Co-Delivery of a Novel Lipidated TLR7/8 Agonist and Hemagglutinin-Based Influenza Antigen Using Silica Nanoparticles Promotes Enhanced Immune Responses
Abstract
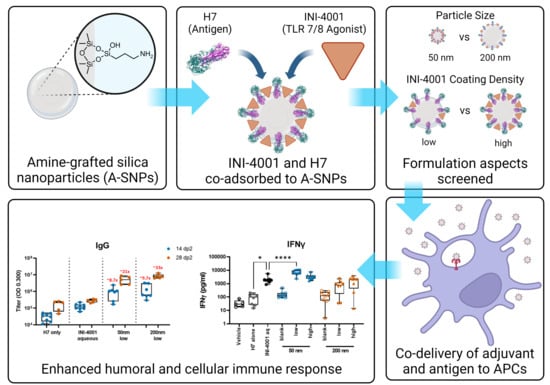
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of A-SNP
2.3. Co-Adsorption of Adjuvant (INI-4001) and Antigen (H7) onto A-SNP
2.4. Characterization of INI-4001/A-SNP Formulations
2.4.1. Size Distribution by DLS and Transmission Electron Microscopy (TEM)
2.4.2. Zeta Potential and pH Measurements
2.4.3. Quantitative Analysis and Determination of Adsorption of INI-4001 and H7 onto A-SNP
2.5. Determination of INI-4001 Release Kinetics from INI-4001/A-SNP Formulations in Plasma
2.5.1. Development and Optimization of a Bioanalytical Method for Quantitation of INI-4001 in Biological Samples
2.5.2. In Vitro Release Studies of INI-4001 from 50 nm Low- and High-Coating-Density INI-4001/A-SNP Formulations
2.5.3. Mathematical Modeling of the Desorption Profile of INI-4001
2.6. Isolation of Human PBMCs and Cytokine Analysis
2.7. Isolation of Murine BMDCs and Cytokine Analysis
2.8. In Vivo Vaccine Studies
2.8.1. H7-Specific Antibody ELISAs
2.8.2. Splenocyte and dLNs Cell Restimulation and Cytokine Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of INI-4001/A-SNP Formulations
3.2. INI-4001 Shows Slow-Release Kinetics in Plasma When Adsorbed onto A-SNP
3.3. INI-4001-Coating Density and A-SNP Size Affect IFN-α and TNF-α Induction in Human PBMCs
3.4. Evaluation of INI-4001/A-SNP Formulations in mBMDCs
3.5. INI-4001/A-SNP Formulations Enhance Humoral and Cellular Immune Responses
3.5.1. Humoral Responses Using 14-Day Regimen
3.5.2. Humoral Responses Using 28-Day Regimen
3.5.3. Antigen-Specific Splenic T Cell Responses Using the 14-Day Regimen
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Excler, J.-L.; Saville, M.; Berkley, S.; Kim, J.H. Vaccine development for emerging infectious diseases. Nat. Med. 2021, 27, 591–600. [Google Scholar] [CrossRef]
- Roth, G.A.; Picece, V.C.T.M.; Ou, B.S.; Luo, W.; Pulendran, B.; Appel, E.A. Designing spatial and temporal control of vaccine responses. Nat. Rev. Mater. 2021, 7, 174–195. [Google Scholar] [CrossRef] [PubMed]
- Pati, R.; Shevtsov, M.; Sonawane, A. Nanoparticle Vaccines Against Infectious Diseases. Front. Immunol. 2018, 9, 2224. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.H.; Kroll, A.V.; Zhang, L. Nanoparticle-Based Manipulation of Antigen-Presenting Cells for Cancer Immunotherapy. Small 2015, 11, 5483–5496. [Google Scholar] [CrossRef]
- Isaacs, A.; Li, Z.; Cheung, S.T.M.; Wijesundara, D.K.; McMillan, C.L.D.; Modhiran, N.; Young, P.R.; Ranasinghe, C.; Watterson, D.; Chappell, K.J. Adjuvant Selection for Influenza and RSV Prefusion Subunit Vaccines. Vaccines 2021, 9, 71. [Google Scholar] [CrossRef]
- Dowling, D.J. Recent Advances in the Discovery and Delivery of TLR7/8 Agonists as Vaccine Adjuvants. ImmunoHorizons 2018, 2, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.M.; Cybulski, V.; Whitacre, M.; Bess, L.S.; Livesay, M.T.; Walsh, L.; Burkhart, D.; Bazin, H.G.; Evans, J.T. Novel Lipidated Imidazoquinoline TLR7/8 Adjuvants Elicit Influenza-Specific Th1 Immune Responses and Protect against Heterologous H3N2 Influenza Challenge in Mice. Front. Immunol. 2020, 11, 406. [Google Scholar] [CrossRef]
- Toy, R.; Keenum, M.C.; Pradhan, P.; Phang, K.; Chen, P.; Chukwu, C.; Nguyen, L.A.H.; Liu, J.; Jain, S.; Kozlowski, G.; et al. TLR7 and RIG-I dual-adjuvant loaded nanoparticles drive broadened and synergistic responses in dendritic cells in vitro and generate unique cellular immune responses in influenza vaccination. J. Control. Release 2021, 330, 866–877. [Google Scholar] [CrossRef]
- Spitaels, J.; Roose, K.; Saelens, X. Influenza and Memory T Cells: How to Awake the Force. Vaccines 2016, 4, 33. [Google Scholar] [CrossRef]
- Nagashima, K.A.; Mousa, J.J. Next-Generation Influenza HA Immunogens and Adjuvants in Pursuit of a Broadly Protective Vaccine. Viruses 2021, 13, 546. [Google Scholar] [CrossRef]
- Goldinger, S.M.; Dummer, R.; Baumgaertner, P.; Mihic-Probst, D.; Schwarz, K.; Hammann-Haenni, A.; Willers, J.; Geldhof, C.; Prior, J.O.; Kündig, T.M.; et al. Nano-particle vaccination combined with TLR-7 and -9 ligands triggers memory and effector CD8+ T-cell responses in melanoma patients. Eur. J. Immunol. 2012, 42, 3049–3061. [Google Scholar] [CrossRef] [PubMed]
- Moody, M.A.; Santra, S.; Vandergrift, N.A.; Sutherland, L.L.; Gurley, T.C.; Drinker, M.S.; Allen, A.A.; Xia, S.M.; Meyerhoff, R.R.; Parks, R.; et al. Toll-like receptor 7/8 (TLR7/8) and TLR9 agonists cooperate to enhance HIV-1 envelope antibody responses in rhesus macaques. J. Virol. 2014, 88, 3329–3339. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.A.A.; Gale, E.C.; Roth, G.A.; Maikawa, C.L.; Correa, S.; Yu, A.C.; Appel, E.A. Nanoparticles Presenting Potent TLR7/8 Agonists Enhance Anti-PD-L1 Immunotherapy in Cancer Treatment. Biomacromolecules 2020, 21, 3704–3712. [Google Scholar] [CrossRef] [PubMed]
- Bhagchandani, S.; Johnson, J.A.; Irvine, D.J. Evolution of Toll-like receptor 7/8 agonist therapeutics and their delivery approaches: From antiviral formulations to vaccine adjuvants. Adv. Drug Deliv. Rev. 2021, 175, 113803. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Mendez, N.; Echeagaray, O.H.; Weeks, J.; Wang, J.; Vallez, C.N.; Gude, N.; Trogler, W.C.; Carson, D.A.; Hayashi, T.; et al. Conjugation of a Small-Molecule TLR7 Agonist to Silica Nanoshells Enhances Adjuvant Activity. ACS Appl. Mater. Interfaces 2019, 11, 26637–26647. [Google Scholar] [CrossRef] [PubMed]
- Stickdorn, J.; Stein, L.; Arnold-Schild, D.; Hahlbrock, J.; Medina-Montano, C.; Bartneck, J.; Ziß, T.; Montermann, E.; Kappel, C.; Hobernik, D.; et al. Systemically Administered TLR7/8 Agonist and Antigen-Conjugated Nanogels Govern Immune Responses against Tumors. ACS Nano 2022, 16, 4426–4443. [Google Scholar] [CrossRef] [PubMed]
- Lathrop, S.K.; Massena, C.J.; Davison, C.J.; Schoener, R.; Burkhart, D.J.; Evans, J.T. Co-delivery of antigens with TLR7/8 agonists leads to an improved Th1-mediated immune response to subunit vaccines. J. Immunol. 2021, 206, 102.09. [Google Scholar] [CrossRef]
- Short, K.K.; Lathrop, S.K.; Davison, C.J.; Partlow, H.A.; Kaiser, J.A.; Tee, R.D.; Lorentz, E.B.; Evans, J.T.; Burkhart, D.J. Using Dual Toll-like Receptor Agonism to Drive Th1-Biased Response in a Squalene- and α-Tocopherol-Containing Emulsion for a More Effective SARS-CoV-2 Vaccine. Pharmaceutics 2022, 14, 1455. [Google Scholar] [CrossRef]
- Lin, X.; Wu, W.; Fu, J.; Yang, Y.; Guo, B.; Yu, C.; Song, H. Asymmetric Silica Nanoparticles with Tailored Spiky Coverage Derived from Silica–Polymer Cooperative Assembly for Enhanced Hemocompatibility and Gene Delivery. ACS Appl. Mater. Interfaces 2021, 13, 50695–50704. [Google Scholar] [CrossRef]
- Abdelwahab, W.M.; Riffey, A.; Buhl, C.; Johnson, C.; Ryter, K.; Evans, J.T.; Burkhart, D.J. Co-adsorption of synthetic Mincle agonists and antigen to silica nanoparticles for enhanced vaccine activity: A formulation approach to co-delivery. Int. J. Pharm. 2021, 593, 120119. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. J. Nanomater. 2019, 2019, 3702518. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Khashab, N.M. Degradability and Clearance of Silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide, and Mesoporous Silica Nanoparticles. Adv. Mater. 2017, 29, 1604634. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef]
- Phillips, E.; Penate-Medina, O.; Zanzonico, P.B.; Carvajal, R.D.; Mohan, P.; Ye, Y.; Humm, J.; Gönen, M.; Kalaigian, H.; Schöder, H.; et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Transl. Med. 2014, 6, 260ra149. [Google Scholar] [CrossRef] [PubMed]
- Bukara, K.; Schueller, L.; Rosier, J.; Martens, M.A.; Daems, T.; Verheyden, L.; Eelen, S.; Van Speybroeck, M.; Libanati, C.; Martens, J.A.; et al. Ordered mesoporous silica to enhance the bioavailability of poorly water-soluble drugs: Proof of concept in man. Eur. J. Pharm. Biopharm. 2016, 108, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Schudel, A.; Francis, D.M.; Thomas, S.N. Material design for lymph node drug delivery. Nat. Rev. Mater. 2019, 4, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Querol, E.; Rosales, C. Control of Phagocytosis by Microbial Pathogens. Front. Immunol. 2017, 8, 1368. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Standley, S.M.; Goh, S.L.; Fréchet, J.M.J. Enhanced antigen presentation and immunostimulation of dendritic cells using acid-degradable cationic nanoparticles. J. Control. Release 2005, 105, 199–212. [Google Scholar] [CrossRef]
- Bazin, H.G.; Bess, L.S.; Livesay, M.T.; Li, Y.; Cybulski, V.; Miller, S.M.; Johnson, D.A.; Evans, J.T. Optimization of 8-oxoadenines with toll-like-receptor 7 and 8 activity. Bioorg. Med. Chem. Lett. 2020, 30, 126984. [Google Scholar] [CrossRef]
- Krammer, F.; Pica, N.; Hai, R.; Margine, I.; Palese, P. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J. Virol. 2013, 87, 6542–6550. [Google Scholar] [CrossRef]
- Krammer, F.; Jul-Larsen, A.; Margine, I.; Hirsh, A.; Sjursen, H.; Zambon, M.; Cox, R.J. An H7N1 influenza virus vaccine induces broadly reactive antibody responses against H7N9 in humans. Clin. Vaccine Immunol. 2014, 21, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.S.; Adamcakova-Dodd, A.; Lehman, S.E.; Wongrakpanich, A.; Thorne, P.S.; Larsen, S.C.; Salem, A.K. Amine modification of nonporous silica nanoparticles reduces inflammatory response following intratracheal instillation in murine lungs. Toxicol. Lett. 2016, 241, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Sodipo, B.K.; Aziz, A.A. One minute synthesis of amino-silane functionalized superparamagnetic iron oxide nanoparticles by sonochemical method. Ultrason. Sonochem. 2018, 40, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. Aaps J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.N.; King, D.; Macparland, S.A.; McGrath, J.S.; Reddy, S.B.; Bursey, F.R.; Michalak, T.I. Hepatitis C virus replicates in the same immune cell subsets in chronic hepatitis C and occult infection. Gastroenterology 2008, 134, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Rao, S.P.; Takabayashi, K.; Van Uden, J.H.; Kornbluth, R.S.; Baird, S.M.; Taylor, M.W.; Carson, D.A.; Catanzaro, A.; Raz, E. Enhancement of innate immunity against Mycobacterium avium infection by immunostimulatory DNA is mediated by indoleamine 2,3-dioxygenase. Infect. Immun. 2001, 69, 6156–6164. [Google Scholar] [CrossRef] [PubMed]
- Auderset, F.; Belnoue, E.; Mastelic-Gavillet, B.; Lambert, P.-H.; Siegrist, C.-A. A TLR7/8 Agonist-Including DOEPC-Based Cationic Liposome Formulation Mediates Its Adjuvanticity through the Sustained Recruitment of Highly Activated Monocytes in a Type I IFN-Independent but NF-κB-Dependent Manner. Front. Immunol. 2020, 11, 580974. [Google Scholar] [CrossRef]
- Croissant, J.G.; Brinker, C.J. Biodegradable Silica-Based Nanoparticles: Dissolution Kinetics and Selective Bond Cleavage. Enzymes 2018, 43, 181–214. [Google Scholar] [CrossRef]
- Soto-Cantu, E.; Cueto, R.; Koch, J.; Russo, P.S. Synthesis and Rapid Characterization of Amine-Functionalized Silica. Langmuir 2012, 28, 5562–5569. [Google Scholar] [CrossRef]
- Jang, H.; Meyers, L.M.; Boyle, C.; De Groot, A.S.; Moise, L.; Ross, T.M. Immune-engineered H7N9 influenza hemagglutinin improves protection against viral influenza virus challenge. Hum. Vaccines Immunother. 2020, 16, 2042–2050. [Google Scholar] [CrossRef]
- Strixner, T.; Kulozik, U. Handbook of Food Proteins; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1–432. [Google Scholar] [CrossRef]
- Chen, C.; Zheng, H.; Xu, J.; Shi, X.; Li, F.; Wang, X. Sustained-release study on Exenatide loaded into mesoporous silica nanoparticles: In vitro characterization and in vivo evaluation. Daru 2017, 25, 20. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.; Gadag, S.; Cheruku, S.P.; Raichur, A.M.; Day, C.M.; Garg, S.; Manandhar, S.; Pai, K.S.R.; Suresh, A.; Mehta, C.H.; et al. Chitosan-glucuronic acid conjugate coated mesoporous silica nanoparticles: A smart pH-responsive and receptor-targeted system for colorectal cancer therapy. Carbohydr. Polym. 2021, 261, 117893. [Google Scholar] [CrossRef] [PubMed]
- Braun, K.; Pochert, A.; Gerber, M.; Raber, H.F.; Lindén, M. Influence of mesopore size and peptide aggregation on the adsorption and release of a model antimicrobial peptide onto/from mesoporous silica nanoparticles in vitro. Mol. Syst. Des. Eng. 2017, 2, 393–400. [Google Scholar] [CrossRef]
- Bruschi, M.L. Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- Shinchi, H.; Crain, B.; Yao, S.; Chan, M.; Zhang, S.S.; Ahmadiiveli, A.; Suda, Y.; Hayashi, T.; Cottam, H.B.; Carson, D.A. Enhancement of the Immunostimulatory Activity of a TLR7 Ligand by Conjugation to Polysaccharides. Bioconj. Chem. 2015, 26, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Seydoux, E.; Rothen-Rutishauser, B.; Nita, I.M.; Balog, S.; Gazdhar, A.; Stumbles, P.A.; Petri-Fink, A.; Blank, F.; von Garnier, C. Size-dependent accumulation of particles in lysosomes modulates dendritic cell function through impaired antigen degradation. Int. J. Nanomed. 2014, 9, 3885–3902. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, Y.; Zhang, P.; Xing, H.; Zhao, S.; Song, Y.; Wan, D.; Yu, J. Targeting toll-like receptor 7/8 for immunotherapy: Recent advances and prospectives. Biomark. Res. 2022, 10, 89. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Zhang, Y.; Zhang, G.; Kang, Y.; Chen, A.; Feng, X.; Shao, L. The toxicity of silica nanoparticles to the immune system. Nanomedicine 2018, 13, 1939–1962. [Google Scholar] [CrossRef]
- Cervantes, J.L.; Weinerman, B.; Basole, C.; Salazar, J.C. TLR8: The forgotten relative revindicated. Cell. Mol. Immunol. 2012, 9, 434–438. [Google Scholar] [CrossRef]
- Russell, R.F.; McDonald, J.U.; Lambert, L.; Tregoning, J.S. Use of the Microparticle Nanoscale Silicon Dioxide as an Adjuvant To Boost Vaccine Immune Responses against Influenza Virus in Neonatal Mice. J. Virol. 2016, 90, 4735–4744. [Google Scholar] [CrossRef]
- Fornefett, J.; Krause, J.; Klose, K.; Fingas, F.; Hassert, R.; Benga, L.; Grunwald, T.; Müller, U.; Schrödl, W.; Baums, C.G. Comparative analysis of humoral immune responses and pathologies of BALB/c and C57BL/6 wildtype mice experimentally infected with a highly virulent Rodentibacter pneumotropicus (Pasteurella pneumotropica) strain. BMC Microbiol. 2018, 18, 45. [Google Scholar] [CrossRef]
- Schüler, T.; Qin, Z.; Ibe, S.; Noben-Trauth, N.; Blankenstein, T. T helper cell type 1-associated and cytotoxic T lymphocyte-mediated tumor immunity is impaired in interleukin 4-deficient mice. J. Exp. Med. 1999, 189, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M.I.; Catalan-Dibene, J.; Zlotnik, A. B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine 2015, 74, 318–326. [Google Scholar] [CrossRef] [PubMed]







| Formulation | A-SNP Conc. (mg/mL) | INI-4001 Conc. (µM) | INI-4001-Coating Density nmol/cm2 | TEM Size (nm) a | Z-Average Diameter (nm) | PDI | Zeta Potential (mV) b | pH |
|---|---|---|---|---|---|---|---|---|
| A-SNP-50 | 10.0 | --- | --- | 48.9 ± 2.5 | 215 | 0.270 | 46.7 ± 5.4 | 5.9 |
| A-SNP-200 | 40.0 | --- | --- | 199.6 ± 15.3 | 997 | 0.422 | 61.5 ± 4.9 | 6.1 |
| INI-4001 aqueous control | NA | 226.3 | --- | --- | 73 | 0.163 | −62.2 ± 6.8 | 8.2 |
| INI-4001/A-SNP-50 high | 1.0 | 221.2 | 0.20 | 55.9 ± 2.9 | 246 | 0.256 | −20.1 ± 6.2 | 6.2 |
| INI-4001/A-SNP-50 low | 10.0 | 259.8 | 0.02 | 48.3 ± 2.4 | 1107 | 0.334 | 26.6 ± 4.8 | 5.8 |
| INI-4001/A-SNP-200 high | 4.0 | 277.6 | 0.21 | 209.3 ± 10.0 | 631 | 0.249 | −34.1 ± 5.4 | 6.0 |
| INI-4001/A-SNP-200 low | 40.0 | 242.6 | 0.02 | 209.8 ± 18.3 | 1305 | 0.608 | 47.0 ± 3.9 | 6.0 |
| Formulation | INI-4001 % Adsorption a | H7 % Adsorption a |
|---|---|---|
| A-SNP-50 blank | --- | 97.7 ± 3.1 |
| A-SNP-200 blank | --- | 98.2 ± 2.4 |
| INI-4001/A-SNP-50 high | 101.4 ± 1.9 | 96.7 ± 4.4 |
| INI-4001/A-SNP-50 low | 95.4 ± 1.7 | 94.1 ± 7.6 |
| INI-4001/A-SNP-200 high | 101.1 ± 3.0 | 97.7 ± 2.0 |
| INI-4001/A-SNP-200 low | 100.7 ± 1.8 | 97.8 ± 3.2 |
| Formulation | Zero-Order (R2) | First Order (R2) | Higuchi (R2) | Korsmeyer–Peppas | |
|---|---|---|---|---|---|
| R2 | n | ||||
| INI-4001/A-SNP high | 0.9520 | 0.9717 | 0.9299 | 0.9866 | 0.738 |
| INI-4001/A-SNP low | 0.5178 | 0.3111 | 0.4811 | 0.8325 | 0.260 |
| 50 nm INI-4001/A-SNP | 200 nm INI-4001/A-SNP | ||||
|---|---|---|---|---|---|
| Cytokine | Low | High | Low | High | Aqueous INI-4001 |
| IFN-α | 3.2 ± 0.7 | 40.2 ± 30.9 | 13.6 ± 7.6 | 15.0 ± 8.8 | 34.0 ± 10.3 |
| TNF-α | 4.6 ± 1.5 * | 132.1 ± 67.2 | 1.6 ± 0.8 * | 14.5 ± 5.6 | 21.4 ± 8.7 |
| 50 nm INI-4001/A-SNP | 200 nm INI-4001/A-SNP | ||||
|---|---|---|---|---|---|
| Cytokine | Low | High | Low | High | Aqueous INI-4001 |
| IL-6 | 40 ± 10 * | 133 ± 23 | 20 ± 2 * | 72 ± 15 | 19 ± 3 |
| TNF-α | 49 ± 15 * | 117 ± 13 | 35 ± 5 | 95 ± 25 | 48 ± 12 |
| IL-12 | 62 ± 31 * | 58 ± 20 | 22 ± 3 | 52 ± 27 | <1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelwahab, W.M.; Auclair, S.; Borgogna, T.; Siram, K.; Riffey, A.; Bazin, H.G.; Cottam, H.B.; Hayashi, T.; Evans, J.T.; Burkhart, D.J. Co-Delivery of a Novel Lipidated TLR7/8 Agonist and Hemagglutinin-Based Influenza Antigen Using Silica Nanoparticles Promotes Enhanced Immune Responses. Pharmaceutics 2024, 16, 107. https://doi.org/10.3390/pharmaceutics16010107
Abdelwahab WM, Auclair S, Borgogna T, Siram K, Riffey A, Bazin HG, Cottam HB, Hayashi T, Evans JT, Burkhart DJ. Co-Delivery of a Novel Lipidated TLR7/8 Agonist and Hemagglutinin-Based Influenza Antigen Using Silica Nanoparticles Promotes Enhanced Immune Responses. Pharmaceutics. 2024; 16(1):107. https://doi.org/10.3390/pharmaceutics16010107
Chicago/Turabian StyleAbdelwahab, Walid M., Sarah Auclair, Timothy Borgogna, Karthik Siram, Alexander Riffey, Hélène G. Bazin, Howard B. Cottam, Tomoko Hayashi, Jay T. Evans, and David J. Burkhart. 2024. "Co-Delivery of a Novel Lipidated TLR7/8 Agonist and Hemagglutinin-Based Influenza Antigen Using Silica Nanoparticles Promotes Enhanced Immune Responses" Pharmaceutics 16, no. 1: 107. https://doi.org/10.3390/pharmaceutics16010107
APA StyleAbdelwahab, W. M., Auclair, S., Borgogna, T., Siram, K., Riffey, A., Bazin, H. G., Cottam, H. B., Hayashi, T., Evans, J. T., & Burkhart, D. J. (2024). Co-Delivery of a Novel Lipidated TLR7/8 Agonist and Hemagglutinin-Based Influenza Antigen Using Silica Nanoparticles Promotes Enhanced Immune Responses. Pharmaceutics, 16(1), 107. https://doi.org/10.3390/pharmaceutics16010107