Cancer Vaccines: From the State of the Art to the Most Promising Frontiers in the Treatment of Colorectal Cancer
Abstract
:1. Introduction
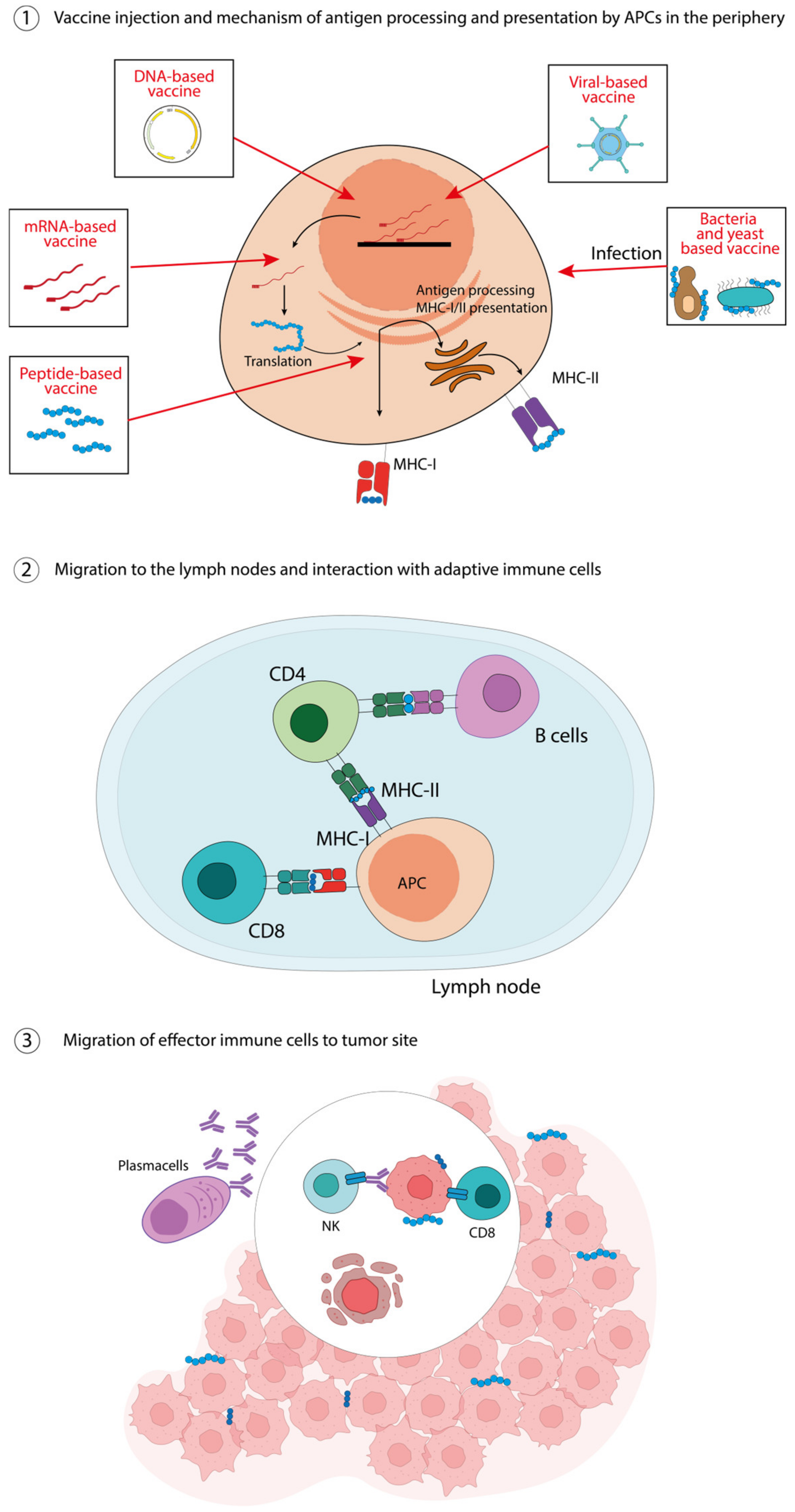
2. How Do Cancer Vaccines Work?
3. CRC-Targeting Vaccines
3.1. Peptide-Based Vaccines for CRC
3.2. Nucleic Acid-Based Vaccines for CRC
3.3. Virus-, Bacteria-, and Yeast-Based Vector Vaccines for CRC
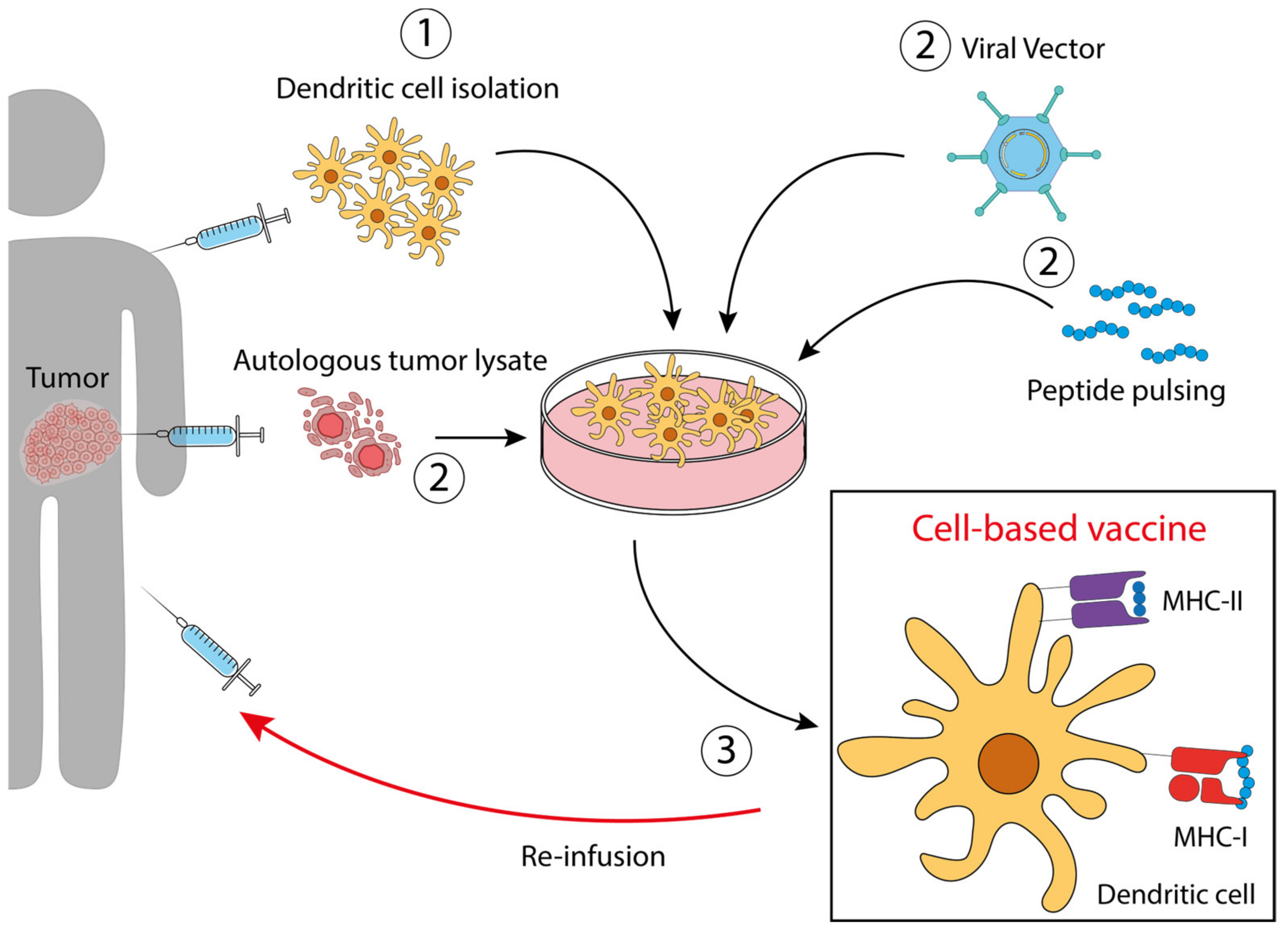
3.4. Dendritic Cell (DC)-Based Vaccines for CRC
- DCs loaded with autologous tumor lysate [58]. DCs treated with tumor lysates derived from needle-core biopsies were injected in patients with resectable metastatic colon cancer. This therapeutic vaccine resulted in an improved disease-free survival. NCT01348256.
- DCs modified to express tumor antigens [25]. Dendritic cells engineered with the fowlpox virus encoding CEA and MUC1 and costimulatory molecules. The study aimed to compare DCs and poxvector vaccines against CEA and MUC1, reaching the conclusion that both had similar activity, with superior survival of the vaccinated patients compared with the contemporary unvaccinated group. NCT00103142.
- DCs pulsed with CEA peptide [17]. Ten patients were vaccinated intradermally and intravenously with CEA peptide-pulsed mature DCs three times prior to resection of liver metastases. High numbers of CEA-specific T-cells were detected in post-treatment DTH (delayed-type hypersensitivity) biopsies in 7 out of 10 patients, which produced high amounts of IFNγ upon stimulation.
4. Use of Nanotechnologies to Improve CRC Vaccines Efficacy
5. CRC-Targeting Vaccines under Clinical Trial
6. B-Cell Vaccines: A Possible Route for Cell-Based Vaccines?
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.; Weber, J.S.; et al. Five-Year Survival Outcomes for Patients with Advanced Melanoma Treated with Pembrolizumab in KEYNOTE-001. Ann. Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef]
- Punekar, S.R.; Shum, E.; Grello, C.M.; Lau, S.C.; Velcheti, V. Immunotherapy in Non-Small Cell Lung Cancer: Past, Present, and Future Directions. Front. Oncol. 2022, 12, 3576. [Google Scholar] [CrossRef]
- Alberti, A.; Bossi, P. Immunotherapy for Cutaneous Squamous Cell Carcinoma: Results and Perspectives. Front. Oncol. 2022, 11, 5455. [Google Scholar] [CrossRef]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic Cancer Vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Sarobe, P.; Hervás-Stubbs, S.; Melero, I. Advances in Immunotherapy for Hepatocellular Carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543. [Google Scholar] [CrossRef]
- Takei, S.; Kawazoe, A.; Shitara, K. The New Era of Immunotherapy in Gastric Cancer. Cancers 2022, 14, 1054. [Google Scholar] [CrossRef]
- Valencia, G.A.; Rioja, P.; Morante, Z.; Ruiz, R.; Fuentes, H.; Castaneda, C.A.; Vidaurre, T.; Neciosup, S.; Gomez, H.L. Immunotherapy in Triple-Negative Breast Cancer: A Literature Review and New Advances. World J. Clin. Oncol. 2022, 13, 219. [Google Scholar] [CrossRef]
- Weng, J.; Li, S.; Zhu, Z.; Liu, Q.; Zhang, R.; Yang, Y.; Li, X. Exploring Immunotherapy in Colorectal Cancer. J. Hematol. Oncol. 2022, 15, 95. [Google Scholar] [CrossRef] [PubMed]
- Johdi, N.A.; Sukor, N.F. Colorectal Cancer Immunotherapy: Options and Strategies. Front. Immunol. 2020, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- Kerr, M.D.; McBride, D.A.; Chumber, A.K.; Shah, N.J. Combining Therapeutic Vaccines with Chemo- and Immunotherapies in the Treatment of Cancer. Expert Opin. Drug. Discov. 2020, 16, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Finn, O.J. The Dawn of Vaccines for Cancer Prevention. Nat. Rev. Immunol. 2018, 18, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fu, M.; Wang, M.; Wan, D.; Wei, Y.; Wei, X. Cancer Vaccines as Promising Immuno-Therapeutics: Platforms and Current Progress. J. Hematol. Oncol. 2022, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Blass, E.; Ott, P.A. Advances in the Development of Personalized Neoantigen-Based Therapeutic Cancer Vaccines. Nat. Rev. Clin. Oncol. 2021, 18, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Mo, Y.; Wang, Y.; Wu, P.; Zhang, Y.; Xiong, F.; Guo, C.; Wu, X.; Li, Y.; Li, X.; et al. Neoantigen Vaccine: An Emerging Tumor Immunotherapy. Mol. Cancer 2019, 18, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staff, C.; Mozaffari, F.; Haller, K.B.; Blomberg, P.; Frödin, J.-E.; Wahren, B.; Mellstedt, H.; Liljefors, M. DNA Immunization Targeting Carcinoembryonic Antigen in Colorectal Cancer Patients. Glob. Vaccines Immunol. 2017, 2, 1–13. [Google Scholar] [CrossRef]
- Lesterhuis, W.J.; de Vries, I.J.M.; Schuurhuis, D.H.; Boullart, A.C.I.; Jacobs, J.F.M.; de Boer, A.J.; Scharenborg, N.M.; Brouwer, H.M.H.; van de Rakt, M.W.M.M.; Figdor, C.G.; et al. Vaccination of Colorectal Cancer Patients with CEA-Loaded Dendritic Cells: Antigen-Specific T Cell Responses in DTH Skin Tests. Ann. Oncol. 2006, 17, 974–980. [Google Scholar] [CrossRef]
- Lesterhuis, W.J.; De Vries, I.J.M.; Aarntzen, E.A.; De Boer, A.; Scharenborg, N.M.; Van De Rakt, M.; Van Spronsen, D.J.; Preijers, F.W.; Figdor, C.G.; Adema, G.J.; et al. A Pilot Study on the Immunogenicity of Dendritic Cell Vaccination during Adjuvant Oxaliplatin/Capecitabine Chemotherapy in Colon Cancer Patients. Br. J. Cancer 2010, 103, 1415–1421. [Google Scholar] [CrossRef]
- Crosby, E.J.; Hobeika, A.C.; Niedzwiecki, D.; Rushing, C.; Hsu, D.; Berglund, P.; Smith, J.; Osada, T.; Gwin, W.R., III; Hartman, Z.C.; et al. Original Research: Long-Term Survival of Patients with Stage III Colon Cancer Treated with VRP-CEA(6D), an Alphavirus Vector That Increases the CD8+ Effector Memory T Cell to Treg Ratio. J. Immunother. Cancer 2020, 8, e001662. [Google Scholar] [CrossRef]
- Nagasaka, M. ES28.04 Emerging Mechanisms to Target KRAS Directly. J. Thorac. Oncol. 2021, 16, S96–S97. [Google Scholar] [CrossRef]
- Cafri, G.; Gartner, J.J.; Zaks, T.; Hopson, K.; Levin, N.; Paria, B.C.; Parkhurst, M.R.; Yossef, R.; Lowery, F.J.; Jafferji, M.S.; et al. MRNA Vaccine-Induced Neoantigen-Specific T Cell Immunity in Patients with Gastrointestinal Cancer. J. Clin. Investig. 2020, 130, 5976–5988. [Google Scholar] [CrossRef] [PubMed]
- Toubaji, A.; Achtar, M.; Provenzano, M.; Herrin, V.E.; Behrens, R.; Hamilton, M.; Bernstein, S.; Venzon, D.; Gause, B.; Marincola, F.; et al. Pilot Study of Mutant Ras Peptide-Based Vaccine as an Adjuvant Treatment in Pancreatic and Colorectal Cancers. Cancer Immunol. Immunother. 2008, 57, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; McKolanis, J.R.; Dzubinski, L.A.; Islam, K.; Potter, D.M.; Salazar, A.M.; Schoen, R.E.; Finn, O.J. MUC1 Vaccine for Individuals with Advanced Adenoma of the Colon: A Cancer Immunoprevention Feasibility Study. Cancer Prev. Res. 2013, 6, 18–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohmueller, J.J.; Sato, S.; Popova, L.; Chu, I.M.; Tucker, M.A.; Barberena, R.; Innocenti, G.M.; Cudic, M.; Ham, J.D.; Cheung, W.C.; et al. Antibodies Elicited by the First Non-Viral Prophylactic Cancer Vaccine Show Tumor-Specificity and Immunotherapeutic Potential. Sci. Rep. 2016, 6, 31740. [Google Scholar] [CrossRef] [Green Version]
- Morse, M.A.; Niedzwiecki, D.; Marshall, J.L.; Garrett, C.; Chang, D.Z.; Aklilu, M.; Crocenzi, T.S.; Cole, D.J.; Dessureault, S.; Hobeika, A.C.; et al. A Randomized Phase II Study of Immunization with Dendritic Cells Modified with Poxvectors Encoding CEA and MUC1 Compared with the Same Poxvectors plus GM-CSF for Resected Metastatic Colorectal Cancer. Ann. Surg. 2013, 258, 879–886. [Google Scholar] [CrossRef] [Green Version]
- Kanekiyo, S.; Hazama, S.; Takenouchi, H.; Nakajima, M.; Shindo, Y.; Matsui, H.; Tokumitsu, Y.; Tomochika, S.; Tsunedomi, R.; Tokuhisa, Y.; et al. IgG Response to MHC Class I Epitope Peptides Is a Quantitative Predictive Biomarker in the Early Course of Treatment of Colorectal Cancer Using Therapeutic Peptides. Oncol. Rep. 2018, 39, 2385–2392. [Google Scholar] [CrossRef]
- Miyagi, Y.; Imai, N.; Sasatomi, T.; Yamada, A.; Mine, T.; Katagiri, K.; Nakagawa, M.; Muto, A.; Okouchi, S.; Isomoto, H.; et al. Induction of Cellular Immune Responses to Tumor Cells and Peptides in Colorectal Cancer Patients by Vaccination with SART3 Peptides1. Clin. Cancer Res. 2001, 7, 3950–3962. [Google Scholar]
- Moulton, H.M.; Yoshihara, P.M.; Mason, D.H.; Iversen, P.L.; Triozzi, P.L. Active Specific Immunotherapy with a β-Human Chorionic Gonadotropin Peptide Vaccine in Patients with Metastatic Colorectal Cancer|Clinical Cancer Research|American Association for Cancer Research. Clin. Cancer Res. 2002, 8, 2044–2051. [Google Scholar]
- Tsuruma, T.; Hata, F.; Torigoe, T.; Furuhata, T.; Idenoue, S.; Kurotaki, T.; Yamamoto, M.; Yagihashi, A.; Ohmura, T.; Yamaguchi, K.; et al. Phase I Clinical Study of Anti-Apoptosis Protein, Survivin-Derived Peptide Vaccine Therapy for Patients with Advanced or Recurrent Colorectal Cancer. J. Transl. Med. 2004, 2, 19. [Google Scholar] [CrossRef] [Green Version]
- Pham, T.; Pereira, L.; Roth, S.; Galletta, L.; Link, E.; Akhurst, T.; Solomon, B.; Michael, M.; Darcy, P.; Sampurno, S.; et al. First-in-Human Phase I Clinical Trial of a Combined Immune Modulatory Approach Using TetMYB Vaccine and Anti-PD-1 Antibody in Patients with Advanced Solid Cancer Including Colorectal or Adenoid Cystic Carcinoma: The MYPHISMO Study Protocol (NCT03287427). Contemp. Clin. Trials Commun. 2019, 16, 100409. [Google Scholar] [CrossRef]
- Besneux, M.; Greenshields-Watson, A.; Scurr, M.J.; MacLachlan, B.J.; Christian, A.; Davies, M.M.; Hargest, R.; Phillips, S.; Godkin, A.; Gallimore, A. The Nature of the Human T Cell Response to the Cancer Antigen 5T4 Is Determined by the Balance of Regulatory and Inflammatory T Cells of the Same Antigen-Specificity: Implications for Vaccine Design. Cancer Immunol. Immunother. 2019, 68, 247–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, J.; Cen, P. TroVax in Colorectal Cancer. Hum. Vaccin. Immunother. 2014, 10, 3196–3200. [Google Scholar] [CrossRef] [Green Version]
- Hattori, T.; Mine, T.; Komatsu, N.; Yamada, A.; Itoh, K.; Shiozaki, H.; Okuno, K. Immunological Evaluation of Personalized Peptide Vaccination in Combination with UFT and UZEL for Metastatic Colorectal Carcinoma Patients. Cancer Immunol. Immunother. 2009, 58, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Bekaii-Saab, T.; Wesolowski, R.; Ahn, D.H.; Wu, C.; Mortazavi, A.; Lustberg, M.; Ramaswamy, B.; Fowler, J.; Wei, L.; Overholser, J.; et al. Phase I Immunotherapy Trial with Two Chimeric HER-2 B-Cell Peptide Vaccines Emulsified in Montanide ISA 720VG and Nor-MDP Adjuvant in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2019, 25, 3495–3507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castle, J.C.; Kreiter, S.; Diekmann, J.; Löwer, M.; Van De Roemer, N.; De Graaf, J.; Selmi, A.; Diken, M.; Boegel, S.; Paret, C.; et al. Exploiting the Mutanome for Tumor Vaccination. Cancer Res. 2012, 72, 1081–1091. [Google Scholar] [CrossRef] [Green Version]
- Kumai, T.; Yamaki, H.; Kono, M.; Hayashi, R.; Wakisaka, R.; Komatsuda, H. Antitumor Peptide-Based Vaccine in the Limelight. Vaccines 2022, 10, 70. [Google Scholar] [CrossRef]
- Jensen, S.M.; Twitty, C.G.; Maston, L.D.; Antony, P.A.; Lim, M.; Hu, H.-M.; Petrausch, U.; Restifo, N.P.; Fox, B.A. Increased Frequency of Suppressive Regulatory T Cells and T Cell-Mediated Antigen Loss Results in Murine Melanoma Recurrence. J. Immunol. 2012, 189, 767–776. [Google Scholar] [CrossRef] [Green Version]
- Bijker, M.S.; van den Eeden, S.J.F.; Franken, K.L.; Melief, C.J.M.; van der Burg, S.H.; Offringa, R. Superior Induction of Anti-Tumor CTL Immunity by Extended Peptide Vaccines Involves Prolonged, DC-Focused Antigen Presentation. Eur. J. Immunol. 2008, 38, 1033–1042. [Google Scholar] [CrossRef]
- Ivanova, M.; Venetis, K.; Guerini-Rocco, E.; Bottiglieri, L.; Mastropasqua, M.G.; Garrone, O.; Fusco, N.; Ghidini, M. HER2 in Metastatic Colorectal Cancer: Pathology, Somatic Alterations, and Perspectives for Novel Therapeutic Schemes. Life 2022, 12, 1403. [Google Scholar] [CrossRef]
- Terbuch, A.; Lopez, J. Next Generation Cancer Vaccines—Make It Personal! Vaccines 2018, 6, 52. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.A. A Comparison of Plasmid DNA and MRNA as Vaccine Technologies. Vaccines 2019, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Jahanafrooz, Z.; Baradaran, B.; Mosafer, J.; Hashemzaei, M.; Rezaei, T.; Mokhtarzadeh, A.; Hamblin, M.R. Comparison of DNA and MRNA Vaccines against Cancer. Drug Discov. Today 2020, 25, 552–560. [Google Scholar] [CrossRef]
- Fan, X.; Wang, Y.; Jiang, T.; Liu, T.; Jin, Y.; Du, K.; Niu, Y.; Zhang, C.; Liu, Z.; Lei, Y.; et al. B-Myb Accelerates Colorectal Cancer Progression through Reciprocal Feed-Forward Transactivation of E2F2. Oncogene 2021, 40, 5613–5625. [Google Scholar] [CrossRef]
- Bauman, J.; Burris, H.; Clarke, J.; Patel, M.; Cho, D.; Gutierrez, M.; Julian, R.; Scott, A.; Cohen, P.; Frederick, J.; et al. 798 Safety, Tolerability, and Immunogenicity of MRNA-4157 in Combination with Pembrolizumab in Subjects with Unresectable Solid Tumors (KEYNOTE-603): An Update. J. Immunother. Cancer 2020, 8, A477.1. [Google Scholar] [CrossRef]
- Lin, I.Y.C.; Van, T.T.H.; Smooker, P.M. Live-Attenuated Bacterial Vectors: Tools for Vaccine and Therapeutic Agent Delivery. Vaccines 2015, 3, 940–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasinskas, R.W.; Forbes, N.S. Salmonella Typhimurium Lacking Ribose Chemoreceptors Localize in Tumor Quiescence and Induce Apoptosis. Cancer Res. 2007, 67, 3201–3209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.A.; Shabahang, S.; Timiryasova, T.M.; Zhang, Q.; Beltz, R.; Gentschev, I.; Goebel, W.; Szalay, A.A. Visualization of Tumors and Metastases in Live Animals with Bacteria and Vaccinia Virus Encoding Light-Emitting Proteins. Nat. Biotechnol. 2004, 22, 313–320. [Google Scholar] [CrossRef]
- Baban, C.K.; Cronin, M.; O’Hanlon, D.; O’Sullivan, G.C.; Tangney, M. Bacteria as Vectors for Gene Therapy of Cancer. Bioeng. Bugs 2010, 1, 385–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullard, A. The Cancer Vaccine Resurgence. Nat. Rev. Drug. Discov. 2016, 15, 663–665. [Google Scholar] [CrossRef] [PubMed]
- Oladejo, M.; Paterson, Y.; Wood, L.M. Clinical Experience and Recent Advances in the Development of Listeria-Based Tumor Immunotherapies. Front. Immunol. 2021, 12, 773. [Google Scholar] [CrossRef]
- Ferreira, R.; Limeta, A.; Nielsen, J. Tackling Cancer with Yeast-Based Technologies. Trends Biotechnol. 2019, 37, 592–603. [Google Scholar] [CrossRef]
- Wang, L.; Yang, M.; Luo, S.; Yang, G.; Lu, X.; Lu, J.; Chen, J. Oral Vaccination of Recombinant Saccharomyces Cerevisiae Expressing ORF132 Induces Protective Immunity against Cyprinid Herpesvirus-2. Vaccines 2023, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.M.V.; Carmo, T.S.; Carvalho, L.S.; Bahia, F.M.; Parachin, N.S. Comparison of Yeasts as Hosts for Recombinant Protein Production. Microorganisms 2018, 6, 38. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Kumar, P. Yeast-Based Vaccines: New Perspective in Vaccine Development and Application. FEMS Yeast Res. 2019, 19, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Jiang, S.; Wang, Y. Recent Advances in the Production of Recombinant Subunit Vaccines in Pichia Pastoris. Bioengineered 2016, 7, 155–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ardiani, A.; Higgins, J.P.; Hodge, J.W. Vaccines Based on Whole Recombinant Saccharomyces Cerevisiae Cells. FEMS Yeast Res. 2010, 10, 1060–1069. [Google Scholar] [CrossRef]
- Binnewies, M.; Mujal, A.M.; Pollack, J.L.; Combes, A.J.; Hardison, E.A.; Barry, K.C.; Tsui, J.; Ruhland, M.K.; Kersten, K.; Abushawish, M.A.; et al. Unleashing Type-2 Dendritic Cells to Drive Protective Antitumor CD4+ T Cell Immunity. Cell 2019, 177, 556–571.e16. [Google Scholar] [CrossRef]
- Rodriguez, J.; Castañón, E.; Perez-Gracia, J.L.; Rodriguez, I.; Viudez, A.; Alfaro, C.; Oñate, C.; Perez, G.; Rotellar, F.; Inogés, S.; et al. A Randomized Phase II Clinical Trial of Dendritic Cell Vaccination Following Complete Resection of Colon Cancer Liver Metastasis 11 Medical and Health Sciences 1112 Oncology and Carcinogenesis. J. Immunother. Cancer 2018, 6, 96. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Moynihan, K.D.; Zheng, Y.; Szeto, G.L.; Li, A.V.; Huang, B.; Van Egeren, D.S.; Park, C.; Irvine, D.J. Structure-Based Programming of Lymph-Node Targeting in Molecular Vaccines. Nature 2014, 507, 519–522. [Google Scholar] [CrossRef] [Green Version]
- Batty, C.J.; Tiet, P.; Bachelder, E.M.; Ainslie, K.M. Drug Delivery for Cancer Immunotherapy and Vaccines. Pharm. Nanotechnol. 2018, 6, 232–244. [Google Scholar] [CrossRef]
- Sixt, M.; Kanazawa, N.; Selg, M.; Samson, T.; Roos, G.; Reinhardt, D.P.; Pabst, R.; Lutz, M.B.; Sorokin, L. The Conduit System Transports Soluble Antigens from the Afferent Lymph to Resident Dendritic Cells in the T Cell Area of the Lymph Node. Immunity 2005, 22, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Rohner, N.A.; Thomas, S.N. Flexible Macromolecule versus Rigid Particle Retention in the Injected Skin and Accumulation in Draining Lymph Nodes Are Differentially Influenced by Hydrodynamic Size. ACS Biomater. Sci. Eng. 2017, 3, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Samaridou, E.; Heyes, J.; Lutwyche, P. Lipid Nanoparticles for Nucleic Acid Delivery: Current Perspectives. Adv. Drug Deliv. Rev. 2020, 154–155, 37–63. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 MRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Lei, S.; Zhang, X.; Men, K.; Gao, Y.; Yang, X.; Wu, S.; Duan, X.; Wei, Y.; Tong, R. Efficient Colorectal Cancer Gene Therapy with IL-15 MRNA Nanoformulation. Mol. Pharm. 2020, 17, 3378–3391. [Google Scholar] [CrossRef]
- Patel, M.R.; Bauer, T.M.; Jimeno, A.; Wang, D.; LoRusso, P.; Do, K.T.; Stemmer, S.M.; Maurice-Dror, C.; Geva, R.; Zacharek, S.; et al. A Phase I Study of MRNA-2752, a Lipid Nanoparticle Encapsulating MRNAs Encoding Human OX40L, IL-23, and IL-36γ, for Intratumoral (ITu) Injection Alone and in Combination with Durvalumab. J. Clin. Oncol. 2020, 38, 3092. [Google Scholar] [CrossRef]
- O’Neill, C.L.; Shrimali, P.C.; Clapacs, Z.P.; Files, M.A.; Rudra, J.S. Peptide-Based Supramolecular Vaccine Systems. Acta Biomater. 2021, 133, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.O.; Zha, L.; Cabral-Miranda, G.; Bachmann, M.F. Major Findings and Recent Advances in Virus-like Particle (VLP)-Based Vaccines. Semin. Immunol. 2017, 34, 123–132. [Google Scholar] [CrossRef]
- Neek, M.; Kim, T.I.; Wang, S.W. Protein-Based Nanoparticles in Cancer Vaccine Development. Nanomedicine 2019, 15, 164–174. [Google Scholar] [CrossRef]
- Tesarova, B.; Musilek, K.; Rex, S.; Heger, Z. Taking Advantage of Cellular Uptake of Ferritin Nanocages for Targeted Drug Delivery. J. Control. Release 2020, 325, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Ali, N. Nanovaccine: An Emerging Strategy. Expert Rev. Vaccines 2021, 20, 1273–1290. [Google Scholar] [CrossRef] [PubMed]
- Simón-Vázquez, R.; Peleteiro, M.; González-Fernández, Á. Polymeric Nanostructure Vaccines: Applications and Challenges. Expert Opin. Drug Deliv. 2020, 17, 1007–1023. [Google Scholar] [CrossRef] [PubMed]
- González-Ballesteros, N.; Diego-González, L.; Lastra-Valdor, M.; Rodríguez-Argüelles, M.C.; Grimaldi, M.; Cavazza, A.; Bigi, F.; Simón-Vázquez, R. Immunostimulant and Biocompatible Gold and Silver Nanoparticles Synthesized Using the Ulva intestinalis L. Aqueous Extract. J. Mater. Chem. B 2019, 7, 4677–4691. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.A. Gold Nanoparticles for Preparation of Antibodies and Vaccines against Infectious Diseases. Expert Rev. Vaccines 2020, 19, 465–477. [Google Scholar] [CrossRef]
- Brar, B.; Ranjan, K.; Palria, A.; Kumar, R.; Ghosh, M.; Sihag, S.; Minakshi, P. Nanotechnology in Colorectal Cancer for Precision Diagnosis and Therapy. Front. Nanotechnol. 2021, 3, 699266. [Google Scholar] [CrossRef]
- Schultze, J.L.; Michalak, S.; Seamon, M.J.; Dranoff, G.; Jung, K.; Daley, J.; Delgado, J.C.; Gribben, J.G.; Nadler, L.M. CD40-Activated Human B Cells: An Alternative Source of Highly Efficient Antigen Presenting Cells to Generate Autologous Antigen-Specific T Cells for Adoptive Immunotherapy. J. Clin. Investig. 1997, 100, 2757. [Google Scholar] [CrossRef]
- Lapointe, R.; Bellemare-Pelletier, A.; Housseau, F.; Thibodeau, J.; Hwu, P. CD40-Stimulated B Lymphocytes Pulsed with Tumor Antigens Are Effective Antigen-Presenting Cells That Can Generate Specific T Cells. Cancer Res 2003;63:2836–43. Cancer Res. 2003, 63, 2836–2843. [Google Scholar]
- Von Bergwelt-Baildon, M.; Schultze, J.L.; Maecker, B.; Menezes, I.; Nadler, L.M.; Lapointe, R.; Thibodeau, J.; Hwu, P. Correspondence Re R. Lapointe et al., CD40-Stimulated B Lymphocytes Pulsed with Tumor Antigens Are Effective Antigen-Presenting Cells That Can Generate Specific T Cells. Cancer Res. 2004, 64, 4055–4057. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Zhao, S.; Li, W.; Dong, H.; Zhou, M.; Cao, M.; Hu, H.M.; Wang, L.X. Therapeutic Antitumor Efficacy of B Cells Loaded with Tumor-Derived Autophagasomes Vaccine (DRibbles). J. Immunother. 2014, 37, 383–393. [Google Scholar] [CrossRef]
- Li, Q.; Lao, X.; Pan, Q.; Ning, N.; Yet, J.; Xu, Y.; Li, S.; Chang, A.E. Adoptive Transfer of Tumor Reactive B Cells Confers Host T-Cell Immunity and Tumor Regression. Clin. Cancer Res. 2011, 17, 4987–4995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oxley, K.L.; Hanson, B.M.; Zani, A.N.; Bishop, G.A. Activated B Lymphocytes and Tumor Cell Lysate as an Effective Cellular Cancer Vaccine. Cancer Immunol. Immunother. 2021, 70, 3093–3103. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Kwon, W.S.; Kim, H.S.; Jung, M.; Kim, S.; Park, M.; Kim, W.; Choi, K.-Y.; Oh, T.; Kang, C.-Y.; et al. First-in-Human Phase I Study of BVAC-B Cell Therapy in HER2-Positive Advanced Gastric Cancer. J. Clin. Oncol. 2020, 38, 4534. [Google Scholar] [CrossRef]
- Castle, J.C.; Uduman, M.; Pabla, S.; Stein, R.B.; Buell, J.S. Mutation-Derived Neoantigens for Cancer Immunotherapy. Front. Immunol. 2019, 10, 1856. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, L.Y.; Wu, X.J.; Ye, S.B.; Zhang, R.; Li, Z.L.; Liao, W.; Pan, Z.Z.; Zheng, L.M.; Zhang, X.S.; Wang, Z.; et al. Tumor-Induced Myeloid-Derived Suppressor Cells Promote Tumor Progression through Oxidative Metabolism in Human Colorectal Cancer. J. Transl. Med. 2015, 13, 47. [Google Scholar] [CrossRef] [Green Version]
- Bai, J.; Chen, H.; Bai, X. Relationship between Microsatellite Status and Immune Microenvironment of Colorectal Cancer and Its Application to Diagnosis and Treatment. J. Clin. Lab. Anal. 2021, 35, e23810. [Google Scholar] [CrossRef]
- Gagne, M.; Moliva, J.I.; Foulds, K.E.; Andrew, S.F.; Flynn, B.J.; Werner, A.P.; Wagner, D.A.; Teng, I.T.; Lin, B.C.; Moore, C.; et al. MRNA-1273 or MRNA-Omicron Boost in Vaccinated Macaques Elicits Similar B Cell Expansion, Neutralizing Responses, and Protection from Omicron. Cell 2022, 185, 1556–1571.e18. [Google Scholar] [CrossRef]
- Szeto, G.L.; Van Egeren, D.; Worku, H.; Sharei, A.; Alejandro, B.; Park, C.; Frew, K.; Brefo, M.; Mao, S.; Heimann, M.; et al. Microfluidic Squeezing for Intracellular Antigen Loading in Polyclonal B-Cells as Cellular Vaccines. Sci. Rep. 2015, 5, 10276. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Ferreras, R.; Osuna-Perez, J.; Ramirez-Santiago, G.; Mendez-Perez, A.; Acosta-Moreno, A.M.; Del Campo, L.; Gomez-Sanchez, M.J.; Iborra, M.; Herrero-Fernandez, B.; Gonzalez-Granado, J.M.; et al. Bacteria-Instructed B Cells Cross-Prime Naïve CD8+ T Cells Triggering Effective Cytotoxic Responses. EMBO Rep. 2023, 24, e56131. [Google Scholar] [CrossRef]
| Antigen | TAAs or TSAs | Expression in Tumor | Vaccine | Ref. |
|---|---|---|---|---|
| CEA (carcinoembryonic antigen) | TAA | Increased expression of CEA is associated with adenoma carcinoma, mostly CRC | mRNA, DC-loaded cells, DNA vaccine, viral vector | [16,17,18,19] |
| RAS | TSA | Mutated in 50% of CRC patients | Peptide, mRNA | [20,21,22] |
| MUC1 (mucin-1) | TAA | Overexpressed and hypoglycosylated | Peptide, DC-based | [23,24,25] |
| RNF43 (ring finger protein 43) TOMM34 (translocase of the outer mitochondrial membrane 34) VEGFR (vascular endothelial growth factor receptor) | TAA | CTL-inducing peptide | Peptide | [26] |
| SART3 (squamous cell carcinoma antigen recognized by T cell 3) | TAA | Overexpressed in the majority of colorectal cancers | Peptide | [27] |
| β-hCG (beta-human chorionic gonadotropin) | TAA | Expressed at the invasive front of CRC and correlated with poor prognosis | Peptide | [28] |
| Survivin-2B | TAA | Overexpressed on both cancer and endothelial cells of the tumor vasculature also in CRC | Peptide | [29] |
| MYB | TAA | Transcription factor that is overexpressed in CRC | Plasmid DNA | [30] |
| 5T4 glycoprotein | TAA | Overexpressed in adenocarcinomas, included CRC | Peptide, Viral vector | [31,32] |
| Her2 | TAA | Gene alterations in CRC include amplification and missense mutations, often mirrored by protein overexpression | Peptide | [33,34] |
| Recruitment Status | Vaccine | Number of Studies | Phase 1 | Phase 1|2 | Phase 2 | Phase 2|3 | Phase 3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recruiting | Peptides | 9 | 4 | NCT05130060 NCT04117087 NCT02600949 NCT04853017 | 2 | NCT04046445 NCT03953235 | 2 | NCT04912765 NCT05243862 | 1 | NCT05141721 | - | |
| Cell-based | 2 | 1 | NCT04147078 | - | 1 | NCT02919644 | - | - | ||||
| Nucleic acids | 1 | 1 | NCT04147078 | - | - | - | - | |||||
| vector | 1 | - | - | 1 | NCT04111172 | - | - | |||||
| Not yet recruiting | Peptides | 3 | 1 | NCT04799431 | 1 | NCT05589597 | 1 | NCT05350501 | - | - | ||
| Cell-based | 1 | 1 | NCT05235607 | - | - | - | - | |||||
| Active, not recruiting | Peptides | 2 | - | 2 | NCT03639714 NCT03761914 | - | - | - | ||||
| Cell-based | 3 | 2 | NCT03730948 NCT05238558 | 1 | NCT01885702 | - | - | - | ||||
| Nucleic acids | 1 | 1 | NCT03287427 | - | - | - | - | |||||
| vector | 2 | - | 1 | NCT03563157 | 1 | NCT04491955 | - | - | ||||
| Terminated | Peptides | 5 | 1 | NCT00091286 | 2 | NCT00677612 NCT00677287 | 2 | NCT00012246 NCT01322815 | - | - | ||
| Cell-based | 3 | 1 | NCT01952730 | - | 2 | NCT00176761 NCT01505166 | - | - | ||||
| vector | 4 | 2 | NCT00088933 NCT02714374 | - | 1 | NCT03050814 | - | 1 | NCT01309126 | |||
| Completed | Peptides | 13 | 6 | NCT00641615 NCT00006387 NCT00128622 NCT00020267 NCT01522820 NCT00019006 | 4 | NCT03391232 NCT00019591 NCT00785122 NCT00861107 | 3 | NCT00773097 NCT00019084 NCT00019331 | - | - | ||
| Cell-based | 16 | 6 | NCT00558051 NCT01966289 NCT00656123 NCT01671592 NCT00027534 NCT00004604 | 5 | NCT03152565 NCT00228189 NCT00016133 NCT02176746 NCT01065441 | 5 | NCT02981524 NCT02380443 NCT00103142 NCT01413295 NCT00002475 | - | - | |||
| Nucleic acids | 2 | 1 | NCT03948763 | 1 | NCT01064375 | - | - | - | ||||
| vector | 7 | 2 | NCT01890213 NCT00924092 | 2 | NCT00088413 NCT00529984 | 2 | NCT04591379 NCT00259844 | - | 1 | NCT00427570 | ||
| Cell-based | 4 | 1 | NCT00780988 | - | 2 | NCT02615574 NCT03524274 | 1 | NCT01741038 | - | |||
| vector | 1 | - | 1 | - | - | - | ||||||
| Unknown status | Peptides | 2 | 2 | NCT03689192 NCT03552718 | - | - | - | - | ||||
| Cell-based | 4 | - | 2 | NCT00854971 NCT00722228 | 1 | NCT01348256 | - | 1 | NCT02503150 | |||
| vector | 2 | - | 1 | NCT00007826 | 1 | NCT00027833 | - | - | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinis, E.; Ricci, C.; Trevisan, C.; Tomadini, G.; Tonon, S. Cancer Vaccines: From the State of the Art to the Most Promising Frontiers in the Treatment of Colorectal Cancer. Pharmaceutics 2023, 15, 1969. https://doi.org/10.3390/pharmaceutics15071969
Martinis E, Ricci C, Trevisan C, Tomadini G, Tonon S. Cancer Vaccines: From the State of the Art to the Most Promising Frontiers in the Treatment of Colorectal Cancer. Pharmaceutics. 2023; 15(7):1969. https://doi.org/10.3390/pharmaceutics15071969
Chicago/Turabian StyleMartinis, Eleonora, Carolina Ricci, Caterina Trevisan, Gaia Tomadini, and Silvia Tonon. 2023. "Cancer Vaccines: From the State of the Art to the Most Promising Frontiers in the Treatment of Colorectal Cancer" Pharmaceutics 15, no. 7: 1969. https://doi.org/10.3390/pharmaceutics15071969
APA StyleMartinis, E., Ricci, C., Trevisan, C., Tomadini, G., & Tonon, S. (2023). Cancer Vaccines: From the State of the Art to the Most Promising Frontiers in the Treatment of Colorectal Cancer. Pharmaceutics, 15(7), 1969. https://doi.org/10.3390/pharmaceutics15071969








