Modulation of Human Dendritic Cell Functions by Phosphodiesterase-4 Inhibitors: Potential Relevance for the Treatment of Respiratory Diseases
Abstract
:1. Introduction
2. Overview of PDE4 Inhibitors
3. DC Subtypes and Functions in the Respiratory Tract
3.1. The Biology of DCs
3.1.1. DC Subpopulations
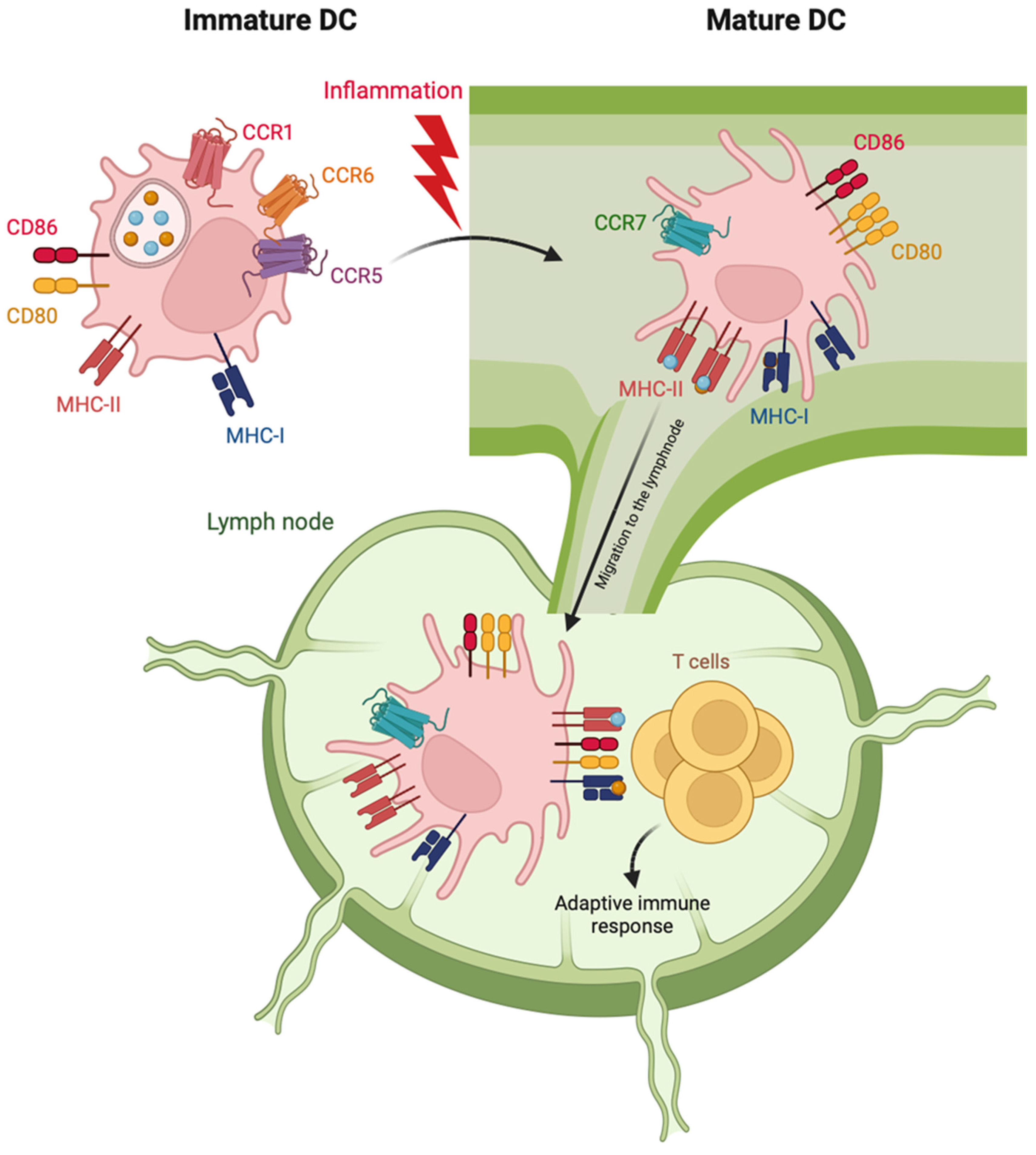
3.1.2. DC Maturation
3.1.3. Tolerogenic DCs
3.2. DCs in the Respiratory Tract
4. DC Regulation by PDE4 Inhibitors
4.1. DC Phenotype
4.2. DC Immune Functions
4.2.1. Production of Pro-Inflammatory and T-Polarizing Mediators
4.2.2. T Cell Activation
5. Pathological Role of DCs in Respiratory Diseases and Implications for PDE4 Inhibitor Therapy
5.1. COPD
5.2. Asthma
5.3. Coronavirus Disease 2019 (COVID-19)
5.4. Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALI | acute lung injury |
| ARDS | acute respiratory distress syndrome |
| BATF3 | Basic Leucine Zipper ATF-Like Transcription Factor 3 |
| cAMP | cyclic AMP |
| cDC | conventional dendritic cell |
| COPD | chronic obstructive pulmonary disease |
| DC | dendritic cell |
| GrB | Granzyme B |
| HMGB1 | high mobility group box 1 |
| IBMX | 3-isobutyl-1-methyl-xanthine |
| ID2 | inhibitor of DNA binding 2 |
| IDO | indoleamine 2,3-dioxygenase |
| IKZF1 | Ikaros family zinc finger 1 |
| IL | interleukin |
| IPF | idiopathic pulmonary fibrosis |
| IRF | interferon responsive factor |
| LPS | lipopolysaccharide |
| moDC | monocyte-derived dendritic cell |
| MHC | major histocompatibility complex |
| NECA | N-ethylcarboxamidoadenosine |
| pDC | plasmacytoid dendritic cell |
| PDE | phosphodiesterase |
| SARS | severe acute respiratory syndrome coronavirus |
| TCF4 | transcription factor |
| TGF | tumor growth factor |
| Th | T helper |
| TNF | tumor necrosis factor |
| Treg | T regulatory |
| TSLP | thymic stromal lymphopoietin |
| TSP-1 | thrombospondin-1 |
| VEGF | vascular endothelial growth factor |
References
- Serezani, C.H.; Ballinger, M.N.; Aronoff, D.M.; Peters-Golden, M. Cyclic AMP: Master regulator of innate immune cell function. Am. J. Respir. Cell. Mol. Biol. 2008, 39, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.P.; Negreiros-Lima, G.L.; Lima, K.M.; Silva, P.M.R.E.; Pinho, V.; Teixeira, M.M.; Sousa, L.P. Blame the signaling: Role of cAMP for the resolution of inflammation. Pharmacol. Res. 2020, 159, 105030. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zuo, J.; Tang, W. Phosphodiesterase-4 Inhibitors for the Treatment of Inflammatory Diseases. Front. Pharmacol. 2018, 9, 1048. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, M.; Cao, Z.; Qiu, P.; Song, G. Phosphodiesterase-4 inhibitors: A review of current developments (2013-2021). Expert. Opin. Ther. Pat. 2022, 32, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Sozzani, S.; Del Prete, A.; Bosisio, D. Dendritic cell recruitment and activation in autoimmunity. J. Autoimmun. 2017, 85, 126–140. [Google Scholar] [CrossRef]
- Heystek, H.C.; Thierry, A.C.; Soulard, P.; Moulon, C. Phosphodiesterase 4 inhibitors reduce human dendritic cell inflammatory cytokine production and Th1-polarizing capacity. Int. Immunol. 2003, 15, 827–835. [Google Scholar] [CrossRef]
- Crocetti, L.; Floresta, G.; Cilibrizzi, A.; Giovannoni, M.P. An Overview of PDE4 Inhibitors in Clinical Trials: 2010 to Early 2022. Molecules 2022, 27, 4964. [Google Scholar] [CrossRef]
- Naseem, S.; Hassan, M.; Akhtar, S.N.; Syed, F.; Khan, N.U.; Usman, M. Effectiveness of Roflumilast in Treating Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Cureus 2022, 14, e22843. [Google Scholar] [CrossRef]
- Lipari, M.; Benipal, H.; Kale-Pradhan, P. Roflumilast in the management of chronic obstructive pulmonary disease. Am. J. Health Syst. Pharm. 2013, 70, 2087–2095. [Google Scholar] [CrossRef]
- Singh, D.; Lea, S.; Mathioudakis, A.G. Inhaled Phosphodiesterase Inhibitors for the Treatment of Chronic Obstructive Pulmonary Disease. Drugs 2021, 81, 1821–1830. [Google Scholar] [CrossRef]
- Facchinetti, F.; Civelli, M.; Singh, D.; Papi, A.; Emirova, A.; Govoni, M. Tanimilast, A Novel Inhaled Pde4 Inhibitor for the Treatment of Asthma and Chronic Obstructive Pulmonary Disease. Front. Pharmacol. 2021, 12, 740803. [Google Scholar] [CrossRef] [PubMed]
- Lea, S.; Metryka, A.; Li, J.; Higham, A.; Bridgewood, C.; Villetti, G.; Civelli, M.; Facchinetti, F.; Singh, D. The modulatory effects of the PDE4 inhibitors CHF6001 and roflumilast in alveolar macrophages and lung tissue from COPD patients. Cytokine 2019, 123, 154739. [Google Scholar] [CrossRef] [PubMed]
- Moretto, N.; Caruso, P.; Bosco, R.; Marchini, G.; Pastore, F.; Armani, E.; Amari, G.; Rizzi, A.; Ghidini, E.; De Fanti, R.; et al. CHF6001 I: A novel highly potent and selective phosphodiesterase 4 inhibitor with robust anti-inflammatory activity and suitable for topical pulmonary administration. J. Pharmacol. Exp. Ther. 2015, 352, 559–567. [Google Scholar] [CrossRef]
- Bondarev, A.D.; Attwood, M.M.; Jonsson, J.; Chubarev, V.N.; Tarasov, V.V.; Liu, W.; Schiöth, H.B. Recent developments of phosphodiesterase inhibitors: Clinical trials, emerging indications and novel molecules. Front. Pharmacol. 2022, 13, 1057083. [Google Scholar] [CrossRef]
- Goonathilake, M.R.; Waqar, S.; George, S.; Jean-Baptiste, W.; Yusuf Ali, A.; Inyang, B.; Koshy, F.S.; George, K.; Poudel, P.; Chalasani, R.; et al. Can Phosphodiesterase 4 Inhibitor Therapy Be Used in Respiratory Diseases Other Than Chronic Obstructive Pulmonary Disease? Cureus 2022, 14, e27132. [Google Scholar] [CrossRef] [PubMed]
- Rennard, S.; Knobil, K.; Rabe, K.F.; Morris, A.; Schachter, N.; Locantore, N.; Canonica, W.G.; Zhu, Y.; Barnhart, F. The efficacy and safety of cilomilast in COPD. Drugs 2008, 68 (Suppl. 2), 3–57. [Google Scholar] [CrossRef]
- Zuo, H.; Cattani-Cavalieri, I.; Musheshe, N.; Nikolaev, V.O.; Schmidt, M. Phosphodiesterases as therapeutic targets for respiratory diseases. Pharmacol. Ther. 2019, 197, 225–242. [Google Scholar] [CrossRef]
- Contreras, S.; Milara, J.; Morcillo, E.; Cortijo, J. Selective Inhibition of Phosphodiesterases 4A, B, C and D Isoforms in Chronic Respiratory Diseases: Current and Future Evidences. Curr. Pharm. Des. 2017, 23, 2073–2083. [Google Scholar] [CrossRef]
- Beghè, B.; Rabe, K.F.; Fabbri, L.M. Phosphodiesterase-4 inhibitor therapy for lung diseases. Am. J. Respir. Crit. Care Med. 2013, 188, 271–278. [Google Scholar] [CrossRef]
- Kawamatawong, T. Phosphodiesterase-4 Inhibitors for Non-COPD Respiratory Diseases. Front. Pharmacol. 2021, 12, 518345. [Google Scholar] [CrossRef]
- Spina, D. Phosphodiesterase-4 inhibitors in the treatment of inflammatory lung disease. Drugs 2003, 63, 2575–2594. [Google Scholar] [CrossRef]
- Puhr, S.; Lee, J.; Zvezdova, E.; Zhou, Y.J.; Liu, K. Dendritic cell development-History, advances, and open questions. Semin. Immunol. 2015, 27, 388–396. [Google Scholar] [CrossRef]
- Guilliams, M.; Dutertre, C.A.; Scott, C.L.; McGovern, N.; Sichien, D.; Chakarov, S.; Van Gassen, S.; Chen, J.; Poidinger, M.; De Prijck, S.; et al. Unsupervised High-Dimensional Analysis Aligns Dendritic Cells across Tissues and Species. Immunity 2016, 45, 669–684. [Google Scholar] [CrossRef]
- Merad, M.; Sathe, P.; Helft, J.; Miller, J.; Mortha, A. The dendritic cell lineage: Ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 2013, 31, 563–604. [Google Scholar] [CrossRef]
- Segura, E. Human dendritic cell subsets: An updated view of their ontogeny and functional specialization. Eur. J. Immunol. 2022, 52, 1759–1767. [Google Scholar] [CrossRef]
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef]
- Dutertre, C.A.; Becht, E.; Irac, S.E.; Khalilnezhad, A.; Narang, V.; Khalilnezhad, S.; Ng, P.Y.; van den Hoogen, L.L.; Leong, J.Y.; Lee, B.; et al. Single-Cell Analysis of Human Mononuclear Phagocytes Reveals Subset-Defining Markers and Identifies Circulating Inflammatory Dendritic Cells. Immunity 2019, 51, 573–589.e578. [Google Scholar] [CrossRef]
- Brown, C.C.; Gudjonson, H.; Pritykin, Y.; Deep, D.; Lavallée, V.P.; Mendoza, A.; Fromme, R.; Mazutis, L.; Ariyan, C.; Leslie, C.; et al. Transcriptional Basis of Mouse and Human Dendritic Cell Heterogeneity. Cell 2019, 179, 846–863.e824. [Google Scholar] [CrossRef] [PubMed]
- Bourdely, P.; Anselmi, G.; Vaivode, K.; Ramos, R.N.; Missolo-Koussou, Y.; Hidalgo, S.; Tosselo, J.; Nuñez, N.; Richer, W.; Vincent-Salomon, A.; et al. Transcriptional and Functional Analysis of CD1c. Immunity 2020, 53, 335–352.e338. [Google Scholar] [CrossRef] [PubMed]
- Cytlak, U.; Resteu, A.; Pagan, S.; Green, K.; Milne, P.; Maisuria, S.; McDonald, D.; Hulme, G.; Filby, A.; Carpenter, B.; et al. Differential IRF8 Transcription Factor Requirement Defines Two Pathways of Dendritic Cell Development in Humans. Immunity 2020, 53, 353–370.e358. [Google Scholar] [CrossRef]
- Hambleton, S.; Salem, S.; Bustamante, J.; Bigley, V.; Boisson-Dupuis, S.; Azevedo, J.; Fortin, A.; Haniffa, M.; Ceron-Gutierrez, L.; Bacon, C.M.; et al. IRF8 mutations and human dendritic-cell immunodeficiency. N. Engl. J. Med. 2011, 365, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Cisse, B.; Caton, M.L.; Lehner, M.; Maeda, T.; Scheu, S.; Locksley, R.; Holmberg, D.; Zweier, C.; den Hollander, N.S.; Kant, S.G.; et al. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell 2008, 135, 37–48. [Google Scholar] [CrossRef]
- Cytlak, U.; Resteu, A.; Bogaert, D.; Kuehn, H.S.; Altmann, T.; Gennery, A.; Jackson, G.; Kumanovics, A.; Voelkerding, K.V.; Prader, S.; et al. Ikaros family zinc finger 1 regulates dendritic cell development and function in humans. Nat. Commun. 2018, 9, 1239. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, A.; Lutz, K.; Winheim, E.; Krug, A.B. What Makes a pDC: Recent Advances in Understanding Plasmacytoid DC Development and Heterogeneity. Front. Immunol. 2019, 10, 1222. [Google Scholar] [CrossRef]
- Segura, E.; Touzot, M.; Bohineust, A.; Cappuccio, A.; Chiocchia, G.; Hosmalin, A.; Dalod, M.; Soumelis, V.; Amigorena, S. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity 2013, 38, 336–348. [Google Scholar] [CrossRef]
- Boltjes, A.; van Wijk, F. Human dendritic cell functional specialization in steady-state and inflammation. Front. Immunol. 2014, 5, 131. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Tiberio, L.; Del Prete, A.; Schioppa, T.; Sozio, F.; Bosisio, D.; Sozzani, S. Chemokine and chemotactic signals in dendritic cell migration. Cell. Mol. Immunol. 2018, 15, 346–352. [Google Scholar] [CrossRef]
- Reis e Sousa, C. Activation of dendritic cells: Translating innate into adaptive immunity. Curr. Opin. Immunol. 2004, 16, 21–25. [Google Scholar] [CrossRef]
- See, P.; Dutertre, C.A.; Chen, J.; Günther, P.; McGovern, N.; Irac, S.E.; Gunawan, M.; Beyer, M.; Händler, K.; Duan, K.; et al. Mapping the human DC lineage through the integration of high-dimensional techniques. Science 2017, 356, eaag3009. [Google Scholar] [CrossRef]
- Segura, E.; Valladeau-Guilemond, J.; Donnadieu, M.H.; Sastre-Garau, X.; Soumelis, V.; Amigorena, S. Characterization of resident and migratory dendritic cells in human lymph nodes. J. Exp. Med. 2012, 209, 653–660. [Google Scholar] [CrossRef]
- Sozzani, S.; Vermi, W.; Del Prete, A.; Facchetti, F. Trafficking properties of plasmacytoid dendritic cells in health and disease. Trends Immunol. 2010, 31, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Bourque, J.; Hawiger, D. Activation, Amplification, and Ablation as Dynamic Mechanisms of Dendritic Cell Maturation. Biology 2023, 12, 716. [Google Scholar] [CrossRef] [PubMed]
- Wimmers, F.; Subedi, N.; van Buuringen, N.; Heister, D.; Vivié, J.; Beeren-Reinieren, I.; Woestenenk, R.; Dolstra, H.; Piruska, A.; Jacobs, J.F.M.; et al. Single-cell analysis reveals that stochasticity and paracrine signaling control interferon-alpha production by plasmacytoid dendritic cells. Nat. Commun. 2018, 9, 3317. [Google Scholar] [CrossRef] [PubMed]
- Bardou, M.; Postat, J.; Loaec, C.; Lemaître, F.; Ronteix, G.; Garcia, Z.; Bousso, P. Quorum sensing governs collective dendritic cell activation in vivo. EMBO J. 2021, 40, e107176. [Google Scholar] [CrossRef]
- Maney, N.J.; Reynolds, G.; Krippner-Heidenreich, A.; Hilkens, C.M.U. Dendritic cell maturation and survival are differentially regulated by TNFR1 and TNFR2. J. Immunol. 2014, 193, 4914–4923. [Google Scholar] [CrossRef]
- Pang, I.K.; Ichinohe, T.; Iwasaki, A. IL-1R signaling in dendritic cells replaces pattern-recognition receptors in promoting CD8+ T cell responses to influenza A virus. Nat. Immunol. 2013, 14, 246–253. [Google Scholar] [CrossRef]
- Iberg, C.A.; Hawiger, D. Natural and Induced Tolerogenic Dendritic Cells. J. Immunol. 2020, 204, 733–744. [Google Scholar] [CrossRef]
- Iberg, C.A.; Jones, A.; Hawiger, D. Dendritic Cells As Inducers of Peripheral Tolerance. Trends Immunol. 2017, 38, 793–804. [Google Scholar] [CrossRef]
- Manicassamy, S.; Pulendran, B. Dendritic cell control of tolerogenic responses. Immunol. Rev. 2011, 241, 206–227. [Google Scholar] [CrossRef]
- Loschko, J.; Heink, S.; Hackl, D.; Dudziak, D.; Reindl, W.; Korn, T.; Krug, A.B. Antigen targeting to plasmacytoid dendritic cells via Siglec-H inhibits Th cell-dependent autoimmunity. J. Immunol. 2011, 187, 6346–6356. [Google Scholar] [CrossRef] [PubMed]
- Chappell, C.P.; Giltiay, N.V.; Draves, K.E.; Chen, C.; Hayden-Ledbetter, M.S.; Shlomchik, M.J.; Kaplan, D.H.; Clark, E.A. Targeting antigens through blood dendritic cell antigen 2 on plasmacytoid dendritic cells promotes immunologic tolerance. J. Immunol. 2014, 192, 5789–5801. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Wentrup, F.; Benitez-Ribas, D.; Tacken, P.J.; Punt, C.J.; Figdor, C.G.; de Vries, I.J.; Adema, G.J. Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-alpha production. Blood 2008, 111, 4245–4253. [Google Scholar] [CrossRef]
- Morante-Palacios, O.; Fondelli, F.; Ballestar, E.; Martínez-Cáceres, E.M. Tolerogenic Dendritic Cells in Autoimmunity and Inflammatory Diseases. Trends Immunol. 2021, 42, 59–75. [Google Scholar] [CrossRef]
- Granot, T.; Senda, T.; Carpenter, D.J.; Matsuoka, N.; Weiner, J.; Gordon, C.L.; Miron, M.; Kumar, B.V.; Griesemer, A.; Ho, S.H.; et al. Dendritic Cells Display Subset and Tissue-Specific Maturation Dynamics over Human Life. Immunity 2017, 46, 504–515. [Google Scholar] [CrossRef]
- Vermaelen, K.; Pauwels, R. Pulmonary dendritic cells. Am. J. Respir. Crit. Care Med. 2005, 172, 530–551. [Google Scholar] [CrossRef] [PubMed]
- Sertl, K.; Takemura, T.; Tschachler, E.; Ferrans, V.J.; Kaliner, M.A.; Shevach, E.M. Dendritic cells with antigen-presenting capability reside in airway epithelium, lung parenchyma, and visceral pleura. J. Exp. Med. 1986, 163, 436–451. [Google Scholar] [CrossRef]
- Sung, S.S.; Fu, S.M.; Rose, C.E.; Gaskin, F.; Ju, S.T.; Beaty, S.R. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J. Immunol. 2006, 176, 2161–2172. [Google Scholar] [CrossRef]
- Blank, F.; Wehrli, M.; Lehmann, A.; Baum, O.; Gehr, P.; von Garnier, C.; Rothen-Rutishauser, B.M. Macrophages and dendritic cells express tight junction proteins and exchange particles in an in vitro model of the human airway wall. Immunobiology 2011, 216, 86–95. [Google Scholar] [CrossRef]
- Dieu, M.C.; Vanbervliet, B.; Vicari, A.; Bridon, J.M.; Oldham, E.; Aït-Yahia, S.; Brière, F.; Zlotnik, A.; Lebecque, S.; Caux, C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 1998, 188, 373–386. [Google Scholar] [CrossRef]
- McWilliam, A.S.; Nelson, D.; Thomas, J.A.; Holt, P.G. Rapid dendritic cell recruitment is a hallmark of the acute inflammatory response at mucosal surfaces. J. Exp. Med. 1994, 179, 1331–1336. [Google Scholar] [CrossRef]
- Peters, M.; Peters, K.; Bufe, A. Regulation of lung immunity by dendritic cells: Implications for asthma, chronic obstructive pulmonary disease and infectious disease. Innate Immun. 2019, 25, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Gianello, V.; Salvi, V.; Parola, C.; Moretto, N.; Facchinetti, F.; Civelli, M.; Villetti, G.; Bosisio, D.; Sozzani, S. The PDE4 inhibitor CHF6001 modulates pro-inflammatory cytokines, chemokines and Th1- and Th17-polarizing cytokines in human dendritic cells. Biochem. Pharmacol. 2019, 163, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.O.; Schioppa, T.; Tiberio, L.; Facchinetti, F.; Villetti, G.; Civelli, M.; Del Prete, A.; Sozio, F.; Gaudenzi, C.; Passari, M.; et al. The PDE4 Inhibitor Tanimilast Blunts Proinflammatory Dendritic Cell Activation by SARS-CoV-2 ssRNAs. Front. Immunol. 2021, 12, 797390. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.O.; Salvi, V.; Tiberio, L.; Facchinetti, F.; Govoni, M.; Villetti, G.; Civelli, M.; Barbazza, I.; Gaudenzi, C.; Passari, M.; et al. The PDE4 inhibitor tanimilast shows distinct immunomodulatory properties associated with a type 2 endotype and CD141 upregulation. J. Transl. Med. 2022, 20, 203. [Google Scholar] [CrossRef]
- Oehrl, S.; Prakash, H.; Ebling, A.; Trenkler, N.; Wölbing, P.; Kunze, A.; Döbel, T.; Schmitz, M.; Enk, A.; Schäkel, K. The phosphodiesterase 4 inhibitor apremilast inhibits Th1 but promotes Th17 responses induced by 6-sulfo LacNAc (slan) dendritic cells. J. Dermatol. Sci. 2017, 87, 110–115. [Google Scholar] [CrossRef]
- Garay, J.; D’Angelo, J.A.; Park, Y.; Summa, C.M.; Aiken, M.L.; Morales, E.; Badizadegan, K.; Fiebiger, E.; Dickinson, B.L. Crosstalk between PKA and Epac regulates the phenotypic maturation and function of human dendritic cells. J. Immunol. 2010, 185, 3227–3238. [Google Scholar] [CrossRef]
- Vlad, G.; Chang, C.C.; Colovai, A.I.; Vasilescu, E.R.; Cortesini, R.; Suciu-Foca, N. Membrane and soluble ILT3 are critical to the generation of T suppressor cells and induction of immunological tolerance. Int. Rev. Immunol. 2010, 29, 119–132. [Google Scholar] [CrossRef]
- Planès, R.; BenMohamed, L.; Leghmari, K.; Delobel, P.; Izopet, J.; Bahraoui, E. HIV-1 Tat protein induces PD-L1 (B7-H1) expression on dendritic cells through tumor necrosis factor alpha- and toll-like receptor 4-mediated mechanisms. J. Virol. 2014, 88, 6672–6689. [Google Scholar] [CrossRef]
- Sunagawa, M.; Shimada, S.; Hanashiro, K.; Nakamura, M.; Kosugi, T. Elevation of intracellular cAMP up-regulated thrombomodulin mRNA in cultured vascular endothelial cells derived from spontaneous type-II diabetes mellitus model rat. Endothelium 2006, 13, 325–333. [Google Scholar] [CrossRef]
- Weiler-Guettler, H.; Yu, K.; Soff, G.; Gudas, L.J.; Rosenberg, R.D. Thrombomodulin gene regulation by cAMP and retinoic acid in F9 embryonal carcinoma cells. Proc. Natl. Acad. Sci. USA 1992, 89, 2155–2159. [Google Scholar] [CrossRef]
- Abeyama, K.; Stern, D.M.; Ito, Y.; Kawahara, K.; Yoshimoto, Y.; Tanaka, M.; Uchimura, T.; Ida, N.; Yamazaki, Y.; Yamada, S.; et al. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J. Clin. Invest. 2005, 115, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Van de Wouwer, M.; Plaisance, S.; De Vriese, A.; Waelkens, E.; Collen, D.; Persson, J.; Daha, M.R.; Conway, E.M. The lectin-like domain of thrombomodulin interferes with complement activation and protects against arthritis. J. Thromb. Haemost. 2006, 4, 1813–1824. [Google Scholar] [CrossRef]
- Jongbloed, S.L.; Kassianos, A.J.; McDonald, K.J.; Clark, G.J.; Ju, X.; Angel, C.E.; Chen, C.J.; Dunbar, P.R.; Wadley, R.B.; Jeet, V.; et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J. Exp. Med. 2010, 207, 1247–1260. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Taguchi, O.; Toda, M.; Ruiz, D.B.; Bernabe, P.G.; D’Alessandro-Gabazza, C.N.; Miyake, Y.; Kobayashi, T.; Aoki, S.; Chiba, F.; et al. Inhibition of allergic bronchial asthma by thrombomodulin is mediated by dendritic cells. Am. J. Respir. Crit. Care Med. 2011, 183, 31–42. [Google Scholar] [CrossRef]
- Chu, C.C.; Ali, N.; Karagiannis, P.; Di Meglio, P.; Skowera, A.; Napolitano, L.; Barinaga, G.; Grys, K.; Sharif-Paghaleh, E.; Karagiannis, S.N.; et al. Resident CD141 (BDCA3)+ dendritic cells in human skin produce IL-10 and induce regulatory T cells that suppress skin inflammation. J. Exp. Med. 2012, 209, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; D’Alessandro-Gabazza, C.N.; Takagi, T.; Chelakkot-Govindalayathila, A.L.; Taguchi, O.; Roeen, Z.; Munesue, S.; Yamamoto, Y.; Yamamoto, H.; Gabazza, E.C.; et al. Thrombomodulin modulates dendritic cells via both antagonism of high mobility group protein B1 and an independent mechanism. Allergol. Int. 2014, 63, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.I.; Becker, C.; Metang, P.; Marches, F.; Wang, Y.; Toshiyuki, H.; Banchereau, J.; Merad, M.; Palucka, A.K. Human CD141+ dendritic cells induce CD4+ T cells to produce type 2 cytokines. J. Immunol. 2014, 193, 4335–4343. [Google Scholar] [CrossRef]
- Agrawal, A.; Agrawal, S.; Gupta, S. Role of Dendritic Cells in Inflammation and Loss of Tolerance in the Elderly. Front. Immunol. 2017, 8, 896. [Google Scholar] [CrossRef]
- Baban, B.; Chandler, P.R.; Sharma, M.D.; Pihkala, J.; Koni, P.A.; Munn, D.H.; Mellor, A.L. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J. Immunol. 2009, 183, 2475–2483. [Google Scholar] [CrossRef]
- Doyen, V.; Rubio, M.; Braun, D.; Nakajima, T.; Abe, J.; Saito, H.; Delespesse, G.; Sarfati, M. Thrombospondin 1 is an autocrine negative regulator of human dendritic cell activation. J. Exp. Med. 2003, 198, 1277–1283. [Google Scholar] [CrossRef]
- Lee, C.G.; Link, H.; Baluk, P.; Homer, R.J.; Chapoval, S.; Bhandari, V.; Kang, M.J.; Cohn, L.; Kim, Y.K.; McDonald, D.M.; et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat. Med. 2004, 10, 1095–1103. [Google Scholar] [CrossRef]
- Matsumoto, K.; Fukuda, S.; Nakamura, Y.; Saito, H. Amphiregulin production by human eosinophils. Int. Arch. Allergy Immunol. 2009, 149 (Suppl. 1), 39–44. [Google Scholar] [CrossRef] [PubMed]
- Kataru, R.P.; Jung, K.; Jang, C.; Yang, H.; Schwendener, R.A.; Baik, J.E.; Han, S.H.; Alitalo, K.; Koh, G.Y. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood 2009, 113, 5650–5659. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, L.A.; Sonnenberg, G.F.; Abt, M.C.; Alenghat, T.; Ziegler, C.G.; Doering, T.A.; Angelosanto, J.M.; Laidlaw, B.J.; Yang, C.Y.; Sathaliyawala, T.; et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 2011, 12, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.; Charbonnier, A.S.; Duez, C.; Jacquet, A.; Stewart, G.A.; Tonnel, A.B.; Pestel, J. Th2 polarization by Der p 1--pulsed monocyte-derived dendritic cells is due to the allergic status of the donors. Blood 2001, 98, 1135–1141. [Google Scholar] [CrossRef]
- Jirapongsananuruk, O.; Hofer, M.F.; Trumble, A.E.; Norris, D.A.; Leung, D.Y. Enhanced expression of B7.2 (CD86) in patients with atopic dermatitis: A potential role in the modulation of IgE synthesis. J. Immunol. 1998, 160, 4622–4627. [Google Scholar] [CrossRef]
- Gagliardi, M.C.; Sallusto, F.; Marinaro, M.; Langenkamp, A.; Lanzavecchia, A.; De Magistris, M.T. Cholera toxin induces maturation of human dendritic cells and licences them for Th2 priming. Eur. J. Immunol. 2000, 30, 2394–2403. [Google Scholar] [CrossRef] [PubMed]
- Gosset, P.; Bureau, F.; Angeli, V.; Pichavant, M.; Faveeuw, C.; Tonnel, A.B.; Trottein, F. Prostaglandin D2 affects the maturation of human monocyte-derived dendritic cells: Consequence on the polarization of naive Th cells. J. Immunol. 2003, 170, 4943–4952. [Google Scholar] [CrossRef]
- Kaliński, P.; Hilkens, C.M.; Snijders, A.; Snijdewint, F.G.; Kapsenberg, M.L. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J. Immunol. 1997, 159, 28–35. [Google Scholar] [CrossRef]
- la Sala, A.; Ferrari, D.; Corinti, S.; Cavani, A.; Di Virgilio, F.; Girolomoni, G. Extracellular ATP induces a distorted maturation of dendritic cells and inhibits their capacity to initiate Th1 responses. J. Immunol. 2001, 166, 1611–1617. [Google Scholar] [CrossRef]
- Bros, M.; Montermann, E.; Cholaszczyńska, A.; Reske-Kunz, A.B. The phosphodiesterase 4 inhibitor roflumilast augments the Th17-promoting capability of dendritic cells by enhancing IL-23 production, and impairs their T cell stimulatory activity due to elevated IL-10. Int. Immunopharmacol. 2016, 35, 174–184. [Google Scholar] [CrossRef]
- Lee, J.; Zhang, J.; Chung, Y.J.; Kim, J.H.; Kook, C.M.; González-Navajas, J.M.; Herdman, D.S.; Nürnberg, B.; Insel, P.A.; Corr, M.; et al. Inhibition of IRF4 in dendritic cells by PRR-independent and -dependent signals inhibit Th2 and promote Th17 responses. Elife 2020, 9, e49416. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, T.H. Fundamental role of dendritic cells in inducing Th2 responses. Korean J. Intern. Med. 2018, 33, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Challier, J.; Bruniquel, D.; Sewell, A.K.; Laugel, B. Adenosine and cAMP signalling skew human dendritic cell differentiation towards a tolerogenic phenotype with defective CD8(+) T-cell priming capacity. Immunology 2013, 138, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, C.M.; Snelgrove, R.J. Type 2 immunity: Expanding our view. Sci. Immunol. 2018, 3. [Google Scholar] [CrossRef]
- Allen, J.E.; Wynn, T.A. Evolution of Th2 immunity: A rapid repair response to tissue destructive pathogens. PLoS Pathog. 2011, 7, e1002003. [Google Scholar] [CrossRef]
- Chinn, A.M.; Insel, P.A. Cyclic AMP in dendritic cells: A novel potential target for disease-modifying agents in asthma and other allergic disorders. Br. J. Pharmacol. 2020, 177, 3363–3377. [Google Scholar] [CrossRef]
- Caramori, G.; Casolari, P.; Barczyk, A.; Durham, A.L.; Di Stefano, A.; Adcock, I. COPD immunopathology. Semin. Immunopathol. 2016, 38, 497–515. [Google Scholar] [CrossRef]
- Demedts, I.K.; Bracke, K.R.; Van Pottelberge, G.; Testelmans, D.; Verleden, G.M.; Vermassen, F.E.; Joos, G.F.; Brusselle, G.G. Accumulation of dendritic cells and increased CCL20 levels in the airways of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2007, 175, 998–1005. [Google Scholar] [CrossRef]
- Yan, L.; Wu, X.; Wu, P.; Su, B.; Xiong, Y.; Rao, Y.; Chen, X.; Huang, W.; Cui, T. Increased expression of Clec9A on cDC1s associated with cytotoxic CD8. Clin. Immunol. 2022, 242, 109082. [Google Scholar] [CrossRef]
- Naessens, T.; Morias, Y.; Hamrud, E.; Gehrmann, U.; Budida, R.; Mattsson, J.; Baker, T.; Skogberg, G.; Israelsson, E.; Thörn, K.; et al. Human Lung Conventional Dendritic Cells Orchestrate Lymphoid Neogenesis during Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2020, 202, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Emirova, A.; Francisco, C.; Santoro, D.; Govoni, M.; Nandeuil, M.A. Efficacy and safety of CHF6001, a novel inhaled PDE4 inhibitor in COPD: The PIONEER study. Respir. Res. 2020, 21, 246. [Google Scholar] [CrossRef] [PubMed]
- Boswell-Smith, V.; Spina, D. PDE4 inhibitors as potential therapeutic agents in the treatment of COPD-focus on roflumilast. Int. J. Chron. Obstruct Pulmon Dis. 2007, 2, 121–129. [Google Scholar]
- Leclerc, O.; Lagente, V.; Planquois, J.M.; Berthelier, C.; Artola, M.; Eichholtz, T.; Bertrand, C.P.; Schmidlin, F. Involvement of MMP-12 and phosphodiesterase type 4 in cigarette smoke-induced inflammation in mice. Eur. Respir. J. 2006, 27, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Martorana, P.A.; Beume, R.; Lucattelli, M.; Wollin, L.; Lungarella, G. Roflumilast fully prevents emphysema in mice chronically exposed to cigarette smoke. Am. J. Respir. Crit. Care Med. 2005, 172, 848–853. [Google Scholar] [CrossRef]
- Gamble, E.; Grootendorst, D.C.; Brightling, C.E.; Troy, S.; Qiu, Y.; Zhu, J.; Parker, D.; Matin, D.; Majumdar, S.; Vignola, A.M.; et al. Antiinflammatory effects of the phosphodiesterase-4 inhibitor cilomilast (Ariflo) in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2003, 168, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Grootendorst, D.C.; Gauw, S.A.; Verhoosel, R.M.; Sterk, P.J.; Hospers, J.J.; Bredenbröker, D.; Bethke, T.D.; Hiemstra, P.S.; Rabe, K.F. Reduction in sputum neutrophil and eosinophil numbers by the PDE4 inhibitor roflumilast in patients with COPD. Thorax 2007, 62, 1081–1087. [Google Scholar] [CrossRef]
- Oishi, K.; Matsunaga, K.; Shirai, T.; Hirai, K.; Gon, Y. Role of Type2 Inflammatory Biomarkers in Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2020, 9, 2670. [Google Scholar] [CrossRef]
- Rennard, S.I.; Calverley, P.M.; Goehring, U.M.; Bredenbröker, D.; Martinez, F.J. Reduction of exacerbations by the PDE4 inhibitor roflumilast--the importance of defining different subsets of patients with COPD. Respir. Res. 2011, 12, 18. [Google Scholar] [CrossRef]
- Buc, M.; Dzurilla, M.; Vrlik, M.; Bucova, M. Immunopathogenesis of bronchial asthma. Arch. Immunol. Ther. Exp. 2009, 57, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, M.E.; Lee, F.E.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef]
- Dua, B.; Tang, W.; Watson, R.; Gauvreau, G.; O’Byrne, P.M. Myeloid dendritic cells type 2 after allergen inhalation in asthmatic subjects. Clin. Exp. Allergy 2014, 44, 921–929. [Google Scholar] [CrossRef]
- Dua, B.; Smith, S.; Kinoshita, T.; Imaoka, H.; Gauvreau, G.; O’Byrne, P. Myeloid dendritic cells type 2 in allergic asthma. Allergy 2013, 68, 1322–1326. [Google Scholar] [CrossRef]
- Voskamp, A.L.; Tak, T.; Gerdes, M.L.; Menafra, R.; Duijster, E.; Kielbasa, S.M.; Kormelink, T.G.; Stam, K.A.; van Hengel, O.R.J.; de Jong, N.W.; et al. Inflammatory and tolerogenic myeloid cells determine outcome following human allergen challenge. J. Exp. Med. 2023, 220. [Google Scholar] [CrossRef]
- Nakano, H.; Free, M.E.; Whitehead, G.S.; Maruoka, S.; Wilson, R.H.; Nakano, K.; Cook, D.N. Pulmonary CD103(+) dendritic cells prime Th2 responses to inhaled allergens. Mucosal Immunol. 2012, 5, 53–65. [Google Scholar] [CrossRef]
- Plantinga, M.; Guilliams, M.; Vanheerswynghels, M.; Deswarte, K.; Branco-Madeira, F.; Toussaint, W.; Vanhoutte, L.; Neyt, K.; Killeen, N.; Malissen, B.; et al. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity 2013, 38, 322–335. [Google Scholar] [CrossRef]
- Medoff, B.D.; Seung, E.; Hong, S.; Thomas, S.Y.; Sandall, B.P.; Duffield, J.S.; Kuperman, D.A.; Erle, D.J.; Luster, A.D. CD11b+ myeloid cells are the key mediators of Th2 cell homing into the airway in allergic inflammation. J. Immunol. 2009, 182, 623–635. [Google Scholar] [CrossRef]
- Jirmo, A.C.; Grychtol, R.; Gaedcke, S.; Liu, B.; DeStefano, S.; Happle, C.; Halle, O.; Monteiro, J.T.; Habener, A.; Breiholz, O.D.; et al. Single cell RNA sequencing reveals distinct clusters of Irf8-expressing pulmonary conventional dendritic cells. Front. Immunol. 2023, 14, 1127485. [Google Scholar] [CrossRef]
- Huang, H.; Dawicki, W.; Lu, M.; Nayyar, A.; Zhang, X.; Gordon, J.R. Regulatory dendritic cell expression of MHCII and IL-10 are jointly requisite for induction of tolerance in a murine model of OVA-asthma. Allergy 2013, 68, 1126–1135. [Google Scholar] [CrossRef]
- Koya, T.; Matsuda, H.; Takeda, K.; Matsubara, S.; Miyahara, N.; Balhorn, A.; Dakhama, A.; Gelfand, E.W. IL-10-treated dendritic cells decrease airway hyperresponsiveness and airway inflammation in mice. J. Allergy Clin. Immunol. 2007, 119, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Nayyar, A.; Dawicki, W.; Huang, H.; Lu, M.; Zhang, X.; Gordon, J.R. Induction of prolonged asthma tolerance by IL-10-differentiated dendritic cells: Differential impact on airway hyperresponsiveness and the Th2 immunoinflammatory response. J. Immunol. 2012, 189, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Peng, Y.; Gu, Y.; Zhong, Y.; Su, C.; Liu, L.; Chai, D.; Song, T.; Zhao, N.; Yan, X.; et al. Resveratrol pretreatment mitigates LPS-induced acute lung injury by regulating conventional dendritic cells’ maturation and function. Open. Life Sci. 2021, 16, 1064–1081. [Google Scholar] [CrossRef]
- Dong, L.; He, H.L.; Lu, X.M.; Yang, Y.; Qiu, H.B. Modulation of FLT3 signaling targets conventional dendritic cells to attenuate acute lung injury. APMIS 2012, 120, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Chang, W.; Meng, S.; Xu, X.; Xie, J.; Guo, F.; Yang, Y.; Qiu, H.; Liu, L. Mesenchymal stem cells induce dendritic cell immune tolerance via paracrine hepatocyte growth factor to alleviate acute lung injury. Stem Cell. Res. Ther. 2019, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- de Heer, H.J.; Hammad, H.; Soullié, T.; Hijdra, D.; Vos, N.; Willart, M.A.; Hoogsteden, H.C.; Lambrecht, B.N. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J. Exp. Med. 2004, 200, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.F.; Song, Y.H.; Chen, J.C.; Chen, J.Q.; Wang, P. Upregulation of phosphodiesterase-4 in the lung of allergic rats. Am. J. Respir. Crit. Care Med. 2005, 171, 823–828. [Google Scholar] [CrossRef]
- Jin, S.L.; Goya, S.; Nakae, S.; Wang, D.; Bruss, M.; Hou, C.; Umetsu, D.; Conti, M. Phosphodiesterase 4B is essential for T(H)2-cell function and development of airway hyperresponsiveness in allergic asthma. J. Allergy Clin. Immunol. 2010, 126, 1252–1259.e1212. [Google Scholar] [CrossRef]
- Mokry, J.; Joskova, M.; Mokra, D.; Christensen, I.; Nosalova, G. Effects of selective inhibition of PDE4 and PDE7 on airway reactivity and cough in healthy and ovalbumin-sensitized guinea pigs. Adv. Exp. Med. Biol. 2013, 756, 57–64. [Google Scholar] [CrossRef]
- Lee, J.; Kim, T.H.; Murray, F.; Li, X.; Choi, S.S.; Broide, D.H.; Corr, M.; Webster, N.J.; Insel, P.A.; Raz, E. Cyclic AMP concentrations in dendritic cells induce and regulate Th2 immunity and allergic asthma. Proc. Natl. Acad. Sci. USA 2015, 112, 1529–1534. [Google Scholar] [CrossRef]
- Gauvreau, G.M.; Boulet, L.P.; Schmid-Wirlitsch, C.; Côté, J.; Duong, M.; Killian, K.J.; Milot, J.; Deschesnes, F.; Strinich, T.; Watson, R.M.; et al. Roflumilast attenuates allergen-induced inflammation in mild asthmatic subjects. Respir. Res. 2011, 12, 140. [Google Scholar] [CrossRef] [PubMed]
- West, E.E.; Kashyap, M.; Leonard, W.J. TSLP: A Key Regulator of Asthma Pathogenesis. Drug. Discov. Today Dis. Mech. 2012, 9. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.K.; Fan, C.; Xiang, C.G.; Wu, B.; Lu, H.M.; Feng, C.L.; Yang, X.Q.; Li, H.; Tang, W. Inhibition of PDE4 by apremilast attenuates skin fibrosis through directly suppressing activation of M1 and T cells. Acta Pharmacol. Sin. 2022, 43, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; An, T.J.; Rhee, C.K.; Park, C.K.; Kim, J.H.; Yoon, H. The effect and associated mechanism of action of phosphodiesterase 4 (PDE4) inhibitor on CD4+ lymphocyte proliferation. Clin. Exp. Pharmacol. Physiol. 2021, 48, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Kortum, R.L.; Samelson, L.E. Priming the pump: Adhesion enhances T cell antigen receptor-induced signaling. Immunity 2009, 30, 3–5. [Google Scholar] [CrossRef]
- Park, C.K.; An, T.J.; Kim, J.H.; Rhee, C.K.; Yoon, H.K. Synergistic effect of roflumilast with dexamethasone in a neutrophilic asthma mouse model. Clin. Exp. Pharmacol. Physiol. 2022, 49, 624–632. [Google Scholar] [CrossRef]
- Roeen, Z.; Toda, M.; D’Alessandro-Gabazza, C.N.; Onishi, M.; Kobayashi, T.; Yasuma, T.; Urawa, M.; Taguchi, O.; Gabazza, E.C. Thrombomodulin inhibits the activation of eosinophils and mast cells. Cell. Immunol. 2015, 293, 34–40. [Google Scholar] [CrossRef]
- Haque, S.M.; Ashwaq, O.; Sarief, A.; Azad John Mohamed, A.K. A comprehensive review about SARS-CoV-2. Future Virol. 2020, 15, 625–648. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Girija, A.S.S.; Shankar, E.M.; Larsson, M. Could SARS-CoV-2-Induced Hyperinflammation Magnify the Severity of Coronavirus Disease (CoViD-19) Leading to Acute Respiratory Distress Syndrome? Front. Immunol. 2020, 11, 1206. [Google Scholar] [CrossRef]
- Manson, J.J.; Crooks, C.; Naja, M.; Ledlie, A.; Goulden, B.; Liddle, T.; Khan, E.; Mehta, P.; Martin-Gutierrez, L.; Waddington, K.E.; et al. COVID-19-associated hyperinflammation and escalation of patient care: A retrospective longitudinal cohort study. Lancet Rheumatol. 2020, 2, e594–e602. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.J.; Peltan, I.D.; Jensen, P.; Hoda, D.; Hunter, B.; Silver, A.; Starr, N.; Buckel, W.; Grisel, N.; Hummel, E.; et al. Clinical criteria for COVID-19-associated hyperinflammatory syndrome: A cohort study. Lancet Rheumatol. 2020, 2, e754–e763. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.T.; Krishnan, V.; Chang, C.Y.; Engle, S.M.; Casalini, G.; Rodgers, G.H.; Bivi, N.; Nickoloff, B.J.; Konrad, R.J.; de Bono, S.; et al. Characterization of the cytokine storm reflects hyperinflammatory endothelial dysfunction in COVID-19. J. Allergy Clin. Immunol. 2021, 147, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Meyerowitz, E.A.; Sen, P.; Schoenfeld, S.R.; Neilan, T.G.; Frigault, M.J.; Stone, J.H.; Kim, A.Y.; Mansour, M.K. Immunomodulation as Treatment for Severe Coronavirus Disease 2019: A Systematic Review of Current Modalities and Future Directions. Clin. Infect. Dis. 2021, 72, e1130–e1143. [Google Scholar] [CrossRef]
- Alunno, A.; Najm, A.; Mariette, X.; De Marco, G.; Emmel, J.; Mason, L.; McGonagle, D.G.; Machado, P.M. Immunomodulatory therapies for the treatment of SARS-CoV-2 infection: An update of the systematic literature review to inform EULAR points to consider. RMD Open. 2021, 7. [Google Scholar] [CrossRef]
- Navarro, J.; Punzón, C.; Jiménez, J.L.; Fernández-Cruz, E.; Pizarro, A.; Fresno, M.; Muñoz-Fernández, M.A. Inhibition of phosphodiesterase type IV suppresses human immunodeficiency virus type 1 replication and cytokine production in primary T cells: Involvement of NF-kappaB and NFAT. J. Virol. 1998, 72, 4712–4720. [Google Scholar] [CrossRef]
- Secchiero, P.; Zella, D.; Curreli, S.; Mirandola, P.; Capitani, S.; Gallo, R.C.; Zauli, G. Pivotal role of cyclic nucleoside phosphodiesterase 4 in Tat-mediated CD4+ T cell hyperactivation and HIV type 1 replication. Proc. Natl. Acad. Sci. USA 2000, 97, 14620–14625. [Google Scholar] [CrossRef]
- Dalamaga, M.; Karampela, I.; Mantzoros, C.S. Commentary: Phosphodiesterase 4 inhibitors as potential adjunct treatment targeting the cytokine storm in COVID-19. Metabolism 2020, 109, 154282. [Google Scholar] [CrossRef]
- Kimura, H.; Francisco, D.; Conway, M.; Martinez, F.D.; Vercelli, D.; Polverino, F.; Billheimer, D.; Kraft, M. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J. Allergy Clin. Immunol. 2020, 146, 80–88.e88. [Google Scholar] [CrossRef]
- Sajuthi, S.P.; DeFord, P.; Jackson, N.D.; Montgomery, M.T.; Everman, J.L.; Rios, C.L.; Pruesse, E.; Nolin, J.D.; Plender, E.G.; Wechsler, M.E.; et al. Type 2 and interferon inflammation regulate SARS-CoV-2 entry factor expression in the airway epithelium. Nat. Commun. 2020, 11, 5139. [Google Scholar] [CrossRef]
- Bonser, L.R.; Eckalbar, W.L.; Rodriguez, L.; Shen, J.; Koh, K.D.; Zlock, L.T.; Christenson, S.; Woodruff, P.G.; Finkbeiner, W.E.; Erle, D.J. The type 2 asthma mediator IL-13 inhibits Severe Acute Respiratory Syndrome-Coronavirus-2 infection of bronchial epithelium. Am. J. Respir. Cell. Mol. Biol. 2022, 66, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Herbert, J.M.; Savi, P.; Laplace, M.C.; Lalé, A.; Dol, F.; Dumas, A.; Labit, C.; Minty, A. IL-4 and IL-13 exhibit comparable abilities to reduce pyrogen-induced expression of procoagulant activity in endothelial cells and monocytes. FEBS Lett. 1993, 328, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Shao, Z.; Yamaguchi, K.D.; Takagi, T.; D’Alessandro-Gabazza, C.N.; Taguchi, O.; Salamon, H.; Leung, L.L.; Gabazza, E.C.; Morser, J. Differential gene expression in thrombomodulin (TM.; CD141)(+) and TM(-) dendritic cell subsets. PLoS ONE 2013, 8, e72392. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.K.; Han, G.C.; Kim, M.; Kim, G.; Shin, H.M.; Song, K.H.; Choe, P.G.; Park, W.B.; Kim, E.S.; Kim, H.B.; et al. Aberrant hyperactivation of cytotoxic T-cell as a potential determinant of COVID-19 severity. Int. J. Infect. Dis. 2020, 97, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- De Biasi, S.; Meschiari, M.; Gibellini, L.; Bellinazzi, C.; Borella, R.; Fidanza, L.; Gozzi, L.; Iannone, A.; Lo Tartaro, D.; Mattioli, M.; et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat. Commun. 2020, 11, 3434. [Google Scholar] [CrossRef]
- Sánchez-Cerrillo, I.; Landete, P.; Aldave, B.; Sánchez-Alonso, S.; Sánchez-Azofra, A.; Marcos-Jiménez, A.; Ávalos, E.; Alcaraz-Serna, A.; de Los Santos, I.; Mateu-Albero, T.; et al. COVID-19 severity associates with pulmonary redistribution of CD1c+ DCs and inflammatory transitional and nonclassical monocytes. J. Clin. Invest. 2020, 130, 6290–6300. [Google Scholar] [CrossRef]
- Queiro Silva, R.; Armesto, S.; González Vela, C.; Naharro Fernández, C.; González-Gay, M.A. COVID-19 patients with psoriasis and psoriatic arthritis on biologic immunosuppressant therapy vs apremilast in North Spain. Dermatol. Ther. 2020, 33, e13961. [Google Scholar] [CrossRef]
- Mugheddu, C.; Pizzatti, L.; Sanna, S.; Atzori, L.; Rongioletti, F. COVID-19 pulmonary infection in erythrodermic psoriatic patient with oligodendroglioma: Safety and compatibility of apremilast with critical intensive care management. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e376–e378. [Google Scholar] [CrossRef]
- Santaniello, A.; Vigone, B.; Beretta, L. Letter to the editor: Immunomodulation by phosphodiesterase-4 inhibitor in COVID-19 patients. Metabolism 2020, 110, 154300. [Google Scholar] [CrossRef]
- Matuschak, G.M.; Lechner, A.J. Acute lung injury and the acute respiratory distress syndrome: Pathophysiology and treatment. Mo. Med. 2010, 107, 252–258. [Google Scholar]
- Li, L.; Dong, L.; Zhao, D.; Gao, F.; Yan, J. Classical dendritic cells regulate acute lung inflammation and injury in mice with lipopolysaccharide-induced acute respiratory distress syndrome. Int. J. Mol. Med. 2019, 44, 617–629. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, P.S.; Yu, Q.; Liu, L.; Yang, Y.; Qiu, H.B. Kinetic and distinct distribution of conventional dendritic cells in the early phase of lipopolysaccharide-induced acute lung injury. Mol. Biol. Rep. 2012, 39, 10421–10431. [Google Scholar] [CrossRef] [PubMed]
- von Wulffen, W.; Steinmueller, M.; Herold, S.; Marsh, L.M.; Bulau, P.; Seeger, W.; Welte, T.; Lohmeyer, J.; Maus, U.A. Lung dendritic cells elicited by Fms-like tyrosine 3-kinase ligand amplify the lung inflammatory response to lipopolysaccharide. Am. J. Respir. Crit. Care Med. 2007, 176, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Kosutova, P.; Mikolka, P.; Kolomaznik, M.; Rezakova, S.; Calkovska, A.; Mokra, D. Effects of roflumilast, a phosphodiesterase-4 inhibitor, on the lung functions in a saline lavage-induced model of acute lung injury. Physiol. Res. 2017, 66, S237–S245. [Google Scholar] [CrossRef] [PubMed]
- Rocco, P.R.; Momesso, D.P.; Figueira, R.C.; Ferreira, H.C.; Cadete, R.A.; Légora-Machado, A.; Koatz, V.L.; Lima, L.M.; Barreiro, E.J.; Zin, W.A. Therapeutic potential of a new phosphodiesterase inhibitor in acute lung injury. Eur. Respir. J. 2003, 22, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Imam, F.; Al-Harbi, N.O.; Al-Harbi, M.M.; Qamar, W.; Aljerian, K.; Belali, O.M.; Alsanea, S.; Alanazi, A.Z.; Alhazzani, K. Apremilast ameliorates carfilzomib-induced pulmonary inflammation and vascular injuries. Int. Immunopharmacol. 2019, 66, 260–266. [Google Scholar] [CrossRef]
- Yang, D.; Yang, Y.; Zhao, Y. Ibudilast, a Phosphodiesterase-4 Inhibitor, Ameliorates Acute Respiratory Distress Syndrome in Neonatal Mice by Alleviating Inflammation and Apoptosis. Med. Sci. Monit. 2020, 26, e922281. [Google Scholar] [CrossRef]
- Sharma, G.; Champalal Sharma, D.; Hwei Fen, L.; Pathak, M.; Bethur, N.; Pendharkar, V.; Peiris, M.; Altmeyer, R. Reduction of influenza virus-induced lung inflammation and mortality in animals treated with a phosophodisestrase-4 inhibitor and a selective serotonin reuptake inhibitor. Emerg. Microbes Infect. 2013, 2, e54. [Google Scholar] [CrossRef]
- Park, S.J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012, 148, 421–433. [Google Scholar] [CrossRef]


| Drug Name | Company | Indications | Phase | NCT Number | Comments |
|---|---|---|---|---|---|
| Roflumilast (Daliresp) | AstraZeneca | COPD | n/a | Approved | Approved in EU (2010) and in the USA (2011) for the management of severe COPD. |
| Bronchiec- tasis | II | NCT03988816 | Efficacy on lung function and mucus properties. | ||
| Tanimilast (inhaled) | Chiesi Farmaceutici | COPD | III | NCT04636801 (Pilaster) | Placebo-controlled (Pilaster) and active (Roflumilast)-controlled (Pillar) 52-week studies for efficacy and safety as an add-on to maintenance triple therapy in COPD and chronic bronchitis. |
| III | NCT04636814 (Pillar) | ||||
| Asthma | II | NCT01689571 | Efficacy, tolerability and safety of Tanimilast in asthmatic patients. | ||
| Ensifentrine (RPL554) | Verona Pharma | COPD | II | NCT05270525 | Under evaluation as an add-on to standard of care treatments. |
| Asthma | II | NCT02427165 | |||
| COVID-19 | II | NCT04527471 | |||
| BI 1015550 | Boehringer Ingelheim International | Idiopathic Pulmonary Fibrosis | III | NCT05321069 | Evaluate long-term efficacy and safety of BI 1015550. |
| Apremilast (Otezla) | Amgen | COVID-19 | III | NCT04590586 | Efficacy and safety of Apremilast as add-on to standard of care in hospitalized patients. |
| Cilomilast (Ariflo) | GSK | COPD | III | NCT00103922 | Completed 24-week study for safety and efficacy in COPD patients (development terminated). |
| GSK256066 (inhaled) | GSK | COPD | II | NCT00549679 | Safety and tolerability in mild to moderate COPD patients (development terminated). |
| Tetomilast (OPC-6535) | Otsuka Pharmaceutical | COPD | II | NCT00917150 | 24-month study for efficacy and safety of OPC-6535 in COPD patients (last update 2021). |
| Oglemilast | Forest Laboratories | COPD | II | NCT00671073 | 14-week study for safety and efficacy of a range of Oglemilast doses (last update 2019). |
| Asthma | II | NCT00322686 | Prevention of bronchoconstriction after the administration of allergen in mild asthma patients (last update 2012). | ||
| Revamilast | Glenmark Pharmaceuticals Ltd. India | Asthma | II | NCT01436890 | 12-week study for effects of Revamilast in patients with chronic persistent asthma (last update 2013). |
| Animal Model | Findings | Reference |
|---|---|---|
| Bronchial asthma model (GnasΔCD11c mice) | Adoptive transfer of OVA-loaded DCs from GnasΔCD11c mice induced Th2 response and airway inflammation in WT and GnasΔCD11c mice. Adoptive transfer of 8-CPT-cAMP-treated DCs from GnasΔCD11c mice reduced Th2 development and airway inflammation in recipient mice. | [130] |
| LPS induced ALI in mice | Adoptive transfer of Resveratrol-treated DCs to WT mice before LPS challenge enhanced survival rates, reduced lung tissue damage and lowered the expression of Th17 cells in the lung. | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.O.; Tiberio, L.; Facchinetti, F.; Ripari, G.; Violi, V.; Villetti, G.; Salvi, V.; Bosisio, D. Modulation of Human Dendritic Cell Functions by Phosphodiesterase-4 Inhibitors: Potential Relevance for the Treatment of Respiratory Diseases. Pharmaceutics 2023, 15, 2254. https://doi.org/10.3390/pharmaceutics15092254
Nguyen HO, Tiberio L, Facchinetti F, Ripari G, Violi V, Villetti G, Salvi V, Bosisio D. Modulation of Human Dendritic Cell Functions by Phosphodiesterase-4 Inhibitors: Potential Relevance for the Treatment of Respiratory Diseases. Pharmaceutics. 2023; 15(9):2254. https://doi.org/10.3390/pharmaceutics15092254
Chicago/Turabian StyleNguyen, Hoang Oanh, Laura Tiberio, Fabrizio Facchinetti, Giulia Ripari, Valentina Violi, Gino Villetti, Valentina Salvi, and Daniela Bosisio. 2023. "Modulation of Human Dendritic Cell Functions by Phosphodiesterase-4 Inhibitors: Potential Relevance for the Treatment of Respiratory Diseases" Pharmaceutics 15, no. 9: 2254. https://doi.org/10.3390/pharmaceutics15092254








