Modified mRNA Formulation and Stability for Cardiac and Skeletal Muscle Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Construction of Templates and Synthesis of Synthetic of mRNA and Synthetic modRNA
2.2. BMDM Differentiation and FACS Staining
2.3. Cell Transfections and Bioluminescent Imaging
2.4. In Vivo modRNA Injections
2.5. Quality Control of LNP-RNA Formulations
2.6. Bioluminescence Imaging
3. Results
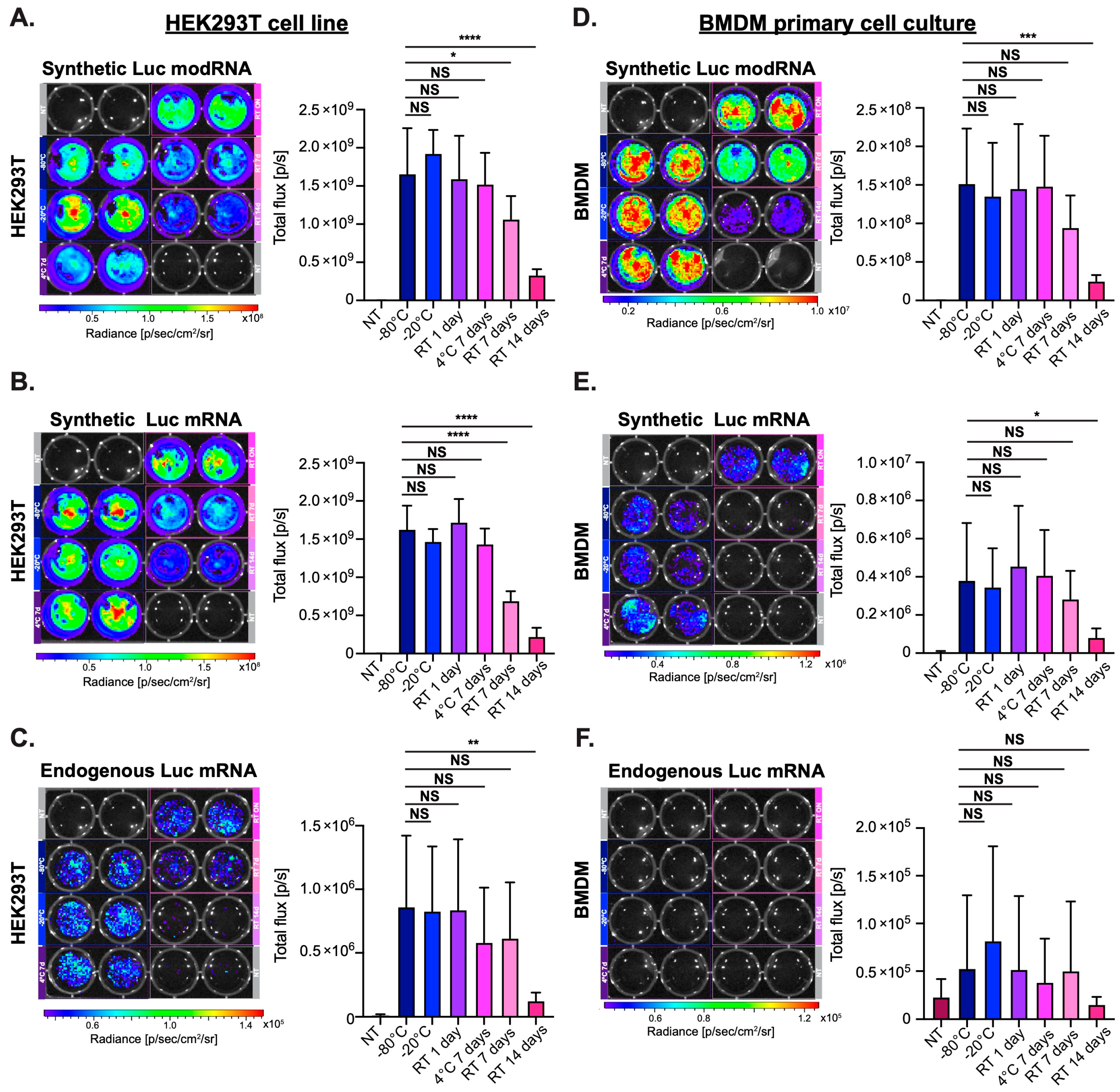
3.1. Synthetic modRNA but Not Synthetic Non-Modified mRNA or Endogenous mRNA Translates Well in Primary Cell Culture
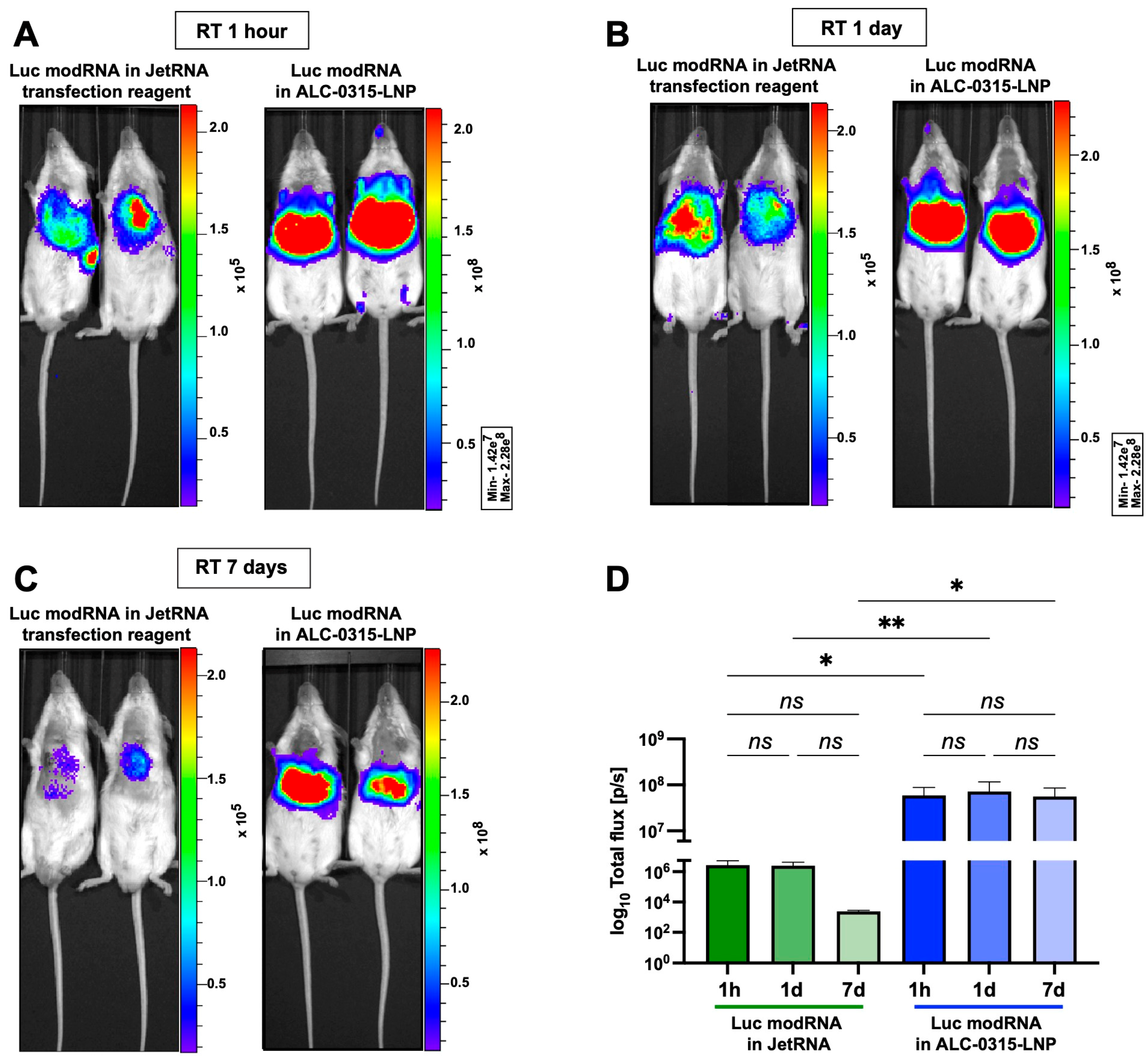
3.2. Naked modRNA Expresses Predominantly in the Injection Area and Keeps Its Integrity When Stored for One Day at Room Temperature (RT) or up to Seven Days at 4 °C
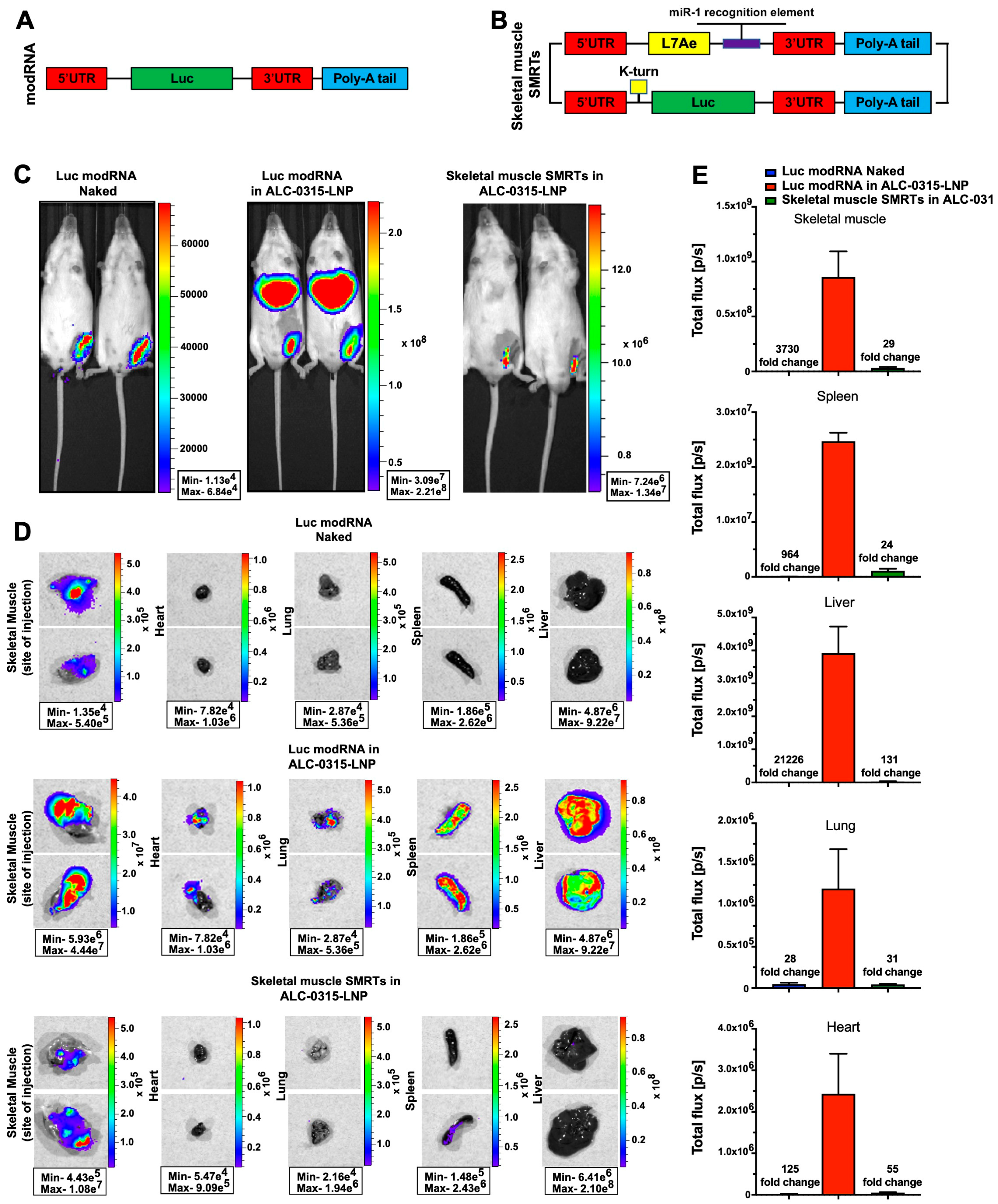
3.3. Direct Myocardial Injection of ALC-0315-Encapsulated modRNA Causes Protein Translation at the Injection Site and in Major Mouse Organs
3.4. Direct Intramuscular Injection of LNP-Encapsulated Skeletal Muscle SMRTs Allows Efficient Translation Primarily at the Site of Injection, and Not in Other Mouse Organs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pilishvili, T.; Gierke, R.; Fleming-Dutra, K.E.; Farrar, J.L.; Mohr, N.M.; Talan, D.A.; Krishnadasan, A.; Harland, K.K.; Smithline, H.A.; Hou, P.C.; et al. Effectiveness of mRNA COVID-19 Vaccine among U.S. Health Care Personnel. N. Engl. J. Med. 2021, 385, e90. [Google Scholar] [CrossRef]
- El Sahly, H.M.; Baden, L.R.; Essink, B.; Doblecki-Lewis, S.; Martin, J.M.; Anderson, E.J.; Campbell, T.B.; Clark, J.; Jackson, L.A.; Fichtenbaum, C.J.; et al. Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. N. Engl. J. Med. 2021, 385, 1774–1785. [Google Scholar] [CrossRef]
- Zangi, L.; Oliveira, M.S.; Ye, L.Y.; Ma, Q.; Sultana, N.; Hadas, Y.; Chepurko, E.; Später, D.; Zhou, B.; Chew, W.L.; et al. Insulin-Like Growth Factor 1 Receptor-Dependent Pathway Drives Epicardial Adipose Tissue Formation After Myocardial Injury. Circulation 2017, 135, 59–72. [Google Scholar] [CrossRef]
- Zangi, L.; Lui, K.O.; von Gise, A.; Ma, Q.; Ebina, W.; Ptaszek, L.M.; Später, D.; Xu, H.; Tabebordbar, M.; Gorbatov, R.; et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat. Biotechnol. 2013, 31, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, I.C.; Eltoukhy, A.A.; Fish, K.M.; Nonnenmacher, M.; Ishikawa, K.; Chen, J.; Hajjar, R.J.; Anderson, D.G.; Costa, K.D. Myocardial Delivery of Lipidoid Nanoparticle Carrying modRNA Induces Rapid and Transient Expression. Mol. Ther. 2016, 24, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Magadum, A.; Hadas, Y.; Kondrat, J.; Singh, N.; Youssef, E.; Calderon, D.; Chepurko, E.; Dubois, N.; Hajjar, R.J.; et al. Optimizing Cardiac Delivery of Modified mRNA. Mol. Ther. 2017, 25, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Kondrat, J.; Sultana, N.; Zangi, L. Synthesis of Modified mRNA for Myocardial Delivery. Methods Mol. Biol. 2017, 1521, 127–138. [Google Scholar] [CrossRef]
- Huang, C.-L.; Leblond, A.-L.; Turner, E.C.; Kumar, A.H.; Martin, K.; Whelan, D.; O’sullivan, D.M.; Caplice, N.M. Synthetic Chemically Modified mRNA-Based Delivery of Cytoprotective Factor Promotes Early Cardiomyocyte Survival Post-Acute Myocardial Infarction. Mol. Pharm. 2015, 12, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of Pseudouridine Into mRNA Yields Superior Nonimmunogenic Vector With Increased Translational Capacity and Biological Stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef]
- Hadas, Y.; Vincek, A.S.; Youssef, E.; Żak, M.M.; Chepurko, E.; Sultana, N.; Sharkar, M.T.K.; Guo, N.; Komargodski, R.; Kurian, A.A.; et al. Altering Sphingolipid Metabolism Attenuates Cell Death and Inflammatory Response After Myocardial Infarction. Circulation 2020, 141, 916–930. [Google Scholar] [CrossRef]
- Magadum, A.; Singh, N.; Kurian, A.A.; Sharkar, M.T.K.; Chepurko, E.; Zangi, L. Ablation of a Single N-Glycosylation Site in Human FSTL 1 Induces Cardiomyocyte Proliferation and Cardiac Regeneration. Mol. Ther. Nucleic Acids 2018, 13, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Magadum, A.; Singh, N.; Kurian, A.A.; Munir, I.; Mehmood, T.; Brown, K.; Sharkar, M.T.K.; Chepurko, E.; Sassi, Y.; Oh, J.G.; et al. Pkm2 Regulates Cardiomyocyte Cell Cycle and Promotes Cardiac Regeneration. Circulation 2020, 141, 1249–1265. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Hadas, Y.; Kurian, A.A.; Żak, M.M.; Yoo, J.; Mahmood, A.; Girard, H.; Komargodski, R.; Io, T.; Santini, M.P.; et al. Direct reprogramming induces vascular regeneration post muscle ischemic injury. Mol. Ther. 2021, 29, 3042–3058. [Google Scholar] [CrossRef]
- Magadum, A.; Singh, N.; Kurian, A.A.; Sharkar, M.T.K.; Sultana, N.; Chepurko, E.; Kaur, K.; Żak, M.M.; Hadas, Y.; Lebeche, D.; et al. Therapeutic Delivery of Pip4k2c-Modified mRNA Attenuates Cardiac Hypertrophy and Fibrosis in the Failing Heart. Adv. Sci. 2021, 8, 2004661. [Google Scholar] [CrossRef]
- Uddin, M.N.; Roni, M.A. Challenges of Storage and Stability of mRNA-Based COVID-19 Vaccines. Vaccines 2021, 9, 1033. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. More Flexible Storage Conditions for BioNTech/Pfizer’s COVID-19 Vaccine. Available online: https://www.ema.europa.eu/en/news/more-flexible-storage-conditions-biontechpfizers-covid-19-vaccine (accessed on 8 June 2023).
- Yonker, L.M.; Swank, Z.; Bartsch, Y.C.; Burns, M.D.; Kane, A.; Boribong, B.P.; Davis, J.P.; Loiselle, M.; Novak, T.; Senussi, Y.; et al. Circulating Spike Protein Detected in Post–COVID-19 mRNA Vaccine Myocarditis. Circulation 2023, 147, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, Y.; Zhao, Y.; Lung, D.C.; Ye, Z.; Song, W.; Liu, F.-F.; Cai, J.-P.; Wong, W.-M.; Yip, C.C.-Y.; et al. Intravenous Injection of Coronavirus Disease 2019 (COVID-19) mRNA Vaccine Can Induce Acute Myopericarditis in Mouse Model. Clin. Infect. Dis. 2021, 74, 1933–1950. [Google Scholar] [CrossRef]
- Halsell, J.S.; Riddle, J.R.; Atwood, J.E.; Gardner, P.; Shope, R.; Poland, G.A.; Gray, G.C.; Ostroff, S.; Eckart, R.E.; Hospenthal, D.R.; et al. Myopericarditis Following Smallpox Vaccination Among Vaccinia-Naive US Military Personnel. JAMA 2003, 289, 3283–3289. [Google Scholar] [CrossRef] [PubMed]
- Keinath, K.; Church, T.; Kurth, B.; Hulten, E. Myocarditis secondary to smallpox vaccination. BMJ Case Rep. 2018, 2018, bcr2017223523. [Google Scholar] [CrossRef]
- Mei, R.; Raschi, E.; Forcesi, E.; Diemberger, I.; De Ponti, F.; Poluzzi, E. Myocarditis and pericarditis after immunization: Gaining insights through the Vaccine Adverse Event Reporting System. Int. J. Cardiol. 2018, 273, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Magadum, A.; Kurian, A.A.; Chepurko, E.; Sassi, Y.; Hajjar, R.J.; Zangi, L. Specific Modified mRNA Translation System. Circulation 2020, 142, 2485–2488. [Google Scholar] [CrossRef]
- Chen, J.-F.; Mandel, E.M.; Thomson, J.M.; Wu, Q.; Callis, T.E.; Hammond, S.M.; Conlon, F.L.; Wang, D.-Z. The Role of MicroRNA-1 and MicroRNA-133 in Skeletal Muscle Proliferation and Differentiation. Nat. Genet. 2005, 38, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Sharkar, M.T.K.; Hadas, Y.; Chepurko, E.; Zangi, L. In Vitro Synthesis of Modified RNA for Cardiac Gene Therapy. Methods Mol. Biol. 2020, 2158, 281–294. [Google Scholar] [CrossRef]
- Kaur, K.; Sultana, N.; Hadas, Y.; Magadum, A.; Sharkar, M.T.K.; Chepurko, E.; Zangi, L. Delivery of Modified mRNA in a Myocardial Infarction Mouse Model. J. Vis. Exp. 2020, 160, e60832. [Google Scholar] [CrossRef]
- Malone, R.W.; Felgner, P.L.; Verma, I.M. Cationic liposome-mediated RNA transfection. Proc. Natl. Acad. Sci. USA 1989, 86, 6077–6081. [Google Scholar] [CrossRef] [PubMed]
- Auer, H.; Mobley, J.; Ayers, L.; Bowen, J.; Chuaqui, R.; Johnson, L.; Livolsi, V.; Lubensky, I.; McGarvey, D.; Monovich, L.; et al. The effects of frozen tissue storage conditions on the integrity of RNA and protein. Biotech. Histochem. 2014, 89, 518–528. [Google Scholar] [CrossRef]
- Halfon, P.; Khiri, H.; Gerolami, V.; Bourliere, M.; Feryn, J.M.; Reynier, P.; Gauthier, A.; Cartouzou, G. Impact of various handling and storage conditions on quantitative detection of hepatitis C virus RNA. J. Hepatol. 1996, 25, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Seelenfreund, E.; Robinson, W.A.; Amato, C.M.; Tan, A.-C.; Kim, J.; Robinson, S.E. Long Term Storage of Dry versus Frozen RNA for Next Generation Molecular Studies. PLoS ONE 2014, 9, e111827. [Google Scholar] [CrossRef]
- Collén, A.; Bergenhem, N.; Carlsson, L.; Chien, K.R.; Hoge, S.; Gan, L.-M.; Fritsche-Danielson, R. VEGFA mRNA for regenerative treatment of heart failure. Nat. Rev. Drug Discov. 2021, 21, 79–80. [Google Scholar] [CrossRef]
- Rouf, N.Z.; Biswas, S.; Tarannum, N.; Oishee, L.M.; Muna, M.M. Demystifying mRNA vaccines: An emerging platform at the forefront of cryptic diseases. RNA Biol. 2022, 19, 386–410. [Google Scholar] [CrossRef]
- Kim, B.; Hosn, R.R.; Remba, T.; Yun, D.; Li, N.; Abraham, W.; Melo, M.B.; Cortes, M.; Li, B.; Zhang, Y.; et al. Optimization of storage conditions for lipid nanoparticle-formulated self-replicating RNA vaccines. J. Control. Release 2023, 353, 241–253. [Google Scholar] [CrossRef]
- Kobiyama, K.; Ishii, K.J. Making innate sense of mRNA vaccine adjuvanticity. Nat. Immunol. 2022, 23, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A.; et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023, 618, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, J.N. The importance of injecting vaccines into muscle. Different patients need different needle sizes. BMJ 2000, 321, 1237–1238. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Manabe, I.; Oishi, Y. Mechanisms of cooperative cell-cell interactions in skeletal muscle regeneration. Inflamm. Regen. 2022, 42, 48. [Google Scholar] [CrossRef]
- Huang, Q.; Ji, K.; Tian, S.; Wang, F.; Huang, B.; Tong, Z.; Tan, S.; Hao, J.; Wang, Q.; Tan, W.; et al. A single-dose mRNA vaccine provides a long-term protection for hACE2 transgenic mice from SARS-CoV-2. Nat. Commun. 2021, 12, 776. [Google Scholar] [CrossRef]
- Ji, R.-R.; Qu, Y.; Zhu, H.; Yang, Y.; Vogel, A.B.; Sahin, U.; Qin, C.; Hui, A. BNT162b2 Vaccine Encoding the SARS-CoV-2 P2 S Protects Transgenic hACE2 Mice against COVID-19. Vaccines 2021, 9, 324. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żak, M.M.; Kaur, K.; Yoo, J.; Kurian, A.A.; Adjmi, M.; Mainkar, G.; Yoon, S.; Zangi, L. Modified mRNA Formulation and Stability for Cardiac and Skeletal Muscle Delivery. Pharmaceutics 2023, 15, 2176. https://doi.org/10.3390/pharmaceutics15092176
Żak MM, Kaur K, Yoo J, Kurian AA, Adjmi M, Mainkar G, Yoon S, Zangi L. Modified mRNA Formulation and Stability for Cardiac and Skeletal Muscle Delivery. Pharmaceutics. 2023; 15(9):2176. https://doi.org/10.3390/pharmaceutics15092176
Chicago/Turabian StyleŻak, Magdalena M., Keerat Kaur, Jimeen Yoo, Ann Anu Kurian, Matthew Adjmi, Gayatri Mainkar, Seonghun Yoon, and Lior Zangi. 2023. "Modified mRNA Formulation and Stability for Cardiac and Skeletal Muscle Delivery" Pharmaceutics 15, no. 9: 2176. https://doi.org/10.3390/pharmaceutics15092176




