Satisfying QTPP of Erythropoietin Biosimilar by QbD through DoE-Derived Downstream Process Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Screening of Binding and Elution Conditions of EPO
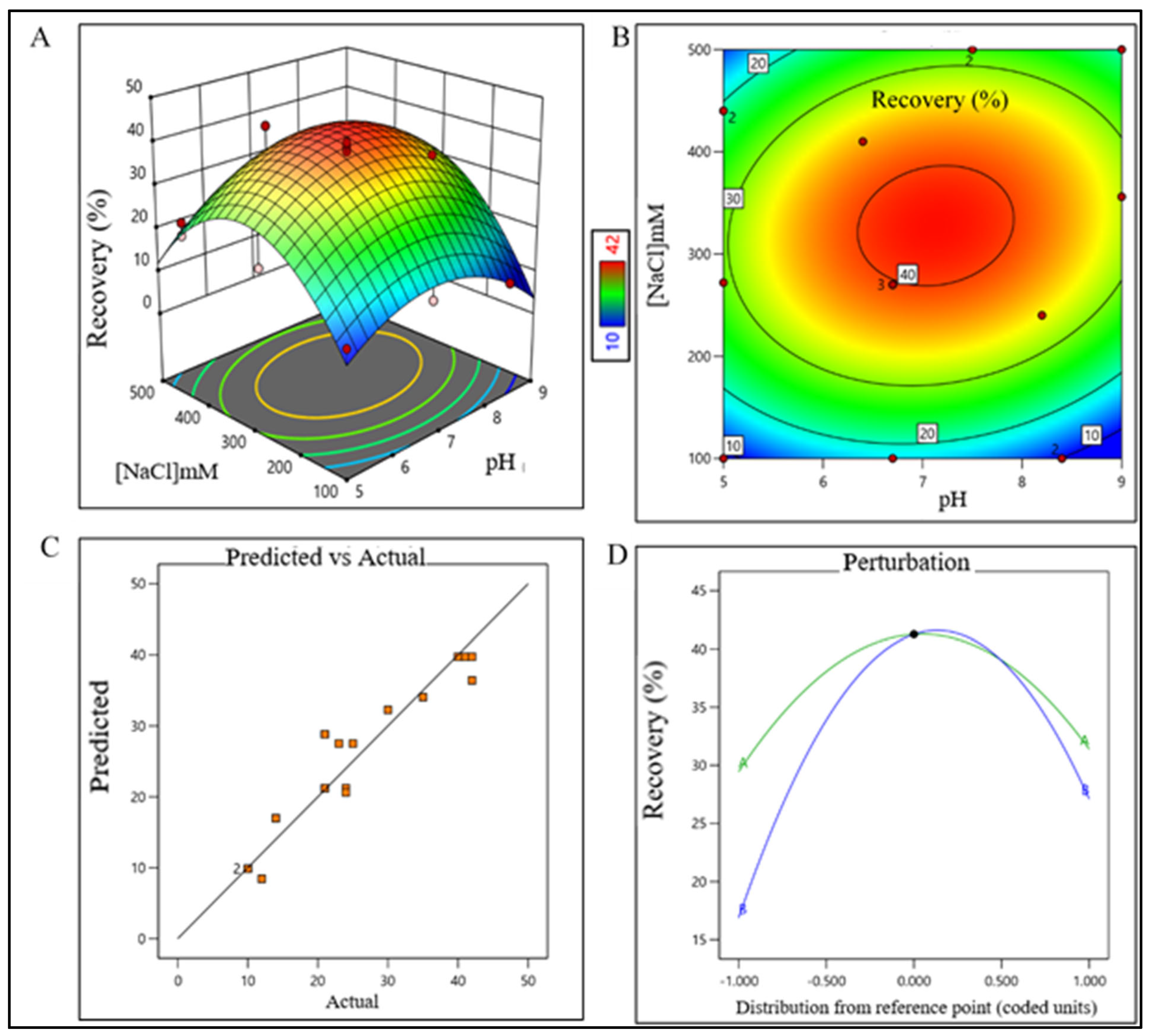
2.2. DoE for the Unit Processes of Purification Train
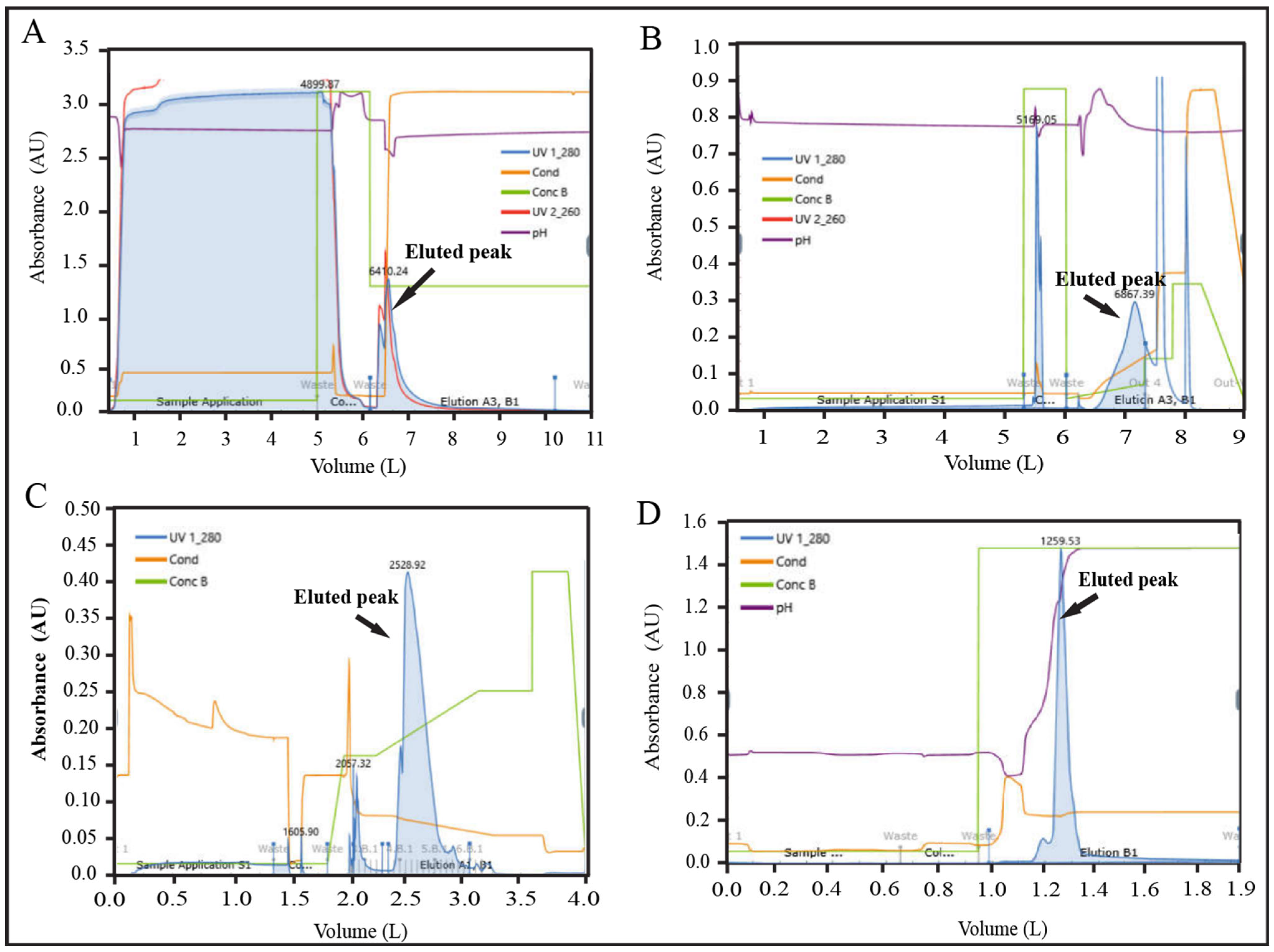
2.3. Scale-Up and Adaptation of the Unit Purification Processes in Dynamic Mode
2.4. Scale-Up and Validation of 10× (500 mL) and 100× (5000 mL) Batches
2.5. Analytical Approach for QTPP Confirmation
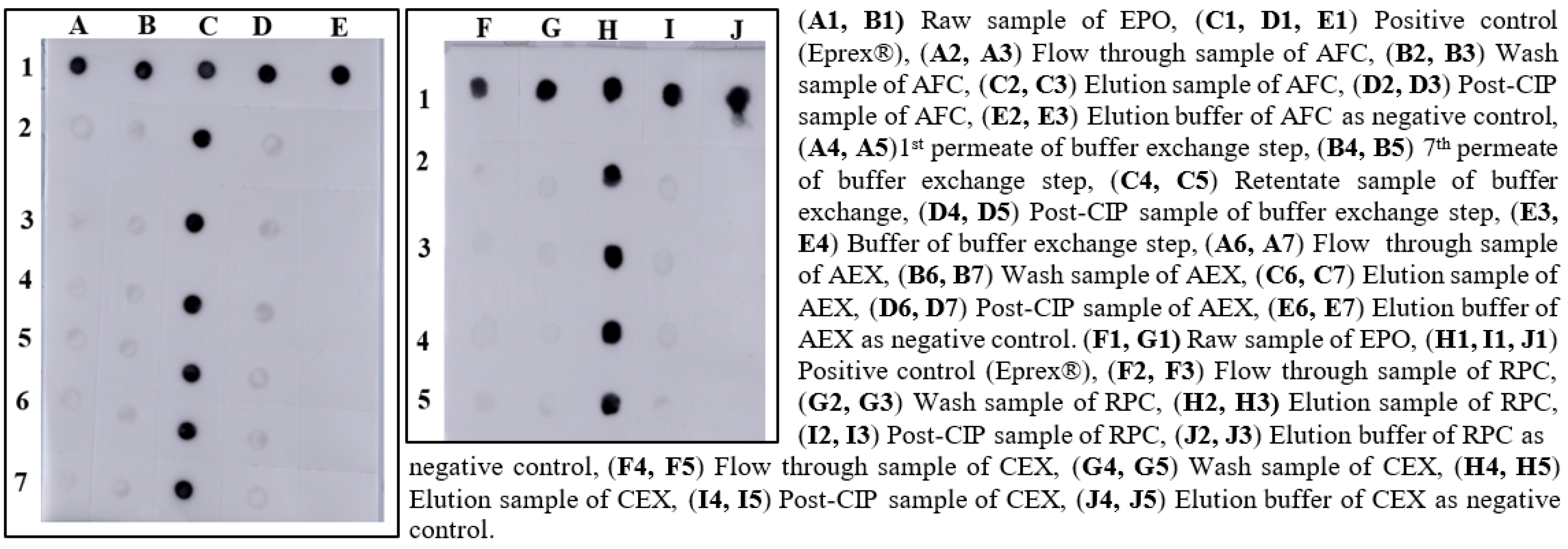
2.5.1. Dot Blotting
2.5.2. Particle Size Distribution
2.5.3. Chromatography for Determination of Assay and Impurities
2.5.4. In Vitro Functional Assay Using Cell Culture
2.5.5. Sterility and Endotoxin Testing
3. Results
3.1. Screening of Binding and Elution Conditions of EPO
3.2. Design of Experiments (DoE) of Purification Process
3.3. Adaptation of Unit Process in Dynamic Mode, and Scale-Up
3.4. Validation of Unit Processes for 10× (500 mL) Batch
3.5. Validation of 100× (5000 mL)
3.6. Analysis for QTPP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ifeanyi, O.E. A Review on Erythropietin in Pregnancy. J. Gynecol. Womens Health 2018, 8, 555740. [Google Scholar] [CrossRef]
- Krantz, S. Erythropoietin. Blood 1991, 77, 419–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varas, N.; Camacho, F.; Sánchez, O. Recombinant Human Erythropoietin. The problem of glycosylation. Lat. Am. J. Biotechnol. Life Sci. 2018, 3, 683–688. [Google Scholar] [CrossRef] [Green Version]
- Lai, P.H.; Everett, R.; Wang, F.F.; Arakawa, T.; Goldwasser, E. Structural Characterization of Human Erythropoietin. J. Biol. Chem. 1986, 261, 3116–3121. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.A.; Codevilla, C.F.; Fronza, M.; Casali, R.G.; Dalmora, S.L. Physico-Chemical Characterization and Biological Evaluation of Recombinant Human Erythropoietin in Pharmaceutical Products. Lat. Am. J. Pharm. 2003, 22, 343–350. [Google Scholar]
- Bunn, H.F. Erythropoietin. Cold Spring Harb. Perspect. Med. 2013, 3, a011619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, M.N.; Sasaki, H.; Lopez, L.; Fukuda, M. Survival of Recombinant Erythropoietin in the Circulation: The Role of Car-bohydrates. Blood 1989, 73, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Egrie, J.C.; Strickland, T.W.; Lane, J.; Aoki, K.; Cohen, A.M.; Smalling, R.; Trail, G.; Lin, F.K.; Browne, J.K.; Hines, D.K. Characterization and Biological Effects of Recombinant Human Erythropoietin. Immunobiology 1986, 172, 213–224. [Google Scholar] [CrossRef]
- Browne, J.K.; Cohen, A.M.; Egrie, J.C.; Lai, P.H.; Lin, F.-K.; Strickland, T.; Watson, E.; Stebbing, N. Erythropoietin: Gene Cloning, Protein Structure, and Biological Properties. Cold Spring Harb. Symp. Quant. Biol. 1986, 51, 693–702. [Google Scholar] [CrossRef]
- Recny, M.A.; Scoble, H.A.; Kim, Y. Structural Characterization of Natural Human Urinary and Recombinant DNA-Derived Erythropoietin. Identification of Des-Arginine 166 Erythropoietin. J. Biol. Chem. 1987, 262, 17156–17163. [Google Scholar] [CrossRef]
- Middleton, S.A.; Barbone, F.P.; Johnson, D.L.; Thurmond, R.L.; You, Y.; McMahon, F.J.; Jin, R.; Livnah, O.; Tullai, J.; Farrell, F.X.; et al. Shared and Unique Determinants of the Erythropoietin (EPO) Receptor Are Important for Binding EPO and EPO Mimetic Peptide. J. Biol. Chem. 1999, 274, 14163–14169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livnah, O.; Johnson, D.L.; Stura, E.A.; Farrell, F.X.; Barbone, F.P.; You, Y.; Liu, K.D.; Goldsmith, M.A.; He, W.; Krause, C.D.; et al. An Antagonist Peptide–EPO Receptor Complex Suggests That Receptor Dimerization Is Not Sufficient for Activation. Nat. Struct. Biol. 1998, 5, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.W.; Lotze, M.T. The Cytokine Handbook, 4th ed.; Academic Press: Amsterdam, The Netherlands, 2003; ISBN 978-0-12-689663-3. [Google Scholar]
- Maxwell, A.P. Novel Erythropoiesis-Stimulating Protein in the Management of the Anemia of Chronic Renal Failure. Kidney Int. 2002, 62, 720–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzo, J.D.; Seidenfeld, J.; Piper, M.; Aronson, N.; Lichtin, A.; Littlewood, T.J. Erythropoietin: A Paradigm for the Development of Practice Guidelines. Hematology 2001, 2001, 10–30. [Google Scholar] [CrossRef] [Green Version]
- Shiferaw, W.S.; Akalu, T.Y.; Aynalem, Y.A. Risk Factors for Anemia in Patients with Chronic Renal Failure: A Systematic Review and Meta-Analysis. Ethiop. J. Health Sci. 2020, 30, 829–842. [Google Scholar] [CrossRef]
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A Single Number for Advocacy and Communication—Worldwide More Than 850 Million Individuals Have Kidney Diseases. Kidney Int. 2019, 96, 1048–1050. [Google Scholar] [CrossRef]
- Chronic Kidney Disease in the United States, US Department of Health and Human Service, Center for Disease Control and Prevention. 2019. Available online: www.cdc.gov/kidneydisease/pdf/2019_National-Chronic-Kidney-Disease-Fact-Sheet.pdf (accessed on 3 January 2023).
- Adera, H.; Hailu, W.; Adane, A.; Tadesse, A. Prevalence of Anemia and Its Associated Factors among Chronic Kidney Disease Patients at University of Gondar Hospital, Northwest Ethiopia: A Hospital-Based Cross Sectional Study. Int. J. Nephrol. Renov. Dis. 2019, 12, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Fior Markets. Erythropoietin Drugs Market by Drug Class (Biologics, Biosimilars), Product (Epoetin Alpha, Epoetin Beta, Others), Application, Regions, Global Industry Analysis, Market Size, Share, Growth, Trends, and Forecast 2018 to 2025; Fior Markets: Pune, India, 2019. [Google Scholar]
- Thaweethamcharoen, T.; Sakulbumrungsil, R.; Nopmaneejumruslers, C.; Vasuvattakul, S. Cost-Utility Analysis of Erythropoietin for Anemia Treatment in Thai End-Stage Renal Disease Patients with Hemodialysis. Value Health Reg. Issues 2014, 3, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Aapro, M.; Cornes, P.; Sun, D.; Abraham, I. Comparative Cost Efficiency Across the European G5 Countries of Originators and a Biosimilar Erythropoiesis-Stimulating Agent to Manage Chemotherapy-Induced Anemia in Patients with Cancer. Ther. Adv. Med. Oncol. 2012, 4, 95–105. [Google Scholar] [CrossRef] [Green Version]
- World Bank Report Global GDP per Capita 2021, GDP per Capita (Current US$). Available online: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD (accessed on 7 January 2023).
- Bolton, G.R.; Violand, B.N.; Wright, R.S.; Sun, S.; Sunasara, K.M.; Watson, K.; Coffman, J.L.; Gallo, C.; Godavarti, R. Addressing the Challenges in Downstream Processing Today and Tomorrow: Newer Classes of Biotherapeutics Will Require Innovations in Processing Technology. BioPharm Int. 2011, 24, s8–s15. [Google Scholar]
- Kumar, V.; Bhalla, A.; Rathore, A.S. Design of Experiments Applications in Bioprocessing: Concepts and Approach. Biotechnol. Prog. 2014, 30, 86–99. [Google Scholar] [CrossRef]
- Committee on Specification for Pharmaceuticals Preparations. Good Manufacturing Practice for Pharmaceuticals Products; WHO Technical Report Series No. 823; World Health Organization: Geneva, Switzerland, 1992; pp. 14–19. [Google Scholar]
- Reis, C.; Gouveia, B.; Rijo, P.; Gonçalo, T. Good Manufacturing Practices for Medicinal Products for Human Use. J. Pharm. Bioallied Sci. 2015, 7, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.H.; Strickland, T.W. Erythropoietin Purification. U.S. Patent US4667016, 20 June 1985. [Google Scholar]
- Beck, A.K.; Withy, R.M. Recombinant Human Erythropoietin. European Patent EP0267678, 14 September 1987. [Google Scholar]
- Sugaya, S.; Ohashi, H.; Shinozawa, T. Expression and Purification of a Recombinant Human Glycoprotein in Mouse Hybridoma SP2/0-AG14 Cells. Biotechnol. Lett. 1997, 19, 185–188. [Google Scholar] [CrossRef]
- Zanette, D.; Soffientini, A.; Sottani, C.; Sarubbi, E. Evaluation of Phenylboronate Agarose for Industrial-Scale Purification of Erythropoietin from Mammalian Cell Cultures. J. Biotechnol. 2003, 101, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hu, Y.; Xu, M.; Zhang, S. An Improved, Inexpensive Procedure for the Large-Scale Purification of Recombinant Human Erythropoietin. Biotechnol. Appl. Biochem. 2004, 40, 89–94. [Google Scholar] [CrossRef]
- Carcagno, C.M.; Criscuolo, M.E.; Melo, C.A.; Vidal, J.A. Methods of Purifying Recombinant Human Erythropoietin from Cell Culture Supernatants. U.S. Patent US7012130, 14 March 2006. [Google Scholar]
- Goletz, S.; Stöckl, L. Process for the Purification of Glycoproteins. U.S. Patent US2012329092, 27 December 2012. [Google Scholar]
- Wojchowski, D.M.; Sue, J.M.; Sytkowski, A.J. Site-Specific Antibodies to Human Erythropoietin: Immunoaffinity Purification of Urinary and Recombinant Hormone. Biochim. Biophys. Acta BBA—Protein Struct. Mol. Enzym. 1987, 913, 170–178. [Google Scholar] [CrossRef]
- Ben Ghanem, A.; Winchenne, J.J.; Lopez, C.; Chretien, S.; Dubarry, M.; Craescu, C.T.; Le Caer, J.P.; Casadevall, N.; Rouger, P.; Cartron, J.P.; et al. Purification and Biological Activity of a Recombinant Human Erythropoietin Produced by Lymphoblastoid Cells. Prep. Biochem. 1994, 24, 127–142. [Google Scholar] [CrossRef]
- Hsu, L.W.; Chang, S.C. Expression System for Producing Recombinant Human Erythropoietin, Method for Purifying Secreted Human Erythropoietin and Uses Thereof. U.S. Patent US6376218, 23 April 2002. [Google Scholar]
- Surabattula, R.; Rao, K.S.; Polavarapu, R. An Optimized Process for Expression, Scale-up and Purification of Recombinant Eryth-ropoietin Produced in Chinese Hamster Ovary Cell Culture. Res. Biotechnol. 2011, 2, 58–74. [Google Scholar]
- Broudy, V.C.; Tait, J.F.; Powell, J.S. Recombinant Human Erythropoietin: Purification and Analysis of Carbohydrate Linkage. Arch. Biochem. Biophys. 1988, 265, 329–336. [Google Scholar] [CrossRef]
- Quelle, F.; Caslake, L.; Burkert, R.; Wojchowski, D. High-Level Expression and Purification of a Recombinant Human Erythropoietin Produced Using a Baculovirus Vector. Blood 1989, 74, 652–657. [Google Scholar] [CrossRef] [Green Version]
- Merrifield, E.H. Purification of Erythropoietin. European Patent EP0358463, 14 March 1990. [Google Scholar]
- Burg, J.; Fuerst, W.; Schneider, W.; Sellinger, K.H.; Wrba, A. Process for Producing Erythropoietin Containing No Animal Proteins. U.S. Patent US6399333, 4 June 2002. [Google Scholar]
- Koh, Y.W.; Lee, S.Y.; Kim, C.H.; Lee, S.H.; Kim, H.N.; Kim, S.Y.; Seong, J.H.; Cho, Y.H. Methods for Purifying Erythropoietin Analogs Having Lower Isoelectric Point. U.S. Patent US2014243510, 28 August 2014. [Google Scholar]
- Bandi, V.K.; Reddy, B.R.B.; Mugthihalli, S.M.; Iyer, P.K.; Pasupuleti, P. Process for the Purification of Erythropoietin and Dar-bepoetin Alfa. European Patent EP3153522, 21 April 2017. [Google Scholar]
- Nag, K.; Islam, M.J.; Rahman Khan, M.M.; Rahman Chowdhury, M.M.; Haq Sarker, M.E.; Kumar, S.; Khan, H.; Chakraborty, S.; Roy, R.; Roy, R.; et al. Development and Qualification of a High-Yield Recombinant Human Erythropoietin Biosimilar. bioRxiv 2023. [Google Scholar] [CrossRef]
- Thorpe, R.; Grampp, G.; Kang, H.-N.; Knezevic, I. Quality Assessment and Its Impact on Clinical Performance of a Biosimilar Erythropoietin: A Simulated Case Study. Biologicals 2019, 62, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Skibeli, V.; Nissen-Lie, G.; Torjesen, P. Sugar Profiling Proves That Human Serum Erythropoietin Differs from Recombinant Human Erythropoietin. Blood 2001, 98, 3626–3634. [Google Scholar] [CrossRef] [PubMed]
- Reichel, C. The Overlooked Difference between Human Endogenous and Recombinant Erythropoietins and Its Implication for Sports Drug Testing and Pharmaceutical Drug Design. Drug Test. Anal. 2011, 3, 883–891. [Google Scholar] [CrossRef]
- Cowper, B.; Li, X.; Yu, L.; Zhou, Y.; Fan, W.; Rao, C. Comprehensive Glycan Analysis of Twelve Recombinant Human Erythropoietin Preparations from Manufacturers in China and Japan. J. Pharm. Biomed. Anal. 2018, 153, 214–220. [Google Scholar] [CrossRef]
- Stalker, D.; Reid, S.; Ramaiya, A.; Wisemandle, W.A.; Martin, N.E. Pharmacokinetic and Pharmacodynamic Equivalence of Epoetin Hospira and Epogen after Single Subcutaneous Doses to Healthy Male Subjects. Clin. Ther. 2016, 38, 1778–1788. [Google Scholar] [CrossRef]
- Oh, M.; Yoon, J.; Cho, D.-Y. Pharmacokinetic and Pharmacodynamic Comparison of Two Recombinant Human Erythropoietin Formulations, PDA10 and Eprex, in Healthy Korean Male Volunteers: A Randomized, Double-Blinded, Single-Dose, Two-Period Crossover Study. Clin. Drug Investig. 2015, 35, 659–664. [Google Scholar] [CrossRef]
- Krivoshiev, S.; Wizemann, V.; Czekalski, S.; Schiller, A.; Pljesa, S.; Wolf-Pflugmann, M.; Siebert-Weigel, M.; Koytchev, R.; Bronn, A.; Epoetin Zeta Study Group. Therapeutic Equivalence of Epoetin Zeta and Alfa, Administered Subcutaneously, for Maintenance Treatment of Renal Anemia. Adv. Ther. 2010, 27, 105–117. [Google Scholar] [CrossRef]
- Sörgel, F.; Thyroff-Friesinger, U.; Vetter, A.; Vens-Cappell, B.; Kinzig, M. Bioequivalence of HX575 (Recombinant Human Epoetin Alfa) and a Comparator Epoetin Alfa after Multiple Subcutaneous Administrations. Pharmacology 2009, 83, 122–130. [Google Scholar] [CrossRef] [Green Version]
- Miao, B.; Isachkina, A.N.; Shutov, E.V.; Selyutin, A.A.; Kvitkova, L.V.; Shilo, V.Y.; Vetchinnikova, O.N.; Alexandrov, I.V.; Perlin, D.V.; Zuev, A.V.; et al. Biosimilar Erythropoietin in Anemia Treatment (BEAT)—Efficacy and Safety of a 1:1 Dose Conversion from EPREX® to EPIAO® in Patients with End-Stage Renal Disease on Hemodialysis: A Prospective, Randomized, Double Blind, Parallel Group Study. Medicine 2022, 101, e31426. [Google Scholar] [CrossRef]
- Fishbane, S.; Singh, B.; Kumbhat, S.; Wisemandle, W.A.; Martin, N.E. Intravenous Epoetin Alfa-Epbx versus Epoetin Alfa for Treatment of Anemia in End-Stage Kidney Disease. Clin. J. Am. Soc. Nephrol. 2018, 13, 1204–1214. [Google Scholar] [CrossRef] [Green Version]
- Lissy, M.; Ode, M.; Roth, K. Comparison of the Pharmacokinetic and Pharmacodynamic Profiles of One US-Marketed and Two European-Marketed Epoetin Alfas: A Randomized Prospective Study. Drugs R&D 2011, 11, 61–75. [Google Scholar] [CrossRef]
- Szabo, Z.; Thayer, J.R.; Reusch, D.; Agroskin, Y.; Viner, R.; Rohrer, J.; Patil, S.P.; Krawitzky, M.; Huhmer, A.; Avdalovic, N.; et al. High Performance Anion Exchange and Hydrophilic Interaction Liquid Chromatography Approaches for Comprehensive Mass Spectrometry-Based Characterization of the N-Glycome of a Recombinant Human Erythropoietin. J. Proteome Res. 2018, 17, 1559–1574. [Google Scholar] [CrossRef] [PubMed]
- Gianoncelli, A.; Bonini, S.A.; Bertuzzi, M.; Guarienti, M.; Vezzoli, S.; Kumar, R.; Delbarba, A.; Mastinu, A.; Sigala, S.; Spano, P.; et al. An Integrated Approach for a Structural and Functional Evaluation of Biosimilars: Implications for Erythropoietin. Biodrugs 2015, 29, 285–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Kort, B.J.; de Jong, G.J.; Somsen, G.W. Profiling of Erythropoietin Products by Capillary Electrophoresis with Native Fluorescence Detection. Electrophoresis 2012, 33, 2996–3001. [Google Scholar] [CrossRef]
- McKoy, J.M.; Stonecash, R.E.; Cournoyer, D.; Rossert, J.; Nissenson, A.R.; Raisch, D.W.; Casadevall, N.; Bennett, C.L. Epoetin-associated Pure Red Cell Aplasia: Past, Present, and Future Considerations. Transfusion 2008, 48, 1754–1762. [Google Scholar] [CrossRef] [Green Version]
- LaCreta, G.; Bucharles, S.G.E.; Sevignani, G.; Riella, M.C.; Nascimento, M.M.D. Pure Red Cell Aplasia and Anti-Erythropoietin Antibodies in Patients on Hemodialysis: A Report of Two Cases and a Literature Review. Braz. J. Nephrol. 2019, 41, 145–151. [Google Scholar] [CrossRef]
- Macdougall, I.C.; Casadevall, N.; Locatelli, F.; Combe, C.; London, G.M.; Di Paolo, S.; Kribben, A.; Fliser, D.; Messner, H.; McNeil, J.; et al. Incidence of erythropoietin antibody-mediated pure red cell aplasia: The Prospective Immunogenicity Surveillance Registry (PRIMS). Nephrol. Dial. Transplant. 2015, 30, 451–460. [Google Scholar] [CrossRef] [Green Version]
- Weise, M.; Bielsky, M.-C.; De Smet, K.; Ehmann, F.; Ekman, N.; Giezen, T.J.; Gravanis, I.; Heim, H.-K.; Heinonen, E.; Ho, K.; et al. Biosimilars: What Clinicians Should Know. Blood 2012, 120, 5111–5117. [Google Scholar] [CrossRef] [Green Version]
- Halim, L.A.; Brinks, V.; Jiskoot, W.; Romeijn, S.; Haselberg, R.; Burns, C.; Wadhwa, M.; Schellekens, H. Quality and Batch-to-Batch Consistency of Original and Biosimilar Epoetin Products. J. Pharm. Sci. 2016, 105, 542–550. [Google Scholar] [CrossRef] [Green Version]
- Nag, K.; Mohiuddin, M.; Mahtab, M.A.; Bachar, S.C.; Rahim, M.A.; Uddin, M.H.; Kumar, S.; Khan, M.M.R.; Sarker, M.E.H.; Chowdhury, M.M.R.; et al. Biosimilarity of GBPD002 compared with Eprex® through clinical evaluation in human. medRxiv 2023. [Google Scholar] [CrossRef]
- A Randomized, Double-blinded, Active Controlled Crossover Clinical Trial to Investigate PK, PD and Safety of GBPD002, ClinicalTrials.gov Identifier: NCT05585658, 20 October 2022. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05585658 (accessed on 4 May 2023).
- Ramakrishnan, R.; Cheung, W.K.; Wacholtz, M.C.; Minton, N.; Jusko, W.J. Pharmacokinetic and Pharmacodynamic Modeling of Recombinant Human Erythropoietin after Single and Multiple Doses in Healthy Volunteers. J. Clin. Pharmacol. 2004, 44, 991–1002. [Google Scholar] [CrossRef] [PubMed]




| References | Process Steps | Process Performance | Limitations |
|---|---|---|---|
| Lai and Strickland et al. (1985) [28] | AEC → RPC →SEC | Not mentioned. | Incomplete and uncharacterized process, SEC is not economic or suitable for scale-up |
| Beck and Withy (1988) [29] | CEC → RPC → SEC | Total yield (%): 60 → 50 → 41 | Incomplete and uncharacterized process. Need to adjust sample pH below 4 resulting in precipitation or degradation, SEC is not economic or suitable for scale-up |
| Sugaya et al. (1997) [30] | AFC → AFC → SEC | Total yield (%): 79 → 54 → 40 | Incomplete and uncharacterized process, SEC is not economic or suitable for scale-up |
| Zanette et al. (2003) [31] | AFC → AEC → SEC | Total yield (%): 76 → 60 → 30 | Incomplete and uncharacterized process. Need to adjust sample pH below 4, resulting precipitation or degradation, SEC is not economic or suitable for scale-up, impurities were not removed based on hydrophobicity |
| Hu et al. (2004) [32] | AEC → HIC → SEC | Total yield (%): 51 → 46 → 43 | AFC is more efficient to enrich glycosylated protein compared to AEC, SEC is not economic or suitable for scale-up, AEC is more suitable to separate charge variant compared to HIC |
| Carcagno et al. (2006) [33] | HIC → AEC → CEC → SEC | Total yield (%): 70 →56 → 40 → 30 | Need to add high molar salt at first step, SEC is not economic or suitable for scale-up, need extra process step to complete the process |
| Goletz and Stöckl (2012) [34] | RPC → AFC → SEC → AEC | Final host cell protein content less than 0.01% | AFC is more efficient to enrich glycosylated protein compared to RPC, SEC is not economic or suitable for scale-up and not suitable after AFC, |
| Wojchowski et al. (1987) [35] | HIC→ AFC | Total yield (%): 100 → 55 | Incomplete and uncharacterized process, no suitability in production scale |
| Ghanem et al. (1994) [36] | AFC → AEC | Total yield (%): 73 → 53 | Incomplete and uncharacterized process, no suitability in production scale |
| Hsu and Chang (2002) [37] | AFC → SEC | in vitro activity: 240 kU/mg | Incomplete and uncharacterized process, not suitable for scale-up, yield was not claimed |
| Surabattula et al. (2011) [38] | AFC → AEC | Final total yield: 42% | Incomplete and uncharacterized process, not suitable for scale-up |
| Broudy et al. (1988) [39] | AFC → AEC → RPC | Total yield (%): 65 → 49 → 35 | After RPC, acetonitrile removal is difficult to formulate the sample, needs other steps which are not performed and characterized |
| Quelle et al. (1989) [40] | AEC → RPC → AFC | Total yield (%): 95 → 85 → 80 | AFC is more efficient to capture glycosylated protein, difficult to formulate the sample after AFC, need other steps which are not performed and characterized |
| Merrifield (1990) [41] | AFC → AFC → AEC | Total yield: 80% (in the first step) | Need to add extra TFF process before each chromatography step, difficult to formulate the sample after AFC, need other steps which are not performed and characterized |
| Burg et al. (2002) [42] | AFC → HIC → HAC → RPC → AEC | Final total yield: 25% | Need other steps to formulate the sample which are not performed and characterized, after RPC, elute is not suitable to proceed for AEC |
| Koh et al. (2014) [43] | AEC → HAC → AEC | Total yield (%): 60.1 → 54.2 → 18.1 | AFC is more suitable for enrichment of glycosylated protein, needs other steps which are not performed and characterized |
| Bandi et al. (2017) [44] | AEC → MMC → AEC → CEC → AFC → AEC → SEC | Purity (%): ≥98% | Need at least three TFF process steps and other steps to formulate the sample which are not characterized, too many process steps increase process cost, SEC is not economic or suitable for scale-up, specific yield was not claimed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nag, K.; Sarker, E.H.; Kumar, S.; Chakraborty, S.; Khan, M.R.; Chowdhury, M.R.; Roy, R.; Roy, R.; Biswas, B.K.; Bappi, E.H.; et al. Satisfying QTPP of Erythropoietin Biosimilar by QbD through DoE-Derived Downstream Process Engineering. Pharmaceutics 2023, 15, 2087. https://doi.org/10.3390/pharmaceutics15082087
Nag K, Sarker EH, Kumar S, Chakraborty S, Khan MR, Chowdhury MR, Roy R, Roy R, Biswas BK, Bappi EH, et al. Satisfying QTPP of Erythropoietin Biosimilar by QbD through DoE-Derived Downstream Process Engineering. Pharmaceutics. 2023; 15(8):2087. https://doi.org/10.3390/pharmaceutics15082087
Chicago/Turabian StyleNag, Kakon, Enamul Haq Sarker, Samir Kumar, Sourav Chakraborty, Maksusdur Rahman Khan, Mashfiqur Rahman Chowdhury, Rony Roy, Ratan Roy, Bipul Kumar Biswas, Emrul Hasan Bappi, and et al. 2023. "Satisfying QTPP of Erythropoietin Biosimilar by QbD through DoE-Derived Downstream Process Engineering" Pharmaceutics 15, no. 8: 2087. https://doi.org/10.3390/pharmaceutics15082087
APA StyleNag, K., Sarker, E. H., Kumar, S., Chakraborty, S., Khan, M. R., Chowdhury, M. R., Roy, R., Roy, R., Biswas, B. K., Bappi, E. H., Mohiuddin, M., & Sultana, N. (2023). Satisfying QTPP of Erythropoietin Biosimilar by QbD through DoE-Derived Downstream Process Engineering. Pharmaceutics, 15(8), 2087. https://doi.org/10.3390/pharmaceutics15082087






