Antispasmodic Effect of Valeriana pilosa Root Essential Oil and Potential Mechanisms of Action: Ex Vivo and In Silico Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Drugs, and Solutions
2.2. Plant Material
2.3. Gas Chromatography Analysis (GC) and Gas Chromatography–Mass Spectrometry (GC–MS) of Valeriana Pilosa Essential Oil (VPEO)
2.4. Animals
2.5. Preparation of Rat Ileum
2.6. Ex Vivo Experimental Protocol
2.6.1. Effect of VPEO on the Basal Tone of Rat Ileum
2.6.2. Spasmolytic Effect in Precontracted Rat Ileum
2.6.3. Antispasmodic VPEO Effect in the Dose–Response Curves of ACh, KCl, and BaCl2
2.6.4. Role of Extracellular Ca2+ Influx in the Intestinal VPEO-Mediated Relaxation
2.6.5. Effect of VPEO on Muscarinic Receptors
2.6.6. Effects of VPEO on Voltage-Gated Calcium Channel
2.6.7. Effects of VPEO on Potassium Channel Blockers
2.7. In Silico Studies
2.8. Statistical Analysis
3. Results
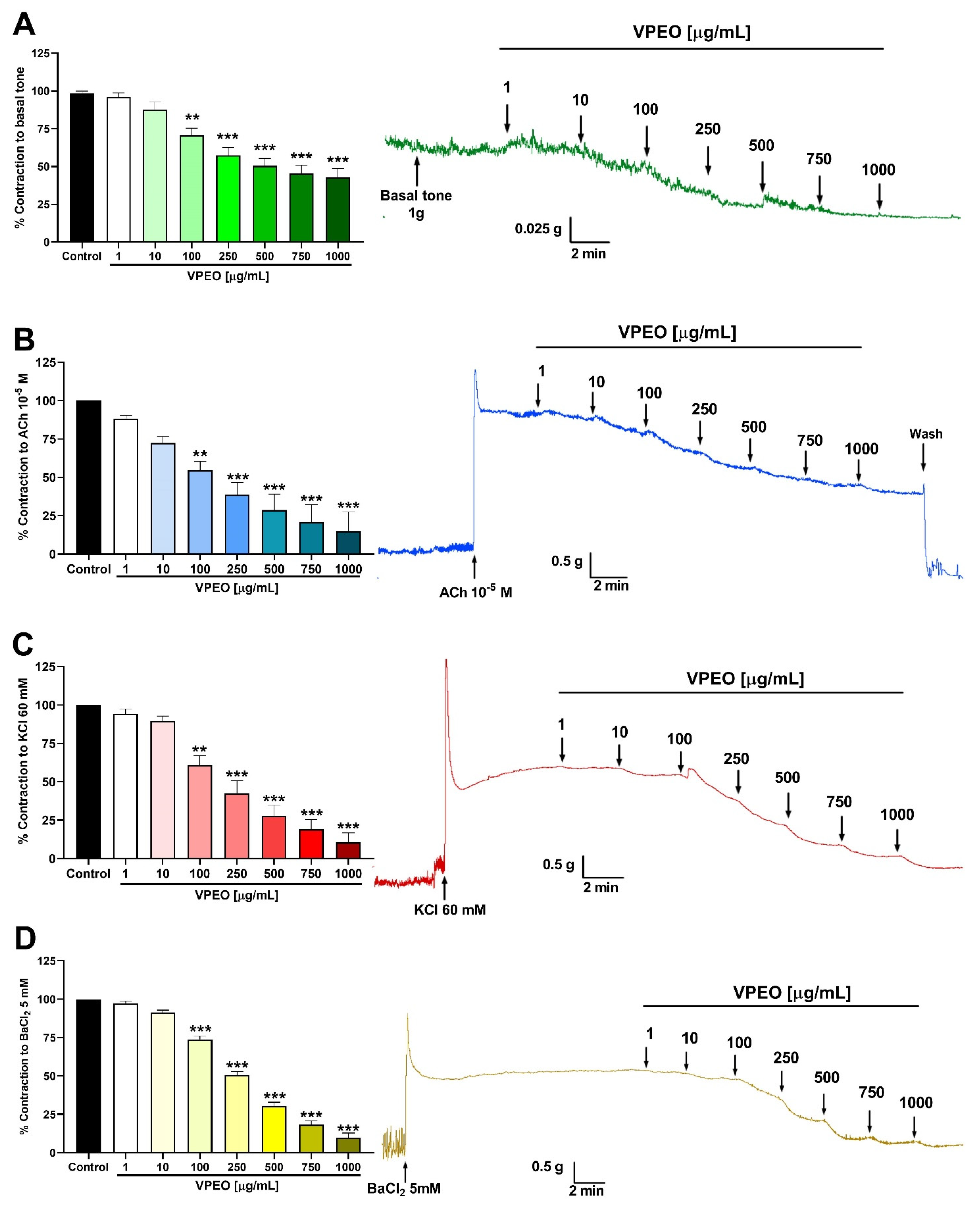
3.1. Spasmolytic Activity of VPEO on Rat Ileum
3.2. Antispasmodic Activity of VPEO on Rat Ileum
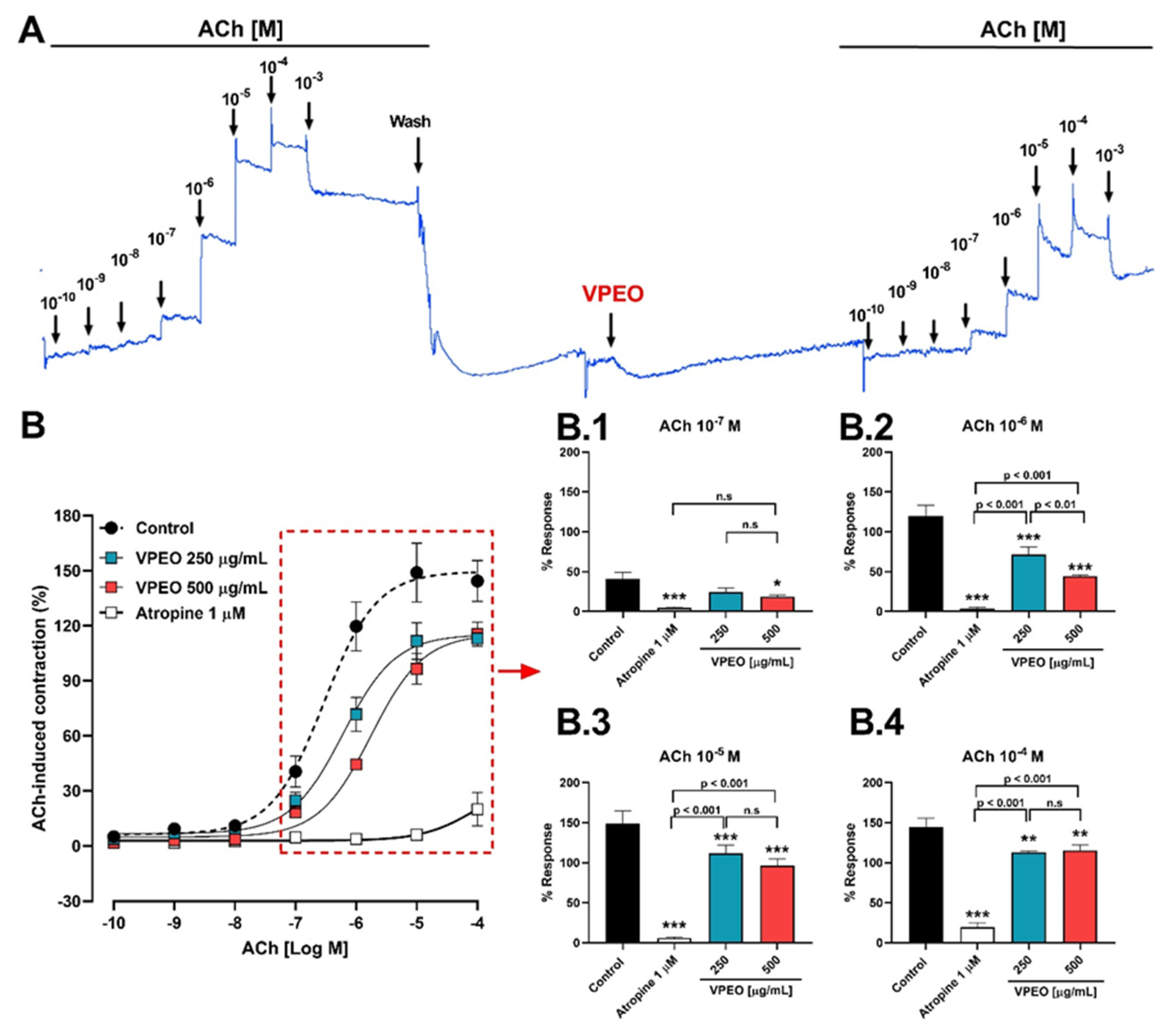
3.2.1. Role of Muscarinic Acetylcholine Receptors
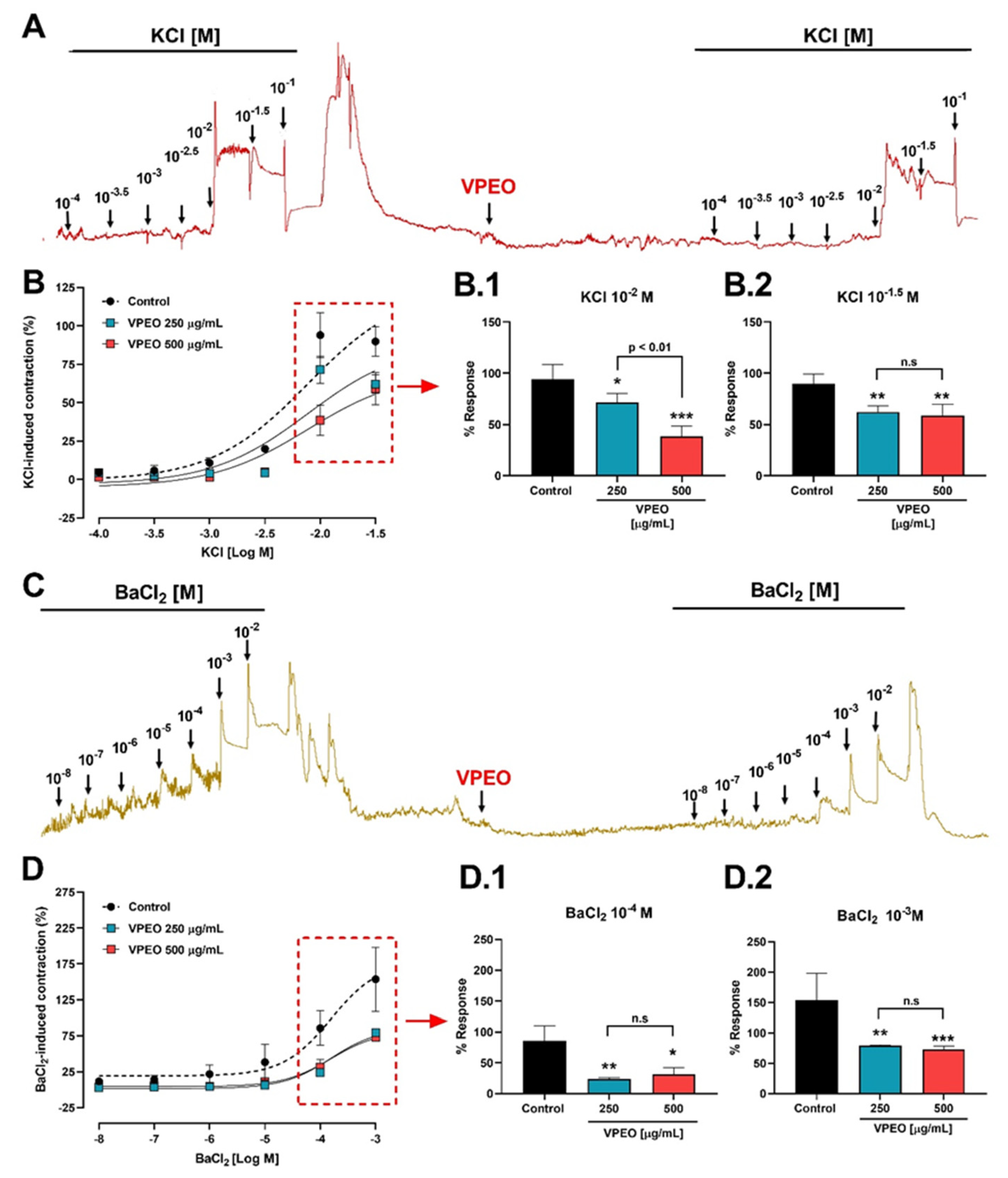
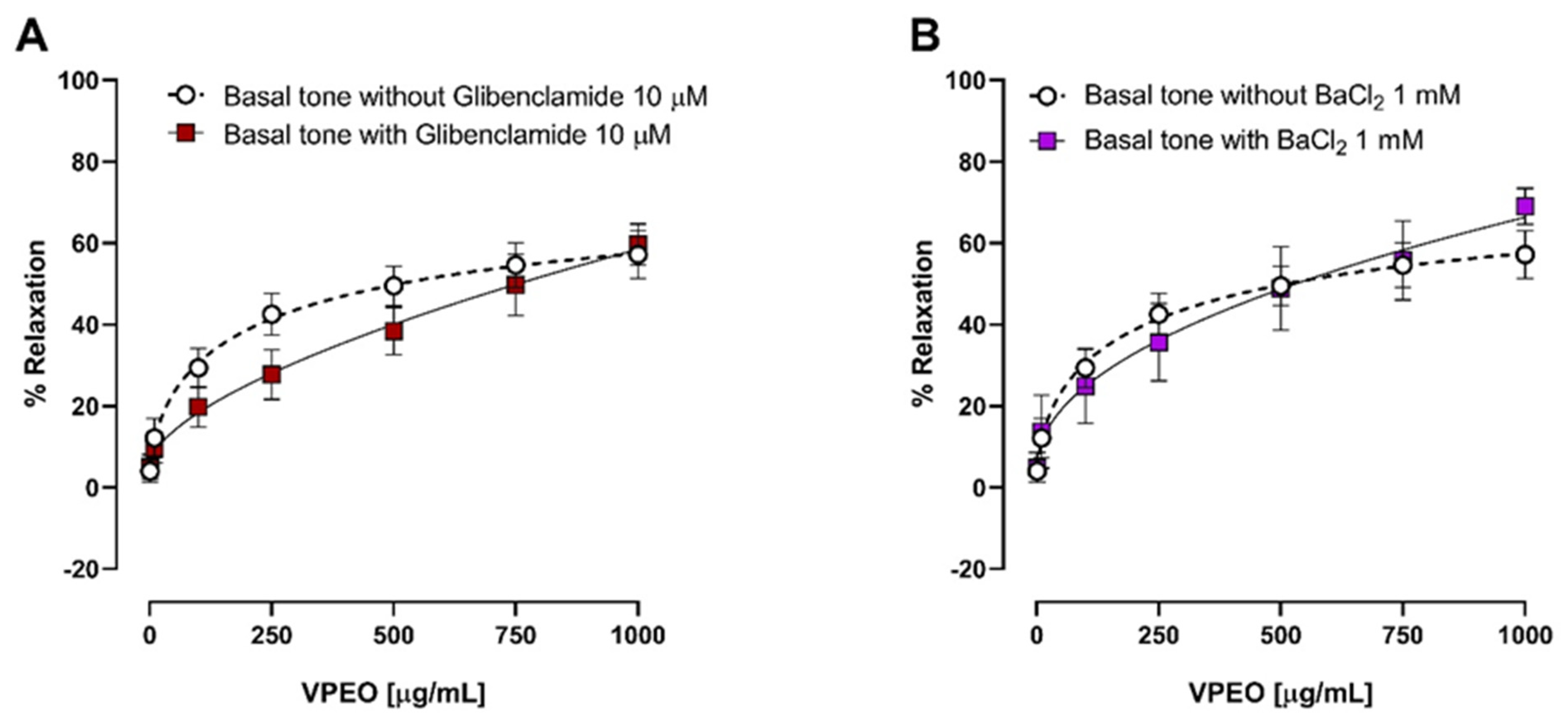
3.2.2. Effect of VPEO on Intestinal Sections Precontracted with KCl or BaCl2
3.3. Determination of the Mechanisms of Action Underlying the VPEO Effect
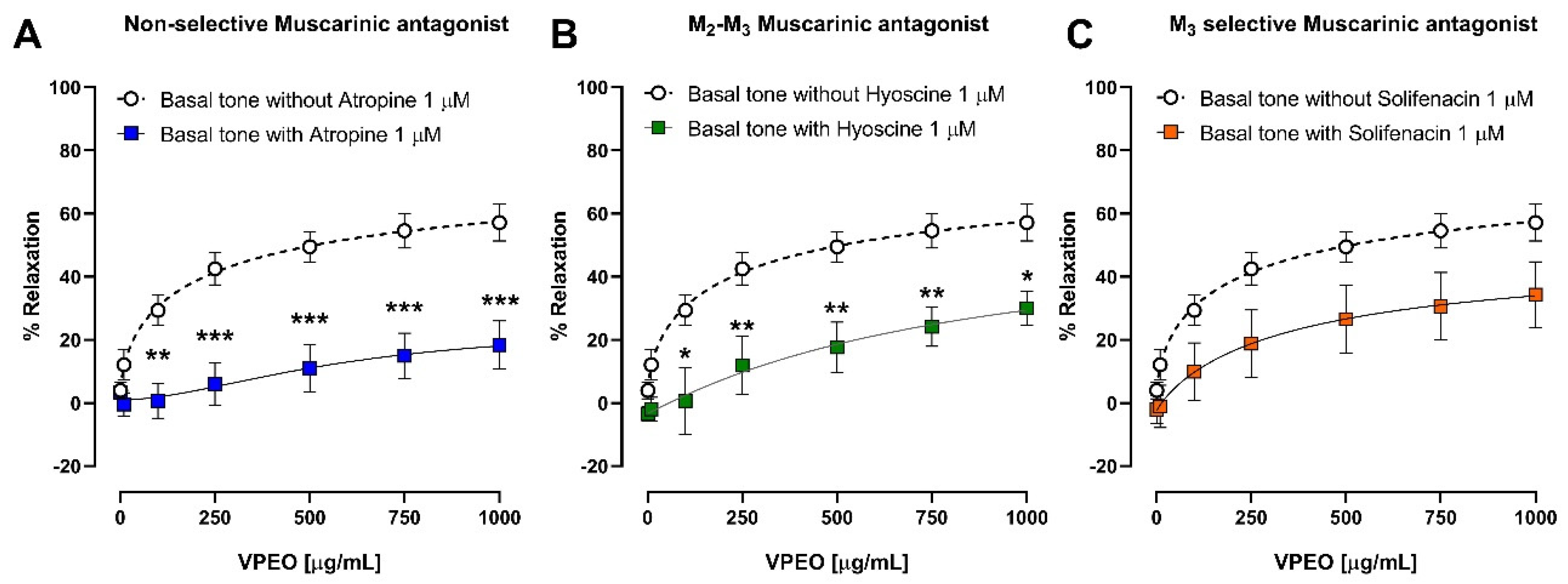
3.3.1. Effect of VPEO on Muscarinic Receptors
3.3.2. Extracellular Ca2+ Dependence of VPEO Effect
3.3.3. Effect of Two K⁺ Channel Blockers on the Relaxation in Precontracted Ileum
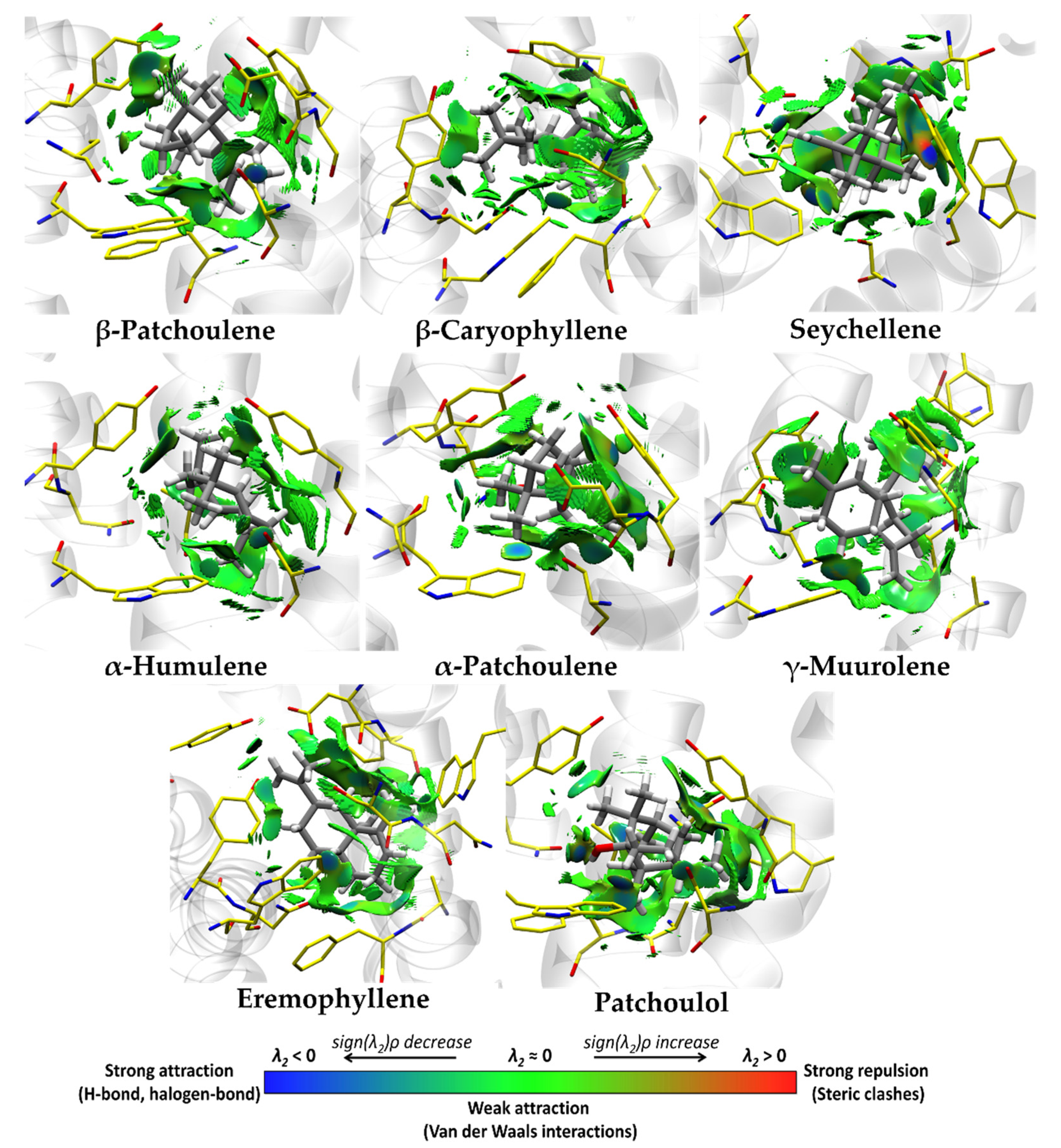
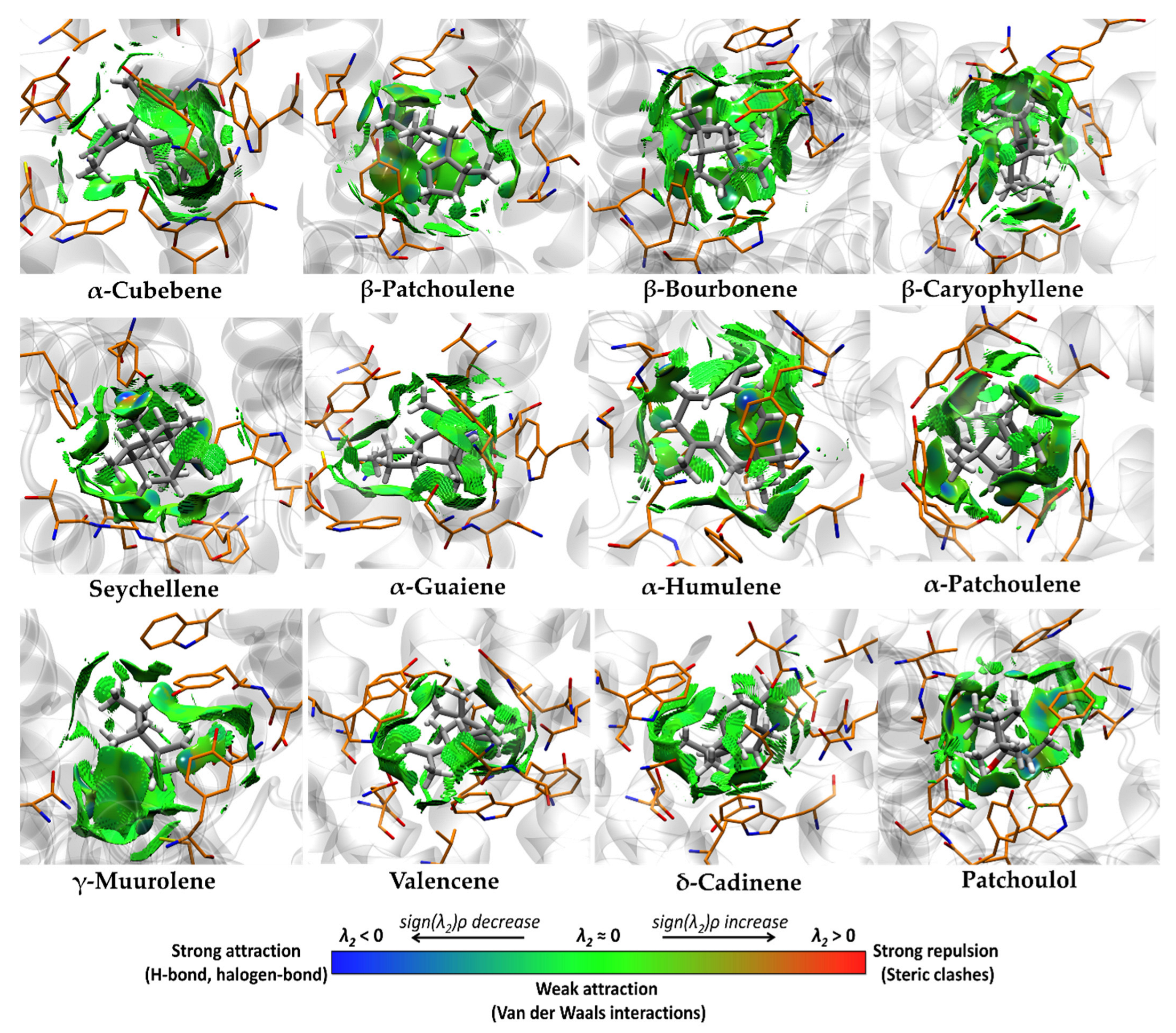
3.4. Molecular Docking and Ligand Efficiency Analysis of VPEO
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drossman, D.A. History of functional gastrointestinal symptoms and disorders and chronicle of the Rome Foundation. In Rome IV Functional Gastrointestinal Disorders: Disorders of Gut-Brain Interaction; Drossman, D.A., Chang, L.C., Kellow, W.J., Tack, J., Whitehead, W.E., Eds.; The Rome Foundation: Raleigh, NC, USA, 2016; pp. 549–576. [Google Scholar]
- Canavan, C.; West, J.; Card, T. Review article: The economic impact of the irritable bowel syndrome. Aliment. Pharmacol. Ther. 2014, 40, 1023–1034. [Google Scholar] [CrossRef] [Green Version]
- Tack, J.; Stanghellini, V.; Mearin, F.; Yiannakou, Y.; Layer, P.; Coffin, B.; Simren, M.; Mackinnon, J.; Wiseman, G.; Marciniak, A.; et al. Economic burden of moderate to severe irritable bowel syndrome with constipation in six European countries. BMC Gastroenterol. 2019, 19, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Wong, R.K.; Drossman, D.A. Quality of life measures in irritable bowel syndrome. Expert Rev. Gastroenterol. Hepatol. 2010, 4, 277–284. [Google Scholar] [CrossRef]
- Clark, A.M. Natural products as a resource for new drugs. Pharm. Res. 1996, 13, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Heghes, S.C.; Vostinaru, O.; Rus, L.M.; Mogosan, C.; Iuga, C.A.; Filip, L. Antispasmodic Effect of Essential Oils and Their Constituents: A Review. Molecules 2019, 24, 1675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazar, V.; Holban, A.M.; Curutiu, C.; Ditu, L.M. Modulation of Gut Microbiota by Essential Oils and Inorganic Nanoparticles: Impact in Nutrition and Health. Front. Nutr. 2022, 9, 920413. [Google Scholar] [CrossRef]
- Waclawiková, B.; Bullock, A.; Schwalbe, M.; Aranzamendi, C.; Nelemans, S.A.; van Dijk, G.; El Aidy, S. Gut bacteria-derived 5-hydroxyindole is a potent stimulant of intestinal motility via its action on L-type calcium channels. PLoS Biol. 2021, 19, e3001070. [Google Scholar] [CrossRef]
- Kang, S.; Park, M.Y.; Brooks, I.; Lee, J.; Kim, S.H.; Kim, J.Y.; Oh, B.; Kim, J.W.; Kwon, O. Spore-forming Bacillus coagulans SNZ 1969 improved intestinal motility and constipation perception mediated by microbial alterations in healthy adults with mild intermittent constipation: A randomized controlled trial. Food Res. Int. 2021, 146, 110428. [Google Scholar] [CrossRef]
- Blakeney, B.A.; Crowe, M.S.; Mahavadi, S.; Murthy, K.S.; Grider, J.R. Branched short-chain fatty acid isovaleric acid causes colonic smooth muscle relaxation via cAMP/PKA pathway. Dig. Dis. Sci. 2019, 64, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Waclawiková, B.; Codutti, A.; Alim, K.; El Aidy, S. Gut microbiota-motility interregulation: Insights from in vivo, ex vivo and in silico studies. Gut Microbes 2022, 14, 1997296. [Google Scholar] [CrossRef] [PubMed]
- Galan, A.; Sanchez, I.; Montoya, J.; Linares, E.; Campos, J.; Vicente, J. La vegetacion del norte del Peru: De los bosques a la jalca en Cajamarca. Acta Bot. Malaci. 2015, 40, 157–190. [Google Scholar] [CrossRef]
- Seminario-Cunya, J.; Rumay-Sanchez, L.; Seminario-Cunya, A. Biología de Valeriana pilosa R. &. P. (Valerianaceae): Una especie en peligro de extinción de las altas montañas de Peru. Bol. Latinoam. Caribe Plants Med. Aromat. 2016, 15, 337–351. [Google Scholar]
- Rojo, L.; Benites, J.; Rodriguez, A.; Venancio, F.; Ramalho, L.; Teixeira, A.; Feio, S.; do Ceu Costa, M. Composition and antimicrobial screening of the essential oil of Acantholippia deserticola (Phil.ex F. Phil.) Moldenke. J. Essent. Oil Res. 2006, 18, 695–697. [Google Scholar] [CrossRef]
- Rojo, L.E.; Benites, J.; López, J.; Rojas, M.; Díaz, P.; Pastene, E.; Ordoñez, J. Comparative study on the antioxidant effects and phenolic content of twelve highly consumed medicinal plants from South American Andes. Bol. Latinoam. Caribe Plants Med. Aromat. 2009, 8, 498–508. [Google Scholar]
- Benites, J.; Moiteiro, C.; Miguel, G.; Rojo, L.; López, J.; Venâncio, F.; Ramalho, L.; Feio, S.; Dandlen, S.; Casanova, H.; et al. Composition and biological activity of the essential oil of Peruvian Lantana camara. J. Chil. Chem. Soc. 2009, 54, 379–384. [Google Scholar] [CrossRef]
- Benites, J.; López, J.; Rojo, L.; Díaz, P.; Rojas, M.; Venâncio, F.; Moiteiro, M. Chemical composition of the essential oil of the leaves and stems of Xenophillum poposum. Chem. Nat. Compound. 2011, 46, 988–989. [Google Scholar] [CrossRef]
- Benites, J.; Moiteiro, C.; Figueiredo, A.C.; Rijo, P.; Buc-Calderon, P.; Bravo, F.; Gajardo, S.; Sánchez, I.; Torres, I.; Ganoza, M. Chemical composition and antimicrobial activity of essential oil of Peruvian Dalea strobilacea Barneby. Bol. Latinoam. Caribe Plants Med. Aromat. 2016, 15, 429–435. [Google Scholar]
- Benites, J.; Ríos, D.; Guerrero-Castilla, A.; Enríquez, C.; Zavala, E.; Ybañez-Julca, R.O.; Quispe-Díaz, I.; Jara-Aguilar, R.; Buc Calderon, P. Chemical Composition and Assessment of Antimicrobial, Antioxidant and Antiproliferative Activities of Essential oil from Clinopodium sericeum, a Peruvian Medicinal Plant. Rec. Nat. Prod. 2021, 15, 175–186. [Google Scholar] [CrossRef]
- Minchán-Herrera, P.; Ybañez-Julca, R.O.; Quispe-Díaz, I.M.; Venegas-Casanova, E.A.; Jara-Aguilar, R.; Salas, F.; Zevallos-Escobar, L.; Yáñez, O.; Pino-Rios, R.; Buc Calderon, P.; et al. Valeriana pilosa Roots Essential Oil: Chemical Composition, Antioxidant Activities and Molecular Docking Studies on Enzymes involved in Redox Biological Processes. Antioxidants 2022, 11, 1337. [Google Scholar] [CrossRef]
- AVMA (American Veterinary Medical Association). AVMA Guidelines for the Euthanasia of Animals, 2020 ed.; AMVA: Schaumburg, IL, USA, 2020; Available online: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf (accessed on 26 March 2021).
- Ybañez-Julca, R.O.; Asunción-Alvarez, D.; Quispe-Díaz, I.M.; Palacios, J.; Bórquez, J.; Simirgiotis, M.J.; Perveen, S.; Nwokocha, C.R.; Cifuentes, F.; Paredes, A. Metabolomic Profiling of Mango (Mangifera indica Linn) Leaf Extract and Its Intestinal Protective Effect and Antioxidant Activity in Different Biological Models. Molecules 2020, 25, 5149. [Google Scholar] [CrossRef] [PubMed]
- Hassan Gilani, A.U.; Aziz, N.; Ahmad, M.; Alam, M.T.; Rizwani, G.H. Spasmogenic and Spasimolytic Constituents in Sida Pakistanica. Pharm. Biol. 1999, 37, 173–180. [Google Scholar] [CrossRef]
- Ryoo, S.B.; Oh, H.K.; Moon, S.H.; Choe, E.K.; Yu, S.A.; Park, S.H.; Park, K.J. Electrophysiological and Mechanical Characteristics in Human Ileal Motility: Recordings of Slow Waves Conductions and Contractions, In Vitro. Korean J. Physiol. Pharmacol. 2015, 19, 533–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Song, J.; Bai, T.; Lu, X.; Yang, G.; Qian, W.; Wang, R.; Hou, X. Effects of Buscopan on Human Gastrointestinal Smooth Muscle Activity in an Ex Vivo Model: Are There Any Differences for Various Sections? Eur. J. Pharmacol. 2016, 780, 180–187. [Google Scholar] [CrossRef]
- Veer, V.; Chess-Williams, R.; Moro, C. Antimuscarinic Actions on Bladder Urothelium and Lamina Propria Contractions Are Similar to Those Observed in Detrusor Smooth Muscle Preparations. Neurourol. Urodyn. 2023, 42, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Gaion, R.M.; Trento, M. Prostacyclin-Induced Contraction of Guinea-Pig Ileum: Influence of Drugs Affecting Calcium Flux in the Smooth Muscle. Eur. J. Pharmacol. 1984, 102, 529–533. [Google Scholar] [CrossRef] [PubMed]
- York, N.W.; Parker, H.; Xie, Z.; Tyus, D.; Waheed, M.A.; Yan, Z.; Grange, D.K.; Remedi, M.S.; England, S.K.; Hu, H.; et al. Kir6.1- and SUR2-Dependent KATP Overactivity Disrupts Intestinal Motility in Murine Models of Cantú Syndrome. JCI Insight 2020, 5, e141443. [Google Scholar] [CrossRef] [PubMed]
- Krapivinsky, G.; Medina, I.; Eng, L.; Krapivinsky, L.; Yang, Y.; Clapham, D.E. A Novel Inward Rectifier K+ Channel with Unique Pore Properties. Neuron 1998, 20, 995–1005. [Google Scholar] [CrossRef] [Green Version]
- Haga, K.; Kruse, A.C.; Asada, H.; Yurugi-Kobayashi, T.; Shiroishi, M.; Zhang, C.; Weis, W.I.; Okada, T.; Kobilka, B.K.; Haga, T.; et al. Structure of the human M2 muscarinic acetylcholine receptor bound to an antagonist. Nature 2012, 482, 547–551. [Google Scholar] [CrossRef] [Green Version]
- Kruse, A.C.; Hu, J.; Pan, A.C.; Arlow, D.H.; Rosenbaum, D.M.; Rosemond, E.; Green, H.F.; Liu, T.; Chae, P.S.; Dror, R.O.; et al. Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature 2012, 482, 552–556. [Google Scholar] [CrossRef] [Green Version]
- Findeisen, F.; Campiglio, M.; Jo, H.; Abderemane-Ali, F.; Rumpf, C.H.; Pope, L.; Rossen, N.D.; Flucher, B.E.; DeGrado, W.F.; Minor, D.L., Jr. Stapled Voltage-Gated Calcium Channel (CaV) α-Interaction Domain (AID) Peptides Act As Selective Protein-Protein Interaction Inhibitors of CaV Function. ACS Chem. Neurosci. 2017, 8, 1313–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trott, O.; Olson, A. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Model. 1999, 17, 55–84. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. J. Mol. Model. 2007, 13, 1173–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Řezáč, J.; Hobza, P. Advanced corrections of hydrogen bonding and dispersion for semiempirical quantum mechanical methods. J. Chem. Theory Comput. 2012, 8, 141–151. [Google Scholar] [CrossRef]
- Madhavi Sastry, G.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Dassault Systèmes BIOVIA. Discovery Studio Modeling Environment; Dassault Systèmes BIOVIA: San Diego, CA, USA, 2017. [Google Scholar]
- Rauf, A.; Akram, M.; Semwal, P.; Mujawah, A.A.H.; Muhammad, N.; Riaz, Z.; Munir, N.; Piotrovsky, D.; Vdovina, I.; Bouyahya, A.; et al. Antispasmodic Potential of Medicinal Plants: A Comprehensive Review. Oxidative Med. Cell. Longev. 2021, 2021, 4889719. [Google Scholar] [CrossRef]
- Bezerra, M.A.C.; Leal-Cardoso, J.H.; Coelho-de-Souza, A.N.; Criddle, D.N.; Fonteles, M.C. Myorelaxant and antispasmodic effects of the essential oil of Alpinia speciosa on rat ileum. Phytother. Res. 2000, 14, 549–551. [Google Scholar] [CrossRef]
- Tanahashi, Y.; Komori, S.; Matsuyama, H.; Kitazawa, T.; Unno, T. Functions of Muscarinic Receptor Subtypes in Gastrointestinal Smooth Muscle: A Review of Studies with Receptor-Knockout Mice. Int. J. Mol. Sci. 2021, 22, 926. [Google Scholar] [CrossRef]
- Caulfield, M.; Birdsall, N. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol. Rev. 1998, 50, 279–290. [Google Scholar]
- Caulfield, M.P. Muscarinic receptors-characterization, coupling and function. Pharmacol. Ther. 1993, 58, 319–379. [Google Scholar] [CrossRef]
- Sol, I.; Yang, D.K.; Kim, H.J.; Min, K.W.; Kang, T.M.; Kim, S.J.; Kim, K.W.; Park, K.H.; Jeon, J.H.; Choi, K.H.; et al. Five subtypes of muscarinic receptors are expressed in gastric smooth muscles of guinea pig. Exp. Mol. Med. 2003, 35, 46–52. [Google Scholar]
- Bolton, T.B.; Prestwich, S.A.; Zholos, A.V.; Gordienko, D.V. Excitation-contraction coupling in gastrointestinal and other smooth muscles. Annu. Rev. Physiol. 1999, 61, 85–115. [Google Scholar] [CrossRef]
- Ehlert, F.J.; Ostrom, R.S.; Sawyer, G.W. Subtypes of the muscarinic receptor in smooth muscle. Life Sci. 1997, 61, 1729–1740. [Google Scholar] [CrossRef]
- Sawyer, G.W.; Ehlert, F.J. Muscarinic M-3 receptor inactivation reveals a pertussis toxin-sensitive contractile response in the guinea pig colon: Evidence for M-2/M-3 receptor interactions. J. Pharmacol. Exp. Ther. 1999, 289, 464–476. [Google Scholar] [PubMed]
- Zholos, A.V.; Bolton, T.B. Muscarinic receptor subtypes controlling the cationic current in guinea-pig ileal smooth muscle. Br. J. Pharmacol. 1997, 122, 885–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komori, S.; Unno, T.; Nakayama, T.; Ohashi, H. M2 and M3 muscarinic receptors couple, respectively, with activation of nonselective cationic channels and potassium channels in intestinal smooth muscle cells. Jpn. J. Pharmacol. 1998, 76, 213–218. [Google Scholar] [CrossRef]
- Pucovsky, V.; Zholos, A.V.; Bolton, T.B. Muscarinic cation current and suppression of Ca2+ current in guinea pig ileal smooth muscle cells. Eur. J. Pharmacol. 1998, 346, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Serio, R.; Mulè, F. Involvement of cholinergic nicotinic receptors in the menthol-induced gastric relaxation. Eur. J. Pharmacol. 2014, 745, 129–134. [Google Scholar] [CrossRef]
- Sadraei, H.; Asghari, G.; Kasiri, F. Comparison of antispasmodic effects of Dracocephalum kotschyi essential oil, limonene and α-terpineol. Res. Pharm. Sci. 2015, 10, 109–116. [Google Scholar]
- Lozon, Y.; Sultan, A.; Lansdell, S.J.; Prytkova, T.; Sadek, B.; Yang, K.-H.S.; Howarth, F.C.; Millar, N.S.; Oz, M. Inhibition of human α7 nicotinic acetylcholine receptors by cyclic monoterpene carveol. Eur. J. Pharmacol. 2016, 776, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Ratz, P.H.; Berg, K.M.; Urban, N.H.; Miner, A.S. Regulation of Smooth Muscle Calcium Sensitivity: KCl as a Calcium-Sensitizing Stimulus. Am. J. Physiol.-Cell Physiol. 2005, 288, 769–783. [Google Scholar] [CrossRef] [Green Version]
- Mori, M.X.; Itsuki, K.; Hase, H.; Sawamura, S.; Kurokawa, T.; Mori, Y.; Inoue, R. Dynamics of receptor-operated Ca2+ currents through TRPC channels controlled via the PI(4,5)P2-PLC signaling pathway. Front. Pharmacol. 2015, 6, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.Y.; Peng, C.; Xie, X.F.; Xiong, L.; Zhang, S.Y.; Cao, X.Y. Patchouli alcohol isolated from Pogostemon cablin mediates endothelium-independent vasorelaxation by blockade of Ca2+ channels in rat isolated thoracic aorta. J. Ethnopharmacol. 2018, 220, 188–196. [Google Scholar] [CrossRef]
- Chen, F.; Xu, Y.; Wang, J.; Yang, X.; Cao, H.; Huang, P. Relaxation Effect of Patchouli Alcohol in Rat Corpus Cavernous and Its Underlying Mechanisms. Evid. Based Complement. Altern. Med. 2020, 2020, 3109069. [Google Scholar] [CrossRef]
- Mathela, C.S.; Chanotiya, C.S.; Sammal, S.S.; Pant, A.K.; Pandey, S. Compositional diversity of terpenoids in Evidence-Based Complementary and Alternative Medicine the Himalayans Valeriana genera. Chem. Biodivers. 2005, 2, 1174–1182. [Google Scholar] [CrossRef]
- Bashir, S.; Memon, R.; Gilani, A.H. Antispasmodic and Antidiarrheal Activities of Valeriana hardwickii Wall. Rhizome are Putatively Mediated through Calcium Channel Blockade. Evid. Based Complement. Altern. Med. 2011, 2011, 304960. [Google Scholar] [CrossRef] [Green Version]
- Vogalis, F. Potassium channels in gastrointestinal smooth muscle. J. Auton. Pharmacol. 2000, 20, 207–219. [Google Scholar] [CrossRef]
- McHugh, D.; Beech, D.J. Inhibition of delayed rectifier K(+)-current by levcromakalim in single intestinal smooth muscle cells: Effects of cations and dependence on K(+)-flux. Br. J. Pharmacol. 1995, 114, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Cornejo, I.; Villanueva, S.; Burgos, J.; López-Cayuqueo, K.I.; Chambrey, R.; Julio-Kalajzić, F.; Buelvas, N.; Niemeyer, M.I.; Figueiras-Fierro, D.; Brown, P.D.; et al. Tissue Distribution of Kir7.1 Inwardly Rectifying K+ Channel Probed in a Knock-in Mouse Expressing a Haemagglutinin-Tagged Protein. Front. Physiol. 2018, 9, 428. [Google Scholar] [CrossRef] [Green Version]
- Gilani, A.H.; Khan, A.U.; Jabeen, Q.; Subhan, F.; Ghafar, R. Antispasmodic and blood pressure lowering effects of Valeriana wallichii are mediated through K+ channel activation. J. Ethnopharmacol. 2005, 100, 347–352. [Google Scholar] [CrossRef]
- Martínez-Pérez, E.F.; Juárez, Z.N.; Hernández, L.R.; Bach, H. Natural Antispasmodics: Source, Stereochemical Configuration, and Biological Activity. BioMed Res. Int. 2018, 2018, 3819714. [Google Scholar] [CrossRef] [Green Version]
- Xiannenas, I.; Papaneophytou, C.P.; Tsalie, E.; Pappas, I.; Triantafillou, E.; Tontis, D.; Kontopidis, G.A. Dietary Supplementation of Benzoic Acid and Essential Oil Compounds Affects Buffering Capacity of the Feeds, Performance of Turkey Poults and Their Antioxidant Status, pH in the Digestive Tract, Intestinal Microbiota and Morphology. Asian-Australas. J. Anim. Sci. 2014, 27, 225–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araruna, M.E.; Serafim, C.; Alves Júnior, E.; Hiruma-Lima, C.; Diniz, M.; Batista, L. Intestinal Anti-Inflammatory Activity of Terpenes in Experimental Models (2010–2020): A Review. Molecules 2020, 25, 5430. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Tang, J.; Hu, Y.; Zhang, W. Role of gut microbiota-derived signals in the regulation of gastrointestinal motility. Front. Med. 2022, 9, 961703. [Google Scholar] [CrossRef] [PubMed]

| Protein | PDBID | Center Grid Box | ||
|---|---|---|---|---|
| x | y | z | ||
| M2 Muscarinic Acetylcholine Receptor | 3UON | 7.79 | 0.25 | −3.95 |
| M3 Muscarinic Acetylcholine Receptor | 4DAJ | −14.79 | −7.50 | −42.53 |
| Cav1.2 L-type voltage-gated calcium channel | 5V2P | 7.76 | 42.10 | 123.95 |
| N° | Components | M2 | M3 | Cav1.2 |
|---|---|---|---|---|
| 1 | Isovaleric acid | −4.8 | −4.5 | −4.2 |
| 2 | Tricyclene | −6.8 | −6.5 | −4.4 |
| 3 | α-Thujene | −6.5 | −6.7 | −4.6 |
| 4 | α-Pinene | −6.5 | −6.8 | −4.3 |
| 5 | Camphene | −6.9 | −6.7 | −4.6 |
| 6 | 3-Methyl valeric acid | −4.7 | −4.8 | −4.4 |
| 7 | Sabinene | −6.5 | −6.8 | −4.7 |
| 8 | 1-Octen-3-ol | −5.2 | −5.1 | −3.7 |
| 9 | β-Pinene | −6.6 | −6.8 | −4.4 |
| 10 | Myrcene | −6 | −5.9 | −3.7 |
| 11 | Limonene | −6.5 | −6.5 | −4.5 |
| 12 | p-Cymene | −6.6 | −6.5 | −4.6 |
| 13 | 1,8-Cineole | −6.7 | −6.7 | −4.4 |
| 14 | Linalool | −6 | −5.9 | −4.5 |
| 15 | Isopentyl isovalerate | −5.8 | −6.0 | −4.3 |
| 16 | Camphor | −6.9 | −6.7 | −4.2 |
| 17 | Menthone | −6.9 | −6.7 | −4.4 |
| 18 | Isomenthone | −6.9 | −6.7 | −4.4 |
| 19 | Borneol | −6.8 | −6.5 | −4.1 |
| 20 | Neomenthol | −6.8 | −6.6 | −4.2 |
| 21 | Menthol | −6.4 | −6.3 | −4.2 |
| 22 | Carvone | −6.9 | −6.9 | −4.7 |
| 23 | Menthyl acetate | −7.5 | −7.4 | −4.7 |
| 24 | α-Cubebene | −8.5 | −9.0 | −5.5 |
| 25 | Cyclosativene | −8.5 | −8.9 | −5.4 |
| 26 | α-Copaene | −8.2 | −8.2 | −5.3 |
| 27 | β-Patchoulene | −9.1 | −9.4 | −5.8 |
| 28 | β-Bourbonene | −8.9 | −9.1 | −5.1 |
| 29 | β-Elemene | −8.1 | −8.0 | −5 |
| 30 | β-Caryophyllene | −9.0 | −9.1 | −5.6 |
| 31 | Seychellene | −7.9 | −8.4 | −5.4 |
| 32 | α-Guaiene | −8.6 | −9.0 | −5.7 |
| 33 | α-Humulene | −8.6 | −9.1 | −5.3 |
| 34 | allo-Aromadendrene | −8.3 | −8.6 | −5.4 |
| 35 | α-Patchoulene | −8.3 | −8.7 | −5.5 |
| 36 | γ-Muurolene | −9.1 | −9.2 | −5.5 |
| 37 | Germacrene-D | −8.6 | −8.8 | −5.2 |
| 38 | Valencene | −8.5 | −9.0 | −5.6 |
| 39 | Eremophyllene | −9.1 | −8.9 | −5.5 |
| 40 | γ-Cadinene | −8.6 | −8.6 | −5.5 |
| 41 | 7-epi-α-Selinene | −8.3 | −8.8 | −5.4 |
| 42 | δ-Cadinene | −8.8 | −9.0 | −5.4 |
| 43 | Spathulenol | −8.6 | −8.6 | −5.4 |
| 44 | β-Caryophyllene oxide | −8.1 | −8.3 | −5.5 |
| 45 | T-Cadinol | −8.5 | −8.8 | −5.4 |
| 46 | δ-Cadinol | −8.4 | −8.8 | −5.4 |
| 47 | Patchoulol | −7.8 | −8.6 | −5.2 |
| Proteins | ∆Ebinding (kcal·mol−1) | LE (kcal·mol−1) | IEnorm.binding (kcal·mol−1) |
|---|---|---|---|
| M2 Muscarinic Acetylcholine Receptor | −7.67 | 0.55 | −2.06 |
| M3 M3 Muscarinic Acetylcholine Receptor | −8.03 | 0.58 | −2.15 |
| Cav1.2 (L-type VGCC) | −5.11 | 0.37 | −1.37 |
| N° | Components | ∆Ebinding (kcal·mol−1) | Kd | LE (kcal·mol−1) | BEI (kDa) | LLE | IEnorm.binding (kcal·mol−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M2 | M3 | M2 | M3 | M2 | M3 | M2 | M3 | M2 | M3 | M2 | M3 | ||
| 24 | α-Cubebene | −8.5 | −9.0 | 5.90 × 10−7 | 2.54 × 10−7 | 0.60 | 0.60 | 30.5 | 32.3 | 2.0 | 2.3 | −2.2 | −2.3 |
| 27 | β-Patchoulene | −9.1 | −9.4 | 2.14 × 10−7 | 1.29 × 10−7 | 0.61 | 0.63 | 32.6 | 33.7 | 2.1 | 2.3 | −2.3 | −2.4 |
| 28 | β-Bourbonene | −8.9 | −9.1 | 3.00 × 10−7 | 2.14 × 10−7 | 0.59 | 0.61 | 31.9 | 32.6 | 2.3 | 2.4 | −2.3 | −2.3 |
| 30 | β-Caryophyllene | −9.0 | −9.1 | 2.54 × 10−7 | 2.14 × 10−7 | 0.60 | 0.61 | 32.0 | 32.3 | 1.8 | 1.9 | −2.3 | −2.3 |
| 31 | Seychellene * | −7.9 | −8.4 | 1.62 × 10−6 | 6.98 × 10−7 | 0.53 | 0.56 | 28.3 | 30.1 | 1.4 | 1.7 | −2.0 | −2.2 |
| 32 | α-Guaiene | −8.6 | −9.0 | 4.98 × 10−7 | 2.54 × 10−7 | 0.60 | 0.60 | 30.8 | 32.3 | 1.6 | 1.9 | −2.2 | −2.3 |
| 33 | α-Humulene * | −8.6 | −9.1 | 4.98 × 10−7 | 2.14 × 10−7 | 0.57 | 0.61 | 30.8 | 32.6 | 1.3 | 1.6 | −2.2 | −2.3 |
| 35 | α-Patchoulene * | −8.3 | −8.7 | 8.26 × 10−7 | 4.21 × 10−7 | 0.55 | 0.58 | 29.8 | 31.2 | 1.7 | 2.0 | −2.1 | −2.2 |
| 36 | γ-Muurolene | −9.1 | −9.2 | 2.14 × 10−7 | 1.81 × 10−7 | 0.61 | 0.61 | 32.6 | 33.0 | 2.1 | 2.2 | −2.3 | −2.4 |
| 38 | Valencene | −8.5 | −9.0 | 5.90 × 10−7 | 2.54 × 10−7 | 0.60 | 0.60 | 30.5 | 32.3 | 1.5 | 1.9 | −2.2 | −2.3 |
| 39 | Eremophyllene | −9.1 | −8.9 | 2.14 × 10−7 | 3.00 × 10−7 | 0.60 | 0.60 | 32.6 | 31.9 | 1.9 | 1.8 | −2.3 | −2.3 |
| 42 | δ-Cadinene | −8.8 | −9.0 | 3.55 × 10−7 | 2.54 × 10−7 | 0.60 | 0.60 | 31.6 | 32.3 | 1.7 | 1.9 | −2.3 | −2.3 |
| 47 | Patchoulol * | −7.8 | −8.6 | 1.92 × 10−6 | 4.98 × 10−7 | 0.49 | 0.54 | 25.7 | 28.3 | 2.1 | 2.7 | −2.0 | −2.2 |
| Interacting Amino Acids in the Binding Pocket of M2R | |
|---|---|
| Compound | Amino Acids (Distance in Å) |
| β-Patchoulene | Ala194 (5.43), Cys429 (4.53), Tyr104 (3.57), Phe195 (5.17), Trp400 (4.62), Tyr403 (3.77), Tyr426 (4.49). |
| β-Caryophyllene | Ala194 (3.83), Tyr104 (4.57), Trp155 (4.66), Trp400 (5.29), Tyr403 (4.51). |
| Seychellene * | Ala191 (4.22), Ala194 (4.77), Tyr104 (3.99), Trp155 (4.75), Phe181 (4.97), Trp400 (4.90), Tyr403 (4.86). |
| α-Humulene * | Ala194 (5.44), Tyr104 (4.80), Trp400 (5.20), Tyr403 (4.78). |
| α-Patchoulene * | Cys429 (5.17), Tyr104 (5.14), Phe181 (5.46), Trp400 (4.90), Tyr403 (5.38). |
| γ-Muurolene | Ala191 (5.19), Ala194 (4.98), Cys429 (5.20), Tyr104 (5.04), Phe181 (4.80), Phe195 (5.25), Trp400 (5.20), Tyr403 (4.43), Tyr426 (4.70). |
| Eremophyllene | Val111 (5.15), Ala194 (3.92), Tyr104 (5.15), Trp155 (4.26), Trp400 (5.09), Tyr403 (4.12), Tyr426 (4.44). |
| Patchoulol * | Asn404 (2.08) **, Ala191 (3.97), Ala194 (4.31), Tyr104 (4.26), Trp155 (4.18), Phe195 (4.82), Trp400 (4.86), Tyr403 (4.90). |
| Interacting Amino Acids in the Binding Pocket of M3R | |
|---|---|
| Compound | Amino Acids (Distance in Å) |
| α-Cubebene | Trp503 (3.65), Ala235 (4.43), Ala238 (4.30), Cys532 (3.89), Tyr148 (5.34), Trp199 (4.60), Trp503 (4.59), Tyr506 (4.85). |
| β-Patchoulene | Tyr506 (2.76), Ala238 (3.96), Cys532 (4.04), Tyr148 (3.64), Phe239 (5.47), Trp503 (4.39), Tyr506 (5.02). |
| β-Bourbonene | Ala235 (4.36), Ala238 (4.16), Cys532 (4.77), Tyr148 (5.22), Trp503 (5.24), Tyr506 (4.78). |
| β-Caryophyllene | Ala238 (4.70), Val155 (4.08), Tyr148 (5.18), Trp199 (4.23), Trp503 (4.88), Tyr506 (4.25), Tyr529 (5.34). |
| Seychellene * | Ala235 (4.08), Ala238 (3.79), Tyr148 (4.35), Trp199 (4.21), Trp503 (4.04), Tyr506 (4.77). |
| α-Guaiene | Trp503 (3.71), Ala235 (3.68), Ala238 (5.23), Cys532 (4.46), Val510 (4.30), Tyr148 (5.06), Trp199 (5.05), Trp503 (4.47), Tyr506 (4.23). |
| α-Humulene * | Ala235 (3.97), Cys532 (3.57), Val155 (4.38), Trp503 (3.59), Tyr506 (3.87), Tyr529 (4.68). |
| α-Patchoulene * | Tyr506 (2.57), Cys532 (4.03, Tyr148 (4.16), Trp503 (4.19), Tyr529 (4.19). |
| γ-Muurolene | Cys532 (4.01), Tyr148 (3.73), Trp199 (5.01), Trp503 (4.31), Tyr506 (4.37), Tyr529 (4.69). |
| Valencene | Ala238 (3.94), Cys532 (4.43), Val155 (4.14), Tyr148 (4.10), Trp199 (4.40), Trp503 (5.17), Tyr506 (4.32), Tyr529 (4.76). |
| δ-Cadinene | Ala235 (3.67), Ala238 (4.65), Cys532 (3.62), Tyr148 (5.36), Trp199 (5.46), Trp503 (4.34), Tyr506 (5.01). |
| Patchoulol * | Asn507 (2.58) **, Ala235 (3.83), Ala238 (3.61), Tyr148 (4.54), Trp199 (4.64), Phe239 (5.23), Trp503 (4.40), Tyr506 (4.66). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ybañez-Julca, R.O.; Pino-Ríos, R.; Quispe-Díaz, I.M.; Asunción-Alvarez, D.; Acuña-Tarrillo, E.E.; Mantilla-Rodríguez, E.; Minchan-Herrera, P.; Catalán, M.A.; Zevallos-Escobar, L.; Vásquez-Corales, E.; et al. Antispasmodic Effect of Valeriana pilosa Root Essential Oil and Potential Mechanisms of Action: Ex Vivo and In Silico Studies. Pharmaceutics 2023, 15, 2072. https://doi.org/10.3390/pharmaceutics15082072
Ybañez-Julca RO, Pino-Ríos R, Quispe-Díaz IM, Asunción-Alvarez D, Acuña-Tarrillo EE, Mantilla-Rodríguez E, Minchan-Herrera P, Catalán MA, Zevallos-Escobar L, Vásquez-Corales E, et al. Antispasmodic Effect of Valeriana pilosa Root Essential Oil and Potential Mechanisms of Action: Ex Vivo and In Silico Studies. Pharmaceutics. 2023; 15(8):2072. https://doi.org/10.3390/pharmaceutics15082072
Chicago/Turabian StyleYbañez-Julca, Roberto O., Ricardo Pino-Ríos, Iván M. Quispe-Díaz, Daniel Asunción-Alvarez, Edwin E. Acuña-Tarrillo, Elena Mantilla-Rodríguez, Patricia Minchan-Herrera, Marcelo A. Catalán, Liz Zevallos-Escobar, Edison Vásquez-Corales, and et al. 2023. "Antispasmodic Effect of Valeriana pilosa Root Essential Oil and Potential Mechanisms of Action: Ex Vivo and In Silico Studies" Pharmaceutics 15, no. 8: 2072. https://doi.org/10.3390/pharmaceutics15082072
APA StyleYbañez-Julca, R. O., Pino-Ríos, R., Quispe-Díaz, I. M., Asunción-Alvarez, D., Acuña-Tarrillo, E. E., Mantilla-Rodríguez, E., Minchan-Herrera, P., Catalán, M. A., Zevallos-Escobar, L., Vásquez-Corales, E., Yáñez, O., Gutiérrez-Alvarado, W. O., & Benites, J. (2023). Antispasmodic Effect of Valeriana pilosa Root Essential Oil and Potential Mechanisms of Action: Ex Vivo and In Silico Studies. Pharmaceutics, 15(8), 2072. https://doi.org/10.3390/pharmaceutics15082072









