A Drug-Eluting Injectable NanoGel for Localized Delivery of Anticancer Drugs to Solid Tumors
Abstract
:1. Introduction
2. Methods
2.1. Formulation & Naming System
2.2. STH Formulation
2.3. Zeta Potential
2.4. X-ray Diffraction
2.5. Scanning Electron Microscopy
2.6. In Vitro Release Study in Well plates
2.7. In Vitro Drug Toxicity Studies in 2D and 3D Cultures
2.8. Free Dox Cell Viability
2.9. Dox-Loaded STH-Treated Cell Viability
2.10. Three-Dimensional (3D) Microfluidic Culture
2.11. Cell Encapsulation in Collagen
2.12. STH Injection in a Microfluidic Device
2.13. Diffusion Test with the Microfluidic Device
2.14. COMSOL Brain-Tumor-on-a-Chip Dox Diffusion Simulation
2.15. In Vivo Antitumor Activity of Dox-Loaded STHs
2.15.1. Rat Glioblastoma Model and STH Implantation
2.15.2. Mice Breast Tumor Model and STH Implantation
2.15.3. In Vivo Fluorescent Imaging
2.15.4. Histopathological Studies
2.15.5. Mitochondrial Preparation and SDH Assay
2.16. Statistical Analyses
3. Results and Discussion
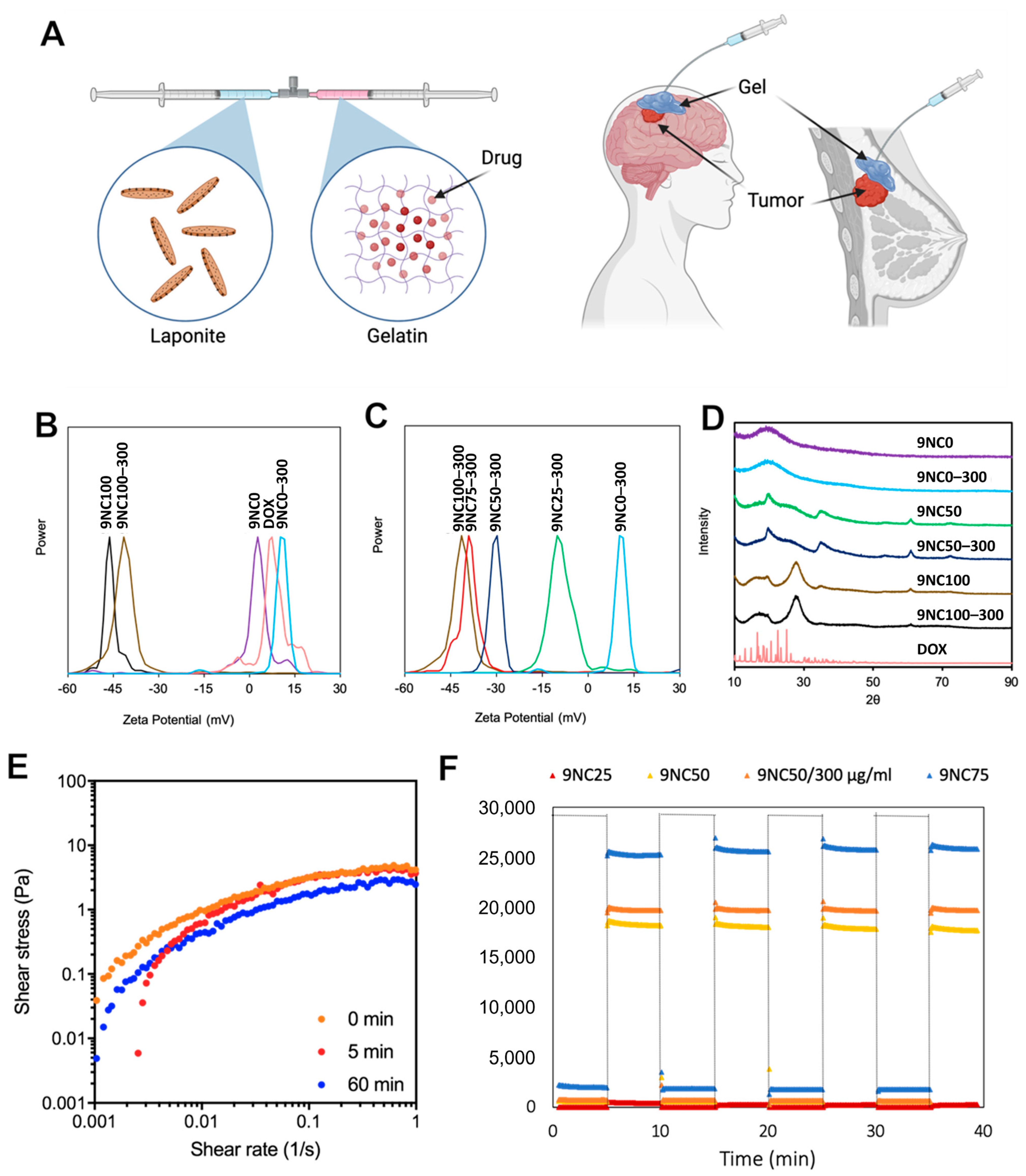
3.1. Characterization of Dox-Loaded STH
3.1.1. Zeta Potential
3.1.2. Powder X-ray Diffraction
3.1.3. Viscoelasticity and Recovery Analysis
3.1.4. Scanning Electron Microscopy
3.2. In Vitro Enzymatic Degradation and Swelling Test
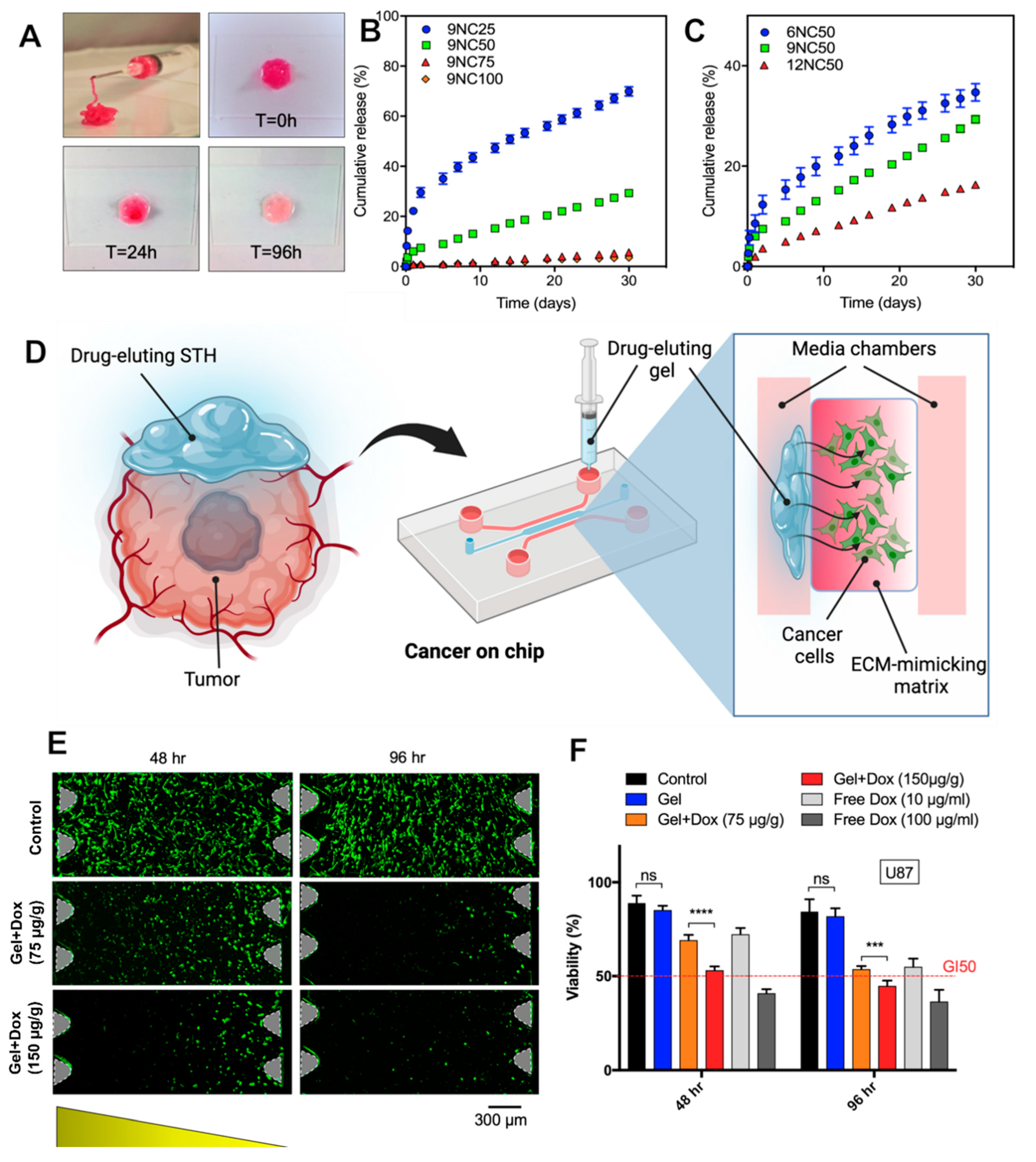
3.3. In Vitro Release Study
3.4. In Vitro Cytotoxicity Studies
3.4.1. MCF7 and U87 Human Glioblastoma Cell Viability
3.4.2. In Vitro Brain-Tumor-on-a-Chip Antitumor Activity Study
3.4.3. Cancer-on-a-Chip Model
3.4.4. Computational and Experimental Dox Diffusion Analysis of Brain-Tumor-on-a-Chip
3.5. In Vivo Antitumor Activity of Dox-Loaded STHs
3.5.1. In Vivo Antitumor Activity of Dox-Loaded STHs in Breast Tumor Models
3.5.2. In Vivo Antitumor Activity of Dox-Loaded STHs in Brain Tumor Models
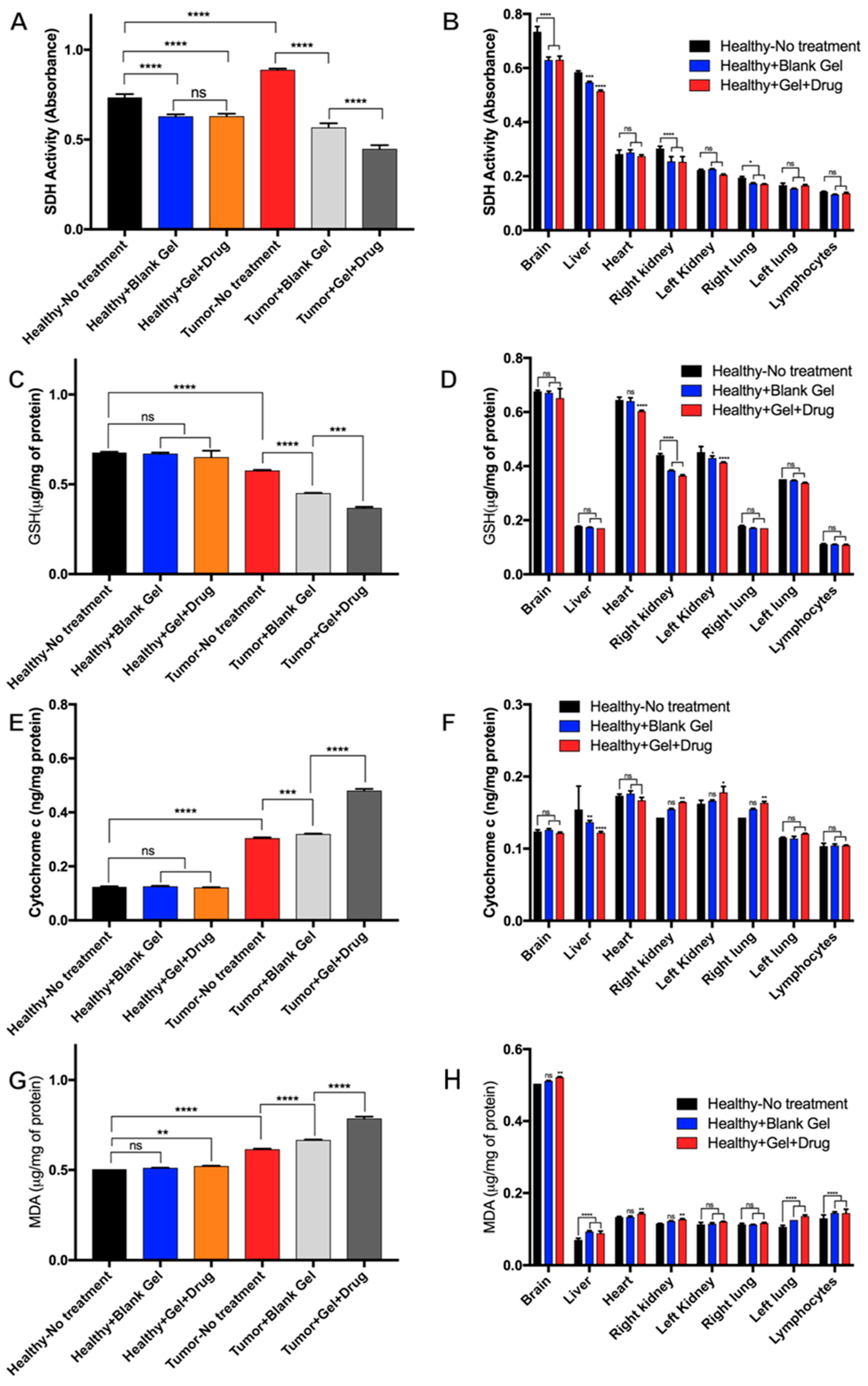
3.5.3. Mitochondrial Evaluation of Dox-Loaded STH Cytotoxicity
3.5.4. Histopathological Evaluation of Dox-Loaded STH Tissue Distribution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in Biomaterials for Drug Delivery. Adv. Mater. 2018, 30, 1705328. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Nazemi, Z.; Salehi, A.O.M.; Seyfoori, A.; John, J.V.; Nourbakhsh, M.S.; Akbari, M. Cellulose-Based Composite Scaffolds for Bone Tissue Engineering and Localized Drug Delivery. Bioact. Mater. 2023, 20, 137–163. [Google Scholar] [CrossRef]
- Askari, E.; Seyfoori, A.; Amereh, M.; Gharaie, S.S.; Ghazali, H.S.; Ghazali, Z.S.; Khunjush, B.; Akbari, M. Stimuli-Responsive Hydrogels for Local Post-Surgical Drug Delivery. Gels 2020, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Luo, Q.; Wang, Y.; Li, X.; Shen, Z.; Zhu, W. An Injectable Drug-Loaded Hydrogel Based on a Supramolecular Polymeric Prodrug. Chem. Commun. 2015, 51, 14644–14647. [Google Scholar] [CrossRef]
- Talebian, S.; Foroughi, J.; Wade, S.J.; Vine, K.L.; Dolatshahi-Pirouz, A.; Mehrali, M.; Conde, J.; Wallace, G.G. Biopolymers for Antitumor Implantable Drug Delivery Systems: Recent Advances and Future Outlook. Adv. Mater. 2018, 30, 1706665. [Google Scholar] [CrossRef]
- Bu, L.L.; Yan, J.; Wang, Z.; Ruan, H.; Chen, Q.; Gunadhi, V.; Bell, R.B.; Gu, Z. Advances in Drug Delivery for Post-Surgical Cancer Treatment. Biomaterials 2019, 219, 119182. [Google Scholar] [CrossRef]
- D’Amico, R.S.; Aghi, M.K.; Vogelbaum, M.A.; Bruce, J.N. Convection-Enhanced Drug Delivery for Glioblastoma: A Review. J. Neurooncol. 2021, 151, 415–427. [Google Scholar] [CrossRef]
- Marques, A.C.; Costa, P.J.; Velho, S.; Amaral, M.H. Stimuli-Responsive Hydrogels for Intratumoral Drug Delivery. Drug Discov. Today 2021, 26, 2397–2405. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, Y.; Yang, R.; Ouyang, Z.; Yu, H.; Wang, H.; Shi, X.; Shen, M. Tumor-Anchoring Drug-Loaded Fibrous Microspheres for MR Imaging-Guided Local Chemotherapy and Metastasis Inhibition. Adv. Fiber Mater. 2022, 4, 807–819. [Google Scholar] [CrossRef]
- Li, W.; Chen, J.; Zhao, S.; Huang, T.; Ying, H.; Trujillo, C.; Molinaro, G.; Zhou, Z.; Jiang, T.; Liu, W.; et al. High Drug-Loaded Microspheres Enabled by Controlled in-Droplet Precipitation Promote Functional Recovery after Spinal Cord Injury. Nat. Commun. 2022, 13, 1262. [Google Scholar] [CrossRef]
- Gharaie, S.S.; Dabiri, S.M.H.; Akbari, M. Smart Shear-Thinning Hydrogels as Injectable Drug Delivery Systems. Polymer 2018, 10, 1317. [Google Scholar] [CrossRef] [PubMed]
- Avery, R.K.; Albadawi, H.; Akbari, M.; Zhang, Y.S.; Duggan, M.J.; Sahani, D.V.; Olsen, B.D.; Khademhosseini, A.; Oklu, R. An Injectable Shear-Thinning Biomaterial for Endovascular Embolization. Sci. Transl. Med. 2016, 8, 365ra156. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Lee, D.S. Injectable Biodegradable Hydrogels. Macromol. Biosci. 2010, 10, 563–579. [Google Scholar] [CrossRef]
- Uman, S.; Dhand, A.; Burdick, J.A. Recent Advances in Shear-Thinning and Self-Healing Hydrogels for Biomedical Applications. J. Appl. Polym. Sci. 2020, 137, 48668. [Google Scholar] [CrossRef]
- Guvendiren, M.; Lu, H.D.; Burdick, J.A. Shear-Thinning Hydrogels for Biomedical Applications. Soft Matter 2011, 8, 260–272. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite Hydrogels for Biomedical Applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Godau, B.; Stefanek, E.; Libert-Scott, E.; Ahadian, S.; Akbari, M. Non-Destructive Mechanical Assessment for Optimization of 3D Bioprinted Soft Tissue Scaffolds. iScience 2022, 25, 104251. [Google Scholar] [CrossRef]
- Krishnadoss, V.; Kanjilal, B.; Hesketh, A.; Miller, C.; Mugweru, A.; Akbard, M.; Khademhosseini, A.; Leijten, J.; Noshadi, I. In Situ 3D Printing of Implantable Energy Storage Devices. Chem. Eng. J. 2021, 409, 128213. [Google Scholar] [CrossRef]
- Kadumudi, F.B.; Jahanshahi, M.; Mehrali, M.; Zsurzsan, T.-G.; Taebnia, N.; Hasany, M.; Mohanty, S.; Knott, A.; Godau, B.; Akbari, M.; et al. A Protein-Based, Water-Insoluble, and Bendable Polymer with Ionic Conductivity: A Roadmap for Flexible and Green Electronics. Adv. Sci. 2019, 6, 1801241. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Y.; Guo, R.; Huang, Y.; Wen, S.; Shen, M.; Wang, J.; Shi, X. Laponite Nanodisks as an Efficient Platform for Doxorubicin Delivery to Cancer Cells. Langmuir 2013, 29, 5030–5036. [Google Scholar] [CrossRef]
- Gharaie, S.S. Silicate Based Hydrogels for Tissue Engineering and Drug Delivery Applications. Doctoral Thesis, University of Victoria, Victoria, BC, Canada, 2021. [Google Scholar]
- Samiei, E.; Seyfoori, A.; Toyota, B.; Ghavami, S.; Akbari, M. Investigating Programmed Cell Death and Tumor Invasion in a Three-Dimensional (3D) Microfluidic Model of Glioblastoma. Int. J. Mol. Sci. 2020, 21, 3162. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, D.; Lecomte, R.; Tsanaclis, A.M.; Larouche, A.; Fortin, D. Standardization and Detailed Characterization of the Syngeneic Fischer/F98 Glioma Model. Can. J. Neurol. Sci. 2007, 34, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Blanton, T.N.; Majumdar, D. Microstructure of Clay-Polymer Composites. Adv. X-ray Anal. 2000, 42, 562–568. [Google Scholar]
- Cidonio, G.; Alcala-Orozco, C.R.; Lim, K.S.; Glinka, M.; Mutreja, I.; Kim, Y.H.; Dawson, J.I.; Woodfield, T.B.F.; Oreffo, R.O.C. Osteogenic and Angiogenic Tissue Formation in High Fidelity Nanocomposite Laponite-Gelatin Bioinks. Biofabrication 2019, 11, 035027. [Google Scholar] [CrossRef]
- López-Angulo, D.; Bittante, A.M.Q.B.; Luciano, C.G.; Ayala-Valencia, G.; Flaker, C.H.C.; Djabourov, M.; José do Amaral Sobral, P. Effect of Laponite® on the Structure, Thermal Stability and Barrier Properties of Nanocomposite Gelatin Films. Food Biosci. 2020, 35, 100596. [Google Scholar] [CrossRef]
- Alhowail, A.; Almogbel, Y. Metformin Administration Increases the Survival Rate of Doxorubicin-Treated Mice. Pharmazie 2019, 74, 737–739. [Google Scholar] [CrossRef]
- Omuro, A. Glioblastoma and Other Malignant Gliomas. JAMA 2013, 310, 1842. [Google Scholar] [CrossRef]
- Ozeki, T.; Kaneko, D.; Hashizawa, K.; Imai, Y.; Tagami, T.; Okada, H. Improvement of Survival in C6 Rat Glioma Model by a Sustained Drug Release from Localized PLGA Microspheres in a Thermoreversible Hydrogel. Int. J. Pharm. 2012, 427, 299–304. [Google Scholar] [CrossRef]
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local Drug Delivery Strategies for Cancer Treatment: Gels, Nanoparticles, Polymeric Films, Rods, and Wafers. J. Control. Release 2012, 159, 14–26. [Google Scholar] [CrossRef]
- Ajaykumar, C.; Ajaykumar, C. Overview on the Side Effects of Doxorubicin. In Advances in Precision Medicine Oncology; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Abramczyk, H.; Brozek-Pluska, B.; Kopeć, M. Double Face of Cytochrome c in Cancers by Raman Imaging. Sci. Rep. 2022, 12, 2120. [Google Scholar] [CrossRef]
- Dirks-Naylor, A.J.; Kouzi, S.A.; Bero, J.D.; Phan, D.T.; Taylor, H.N.; Whitt, S.D.; Mabolo, R. Doxorubicin Alters the Mitochondrial Dynamics Machinery and Mitophagy in the Liver of Treated Animals. Fundam. Clin. Pharmacol. 2014, 28, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Dharmajaya, R.; Sari, D.K. Malondialdehyde Value as Radical Oxidative Marker and Endogenous Antioxidant Value Analysis in Brain Tumor. Ann. Med. Surg. 2022, 77, 103231. [Google Scholar] [CrossRef] [PubMed]
- Ergül, M.; Taşkiran, A.Ş. Thiamine Protects Glioblastoma Cells against Glutamate Toxicity by Suppressing Oxidative/Endoplasmic Reticulum Stress. Chem. Pharm. Bull. 2021, 69, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, B. Doxorubicin Induces Cardiotoxicity through Upregulation of Death Receptors Mediated Apoptosis in Cardiomyocytes. Sci. Rep. 2017, 7, 44735. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godau, B.; Samimi, S.; Seyfoori, A.; Samiei, E.; Khani, T.; Naserzadeh, P.; Najafabadi, A.H.; Lesha, E.; Majidzadeh-A, K.; Ashtari, B.; et al. A Drug-Eluting Injectable NanoGel for Localized Delivery of Anticancer Drugs to Solid Tumors. Pharmaceutics 2023, 15, 2255. https://doi.org/10.3390/pharmaceutics15092255
Godau B, Samimi S, Seyfoori A, Samiei E, Khani T, Naserzadeh P, Najafabadi AH, Lesha E, Majidzadeh-A K, Ashtari B, et al. A Drug-Eluting Injectable NanoGel for Localized Delivery of Anticancer Drugs to Solid Tumors. Pharmaceutics. 2023; 15(9):2255. https://doi.org/10.3390/pharmaceutics15092255
Chicago/Turabian StyleGodau, Brent, Sadaf Samimi, Amir Seyfoori, Ehsan Samiei, Tahereh Khani, Parvaneh Naserzadeh, Alireza Hassani Najafabadi, Emal Lesha, Keivan Majidzadeh-A, Behnaz Ashtari, and et al. 2023. "A Drug-Eluting Injectable NanoGel for Localized Delivery of Anticancer Drugs to Solid Tumors" Pharmaceutics 15, no. 9: 2255. https://doi.org/10.3390/pharmaceutics15092255
APA StyleGodau, B., Samimi, S., Seyfoori, A., Samiei, E., Khani, T., Naserzadeh, P., Najafabadi, A. H., Lesha, E., Majidzadeh-A, K., Ashtari, B., Charest, G., Morin, C., Fortin, D., & Akbari, M. (2023). A Drug-Eluting Injectable NanoGel for Localized Delivery of Anticancer Drugs to Solid Tumors. Pharmaceutics, 15(9), 2255. https://doi.org/10.3390/pharmaceutics15092255









