Targeted Bioluminescent Imaging of Pancreatic Ductal Adenocarcinoma Using Nanocarrier-Complexed EGFR-Binding Affibody–Gaussia Luciferase Fusion Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmid Construction
2.2. Expression and Purification of Recombinant Proteins
2.3. G5-PAMAM–ZEGFR-GLuc Complex Formulation
2.4. Cell Lines and Cell Lysate Preparation
2.5. EGFR Selectivity in Cells
2.6. Fluorescent Tagging and Microscopy
2.7. Mouse Models and Animal Studies
2.8. Statistics
3. Results and Discussion
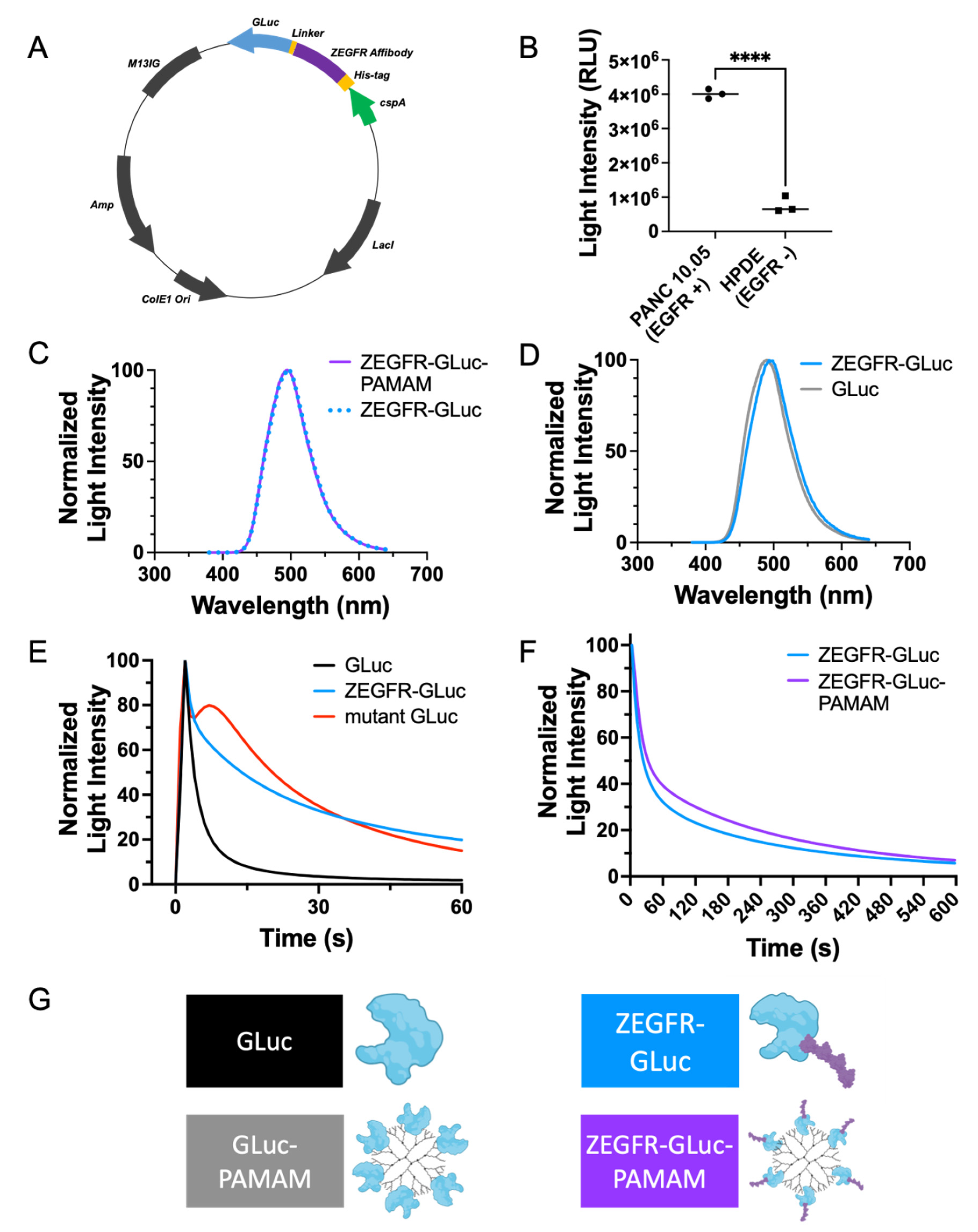
3.1. Development of a Fusion Protein and Protein–Dendrimer Complex
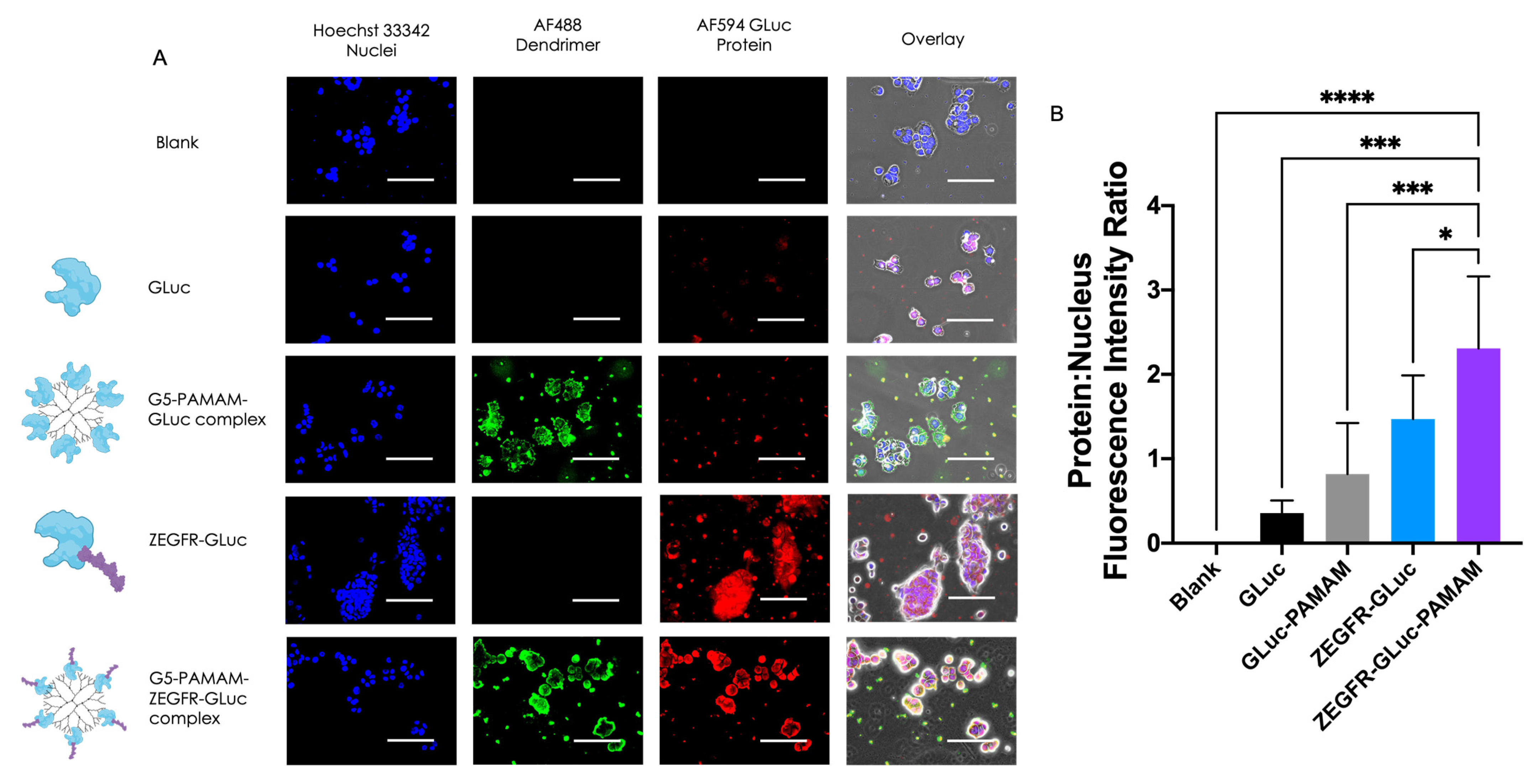
3.2. In Vitro Delivery in Pancreatic Cancer Cells
3.3. In Vivo Delivery in Pancreatic Cancer Xenograft Mouse Models
3.4. In Vivo Tumor Margin Delineation and Location of Metastatic Cancer Cells
3.5. Additional Capabilities and Applications of Targeted Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Condeelis, J.; Weissleder, R. In Vivo Imaging in Cancer. Cold Spring Harb. Perspect. Biol. 2010, 2, a003848. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Yuste, R. In vivo imaging of neural activity. Nat. Methods 2017, 14, 349–359. [Google Scholar] [CrossRef] [Green Version]
- Isherwood, B.; Timpson, P.; Mcghee, E.J.; Anderson, K.I.; Canel, M.; Serrels, A.; Brunton, V.G.; Carragher, N.O. Live cell in vitro and in vivo imaging applications: Accelerating drug discovery. Pharmaceutics 2011, 3, 141–170. [Google Scholar] [CrossRef] [Green Version]
- Hunter, P. Illuminating human disease. EMBO Rep. 2019, 20, e49195. [Google Scholar] [CrossRef]
- Badr, C.E.; Tannous, B.A. Bioluminescence imaging: Progress and applications. Trends Biotechnol. 2011, 29, 624–633. [Google Scholar] [CrossRef] [Green Version]
- Mezzanotte, L.; van ‘t Root, M.; Karatas, H.; Goun, E.A.; Löwik, C.W.G.M. In Vivo Molecular Bioluminescence Imaging: New Tools and Applications. Trends Biotechnol. 2017, 35, 640–652. [Google Scholar] [CrossRef]
- Tung, J.K.; Berglund, K.; Gutekunst, C.-A.; Hochgeschwender, U.; Gross, R.E. Bioluminescence imaging in live cells and animals. Neurophotonics 2016, 3, 025001. [Google Scholar] [CrossRef]
- Joda, H.; Moutsiopoulou, A.; Stone, G.; Daunert, S.; Deo, S. Design of Gaussia luciferase-based bioluminescent stem-loop probe for sensitive detection of HIV-1 nucleic acids. Analyst 2018, 143, 3374–3381. [Google Scholar] [CrossRef]
- Yeh, H.W.; Xiong, Y.; Wu, T.; Chen, M.; Ji, A.; Li, X.; Ai, H.W. ATP-Independent Bioluminescent Reporter Variants to Improve in Vivo Imaging. ACS Chem. Biol. 2019, 14, 959–965. [Google Scholar] [CrossRef]
- Dragulescu-Andrasi, A.; Chan, C.T.; De, A.; Massoud, T.F.; Gambhir, S.S. Bioluminescence resonance energy transfer (BRET) imaging of protein-protein interactions within deep tissues of living subjects. Proc. Natl. Acad. Sci. USA 2011, 108, 12060–12065. [Google Scholar] [CrossRef]
- Lu, L.; Li, B.; Ding, S.; Fan, Y.; Wang, S.; Sun, C.; Zhao, M.; Zhao, C.X.; Zhang, F. NIR-II bioluminescence for in vivo high contrast imaging and in situ ATP-mediated metastases tracing. Nat. Commun. 2020, 11, 4192. [Google Scholar] [CrossRef]
- Nishiguchi, T.; Yamada, T.; Nasu, Y.; Ito, M.; Yoshimura, H.; Ozawa, T. Development of red-shifted mutants derived from luciferase of Brazilian click beetle Pyrearinus termitilluminans. J. Biomed. Opt. 2015, 20, 101205. [Google Scholar] [CrossRef]
- Zambito, G.; Hall, M.P.; Wood, M.G.; Gaspar, N.; Ridwan, Y.; Stellari, F.F.; Shi, C.; Kirkland, T.A.; Encell, L.P.; Löwik, C.; et al. Red-shifted click beetle luciferase mutant expands the multicolor bioluminescent palette for deep tissue imaging. iScience 2021, 24, 101986. [Google Scholar] [CrossRef]
- Dorsaz, S.; Coste, A.T.; Sanglard, D. Red-shifted firefly luciferase optimized for Candida albicans in vivo bioluminescence imaging. Front. Microbiol. 2017, 8, 1478. [Google Scholar] [CrossRef] [Green Version]
- Calabretta, M.M.; Gregucci, D.; Martínez-Pérez-Cejuela, H.; Michelini, E. A Luciferase Mutant with Improved Brightness and Stability for Whole-Cell Bioluminescent Biosensors and In Vitro Biosensing. Biosensors 2022, 12, 742. [Google Scholar] [CrossRef]
- Han, X.J.; Wei, Y.F.; Wan, Y.Y.; Jiang, L.P.; Zhang, J.F.; Xin, H.B. Development of a novel liposomal nanodelivery system for bioluminescence imaging and targeted drug delivery in ErbB2-overexpressing metastatic ovarian carcinoma. Int. J. Mol. Med. 2014, 34, 1225–1232. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.X.; Lin, Y.Y.; Zhao, C.F.; Chen, W.B.; Liu, Q.C.; Li, Q.W.; Gao, F. Pancreatic cancer: A review of epidemiology, trend, and risk factors. World J. Gastroenterol. 2021, 27, 4298–4321. [Google Scholar] [CrossRef]
- Gheorghe, G.; Bungau, S.; Ilie, M.; Behl, T.; Vesa, C.M.; Brisc, C.; Bacalbasa, N.; Turi, V.; Costache, R.S.; Diaconu, C.C. Early diagnosis of pancreatic cancer: The key for survival. Diagnostics 2020, 10, 869. [Google Scholar] [CrossRef]
- American Cancer Society Cancer Statistics Center. Available online: http://cancerstatisticscenter.cancer.org (accessed on 10 July 2023).
- Wood, L.D.; Canto, M.I.; Jaffee, E.M.; Simeone, D.M. Pancreatic Cancer: Pathogenesis, Screening, Diagnosis, and Treatment. Gastroenterology 2022, 163, 386–402.e1. [Google Scholar] [CrossRef]
- Grapa, C.M.; Mocan, T.; Gonciar, D.; Zdrehus, C.; Mosteanu, O.; Pop, T.; Mocan, L. Epidermal Growth Factor Receptor and Its Role in Pancreatic Cancer Treatment Mediated by Nanoparticles. Int. J. Nanomed. 2019, 14, 9693–9706. [Google Scholar] [CrossRef] [Green Version]
- Oliveira-Cunha, M.; Newman, W.G.; Siriwardena, A.K. Epidermal growth factor receptor in pancreatic cancer. Cancers 2011, 3, 1513–1526. [Google Scholar] [CrossRef]
- Ståhl, S.; Gräslund, T.; Eriksson Karlström, A.; Frejd, F.Y.; Nygren, P.-Å.; Löfblom, J. Affibody Molecules in Biotechnological and Medical Applications. Trends Biotechnol. 2017, 35, 691–712. [Google Scholar] [CrossRef]
- Yu, X.; Yang, Y.-P.; Dikici, E.; Deo, S.K.; Daunert, S. Beyond Antibodies as Binding Partners: The Role of Antibody Mimetics in Bioanalysis. Annu. Rev. Anal. Chem. 2017, 10, 293–320. [Google Scholar] [CrossRef] [Green Version]
- Tannous, B.A.; Kim, D.E.; Fernandez, J.L.; Weissleder, R.; Breakefield, X.O. Codon-optimized gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol. Ther. 2005, 11, 435–443. [Google Scholar] [CrossRef]
- Maguire, C.A.; Deliolanis, N.C.; Pike, L.; Niers, J.M.; Tjon-Kon-Fat, L.A.; Sena-Esteves, M.; Tannous, B.A. Gaussia luciferase variant for high-throughput functional screening applications. Anal. Chem. 2009, 81, 7102–7106. [Google Scholar] [CrossRef] [Green Version]
- El-Amouri, S.S.; Cao, P.; Miao, C.; Pan, D. Secreted Luciferase for In Vivo Evaluation of Systemic Protein Delivery in Mice. Mol. Biotechnol. 2013, 53, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Hunt, E.A.; Moutsiopoulou, A.; Ioannou, S.; Ahern, K.; Woodward, K.; Dikici, E.; Daunert, S.; Deo, S.K. Truncated Variants of Gaussia Luciferase with Tyrosine Linker for Site-Specific Bioconjugate Applications. Sci. Rep. 2016, 6, 26814. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.B.; Suzuki, H.; Sato, M.; Tao, H. Superluminescent variants of marine luciferases for bioassays. Anal. Chem. 2011, 83, 8732–8740. [Google Scholar] [CrossRef]
- Adamczyk, K.A.; Klein-Scory, S.; Tehrani, M.M.; Warnken, U.; Schmiegel, W.; Schnölzer, M.; Schwarte-Waldhoff, I. Characterization of soluble and exosomal forms of the EGFR released from pancreatic cancer cells. Life Sci. 2011, 89, 304–312. [Google Scholar] [CrossRef]
- Leroux, C.; Konstantinidou, G. Targeted therapies for pancreatic cancer: Overview of current treatments and new opportunities for personalized oncology. Cancers 2021, 13, 799. [Google Scholar] [CrossRef]
- Philip, P.A.; Lutz, M.P. Targeting Epidermal Growth Factor Receptor–Related Signaling Pathways in Pancreatic Cancer. Pancreas 2015, 44, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Haggblad Sahlberg, S.; Spiegelberg, D.; Lennartsson, J.; Nygren, P.; Glimelius, B.; Stenerlow, B. The effect of a dimeric Affibody molecule (ZEGFR:1907)2 targeting EGFR in combination with radiation in colon cancer cell lines. Int. J. Oncol. 2011, 40, 176–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazarova, A.; Yakimova, L.; Mostovaya, O.; Kulikova, T.; Mikhailova, O.; Evtugyn, G.; Ganeeva, I.; Bulatov, E.; Stoikov, I. Encapsulation of the quercetin with interpolyelectrolyte complex based on pillar[5]arenes. J. Mol. Liq. 2022, 368, 120807. [Google Scholar] [CrossRef]
- Smith, B.R.; Gambhir, S.S. Nanomaterials for in Vivo Imaging. Chem. Rev. 2017, 117, 901–986. [Google Scholar] [CrossRef]
- Taghavi Pourianazar, N.; Mutlu, P.; Gunduz, U. Bioapplications of poly(amidoamine) (PAMAM) dendrimers in nanomedicine. J. Nanopart. Res. 2014, 16, 2342. [Google Scholar] [CrossRef]
- Wang, B.; Sun, Y.; Davis, T.P.; Ke, P.C.; Wu, Y.; Ding, F. Understanding Effects of PAMAM Dendrimer Size and Surface Chemistry on Serum Protein Binding with Discrete Molecular Dynamics Simulations. ACS Sustain. Chem. Eng. 2018, 6, 11704–11715. [Google Scholar] [CrossRef]
- Liu, Z.-J.J.; Daftarian, P.; Kovalski, L.; Wang, B.; Tian, R.; Castilla, D.M.; Dikici, E.; Perez, V.L.; Deo, S.; Daunert, S.; et al. Directing and Potentiating Stem Cell-Mediated Angiogenesis and Tissue Repair by Cell Surface E-Selectin Coating. PLoS ONE 2016, 11, e0154053. [Google Scholar] [CrossRef] [Green Version]
- Hersh, J.; Condor Capcha, J.M.; Iansen Irion, C.; Lambert, G.; Noguera, M.; Singh, M.; Kaur, A.; Dikici, E.; Jiménez, J.J.; Shehadeh, L.A.; et al. Peptide-functionalized dendrimer nanocarriers for targeted microdystrophin gene delivery. Pharmaceutics 2021, 13, 2159. [Google Scholar] [CrossRef]
- Tummers, W.S.; Miller, S.E.; Teraphongphom, N.T.; van den Berg, N.S.; Hasan, A.; Longacre, T.A.; Fisher, G.A.; Bonsing, B.A.; Vahrmeijer, A.L.; Gambhir, S.S.; et al. Detection of visually occult metastatic lymph nodes using molecularly targeted fluorescent imaging during surgical resection of pancreatic cancer. HPB 2019, 21, 883–890. [Google Scholar] [CrossRef]
- Mondal, G.; Kumar, V.; Shukla, S.K.; Singh, P.K.; Mahato, R.I. EGFR-Targeted Polymeric Mixed Micelles Carrying Gemcitabine for Treating Pancreatic Cancer. Biomacromolecules 2016, 17, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Mondal, G.; Almawash, S.; Chaudhary, A.K.; Mahato, R.I. EGFR-Targeted Cationic Polymeric Mixed Micelles for Codelivery of Gemcitabine and miR-205 for Treating Advanced Pancreatic Cancer. Mol. Pharm. 2017, 14, 3121–3133. [Google Scholar] [CrossRef] [PubMed]
- Patra, C.R.; Bhattacharya, R.; Wang, E.; Katarya, A.; Lau, J.S.; Dutta, S.; Muders, M.; Wang, S.; Buhrow, S.A.; Safgren, S.L.; et al. Targeted delivery of gemcitabine to pancreatic adenocarcinoma using cetuximab as a targeting agent. Cancer Res. 2008, 68, 1970–1978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahajan, U.M.; Li, Q.; Alnatsha, A.; Maas, J.; Orth, M.; Maier, S.H.; Peterhansl, J.; Regel, I.; Sendler, M.; Wagh, P.R.; et al. Tumor-Specific Delivery of 5-Fluorouracil–Incorporated Epidermal Growth Factor Receptor–Targeted Aptamers as an Efficient Treatment in Pancreatic Ductal Adenocarcinoma Models. Gastroenterology 2021, 161, 996–1010.e1. [Google Scholar] [CrossRef]
- Singh, A.; Xu, J.; Mattheolabakis, G.; Amiji, M. EGFR-targeted gelatin nanoparticles for systemic administration of gemcitabine in an orthotopic pancreatic cancer model. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 589–600. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Mao, H.; Wang, Y.A.; Cao, Z.; Peng, X.; Wang, X.; Duan, H.; Ni, C.; Yuan, Q.; Adams, G.; et al. Single Chain Epidermal Growth Factor Receptor Antibody Conjugated Nanoparticles for in vivo Tumor Targeting and Imaging. Small 2008, 5, 235–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katti, G.; Arshiya Ara, S.; Shireen, A. Magnetic Resonance Imaging (MRI)—A Review. Int. J. Dent. Clin. 2011, 3, 65–70. [Google Scholar]
- Oldendorf, M.D.; Oldendorf, W., Jr. Advantages and Disadvantages of MRI. In Basics of Magnetic Resonance Imaging; Springer: Berlin/Heidelberg, Germany, 1988; pp. 125–138. ISBN 978-1-4612-9234-0. [Google Scholar]
- Zhang, L.; Sanagapalli, S.; Stoita, A. Challenges in diagnosis of pancreatic cancer. World J. Gastroenterol. 2018, 24, 2047–2060. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.W.; Huang, V.; Arguelles, G.R.; Zhou, C.; Cho, S.S.; Harmsen, S.; Lee, J.Y.K. Applications of indocyanine green in brain tumor surgery: Review of clinical evidence and emerging technologies. Neurosurg. Focus 2021, 50, E4. [Google Scholar] [CrossRef]
- Ghoroghchian, P.P.; Therien, M.J.; Hammer, D.A. In vivo fluorescence imaging: A personal perspective. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 156–167. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Chen, G.; Zhang, Y.; Wu, F.; Wang, Q. Advanced fluorescence imaging technology in the near-infrared-II window for biomedical applications. J. Am. Chem. Soc. 2020, 142, 14789–14804. [Google Scholar] [CrossRef]
- Refaat, A.; Yap, M.L.; Pietersz, G.; Walsh, A.P.G.; Zeller, J.; del Rosal, B.; Wang, X.; Peter, K. In vivo fluorescence imaging: Success in preclinical imaging paves the way for clinical applications. J. Nanobiotechnol. 2022, 20, 450. [Google Scholar] [CrossRef] [PubMed]
- Sexton, K.; Tichauer, K.; Samkoe, K.S.; Gunn, J.; Hoopes, P.J.; Pogue, B.W. Fluorescent Affibody Peptide Penetration in Glioma Margin Is Superior to Full Antibody. PLoS ONE 2013, 8, e60390. [Google Scholar] [CrossRef] [Green Version]
- Tolmachev, V.; Friedman, M.; Sandström, M.; Eriksson, T.L.J.; Rosik, D.; Hodik, M.; Ståhl, S.; Frejd, F.Y.; Orlova, A. Affibody molecules for epidermal growth factor receptor targeting in vivo: Aspects of dimerization and labeling chemistry. J. Nucl. Med. 2009, 50, 274–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hersh, J.; Broyles, D.; Capcha, J.M.C.; Dikici, E.; Shehadeh, L.A.; Daunert, S.; Deo, S. Peptide-Modified Biopolymers for Biomedical Applications. ACS Appl. Bio Mater. 2021, 4, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef] [Green Version]
- Yun, Y.; Cho, Y.W.; Park, K. Nanoparticles for oral delivery: Targeted nanoparticles with peptidic ligands for oral protein delivery. Adv. Drug Deliv. Rev. 2013, 65, 822–832. [Google Scholar] [CrossRef] [Green Version]
- Srinivasarao, M.; Low, P.S. Ligand-Targeted Drug Delivery. Chem. Rev. 2017, 117, 12133–12164. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mukherjee, S.; Abourehab, M.A.S.; Sahebkar, A.; Kesharwani, P. Exploring dendrimer-based drug delivery systems and their potential applications in cancer immunotherapy. Eur. Polym. J. 2022, 177, 111471. [Google Scholar] [CrossRef]
- Gauro, R.; Nandave, M.; Jain, V.K.; Jain, K. Advances in dendrimer-mediated targeted drug delivery to the brain. J. Nanopart. Res. 2021, 23, 76. [Google Scholar] [CrossRef]
- Abedi-Gaballu, F.; Dehghan, G.; Ghaffari, M.; Yekta, R.; Abbaspour-Ravasjani, S.; Baradaran, B.; Ezzati Nazhad Dolatabadi, J.; Hamblin, M.R. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today 2018, 12, 177–190. [Google Scholar] [CrossRef]
- Fatemi, S.M.; Fatemi, S.J.; Abbasi, Z. PAMAM dendrimer-based macromolecules and their potential applications: Recent advances in theoretical studies. Polym. Bull. 2020, 77, 6671–6691. [Google Scholar] [CrossRef]
- De Araújo, R.V.; da Silva Santos, S.; Ferreira, E.I.; Giarolla, J. New advances in general biomedical applications of PAMAM dendrimers. Molecules 2018, 23, 2849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Dąbkowska, M.; Łuczkowska, K.; Rogińska, D.; Sobuś, A.; Wasilewska, M.; Ulańczyk, Z.; Machaliński, B. Novel design of (PEG-ylated)PAMAM-based nanoparticles for sustained delivery of BDNF to neurotoxin-injured differentiated neuroblastoma cells. J. Nanobiotechnol. 2020, 18, 120. [Google Scholar] [CrossRef]
- Márquez-Miranda, V.; Abrigo, J.; Rivera, J.C.; Araya-Durán, I.; Aravena, J.; Simon, F.; Pacheco, N.; González-Nilo, F.D.; Cabello-Verrugio, C. The complex of PAMAM-OH dendrimer with angiotensin (1–7) prevented the disuse-induced skeletal muscle atrophy in mice. Int. J. Nanomed. 2017, 12, 1985–1999. [Google Scholar] [CrossRef] [Green Version]
- Virostko, J.; Chen, Z.; Fowler, M.; Poffenberger, G.; Powers, A.C.; Duco Jansen, E. Factors influencing quantification of in vivo bioluminescence imaging: Application to assessment of pancreatic islet transplants. Mol. Imaging 2004, 3, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Debie, P.; Hernot, S. Emerging Fluorescent Molecular Tracers to Guide Intra-Operative Surgical Decision-Making. Front. Pharmacol. 2019, 10, 510. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Angelova, A.; Garamus, V.M.; Angelov, B.; Tu, S.; Kong, L.; Zhang, X.; Li, N.; Zou, A. Mitochondrial Voltage-Dependent Anion Channel 1-Hexokinase-II Complex-Targeted Strategy for Melanoma Inhibition Using Designed Multiblock Peptide Amphiphiles. ACS Appl. Mater. Interfaces 2021, 13, 35281–35293. [Google Scholar] [CrossRef]
- Todaro, B.; Ottalagana, E.; Luin, S. Targeting Peptides: The New Generation of Targeted Drug Delivery Systems. Pharmaceutics 2023, 15, 1648. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hersh, J.; Yang, Y.-P.; Roberts, E.; Bilbao, D.; Tao, W.; Pollack, A.; Daunert, S.; Deo, S.K. Targeted Bioluminescent Imaging of Pancreatic Ductal Adenocarcinoma Using Nanocarrier-Complexed EGFR-Binding Affibody–Gaussia Luciferase Fusion Protein. Pharmaceutics 2023, 15, 1976. https://doi.org/10.3390/pharmaceutics15071976
Hersh J, Yang Y-P, Roberts E, Bilbao D, Tao W, Pollack A, Daunert S, Deo SK. Targeted Bioluminescent Imaging of Pancreatic Ductal Adenocarcinoma Using Nanocarrier-Complexed EGFR-Binding Affibody–Gaussia Luciferase Fusion Protein. Pharmaceutics. 2023; 15(7):1976. https://doi.org/10.3390/pharmaceutics15071976
Chicago/Turabian StyleHersh, Jessica, Yu-Ping Yang, Evan Roberts, Daniel Bilbao, Wensi Tao, Alan Pollack, Sylvia Daunert, and Sapna K. Deo. 2023. "Targeted Bioluminescent Imaging of Pancreatic Ductal Adenocarcinoma Using Nanocarrier-Complexed EGFR-Binding Affibody–Gaussia Luciferase Fusion Protein" Pharmaceutics 15, no. 7: 1976. https://doi.org/10.3390/pharmaceutics15071976
APA StyleHersh, J., Yang, Y.-P., Roberts, E., Bilbao, D., Tao, W., Pollack, A., Daunert, S., & Deo, S. K. (2023). Targeted Bioluminescent Imaging of Pancreatic Ductal Adenocarcinoma Using Nanocarrier-Complexed EGFR-Binding Affibody–Gaussia Luciferase Fusion Protein. Pharmaceutics, 15(7), 1976. https://doi.org/10.3390/pharmaceutics15071976







