A Novel Platform of MOF for Sonodynamic Therapy Advanced Therapies
Abstract
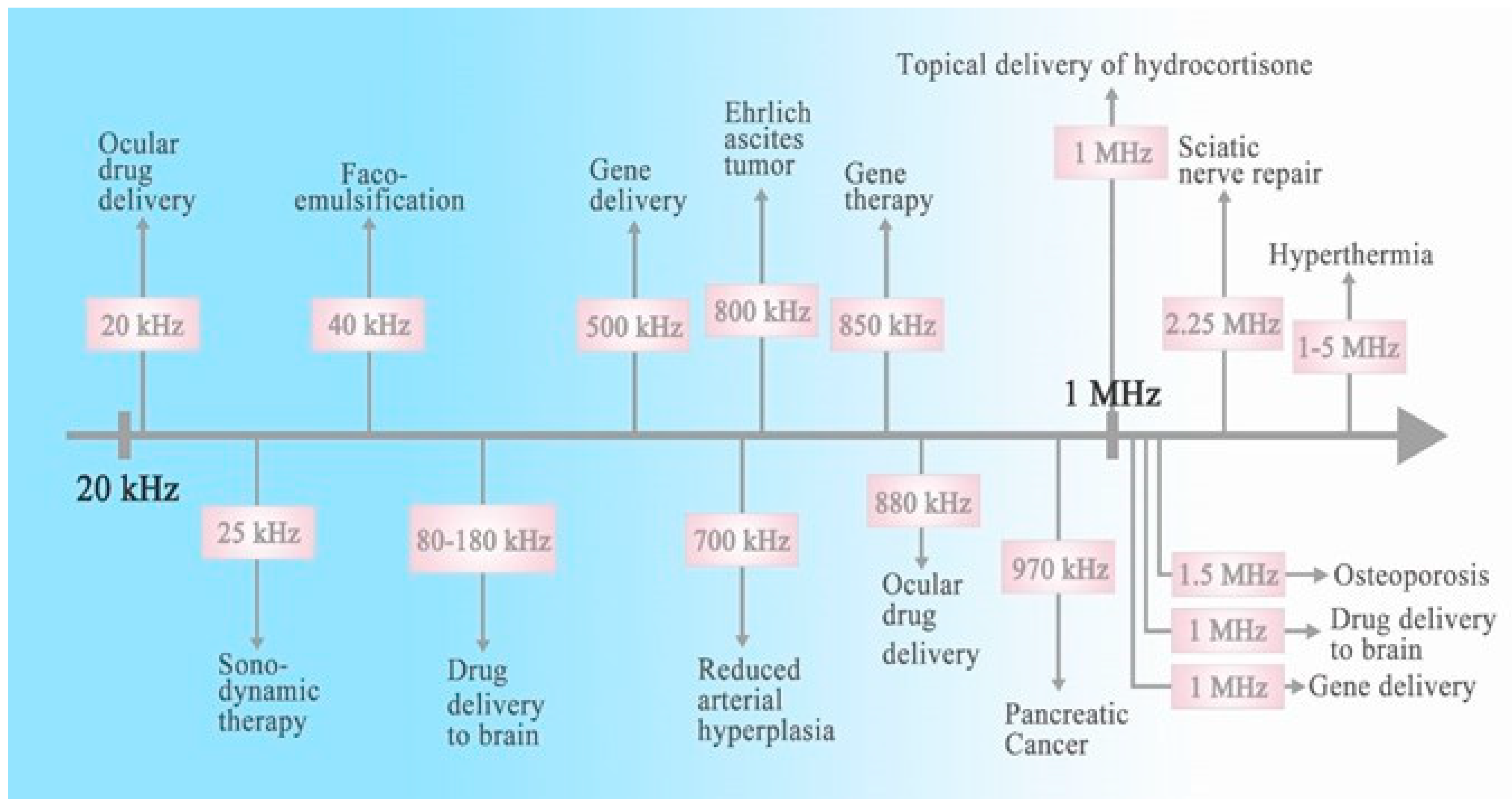
:1. Introduction
2. Application of MOFs in SDT
2.1. Application of MOFs as Sonosensitizer Carriers in SDT
| No. | Composition | MOFs | Synthesis Method | Grain Size | Treatment | Ref. |
|---|---|---|---|---|---|---|
| 1 | O2@Hb@ZIF-8 | ZIF-8 | One Pot Method | 200 nm | 4T1 | [96] |
| 2 | DOX/Ce6@ZIF-8@PDA | ZIF-8 | One Pot Method | 135 nm | 4T1 | [97] |
| 3 | ZDC@M | ZIF-8 | One Pot Method | 240 nm | 4T1 | [98] |
| 4 | GSNO/Ce6@ZIF-8@Cytomembrane | ZIF-8 | One Pot Method | 179 nm | 4T1 | [99] |
| 5 | Zr-MOF@AIPH | Zr-MOF | ~138 nm | PancO2 | [100] | |
| 6 | ZIF-8@mSiO2 | ZIF-8 | One Pot Method | 180–190 nm | 4T1 | [101] |
| 7 | MIL@Ag-PEG | Ti-MOF (MIL) | Solvent heat method | 230 nm | A549 | [102] |
| 8 | PMCS | ZIF-8 | One Pot Method | 110 nm | 4T1 | [103] |
| 9 | cMn-MOF@CM | Mn-MOF | Solvent heat method | 87.1 ± 1.0 nm | B16/H22 | [104] |
| 10 | Mn-TCPP | Mn-MOF | Solvent heat method | 70 nm | H22/4T1 | [105] |
| 11 | DOX@FeCPs | Fe-HMME | self-assemble | ~70 nm | 4T1/CT26 | [106] |
| 12 | PL-PEG-PCN | PCN-222 | Solvent heat method | ~185 nm | MCF-7 | [107] |
| 13 | DOX@PCN-224/Pt | PCN-224 | Solvent heat method | 100 nm | CT16/SKOV3 | [108] |
| 14 | AIPH@Cu-MOF | Cu-MOF | 259.4 × 118.9 nm | Panc02 | [109] | |
| 15 | D-MOF(Ti) | Ti-MOF (MIL) | Solvent heat method | 120 nm | 4T1 | [110] |
| 16 | MOF@MP-RGD | UiO-66 | Solvent heat method | <200 nm | MDA-MB-231 | [111] |
| 17 | MPG | Fe-MIL-88B-NH2 | Solvent heat method | ~200 nm | 4T1 | [112] |
| 18 | Cu-MOF/Ce6 | Cu-MOF | One Pot Method | 260 nm | MCF-7 | [113] |
| 19 | Pt@ZIF-90@Gem@IR780 | ZIF-90 | ~150 nm | BxPC-3 | [114] |
2.2. Application of MOFs as Sonosensitizer in SDT
2.3. Combined Treatment of SDT Based on MOF-Sonosensitizer
3. Conclusions and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Biankin, A.V.; Piantadosi, S.; Hollingsworth, S.J. Patient-centric trials for therapeutic development in precision oncology. Nature 2015, 526, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Levinson, A.D. Cancer Therapy Reform. Science 2010, 328, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obenauf, A.C.; Zou, Y.; Ji, A.L.; Vanharanta, S.; Shu, W.; Shi, H.; Kong, X.; Bosenberg, M.C.; Wiesner, T.; Rosen, N.; et al. Therapy-induced tumour secretomes promote resistance and tumour progression. Nature 2015, 520, 368–372. [Google Scholar] [CrossRef] [Green Version]
- Scheel, C.; NgEaton, E.; Li, S.J.; Chaffer, C.L.; Reinhardt, F.; Kah, K.J.; Bell, G.; Guo, W.; Rubin, J.; Richardson, A.; et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell 2011, 145, 926–940. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, B.C.; Chang, C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef] [Green Version]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Schumacker, P.T. Reactive oxygen species in cancer: A dance with the devil. Cancer Cell. 2015, 27, 156–157. [Google Scholar] [CrossRef] [Green Version]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Ramsey, M.R.; Sharpless, N.E. ROS as a tumour suppressor? Nat. Cell Biol. 2006, 8, 1213–1215. [Google Scholar] [CrossRef]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, A.; Ohtani, N.; Yamakoshi, K.; Iida, S.; Tahara, H.; Nakayama, K.; Nakayama, K.I.; Ide, T.; Saya, H.; Hara, E. Mitogenic signalling and the p16INK4a-Rb pathway cooperate to enforce irreversible cellular senescence. Nat. Cell Biol. 2006, 8, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Graham, C.H. Chemosensitization of cancer by nitric oxide. Curr. Pharm. Des. 2008, 14, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, S.; Clement, M.V. Tumor intracellular redox status and drug resistance—Serendipity or a causal relationship? Curr. Pharm. Des. 2004, 10, 1969–1977. [Google Scholar] [CrossRef]
- Toyokuni, S.; Okamoto, K.; Yodoi, J.; Hiai, H. Persistent oxidative stress in cancer. FEBS Lett. 1995, 358, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Szatrowski, T.P.; Nathan, C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991, 51, 794–798. [Google Scholar]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef] [Green Version]
- Kawanishi, S.; Hiraku, Y.; Pinlaor, S.; Ma, N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol. Chem. 2006, 387, 365–372. [Google Scholar] [CrossRef]
- Tiligada, E. Chemotherapy: Induction of stress responses. Endocr. Relat. Cancer 2006, 13 (Suppl. S1), S115–S124. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug. Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Felsher, D.W. Cancer revoked: Oncogenes as therapeutic targets. Nat. Rev. Cancer 2003, 3, 375–380. [Google Scholar] [CrossRef]
- Moan, J.; Berg, K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem. Photobiol. 1991, 53, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.C. Photodynamic therapy for cancer: Principles. Can. J. Gastroenterol. 2002, 16, 393–396. [Google Scholar] [CrossRef]
- Hong, G.; Lee, J.C.; Robinson, J.T.; Raaz, U.; Xie, L.; Huang, N.F.; Cooke, J.P.; Dai, H. Multifunctional in vivo vascular imaging using near-infrared II fluorescence. Nat. Med. 2012, 18, 1841–1846. [Google Scholar] [CrossRef]
- Bailey, M.R.; Khokhlova, V.A.; Sapozhnikov, O.A.; Kargl, S.G.; Crum, L.A. Physical mechanisms of the therapeutic effect of ultrasound. Acoust. Phys. 2003, 49, 369–388. [Google Scholar] [CrossRef]
- Schuster, A.; Schwab, T.; Bischof, M.; Klotz, M.; Lemor, R.; Degel, C.; Schafer, K.H. Cell specific ultrasound effects are dose and frequency dependent. Ann. Anat. 2013, 195, 57–67. [Google Scholar] [CrossRef]
- Liberman, A.; Wang, J.; Lu, N.; Viveros, R.D.; Allen, C.A.; Mattrey, R.F.; Blair, S.L.; Trogler, W.C.; Kim, M.J.; Kummel, A.C. Mechanically Tunable Hollow Silica Ultrathin Nanoshells for Ultrasound Contrast Agents. Adv. Funct. Mater. 2015, 25, 4049–4057. [Google Scholar] [CrossRef] [Green Version]
- Huynh, E.; Leung, B.Y.; Helfield, B.L.; Shakiba, M.; Gandier, J.A.; Jin, C.S.; Master, E.R.; Wilson, B.C.; Goertz, D.E.; Zheng, G. In situ conversion of porphyrin microbubbles to nanoparticles for multimodality imaging. Nat. Nanotechnol. 2015, 10, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Wang, W.; Kong, W.; Chen, Y. Organic-Inorganic Hybrid Hollow Mesoporous Organosilica Nanoparticles for Efficient Ultrasound-Based Imaging and Controlled Drug Release. J. Nanomater. 2014, 2014, 115. [Google Scholar] [CrossRef] [Green Version]
- Umemura, S.; Yumita, N.; Nishigaki, R.; Umemura, K. Mechanism of cell damage by ultrasound in combination with hematoporphyrin. Jpn. J. Cancer Res. 1990, 81, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Deng, X.; Ma, P.; Cheng, Z.; Lin, J. Recent Advances in Nanomaterial-Assisted Combinational Sonodynamic Cancer Therapy. Adv. Mater. 2020, 32, e2003214. [Google Scholar] [CrossRef]
- Son, S.; Kim, J.H.; Wang, X.; Zhang, C.; Yoon, S.A.; Shin, J.; Sharma, A.; Lee, M.H.; Cheng, L.; Wu, J.; et al. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem. Soc. Rev. 2020, 49, 3244–3261. [Google Scholar] [CrossRef]
- Lin, X.; Song, J.; Chen, X.; Yang, H. Ultrasound-Activated Sensitizers and Applications. Angew. Chem. Int. Ed. Engl. 2020, 59, 14212–14233. [Google Scholar] [CrossRef]
- Harada, Y.; Ogawa, K.; Irie, Y.; Endo, H.; Feril, L.B., Jr.; Uemura, T.; Tachibana, K. Ultrasound activation of TiO2 in melanoma tumors. J. Control. Release 2011, 149, 190–195. [Google Scholar] [CrossRef]
- Qian, X.; Zheng, Y.; Chen, Y. Micro/Nanoparticle-Augmented Sonodynamic Therapy (SDT): Breaking the Depth Shallow of Photoactivation. Adv. Mater. 2016, 28, 8097–8129. [Google Scholar] [CrossRef]
- Karami, A.; Mohamed, O.; Ahmed, A.; Husseini, G.A.; Sabouni, R. Recent Advances in Metal-Organic Frameworks as Anticancer Drug Delivery Systems: A Review. Anticancer Agents Med. Chem. 2021, 21, 2487–2504. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wang, T.; Yuan, S.; Wang, M.; Qi, W.; Su, R.; He, Z. A tumor-sensitive biological metal-organic complex for drug delivery and cancer therapy. J. Mater. Chem. B 2020, 8, 7189–7196. [Google Scholar] [CrossRef]
- Seo, P.W.; Khan, N.A.; Jhung, S.H. Removal of nitroimidazole antibiotics from water by adsorption over metal–organic frameworks modified with urea or melamine. Chem. Eng. J. 2017, 315, 92–100. [Google Scholar] [CrossRef]
- Ahmed, I.; Jhung, S.H. Adsorptive desulfurization and denitrogenation using metal-organic frameworks. J. Hazard. Mater. 2016, 301, 259–276. [Google Scholar] [CrossRef]
- Furukawa, H.; Gandara, F.; Zhang, Y.B.; Jiang, J.; Queen, W.L.; Hudson, M.R.; Yaghi, O.M. Water adsorption in porous metal-organic frameworks and related materials. J. Am. Chem. Soc. 2014, 136, 4369–4381. [Google Scholar] [CrossRef]
- Ma, D.; Li, Z.; Zhu, J.; Zhou, Y.; Chen, L.; Mai, X.; Liufu, M.L.; Wu, Y.B.; Li, Y.W. Inverse and highly selective separation of CO2/C2H2 on a thulium–organic framework. J. Mater. Chem. A 2020, 8, 11933–11937. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Zeng, G.; Chen, X.; Leng, L.; Li, H. Synthesis and applications of novel graphitic carbon nitride/metal-organic frameworks mesoporous photocatalyst for dyes removal. Appl. Catal. 2015, 174, 445–454. [Google Scholar] [CrossRef]
- Hu, M.L.; Safarifard, V.; Doustkhah, E.; Rostamnia, S.; Morsali, A.; Nouruzi, N.; Beheshti, S.; Akhbari, K. Taking organic reactions over metal-organic frameworks as heterogeneous catalysis. Microporous Mesoporous Mater. 2018, 256, 111–127. [Google Scholar] [CrossRef]
- Gao, D.; Ding, T.; Yan, W.W.; Zheng, L.N.; Xie, K.F.; Gao, Z.W. Two Structurally Similar Co5 Cluster-Based Metal-Organic Frameworks Containing Open Metal Sites for Efficient C2H2/CO2 Separation. Inorg. Chem. 2022, 61, 20026–20034. [Google Scholar] [CrossRef]
- Bavykina, A.; Kolobov, N.; Khan, I.S.; Bau, J.A.; Ramirez, A.; Gascon, J. Metal-Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020, 120, 8468–8535. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Li, F.; Sun, L. Metal-organic frameworks and their derivatives as electrocatalysts for the oxygen evolution reaction. Chem. Soc. Rev. 2021, 50, 2663–2695. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Qu, C.; Guo, W.; Zou, R.; Xu, Q. Pristine Metal-Organic Frameworks and their Composites for Energy Storage and Conversion. Adv. Mater. 2018, 30, e1702891. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, S.; Day, G.; Wang, X.; Yang, X.; Zhou, H.C. Luminescent sensors based on metal-organic frameworks. Coord. Chem. Rev. 2017, 354, 28–45. [Google Scholar] [CrossRef]
- Zeng, L.; Guo, X.; He, C.; Duan, C. Metal–Organic Frameworks: Versatile Materials for Heterogeneous Photocatalysis. ACS Catal. 2016, 6, 7935–7947. [Google Scholar]
- Taddei, M. When defects turn into virtues: The curious case of zirconium-based metal-organic frameworks. Coord. Chem. Rev. 2017, 343, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Dong, J.P.; Zhou, Z.; Wang, R.; Wang, L.Y.; Zang, S.Q. Robust lanthanide metal–organic frameworks with “all-in-one” multifunction: Efficient gas adsorption and separation, tunable light emission and luminescence sensing. J. Mater. Chem. C 2021, 9, 3429–3439. [Google Scholar] [CrossRef]
- Yang, X.; Xu, Q. Bimetallic Metal–Organic Frameworks for Gas Storage and Separation. Cryst. Growth Des. 2017, 17, 1450–1455. [Google Scholar] [CrossRef]
- Howarth, A.J.; Katz, M.J.; Wang, T.C.; Platero-Prats, A.E.; Chapman, K.W.; Hupp, J.T.; Farha, O.K. High efficiency adsorption and removal of selenate and selenite from water using metal-organic frameworks. J. Am. Chem. Soc. 2015, 137, 7488–7494. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Li, B.; Shi, Z. Multi-functional sites catalysts based on post-synthetic modification of metal-organic frameworks. Chin. Chem. Lett. 2018, 29, 123–126. [Google Scholar] [CrossRef]
- Wang, C.C.; Du, X.D.; Li, J.; Guo, X.X.; Wang, P.; Zhang, J. Photocatalytic Cr(VI) reduction in metal-organic frameworks: A mini-review. Appl. Catal. 2016, 193, 198–216. [Google Scholar] [CrossRef]
- Zhao, D.; Wan, X.; Song, H.; Hao, L.; Su, Y.; Lv, Y. Metal–organic frameworks (MOFs) combined with ZnO quantum dots as a fluorescent sensing platform for phosphate. Sens. Actuators B Chem. 2014, 197, 50–57. [Google Scholar] [CrossRef]
- Langmi, H.W.; Ren, J.; North, B.; Mathe, M.; Bessarabov, D. Hydrogen Storage in Metal-Organic Frameworks: A Review. Electrochim. Acta 2014, 128, 368–392. [Google Scholar] [CrossRef]
- Bai, Y.; Dou, Y.; Xie, L.H.; Rutledge, W.; Li, J.R.; Zhou, H.C. Zr-based metal-organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef]
- Sholl, D.S.; Lively, R.P. Defects in Metal-Organic Frameworks: Challenge or Opportunity? J. Phys. Chem. Lett. 2015, 6, 3437–3444. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Yang, D.H.; Xu, J.; Hu, T.L.; Bu, X.H. Flexible Metal-Organic Frameworks: Recent Advances and Potential Applications. Adv. Mater. 2015, 27, 5432–5441. [Google Scholar] [CrossRef] [PubMed]
- Gandara-Loe, J.; Pastor-Perez, L.; Bobadilla, L.F.; Odriozola, J.A.; Reina, T.R. Understanding the opportunities of metal–organic frameworks (MOFs) for CO2 capture and gas-phase CO2 conversion processes: A comprehensive overview. React. Chem. Eng. 2021, 6, 787–814. [Google Scholar] [CrossRef]
- Lv, Y.; Tan, X.; Svec, F. Preparation and applications of monolithic structures containing metal-organic frameworks. J. Sep. Sci. 2017, 40, 272–287. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Desai, A.V.; Ghosh, S.K. Ionic metal-organic frameworks (iMOFs): Design principles and applications. Coord. Chem. Rev. 2016, 307, 313–341. [Google Scholar] [CrossRef]
- Hendon, C.H.; Rieth, A.J.; Korzynski, M.D.; Dinca, M. Grand Challenges and Future Opportunities for Metal-Organic Frameworks. ACS Cent. Sci. 2017, 3, 554–563. [Google Scholar] [CrossRef]
- Chen, L.; Luque, R.; Li, Y. Controllable design of tunable nanostructures inside metal-organic frameworks. Chem. Soc. Rev. 2017, 46, 4614–4630. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, D.; Xu, Z.; Liu, B.; Boubeche, M.; Chen, Z.; Wang, Y.; Luo, H.; Yan, K. Facile synthesis of Ni-, Co-, Cu-metal organic frameworks electrocatalyst boosting for hydrogen evolution reaction. J. Mater. Sci. Technol. 2021, 72, 172–179. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, T.; Wang, L.; Zhang, T. Metal-Organic Frameworks-Derived Hierarchical Co3O4 Structures as Efficient Sensing Materials for Acetone Detection. ACS Appl. Mater. Interfaces 2018, 10, 9765–9773. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.A.; Keitz, B.K.; Oktawiec, J.; Mason, J.A.; Runcevski, T.; Xiao, D.J.; Darago, L.E.; Crocella, V.; Bordiga, S.; Long, J.R. A spin transition mechanism for cooperative adsorption in metal-organic frameworks. Nature 2017, 550, 96–100. [Google Scholar] [CrossRef]
- Li, B.; Chrzanowski, M.; Zhang, Y.; Ma, S. Applications of metal-organic frameworks featuring multi-functional sites. Coord. Chem. Rev. 2016, 307, 106–129. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.A.; Jhung, S.H. Synthesis of metal-organic frameworks (MOFs) with microwave or ultrasound: Rapid reaction, phase-selectivity, and size reduction. Coord. Chem. Rev. 2015, 285, 11–23. [Google Scholar] [CrossRef]
- Bon, V.; Senkovska, I.; Kaskel, S. Metal-Organic Frameworks. In Nanoporous Materials for Gas Storage; Springer: Singapore, 2019; pp. 137–172. [Google Scholar]
- Li, B.; Suo, T.; Xie, S.; Xia, A.; Ma, Y.; Huang, H.; Zhang, X.; Hu, Q. Rational design, synthesis, and applications of carbon dots@metal–organic frameworks (CD@MOF) based sensors. Trends Anal. Chem. 2021, 135, 116163. [Google Scholar] [CrossRef]
- Pettinari, C.; Marchetti, F.; Mosca, N.; Tosi, G.; Drozdov, A. Application of metal-organic frameworks. Polym. Int. 2017, 66, 731–744. [Google Scholar] [CrossRef]
- Qin, W.; Gu, Y.; Wang, G.; Wu, T.; Zhang, H.; Tang, X.; Zhang, Y.; Zhao, H. Zirconium metal organic frameworks-based DGT technique for in situ measurement of dissolved reactive phosphorus in waters. Water Res. 2018, 147, 223–232. [Google Scholar] [CrossRef]
- Cheng, M.; Lai, C.; Liu, Y.; Zeng, G.; Huang, D.; Zhang, C.; Qin, L.; Hu, L.; Zhou, C.; Xiong, W. Metal-organic frameworks for highly efficient heterogeneous Fenton-like catalysis. Coord. Chem. Rev. 2018, 368, 80–92. [Google Scholar] [CrossRef]
- Dubinsky, T.J.; Cuevas, C.; Dighe, M.K.; Kolokythas, O.; Hwang, J.H. High-intensity focused ultrasound: Current potential and oncologic applications. Am. J. Roentgenol. 2008, 190, 191–199. [Google Scholar] [CrossRef]
- Mitragotri, S. Healing sound: The use of ultrasound in drug delivery and other therapeutic applications. Nat. Rev. Drug Discov. 2005, 4, 255–260. [Google Scholar] [CrossRef]
- Xing, X.; Zhao, S.; Xu, T.; Huang, L.; Zhang, Y.; Lan, M.; Lin, C.; Zheng, X.; Wang, P. Advances and perspectives in organic sonosensitizers for sonodynamic therapy. Coord. Chem. Rev. 2021, 445, 214087. [Google Scholar] [CrossRef]
- Yin, H.; Chang, N.; Xu, S.; Wan, M. Sonoluminescence characterization of inertial cavitation inside a BSA phantom treated by pulsed HIFU. Ultrason. Sonochem. 2016, 32, 158–164. [Google Scholar] [CrossRef]
- Canavese, G.; Ancona, A.; Racca, L.; Canta, M.; Dumontel, B.; Barbaresco, F.; Limongi, T.; Cauda, V. Nanoparticle-assisted ultrasound: A special focus on sonodynamic therapy against cancer. Chem. Eng. J. 2018, 340, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, I.; Sostaric, J.Z.; Riesz, P. Sonodynamic therapy—A review of the synergistic effects of drugs and ultrasound. Ultrason. Sonochem. 2004, 11, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.F. High intensity focused ultrasound in clinical tumor ablation. World J. Clin. Oncol. 2011, 2, 8–27. [Google Scholar] [CrossRef]
- Zhou, Q.L.; Chen, Z.Y.; Wang, Y.X.; Yang, F.; Lin, Y.; Liao, Y.Y. Ultrasound-mediated local drug and gene delivery using nanocarriers. BioMed Res. Int. 2014, 2014, 963891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, G.Y.; Liu, Y.; Chen, B.W.; Liu, Y.Y.; Wang, Y.S.; Zhang, N. Recent advances of sonodynamic therapy in cancer treatment. Cancer Biol. Med. 2016, 13, 325–338. [Google Scholar] [CrossRef] [Green Version]
- Sazgarnia, A.; Shanei, A.; Eshghi, H.; Hassanzadeh, K.M.; Esmaily, H.; Shanei, M.M. Detection of sonoluminescence signals in a gel phantom in the presence of Protoporphyrin IX conjugated to gold nanoparticles. Ultrasonics 2013, 53, 29–35. [Google Scholar] [CrossRef]
- Serpe, L.; Foglietta, F.; Canaparo, R. Nanosonotechnology: The next challenge in cancer sonodynamic therapy. Nanotechnol. Rev. 2012, 1, 173–182. [Google Scholar] [CrossRef]
- Bai, W.K.; Shen, E.; Hu, B. The induction of the apoptosis of cancer cell by sonodynamic therapy: A review. Chin. J. Cancer Res. 2012, 24, 368–373. [Google Scholar] [CrossRef]
- Trendowski, M. The promise of sonodynamic therapy. Cancer Metastasis Rev. 2014, 33, 143–160. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Lu, C.T.; Zhou, Z.C.; Jin, Z.; Zhang, L.; Sun, C.Z.; Xu, Y.Y.; Gao, H.S.; Tian, J.L.; Gao, F.H.; et al. Enhancing chemotherapeutic drug inhibition on tumor growth by ultrasound: An in vivo experiment. J. Drug Target. 2011, 19, 154–160. [Google Scholar] [CrossRef]
- Huang, P.; Qian, X.; Chen, Y.; Yu, L.; Lin, H.; Wang, L.; Zhu, Y.; Shi, J. Metalloporphyrin-Encapsulated Biodegradable Nanosystems for Highly Efficient Magnetic Resonance Imaging-Guided Sonodynamic Cancer Therapy. J. Am. Chem. Soc. 2017, 139, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, I.B.; Silva, T.G.D.; Militão, G.C.G.; Soares, T.A.; Rodrigues, N.M.; Rodrigues, M.O.; Costa, N.B.D.; Freire, R.O.; Junior, S.A. Cytotoxicity and slow release of the anti-cancer drug doxorubicin from ZIF-8. RSC Adv. 2012, 2, 9437–9442. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Y.; Liu, L.; Wan, W.; Guo, P.; Nystrom, A.M.; Zou, X. One-pot Synthesis of Metal-Organic Frameworks with Encapsulated Target Molecules and Their Applications for Controlled Drug Delivery. J. Am. Chem. Soc. 2016, 138, 962–968. [Google Scholar] [CrossRef]
- Lawson, H.D.; Walton, S.P.; Chan, C. Metal-Organic Frameworks for Drug Delivery: A Design Perspective. ACS Appl. Mater. Interfaces 2021, 13, 7004–7020. [Google Scholar] [CrossRef]
- Yuan, M.; Liang, S.; Zhou, Y.; Xiao, X.; Liu, B.; Yang, C.; Ma, P.; Cheng, Z.; Lin, J. A Robust Oxygen-Carrying Hemoglobin-Based Natural Sonosensitizer for Sonodynamic Cancer Therapy. Nano Lett. 2021, 21, 6042–6050. [Google Scholar] [CrossRef]
- Zhong, L.; Yang, T.; Li, P.; Shi, L.; Lai, J.; Gu, L. Metal-Organic Framework-Based Nanotherapeutics With Tumor Hypoxia-Relieving Ability for Synergistic Sonodynamic/Chemo-therapy. Front. Mater. 2022, 9, 841503. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, M.; Yu, Z.; Pham, T.T.H.; Mo, S.; He, Y.; Liang, T.; Cao, W.; Han, C. Biomimetic cytomembrane-coated ZIF-8-loaded DMDD nanoparticle and sonodynamic co-therapy for cancer. Ann. Transl. Med. 2022, 10, 971. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Hu, Y.G.; Li, C.; Hou, X.L.; Cheng, K.; Zhang, B.; Zhang, R.Y.; Li, D.Y.; Liu, S.J.; Liu, B.; et al. A pH/Ultrasound dual-response biomimetic nanoplatform for nitric oxide gas-sonodynamic combined therapy and repeated ultrasound for relieving hypoxia. Biomaterials 2020, 230, 119636. [Google Scholar] [CrossRef]
- Zhang, C.; Xin, L.; Li, J.; Cao, J.; Sun, Y.; Wang, X.; Luo, J.; Zeng, Y.; Li, Q.; Zhang, Y.; et al. Metal-Organic Framework (MOF)-Based Ultrasound-Responsive Dual-Sonosensitizer Nanoplatform for Hypoxic Cancer Therapy. Adv. Healthc. Mater. 2022, 11, e2101946. [Google Scholar] [CrossRef]
- Wang, W.; Xu, B.; Pan, X.; Zhang, J.; Liu, H. Solvent-Dependent Adsorption-Driven Mechanism for MOFs-Based Yolk-Shell Nanostructures. Angew. Chem. Int. Ed. Engl. 2021, 60, 7802–7808. [Google Scholar] [CrossRef]
- Meng, X.; Sun, S.; Gong, C.; Yang, J.; Yang, Z.; Zhang, X.; Dong, H. Ag-Doped Metal-Organic Frameworks’ Heterostructure for Sonodynamic Therapy of Deep-Seated Cancer and Bacterial Infection. ACS Nano 2022, 17, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Bai, L.; Wang, H.; Wu, Q.; Wang, H.; Liu, S.; Xu, B.; Shi, X.; Liu, H. Metal-Organic-Framework-Derived Carbon Nanostructure Augmented Sonodynamic Cancer Therapy. Adv. Mater. 2018, 30, e1800180. [Google Scholar] [CrossRef]
- Zhan, G.; Xu, Q.; Zhang, Z.; Wei, Z.; Yong, T.; Bie, N.; Zhang, X.; Li, X.; Li, J.; Gan, L.; et al. Biomimetic sonodynamic therapy-nanovaccine integration platform potentiates Anti-PD-1 therapy in hypoxic tumors. Nano Today 2021, 38, 101195. [Google Scholar] [CrossRef]
- Xu, Q.; Zhan, G.; Zhang, Z.; Yong, T.; Yang, X.; Gan, L. Manganese porphyrin-based metal-organic framework for synergistic sonodynamic therapy and ferroptosis in hypoxic tumors. Theranostics 2021, 11, 1937–1952. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yu, N.; Zhang, J.; Wang, Z.; Geng, P.; Wen, M.; Li, M.; Zhang, H.; Chen, Z. Biocompatible Fe-Hematoporphyrin coordination nanoplatforms with efficient sonodynamic-chemo effects on deep-seated tumors. Biomaterials 2020, 257, 120239. [Google Scholar] [CrossRef]
- Hoang, Q.T.; Kim, M.; Kim, B.C.; Lee, C.Y.; Shim, M.S. Pro-oxidant drug-loaded porphyrinic zirconium metal-organic-frameworks for cancer-specific sonodynamic therapy. Colloids Surf. B Biointerfaces 2022, 209, 112189. [Google Scholar] [CrossRef]
- Ren, Q.; Yu, N.; Wang, L.; Wen, M.; Geng, P.; Jiang, Q.; Li, M.; Chen, Z. Nanoarchitectonics with metal-organic frameworks and platinum nanozymes with improved oxygen evolution for enhanced sonodynamic/chemo-therapy. J. Colloid Interface Sci. 2022, 614, 147–159. [Google Scholar] [CrossRef]
- Sun, Y.; Cao, J.; Wang, X.; Zhang, C.; Luo, J.; Zeng, Y.; Zhang, C.; Li, Q.; Zhang, Y.; Xu, W.; et al. Hypoxia-Adapted Sono-chemodynamic Treatment of Orthotopic Pancreatic Carcinoma Using Copper Metal-Organic Frameworks Loaded with an Ultrasound-Induced Free Radical Initiator. ACS Appl. Mater. Interfaces 2021, 13, 38114–38126. [Google Scholar] [CrossRef]
- Liang, S.; Xiao, X.; Bai, L.; Liu, B.; Yuan, M.; Ma, P.; Pang, M.; Cheng, Z.; Lin, J. Conferring Ti-Based MOFs with Defects for Enhanced Sonodynamic Cancer Therapy. Adv. Mater. 2021, 33, e2100333. [Google Scholar] [CrossRef]
- Niu, H.; Chen, J.; Jin, J.; Qi, X.; Bai, K.; Shu, C.; Wu, A.; Xiao, Y.; Wu, C.; Bu, H.; et al. Engineering metalloporphyrin-integrated nanosystems for targeted sono-/chemo-dynamic therapy of leptomeningeal carcinomatosis through intrathecal administration. Chem. Eng. J. 2022, 437, 135373. [Google Scholar] [CrossRef]
- Hu, C.; Wang, J.; Liu, S.; Cai, L.; Zhou, Y.; Liu, X.; Wang, M.; Liu, Z.; Pang, M. Urchin-Shaped Metal Organic/Hydrogen-Bonded Framework Nanocomposite as a Multifunctional Nanoreactor for Catalysis-Enhanced Synergetic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 4825–4834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Meng, X.; Yang, Z.; Dong, H.; Zhang, X. Enhanced cancer therapy by hypoxia-responsive copper metal-organic frameworks nanosystem. Biomaterials 2020, 258, 120278. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bao, Y.; Song, Y.; Zhang, C.; Qiu, F.; Sun, Y.; Xin, L.; Cao, J.; Jiang, Y.; Luo, J.; et al. Hypoxia-alleviated nanoplatform to enhance chemosensitivity and sonodynamic effect in pancreatic cancer. Cancer Lett. 2021, 520, 100–108. [Google Scholar] [CrossRef] [PubMed]

























Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, D.; Huang, J.; Jiang, C.; Zhou, L.; Zheng, M.; Nezamzadeh-Ejhieh, A.; Qi, N.; Lu, C.; Liu, J. A Novel Platform of MOF for Sonodynamic Therapy Advanced Therapies. Pharmaceutics 2023, 15, 2071. https://doi.org/10.3390/pharmaceutics15082071
Liao D, Huang J, Jiang C, Zhou L, Zheng M, Nezamzadeh-Ejhieh A, Qi N, Lu C, Liu J. A Novel Platform of MOF for Sonodynamic Therapy Advanced Therapies. Pharmaceutics. 2023; 15(8):2071. https://doi.org/10.3390/pharmaceutics15082071
Chicago/Turabian StyleLiao, Donghui, Jiefeng Huang, Chenyi Jiang, Luyi Zhou, Mingbin Zheng, Alireza Nezamzadeh-Ejhieh, Na Qi, Chengyu Lu, and Jianqiang Liu. 2023. "A Novel Platform of MOF for Sonodynamic Therapy Advanced Therapies" Pharmaceutics 15, no. 8: 2071. https://doi.org/10.3390/pharmaceutics15082071
APA StyleLiao, D., Huang, J., Jiang, C., Zhou, L., Zheng, M., Nezamzadeh-Ejhieh, A., Qi, N., Lu, C., & Liu, J. (2023). A Novel Platform of MOF for Sonodynamic Therapy Advanced Therapies. Pharmaceutics, 15(8), 2071. https://doi.org/10.3390/pharmaceutics15082071







