Meropenem PK/PD Variability and Renal Function: “We Go Together”
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Demographic and Clinical Data
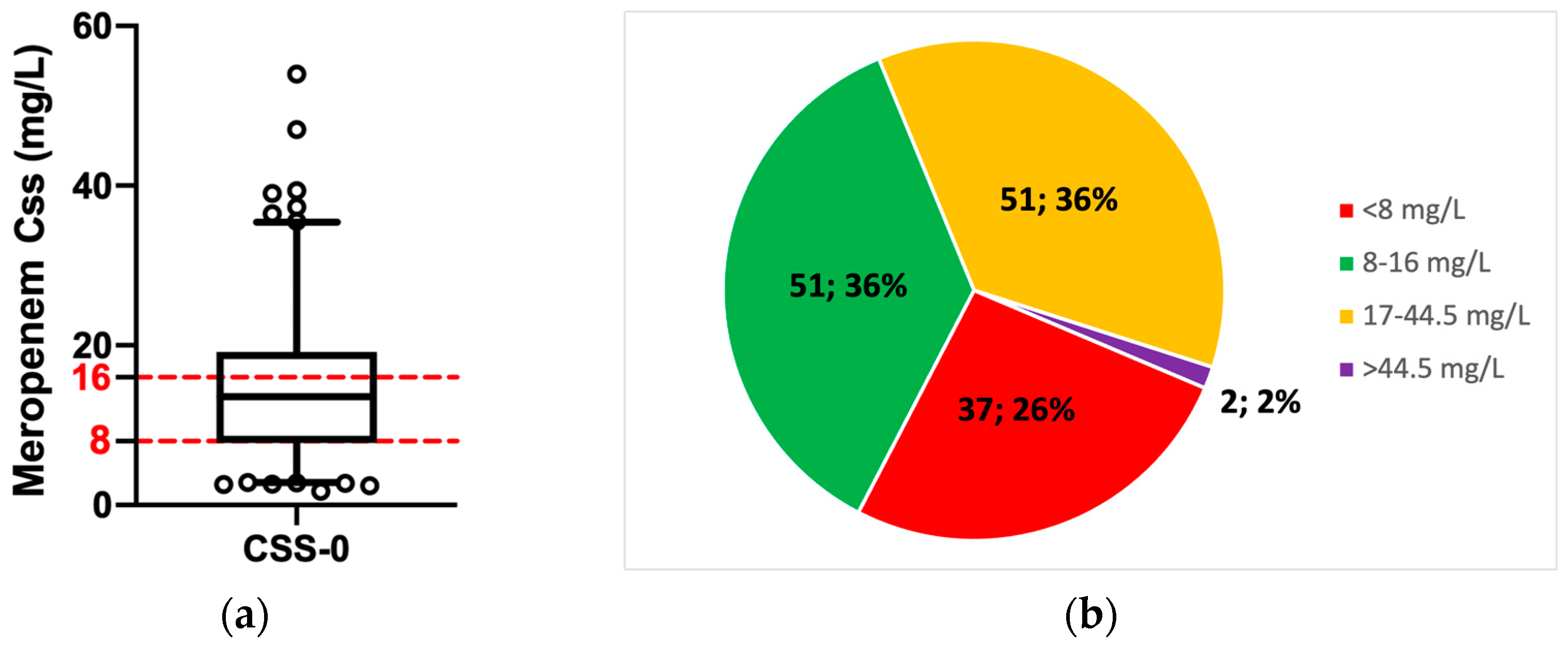
3.2. Patients’ Exposure to Meropenem
3.3. Meropenem Exposure and Renal Function
3.4. Meropenem Exposure and Other Clinical Data (Sex, BMI, and Age)
3.5. The Support of the Therapeutic Drug Monitoring in the Meropenem Dosing Management
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roberts, J.A.; Abdul-Aziz, M.-H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Hope, W.W.; Roberts, J.A. Applying Pharmacokinetic/Pharmacodynamic Principles in Critically Ill Patients: Optimizing Efficacy and Reducing Resistance Development. Semin. Respir. Crit. Care Med. 2015, 36, 136–153. [Google Scholar] [CrossRef]
- Fawaz, S.; Barton, S.; Whitney, L.; Swinden, J.; Nabhani-Gebara, S. Stability of Meropenem After Reconstitution for Administration by Prolonged Infusion. Hosp. Pharm. 2019, 54, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chen, F.; Lei, J.; Zhou, B. Clinical outcomes of continuous vs intermittent meropenem infusion for the treatment of sepsis: A systematic review and meta-analysis. Adv. Clin. Exp. Med. 2020, 29, 993–1000. [Google Scholar] [CrossRef]
- Taccone, F.S.; Laterre, P.-F.; Dugernier, T.; Spapen, H.; Delattre, I.; Witebolle, X.; De Backer, D.; Layeux, B.; Wallemacq, P.; Vincent, J.-L.; et al. Insufficient β-lactam concentrations in the early phase of severe sepsis and septic shock. Crit. Care 2010, 14, R126. [Google Scholar] [CrossRef]
- Ehmann, L.; Zoller, M.; Minichmayr, I.K.; Scharf, C.; Maier, B.; Schmitt, M.V.; Hartung, N.; Huisinga, W.; Vogeser, M.; Frey, L.; et al. Role of renal function in risk assessment of target non-attainment after standard dosing of meropenem in critically ill patients: A prospective observational study. Crit. Care 2017, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef]
- Bauer, M.; Gerlach, H.; Vogelmann, T.; Preissing, F.; Stiefel, J.; Adam, D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019—Results from a systematic review and meta-analysis. Crit. Care 2020, 24, 239. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.-H.; Alffenaar, J.-W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper#. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- Schoenenberger-Arnaiz, J.A.; Ahmad-Diaz, F.; Miralbes-Torner, M.; Aragones-Eroles, A.; Cano-Marron, M.; Palomar-Martinez, M. Usefulness of therapeutic drug monitoring of piperacillin and meropenem in routine clinical practice: A prospective cohort study in critically ill patients. Eur. J. Hosp. Pharm. 2020, 27, e30–e35. [Google Scholar] [CrossRef]
- Mangalore, R.P.; Peel, T.N.; A Udy, A.; Peleg, A.Y. The clinical application of beta-lactam antibiotic therapeutic drug monitoring in the critical care setting. J. Antimicrob. Chemother. 2023, dkad223. [Google Scholar] [CrossRef] [PubMed]
- Mendez, A.S.; Steppe, M.; Schapoval, E.E. Validation of HPLC and UV spectrophotometric methods for the determination of meropenem in pharmaceutical dosage form. J. Pharm. Biomed. Anal. 2003, 33, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Pea, F.; Viale, P.; Cojutti, P.; Furlanut, M. Dosing Nomograms for Attaining Optimum Concentrations of Meropenem by Continuous Infusion in Critically Ill Patients with Severe Gram-Negative Infections: A Pharmacokinetics/Pharmacodynamics-Based Approach. Antimicrob. Agents Chemother. 2012, 56, 6343–6348. [Google Scholar] [CrossRef] [PubMed]
- Pea, F.; Cojutti, P.; Sbrojavacca, R.; Cadeo, B.; Cristini, F.; Bulfoni, A.; Furlanut, M. TDM-Guided Therapy with Daptomycin and Meropenem in a Morbidly Obese, Critically Ill Patient. Ann. Pharmacother. 2011, 45, 1022. [Google Scholar] [CrossRef]
- Novy, E.; Martinière, H.; Roger, C. The Current Status and Future Perspectives of Beta-Lactam Therapeutic Drug Monitoring in Critically Ill Patients. Antibiotics 2023, 12, 681. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. Data from the EUCAST MIC Distribution. Available online: http://www.eucast.org (accessed on 26 July 2023).
- Guilhaumou, R.; Benaboud, S.; Bennis, Y.; Dahyot-Fizelier, C.; Dailly, E.; Gandia, P.; Goutelle, S.; Lefeuvre, S.; Mongardon, N.; Roger, C.; et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients—Guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique—SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation—SFAR). Crit. Care 2019, 23, 104. [Google Scholar] [CrossRef]
- Gatti, M.; Cojutti, P.G.; Bartoletti, M.; Tonetti, T.; Bianchini, A.; Ramirez, S.; Pizzilli, G.; Ambretti, S.; Giannella, M.; Mancini, R.; et al. Expert clinical pharmacological advice may make an antimicrobial TDM program for emerging candidates more clinically useful in tailoring therapy of critically ill patients. Crit. Care 2022, 26, 178. [Google Scholar] [CrossRef]
- de With, K.; Allerberger, F.; Amann, S.; Apfalter, P.; Brodt, H.-R.; Eckmanns, T.; Fellhauer, M.; Geiss, H.K.; Janata, O.; Krause, R.; et al. Strategies to enhance rational use of antibiotics in hospital: A guideline by the German Society for Infectious Diseases. Infection 2016, 44, 395–439. [Google Scholar] [CrossRef]
- Shimasaki, T.; Seekatz, A.; Bassis, C.; Rhee, Y.; Yelin, R.D.; Fogg, L.; Dangana, T.; Cisneros, E.C.; A Weinstein, R.; Okamoto, K.; et al. Increased Relative Abundance of Klebsiella pneumoniae Carbapenemase-producing Klebsiella pneumoniae Within the Gut Microbiota Is Associated with Risk of Bloodstream Infection in Long-term Acute Care Hospital Patients. Clin. Infect. Dis. 2019, 68, 2053–2059. [Google Scholar] [CrossRef]
- Mitsacos, A.; Reisine, H.; Highstein, S.M. The superior vestibular nucleus: An intracellular hrp study in the cat. II. Non-vestibulo-ocular neurons. J. Comp. Neurol. 1983, 215, 92–107. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Wang, T.; Di Zhang, D.; You, H.; Dong, Y.M.; Liu, Y.M.; Du, Q.M.; Sun, D.M.; Zhang, T.; Dong, Y.P. Therapeutic Drug Monitoring Coupled With Bayesian Forecasting Could Prevent Vancomycin-Associated Nephrotoxicity in Renal Insufficiency Patients: A Prospective Study and Pharmacoeconomic Analysis. Ther. Drug Monit. 2020, 42, 600–609. [Google Scholar] [CrossRef]
- Conti, R.M.; Padula, W.V.; Becker, R.V.; Salamone, S. The cost-effectiveness of therapeutic drug monitoring for the prescription drug-based treatment of chronic myeloid leukemia. J. Manag. Care Spéc. Pharm. 2021, 27, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Clementi, E.; Perrone, V.; Cattaneo, D.; Radice, S.; Federici, A.B.; Gismondo, M.R.; Medaglia, M.; Vimercati, S.; Pallone, E.; Degli Esposti, L.; et al. Impact of therapeutic drug monitoring of antiretroviral drugs in routine clinical management of patients infected with human immunodeficiency virus and related health care costs: A real-life study in a large cohort of patients. Clin. Outcomes Res. 2014, 6, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Touw, D.J.; Neef, C.; Thomson, A.H.; Vinks, A.A. Cost-Effectiveness of Therapeutic Drug Monitoring Committee of the International Association for Therapeutic Drug Monitoring and Clinical Toxicology. Cost-Effectiveness of Therapeutic Drug Monitoring: A systematic review. Ther. Drug Monit. 2005, 27, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; Varghese, J.M.; Altukroni, M.; Briscoe, S.; McWhinney, B.C.; Ungerer, J.P.; Lipman, J.; Roberts, J.A. Subtherapeutic Initial β-Lactam Concentrations in Select Critically Ill Patients: Association between augmented renal clearance and low trough drug concentrations. Chest 2012, 142, 30–39. [Google Scholar] [CrossRef]
- Im, Y.; Kang, D.; Ko, R.-E.; Lee, Y.J.; Lim, S.Y.; Park, S.; Na, S.J.; Chung, C.R.; Park, M.H.; Oh, D.K.; et al. Time-to-antibiotics and clinical outcomes in patients with sepsis and septic shock: A prospective nationwide multicenter cohort study. Crit. Care 2022, 26, 19. [Google Scholar] [CrossRef]
- Ferrer, R.; Martin-Loeches, I.; Phillips, G.; Osborn, T.M.; Townsend, S.; Dellinger, R.P.; Artigas, A.; Schorr, C.; Levy, M.M. Empiric Antibiotic Treatment Reduces Mortality in Severe Sepsis and Septic Shock from the First Hour: Results from a guideline-based performance improvement program. Crit. Care Med. 2014, 42, 1749–1755. [Google Scholar] [CrossRef]
- Gaieski, D.F.; Mikkelsen, M.E.; Band, R.A.; Pines, J.M.; Massone, R.; Furia, F.F.; Shofer, F.S.; Goyal, M. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department*. Crit. Care Med. 2010, 38, 1045–1053. [Google Scholar] [CrossRef]
- Moser, S.; Rehm, S.; Guertler, N.; Hinic, V.; Dräger, S.; Bassetti, S.; Rentsch, K.M.; Sendi, P.; Osthoff, M. Probability of pharmacological target attainment with flucloxacillin in Staphylococcus aureus bloodstream infection: A prospective cohort study of unbound plasma and individual MICs. J. Antimicrob. Chemother. 2021, 76, 1845–1854. [Google Scholar] [CrossRef]
- Richter, D.C.; Frey, O.; Röhr, A.; Roberts, J.A.; Köberer, A.; Fuchs, T.; Papadimas, N.; Heinzel-Gutenbrunner, M.; Brenner, T.; Lichtenstern, C.; et al. Therapeutic drug monitoring-guided continuous infusion of piperacillin/tazobactam significantly improves pharmacokinetic target attainment in critically ill patients: A retrospective analysis of four years of clinical experience. Infection 2019, 47, 1001–1011. [Google Scholar] [CrossRef]
- Scharf, C.; Paal, M.; Schroeder, I.; Vogeser, M.; Draenert, R.; Irlbeck, M.; Zöller, M.; Liebchen, U. Therapeutic Drug Monitoring of Meropenem and Piperacillin in Critical Illness—Experience and Recommendations from One Year in Routine Clinical Practice. Antibiotics 2020, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.; Taccone, F.S.; Roberts, J.A.; Jacobs, F.; Cotton, F.; Wolff, F.; Creteur, J.; Vincent, J.-L.; Hites, M. β-Lactam Dosage Regimens in Septic Patients with Augmented Renal Clearance. Antimicrob. Agents Chemother. 2018, 62, e02534-17. [Google Scholar] [CrossRef] [PubMed]
- Tamatsukuri, T.; Ohbayashi, M.; Kohyama, N.; Kobayashi, Y.; Yamamoto, T.; Fukuda, K.; Nakamura, S.; Miyake, Y.; Dohi, K.; Kogo, M. The exploration of population pharmacokinetic model for meropenem in augmented renal clearance and investigation of optimum setting of dose. J. Infect. Chemother. 2018, 24, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Troisi, C.; Cojutti, P.G.; Rinaldi, M.; Laici, C.; Siniscalchi, A.; Viale, P.; Pea, F. Measuring Creatinine Clearance Is the Most Accurate Way for Calculating the Proper Continuous Infusion Meropenem Dose for Empirical Treatment of Severe Gram-Negative Infections among Critically Ill Patients. Pharmaceutics 2023, 15, 551. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, A.; Broek, P.V.D.; Ewoldt, T.M.J.; Muller, A.E.; Endeman, H.; Koch, B.C.P. Barriers and Facilitators in the Clinical Implementation of Beta-Lactam Therapeutic Drug Monitoring in Critically Ill Patients: A Critical Review. Ther. Drug Monit. 2022, 44, 112–120. [Google Scholar] [CrossRef]
- Giuliano, S.; Angelini, J.; D’elia, D.; Geminiani, M.; Barison, R.D.; Giacinta, A.; Sartor, A.; Campanile, F.; Curcio, F.; Cotta, M.O.; et al. Ampicillin and Ceftobiprole Combination for the Treatment of Enterococcus faecalis Invasive Infections: “The Times They Are A-Changin”. Antibiotics 2023, 12, 879. [Google Scholar] [CrossRef]
- E Franks, C.; Scott, M.G. On the Basis of Race: The Utility of a Race Factor in Estimating Glomerular Filtration. J. Appl. Lab. Med. 2021, 6, 155–166. [Google Scholar] [CrossRef]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A More Accurate Method to Estimate Glomerular Filtration Rate from Serum Creatinine: A New Prediction Equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef]
- Pai, M.P.; Cojutti, P.; Pea, F. Pharmacokinetics and Pharmacodynamics of Continuous Infusion Meropenem in Overweight, Obese, and Morbidly Obese Patients with Stable and Unstable Kidney Function: A Step Toward Dose Optimization for the Treatment of Severe Gram-Negative Bacterial Infections. Clin. Pharmacokinet. 2015, 54, 933–941. [Google Scholar] [CrossRef]





| Variable | n (%) or Median (IQR) |
|---|---|
| Sex (male/female) | 92/52 (63.9/36.1) |
| Age (years) | 72 (60–79) |
| Weight (kg) | 75 (65–85) |
| Height (cm) | 170 (164–178) |
| BMI (kg/m2) | 25 (23–28) |
| Serum total protein (g/L) | 56.0 (51.0–61.2) |
| Serum albumin (g/L) | 28.5 (24.0–32.4) |
| Alanine aminotransferase (UI) | 23.0 (13.2–42.5) |
| Serum creatinine (mg/dL) | 0.90 (0.63–1.42) |
| Patients with eCLCr 1 < 10 mL/min | 0 (0) |
| eCLCr 1 in < 10 mL/min group | - |
| Patients with eCLCr 1 10–25 mL/min | 14 (10.1) |
| eCLCr 1 in 10–25 mL/min group | 16.25 (14.15–19.43) |
| Patients with eCLCr 1 26–50 mL/min | 30 (21.6) |
| eCLCr 1 in 26–50 mL/min group | 30.15 (33.05–45.75) |
| Patients with eCLCr 1 50–120 mL/min | 62 (44.6) |
| eCLCr 1 in 50–120 mL/min group | 81.05 (65.73–98.63) |
| Patients with eCLCr 1 >120 mL/min | 33 (23.7) |
| eCLCr 1 in >120 mL/min group | 159.10 (136.60–197.30) |
| C-reactive protein (mg/L) | 58.3 (22.1–123.7) |
| Time between initiation therapy with meropenem and first TDM (hours) | 96 (72–120) |
| Variable 1 | Patients under the PK/PD Target (n = 37) | Patients on PK/PD Target (n = 51) | Patients over the PK/PD Target (n = 53) | p-Value |
|---|---|---|---|---|
| Sex (male/female) | 24/13 (65/35) | 35/16 (69/31) | 31/22 (58/42) | 0.55 |
| Age (years) | 65 (52–75) | 71 (56–78) | 75 (65–81) | 0.002 |
| Weight (kg) | 75 (65.5–84.75) | 78 (65–87) | 70 (64.25–85) | 0.50 |
| Height (cm) | 173 (163–179) | 172 (165–179) | 170 (163–175) | 0.24 |
| BMI (kg/m2) | 25.3 (23.1–27.7) | 25.7 (24.1–28.9) | 24.4 (23.2–27.7) | 0.54 |
| Serum total protein (g/L) | 30.0 (26.4–33.5) | 29.0 (25.0–34.0) | 27.0 (23.0–30.7) | 0.09 |
| Serum albumin (g/L) | 55.0 (52.0–63.0) | 56.5 (52.5–65.2) | 54.0 (49.0–58.0) | 0.10 |
| Alanine aminotransferase (UI) | 21.0 (10.0–48.0) | 25 (15.0–43.0) | 28.0 (14.2–41.0) | 0.68 |
| Serum creatinine (mg/dL) | 0.74 (1.01–0.58) | 0.76 (0.56–1.18) | 1.3 (0.95–1.97) | <0.001 |
| eCLCr 1 (mL/min) | 104.9 (68.9–136.5) | 96.6 (47.3–139.2) | 53.1 (33.8–79.5) | <0.001 |
| C-reactive protein (mg/L) | 27.1 (7.7–89.1) | 59.3 (21.5–111.8) | 75.1 (32.9–164.8) | 0.005 |
| Time between initiation therapy with meropenem and first TDM (hours) | 72 (72–120) | 96 (72–96) | 96 (72–120) | 0.80 |
| Meropenem daily dose (g) | 3 (2–3) | 3 (2–4) | 3 (2–4) | 0.39 |
| Serum meropenem concentration/daily dose ratio (C/D) | 2.3 (1.0–2.6) | 3.7 (2.6–6.7) | 8.5 (5.9–10.8) | <0.001 |
| Serum meropenem concentration/kg (C/kg) | 0.08 (0.01–0.10) | 0.16 (0.12–0.20) | 0.33 (0.23–0.4) | <0.001 |
| Serum meropenem concentration normalized for daily dose and weight (C/D/kg) | 0.03 (0.01–0.04) | 0.05 (0.03–0.09) | 0.11 (0.08–0.14) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelini, J.; Giuliano, S.; Flammini, S.; Pagotto, A.; Lo Re, F.; Tascini, C.; Baraldo, M. Meropenem PK/PD Variability and Renal Function: “We Go Together”. Pharmaceutics 2023, 15, 2238. https://doi.org/10.3390/pharmaceutics15092238
Angelini J, Giuliano S, Flammini S, Pagotto A, Lo Re F, Tascini C, Baraldo M. Meropenem PK/PD Variability and Renal Function: “We Go Together”. Pharmaceutics. 2023; 15(9):2238. https://doi.org/10.3390/pharmaceutics15092238
Chicago/Turabian StyleAngelini, Jacopo, Simone Giuliano, Sarah Flammini, Alberto Pagotto, Francesco Lo Re, Carlo Tascini, and Massimo Baraldo. 2023. "Meropenem PK/PD Variability and Renal Function: “We Go Together”" Pharmaceutics 15, no. 9: 2238. https://doi.org/10.3390/pharmaceutics15092238





