Natural Polymeric Nanobiocomposites for Anti-Cancer Drug Delivery Therapeutics: A Recent Update
Abstract
:1. Introduction
2. Cancer
2.1. General Features
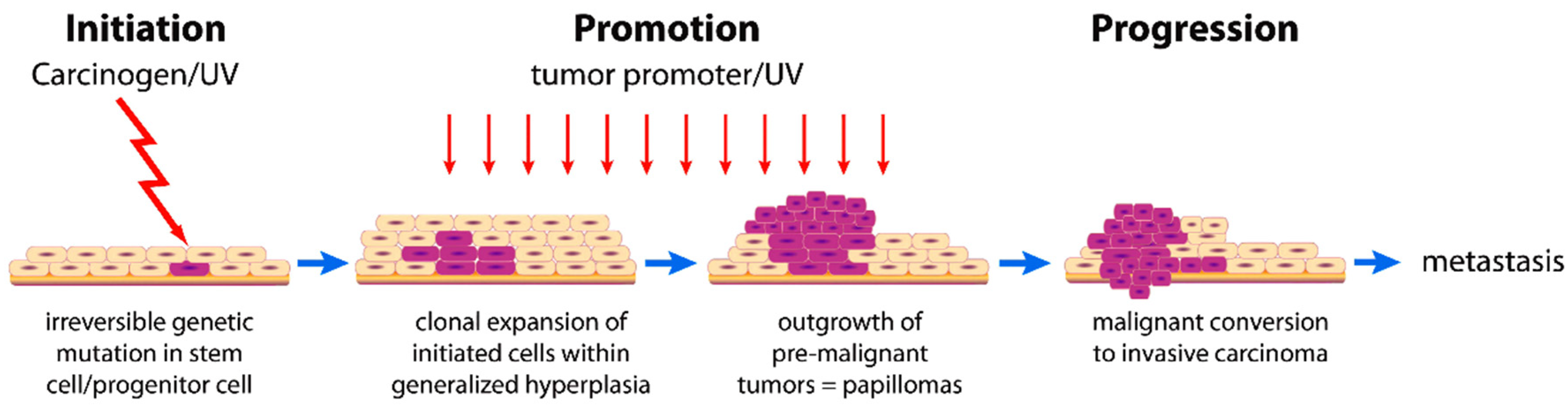
2.2. Cancer Development
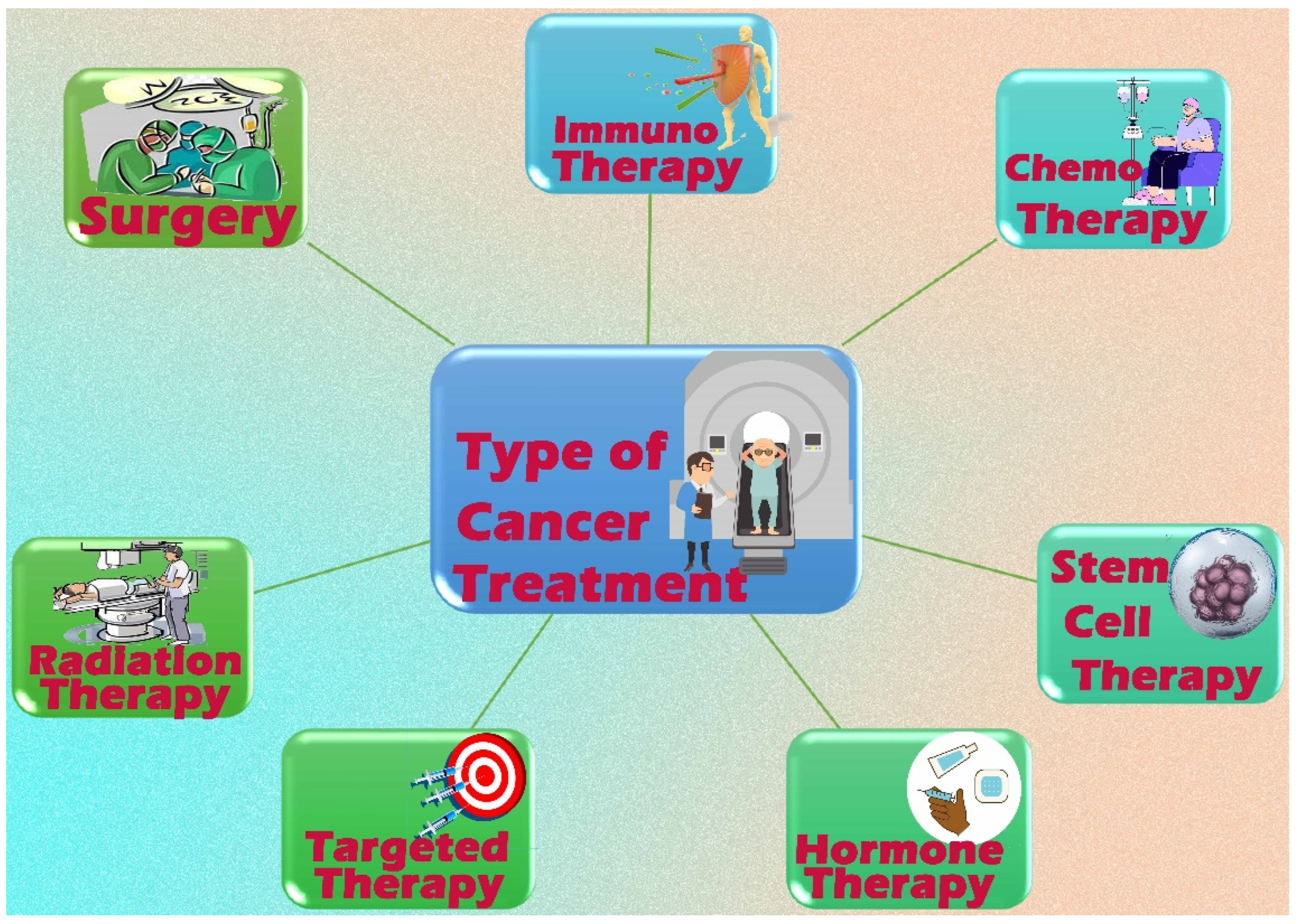
2.3. Cancer Treatment
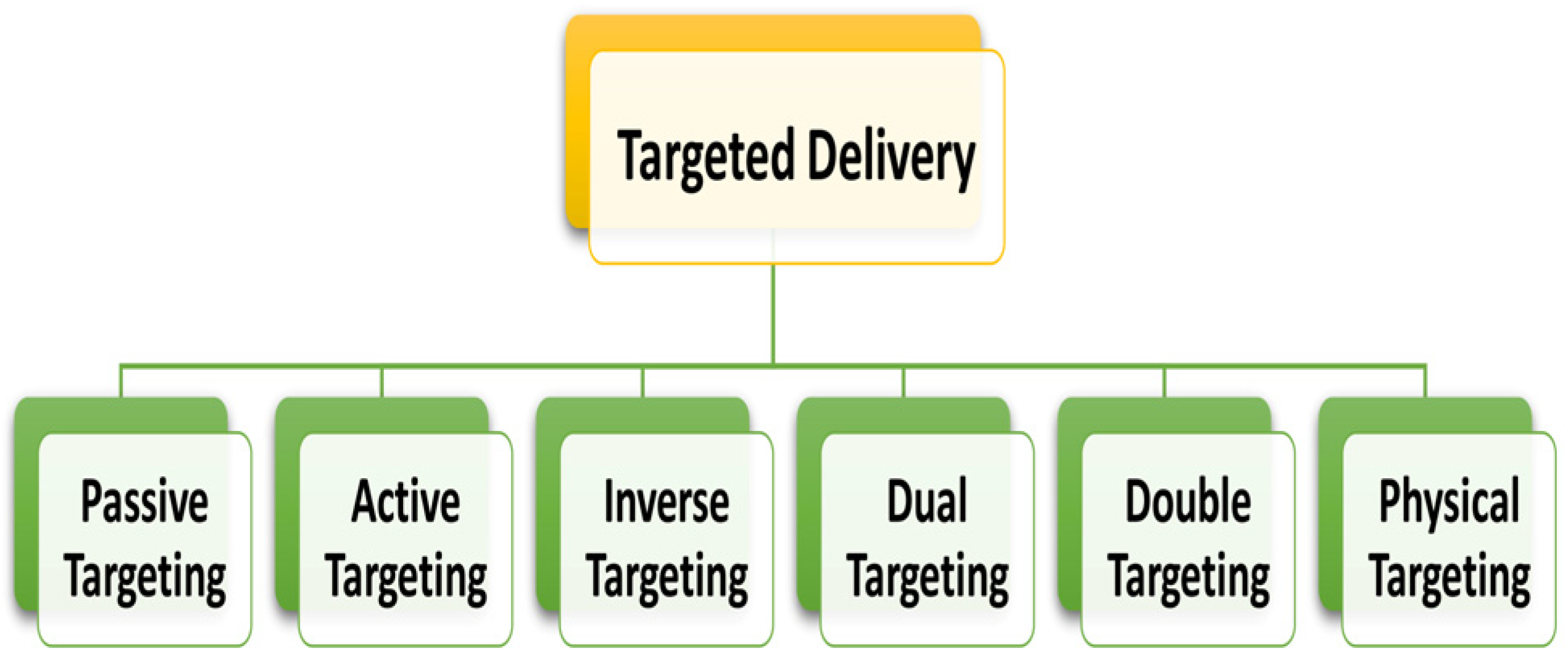
2.4. Approaches for Drug Delivery in Cancer Therapy: General Considerations
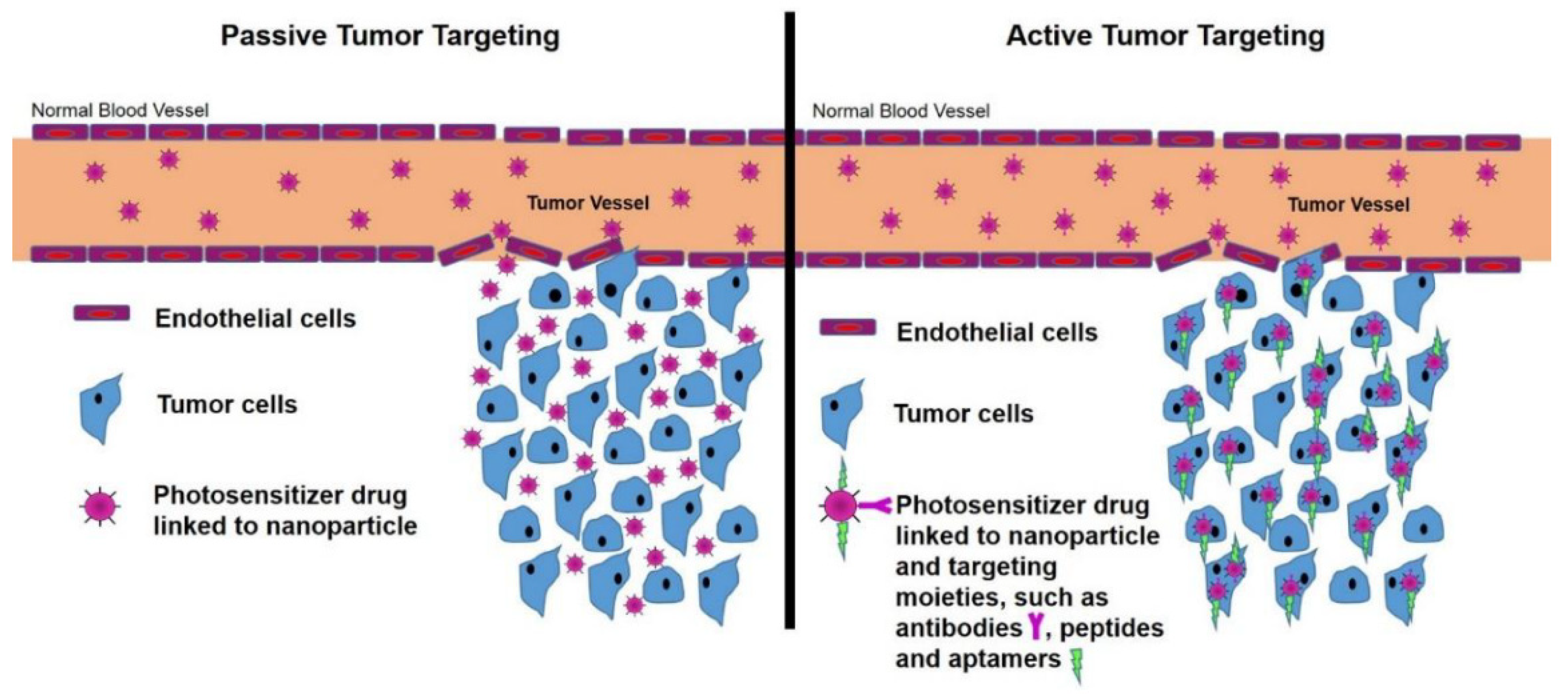
2.4.1. Passive Targeting
2.4.2. Active Targeting
2.5. Nanoscale Drug-Delivery Systems for Treating Cancer
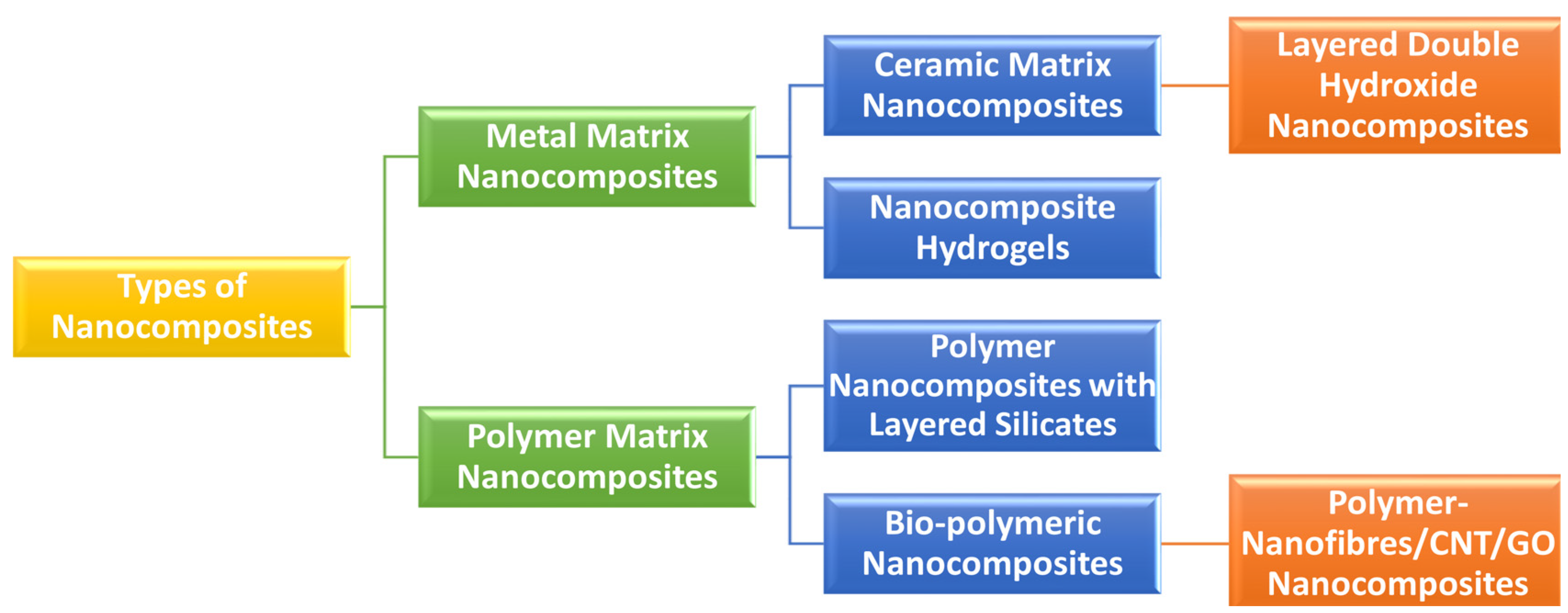
3. Definition and Classifications of Nanobiocomposites
4. Manufacturing/Processing of Nanocomposites
4.1. Solution Mixing
4.2. Melt Mixing
4.3. Extrusion
4.4. In Situ Polymerization
4.5. Sol–Gel Process
4.6. Electrospinning
4.7. Resin Injection Methods or Resin Transfer Molding
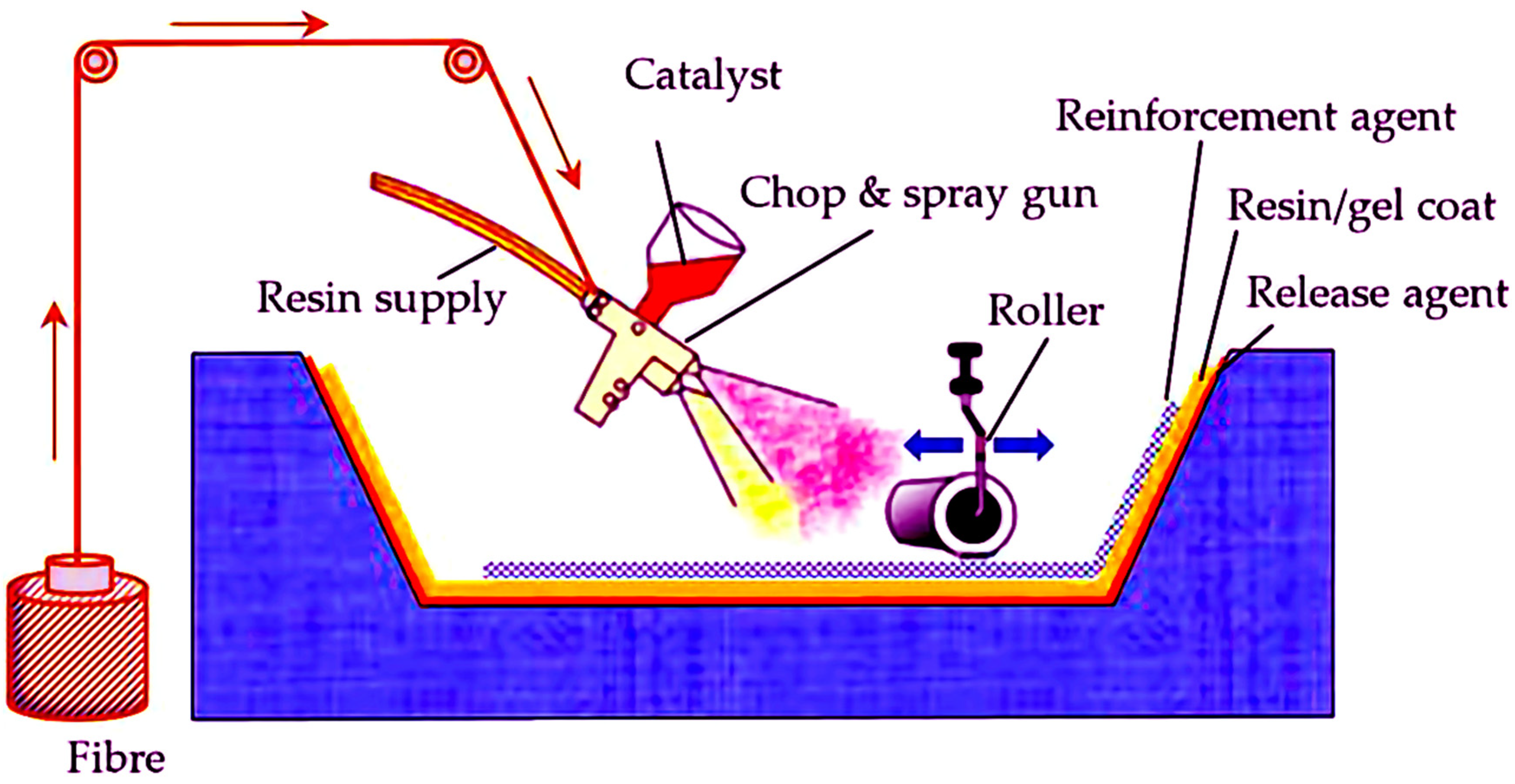
4.8. Spray-Up and Hand Lay-Up Methods
4.9. Other Methods
4.10. Rational for the Uses of Natural Polymers to Construct Nanobiocomposites
5. Different Natural Polymeric Nanobiocomposites for Delivery of Anti-Cancer Drugs
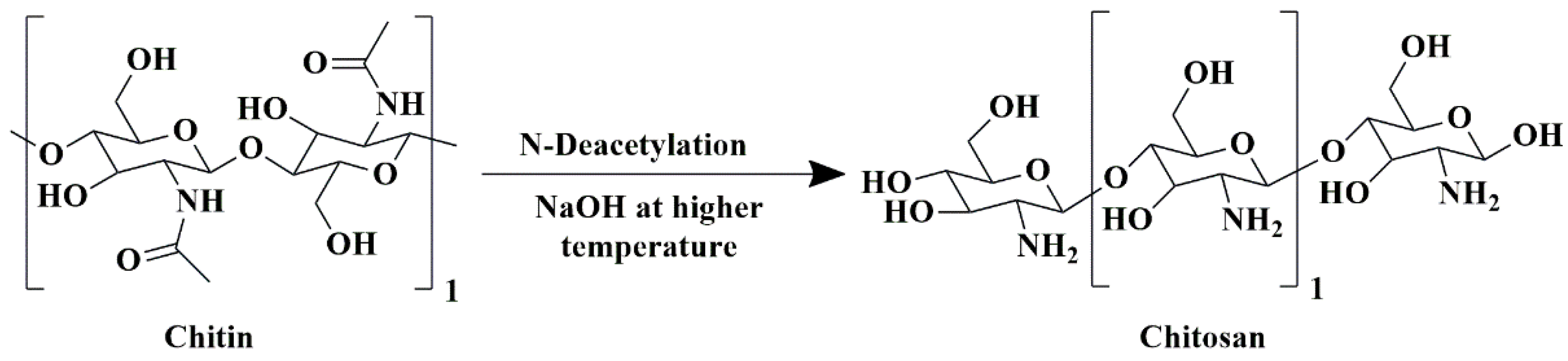
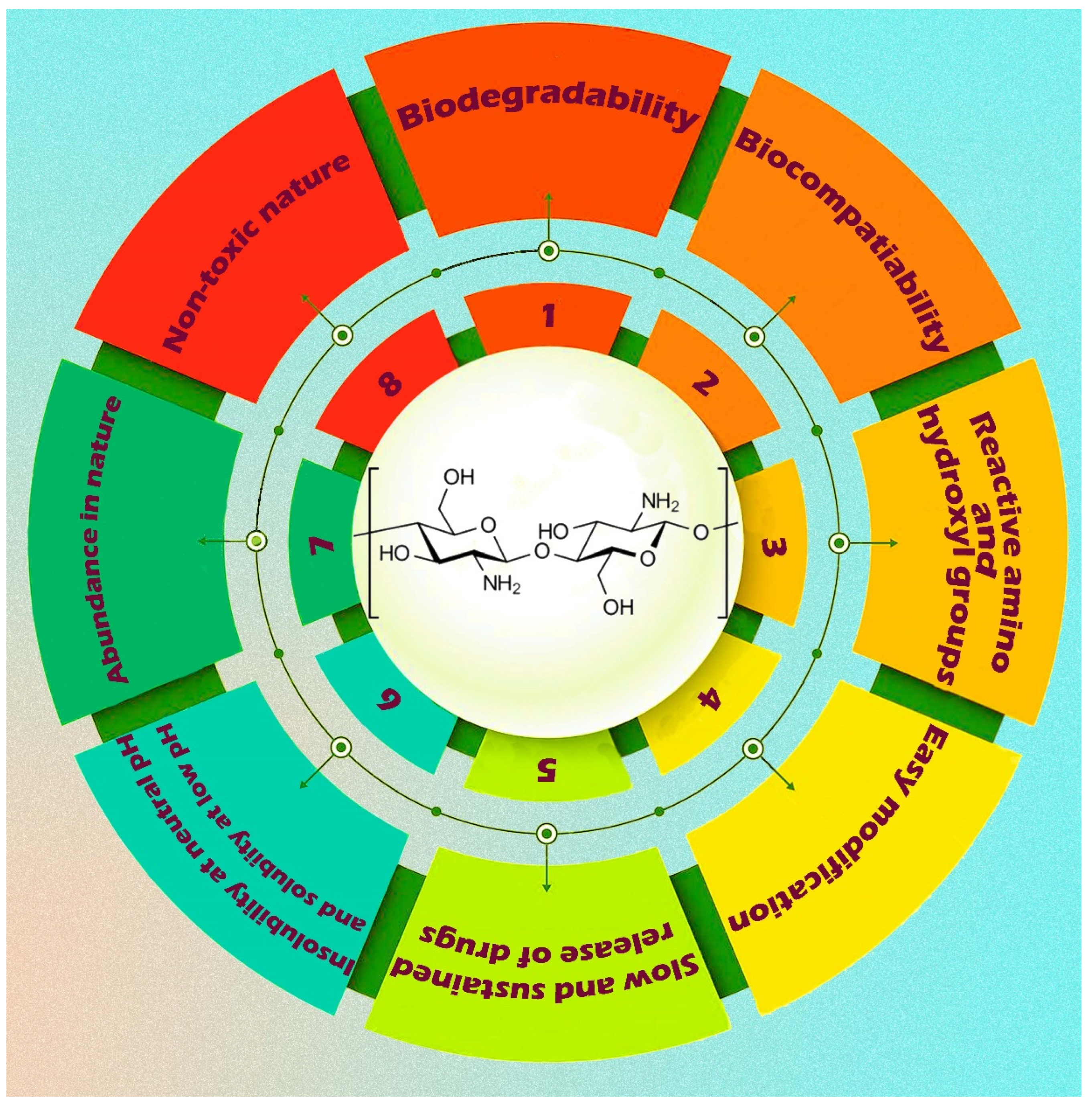
5.1. Chitosan-Based Nanobiocomposites
5.2. Alginate-Based Nanobiocomposites
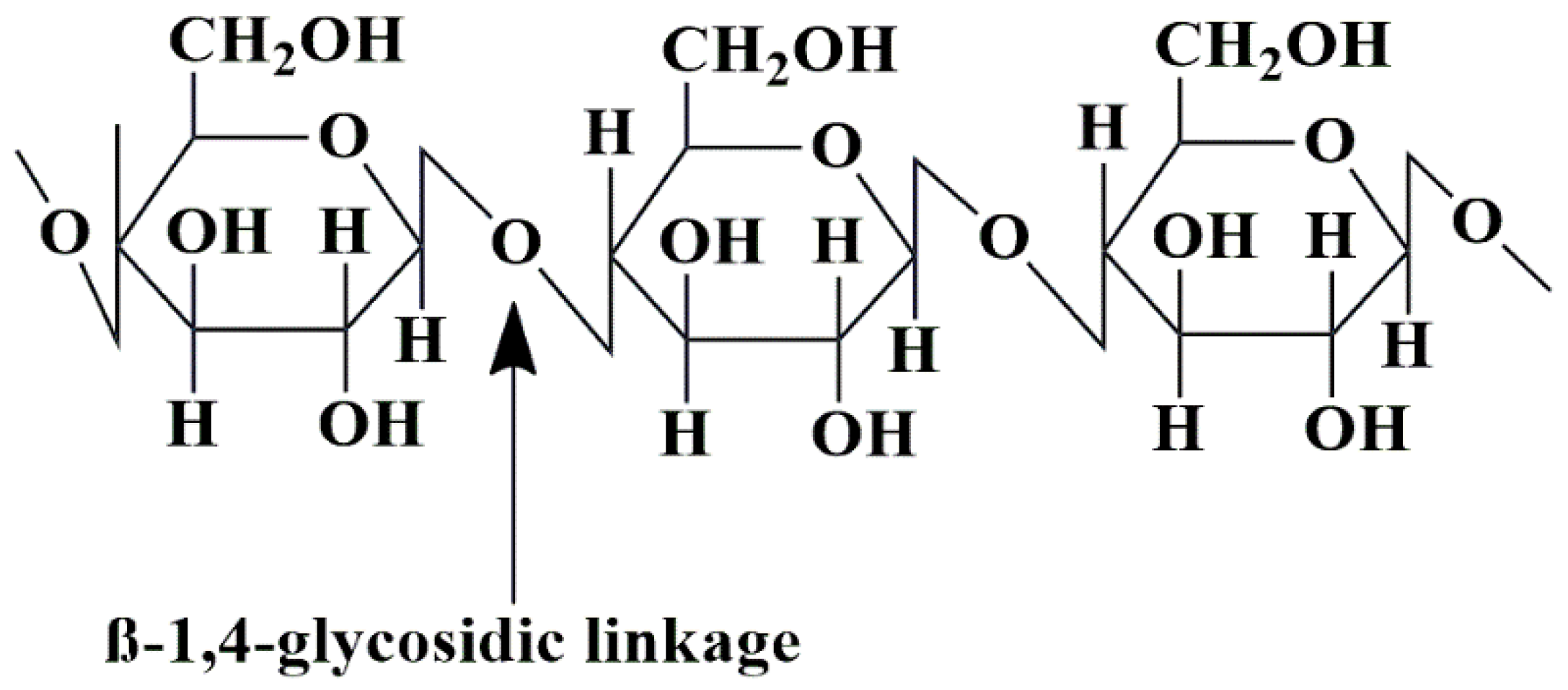
5.3. Cellulose-Based Nanobiocomposites
5.4. Starch-Based Nanobiocomposites
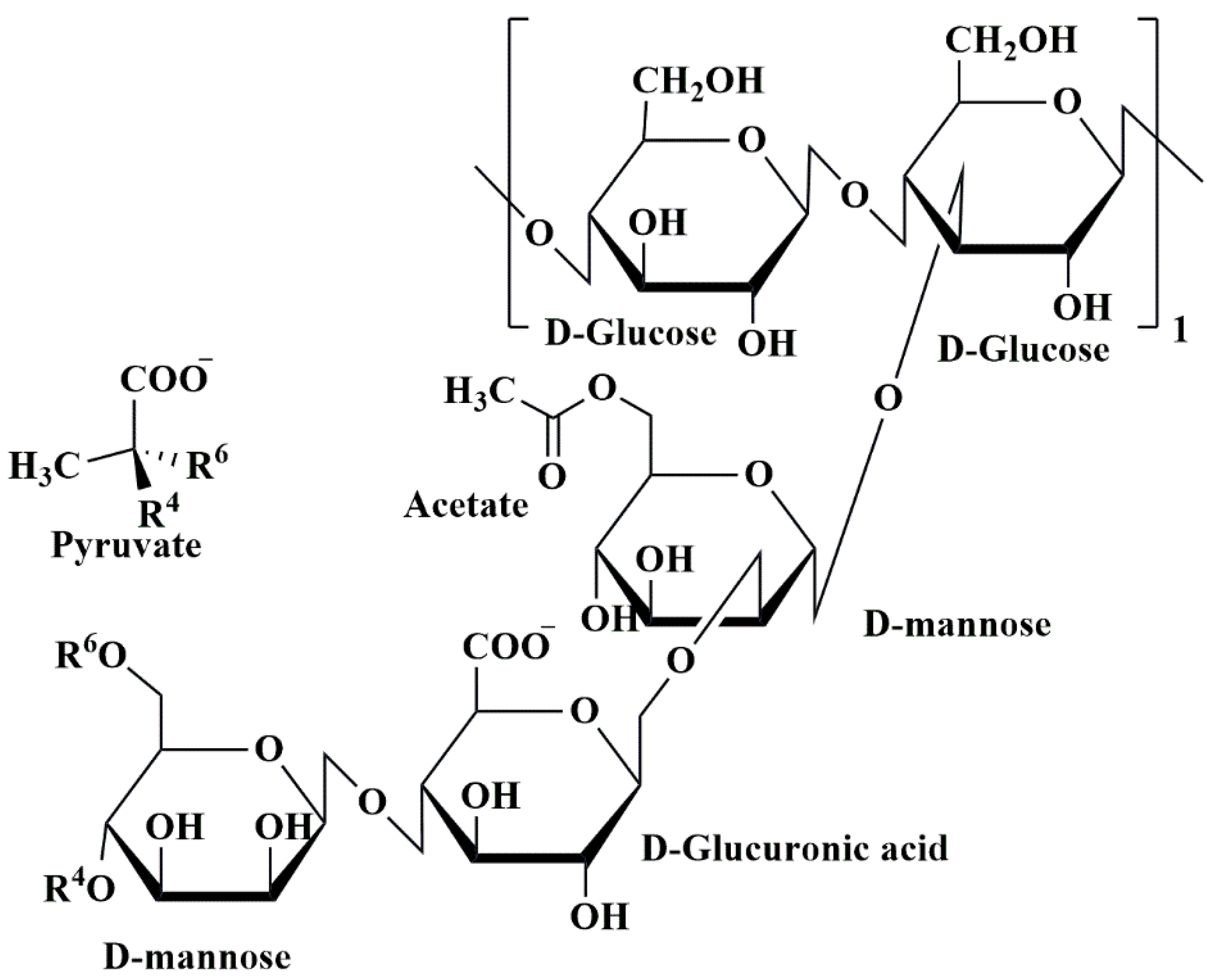
5.5. Xanthan Gum-Based Nanobiocomposites
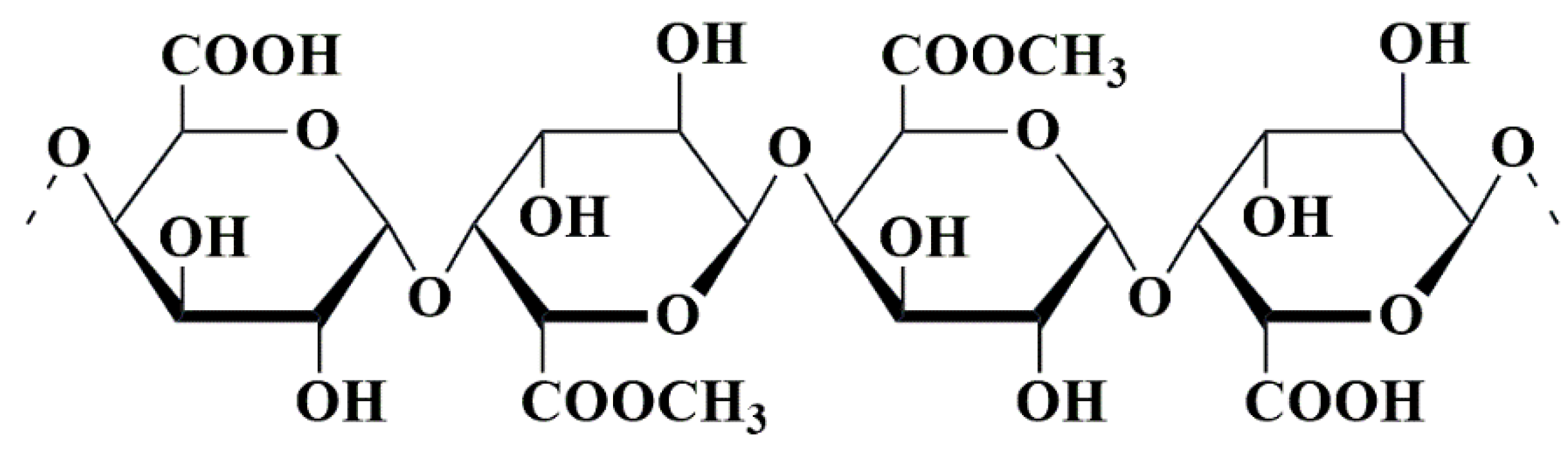
5.6. Pectin-Based Nanobiocomposites
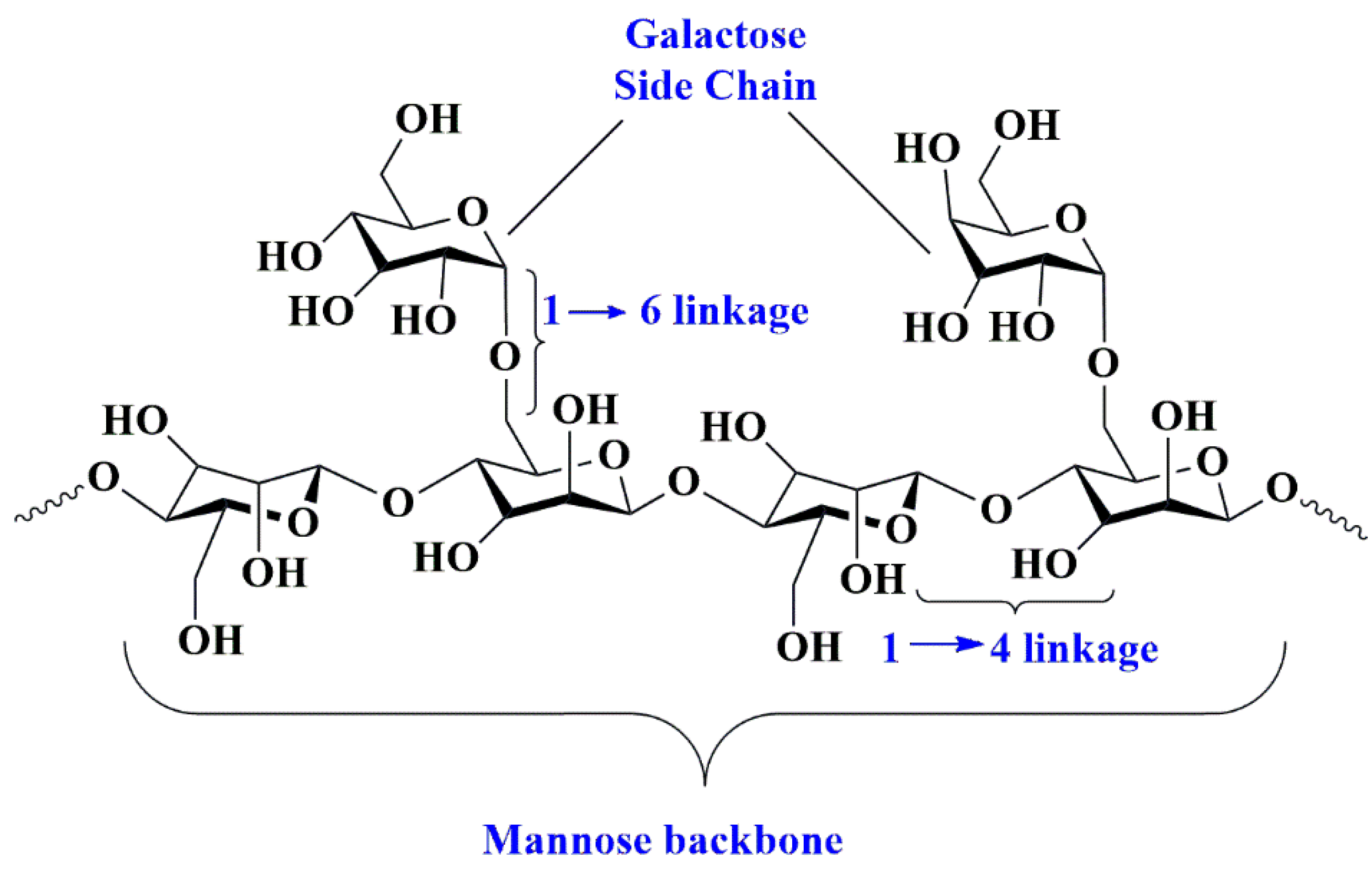
5.7. Guar Gum-Based Nanobiocomposites
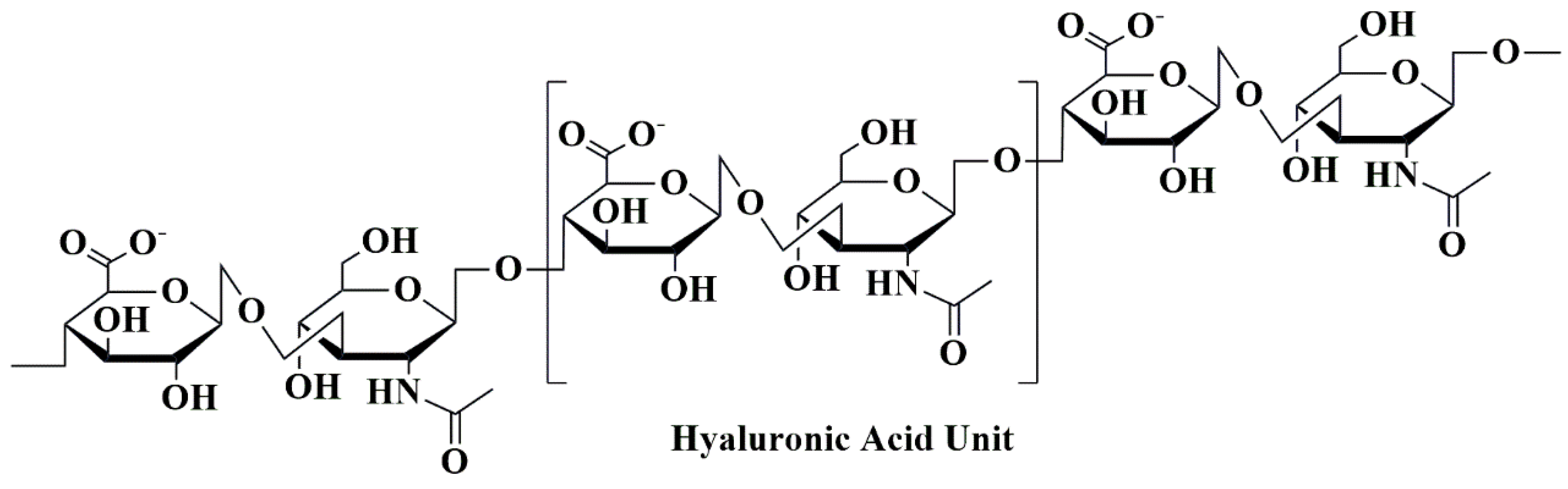
5.8. Hyaluronic Acid-Based Nanobiocomposites
5.9. Gelatin-Based Nanobiocomposites
5.10. Albumin-Based Nanobiocomposites
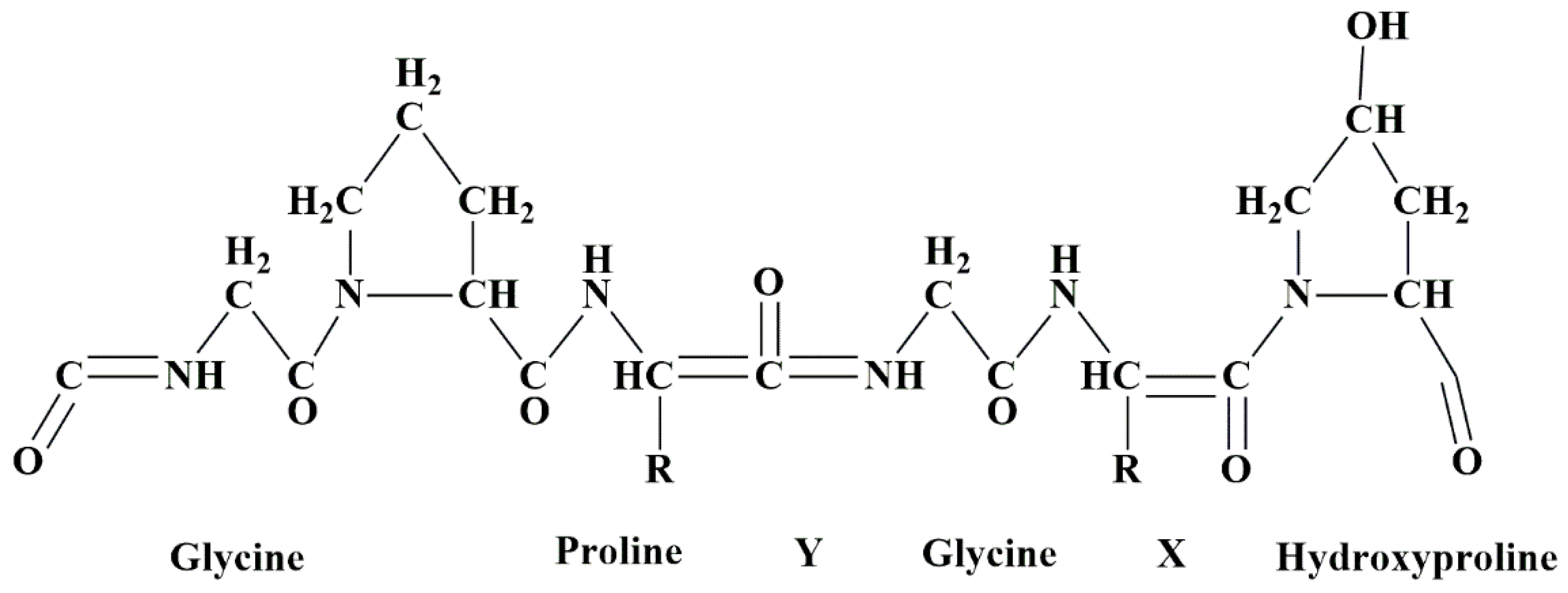
5.11. Collagen-Based Nanobiocomposites
5.12. Miscellaneous
6. Challenges and Future Perspectives of Natural Polymeric Nanobiocomposites for the Delivery of Anti-Cancer Drugs
- (a)
- Nanomaterials’ physicochemical characteristics are fundamental in deciding their biocompatibility and toxicity in biological systems;
- (b)
- The vigilant fabrication of nanomaterials for drug delivery is compulsory to check the latent toxicity of nanocarriers to the human body;
- (c)
- The lack of a correlation between in vitro drug release profiles with in vivo data is a noteworthy and unrelenting problem;
- (d)
- The regulatory judgments concerning nano-formulated drugs are contingent on individual evaluations of benefits and risks, resulting in a protracted assessment process that delays commercialization. Moreover, the challenges in obtaining approval will likely rise due to the development of multifunctional nanoplatforms.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, F.-M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Ma, B.; Hu, K.; Yuan, B.; Sun, X.; Song, X.; Tang, Z.; Lin, H.; Zhu, X.; Zheng, Y. Evidence-based biomaterials research. Bioact. Mater. 2022, 15, 495–503. [Google Scholar] [CrossRef]
- Tang, X.; Alavi, S. Recent advances in starch, polyvinyl alcohol based polymer blends, nanocomposites and their biodegradability. Carbohydr. Polym. 2011, 85, 7–16. [Google Scholar] [CrossRef]
- Zafar, R.; Zia, K.M.; Tabasum, S.; Jabeen, F.; Noreen, A.; Zuber, M. Polysaccharide based bionanocomposites, properties and applications: A review. Int. J. Biol. Macromol. 2016, 92, 1012–1024. [Google Scholar] [CrossRef]
- Gleiter, H. Nanostructured materials: Basic concepts and microstructure. Acta Mater. 2000, 48, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible polymer nanoparticles for drug delivery applications in cancer and neurodegenerative disorder therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Gu, F.; Chan, J.; Wang, A.; Langer, R.; Farokhzad, O. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-S.; Hu, S.-H.; Liao, B.-J.; Chang, Y.-C.; Chen, S.-Y. Enhancement of cancer therapy efficacy by trastuzumab-conjugated and pH-sensitive nanocapsules with the simultaneous encapsulation of hydrophilic and hydrophobic compounds. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 99–107. [Google Scholar] [CrossRef]
- Deng, S.; Gigliobianco, M.R.; Censi, R.; Di Martino, P. Polymeric nanocapsules as nanotechnological alternative for drug delivery system: Current status, challenges and opportunities. Nanomaterials 2020, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, S.; Bolzinger, M.-A.; Jordheim, L.P.; Chevalier, Y.; Fessi, H.; Almouazen, E. Polymeric nanocapsules as drug carriers for sustained anticancer activity of calcitriol in breast cancer cells. Int. J. Pharm. 2018, 550, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Trindade, I.C.; Pound-Lana, G.; Pereira, D.G.S.; de Oliveira, L.A.M.; Andrade, M.S.; Vilela, J.M.C.; Postacchini, B.B.; Mosqueira, V.C.F. Mechanisms of interaction of biodegradable polyester nanocapsules with non-phagocytic cells. Eur. J. Pharm. Sci. 2018, 124, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Balan, V.; Dodi, G.; Tudorachi, N.; Ponta, O.; Simon, V.; Butnaru, M.; Verestiuc, L. Doxorubicin-loaded magnetic nanocapsules based on N-palmitoyl chitosan and magnetite: Synthesis and characterization. Chem. Eng. J. 2015, 279, 188–197. [Google Scholar] [CrossRef]
- Jurgons, R.; Seliger, C.; Hilpert, A.; Trahms, L.; Odenbach, S.; Alexiou, C. Drug loaded magnetic nanoparticles for cancer therapy. J. Phys. Condens. Matter 2006, 18, S2893. [Google Scholar] [CrossRef]
- Dabholkar, N.; Waghule, T.; Rapalli, V.K.; Gorantla, S.; Alexander, A.; Saha, R.N.; Singhvi, G. Lipid shell lipid nanocapsules as smart generation lipid nanocarriers. J. Mol. Liq. 2021, 339, 117145. [Google Scholar] [CrossRef]
- Rochín-Wong, S.; Vélaz-Rivas, I. Lipid and Polymeric Nanocapsules; IntechOpen: London, UK, 2022. [Google Scholar]
- Tsakiris, N.; Papavasileiou, M.; Bozzato, E.; Lopes, A.; Vigneron, A.M.; Préat, V. Combinational drug-loaded lipid nanocapsules for the treatment of cancer. Int. J. Pharm. 2019, 569, 118588. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, D.B.; Dunne, M.; Stewart, J.; Allen, C.; Jaffray, D.A. Cellular uptake and transport of gold nanoparticles incorporated in a liposomal carrier. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 161–169. [Google Scholar] [CrossRef]
- Lee, J.; Chatterjee, D.K.; Lee, M.H.; Krishnan, S. Gold nanoparticles in breast cancer treatment: Promise and potential pitfalls. Cancer Lett. 2014, 347, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Sekar, R.; Basavegowda, N.; Thathapudi, J.J.; Sekhar, M.R.; Joshi, P.; Somu, P.; Baek, K.-H. Recent Progress of Gold-Based Nanostructures towards Future Emblem of Photo-Triggered Cancer Theranostics: A Special Focus on Combinatorial Phototherapies. Pharmaceutics 2023, 15, 433. [Google Scholar] [CrossRef]
- Yang, W.; Liang, H.; Ma, S.; Wang, D.; Huang, J. Gold nanoparticle based photothermal therapy: Development and application for effective cancer treatment. Sustain. Mater. Technol. 2019, 22, e00109. [Google Scholar] [CrossRef]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef]
- Li, B.; Sun, L.; Li, T.; Zhang, Y.; Niu, X.; Xie, M.; You, Z. Ultra-small gold nanoparticles self-assembled by gadolinium ions for enhanced photothermal/photodynamic liver cancer therapy. J. Mater. Chem. B 2021, 9, 1138–1150. [Google Scholar] [CrossRef] [PubMed]
- Shindu, C.T.; Harshita; Pawan, K.M.; Sushama, T. Ceramic nanoparticles: Fabrication methods and applications in drug delivery. Curr. Pharm. Des. 2015, 21, 6165–6188. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Vega, A.-I.; Gomez-Quintero, T.; Nunez-Anita, R.-E.; Acosta-Torres, L.-S.; Castaño, V. Polymeric and ceramic nanoparticles in biomedical applications. J. Nanotechnol. 2012, 2012, 936041. [Google Scholar] [CrossRef] [Green Version]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Deng, Z.; Liu, L.; Zhang, W.; Wang, C. Plant-derived nanovesicles: A novel form of nanomedicine. Front. Bioeng. Biotechnol. 2020, 8, 584391. [Google Scholar] [CrossRef]
- Wong, B.S.; Yoong, S.L.; Jagusiak, A.; Panczyk, T.; Ho, H.K.; Ang, W.H.; Pastorin, G. Carbon nanotubes for delivery of small molecule drugs. Adv. Drug Deliv. Rev. 2013, 65, 1964–2015. [Google Scholar] [CrossRef]
- Mishra, D.K.; Yadav, K.S.; Prabhakar, B.; Gaud, R. Nanocomposite for cancer targeted drug delivery. In Applications of Nanocomposite Materials in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 323–337. [Google Scholar]
- Reinhardt, L.S.; de Barros Dias, M.C.H.; Gnoatto, J.; Wawruszak, A.; Hałasa, M.; Arantes, P.R.; Rowan, N.J.; Moura, D.J. Polymeric Nanocomposites for Cancer-Targeted Drug Delivery. In Polymeric and Natural Composites; Springer: Berlin/Heidelberg, Germany, 2022; pp. 241–270. [Google Scholar]
- Kumar, S.K.; Krishnamoorti, R. Nanocomposites: Structure, phase behavior, and properties. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 37–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.; Chen, S.; Yang, J.; Li, Z.; Wang, H. Functionalized bacterial cellulose derivatives and nanocomposites. Carbohydr. Polym. 2014, 101, 1043–1060. [Google Scholar] [CrossRef]
- Kasirga, Y.; Oral, A.; Caner, C. Preparation and characterization of chitosan/montmorillonite-K10 nanocomposites films for food packaging applications. Polym. Compos. 2012, 33, 1874–1882. [Google Scholar] [CrossRef]
- Zheng, Y.; Monty, J.; Linhardt, R.J. Polysaccharide-based nanocomposites and their applications. Carbohydr. Res. 2015, 405, 23–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of xanthan gum as polysaccharide in tissue engineering: A review. Carbohydr. Polym. 2018, 180, 128–144. [Google Scholar] [CrossRef]
- Camargo, P.H.C.; Satyanarayana, K.G.; Wypych, F. Nanocomposites: Synthesis, structure, properties and new application opportunities. Mater. Res. 2009, 12, 1–39. [Google Scholar] [CrossRef] [Green Version]
- Mclaughlin, S.; Podrebarac, J.; Ruel, M.; Suuronen, E.J.; McNeill, B.; Alarcon, E.I. Nano-engineered biomaterials for tissue regeneration: What has been achieved so far? Front. Mater. 2016, 3, 27. [Google Scholar] [CrossRef] [Green Version]
- Melchor-Martínez, E.M.; Torres Castillo, N.E.; Macias-Garbett, R.; Lucero-Saucedo, S.L.; Parra-Saldívar, R.; Sosa-Hernández, J.E. Modern world applications for nano-bio materials: Tissue engineering and COVID-19. Front. Bioeng. Biotechnol. 2021, 9, 597958. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, E.; Medina-Cruz, D.; Kalantari, K.; Taymoori, A.; Soltantabar, P.; Webster, T.J. Electroconductive nanobiomaterials for tissue engineering and regenerative medicine. Bioelectricity 2020, 2, 120–149. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Banerjee, S. Anticancer Potential and Molecular Mechanisms of Cinnamaldehyde and Its Congeners Present in the Cinnamon Plant. Physiologia 2023, 3, 173–207. [Google Scholar] [CrossRef]
- Bose, S.; Banerjee, S.; Mondal, A.; Chakraborty, U.; Pumarol, J.; Croley, C.R.; Bishayee, A. Targeting the JAK/STAT signaling pathway using phytocompounds for cancer prevention and therapy. Cells 2020, 9, 1451. [Google Scholar] [CrossRef]
- Bose, S.; Banerjee, S.; Verma, D.K.; Thakur, M.; Singh, S.; González, M.L.C.; Aguilar, C.N. Mechanism, regulation, and inhibition of alkaloids in cancer therapy targeting JAK/STAT pathway. In Innovations in Fermentation and Phytopharmaceutical Technologies; Elsevier: Amsterdam, The Netherlands, 2022; pp. 247–270. [Google Scholar]
- Mondal, A.; Banerjee, S.; Bose, S.; Das, P.P.; Sandberg, E.N.; Atanasov, A.G.; Bishayee, A. Cancer preventive and therapeutic potential of banana and its bioactive constituents: A systematic, comprehensive, and mechanistic review. Front. Oncol. 2021, 11, 697143. [Google Scholar] [CrossRef]
- Mondal, A.; Banerjee, S.; Bose, S.; Mazumder, S.; Haber, R.A.; Farzaei, M.H.; Bishayee, A. Garlic constituents for cancer prevention and therapy: From phytochemistry to novel formulations. Pharmacol. Res. 2022, 175, 105837. [Google Scholar] [CrossRef]
- Padhy, I.; Paul, P.; Sharma, T.; Banerjee, S.; Mondal, A. Molecular mechanisms of action of Eugenol in cancer: Recent trends and advancement. Life 2022, 12, 1795. [Google Scholar] [CrossRef]
- Pecorino, L. Molecular Biology of Cancer: Mechanisms, Targets, and Therapeutics; Oxford University Press: Oxford, UK, 2021. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.; Barzi, A.; Jemal, A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 177–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [Green Version]
- Antoni, S.; Soerjomataram, I.; Møller, B.; Bray, F.; Ferlay, J. An assessment of GLOBOCAN methods for deriving national estimates of cancer incidence. Bull. World Health Organ. 2016, 94, 174. [Google Scholar] [CrossRef]
- Torre, L.; Bray, F.; Siegel, R.; Ferlay, J.; Lortet Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2012, 65, 87–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, J.S.; Jones, P.A. Cancer genetics and epigenetics: Two sides of the same coin? Cancer Cell 2012, 22, 9–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, V.L.d.; Leitão, A.; Reina, L.d.C.B.; Montanari, C.A.; Donnici, C.L.; Lopes, M.T.P. Câncer e agentes antineoplásicos ciclo-celular específicos e ciclo-celular não específicos que interagem com o DNA: Uma introdução. Química Nova 2005, 28, 118–129. [Google Scholar] [CrossRef] [Green Version]
- Rundhaug, J.E.; Fischer, S.M. Molecular mechanisms of mouse skin tumor promotion. Cancers 2010, 2, 436–482. [Google Scholar] [CrossRef] [Green Version]
- Alibek, K.; Kakpenova, A.; Baiken, Y. Role of infectious agents in the carcinogenesis of brain and head and neck cancers. Infect. Agents Cancer 2013, 8, 7. [Google Scholar] [CrossRef] [Green Version]
- Wroblewski, L.E.; Peek, R.M. Helicobacter pylori in gastric carcinogenesis: Mechanisms. Gastroenterol. Clin. 2013, 42, 285–298. [Google Scholar] [CrossRef] [Green Version]
- Palumbo, M.O.; Kavan, P.; Miller, W.H., Jr.; Panasci, L.; Assouline, S.; Johnson, N.; Cohen, V.; Patenaude, F.; Pollak, M.; Jagoe, R.T. Systemic cancer therapy: Achievements and challenges that lie ahead. Front. Pharmacol. 2013, 4, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramakrishnan, R.; Gabrilovich, D.I. Novel mechanism of synergistic effects of conventional chemotherapy and immune therapy of cancer. Cancer Immunol. Immunother. 2013, 62, 405–410. [Google Scholar] [CrossRef]
- Luo, D.; Carter, K.A.; Miranda, D.; Lovell, J.F. Chemophototherapy: An emerging treatment option for solid tumors. Adv. Sci. 2017, 4, 1600106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salave, S.; Rana, D.; Sharma, A.; Bharathi, K.; Gupta, R.; Khode, S.; Benival, D.; Kommineni, N. Polysaccharide based implantable drug delivery: Development strategies, regulatory requirements, and future perspectives. Polysaccharides 2022, 3, 625–654. [Google Scholar] [CrossRef]
- McLeod, H.L. Cancer pharmacogenomics: Early promise, but concerted effort needed. Science 2013, 339, 1563–1566. [Google Scholar] [CrossRef] [Green Version]
- Kim, J. Categorizing accident sequences in the external radiotherapy for risk analysis. Radiat. Oncol. J. 2013, 31, 88. [Google Scholar] [CrossRef] [PubMed]
- Chabner, B.A.; Roberts Jr, T.G. Chemotherapy and the war on cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef]
- Franceschini, G.; Terribile, D.; Magno, S.; Fabbri, C.; D’Alba, P.; Chiesa, F.; Di Leone, A.; Masetti, R. Update in the treatment of locally advanced breast cancer: A multidisciplinary approach. Eur. Rev. Med. Pharmacol. Sci. 2007, 11, 283–289. [Google Scholar]
- Lomax, M.; Folkes, L.; O’neill, P. Biological consequences of radiation-induced DNA damage: Relevance to radiotherapy. Clin. Oncol. 2013, 25, 578–585. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Sambi, M.; DeCarlo, A.; Burov, S.V.; Akasov, R.; Markvicheva, E.; Malardier-Jugroot, C.; Szewczuk, M.R. Functionalized folic acid-conjugated amphiphilic alternating copolymer actively targets 3D multicellular tumour spheroids and delivers the hydrophobic drug to the inner core. Nanomaterials 2018, 8, 588. [Google Scholar] [CrossRef] [Green Version]
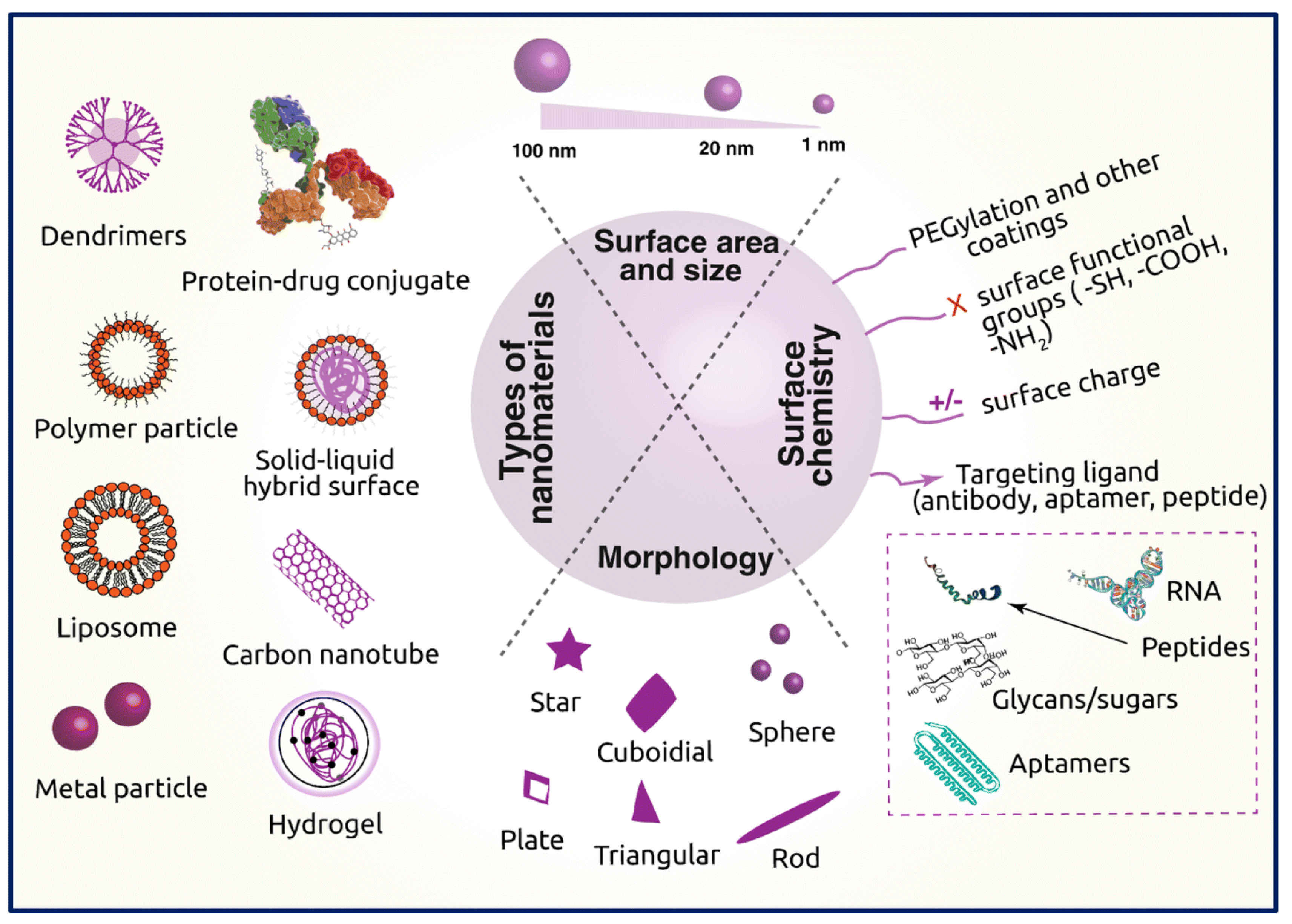
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of physico-chemical properties of nanoparticles on their intracellular uptake. Int. J. Mol. Sci. 2020, 21, 8019. [Google Scholar] [CrossRef] [PubMed]
- Sambi, M.; DeCarlo, A.; Malardier-Jugroot, C.; Szewczuk, M.R. Next-generation multimodality of nanomedicine therapy: Size and structure dependence of folic acid conjugated copolymers actively target cancer cells in disabling cell division and inducing apoptosis. Cancers 2019, 11, 1698. [Google Scholar] [CrossRef] [Green Version]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, L.; Liu, J.; Shi, J. Cancer cell nucleus-targeting nanocomposites for advanced tumor therapeutics. Chem. Soc. Rev. 2018, 47, 6930–6946. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.H.; Park, K. Targeted drug delivery to tumors: Myths, reality and possibility. J. Control. Release 2011, 153, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torchilin, V.P. Passive and active drug targeting: Drug delivery to tumors as an example. In Drug Delivivery; Springer: Berlin/Heidelberg, Germany, 2010; pp. 3–53. [Google Scholar]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [Green Version]
- Yingchoncharoen, P.; Kalinowski, D.S.; Richardson, D.R. Lipid-based drug delivery systems in cancer therapy: What is available and what is yet to come. Pharmacol. Rev. 2016, 68, 701–787. [Google Scholar] [CrossRef] [Green Version]
- M. Rabanel, J.; Aoun, V.; Elkin, I.; Mokhtar, M.; Hildgen, P. Drug-loaded nanocarriers: Passive targeting and crossing of biological barriers. Curr. Med. Chem. 2012, 19, 3070–3102. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Yu, C. Advances in silica based nanoparticles for targeted cancer therapy. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 317–332. [Google Scholar] [CrossRef]
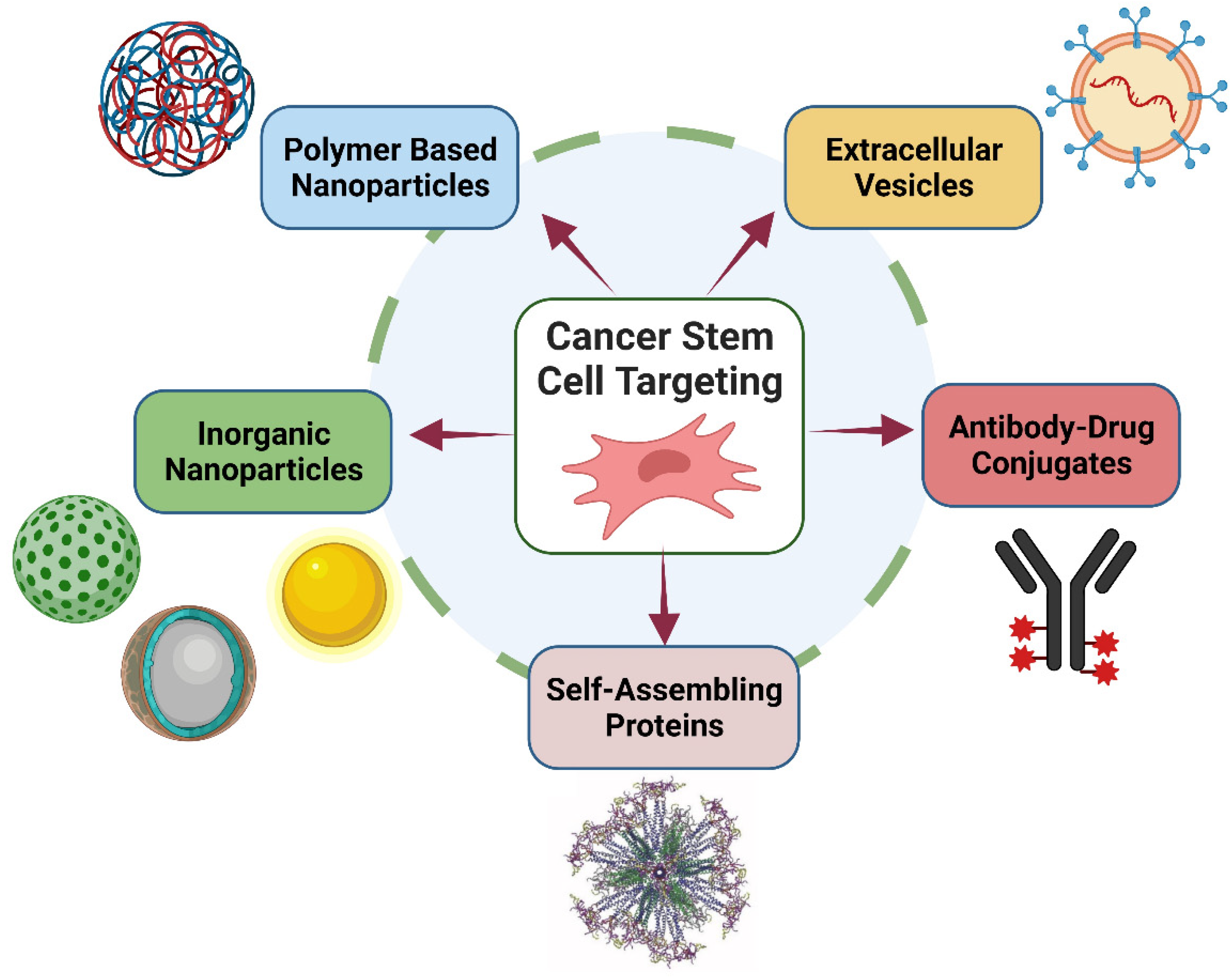
- Ertas, Y.N.; Abedi Dorcheh, K.; Akbari, A.; Jabbari, E. Nanoparticles for targeted drug delivery to cancer stem cells: A review of recent advances. Nanomaterials 2021, 11, 1755. [Google Scholar] [CrossRef]
- Sun, X.; Liu, C.; Omer, A.; Lu, W.; Zhang, S.; Jiang, X.; Wu, H.; Yu, D.; Ouyang, X.-k. pH-sensitive ZnO/carboxymethyl cellulose/chitosan bio-nanocomposite beads for colon-specific release of 5-fluorouracil. Int. J. Biol. Macromol. 2019, 128, 468–479. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current trends in the use of liposomes for tumor targeting. Nanomedicine 2013, 8, 1509–1528. [Google Scholar] [CrossRef] [Green Version]
- Bazak, R.; Houri, M.; El Achy, S.; Hussein, W.; Refaat, T. Passive targeting of nanoparticles to cancer: A comprehensive review of the literature. Mol. Clin. Oncol. 2014, 2, 904–908. [Google Scholar] [CrossRef] [Green Version]
- Jabir, N.R.; Tabrez, S.; Ashraf, G.M.; Shakil, S.; Damanhouri, G.A.; Kamal, M.A. Nanotechnology-based approaches in anticancer research. Int. J. Nanomed. 2012, 7, 4391–4408. [Google Scholar]
- Sun, B.; Rachmawati, H.; Feng, S.-S. -Antibody-Conjugated Nanoparticles of Biodegradable Polymers for Targeted Drug Delivery. In Bionanotechnology II; CRC Press: Boca Raton, FL, USA, 2011; pp. 174–199. [Google Scholar]
- Wang, M.; Thanou, M. Targeting nanoparticles to cancer. Pharmacol. Res. 2010, 62, 90–99. [Google Scholar] [CrossRef]
- Ma, X.; Huang, G.; Wang, Y.; Gao, J. Targeted Nanomedicines: Challenges and Opportunities. In Functional Polymers for Nanomedicine; Royal Society of Chemistry: London, UK, 2013; p. 20. [Google Scholar]
- Kruger, C.A.; Abrahamse, H. Utilisation of targeted nanoparticle photosensitiser drug delivery systems for the enhancement of photodynamic therapy. Molecules 2018, 23, 2628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafiei, P.; Haddadi, A. Pharmacokinetic consequences of PLGA nanoparticles in docetaxel drug delivery. Pharm. Nanotechnol. 2017, 5, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, Z.; Ahmed, N.; Rehman, A.; Khan, G.M. Lipid polymer hybrid carrier systems for cancer targeting: A review. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 86–100. [Google Scholar] [CrossRef]
- Bahrami, B.; Hojjat-Farsangi, M.; Mohammadi, H.; Anvari, E.; Ghalamfarsa, G.; Yousefi, M.; Jadidi-Niaragh, F. Nanoparticles and targeted drug delivery in cancer therapy. Immunol. Lett. 2017, 190, 64–83. [Google Scholar] [CrossRef] [PubMed]
- Vienna, S.; Wien, S. Cancer nanotechnology. Cancer 2015, 6, 1–23. [Google Scholar]
- Ragelle, H.; Danhier, F.; Préat, V.; Langer, R.; Anderson, D.G. Nanoparticle-based drug delivery systems: A commercial and regulatory outlook as the field matures. Expert Opin. Drug Deliv. 2017, 14, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, R.H.; Patravale, V.B.; Joshi, M.D. Polymeric nanoparticles for targeted treatment in oncology: Current insights. Int. J. Nanomed. 2015, 10, 1001. [Google Scholar]
- Guterres, S.S.; Alves, M.P.; Pohlmann, A.R. Polymeric nanoparticles, nanospheres and nanocapsules, for cutaneous applications. Drug Target Insights 2007, 2, 117739280700200002. [Google Scholar] [CrossRef]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 147–166. [Google Scholar] [CrossRef]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef] [Green Version]
- Frasco, M.F.; Almeida, G.M.; Santos-Silva, F.; Pereira, M.d.C.; Coelho, M.A. Transferrin surface-modified PLGA nanoparticles-mediated delivery of a proteasome inhibitor to human pancreatic cancer cells. J. Biomed. Mater. Res. Part A 2015, 103, 1476–1484. [Google Scholar] [CrossRef]
- Winer, I.; Wang, S.; Lee, Y.-E.K.; Fan, W.; Gong, Y.; Burgos-Ojeda, D.; Spahlinger, G.; Kopelman, R.; Buckanovich, R.J. F3-Targeted Cisplatin-Hydrogel Nanoparticles as an Effective Therapeutic That Targets Both Murine and Human Ovarian Tumor Endothelial Cells In vivoF3-Cisplatin-Nanoparticle Therapy. Cancer Res. 2010, 70, 8674–8683. [Google Scholar] [CrossRef] [Green Version]
- Khalid, M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar]
- Iftekhar, A. Biomedical composites. In Standard Handbook of Biomedical Engineering and Design; McGraw Hill: New York, NY, USA, 2004; pp. 1–17. [Google Scholar]
- Sen, M. Nanocomposite materials. In Nanotechnology and the Environment; IntechOpen: London, UK, 2020; pp. 1–12. [Google Scholar]
- Mousa, M.; Dong, Y. Polymeric materials as bionanocomposites. In Bionanocomposites; Elsevier: Amsterdam, The Netherlands, 2020; pp. 335–365. [Google Scholar]
- Ullah, H.; Wahid, F.; Santos, H.A.; Khan, T. Advances in biomedical and pharmaceutical applications of functional bacterial cellulose-based nanocomposites. Carbohydr. Polym. 2016, 150, 330–352. [Google Scholar] [CrossRef]
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, structures, and advanced applications of nanocomposites from biorenewable resources. Chem. Rev. 2020, 120, 9304–9362. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Verma, A.; Sharma, B.; Chaudhary, J.; Tamulevicius, S.; Thakur, V.K. Recent developments in recycling of polystyrene based plastics. Curr. Opin. Green Sustain. Chem. 2018, 13, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Gandini, A.; Lacerda, T.M.; Carvalho, A.J.; Trovatti, E. Progress of polymers from renewable resources: Furans, vegetable oils, and polysaccharides. Chem. Rev. 2016, 116, 1637–1669. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Govender, P.P.; Mamo, M.A.; Tamulevicius, S.; Thakur, V.K. Recent progress in gelatin hydrogel nanocomposites for water purification and beyond. Vacuum 2017, 146, 396–408. [Google Scholar] [CrossRef] [Green Version]
- Fawaz, J.; Mittal, V. Synthesis of polymer nanocomposites: Review of various techniques. In Synthesis Techniques for Polymer Nanocomposites; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–30. [Google Scholar]
- Bledzki, A.; Mamun, A.; Faruk, O. Abaca fibre reinforced PP composites and comparison with jute and flax fibre PP composites. Express Polym. Lett. 2007, 1, 755–762. [Google Scholar] [CrossRef]
- Thomas, S.; Bandyopadhyay, S.; Thomas, S.; Stevens, R. Polymer Nanocomposites:-preparation, properties and applications. Gummi Fascrn Kunststoffe 2006, 9, 49–56. [Google Scholar]
- Neibolts, N.; Platnieks, O.; Gaidukovs, S.; Barkane, A.; Thakur, V.; Filipova, I.; Mihai, G.; Zelca, Z.; Yamaguchi, K.; Enachescu, M. Needle-free electrospinning of nanofibrillated cellulose and graphene nanoplatelets based sustainable poly (butylene succinate) nanofibers. Mater. Today Chem. 2020, 17, 100301. [Google Scholar] [CrossRef]
- Wróblewska-Krepsztul, J.; Rydzkowski, T.; Borowski, G.; Szczypiński, M.; Klepka, T.; Thakur, V.K. Recent progress in biodegradable polymers and nanocomposite-based packaging materials for sustainable environment. Int. J. Polym. Anal. Charact. 2018, 23, 383–395. [Google Scholar] [CrossRef]
- Ngo, T. Introduction to Composite Materials, Composite and Nano composite Materials. In Composite and Nanocomposite Materials—From Knowledge to Industrial Applications; InTech Open: London, UK, 2020. [Google Scholar]
- Shariatinia, Z. Pharmaceutical applications of natural polysaccharides. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 15–57. [Google Scholar]
- Yang, F.; Lu, J.; Ke, Q.; Peng, X.; Guo, Y.; Xie, X. Magnetic mesoporous calcium sillicate/chitosan porous scaffolds for enhanced bone regeneration and photothermal-chemotherapy of osteosarcoma. Sci. Rep. 2018, 8, 7345. [Google Scholar] [CrossRef] [Green Version]
- Bianco, A.; Kostarelos, K.; Prato, M. Applications of carbon nanotubes in drug delivery. Curr. Opin. Chem. Biol. 2005, 9, 674–679. [Google Scholar] [CrossRef]
- de Oliveira, A.D.; Beatrice, C.A.G. Polymer nanocomposites with different types of nanofiller. In Nanocomposites-Recent Evolutions; InTech Open: London, UK, 2018; pp. 103–104. [Google Scholar]
- Jafarizad, A.; Aghanejad, A.; Sevim, M.; Metin, Ö.; Barar, J.; Omidi, Y.; Ekinci, D. Gold nanoparticles and reduced graphene oxide-gold nanoparticle composite materials as covalent drug delivery systems for breast cancer treatment. ChemistrySelect 2017, 2, 6663–6672. [Google Scholar] [CrossRef]
- Li, X.; Xu, W.; Xin, Y.; Yuan, J.; Ji, Y.; Chu, S.; Liu, J.; Luo, Q. Supramolecular polymer nanocomposites for biomedical applications. Polymers 2021, 13, 513. [Google Scholar] [CrossRef]
- Sharma, A.K.; Kaith, B.S. Polymer nanocomposite matrices: Classification, synthesis methods, and applications. In Handbook of Polymer and Ceramic Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2021; pp. 403–428. [Google Scholar]
- Navya, P.; Kaphle, A.; Srinivas, S.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Idrees, H.; Zaidi, S.Z.J.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S.-u. A review of biodegradable natural polymer-based nanoparticles for drug delivery applications. Nanomaterials 2020, 10, 1970. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.C.; Mascarenhas-Melo, F.; Pedrosa, K.; Lopes, D.; Lopes, J.; Macário-Soares, A.; Peixoto, D.; Giram, P.S.; Veiga, F.; Paiva-Santos, A.C. Polymer-based biomaterials for pharmaceutical and biomedical applications: A focus on topical drug administration. Eur. Polym. J. 2023, 187, 111868. [Google Scholar] [CrossRef]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; classification, chemical properties, and future perspective applications in fields of pharmacology and biological medicine (a review of current applications and upcoming potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef]
- Han, H.; Lee, K. Systematic approach to mimic phenolic natural polymers for biofabrication. Polymers 2022, 14, 1282. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Wilson, L.D. A review on the design and hydration properties of natural polymer-based hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Kim, K.H.; Yoon, H.; Kim, H. Chemical design of functional polymer structures for biosensors: From nanoscale to macroscale. Polymers 2018, 10, 551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chelu, M.; Musuc, A.M. Polymer Gels: Classification and Recent Developments in Biomedical Applications. Gels 2023, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Dumontel, B.; Conejo-Rodríguez, V.; Vallet-Regí, M.; Manzano, M. Natural Biopolymers as Smart Coating Materials of Mesoporous Silica Nanoparticles for Drug Delivery. Pharmaceutics 2023, 15, 447. [Google Scholar] [CrossRef] [PubMed]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and chitosan: Production and application of versatile biomedical nanomaterials. Int. J. Adv. Res. 2016, 4, 411. [Google Scholar]
- Periayah, M.H.; Halim, A.S.; Saad, A.Z.M. Chitosan: A promising marine polysaccharide for biomedical research. Pharmacogn. Rev. 2016, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Raafat, D.; Sahl, H.G. Chitosan and its antimicrobial potential–a critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Bary, A.S.; Tolan, D.A.; Nassar, M.Y.; Taketsugu, T.; El-Nahas, A.M. Chitosan, magnetite, silicon dioxide, and graphene oxide nanocomposites: Synthesis, characterization, efficiency as cisplatin drug delivery, and DFT calculations. Int. J. Biol. Macromol. 2020, 154, 621–633. [Google Scholar] [CrossRef]
- Manivasagan, P.; Quang Bui, N.; Bharathiraja, S.; Santha Moorthy, M.; Oh, Y.-O.; Song, K.; Seo, H.; Yoon, M.; Oh, J. Multifunctional biocompatible chitosan-polypyrrole nanocomposites as novel agents for photoacoustic imaging-guided photothermal ablation of cancer. Sci. Rep. 2017, 7, 43593. [Google Scholar] [CrossRef] [Green Version]
- Hauksdóttir, H.L.; Webster, T.J. Selenium and iron oxide nanocomposites for magnetically-targeted anti-cancer applications. J. Biomed. Nanotechnol. 2018, 14, 510–525. [Google Scholar] [CrossRef]
- Ignjatović, N.L.; Penov-Gaši, K.M.; Ajduković, J.J.; Kojić, V.V.; Marković, S.B.; Uskoković, D.P. The effect of the androstane lung cancer inhibitor content on the cell-selective toxicity of hydroxyapatite-chitosan-PLGA nanocomposites. Mater. Sci. Eng. C 2018, 89, 371–377. [Google Scholar] [CrossRef]
- Nejadshafiee, V.; Naeimi, H.; Goliaei, B.; Bigdeli, B.; Sadighi, A.; Dehghani, S.; Lotfabadi, A.; Hosseini, M.; Nezamtaheri, M.S.; Amanlou, M. Magnetic bio-metal–organic framework nanocomposites decorated with folic acid conjugated chitosan as a promising biocompatible targeted theranostic system for cancer treatment. Mater. Sci. Eng. C 2019, 99, 805–815. [Google Scholar] [CrossRef]
- Kamel, E.M.; Ahmed, O.M.; Abd El-Salam, H. Fabrication of facile polymeric nanocomposites based on chitosan-gr-P2-aminothiophenol for biomedical applications. Int. J. Biol. Macromol. 2020, 165, 2649–2659. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Y.; Chen, Z.; Geng, Y.; Xie, X.; Shen, X.; Li, T.; Li, S.; Wu, C.; Liu, Y. Chemo-photodynamic combined gene therapy and dual-modal cancer imaging achieved by pH-responsive alginate/chitosan multilayer-modified magnetic mesoporous silica nanocomposites. Biomater. Sci. 2017, 5, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Rajan, M.; Murugan, M.; Ponnamma, D.; Sadasivuni, K.K.; Munusamy, M.A. Poly-carboxylic acids functionalized chitosan nanocarriers for controlled and targeted anti-cancer drug delivery. Biomed. Pharmacother. 2016, 83, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, D.; Ranjithkumar, R.; Chandarshekar, B.; Bhuvaneshwari, V. Bio-inspired synthesis of chitosan/copper oxide nanocomposite using rutin and their anti-proliferative activity in human lung cancer cells. Int. J. Biol. Macromol. 2019, 141, 476–483. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.M.; Elella, M.H.A.; Mohamed, R.R. Green synthesis of quaternized chitosan/silver nanocomposites for targeting mycobacterium tuberculosis and lung carcinoma cells (A-549). Int. J. Biol. Macromol. 2020, 142, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Prabha, G.; Raj, V. Preparation and characterization of polymer nanocomposites coated magnetic nanoparticles for drug delivery applications. J. Magn. Magn. Mater. 2016, 408, 26–34. [Google Scholar] [CrossRef]
- Rasoulzadehzali, M.; Namazi, H. Facile preparation of antibacterial chitosan/graphene oxide-Ag bio-nanocomposite hydrogel beads for controlled release of doxorubicin. Int. J. Biol. Macromol. 2018, 116, 54–63. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, F.; Peng, H.; Zhang, Y.; Li, Y.; Xu, Y.; Xie, J. Layer-by-layer modification of magnetic graphene oxide by chitosan and sodium alginate with enhanced dispersibility for targeted drug delivery and photothermal therapy. Colloids Surf. B: Biointerfaces 2019, 176, 462–470. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Hosseinzadeh, H.; Pashaei, S.; Khodaparast, Z. Synthesis of stimuli-responsive chitosan nanocomposites via RAFT copolymerization for doxorubicin delivery. Int. J. Biol. Macromol. 2019, 121, 677–685. [Google Scholar] [CrossRef]
- Dhanavel, S.; Nivethaa, E.; Narayanan, V.; Stephen, A. In vitro cytotoxicity study of dual drug loaded chitosan/palladium nanocomposite towards HT-29 cancer cells. Mater. Sci. Eng. C 2017, 75, 1399–1410. [Google Scholar] [CrossRef]
- Bao, H.; Pan, Y.; Ping, Y.; Sahoo, N.G.; Wu, T.; Li, L.; Li, J.; Gan, L.H. Chitosan-functionalized graphene oxide as a nanocarrier for drug and gene delivery. Small 2011, 7, 1569–1578. [Google Scholar] [CrossRef]
- Parida, U.K.; Nayak, A.K.; Binhani, B.K.; Nayak, P. Synthesis and characterization of chitosan-polyvinyl alcohol blended with cloisite 30B for controlled release of the anticancer drug curcumin. J. Biomater. Nanobiotechnol. 2011, 2, 414. [Google Scholar] [CrossRef] [Green Version]
- Das, R.K.; Kasoju, N.; Bora, U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Nanda, R.; Sasmal, A.; Nayak, P. Preparation and characterization of chitosan–polylactide composites blended with Cloisite 30B for control release of the anticancer drug paclitaxel. Carbohydr. Polym. 2011, 83, 988–994. [Google Scholar] [CrossRef]
- Tan, L.; Huang, R.; Li, X.; Liu, S.; Shen, Y.-M.; Shao, Z. Chitosan-based core-shell nanomaterials for pH-triggered release of anticancer drug and near-infrared bioimaging. Carbohydr. Polym. 2017, 157, 325–334. [Google Scholar] [CrossRef] [PubMed]
- George, D.; Maheswari, P.U.; Sheriffa Begum, K.M.M.; Arthanareeswaran, G. Biomass-derived dialdehyde cellulose cross-linked chitosan-based nanocomposite hydrogel with phytosynthesized zinc oxide nanoparticles for enhanced curcumin delivery and bioactivity. J. Agric. Food Chem. 2019, 67, 10880–10890. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Bose, S.; Banerjee, S.; Patra, J.K.; Malik, J.; Mandal, S.K.; Kilpatrick, K.L.; Das, G.; Kerry, R.G.; Fimognari, C. Marine cyanobacteria and microalgae metabolites—A rich source of potential anticancer drugs. Mar. Drugs 2020, 18, 476. [Google Scholar] [CrossRef]
- Zhang, S.; Qamar, S.A.; Junaid, M.; Munir, B.; Badar, Q.; Bilal, M. Algal Polysaccharides-Based Nanoparticles for Targeted Drug Delivery Applications. Starch-Stärke 2022, 74, 2200014. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Zhang, C.; Kang, H.; Xia, Y.; Sui, K.; Liu, R. Gelation of Na-alginate aqueous solution: A study of sodium ion dynamics via NMR relaxometry. Carbohydr. Polym. 2017, 169, 206–212. [Google Scholar] [CrossRef]
- Iliescu, R.I.; Andronescu, E.; Ghitulica, C.D.; Voicu, G.; Ficai, A.; Hoteteu, M. Montmorillonite–alginate nanocomposite as a drug delivery system–incorporation and in vitro release of irinotecan. Int. J. Pharm. 2014, 463, 184–192. [Google Scholar] [CrossRef]
- Azhar, F.F.; Olad, A. A study on sustained release formulations for oral delivery of 5-fluorouracil based on alginate–chitosan/montmorillonite nanocomposite systems. Appl. Clay Sci. 2014, 101, 288–296. [Google Scholar] [CrossRef]
- Son, K.D.; Kim, Y.-J. Anticancer activity of drug-loaded calcium phosphate nanocomposites against human osteosarcoma. Biomater. Res. 2017, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mahkam, M.; Zeynabad, F.B.; Alizadeh, E.; Rahimi, M.; Rahimi, F.; Salehi, R. Novel methotrexate-ciprofloxacin loaded alginate-clay based nanocomposite as anticancer and antibacterial co-drug delivery system. Adv. Pharm. Bull. 2021, 11, 477. [Google Scholar] [CrossRef] [PubMed]
- Hosny, K.M.; Alkhalidi, H.M.; Alharbi, W.S.; Md, S.; Sindi, A.M.; Ali, S.A.; Bakhaidar, R.B.; Almehmady, A.M.; Alfayez, E.; Kurakula, M. Recent trends in assessment of cellulose derivatives in designing novel and nanoparticulate-based drug delivery systems for improvement of oral health. Polymers 2022, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Sunasee, R.; Hemraz, U.D. Synthetic strategies for the fabrication of cationic surface-modified cellulose nanocrystals. Fibers 2018, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Metaxa, A.-F.; Kordas, G. Modified polysaccharides for drug delivery. In Polysaccharides; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1805–1835. [Google Scholar]
- Madusanka, N.; de Silva, K.N.; Amaratunga, G. A curcumin activated carboxymethyl cellulose–montmorillonite clay nanocomposite having enhanced curcumin release in aqueous media. Carbohydr. Polym. 2015, 134, 695–699. [Google Scholar] [CrossRef]
- Rasoulzadeh, M.; Namazi, H. Carboxymethyl cellulose/graphene oxide bio-nanocomposite hydrogel beads as anticancer drug carrier agent. Carbohydr. Polym. 2017, 168, 320–326. [Google Scholar] [CrossRef]
- Javanbakht, S.; Namazi, H. Doxorubicin loaded carboxymethyl cellulose/graphene quantum dot nanocomposite hydrogel films as a potential anticancer drug delivery system. Mater. Sci. Eng. C 2018, 87, 50–59. [Google Scholar] [CrossRef]
- Mahdi Eshaghi, M.; Pourmadadi, M.; Rahdar, A.; Díez-Pascual, A.M. Novel Carboxymethyl Cellulose-Based Hydrogel with Core–Shell Fe3O4@ SiO2 Nanoparticles for Quercetin Delivery. Materials 2022, 15, 8711. [Google Scholar] [CrossRef] [PubMed]
- Abbasian, M.; Mahmoodzadeh, F.; Salehi, R. Chemotherapy of breast cancer cells using novel pH-responsive cellulose-based nanocomposites. Adv. Pharm. Bull. 2019, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Yusefi, M.; Lee-Kiun, M.S.; Shameli, K.; Teow, S.-Y.; Ali, R.R.; Siew, K.-K.; Chan, H.-Y.; Wong, M.M.-T.; Lim, W.-L.; Kuča, K. 5-Fluorouracil loaded magnetic cellulose bionanocomposites for potential colorectal cancer treatment. Carbohydr. Polym. 2021, 273, 118523. [Google Scholar] [CrossRef]
- Shahzadi, I.; Islam, M.; Saeed, H.; Shahzadi, A.; Haider, J.; Haider, A.; Imran, M.; Rathore, H.A.; Ul-Hamid, A.; Nabgan, W. Facile synthesis of copolymerized cellulose grafted hydrogel doped calcium oxide nanocomposites with improved antioxidant activity for anti-arthritic and controlled release of doxorubicin for anti-cancer evaluation. Int. J. Biol. Macromol. 2023, 235, 123874. [Google Scholar] [CrossRef] [PubMed]
- Gholamali, I.; Yadollahi, M. Doxorubicin-loaded carboxymethyl cellulose/Starch/ZnO nanocomposite hydrogel beads as an anticancer drug carrier agent. Int. J. Biol. Macromol. 2020, 160, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.B.; Francis, A.P.; Devasena, T. Chitosan–starch nanocomposite particles as a drug carrier for the delivery of bis-desmethoxy curcumin analog. Carbohydr. Polym. 2014, 114, 170–178. [Google Scholar] [CrossRef]
- Raj, V.; Prabha, G. Synthesis, characterization and in vitro drug release of cisplatin loaded Cassava starch acetate–PEG/gelatin nanocomposites. J. Assoc. Arab Univ. Basic Appl. Sci. 2016, 21, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Saikia, C.; Das, M.K.; Ramteke, A.; Maji, T.K. Evaluation of folic acid tagged aminated starch/ZnO coated iron oxide nanoparticles as targeted curcumin delivery system. Carbohydr. Polym. 2017, 157, 391–399. [Google Scholar] [CrossRef]
- Meneguin, A.B.; Cury, B.S.F.; Dos Santos, A.M.; Franco, D.F.; Barud, H.S.; da Silva Filho, E.C. Resistant starch/pectin free-standing films reinforced with nanocellulose intended for colonic methotrexate release. Carbohydr. Polym. 2017, 157, 1013–1023. [Google Scholar] [CrossRef]
- Hasanin, M.; Al Abboud, M.A.; Alawlaqi, M.M.; Abdelghany, T.M.; Hashem, A.H. Ecofriendly synthesis of biosynthesized copper nanoparticles with starch-based nanocomposite: Antimicrobial, antioxidant, and anticancer activities. Biol. Trace Elem. Res. 2021, 200, 2099–2112. [Google Scholar] [CrossRef]
- Fang, K.; Li, K.; Yang, T.; Li, J.; He, W. Starch-based magnetic nanocomposite as an efficient absorbent for anticancer drug removal from aqueous solution. Int. J. Biol. Macromol. 2021, 184, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Massoumi, B.; Mozaffari, Z.; Jaymand, M. A starch-based stimuli-responsive magnetite nanohydrogel as de novo drug delivery system. Int. J. Biol. Macromol. 2018, 117, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Petri, D.F. Xanthan gum: A versatile biopolymer for biomedical and technological applications. J. Appl. Polym. Sci. 2015, 132, 23. [Google Scholar] [CrossRef] [Green Version]
- Alhalmi, A.; Alzubaidi, N.; Altowairi, M.; Almoiliqy, M.; Sharma, B. Xanthan gum; its biopharmaceutical applications: An overview. Word J. Biopharm. Appl. Sci 2018, 7, 1536–1548. [Google Scholar]
- Afnan, U.; Sharma, K.; Sehgal, R.; Kumar, V. Xanthan gum-based nanocarriers for therapeutic delivery. In Polymeric Nanosystems; Elsevier: Amsterdam, The Netherlands, 2023; pp. 333–365. [Google Scholar]
- Djekic, L. Novel Mucoadhesive Polymers for Nasal Drug Delivery. In Nasal Drug Delivery: Formulations, Developments, Challenges, and Solutions; Springer: Berlin/Heidelberg, Germany, 2023; pp. 189–234. [Google Scholar]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and pectin-based composite materials: Beyond food texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef] [Green Version]
- Mellinas, C.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Recent trends in the use of pectin from agro-waste residues as a natural-based biopolymer for food packaging applications. Materials 2020, 13, 673. [Google Scholar] [CrossRef] [Green Version]
- Sundar Raj, A.; Rubila, S.; Jayabalan, R.; Ranganathan, T. A Review on Pectin: Chemistry due to General Properties of Pectin and its Pharmaceutical Uses. Sci. Rep. 2012, 1, 550. [Google Scholar] [CrossRef]
- Minzanova, S.T.; Mironov, V.F.; Arkhipova, D.M.; Khabibullina, A.V.; Mironova, L.G.; Zakirova, Y.M.; Milyukov, V.A. Biological activity and pharmacological application of pectic polysaccharides: A review. Polymers 2018, 10, 1407. [Google Scholar] [CrossRef] [Green Version]
- Rascón-Chu, A.; Díaz-Baca, J.; Carvajal-Millán, E.; López-Franco, Y.; Lizardi-Mendoza, J. New use for an “old” polysaccharide: Pectin-based composite materials. In Handbook of Sustainable Polymers: Structure and Chemistry; Thakur, V.K., Thakur, M.K., Eds.; Taylor Francis Group: Abingdon, UK, 2016; pp. 72–107. [Google Scholar]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-related gelling of pectins and linking with other natural compounds: A review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef] [Green Version]
- Odun-Ayo, F.; Reddy, L. Potential Biomedical Applications of Modified Pectin as a Delivery System for Bioactive Substances. Polysaccharides 2023, 4, 1–32. [Google Scholar] [CrossRef]
- Mountzouris, K.C. Nutritional strategies targeting the beneficial modulation of the intestinal microflora with relevance to food safety: The role of probiotics and prebiotics. In Proceedings of the Food Safety: A Practical and Case Study Approach; Springer: Berlin/Heidelberg, Germany, 2007; pp. 133–152. [Google Scholar]
- Orii, S.; Yamaguchi, T.; Anzai, H.; Saito, S.; Chiba, T.; Suzuki, K. Chemoprevention for colorectal tumorigenesis associated with chronic colitis in mice via apoptosis. J. Exp. Clin. Cancer Res. CR 2003, 22, 41–46. [Google Scholar]
- Wang, S.; Meng, Y.; Li, J.; Liu, J.; Liu, Z.; Li, D. A novel and simple oral colon-specific drug delivery system based on the pectin/modified nano-carbon sphere nanocomposite gel films. Int. J. Biol. Macromol. 2020, 157, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M. Prospective of guar gum and its derivatives as controlled drug delivery systems. Int. J. Biol. Macromol. 2011, 49, 117–124. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Shah, P.A.; Shrivastav, P.S. Guar gum: Versatile natural polymer for drug delivery applications. Eur. Polym. J. 2019, 112, 722–735. [Google Scholar] [CrossRef]
- Bauer, E.; Williams, B.A.; Smidt, H.; Mosenthin, R.; Verstegen, M.W. Influence of dietary components on development of the microbiota in single-stomached species. Nutr. Res. Rev. 2006, 19, 63–78. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, M.; Tao, Y.; Wang, T.; Wang, R.; Yong, Q. Assessing the in vitro digestion of Sesbania gum, a galactomannan from S. cannabina, and subsequent impact on the fecal microbiota. J. Funct. Foods 2021, 87, 104766. [Google Scholar] [CrossRef]
- Elsaeed, S.M.; Zaki, E.G.; Omar, W.A.; Ashraf Soliman, A.; Attia, A.M. Guar gum-based hydrogels as potent green polymers for enhanced oil recovery in high-salinity reservoirs. ACS Omega 2021, 6, 23421–23431. [Google Scholar] [CrossRef]
- Tripathi, J.; Ambolikar, R.; Gupta, S.; Jain, D.; Bahadur, J.; Variyar, P.S. Methylation of guar gum for improving mechanical and barrier properties of biodegradable packaging films. Sci. Rep. 2019, 9, 14505. [Google Scholar] [CrossRef] [Green Version]
- Abdel Rahim, S.; Elkordy, A. Application of Xanthan Gum as a Sustained Release Agent; Nova Science Publishers, Inc.: New York, NY, USA, 2016. [Google Scholar]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications—A review. J. Food Sci. Technol. 2014, 51, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, T.; Kumar, S.S. Pharmaceutical and pharmacological profile of guar gum an overview. Int. J. Pharm. Pharm. Sci. 2011, 3, 38–40. [Google Scholar]
- Raymond, P.; Rowe, C.; Sheskey, J.P.; Owen, S.C. Handbook of Pharmaceutical Excipients; Pharmaceutical Press: London, UK, 2006. [Google Scholar]
- Khullar, P.; Khar, R.; Agarwal, S. Guar gum as a hydrophilic matrix for preparation of theophylline controlled-release dosage form. Indian J. Pharm. Sci. 1999, 61, 342. [Google Scholar]
- Krishnaiah, Y.; Satyanarayana, V.; Kumar, B.D.; Karthikeyan, R. In vitro drug release studies on guar gum-based colon targeted oral drug delivery systems of 5-fluorouracil. Eur. J. Pharm. Sci. 2002, 16, 185–192. [Google Scholar] [CrossRef]
- Toti, U.S.; Aminabhavi, T.M. Modified guar gum matrix tablet for controlled release of diltiazem hydrochloride. J. Control. Release 2004, 95, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Tuğcu-Demiröz, F.; Acartürk, F.; Takka, S.; Konuş-Boyunağa, Ö. In-vitro and in-vivo evaluation of mesalazine–guar gum matrix tablets for colonic drug delivery. J. Drug Target. 2004, 12, 105–112. [Google Scholar] [CrossRef]
- Prasad, Y.R.; Krishnaiah, Y.; Satyanarayana, S. In vitro evaluation of guar gum as a carrier for colon-specific drug delivery. J. Control. Release 1998, 51, 281–287. [Google Scholar] [CrossRef]
- Gamal-Eldeen, A.M.; Amer, H.; Helmy, W.A. Cancer chemopreventive and anti-inflammatory activities of chemically modified guar gum. Chem. -Biol. Interact. 2006, 161, 229–240. [Google Scholar] [CrossRef]
- Pina, S.; Oliveira, J.M.; Reis, R.L. Natural-based nanocomposites for bone tissue engineering and regenerative medicine: A review. Adv. Mater. 2015, 27, 1143–1169. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.; Sun, Y.; Dong, W.; Zhou, H.; Guo, S.; Zhang, L.; Wang, X.; Wan, M.; Zong, Y. Enhanced anti-tumor efficacy of hyaluronic acid modified nanocomposites combined with sonochemotherapy against subcutaneous and metastatic breast tumors. Nanoscale 2019, 11, 11470–11483. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.M.; Nagappan, S.; Seo, D.J.; Ha, C.-S. pH sensitive halloysite-sodium hyaluronate/poly (hydroxyethyl methacrylate) nanocomposites for colon cancer drug delivery. Appl. Clay Sci. 2014, 97, 33–42. [Google Scholar] [CrossRef]
- Foox, M.; Zilberman, M. Drug delivery from gelatin-based systems. Expert Opin. Drug Deliv. 2015, 12, 1547–1563. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wei, L.; Xiao, Y.; Bi, H.; Yang, H.; Du, Y. Physicochemical and functional properties of gelatin extracted from Yak skin. Int. J. Biol. Macromol. 2017, 95, 1246–1253. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Control. Release 2013, 172, 1075–1091. [Google Scholar] [CrossRef]
- Santoro, M.; Tatara, A.M.; Mikos, A.G. Gelatin carriers for drug and cell delivery in tissue engineering. J. Control. Release 2014, 190, 210–218. [Google Scholar] [CrossRef] [Green Version]
- Rozga, J.; Piatek, T.; Malkowski, P. Human albumin: Old, new, and emerging applications. Ann Transpl. 2013, 18, 205–217. [Google Scholar]
- Quinlan, G.J.; Martin, G.S.; Evans, T.W. Albumin: Biochemical properties and therapeutic potential. Hepatology 2005, 41, 1211–1219. [Google Scholar] [CrossRef]
- Raval, N.; Mistry, T.; Acharya, N.; Acharya, S. Development of glutathione-conjugated asiatic acid-loaded bovine serum albumin nanoparticles for brain-targeted drug delivery. J. Pharm. Pharmacol. 2015, 67, 1503–1511. [Google Scholar] [CrossRef]
- Woods, A.; Patel, A.; Spina, D.; Riffo-Vasquez, Y.; Babin-Morgan, A.; De Rosales, R.; Sunassee, K.; Clark, S.; Collins, H.; Bruce, K. In vivo biocompatibility, clearance, and biodistribution of albumin vehicles for pulmonary drug delivery. J. Control. Release 2015, 210, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mienaltowski, M.J.; Birk, D.E. Structure, physiology, and biochemistry of collagens. In Progress in Heritable Soft Connective Tissue Diseases; Springer: Berlin/Heidelberg, Germany, 2014; pp. 5–29. [Google Scholar]
- Lin, W.; Klein, J. Recent progress in cartilage lubrication. Adv. Mater. 2021, 33, 2005513. [Google Scholar]
- Meghezi, S.; Seifu, D.G.; Bono, N.; Unsworth, L.; Mequanint, K.; Mantovani, D. Engineering 3D cellularized collagen gels for vascular tissue regeneration. JoVE 2015, 100, e52812. [Google Scholar]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, Z.; Dong, Y. Collagen-Based Biomaterials for Tissue Engineering. ACS Biomater. Sci. Eng. 2023, 9, 1132–1150. [Google Scholar] [CrossRef] [PubMed]
- Pande, V.; Sanklecha, V. Bionanocomposite: A review. Austin J. Nanomed. Nanotechnol. 2017, 5, 1045. [Google Scholar]
- Wang, J.; Sui, L.; Huang, J.; Miao, L.; Nie, Y.; Wang, K.; Yang, Z.; Huang, Q.; Gong, X.; Nan, Y. MoS2-based nanocomposites for cancer diagnosis and therapy. Bioact. Mater. 2021, 6, 4209–4242. [Google Scholar] [CrossRef]
- Carraher Jr, C.E.; Roner, M.R.; Campbell, A.G.; Moric-Johnson, A.; Miller, L.; Slawek, P.; Mosca, F. Group IVB metallocene polyesters containing camphoric acid and preliminary cancer cell activity. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 469–479. [Google Scholar] [CrossRef]
- Din, F.u.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Zhou, Y.; Xiang, Y.; Shu, M.; Chen, H.; Yang, B.; Liao, X. Polyurethane/doxorubicin nanoparticles based on electrostatic interactions as pH-sensitive drug delivery carriers. Polym. Int. 2018, 67, 1186–1193. [Google Scholar] [CrossRef]
- Liang, P.; Wang, C.Q.; Chen, H.; Zhuo, R.X.; Cheng, S.X. Multi-functional heparin–biotin/heparin/calcium carbonate/calcium phosphate nanoparticles for targeted co-delivery of gene and drug. Polym. Int. 2015, 64, 647–653. [Google Scholar] [CrossRef]
- Su, T.; Cheng, F.; Lin, S.; Xiao, T.; Zhu, Y.; Cao, J.; He, B. Reduction-induced decomposition and self-aggregation strategy to induce reactive oxygen species generation for cancer therapy. ACS Appl. Bio Mater. 2018, 1, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Wu, M.; Han, L.; Song, Y.; Yuan, S.; Zhang, Y.; Wu, Z.; Wu, Z.; Qi, X. Surface partially neutralized dendtric polymer demonstrating proton-triggered self-assembled aggregation for tumor therapy. Eur. Polym. J. 2018, 103, 59–67. [Google Scholar] [CrossRef]
- Bonadies, I.; Maglione, L.; Ambrogi, V.; Paccez, J.D.; Zerbini, L.F.; e Silva, L.F.R.; Picanço, N.S.; Tadei, W.P.; Grafova, I.; Grafov, A. Electrospun core/shell nanofibers as designed devices for efficient Artemisinin delivery. Eur. Polym. J. 2017, 89, 211–220. [Google Scholar] [CrossRef]
- Feldman, D. Polymers and polymer nanocomposites for cancer therapy. Appl. Sci. 2019, 9, 3899. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, F.N.; Aristizabal Bedoya, D.; Strumia, M.C.; Macchione, M.A. Nanocomposites for Cancer Targeted Drug Delivery Therapeutics. In Biomedical Composites, Perspectives and Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 201–222. [Google Scholar]

| Nanobiocomposites for Anti-Cancer Drug Delivery | Drugs | References |
|---|---|---|
| Ag2S(DOX)@CS nanospheres | Doxorubicin | [150] |
| Stimuli-responsive hydrogel nanocomposite with magnetic Fe3O4 nanoparticles | Doxorubicin | [144] |
| Biomass-derived dialdehyde cellulose cross-linked chitosan-based nanocomposite hydrogel with phytosynthesized zinc oxide nanoparticles (ZnO NPs) | Curcumin | [151] |
| Nanobiocomposites for Anti-Cancer Drug Delivery | Drugs | References |
|---|---|---|
| CaP nanocomposites with alginate as a polymer template | Chlorogenic acid (CG-NP), caffeic acid (CA-NP), or cisplatin (CP-NP) | [158] |
| Alginate/clay/imidazolium-based ionic liquid-based nanocomposites | Methotrexate | [159] |
| Nanobiocomposites for Anti-Cancer Drug Delivery | Drugs | References |
|---|---|---|
| Nanocomposite of carboxymethyl cellulose (CMC) and core-shell nanoparticles of Fe3O4@SiO2 | Quercetin | [168] |
| The cellulose-based nanocomposite of magnetic (Fe3O4), zinc oxide (ZnO) | Doxorubicin | [169] |
| Carboxymethyl cellulose/graphene quantum dot nanocomposite | Doxorubicin | [167] |
| Nanocomposites of Fe3O4-supported on rice straw cellulose | 5-fluorouracil | [170] |
| ZnO/carboxymethyl cellulose/chitosan bio-nanocomposite | 5-fluorouracil | [78] |
| Cellulose grafted hydrogel doped calcium oxide nanocomposites | Doxorubicin | [171] |
| Nanocomposite of carboxymethyl cellulose (CMC) and core-shell nanoparticles of Fe3O4@SiO2 | Quercetin | [168] |
| Nanobiocomposites for Anti-Cancer Drug Delivery | Drugs | References |
|---|---|---|
| Nanocomposite of myco-synthesized copper nanoparticles (CuNPs) and starch | Copper | [177] |
| Carboxymethyl cassava starch (CMCS)-functionalized magnetic nanoparticles | Doxorubicin hydrochloride | [178] |
| Starch-based stimuli-responsive magnetite nanohydrogel (MNHG), namely Fe3O4-g-[poly(N-isopropylacrylamide-co-maleic anhydride)]@strach; Fe3O4-g-(PNIPAAm-co-PMA)@starch | Doxorubicin | [179] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondal, A.; Nayak, A.K.; Chakraborty, P.; Banerjee, S.; Nandy, B.C. Natural Polymeric Nanobiocomposites for Anti-Cancer Drug Delivery Therapeutics: A Recent Update. Pharmaceutics 2023, 15, 2064. https://doi.org/10.3390/pharmaceutics15082064
Mondal A, Nayak AK, Chakraborty P, Banerjee S, Nandy BC. Natural Polymeric Nanobiocomposites for Anti-Cancer Drug Delivery Therapeutics: A Recent Update. Pharmaceutics. 2023; 15(8):2064. https://doi.org/10.3390/pharmaceutics15082064
Chicago/Turabian StyleMondal, Arijit, Amit Kumar Nayak, Prithviraj Chakraborty, Sabyasachi Banerjee, and Bankim Chandra Nandy. 2023. "Natural Polymeric Nanobiocomposites for Anti-Cancer Drug Delivery Therapeutics: A Recent Update" Pharmaceutics 15, no. 8: 2064. https://doi.org/10.3390/pharmaceutics15082064








