Development of Blueberry-Derived Extracellular Nanovesicles for Immunomodulatory Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Preparation of Plant-Derived Nanovesicles
2.4. Attenuated Total Reflection Fourier-Transform Infrared Spectroscopy (ATR-FTIR)
2.5. Nanoparticle Tracking Analysis (NTA)
2.6. Transmission Electron Microscopy (TEM)
2.7. Protein Extraction of Plant Samples
2.8. Aptamer Binding Assay Using Fluorescence Polarization
2.9. DiD-Labeled Aptamer-Conjugated BENVs
2.10. Cellular Uptake
2.11. Transport Study
2.12. Preparation of Drug-Loaded BENVs
2.13. Cell Viability Assay
2.14. Evaluation of BENVs’ Capability in Immune Modulation
2.15. Immunoblotting Analysis
2.16. Statistical Analysis
3. Results
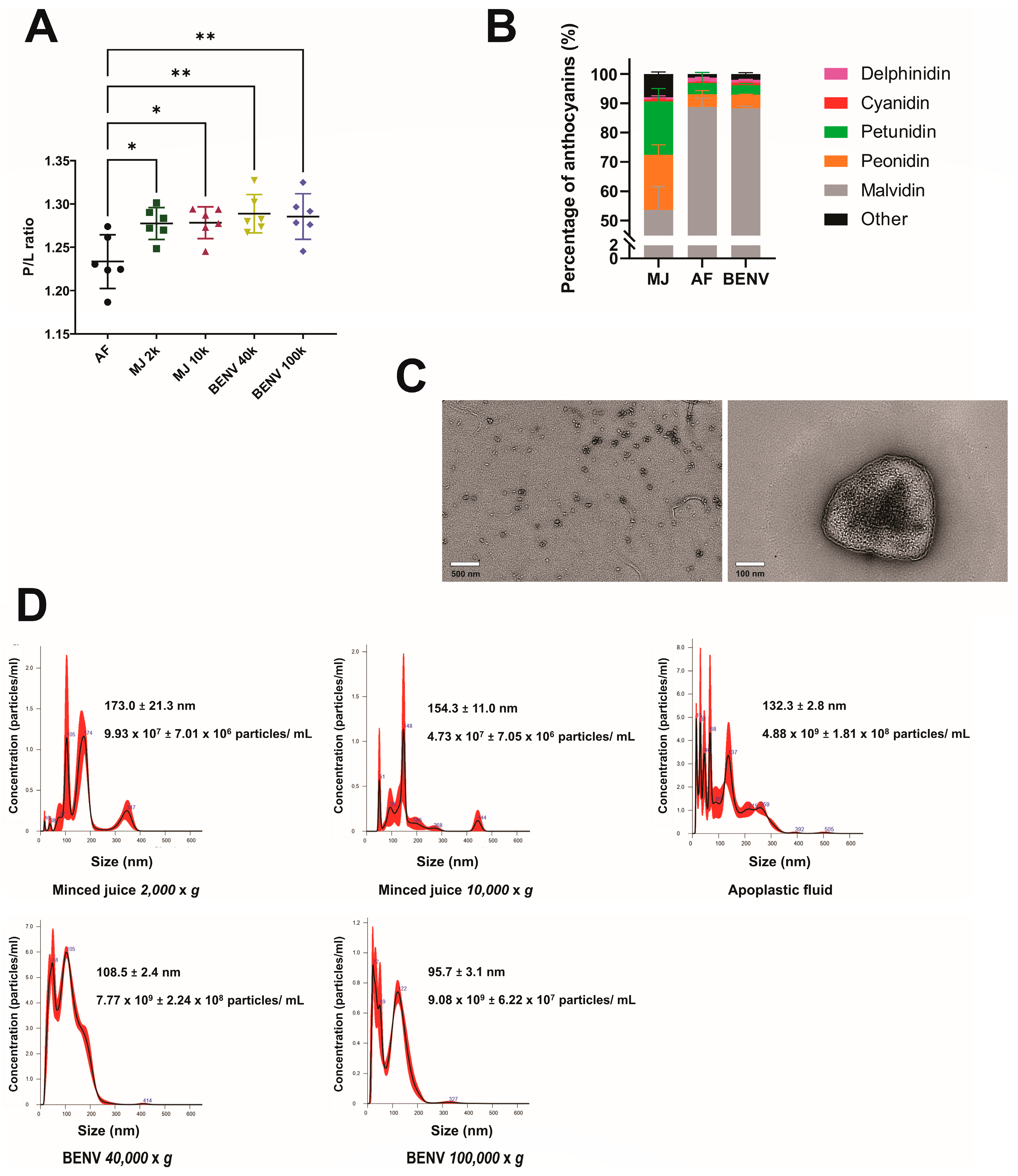
3.1. Preparation and Characterization of Blueberry-Derived Extracellular Nanovesicles (BENVs)
3.2. A putative “Universal” Biomarker of Plant-Derived Extracellular Nanovesicles
3.3. BENVs as a Nanocarrier for Drug Delivery
3.4. Immunomodulatory Effects of BENVs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hansen, L.L.; Nielsen, M.E. Plant exosomes: Using an unconventional exit to prevent pathogen entry? J. Exp. Bot. 2018, 69, 59–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dad, H.A.; Gu, T.-W.; Zhu, A.-Q.; Huang, L.-Q.; Peng, L.-H. Plant exosome-like nanovesicles: Emerging therapeutics and drug delivery nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, O.; Fontana, S.; Monteleone, F.; Taverna, S.; Di Bella, M.A.; Di Vizio, D.; Alessandro, R. Exosomes from metastatic cancer cells transfer amoeboid phenotype to non-metastatic cells and increase endothelial permeability: Their emerging role in tumor heterogeneity. Sci. Rep. 2017, 7, 4711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Ren, Y.; Mu, J.; Egilmez, N.K.; Zhuang, X.; Deng, Z.; Zhang, L.; Yan, J.; Miller, D.; Zhang, H.-G. Grapefruit-derived nanovectors use an activated leukocyte trafficking pathway to deliver therapeutic agents to inflammatory tumor sites. Cancer Res. 2015, 75, 2520–2529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutter, B.D.; Innes, R.W. Extracellular vesicles as key mediators of plant–microbe interactions. Curr. Opin. Plant Biol. 2018, 44, 16–22. [Google Scholar] [CrossRef]
- Turhan, E.; Karni, L.; Aktas, H.; Deventurero, G.; Chang, D.; Bar-Tal, A.; Aloni, B. Apoplastic anti-oxidants in pepper (Capsicum annuum L.) fruit and their relationship to blossom-end rot. J. Hortic. Sci. Biotechnol. 2006, 81, 661–667. [Google Scholar] [CrossRef]
- Kim, S.G.; Wang, Y.; Lee, K.H.; Park, Z.-Y.; Park, J.; Wu, J.; Kwon, S.J.; Lee, Y.-H.; Agrawal, G.K.; Rakwal, R. In-depth insight into in vivo apoplastic secretome of rice-Magnaporthe oryzae interaction. J. Proteomics 2013, 78, 58–71. [Google Scholar] [CrossRef]
- Witzel, K.; Shahzad, M.; Matros, A.; Mock, H.-P.; Mühling, K.H. Comparative evaluation of extraction methods for apoplastic proteins from maize leaves. Plant Methods 2011, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- Lohaus, G.; Pennewiss, K.; Sattelmacher, B.; Hussmann, M.; Hermann Muehling, K. Is the infiltration-centrifugation technique appropriate for the isolation of apoplastic fluid? A critical evaluation with different plant species. Physiol. Plant. 2001, 111, 457–465. [Google Scholar] [CrossRef]
- Ruan, Y.; Mate, C.; Patrick, J.; Brady, C. Non-destructive collection of apoplast fluid from developing tomato fruit using a pressure dehydration procedure. Funct. Plant Biol. 1995, 22, 761–769. [Google Scholar] [CrossRef]
- Almeida, D.P.; Huber, D.J. Apoplastic pH and inorganic ion levels in tomato fruit: A potential means for regulation of cell wall metabolism during ripening. Physiol. Plant. 1999, 105, 506–512. [Google Scholar] [CrossRef]
- Almeida, D.P.; Huber, D.J. Autolysis of cell walls from polygalacturonase-antisense tomato fruit in simulated apoplastic solutions. Plant Physiol. Biochem. 2011, 49, 617–622. [Google Scholar] [CrossRef]
- Bernstein, L. Method for determining solutes in the cell walls of leaves. Plant Physiol. 1971, 47, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Long, J.M.; Widders, I.E. Quantification of apoplastic potassium content by elution analysis of leaf lamina tissue from pea (Pisum sativum L. cv Argenteum). Plant Physiol. 1990, 94, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Maksimović, J.J.D.; Živanović, B.D.; Maksimović, V.M.; Mojović, M.D.; Nikolic, M.T.; Vučinić, Ž.B. Filter strip as a method of choice for apoplastic fluid extraction from maize roots. Plant Sci. 2014, 223, 49–58. [Google Scholar] [CrossRef]
- Gupta, R.; Lee, S.E.; Agrawal, G.K.; Rakwal, R.; Park, S.; Wang, Y.; Kim, S.T. Understanding the plant-pathogen interactions in the context of proteomics-generated apoplastic proteins inventory. Front. Plant Sci. 2015, 6, 352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prado, N.; de Dios Alché, J.; Casado-Vela, J.; Mas, S.; Villalba, M.; Rodríguez, R.; Batanero, E. Nanovesicles are secreted during pollen germination and pollen tube growth: A possible role in fertilization. Mol. Plant 2014, 7, 573–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Movahed, N.; Cabanillas, D.G.; Wan, J.; Vali, H.; Laliberté, J.-F.; Zheng, H. Turnip mosaic virus components are released into the extracellular space by vesicles in infected leaves. Plant Physiol. 2019, 180, 1375–1388. [Google Scholar] [CrossRef]
- Rutter, B.D.; Innes, R.W. Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins. Plant Physiol. 2017, 173, 728–741. [Google Scholar] [CrossRef] [Green Version]
- Regente, M.; Pinedo, M.; San Clemente, H.; Balliau, T.; Jamet, E.; De La Canal, L. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J. Exp. Bot. 2017, 68, 5485–5495. [Google Scholar] [CrossRef] [Green Version]
- Pinedo, M.; de la Canal, L.; de Marcos Lousa, C. A call for Rigor and standardization in plant extracellular vesicle research. J. Extracell. Vesicles 2021, 10, e12048. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Dico, A.L.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di Bella, M.A. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget 2015, 6, 19514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.-B.; Wang, B.; Zhang, L. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Zhuang, X.; Deng, Z.-B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pocsfalvi, G.; Turiák, L.; Ambrosone, A.; Del Gaudio, P.; Puska, G.; Fiume, I.; Silvestre, T.; Vékey, K. Physiochemical and protein datasets related to citrus juice sac cells-derived nanovesicles and microvesicles. Data Brief 2019, 22, 251–254. [Google Scholar] [CrossRef]
- Oancea, S.; Oprean, L. Anthocyanins, from Biosynthesis in Plants to Human Health Benefits. Acta Univ. Cinbinesis Ser. E Food Technol. 2011, 15, 3–16. [Google Scholar]
- Wada, H.; Shackel, K.A.; Matthews, M.A. Fruit ripening in Vitis vinifera: Apoplastic solute accumulation accounts for pre-veraison turgor loss in berries. Planta 2008, 227, 1351–1361. [Google Scholar] [CrossRef]
- Welbaum, G.E.; Meinzer, F.C. Compartmentation of solutes and water in developing sugarcane stalk tissue. Plant Physiol. 1990, 93, 1147–1153. [Google Scholar] [CrossRef] [Green Version]
- Mihály, J.; Deák, R.; Szigyártó, I.C.; Bóta, A.; Beke-Somfai, T.; Varga, Z. Characterization of extracellular vesicles by IR spectroscopy: Fast and simple classification based on amide and C H stretching vibrations. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 459–466. [Google Scholar] [CrossRef]
- Tran, P.H.L.; Wang, T.; Yin, W.; Tran, T.T.D.; Barua, H.T.; Zhang, Y.; Midge, S.B.; Nguyen, T.N.G.; Lee, B.-J.; Duan, W. Development of a nanoamorphous exosomal delivery system as an effective biological platform for improved encapsulation of hydrophobic drugs. Int. J. Pharm. 2019, 566, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Masuda, K. Class II chitinase accumulated in the bark tissue involves with the cold hardiness of shoot stems in highbush blueberry (Vaccinium corymbosum L.). Sci. Hortic. 2009, 120, 230–236. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, Z.; An, Y.; Zhang, W.; Zhang, H.; Liu, D.; Yu, C.; Duan, W.; Yang, C.J. Selection of DNA aptamers against epithelial cell adhesion molecule for cancer cell imaging and circulating tumor cell capture. Anal. Chem. 2013, 85, 4141–4149. [Google Scholar] [CrossRef]
- Nguyen, T.N.-G.; Tran, P.H.-L.; Tran, T.V.; Vo, T.V.; Truong-DinhTran, T. Development of a modified–solid dispersion in an uncommon approach of melting method facilitating properties of a swellable polymer to enhance drug dissolution. Int. J. Pharm. 2015, 484, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, R.; Tsao, R. Anthocyanin-rich phenolic extracts of purple root vegetables inhibit pro-inflammatory cytokines induced by H2O2 and enhance antioxidant enzyme activities in Caco-2 cells. J. Funct. Foods 2016, 22, 363–375. [Google Scholar] [CrossRef]
- Misra, B.B. The black-box of plant apoplast lipidomes. Front. Plant Sci. 2016, 7, 323. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Feng, S.; Wang, X.; Long, K.; Luo, Y.; Wang, Y.; Ma, J.; Tang, Q.; Jin, L.; Li, X. Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. PeerJ 2018, 6, e5186. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Choi, H.-K.; Kim, Y.-K.; Lee, H.-J. Delphinidin and Its Glycosides’ War on Cancer: Preclinical Perspectives. Int. J. Mol. Sci. 2021, 22, 11500. [Google Scholar] [CrossRef] [PubMed]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jao, D.; Xue, Y.; Medina, J.; Hu, X. Protein-Based Drug-Delivery Materials. Materials 2017, 10, 517. [Google Scholar] [CrossRef] [Green Version]
- Regente, M.; Corti-Monzón, G.; Maldonado, A.M.; Pinedo, M.; Jorrín, J.; de la Canal, L. Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett. 2009, 583, 3363–3366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regente, M.; Pinedo, M.; Elizalde, M.; de la Canal, L. Apoplastic exosome-like vesicles: A new way of protein secretion in plants? Plant Signal. Behav. 2012, 7, 544–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Y.; Gao, J.; He, Y.; Jiang, L. Plant extracellular vesicles. Protoplasma 2020, 257, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qiu, Y.; Xu, K. Characterization of GFP-AtPEN1 as a marker protein for extracellular vesicles isolated from Nicotiana benthamiana leaves. Plant Signal. Behav. 2020, 15, 1791519. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Yu, J. Exosome-like nanoparticles from ginger rhizomes inhibited NLRP3 inflammasome activation. Mol. Pharm. 2019, 16, 2690–2699. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y. Inhibitory Effects of Shiitake-Derived Exosome-like Nanoparticles on NLRP3 Inflammasome Activation. Master’s Thesis, University of Nebraska, Lincoln, NB, USA, 2019. [Google Scholar]
- Pocsfalvi, G.; Turiák, L.; Ambrosone, A.; Del Gaudio, P.; Puska, G.; Fiume, I.; Silvestre, T.; Vákey, K. Dissection of protein cargo of citrus fruit juice sac cells-derived vesicles reveals heterogeneous transport and extracellular vesicles subpopulations. bioRxiv 2018. [Google Scholar] [CrossRef]
- Lado, J.; Rodrigo, M.J.; Zacarías, L. Analysis of ethylene biosynthesis and perception during postharvest cold storage of Marsh and Star Ruby grapefruits. Food Sci. Technol. Int. 2015, 21, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Porat, R.; Lers, A.; Dori, S.; Cohen, L.; Weiss, B.; Daus, A.; Wilson, C.; Droby, S. Induction of chitinase and β-1, 3-endoglucanase proteins by UV irradiation and wounding in grapefruit peel tissue. Phytoparasitica 1999, 27, 233–238. [Google Scholar] [CrossRef]
- Velazhahan, R.; Jayaraj, J.; Liang, G.; Muthukrishnan, S. Partial purification and N-terminal amino acid sequencing of a β-1, 3-glucanase from sorghum leaves. Biol. Plant. 2003, 46, 29–33. [Google Scholar] [CrossRef]
- Krishnaveni, S.; Muthukrishnan, S.; Liang, G.; Wilde, G.; Manickam, A. Induction of chitinases and β-1, 3-glucanases in resistant and susceptible cultivars of sorghum in response to insect attack, fungal infection and wounding. Plant Sci. 1999, 144, 9–16. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Vashisht, D.; Cletus, J.; Sakthivel, N. Plant β-1, 3-glucanases: Their biological functions and transgenic expression against phytopathogenic fungi. Biotechnol. Lett. 2012, 34, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Boevink, P.C. Exchanging missives and missiles: The roles of extracellular vesicles in plant–pathogen interactions. J. Exp. Bot. 2017, 68, 5411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, Y.; Wang, L.; Zhu, C.; Zheng, Q.; Wang, G.; Tong, J.; Fang, Y.; Xia, Y.; Cheng, G.; He, X. Aptamer-conjugated extracellular nanovesicles for targeted drug delivery. Cancer Res. 2018, 78, 798–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Tang, C.; Zhao, L.; Xu, L.; Zhou, W.; Dong, Z.; Yang, Y.; Xie, Q.; Fang, X. Aptamer-based fluorescence polarization assay for separation-free exosome quantification. Nanoscale 2019, 11, 10106–10113. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, H.; Yu, M.; Fang, J. Targeted Delivery of Doxorubicin Using a Colorectal Cancer-Specific ssDNA Aptamer. Anat. Rec. 2014, 297, 2280–2288. [Google Scholar] [CrossRef]
- Guardamagna, I.; Lonati, L.; Savio, M.; Stivala, L.A.; Ottolenghi, A.; Baiocco, G. An Integrated Analysis of the Response of Colorectal Adenocarcinoma Caco-2 Cells to X-Ray Exposure. Front. Oncol. 2021, 11, 688919. [Google Scholar] [CrossRef]
- van den Boorn, J.G.; Schlee, M.; Coch, C.; Hartmann, G. SiRNA delivery with exosome nanoparticles. Nat. Biotechnol. 2011, 29, 325. [Google Scholar] [CrossRef]
- Hubatsch, I.; Ragnarsson, E.G.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Agrawal, A.K.; Gupta, R. Exosomes for the enhanced tissue bioavailability and efficacy of curcumin. AAPS J. 2017, 19, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.J.; Zhao, Y.; Jin, Y.S.; Li, S.M.; Bago, J.R.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Macrophage-Derived Extracellular Vesicles as Drug Delivery Systems for Triple Negative Breast Cancer (TNBC) Therapy. J. Neuroimmune Pharmacol. 2020, 15, 487–500. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Shen, Y.; Mahalingam, R.; Jasti, B.; Li, X. Polymers in Oral Modified Release Systems; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Wen, H.; Park, K. Oral Controlled Release Formulation Design and Drug Delivery: Theory to Practice; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Ileri Ercan, N. Understanding Interactions of Curcumin with Lipid Bilayers: A Coarse-Grained Molecular Dynamics Study. J. Chem. Inf. Model. 2019, 59, 4413–4426. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Jia, L.; Zhou, H.-M.; Liu, Y.; Zhong, L.-F. Mitochondrial and nuclear DNA damage induced by curcumin in human hepatoma G2 cells. Toxicol. Sci. 2006, 91, 476–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Lázaro, M. Anticancer and carcinogenic properties of curcumin: Considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol. Nutr. Food Res. 2008, 52, S103–S127. [Google Scholar] [CrossRef] [PubMed]
- Syng-Ai, C.; Kumari, A.L.; Khar, A. Effect of curcumin on normal and tumor cells: Role of glutathione and bcl-2. Mol. Cancer Ther. 2004, 3, 1101–1108. [Google Scholar] [CrossRef]
- López-Lázaro, M.; Willmore, E.; Jobson, A.; Gilroy, K.L.; Curtis, H.; Padget, K.; Austin, C.A. Curcumin induces high levels of topoisomerase I− and II− DNA complexes in K562 leukemia cells. J. Nat. Prod. 2007, 70, 1884–1888. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [Green Version]
- Cross, C.; Halliwell, B.; Allen, A. Antioxidant protection: A function of tracheobronchial and gastrointestinal mucus. Lancet 1984, 323, 1328–1330. [Google Scholar] [CrossRef]
- Yi, W.; Akoh, C.C.; Fischer, J.; Krewer, G. Absorption of anthocyanins from blueberry extracts by caco-2 human intestinal cell monolayers. J. Agric. Food Chem. 2006, 54, 5651–5658. [Google Scholar] [CrossRef]
- Richter, S.; Kientz, M.; Brumm, S.; Nielsen, M.E.; Park, M.; Gavidia, R.; Krause, C.; Voss, U.; Beckmann, H.; Mayer, U. Delivery of endocytosed proteins to the cell–division plane requires change of pathway from recycling to secretion. eLife 2014, 3, e02131. [Google Scholar] [CrossRef]
- Theos, A.C.; Truschel, S.T.; Tenza, D.; Hurbain, I.; Harper, D.C.; Berson, J.F.; Thomas, P.C.; Raposo, G.; Marks, M.S. A lumenal domain-dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev. Cell 2006, 10, 343–354. [Google Scholar] [CrossRef] [Green Version]
- Delaunois, B.; Jeandet, P.; Clément, C.; Baillieul, F.; Dorey, S.; Cordelier, S. Uncovering plant-pathogen crosstalk through apoplastic proteomic studies. Front. Plant Sci. 2014, 5, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, F.-y.; Blackburn, K.; Lin, Y.-m.; Goshe, M.B.; Williamson, J.D. Absolute protein quantification by LC/MSE for global analysis of salicylic acid-induced plant protein secretion responses. J. Proteome Res. 2008, 8, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Kaffarnik, F.A.; Jones, A.M.; Rathjen, J.P.; Peck, S.C. Effector proteins of the bacterial pathogen Pseudomonas syringae alter the extracellular proteome of the host plant, Arabidopsis thaliana. Mol. Cell. Proteomics 2009, 8, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Bokhari, S.A.; Dong, C.-J.; Liu, J.-Y. Comparative proteomics analysis of the root apoplasts of rice seedlings in response to hydrogen peroxide. PLoS ONE 2011, 6, e16723. [Google Scholar] [CrossRef]
- Ebrahim, S.; Usha, K.; Singh, B. Pathogenesis-related (PR)-proteins: Chitinase and β-1, 3-glucanase in defense mechanism against malformation in mango (Mangifera indica L.). Sci. Hortic. 2011, 130, 847–852. [Google Scholar] [CrossRef]
- Miles, T.D.; Schilder, A.C. Host defenses associated with fruit infection by Colletotrichum species with an emphasis on anthracnose of blueberries. Plant Health Progress 2013, 14, 30. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Caballero, C.; Romero, I.; Goni, O.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. Characterization of an antifungal and cryoprotective class I chitinase from table grape berries (Vitis vinifera Cv. Cardinal). J. Agric. Food Chem. 2009, 57, 8893–8900. [Google Scholar] [CrossRef] [PubMed]
- Romero, I.; Fernandez-Caballero, C.; Goñi, O.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. Functionality of a class I beta-1, 3-glucanase from skin of table grapes berries. Plant Sci. 2008, 174, 641–648. [Google Scholar] [CrossRef]
- Hamel, F.; Bellemare, G. Characterization of a class I chitinase gene and of wound-inducible, root and flower-specific chitinase expression inBrassica napus. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 1995, 1263, 212–220. [Google Scholar] [CrossRef]
- Ding, C.-K.; Wang, C.; Gross, K.C.; Smith, D.L. Jasmonate and salicylate induce the expression of pathogenesis-related-protein genes and increase resistance to chilling injury in tomato fruit. Planta 2002, 214, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Neale, A.D.; Wahleithner, J.A.; Lund, M.; Bonnett, H.T.; Kelly, A.; Meeks-Wagner, D.R.; Peacock, W.J.; Dennis, E.S. Chitinase, beta-1, 3-glucanase, osmotin, and extensin are expressed in tobacco explants during flower formation. Plant Cell 1990, 2, 673–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, S.P.; Jacobs, A.K.; Dry, I.B. A class IV chitinase is highly expressed in grape berries during ripening. Plant Physiol. 1997, 114, 771–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-T.; Leubner-Metzger, G.; Meins, F., Jr.; Bradford, K.J. Class I β-1, 3-glucanase and chitinase are expressed in the micropylar endosperm of tomato seeds prior to radicle emergence. Plant Physiol. 2001, 126, 1299–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasprzewska, A. Plant chitinases-regulation and function. Cell. Mol. Biol. Lett. 2003, 8, 809–824. [Google Scholar] [PubMed]
- Pereira, C.; Pereira, S.; Pissarra, J. Delivering of proteins to the plant vacuole—An update. Int. J. Mol. Sci. 2014, 15, 7611–7623. [Google Scholar] [CrossRef] [Green Version]
- Isayenkov, S. Plant vacuoles: Physiological roles and mechanisms of vacuolar sorting and vesicular trafficking. Cytol. Genet. 2014, 48, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Stigliano, E.; Di Sansebastiano, G.-P.; Neuhaus, J.-M. Contribution of chitinase A’s C-terminal vacuolar sorting determinant to the study of soluble protein compartmentation. Int. J. Mol. Sci. 2014, 15, 11030–11039. [Google Scholar] [CrossRef] [Green Version]
- Meins, F.; Sperisen, C.; Neuhaus, J.-M.; Ryals, J. The primary structure of plant pathogenesis-related glucanohydrolases and their genes. In Genes Involved in Plant Defense; Springer: Vienna, Austria, 1992; pp. 245–282. [Google Scholar]
- Li, Z.; Wang, H.; Yin, H.; Bennett, C.; Zhang, H.-G.; Guo, P. Arrowtail RNA for Ligand Display on Ginger Exosome-like Nanovesicles to Systemic Deliver siRNA for Cancer Suppression. Sci. Rep. 2018, 8, 14644. [Google Scholar] [CrossRef] [Green Version]
- Lea, T. Caco-2 cell line. In The Impact of Food Bioactives on Health; Springer: Cham, Switzerland, 2015; pp. 103–111. [Google Scholar]
- Vashisht, M.; Rani, P.; Onteru, S.K.; Singh, D. Curcumin encapsulated in milk exosomes resists human digestion and possesses enhanced intestinal permeability in vitro. Appl. Biochem. Biotechnol. 2017, 183, 993–1007. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Y.; Wang, H.; Zhu, Z.; Xiao, Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J. Cell. Biochem. 2010, 111, 488–496. [Google Scholar] [CrossRef]
- Chakrabarti, R.; Lee, M.; Higgs, H.N. Multiple roles for actin in secretory and endocytic pathways. Curr. Biol. 2021, 31, R603–R618. [Google Scholar] [CrossRef] [PubMed]
- Rennick, J.J.; Johnston, A.P.; Parton, R.G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 2021, 16, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Hua, S. Advances in Oral Drug Delivery for Regional Targeting in the Gastrointestinal Tract—Influence of Physiological, Pathophysiological and Pharmaceutical Factors. Front. Pharmacol. 2020, 11, 00524. [Google Scholar] [CrossRef] [PubMed]
- Ghiasi, M.R.; Rahimi, E.; Amirkhani, Z.; Salehi, R. Leucine-rich Repeat-containing G-protein Coupled Receptor 5 Gene Overexpression of the Rat Small Intestinal Progenitor Cells in Response to Orally Administered Grape Exosome-like Nanovesicles. Adv. Biomed. Res. 2018, 7, 125. [Google Scholar]
- Chen, Y.; Wu, Q.; Zhang, Z.; Yuan, L.; Liu, X.; Zhou, L. Preparation of curcumin-loaded liposomes and evaluation of their skin permeation and pharmacodynamics. Molecules 2012, 17, 5972–5987. [Google Scholar] [CrossRef]
- Jin, H.-H.; Lu, Q.; Jiang, J.-G. Curcumin liposomes prepared with milk fat globule membrane phospholipids and soybean lecithin. J. Dairy Sci. 2016, 99, 1780–1790. [Google Scholar] [CrossRef] [Green Version]
- Lyu, Y.; Xiang, N.; Mondal, J.; Zhu, X.; Narsimhan, G. Characterization of interactions between curcumin and different types of lipid bilayers by molecular dynamics simulation. J. Phys. Chem. B 2018, 122, 2341–2354. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.; Fritz, M.; Brender, J.R.; Smith, P.E.; Lee, D.-K.; Ramamoorthy, A. Determining the effects of lipophilic drugs on membrane structure by solid-state NMR spectroscopy: The case of the antioxidant curcumin. J. Am. Chem. Soc. 2009, 131, 4490–4498. [Google Scholar] [CrossRef] [Green Version]
- Antimisiaris, S.G.; Mourtas, S.; Marazioti, A. Exosomes and exosome-inspired vesicles for targeted drug delivery. Pharmaceutics 2018, 10, 218. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.-G. A novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Kalaydina, R.-V.; Bajwa, K.; Qorri, B.; Decarlo, A.; Szewczuk, M.R. Recent advances in “smart” delivery systems for extended drug release in cancer therapy. Int. J. Nanomed. 2018, 13, 4727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2019, 11, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.J.; van der Velpen, V.; Berends, L.; Jennings, A.; Feelisch, M.; Umpleby, A.M.; Evans, M.; Fernandez, B.O.; Meiss, M.S.; Minnion, M. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome—Results from a 6-month, double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1535–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lätti, A.K.; Riihinen, K.R.; Kainulainen, P.S. Analysis of anthocyanin variation in wild populations of bilberry (Vaccinium myrtillus L. ) in Finland. J. Agric. Food Chem. 2007, 56, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Hassanali, S.; Nugent, C.; Wen, L.; Hamilton-Williams, E.; Dias, P.; Dai, Y.D. Insulinoma-released exosomes or microparticles are immunostimulatory and can activate autoreactive T cells spontaneously developed in nonobese diabetic mice. J. Immunol. 2011, 187, 1591–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estelles, A.; Sperinde, J.; Roulon, T.; Aguilar, B.; Bonner, C.; LePecq, J.B.; Delcayre, A. Exosome nanovesicles displaying G protein-coupled receptors for drug discovery. Int. J. Nanomed. 2007, 2, 751. [Google Scholar]
- Mitchell, J.P.; Court, J.; Mason, M.D.; Tabi, Z.; Clayton, A. Increased exosome production from tumour cell cultures using the Integra CELLine Culture System. J. Immunol. Methods 2008, 335, 98–105. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.N.-G.; Pham, C.V.; Chowdhury, R.; Patel, S.; Jaysawal, S.K.; Hou, Y.; Xu, H.; Jia, L.; Duan, A.; Tran, P.H.-L.; et al. Development of Blueberry-Derived Extracellular Nanovesicles for Immunomodulatory Therapy. Pharmaceutics 2023, 15, 2115. https://doi.org/10.3390/pharmaceutics15082115
Nguyen TN-G, Pham CV, Chowdhury R, Patel S, Jaysawal SK, Hou Y, Xu H, Jia L, Duan A, Tran PH-L, et al. Development of Blueberry-Derived Extracellular Nanovesicles for Immunomodulatory Therapy. Pharmaceutics. 2023; 15(8):2115. https://doi.org/10.3390/pharmaceutics15082115
Chicago/Turabian StyleNguyen, Tuong Ngoc-Gia, Cuong Viet Pham, Rocky Chowdhury, Shweta Patel, Satendra Kumar Jaysawal, Yingchun Hou, Huo Xu, Lee Jia, Andrew Duan, Phuong Ha-Lien Tran, and et al. 2023. "Development of Blueberry-Derived Extracellular Nanovesicles for Immunomodulatory Therapy" Pharmaceutics 15, no. 8: 2115. https://doi.org/10.3390/pharmaceutics15082115
APA StyleNguyen, T. N.-G., Pham, C. V., Chowdhury, R., Patel, S., Jaysawal, S. K., Hou, Y., Xu, H., Jia, L., Duan, A., Tran, P. H.-L., & Duan, W. (2023). Development of Blueberry-Derived Extracellular Nanovesicles for Immunomodulatory Therapy. Pharmaceutics, 15(8), 2115. https://doi.org/10.3390/pharmaceutics15082115





