Advancing Treatment Strategies: A Comprehensive Review of Drug Delivery Innovations for Chronic Inflammatory Respiratory Diseases
Abstract
:1. Introduction
2. Nanoparticle-Based Drug Delivery Systems
3. Inhaled Corticosteroids (ICSs)
4. Novel Biologicals
5. Gene Therapy
6. Personalized Medicine
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reddel, H.K.; Bacharier, L.B.; Bateman, E.D.; Brightling, C.E.; Brusselle, G.G.; Buhl, R.; Cruz, A.A.; Duijts, L.; Drazen, J.M.; FitzGerald, J.M.; et al. Global Initiative for Asthma Strategy 2021: Executive summary and rationale for key changes. Eur. Respir. J. 2021, 59, 2102730. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.; Pier, J.; Litonjua, A.A. Asthma epidemiology and risk factors. Semin. Immunopathol. 2020, 42, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Raherison, C.; Girodet, P.-O. Epidemiology of COPD. Eur. Respir. Rev. 2009, 18, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.L.; Grayson, M.H.; Strothman, K. Advances in asthma: New understandings of asthma’s natural history, risk factors, underlying mechanisms, and clinical management. J. Allergy Clin. Immunol. 2021, 148, 1430–1441. [Google Scholar] [CrossRef] [PubMed]
- Christenson, S.A.; Smith, S.A.; Bafadhel, M.; Putcha, N. Chronic obstructive pulmonary disease. Lancet 2022, 399, 2227–2242. [Google Scholar] [CrossRef]
- Martinez, F.J.; Collard, H.R.; Pardo, A.; Raghu, G.; Richeldi, L.; Selman, M.; Swigris, J.J.; Taniguchi, H.; Wells, A.U. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Prim. 2017, 3, 17074. [Google Scholar] [CrossRef]
- Porsbjerg, C.M.; Sverrild, A.; Lloyd, C.M.; Menzies-Gow, A.N.; Bel, E.H. Anti-alarmins in asthma: Targeting the airway epithelium with next-generation biologics. Eur. Respir. J. 2020, 56, 2000260. [Google Scholar] [CrossRef]
- Brightling, C.; Greening, N. Airway inflammation in COPD: Progress to precision medicine. Eur. Respir. J. 2019, 54, 1900651. [Google Scholar] [CrossRef]
- Wang, J.; Hu, K.; Cai, X.; Yang, B.; He, Q.; Wang, J.; Weng, Q. Targeting PI3K/AKT signaling for treatment of idiopathic pulmonary fibrosis. Acta Pharm. Sin. B 2022, 12, 18–32. [Google Scholar] [CrossRef]
- Forest, V.; Pourchez, J. Nano-delivery to the lung—By inhalation or other routes and why nano when micro is largely sufficient? Adv. Drug Deliv. Rev. 2022, 183, 114173. [Google Scholar] [CrossRef]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, J.; Wu, J.; Suk, J.S. Enhancing nanoparticle penetration through airway mucus to improve drug delivery efficacy in the lung. Expert Opin. Drug Deliv. 2021, 18, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Yang, G.; Choi, E.; Ryu, J.-H. Mesoporous silica nanoparticle-supported nanocarriers with enhanced drug loading, encapsulation stability, and targeting efficiency. Biomater. Sci. 2022, 10, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.-A. Budesonide/Glycopyrronium/Formoterol: A Review in COPD. Drugs 2021, 81, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, M.M.; Dixon, A.E.; Krishnan, J.A.; Lemanske, R.F.; Pace, W.; Schatz, M. Managing Asthma in Adolescents and Adults: 2020 Asthma Guideline Update from the National Asthma Education and Prevention Program. JAMA 2020, 324, 2301–2317. [Google Scholar] [CrossRef]
- Werder, R.B.; Liu, T.; Abo, K.M.; Lindstrom-Vautrin, J.; Villacorta-Martin, C.; Huang, J.; Hinds, A.; Boyer, N.; Bullitt, E.; Liesa, M.; et al. CRISPR interference interrogation of COPD GWAS genes reveals the functional significance of desmoplakin in iPSC-derived alveolar epithelial cells. Sci. Adv. 2022, 8, eabo6566. [Google Scholar] [CrossRef]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef]
- Wouters, E.F.; Wouters, B.B.; Augustin, I.M.; Houben-Wilke, S.; Vanfleteren, L.E.; Franssen, F.M. Personalised pulmonary rehabilitation in COPD. Eur. Respir. Rev. 2018, 27, 170125. [Google Scholar] [CrossRef]
- Kaur, R.; Chupp, G. Phenotypes and endotypes of adult asthma: Moving toward precision medicine. J. Allergy Clin. Immunol. 2019, 144, 1–12. [Google Scholar] [CrossRef]
- Castaldi, P.J.; Yun, J.; Estepar, R.S.J.; Ross, J.C.; Cho, M.H.; Hersh, C.P.; Kinney, G.L.; Young, K.A.; Regan, E.A.; Lynch, D.A.; et al. Machine Learning Characterization of COPD Subtypes. Chest 2020, 157, 1147–1157. [Google Scholar] [CrossRef]
- Passi, M.; Shahid, S.; Chockalingam, S.; Sundar, I.K.; Packirisamy, G. Conventional and Nanotechnology Based Approaches to Combat Chronic Obstructive Pulmonary Disease: Implications for Chronic Airway Diseases. Int. J. Nanomed. 2020, 15, 3803–3826. [Google Scholar] [CrossRef]
- Yhee, J.Y.; Im, J.; Nho, R.S. Advanced Therapeutic Strategies for Chronic Lung Disease Using Nanoparticle-Based Drug Delivery. J. Clin. Med. 2016, 5, 82. [Google Scholar] [CrossRef]
- Pramanik, S.; Mohanto, S.; Manne, R.; Rajendran, R.R.; Deepak, A.; Edapully, S.J.; Patil, T.; Katari, O. Nanoparticle-Based Drug Delivery System: The Magic Bullet for the Treatment of Chronic Pulmonary Diseases. Mol. Pharm. 2021, 18, 3671–3718. [Google Scholar] [CrossRef] [PubMed]
- Dymek, M.; Sikora, E. Liposomes as biocompatible and smart delivery systems—The current state. Adv. Colloid Interface Sci. 2022, 309, 102757. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef] [PubMed]
- Thorley, A.J.; Tetley, T.D. New perspectives in nanomedicine. Pharmacol. Ther. 2013, 140, 176–185. [Google Scholar] [CrossRef]
- Pohlit, H.; Bellinghausen, I.; Frey, H.; Saloga, J. Recent advances in the use of nanoparticles for allergen-specific immunotherapy. Allergy 2017, 72, 1461–1474. [Google Scholar] [CrossRef]
- Kularatne, S.A.; Low, P.S. Targeting of Nanoparticles: Folate Receptor. Cancer Nanotechnol. 2010, 624, 249–265. [Google Scholar] [CrossRef]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef]
- Xu, H.; Yao, Q.; Cai, C.; Gou, J.; Zhang, Y.; Zhong, H.; Tang, X. Amphiphilic poly(amino acid) based micelles applied to drug delivery: The in vitro and in vivo challenges and the corresponding potential strategies. J. Control. Release 2015, 199, 84–97. [Google Scholar] [CrossRef]
- Sahib, M.; Darwis, Y.; Peh, K.K.; Abdulameer, S.; Tan, Y.T.F. Rehydrated sterically stabilized phospholipid nanomicelles of budesonide for nebulization: Physicochemical characterization and in vitro, in vivo evaluations. Int. J. Nanomed. 2011, 6, 2351–2366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xu, Q.; Huang, T.; Ling, D.; Gao, J. New Insights into Biocompatible Iron Oxide Nanoparticles: A Potential Booster of Gene Delivery to Stem Cells. Small 2020, 16, e2001588. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Acosta, J.R.; Iriarte-Mesa, C.; Ortega, G.A.; Díaz-García, A.M. DNA–Iron Oxide Nanoparticles Conjugates: Functional Magnetic Nanoplatforms in Biomedical Applications. Top. Curr. Chem. 2020, 378, 1–29. [Google Scholar] [CrossRef]
- Wei, H.; Hu, Y.; Wang, J.; Gao, X.; Qian, X.; Tang, M. Superparamagnetic Iron Oxide Nanoparticles: Cytotoxicity, Metabolism, and Cellular Behavior in Biomedicine Applications. Int. J. Nanomed. 2021, 16, 6097–6113. [Google Scholar] [CrossRef]
- Halwani, R.; Shaik, A.S.; Ratemi, E.; Afzal, S.; Kenana, R.; Al-Muhsen, S.; Al Faraj, A. A novel anti-IL4Rα nanoparticle efficiently controls lung inflammation during asthma. Exp. Mol. Med. 2016, 48, e262. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, W.; Wang, H.; Yue, J.; Mao, Y.; Zhou, W.; Kong, X.; Guo, Q.; Zhang, L.; Xu, P.; et al. Anti-ST2 Nanoparticle Alleviates Lung Inflammation by Targeting ILC2s-CD4+T Response. Int. J. Nanomed. 2020, 15, 9745–9758. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Wei, T.; Farbiak, L.; Johnson, L.T.; Dilliard, S.A.; Siegwart, D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 2020, 15, 313–320. [Google Scholar] [CrossRef]
- Zuo, X.; Guo, X.; Gu, Y.; Zheng, H.; Zhou, Z.; Wang, X.; Jiang, S.; Wang, G.; Xu, C.; Wang, F. Recent Advances in Nanomaterials for Asthma Treatment. Int. J. Mol. Sci. 2022, 23, 14427. [Google Scholar] [CrossRef]
- Soares, D.C.F.; Domingues, S.C.; Viana, D.B.; Tebaldi, M.L. Polymer-hybrid nanoparticles: Current advances in biomedical applications. Biomed. Pharmacother. 2020, 131, 110695. [Google Scholar] [CrossRef]
- Pei, W.; Li, X.; Bi, R.; Zhang, X.; Zhong, M.; Yang, H.; Zhang, Y.; Lv, K. Exosome membrane-modified M2 macrophages targeted nanomedicine: Treatment for allergic asthma. J. Control. Release 2021, 338, 253–267. [Google Scholar] [CrossRef]
- Dauletbaev, N.; Cammisano, M.; Herscovitch, K.; Lands, L.C. Stimulation of the RIG-I/MAVS Pathway by Polyinosinic:Polycytidylic Acid Upregulates IFN-β in Airway Epithelial Cells with Minimal Costimulation of IL-8. J. Immunol. 2015, 195, 2829–2841. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, I.; Costabile, G.; Durantie, E.; Brocca, P.; Rondelli, V.; Russo, A.; Russo, G.; Miro, A.; Quaglia, F.; Petri-Fink, A.; et al. Hybrid Lipid/Polymer Nanoparticles for Pulmonary Delivery of siRNA: Development and Fate Upon In Vitro Deposition on the Human Epithelial Airway Barrier. J. Aerosol Med. Pulm. Drug Deliv. 2018, 31, 170–181. [Google Scholar] [CrossRef]
- Dormenval, C.; Lokras, A.; Cano-Garcia, G.; Wadhwa, A.; Thanki, K.; Rose, F.; Thakur, A.; Franzyk, H.; Foged, C. Identification of Factors of Importance for Spray Drying of Small Interfering RNA-Loaded Lipidoid-Polymer Hybrid Nanoparticles for Inhalation. Pharm. Res. 2019, 36, 142. [Google Scholar] [CrossRef]
- Bai, X.; Zhao, G.; Chen, Q.; Li, Z.; Gao, M.; Ho, W.; Xu, X.; Zhang, X.-Q. Inhaled siRNA nanoparticles targeting IL11 inhibit lung fibrosis and improve pulmonary function post-bleomycin challenge. Sci. Adv. 2022, 8, eabn7162. [Google Scholar] [CrossRef]
- Tagalakis, A.D.; McAnulty, R.J.; Devaney, J.; Bottoms, S.E.; Wong, J.B.; Elbs, M.; Writer, M.J.; Hailes, H.C.; Tabor, A.B.; O’Callaghan, C.; et al. A Receptor-targeted Nanocomplex Vector System Optimized for Respiratory Gene Transfer. Mol. Ther. 2008, 16, 907–915. [Google Scholar] [CrossRef]
- Blank, F.; Fytianos, K.; Seydoux, E.; Rodriguez-Lorenzo, L.; Petri-Fink, A.; von Garnier, C.; Rothen-Rutishauser, B. Interaction of biomedical nanoparticles with the pulmonary immune system. J. Nanobiotechnol. 2017, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; He, Q.; Liu, J.; Chen, Y.; Ma, M.; Zhang, L.; Shi, J. Nuclear-Targeted Drug Delivery of TAT Peptide-Conjugated Monodisperse Mesoporous Silica Nanoparticles. J. Am. Chem. Soc. 2012, 134, 5722–5725. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, A.; Sancenón, F.; Martínez-Máñez, R. Mesoporous silica nanoparticles for pulmonary drug delivery. Adv. Drug Deliv. Rev. 2021, 177, 113953. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.E.; Lee, Y.; Kim, M.; Lee, S.; Jon, S.; Lee, S.-H. Bilirubin nanoparticles ameliorate allergic lung inflammation in a mouse model of asthma. Biomaterials 2017, 140, 37–44. [Google Scholar] [CrossRef]
- Kabir, E.; Kumar, V.; Kim, K.-H.; Yip, A.C.; Sohn, J. Environmental impacts of nanomaterials. J. Environ. Manag. 2018, 225, 261–271. [Google Scholar] [CrossRef]
- Ali, M. What function of nanoparticles is the primary factor for their hyper-toxicity? Adv. Colloid Interface Sci. 2023, 314, 102881. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.-H.; Chen, Y.; Zha, H.-X.; Song, L.-J.; Li, C.-Y.; Li, J.-Q.; Xia, X.-H. Determination, characterization and cytotoxicity on HELF cells of ZnO nanoparticles. Colloids Surf. B Biointerfaces 2010, 76, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Wongrakpanich, A.; Mudunkotuwa, I.A.; Geary, S.M.; Morris, A.S.; Mapuskar, K.A.; Spitz, D.R.; Grassian, V.H.; Salem, A.K. Size-dependent cytotoxicity of copper oxide nanoparticles in lung epithelial cells. Environ. Sci. Nano 2016, 3, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lin, Z.; Wang, T.; Yao, Z.; Qin, M.; Zheng, S.; Lu, W. Where does the toxicity of metal oxide nanoparticles come from: The nanoparticles, the ions, or a combination of both? J. Hazard. Mater. 2016, 308, 328–334. [Google Scholar] [CrossRef]
- Cheng, H.; Cui, Z.; Guo, S.; Zhang, X.; Huo, Y.; Mao, S. Mucoadhesive versus mucopenetrating nanoparticles for oral delivery of insulin. Acta Biomater. 2021, 135, 506–519. [Google Scholar] [CrossRef]
- Netsomboon, K.; Bernkop-Schnürch, A. Mucoadhesive vs. mucopenetrating particulate drug delivery. Eur. J. Pharm. Biopharm. 2016, 98, 76–89. [Google Scholar] [CrossRef]
- da Silva, A.L.; de Oliveira, G.P.; Kim, N.; Cruz, F.F.; Kitoko, J.Z.; Blanco, N.G.; Martini, S.V.; Hanes, J.; Rocco, P.R.M.; Suk, J.S.; et al. Nanoparticle-based thymulin gene therapy therapeutically reverses key pathology of experimental allergic asthma. Sci. Adv. 2020, 6, eaay7973. [Google Scholar] [CrossRef]
- Hou, C.; Bai, H.; Wang, Z.; Qiu, Y.; Kong, L.-L.; Sun, F.; Wang, D.; Yin, H.; Zhang, X.; Mu, H.; et al. A hyaluronan-based nanosystem enables combined anti-inflammation of mTOR gene silencing and pharmacotherapy. Carbohydr. Polym. 2018, 195, 339–348. [Google Scholar] [CrossRef]
- Kubczak, M.; Michlewska, S.; Bryszewska, M.; Aigner, A.; Ionov, M. Nanoparticles for local delivery of siRNA in lung therapy. Adv. Drug Deliv. Rev. 2021, 179, 114038. [Google Scholar] [CrossRef]
- Latorre, M.; Bacci, E.; Seccia, V.; Bartoli, M.L.; Cardini, C.; Cianchetti, S.; Cristofani, L.; Di Franco, A.; Miccoli, M.; Puxeddu, I.; et al. Upper and lower airway inflammation in severe asthmatics: A guide for a precision biologic treatment. Ther. Adv. Respir. Dis. 2020, 14, 1753466620965151. [Google Scholar] [CrossRef]
- Aalbers, R.; Vogelmeier, C.; Kuna, P. Achieving asthma control with ICS/LABA: A review of strategies for asthma management and prevention. Respir. Med. 2016, 111, 1–7. [Google Scholar] [CrossRef]
- Evans, D.J.; Kew, K.M.; Anderson, D.E.; Boyter, A.C. Long-acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus higher dose ICS for adults with asthma. Cochrane Database Syst. Rev. 2015, 2015, CD011437. [Google Scholar] [CrossRef] [PubMed]
- Dorinsky, P.; DePetrillo, P.; DeAngelis, K.; Trivedi, R.; Darken, P.; Gillen, M. Relative Bioavailability of Budesonide/Glycopyrrolate/Formoterol Fumarate Metered Dose Inhaler Administered with and without a Spacer: Results of a Phase I, Randomized, Crossover Trial in Healthy Adults. Clin. Ther. 2020, 42, 634–648. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, T.W.; Colice, G.; Grigg, J.; van Aalderen, W.; Martin, R.J.; Israel, E.; Postma, D.S.; Roche, N.; Phipatanakul, W.; Hillyer, E.V.; et al. Real-Life Outcomes for Patients with Asthma Prescribed Spacers for Use with Either Extrafine- or Fine-Particle Inhaled Corticosteroids. J. Allergy Clin. Immunol. Pract. 2017, 5, 1040–1049.e4. [Google Scholar] [CrossRef] [PubMed]
- Postma, D.S.; Dekhuijzen, R.; van der Molen, T.; Martin, R.J.; van Aalderen, W.; Roche, N.; Guilbert, T.W.; Israel, E.; van Eickels, D.; Khalid, J.M.; et al. Asthma-Related Outcomes in Patients Initiating Extrafine Ciclesonide or Fine-Particle Inhaled Corticosteroids. Allergy Asthma Immunol. Res. 2017, 9, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Sonnappa, S.; McQueen, B.; Postma, D.S.; Martin, R.J.; Roche, N.; Grigg, J.; Guilbert, T.; Gouder, C.; Pizzichini, E.; Niimi, A.; et al. Extrafine Versus Fine Inhaled Corticosteroids in Relation to Asthma Control: A Systematic Review and Meta-Analysis of Observational Real-Life Studies. J. Allergy Clin. Immunol. Pract. 2018, 6, 907–915.e7. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; De Simoni, A.; Wileman, V.; Holliday, L.; Newby, C.J.; Chisari, C.; Ali, S.; Zhu, N.; Padakanti, P.; Pinprachanan, V.; et al. Digital interventions to improve adherence to maintenance medication in asthma. Cochrane Database Syst. Rev. 2022, 6, CD013030. [Google Scholar] [CrossRef]
- de Boer, A.H.; Hagedoorn, P.; Hoppentocht, M.; Buttini, F.; Grasmeijer, F.; Frijlink, H.W. Dry powder inhalation: Past, present and future. Expert Opin. Drug Deliv. 2016, 14, 499–512. [Google Scholar] [CrossRef]
- Gaikwad, S.S.; Pathare, S.R.; More, M.A.; Waykhinde, N.A.; Laddha, U.D.; Salunkhe, K.S.; Kshirsagar, S.J.; Patil, S.S.; Ramteke, K.H. Dry Powder Inhaler with the technical and practical obstacles, and forthcoming platform strategies. J. Control. Release 2023, 355, 292–311. [Google Scholar] [CrossRef]
- Bremner, P.R.; Birk, R.; Brealey, N.; Ismaila, A.S.; Zhu, C.Q.; Lipson, D.A. Single-inhaler fluticasone furoate/umeclidinium/vilanterol versus fluticasone furoate/vilanterol plus umeclidinium using two inhalers for chronic obstructive pulmonary disease: A randomized non-inferiority study. Respir. Res. 2018, 19, 19. [Google Scholar] [CrossRef]
- Chapman, K.R.; Hurst, J.R.; Frent, S.-M.; Larbig, M.; Fogel, R.; Guerin, T.; Banerji, D.; Patalano, F.; Goyal, P.; Pfister, P.; et al. Long-Term Triple Therapy De-escalation to Indacaterol/Glycopyrronium in Patients with Chronic Obstructive Pulmonary Disease (SUNSET): A Randomized, Double-Blind, Triple-Dummy Clinical Trial. Am. J. Respir. Crit. Care Med. 2018, 198, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, G.T.; Rabe, K.F.; Martinez, F.J.; Fabbri, L.M.; Wang, C.; Ichinose, M.; Bourne, E.; Ballal, S.; Darken, P.; DeAngelis, K.; et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): A double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir. Med. 2018, 6, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-D.; Xie, C.-M.; Yunus, F.; Itoh, Y.; Ling, X.; Yu, W.-C.; Kiatboonsri, S. Efficacy and tolerability of budesonide/formoterol added to tiotropium compared with tiotropium alone in patients with severe or very severe COPD: A randomized, multicentre study in East Asia. Respirology 2016, 21, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Lipson, D.A.; Barnacle, H.; Birk, R.; Brealey, N.; Locantore, N.; Lomas, D.A.; Ludwig-Sengpiel, A.; Mohindra, R.; Tabberer, M.; Zhu, C.-Q.; et al. FULFIL Trial: Once-Daily Triple Therapy for Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2017, 196, 438–446. [Google Scholar] [CrossRef]
- Lipson, D.A.; Barnhart, F.; Brealey, N.; Brooks, J.; Criner, G.J.; Day, N.C.; Dransfield, M.T.; Halpin, D.M.; Han, M.K.; Jones, C.E.; et al. Once-Daily Single-Inhaler Triple versus Dual Therapy in Patients with COPD. N. Engl. J. Med. 2018, 378, 1671–1680. [Google Scholar] [CrossRef]
- Martin, A.R.; Finlay, W.H. Nebulizers for drug delivery to the lungs. Expert Opin. Drug Deliv. 2015, 12, 889–900. [Google Scholar] [CrossRef]
- Khan, I.; Hussein, S.; Houacine, C.; Sadozai, S.K.; Islam, Y.; Bnyan, R.; Elhissi, A.; Yousaf, S. Fabrication, characterization and optimization of nanostructured lipid carrier formulations using Beclomethasone dipropionate for pulmonary drug delivery via medical nebulizers. Int. J. Pharm. 2021, 598, 120376. [Google Scholar] [CrossRef]
- Dhayanandamoorthy, Y.; Antoniraj, M.G.; Kandregula, C.A.B.; Kandasamy, R. Aerosolized hyaluronic acid decorated, ferulic acid loaded chitosan nanoparticle: A promising asthma control strategy. Int. J. Pharm. 2020, 591, 119958. [Google Scholar] [CrossRef]
- Montoro, J.; Antolín-Amérigo, D.; Izquierdo-Domínguez, A.; Zapata, J.; González, G.; Valero, A. Impact of Asthma Inhalers on Global Climate: A Systematic Review of Their Carbon Footprint and Clinical Outcomes in Spain. J. Investig. Allergol. Clin. Immunol. 2023, 33, 250–262. [Google Scholar] [CrossRef]
- Kaur, I.; Aggarwal, B.; Gogtay, J. Integration of dose counters in pressurized metered-dose inhalers for patients with asthma and chronic obstructive pulmonary disease: Review of evidence. Expert Opin. Drug Deliv. 2015, 12, 1301–1310. [Google Scholar] [CrossRef]
- Komalla, V.; Wong, C.Y.J.; Sibum, I.; Muellinger, B.; Nijdam, W.; Chaugule, V.; Soria, J.; Ong, H.X.; Buchmann, N.A.; Traini, D. Advances in soft mist inhalers. Expert Opin. Drug Deliv. 2023. [Google Scholar] [CrossRef] [PubMed]
- Agusti, A.; Fabbri, L.M.; Singh, D.; Vestbo, J.; Celli, B.; Franssen, F.M.; Rabe, K.F.; Papi, A. Inhaled corticosteroids in COPD: Friend or foe? Eur. Respir. J. 2018, 52, 1801219. [Google Scholar] [CrossRef] [PubMed]
- Mattishent, K.; Thavarajah, M.; Blanco, P.; Gilbert, D.; Wilson, A.M.; Loke, Y.K. Meta-Review: Adverse Effects of Inhaled Corticosteroids Relevant to Older Patients. Drugs 2014, 74, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Cazzola, M.; Matera, M.G.; Rogliani, P. Adding a LAMA to ICS/LABA Therapy: A Meta-analysis of Triple Combination Therapy in COPD. Chest 2019, 155, 758–770. [Google Scholar] [CrossRef]
- Fitzpatrick, A.M.; Jackson, D.J.; Mauger, D.T.; Boehmer, S.J.; Phipatanakul, W.; Sheehan, W.J.; Moy, J.N.; Paul, I.M.; Bacharier, L.B.; Cabana, M.D.; et al. Individualized therapy for persistent asthma in young children. J. Allergy Clin. Immunol. 2016, 138, 1608–1618.e12. [Google Scholar] [CrossRef]
- Casula, L.; Sinico, C.; Valenti, D.; Pini, E.; Pireddu, R.; Schlich, M.; Lai, F.; Fadda, A.M. Delivery of beclomethasone dipropionate nanosuspensions with an electronic cigarette. Int. J. Pharm. 2021, 596, 120293. [Google Scholar] [CrossRef]
- Brandsma, C.; Berge, M.V.D.; Hackett, T.; Brusselle, G.; Timens, W. Recent advances in chronic obstructive pulmonary disease pathogenesis: From disease mechanisms to precision medicine. J. Pathol. 2020, 250, 624–635. [Google Scholar] [CrossRef]
- Werder, R.B.; Ullah, A.; Rahman, M.M.; Simpson, J.; Lynch, J.P.; Collinson, N.; Rittchen, S.; Rashid, R.B.; Sikder, A.A.; Handoko, H.Y.; et al. Targeting the P2Y13 Receptor Suppresses IL-33 and HMGB1 Release and Ameliorates Experimental Asthma. Am. J. Respir. Crit. Care Med. 2022, 205, 300–312. [Google Scholar] [CrossRef]
- Chantveerawong, T.; Sangkangjanavanich, S.; Chiewchalermsri, C.; Pradubpongsa, P.; Mitthamsiri, W.; Jindarat, S.; Wang, M.; Akdis, M.; Sokolowska, M.; Akdis, C.A.; et al. Increased circulating CRTH2+Tregs are associated with asthma control and exacerbation. Allergy 2022, 77, 681–685. [Google Scholar] [CrossRef]
- Burgess, J.K.; Jonker, M.R.; Berg, M.; Hacken, N.T.H.T.; Meyer, K.B.; Berge, M.v.D.; Nawijn, M.C.; Heijink, I.H. Periostin: Contributor to abnormal airway epithelial function in asthma? Eur. Respir. J. 2021, 57, 2001286. [Google Scholar] [CrossRef]
- Toki, S.; Goleniewska, K.; Zhang, J.; Zhou, W.; Newcomb, D.C.; Zhou, B.; Kita, H.; Boyd, K.L.; Peebles, R.S. TSLP and IL-33 reciprocally promote each other’s lung protein expression and ILC2 receptor expression to enhance innate type-2 airway inflammation. Allergy 2020, 75, 1606–1617. [Google Scholar] [CrossRef] [PubMed]
- Kupczyk, M.; Kuna, P. Targeting the PGD2/CRTH2/DP1 Signaling Pathway in Asthma and Allergic Disease: Current Status and Future Perspectives. Drugs 2017, 77, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Xiong, Y.; Li, W.; Cui, X.; Cheng, X.; Leng, Q.; He, R. IL-37 inhibits IL-4/IL-13-induced CCL11 production and lung eosinophilia in murine allergic asthma. Allergy 2018, 73, 1642–1652. [Google Scholar] [CrossRef]
- Tsai, M.-L.; Tsai, Y.-G.; Lin, Y.-C.; Hsu, Y.-L.; Chen, Y.-T.; Tsai, M.-K.; Liao, W.-T.; Lin, Y.-C.; Hung, C.-H. IL-25 Induced ROS-Mediated M2 Macrophage Polarization via AMPK-Associated Mitophagy. Int. J. Mol. Sci. 2021, 23, 3. [Google Scholar] [CrossRef]
- Jember, A.G.; Zuberi, R.; Liu, F.T.; Croft, M. Development of Allergic Inflammation in a Murine Model of Asthma Is Dependent on the Costimulatory Receptor Ox40. J. Exp. Med. 2001, 193, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Riemma, M.A.; Cerqua, I.; Romano, B.; Irollo, E.; Bertolino, A.; Camerlingo, R.; Granato, E.; Rea, G.; Scala, S.; Terlizzi, M.; et al. Sphingosine-1-phosphate/TGF-β axis drives epithelial mesenchymal transition in asthma-like disease. Br. J. Pharmacol. 2022, 179, 1753–1768. [Google Scholar] [CrossRef]
- Wang, J.; Lai, X.; Yao, S.; Chen, H.; Cai, J.; Luo, Y.; Wang, Y.; Qiu, Y.; Huang, Y.; Wei, X.; et al. Nestin promotes pulmonary fibrosis via facilitating recycling of TGF-β receptor I. Eur. Respir. J. 2022, 59, 2003721. [Google Scholar] [CrossRef]
- Wollin, L.; Wex, E.; Pautsch, A.; Schnapp, G.; Hostettler, K.E.; Stowasser, S.; Kolb, M. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur. Respir. J. 2015, 45, 1434–1445. [Google Scholar] [CrossRef]
- Aumiller, V.; Balsara, N.; Wilhelm, J.; Günther, A.; Königshoff, M. WNT/β-Catenin Signaling Induces IL-1β Expression by Alveolar Epithelial Cells in Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2013, 49, 96–104. [Google Scholar] [CrossRef]
- Craig, V.J.; Zhang, L.; Hagood, J.S.; Owen, C.A. Matrix Metalloproteinases as Therapeutic Targets for Idiopathic Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2015, 53, 585–600. [Google Scholar] [CrossRef]
- Davies, J.C.; Wainwright, C.E.; Canny, G.J.; Chilvers, M.A.; Howenstine, M.S.; Munck, A.; Mainz, J.G.; Rodriguez, S.; Li, H.; Yen, K.; et al. Efficacy and Safety of Ivacaftor in Patients Aged 6 to 11 Years with Cystic Fibrosis with aG551DMutation. Am. J. Respir. Crit. Care Med. 2013, 187, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Van Goor, F.; Hadida, S.; Grootenhuis, P.D.J.; Burton, B.; Stack, J.H.; Straley, K.S.; Decker, C.J.; Miller, M.; McCartney, J.; Olson, E.R.; et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc. Natl. Acad. Sci. USA 2011, 108, 18843–18848. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, C.E.; Elborn, J.S.; Ramsey, B.W.; Marigowda, G.; Huang, X.; Cipolli, M.; Colombo, C.; Davies, J.C.; De Boeck, K.; Flume, P.A.; et al. Lumacaftor–Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N. Engl. J. Med. 2015, 373, 220–231. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Gokarn, Y.; Mitragotri, S. Non-invasive delivery strategies for biologics. Nat. Rev. Drug Discov. 2019, 18, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Zhang, X.; Zeng, Y.; Lin, D.; Wu, J. Recent applications and strategies in nanotechnology for lung diseases. Nano Res. 2021, 14, 2067–2089. [Google Scholar] [CrossRef]
- Tay, H.L.; Foster, P.S. Biologics or immunotherapeutics for asthma? Pharmacol. Res. 2020, 158, 104782. [Google Scholar] [CrossRef]
- Wollin, L.; Distler, J.H.; Redente, E.F.; Riches, D.W.H.; Stowasser, S.; Schlenker-Herceg, R.; Maher, T.M.; Kolb, M. Potential of nintedanib in treatment of progressive fibrosing interstitial lung diseases. Eur. Respir. J. 2019, 54, 1900161. [Google Scholar] [CrossRef]
- Roth-Walter, F.; Adcock, I.M.; Benito-Villalvilla, C.; Bianchini, R.; Bjermer, L.; Caramori, G.; Cari, L.; Chung, K.F.; Diamant, Z.; Eguiluz-Gracia, I.; et al. Comparing biologicals and small molecule drug therapies for chronic respiratory diseases: An EAACI Taskforce on Immunopharmacology position paper. Allergy 2019, 74, 432–448. [Google Scholar] [CrossRef]
- Häußermann, S.; Arendsen, L.J.; Pritchard, J.N. Smart dry powder inhalers and intelligent adherence management. Adv. Drug Deliv. Rev. 2022, 191, 114580. [Google Scholar] [CrossRef] [PubMed]
- Drazen, J.M.; Harrington, D. New Biologics for Asthma. N. Engl. J. Med. 2018, 378, 2533–2534. [Google Scholar] [CrossRef]
- McGregor, M.C.; Krings, J.G.; Nair, P.; Castro, M. Role of Biologics in Asthma. Am. J. Respir. Crit. Care Med. 2019, 199, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Celli, B.R.; Wechsler, M.E.; Abdulai, R.M.; Luo, X.; Boomsma, M.M.; Staudinger, H.; Horowitz, J.E.; Baras, A.; Ferreira, M.A.; et al. Safety and efficacy of itepekimab in patients with moderate-to-severe COPD: A genetic association study and randomised, double-blind, phase 2a trial. Lancet Respir. Med. 2021, 9, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, C.E.; High, K.A.; Joung, J.K.; Kohn, D.B.; Ozawa, K.; Sadelain, M. Gene therapy comes of age. Science 2018, 359, eaan4672. [Google Scholar] [CrossRef] [PubMed]
- Robbins, P.D.; Ghivizzani, S.C. Viral vectors for gene therapy. Pharmacol. Ther. 1998, 80, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Ping, Y. Delivery of genome-editing biomacromolecules for treatment of lung genetic disorders. Adv. Drug Deliv. Rev. 2021, 168, 196–216. [Google Scholar] [CrossRef]
- Chiang, P.-C.; Chen, J.-C.; Chen, L.-C.; Kuo, M.-L. Adeno-Associated Virus-Mediated Interleukin-12 Gene Expression Alleviates Lung Inflammation and Type 2 T-Helper-Responses in Ovalbumin-Sensitized Asthmatic Mice. Hum. Gene Ther. 2022, 33, 1052–1061. [Google Scholar] [CrossRef]
- Do, D.C.; Mu, J.; Ke, X.; Sachdeva, K.; Qin, Z.; Wan, M.; Ishmael, F.T.; Gao, P. miR-511-3p protects against cockroach allergen–induced lung inflammation by antagonizing CCL2. J. Clin. Investig. 2019, 4, 126832. [Google Scholar] [CrossRef]
- Li, R.; Wang, F.; Wei, J.; Lin, Y.; Tang, G.; Rao, L.; Ma, L.; Xu, Q.; Wu, J.; Lv, Q.; et al. The Role of Macrophage Migration Inhibitory Factor (MIF) in Asthmatic Airway Remodeling. Allergy Asthma Immunol. Res. 2021, 13, 88–105. [Google Scholar] [CrossRef]
- Ustiugova, A.S.; Dvorianinova, E.M.; Melnikova, N.V.; Dmitriev, A.A.; Kuprash, D.V.; Afanasyeva, M.A. CRISPR/Cas9 genome editing demonstrates functionality of the autoimmunity-associated SNP rs12946510. Biochim. Biophys. Acta 2023, 1869, 166599. [Google Scholar] [CrossRef]
- Shen, S.; Sanchez, M.E.; Blomenkamp, K.; Corcoran, E.M.; Marco, E.; Yudkoff, C.J.; Jiang, H.; Teckman, J.H.; Bumcrot, D.; Albright, C.F. Amelioration of Alpha-1 Antitrypsin Deficiency Diseases with Genome Editing in Transgenic Mice. Hum. Gene Ther. 2018, 29, 861–873. [Google Scholar] [CrossRef]
- Zieger, M.; Borel, F.; Greer, C.; Gernoux, G.; Blackwood, M.; Flotte, T.R.; Mueller, C. Liver-directed SERPINA1 gene therapy attenuates progression of spontaneous and tobacco smoke-induced emphysema in α1-antitrypsin null mice. Mol. Ther. Methods Clin. Dev. 2022, 25, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Bjursell, M.; Porritt, M.J.; Ericson, E.; Taheri-Ghahfarokhi, A.; Clausen, M.; Magnusson, L.; Admyre, T.; Nitsch, R.; Mayr, L.; Aasehaug, L.; et al. Therapeutic Genome Editing with CRISPR/Cas9 in a Humanized Mouse Model Ameliorates α1-antitrypsin Deficiency Phenotype. Ebiomedicine 2018, 29, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Cooney, A.L.; Thornell, I.M.; Singh, B.K.; Shah, V.S.; Stoltz, D.A.; McCray, P.B.; Zabner, J.; Sinn, P.L. A Novel AAV-mediated Gene Delivery System Corrects CFTR Function in Pigs. Am. J. Respir. Cell Mol. Biol. 2019, 61, 747–754. [Google Scholar] [CrossRef]
- Moss, R.B.; Milla, C.; Colombo, J.; Accurso, F.; Zeitlin, P.L.; Clancy, J.P.; Spencer, L.T.; Pilewski, J.; Waltz, D.A.; Dorkin, H.L.; et al. Repeated Aerosolized AAV-CFTR for Treatment of Cystic Fibrosis: A Randomized Placebo-Controlled Phase 2B Trial. Hum. Gene Ther. 2007, 18, 726–732. [Google Scholar] [CrossRef]
- Geurts, M.H.; de Poel, E.; Amatngalim, G.D.; Oka, R.; Meijers, F.M.; Kruisselbrink, E.; van Mourik, P.; Berkers, G.; Groot, K.M.d.W.-D.; Michel, S.; et al. CRISPR-Based Adenine Editors Correct Nonsense Mutations in a Cystic Fibrosis Organoid Biobank. Cell Stem Cell 2020, 26, 503–510.e7. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sharma, A.R.; Bhattacharya, M.; Lee, S.-S.; Chakraborty, C. CRISPR-Cas9: A Preclinical and Clinical Perspective for the Treatment of Human Diseases. Mol. Ther. 2021, 29, 571–586. [Google Scholar] [CrossRef]
- Zimmermann, C.M.; Baldassi, D.; Chan, K.; Adams, N.B.; Neumann, A.; Porras-Gonzalez, D.L.; Wei, X.; Kneidinger, N.; Stoleriu, M.G.; Burgstaller, G.; et al. Spray drying siRNA-lipid nanoparticles for dry powder pulmonary delivery. J. Control. Release 2022, 351, 137–150. [Google Scholar] [CrossRef]
- Duan, L.; Xu, L.; Xu, X.; Qin, Z.; Zhou, X.; Xiao, Y.; Liang, Y.; Xia, J. Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale 2021, 13, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, C.C.; Amatullah, H.; Vaswani, C.M.; Maron-Gutierrez, T.; Kim, M.; Mei, S.H.; Szaszi, K.; Monteiro, A.P.T.; Varkouhi, A.K.; Herreroz, R.; et al. Mesenchymal Stromal (stem) Cell (MSC) therapy modulates miR-193b-5p expression to attenuate sepsis-induced acute lung injury. Eur. Respir. J. 2022, 59, 2004216. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.M.; Obeidat, M.; Sadatsafavi, M.; Sin, D.D. Introduction to precision medicine in COPD. Eur. Respir. J. 2019, 53, 1802460. [Google Scholar] [CrossRef]
- Ramsheh, M.Y.; Haldar, K.; Esteve-Codina, A.; Purser, L.F.; Richardson, M.; Müller-Quernheim, J.; Greulich, T.; Nowinski, A.; Barta, I.; Stendardo, M.; et al. Lung microbiome composition and bronchial epithelial gene expression in patients with COPD versus healthy individuals: A bacterial 16S rRNA gene sequencing and host transcriptomic analysis. Lancet Microbe 2021, 2, e300–e310. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.T.-J.; Cant, E.; Keir, H.R.; Barton, A.K.; Kuzmanova, E.; Shuttleworth, M.; Pollock, J.; Finch, S.; Polverino, E.; Bottier, M.; et al. Endotyping Chronic Obstructive Pulmonary Disease, Bronchiectasis, and the “Chronic Obstructive Pulmonary Disease–Bronchiectasis Association”. Am. J. Respir. Crit. Care Med. 2022, 206, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Murphy, V.E.; Porsbjerg, C.M.; Robijn, A.L.; Gibson, P.G. Biomarker-guided management reduces exacerbations in non-eosinophilic asthma in pregnancy: A secondary analysis of a randomized controlled trial. Respirology 2020, 25, 719–725. [Google Scholar] [CrossRef]
- Malinovschi, A. Limited use of biomarker-guided therapy in mild asthma. Lancet Respir. Med. 2020, 8, 648–649. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.; Cao, H.; FitzGerald, J.M.; Iannotti, N.; Yang, E.; Kocks, J.W.H.; Kostikas, K.; Price, D.; Reddel, H.K.; Tsiligianni, I.; et al. Artificial Intelligence/Machine Learning in Respiratory Medicine and Potential Role in Asthma and COPD Diagnosis. J. Allergy Clin. Immunol. Pract. 2021, 9, 2255–2261. [Google Scholar] [CrossRef] [PubMed]
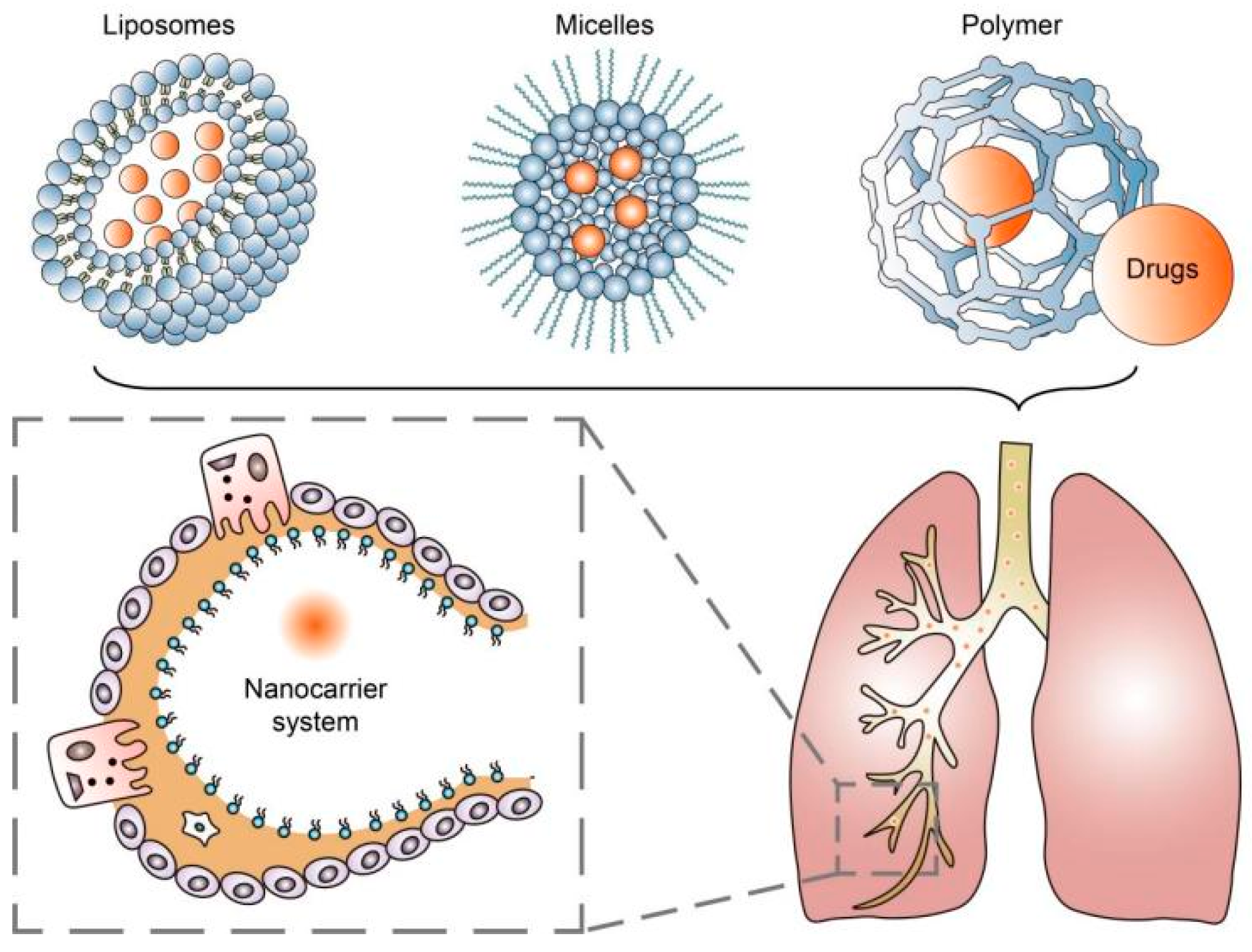


| Diseases | Type of Nanoparticles | Drugs | Target Ligands | Targets | References |
|---|---|---|---|---|---|
| Asthma | SPION | None | IL4Rα monoclonal antibody | ASMs | [35] |
| SPION | None | Anti-ST2 blocking antibodies | Inflammatory lung tissue | [36] | |
| PLGA-based nanoparticles | Smart silencer of Dnmt3aos | Exosome membrane of M2 macrophages | M2 macrophages | [40] | |
| LNP | Polyinosinic-polycytidylic acid | None | Lung epithelial cells | [41] | |
| COPD | HNP | siRNA against SCNN1A and SCNN1B | None | Lung epithelial cells | [42] |
| LNP | siRNA against TNF-α | None | None | [43] | |
| IPF | LNP | siRNA against IL-11 | None | MLFs | [44] |
| CF | LNP | Plasmid DNA | ICAM-1 targeting peptide | Lung epithelial cells | [45] |
| Type of Inhaler | Subtype | Characteristics | Advantages | Limitations | References |
|---|---|---|---|---|---|
| Nebulizers | Jet (or pneumatic) | Use compressed air or oxygen to convert liquid medication into a fine mist for inhalation. | Versatile and suitable for all ages. | Longer administration times, produce noise and vibration, require power sources, and need regular maintenance. | [76] |
| Ultrasonic | Use high-frequency vibrations to convert liquid medication into a fine mist for inhalation. | Portable and compact, have faster administration times, operate quietly. | Not suitable for medications that are heat-sensitive or contain suspensions. | [77] | |
| Mesh | Use a vibrating mesh or perforated plate to generate a fine aerosol mist from liquid medication. | Portable, lightweight, and operate silently with faster administration times. | Have limitations in delivering higher viscosity medications or large medication volumes. | [78] | |
| Dry powder inhalers (DPIs) | Single- and multi-unit doses | Deliver medication directly to the lungs in a powdered form. | Breath-activated, portable, and do not require coordination between inhalation and device activation. | Require adequate inspiratory flow for optimal drug delivery, and can be used only with specific types of dry powder medications. | [79] |
| Pressurized metered-dose inhalers (pMDIs) | Single and combined drugs | Deliver medication in a pressurized aerosol form using propellants. | Deliver a consistent dose, require minimal preparation time. | The presence of propellants and the inability to assess remaining medication levels easily. | [80] |
| Soft mist inhalers (SMIs) | None | Deliver medication as a slow-moving aerosol mist. | Provide consistent and precise dosing, generate a slow-moving mist suitable for patients with diverse inspiratory abilities, and are equipped with dose counters to monitor medication levels. | Potential clogging if not used properly, higher cost compared with other inhalers, and limited availability of medications in soft mist formulation. | [81] |
| Chronic Inflammatory Respiratory Diseases | Targets | Mechanism/Effect/Receptor | Research Progress | References |
|---|---|---|---|---|
| Asthma | IL-33 | When IL-33 binds to its receptor ST2, it can trigger inflammation and airway hyperresponsiveness. | The knockdown of P2Y13-R can regulate the release of IL-33 and prevent experimental asthma. | [88] |
| TSLP | A cytokine involved in regulating the immune system. | TSLP can promote the activation of ILC2 and induce congenital allergic inflammation. | [91] | |
| CRTH2 | A receptor mainly expressed on Th2 cells. | A CRTH2 antagonist (OC000459) can effectively reduce the increase in eosinophils and swelling of nasal mucosa. CRTH2 and TP antagonists have been registered for clinical use in asthma. | [92] | |
| IL-4/IL-13 | Two cytokines involved in regulating immune responses. | IL-4/IL-13 stimulate CCL-11 production to alleviate HDM-induced asthma. | [93] | |
| IL-25 | Also known as IL-17E, a cytokine primarily expressed in respiratory epithelial cells. | IL-25 induces excessive production of ROS through AMPK-related mitochondrial autophagy, leading to airway inflammation and remodeling in asthma. | [94] | |
| OX40 | A co-stimulatory molecule that plays an important role in T-cell activation. | OX40-deficient mice exhibit reduced lung inflammation and weakened airway hyperresponsiveness | [95] | |
| S1P | A physiologically active lipid molecule that plays an important role in the immune system. | Bronchial specimens harvested from S1P-overexpressing mice showed overexpression of EMT-related markers and bronchial hyperresponsiveness. | [96] | |
| IPF | TGF-β | An important growth factor that plays an important role in the pathological process of IPF. | Nestin knockdown inhibited TGF-β signaling by suppressing the recycling of TβRI to the cell surface. | [97] |
| IL-11 | IL-11 activates multiple signal transduction pathways by binding to its receptor, IL-11Rα, thereby promoting the activation and proliferation of fibroblasts. | An inhalable and mucus-penetrative nanoparticle (NP) system incorporating siRNA against IL11 (siIL11@PPGC NPs) hindered fibroblast differentiation and reduced ECM deposition via inhibition of ERK and SMAD2. | [44] | |
| PDGF | A cell growth factor that is involved in fibrocyte proliferation, inflammatory response, and the occurrence of IPF. | Nintedanib, a potent small-molecule inhibitor of the receptor tyrosine kinases PDGF receptor, has shown consistent anti-fibrotic and anti-inflammatory activity in animal models of lung fibrosis. | [98] | |
| Wnt/β-catenin | An important signaling pathway involved in biological processes such as cell proliferation and differentiation. | Activation of Wnt/β-catenin led to a significant increase in IL-1β and IL-6 in mice. | [99] | |
| MMPs | MMPs are involved in the process of lung tissue remodeling and fibrosis. | Clinical research reports show a significant increase in MMP levels in blood and lung samples from patients with IPF. Most MMPs can promote the development of IPF in mouse models. | [100] | |
| CF | CFTR | The CFTR protein forms a channel on the cell membrane that primarily regulates chloride ion (Cl−) transport, maintaining water and salt balance. When the CFTR gene mutates, it can affect the production and excretion of mucus. | Currently available CFTR modulators: ivacaftor, lumacaftor, Orkambi (a combination of lumacaftor and ivacaftor). | [101,102,103] |
| Chronic Inflammatory Diseases | Gene | Research Progress | References |
|---|---|---|---|
| Asthma | IL-12 | Overexpression of single chain IL-12 (scIL-12) through rAAV vector significantly suppressed the total number of cells and eosinophil infiltration as well as the mucus secretion in mice. | [116] |
| CTNNAL1 | Airway hyperresponsiveness and inflammation were significantly attenuated in mice pretransduced with AAV-miR-511-3p. | [117] | |
| MIF | Intratracheal adeno-associated virus (AAV) vector (MIF-mutant AAV57) could reduce airway remodeling in asthmatic mice. | [118] | |
| SNP rs12946510 | CRISPR–Cas9 genome editing demonstrated that the SNP of rs12946510 was associated with asthma. | [119] | |
| AATD (An important cause of COPD) | SERPINA1 | The systemic delivery of AAV8-CRISPR, targeting exon 2 of hSERPINA1, and the AAV, which provided the donor template to correct the Z mutation, could both restore modest levels of wildtype AAT-M in a mouse model of AATD. | [120] |
| rAAV-mediated SERPINA1 gene augmentation largely preserved lung tissue elasticity and alveolar wall integrity in mice models. | [121] | ||
| The therapeutic application of CRISPR–Cas9 for genome editing in a humanized mouse model successfully mitigated the phenotype of AATD. | [122] | ||
| CF | CFTR | Complementing CFTR in CF pigs with AAV rescued the anion transport defect in a large-animal CF model. | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wang, P.; Shao, Y.; He, D. Advancing Treatment Strategies: A Comprehensive Review of Drug Delivery Innovations for Chronic Inflammatory Respiratory Diseases. Pharmaceutics 2023, 15, 2151. https://doi.org/10.3390/pharmaceutics15082151
Wang J, Wang P, Shao Y, He D. Advancing Treatment Strategies: A Comprehensive Review of Drug Delivery Innovations for Chronic Inflammatory Respiratory Diseases. Pharmaceutics. 2023; 15(8):2151. https://doi.org/10.3390/pharmaceutics15082151
Chicago/Turabian StyleWang, Junming, Pengfei Wang, Yiru Shao, and Daikun He. 2023. "Advancing Treatment Strategies: A Comprehensive Review of Drug Delivery Innovations for Chronic Inflammatory Respiratory Diseases" Pharmaceutics 15, no. 8: 2151. https://doi.org/10.3390/pharmaceutics15082151
APA StyleWang, J., Wang, P., Shao, Y., & He, D. (2023). Advancing Treatment Strategies: A Comprehensive Review of Drug Delivery Innovations for Chronic Inflammatory Respiratory Diseases. Pharmaceutics, 15(8), 2151. https://doi.org/10.3390/pharmaceutics15082151





