Breast Milk Mesenchymal Stem Cells and/or Derived Exosomes Mitigated Adenine-Induced Nephropathy via Modulating Renal Autophagy and Fibrotic Signaling Pathways and Their Epigenetic Regulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Isolation and Identification of Breast Milk Mesenchymal Stem Cells (Br-MSCs)
2.3. Isolation, Identification, and Electron Microscopy of Breast Milk Mesenchymal Stem Cell Exosomes
2.4. Experimental Design
2.5. Administration of Br-MSCs and Br-MSCs-EXOs
2.6. Biochemical Analysis
2.7. Real-Time PCR
2.8. Histopathological Examination
2.9. Immunohistochemical Analysis
2.10. Statistical Analysis
3. Results
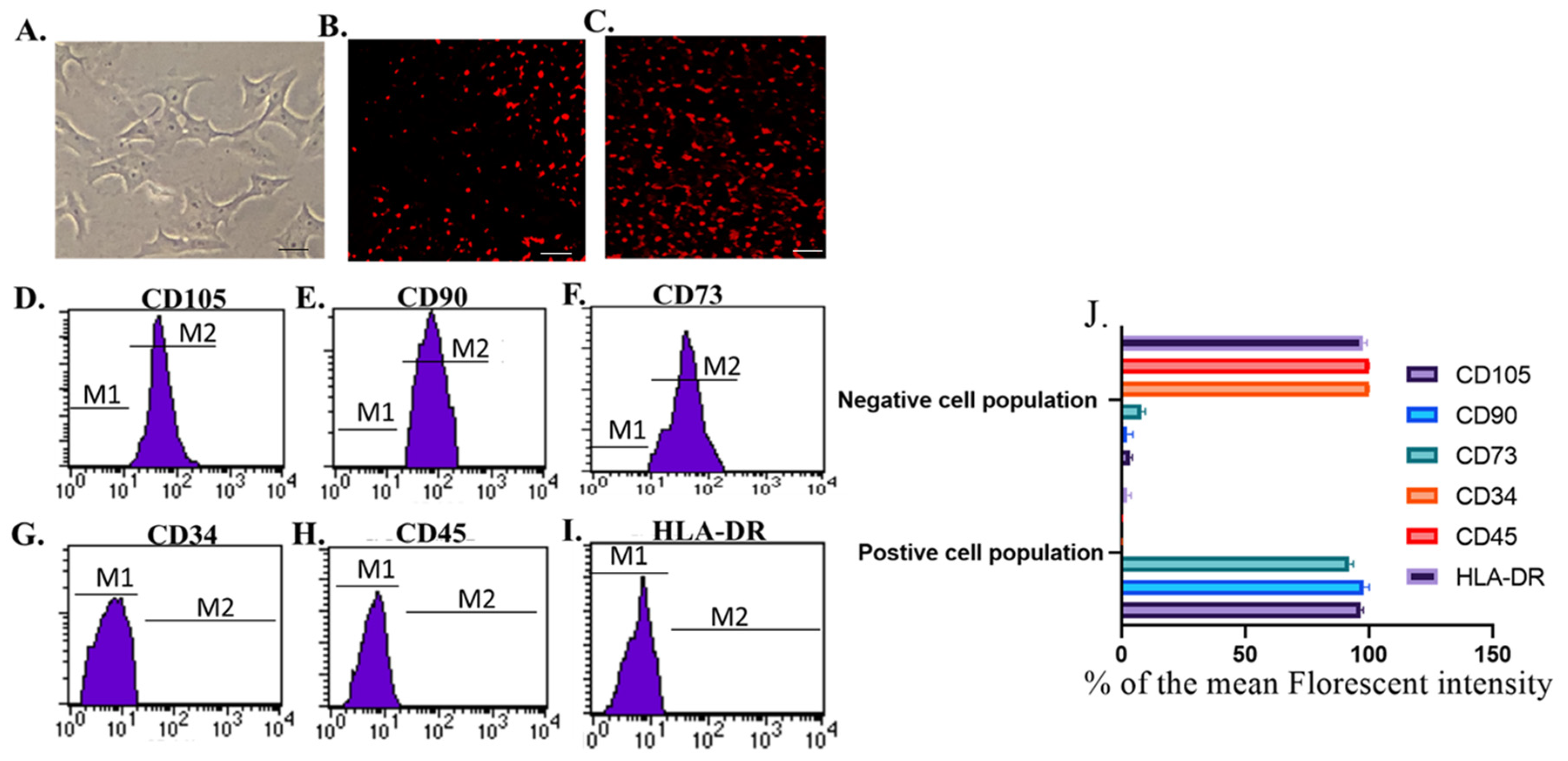
3.1. Identification and Homing of Br-MSCs and Their Derived Exosomes
3.2. Br-MSCs and/or Their Derived EXOs Improved Renal Function Tests
3.3. Br-MSCs and/or Their Derived EXOs’ Impact on Renal Oxidant/Antioxidant Markers
3.4. Br-MSCs and/or Their Derived EXOs’ Impact on Renal Expression of mir-34a/SNHG-7 /AMPK/ULK-1–AKT/mTOR Signaling Pathway
3.5. Br-MSCs and/or Their Derived EXOs Affect Renal Histopathology
3.6. Br-MSCs and/or Their Derived EXOs Affect Renal Beclin-1 mRNA and Protein Expression
3.7. Br-MSCs and/or Their Derived EXOs Affect Renal LC3-II mRNA and Protein Expression
3.8. Br-MSCs and/or Their Derived EXOs Affect Renal P62 mRNA and Protein Expression
3.9. Expression Pattern of Antifibrotic microRNAs and Effect of Br-MSCs and/or Their Derived EXOs on Their Expression
3.10. Br-MSCs and/or Their Derived EXOs’ Effect on the Renal TGF-β/Smad/Fibrotic Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, Y.; Bowe, B.; Mokdad, A.H.; Xian, H.; Yan, Y.; Li, T.; Maddukuri, G.; Tsai, C.-Y.; Floyd, T.; Al-Aly, Z. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018, 94, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Anderson, M.D.; Sutton, T.A. Pathophysiology of acute kidney injury. Compr. Physiol. 2012, 2, 1303–1353. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Daneshpajouhnejad, P.; Hashmi, M.F.; Bashir, K. Acute Kidney Injury. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kusaba, T.; Lalli, M.; Kramann, R.; Kobayashi, A.; Humphreys, B.D. Differentiated kidney epithelial cells repair injured proximal tubule. Proc. Natl. Acad. Sci. USA 2014, 111, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.-C.; Wu, C.-H.; Huang, T.-M.; Wang, C.-Y.; Lai, C.-F.; Shiao, C.-C.; Chang, C.-H.; Lin, S.-L.; Chen, Y.-Y.; Chen, Y.-M. Long-term risk of coronary events after AKI. J. Am. Soc. Nephrol. 2014, 25, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Bucaloiu, I.D.; Kirchner, H.L.; Norfolk, E.R.; Hartle, J.E.; Perkins, R.M. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. 2012, 81, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Kida, Y.; Tchao, B.N.; Yamaguchi, I. Peritubular capillary rarefaction: A new therapeutic target in chronic kidney disease. Pediatr. Nephrol. 2014, 29, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Di Gregorio, J.; Robuffo, I.; Spalletta, S.; Giambuzzi, G.; De Iuliis, V.; Toniato, E.; Martinotti, S.; Conti, P.; Flati, V. The Epithelial-to-Mesenchymal Transition as a Possible Therapeutic Target in Fibrotic Disorders. Front. Cell Dev. Biol. 2020, 8, 607483. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA 2019, 322, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bi, X.; Xiong, J.; Han, W.; Xiao, T.; Xu, X.; Yang, K.; Liu, C.; Jiang, W.; He, T.; et al. MicroRNA-34a Promotes Renal Fibrosis by Downregulation of Klotho in Tubular Epithelial Cells. Mol. Ther. 2019, 27, 1051–1065. [Google Scholar] [CrossRef]
- Tian, F.; Wang, J.; Zhang, Z.; Yang, J. LncRNA SNHG7/miR-34a-5p/SYVN1 axis plays a vital role in proliferation, apoptosis and autophagy in osteoarthritis. Biol. Res. 2020, 53, 9. [Google Scholar] [CrossRef]
- Li, Z.-h.; Yu, N.-s.; Deng, Q.; Zhang, Y.; Hu, Y.-y.; Liu, G.; Huang, K. LncRNA SNHG7 Mediates the Chemoresistance and Stemness of Breast Cancer by Sponging miR-34a. Front. Oncol. 2020, 10, 592757. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y. AMPK and Autophagy. Autophagy: Biol. Dis. 2019, 1206, 85–108. [Google Scholar] [CrossRef]
- Tang, C.; Livingston, M.J.; Liu, Z.; Dong, Z. Autophagy in kidney homeostasis and disease. Nat. Rev. Nephrol. 2020, 16, 489–508. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, G.P.; Chandrashekar, K.; Juncos, L.A.; Shah, S.V. Autophagy Function and Regulation in Kidney Disease. Biomolecules 2020, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Takabatake, Y.; Kimura, T.; Takahashi, A.; Namba, T.; Matsuda, J.; Minami, S.; Kaimori, J.-y.; Matsui, I.; Kitamura, H.; et al. Time-dependent dysregulation of autophagy: Implications in aging and mitochondrial homeostasis in the kidney proximal tubule. Autophagy 2016, 12, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cecconi, F.; Choi, A.M.; Chu, C.T.; Codogno, P.; Colombo, M.I.; et al. Molecular definitions of autophagy and related processes. EMBO J. 2017, 36, 1811–1836. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.K.; Levin, A.; Tonelli, M.; Okpechi, I.G.; Feehally, J.; Harris, D.; Jindal, K.; Salako, B.L.; Rateb, A.; Osman, M.A.; et al. Assessment of Global Kidney Health Care Status. JAMA 2017, 317, 1864. [Google Scholar] [CrossRef]
- Wong, G.; Turner, R.M.; Chapman, J.R.; Howell, M.; Lim, W.H.; Webster, A.C.; Craig, J.C. Time on Dialysis and Cancer Risk After Kidney Transplantation. Transplantation 2013, 95, 114–121. [Google Scholar] [CrossRef]
- Togel, F.; Hu, Z.; Weiss, K.; Isaac, J.; Lange, C.; Westenfelder, C. Amelioration of Acute Renal Failure by Stem Cell Therapy—Paracrine Secretion Versus Transdifferentiation into Resident Cells. J. Am. Soc. Nephrol. 2005, 16, 1153–1155. [Google Scholar] [CrossRef]
- Jeong, J.-O.; Han, J.W.; Kim, J.-M.; Cho, H.-J.; Park, C.; Lee, N.; Kim, D.-W.; Yoon, Y.-S. Malignant Tumor Formation After Transplantation of Short-Term Cultured Bone Marrow Mesenchymal Stem Cells in Experimental Myocardial Infarction and Diabetic Neuropathy. Circ. Res. 2011, 108, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guo, L.; Ge, J.; Yu, L.; Cai, T.; Tian, R.; Jiang, Y.; Zhao, R.C.H.; Wu, Y. Excess Integrins Cause Lung Entrapment of Mesenchymal Stem Cells. Stem Cells 2015, 33, 3315–3326. [Google Scholar] [CrossRef] [PubMed]
- Grange, C.; Skovronova, R.; Marabese, F.; Bussolati, B. Stem Cell-Derived Extracellular Vesicles and Kidney Regeneration. Cells 2019, 8, 1240. [Google Scholar] [CrossRef] [PubMed]
- Cregan, M.D.; Fan, Y.; Appelbee, A.; Brown, M.L.; Klopcic, B.; Koppen, J.; Mitoulas, L.R.; Piper, K.M.; Choolani, M.A.; Chong, Y.S.; et al. Identification of nestin-positive putative mammary stem cells in human breastmilk. Cell Tissue Res. 2007, 329, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chong, Y.S.; Choolani, M.A.; Cregan, M.D.; Chan, J.K. Unravelling the mystery of stem/progenitor cells in human breast milk. PLoS ONE 2010, 5, e14421. [Google Scholar] [CrossRef] [PubMed]
- Hassiotou, F.; Beltran, A.; Chetwynd, E.; Stuebe, A.M.; Twigger, A.J.; Metzger, P.; Trengove, N.; Lai, C.T.; Filgueira, L.; Blancafort, P.; et al. Breastmilk is a novel source of stem cells with multilineage differentiation potential. Stem Cells 2012, 30, 2164–2174. [Google Scholar] [CrossRef] [PubMed]
- Arvola, M.; Gustafsson, E.; Svensson, L.; Jansson, L.; Holmdahl, R.; Heyman, B.; Okabe, M.; Mattsson, R. Immunoglobulin-secreting cells of maternal origin can be detected in B cell-deficient mice. Biol. Reprod. 2000, 63, 1817–1824. [Google Scholar] [CrossRef]
- Zhou, L.; Yoshimura, Y.; Huang, Y.; Suzuki, R.; Yokoyama, M.; Okabe, M.; Shimamura, M. Two independent pathways of maternal cell transmission to offspring: Through placenta during pregnancy and by breast-feeding after birth. Immunology 2000, 101, 570–580. [Google Scholar] [CrossRef]
- Imperato, S.; Mistretta, C.; Marone, M.; Migliaccio, I.; Pulcinelli, I.; Faraone Mennella, M.R. Automodified Poly(ADP-Ribose) Polymerase Analysisto Monitor DNA Damagein Peripheral Lymphocytes of Floriculturists Occupationally Exposed to Pesticides. Cells 2019, 8, 137. [Google Scholar] [CrossRef]
- Liew, L.C.; Katsuda, T.; Gailhouste, L.; Nakagama, H.; Ochiya, T. Mesenchymal stem cell-derived extracellular vesicles: A glimmer of hope in treating Alzheimer’s disease. Int. Immunol. 2017, 29, 11–19. [Google Scholar] [CrossRef]
- Huang, Y.; Lei, Y.A.N.; Zheng, Z.; Wang, X.; Hu, M.; Liu, R.; Yu, X. Rosiglitazone alleviates injury in rats with adenine-induced chronic kidney disease. Mol. Med. Rep. 2013, 8, 1831–1835. [Google Scholar] [CrossRef][Green Version]
- Patki, S.; Kadam, S.; Chandra, V.; Bhonde, R. Human breast milk is a rich source of multipotent mesenchymal stem cells. Hum. Cell 2010, 23, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Khamis, T.; Abdelalim, A.F.; Saeed, A.A.; Edress, N.M.; Nafea, A.; Ebian, H.F.; Algendy, R.; Hendawy, D.M.; Arisha, A.H.; Abdallah, S.H. Breast milk MSCs upregulated β-cells PDX1, Ngn3, and PCNA expression via remodeling ER stress/inflammatory/apoptotic signaling pathways in type 1 diabetic rats. Eur. J. Pharmacol. 2021, 905, 174188. [Google Scholar] [CrossRef]
- Khamis, T.; Abdelalim, A.F.; Abdallah, S.H.; Saeed, A.A.; Edress, N.M.; Arisha, A.H. Early intervention with breast milk mesenchymal stem cells attenuates the development of diabetic-induced testicular dysfunction via hypothalamic Kisspeptin/Kiss1r-GnRH/GnIH system in male rats. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2020, 1866, 165577. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska, S.; Del Fattore, A.; Karnas, E.; Frontczak-Baniewicz, M.; Kozlowska, H.; Muraca, M.; Janowski, M.; Lukomska, B. Imaging of extracellular vesicles derived from human bone marrow mesenchymal stem cells using fluorescent and magnetic labels. Int. J. Nanomed. 2018, 13, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, Y.; Bao, S.; Huang, H.; Liu, W.; Guo, W. Bone marrow mesenchymal stem cell-derived exosomal microRNA-381-3p alleviates vascular calcification in chronic kidney disease by targeting NFAT5. Cell Death Dis. 2022, 13, 278. [Google Scholar] [CrossRef] [PubMed]
- Khamis, T.; Abdelalim, A.F.; Abdallah, S.H.; Saeed, A.A.; Edress, N.M.; Arisha, A.H. Breast milk MSCs transplantation attenuates male diabetic infertility via immunomodulatory mechanism in rats. Adv. Anim. Vet. Sci. 2019, 7, 145–153. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Khamis, T.; Alsemeh, A.E.; Abdullah, D.M. Sacubitril/valsartan (LCZ696) ameliorates hyperthyroid-induced cardiac hypertrophy in male rats through modulation of miR-377, let-7 b, autophagy, and fibrotic signaling pathways. Sci. Rep. 2022, 12, 14654. [Google Scholar] [CrossRef]
- Ali, R.; Khamis, T.; Enan, G.; El-Didamony, G.; Sitohy, B.; Abdel-Fattah, G. The Healing Capability of Clove Flower Extract (CFE) in Streptozotocin-Induced (STZ-Induced) Diabetic Rat Wounds Infected with Multidrug Resistant Bacteria. Molecules 2022, 27, 2270. [Google Scholar] [CrossRef]
- Layton, C.; Suvarna, K. Bancroft’s Theory and Practise of Histological Techniques, 7th ed; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Forbes, J.M.; Cooper, M.E. Mechanisms of Diabetic Complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef] [PubMed]
- Birtwistle, L.; Chen, X.-M.; Pollock, C. Mesenchymal Stem Cell-Derived Extracellular Vesicles to the Rescue of Renal Injury. Int. J. Mol. Sci. 2021, 22, 6596. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, K.; Wu, S.; Cui, M.; Xu, T. Focus on Mesenchymal Stem Cell-Derived Exosomes: Opportunities and Challenges in Cell-Free Therapy. Stem Cells Int. 2017, 2017, 6305295. [Google Scholar] [CrossRef] [PubMed]
- Mendt, M.; Rezvani, K.; Shpall, E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019, 54, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.M.; Harting, M.T.; Jimenez, F.; Monzon-Posadas, W.O.; Xue, H.; Savitz, S.I.; Laine, G.A.; Cox, C.S., Jr. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev. 2009, 18, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Gil-Kulik, P.; Leśniewski, M.; Bieńko, K.; Wójcik, M.; Więckowska, M.; Przywara, D.; Petniak, A.; Kondracka, A.; Świstowska, M.; Szymanowski, R.; et al. Influence of Perinatal Factors on Gene Expression of IAPs Family and Main Factors of Pluripotency: OCT4 and SOX2 in Human Breast Milk Stem Cells—A Preliminary Report. Int. J. Mol. Sci. 2023, 24, 2476. [Google Scholar] [CrossRef]
- Andrianova, N.V.; Zorov, D.B.; Plotnikov, E.Y. Targeting Inflammation and Oxidative Stress as a Therapy for Ischemic Kidney Injury. Biochemistry 2020, 85, 1591–1602. [Google Scholar] [CrossRef]
- Ueda, N. A Rheostat of Ceramide and Sphingosine-1-Phosphate as a Determinant of Oxidative Stress-Mediated Kidney Injury. Int. J. Mol. Sci. 2022, 23, 4010. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Aparicio-Trejo, O.E.; Ortega-Lozano, A.J.; Pedraza-Chaverri, J. Redox signaling pathways in unilateral ureteral obstruction (UUO)-induced renal fibrosis. Free Radic. Biol. Med. 2021, 172, 65–81. [Google Scholar] [CrossRef]
- Ali, B.H.; Al-Husseni, I.; Beegam, S.; Al-Shukaili, A.; Nemmar, A.; Schierling, S.; Queisser, N.; Schupp, N. Effect of Gum Arabic on Oxidative Stress and Inflammation in Adenine–Induced Chronic Renal Failure in Rats. PLoS ONE 2013, 8, e55242. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-H.; Tseng, W.-C.; Yang, C.-Y.; Tarng, D.-C. The Anti-Inflammatory, Anti-Oxidative, and Anti-Apoptotic Benefits of Stem Cells in Acute Ischemic Kidney Injury. Int. J. Mol. Sci. 2019, 20, 3529. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chen, N.; Xia, C.; Wang, H.; Shao, L.; Zhou, C.; Wang, J. Mesenchymal Stem Cell Therapy in Kidney Diseases: Potential and Challenges. Cell Transplant. 2023, 32, 096368972311642. [Google Scholar] [CrossRef] [PubMed]
- Gregorini, M.; Corradetti, V.; Pattonieri, E.F.; Rocca, C.; Milanesi, S.; Peloso, A.; Canevari, S.; De Cecco, L.; Dugo, M.; Avanzini, M.A.; et al. Perfusion of isolated rat kidney with Mesenchymal Stromal Cells/Extracellular Vesicles prevents ischaemic injury. J. Cell. Mol. Med. 2017, 21, 3381–3393. [Google Scholar] [CrossRef] [PubMed]
- Baniene, R.; Trumbeckas, D.; Kincius, M.; Pauziene, N.; Raudone, L.; Jievaltas, M.; Trumbeckaite, S. Short ischemia induces rat kidney mitochondria dysfunction. J. Bioenerg. Biomembr. 2016, 48, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Cheng, Y.; Gao, H.; Zhuang, J.; Zhang, W.; Bian, Q.; Wang, F.; Du, Y.; Li, Z.; Kong, D.; et al. Tracking of Mesenchymal Stem Cell-Derived Extracellular Vesicles Improving Mitochondrial Function in Renal Ischemia–Reperfusion Injury. ACS Nano 2020, 14, 4014–4026. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, M.; Wan, X.; Jin, X.; Chen, S.; Yu, C.; Li, Y. Effect of miR-34a in regulating steatosis by targeting PPARα expression in nonalcoholic fatty liver disease. Sci. Rep. 2015, 5, 13729. [Google Scholar] [CrossRef]
- Chen, P.; Chen, F.; Lei, J.; Li, Q.; Zhou, B. Activation of the miR-34a-Mediated SIRT1/mTOR Signaling Pathway by Urolithin A Attenuates d-Galactose-Induced Brain Aging in Mice. Neurotherapeutics 2019, 16, 1269–1282. [Google Scholar] [CrossRef]
- Najafi, S.; Ghafouri-Fard, S.; Hussen, B.M.; Jamal, H.H.; Taheri, M.; Hallajnejad, M. Oncogenic Roles of Small Nucleolar RNA Host Gene 7 (SNHG7) Long Noncoding RNA in Human Cancers and Potentials. Front. Cell Dev. Biol. 2022, 9, 809345. [Google Scholar] [CrossRef]
- Xie, Z.; Wu, Y.; Liu, S.; Lai, Y.; Tang, S. LncRNA-SNHG7/miR-29b/DNMT3A axis affects activation, autophagy and proliferation of hepatic stellate cells in liver fibrosis. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101469. [Google Scholar] [CrossRef]
- Song, Y.; Tao, Q.; Yu, L.; Li, L.; Bai, T.; Song, X.; Hu, H.; Li, Y.; Tan, X. Activation of autophagy contributes to the renoprotective effect of postconditioning on acute kidney injury and renal fibrosis. Biochem. Biophys. Res. Commun. 2018, 504, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Wang, F.; Gao, R.; Wu, J.; Ou, Y.; Chen, X.; Wang, T.; Zhou, X.; Zhu, W.; Li, P.; et al. Autophagy inhibition of hsa-miR-19a-3p/19b-3p by targeting TGF-β R II during TGF-β1-induced fibrogenesis in human cardiac fibroblasts. Sci. Rep. 2016, 6, 24747. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, Z.-D.; Han, L.; Zhang, J.; Lee, S.-W.; Wang, W.; Lee, H.; Zhuang, L.; Chen, J.; Lin, H.-K.; et al. LncRNA NBR2 engages a metabolic checkpoint by regulating AMPK under energy stress. Nat. Cell Biol. 2016, 18, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xie, Y.; Zhou, Z.; Wu, Z.; Dai, X.; Xu, B. Curcumin Regulates the Progression of Colorectal Cancer via LncRNA NBR2/AMPK Pathway. Technol. Cancer Res. Treat. 2019, 18, 153303381987078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, C.; Shen, M.; Yang, M.; Jin, Z.; Ding, L.; Jiang, W.; Yang, J.; Chen, H.; Cao, F.; et al. Autophagy mediates the beneficial effect of hypoxic preconditioning on bone marrow mesenchymal stem cells for the therapy of myocardial infarction. Stem Cell Res. Ther. 2017, 8, 89. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2014, 22, 377–388. [Google Scholar] [CrossRef]
- Hu, C.; Zhao, L.; Wu, D.; Li, L. Modulating autophagy in mesenchymal stem cells effectively protects against hypoxia- or ischemia-induced injury. Stem Cell Res. Ther. 2019, 10, 120. [Google Scholar] [CrossRef]
- Humphreys, B.D. Mechanisms of Renal Fibrosis. Annu. Rev. Physiol. 2018, 80, 309–326. [Google Scholar] [CrossRef]
- Hu, L.; Ding, M.; He, W. Emerging Therapeutic Strategies for Attenuating Tubular EMT and Kidney Fibrosis by Targeting Wnt/β-Catenin Signaling. Front. Pharmacol. 2021, 12, 830340. [Google Scholar] [CrossRef]
- Hu, S.; Hu, H.; Wang, R.; He, H.; Shui, H. microRNA-29b prevents renal fibrosis by attenuating renal tubular epithelial cell–mesenchymal transition through targeting the PI3K/AKT pathway. Int. Urol. Nephrol. 2021, 53, 1941–1950. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Z.; Heng, Y.; Miao, C. MicroRNA-181 exerts an inhibitory role during renal fibrosis by targeting early growth response factor-1 and attenuating the expression of profibrotic markers. Mol. Med. Rep. 2019, 19, 3305–3313. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, C.; Sun, Y.; Chen, Y.; Xue, D. Let-7c-5p Is Involved in Chronic Kidney Disease by Targeting TGF-β Signaling. BioMed Res. Int. 2020, 2020, 6960941. [Google Scholar] [CrossRef]
- Hertel, J.; Bartschat, S.; Wintsche, A.; Otto, C.; Stadler, P.F. Evolution of the let-7 microRNA family. RNA Biol. 2012, 9, 231–241. [Google Scholar] [CrossRef]
- Grange, C.; Tritta, S.; Tapparo, M.; Cedrino, M.; Tetta, C.; Camussi, G.; Brizzi, M.F. Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Sci. Rep. 2019, 9, 4468. [Google Scholar] [CrossRef]










| Gene | Forward Primer | Reverse Primer | bp | Accession No. | References |
|---|---|---|---|---|---|
| TGF-β1 | AGGGCTACCATGCCAACTTC | CCACGTAGTAGACGATGGGC | 168 | NM_021578.2 | [40] |
| Smad-7 | GAGTCTCGGAGGAAGAGGCT | CTGCTCGCATAAGCTGCTGG | 84 | NM_030858.2 | [40] |
| Smad-3 | CTGGGCAAGTTCTCCAGAGTT | AAGGGCAGGATGGACGACAT | 148 | NM_013095.3 | [40] |
| Beclin-1 | GAATGGAGGGGTCTAAGGCG | CTTCCTCCTGGCTCTCTCT | 180 | NM_001034117.1 | [40] |
| LC-3 | GAAATGGTCACCCCACGAGT | ACACAGTTTTCCCATGCCCA | 147 | NM_012823.2 | [40] |
| mTOR | GCAATGGGCACGAGTTTGTT | AGTGTGTTCACCAGGCCAAA | 94 | NM_019906.2 | [40] |
| P62 | GGAAGCTGAAACATGGGCAC | CCAAGGGTCCACCTGAACAA | 183 | NM_181550.2 | [40] |
| SNHG-7 | TGGCAGTGTCTTAGCTGGTT | AACGTGCAGCACTTCTAGGG | 81 | NR_031850.1 | |
| Coli-1 | GCAATGCTGAATCGTCCCAC | CAGCACAGGCCCTCAAAAAC | 176 | NM_053304.1 | [41] |
| AMPK | GCGTGTGAAGATCGGACACT | TGCCACTTTATGGCCTGTCA | 103 | NM_023991.1 | |
| AKT-1 | GAAGGAGAAGGCCACAGGTC | TTCTGCAGGACACGGTTCTC | 111 | NM_033230.3 | |
| ULK-1 | CGTACACTGCCTGACCTCTC | AGAGGCCTGTGTCCCAAATG | 162 | NM_001108341.1 | |
| rat Gapdh | GGCACAGTCAAGGCTGAGAATG | ATGGTGGTGAAGACGCCAGTA | 143 | NM_017008.4 | [35] |
| human Gapdh | GGAGTCAACGGATTTGGTCGT | ACGGTGCCATGGAATTTGC | 161 | NM_002046.7 | [35] |
| mir-29b | AACACGCCTGGTTTCACATG | GTCGTATCCAGTGCAGGGT | |||
| mir-34a | AACACGCTGGCAGTGTCTTA | GTCGTATCCAGTGCAGGGT | |||
| mir-181 | AACACGCAACATTCAACGCT | GTCGTATCCAGTGCAGGGT | |||
| Let-7b | AACACGCTGAGGTAGTAGGTT | GTCGTATCCAGTGCAGGGT | [40] | ||
| U6 | GCTCGCTTCGGCAGCACA | GAGGTATTCGCACCAGAGGA | [40] | ||
| mir-29b stem-Loop primer | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCTAAGC | ||||
| mir-34a stem-Loop primer | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACAACC | ||||
| mir-181 stem-Loop primer | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACTCAC | ||||
| Let-7b stem-Loop primer | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAACCAC | [40] | |||
| U6 stem-Loop primer | AACGCTTCACGAATTTGCGTG | [40] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khamis, T.; Alsemeh, A.E.; Alanazi, A.; Eltaweel, A.M.; Abdel-Ghany, H.M.; Hendawy, D.M.; Abdelkhalek, A.; Said, M.A.; Awad, H.H.; Ibrahim, B.H.; et al. Breast Milk Mesenchymal Stem Cells and/or Derived Exosomes Mitigated Adenine-Induced Nephropathy via Modulating Renal Autophagy and Fibrotic Signaling Pathways and Their Epigenetic Regulations. Pharmaceutics 2023, 15, 2149. https://doi.org/10.3390/pharmaceutics15082149
Khamis T, Alsemeh AE, Alanazi A, Eltaweel AM, Abdel-Ghany HM, Hendawy DM, Abdelkhalek A, Said MA, Awad HH, Ibrahim BH, et al. Breast Milk Mesenchymal Stem Cells and/or Derived Exosomes Mitigated Adenine-Induced Nephropathy via Modulating Renal Autophagy and Fibrotic Signaling Pathways and Their Epigenetic Regulations. Pharmaceutics. 2023; 15(8):2149. https://doi.org/10.3390/pharmaceutics15082149
Chicago/Turabian StyleKhamis, Tarek, Amira Ebrahim Alsemeh, Asma Alanazi, Asmaa Monir Eltaweel, Heba M. Abdel-Ghany, Doaa M. Hendawy, Adel Abdelkhalek, Mahmoud A. Said, Heba H. Awad, Basma Hamed Ibrahim, and et al. 2023. "Breast Milk Mesenchymal Stem Cells and/or Derived Exosomes Mitigated Adenine-Induced Nephropathy via Modulating Renal Autophagy and Fibrotic Signaling Pathways and Their Epigenetic Regulations" Pharmaceutics 15, no. 8: 2149. https://doi.org/10.3390/pharmaceutics15082149
APA StyleKhamis, T., Alsemeh, A. E., Alanazi, A., Eltaweel, A. M., Abdel-Ghany, H. M., Hendawy, D. M., Abdelkhalek, A., Said, M. A., Awad, H. H., Ibrahim, B. H., Mekawy, D. M., Pascu, C., Florin, C., & Arisha, A. H. (2023). Breast Milk Mesenchymal Stem Cells and/or Derived Exosomes Mitigated Adenine-Induced Nephropathy via Modulating Renal Autophagy and Fibrotic Signaling Pathways and Their Epigenetic Regulations. Pharmaceutics, 15(8), 2149. https://doi.org/10.3390/pharmaceutics15082149






