Targeted Glioma Therapy—Clinical Trials and Future Directions
Abstract
:1. Introduction
2. Conventional Standard of Care for Glioma Therapy
3. Delivery of Therapeutic Drugs to the Brain and Associated Difficulties
3.1. Blood–Brain Barrier as an Obstacle for Drug Delivery
3.2. Targeted Drug Delivery to the Brain
3.2.1. Passive Targeting
3.2.2. Mechanical Targeting (Local Delivery)
3.2.3. Active Targeting
4. Drug Delivery Systems for Active Targeting of Brain Tumors
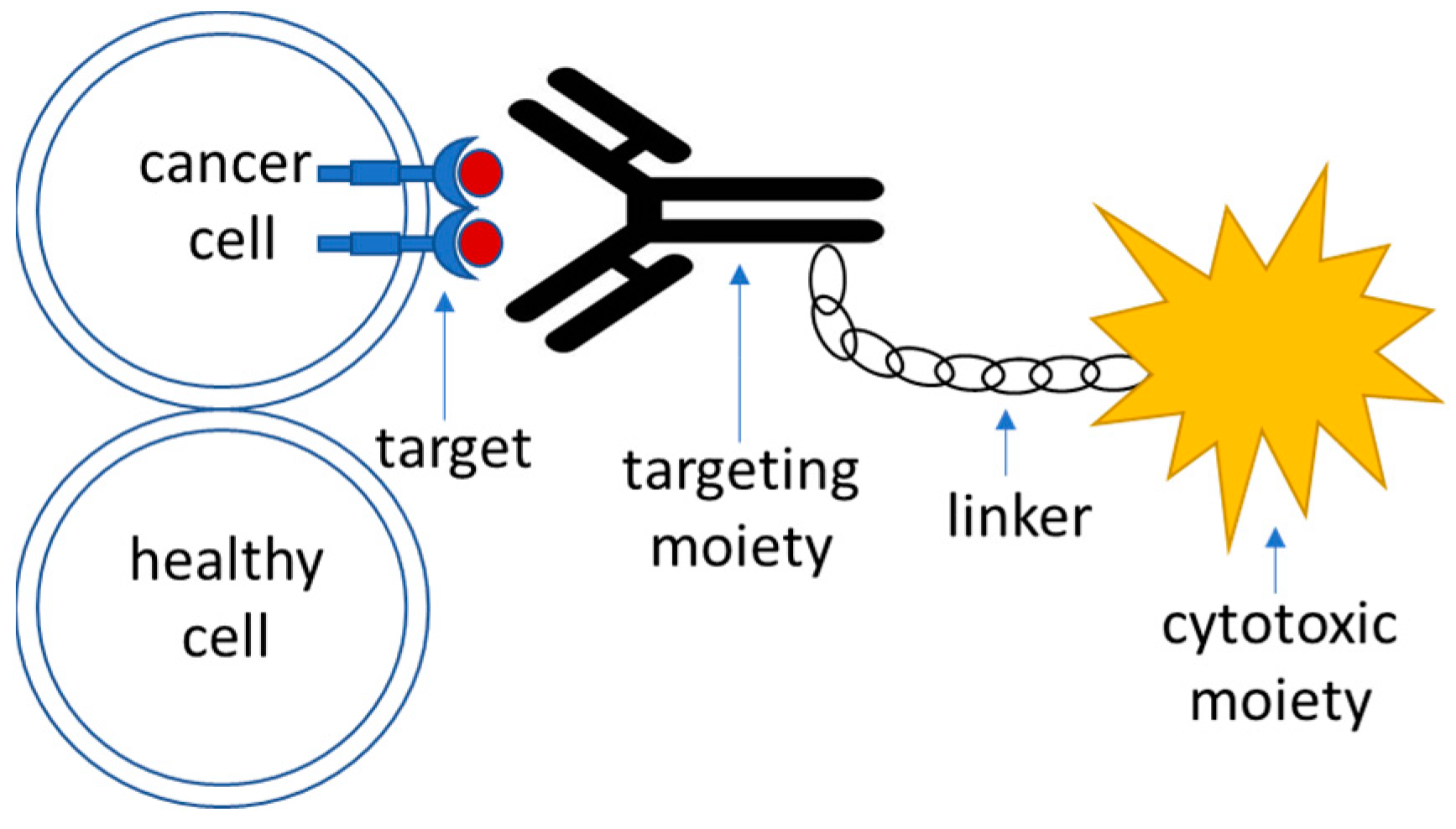
4.1. General Scheme for Drug Delivery Systems That Use Active Targeting
- Target—this is a moiety that is specific to the cell of interest—in this case, a tumor cell. It is required to be expressed or significantly overrepresented in the tumor over healthy cells; high-level expression is advantageous, although not necessary [90]. It could also represent a molecule within an altered biochemical pathway that is specific to a tumor cell.
- Recognition moiety—a part of the drug delivery system that recognizes the target specifically and allows address delivery to a specified tumor site with minimal toxicity and off-target effects.
- Linker—an engineered connective unit that ties the recognition part of the drug delivery system to the payload.
- Payload—a cytotoxic agent that acts upon delivery to the tumor cells either directly or indirectly through the mediation of the host’s immune response.
4.2. Targets
4.3. Recognition Moiety
- Monoclonal antibodies and their derivatives (such as antibody fragments (Fabs), single chain variable fragments (scFvs), and bispecific antibodies) [99]. The most frequently used format for the development of antibody–drug conjugates is IgG1 immunoglobulin, as it is both easy to manufacture as well as the fact that it shows a strong cytotoxic effect [100]. To promote the cell-killing effect, engineered antibodies could work as both internalizing moieties that release an active drug within the cell of interest [101] or non-internalizing units that release toxic payload in the extracellular matrix of the tumor site affecting malignant cells [102,103,104,105,106]. Monoclonal antibodies could also promote an antiproliferative effect on cancer cells via the receptor blockade, which leads to the depletion of signaling and subsequent cell death [91,107], combining recognition and cytotoxic function in one molecular unit.
- Peptides as recognition moieties in the targeted drugs are presented as either cell-targeting or cell-penetrating units [108]. While the first group’s mechanism of action is similar to that of antibody–drug conjugates, the latter’s is not yet fully understood [95,96]. The uptake and delivery of cytotoxic drugs with the help of peptides as a recognition particle have been shown in preclinical [88] and clinical research [67].
- Small molecules as inhibitors of intrinsic pathways are often used as therapeutic agents in glioma treatment [109,110], as the dysregulation of such pathways (e.g., signaling pathways involving RTKs, PI3K, p53, etc.) has a major role in GBM development [6]. Preclinical data on efficacy and targeted distribution are reviewed elsewhere in detail [110]; however, clinical trials of such compounds often result in disappointing efficacy in the treatment of GBM (see below).
- Aptamers could also be used as a recognition unit of a targeted drug delivery system. Aptamers are short nucleic acid sequences that could be selected based on their affinity towards tumor cells via the systematic evolution of ligands by an exponential enrichment (SELEX) process [111]. It was shown through in vitro [112] and in vivo [113] studies that the PDGFRβ-specific aptamer Gint4.1 can promote cytotoxicity and the delivery of other pharmaceutically active compounds within GBM models.
4.4. Linker
4.5. Payload (Cytotoxic Agent)
- Microtubule assembly inhibitors: auristatins that inhibit tubulin polymerase and promote cell cycle arrest [137] such as monomethyl auristatin E (MMAE) [138] and monomethyl auristatin F (MMAF) [139], or maytansines and their derivatives that bind to tubulin and therefore interfere with the assembly of microtubules [140]. Common examples include mertansine (DM1) [141,142,143] or ravtansine (DM4) [144,145] as parts of the cytotoxic agents in targeted therapeutic drugs.
- Bacterial toxins—toxic compounds produced by Pseudomonas aeruginosa (Pseudomonas Exotoxin A) and Corynebacterium diphtheria (Diphtheria toxin) are the most commonly used toxins of bacterial origin that are utilized for cancer therapies [146]. Both toxins act upon binding and irreversibly modify the eukaryotic EF2 elongation factor, which results in impaired protein synthesis and cell death [147,148]. In the treatment of GBM, a targeted drug D2C7-IT, comprising of the EGFR-targeted recognition moiety linked to a recombinant pseudomonas exotoxin A, showed promising results in preclinical studies [149] and now patients are being recruited for the evaluation of the drug’s safety and efficacy in Phase I and II clinical trials NCT04547777 and NCT05734560;
- Radioligands fused with peptides or antibodies with tumor specificity are used as a cytotoxic moiety for the targeted delivery of drugs to the tumor sites [71,72,150,151], which allows tumor-specific distribution of the radioactivity as compared to standard beam irradiation in the standard-of-care Stupp protocol [3].
- Photodynamic therapy (PDT) is a therapeutic approach that requires the incorporation of non-toxic and inactive photosensitizer molecules into the cells of interest with subsequent light irradiation, in which the therapeutic molecules become activated. Upon irradiation, photosensitizer molecules transfer light energy and excitate molecular oxygen present in the surrounding tissues to a triplet or singlet state. In the triplet state, oxygen is capable of the generation of reactive oxygen species that react with molecules containing double bonds, leading to their damage and subsequent cell death [152]. In glioma studies and treatment, PDT is implemented via placing fiberoptics at the site of the tumor in order to irradiate cells within the brain that have obtained photosensitizer molecules [153,154]. The clinical application of PDT in glioblastoma patients showed promising results as an intraoperative strategy, with a median PFS of 17.1 months and median OS of 23.1 months [155]. PDT showed a significant improvement in patients’ overall survival compared with the Stupp protocol in patients with non-resectable brain tumors [153] and in clinical trials evaluating the efficacy of PDT in patients with newly diagnosed glioblastoma in intra- and postoperative settings [156,157].
- Oncolytic virotherapy is a novel approach for the targeted therapy of neoplasms that utilizes viruses for the elimination of tumor cells. While the exact mechanisms and the nature of tropism for such viruses might still be unknown, the main theories involve either direct killing of the targeted cells or indirect modulation of the host’s immune system response in order to mediate immunogenic cytotoxicity [158,159]. Clinical trials that have tested oncolytic virotherapy showed increased overall survival as compared with historical controls for polio-rhinovirus chimera PVSRIPO [160]; tumor reduction and safety were observed for tumor-replicative adenovirus DNX2401 with a median overall survival at 13 months for patients who received the treatment intratumorally [161]. For residual and recurrent glioblastoma, the use of a triple-mutated herpes simplex virus showed a median overall survival of 20.2 months after initial tumor resection, which is favorable to any other treatment [162]. This result leads to the governmental approval of an oncolytic virus as a therapeutic modality in Japan (clinical trial registry number UMIN000015995) [162].
5. Strategies in Clinical Approaches towards the Treatment of GBM
5.1. Targeting Receptor Tyrosine Kinases
5.1.1. EGFR
5.1.2. PDGFR
5.1.3. VEGF/VEGFR
5.1.4. c-MET Pathway
5.1.5. FGFR
5.1.6. Multikinase Inhibitors
5.2. Targeting Immune Checkpoints
5.2.1. PD-1/PD-L1
5.2.2. CTLA-4
5.2.3. TIM-3
5.2.4. LAG-3
5.3. Targeting Extracellular Matrix (ECM) Components in Glioma Microenvironment
6. Future Prospects and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BBB | Blood–brain barrier |
| CNS | Central Nervous System |
| CTLA-4 | Cytotoxic T-Lymphocyte-Associated Protein 4 |
| DM1 | mertansine |
| DM4 | ravtansine |
| DSPE-PEG | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)] |
| ECM | Extracellular Matrix |
| EGFR | Epidermal Growth Factor Receptor |
| EPR | Enhanced Permeation and Retention |
| FDA | Food and Drug Administration |
| FGFR | Fibroblast Growth Factor Receptor |
| GBM | glioblastoma multiforme |
| HGF | Hepatocyte Growth Factor |
| HGG | High-Grade Gliomas |
| LGG | Low-Grade Gliomas |
| MDR | Multidrug Resistance |
| MET/c-MET | Mesenchymal–Epithelial Transition factor |
| MMAE | Monomethyl Auristatin E |
| MMAF | Monomethyl Auristatin F |
| MRP | MDR-Associated Protein |
| OS | Overall Survival |
| OS12 | 12-month OS |
| PBS | Phosphate-Buffered Saline |
| PD-1 | Programmed Cell Death Protein 1 |
| PDGFR | Platelet-Derived Growth Factor Receptor |
| PD-L1 | Programmed Cell Death Ligand 1 |
| PDT | Photodynamic Therapy |
| PFS | Progression-Free Survival |
| PFS12 | 12-month PFS |
| PFS6 | 6-month PFS |
| RT | Radiotherapy |
| RTK | Receptor Tyrosine Kinase |
| SELEX | Systematic Evolution of Ligands by Exponential Enrichment |
| SRS | Stereotactic Radiosurgery |
| TKI | Tyrosine Kinase Inhibitor |
| TMZ | Temozolomide |
| VEGF | Vascular Endothelial Growth Factor |
| VEGFR | Vascular Endothelial Growth Factor Receptor |
| WHO | World Health Organization |
References
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro Oncol. 2021, 23, 1–105. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.; Rosenthal, M.A. Survival Comparison between Glioblastoma Multiforme and Other Incurable Cancers. J. Clin. Neurosci. 2010, 17, 417–421. [Google Scholar] [CrossRef]
- Verhaak, R.G.W.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.W.; Verhaak, R.G.W.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The Somatic Genomic Landscape of Glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, B.; Hu, X.; Kim, H.; Squatrito, M.; Scarpace, L.; deCarvalho, A.C.; Lyu, S.; Li, P.; Li, Y.; et al. Tumor Evolution of Glioma Intrinsic Gene Expression Subtype Associates with Immunological Changes in the Microenvironment. Cancer Cell 2017, 32, 42–56.e6. [Google Scholar] [CrossRef]
- Verdugo, E.; Puerto, I.; Medina, M.Á. An Update on the Molecular Biology of Glioblastoma, with Clinical Implications and Progress in Its Treatment. Cancer Commun. 2022, 42, 1083–1111. [Google Scholar] [CrossRef]
- Lee, E.; Yong, R.L.; Paddison, P.; Zhu, J. Comparison of Glioblastoma (GBM) Molecular Classification Methods. Semin. Cancer Biol. 2018, 53, 201–211. [Google Scholar] [CrossRef]
- Zhang, P.; Xia, Q.; Liu, L.; Li, S.; Dong, L. Current Opinion on Molecular Characterization for GBM Classification in Guiding Clinical Diagnosis, Prognosis, and Therapy. Front. Mol. Biosci. 2020, 7, 562798. [Google Scholar] [CrossRef]
- Guo, X.; Shi, Y.; Liu, D.; Li, Y.; Chen, W.; Wang, Y.; Wang, Y.; Xing, H.; Xia, Y.; Li, J.; et al. Clinical Updates on Gliomas and Implications of the 5th Edition of the WHO Classification of Central Nervous System Tumors. Front. Oncol. 2023, 13, 1131642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Xu, X.; Zhu, L.; Li, S.; Chen, R.; Lv, N.; Li, Z.; Wang, J.; Li, Q.; Zhou, W.; et al. A Novel Molecular Classification Method for Glioblastoma Based on Tumor Cell Differentiation Trajectories. Stem Cells Int. 2023, 2023, e2826815. [Google Scholar] [CrossRef]
- El Atat, O.; Naser, R.; Abdelkhalek, M.; Habib, R.A.; El Sibai, M. Molecular Targeted Therapy: A New Avenue in Glioblastoma Treatment (Review). Oncol. Lett. 2023, 25, 46. [Google Scholar] [CrossRef]
- Burnet, N.G.; Jefferies, S.J.; Benson, R.J.; Hunt, D.P.; Treasure, F.P. Years of Life Lost (YLL) from Cancer Is an Important Measure of Population Burden—And Should Be Considered When Allocating Research Funds. Br. J. Cancer 2005, 92, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. Glioblastoma: Overview of Disease and Treatment. Clin. J. Oncol. Nurs. 2016, 20, S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Vaz-Salgado, M.A.; Villamayor, M.; Albarrán, V.; Alía, V.; Sotoca, P.; Chamorro, J.; Rosero, D.; Barrill, A.M.; Martín, M.; Fernandez, E.; et al. Recurrent Glioblastoma: A Review of the Treatment Options. Cancers 2023, 15, 4279. [Google Scholar] [CrossRef]
- Leone, A.; Colamaria, A.; Fochi, N.P.; Sacco, M.; Landriscina, M.; Parbonetti, G.; de Notaris, M.; Coppola, G.; De Santis, E.; Giordano, G.; et al. Recurrent Glioblastoma Treatment: State of the Art and Future Perspectives in the Precision Medicine Era. Biomedicines 2022, 10, 1927. [Google Scholar] [CrossRef]
- Ameratunga, M.; Pavlakis, N.; Wheeler, H.; Grant, R.; Simes, J.; Khasraw, M. Anti-angiogenic Therapy for High-grade Glioma. Cochrane Database Syst. Rev. 2018, 5, 610–620. [Google Scholar] [CrossRef]
- Cohen, M.H.; Shen, Y.L.; Keegan, P.; Pazdur, R. FDA Drug Approval Summary: Bevacizumab (Avastin) as Treatment of Recurrent Glioblastoma Multiforme. Oncologist 2009, 14, 1131–1138. [Google Scholar] [CrossRef]
- Jakola, A.S.; Skjulsvik, A.J.; Myrmel, K.S.; Sjåvik, K.; Unsgård, G.; Torp, S.H.; Aaberg, K.; Berg, T.; Dai, H.Y.; Johnsen, K.; et al. Surgical Resection versus Watchful Waiting in Low-Grade Gliomas. Ann. Oncol. 2017, 28, 1942–1948. [Google Scholar] [CrossRef]
- Shaw, E.G.; Berkey, B.; Coons, S.W.; Brachman, D.; Buckner, J.C.; Stelzer, K.J.; Barger, G.R.; Brown, P.D.; Gilbert, M.R.; Mehta, M. Initial Report of Radiation Therapy Oncology Group (RTOG) 9802: Prospective Studies in Adult Low-Grade Glioma (LGG). J. Clin. Oncol. 2006, 24, 1500. [Google Scholar] [CrossRef]
- Lapointe, S.; Perry, A.; Butowski, N.A. Primary Brain Tumours in Adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Baumert, B.G.; Hegi, M.E.; van den Bent, M.J.; von Deimling, A.; Gorlia, T.; Hoang-Xuan, K.; Brandes, A.A.; Kantor, G.; Taphoorn, M.J.B.; Hassel, M.B.; et al. Temozolomide Chemotherapy versus Radiotherapy in High-Risk Low-Grade Glioma (EORTC 22033-26033): A Randomised, Open-Label, Phase 3 Intergroup Study. Lancet Oncol. 2016, 17, 1521–1532. [Google Scholar] [CrossRef]
- Buckner, J.C.; Shaw, E.G.; Pugh, S.L.; Chakravarti, A.; Gilbert, M.R.; Barger, G.R.; Coons, S.; Ricci, P.; Bullard, D.; Brown, P.D.; et al. Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. N. Engl. J. Med. 2016, 374, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Lassaletta, A.; Scheinemann, K.; Zelcer, S.M.; Hukin, J.; Wilson, B.A.; Jabado, N.; Carret, A.S.; Lafay-Cousin, L.; Larouche, V.; Hawkins, C.E.; et al. Phase II Weekly Vinblastine for Chemotherapy-Naïve Children with Progressive Low-Grade Glioma: A Canadian Pediatric Brain Tumor Consortium Study. J. Clin. Oncol. 2016, 34, 3537–3543. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.P.; Adamson, D.C. Current FDA-Approved Therapies for High-Grade Malignant Gliomas. Biomedicines 2021, 9, 324. [Google Scholar] [CrossRef]
- Hochberg, F.H.; Linggood, R.; Wolfson, L.; Baker, W.H.; Kornblith, P. Quality and Duration of Survival in Glioblastoma Multiforme: Combined Surgical, Radiation, and Lomustine Therapy. JAMA 1979, 241, 1016–1018. [Google Scholar] [CrossRef]
- Walker, M.D.; Alexander, E.; Hunt, W.E.; MacCarty, C.S.; Mahaley, M.S.; Mealey, J.; Norrell, H.A.; Owens, G.; Ransohoff, J.; Wilson, C.B.; et al. Evaluation of BCNU and/or Radiotherapy in the Treatment of Anaplastic Gliomas: A Cooperative Clinical Trial. J. Neurosurg. 1978, 49, 333–343. [Google Scholar] [CrossRef]
- Westphal, M.; Hilt, D.C.; Bortey, E.; Delavault, P.; Olivares, R.; Warnke, P.C.; Whittle, I.R.; Jääskeläinen, J.; Ram, Z. A Phase 3 Trial of Local Chemotherapy with Biodegradable Carmustine (BCNU) Wafers (Gliadel Wafers) in Patients with Primary Malignant Glioma. Neuro Oncol. 2003, 5, 79–88. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.A.; Kesari, S.; Steinberg, D.M.; Toms, S.A.; Taylor, L.P.; Lieberman, F.; Silvani, A.; Fink, K.L.; et al. Maintenance Therapy with Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA 2015, 314, 2535–2543. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Huang, H.J.; Nagane, M.; Ji, X.D.; Wang, D.; Shih, C.C.; Arap, W.; Huang, C.M.; Cavenee, W.K. Suppression of Glioblastoma Angiogenicity and Tumorigenicity by Inhibition of Endogenous Expression of Vascular Endothelial Growth Factor. Proc. Natl. Acad. Sci. USA 1996, 93, 8502–8507. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A Randomized Trial of Bevacizumab for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Umemura, Y.; Leung, D. Bevacizumab and Glioblastoma: Past, Present, and Future Directions. Cancer J. 2018, 24, 180–186. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The Blood–Brain Barrier: Structure, Regulation, and Drug Delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-Y.; Wang, Z.-B.; Zhang, L.-C.; Wei, X.; Li, L. Tight Junction in Blood-Brain Barrier: An Overview of Structure, Regulation, and Regulator Substances. CNS Neurosci. Ther. 2012, 18, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson, P.T.; Davis, T.P. Targeting Blood–Brain Barrier Changes during Inflammatory Pain: An Opportunity for Optimizing CNS Drug Delivery. Ther. Deliv. 2011, 2, 1015–1041. [Google Scholar] [CrossRef]
- Pardridge, W.M. CNS Drug Design Based on Principles of Blood-Brain Barrier Transport. J. Neurochem. 1998, 70, 1781–1792. [Google Scholar] [CrossRef]
- Pulgar, V.M. Transcytosis to Cross the Blood Brain Barrier, New Advancements and Challenges. Front. Neurosci. 2019, 12, 1019. [Google Scholar] [CrossRef]
- Hersh, D.S.; Wadajkar, A.S.; Roberts, N.B.; Perez, J.G.; Connolly, N.P.; Frenkel, V.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J. Evolving Drug Delivery Strategies to Overcome the Blood Brain Barrier. Curr. Pharm. Des. 2016, 22, 1177–1193. [Google Scholar] [CrossRef]
- Patel, M.M.; Goyal, B.R.; Bhadada, S.V.; Bhatt, J.S.; Amin, A.F. Getting into the Brain. CNS Drugs 2009, 23, 35–58. [Google Scholar] [CrossRef]
- Cecchelli, R.; Berezowski, V.; Lundquist, S.; Culot, M.; Renftel, M.; Dehouck, M.-P.; Fenart, L. Modelling of the Blood–Brain Barrier in Drug Discovery and Development. Nat. Rev. Drug Discov. 2007, 6, 650–661. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yao, Y.; Tsirka, S.E.; Cao, Y. Cell-Culture Models of the Blood–Brain Barrier. Stroke 2014, 45, 2514–2526. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Wang, K.; Wang, Y.; Wang, S.; Li, J.; Lou, J.; Ye, L.; Yan, X.; Lu, W.; Huang, R. Enhanced Blood–Brain Barrier Penetration and Glioma Therapy Mediated by a New Peptide Modified Gene Delivery System. Biomaterials 2015, 37, 345–352. [Google Scholar] [CrossRef]
- Omidi, Y.; Kianinejad, N.; Kwon, Y.; Omidian, H. Drug Delivery and Targeting to Brain Tumors: Considerations for Crossing the Blood-Brain Barrier. Expert Rev. Clin. Pharmacol. 2021, 14, 357–381. [Google Scholar] [CrossRef]
- Spiegl-Kreinecker, S.; Buchroithner, J.; Elbling, L.; Steiner, E.; Wurm, G.; Bodenteich, A.; Fischer, J.; Micksche, M.; Berger, W. Expression and Functional Activity of the ABC-Transporter Proteins P-Glycoprotein and Multidrug-Resistance Protein 1 in Human Brain Tumor Cells and Astrocytes. J. Neurooncol. 2002, 57, 27–36. [Google Scholar] [CrossRef] [PubMed]
- De Trizio, I.; Errede, M.; d’Amati, A.; Girolamo, F.; Virgintino, D. Expression of P-Gp in Glioblastoma: What We Can Learn from Brain Development. Curr. Pharm. Des. 2020, 26, 1428–1437. [Google Scholar] [CrossRef]
- Chernov, A.N.; Alaverdian, D.A.; Galimova, E.S.; Renieri, A.; Frullanti, E.; Meloni, I.; Shamova, O.V. The Phenomenon of Multidrug Resistance in Glioblastomas. Hematol. Oncol. Stem Cell Ther. 2021, 15, 1–7. [Google Scholar] [CrossRef]
- Callaghan, R.; Luk, F.; Bebawy, M. Inhibition of the Multidrug Resistance P-Glycoprotein: Time for a Change of Strategy? Drug Metab. Dispos. 2014, 42, 623–631. [Google Scholar] [CrossRef]
- Clark, D.E. In Silico Prediction of Blood–Brain Barrier Permeation. Drug Discov. Today 2003, 8, 927–933. [Google Scholar] [CrossRef]
- Gleeson, M.P. Generation of a Set of Simple, Interpretable ADMET Rules of Thumb. J. Med. Chem. 2008, 51, 817–834. [Google Scholar] [CrossRef]
- Hervé, F.; Ghinea, N.; Scherrmann, J.-M. CNS Delivery Via Adsorptive Transcytosis. AAPS J. 2008, 10, 455–472. [Google Scholar] [CrossRef]
- Lalatsa, A.; Schätzlein, A.G.; Uchegbu, I.F. Drug Delivery across the Blood-Brain Barrier. In Comprehensive Biotechnology; Moo-Young, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 5, pp. 657–667. ISBN 978-0-444-53352-4. [Google Scholar]
- Serrano Lopez, D.R.; Lalatsa, A. Peptide Pills for Brain Diseases? Reality and Future Perspectives. Ther. Deliv. 2013, 4, 479–501. [Google Scholar] [CrossRef] [PubMed]
- Laquintana, V.; Trapani, A.; Denora, N.; Wang, F.; Gallo, J.M.; Trapani, G. New Strategies to Deliver Anticancer Drugs to Brain Tumors. Expert Opin. Drug Deliv. 2009, 6, 1017–1032. [Google Scholar] [CrossRef]
- Ostermann, S.; Csajka, C.; Buclin, T.; Leyvraz, S.; Lejeune, F.; Decosterd, L.A.; Stupp, R. Plasma and Cerebrospinal Fluid Population Pharmacokinetics of Temozolomide in Malignant Glioma Patients. Clin. Cancer Res. 2004, 10, 3728–3736. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Gu, B.; Xie, C.; Li, J.; Liu, Y.; Lu, W. Cyclic RGD Conjugated Poly(Ethylene Glycol)-Co-Poly(Lactic Acid) Micelle Enhances Paclitaxel Anti-Glioblastoma Effect. J. Control. Release 2010, 143, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, M.S.; Upadhyay, U.; Goodwin, R.; Tyler, B.; Brem, H. Local Delivery of Doxorubicin for the Treatment of Malignant Brain Tumors in Rats. Anticancer Res. 2005, 25, 3825–3831. [Google Scholar]
- Kondo, Y.; Kondo, S.; Tanaka, Y.; Haqqi, T.; Barna, B.P.; Cowell, J.K. Inhibition of Telomerase Increases the Susceptibility of Human Malignant Glioblastoma Cells to Cisplatin-Induced Apoptosis. Oncogene 1998, 16, 2243–2248. [Google Scholar] [CrossRef]
- Wang, P.P.; Frazier, J.; Brem, H. Local Drug Delivery to the Brain. Adv. Drug Deliv. Rev. 2002, 54, 987–1013. [Google Scholar] [CrossRef]
- Dufès, C.; Al Robaian, M.; Somani, S. Transferrin and the Transferrin Receptor for the Targeted Delivery of Therapeutic Agents to the Brain and Cancer Cells. Ther. Deliv. 2013, 4, 629–640. [Google Scholar] [CrossRef]
- Li, Y.; He, H.; Jia, X.; Lu, W.-L.; Lou, J.; Wei, Y. A Dual-Targeting Nanocarrier Based on Poly(Amidoamine) Dendrimers Conjugated with Transferrin and Tamoxifen for Treating Brain Gliomas. Biomaterials 2012, 33, 3899–3908. [Google Scholar] [CrossRef]
- Ruan, S.; Yuan, M.; Zhang, L.; Hu, G.; Chen, J.; Cun, X.; Zhang, Q.; Yang, Y.; He, Q.; Gao, H. Tumor Microenvironment Sensitive Doxorubicin Delivery and Release to Glioma Using Angiopep-2 Decorated Gold Nanoparticles. Biomaterials 2015, 37, 425–435. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L. Modern Methods for Delivery of Drugs across the Blood–Brain Barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Gorlia, T.; Erridge, S.C.; Perry, J.; Hong, Y.-K.; Aldape, K.D.; Lhermitte, B.; Pietsch, T.; Grujicic, D.; et al. Cilengitide Combined with Standard Treatment for Patients with Newly Diagnosed Glioblastoma with Methylated MGMT Promoter (CENTRIC EORTC 26071-22072 Study): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2014, 15, 1100–1108. [Google Scholar] [CrossRef]
- Gao, H.; Qian, J.; Cao, S.; Yang, Z.; Pang, Z.; Pan, S.; Fan, L.; Xi, Z.; Jiang, X.; Zhang, Q. Precise Glioma Targeting of and Penetration by Aptamer and Peptide Dual-Functioned Nanoparticles. Biomaterials 2012, 33, 5115–5123. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, T.T.; Mulholland, P.; Neyns, B.; Nabors, L.B.; Campone, M.; Wick, A.; Mason, W.; Mikkelsen, T.; Phuphanich, S.; Ashby, L.S.; et al. Phase III Randomized Trial Comparing the Efficacy of Cediranib as Monotherapy, and in Combination with Lomustine, Versus Lomustine Alone in Patients with Recurrent Glioblastoma. J. Clin. Oncol. 2013, 31, 3212–3218. [Google Scholar] [CrossRef] [PubMed]
- Drappatz, J.; Brenner, A.; Wong, E.T.; Eichler, A.; Schiff, D.; Groves, M.D.; Mikkelsen, T.; Rosenfeld, S.; Sarantopoulos, J.; Meyers, C.A.; et al. Phase I Study of GRN1005 in Recurrent Malignant Glioma. Clin. Cancer Res. 2013, 19, 1567–1576. [Google Scholar] [CrossRef]
- Gan, H.K.; Burgess, A.W.; Clayton, A.H.A.; Scott, A.M. Targeting of a Conformationally Exposed, Tumor-Specific Epitope of EGFR as a Strategy for Cancer Therapy. Cancer Res. 2012, 72, 2924–2930. [Google Scholar] [CrossRef]
- Neyns, B.; Sadones, J.; Joosens, E.; Bouttens, F.; Verbeke, L.; Baurain, J.-F.; D’Hondt, L.; Strauven, T.; Chaskis, C.; Veld, P.I.; et al. Stratified Phase II Trial of Cetuximab in Patients with Recurrent High-Grade Glioma. Ann. Oncol. 2009, 20, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- van den Bent, M.; Azaro, A.; De Vos, F.; Sepulveda, J.; Yung, W.K.A.; Wen, P.Y.; Lassman, A.B.; Joerger, M.; Tabatabai, G.; Rodon, J.; et al. A Phase Ib/II, Open-Label, Multicenter Study of INC280 (Capmatinib) Alone and in Combination with Buparlisib (BKM120) in Adult Patients with Recurrent Glioblastoma. J. Neurooncol. 2020, 146, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Loya, J.; Zhang, C.; Cox, E.; Achrol, A.S.; Kesari, S. Biological Intratumoral Therapy for the High-Grade Glioma Part I: Intratumoral Delivery and Immunotoxins. CNS Oncol. 2019, 8, CNS38. [Google Scholar] [CrossRef] [PubMed]
- Loya, J.; Zhang, C.; Cox, E.; Achrol, A.S.; Kesari, S. Biological Intratumoral Therapy for the High-Grade Glioma Part II: Vector- and Cell-Based Therapies and Radioimmunotherapy. CNS Oncol. 2019, 8, CNS40. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, A.J. Intracerebroventricular Drug Administration. Transl. Clin. Pharmacol. 2017, 25, 117. [Google Scholar] [CrossRef]
- Cohen-Pfeffer, J.L.; Gururangan, S.; Lester, T.; Lim, D.A.; Shaywitz, A.J.; Westphal, M.; Slavc, I. Intracerebroventricular Delivery as a Safe, Long-Term Route of Drug Administration. Pediatr. Neurol. 2017, 67, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Slavc, I.; Cohen-Pfeffer, J.L.; Gururangan, S.; Krauser, J.; Lim, D.A.; Maldaun, M.; Schwering, C.; Shaywitz, A.J.; Westphal, M. Best Practices for the Use of Intracerebroventricular Drug Delivery Devices. Mol. Genet. Metab. 2018, 124, 184–188. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.S.; Khatri, D.; Reichman, N.; Patel, N.V.; Wong, T.; Fralin, S.R.; Li, M.; Ellis, J.A.; Ortiz, R.; Langer, D.J.; et al. Super Selective Intra-Arterial Cerebral Infusion of Modern Chemotherapeutics after Blood–Brain Barrier Disruption: Where Are We Now, and Where We Are Going. J. Neurooncol. 2020, 147, 261–278. [Google Scholar] [CrossRef]
- Torchilin, V. Tumor Delivery of Macromolecular Drugs Based on the EPR Effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. The Enhanced Permeability and Retention (EPR) Effect in Tumor Vasculature: The Key Role of Tumor-Selective Macromolecular Drug Targeting. Adv. Enzyme Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef]
- Lammers, T.; Kiessling, F.; Hennink, W.E.; Storm, G. Drug Targeting to Tumors: Principles, Pitfalls and (Pre-) Clinical Progress. J. Control. Release 2012, 161, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F. To Exploit the Tumor Microenvironment: Since the EPR Effect Fails in the Clinic, What Is the Future of Nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef]
- Xu, H.-L.; Yang, J.-J.; ZhuGe, D.-L.; Lin, M.-T.; Zhu, Q.-Y.; Jin, B.-H.; Tong, M.-Q.; Shen, B.-X.; Xiao, J.; Zhao, Y.-Z. Glioma-Targeted Delivery of a Theranostic Liposome Integrated with Quantum Dots, Superparamagnetic Iron Oxide, and Cilengitide for Dual-Imaging Guiding Cancer Surgery. Adv. Healthc. Mater. 2018, 7, 1701130. [Google Scholar] [CrossRef] [PubMed]
- Brem, H.; Mahaley, M.S.; Vick, N.A.; Black, K.L.; Schold, S.C.; Burger, P.C.; Friedman, A.H.; Ciric, I.S.; Eller, T.W.; Cozzens, J.W.; et al. Interstitial Chemotherapy with Drug Polymer Implants for the Treatment of Recurrent Gliomas. J. Neurosurg. 1991, 74, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Brem, S.; Tyler, B.; Li, K.; Pradilla, G.; Legnani, F.; Caplan, J.; Brem, H. Local Delivery of Temozolomide by Biodegradable Polymers Is Superior to Oral Administration in a Rodent Glioma Model. Cancer Chemother. Pharmacol. 2007, 60, 643–650. [Google Scholar] [CrossRef]
- Shi, M.; Sanche, L. Convection-Enhanced Delivery in Malignant Gliomas: A Review of Toxicity and Efficacy. J. Oncol. 2019, 2019, e9342796. [Google Scholar] [CrossRef] [PubMed]
- Voulgaris, S.; Partheni, M.; Karamouzis, M.; Dimopoulos, P.; Papadakis, N.; Kalofonos, H.P. Intratumoral Doxorubicin in Patients with Malignant Brain Gliomas. Am. J. Clin. Oncol. 2002, 25, 60. [Google Scholar] [CrossRef]
- Weinberg, B.D.; Blanco, E.; Gao, J. Polymer Implants for Intratumoral Drug Delivery and Cancer Therapy. J. Pharm. Sci. 2008, 97, 1681–1702. [Google Scholar] [CrossRef]
- Kim, J.; Song, S.; Gwak, M.; Cho, H.; Yun, W.S.; Hwang, N.; Kim, J.; Lee, J.S.; Kim, D.-H.; Kim, H.; et al. Micro-Syringe Chip-Guided Intratumoral Administration of Lipid Nanoparticles for Targeted Anticancer Therapy. Biomater. Res. 2023, 27, 102. [Google Scholar] [CrossRef]
- Gu, G.; Xia, H.; Hu, Q.; Liu, Z.; Jiang, M.; Kang, T.; Miao, D.; Tu, Y.; Pang, Z.; Song, Q.; et al. PEG-Co-PCL Nanoparticles Modified with MMP-2/9 Activatable Low Molecular Weight Protamine for Enhanced Targeted Glioblastoma Therapy. Biomaterials 2013, 34, 196–208. [Google Scholar] [CrossRef]
- Škrlj, N.; Drevenšek, G.; Hudoklin, S.; Romih, R.; Čurin Šerbec, V.; Dolinar, M. Recombinant Single-Chain Antibody with the Trojan Peptide Penetratin Positioned in the Linker Region Enables Cargo Transfer Across the Blood–Brain Barrier. Appl. Biochem. Biotechnol. 2013, 169, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Teicher, B.A.; Hassan, R. Antibody–Drug Conjugates for Cancer Therapy. Lancet Oncol. 2016, 17, e254–e262. [Google Scholar] [CrossRef]
- Qin, A.; Musket, A.; Musich, P.R.; Schweitzer, J.B.; Xie, Q. Receptor Tyrosine Kinases as Druggable Targets in Glioblastoma: Do Signaling Pathways Matter? Neuro Oncol. Adv. 2021, 3, vdab133. [Google Scholar] [CrossRef] [PubMed]
- McLendon, R.; Friedman, A.; Bigner, D.; Van Meir, E.G.; Brat, D.J.; Mastrogianakis, G.M.; Olson, J.J.; Mikkelsen, T.; Lehman, N.; Aldape, K.; et al. Comprehensive Genomic Characterization Defines Human Glioblastoma Genes and Core Pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef]
- Lemmon, M.A.; Schlessinger, J. Cell Signaling by Receptor Tyrosine Kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef] [PubMed]
- Snuderl, M.; Fazlollahi, L.; Le, L.P.; Nitta, M.; Zhelyazkova, B.H.; Davidson, C.J.; Akhavanfard, S.; Cahill, D.P.; Aldape, K.D.; Betensky, R.A.; et al. Mosaic Amplification of Multiple Receptor Tyrosine Kinase Genes in Glioblastoma. Cancer Cell 2011, 20, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Dine, J.; Gordon, R.; Shames, Y.; Kasler, M.K.; Barton-Burke, M. Immune Checkpoint Inhibitors: An Innovation in Immunotherapy for the Treatment and Management of Patients with Cancer. Asia Pac. J. Oncol. Nurs. 2017, 4, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Ghouzlani, A.; Kandoussi, S.; Tall, M.; Reddy, K.P.; Rafii, S.; Badou, A. Immune Checkpoint Inhibitors in Human Glioma Microenvironment. Front. Immunol. 2021, 12, 679425. [Google Scholar] [CrossRef]
- Singh, S.; Hassan, D.; Aldawsari, H.M.; Molugulu, N.; Shukla, R.; Kesharwani, P. Immune Checkpoint Inhibitors: A Promising Anticancer Therapy. Drug Discov. Today 2020, 25, 223–229. [Google Scholar] [CrossRef]
- Wang, W.; Wang, E.; Balthasar, J. Monoclonal Antibody Pharmacokinetics and Pharmacodynamics. Clin. Pharmacol. Ther. 2008, 84, 548–558. [Google Scholar] [CrossRef]
- Chacko, A.-M.; Li, C.; Pryma, D.A.; Brem, S.; Coukos, G.; Muzykantov, V.R. Targeted Delivery of Antibody-Based Therapeutic and Imaging Agents to CNS Tumors: Crossing the Blood-Brain-Barrier Divide. Expert Opin. Drug Deliv. 2013, 10, 907–926. [Google Scholar] [CrossRef]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Strategies and Challenges for the next Generation of Antibody–Drug Conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef]
- Chari, R.V.J. Targeted Cancer Therapy: Conferring Specificity to Cytotoxic Drugs. Acc. Chem. Res. 2008, 41, 98–107. [Google Scholar] [CrossRef]
- Fuchigami, H.; Manabe, S.; Yasunaga, M.; Matsumura, Y. Chemotherapy Payload of Anti-Insoluble Fibrin Antibody-Drug Conjugate Is Released Specifically upon Binding to Fibrin. Sci. Rep. 2018, 8, 14211. [Google Scholar] [CrossRef]
- Gébleux, R.; Stringhini, M.; Casanova, R.; Soltermann, A.; Neri, D. Non-Internalizing Antibody-Drug Conjugates Display Potent Anti-Cancer Activity upon Proteolytic Release of Monomethyl Auristatin E in the Sub-Endothelial Extracellular Matrix. Int. J. Cancer 2017, 140, 1670–1679. [Google Scholar] [CrossRef]
- Perrino, E.; Steiner, M.; Krall, N.; Bernardes, G.J.L.; Pretto, F.; Casi, G.; Neri, D. Curative Properties of Noninternalizing Antibody–Drug Conjugates Based on Maytansinoids. Cancer Res. 2014, 74, 2569–2578. [Google Scholar] [CrossRef]
- Yasunaga, M.; Manabe, S.; Tarin, D.; Matsumura, Y. Cancer-Stroma Targeting Therapy by Cytotoxic Immunoconjugate Bound to the Collagen 4 Network in the Tumor Tissue. Bioconjug. Chem. 2011, 22, 1776–1783. [Google Scholar] [CrossRef]
- Yasunaga, M.; Manabe, S.; Matsumura, Y. New Concept of Cytotoxic Immunoconjugate Therapy Targeting Cancer-Induced Fibrin Clots. Cancer Sci. 2011, 102, 1396–1402. [Google Scholar] [CrossRef]
- Alexandru, O.; Horescu, C.; Sevastre, A.-S.; Cioc, C.; Baloi, C.; Oprita, A.; Dricu, A. Receptor Tyrosine Kinase Targeting in Glioblastoma: Performance, Limitations and Future Approaches. Contemp. Oncol. 2020, 24, 55–66. [Google Scholar] [CrossRef]
- Ma, L.; Wang, C.; He, Z.; Cheng, B.; Zheng, L.; Huang, K. Peptide-Drug Conjugate: A Novel Drug Design Approach. Curr. Med. Chem. 2017, 24, 3373–3396. [Google Scholar] [CrossRef]
- Huang, W.; Hao, Z.; Mao, F.; Guo, D. Small Molecule Inhibitors in Adult High-Grade Glioma: From the Past to the Future. Front. Oncol. 2022, 12, 911876. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, W.; Sun, T.; Wang, L.; Du, C.; Hu, Y.; Liu, W.; Feng, F.; Chen, Y.; Sun, H. Therapeutic Strategies of Glioblastoma (GBM): The Current Advances in the Molecular Targets and Bioactive Small Molecule Compounds. Acta Pharm. Sin. B 2022, 12, 1781–1804. [Google Scholar] [CrossRef] [PubMed]
- Cesarini, V.; Scopa, C.; Silvestris, D.A.; Scafidi, A.; Petrera, V.; Del Baldo, G.; Gallo, A. Aptamer-Based In Vivo Therapeutic Targeting of Glioblastoma. Molecules 2020, 25, 4267. [Google Scholar] [CrossRef] [PubMed]
- Camorani, S.; Esposito, C.L.; Rienzo, A.; Catuogno, S.; Iaboni, M.; Condorelli, G.; de Franciscis, V.; Cerchia, L. Inhibition of Receptor Signaling and of Glioblastoma-Derived Tumor Growth by a Novel PDGFRβ Aptamer. Mol. Ther. 2014, 22, 828–841. [Google Scholar] [CrossRef] [PubMed]
- Monaco, I.; Camorani, S.; Colecchia, D.; Locatelli, E.; Calandro, P.; Oudin, A.; Niclou, S.; Arra, C.; Chiariello, M.; Cerchia, L.; et al. Aptamer Functionalization of Nanosystems for Glioblastoma Targeting through the Blood–Brain Barrier. J. Med. Chem. 2017, 60, 4510–4516. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Wang, X.; Sun, H.; Zhang, J.; Deng, C.; Zhong, Z. Cyclic RGD-Peptide-Functionalized Polylipopeptide Micelles for Enhanced Loading and Targeted Delivery of Monomethyl Auristatin E. Mol. Pharm. 2018, 15, 4854–4861. [Google Scholar] [CrossRef] [PubMed]
- Sievers, E.L.; Senter, P.D. Antibody-Drug Conjugates in Cancer Therapy. Annu. Rev. Med. 2013, 64, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Dai, W.; He, B.; Zhang, H.; Wang, X.; Wang, Y.; Zhang, Q. Current Multistage Drug Delivery Systems Based on the Tumor Microenvironment. Theranostics 2017, 7, 538–558. [Google Scholar] [CrossRef] [PubMed]
- Pillay, C.S.; Elliott, E.; Dennison, C. Endolysosomal Proteolysis and Its Regulation. Biochem. J. 2002, 363, 417–429. [Google Scholar] [CrossRef]
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and Regulators of Intracellular pH. Nat. Rev. Mol. Cell Biol. 2010, 11, 50–61. [Google Scholar] [CrossRef]
- Dubowchik, G.M.; Firestone, R.A. Cathepsin B-Sensitive Dipeptide Prodrugs. 1. A Model Study of Structural Requirements for Efficient Release of Doxorubicin. Bioorg. Med. Chem. Lett. 1998, 8, 3341–3346. [Google Scholar] [CrossRef]
- Jeffrey, S.C.; Andreyka, J.B.; Bernhardt, S.X.; Kissler, K.M.; Kline, T.; Lenox, J.S.; Moser, R.F.; Nguyen, M.T.; Okeley, N.M.; Stone, I.J.; et al. Development and Properties of β-Glucuronide Linkers for Monoclonal Antibody−Drug Conjugates. Bioconjug. Chem. 2006, 17, 831–840. [Google Scholar] [CrossRef]
- Tranoy-Opalinski, I.; Legigan, T.; Barat, R.; Clarhaut, J.; Thomas, M.; Renoux, B.; Papot, S. β-Glucuronidase-Responsive Prodrugs for Selective Cancer Chemotherapy: An Update. Eur. J. Med. Chem. 2014, 74, 302–313. [Google Scholar] [CrossRef]
- Van Heeswijk, W.A.R.; Hoes, C.J.T.; Stoffer, T.; Eenink, M.J.D.; Potman, W.; Feijen, J. The Synthesis and Characterization of Polypeptide-Adriamycin Conjugates and Its Complexes with Adriamycin. Part I. J. Control. Release 1985, 1, 301–315. [Google Scholar] [CrossRef]
- Wu, G.; Lupton, J.R.; Turner, N.D.; Fang, Y.-Z.; Yang, S. Glutathione Metabolism and Its Implications for Health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Alas, M.; Saghaeidehkordi, A.; Kaur, K. Peptide–Drug Conjugates with Different Linkers for Cancer Therapy. J. Med. Chem. 2021, 64, 216–232. [Google Scholar] [CrossRef] [PubMed]
- Ashman, N.; Bargh, J.D.; Spring, D.R. Non-Internalising Antibody–Drug Conjugates. Chem. Soc. Rev. 2022, 51, 9182–9202. [Google Scholar] [CrossRef] [PubMed]
- Bargh, J.D.; Isidro-Llobet, A.; Parker, J.S.; Spring, D.R. Cleavable Linkers in Antibody–Drug Conjugates. Chem. Soc. Rev. 2019, 48, 4361–4374. [Google Scholar] [CrossRef]
- Ferreira, N.N.; Granja, S.; Boni, F.I.; Ferreira, L.M.B.; Reis, R.M.; Baltazar, F.; Gremião, M.P.D. A Novel Strategy for Glioblastoma Treatment Combining Alpha-Cyano-4-Hydroxycinnamic Acid with Cetuximab Using Nanotechnology-Based Delivery Systems. Drug Deliv. Transl. Res. 2020, 10, 594–609. [Google Scholar] [CrossRef]
- Singh, I.; Swami, R.; Jeengar, M.K.; Khan, W.; Sistla, R. P-Aminophenyl-α-d-Mannopyranoside Engineered Lipidic Nanoparticles for Effective Delivery of Docetaxel to Brain. Chem. Phys. Lipids 2015, 188, 1–9. [Google Scholar] [CrossRef]
- Li, J.; Zeng, H.; You, Y.; Wang, R.; Tan, T.; Wang, W.; Yin, L.; Zeng, Z.; Zeng, Y.; Xie, T. Active Targeting of Orthotopic Glioma Using Biomimetic Liposomes Co-Loaded Elemene and Cabazitaxel Modified by Transferritin. J. Nanobiotechnol. 2021, 19, 289. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, Y.; Meng, F.; Deng, C.; Cheng, R.; Zhang, J.; Feijen, J.; Zhong, Z. Highly Efficacious and Specific Anti-Glioma Chemotherapy by Tandem Nanomicelles Co-Functionalized with Brain Tumor-Targeting and Cell-Penetrating Peptides. J. Control. Release 2018, 278, 1–8. [Google Scholar] [CrossRef]
- Kaluzova, M.; Bouras, A.; Machaidze, R.; Hadjipanayis, C.G. Targeted Therapy of Glioblastoma Stem-like Cells and Tumor Non-Stem Cells Using Cetuximab-Conjugated Iron-Oxide Nanoparticles. Oncotarget 2015, 6, 8788–8806. [Google Scholar] [CrossRef]
- Peng, L.; Liang, Y.; Zhong, X.; Liang, Z.; Tian, Y.; Li, S.; Liang, J.; Wang, R.; Zhong, Y.; Shi, Y.; et al. Aptamer-Conjugated Gold Nanoparticles Targeting Epidermal Growth Factor Receptor Variant III for the Treatment of Glioblastoma. Int. J. Nanomedicine 2020, 15, 1363–1372. [Google Scholar] [CrossRef]
- Ducry, L.; Stump, B. Antibody−Drug Conjugates: Linking Cytotoxic Payloads to Monoclonal Antibodies. Bioconjug. Chem. 2010, 21, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Cheetham, A.G.; Lock, L.L.; Cui, H. Cellular Uptake and Cytotoxicity of Drug–Peptide Conjugates Regulated by Conjugation Site. Bioconjug. Chem. 2013, 24, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, X.; Gong, M.; Zhang, J. Delivery of a Peptide-Drug Conjugate Targeting the Blood Brain Barrier Improved the Efficacy of Paclitaxel against Glioma. Oncotarget 2016, 7, 79401–79407. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Yan, Z.; Jin, K.; Pang, Q.; Jiang, T.; Lu, H.; Liu, X.; Pang, Z.; Yu, L.; Jiang, X. Precise Glioblastoma Targeting by AS1411 Aptamer-Functionalized Poly (l-γ-Glutamylglutamine)–Paclitaxel Nanoconjugates. J. Colloid Interface Sci. 2017, 490, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Sapra, P.; Shor, B. Monoclonal Antibody-Based Therapies in Cancer: Advances and Challenges. Pharmacol. Ther. 2013, 138, 452–469. [Google Scholar] [CrossRef] [PubMed]
- Cooper, B.M.; Iegre, J.; Donovan, D.H.O.; Halvarsson, M.Ö.; Spring, D.R. Peptides as a Platform for Targeted Therapeutics for Cancer: Peptide–Drug Conjugates (PDCs). Chem. Soc. Rev. 2021, 50, 1480–1494. [Google Scholar] [CrossRef]
- Doronina, S.O.; Mendelsohn, B.A.; Bovee, T.D.; Cerveny, C.G.; Alley, S.C.; Meyer, D.L.; Oflazoglu, E.; Toki, B.E.; Sanderson, R.J.; Zabinski, R.F.; et al. Enhanced Activity of Monomethylauristatin F through Monoclonal Antibody Delivery: Effects of Linker Technology on Efficacy and Toxicity. Bioconjug. Chem. 2006, 17, 114–124. [Google Scholar] [CrossRef]
- Lopus, M.; Oroudjev, E.; Wilson, L.; Wilhelm, S.; Widdison, W.; Chari, R.; Jordan, M.A. Maytansine and Cellular Metabolites of Antibody-Maytansinoid Conjugates Strongly Suppress Microtubule Dynamics by Binding to Microtubules. Mol. Cancer Ther. 2010, 9, 2689–2699. [Google Scholar] [CrossRef]
- Hamblett, K.J.; Kozlosky, C.J.; Siu, S.; Chang, W.S.; Liu, H.; Foltz, I.N.; Trueblood, E.S.; Meininger, D.; Arora, T.; Twomey, B.; et al. AMG 595, an Anti-EGFRvIII Antibody–Drug Conjugate, Induces Potent Antitumor Activity against EGFRvIII-Expressing Glioblastoma. Mol. Cancer Ther. 2015, 14, 1614–1624. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Cao, J.; Meng, Q.; Li, X.; Zhang, Y.; Lam, K.S.; Hong, A.; Liu, R.; Chen, X. Development and Characterization of a Novel Peptide—Drug Conjugate with DM1 for Treatment of FGFR2-Positive Tumors. Biomedicines 2021, 9, 849. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Yu, Q.-L.; Luan, W.-J.; Li, T.-F.; Xiao, Y.-N.; Zhang, L.; Li, Y.; Rong, R.; Ren, C.-G. LWJ-M30, a Conjugate of DM1 and B6, for the Targeted Therapy of Colorectal Cancer with Improved Therapeutic Effects. RSC Adv. 2023, 13, 10840–10846. [Google Scholar] [CrossRef] [PubMed]
- Gazzah, A.; Bedard, P.L.; Hierro, C.; Kang, Y.-K.; Razak, A.A.; Ryu, M.-H.; Demers, B.; Fagniez, N.; Henry, C.; Hospitel, M.; et al. Safety, Pharmacokinetics, and Antitumor Activity of the Anti-CEACAM5-DM4 Antibody–Drug Conjugate Tusamitamab Ravtansine (SAR408701) in Patients with Advanced Solid Tumors: First-in-Human Dose-Escalation Study. Ann. Oncol. 2022, 33, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Pouzin, C.; Gibiansky, L.; Fagniez, N.; Chadjaa, M.; Tod, M.; Nguyen, L. Integrated Multiple Analytes and Semi-Mechanistic Population Pharmacokinetic Model of Tusamitamab Ravtansine, a DM4 Anti-CEACAM5 Antibody-Drug Conjugate. J. Pharmacokinet. Pharmacodyn. 2022, 49, 381–394. [Google Scholar] [CrossRef]
- Zahaf, N.-I.; Schmidt, G. Bacterial Toxins for Cancer Therapy. Toxins 2017, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.M.; Pappenheimer, A.M., Jr.; Brown, R.; Kurnick, J.T. Studies on the mode of action of diphtheria toxin: VII: Toxin-stimulated hydrolysis of nicotinamide adenine dinucleotide in mammalian cell extracts. J. Exp. Med. 1969, 129, 1–21. [Google Scholar] [CrossRef]
- Siegall, C.B.; Chaudhary, V.K.; FitzGerald, D.J.; Pastan, I. Functional Analysis of Domains II, Ib, and III of Pseudomonas Exotoxin. J. Biol. Chem. 1989, 264, 14256–14261. [Google Scholar] [CrossRef]
- Bao, X.; Pastan, I.; Bigner, D.D.; Chandramohan, V. EGFR/EGFRvIII-Targeted Immunotoxin Therapy for the Treatment of Glioblastomas via Convection-Enhanced Delivery. Recept. Clin. Investig. 2016, 3, e1430. [Google Scholar] [CrossRef]
- Cordier, D.; Krolicki, L.; Morgenstern, A.; Merlo, A. Targeted Radiolabeled Compounds in Glioma Therapy. Semin. Nucl. Med. 2016, 46, 243–249. [Google Scholar] [CrossRef]
- Steiner, M.; Neri, D. Antibody-Radionuclide Conjugates for Cancer Therapy: Historical Considerations and New Trends. Clin. Cancer Res. 2011, 17, 6406–6416. [Google Scholar] [CrossRef]
- Hirschberg, H.; Berg, K.; Peng, Q. Photodynamic Therapy Mediated Immune Therapy of Brain Tumors. Neuroimmunol. Neuroinflamm. 2018, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Beck, T.J.; Kreth, F.W.; Beyer, W.; Mehrkens, J.H.; Obermeier, A.; Stepp, H.; Stummer, W.; Baumgartner, R. Interstitial Photodynamic Therapy of Nonresectable Malignant Glioma Recurrences Using 5-Aminolevulinic Acid Induced Protoporphyrin IX. Lasers Surg. Med. 2007, 39, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Madsen, S.J.; Angell-Petersen, E.; Spetalen, S.; Carper, S.W.; Ziegler, S.A.; Hirschberg, H. Photodynamic Therapy of Newly Implanted Glioma Cells in the Rat Brain. Lasers Surg. Med. 2006, 38, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C.; Vermandel, M.; Leroy, H.-A.; Quidet, M.; Lecomte, F.; Delhem, N.; Mordon, S.; Reyns, N. INtraoperative photoDYnamic Therapy for GliOblastomas (INDYGO): Study Protocol for a Phase I Clinical Trial. Neurosurgery 2019, 84, E414. [Google Scholar] [CrossRef] [PubMed]
- Muragaki, Y.; Akimoto, J.; Maruyama, T.; Iseki, H.; Ikuta, S.; Nitta, M.; Maebayashi, K.; Saito, T.; Okada, Y.; Kaneko, S.; et al. Phase II Clinical Study on Intraoperative Photodynamic Therapy with Talaporfin Sodium and Semiconductor Laser in Patients with Malignant Brain Tumors: Clinical Article. J. Neurosurg. 2013, 119, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Nitta, M.; Muragaki, Y.; Maruyama, T.; Iseki, H.; Komori, T.; Ikuta, S.; Saito, T.; Yasuda, T.; Hosono, J.; Okamoto, S.; et al. Role of Photodynamic Therapy Using Talaporfin Sodium and a Semiconductor Laser in Patients with Newly Diagnosed Glioblastoma. J. Neurosurg. 2018, 131, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.; Yusubalieva, G.M.; Baklaushev, V.P.; Chumakov, P.M.; Lipatova, A.V. Recent Developments in Glioblastoma Therapy: Oncolytic Viruses and Emerging Future Strategies. Viruses 2023, 15, 547. [Google Scholar] [CrossRef]
- Li, J.; Meng, Q.; Zhou, X.; Zhao, H.; Wang, K.; Niu, H.; Wang, Y. Gospel of Malignant Glioma: Oncolytic Virus Therapy. Gene 2022, 818, 146217. [Google Scholar] [CrossRef]
- Desjardins, A.; Gromeier, M.; Herndon, J.E.; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.M.; Nair, S.; et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N. Engl. J. Med. 2018, 379, 150–161. [Google Scholar] [CrossRef]
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, W.K.A.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018, 36, 1419–1427. [Google Scholar] [CrossRef]
- Todo, T.; Ito, H.; Ino, Y.; Ohtsu, H.; Ota, Y.; Shibahara, J.; Tanaka, M. Intratumoral Oncolytic Herpes Virus G47∆ for Residual or Recurrent Glioblastoma: A Phase 2 Trial. Nat. Med. 2022, 28, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, G.J.L.; Casi, G.; Trüssel, S.; Hartmann, I.; Schwager, K.; Scheuermann, J.; Neri, D. A Traceless Vascular-Targeting Antibody–Drug Conjugate for Cancer Therapy. Angew. Chem. Int. Ed. 2012, 51, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Jain, R.K.; Batchelor, T.T. New Directions in Anti-Angiogenic Therapy for Glioblastoma. Neurotherapeutics 2017, 14, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Day, S.E.; Waziri, A. Clinical Trials of Small Molecule Inhibitors in High-Grade Glioma. Neurosurg. Clin. N. Am. 2012, 23, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, T.T.; Sorensen, A.G.; di Tomaso, E.; Zhang, W.-T.; Duda, D.G.; Cohen, K.S.; Kozak, K.R.; Cahill, D.P.; Chen, P.-J.; Zhu, M.; et al. AZD2171, a Pan-VEGF Receptor Tyrosine Kinase Inhibitor, Normalizes Tumor Vasculature and Alleviates Edema in Glioblastoma Patients. Cancer Cell 2007, 11, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Clara, C.A.; Marie, S.K.N.; de Almeida, J.R.W.; Wakamatsu, A.; Oba-Shinjo, S.M.; Uno, M.; Neville, M.; Rosemberg, S. Angiogenesis and Expression of PDGF-C, VEGF, CD105 and HIF-1α in Human Glioblastoma. Neuropathology 2014, 34, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Puputti, M.; Tynninen, O.; Sihto, H.; Blom, T.; Mäenpää, H.; Isola, J.; Paetau, A.; Joensuu, H.; Nupponen, N.N. Amplification of KIT, PDGFRA, VEGFR2, and EGFR in Gliomas. Mol. Cancer Res. 2006, 4, 927–934. [Google Scholar] [CrossRef]
- Kwak, Y.; Kim, S.-I.; Park, C.-K.; Paek, S.H.; Lee, S.-T.; Park, S.-H. C-MET Overexpression and Amplification in Gliomas. Int. J. Clin. Exp. Pathol. 2015, 8, 14932–14938. [Google Scholar]
- Xie, Q.; Bradley, R.; Kang, L.; Koeman, J.; Ascierto, M.L.; Worschech, A.; De Giorgi, V.; Wang, E.; Kefene, L.; Su, Y.; et al. Hepatocyte Growth Factor (HGF) Autocrine Activation Predicts Sensitivity to MET Inhibition in Glioblastoma. Proc. Natl. Acad. Sci. USA 2012, 109, 570–575. [Google Scholar] [CrossRef]
- Cruz Da Silva, E.; Mercier, M.-C.; Etienne-Selloum, N.; Dontenwill, M.; Choulier, L. A Systematic Review of Glioblastoma-Targeted Therapies in Phases II, III, IV Clinical Trials. Cancers 2021, 13, 1795. [Google Scholar] [CrossRef]
- Zhang, H.; Berezov, A.; Wang, Q.; Zhang, G.; Drebin, J.; Murali, R.; Greene, M.I. ErbB Receptors: From Oncogenes to Targeted Cancer Therapies. J. Clin. Investig. 2007, 117, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Oda, K.; Matsuoka, Y.; Funahashi, A.; Kitano, H. A Comprehensive Pathway Map of Epidermal Growth Factor Receptor Signaling. Mol. Syst. Biol. 2005, 1, 2005.0010. [Google Scholar] [CrossRef] [PubMed]
- Hatanpaa, K.J.; Burma, S.; Zhao, D.; Habib, A.A. Epidermal Growth Factor Receptor in Glioma: Signal Transduction, Neuropathology, Imaging, and Radioresistance. Neoplasia 2010, 12, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dutra, A.; Pak, E.; Labrie, J.E., III; Gerstein, R.M.; Pandolfi, P.P.; Recht, L.D.; Ross, A.H. EGFRvIII Expression and PTEN Loss Synergistically Induce Chromosomal Instability and Glial Tumors. Neuro Oncol. 2009, 11, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Mazzoleni, S.; Politi, L.S.; Pala, M.; Cominelli, M.; Franzin, A.; Sergi Sergi, L.; Falini, A.; De Palma, M.; Bulfone, A.; Poliani, P.L.; et al. Epidermal Growth Factor Receptor Expression Identifies Functionally and Molecularly Distinct Tumor-Initiating Cells in Human Glioblastoma Multiforme and Is Required for Gliomagenesis. Cancer Res. 2010, 70, 7500–7513. [Google Scholar] [CrossRef]
- Pang, L.Y.; Saunders, L.; Argyle, D.J. Epidermal Growth Factor Receptor Activity Is Elevated in Glioma Cancer Stem Cells and Is Required to Maintain Chemotherapy and Radiation Resistance. Oncotarget 2017, 8, 72494–72512. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Kesari, S. Malignant Gliomas in Adults. N. Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef]
- Rosenthal, M.; Curry, R.; Reardon, D.A.; Rasmussen, E.; Upreti, V.V.; Damore, M.A.; Henary, H.A.; Hill, J.S.; Cloughesy, T. Safety, Tolerability, and Pharmacokinetics of Anti-EGFRvIII Antibody–Drug Conjugate AMG 595 in Patients with Recurrent Malignant Glioma Expressing EGFRvIII. Cancer Chemother. Pharmacol. 2019, 84, 327–336. [Google Scholar] [CrossRef]
- Whittle, J.R.; Lickliter, J.D.; Gan, H.K.; Scott, A.M.; Simes, J.; Solomon, B.J.; MacDiarmid, J.A.; Brahmbhatt, H.; Rosenthal, M.A. First in Human Nanotechnology Doxorubicin Delivery System to Target Epidermal Growth Factor Receptors in Recurrent Glioblastoma. J. Clin. Neurosci. 2015, 22, 1889–1894. [Google Scholar] [CrossRef]
- Kasenda, B.; König, D.; Manni, M.; Ritschard, R.; Duthaler, U.; Bartoszek, E.; Bärenwaldt, A.; Deuster, S.; Hutter, G.; Cordier, D.; et al. Targeting Immunoliposomes to EGFR-Positive Glioblastoma. ESMO Open 2022, 7, 100365. [Google Scholar] [CrossRef]
- Lassman, A.B.; van den Bent, M.J.; Gan, H.K.; Reardon, D.A.; Kumthekar, P.; Butowski, N.; Lwin, Z.; Mikkelsen, T.; Nabors, L.B.; Papadopoulos, K.P.; et al. Safety and Efficacy of Depatuxizumab Mafodotin + Temozolomide in Patients with EGFR-Amplified, Recurrent Glioblastoma: Results from an International Phase I Multicenter Trial. Neuro Oncol. 2019, 21, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Narita, Y.; Muragaki, Y.; Kagawa, N.; Asai, K.; Nagane, M.; Matsuda, M.; Ueki, K.; Kuroda, J.; Date, I.; Kobayashi, H.; et al. Safety and Efficacy of Depatuxizumab Mafodotin in Japanese Patients with Malignant Glioma: A Nonrandomized, Phase 1/2 Trial. Cancer Sci. 2021, 112, 5020–5033. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bent, M.; Eoli, M.; Sepulveda, J.M.; Smits, M.; Walenkamp, A.; Frenel, J.-S.; Franceschi, E.; Clement, P.M.; Chinot, O.; De Vos, F.; et al. INTELLANCE 2/EORTC 1410 Randomized Phase II Study of Depatux-M Alone and with Temozolomide vs Temozolomide or Lomustine in Recurrent EGFR Amplified Glioblastoma. Neuro Oncol. 2020, 22, 684–693. [Google Scholar] [CrossRef]
- Gan, H.K.; Reardon, D.A.; Lassman, A.B.; Merrell, R.; van den Bent, M.; Butowski, N.; Lwin, Z.; Wheeler, H.; Fichtel, L.; Scott, A.M.; et al. Safety, Pharmacokinetics, and Antitumor Response of Depatuxizumab Mafodotin as Monotherapy or in Combination with Temozolomide in Patients with Glioblastoma. Neuro Oncol. 2018, 20, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.C.; Boghaert, E.R.; Vaidya, K.S.; Mitten, M.J.; Norvell, S.; Falls, H.D.; DeVries, P.J.; Cheng, D.; Meulbroek, J.A.; Buchanan, F.G.; et al. ABT-414, an Antibody–Drug Conjugate Targeting a Tumor-Selective EGFR Epitope. Mol. Cancer Ther. 2016, 15, 661–669. [Google Scholar] [CrossRef]
- Desjardins, A.; Chandramohan, V.; Landi, D.B.; Johnson, M.O.; Khasraw, M.; Peters, K.B.; Low, J.; Herndon, J.E.; Threatt, S.; Bullock, C.A.; et al. A Phase 1 Trial of D2C7-It in Combination with an Fc-Engineered Anti-CD40 Monoclonal Antibody (2141-V11) Administered Intratumorally via Convection-Enhanced Delivery for Adult Patients with Recurrent Malignant Glioma (MG). J. Clin. Oncol. 2022, 40, e14015. [Google Scholar] [CrossRef]
- Sternjak, A.; Lee, F.; Thomas, O.; Balazs, M.; Wahl, J.; Lorenczewski, G.; Ullrich, I.; Muenz, M.; Rattel, B.; Bailis, J.M.; et al. Preclinical Assessment of AMG 596, a Bispecific T-Cell Engager (BiTE) Immunotherapy Targeting the Tumor-Specific Antigen EGFRvIII. Mol. Cancer Ther. 2021, 20, 925–933. [Google Scholar] [CrossRef]
- Combs, S.E.; Schulz-Ertner, D.; Hartmann, C.; Welzel, T.; Timke, C.; Herfarth, K.; von Deimling, A.; Edler, L.; Platten, M.; Wick, W.; et al. Phase I/II Study of Cetuximab plus Temozolomide as Radiochemotherapy for Primary Glioblastoma (GERT)—Eudract Number 2005–003911–63; NCT00311857. J. Clin. Oncol. 2008, 26, 2077. [Google Scholar] [CrossRef]
- Hasselbalch, B.; Lassen, U.; Hansen, S.; Holmberg, M.; Sørensen, M.; Kosteljanetz, M.; Broholm, H.; Stockhausen, M.-T.; Poulsen, H.S. Cetuximab, Bevacizumab, and Irinotecan for Patients with Primary Glioblastoma and Progression after Radiation Therapy and Temozolomide: A Phase II Trial. Neuro Oncol. 2010, 12, 508–516. [Google Scholar] [CrossRef]
- Sathornsumetee, S.; Desjardins, A.; Vredenburgh, J.J.; McLendon, R.E.; Marcello, J.; Herndon, J.E.; Mathe, A.; Hamilton, M.; Rich, J.N.; Norfleet, J.A.; et al. Phase II Trial of Bevacizumab and Erlotinib in Patients with Recurrent Malignant Glioma. Neuro Oncol. 2010, 12, 1300–1310. [Google Scholar] [CrossRef]
- Reardon, D.A.; Desjardins, A.; Vredenburgh, J.J.; Gururangan, S.; Friedman, A.H.; Herndon, J.E.; Marcello, J.; Norfleet, J.A.; McLendon, R.E.; Sampson, J.H.; et al. Phase 2 Trial of Erlotinib plus Sirolimus in Adults with Recurrent Glioblastoma. J. Neurooncol. 2010, 96, 219–230. [Google Scholar] [CrossRef]
- Wen, P.Y.; Chang, S.M.; Lamborn, K.R.; Kuhn, J.G.; Norden, A.D.; Cloughesy, T.F.; Robins, H.I.; Lieberman, F.S.; Gilbert, M.R.; Mehta, M.P.; et al. Phase I/II Study of Erlotinib and Temsirolimus for Patients with Recurrent Malignant Gliomas: North American Brain Tumor Consortium Trial 04-02. Neuro Oncol. 2014, 16, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Kesavabhotla, K.; Schlaff, C.D.; Shin, B.; Mubita, L.; Kaplan, R.; Tsiouris, A.J.; Pannullo, S.C.; Christos, P.; Lavi, E.; Scheff, R.; et al. Phase I/II Study of Oral Erlotinib for Treatment of Relapsed/Refractory Glioblastoma Multiforme and Anaplastic Astrocytoma. J. Exp. Ther. Oncol. 2012, 10, 71–81. [Google Scholar]
- Prados, M.D.; Chang, S.M.; Butowski, N.; DeBoer, R.; Parvataneni, R.; Carliner, H.; Kabuubi, P.; Ayers-Ringler, J.; Rabbitt, J.; Page, M.; et al. Phase II Study of Erlotinib Plus Temozolomide During and After Radiation Therapy in Patients with Newly Diagnosed Glioblastoma Multiforme or Gliosarcoma. J. Clin. Oncol. 2009, 27, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Peereboom, D.M.; Ahluwalia, M.S.; Ye, X.; Supko, J.G.; Hilderbrand, S.L.; Phuphanich, S.; Nabors, L.B.; Rosenfeld, M.R.; Mikkelsen, T.; Grossman, S.A.; et al. NABTT 0502: A Phase II and Pharmacokinetic Study of Erlotinib and Sorafenib for Patients with Progressive or Recurrent Glioblastoma Multiforme. Neuro Oncol. 2013, 15, 490–496. [Google Scholar] [CrossRef] [PubMed]
- van den Bent, M.J.; Brandes, A.A.; Rampling, R.; Kouwenhoven, M.C.M.; Kros, J.M.; Carpentier, A.F.; Clement, P.M.; Frenay, M.; Campone, M.; Baurain, J.-F.; et al. Randomized Phase II Trial of Erlotinib Versus Temozolomide or Carmustine in Recurrent Glioblastoma: EORTC Brain Tumor Group Study 26034. J. Clin. Oncol. 2009, 27, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.L.; Molinaro, A.M.; Phillips, J.J.; Butowski, N.A.; Chang, S.M.; Perry, A.; Costello, J.F.; DeSilva, A.A.; Rabbitt, J.E.; Prados, M.D. A Single-Institution Phase II Trial of Radiation, Temozolomide, Erlotinib, and Bevacizumab for Initial Treatment of Glioblastoma. Neuro Oncol. 2014, 16, 984–990. [Google Scholar] [CrossRef]
- Brown, N.; McBain, C.; Nash, S.; Hopkins, K.; Sanghera, P.; Saran, F.; Phillips, M.; Dungey, F.; Clifton-Hadley, L.; Wanek, K.; et al. Multi-Center Randomized Phase II Study Comparing Cediranib plus Gefitinib with Cediranib plus Placebo in Subjects with Recurrent/Progressive Glioblastoma. PLoS ONE 2016, 11, e0156369. [Google Scholar] [CrossRef]
- Hegi, M.E.; Diserens, A.-C.; Bady, P.; Kamoshima, Y.; Kouwenhoven, M.C.M.; Delorenzi, M.; Lambiv, W.L.; Hamou, M.-F.; Matter, M.S.; Koch, A.; et al. Pathway Analysis of Glioblastoma Tissue after Preoperative Treatment with the EGFR Tyrosine Kinase Inhibitor Gefitinib--a Phase II Trial. Mol. Cancer Ther. 2011, 10, 1102–1112. [Google Scholar] [CrossRef]
- Westphal, M.; Heese, O.; Steinbach, J.P.; Schnell, O.; Schackert, G.; Mehdorn, M.; Schulz, D.; Simon, M.; Schlegel, U.; Senft, C.; et al. A Randomised, Open Label Phase III Trial with Nimotuzumab, an Anti-Epidermal Growth Factor Receptor Monoclonal Antibody in the Treatment of Newly Diagnosed Adult Glioblastoma. Eur. J. Cancer 2015, 51, 522–532. [Google Scholar] [CrossRef]
- Nitta, Y.; Shimizu, S.; Shishido-Hara, Y.; Suzuki, K.; Shiokawa, Y.; Nagane, M. Nimotuzumab Enhances Temozolomide-Induced Growth Suppression of Glioma Cells Expressing Mutant EGFR in Vivo. Cancer Med. 2016, 5, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.T.; Miranda, N.; Jorrín, E.; Chon, I.; Marinello, J.J.; Alert, J.; Lorenzo-Luaces, P.; Crombet, T. Nimotuzumab in Combination with Radiotherapy in High Grade Glioma Patients. Cancer Biol. Ther. 2014, 15, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Yoo, J.; Kim, M.-S.; Hur, M.; Lee, E.H.; Hur, H.-S.; Lee, J.-C.; Lee, S.-N.; Park, T.W.; Lee, K.; et al. GC1118, an Anti-EGFR Antibody with a Distinct Binding Epitope and Superior Inhibitory Activity against High-Affinity EGFR Ligands. Mol. Cancer Ther. 2016, 15, 251–263. [Google Scholar] [CrossRef]
- Chakravarti, A.; Wang, M.; Robins, H.I.; Lautenschlaeger, T.; Curran, W.J.; Brachman, D.G.; Schultz, C.J.; Choucair, A.; Dolled-Filhart, M.; Christiansen, J.; et al. RTOG 0211: A Phase 1/2 Study of Radiation Therapy with Concurrent Gefitinib for Newly Diagnosed Glioblastoma Patients. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Uhm, J.H.; Ballman, K.V.; Wu, W.; Giannini, C.; Krauss, J.C.; Buckner, J.C.; James, C.D.; Scheithauer, B.W.; Behrens, R.J.; Flynn, P.J.; et al. Phase II Evaluation of Gefitinib in Patients with Newly Diagnosed Grade 4 Astrocytoma: Mayo/North Central Cancer Treatment Group Study N0074. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Cantanhede, I.G.; de Oliveira, J.R.M. PDGF Family Expression in Glioblastoma Multiforme: Data Compilation from Ivy Glioblastoma Atlas Project Database. Sci. Rep. 2017, 7, 15271. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y. Multifarious Functions of PDGFs and PDGFRs in Tumor Growth and Metastasis. Trends Mol. Med. 2013, 19, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Nazarenko, I.; Hede, S.-M.; He, X.; Hedrén, A.; Thompson, J.; Lindström, M.S.; Nistér, M. PDGF and PDGF Receptors in Glioma. Upsala J. Med. Sci. 2012, 117, 99–112. [Google Scholar] [CrossRef]
- Plate, K.H.; Breier, G.; Farrell, C.L.; Risau, W. Platelet-Derived Growth Factor Receptor-Beta Is Induced during Tumor Development and Upregulated during Tumor Progression in Endothelial Cells in Human Gliomas. Lab. Investig. 1992, 67, 529–534. [Google Scholar]
- Lokker, N.A.; Sullivan, C.M.; Hollenbach, S.J.; Israel, M.A.; Giese, N.A. Platelet-Derived Growth Factor (PDGF) Autocrine Signaling Regulates Survival and Mitogenic Pathways in Glioblastoma Cells: Evidence That the Novel PDGF-C and PDGF-D Ligands May Play a Role in the Development of Brain Tumors. Cancer Res. 2002, 62, 3729–3735. [Google Scholar]
- Vassbotn, F.S.; Östman, A.; Langeland, N.; Holmsen, H.; Westermark, B.; Heldin, C.-H.; Nistér, M. Activated Platelet-Derived Growth Factor Autocrine Pathway Drives the Transformed Phenotype of a Human Glioblastoma Cell Line. J. Cell. Physiol. 1994, 158, 381–389. [Google Scholar] [CrossRef]
- Phuphanich, S.; Raizer, J.; Chamberlain, M.; Canelos, P.; Narwal, R.; Hong, S.; Miday, R.; Nade, M.; Laubscher, K. Phase II Study of MEDI-575, an Anti-Platelet-Derived Growth Factor-α Antibody, in Patients with Recurrent Glioblastoma. J. Neurooncol. 2017, 131, 185–191. [Google Scholar] [CrossRef]
- Jain, R.K.; di Tomaso, E.; Duda, D.G.; Loeffler, J.S.; Sorensen, A.G.; Batchelor, T.T. Angiogenesis in Brain Tumours. Nat. Rev. Neurosci. 2007, 8, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, I.H.; O’Donovan, D.G.; Brenchley, P.E.C.; Reid, H.; Roberts, I.S.D. Vascular Endothelial Growth Factor Expression Correlates with Tumour Grade and Vascularity in Gliomas. Histopathology 2001, 39, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R.; Ohara, K.; Sasaki, H.; Morimoto, Y.; Yoshida, K.; Toda, M. Histopathological Vascular Investigation of the Peritumoral Brain Zone of Glioblastomas. J. Neurooncol. 2018, 136, 233–241. [Google Scholar] [CrossRef]
- Roskoski, R. Vascular Endothelial Growth Factor (VEGF) Signaling in Tumor Progression. Crit. Rev. Oncol. Hematol. 2007, 62, 179–213. [Google Scholar] [CrossRef] [PubMed]
- Hamerlik, P.; Lathia, J.D.; Rasmussen, R.; Wu, Q.; Bartkova, J.; Lee, M.; Moudry, P.; Bartek, J.; Fischer, W.; Lukas, J.; et al. Autocrine VEGF-VEGFR2-Neuropilin-1 Signaling Promotes Glioma Stem-like Cell Viability and Tumor Growth. J. Exp. Med. 2012, 209, 507–520. [Google Scholar] [CrossRef]
- Tamura, R.; Tanaka, T.; Akasaki, Y.; Murayama, Y.; Yoshida, K.; Sasaki, H. The Role of Vascular Endothelial Growth Factor in the Hypoxic and Immunosuppressive Tumor Microenvironment: Perspectives for Therapeutic Implications. Med. Oncol. 2019, 37, 2. [Google Scholar] [CrossRef]
- Brandes, A.A.; Gil-Gil, M.; Saran, F.; Carpentier, A.F.; Nowak, A.K.; Mason, W.; Zagonel, V.; Dubois, F.; Finocchiaro, G.; Fountzilas, G.; et al. A Randomized Phase II Trial (TAMIGA) Evaluating the Efficacy and Safety of Continuous Bevacizumab Through Multiple Lines of Treatment for Recurrent Glioblastoma. Oncology 2019, 24, 521–528. [Google Scholar] [CrossRef]
- Wirsching, H.-G.; Tabatabai, G.; Roelcke, U.; Hottinger, A.F.; Jörger, F.; Schmid, A.; Plasswilm, L.; Schrimpf, D.; Mancao, C.; Capper, D.; et al. Bevacizumab plus Hypofractionated Radiotherapy versus Radiotherapy Alone in Elderly Patients with Glioblastoma: The Randomized, Open-Label, Phase II ARTE Trial. Ann. Oncol. 2018, 29, 1423–1430. [Google Scholar] [CrossRef]
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus Radiotherapy–Temozolomide for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Friedman, H.S.; Prados, M.D.; Wen, P.Y.; Mikkelsen, T.; Schiff, D.; Abrey, L.E.; Yung, W.K.A.; Paleologos, N.; Nicholas, M.K.; Jensen, R.; et al. Bevacizumab Alone and in Combination with Irinotecan in Recurrent Glioblastoma. J. Clin. Oncol. 2009, 27, 4733–4740. [Google Scholar] [CrossRef] [PubMed]
- Tsien, C.I.; Pugh, S.L.; Dicker, A.P.; Raizer, J.J.; Matuszak, M.M.; Lallana, E.C.; Huang, J.; Algan, O.; Deb, N.; Portelance, L.; et al. NRG Oncology/RTOG1205: A Randomized Phase II Trial of Concurrent Bevacizumab and Reirradiation Versus Bevacizumab Alone as Treatment for Recurrent Glioblastoma. J. Clin. Oncol. 2023, 41, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Herrlinger, U.; Schäfer, N.; Steinbach, J.P.; Weyerbrock, A.; Hau, P.; Goldbrunner, R.; Friedrich, F.; Rohde, V.; Ringel, F.; Schlegel, U.; et al. Bevacizumab Plus Irinotecan Versus Temozolomide in Newly Diagnosed O6-Methylguanine–DNA Methyltransferase Nonmethylated Glioblastoma: The Randomized GLARIUS Trial. J. Clin. Oncol. 2016, 34, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Wick, W.; Gorlia, T.; Bendszus, M.; Taphoorn, M.; Sahm, F.; Harting, I.; Brandes, A.A.; Taal, W.; Domont, J.; Idbaih, A.; et al. Lomustine and Bevacizumab in Progressive Glioblastoma. N. Engl. J. Med. 2017, 377, 1954–1963. [Google Scholar] [CrossRef]
- Schiff, D.; Kesari, S.; de Groot, J.; Mikkelsen, T.; Drappatz, J.; Coyle, T.; Fichtel, L.; Silver, B.; Walters, I.; Reardon, D. Phase 2 Study of CT-322, a Targeted Biologic Inhibitor of VEGFR-2 Based on a Domain of Human Fibronectin, in Recurrent Glioblastoma. Investig. New Drugs 2015, 33, 247–253. [Google Scholar] [CrossRef]
- Vredenburgh, J.J.; Desjardins, A.; Herndon, J.E.; Marcello, J.; Reardon, D.A.; Quinn, J.A.; Rich, J.N.; Sathornsumetee, S.; Gururangan, S.; Sampson, J.; et al. Bevacizumab Plus Irinotecan in Recurrent Glioblastoma Multiforme. J. Clin. Oncol. 2007, 25, 4722–4729. [Google Scholar] [CrossRef]
- Mamluk, R.; Carvajal, I.M.; Morse, B.A.; Wong, H.; Abramowitz, J.; Aslanian, S.; Lim, A.-C.; Gokemeijer, J.; Storek, M.J.; Lee, J.; et al. Anti-Tumor Effect of CT-322 as an Adnectin Inhibitor of Vascular Endothelial Growth Factor Receptor-2. MAbs 2010, 2, 199–208. [Google Scholar] [CrossRef]
- Garcia-Navarrete, R.; Garcia, E.; Arrieta, O.; Sotelo, J. Hepatocyte Growth Factor in Cerebrospinal Fluid Is Associated with Mortality and Recurrence of Glioblastoma, and Could Be of Prognostic Value. J. Neurooncol. 2010, 97, 347–351. [Google Scholar] [CrossRef]
- Jahangiri, A.; Chin, A.T.; Flanigan, P.M.; Chen, R.; Bankiewicz, K.; Aghi, M.K. Convection-Enhanced Delivery in Glioblastoma: A Review of Preclinical and Clinical Studies. J. Neurosurg. 2017, 126, 191–200. [Google Scholar] [CrossRef]
- Johnson, J.; Ascierto, M.L.; Mittal, S.; Newsome, D.; Kang, L.; Briggs, M.; Tanner, K.; Marincola, F.M.; Berens, M.E.; Vande Woude, G.F.; et al. Genomic Profiling of a Hepatocyte Growth Factor-Dependent Signature for MET-Targeted Therapy in Glioblastoma. J. Transl. Med. 2015, 13, 306. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.-S.; Song, S.-Y.; Kim, D.-H.; Joo, K.M.; Yoo, J.-S.; Koh, J.S.; Dong, S.M.; Suh, Y.-L.; Lee, J.-I.; Park, K.; et al. Prognostic Significance of C-Met Expression in Glioblastomas. Cancer 2009, 115, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, A.; Glas, M.; Lal, B.; Ying, M.; Sang, Y.; Xia, S.; Trageser, D.; Guerrero-Cázares, H.; Eberhart, C.G.; et al. C-Met Signaling Induces a Reprogramming Network and Supports the Glioblastoma Stem-like Phenotype. Proc. Natl. Acad. Sci. USA 2011, 108, 9951–9956. [Google Scholar] [CrossRef]
- Lu, K.V.; Chang, J.P.; Parachoniak, C.A.; Pandika, M.M.; Aghi, M.K.; Meyronet, D.; Isachenko, N.; Fouse, S.D.; Phillips, J.J.; Cheresh, D.A.; et al. VEGF Inhibits Tumor Cell Invasion and Mesenchymal Transition through a MET/VEGFR2 Complex. Cancer Cell 2012, 22, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Cloughesy, T.; Finocchiaro, G.; Belda-Iniesta, C.; Recht, L.; Brandes, A.A.; Pineda, E.; Mikkelsen, T.; Chinot, O.L.; Balana, C.; Macdonald, D.R.; et al. Randomized, Double-Blind, Placebo-Controlled, Multicenter Phase II Study of Onartuzumab Plus Bevacizumab Versus Placebo Plus Bevacizumab in Patients with Recurrent Glioblastoma: Efficacy, Safety, and Hepatocyte Growth Factor and O6-Methylguanine–DNA Methyltransferase Biomarker Analyses. J. Clin. Oncol. 2017, 35, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Schiff, D.; Cloughesy, T.F.; Raizer, J.J.; Laterra, J.; Smitt, M.; Wolf, M.; Oliner, K.S.; Anderson, A.; Zhu, M.; et al. A Phase II Study Evaluating the Efficacy and Safety of AMG 102 (Rilotumumab) in Patients with Recurrent Glioblastoma. Neuro Oncol. 2011, 13, 437–446. [Google Scholar] [CrossRef]
- Babina, I.S.; Turner, N.C. Advances and Challenges in Targeting FGFR Signalling in Cancer. Nat. Rev. Cancer 2017, 17, 318–332. [Google Scholar] [CrossRef]
- Helsten, T.; Elkin, S.; Arthur, E.; Tomson, B.N.; Carter, J.; Kurzrock, R. The FGFR Landscape in Cancer: Analysis of 4853 Tumors by Next-Generation Sequencing. Clin. Cancer Res. 2016, 22, 259–267. [Google Scholar] [CrossRef]
- Imamura, T. Physiological Functions and Underlying Mechanisms of Fibroblast Growth Factor (FGF) Family Members: Recent Findings and Implications for Their Pharmacological Application. Biol. Pharm. Bull. 2014, 37, 1081–1089. [Google Scholar] [CrossRef]
- Qin, A.; Johnson, A.; Ross, J.S.; Miller, V.A.; Ali, S.M.; Schrock, A.B.; Gadgeel, S.M. Detection of Known and Novel FGFR Fusions in Non-Small Cell Lung Cancer by Comprehensive Genomic Profiling. J. Thorac. Oncol. 2019, 14, 54–62. [Google Scholar] [CrossRef]
- Tiong, K.H.; Mah, L.Y.; Leong, C.-O. Functional Roles of Fibroblast Growth Factor Receptors (FGFRs) Signaling in Human Cancers. Apoptosis 2013, 18, 1447–1468. [Google Scholar] [CrossRef] [PubMed]
- Day, E.K.; Sosale, N.G.; Xiao, A.; Zhong, Q.; Purow, B.; Lazzara, M.J. Glioblastoma Cell Resistance to EGFR and MET Inhibition Can Be Overcome via Blockade of FGFR-SPRY2 Bypass Signaling. Cell Rep. 2020, 30, 3383–3396.e7. [Google Scholar] [CrossRef]
- Guagnano, V.; Kauffmann, A.; Wöhrle, S.; Stamm, C.; Ito, M.; Barys, L.; Pornon, A.; Yao, Y.; Li, F.; Zhang, Y.; et al. FGFR Genetic Alterations Predict for Sensitivity to NVP-BGJ398, a Selective Pan-FGFR Inhibitor. Cancer Discov. 2012, 2, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Gavine, P.R.; Mooney, L.; Kilgour, E.; Thomas, A.P.; Al-Kadhimi, K.; Beck, S.; Rooney, C.; Coleman, T.; Baker, D.; Mellor, M.J.; et al. AZD4547: An Orally Bioavailable, Potent, and Selective Inhibitor of the Fibroblast Growth Factor Receptor Tyrosine Kinase Family. Cancer Res. 2012, 72, 2045–2056. [Google Scholar] [CrossRef] [PubMed]
- Perera, T.P.S.; Jovcheva, E.; Mevellec, L.; Vialard, J.; De Lange, D.; Verhulst, T.; Paulussen, C.; Van De Ven, K.; King, P.; Freyne, E.; et al. Discovery and Pharmacological Characterization of JNJ-42756493 (Erdafitinib), a Functionally Selective Small-Molecule FGFR Family Inhibitor. Mol. Cancer Ther. 2017, 16, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, A.L.; Fucci, A.; Frattini, V.; Labussiere, M.; Mokhtari, K.; Zoppoli, P.; Marie, Y.; Bruno, A.; Boisselier, B.; Giry, M.; et al. Detection, Characterization and Inhibition of FGFR-TACC Fusions in IDH Wild Type Glioma. Clin. Cancer Res. 2015, 21, 3307–3317. [Google Scholar] [CrossRef]
- Reardon, D.A.; Dresemann, G.; Taillibert, S.; Campone, M.; van den Bent, M.; Clement, P.; Blomquist, E.; Gordower, L.; Schultz, H.; Raizer, J.; et al. Multicentre Phase II Studies Evaluating Imatinib plus Hydroxyurea in Patients with Progressive Glioblastoma. Br. J. Cancer 2009, 101, 1995–2004. [Google Scholar] [CrossRef]
- Dresemann, G.; Weller, M.; Rosenthal, M.A.; Wedding, U.; Wagner, W.; Engel, E.; Heinrich, B.; Mayer-Steinacker, R.; Karup-Hansen, A.; Fluge, Ø.; et al. Imatinib in Combination with Hydroxyurea versus Hydroxyurea Alone as Oral Therapy in Patients with Progressive Pretreated Glioblastoma Resistant to Standard Dose Temozolomide. J. Neurooncol. 2010, 96, 393–402. [Google Scholar] [CrossRef]
- Raymond, E.; Brandes, A.A.; Dittrich, C.; Fumoleau, P.; Coudert, B.; Clement, P.M.J.; Frenay, M.; Rampling, R.; Stupp, R.; Kros, J.M.; et al. Phase II Study of Imatinib in Patients with Recurrent Gliomas of Various Histologies: A European Organisation for Research and Treatment of Cancer Brain Tumor Group Study. J. Clin. Oncol. 2008, 26, 4659–4665. [Google Scholar] [CrossRef]
- Galanis, E.; Anderson, S.K.; Twohy, E.L.; Carrero, X.W.; Dixon, J.G.; Tran, D.D.; Jeyapalan, S.A.; Anderson, D.M.; Kaufmann, T.J.; Feathers, R.W.; et al. A Phase I and Randomized Placebo-Controlled Phase II Trial of Bevacizumab plus Dasatinib in Patients with Recurrent Glioblastoma (GBM): Alliance/NCCTG N0872. Cancer 2019, 125, 3790–3800. [Google Scholar] [CrossRef]
- Lassman, A.B.; Pugh, S.L.; Gilbert, M.R.; Aldape, K.D.; Geinoz, S.; Beumer, J.H.; Christner, S.M.; Komaki, R.; DeAngelis, L.M.; Gaur, R.; et al. Phase 2 Trial of Dasatinib in Target-Selected Patients with Recurrent Glioblastoma (RTOG 0627). Neuro Oncol. 2015, 17, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, E.; Stupp, R.; van den Bent, M.J.; van Herpen, C.; Laigle Donadey, F.; Gorlia, T.; Hegi, M.; Lhermitte, B.; Strauss, L.C.; Allgeier, A.; et al. EORTC 26083 Phase I/II Trial of Dasatinib in Combination with CCNU in Patients with Recurrent Glioblastoma. Neuro Oncol. 2012, 14, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, T.T.; Gerstner, E.R.; Ye, X.; Desideri, S.; Duda, D.G.; Peereboom, D.; Lesser, G.J.; Chowdhary, S.; Wen, P.Y.; Grossman, S.; et al. Feasibility, Phase I, and Phase II Studies of Tandutinib, an Oral Platelet-Derived Growth Factor Receptor-β Tyrosine Kinase Inhibitor, in Patients with Recurrent Glioblastoma. Neuro Oncol. 2017, 19, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Odia, Y.; Sul, J.; Shih, J.H.; Kreisl, T.N.; Butman, J.A.; Iwamoto, F.M.; Fine, H.A. A Phase II Trial of Tandutinib (MLN 518) in Combination with Bevacizumab for Patients with Recurrent Glioblastoma. CNS Oncol. 2016, 5, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Balaña, C.; Gil, M.J.; Perez, P.; Reynes, G.; Gallego, O.; Ribalta, T.; Capellades, J.; Gonzalez, S.; Verger, E. Sunitinib Administered Prior to Radiotherapy in Patients with Non-Resectable Glioblastoma: Results of a Phase II Study. Targ. Oncol. 2014, 9, 321–329. [Google Scholar] [CrossRef]
- Hutterer, M.; Nowosielski, M.; Haybaeck, J.; Embacher, S.; Stockhammer, F.; Gotwald, T.; Holzner, B.; Capper, D.; Preusser, M.; Marosi, C.; et al. A Single-Arm Phase II Austrian/German Multicenter Trial on Continuous Daily Sunitinib in Primary Glioblastoma at First Recurrence (SURGE 01-07). Neuro Oncol. 2014, 16, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Pan, E.; Yu, D.; Yue, B.; Potthast, L.; Chowdhary, S.; Smith, P.; Chamberlain, M. A Prospective Phase II Single-Institution Trial of Sunitinib for Recurrent Malignant Glioma. J. Neurooncol. 2012, 110, 111–118. [Google Scholar] [CrossRef]
- Lee, E.Q.; Muzikansky, A.; Reardon, D.A.; Dietrich, J.; Nayak, L.; Duda, D.G.; Chukwueke, U.N.; Beroukhim, R.; Doherty, L.M.; Kane, C.; et al. Phase II Trial of Ponatinib in Patients with Bevacizumab-Refractory Glioblastoma. J. Clin. Oncol. 2018, 36, 2032. [Google Scholar] [CrossRef]
- O’Hare, T.; Shakespeare, W.C.; Zhu, X.; Eide, C.A.; Rivera, V.M.; Wang, F.; Adrian, L.T.; Zhou, T.; Huang, W.-S.; Xu, Q.; et al. AP24534, a Pan-BCR-ABL Inhibitor for Chronic Myeloid Leukemia, Potently Inhibits the T315I Mutant and Overcomes Mutation-Based Resistance. Cancer Cell 2009, 16, 401–412. [Google Scholar] [CrossRef]
- Brave, S.R.; Ratcliffe, K.; Wilson, Z.; James, N.H.; Ashton, S.; Wainwright, A.; Kendrew, J.; Dudley, P.; Broadbent, N.; Sproat, G.; et al. Assessing the Activity of Cediranib, a VEGFR-2/3 Tyrosine Kinase Inhibitor, against VEGFR-1 and Members of the Structurally Related PDGFR Family. Mol. Cancer Ther. 2011, 10, 861–873. [Google Scholar] [CrossRef]
- Drake, C.G.; Jaffee, E.; Pardoll, D.M. Mechanisms of Immune Evasion by Tumors. In Advances in Immunology; Academic Press: Cambridge, MA, USA, 2006; Volume 90, pp. 51–81. [Google Scholar]
- Armand, P. Immune Checkpoint Blockade in Hematologic Malignancies. Blood 2015, 125, 3393–3400. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Liu, B.; Sun, Q.; Xiong, X.; Chen, Q. Immune Checkpoint Targeted Therapy in Glioma: Status and Hopes. Front. Immunol. 2020, 11, 578877. [Google Scholar] [CrossRef] [PubMed]
- Cloughesy, T.F.; Mochizuki, A.Y.; Orpilla, J.R.; Hugo, W.; Lee, A.H.; Davidson, T.B.; Wang, A.C.; Ellingson, B.M.; Rytlewski, J.A.; Sanders, C.M.; et al. Neoadjuvant Anti-PD-1 Immunotherapy Promotes a Survival Benefit with Intratumoral and Systemic Immune Responses in Recurrent Glioblastoma. Nat. Med. 2019, 25, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Nayak, L.; Molinaro, A.M.; Peters, K.; Clarke, J.L.; Jordan, J.T.; de Groot, J.; Nghiemphu, L.; Kaley, T.; Colman, H.; McCluskey, C.; et al. Randomized Phase II and Biomarker Study of Pembrolizumab plus Bevacizumab versus Pembrolizumab Alone for Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2021, 27, 1048–1057. [Google Scholar] [CrossRef]
- Reardon, D.A.; Kim, T.M.; Frenel, J.-S.; Simonelli, M.; Lopez, J.; Subramaniam, D.S.; Siu, L.L.; Wang, H.; Krishnan, S.; Stein, K.; et al. Treatment with Pembrolizumab in Programmed Death Ligand 1–Positive Recurrent Glioblastoma: Results from the Multicohort Phase 1 KEYNOTE-028 Trial. Cancer 2021, 127, 1620–1629. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Brandes, A.A.; Omuro, A.; Mulholland, P.; Lim, M.; Wick, A.; Baehring, J.; Ahluwalia, M.S.; Roth, P.; Bähr, O.; et al. Effect of Nivolumab vs Bevacizumab in Patients with Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Weller, M.; Idbaih, A.; Steinbach, J.; Finocchiaro, G.; Raval, R.R.; Ansstas, G.; Baehring, J.; Taylor, J.W.; Honnorat, J.; et al. Phase III Trial of Chemoradiotherapy with Temozolomide plus Nivolumab or Placebo for Newly Diagnosed Glioblastoma with Methylated MGMT Promoter. Neuro Oncol. 2022, 24, 1935–1949. [Google Scholar] [CrossRef]
- Omuro, A.; Brandes, A.A.; Carpentier, A.F.; Idbaih, A.; Reardon, D.A.; Cloughesy, T.; Sumrall, A.; Baehring, J.; van den Bent, M.; Bähr, O.; et al. Radiotherapy Combined with Nivolumab or Temozolomide for Newly Diagnosed Glioblastoma with Unmethylated MGMT Promoter: An International Randomized Phase III Trial. Neuro Oncol. 2023, 25, 123–134. [Google Scholar] [CrossRef]
- Nayak, L.; Standifer, N.; Dietrich, J.; Clarke, J.L.; Dunn, G.P.; Lim, M.; Cloughesy, T.; Gan, H.K.; Flagg, E.; George, E.; et al. Circulating Immune Cell and Outcome Analysis from the Phase II Study of PD-L1 Blockade with Durvalumab for Newly Diagnosed and Recurrent Glioblastoma. Clin. Cancer Res. 2022, 28, 2567–2578. [Google Scholar] [CrossRef]
- Fong, B.; Jin, R.; Wang, X.; Safaee, M.; Lisiero, D.N.; Yang, I.; Li, G.; Liau, L.M.; Prins, R.M. Monitoring of Regulatory T Cell Frequencies and Expression of CTLA-4 on T Cells, before and after DC Vaccination, Can Predict Survival in GBM Patients. PLoS ONE 2012, 7, e32614. [Google Scholar] [CrossRef]
- Belcaid, Z.; Phallen, J.A.; Zeng, J.; See, A.P.; Mathios, D.; Gottschalk, C.; Nicholas, S.; Kellett, M.; Ruzevick, J.; Jackson, C.; et al. Focal Radiation Therapy Combined with 4-1BB Activation and CTLA-4 Blockade Yields Long-Term Survival and a Protective Antigen-Specific Memory Response in a Murine Glioma Model. PLoS ONE 2014, 9, e101764. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Gokhale, P.C.; Klein, S.R.; Ligon, K.L.; Rodig, S.J.; Ramkissoon, S.H.; Jones, K.L.; Conway, A.S.; Liao, X.; Zhou, J.; et al. Glioblastoma Eradication Following Immune Checkpoint Blockade in an Orthotopic, Immunocompetent Model. Cancer Immunol. Res. 2016, 4, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, D.A.; Chang, A.L.; Dey, M.; Balyasnikova, I.V.; Kim, C.K.; Tobias, A.; Cheng, Y.; Kim, J.W.; Qiao, J.; Zhang, L.; et al. Durable Therapeutic Efficacy Utilizing Combinatorial Blockade against IDO, CTLA-4, and PD-L1 in Mice with Brain Tumors. Clin. Cancer Res. 2014, 20, 5290–5301. [Google Scholar] [CrossRef] [PubMed]
- Duerinck, J.; Schwarze, J.K.; Awada, G.; Tijtgat, J.; Vaeyens, F.; Bertels, C.; Geens, W.; Klein, S.; Seynaeve, L.; Cras, L.; et al. Intracerebral Administration of CTLA-4 and PD-1 Immune Checkpoint Blocking Monoclonal Antibodies in Patients with Recurrent Glioblastoma: A Phase I Clinical Trial. J. Immunother. Cancer 2021, 9, e002296. [Google Scholar] [CrossRef]
- Das, M.; Zhu, C.; Kuchroo, V.K. Tim-3 and Its Role in Regulating Anti-Tumor Immunity. Immunol. Rev. 2017, 276, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, Z.; Zhang, C.; Liu, X.; Cai, J.; Wang, Z.; Hu, H.; Wu, F.; Bao, Z.; Liu, Y.; et al. Molecular and Clinical Characterization of TIM-3 in Glioma through 1024 Samples. OncoImmunology 2017, 6, e1328339. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Patel, M.A.; Mangraviti, A.; Kim, E.S.; Theodros, D.; Velarde, E.; Liu, A.; Sankey, E.W.; Tam, A.; Xu, H.; et al. Combination Therapy with Anti-PD-1, Anti-TIM-3, and Focal Radiation Results in Regression of Murine Gliomas. Clin. Cancer Res. 2017, 23, 124–136. [Google Scholar] [CrossRef]
- Triebel, F.; Jitsukawa, S.; Baixeras, E.; Roman-Roman, S.; Genevee, C.; Viegas-Pequignot, E.; Hercend, T. LAG-3, a Novel Lymphocyte Activation Gene Closely Related to CD4. J. Exp. Med. 1990, 171, 1393–1405. [Google Scholar] [CrossRef]
- Workman, C.J.; Rice, D.S.; Dugger, K.J.; Kurschner, C.; Vignali, D.A.A. Phenotypic Analysis of the Murine CD4-Related Glycoprotein, CD223 (LAG-3). Eur. J. Immunol. 2002, 32, 2255–2263. [Google Scholar] [CrossRef]
- Yang, K.; Wu, Z.; Zhang, H.; Zhang, N.; Wu, W.; Wang, Z.; Dai, Z.; Zhang, X.; Zhang, L.; Peng, Y.; et al. Glioma Targeted Therapy: Insight into Future of Molecular Approaches. Mol. Cancer 2022, 21, 39. [Google Scholar] [CrossRef]
- Harris-Bookman, S.; Mathios, D.; Martin, A.M.; Xia, Y.; Kim, E.; Xu, H.; Belcaid, Z.; Polanczyk, M.; Barberi, T.; Theodros, D.; et al. Expression of LAG-3 and Efficacy of Combination Treatment with Anti-LAG-3 and Anti-PD-1 Monoclonal Antibodies in Glioblastoma. Int. J. Cancer 2018, 143, 3201–3208. [Google Scholar] [CrossRef] [PubMed]
- Mair, M.J.; Kiesel, B.; Feldmann, K.; Widhalm, G.; Dieckmann, K.; Wöhrer, A.; Müllauer, L.; Preusser, M.; Berghoff, A.S. LAG-3 Expression in the Inflammatory Microenvironment of Glioma. J. Neurooncol. 2021, 152, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Rosenfeld, J.A.; Singer, E.A.; Bhanot, G.; Ganesan, S. Genomic and Immunologic Correlates of LAG-3 Expression in Cancer. OncoImmunology 2020, 9, 1756116. [Google Scholar] [CrossRef]
- Sood, D.; Tang-Schomer, M.; Pouli, D.; Mizzoni, C.; Raia, N.; Tai, A.; Arkun, K.; Wu, J.; Black, L.D.; Scheffler, B.; et al. 3D Extracellular Matrix Microenvironment in Bioengineered Tissue Models of Primary Pediatric and Adult Brain Tumors. Nat. Commun. 2019, 10, 4529. [Google Scholar] [CrossRef]
- Mohiuddin, E.; Wakimoto, H. Extracellular Matrix in Glioblastoma: Opportunities for Emerging Therapeutic Approaches. Am. J. Cancer Res. 2021, 11, 3742–3754. [Google Scholar] [PubMed]
- Brack, S.S.; Silacci, M.; Birchler, M.; Neri, D. Tumor-Targeting Properties of Novel Antibodies Specific to the Large Isoform of Tenascin-C. Clin. Cancer Res. 2006, 12, 3200–3208. [Google Scholar] [CrossRef]
- Nandhu, M.S.; Behera, P.; Bhaskaran, V.; Longo, S.L.; Barrera-Arenas, L.M.; Sengupta, S.; Rodriguez-Gil, D.J.; Chiocca, E.A.; Viapiano, M.S. Development of a Function-Blocking Antibody against Fibulin-3 as Targeted Reagent for Glioblastoma. Clin. Cancer Res. 2018, 24, 821–833. [Google Scholar] [CrossRef]
- Czabanka, M.; Parmaksiz, G.; Bayerl, S.H.; Nieminen, M.; Trachsel, E.; Menssen, H.D.; Erber, R.; Neri, D.; Vajkoczy, P. Microvascular Biodistribution of L19-SIP in Angiogenesis Targeting Strategies. Eur. J. Cancer 2011, 47, 1276–1284. [Google Scholar] [CrossRef]
- Spaeth, N.; Wyss, M.T.; Pahnke, J.; Biollaz, G.; Trachsel, E.; Drandarov, K.; Treyer, V.; Weber, B.; Neri, D.; Buck, A. Radioimmunotherapy Targeting the Extra Domain B of Fibronectin in C6 Rat Gliomas: A Preliminary Study about the Therapeutic Efficacy of Iodine-131-Labeled SIP(L19). Nucl. Med. Biol. 2006, 33, 661–666. [Google Scholar] [CrossRef]
- Sette, P.; Amankulor, N.; Li, A.; Marzulli, M.; Leronni, D.; Zhang, M.; Goins, W.F.; Kaur, B.; Bolyard, C.; Cripe, T.P.; et al. GBM-Targeted oHSV Armed with Matrix Metalloproteinase 9 Enhances Anti-Tumor Activity and Animal Survival. Mol. Ther. Oncolytics 2019, 15, 214–222. [Google Scholar] [CrossRef]
- Nam, L.; Coll, C.; Erthal, L.C.S.; De la Torre, C.; Serrano, D.; Martínez-Máñez, R.; Santos-Martínez, M.J.; Ruiz-Hernández, E. Drug Delivery Nanosystems for the Localized Treatment of Glioblastoma Multiforme. Materials 2018, 11, 779. [Google Scholar] [CrossRef] [PubMed]
- Neftel, C.; Laffy, J.; Filbin, M.G.; Hara, T.; Shore, M.E.; Rahme, G.J.; Richman, A.R.; Silverbush, D.; Shaw, M.L.; Hebert, C.M.; et al. An Integrative Model of Cellular States, Plasticity, and Genetics for Glioblastoma. Cell 2019, 178, 835–849.e21. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.P.; Tirosh, I.; Trombetta, J.J.; Shalek, A.K.; Gillespie, S.M.; Wakimoto, H.; Cahill, D.P.; Nahed, B.V.; Curry, W.T.; Martuza, R.L.; et al. Single-Cell RNA-Seq Highlights Intratumoral Heterogeneity in Primary Glioblastoma. Science 2014, 344, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Perrin, S.L.; Samuel, M.S.; Koszyca, B.; Brown, M.P.; Ebert, L.M.; Oksdath, M.; Gomez, G.A. Glioblastoma Heterogeneity and the Tumour Microenvironment: Implications for Preclinical Research and Development of New Treatments. Biochem. Soc. Trans. 2019, 47, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Sestito, S.; Runfola, M.; Tonelli, M.; Chiellini, G.; Rapposelli, S. New Multitarget Approaches in the War Against Glioblastoma: A Mini-Perspective. Front. Pharmacol. 2018, 9, 874. [Google Scholar] [CrossRef]
- Bausart, M.; Préat, V.; Malfanti, A. Immunotherapy for Glioblastoma: The Promise of Combination Strategies. J. Exp. Clin. Cancer Res. 2022, 41, 35. [Google Scholar] [CrossRef]
- Grossman, S.A.; Ellsworth, S.G. Published Glioblastoma Clinical Trials from 1980 to 2013: Lessons from the Past and for the Future. J. Clin. Oncol. 2016, 34, e13522. [Google Scholar] [CrossRef]
- Mandel, J.J.; Yust-Katz, S.; Patel, A.J.; Cachia, D.; Liu, D.; Park, M.; Yuan, Y.; Kent, T.A.; de Groot, J.F. Inability of Positive Phase II Clinical Trials of Investigational Treatments to Subsequently Predict Positive Phase III Clinical Trials in Glioblastoma. Neuro Oncol. 2018, 20, 113–122. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
| Altered RTK | Occurrence in GBM and Type of Alteration |
|---|---|
| EGFR | A total of 57% of GBM cases show mutation, rearrangement, alternatively spliced isoforms or amplification [6]; around 20% of cases show mutant variant EGFRvIII [92] |
| PDGFR | A total of 13% of GBM harbor PDGFRA amplification or point mutations [6,92], PDGFRB showed overexpression on GBM endothelial cells [166] |
| VEGFR | VEGF is present in 64% of GBM [167] with 6–17% accounting for VEGFR2 amplification [168] |
| c-MET | A total of 1.6–13.1% of GBM cases harbor c-MET overexpression [6,169]; in around 4% of GBM, c-MET amplification is present [92,170] |
| FGFR | A total of 3.2% of GBM has amplification or point mutation in FGFR2 and FGFR3 [6] |
| Trial Code (Study Period) | Recognition Moiety | Linker | Payload | Type of Intervention (Outcome) |
|---|---|---|---|---|
| Depatuximab–mafodotin | ||||
| NCT02573324 (December 2015–April 2022) | anti-EGFR/vIII mAb | maleimidocaproyl | MMAF | Combined with radiotherapy and temozolomide (RT/TMZ) vs placebo + RT/TMZ (N = 619, PFS = 8.0 vs. 6.3 months (p = 0.029), OS = 18.9 vs. 18.7 months (p = 0.633)) |
| AMG-595 | ||||
| NCT01475006 (February 2012–April 2016) | anti-EGFRvIII mAb | maleimidocaproyl | DM1 | Monotherapy (no survival data available, [179]) |
| D2C7-IT | ||||
| NCT04547777 (July 2021–December 2025) | Bispecific anti-EGFRvIII/CD3 Ab | PCR fused | Pseudomonas Exotoxin A | Combined with anti-CD40 mAb (no survival data available) |
| NCT05734560 (February 2023–February 2028) | Bispecific anti-EGFRvIII/CD3 Ab | PCR fused | Pseudomonas Exotoxin A | Combined with anti-CD40 mAb (no survival data available) |
| EGFR(V)-EDV-Dox | ||||
| NCT02766699 (October 2016–June 2020) | Panitumumab scFv (anti-EGFR) | Anti-O-polysaccharide antibody to the component of the drug delivery system (Minicell) + G4S linker | doxorubicin | Monotherapy with pretreatment (N = 14, PFS = 1.6 months, OS = 9.7 months, [180]) |
| C225-ILs-dox | ||||
| NCT03603379 (November 2018–November 2020) | Cetuximab Fab (anti-EGFR) | Maleimide groups | doxorubicin | Monotherapy (N = 9, PFS = 1.5 months, OS = 8 months, [181]) |
| Trial Code (Study Period) | Recognition Moiety/Payload | Type of Intervention (Outcome) |
|---|---|---|
| RO7428731 | ||
| NCT05187624 (April 2022–February 2025) | bispecific anti-EGFRvIII/CD3 Ab | Monotherapy (no survival data available) |
| AMG-596 | ||
| NCT03296696 (April 2018–August 2021) | bispecific anti-EGFRvIII/CD3 Ab | Monotherapy (no survival data available, [188]) |
| Cetuximab | ||
| NCT00311857 (February 2006–September 2006) | anti-EGFR mAb | Combined with RT/TMZ (N = 17, PFS6 = 81% PFS12 = 37%, OS12 = 87% [189]) |
| NCT00463073 (August 2006–December 2008) | anti-EGFR mAb | Combined with bevacizumab and irinotecan (N = 43, PFS = 16 weeks, OS = 30 weeks, [190]) |
| Panitumumab | ||
| NCT01017653 (February 2010–October 2011) | anti-EGFR mAb | Combined with irinotecan (N = 16, PFS6 = 12.5%, OS = 4.6 months; study terminated due not reaching the benchmark efficacy rule) |
| Nimotuzumab | ||
| NCT00600054 (October 2007–December 2010) | anti-EGFR mAb | Combined with RT/TMZ vs. RT/TMZ (insignificant data on PFS/OS in experimental vs. control groups) |
| GC1118 | ||
| NCT03618667 (April 2018–July 2021) | anti-EGFR mAb | Monotherapy (no survival data available) |
| Sym004 | ||
| NCT02540161 (February 2016–April 2020) | mixture of two anti-EGFR mAbs | Monotherapy (N = 43, PFS: non-bevacizumab failures (18 mg/kg) = 1.81 months, bevacizumab failures (18 mg/kg) = 3.91 months, non-bevacizumab failures (24 mg/kg) = 3.55 months, bevacizumab failures (24 mg/kg) = 2.00 months; OS: non-bevacizumab failures (18 mg/kg) = 7.54 months, bevacizumab failures (18 mg/kg) = 5.51 months, non-bevacizumab failures (24 mg/kg) = 9.95 months, bevacizumab failures (24 mg/kg) = 5.39 months) |
| Erlotinib | ||
| NCT00671970 (February 2007–April 2010) | EGFR-specific TKI | Combined with bevacizumab (N (Anaplastic glioma) = 32, PFS = 23.4 weeks, OS = 71.3 weeks; N (GBM) = 25, PFS = 18 weeks, OS = 44.6 weeks [191]) |
| NCT00124657 (March 2005–September 2014) | EGFR-specific TKI | Monotherapy (N = 8 (Phase I, anaplastic astrocytoma), 1-year PFS = 0.75 years; N = 12 (Phase I, GBM), 1-year PFS = 0.33 years; N = 20 (Phase II, anaplastic astrocytoma), 1-year PFS = 0.45 years, 2-year PFS 24 = 0.15 years; N = 21 (Phase II, GBM), 1-year PFS = 0.19 years, 2-year PFS = 0.19 years) |
| NCT00672243 (April 2007–December 2009) | EGFR-specific TKI | Combined with sirolimus (N = 32, PFS = 6.9 weeks, OS = 33.8 weeks [192]) |
| NCT00112736 (April 2005–April 2014) | EGFR-specific TKI | Combined with temsirolimus (N = 16 (Anaplastic glioma), PFS6 = 8%; N = 42 (GBM), PFS6 = 13% [193]) |
| NCT00301418 (March 2006–May 2014) | EGFR-specific TKI | Monotherapy (N = 11, PFS = 1.9 months, OS = 6.9 months [194]) |
| NCT00720356 (July 2009–July 2018) | EGFR-specific TKI | Combined with bevacizumab (N = 46, OS = 13.2 months) |
| NCT00187486 (August 2004–March 2011) | EGFR-specific TKI | Combined with RT/TMZ (N = 65, PFS = 8.2 months, OS = 19.3 months [195]) |
| NCT00445588 (January 2007–August 2009) | EGFR-specific TKI | Combined with sorafenib (N = 56, PFS = 2.5 months, OS = 5.7 months [196]) |
| NCT00086879 (May 2004–March 2011) | EGFR-specific TKI | Monotherapy vs. TMZ or monotherapy vs. carmustine (N = 54, PFS6 (erlotinib arm) = 11.4%; N = 56, PFS6 (control arm) = 24% [197]) |
| NCT00525525 (September 2007–May 2013) | EGFR-specific TKI | Combined with bevacizumab + TMZ in adjuvant therapy (N = 59, PFS = 13.5 months, OS = 19.8 months [198]) |
| Gefitinib | ||
| NCT01310855 (May 2011–January 2014) | EGFR-specific TKI | cediranib + gefitinib vs. cediranib + placebo (N = 97, PFS = 3.6 vs. 2.8 months, OS = 7.2 vs. 5.5 months [199]) |
| NCT00250887 (July 2005–May 2007) | EGFR-specific TKI | Monotherapy (N = 22, OS = 8.8 months [200]) |
| ERAS-801 | ||
| NCT05222802 (February 2022–September 2025) | EGFR-specific TKI | Monotherapy (no survival data available) |
| BDTX-1535 | ||
| NCT05256290 (February 2022–March 2025) | Mutant-selective EGFR TKI | Monotherapy vs. combined with TMZ (no survival data available) |
| Trial Code (Study Period) | Recognition Moiety/Payload | Type of Intervention (Outcome) |
|---|---|---|
| MEDI-575 | ||
| NCT01268566 (December 2010–April 2017) | anti-PDGFRα mAb | Monotherapy (N = 56, PFS = 1.4 months, OS = 9.7 months, [213]) |
| Olaratumab | ||
| NCT00895180 (May 2009–December 2017) | anti-PDGFRα mAb | Monotherapy (N = 40, PFS6 = 7.5%, OS = 34.3 weeks) |
| Trial Code (Study Period) | Recognition Moiety/Payload | Type of Intervention (Outcome) |
|---|---|---|
| Bevacizumab | ||
| NCT01860638 (May 2013–April 2018) | anti-VEGF-A mAb | Treatment with the Stupp protocol, combined with lomustine in the first progression and with chemotherapy in the second progression (N = 296, PFS (first progression) = 2.3 vs. 1.8 months, OS (first progression) = 6.4 vs. 5.5 months (bevacizumab + lomustine vs. lomustine + placebo), PFS (second progression) = 2.0 vs. 2.2 months (bevacizumab + chemotherapy vs. chemotherapy), [220]) |
| NCT01443676 (September 2011–November 2016) | anti-VEGF-A mAb | Combined with RT vs. RT alone (N = 75, PFS = 7.6 vs. 4.8 months, OS = 12.1 vs. 12.2 months, (bevacizumab + RT vs. RT), [221]) |
| NCT00943826 (July 2009–September 2017) | anti-VEGF-A mAb | Combined with RT/TMZ vs. RT/TMZ (N = 921, PFS = 10.6 vs. 6.2 months, OS = 16.8 vs. 16.7 months (bevacizumab + RT/TMZ vs. RT/TMZ), [222]) |
| NCT00345163 (June 2006–May 2017) | anti-VEGF-A mAb | Monotherapy vs. combined with irinotecan (N = 167, PFS = 4.2 vs. 5.6 months, OS = 9.2 vs. 8.7 months, PFS6 = 42.6% vs. 50.3% (bevacizumab vs. bevacizumab + irinotecan), [223]) |
| NCT01730950 (November 2012–December 2022) | anti-VEGF-A mAb | Monotherapy vs. combined with RT (N = 182, PFS = 7.1 vs. 3.8 months, OS = 10.1 vs. 9.7 months [224]) |
| NCT00967330 (August 2009–November 2015) | anti-VEGF-A mAb | Combined with RT, then adjuvant therapy combined with irinotecan vs. RT/TMZ (N = 182, PFS = 5.99 vs. 9.7 months, OS = 16.6 vs. 17.5 months (RT/TMZ vs. RT + bevacizumab and irinotecan, [225]) |
| NCT01290939 (February 2011–February 2021) | anti-VEGF-A mAb | Monotherapy vs. combined with lomustine (N = 437, PFS = 1.5 vs. 4.2 months, OS = 8.6 vs. 9.1 months; (bevacizumab vs. combined with lomustine, [226]) |
| NCT00884741 (April 2009–July 2019) | anti-VEGF-A mAb | Combined with TMZ as adjuvant therapy vs. RT/TMZ (N = 637, PFS = 10.7 vs. 7.3 months, OS = 15.7 vs. 16.1 months, (bevacizumab + RT/TMZ vs. RT/TMZ, [32]) |
| CT-322 | ||
| NCT00562419 (November 2007–October 2010) | VEGFR-2 specific extracellular modified fibronectin 10th type 3 | Monotherapy vs. combined with irinotecan (N = 63; Monotherapy cohort: PFS6 (1 mg/kg) = 18.6%, PFS6 (2 mg/kg) = 0.0%; Combined with irinotecan: PFS6 (1 mg/kg) = 64.3%, PFS6 (2 mg/kg) = 42.1%, [227]) |
| Trial Code (Study Period) | Recognition Moiety/Payload | Type of Intervention (Outcome) |
|---|---|---|
| Onartuzumab | ||
| NCT01632228 (July 2012–February 2018) | anti-cMET mAb | Combined with bevacizumab vs. bevacizumab + placebo (N = 64; PFS = 3.9 vs. 2.9 months (p = 0.7774), OS = 8.8 vs. 12.6 months (p = 0.1389); (combined with bevacizumab vs. bevacizumab + placebo), [236]) |
| Rilotumumab | ||
| NCT01113398 (April 2010–December 2015) | anti-HGF mAb | Combined with bevacizumab, (N = 60; PFS = 4.1 vs. 4.3 weeks, OS = 6.5 vs. 5.4 months (10 vs. 20 mg/kg cohorts), [237]) |
| Trial Code (Study Period) | Recognition Moiety/Payload | Type of Intervention (Outcome) |
|---|---|---|
| Infigratinib | ||
| NCT01975701 (November 2013–December 2019) | pan-FGFR TKI | Monotherapy (N = 26; PFS = 1.7 months, OS = 6.7 months) |
| Trial Code (Study Period) | Recognition Moiety/Payload | Type of Intervention (Outcome) |
|---|---|---|
| Imatinib | ||
| NCT00290771 (February 2006–May 2011) | PDGFRα/β, BCR-Abl, c-Kit multitarget TKI | Combined with hydroxyurea (N = 231, PFS = 5.6 weeks, OS = 26 weeks [248]) |
| NCT00154375 (September 2005–April 2011) | PDGFRα/β, BCR-Abl, c-Kit multitarget TKI | Combined with hydroxyurea vs. only hydroxyurea (N = 240, PFS = 6 vs. 6 weeks (imatinib + hydroxyurea vs. hydroxyurea alone); OS = 21 vs. 19 weeks (imatinib + hydroxyurea vs. hydroxyurea alone) [249]) |
| NCT00039364 (January 2003–July 2012) | PDGFRα/β, BCR-Abl, c-Kit multitarget TKI | Monotherapy (N = 50 (GBM), PFS = 1.8 months, OS = 5.9 months; N = 25 (anaplastic astrocytoma), PFS = 1.8 months, OS = 5.0 months; N = 35 (oligodenrdroglioma), PFS = 1.9 months, OS = 5.3 months [250]) |
| Dasatinib | ||
| NCT00892177 (May 2009–October 2019) | PDGFRβ, EPHA2, BCR-Abl, c-Kit and SRC multitarget TKI | Combined with bevacizumab vs. bevacizumab alone (N = 121, PFS6 = 28.9% vs. 18.4% for dasatinib/bevacizumab vs. bevacizumab alone, no statistical significance; OS = 7.3 vs. 7.7 months for dasatinib/bevacizumab vs. bevacizumab alone, no statistical significance [251]) |
| NCT00423735 (January 2007–July 2019) | PDGFRβ, EPHA2, BCR-Abl, c-Kit and SRC multitarget TKI | Monotherapy (N = 50, PFS = 1.7 months, OS = 7.96 months [252]) |
| NCT00948389 (July 2009–August 2012) | PDGFRβ, EPHA2, BCR-Abl, c-Kit and SRC multitarget TKI | Combined with lomustine (N = 26, PFS = 1.35 months, OS = 6.4 months [253]) |
| NCT00869401 (March 2009–February 2020) | PDGFRβ, EPHA2, BCR-Abl, c-Kit and SRC multitarget TKI | Combined with RT/TMZ vs. RT/TMZ (N = 204, PFS: 15.6 vs. 19.3 months; OS = 6.2 vs. 7.8 months, no statistical significance) |
| Tandutinib | ||
| NCT00379080 (September 2009–April 2017) | PDGFRβ, FLT3, c-Kit multitarget TKI | Monotherapy (N = 31, PFS = 1.9 months, OS = 8.8 months [254]) |
| NCT00667394 (April 2008–November 2015) | PDGFRβ, FLT3, c-Kit multitarget TKI | Combined with bevacizumab (N = 41, PFS = 4.1 months, OS = 11 months [255]) |
| Sunitinib | ||
| NCT01100177 (April 2010–March 2013) | PDGFRα/β, c-Kit, VEGFR1/2/3, FLT3 and RET multitarget TKI | Monotherapy prior, during and after RT (N = 12, PFS = 7.7 weeks, OS = 12.8 weeks [256]) |
| NCT00923117 (June 2009–September 2015) | PDGFRα/β, c-Kit, VEGFR1/2/3, FLT3 and RET multitarget TKI | Monotherapy in patients with/without resistance to bevacizumab (N = 87, PFS (bevacizumab resistant cohort) = 0.92 months, PFS (bevacizumab naïve cohort) = 1.08 months) |
| NCT00535379 (September 2007–August 2010) | PDGFRα/β, c-Kit, VEGFR1/2/3, FLT3 and RET multitarget TKI | Monotherapy (N = 70, PFS = 2.2 months, OS = 9.2 months [257]) |
| NCT00606008 (February 2008–November 2012) | PDGFRα/β, c-Kit, VEGFR1/2/3, FLT3 and RET multitarget TKI | Monotherapy (N = 16 (GBM), PFS = 1.4 months, OS = 12.6 months; N = 14 (AA), PFS = 4.1 months, OS = 12.1 months [258]) |
| NCT00499473 (July 2007–February 2016) | PDGFRα/β, c-Kit, VEGFR1/2/3, FLT3 and RET multitarget TKI | Monotherapy in patients taking/not taking enzyme induced Anticonvulsants (EIAC) (N = 27 (non-EIAC), OS = 5.7 months; N = 4 (EIAC), OS = 12.3 months) |
| Ponatinib | ||
| NCT02478164 (June 2015–July 2018) | BCR-Abl, PDGFRα, VEGFR2, FGFR1, and Src multitarget TKI | Monotherapy (N = 15, PFS = 28 days, OS = 98 days [259]) |
| Cediranib | ||
| NCT01062425 (February 2010–May 2022) | VEGFR, c-Kit, PDGFR multitarget TKI | Combined with RT/TMZ vs. RT/TMZ (N = 261; PFS = 6.2 vs. 2.7 months (p = 0.03), OS = 14.5 vs. 13.8 months (p = 0.44)(Cediranib + RT/TMZ vs. RT/TMZ)) |
| Trial Code (Study Period) | Recognition Moiety/Payload | Type of Intervention (Outcome) |
|---|---|---|
| Pembrolizumab | ||
| NCT02337686 (January 2015–January 2023) | anti-PD-1 mAb | Combined with surgery (N = 18, PFS = 2.4 months vs. 3.3 months (p = 0.03), OS = 7.5 months vs. 13.7 months (p = 0.04) for adjuvant vs. neoadjuvant application [265]) |
| NCT02337491 (January 2015–December 2020) | anti-PD-1 mAb | Monotherapy vs. combined with bevacizumab (N = 80; PFS = 1.4 vs. 4.1 months (p = 0.0026), OS = 10.3 vs. 8.8 months (p = 0.87) (monotherapy vs. combined with bevacizumab) [266]) |
| NCT02054806 (February 2014–May 2021) | anti-PD-1 mAb | Monotherapy vs. combined with bevacizumab (N = 26, PFS = 2.8 months, OS = 13.1 months [267]) |
| Nivolumab | ||
| NCT02017717 (December 2013–June 2022) | anti-PD-1 mAb | Monotherapy vs. bevacizumab (N = 369; PFS = 1.5 vs. 3.5 months, OS = 9.8 vs. 10.0 months, (nivolumab vs. bevacizumab) [268]) |
| NCT02667587 (January 2016–April 2023) | anti-PD-1 mAb | Combined with RT/TMZ vs. RT/TMZ + placebo (N = 716; PFS = 10.6 vs. 10.3 months, OS = 28.9 vs. 32.1 months, (nivolumab + RT/TMZ vs. RT/TMZ + placebo) [269]) |
| NCT02617589 (December 2015–March 2023) | anti-PD-1 mAb | Combined with RT vs. RT/TMZ (N = 560; PFS = 6.0 vs. 6.2 months, OS = 13.4 vs. 14.9 months, (nivolumab + RT vs. RT/TMZ) [270]) |
| Durvalumab | ||
| NCT02336165 (January 2015–October 2022) | anti-PD-L1 mAb | Cohort A (newly diagnosed): Combined with RT, Cohort B (bevacizumab-naïve): Monotherapy; Cohort B2 (bevacizumab-naïve): Combined with 10 mg/kg bevacizumab; Cohort B3 (bevacizumab-naïve): Combined with 3 mg/kg bevacizumab, Cohort C (bevacizumab-refractory): Combined with 10 mg/kg bevacizumab (N (A) = 40, N (B) = 31, N (B2) = 33, N (B3) = 33, N (C) = 22; PFS: A = 4.6, B = 3.0, B2 = 3.7, B3 = 3.7, C = 1.9 months, OS: A = 15.1, B = 6.7, B2 = 8.7, B3 = 9.3, C = 4.5 months [271]) |
| Trial Code (Study Period) | Recognition Moiety/Payload | Type of Intervention (Outcome) |
|---|---|---|
| Tremelimumab | ||
| NCT02794883 (June 2016–April 2022) | Anti-CTLA-4 mAb | Monotherapy (A) vs. durvalumab (B) vs. combined with druvalumab (C) (N = 36; PFS: A = 2.746, B = 4.356, C = 4.913 months; OS: A = 7.246, B = 11.71, C = 7.703 months) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shikalov, A.; Koman, I.; Kogan, N.M. Targeted Glioma Therapy—Clinical Trials and Future Directions. Pharmaceutics 2024, 16, 100. https://doi.org/10.3390/pharmaceutics16010100
Shikalov A, Koman I, Kogan NM. Targeted Glioma Therapy—Clinical Trials and Future Directions. Pharmaceutics. 2024; 16(1):100. https://doi.org/10.3390/pharmaceutics16010100
Chicago/Turabian StyleShikalov, Aleksandr, Igor Koman, and Natalya M. Kogan. 2024. "Targeted Glioma Therapy—Clinical Trials and Future Directions" Pharmaceutics 16, no. 1: 100. https://doi.org/10.3390/pharmaceutics16010100
APA StyleShikalov, A., Koman, I., & Kogan, N. M. (2024). Targeted Glioma Therapy—Clinical Trials and Future Directions. Pharmaceutics, 16(1), 100. https://doi.org/10.3390/pharmaceutics16010100






