube-based hybrid nanogels as the smart anticancer drug carrier. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.; Guo, H.; Ghasemizadeh, A.; Shen, X.; Chintrakulchai, W.; Kobayashi, M.; Toyoda, M.; Ogi, K.; Michinishi, J.; Ohtake, T.; et al. Cancerous pH-responsive polycarboxybetaine-coated lipid nanoparticle for smart delivery of siRNA against subcutaneous tumor model in mice. Cancer Sci. 2022, 113, 4339–4349. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, S.; Zhang, S.; Li, Y.; Li, J.; Dai, Y.; Wang, D. pH-responsive lipid polymer hybrid nanoparticles (LPHNs) based on poly (β-amino ester) as a promising candidate to resist breast cancers. J. Drug. Deliv. Sci. Technol. 2021, 61, 102102. [Google Scholar] [CrossRef]
- Jia, L.; Pang, M.; Fan, M.; Tan, X.; Wang, Y.; Huang, M.; Liu, Y.; Wang, Q.; Zhu, Y.; Yang, X. A pH-responsive Pickering Nanoemulsion for specified spatial delivery of Immune Checkpoint Inhibitor and Chemotherapy agent to Tumors. Theranostics 2020, 10, 9956. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Moitra, P.; Bashir, M.; Kondaiah, P.; Bhattacharya, S. Natural tripeptide capped pH-sensitive gold nanoparticles for efficacious doxorubicin delivery both in vitro and in vivo. Nanoscale 2020, 12, 1067–1074. [Google Scholar] [CrossRef]
- Khodashenas, B.; Ardjmand, M.; Rad, A.; Esfahani, M. Gelatin-coated gold nanoparticles as an effective pH-sensitive methotrexate drug delivery system for breast cancer treatment. Mater. Today Chem. 2021, 20, 100474. [Google Scholar] [CrossRef]
- Gu, J.; Zhao, G.; Yu, J.; Xu, P.; Yan, J.; Jin, Z.; Chen, S.; Wang, Y.; Zhang, L.W.; Wang, Y. Injectable pH-responsive hydrogel for combinatorial chemoimmunotherapy tailored to the tumor microenvironment. J. Nanobiotechnol. 2022, 20, 372. [Google Scholar] [CrossRef]
- Raza, F.; Zhu, Y.; Chen, L.; You, X.; Zhang, J.; Khan, A.; Khan, M.W.; Hasnat, M.; Zafar, H.; Wu, J.; et al. Paclitaxel-loaded pH responsive hydrogel based on self-assembled peptides for tumor targeting. Biomater. Sci. 2019, 7, 2023–2036. [Google Scholar] [CrossRef]
- Cimen, Z.; Babadag, S.; Odabas, S.; Altuntas, S.; Demirel, G.; Demirel, G.B. Injectable and self-healable pH-responsive gelatin–PEG/laponite hybrid hydrogels as long-acting implants for local cancer treatment. ACS Appl. Polym. Mater. 2021, 3, 3504–3518. [Google Scholar] [CrossRef]
- Hu, F.; Zhang, R.; Guo, W.; Yan, T.; He, X.; Hu, F.; Ren, F.; Ma, X.; Lei, J.; Zheng, W. PEGylated-PLGA Nanoparticles Coated with pH-Responsive Tannic Acid–Fe (III) Complexes for Reduced Premature Doxorubicin Release and Enhanced Targeting in Breast Cancer. Mol. Pharm. 2020, 18, 2161–2173. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, P.; Liu, C.; Ma, H.; Huang, H.; Lin, Y.; Wang, C.; Yang, Y. pH-Responsive nanodrug encapsulated by tannic acid complex for controlled drug delivery. RSC Adv. 2017, 7, 2829–2835. [Google Scholar] [CrossRef]
- van der Meel, R.; Sulheim, E.; Shi, Y.; Kiessling, F.; Mulder, W.J.; Lammers, T. Smart cancer nanomedicine. Nat. Nanotechnol. 2019, 14, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Hannig, M.; Joiner, A. The structure, function and properties of the acquired pellicle. Monogr. Oral. Sci. 2006, 19, 29. [Google Scholar]
- Da Costa, G.; Lamy, E.; Capela e Silva, F.; Andersen, J.; Sales Baptista, E.; Coelho, A. Salivary amylase induction by tannin-enriched diets as a possible countermeasure against tannins. J. Chem. Ecol. 2008, 34, 376–387. [Google Scholar] [CrossRef]
- Kandra, L.; Gyémánt, G.; Zajácz, Á.; Batta, G. Inhibitory effects of tannin on human salivary α-amylase. Biochem. Biophys. Res. Commun. 2004, 319, 1265–1271. [Google Scholar] [CrossRef]
- Hannig, C.; Spitzmüller, B.; Al-Ahmad, A.; Hannig, M. Effects of Cistus-tea on bacterial colonization and enzyme activities of the in situ pellicle. J. Dent. 2008, 36, 540–545. [Google Scholar] [CrossRef]
- Wieckiewicz, M.; WBoening, K.; Grychowska, N.; Paradowska-Stolarz, A. Clinical application of chitosan in dental specialities. Mini Rev. Med. Chem. 2017, 17, 401–409. [Google Scholar] [CrossRef]
- Busscher, H.J.; Engels, E.; Dijkstra, R.J.; Van Der Mei, H.C. Influence of a chitosan on oral bacterial adhesion and growth in vitro. Eur. J. Oral. Sci. 2008, 116, 493–495. [Google Scholar] [CrossRef]
- Epstein, J.B.; Villines, D.C.; Baker, S. Efficacy of a glycopolymer-based oral rinse upon pain associated with ulcerative and erosive lesions of the oral mucosa: A within-subject pilot study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz Atay, H. Antibacterial activity of chitosan-based systems. Funct. Chitosan: Drug Deliv. Biomed. Appl. 2019, 457–489. [Google Scholar]
- Moorthy, M.S.; Hoang, G.; Manivasagan, P.; Mondal, S.; Phan, T.T.V.; Kim, H.; Oh, J. Chitosan oligosaccharide coated mesoporous silica nanoparticles for pH-stimuli responsive drug delivery applications. J. Porous Mater. 2019, 26, 217–226. [Google Scholar] [CrossRef]
- Moorthy, M.S.; Bae, J.H.; Kim, M.J.; Kim, S.H.; Ha, C.S. Design of a Novel Mesoporous Organosilica Hybrid Microcarrier: A pH Stimuli-Responsive Dual-Drug-Delivery Vehicle for Intracellular Delivery of Anticancer Agents. Part. Part. Syst. Charact. 2013, 30, 1044–1055. [Google Scholar] [CrossRef]
- Moorthy, M.S.; Park, J.-H.; Bae, J.-H.; Kim, S.-H.; Ha, C.-S. Mesoporous organosilica hybrids with a tunable amphoteric framework for controlled drug delivery. J. Mater. Chem. B 2014, 2, 6487–6499. [Google Scholar] [CrossRef]
- Moorthy, M.S.; Bharathiraja, S.; Manivasagan, P.; Lee, K.D.; Oh, J. Synthesis of surface capped mesoporous silica nanoparticles for pH-stimuli responsive drug delivery applications. Medchemcomm 2017, 8, 1797–1805. [Google Scholar] [CrossRef]
- Lu, K.-Y.; Li, R.; Hsu, C.-H.; Lin, C.-W.; Chou, S.-C.; Tsai, M.-L.; Mi, F.-L. Development of a new type of multifunctional fucoidan-based nanoparticles for anticancer drug delivery. Carbohydr. Polym. 2017, 165, 410–420. [Google Scholar] [CrossRef]
- Popat, A.; Liu, J.; Lu, G.Q.M.; Qiao, S.Z. A pH-responsive drug delivery system based on chitosan coated mesoporous silica nanoparticles. J. Mater. Chem. 2012, 22, 11173–11178. [Google Scholar] [CrossRef]
- Cheng, W.; Tang, K.; Qi, Y.; Sheng, J.; Liu, Z. One-step synthesis of superparamagnetic monodisperse porous Fe3O4 hollow and core-shell spheres. J. Mater. Chem. 2010, 20, 1799–1805. [Google Scholar] [CrossRef]
- Wu, X.; Cheng, Y.; Zheng, R.; Xu, K.; Yan, J.; Song, P.; Wang, Y.; Rauf, A.; Pan, Y.; Zhang, H. Immunomodulation of tumor microenvironment by arginine-loaded iron oxide nanoparticles for gaseous immunotherapy. ACS Appl. Mater. Interfaces 2021, 13, 19825–19835. [Google Scholar] [CrossRef]
- Appleton, J. Arginine: Clinical potential of a semi-essential amino acid. Altern. Med. Rev. 2002, 7, 512–523. [Google Scholar] [PubMed]
- Nascimento, M. Potential uses of arginine in dentistry. Adv. Dent. Res. 2018, 29, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, Z.; Mei, L.; Li, G.; Li, H. Anti-caries effect of arginine-containing formulations in vivo: A systematic review and meta-analysis. Caries Res. 2016, 49, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Van, N.; Carr, J.; Derrick, L.; Kabani, F.; Mallonee, L. The Oral and Systemic Health Benefits of Arginine. Dimens. Dent. Hyg. 2019, 17, 40–43. [Google Scholar]
- Zheng, X.; He, J.; Wang, L.; Zhou, S.; Peng, X.; Huang, S.; Zheng, L.; Cheng, L.; Hao, Y.; Li, J.; et al. Ecological effect of arginine on oral microbiota. Sci. Rep. 2017, 7, 7206. [Google Scholar] [CrossRef]


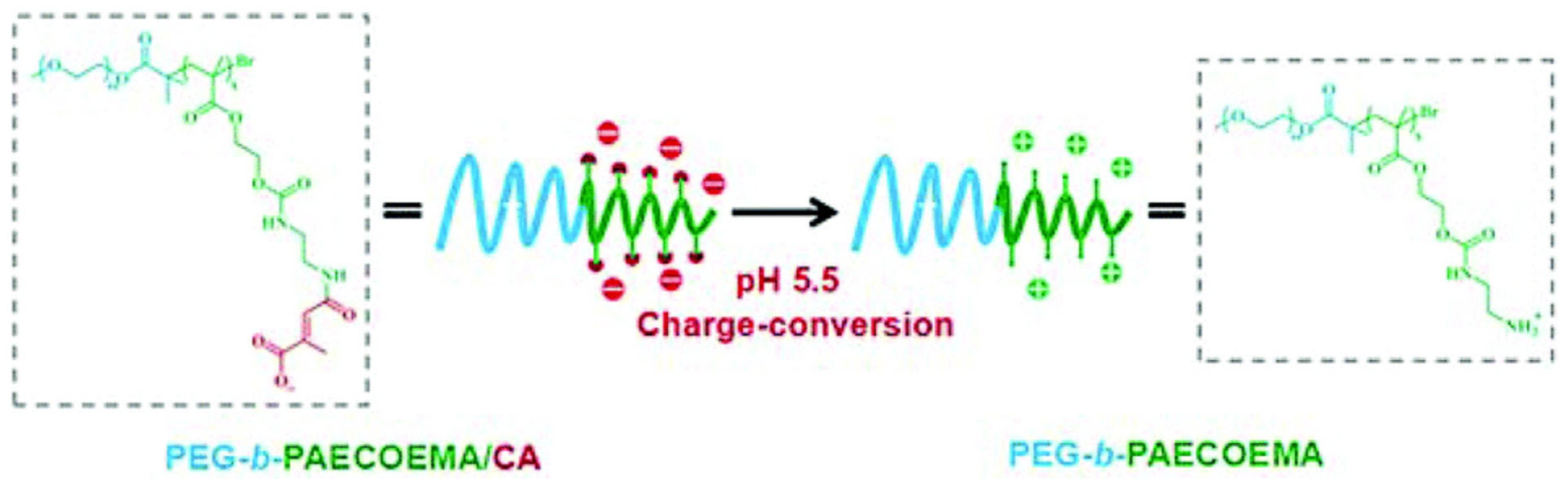
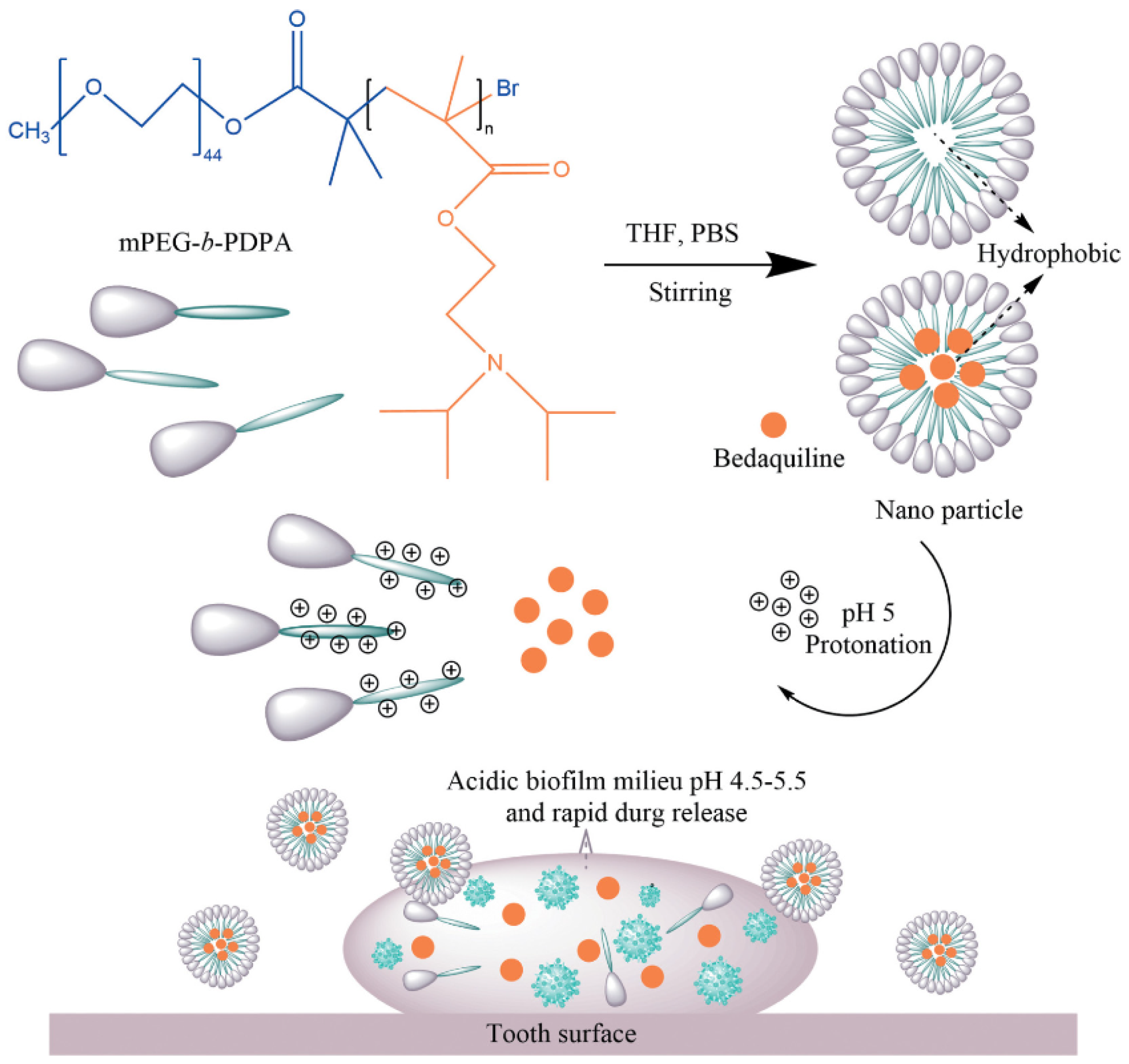
| Carrier Composition | Therapeutic Agent | Pathogen Used for Antibacterial/Antibiofilm Test | pH-Responsive Release Mechanism | Reference |
|---|---|---|---|---|
| PEG-b-PAECOEMA/CA | CHX | S. mutans | PAECOEMA/CA block is pH-sensitive and the degradation of citraconic amides converts the charge of the carrier from negative to positive, thus positively charged CHX is released via charge repulsion | [42] |
| mPEG-b-PDPA | Bedaquiline | S. mutans | The protonation of the PDPA segment converts the hydrophobic core to hydrophilic when the pH falls below the pKa (pH < 6), causing the dismantling of the nano structure and thus releasing the hydrophobic drug [43] | [44] |
| PEG-b-PLL/PBA-d.egradable micelles | NaF and TA | S. mutans | Catechol groups in TA interact with phenylboronic acid (PBA) in the PEG-b-PLL/PBA blocks and form the pH-sensitive boric acid ester link, which is subject to acid cleavage under cariogenic conditions | [45] |
| CaCl2 + PEG-Pasp | Doxycycline (DOXY) | P. intermedia | DOXY-loaded polymeric PEG-PAsp template-assisted CaCO3 mineralized nanoparticles could maintain their mineral structure and keep DOXY from releasing under normal pH in health oral environment, while in acidic pH the DOXY is released due to the dissolution of the CaCO3 mineral cores | [46] |
| PPi-PEGhyd-Far | Farnesal (Far) | S. mutans | Via an acid-labile hydrazone bond | [47] |
| Organic NPs | Inorganic NPs | ||
|---|---|---|---|
| Nanocarrier | Reference | Nanocarrier | Reference |
| Liposome | [137,138,139,140] | Magnetic nanoparticles | [141,142,143] |
| Polymeric micelles | [140,144,145,146] | Metal organic frameworks | [147,148,149] |
| Dendrimers | [150,151] | Carbon nanotubes | [152,153] |
| Solid lipid nanoparticles | [154,155] | Quantum dots | [150] |
| Nano-emulsions | [156] | Gold nanoparticles | [157,158] |
| Hydrogels | [159,160,161] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Vasilev, K.; Zilm, P. pH-Responsive Biomaterials for the Treatment of Dental Caries—A Focussed and Critical Review. Pharmaceutics 2023, 15, 1837. https://doi.org/10.3390/pharmaceutics15071837
He Y, Vasilev K, Zilm P. pH-Responsive Biomaterials for the Treatment of Dental Caries—A Focussed and Critical Review. Pharmaceutics. 2023; 15(7):1837. https://doi.org/10.3390/pharmaceutics15071837
Chicago/Turabian StyleHe, Yanping, Krasimir Vasilev, and Peter Zilm. 2023. "pH-Responsive Biomaterials for the Treatment of Dental Caries—A Focussed and Critical Review" Pharmaceutics 15, no. 7: 1837. https://doi.org/10.3390/pharmaceutics15071837
APA StyleHe, Y., Vasilev, K., & Zilm, P. (2023). pH-Responsive Biomaterials for the Treatment of Dental Caries—A Focussed and Critical Review. Pharmaceutics, 15(7), 1837. https://doi.org/10.3390/pharmaceutics15071837