Fungi Fibrinolytic Compound 1 Plays a Core Role in Modulating Fibrinolysis, Altering Plasma Clot Structure, and Promoting Susceptibility to Lysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasma Preparation
2.3. Turbidity Measurements
2.4. SEM of Plasma Clots
2.5. Laser Confocal Microscopy of Plasma Clots
2.6. Plasma Clot Lysis Assay
2.7. Determination of Plasminogen Activation in Plasma by FGFC1
2.8. Determination of Plasminogen Activation in Plasma by scu-PA Mediated FGFC1
2.9. Determination of scu-PA Activation in Plasma by FGFC1
2.10. Determination of Plasminogen Activation by scu-PA Mediated FGFC1 In Vitro
2.11. Statistical Analysis
3. Results
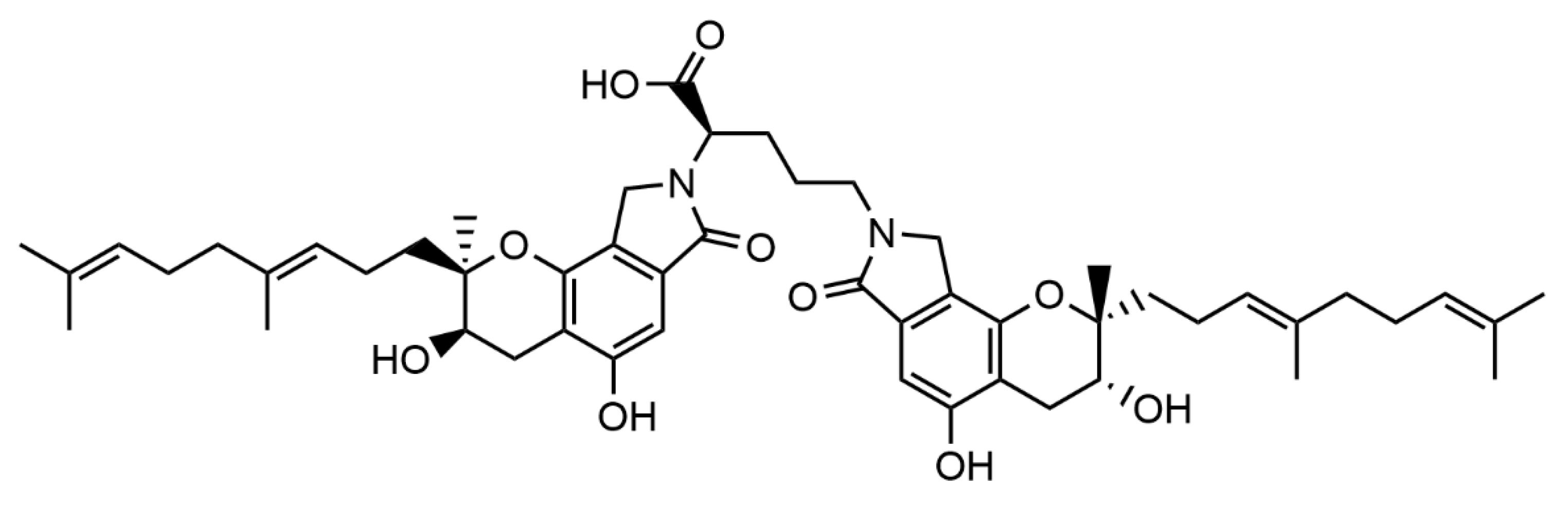
3.1. FGFC1 Effect on Clot Turbidity
3.2. Impacts of FGFC1 on Fibrin Structure by Scanning Electron Microscopy
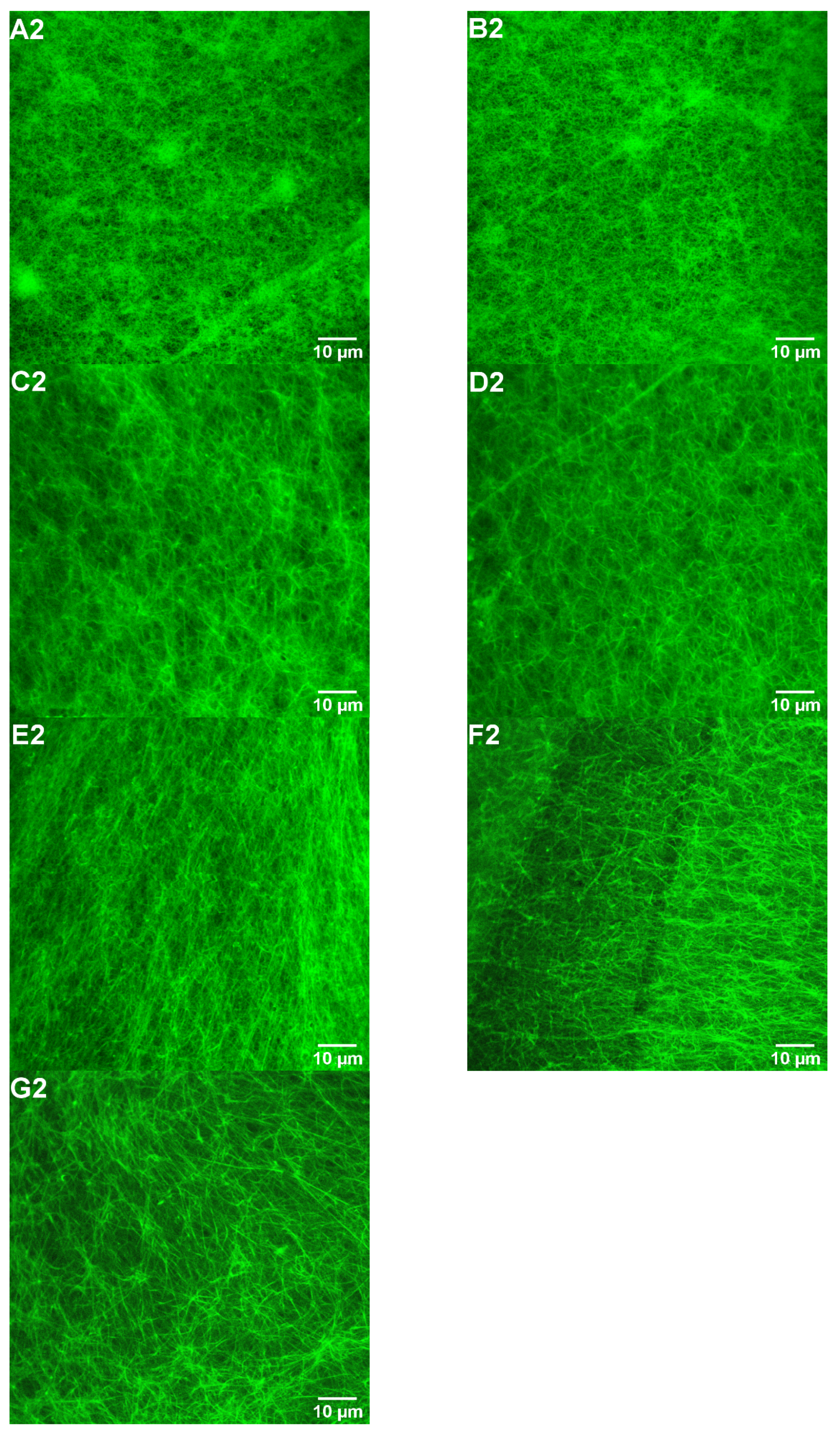
3.3. Impacts of FGFC1 on Fibrin Structure by Confocal Laser Microscopy
3.4. Effects of FGFC1 on Fibrinolysis In Vitro
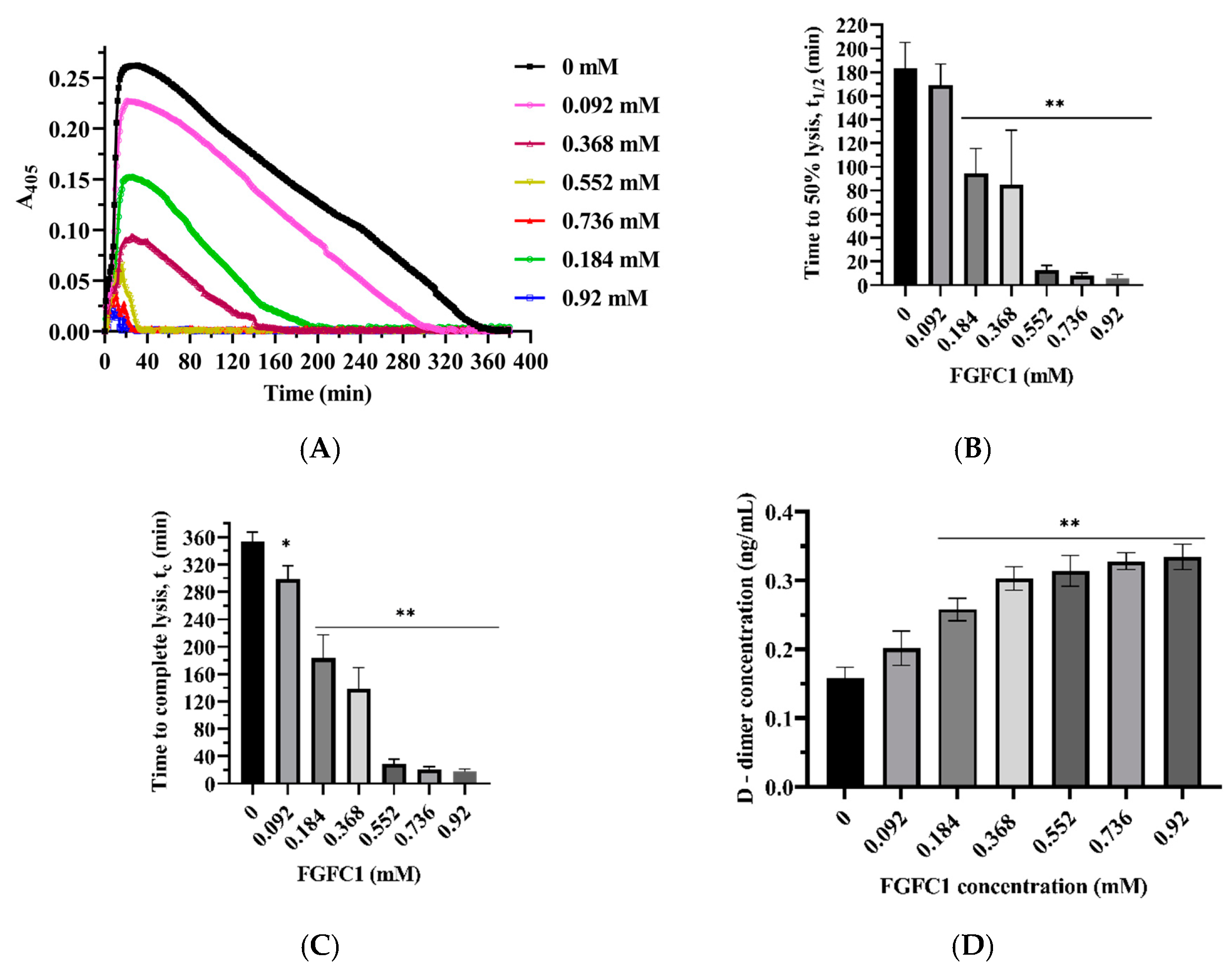
3.4.1. FGFC1 Promotes Fibrinolysis
3.4.2. Effects of FGFC1 on D-Dimer Formation
3.4.3. FGFC1 Influences Fibrin Structure Mediated by scu-PA
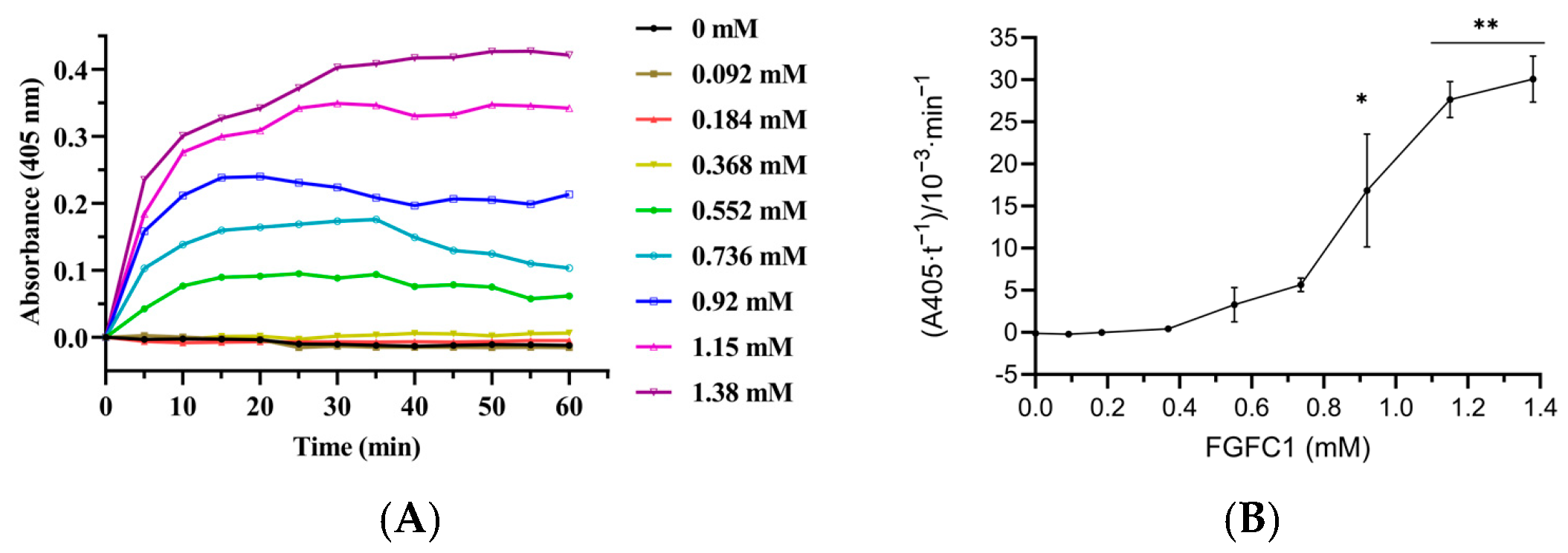
3.5. Effect of FGFC1 on Plasminogen Activation in Plasma
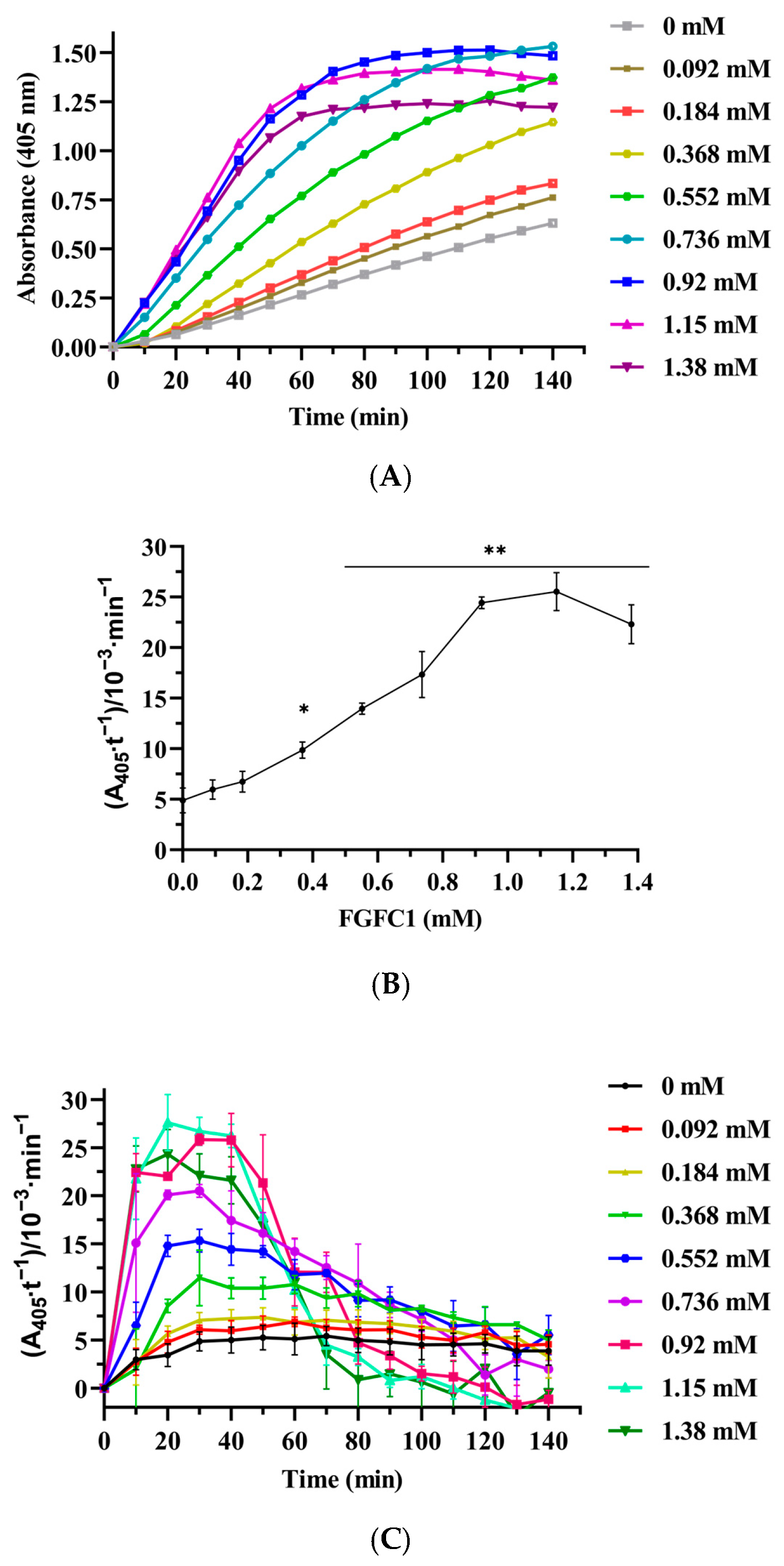
3.6. The Effect of scu-PA-Mediated FGFC1 on Plasminogen Activation in Plasma
3.7. Fibrinolytic Activity of FGFC1 in Plasma
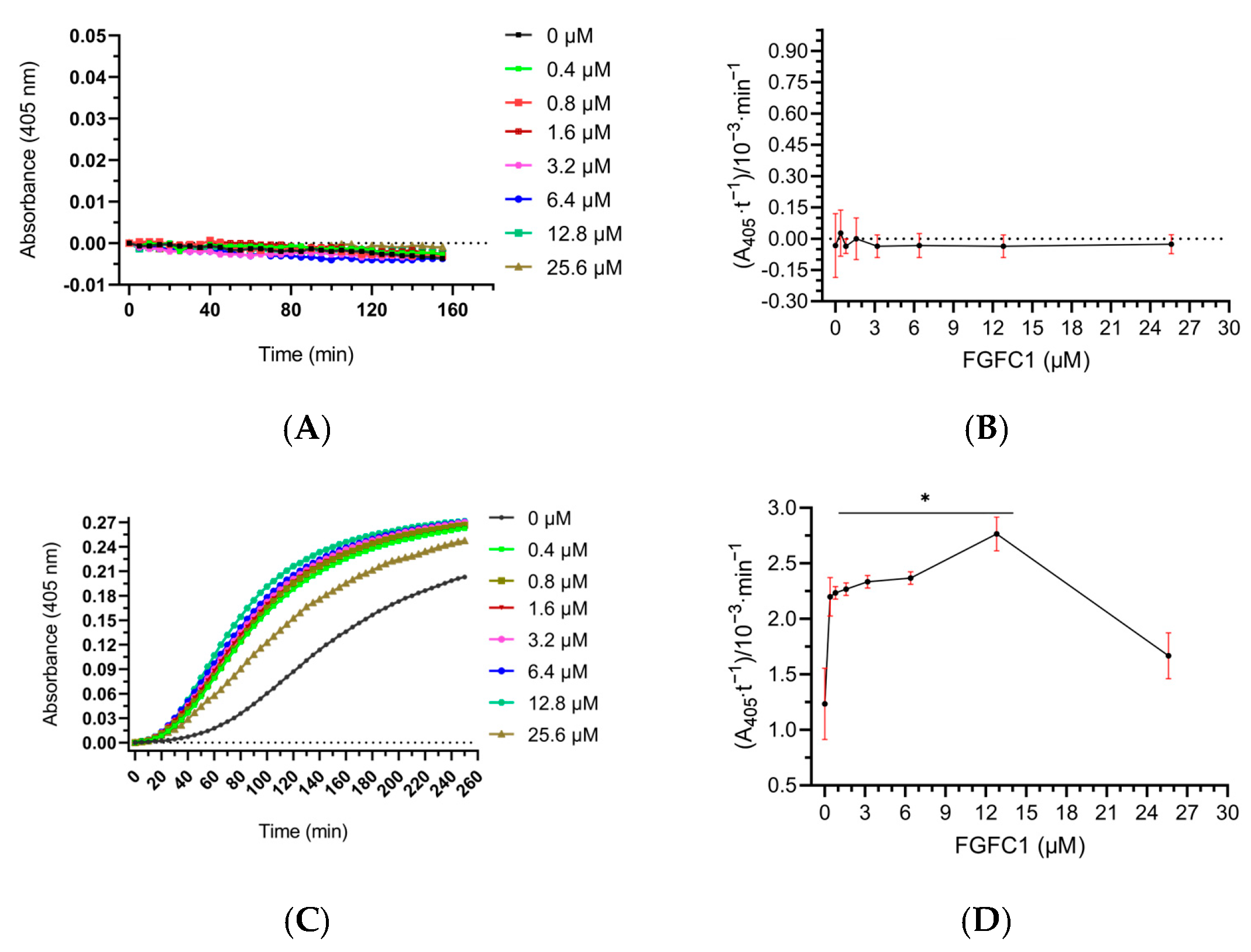
3.8. Effect of FGFC1 on Plasminogen Activation In Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sinegre, T.; Milenkovic, D.; Teissandier, D.; Fully, P.; Bourdin, J.; Morand, C.; Lebreton, A. Impact of epicatechin on fibrin clot structure. Eur. J. Pharmacol. 2021, 893, 173830. [Google Scholar] [CrossRef] [PubMed]
- Poole, L.G.; Kopec, A.K.; Groeneveld, D.J.; Pant, A.; Baker, K.S.; Cline-Fedewa, H.M.; Flick, M.J.; Luyendyk, J.P. Factor XIII cross-links fibrin(ogen) independent of fibrin polymerization in experimental acute liver injury. Blood 2021, 137, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Nellenbach, K.; Kyu, A.; Guzzetta, N.; Brown, A.C. Differential sialic acid content in adult and neonatal fibrinogen mediates differences in clot polymerization dynamics. Blood Adv. 2021, 5, 5202–5214. [Google Scholar] [CrossRef]
- Kamijo, T.; Mukai, S.; Taira, C.; Higuchi, Y.; Okumura, N. γD318Y fibrinogen shows no fibrin polymerization due to defective “A-a” and “B-b” interactions, whereas that of γK321E fibrinogen is nearly normal. Thromb. Res. 2019, 182, 150–158. [Google Scholar] [CrossRef]
- Chapin, J.C.; Hajjar, K.A. Fibrinolysis and the control of blood coagulation. Blood Rev. 2018, 29, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Bialkower, M.; McLiesh, H.; Manderson, C.A.; Tabor, R.F.; Garnier, G. Rapid paper diagnostic for plasma fibrinogen concentration. Analyst 2019, 144, 4848–4857. [Google Scholar] [CrossRef]
- Maners, J.; Gill, D.; Pankratz, N.; Laffan, M.A.; Wolberg, A.S.; de Maat, M.P.; Ligthart, S.; Tang, W.; Ward-Caviness, C.K.; Fornage, M.; et al. A Mendelian randomization of γ′ and total fibrinogen levels in relation to venous thromboembolism and ischemic stroke. Blood 2020, 136, 3062–3069. [Google Scholar] [CrossRef]
- Damiana, T.; Damgaard, D.; Sidelmann, J.J.; Nielsen, C.H.; de Maat, M.P.; Münster, A.M.B.; Palarasah, Y. Citrullination of fibrinogen by peptidylarginine deiminase 2 impairs fibrin clot structure. Clin. Chim. Acta 2019, 501, 6–11. [Google Scholar] [CrossRef]
- Duval, C.; Profumo, A.; Aprile, A.; Salis, A.; Millo, E.; Damonte, G.; Gauer, J.S.; Ariëns, R.A.; Rocco, M. Fibrinogen αC-regions are not directly involved in fibrin polymerization as evidenced by a “Double-Detroit” recombinant fibrinogen mutant and knobs-mimic peptides. J. Thromb. Haemost. 2020, 18, 802–814. [Google Scholar] [CrossRef]
- Asquith, N.L.; Duval, C.; Zhmurov, A.; Baker, S.R.; McPherson, H.R.; Domingues, M.M.; Connell, S.D.; Barsegov, V.; Ariëns, R.A. Fibrin protofibril packing and clot stability are enhanced by extended knob-hole interactions and catch-slip bonds. Blood Adv. 2022, 6, 4015–4027. [Google Scholar] [CrossRef]
- Mihalko, E.; Brown, A.C. Clot Structure and Implications for Bleeding and Thrombosis. Semin. Thromb. Hemost. 2020, 46, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Maksudov, F.; Daraei, A.; Sesha, A.; Marx, K.A.; Guthold, M.; Barsegov, V. Strength, deformability and toughness of uncrosslinked fibrin fibers from theoretical reconstruction of stress-strain curves. Acta Biomater. 2021, 136, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Feller, T.; Connell, S.D.A.; Ariёns, R.A.S. Why fibrin biomechanical properties matter for hemostasis and thrombosis. J. Thromb. Haemost. 2022, 20, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Weisel, J.W.; Litvinov, R.I. Mechanisms of fibrin polymerization and clinical implications. Blood 2013, 121, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Cieslik, J.; Mrozinska, S.; Broniatowska, E.; Undas, A. Altered plasma clot properties increase the risk of recurrent deep vein thrombosis: A cohort study. Blood 2018, 131, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.R.; Zabczyk, M.; Macrae, F.L.; Duval, C.; Undas, A.; Ariëns, R.A. Recurrent venous thromboembolism patients form clots with lower elastic modulus than those formed by patients with non-recurrent disease. J. Thromb. Haemost. 2019, 17, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Siniarski, A.; Baker, S.R.; Duval, C.; Malinowski, K.P.; Gajos, G.; Nessler, J.; Ariëns, R.A. Quantitative analysis of clot density, fibrin fiber radius, and protofibril packing in acute phase myocardial infarction. Thromb. Res. 2021, 205, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.I.; Chan, N.C. Novel antithrombotic strategies for treatment of venous thromboembolism. Blood 2020, 135, 351–359. [Google Scholar] [CrossRef]
- Undas, A.; Ariens, R.A.S. Fibrin clot structure and function: A role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler. Thromb. Vasc. Biol. 2011, 31, E88–E99. [Google Scholar] [CrossRef]
- Collet, J.P.; Allali, Y.; Lesty, C.; Tanguy, M.L.; Silvain, J.; Ankri, A.; Blanchet, B.; Dumaine, R.; Gianetti, J.; Payot, L.; et al. Altered fibrin architecture is associated with hypofibrinolysis and premature coronary atherothrombosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2567–2573. [Google Scholar] [CrossRef]
- Gao, C.L.; Tang, S.M.; Zhang, H.X.; Zhang, H.; Zhang, T.; Bao, B.; Zhu, Y.; Wu, W. A Novel Marine Pyran-Isoindolone Compound Enhances Fibrin Lysis Mediated by Single-Chain Urokinase-Type Plasminogen Activator. Mar. Drugs 2022, 20, 495. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Wu, W.H.; Su, T.W.; Chen, J.; Zhu, Q.; Zhang, C.; Wang, X.; Bao, B. Effects of a novel marine natural product: Pyrano indolone alkaloid fibrinolytic compound on thrombolysis and hemorrhagic activities in vitro and in vivo. Arch. Pharmacal Res. 2015, 38, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.K.; Zhang, B.; Feng, J.W.; Wu, H.; Duan, N.; Zhu, Y.; Zhao, Y.; Shen, S.; Zhang, K.; Wu, W.; et al. FGFC1 Selectively Inhibits Erlotinib-Resistant Non-Small Cell Lung Cancer via Elevation of ROS Mediated by the EGFR/PI3K/Akt/mTOR Pathway. Front. Pharmacol. 2022, 12, 764699. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.L.; Shen, Q.; Tang, P.J.; Cao, Y.; Lin, H.; Li, B.; Sun, P.; Bao, B.; Wu, W. In Vitro Study of the Fibrinolytic Activity via Single Chain Urokinase-Type Plasminogen Activator and Molecular Docking of FGFC1. Molecules 2021, 26, 1816. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wu, W.H.; Zhu, Q.G.; Fu, S.; Wang, X.; Hong, S.; Guo, R.; Bao, B. Identification and Fibrinolytic Evaluation of an Isoindolone Derivative Isolated from a Rare Marine Fungus Stachybotrys longispora FG216. Chin. J. Chem. 2015, 33, 1089–1095. [Google Scholar] [CrossRef]
- Kuter, D.J.; Rosenberg, R.D. The reciprocal relationship of thrombopoietin (c-Mpl ligand) to changes in the platelet mass during busulfan-induced thrombocytopenia in the rabbit. Blood 1995, 85, 2720–2730. [Google Scholar] [CrossRef] [PubMed]
- Pieters, M.; Philippou, H.; Undas, A.; de Lange, Z.; Rijken, D.C.; Mutch, N.J. An international study on the feasibility of a standardized combined plasma clot turbidity and lysis assay: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1007–1012. [Google Scholar] [CrossRef]
- Domingues, M.M.; Macrae, F.L.; Duval, C.; McPherson, H.R.; Bridge, K.I.; Ajjan, R.A.; Ridger, V.C.; Connell, S.D.; Philippou, H.; Ariëns, R.A. Thrombin and fibrinogen γ′ impact clot structure by marked effects on intrafibrillar structure and protofibril packing. Blood 2016, 127, 487–495. [Google Scholar] [CrossRef]
- Mahalakshmi, M.; Hariharan, G.; Brindha, G.R. An efficient wavelet-based optimization algorithm for the solutions of reaction-diffusion equations in biomedicine. Comput. Methods Programs Biomed. 2020, 186, 105218. [Google Scholar] [CrossRef]
- Guo, R.H.; Duan, D.; Hong, S.T.; Zhou, Y.; Wang, F.; Wang, S.; Wu, W.; Bao, B. A marine fibrinolytic compound FGFC1 stimulating enzymatic kinetic parameters of a reciprocal activation system based on a single chain urokinase-type plasminogen activator and plasminogen. Process Biochem. 2018, 68, 190–196. [Google Scholar] [CrossRef]
- Hang, S.J.; Chen, H.; Wu, W.H.; Wang, S.; Fang, Y.; Sheng, R.; Tu, Q.; Guo, R. Progress in Isoindolone Alkaloid Derivatives from Marine Microorganism: Pharmacology, Preparation, and Mechanism. Mar. Drugs 2022, 20, 405. [Google Scholar] [CrossRef] [PubMed]
- Weisel, J.W.; Nagaswami, C. Computer modeling of fibrin polymerization kinetics correlated with electron microscope and turbidity observations: Clot structure and assembly are kinetically controlled. Biophys. J. 1992, 63, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Riedel, T.; Suttnar, J.; Brynda, E.; Houska, M.; Medved, L.; Dyr, J.E. Fibrinopeptides A and B release in the process of surface fibrin formation. Blood 2011, 117, 1700–1706. [Google Scholar] [CrossRef] [PubMed]
- Ferri, F.; Calegari, G.R.; Molteni, M.; Cardinali, B.; Magatti, D.; Rocco, M. Size and Density of Fibers in Fibrin and Other Filamentous Networks from Turbidimetry: Beyond a Revisited Carr–Hermans Method, Accounting for Fractality and Porosity. Macromolecules 2015, 48, 5423–5432. [Google Scholar] [CrossRef]
- Goretzki, L.; Schmitt, M.; Mann, K.; Calvete, J.; Chucholowski, N.; Kramer, M.; Günzler, W.A.; Jänicke, F.; Graeff, H. Effective activation of the proenzyme form of the urokinase-type plasminogen activator (pro-uPA) by the cysteine protease cathepsin L. FEBS Lett. 1992, 297, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Chernysh, I.N.; Weisel, J.W. Dynamic imaging of fibrin network formation correlated with other measures of polymerization. Blood 2008, 111, 4854–4861. [Google Scholar] [CrossRef] [PubMed]
- Abu-Fanne, R.; Stepanova, V.; Litvinov, R.I.; Abdeen, S.; Bdeir, K.; Higazi, M.; Maraga, E.; Nagaswami, C.; Mukhitov, A.R.; Weisel, J.W.; et al. Neutrophil alpha-defensins promote thrombosis in vivo by altering fibrin formation, structure, and stability. Blood 2019, 133, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.M.; Fang, M.H.; Wu, Y.Q. Association of red cell distribution width and D-dimer levels with intracranial hemorrhage in patients with cerebral venous thrombosis. Clin. Neurol. Neurosurg. 2022, 214, 107178. [Google Scholar] [CrossRef]
- Siudut, J.; Natorska, J.; Wypasek, E.; Wiewiórka, Ł.; Ostrowska-Kaim, E.; Wiśniowska-Śmiałek, S.; Plens, K.; Musialek, P.; Legutko, J.; Undas, A. Apolipoproteins and lipoprotein(a) as factors modulating fibrin clot properties in patients with severe aortic stenosis. Atherosclerosis 2022, 344, 49–56. [Google Scholar] [CrossRef]
- Ismail, A.A.; Shaker, B.T.; Bajou, K. The Plasminogen-Activator Plasmin System in Physiological and Pathophysiological Angiogenesis. Int. J. Mol. Sci. 2021, 23, 337. [Google Scholar] [CrossRef]
- Thiebaut, A.M.; Gauberti, M.; Ali, C.; De Lizarrondo, S.M.; Vivien, D.; Yepes, M.; Roussel, B.D. The role of plasminogen activators in stroke treatment: Fibrinolysis and beyond. Lancet Neurol. 2018, 17, 1121–1132. [Google Scholar] [CrossRef]
- Robbie, L.A.; Dummer, S.; Booth, N.A.; Adey, G.D.; Bennett, B. Plasminogen activator inhibitor 2 and urokinase-type plasminogen activator in plasma and leucocytes in patients with severe sepsis. Br. J. Haematol. 2000, 109, 342–348. [Google Scholar] [CrossRef]
- Whyte, C.S.; Morrow, G.B.; Baik, N.; Booth, N.A.; Jalal, M.M.; Parmer, R.J.; Miles, L.A.; Mutch, N.J. Exposure of plasminogen and a novel plasminogen receptor, Plg-RKT, on activated human and murine platelets. Blood 2021, 137, 248–257. [Google Scholar] [CrossRef]
- Lin, Z.H.; Jiang, L.G.; Yuan, C.; Jensen, J.K.; Zhang, X.; Luo, Z.; Furie, B.C.; Furie, B.; Andreasen, P.A.; Huang, M. Structural basis for recognition of urokinase-type plasminogen activator by plasminogen activator inhibitor-1. J. Biol. Chem. 2011, 286, 7027–7032. [Google Scholar] [CrossRef]
- Liu, J.N.; Gurewich, V. Fragment E-2 from fibrin substantially enhances pro-urokinase-induced Glu-plasminogen activation. A kinetic study using the plasmin-resistant mutant pro-urokinase Ala-158-rpro-UK. Biochemistry 1992, 31, 6311–6317. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, C.; Bao, B.; Bao, C.; Wu, W. Fungi Fibrinolytic Compound 1 Plays a Core Role in Modulating Fibrinolysis, Altering Plasma Clot Structure, and Promoting Susceptibility to Lysis. Pharmaceutics 2023, 15, 2320. https://doi.org/10.3390/pharmaceutics15092320
Gao C, Bao B, Bao C, Wu W. Fungi Fibrinolytic Compound 1 Plays a Core Role in Modulating Fibrinolysis, Altering Plasma Clot Structure, and Promoting Susceptibility to Lysis. Pharmaceutics. 2023; 15(9):2320. https://doi.org/10.3390/pharmaceutics15092320
Chicago/Turabian StyleGao, Chunli, Bin Bao, Chunling Bao, and Wenhui Wu. 2023. "Fungi Fibrinolytic Compound 1 Plays a Core Role in Modulating Fibrinolysis, Altering Plasma Clot Structure, and Promoting Susceptibility to Lysis" Pharmaceutics 15, no. 9: 2320. https://doi.org/10.3390/pharmaceutics15092320




