The Effects of Thermocycling on the Physical Properties and Biocompatibilities of Various CAD/CAM Restorative Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Aging Procedure
2.3. Measurements of Physical Properties
Nanoindentation Hardness and Young’s Modulus (Elastic Modulus)
2.4. Analyses of Surface Properties
2.4.1. Energy Dispersive X-ray Spectroscopy (EDS)
2.4.2. Field-Emission Scanning Electron Microscopy (FE-SEM)
2.4.3. Atomic Force Microscopy (AFM)
2.4.4. Contact Angle Measurements
2.5. Analysis of Biological Properties
2.5.1. Cell Culture of Human Gingival Fibroblasts
2.5.2. Cell Viability Tests with the LIVE/DEAD Assay & Cell Counting Kit-8 (CCK-8) Assay
LIVE/DEAD Assay
CCK-8 Assay
2.5.3. Immunocytochemistry of Cell Spreading and Focal Adhesion
3. Results
3.1. Physical Properties
3.1.1. Nanoindentation Hardness
3.1.2. Young’s Modulus (Elastic Modulus)
3.2. Surface Properties
3.2.1. Energy Dispersive X-ray Spectroscopy (EDS)
3.2.2. Field-Emission Scanning Electron Microscopy (FE-SEM)
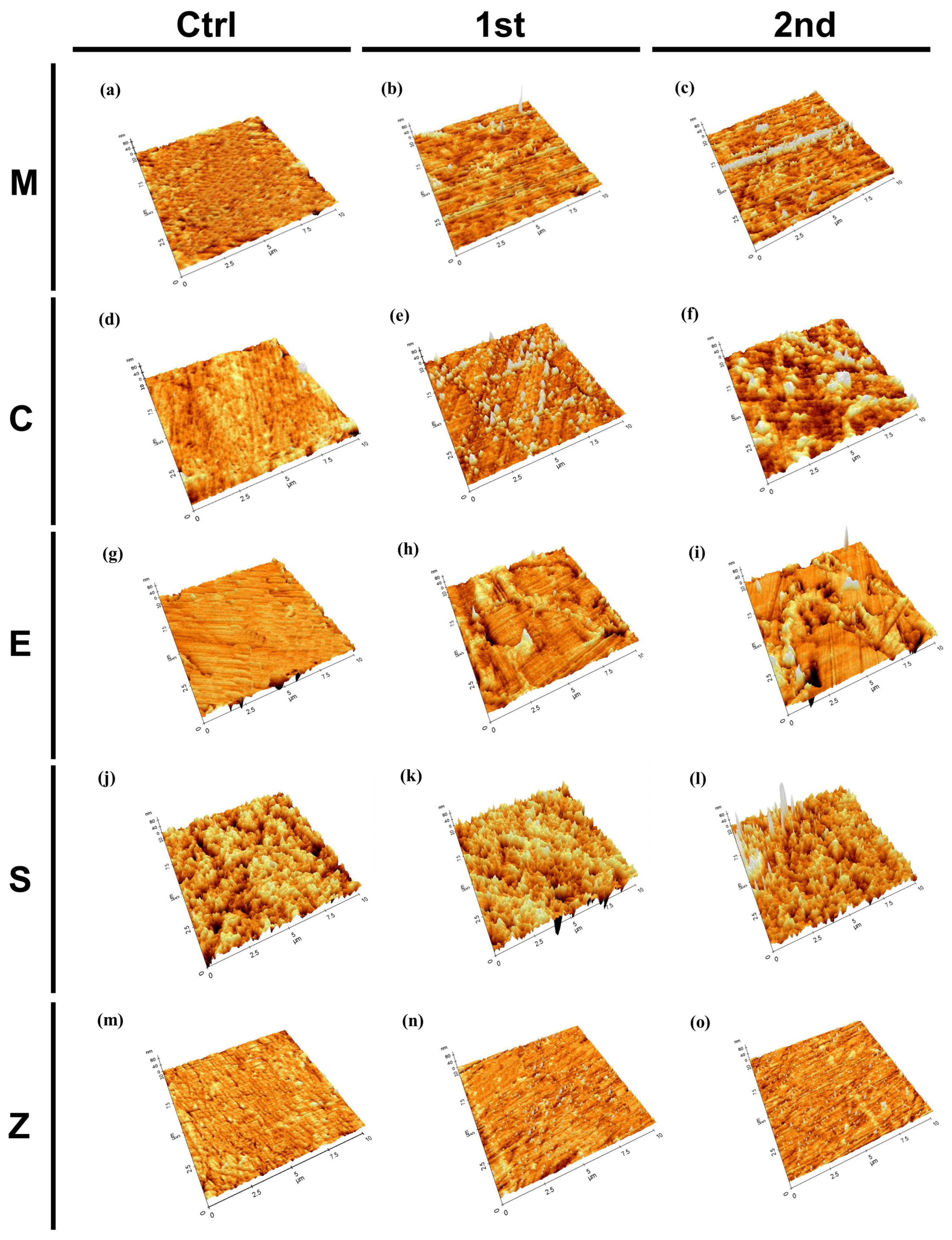
3.2.3. Atomic Force Microscopy (AFM)
3.2.4. Contact Angle Measurements
3.3. Cell Properties
3.3.1. Cell Viability
3.3.2. Immunocytochemistry of Cell Spreading and Focal Adhesion
4. Discussion
5. Conclusions
- i.
- The accelerated aging procedure induced changes in the physical properties of the CAD/CAM materials. The nanoindentation hardnesses and Young’s moduli were decreased after thermocycling aging. Lithium disilicate and hybrid CAD/CAM restorative ceramics tend to be lower than those of highly translucent zirconia before and after aging.
- ii.
- Cell viability and proliferation of the material decreased with aging except for the highly translucent zirconia materials. Significant differences were observed in the viabilities of the HGFs on the CAD/CAM materials. Zirconia-reinforced lithium silicate exhibited significantly lower cell viability than the other materials.
- iii.
- The surface roughness increased with aging for all groups, and the resin nanoceramic showed the highest roughness, while the highly translucent zirconia exhibited the smoothest surface among the materials. After aging, changes were observed in the surface microstructures, compositions, and hydrophilicities.
- iv.
- Cell adhesion and growth on a material surface are influenced by various factors, such as the surface chemical composition, hydrophilicity, roughness, and topography.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paolone, G.; Mandurino, M.; De Palma, F.; Mazzitelli, C.; Scotti, N.; Breschi, L.; Gherlone, E.; Cantatore, G.; Vichi, A. Color Stability of Polymer-Based Composite CAD/CAM Blocks: A Systematic Review. Polymers 2023, 15, 464. [Google Scholar] [CrossRef] [PubMed]
- Kassem, A.S.; Atta, O.; El-Mowafy, O. Fatigue resistance and microleakage of CAD/CAM ceramic and composite molar crowns. J. Prosthodont. 2012, 21, 28–32. [Google Scholar] [CrossRef]
- Okada, K.; Kameya, T.; Ishino, H.; Hayakawa, T. A novel technique for preparing dental CAD/CAM composite resin blocks using the filler press and monomer infiltration method. Dent. Mater. J. 2014, 33, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L.; Berge, H.X.; Condon, J.R. In vitro aging of dental composites in water—Effect of degree of conversion, filler volume, and filler/matrix coupling. J. Biomed. Mater. Res. 1998, 42, 465–472. [Google Scholar] [CrossRef]
- Ruse, N.D.; Sadoun, M.J. Resin-composite blocks for dental CAD/CAM applications. J. Dent. Res. 2014, 93, 1232–1234. [Google Scholar] [CrossRef]
- Choi, B.-J.; Yoon, S.; Im, Y.-W.; Lee, J.-H.; Jung, H.-J.; Lee, H.-H. Uniaxial/biaxial flexure strengths and elastic properties of resin-composite block materials for CAD/CAM. Dent. Mater. 2019, 35, 389–401. [Google Scholar] [CrossRef]
- Castro, E.F.d.; Azevedo, V.L.B.; Nima, G.; Andrade, O.S.d.; Dias, C.T.D.S.; Giannini, M. Adhesion, Mechanical Properties, and Microstructure of Resin-matrix CAD-CAM Ceramics. J. Adhes. Dent. 2020, 22, 421–431. [Google Scholar]
- Belli, R.; Wendler, M.; de Ligny, D.; Cicconi, M.R.; Petschelt, A.; Peterlik, H.; Lohbauer, U. Chairside CAD/CAM materials. Part 1: Measurement of elastic constants and microstructural characterization. Dent. Mater. 2017, 33, 84–98. [Google Scholar] [CrossRef]
- Goujat, A.; Abouelleil, H.; Colon, P.; Jeannin, C.; Pradelle, N.; Seux, D.; Grosgogeat, B. Mechanical properties and internal fit of 4 CAD-CAM block materials. J. Prosthet. Dent. 2018, 119, 384–389. [Google Scholar] [CrossRef]
- Bona, A.D.; Corazza, P.H.; Zhang, Y. Characterization of a polymer-infiltrated ceramic-network material. Dent. Mater. 2014, 30, 564–569. [Google Scholar] [CrossRef]
- Coldea, A.; Swain, M.V.; Thiel, N. Mechanical properties of polymer-infiltrated-ceramic-network materials. Dent. Mater. 2013, 29, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choi, Y.S. Microtensile bond strength and micromorphologic analysis of surface-treated resin nanoceramics. J. Adv. Prosthodont. 2016, 8, 275–284. [Google Scholar] [CrossRef]
- Egilmez, F.; Ergun, G.; Cekic-Nagas, I.; Vallittu, P.K.; Lassila, L.V.J. Does artificial aging affect mechanical properties of CAD/CAM composite materials. J. Prosthodont. Res. 2018, 62, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Lawson, N.C.; Bansal, R.; Burgess, J.O. Wear, strength, modulus and hardness of CAD/CAM restorative materials. Dent. Mater. 2016, 32, e275–e283. [Google Scholar] [CrossRef]
- Nishioka, G.; Prochnow, C.; Firmino, A.; Amaral, M.; Bottino, M.A.; Valandro, L.F. Fatigue strength of several dental ceramics indicated for CAD-CAM monolithic restorations. Braz. Oral Res. 2018, 32, e53. [Google Scholar] [CrossRef] [PubMed]
- Chun, E.P.; Anami, L.C.; Bonfante, E.A.; Bottino, M.A. Microstructural analysis and reliability of monolithic zirconia after simulated adjustment protocols. Dent. Mater. 2017, 33, 934–943. [Google Scholar] [CrossRef]
- Hamza, T.; Alameldin, A.; Elkouedi, A.; Wee, A. Effect of artificial accelerated aging on surface roughness and color stability of different ceramic restorations. Stomatol. Dis. Sci. 2017, 1, 8–13. [Google Scholar] [CrossRef][Green Version]
- Blackburn, C.; Rask, H.; Awada, A. Mechanical properties of resin-ceramic CAD-CAM materials after accelerated aging. J. Prosthet. Dent. 2018, 119, 954–958. [Google Scholar] [CrossRef]
- Pabst, A.M.; Walter, C.; Grassmann, L.; Weyhrauch, M.; Brüllmann, D.D.; Ziebart, T.; Scheller, H.; Lehmann, K.M. Influence of CAD/CAM all-ceramic materials on cell viability, migration ability and adenylate kinase release of human gingival fibroblasts and oral keratinocytes. Clin. Oral Investig. 2014, 18, 1111–1118. [Google Scholar] [CrossRef]
- Urcan, E.; Scherthan, H.; Styllou, M.; Haertel, U.; Hickel, R.; Reichl, F.-X. Induction of DNA double-strand breaks in primary gingival fibroblasts by exposure to dental resin composites. Biomaterials 2010, 31, 2010–2014. [Google Scholar] [CrossRef]
- Jerg, A.; Schulz, S.D.; Tomakidi, P.; Hellwig, E.; Polydorou, O. Modulation of gingival cell response towards dental composites. Dent. Mater. 2018, 34, 412–426. [Google Scholar] [CrossRef]
- Simion, M.; Baldoni, M.; Rossi, P. A study on the attachment of human gingival cell structures to oral implant materials. Int. J. Prosthodont. 1991, 4, 543–547. [Google Scholar] [PubMed]
- Rompen, E.; Domken, O.; Degidi, M.; Farias Pontes, A.E.; Piattelli, A. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: A literature review. Clin. Oral Implants Res. 2006, 17, 55–67. [Google Scholar] [CrossRef]
- Di Nisio, C.; Zara, S.; Cataldi, A.; Di Giacomo, V. 2-Hydroxyethyl methacrylate inflammatory effects in human gingival fibroblasts. Int. Endod. J. 2013, 46, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, A.; Zara, S.; Rapino, M.; Patruno, A.; Di Giacomo, V. Human gingival fibroblasts stress response to HEMA: A role for protein kinase C α. J. Biomed. Mater. Res. A 2013, 101A, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, P.A.N.; Cavalli, V.; Oliveira, M.C.; Barbosa, J.P.; Gomes Boriollo, M.F.; Marcondes Martins, L.R. Influence of surface treatment on the physical properties and biofilm formation of zirconia-reinforced lithium silicate ceramics: In vitro trial. Int. J. Prosthodont. 2021; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Matalon, S.; Safadi, D.; Meirowitz, A.; Ormianer, Z. The effect of aging on the roughness and bacterial adhesion of lithium disilicate and zirconia ceramics. J. Prosthodont. 2021, 30, 440–446. [Google Scholar] [CrossRef]
- Tetè, S.; Zizzari, V.L.; Borelli, B.; De Colli, M.; Zara, S.; Sorrentino, R.; Scarano, A.; Gherlone, E.; Cataldi, A.; Zarone, F. Proliferation and adhesion capability of human gingival fibroblasts onto zirconia, lithium disilicate and feldspathic veneering ceramic in vitro. Dent. Mater. J. 2014, 33, 7–15. [Google Scholar] [CrossRef]
- Al Rezk, F.; Trimpou, G.; Lauer, H.C.; Weigl, P.; Krockow, N. Response of soft tissue to different abutment materials with different surface topographies: A review of the literature. Gen. Dent. 2018, 66, 18–25. [Google Scholar]
- Gale, M.; Darvell, B. Thermal cycling procedures for laboratory testing of dental restorations. J. Dent. 1999, 27, 89–99. [Google Scholar] [CrossRef]
- Palla, E.-S.; Kontonasaki, E.; Kantiranis, N.; Papadopoulou, L.; Zorba, T.; Paraskevopoulos, K.M.; Koidis, P. Color stability of lithium disilicate ceramics after aging and immersion in common beverages. J. Prosthet. Dent. 2018, 119, 632–642. [Google Scholar] [CrossRef]
- Marek, M. Interactions between dental amalgams and the oral environment. Adv. Dent. Res. 1992, 6, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Morena, R.; Beaudreau, G.M.; Lockwood, P.E.; Evans, A.L.; Fairhurst, C.W. Fatigue of dental ceramics in a simulated oral environment. J. Dent. Res. 1986, 65, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Oyafuso, D.K.; Özcan, M.; Bottino, M.A.; Itinoche, M.K. Influence of thermal and mechanical cycling on the flexural strength of ceramics with titanium or gold alloy frameworks. Dent. Mater. 2008, 24, 351–356. [Google Scholar] [CrossRef]
- Morresi, A.L.; D’Amario, M.; Capogreco, M.; Gatto, R.; Marzo, G.; D’Arcangelo, C.; Monaco, A. Thermal cycling for restorative materials: Does a standardized protocol exist in laboratory testing? A literature review. J. Mech. Behav. Biomed. Mater. 2014, 29, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Alamoush, R.A.; Silikas, N.; Salim, N.A.; Al-Nasrawi, S.; Satterthwaite, J.D. Effect of the composition of CAD/CAM composite blocks on mechanical properties. BioMed Res. Int. 2018, 2018, 4893143. [Google Scholar] [CrossRef]
- Porojan, L.; Vasiliu, R.D.; Bîrdeanu, M.I.; Porojan, S.D. Surface characterization and optical properties of reinforced dental glass-ceramics related to artificial aging. Molecules 2020, 25, 3407. [Google Scholar] [CrossRef]
- Toledano, M.; Osorio, R.; Osorio, E.; Fuentes, V.; Prati, C.; García-Godoy, F. Sorption and solubility of resin-based restorative dental materials. J. Dent. Res. 2003, 31, 43–50. [Google Scholar] [CrossRef]
- Rüttermann, S.; Trellenkamp, T.; Bergmann, N.; Raab, W.H.M.; Ritter, H.; Janda, R. A new approach to influence contact angle and surface free energy of resin-based dental restorative materials. Acta Biomater. 2011, 7, 1160–1165. [Google Scholar] [CrossRef]
- Sturz, C.R.; Faber, F.J.; Scheer, M.; Rothamel, D.; Neugebauer, J. Effects of various chair-side surface treatment methods on dental restorative materials with respect to contact angles and surface roughness. Dent. Mater. J. 2015, 34, 796–813. [Google Scholar] [CrossRef]
- Furuhashi, A.; Ayukawa, Y.; Atsuta, I.; Okawachi, H.; Koyano, K. The difference of fibroblast behavior on titanium substrata with different surface characteristics. Odontology 2012, 100, 199–205. [Google Scholar] [CrossRef]
- Yoshinari, M.; Matsuzaka, K.; Inoue, T.; Oda, Y.; Shimono, M.; Health, O.; Center, S. Bio-functionalization of titanium surfaces for dental implants. Mater. Trans. 2002, 43, 2494–2501. [Google Scholar] [CrossRef]
- Yoshinari, M.; Matsuzaka, K.; Inoue, T. Surface modification by cold-plasma technique for dental implants—Bio-functionalization with binding pharmaceuticals. Jpn. Dent. Sci. Rev. 2011, 47, 89–101. [Google Scholar] [CrossRef]
- Rutkunas, V.; Bukelskiene, V.; Sabaliauskas, V.; Balciunas, E.; Malinauskas, M.; Baltriukiene, D. Assessment of human gingival fibroblast interaction with dental implant abutment materials. J. Mater. Sci. Mater. Med. 2015, 26, 169. [Google Scholar] [CrossRef]
- Nothdurft, F.P.; Fontana, D.; Ruppenthal, S.; May, A.; Aktas, C.; Mehraein, Y.; Lipp, P.; Kaestner, L. Differential behavior of fibroblasts and epithelial cells on structured implant abutment materials: A comparison of materials and surface topographies. Clin. Implant Dent. Relat. Res. 2015, 17, 1237–1249. [Google Scholar] [CrossRef]
- Gómez-Florit, M.; Ramis, J.M.; Xing, R.; Taxt-Lamolle, S.; Haugen, H.J.; Lyngstadaas, S.P.; Monjo, M. Differential response of human gingival fibroblasts to titanium- and titanium-zirconium-modified surfaces. J. Periodontal Res. 2014, 49, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, K.; Lopez, B.S.; Hultenby, K.; Wennerberg, A.; Arvidson, K. Attachment and proliferation of human oral fibroblasts to titanium surfaces blasted with TiO2 particles. A scanning electron microscopic and histomorphometric analysis. Clin. Oral Implants Res. 1998, 9, 195–207. [Google Scholar] [CrossRef]
- Hajhamid, B.; Bozec, L.; Tenenbaum, H.; De Souza, G.; Somogyi-Ganss, E. Effect of artificial aging on optical properties and crystalline structure of high-translucency zirconia. J. Prosthodont. 2023; early view. [Google Scholar] [CrossRef]
- Dal Piva, A.; Contreras, L.; Ribeiro, F.; Anami, L.; Camargo, S.; Jorge, A.; Bottino, M. Monolithic ceramics: Effect of finishing techniques on surface properties, bacterial adhesion and cell viability. Oper. Dent. 2018, 43, 315–325. [Google Scholar] [CrossRef]
- Thievessen, I.; Thompson, P.M.; Berlemont, S.; Plevock, K.M.; Plotnikov, S.V.; Zemljic-Harpf, A.; Ross, R.S.; Davidson, M.W.; Danuser, G.; Campbell, S.L.; et al. Vinculin-actin interaction couples actin retrograde flow to focal adhesions, but is dispensable for focal adhesion growth. J. Cell Biol. 2013, 202, 163–177. [Google Scholar] [CrossRef]
- Fischer, N.G.; Wong, J.; Baruth, A.; Cerutis, D.R. Effect of clinically relevant CAD/CAM Zirconia polishing on gingival fibroblast proliferation and focal adhesions. Materials 2017, 10, 1358. [Google Scholar] [CrossRef]
- Kunzler, T.P.; Drobek, T.; Schuler, M.; Spencer, N.D. Systematic study of osteoblast and fibroblast response to roughness by means of surface-morphology gradients. Biomaterials 2007, 28, 2175–2182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, G.; Cai, Y.; Monkley, S.J.; Critchley, D.R.; Sheetz, M.P. Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat. Cell Biol. 2008, 10, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.T.; Sharek, L.; O’Brien, E.T.; Urbina, F.L.; Gupton, S.L.; Superfine, R.; Burridge, K.; Campbell, S.L. Vinculin and metavinculin exhibit distinct effects on focal adhesion properties, cell migration, and mechanotransduction. PLoS ONE 2019, 14, e0221962. [Google Scholar] [CrossRef]
- Zarone, F.; Di Mauro, M.I.; Ausiello, P.; Ruggiero, G.; Sorrentino, R. Current status on lithium disilicate and zirconia: A narrative review. BMC Oral Health 2019, 19, 134. [Google Scholar] [CrossRef]
- Skorulska, A.; Piszko, P.; Rybak, Z.; Szymonowicz, M.; Dobrzyński, M. Review on polymer, ceramic and composite materials for cad/cam indirect restorations in dentistry—Application, mechanical characteristics and comparison. Materials 2021, 14, 1592. [Google Scholar] [CrossRef] [PubMed]






| Product Name | Manufacturer | Shade (Lot No.) | Composition |
|---|---|---|---|
| IPS e.max CAD | Ivoclar Vivadent | 2M2-HT (Y00999, Y26950) | 0.2–2 μm lithium disilicate glass-ceramic (LS2, lithium disilicate) |
| Celtra Duo | Dentsply Sirona | A2-HT (16006746, 16006750) | silica-based glass matrix with 10% dissolved zirconia (ZLS, zirconia reinforced lithium silicate) |
| Vita Enamic | Vita Zahnfabrik | A2-HT (78540, 78880) | 86 wt% mass percentage of inorganic ceramic network with 14 wt% of the organic polymer network infiltrated (PICN, polymer-infiltrated ceramic network) |
| Cerasmart | GC Corporation | A2-HT (1910101) | Polymer matrix (Bis-MEPP, UDMA, DMA) with 71 wt% silica and barium glass nanoparticles (RNC, resin nanoceramic) |
| LavaTM Plus Zirconia | 3 M ESPE | HT (260614) | Yttrium-stabilized zirconium dioxide crystals (Zirconia, high translucency zirconia) |
| Product Name | Material Code | Groups | ||
|---|---|---|---|---|
| Control | 1st Aged | 2nd Aged | ||
| IPS e.max CAD | M | MC | MAF | MAS |
| Celtra Duo | C | CC | CAF | CAS |
| Vita Enamic | E | EC | EAF | EAS |
| Cerasmart | S | SC | SAF | SAS |
| LavaTM plus zirconia | Z | ZC | ZAF | ZAS |
| Elemental Analyses (wt%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Materials | Group | C | O | Al | Si | P | K | Zr | Na | Ba |
| M | MC | 5.90 ± 0.51 a | 54.10 ± 0.28 a | 0.81 ± 0.03 a | 33.70 ± 0.30 a | 1.51 ± 0.05 a | 3.98 ± 0.05 a | - | - | - |
| MAF | 4.11 ± 0.57 b | 55.02 ± 0.33 b | 0.85 ± 0.03 b | 34.59 ± 0.34 b | 1.51 ± 0.09 a | 3.93 ± 0.07 b | - | - | - | |
| MAS | 3.78 ± 0.68 b | 56.62 ± 3.83 c | 0.81 ± 0.08 a | 33.66 ± 3.47 a | 1.51 ± 0.20 a | 3.62 ± 0.64 c | - | - | - | |
| C | CC | 5.63 ± 0.62 a | 45.99 ± 0.49 a | 1.04 ± 0.03 a | 27.52 ± 0.22 a | 1.53 ± 0.07 a | 1.67 ± 0.03 a | 16.33 ± 0.35 a | - | - |
| CAF | 4.32 ±0.93 b | 45.59 ± 0.57 b | 1.07 ± 0.03 b | 28.45 ± 0.42 b | 1.59 ± 0.10 b | 1.66 ± 0.06 a | 17.31 ± 0.32 b | - | - | |
| CAS | 7.30 ± 0.65 c | 52.16 ± 0.42 c | 1.11 ± 0.05 c | 27.65 ± 0.32 a | 2.52 ± 0.10 c | 1.58 ± 0.05 b | 7.69 ± 0.47 c | - | - | |
| E | EC | 19.80 ± 1.14 a | 38.80 ± 0.52 a | 9.42 ± 0.24 a | 22.87 ± 0.63 a | - | - | - | 4.19 ± 0.17 a | - |
| EAF | 18.97 ± 0.96 b | 38.89 ± 0.34 a | 9.58± 0.16 b | 23.37 ± 0.47 b | - | - | - | 4.31 ± 0.08 b | - | |
| EAS | 25.19 ± 4.65 c | 42.70 ± 2.53 b | 7.40 ± 1.36 c | 23.37 ± 0.47 b | - | - | - | 3.24 ± 2.16 c | - | |
| S | SC | 25.45 ± 0.32 a | 33.05 ± 0.40 a | 3.32 ± 0.10 a | 19.61 ± 0.41 a | - | - | - | - | 18.57 ± 0.18 a |
| SAF | 25.92 ± 0.45 b | 32.90 ± 0.78 a | 3.29 ± 0.07 b | 19.73 ± 0.52 a | - | - | - | - | 18.17 ± 0.60 b | |
| SAS | 26.17 ± 0.41 a | 32.97 ± 0.88 a | 3.25 ± 0.05 a | 19.46 ± 0.61 b | - | - | - | - | 18.15 ± 0.61 a | |
| Z | ZC | 6.38 ± 0.32 a | 25.29 ± 0.16 a | 3.03 ± 0.15 a | - | - | - | 65.29 ± 0.16 a | - | - |
| ZAF | 7.05 ± 0.61 b | 25.03 ± 0.28 b | 3.01 ± 0.07 a, b | - | - | - | 64.90 ± 0.39 b | - | - | |
| ZAS | 8.67 ± 0.76 c | 25.38 ± 0.28 a | 2.88 ± 0.25 b | - | - | - | 63.06 ± 0.55 c | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-Y.; Bae, H.-J.; Lee, H.-H.; Lee, J.-H.; Kim, Y.-J.; Choi, Y.-S.; Lee, J.-H.; Shin, S.-Y. The Effects of Thermocycling on the Physical Properties and Biocompatibilities of Various CAD/CAM Restorative Materials. Pharmaceutics 2023, 15, 2122. https://doi.org/10.3390/pharmaceutics15082122
Kim S-Y, Bae H-J, Lee H-H, Lee J-H, Kim Y-J, Choi Y-S, Lee J-H, Shin S-Y. The Effects of Thermocycling on the Physical Properties and Biocompatibilities of Various CAD/CAM Restorative Materials. Pharmaceutics. 2023; 15(8):2122. https://doi.org/10.3390/pharmaceutics15082122
Chicago/Turabian StyleKim, Se-Young, Han-Jin Bae, Hae-Hyoung Lee, Jong-Hyuk Lee, Yu-Jin Kim, Yu-Sung Choi, Jung-Hwan Lee, and Soo-Yeon Shin. 2023. "The Effects of Thermocycling on the Physical Properties and Biocompatibilities of Various CAD/CAM Restorative Materials" Pharmaceutics 15, no. 8: 2122. https://doi.org/10.3390/pharmaceutics15082122
APA StyleKim, S.-Y., Bae, H.-J., Lee, H.-H., Lee, J.-H., Kim, Y.-J., Choi, Y.-S., Lee, J.-H., & Shin, S.-Y. (2023). The Effects of Thermocycling on the Physical Properties and Biocompatibilities of Various CAD/CAM Restorative Materials. Pharmaceutics, 15(8), 2122. https://doi.org/10.3390/pharmaceutics15082122








