Improving Riparin-A Dissolution through a Laponite Based Nanohybrid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Factorial Design for Preliminary Investigation of Riparin-A Solubility
2.3. Solubility Studies
2.4. Preparation of the Physical Mixture and Nanohybrid
2.5. Solid State Characterization of Physical Mixture, Nanohybrid and Raw Materials
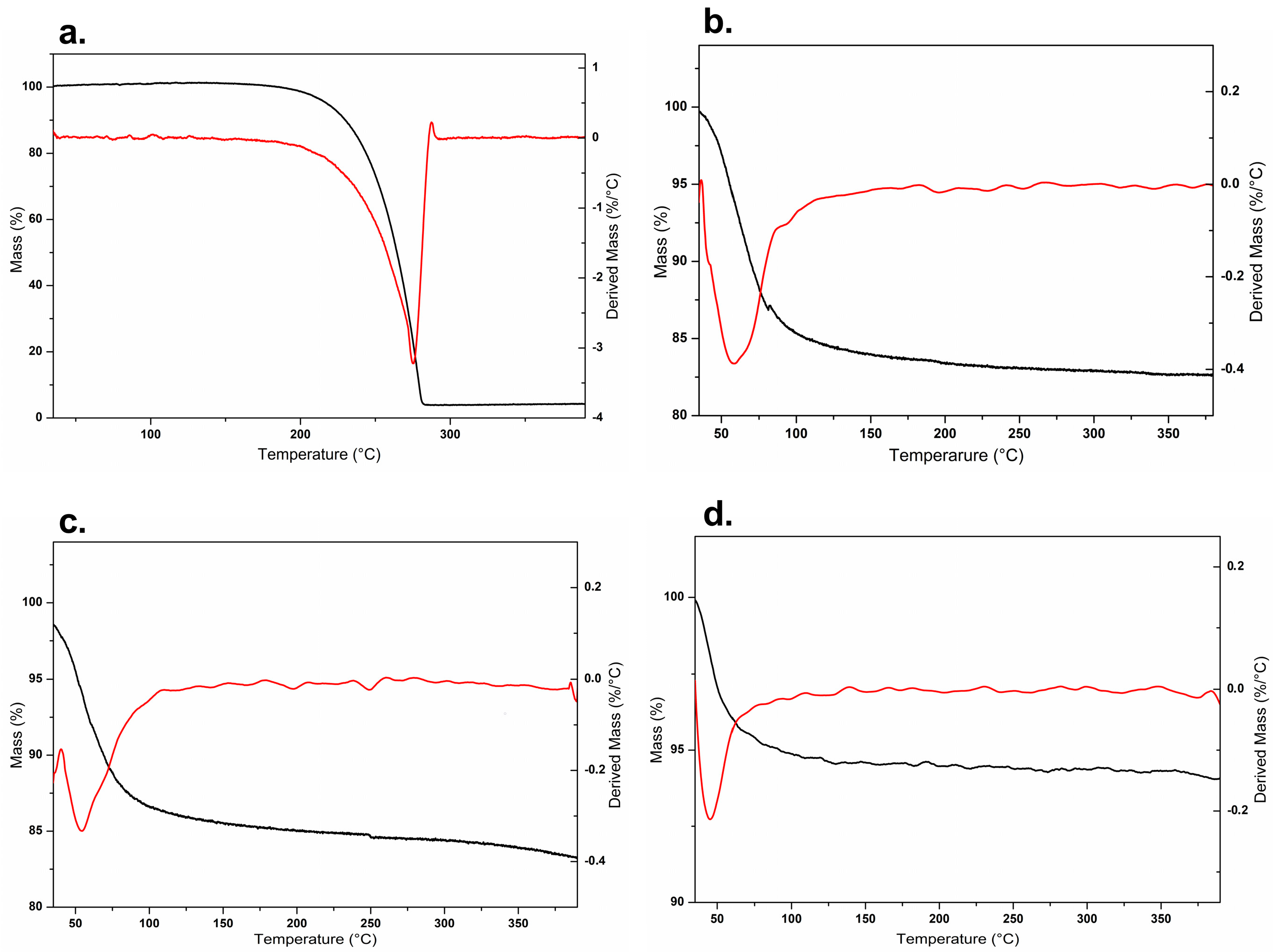
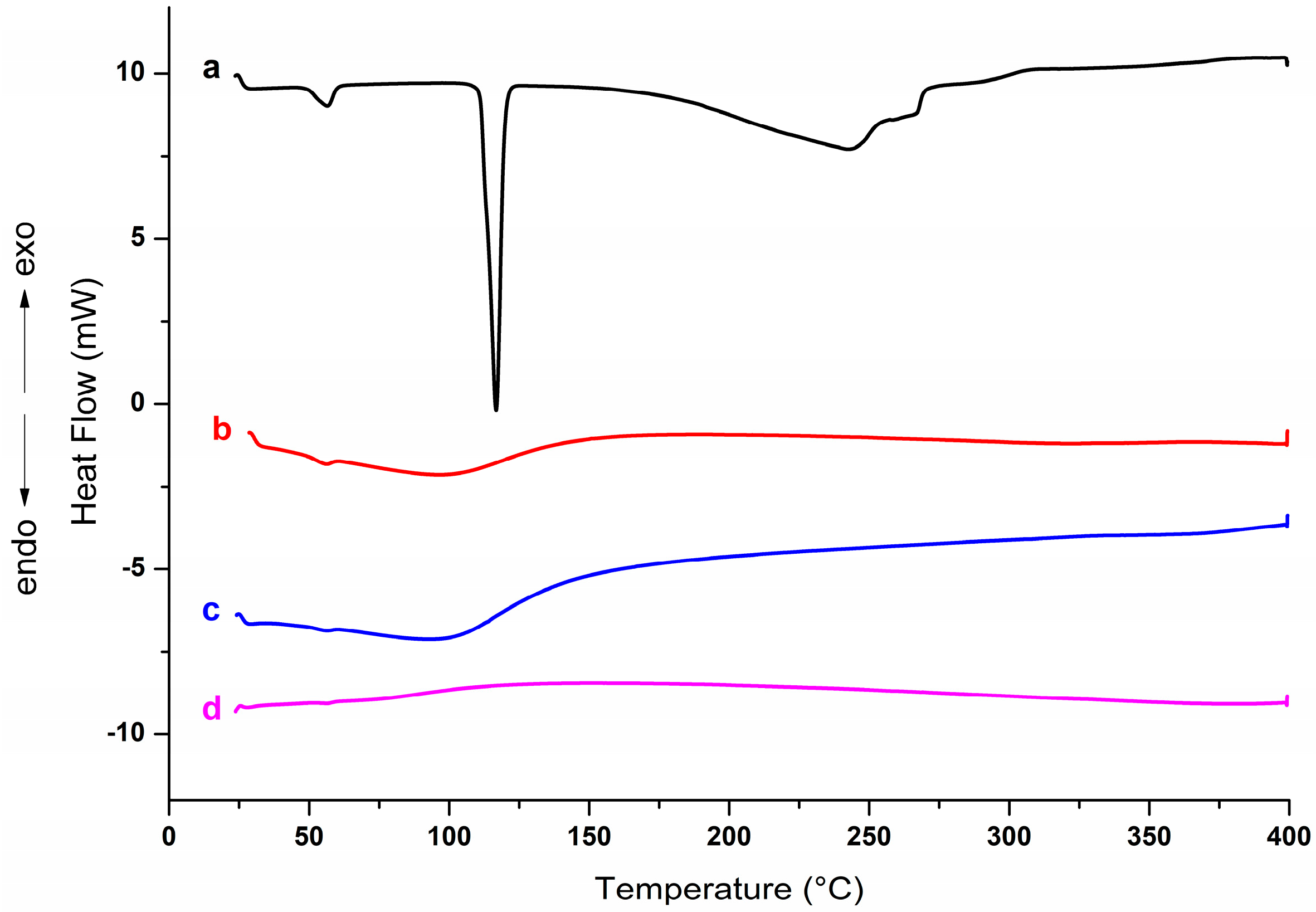
2.5.1. Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
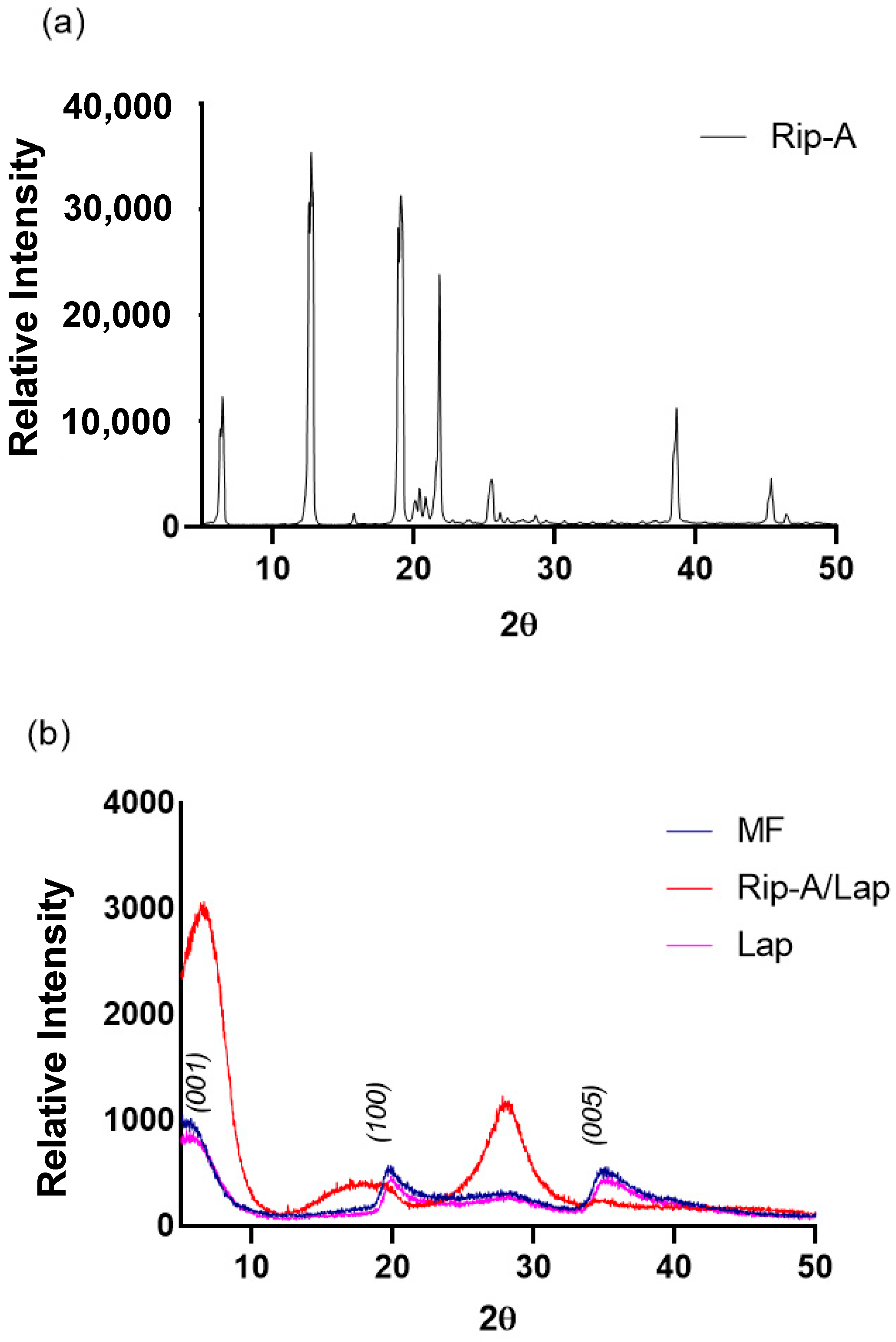
2.5.2. X-ray Powder Diffraction (XRPD)
2.5.3. Fourier-Transform Infrared (FTIR) Spectroscopy
2.6. In Vitro Dissolution Study
2.7. In Vitro Cytotoxicity on Tumor and Normal Cells
2.8. Statistical Analysis
3. Results and Discussion
3.1. Factorial Design for Preliminary Investigation of Riparin—A Solubility
3.2. Solid State Characterization of Physical Mixture, Nanohybrid and Raw Materials
3.2.1. Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
3.2.2. X-ray Powder Diffraction (XRPD)
3.2.3. Fourier-Transform Infrared (FTIR) Spectroscopy
3.3. In Vitro Dissolution Study
3.4. In Vitro Cytotoxicity on Tumor and Normal Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balunas, M.J.; Kinghorn, A.D. Drug Discovery from Medicinal Plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef]
- Barbosa Filho, J.M.; Silva, E.C.; Bhattacharyya, J. Synthesis of Several New Phenylethylamides of Substituted Benzoic Acids. Quim. Nova 1990, 13, 332–334. [Google Scholar]
- Nunes, G.B.L.; Policarpo, P.R.; Costa, L.M.; da Silva, T.G.; Militão, G.C.G.; Câmara, C.A.; Barbosa Filho, J.M.; Gutierrez, S.J.C.; Islam, M.T.; de Freitas, R.M. In Vitro Antioxidant and Cytotoxic Activity of Some Synthetic Riparin-Derived Compounds. Molecules 2014, 19, 4595–4607. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, C.R.d.S.; Bezerra, A.H.; Bezerra, S.R.; Macêdo, N.S.; Oliveira-Tintino, C.D.d.M.; Costa, J.G.M.d.; Coutinho, H.D.M.; dos Santos, H.S.; Cunha, F.A.B. da Bioactivities of Isolated and Synthetic Riparins of Aniba Riparia (NEES) MEZ (LAURACEAE): A Brief Review. Phytochem. Lett. 2022, 52, 149–160. [Google Scholar] [CrossRef]
- Costa, L.M.; Macedo, E.V.; Oliveira, F.A.A.; Ferreira, J.H.L.; Gutierrez, S.J.C.; Peláez, W.J.; Lima, F.C.A.; de Siqueira Júnior, J.P.; Coutinho, H.D.M.; Kaatz, G.W.; et al. Inhibition of the NorA Efflux Pump of Staphylococcus Aureus by Synthetic Riparins. J. Appl. Microbiol. 2016, 121, 1312–1322. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.O.; Damasceno, S.R.B.; Silva, I.S.; Silva, V.G.; Brito, C.F.C.; Teixeira, A.É.A.; Nunes, G.B.L.; Camara, C.A.; Filho, J.M.B.; Gutierrez, S.J.C.; et al. Riparin A, a Compound from Aniba Riparia, Attenuate the Inflammatory Response by Modulation of Neutrophil Migration. Chem. Biol. Interact. 2015, 229, 55–63. [Google Scholar] [CrossRef]
- Araújo, É.J.F.d.; Rezende-Júnior, L.M.; Lima, L.K.F.; Silva-Júnior, M.P.d.; Silva, O.A.; Sousa Neto, B.P.d.; Almeida, A.A.C.d.; Gutierrez, S.J.C.; Tomé, A.d.R.; Lopes, L.d.S.; et al. Pathophysiological Investigations, Anxiolytic Effects and Interaction of a Semisynthetic Riparin with Benzodiazepine Receptors. Biomed. Pharmacother. 2018, 103, 973–981. [Google Scholar] [CrossRef]
- Araújo, É.J.F.d.; Lima, L.K.F.; Silva, O.A.; Júnior, L.M.R.; Gutierrez, S.J.C.; Carvalho, F.A.d.A.; Lima, F.d.C.A.; Pessoa, C.; Freitas, R.M.d.; Ferreira, P.M.P. In Vitro Antioxidant, Antitumor and Leishmanicidal Activity of Riparin A, an Analog of the Amazon Alkamides from Aniba Riparia (Lauraceae). Acta Amaz. 2016, 46, 309–314. [Google Scholar] [CrossRef] [Green Version]
- Nunes, G.B.L.; Costa, L.M.; Gutierrez, S.J.C.; Satyal, P.; de Freitas, R.M. Behavioral Tests and Oxidative Stress Evaluation in Mitochondria Isolated from the Brain and Liver of Mice Treated with Riparin A. Life Sci. 2015, 121, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo, K.A.; Magalhães Costa, R.K.; Rocha, J.A.; Chavez Gutierrez, S.J.; Ramos, R.M.; Muálem de Moraes Alves, M.; Aécio de Amorim Carvalho, F.; Menezes Carvalho, A.L.; Lima, F. das C.A. Antileishmanial Activity of Riparin Structural Analogs of Aniba Riparia: Biological Evaluation, in Silico Adme-Tox, and Molecular Docking. Exp. Parasitol. 2022, 236–237, 108257. [Google Scholar] [CrossRef] [PubMed]
- Araújo, É.J.F.d.; Silva, O.A.; Rezende-Júnior, L.M.; Sousa, I.J.O.; Araújo, D.Y.M.L.d.; Carvalho, R.B.F.d.; Pereira, S.T.; Gutierrez, S.J.C.; Ferreira, P.M.P.; Lima, F.d.C.A. Synthesis, Characterization and Cytotoxic Evaluation of Inclusion Complexes between Riparin A and β-Cyclodextrin. J. Mol. Struct. 2017, 1142, 84–91. [Google Scholar] [CrossRef]
- Jung, H.; Kim, H.-M.; Choy, Y.B.; Hwang, S.-J.; Choy, J.-H. Itraconazole–Laponite: Kinetics and Mechanism of Drug Release. Appl. Clay Sci. 2008, 40, 99–107. [Google Scholar] [CrossRef]
- Lainé, A.-L.; Price, D.; Davis, J.; Roberts, D.; Hudson, R.; Back, K.; Bungay, P.; Flanagan, N. Enhanced Oral Delivery of Celecoxib via the Development of a Supersaturable Amorphous Formulation Utilising Mesoporous Silica and Co-Loaded HPMCAS. Int. J. Pharm. 2016, 512, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Kovvasu, S.P.; Kunamaneni, P.; Yeung, S.; Rueda, J.; Betageri, G. V Formulation of Dronedarone Hydrochloride-Loaded Proliposomes: In Vitro and In Vivo Evaluation Using Caco-2 and Rat Model. AAPS PharmSciTech 2019, 20, 226. [Google Scholar] [CrossRef]
- Cunha, V.R.R.; Lima, F.C.D.A.; Sakai, V.Y.; Véras, L.M.C.; Leite, J.R.S.A.; Petrilli, H.M.; Constantino, V.R.L. LAPONITE®-Pilocarpine Hybrid Material: Experimental and Theoretical Evaluation of Pilocarpine Conformation. RSC Adv. 2017, 7, 27290–27298. [Google Scholar] [CrossRef]
- Yang, H.; Hua, S.; Wang, W.; Wang, A. Composite Hydrogel Beads Based on Chitosan and Laponite: Preparation, Swelling, and Drug Release Behaviour. Iran. Polym. J. 2011, 20, 479–490. [Google Scholar]
- Kiaee, G.; Dimitrakakis, N.; Sharifzadeh, S.; Kim, H.J.; Avery, R.K.; Moghaddam, K.M.; Haghniaz, R.; Yalcintas, E.P.; Barros, N.R.d.; Karamikamkar, S.; et al. Laponite-Based Nanomaterials for Drug Delivery. Adv. Healthc. Mater. 2022, 11, 2102054. [Google Scholar] [CrossRef]
- Tomás, H.; Alves, C.S.; Rodrigues, J. Laponite®: A Key Nanoplatform for Biomedical Applications? Nanomedicine 2018, 14, 2407–2420. [Google Scholar] [CrossRef] [PubMed]
- Aguzzi, C.; Cerezo, P.; Viseras, C.; Caramella, C. Use of Clays as Drug Delivery Systems: Possibilities and Limitations. Appl. Clay Sci. 2007, 36, 22–36. [Google Scholar] [CrossRef]
- Viseras, C.; Cerezo, P.; Sanchez, R.; Salcedo, I.; Aguzzi, C. Current Challenges in Clay Minerals for Drug Delivery. Appl. Clay Sci. 2010, 48, 291–295. [Google Scholar] [CrossRef]
- Gonçalves, M.; Figueira, P.; Maciel, D.; Rodrigues, J.; Qu, X.; Liu, C.; Tomás, H.; Li, Y. PH-Sensitive Laponite(®)/Doxorubicin/Alginate Nanohybrids with Improved Anticancer Efficacy. Acta Biomater. 2014, 10, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Dening, T.J.; Thomas, N.; Rao, S.; van Looveren, C.; Cuyckens, F.; Holm, R.; Prestidge, C.A. Montmorillonite and Laponite Clay Materials for the Solidification of Lipid-Based Formulations for the Basic Drug Blonanserin: In Vitro and in Vivo Investigations. Mol. Pharm. 2018, 15, 4148–4160. [Google Scholar] [CrossRef] [PubMed]
- Úriz, A.; Sanmartín, C.; Plano, D.; de Melo Barbosa, R.; Dreiss, C.A.; González-Gaitano, G. Activity Enhancement of Selective Antitumoral Selenodiazoles Formulated with Poloxamine Micelles. Colloids Surf. B Biointerfaces 2018, 170, 463–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Wang, Y.; Fu, Q.; Guo, Z.; Sun, B.; Liu, W.; Liu, Y.; Mu, S.; Guo, M.; Li, J.; et al. The Role of Particle Size of Glyburide Crystals in Improving Its Oral Absorption. Drug Deliv. Transl. Res. 2017, 7, 428–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, T.; Yamaguchi, M. Host-Guest Interactions between Swelling Clay Minerals and Poorly Water-Soluble Drugs. J. Colloid. Interface Sci. 1991, 146, 556–564. [Google Scholar] [CrossRef]
- Gutierrez, S.J.C. Síntese Do Bowdenol Um Dihidrobenzofuranoide Isolado de Bowdichia Virgilioides e Preparação de Derivados Da Riparina Isolada de Aniba Riparia Com Potencial Atividade Biológica. Ph.D. Thesis, Universidade Federal da Paraíba, João Pessoa, Brazil, 2006. [Google Scholar]
- Willenbacher, N. Unusual Thixotropic Properties of Aqueous Dispersions of Laponite RD. J. Colloid. Interface Sci. 1996, 182, 501–510. [Google Scholar] [CrossRef]
- Poozesh, S.; Bilgili, E. Scale-up of Pharmaceutical Spray Drying Using Scale-up Rules: A Review. Int. J. Pharm. 2019, 562, 271–292. [Google Scholar] [CrossRef]
- Oliveira, M.A.d.; Yoshida, M.I.; Lima Gomes, E.C. de Análise Térmica Aplicada a Fármacos e Formulações Farmacêuticas Na Indústria Farmacêutica. Quim. Nova 2011, 34, 1224–1230. [Google Scholar] [CrossRef]
- Daniel, L.M.; Frost, R.L.; Zhu, H.Y. Edge-Modification of Laponite with Dimethyl-Octylmethoxysilane. J. Colloid. Interface Sci. 2008, 321, 302–309. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Gao, Z.; Liu, K.; Pan, W.-P.; Vaia, R.; Hunter, D.; Singh, A. Thermal Characterization of Organically Modified Montmorillonite. Thermochim. Acta 2001, 367–368, 339–350. [Google Scholar] [CrossRef]
- Capello, C.; Leandro, G.C.; Maduro Campos, C.E.; Hotza, D.; Mattar Carciofi, B.A.; Valencia, G.A. Adsorption and Desorption of Eggplant Peel Anthocyanins on a Synthetic Layered Silicate. J. Food Eng. 2019, 262, 162–169. [Google Scholar] [CrossRef]
- Teixeira-Neto, Â.A.; Izumi, C.M.S.; Temperini, M.L.A.; Ferreira, A.M.d.C.; Constantino, V.R.L. Hybrid Materials Based on Smectite Clays and Nutraceutical Anthocyanins from the Açaí Fruit. Eur. J. Inorg. Chem. 2012, 2012, 5411–5420. [Google Scholar] [CrossRef]
- Zhou, B.; Wu, B.; Wang, J.; Qian, Q.; Wang, J.; Xu, H.; Yang, S.; Feng, P.; Chen, W.; Li, Y.; et al. Drug-Mediation Formation of Nanohybrids for Sequential Therapeutic Delivery in Cancer Cells. Colloids Surf. B Biointerfaces 2018, 163, 284–290. [Google Scholar] [CrossRef]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Khan, K.A. The Concept of Dissolution Efficiency. J. Pharm. Pharmacol. 1975, 27, 48–49. [Google Scholar] [CrossRef] [PubMed]
- Câmara, G.B.M.; Barbosa, R.d.M.; García-Villén, F.; Viseras, C.; Almeida Júnior, R.F.d.; Machado, P.R.L.; Câmara, C.A.; Farias, K.J.S.; de Lima e Moura, T.F.A.; Dreiss, C.A.; et al. Nanocomposite Gels of Poloxamine and Laponite for β-Lapachone Release in Anticancer Therapy. Eur. J. Pharm. Sci. 2021, 163, 105861. [Google Scholar] [CrossRef]
- Mendes, L.P.; Delgado, J.M.F.; Costa, A.D.A.; Vieira, M.S.; Benfica, P.L.; Lima, E.M.; Valadares, M.C. Biodegradable Nanoparticles Designed for Drug Delivery: The Number of Nanoparticles Impacts on Cytotoxicity. Toxicol. Vitr. 2015, 29, 1268–1274. [Google Scholar] [CrossRef] [Green Version]
- Berridge, M.V.; Tan, A.S.; McCoy, K.D.; Wang, R. The Biochemical and Cellular Basis of Cell Proliferation Assays That Use Tetrazolium Salts. Biochemica 1996, 4, 15–20. [Google Scholar]
- Peraro, G.R.; Donzelli, E.H.; Oliveira, P.F.; Tavares, D.C.; Gomes Martins, C.H.; Molina, E.F.; de Faria, E.H. Aminofunctionalized LAPONITE® as a Versatile Hybrid Material for Chlorhexidine Digluconate Incorporation: Cytotoxicity and Antimicrobial Activities. Appl. Clay Sci. 2020, 195, 105733. [Google Scholar] [CrossRef]
- Ghadiri, M.; Chrzanowski, W.; Lee, W.H.; Fathi, A.; Dehghani, F.; Rohanizadeh, R. Physico-Chemical, Mechanical and Cytotoxicity Characterizations of Laponite®/Alginate Nanocomposite. Appl. Clay Sci. 2013, 85, 64–73. [Google Scholar] [CrossRef]
- Massaro, M.; Buscemi, G.; Arista, L.; Biddeci, G.; Cavallaro, G.; D’Anna, F.; Di Blasi, F.; Ferrante, A.; Lazzara, G.; Rizzo, C.; et al. Multifunctional Carrier Based on Halloysite/Laponite Hybrid Hydrogel for Kartogenin Delivery. ACS Med. Chem. Lett. 2019, 10, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Tyson, C.A.; Mitoma, C.; Kalivoda, J. Evaluation of Hepatocytes Isolated by a Nonperfusion Technique in a Prescreen for Cytotoxicity. J. Toxicol. Environ. Health 1980, 6, 197–205. [Google Scholar] [CrossRef] [PubMed]


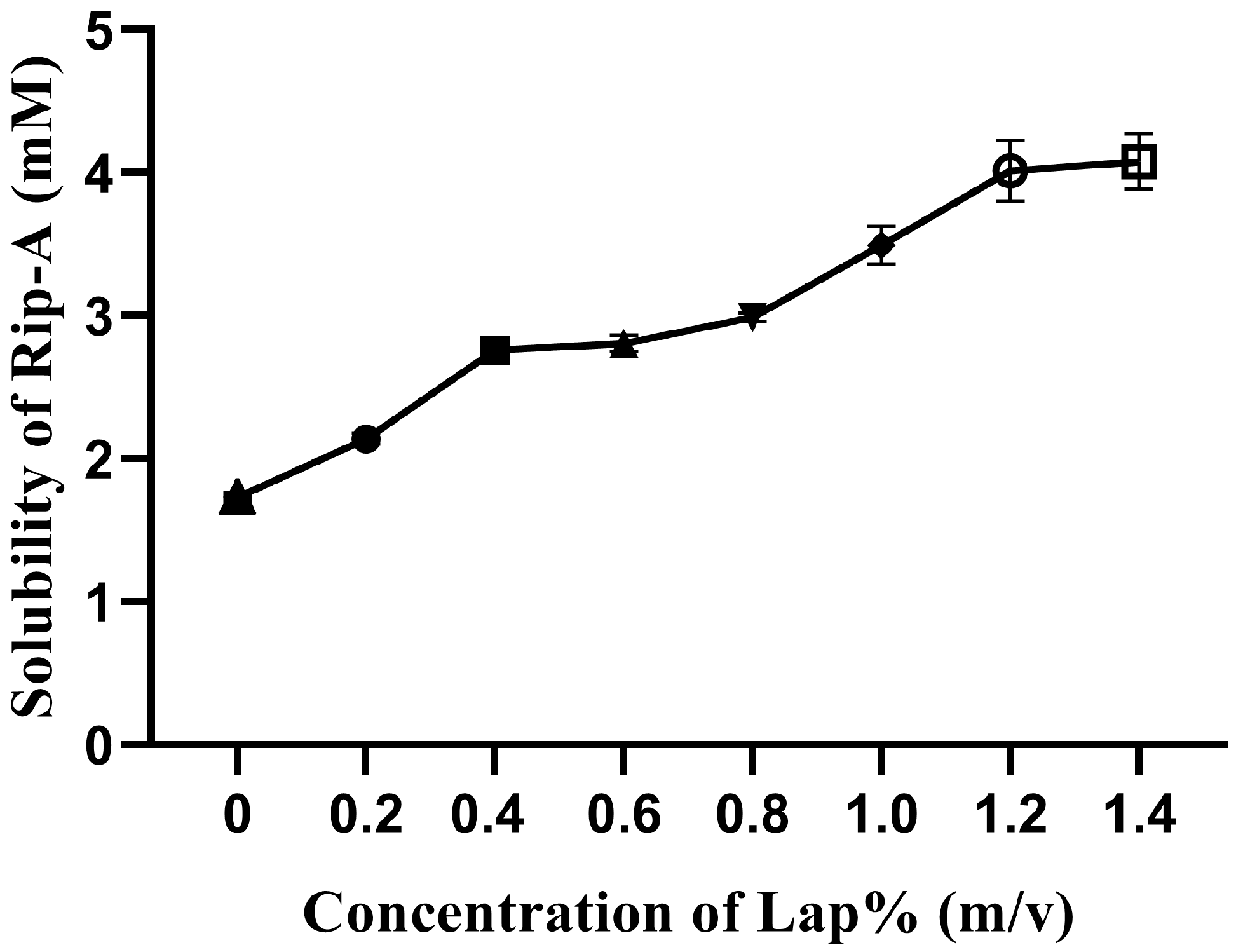



| Factors | Levels |
|---|---|
| A: Laponite concentration (%) | 0.0 0.4 0.8 |
| B: Stirring time (hour) | 24 48 72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, D.M.; Meirelles, L.M.A.; Araujo, P.M.; de Sousa, R.W.R.; Ferreira, P.M.P.; Gutierrez, S.J.C.; de Medeiros, M.d.G.F.; Raffin, F.N. Improving Riparin-A Dissolution through a Laponite Based Nanohybrid. Pharmaceutics 2023, 15, 2136. https://doi.org/10.3390/pharmaceutics15082136
Gomes DM, Meirelles LMA, Araujo PM, de Sousa RWR, Ferreira PMP, Gutierrez SJC, de Medeiros MdGF, Raffin FN. Improving Riparin-A Dissolution through a Laponite Based Nanohybrid. Pharmaceutics. 2023; 15(8):2136. https://doi.org/10.3390/pharmaceutics15082136
Chicago/Turabian StyleGomes, Duanne Mendes, Lyghia Maria Araújo Meirelles, Paulo Monteiro Araujo, Rayran Walter Ramos de Sousa, Paulo Michel Pinheiro Ferreira, Stanley Juan Chavez Gutierrez, Maria das Graças Freire de Medeiros, and Fernanda Nervo Raffin. 2023. "Improving Riparin-A Dissolution through a Laponite Based Nanohybrid" Pharmaceutics 15, no. 8: 2136. https://doi.org/10.3390/pharmaceutics15082136





