Formulation and Evaluation of Transdermal Patches Containing BGP-15
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
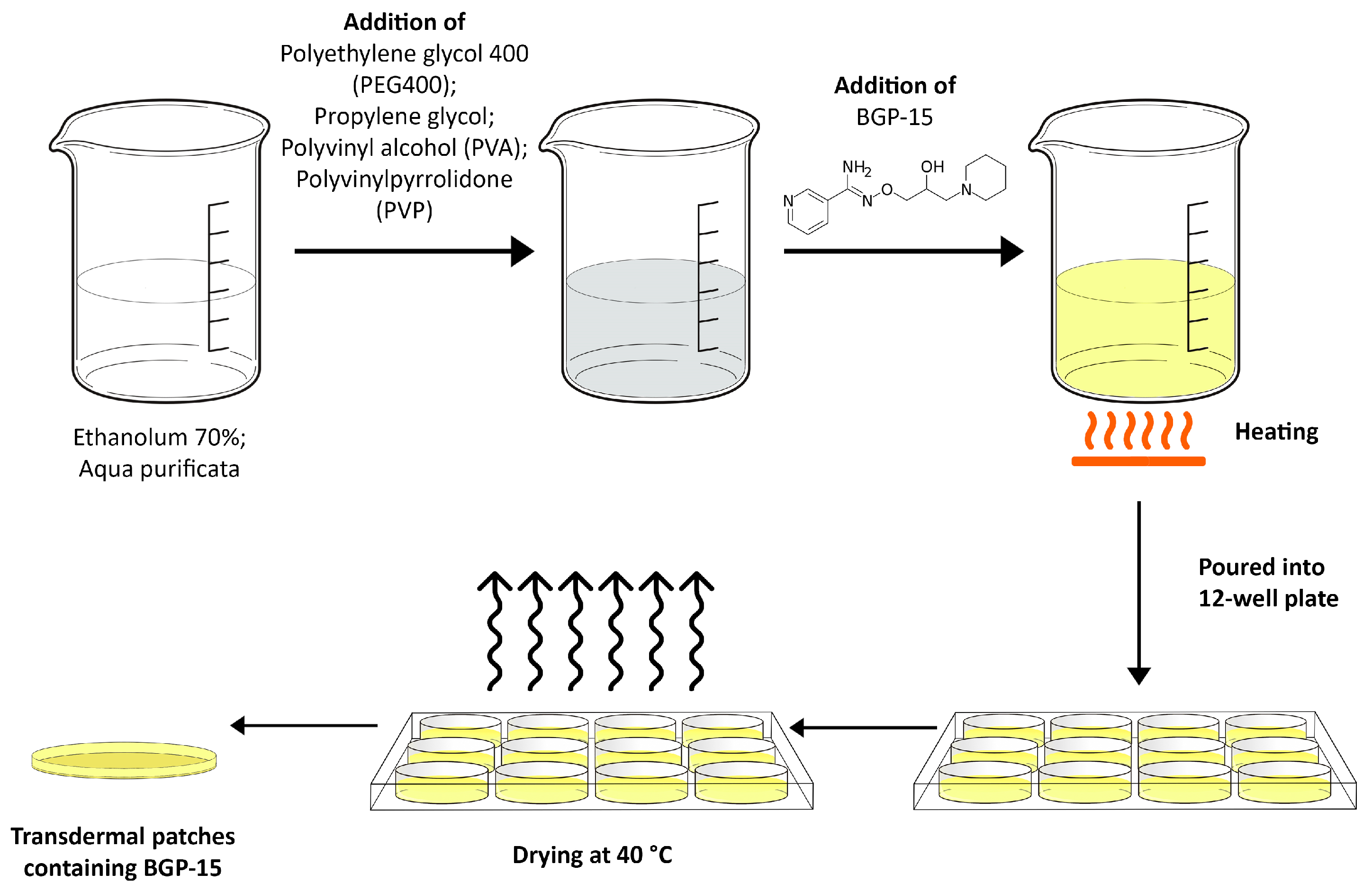
2.2. Formulation of Transdermal Patches
2.3. Design of Experiment
2.3.1. Tensile Strength
2.3.2. Moisture Content
2.3.3. Moisture Uptake
2.4. Penetration Enhancement
2.5. MTT Assay
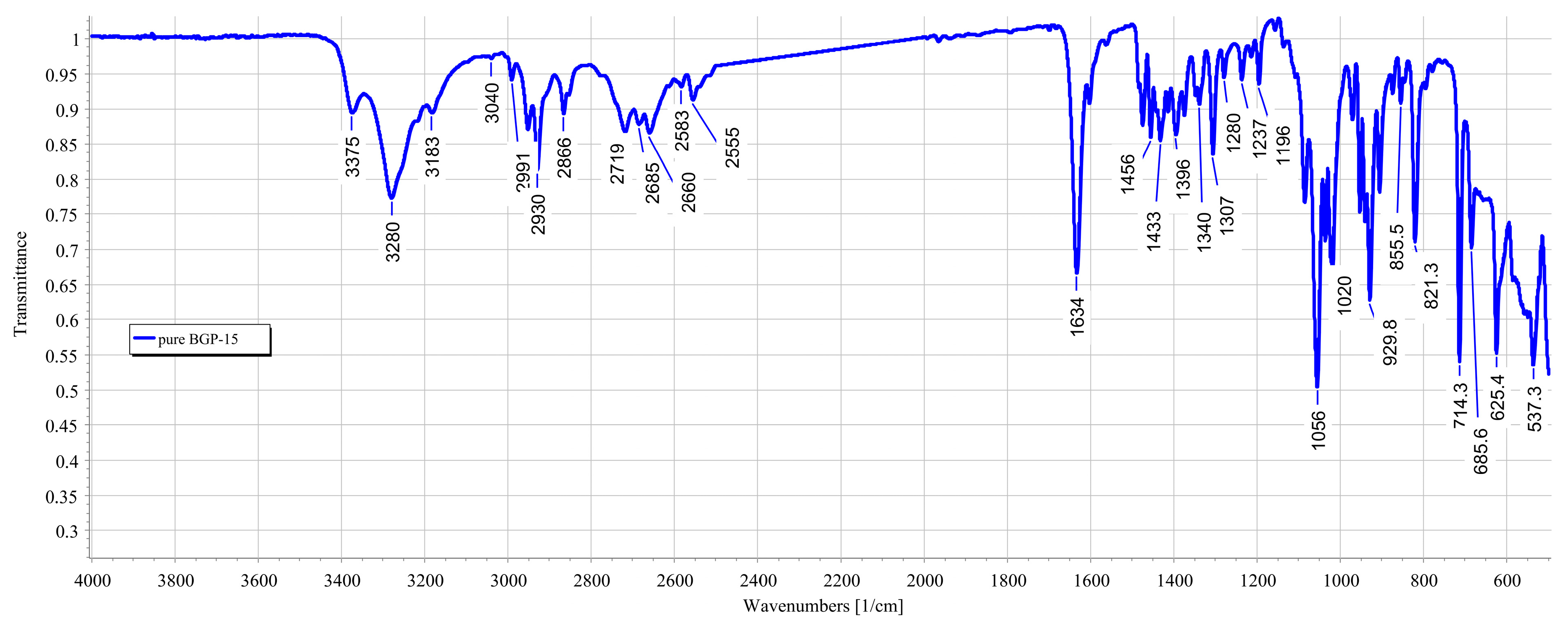
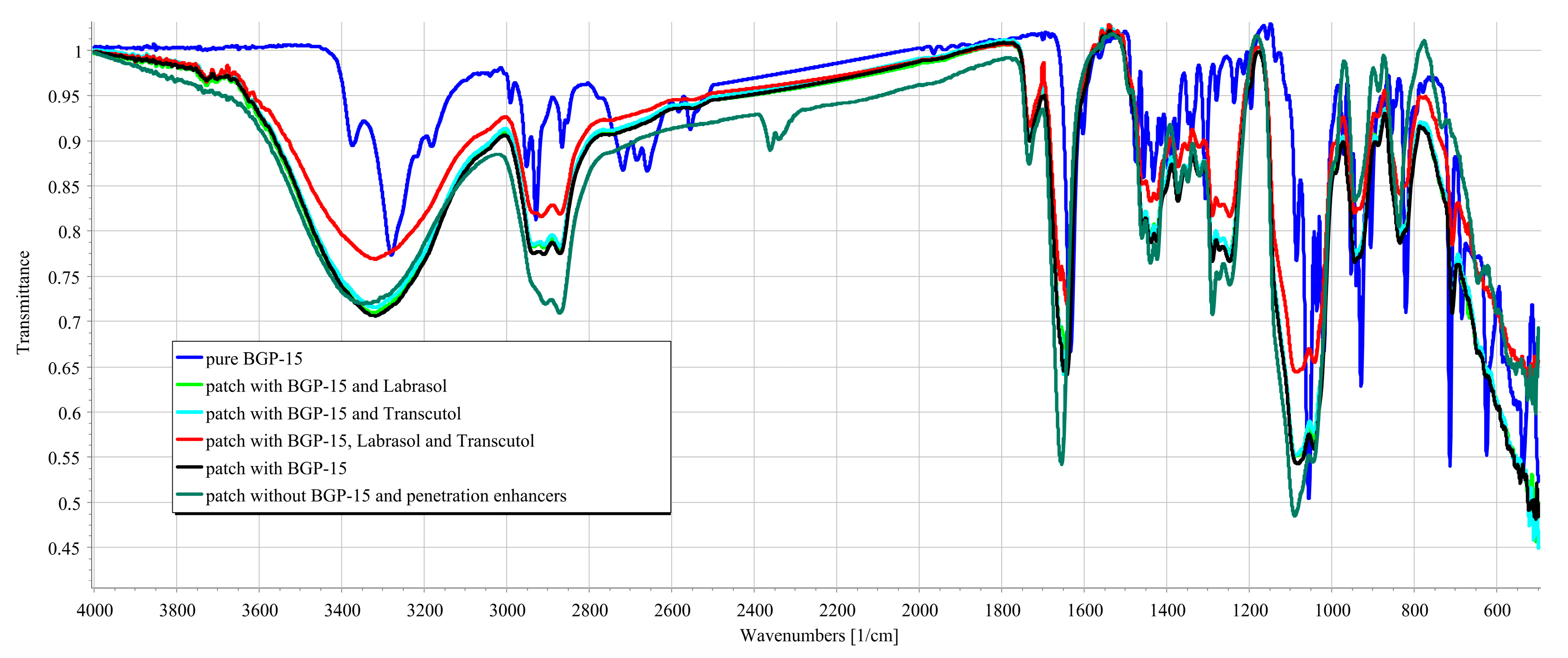
2.6. Fourier-Transform Infrared Spectroscopy (FTIR)
- The active substance (solid BGP-15);
- Transdermal patches with BGP-15, without penetration enhancers;
- The transdermal patches with BGP-15 and the penetration enhancers (Labrasol or Transcutol or the mixture of Labrasol and Transcutol);
- Transdermal patches without penetration enhancers and BGP-15 were obtained by using a JASCO FT/IR-4600 spectrometer with an ATR accessory (Zn/Se ATR PRO ONE Single-Reflection, ABL&E-JASCO, Hungary, Budapest). All the samples were directly placed on the diamond crystal of the equipment. Scanning was run in the wavelength range of 500–4000 cm−1. The spectra were collected from 32 scans to obtain smooth spectra at the spectral resolution of 1 cm−1. Corrections of environmental CO2 and H2O were carried out using the software’s built-in method [34].
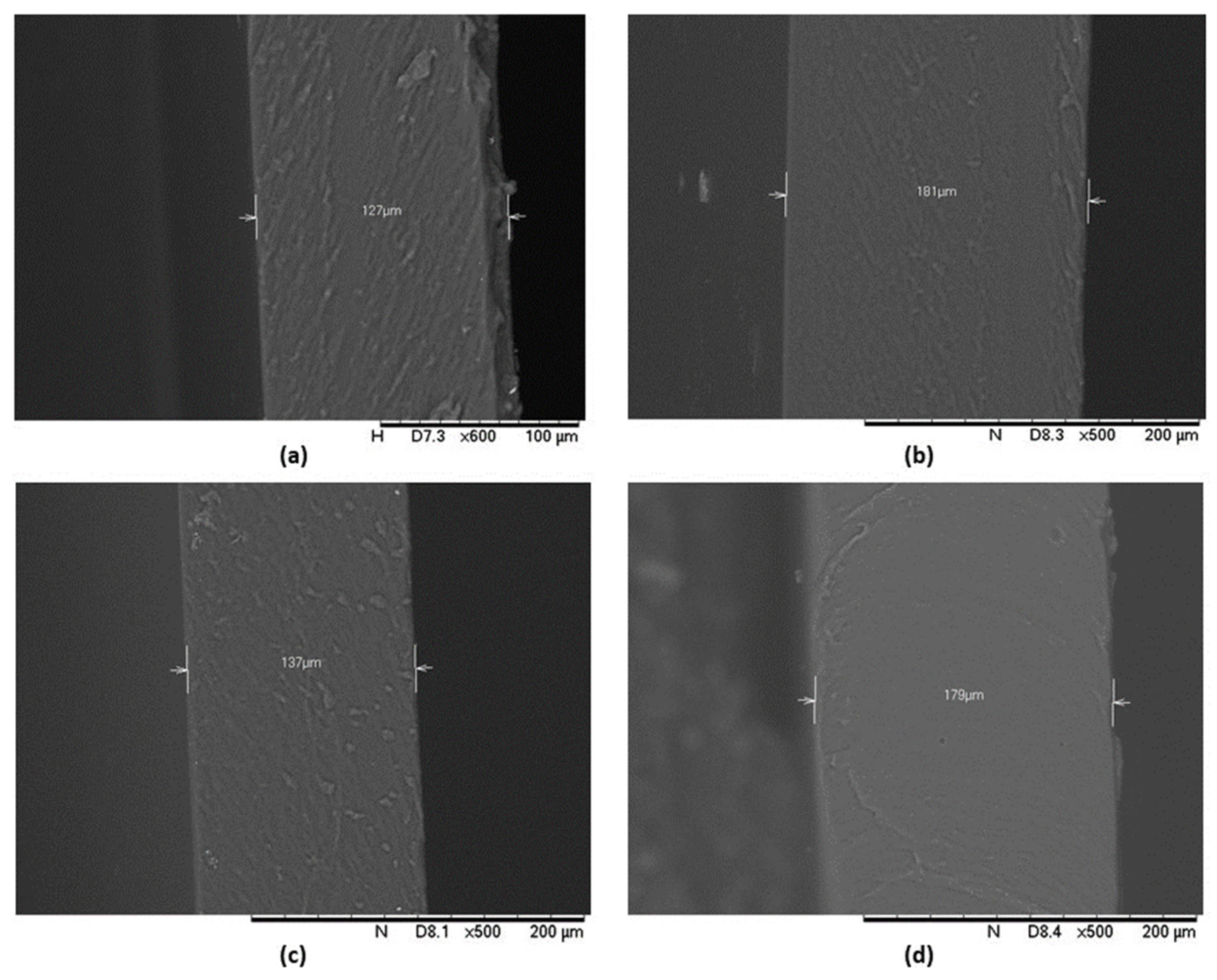
2.7. Scanning Electron Microscopy (SEM)
2.8. In Vitro Release of the Active Ingredient
2.9. In Vitro Permeation Studies
2.10. Determination of BGP-15 in Skin Samples
2.11. Statistical Analysis
3. Results
3.1. Design of Experiment
3.1.1. Tensile Strength
3.1.2. Moisture Content
3.1.3. Moisture Uptake
3.1.4. Incorporating Penetration Enhancers into the Formulation
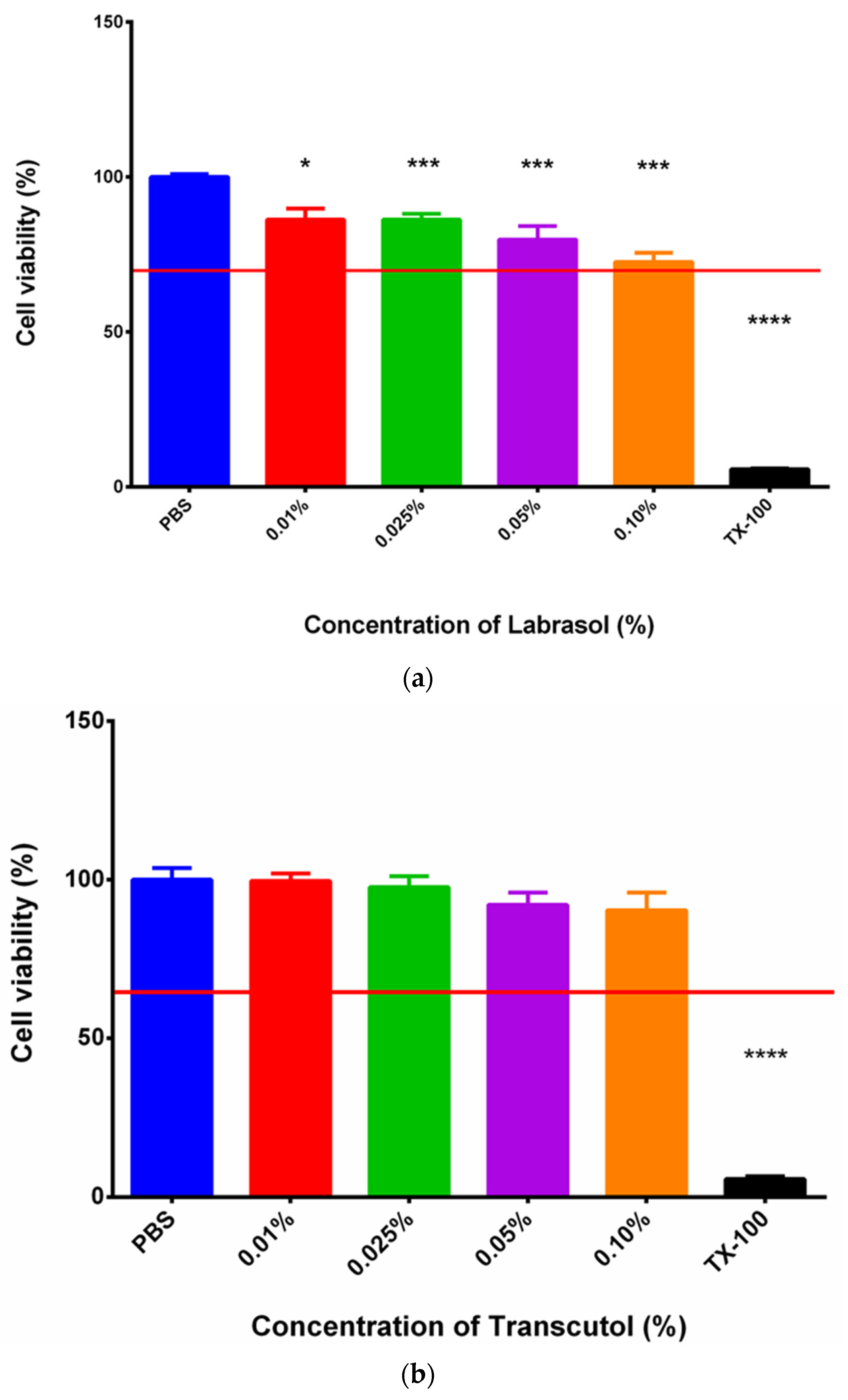
3.2. MTT Assay
3.3. FTIR Measurements
3.4. SEM
3.5. In Vitro Drug Release
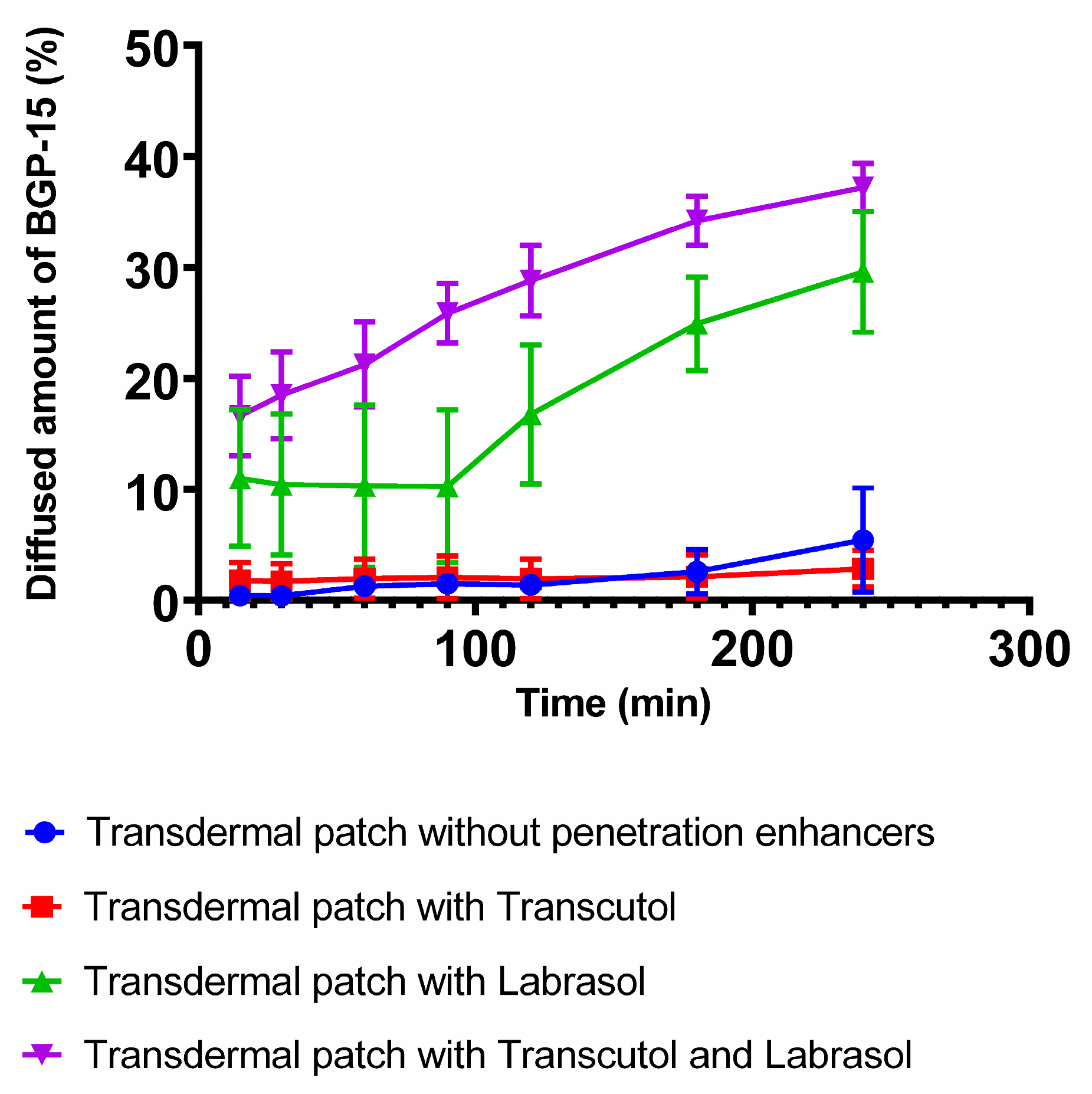
3.6. In Vitro Permeation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al Hanbali, O.A.; Khan, H.M.S.; Sarfraz, M.; Arafat, M.; Ijaz, S.; Hameed, A. Transdermal patches: Design and current approaches to painless drug delivery. Acta Pharm. 2019, 69, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Alkilani, A.; McCrudden, M.T.; Donnelly, R. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Peddapalli, H.; Ganta, R.P.; Boggula, N. Formulation and evaluation of transdermal patches for antianxiety drug. Asian J. Pharm. 2018, 12, 127–136. [Google Scholar]
- Wokovich, A.; Prodduturi, S.; Doub, W.; Hussain, A.; Buhse, L. Transdermal drug delivery system (TDDS) adhesion as a critical safety, efficacy and quality attribute. Eur. J. Pharm. Biopharm. 2006, 64, 1–8. [Google Scholar] [CrossRef]
- Hadžiabdić, J.; Šejto, L.; Rahić, O.; Tucak, A.; Hindija, L.; Sirbubalo, M. Transdermal Patches as Noninvasive Drug Delivery Systems. In Proceedings of the International Conference on Medical and Biological Engineering, Mostar, Bosnia and Herzegovina, 21–24 April 2021; pp. 395–402. [Google Scholar]
- Das, S.; Sarkar, P.; Majee, S.B. Polymers in Matrix Type Transdermal Patch. Int. J. Pharm. Sci. Rev. Res. 2022, 73, 77–86. [Google Scholar] [CrossRef]
- Latif, M.S.; Al-Harbi, F.F.; Nawaz, A.; Rashid, S.A.; Farid, A.; Al Mohaini, M.; Alsalman, A.J.; Al Hawaj, M.A.; Alhashem, Y.N. Formulation and Evaluation of Hydrophilic Polymer Based Methotrexate Patches: In Vitro and In Vivo Characterization. Polymers 2022, 14, 1310. [Google Scholar] [CrossRef]
- Bhatt, P.; Trehan, S.; Inamdar, N.; Mourya, V.K.; Misra, A. Polymers in Drug Delivery: An Update. In Applications of Polymers in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–42. [Google Scholar]
- Valenta, C.; Auner, B.G. The use of polymers for dermal and transdermal delivery. Eur. J. Pharm. Biopharm. 2004, 58, 279–289. [Google Scholar] [CrossRef]
- Yewale, C.; Tandel, H.; Patel, A.; Misra, A. Polymers in Transdermal Drug Delivery. In Applications of Polymers in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2021; pp. 131–158. [Google Scholar]
- Santos, L.F.; Correia, I.J.; Silva, A.S.; Mano, J.F. Biomaterials for drug delivery patches. Eur. J. Pharm. Sci. 2018, 118, 49–66. [Google Scholar] [CrossRef]
- Sa’adon, S.; Abd Razak, S.I.; Ismail, A.E.; Fakhruddin, K. Fabrication of Dual Layer Polyvinyl Alcohol Transdermal Patch: Effect of Freezing-Thawing Cycles on Morphological and Swelling Ability. Procedia Comput. Sci. 2019, 158, 51–57. [Google Scholar] [CrossRef]
- Zahra, F.T.; Quick, Q.; Mu, R. Electrospun PVA Fibers for Drug Delivery: A Review. Polymers 2023, 15, 3837. [Google Scholar] [CrossRef] [PubMed]
- DeMerlis, C.; Schoneker, D. Review of the oral toxicity of polyvinyl alcohol (PVA). Food Chem. Toxicol. 2003, 41, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-C.; He, J.-S.; Tsai, M.-T.; Lin, K.-C. Fabrication of a novel partially dissolving polymer microneedle patch for transdermal drug delivery. J. Mater. Chem. B 2015, 3, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Nokhodchi, A.; Shokri, J.; Dashbolaghi, A.; Hassan-Zadeh, D.; Ghafourian, T.; Barzegar-Jalali, M. The enhancement effect of surfactants on the penetration of lorazepam through rat skin. Int. J. Pharm. 2003, 250, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Hagen, M.; Baker, M. Skin penetration and tissue permeation after topical administration of diclofenac. Curr. Med. Res. Opin. 2017, 33, 1623–1634. [Google Scholar] [CrossRef]
- Som, I.; Bhatia, K.; Yasir, M. Status of surfactants as penetration enhancers in transdermal drug delivery. J. Pharm. Bioallied Sci. 2012, 4, 2–9. [Google Scholar] [CrossRef]
- Korhonen, O.; Pajula, K.; Laitinen, R. Rational excipient selection for co-amorphous formulations. Expert Opin. Drug Deliv. 2017, 14, 551–569. [Google Scholar] [CrossRef]
- Bando, H.; Yamashita, F.; Takakura, Y.; Hashida, M. Skin Penetration Enhancement of Acyclovir by Prodrug-Enhancer Combination. Biol. Pharm. Bull. 1994, 17, 1141–1143. [Google Scholar] [CrossRef]
- Pető, Á.; Kósa, D.; Fehér, P.; Ujhelyi, Z.; Sinka, D.; Vecsernyés, M.; Szilvássy, Z.; Juhász, B.; Csanádi, Z.; Vígh, L.; et al. Pharmacological overview of the BGP-15 chemical agent as a new drug candidate for the treatment of symptoms of metabolic syndrome. Molecules 2020, 25, 429. [Google Scholar] [CrossRef]
- Literati-Nagy, Z.; Tory, K.; Literáti-Nagy, B.; Kolonics, A.; Vígh, L., Jr.; Vígh, L.; Mandl, J.; Szilvássy, Z. A Novel Insulin Sensitizer Drug Candidate—BGP-15—Can Prevent Metabolic Side Effects of Atypical Antipsychotics. Pathol. Oncol. Res. 2012, 18, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Horvath, O.; Ordog, K.; Bruszt, K.; Deres, L.; Gallyas, F.; Sumegi, B.; Toth, K.; Halmosi, R. BGP-15 Protects against Heart Failure by Enhanced Mitochondrial Biogenesis and Decreased Fibrotic Remodelling in Spontaneously Hypertensive Rats. Oxid. Med. Cell. Longev. 2021, 2021, 1250858. [Google Scholar] [CrossRef] [PubMed]
- Farkas, B.; Magyarlaki, M.; Csete, B.; Nemeth, J.; Rabloczky, G.; Bernath, S.; Nagy, P.L.; Sümegi, B. Reduction of acute photodamage in skin by topical application of a novel PARP inhibitor. Biochem. Pharmacol. 2002, 63, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, B.; Larsson, L.; Z’Graggen, W.J. Critical Illness Myopathy: Diagnostic Approach and Resulting Therapeutic Implications. Curr. Treat. Options Neurol. 2022, 24, 173–182. [Google Scholar] [CrossRef]
- Prajapati, S.T.; Patel, C.G.; Patel, C.N. Formulation and Evaluation of Transdermal Patch of Repaglinide. ISRN Pharm. 2011, 2011, 651909. [Google Scholar] [CrossRef]
- Haimhoffer, Á.; Vasvári, G.; Trencsényi, G.; Béresová, M.; Budai, I.; Czomba, Z.; Rusznyák, Á.; Váradi, J.; Bácskay, I.; Ujhelyi, Z.; et al. Process Optimization for the Continuous Production of a Gastroretentive Dosage Form Based on Melt Foaming. AAPS PharmSciTech 2021, 22, 187. [Google Scholar] [CrossRef]
- Głąb, M.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Guigou, M.D.; Makara, A.; Gajda, P.; Jampilek, J.; Tyliszczak, B. Starch Solutions Prepared under Different Conditions as Modifiers of Chitosan/Poly(aspartic acid)-Based Hydrogels. Materials 2021, 14, 4443. [Google Scholar] [CrossRef]
- Thakur, N.; Goswami, M.; Deka Dey, A.; Kaur, B.; Sharma, C.; Kumar, A. Fabrication and Synthesis of Thiococlchicoside Loaded Matrix Type Transdermal Patch. Pharm. Nanotechnol. 2023, 11. [Google Scholar] [CrossRef]
- Suksaeree, J.; Prasomkij, J.; Panrat, K.; Pichayakorn, W. Comparison of Pectin Layers for Nicotine Transdermal Patch Preparation. Adv. Pharm. Bull. 2018, 8, 401–410. [Google Scholar] [CrossRef]
- Kósa, D.; Pető, Á.; Fenyvesi, F.; Váradi, J.; Vecsernyés, M.; Gonda, S.; Vasas, G.; Fehér, P.; Bácskay, I.; Ujhelyi, Z. Formulation of Novel Liquid Crystal (LC) Formulations with Skin-Permeation-Enhancing Abilities of Plantago lanceolata (PL) Extract and Their Assessment on HaCaT Cells. Molecules 2021, 26, 1023. [Google Scholar] [CrossRef]
- Sabir, F.; Qindeel, M.; Rehman, A.U.; Ahmad, N.M.; Khan, G.M.; Csoka, I.; Ahmed, N. An efficient approach for development and optimisation of curcumin-loaded solid lipid nanoparticles’ patch for transdermal delivery. J. Microencapsul. 2021, 38, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Sarwar, H.S.; Sarfraz, M.; Sohail, M.F.; Jalil, A.; Bin Jardan, Y.A.; Arshad, R.; Tahir, I.; Ahmad, Z. Formulation and characterization of thiolated chitosan/polyvinyl acetate based microneedle patch for transdermal delivery of dydrogesterone. Saudi Pharm. J. 2023, 31, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Pető, Á.; Kósa, D.; Haimhoffer, Á.; Fehér, P.; Ujhelyi, Z.; Sinka, D.; Fenyvesi, F.; Váradi, J.; Vecsernyés, M.; Gyöngyösi, A.; et al. Nicotinic amidoxime derivate bgp-15, topical dosage formulation and anti-inflammatory effect. Pharmaceutics 2021, 13, 2037. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Argenziano, M.; Haimhoffer, A.; Bastiancich, C.; Jicsinszky, L.; Caldera, F.; Trotta, F.; Scutera, S.; Alotto, D.; Fumagalli, M.; Musso, T.; et al. In Vitro Enhanced Skin Permeation and Retention of Imiquimod Loaded in β-Cyclodextrin Nanosponge Hydrogel. Pharmaceutics 2019, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Literáti-Nagy, B.; Tory, K.; Peitl, B.; Bajza, Á.; Korányi, L.; Literáti-Nagy, Z.; Hooper, P.L.; Vígh, L.; Szilvássy, Z. Improvement of Insulin Sensitivity by a Novel Drug Candidate, BGP-15, in Different Animal Studies. Metab. Syndr. Relat. Disord. 2014, 12, 125–131. [Google Scholar] [CrossRef]
- Kozma, M.; Bombicz, M.; Varga, B.; Priksz, D.; Gesztelyi, R.; Tarjanyi, V.; Kiss, R.; Szekeres, R.; Takacs, B.; Menes, A.; et al. Cardioprotective Role of BGP-15 in Ageing Zucker Diabetic Fatty Rat (ZDF) Model: Extended Mitochondrial Longevity. Pharmaceutics 2022, 14, 226. [Google Scholar] [CrossRef]
- Priksz, D.; Lampe, N.; Kovacs, A.; Herwig, M.; Bombicz, M.; Varga, B.; Wilisicz, T.; Szilvassy, J.; Posa, A.; Kiss, R.; et al. Nicotinic-acid derivative BGP-15 improves diastolic function in a rabbit model of atherosclerotic cardiomyopathy. Br. J. Pharmacol. 2022, 179, 2240–2258. [Google Scholar] [CrossRef]
- Gao, Y.; Liang, J.; Liu, J.; Xiao, Y. Double-layer weekly sustained release transdermal patch containing gestodene and ethinylestradiol. Int. J. Pharm. 2009, 377, 128–134. [Google Scholar] [CrossRef]
- Kamli, M.; Guettari, M.; Tajouri, T. Structure of polyvinylpyrrolidone in water/ethanol mixture in dilute and semi-dilute regimes: Roles of solvents mixture composition and polymer concentration. J. Mol. Liq. 2023, 382, 122014. [Google Scholar] [CrossRef]
- Lopes, J.F.A.; Simoneau, C. Solubility of Polyvinyl Alcohol in Ethanol. EFSA Support. Publ. 2014, 11, 660E. [Google Scholar] [CrossRef]
- Xiang, A.; Lv, C.; Zhou, H. Changes in Crystallization Behaviors of Poly(Vinyl Alcohol) Induced by Water Content. J. Vinyl Addit. Technol. 2020, 26, 613–622. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices. ISO: Geneva, Switzerland, 2009.
- Taher, M.A.; Elsherbiny, E.A. Impact of Isonicotinic Acid Blending in Chitosan/Polyvinyl Alcohol on Ripening-Dependent Changes of Green Stage Tomato. Polymers 2023, 15, 825. [Google Scholar] [CrossRef]
- Csizmazia, E.; Erős, G.; Berkesi, O.; Berkó, S.; Szabó-Révész, P.; Csányi, E. Pénétration enhancer effect of sucrose laurate and Transcutol on ibuprofen. J. Drug Deliv. Sci. Technol. 2011, 21, 411–415. [Google Scholar] [CrossRef]
- Virani, A.; Puri, V.; Mohd, H.; Michniak-Kohn, B. Effect of Penetration Enhancers on Transdermal Delivery of Oxcarbazepine, an Antiepileptic Drug Using Microemulsions. Pharmaceutics 2023, 15, 183. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm.-Drug Res. 2010, 67, 217–223. [Google Scholar]
- Altun, E.; Yuca, E.; Ekren, N.; Kalaskar, D.M.; Ficai, D.; Dolete, G.; Ficai, A.; Gunduz, O. Kinetic Release Studies of Antibiotic Patches for Local Transdermal Delivery. Pharmaceutics 2021, 13, 613. [Google Scholar] [CrossRef]
- Bom, S.; Santos, C.; Barros, R.; Martins, A.M.; Paradiso, P.; Cláudio, R.; Pinto, P.C.; Ribeiro, H.M.; Marto, J. Effects of Starch Incorporation on the Physicochemical Properties and Release Kinetics of Alginate-Based 3D Hydrogel Patches for Topical Delivery. Pharmaceutics 2020, 12, 719. [Google Scholar] [CrossRef]
- Ruan, J.; Liu, C.; Song, H.; Zhong, T.; Quan, P.; Fang, L. Sustainable and efficient skin absorption behaviour of transdermal drug: The effect of the release kinetics of permeation enhancer. Int. J. Pharm. 2022, 612, 121377. [Google Scholar] [CrossRef]
- Jafri, I.; Shoaib, M.H.; Yousuf, R.I.; Ali, F.R. Effect of permeation enhancers on in vitro release and transdermal delivery of lamotrigine from Eudragit®RS100 polymer matrix-type drug in adhesive patches. Prog. Biomater. 2019, 8, 91–100. [Google Scholar] [CrossRef]
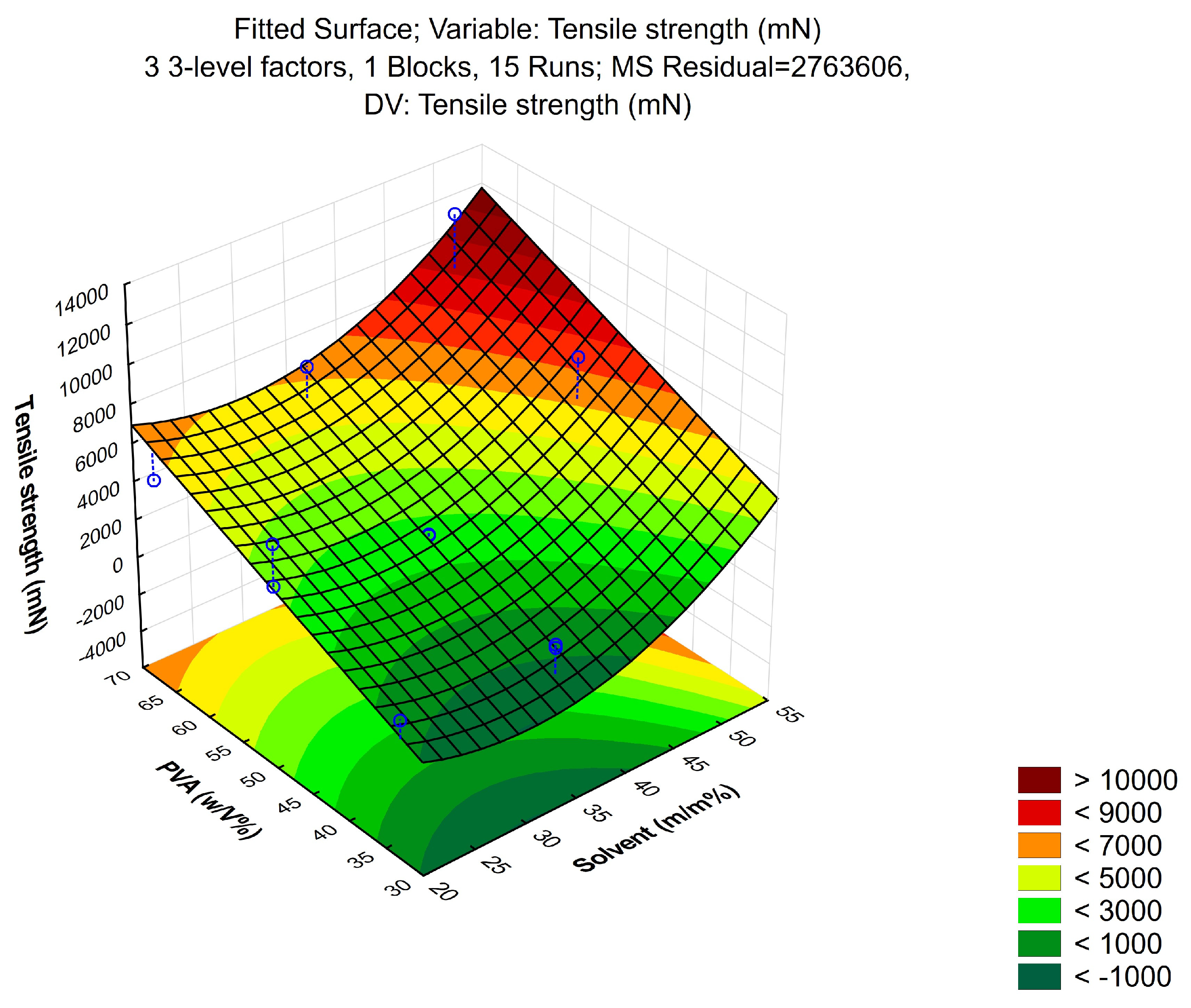
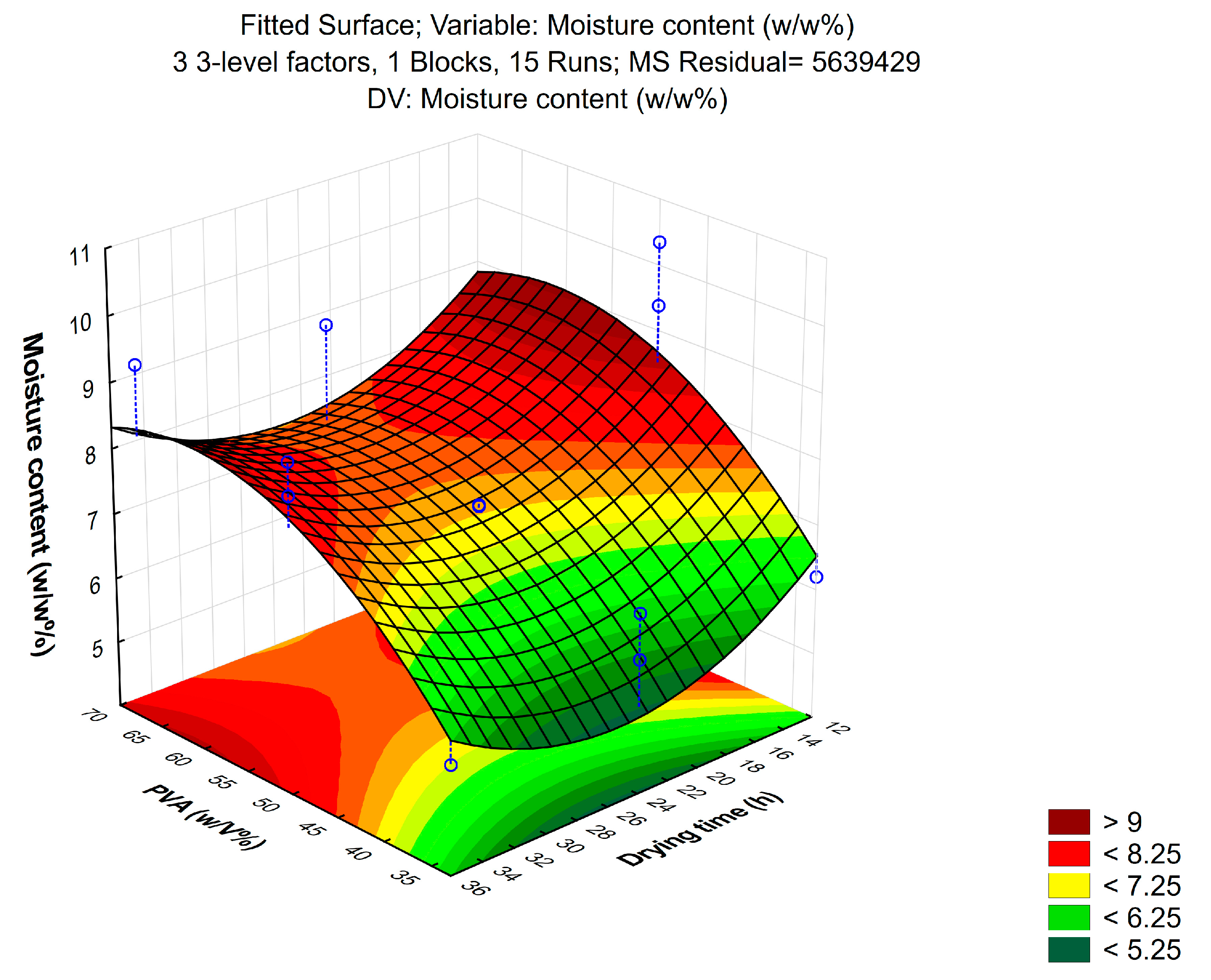

| Composition | Solvent (w/w%) | PVA (w/v%) | Drying Time (h) |
|---|---|---|---|
| 1 | 50 | 33 | 24 |
| 2 | 20 | 33 | 24 |
| 3 | 50 | 67 | 24 |
| 4 | 20 | 67 | 24 |
| 5 | 50 | 50 | 12 |
| 6 | 20 | 50 | 12 |
| 7 | 50 | 50 | 36 |
| 8 | 20 | 50 | 36 |
| 9 | 35 | 33 | 12 |
| 10 | 35 | 67 | 12 |
| 11 | 35 | 33 | 36 |
| 12 | 35 | 67 | 36 |
| 13 | 35 | 50 | 24 |
| 14 | 35 | 50 | 24 |
| 15 | 35 | 50 | 24 |
| Formulation | |
|---|---|
| W.P. | Transdermal patch without any penetration enhancer excipient |
| T | Transdermal patch with 0.1% Transcutol |
| L | Transdermal patch with 0.1% Labrasol |
| T + L | Transdermal patch with 0.1% Transcutol and Labrasol |
| Transdermal Patches | ||||
|---|---|---|---|---|
| Without Penetration Enhancers | With Transcutol | With Labrasol | With Transcutol and Labrasol | |
| Flux (mg/cm2 × h−1) | 0.3843 | 0.2007 | 2.0940 | 2.6324 |
| Kinetic Model 1 | Transdermal Patches | |||
|---|---|---|---|---|
| Without Penetration Enhancers | With Transcutol | With Labrasol | With Transcutol and Labrasol | |
| Zero | 0.8780 | 0.8107 | 0.8977 | 0.9772 |
| First | 0.8741 | 0.8100 | 0.8984 | 0.9857 |
| Korsmeyer–Peppas | 0.8871 | 0.6630 | 0.6253 | 0.9636 |
| Higuchi | 0.7494 | 0.7600 | 0.8668 | 0.9597 |
| Weibull | 0.9097 | 0.6624 | 0.6251 | 0.9578 |
| Formulation | f1 1 | f2 2 |
|---|---|---|
| W.P. vs. T. | 9.45 | 89.48 |
| W.P. vs. L. | 88.53 | 40.39 |
| W.P. vs. T and L. | 92.88 | 30.17 |
| L. vs. T and L. | 37.91 | 49.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bácskay, I.; Hosszú, Z.; Budai, I.; Ujhelyi, Z.; Fehér, P.; Kósa, D.; Haimhoffer, Á.; Pető, Á. Formulation and Evaluation of Transdermal Patches Containing BGP-15. Pharmaceutics 2024, 16, 36. https://doi.org/10.3390/pharmaceutics16010036
Bácskay I, Hosszú Z, Budai I, Ujhelyi Z, Fehér P, Kósa D, Haimhoffer Á, Pető Á. Formulation and Evaluation of Transdermal Patches Containing BGP-15. Pharmaceutics. 2024; 16(1):36. https://doi.org/10.3390/pharmaceutics16010036
Chicago/Turabian StyleBácskay, Ildikó, Zsolt Hosszú, István Budai, Zoltán Ujhelyi, Pálma Fehér, Dóra Kósa, Ádám Haimhoffer, and Ágota Pető. 2024. "Formulation and Evaluation of Transdermal Patches Containing BGP-15" Pharmaceutics 16, no. 1: 36. https://doi.org/10.3390/pharmaceutics16010036
APA StyleBácskay, I., Hosszú, Z., Budai, I., Ujhelyi, Z., Fehér, P., Kósa, D., Haimhoffer, Á., & Pető, Á. (2024). Formulation and Evaluation of Transdermal Patches Containing BGP-15. Pharmaceutics, 16(1), 36. https://doi.org/10.3390/pharmaceutics16010036











