Chitosan and Cyclodextrins—Versatile Materials Used to Create Drug Delivery Systems for Gastrointestinal Cancers
Abstract
:1. Introduction
2. Properties of Interest of the Carbohydrate Materials for Drug Delivery
2.1. Chitosan
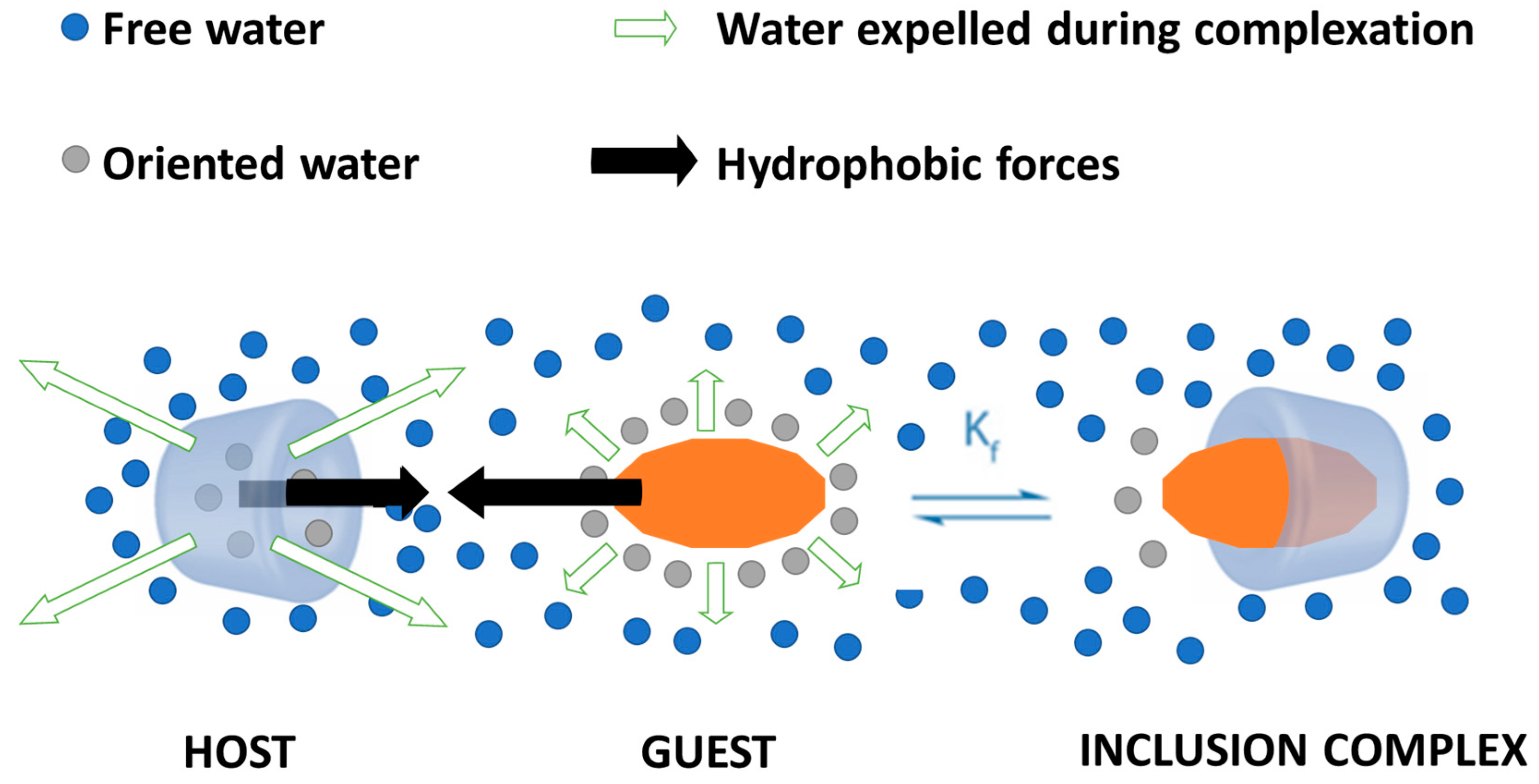
2.2. Cyclodextrins
3. Drug Delivery Systems Based on Chitosan/Cyclodextrins for the Treatment of Gastrointestinal Cancers
3.1. Colorectal Cancer
3.1.1. Drug Delivery Systems Based on Cyclodextrins
3.1.2. Drug Delivery Systems Based on Chitosan
3.2. Liver Cancer
3.2.1. Drug Delivery Systems Based on Cyclodextrins
3.2.2. Drug Delivery Systems Based on Chitosan
3.3. Gastric Cancer
3.4. Pancreatic Cancer
3.4.1. Drug Delivery Systems Based on Cyclodextrins
3.4.2. Drug Delivery Systems Based on Chitosan
3.5. Other Cancers of the Digestive System
3.6. Summative Discussion
4. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Qiu, N.; Li, X.; Liu, J. Application of cyclodextrins in cancer treatment. J. Incl. Phenom. Macrocycl. Chem. 2017, 89, 229–246. [Google Scholar] [CrossRef]
- Xie, Y.; Shi, L.; He, X.; Luo, Y. Gastrointestinal cancers in China, the USA, and Europe. Gastroenterol. Rep. 2021, 9, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lucero-Prisno, D.E.; Zhang, L.; Xu, W.; Wong, S.H.; Ng, S.C.; Wong, M.C.S. Updated epidemiology of gastrointestinal cancers in East Asia. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 271–287. [Google Scholar] [CrossRef] [PubMed]
- Bordry, N.; Astaras, C.; Ongaro, M.; Goossens, N.; Frossard, J.L.; Koessler, T. Recent advances in gastrointestinal cancers. World J. Gastroenterol. 2021, 27, 4493–4503. [Google Scholar] [CrossRef] [PubMed]
- Păduraru, D.N.; Niculescu, A.-G.; Bolocan, A.; Andronic, O.; Grumezescu, A.M.; Bîrlă, R. An Updated Overview of Cyclodextrin-Based Drug Delivery Systems for Cancer Therapy. Pharmaceutics 2022, 14, 1748. [Google Scholar] [CrossRef]
- Narmani, A.; Jafari, S.M. Chitosan-based nanodelivery systems for cancer therapy: Recent advances. Carbohydr. Polym. 2021, 272, 118464. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Chen, B. Combination of drugs and carriers in drug delivery technology and its development. Drug Des. Dev. Ther. 2019, 13, 1401–1408. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Brüßler, J.; Alawak, M.; El-Sayed, M.M.H.; Bakowsky, U.; Shoeib, T. Chemotherapy Based on Supramolecular Chemistry: A Promising Strategy in Cancer Therapy. Pharmaceutics 2019, 11, 292. [Google Scholar] [CrossRef]
- Rezaei, N.; Shahriari, M.; Mehrnejad, F. Recent developments of nanomedicine delivery systems for the treatment of pancreatic cancer. J. Drug Deliv. Sci. Technol. 2023, 79, 104042. [Google Scholar] [CrossRef]
- Asefi, Y.; Fahimi, R.; Ghorbian, S. Synergistic Effect of Vitamin C with Superparamagnetic Iron Oxide Nanoparticles for Inhibiting Proliferation of Gastric Cancer Cells. Biointerfaces Res. Appl. Chem. 2021, 12, 3215–3224. [Google Scholar]
- Souza, M.P.C.d.; Sábio, R.M.; Ribeiro, T.d.C.; Santos, A.M.d.; Meneguin, A.B.; Chorilli, M. Highlighting the impact of chitosan on the development of gastroretentive drug delivery systems. Int. J. Biol. Macromol. 2020, 159, 804–822. [Google Scholar] [CrossRef] [PubMed]
- Shafabakhsh, R.; Yousefi, B.; Asemi, Z.; Nikfar, B.; Mansournia, M.A.; Hallajzadeh, J. Chitosan: A compound for drug delivery system in gastric cancer-a review. Carbohydr. Polym. 2020, 242, 116403. [Google Scholar] [CrossRef] [PubMed]
- Alipanah, H.; Rasti, F.; Zarenezhad, E.; Dehghan, A.; Sahebnazar, B.; Osanloo, M. Comparison of anticancer effects of carvone, carvone-rich essential oils, and chitosan nanoparticles containing each of them. Biointerface Res. Appl. Chem. 2022, 12, 5716–5726. [Google Scholar]
- Rastogi, K.; Vashishtha, R.; Shaloo; Dan, S. Scientific Advances and Pharmacological Applications of Marine Derived-Collagen and Chitosan. Biointerface Res. Appl. Chem. 2022, 12, 3540–3558. [Google Scholar]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Choukaife, H.; Seyam, S.; Alallam, B.; Doolaanea, A.A.; Alfatama, M. Current advances in chitosan nanoparticles based oral drug delivery for colorectal cancer treatment. Int. J. Nanomed. 2022, 17, 3933–3966. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.; Wang, T.; Sun, M.; Chen, X.; Liu, Y. Advances and applications of chitosan-based nanomaterials as oral delivery carriers: A review. Int. J. Biol. Macromol. 2020, 154, 433–445. [Google Scholar] [CrossRef]
- Mallick, S.P.; Panda, S.P.; Gayatri, A.; Kunaal, Y.; Naresh, C.; Suman, D.K.; Samineni, J.; Siddiqui, N.; Singh, B.N. Chitosan oligosaccharide based hydrogel: An insight into the mechanical, drug delivery, and antimicrobial studies. Biointerface Res. Appl. Chem. 2021, 11, 10293–10300. [Google Scholar]
- Abirami, S.; Nagarajan, D.; Samrot, A.V.; Varsini, A.M.; Sugasini, A.; Anand, D.A. Extraction, characterization, and utilization of shrimp waste chitin derived chitosan in antimicrobial activity, seed germination, preservative, and microparticle formulation. Biointerface Res. Appl. Chem. 2021, 11, 8725–8739. [Google Scholar]
- Zaiki, Y.; Iskandar, A.; Wong, T.W. Functionalized chitosan for cancer nano drug delivery. Biotechnol. Adv. 2023, 67, 108200. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Strand, S.P.; Vårum, K.M.; Draget, K.I.; Nordgård, C.T. Chitosan: Gels and Interfacial Properties. Polymers 2015, 7, 552–579. [Google Scholar] [CrossRef]
- Abdelghany, A.M.; Meikhail, M.S.; Hegazy, E.; Badr, S.I.; Agag, D.A. Microbial activity and swelling behavior of chitosan/polyvinyl alcohol/sodium alginate seminatural terpolymer interface containing amoxicillin for wound dressing applications. Biointerface Res. Appl. Chem. 2019, 9, 4368–4373. [Google Scholar]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Ketabchi, N.; Dinarvand, R.; Adabi, M.; Gholami, M.; Firoozi, S.; Amanzadi, B.; Faridi-Majidi, R. Study of third-degree burn wounds debridement and treatment by actinidin enzyme immobilized on electrospun chitosan/PEO nanofibers in rats. Biointerface Res. Appl. Chem. 2021, 11, 10358–10370. [Google Scholar]
- Tiplea, R.E.; Lemnaru, G.-M.; Trusca, R.D.; Holban, A.; Kaya, M.G.A.; Dragu, L.D.; Ficai, D.; Ficai, A.; Bleotu, C. Antimicrobial films based on chitosan, collagen, and zno for skin tissue regeneration. Biointerface Res. Appl. Chem. 2021, 11, 11985–11995. [Google Scholar]
- Abdelghany, S.; Alkhawaldeh, M.; AlKhatib, H.S. Carrageenan-stabilized chitosan alginate nanoparticles loaded with ethionamide for the treatment of tuberculosis. J. Drug Deliv. Sci. Technol. 2017, 39, 442–449. [Google Scholar] [CrossRef]
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The use of chitosan, alginate, and pectin in the biomedical and food sector—biocompatibility, bioadhesiveness, and biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef]
- Ali, D.S.; Othman, H.O.; Anwer, E.T. The Advances in Chitosan-based Drug Delivery Systems for Colorectal Cancer: A Narrative Review. Curr. Pharm. Biotechnol. 2023, 24, 1554–1559. [Google Scholar] [CrossRef]
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-Based Nanoparticles as Effective Drug Delivery Systems—A review. Molecules 2023, 28, 1963. [Google Scholar] [CrossRef]
- Mikušová, V.; Mikuš, P. Advances in Chitosan-Based Nanoparticles for Drug Delivery. Int. J. Mol. Sci. 2021, 22, 9652. [Google Scholar] [CrossRef]
- Lim, C.; Hwang, D.S.; Lee, D.W. Intermolecular interactions of chitosan: Degree of acetylation and molecular weight. Carbohydr. Polym. 2021, 259, 117782. [Google Scholar] [CrossRef] [PubMed]
- Sadreddini, S.; Safaralizadeh, R.; Baradaran, B.; Aghebati-Maleki, L.; Hosseinpour-Feizi, M.A.; Shanehbandi, D.; Jadidi-Niaragh, F.; Sadreddini, S.; Kafil, H.S.; Younesi, V.; et al. Chitosan nanoparticles as a dual drug/siRNA delivery system for treatment of colorectal cancer. Immunol. Lett. 2017, 181, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Gounden, S.; Daniels, A.; Singh, M. Chitosan-modified silver nanoparticles enhance cisplatin activity in breast cancer cells. Biointerface Res. Appl. Chem. 2021, 11, 10572–10584. [Google Scholar]
- Bagheri-Khoulenjani, S.; Taghizadeh, S.M.; Mirzadeh, H. An investigation on the short-term biodegradability of chitosan with various molecular weights and degrees of deacetylation. Carbohydr. Polym. 2009, 78, 773–778. [Google Scholar] [CrossRef]
- Halavach, T.M.; Savchuk, E.S.; Bobovich, A.S.; Dudchik, N.V.; Tsygankow, V.G.; Tarun, E.I.; Yantsevich, A.V.; Kurchenko, V.P.; Kharitonov, V.D.; Asafov, V.A. Antimutagenic and Antibacterial Activity of beta-Cyclodextrin Clathrates with Extensive Hydrolysates of Colostrum and Whey. Biointerface Res. Appl. Chem. 2021, 11, 8626–8638. [Google Scholar] [CrossRef]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins inclusion complex: Preparation methods, analytical techniques and food industry applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef]
- Bakirhan, N.K.; Tok, T.T.; Ozkan, S.A. The redox mechanism investigation of non-small cell lung cancer drug: Erlotinib via theoretical and experimental techniques and its host–guest detection by β-Cyclodextrin nanoparticles modified glassy carbon electrode. Sens. Actuators B Chem. 2019, 278, 172–180. [Google Scholar] [CrossRef]
- Gadade, D.D.; Rathi, P.B.; Sangshetti, J.N.; Kulkarni, D.A. 18—Multifunctional cyclodextrin nanoparticles: A promising theranostic tool for strategic targeting of cancer. In Polysaccharide Nanoparticles; Venkatesan, J., Kim, S.-K., Anil, S., Rekha, P.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 485–515. [Google Scholar]
- Tian, B.; Hua, S.; Liu, J. Cyclodextrin-based delivery systems for chemotherapeutic anticancer drugs: A review. Carbohydr. Polym. 2020, 232, 115805. [Google Scholar] [CrossRef]
- Zhang, D.; Lv, P.; Zhou, C.; Zhao, Y.; Liao, X.; Yang, B. Cyclodextrin-based delivery systems for cancer treatment. Mater. Sci. Eng. C 2019, 96, 872–886. [Google Scholar] [CrossRef]
- Mousazadeh, H.; Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Zarghami, N. Cyclodextrin based natural nanostructured carbohydrate polymers as effective non-viral siRNA delivery systems for cancer gene therapy. J. Control. Release 2021, 330, 1046–1070. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Qiu, N. Cyclodextrin-Based Polymeric Drug Delivery Systems for Cancer Therapy. Polymers 2023, 15, 1400. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Gharibzahedi, S.M.T.; Jafari, S.M. 7—Nanocapsule formation by cyclodextrins. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Jafari, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 187–261. [Google Scholar]
- Leclercq, L. Smart medical textiles based on cyclodextrins for curative or preventive patient care. In Active Coatings for Smart Textiles; Elsevier: Amsterdam, The Netherlands, 2016; pp. 391–427. [Google Scholar]
- Sengupta, P.K.; Bhattacharjee, S.; Chakraborty, S.; Bhowmik, S. Encapsulation of pharmaceutically active dietary polyphenols in cyclodextrin-based nanovehicles: Insights from spectroscopic studies. In Design of Nanostructures for Versatile Therapeutic Applications; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; Chapter 15; pp. 623–645. [Google Scholar]
- Marabada, D.; Li, J.; Wei, S.; Huang, Q.; Wang, Z. Cyclodextrin based nanoparticles for smart drug delivery in colorectal cancer. Chem. Biol. Drug Des. 2023, 102, 1618–1631. [Google Scholar] [CrossRef] [PubMed]
- Adeoye, O.; Cabral-Marques, H. Cyclodextrin nanosystems in oral drug delivery: A mini review. Int. J. Pharm. 2017, 531, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Wankar, J.; Kotla, N.G.; Gera, S.; Rasala, S.; Pandit, A.; Rochev, Y.A. Recent Advances in Host–Guest Self-Assembled Cyclodextrin Carriers: Implications for Responsive Drug Delivery and Biomedical Engineering. Adv. Funct. Mater. 2020, 30, 1909049. [Google Scholar] [CrossRef]
- Sivakumar, M.P.; Peimanfard, S.; Zarrabi, A.; Khosravi, A.; Islami, M. Cyclodextrin-Based Nanosystems as Drug Carriers for Cancer Therapy. Anti-Cancer Agents Med. Chem. 2020, 20, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Kost, B.; Brzeziński, M.; Socka, M.; Baśko, M.; Biela, T. Biocompatible Polymers Combined with Cyclodextrins: Fascinating Materials for Drug Delivery Applications. Molecules 2020, 25, 3404. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, X.; Wang, K.; Lin, S.; Zhu, S.; Dai, Y.; Xia, F. Recent Advances in Cyclodextrin-Based Light-Responsive Supramolecular Systems. Macromol. Rapid Commun. 2018, 39, 1800142. [Google Scholar] [CrossRef]
- Zafar, N.; Fessi, H.; Elaissari, A. Cyclodextrin containing biodegradable particles: From preparation to drug delivery applications. Int. J. Pharm. 2014, 461, 351–366. [Google Scholar] [CrossRef]
- Muankaew, C.; Loftsson, T. Cyclodextrin-based formulations: A non-invasive platform for targeted drug delivery. Basic Clin. Pharmacol. Toxicol. 2018, 122, 46–55. [Google Scholar] [CrossRef]
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Fundamentals and applications of cyclodextrins. In Cyclodextrin Fundamentals, Reactivity and Analysis; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–55. [Google Scholar]
- Haley, R.M.; Gottardi, R.; Langer, R.; Mitchell, M.J. Cyclodextrins in drug delivery: Applications in gene and combination therapy. Drug Deliv. Transl. Res. 2020, 10, 661–677. [Google Scholar] [CrossRef]
- Carneiro, S.B.; Duarte, C.; Ílary, F.; Heimfarth, L.; Quintans, S.; de Souza, J.; Quintans-Júnior, L.J.; Veiga Júnior, V.F.d.; Neves de Lima, Á.A. Cyclodextrin–drug inclusion complexes: In vivo and in vitro approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, T. Cyclodextrins. Ullmann’s Encycl. Ind. Chem. 2000, 11, 23–31. [Google Scholar]
- Del Valle, E.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Leclercq, L. Interactions between cyclodextrins and cellular components: Towards greener medical applications? Beilstein J. Org. Chem. 2016, 12, 2644–2662. [Google Scholar] [CrossRef]
- Radu, C.-D.; Parteni, O.; Ochiuz, L. Applications of cyclodextrins in medical textiles. J. Control. Release 2016, 224, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.C.; Costa, D.; Ferreira, L.; Guerra, C.; Pereira-Silva, M.; Pereira, I.; Peixoto, D.; Ferreira, N.R.; Veiga, F. Cyclodextrin-based delivery systems for in vivo-tested anticancer therapies. Drug Deliv. Transl. Res. 2021, 11, 49–71. [Google Scholar] [CrossRef]
- Wong, K.E.; Ngai, S.C.; Chan, K.-G.; Lee, L.-H.; Goh, B.-H.; Chuah, L.-H. Curcumin nanoformulations for colorectal cancer: A review. Front. Pharmacol. 2019, 10, 152. [Google Scholar] [CrossRef]
- Deokar, S.; Shaikh, K. Exploring Cytotoxic Potential of Ciclopirox on Colorectal Cancer Cells by In-Silico Methodology. Biointerface Res. Appl. Chem. 2021, 12, 7287–7310. [Google Scholar]
- Al-Abboodi, A.S.; Al-Sheikh, W.a.M.; Eid, E.E.M.; Azam, F.; Al-Qubaisi, M.S. Inclusion complex of clausenidin with hydroxypropyl-β-cyclodextrin: Improved physicochemical properties and anti-colon cancer activity. Saudi Pharm. J. 2021, 29, 223–235. [Google Scholar] [CrossRef]
- Altoom, N.; Ibrahim, S.M.; Othman, S.I.; Allam, A.A.; Alqhtani, H.A.; Al-Otaibi, F.S.; Abukhadra, M.R. Characterization of β-cyclodextrin/phillipsite (β-CD/Ph) composite as a potential carrier for oxaliplatin as therapy for colorectal cancer; loading, release, and cytotoxicity. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129144. [Google Scholar] [CrossRef]
- Alfassam, H.E.; Al Othman, S.I.; Al-Waili, M.A.; Allam, A.A.; Abukhadra, M.R. Characterization of β-Cyclodextrin Hybridized Diatomite as Potential Delivery Systems of Oxaliplatin and 5-Fluorouracil Drugs; Equilibrium Modeling of Loading and Release Kinetics. J. Macromol. Sci. Part B 2023, 62, 478–503. [Google Scholar] [CrossRef]
- Akkın, S.; Varan, G.; Aksüt, D.; Malanga, M.; Ercan, A.; Şen, M.; Bilensoy, E. A different approach to immunochemotherapy for colon Cancer: Development of nanoplexes of cyclodextrins and Interleukin-2 loaded with 5-FU. Int. J. Pharm. 2022, 623, 121940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; You, X.; Luo, M.; Zhang, X.; Fang, Y.; Huang, H.; Kang, Y.; Wu, J. Poly(β-cyclodextrin)/platinum prodrug supramolecular nano system for enhanced cancer therapy: Synthesis and in vivo study. Carbohydr. Polym. 2022, 292, 119695. [Google Scholar] [CrossRef] [PubMed]
- Elamin, K.M.; Motoyama, K.; Higashi, T.; Yamashita, Y.; Tokuda, A.; Arima, H. Dual targeting system by supramolecular complex of folate-conjugated methyl-β-cyclodextrin with adamantane-grafted hyaluronic acid for the treatment of colorectal cancer. Int. J. Biol. Macromol. 2018, 113, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zou, Y.; Song, L.; Han, S.; Yang, H.; Chu, D.; Dai, Y.; Ma, J.; O’Driscoll, C.M.; Yu, Z.; et al. A cyclodextrin-based nanoformulation achieves co-delivery of ginsenoside Rg3 and quercetin for chemo-immunotherapy in colorectal cancer. Acta Pharm. Sin. B 2022, 12, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Xiao, F.; Song, L.; Sun, B.; Sun, D.; Chu, D.; Wang, L.; Han, S.; Yu, Z.; O’Driscoll, C.M.; et al. A folate-targeted PEGylated cyclodextrin-based nanoformulation achieves co-delivery of docetaxel and siRNA for colorectal cancer. Int. J. Pharm. 2021, 606, 120888. [Google Scholar] [CrossRef]
- Ünal, S.; Aktaş, Y.; Benito, J.M.; Bilensoy, E. Cyclodextrin nanoparticle bound oral camptothecin for colorectal cancer: Formulation development and optimization. Int. J. Pharm. 2020, 584, 119468. [Google Scholar] [CrossRef]
- Bai, H.; Wang, J.; Phan, C.U.; Chen, Q.; Hu, X.; Shao, G.; Zhou, J.; Lai, L.; Tang, G. Cyclodextrin-based host-guest complexes loaded with regorafenib for colorectal cancer treatment. Nat. Commun. 2021, 12, 759. [Google Scholar] [CrossRef]
- Ameli, H.; Alizadeh, N. Targeted delivery of capecitabine to colon cancer cells using nano polymeric micelles based on beta cyclodextrin. RSC Adv. 2022, 12, 4681–4691. [Google Scholar] [CrossRef]
- Hosseinifar, T.; Sheybani, S.; Abdouss, M.; Hassani Najafabadi, S.A.; Shafiee Ardestani, M. Pressure responsive nanogel base on Alginate-Cyclodextrin with enhanced apoptosis mechanism for colon cancer delivery. J. Biomed. Mater. Res. Part A 2018, 106, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.-J.; Nguyen, D.-T.; Kim, D.; Yoo, S.-Y.; Lee, S.M.; Lee, J.-Y.; Kim, D.-D. Tailoring renal-clearable zwitterionic cyclodextrin for colorectal cancer-selective drug delivery. Nat. Nanotechnol. 2023, 18, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Fai, T.K.; Yee, G.H.; Kumar, P.V.; Elumalai, M. Preparation of Chitosan Particles as a Delivery System for Tetrahydrocurcumin: β-cyclodextrin Inclusive Compound for Colorectal Carcinoma. Curr. Drug Ther. 2021, 16, 430–438. [Google Scholar] [CrossRef]
- Low, Z.X.; Teo, M.Y.; Nordin, F.J.; Dewi, F.R.; Palanirajan, V.K.; In, L.L. Biophysical Evaluation of Water-Soluble Curcumin Encapsulated in β-Cyclodextrins on Colorectal Cancer Cells. Int. J. Mol. Sci. 2022, 23, 12866. [Google Scholar] [CrossRef] [PubMed]
- Vukic, M.D.; Vukovic, N.L.; Popovic, S.L.; Todorovic, D.V.; Djurdjevic, P.M.; Matic, S.D.; Mitrovic, M.M.; Popovic, A.M.; Kacaniova, M.M.; Baskic, D.D. Effect of β-cyclodextrin encapsulation on cytotoxic activity of acetylshikonin against HCT-116 and MDA-MB-231 cancer cell lines. Saudi Pharm. J. 2020, 28, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Madni, A.; Shah, H.; Jan, N.; Shafiq, A.; Basit, A.; Rai, N.; Ali, A.; Khan, M.M. Folate decorated lipid chitosan hybrid nanoparticles of 5-fluorouracil for enhanced anticancer efficacy against colon cancer. Int. J. Biol. Macromol. 2022, 222, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Soe, Z.C.; Poudel, B.K.; Nguyen, H.T.; Thapa, R.K.; Ou, W.; Gautam, M.; Poudel, K.; Jin, S.G.; Jeong, J.-H.; Ku, S.K.; et al. Folate-targeted nanostructured chitosan/chondroitin sulfate complex carriers for enhanced delivery of bortezomib to colorectal cancer cells. Asian J. Pharm. Sci. 2019, 14, 40–51. [Google Scholar] [CrossRef]
- Almeida, A.; Castro, F.; Resende, C.; Lúcio, M.; Schwartz, S.; Sarmento, B. Oral delivery of camptothecin-loaded multifunctional chitosan-based micelles is effective in reduce colorectal cancer. J. Control. Release 2022, 349, 731–743. [Google Scholar] [CrossRef]
- Shirani-Bidabadi, S.; Mirian, M.; Varshosaz, J.; Tavazohi, N.; Sadeghi, H.M.M.; Shariati, L. Gene network analysis of oxaliplatin-resistant colorectal cancer to target a crucial gene using chitosan/hyaluronic acid/protamine polyplexes containing CRISPR-Cas9. Biochim. Biophys. Acta-Gen. Subj. 2023, 1867, 130385. [Google Scholar] [CrossRef]
- Tian, Z.; Wu, X.; Peng, L.; Yu, N.; Gou, G.; Zuo, W.; Yang, J. pH-responsive bufadienolides nanocrystals decorated by chitosan quaternary ammonium salt for treating colon cancer. Int. J. Biol. Macromol. 2023, 242, 124819. [Google Scholar] [CrossRef]
- Narayan, R.; Gadag, S.; Cheruku, S.P.; Raichur, A.M.; Day, C.M.; Garg, S.; Manandhar, S.; Pai, K.S.R.; Suresh, A.; Mehta, C.H.; et al. Chitosan-glucuronic acid conjugate coated mesoporous silica nanoparticles: A smart pH-responsive and receptor-targeted system for colorectal cancer therapy. Carbohydr. Polym. 2021, 261, 117893. [Google Scholar] [CrossRef] [PubMed]
- Hanna, D.H.; Hamed, A.A.; Saad, G.R. Synthesis and characterization of poly(3-hydroxybutyrate)/chitosan-graft poly (acrylic acid) conjugate hyaluronate for targeted delivery of methotrexate drug to colon cancer cells. Int. J. Biol. Macromol. 2023, 240, 124396. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Li, J.; Kong, M.; Liu, Y.; Cheng, X.J.; Li, Y.; Park, H.J.; Chen, X.G. Surface charge effect on mucoadhesion of chitosan based nanogels for local anti-colorectal cancer drug delivery. Colloids Surf. B Biointerfaces 2015, 128, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Bonde, S.; Hatware, K.; Sharma, S.; Anjum, M.M.; Sahu, R.K. Physicochemical characterization, in vitro and in vivo evaluation of chitosan/carrageenan encumbered with Imatinib mesylate-polysarcosine nanoparticles for sustained drug release and enhanced colorectal cancer targeted therapy. Int. J. Biol. Macromol. 2023, 245, 125529. [Google Scholar] [CrossRef] [PubMed]
- Sorasitthiyanukarn, F.N.; Ratnatilaka Na Bhuket, P.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan/alginate nanoparticles as a promising carrier of novel curcumin diethyl diglutarate. Int. J. Biol. Macromol. 2019, 131, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Leonard, T.E.; Liko, A.F.; Gustiananda, M.; Putra, A.B.N.; Juanssilfero, A.B.; Hartrianti, P. Thiolated pectin-chitosan composites: Potential mucoadhesive drug delivery system with selective cytotoxicity towards colorectal cancer. Int. J. Biol. Macromol. 2023, 225, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yusefi, M.; Shameli, K.; Lee-Kiun, M.S.; Teow, S.-Y.; Moeini, H.; Ali, R.R.; Kia, P.; Jie, C.J.; Abdullah, N.H. Chitosan coated magnetic cellulose nanowhisker as a drug delivery system for potential colorectal cancer treatment. Int. J. Biol. Macromol. 2023, 233, 123388. [Google Scholar] [CrossRef]
- Wu, D.; Li, Y.; Zhu, L.; Zhang, W.; Xu, S.; Yang, Y.; Yan, Q.; Yang, G. A biocompatible superparamagnetic chitosan-based nanoplatform enabling targeted SN-38 delivery for colorectal cancer therapy. Carbohydr. Polym. 2021, 274, 118641. [Google Scholar] [CrossRef]
- Wu, D.; Zhu, L.; Li, Y.; Wang, H.; Xu, S.; Zhang, X.; Wu, R.; Yang, G. Superparamagnetic chitosan nanocomplexes for colorectal tumor-targeted delivery of irinotecan. Int. J. Pharm. 2020, 584, 119394. [Google Scholar] [CrossRef]
- Sia, D.; Villanueva, A.; Friedman, S.L.; Llovet, J.M. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology 2017, 152, 745–761. [Google Scholar] [CrossRef]
- Anwanwan, D.; Singh, S.K.; Singh, S.; Saikam, V.; Singh, R. Challenges in liver cancer and possible treatment approaches. Biochim. Biophys. Acta-Rev. Cancer 2020, 1873, 188314. [Google Scholar] [CrossRef] [PubMed]
- Ruman, U.; Fakurazi, S.; Masarudin, M.J.; Hussein, M.Z. Nanocarrier-Based Therapeutics and Theranostics Drug Delivery Systems for Next Generation of Liver Cancer Nanodrug Modalities. Int. J. Nanomed. 2020, 15, 1437–1456. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Du, G.; Cui, Y.; Yu, R.; Hua, C.; Tian, W.; Zhang, Y. pH-sensitive doxorubicin-loaded polymeric nanocomplex based on β-cyclodextrin for liver cancer-targeted therapy. Int. J. Nanomed. 2019, 14, 1997–2010. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, Y.; Lei, M.; Qin, Y.; Wang, Z.; Chen, Z.; Zhang, L.; Zhu, Y. Development of oral curcumin based on pH-responsive transmembrane peptide-cyclodextrin derivative nanoparticles for hepatoma. Carbohydr. Polym. 2022, 277, 118892. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, Z.; Sun, W.; Yang, Y.; Jin, H.; Qiu, L.; Chen, J.; Chen, J. Co-responsive smart cyclodextrin-gated mesoporous silica nanoparticles with ligand-receptor engagement for anti-cancer treatment. Mater. Sci. Eng. C 2019, 103, 109831. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Xu, Y.; Li, Z.; Li, Q. Folic acid-modified β-cyclodextrin nanoparticles as drug delivery to load DOX for liver cancer therapeutics. Soft Mater. 2019, 17, 437–447. [Google Scholar] [CrossRef]
- Li, Y.-N.; Shi, X.; Sun, D.; Han, S.; Zou, Y.; Wang, L.; Yang, L.; Li, Y.; Shi, Y.; Guo, J.; et al. Delivery of melarsoprol using folate-targeted PEGylated cyclodextrin-based nanoparticles for hepatocellular carcinoma. Int. J. Pharm. 2023, 636, 122791. [Google Scholar] [CrossRef]
- Bognanni, N.; Viale, M.; Distefano, A.; Tosto, R.; Bertola, N.; Loiacono, F.; Ponassi, M.; Spinelli, D.; Pappalardo, G.; Vecchio, G. Cyclodextrin Polymers as Delivery Systems for Targeted Anti-Cancer Chemotherapy. Molecules 2021, 26, 6046. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Y.; Wang, J.; Li, H.; Yang, H. Glycyrrhetinic acid-cyclodextrin grafted pullulan nanoparticles loaded doxorubicin as a liver targeted delivery carrier. Int. J. Biol. Macromol. 2022, 216, 789–798. [Google Scholar] [CrossRef]
- Yang, W.; Xue, Y.; Cui, X.; Tang, H.; Li, H. Targeted delivery of doxorubicin to liver used a novel biotinylated β-cyclodextrin grafted pullulan nanocarrier. Colloids Surf. B Biointerfaces 2022, 220, 112934. [Google Scholar] [CrossRef]
- Daga, M.; de Graaf, I.A.M.; Argenziano, M.; Barranco, A.S.M.; Loeck, M.; Al-Adwi, Y.; Cucci, M.A.; Caldera, F.; Trotta, F.; Barrera, G.; et al. Glutathione-responsive cyclodextrin-nanosponges as drug delivery systems for doxorubicin: Evaluation of toxicity and transport mechanisms in the liver. Toxicol. Vitr. 2020, 65, 104800. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Cheng, R.; Gong, T.; Huang, Y.; Li, D.; Zhao, X.; Yu, B.; Su, D.; Song, Z.; Liang, W. β-Cyclodextrin-cholic acid-hyaluronic acid polymer coated Fe3O4-graphene oxide nanohybrids as local chemo-photothermal synergistic agents for enhanced liver tumor therapy. Colloids Surf. B Biointerfaces 2021, 199, 111510. [Google Scholar] [CrossRef] [PubMed]
- Ercan, A.; Çelebier, M.; Oncul, S.; Varan, G.; Kocak, E.; Benito, J.M.; Bilensoy, E. Polycationic cyclodextrin nanoparticles induce apoptosis and affect antitumoral activity in HepG2 cell line: An evaluation at the molecular level. Int. J. Pharm. 2021, 598, 120379. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.-L.; Zheng, R.; Ruan, X.-J.; Zheng, Z.-H.; Cai, H.-J. Chitosan-coated doxorubicin nano-particles drug delivery system inhibits cell growth of liver cancer via p53/PRC1 pathway. Biochem. Biophys. Res. Commun. 2018, 495, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wu, J.; Song, W.; Chen, L.; Zhang, S.; Ji, H.; Liu, J.; Gu, J. Thiolated chitosan nanoparticles for stable delivery and smart release of As2O3 for liver cancer through dual actions. Carbohydr. Polym. 2023, 303, 120462. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Hui, W.; Zhu, S.; He, J.; Liu, Z.; Cheng, J. Carboxymethyl chitosan based redox-responsive micelle for near-infrared fluorescence image-guided photo-chemotherapy of liver cancer. Carbohydr. Polym. 2021, 253, 117284. [Google Scholar] [CrossRef]
- Huang, M.; Liu, J.; Fan, Y.; Sun, J.; Cheng, J.-X.; Zhang, X.-F.; Zhai, B.-T.; Guo, D.-Y. Development of curcumin-loaded galactosylated chitosan-coated nanoparticles for targeted delivery of hepatocellular carcinoma. Int. J. Biol. Macromol. 2023, 253, 127219. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Ratnatilaka Na Bhuket, P.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan/alginate nanoparticles as a promising approach for oral delivery of curcumin diglutaric acid for cancer treatment. Mater. Sci. Eng. C 2018, 93, 178–190. [Google Scholar] [CrossRef]
- Yang, B.; Jiang, J.; Jiang, L.; Zheng, P.; Wang, F.; Zhou, Y.; Chen, Z.; Li, M.; Lian, M.; Tang, S.; et al. Chitosan mediated solid lipid nanoparticles for enhanced liver delivery of zedoary turmeric oil in vivo. Int. J. Biol. Macromol. 2020, 149, 108–115. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Y.; Li, Y.; Li, W.; Zhou, J.; Chen, J.; Shang, Z.; Gu, Q.; Wang, W.; Shen, T.; et al. Micelles modified with a chitosan-derived homing peptide for targeted intracellular delivery of ginsenoside compound K to liver cancer cells. Carbohydr. Polym. 2020, 230, 115576. [Google Scholar] [CrossRef]
- Harisa, G.I.; Badran, M.M.; AlQahtani, S.A.; Alanazi, F.K.; Attia, S.M. Pravastatin chitosan nanogels-loaded erythrocytes as a new delivery strategy for targeting liver cancer. Saudi Pharm. J. 2016, 24, 74–81. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Y.; Li, F.; Bei, S.; Pan, M.; Feng, L. Precise engineering of cholesterol-loaded chitosan micelles as a promising nanocarrier system for co-delivery drug-siRNA for the treatment of gastric cancer therapy. Process Biochem. 2022, 120, 265–274. [Google Scholar] [CrossRef]
- Afzal, A.; Qayyum, M.A.; Shah, M.H. Comparative assessment of trace elements in the blood of gastric cancer patients and healthy subjects. Biointerface Res. Appl. Chem. 2021, 11, 10824–10843. [Google Scholar]
- Gaur, S.; Chen, L.; Yen, T.; Wang, Y.; Zhou, B.; Davis, M.; Yen, Y. Preclinical study of the cyclodextrin-polymer conjugate of camptothecin CRLX101 for the treatment of gastric cancer. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 721–730. [Google Scholar] [CrossRef]
- Bandi, S.P.; Datta, D.; Venuganti, V.V.K. Hydrocaffeic acid-chitosan coating of gastric patch provides long-acting mucoadhesive delivery of model chemotherapeutic agent. Int. J. Pharm. 2023, 631, 122504. [Google Scholar] [CrossRef]
- Jiang, Z.; Chi, J.; Li, H.; Wang, Y.; Liu, W.; Han, B. Effect of chitosan oligosaccharide-conjugated selenium on improving immune function and blocking gastric cancer growth. Eur. J. Pharmacol. 2021, 891, 173673. [Google Scholar] [CrossRef]
- Zhang, E.; Xing, R.; Liu, S.; Li, K.; Qin, Y.; Yu, H.; Li, P. Vascular targeted chitosan-derived nanoparticles as docetaxel carriers for gastric cancer therapy. Int. J. Biol. Macromol. 2019, 126, 662–672. [Google Scholar] [CrossRef]
- Chi, J.; Jiang, Z.; Qiao, J.; Zhang, W.; Peng, Y.; Liu, W.; Han, B. Antitumor evaluation of carboxymethyl chitosan based norcantharidin conjugates against gastric cancer as novel polymer therapeutics. Int. J. Biol. Macromol. 2019, 136, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.; El-Kott, A.F.; El-Kenawy, A.E.; Xue, L. Decorated CuO nanoparticles over chitosan-functionalized magnetic nanoparticles: Investigation of its anti-colon carcinoma and anti-gastric cancer effects. Arab. J. Chem. 2021, 14, 103201. [Google Scholar] [CrossRef]
- Issarachot, O.; Bunlung, S.; Kaewkroek, K.; Wiwattanapatapee, R. Superporous hydrogels based on blends of chitosan and polyvinyl alcohol as a carrier for enhanced gastric delivery of resveratrol. Saudi Pharm. J. 2023, 31, 335–347. [Google Scholar] [CrossRef]
- Catchpole, O.; Mitchell, K.; Bloor, S.; Davis, P.; Suddes, A. Anti-gastrointestinal cancer activity of cyclodextrin-encapsulated propolis. J. Funct. Foods 2018, 41, 1–8. [Google Scholar] [CrossRef]
- Sadoughi, F.; Mansournia, M.A.; Mirhashemi, S.M. The potential role of chitosan-based nanoparticles as drug delivery systems in pancreatic cancer. IUBMB Life 2020, 72, 872–883. [Google Scholar] [CrossRef]
- Higashi, T.; Kogo, T.; Sato, N.; Hirotsu, T.; Misumi, S.; Nakamura, H.; Iohara, D.; Onodera, R.; Motoyama, K.; Arima, H. Efficient Anticancer Drug Delivery for Pancreatic Cancer Treatment Utilizing Supramolecular Polyethylene-Glycosylated Bromelain. ACS Appl. Bio Mater. 2020, 3, 3005–3014. [Google Scholar] [CrossRef]
- Fávaro, W.J.; dos Santos, M.M.; Pereira, M.M.; Garcia, P.V.; Durán, N. Effects of P-MAPA immunotherapy associated with gemcitabine on chemically-induced pancreatic cancer in animal model: New therapeutic perspectives. Biointerface Res. Appl. Chem. 2022, 12, 7540–7555. [Google Scholar]
- Kano, M.T.; Kokuryo, T.; Baba, T.; Yamazaki, K.; Yamaguchi, J.; Sunagawa, M.; Ogura, A.; Watanabe, N.; Onoe, S.; Miyata, K.; et al. Cyclodextrin Conjugated α-Bisabolol Suppresses FAK Phosphorylation and Induces Apoptosis in Pancreatic Cancer. Anticancer Res. 2023, 43, 1009–1016. [Google Scholar] [CrossRef]
- Iacobazzi, R.M.; Cutrignelli, A.; Stefanachi, A.; Porcelli, L.; Lopedota, A.A.; Di Fonte, R.; Lopalco, A.; Serratì, S.; Laquintana, V.; Silvestris, N.; et al. Hydroxy-Propil-β-Cyclodextrin Inclusion Complexes of two Biphenylnicotinamide Derivatives: Formulation and Anti-Proliferative Activity Evaluation in Pancreatic Cancer Cell Models. Int. J. Mol. Sci. 2020, 21, 6545. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Ghosh, H.; Covarrubias-Zambrano, O.; Jain, K.; Swamy, K.V.; Kasi, A.; Hamza, A.; Anant, S.; VanSaun, M.; Weir, S.J.; et al. Anticancer Activity of Novel Difluorinated Curcumin Analog and Its Inclusion Complex with 2-Hydroxypropyl-β-Cyclodextrin against Pancreatic Cancer. Int. J. Mol. Sci. 2023, 24, 6336. [Google Scholar] [CrossRef]
- Dora, C.P.; Trotta, F.; Kushwah, V.; Devasari, N.; Singh, C.; Suresh, S.; Jain, S. Potential of erlotinib cyclodextrin nanosponge complex to enhance solubility, dissolution rate, in vitro cytotoxicity and oral bioavailability. Carbohydr. Polym. 2016, 137, 339–349. [Google Scholar] [CrossRef]
- David, K.I.; Jaidev, L.R.; Sethuraman, S.; Krishnan, U.M. Dual drug loaded chitosan nanoparticles-sugar-coated arsenal against pancreatic cancer. Colloids Surf. B Biointerfaces 2015, 135, 689–698. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Xu, Q.; Xu, S.; Wen, J.; Yu, Z.; Yang, D. Folate-chitosan-gemcitabine core-shell nanoparticles targeted to pancreatic cancer. Chin. J. Cancer Res. 2013, 25, 527–535. [Google Scholar] [CrossRef]
- Zeng, X.; Zhu, X.; Tian, Q.; Tan, X.; Sun, N.; Yan, M.; Zhao, J.; Wu, X.; Li, R.; Zhang, Z.; et al. Celastrol-conjugated chitosan oligosaccharide for the treatment of pancreatic cancer. Drug Deliv. 2022, 29, 89–98. [Google Scholar] [CrossRef]
- Naeeni, N.B.; Tabrizi, M.H.; Karimi, E.; Ghafaripour, H. Synthesis and characterization of liposomal nanoparticles coated with chitosan–folate for efficient delivery of lawsone to pancreatic cancer cells. Polym. Bull. 2023, 1–13. [Google Scholar] [CrossRef]
- Thakkar, A.; Chenreddy, S.; Wang, J.; Prabhu, S. Ferulic acid combined with aspirin demonstrates chemopreventive potential towards pancreatic cancer when delivered using chitosan-coated solid-lipid nanoparticles. Cell Biosci. 2015, 5, 46. [Google Scholar] [CrossRef]
- Deng, L.; Zhu, X.; Yu, Z.; Li, Y.; Qin, L.; Liu, Z.; Feng, L.; Guo, R.; Zheng, Y. Novel T7-Modified pH-Responsive Targeted Nanosystem for Co-Delivery of Docetaxel and Curcumin in the Treatment of Esophageal Cancer. Int. J. Nanomed. 2020, 15, 7745–7762. [Google Scholar] [CrossRef]
- Su, M.; Ren, X.; Du, D.; He, H.; Zhang, D.; Xie, R.; Deng, X.; Zou, C.; Zou, H. Curcumol β-cyclodextrin inclusion complex enhances radiosensitivity of esophageal cancer under hypoxic and normoxic condition. Jpn. J. Radiol. 2023, 41, 1275–1289. [Google Scholar] [CrossRef]
- City of Hope Medical Center. Pilot Trial of CRLX101 in Treatment of Patients with Advanced or Metastatic Stomach, Gastroesophageal, or Esophageal Cancer That Cannot Be Removed by Surgery. 2018. Available online: https://www.clinicaltrials.gov/study/NCT01612546 (accessed on 6 November 2023).
- Hu, A.; Alarfaj, A.A.; Hirad, A.H.; Veeraraghavan, V.P.; Surapaneni, K.M.; Hussein-Al-Ali, S.H.; Natarajan, N.; Elayappan, P.K. Chitosan-sodium alginate-polyethylene glycol-crocin nanocomposite treatment inhibits esophageal cancer KYSE-150 cell growth via inducing apoptotic cell death. Arab. J. Chem. 2022, 15, 103844. [Google Scholar] [CrossRef]
- Mazzarino, L.; Loch-Neckel, G.; Bubniak, L.D.S.; Mazzucco, S.; Santos-Silva, M.C.; Borsali, R.; Lemos-Senna, E. Curcumin-Loaded Chitosan-Coated Nanoparticles as a New Approach for the Local Treatment of Oral Cavity Cancer. J. Nanosci. Nanotechnol. 2015, 15, 781–791. [Google Scholar] [CrossRef]
- Graciano, T.B.; Coutinho, T.S.; Cressoni, C.B.; Freitas, C.d.P.; Pierre, M.B.R.; de Lima Pereira, S.A.; Shimano, M.M.; Cristina da Cunha Frange, R.; Garcia, M.T.J. Using chitosan gels as a toluidine blue O delivery system for photodynamic therapy of buccal cancer: In vitro and in vivo studies. Photodiagnosis Photodyn. Ther. 2015, 12, 98–107. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Vinayagam, R.; Senthilkumar, V.; Paulpandi, M.; Murugan, K.; Xu, B.; Gothandam, K.M.; Kotakadi, V.S.; David, E. Phloretin loaded chitosan nanoparticles augments the pH-dependent mitochondrial-mediated intrinsic apoptosis in human oral cancer cells. Int. J. Biol. Macromol. 2019, 130, 997–1008. [Google Scholar] [CrossRef]
- Sarabia-Vallejo, Á.; Caja, M.D.; Olives, A.I.; Martín, M.A.; Menéndez, J.C. Cyclodextrin Inclusion Complexes for Improved Drug Bioavailability and Activity: Synthetic and Analytical Aspects. Pharmaceutics 2023, 15, 2345. [Google Scholar] [CrossRef]
- Liu, X.; Meng, H. Consideration for the scale-up manufacture of nanotherapeutics—A critical step for technology transfer. VIEW 2021, 2, 20200190. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Nanomaterials Synthesis through Microfluidic Methods: An Updated Overview. Nanomaterials 2021, 11, 864. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Scale-up polymeric-based nanoparticles drug delivery systems: Development and challenges. OpenNano 2022, 7, 100048. [Google Scholar] [CrossRef]





| Drug Delivery System | Carried Agent(s) | Main Observations | Ref. |
|---|---|---|---|
| Hydroxypropyl-β-cyclodextrin | Clausenidin | Greater cytotoxic effect on colon cancer HT-29 cells than the free drug Treated HT-29 cells displayed cell cycle arrest and death by apoptosis Reduced side effects | [65] |
| β-Cyclodextrin/phillipsite composite | Oxaliplatin | Greater cytotoxic effect on colon cancer HCT-116 cells than the free drug Controlled release behavior Safe in normal colorectal cells | [66] |
| Diatomite’s bio-siliceous frustules functionalized with polymeric chains of β-cyclodextrin | Oxaliplatin and 5-fluorouracil | Greater cytotoxic effect on colon cancer HCT-116 cells than free drugs Significant sustained and prolonged drug release | [67] |
| Cyclodextrin nanoplex | 5-fluorouracil and IL-2 | Greater cytotoxic effect on colon cancer CT-26 cells than the free drug solution Suitable intestinal permeability for oral administration | [68] |
| Supramolecular complex composed of [Pt(IV)-SSNPs] based on poly(β-cyclodextrin) | Adamantyl-functionalized platinum(IV) prodrug [Pt(IV)-ADA2] | Effective tumor accumulation and negligible cytotoxicity to major organs Greater cytotoxic effect on colon cancer CT-26 cells than the free drug solution Allows prodrug conversion to cisplatin in the reducing environment of the tumor tissue | [69] |
| Supramolecular complex composed of FA-M-β-CyD and adamantane-grafted hyaluronic acid | - | Greater cytotoxic effect on colon cancer HCT-116 cells than FA-M-β-CyD alone Efficient cellular internalization, resulting in mitophagy-mediated cell death Antiproliferative potential | [70] |
| Folate-targeted PEG-modified amphiphilic cyclodextrin nanoparticles | Rg3 and quercetin | Prolonged blood circulation Enhanced tumor targeting in a colorectal cancer mouse model Lengthened animals survival in combination with anti-PD-L1 | [71] |
| Amphiphilic cationic cyclodextrin nanoparticles modified with PEGylated folate | Docetaxel and siRNA | Significantly retarded tumoral growth Enhanced apoptotic activity of docetaxel with downregulation of RelA expression | [72] |
| Nanoparticles made of two different amphiphilic cyclodextrins coated with polyethylenimine or chitosan | Camptothecin | Greater cytotoxic effect on colon cancer HT29 cells than the free drug Enhanced Caco-2 cell permeability Significantly higher mucosal penetration than the free drug | [73] |
| Channel-type nanoparticles made of mannose-modified γ-cyclodextrin | Regorafenib | Attenuates inflammation and inhibits TAM activation Suppresses tumor cell proliferation and lesion neovascularization, and remodels the TME | [74] |
| Acrylic/maleic copolymer combined with β-cyclodextrin | Capecitabine | pH-responsive delivery system Targeted and controlled drug release | [75] |
| Alginate-based hydrogel crosslinked with modified β-cyclodextrin | 5-Fluorouracil | Greater cytotoxic effect on colon cancer HT-29 cells than the free drug High and rapid accumulation in tumor cells, resulting in apoptosis | [76] |
| PBA-(ZW)-CD | Doxorubicin and ulixertinib | Enhanced tumor accumulation compared to free drugs Improved antitumor efficacy in heterotopic and orthotopic colorectal cancer models Tumor penetration comparable to free drugs | [77] |
| β-Cyclodextrin loaded in chitosan particles | Tetrahydrocurcumin | Immediate cellular uptake in colon cancer Caco-2 cells Displayed a dose-dependent cytotoxic activity | [78] |
| β-Cyclodextrin | Curcumin | Greater cytotoxic effect on SW480 and HCT-116 cells than free curcumin Decreased cancer cell viability, migration rates, and invasion rates Increased apoptosis rates by caspase 3 activation Improved water dispersibility | [79] |
| β-Cyclodextrin | Acetylshikonin | Greater cytotoxic effect on HCT-116 and MDA-MB-231 cells than the free therapeutic agent More pronounced cell cycle arrest and autophagy inhibition Increased accumulation of intracellular ROS | [80] |
| Drug Delivery System | Carried Agent(s) | Main Observations | Ref. |
|---|---|---|---|
| Folate-decorated lipid chitosan hybrid nanoparticles | 5-Fluorouracil | Greater cytotoxic effect on colon cancer HT-29 and HCT-116 cells than the free drug Enhanced cellular uptake Biphasic release pattern: initial burst release followed by a sustained release for 48 h | [81] |
| Folic acid-conjugated chitosan/chondroitin sulfate self-assembled nanoparticles | Bortezomib | Enhanced cellular uptake and apoptosis in folate receptor-expressing colorectal cancer cells than in lung cancer cells pH-dependent release profile | [82] |
| Micelles made of amphiphilic chitosan modified with PEG and oleic acid | Camptothecin | Significant anticancer effects against HCT-116, Caco-2, and HT-29 cells Considerable reduction of tumor incidence and inflammation signs Safety profile for normal tissues | [83] |
| Chitosan-hyaluronic acid-protamine sulfate polyplexes | CRISPR/Cas9 | Efficient gene delivery to HT-29 cells Downregulated ERCC1 and restored drug sensitivity in oxaliplatin-resistant cells | [84] |
| Carboxymethyl dextran–chitosan nanoparticles | Doxorubicin and siRNA | Induced apoptosis and inhibited migration of HCT-116 cells Significantly modified EMT gene expression | [32] |
| Chitosan quaternary ammonium salt | Bufadienolide nanocrystals | Effective targeting of intestinal sites Antitumor activity through Caspase-3 and Bax/Bcl-2 ratio pathways Significant apoptosis induction Enhanced ROS generation | [85] |
| Mesoporous silica nanoparticles capped with chitosan–glucuronic acid | Capecitabine | Higher uptake in HCT-116 cells Reduction in tumors, aberrant crypt foci, dysplasia, and inflammation Alleviation of toxic features | [86] |
| Poly(3-hydroxybutyrate)/chitosan-graft poly (acrylic acid) conjugated with sodium hyaluronate | Methotrexate | Greater cytotoxic effect on colon cancer Caco-2 cells than the free drug Enhanced ROS generation Increased apoptosis rates and elevated levels of DNA breakage inside tumor cells | [87] |
| Nanogels made of chitosan and carboxymethyl chitosan | Doxorubicin | Effective cellular internalization in colorectal cancer cells Prolonged contact time of the formulation onto the intestinal mucosa and an improved local drug concentration | [88] |
| Chitosan–carrageenan nanoparticles | Imatinib mesylate-poly sarcosine | Great potential for active targeting Promising for reducing the dose-dependent toxicity of carried freight | [89] |
| Chitosan–alginate nanoparticles | Curcumin diethyl diglutarate | Significantly enhanced stability, digestibility, bioaccessibility, and cellular uptake in Caco-2 cells | [90] |
| Chitosan–thiolated pectin composite | 5-Fluorouracil | Targeted cytotoxicity towards HT-29 colorectal cells with milder cytotoxicity towards normal HEK-293 cells | [91] |
| Chitosan-coated magnetic cellulose nanowhiskers | 5-Fluorouracil | Appropriate physicochemical properties to ensure a high tumor-penetrating capacity pH-dependent swelling and drug release performance Potent killing effects against colorectal cancer cells | [92] |
| Superparamagnetic chitosan-based nanocomplexes | Poly(L-glutamic acid)-SN-38 prodrug | Significant enhancement of tumor-targeted accumulation and cellular uptake Superior targeting and antitumor efficacy in colorectal cancer model mice | [93] |
| Chitosan-based polyelectrolyte complexes embedded with superparamagnetic nanoparticles | Irinotecan | Greater anti-colon cancer cell efficacy than the free drug Effective internalization by colon tumor cells Favorable tumor-targeting ability under the guidance of a magnetic field | [94] |
| Drug Delivery System | Carried Agent(s) | Main Observations | Ref. |
|---|---|---|---|
| β-Cyclodextrin | Benzimidazole | pH-sensitive drug release with efficient uptake by HepG2 cells Inhibited liver cell proliferation through apoptosis induction | [98] |
| Cyclodextrin derivative (R6H4-CMβCD)-based nanoparticles | Curcumin | pH-sensitive drug release with efficient uptake by hepatoma cells High apoptosis rates in targeted cells with excellent anticancer effects | [99] |
| β-Cyclodextrin-gated mesoporous nanoparticles functionalized with an azobenzene/galactose-grafted polymer | Doxorubicin | Redox-sensitive drug release accelerated under UV irradiation Enhanced cytotoxicity to HepG2 cells compared to HeLa and COS7 cells | [100] |
| Folic acid–polyethylene glycol–β-cyclodextrin nanoparticles | Doxorubicin | Targeted and controlled medicine release to HepG2 cells Good encapsulation efficiency, blood compatibility, enhanced drug solubility | [101] |
| Folate-targeted polyethylene glycol-modified amphiphilic cyclodextrin nanoparticles | Melarsoprol | Achieved cell-specific uptake, cytotoxicity, apoptosis, and migration inhibition in targeted cells Prolonged the survival of mice with orthotopic tumors without causing side toxicity | [102] |
| Cross-linked γ- and β-cyclodextrin polymers | Doxorubicin and oxaliplatin | Greater cytotoxic effect on cancer cells than the free drugs Higher accumulation of the chemotherapeutic inside the cells | [103] |
| Glycyrrhetinic acid–β-cyclodextrin-grafted pullulan nanoparticles | Doxorubicin | High cellular uptake, with significant drug accumulation in the liver and a decreased concentration in the heart and kidneys Slow drug release Better therapeutic outcomes than the free drug | [104] |
| Biotinylated β-cyclodextrin-grafted pullulan | Doxorubicin | High cellular uptake, with significant drug accumulation in the liver and a decreased concentration in the heart and kidneys Sustained drug release Inhibited tumor cell growth | [105] |
| GSH-responsive cyclodextrin-based nanosponges | Doxorubicin | Comparable cytotoxicity and hepatic accumulation to the free drug Contribute to overcoming drug resistance by being taken up by tumor cells through an active mechanism and escaping the efflux drug pump | [106] |
| Magnetite–graphene oxide coated with a β-cyclodextrin–cholic acid–hyaluronic acid polymer | Camptothecin | Strong antitumor effect Induced local hyperthermia that produced tumor cell apoptosis | [107] |
| Polycationic amphiphilic β-cyclodextrin nanoparticles | - | Induced apoptosis and the lowered cell proliferation rate of HepG2 cells Hindered multidrug resistance | [108] |
| Drug Delivery System | Carried Agent(s) | Main Observations | Ref. |
|---|---|---|---|
| Chitosan and folic acid-functionalized chitosan nanoparticles | Doxorubicin | Inhibited tumor cell growth by promoting apoptosis and arresting cell cycle at G2/M phase through the p53/PRC1 pathway | [109] |
| Multifunctional thiolated chitosan derivatives | Arsenic trioxide | Glutathione-sensitive drug release Significantly improved tumor intracellular accumulation of the carried drug High tumor inhibition rate in mice with liver cancer | [110] |
| Micelles based on poly-ε-caprolactone linked to carboxymethyl chitosan through a disulfide bond and functionalized with glycyrrhetinic acid | Doxorubicin and pheophorbide A | Redox-responsive drug release Enhanced intracellular uptake by HepG2 cells Synergistic activity with the carried drugs Enhanced near-infrared imaging performance | [111] |
| Galactosylated chitosan-modified nanoparticles based on PEG-PLGA | Curcumin | Effectively internalized by HepG2 cells Greater inhibition of tumor growth than free curcumin | [112] |
| Chitosan/alginate nanoparticles | Curcumin diglutaric acid | Slow cumulative release of the carried agent in simulated gastrointestinal fluids without enzymes and in body fluid Better anticancer activity against Caco-2, HepG2, and MDA-MB-231 cells compared to the free drug | [113] |
| Chitosan-coated solid lipid nanoparticles | Zedoary turmeric oil | Significantly improved bioavailability Higher liver accumulation than uncoated particles Chitosan coating enhanced the internalization of particles by cells due to charge attraction | [114] |
| Micelles made of deoxycholic acid–O-carboxymethyl chitosan and A54 peptide | Ginsenoside compound K | pH-responsive and sustained drug release Greater cytotoxic effect on colon cancer HepG2 and Huh-7 cells than the free drug Promoted protein expression levels of caspase-3, caspase-9, and poly (ADP-ribose) polymerase | [115] |
| Erythrocytes loaded with chitosan nanogels | Pravastatin | Sustained drug release over 48 h Reduced the cellular viability of HepG2 cells | [116] |
| Drug Delivery System | Carried Agent(s) | Main Observations | Ref. |
|---|---|---|---|
| Cyclodextrin-based polymer nanoparticles | Camptothecin | High in vitro cytotoxicity and strong antitumor activity in vivo Considerably decreased carbonic anhydrase, VEGF, and CD31 protein expression | [119] |
| Cholesterol-loaded chitosan nanoparticles | Salinomycin and siRNA | Superior in vitro cytotoxicity against two gastric carcinoma cells (i.e., SNU-668 and SGC-791) No significant adverse effects | [117] |
| Chitosan–hydrocaffeic acid conjugate gastric patch | Regorafenib | Sustained drug release for 8 days after oral administration Significant reduction in the tumor volume over 7 days | [120] |
| Chitosan oligosaccharide | Selenium | Effectively elevated phagocytosis and increased the secretion of anti-inflammatory cytokines in mouse peritoneal macrophages Possessed a significant immuno-enhancing effect with no cytotoxicity | [121] |
| N-deoxycholic acid–glycol chitosan functionalized with GX1–PEG–deoxycholic acid | Docetaxel | Sustained drug release accelerated by an acidic pH | [122] |
| Carboxymethyl chitosan | Norcantharidin | Upregulated the expression of TNF-α and Bax Downregulated the expression of VEGF, Bcl-2, MMP-2, and MMP-9 Enhanced antitumor efficacy against SGC-7901 cells, inhibiting tumor metastasis and inducing apoptosis in vivo | [123] |
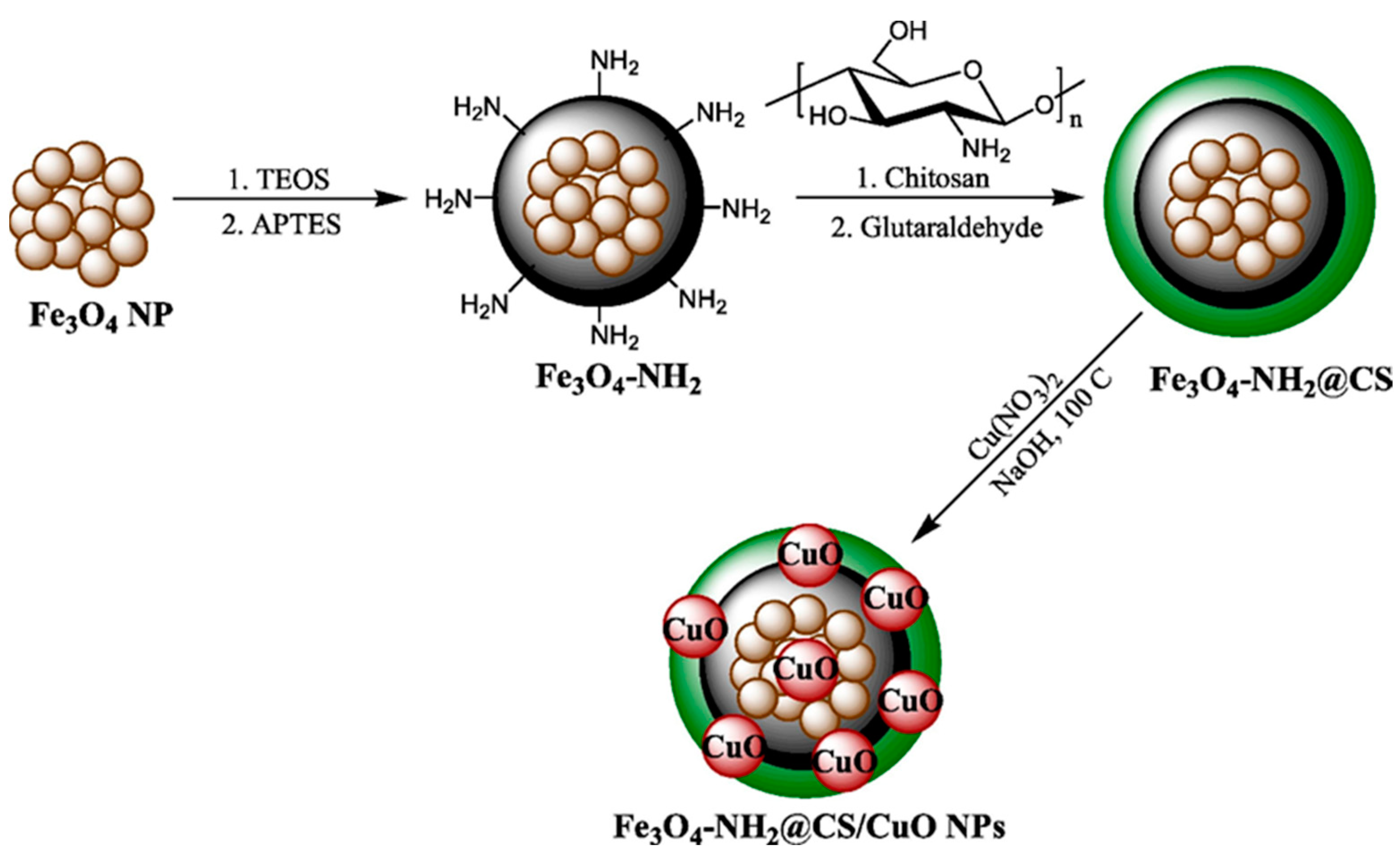
| Chitosan-modified amino-magnetic nanoparticles | Copper oxide nanoparticles | Very low cell viability of human gastric and colorectal carcinoma cell lines | [124] |
| Superporous hydrogels made of chitosan–PVA blends | Resveratrol solid dispersion | Efficient drug release, sustained over 12 h Exhibited anti-inflammatory activity | [125] |
| α-, ß-, and γ-Cyclodextrins | New Zealand propolis | Inhibited the proliferation of four human gastro-intestinal cancer cell lines (i.e., DLD-1, HCT-116, NCI-N87, and KYSE-30) Strongly anti-inflammatory in vitro Strong lipid antioxidant activity | [126] |
| Drug Delivery System | Carried Agent(s) | Main Observations | Ref. |
|---|---|---|---|
| Cyclodextrin | α-Bisabolol | Considerable changes in the cytomorphology of pancreatic tumor cells Reduced tumor volume and lower Ki67-positive cells Induced tumor cell apoptosis and suppressed the phosphorylation of focal adhesion kinase | [130] |
| Hydroxy-propyl-β-cyclodextrin | PTA34 and PTA73 | High antitumor activity towards PDAC cells Strong G2/M phase arrest followed by the induction of apoptosis | [131] |
| Hydroxy-propyl-β-cyclodextrin | Difluorinated curcumin | Inhibited colony and spheroid formation Induced cell cycle and apoptosis in PDAC cell lines | [132] |
| Multisubstituted-PEGylated β-cyclodextrins | Adamantane-modified bromelain | High antitumor activity due to long blood retention and increased tumor accumulation Enhancer of the anticancer effects of conventional chemotherapeutics | [128] |
| β-Cyclodextrin nanosponges | Erlotinib | Higher intracellular uptake and cytotoxicity in MIA PaCa-2 and PANC-1 cells compared to the free drug Reduced dose-related side effects | [133] |
| Drug Delivery System | Carried Agent(s) | Main Observations | Ref. |
|---|---|---|---|
| Chitosan nanoparticles | Quercetin and 5-fluorouracil | Significant toxicity towards MIA PaCa2 pancreatic cancer cells | [134] |
| Folate-functionalized chitosan nanoparticles | Gemcitabine | Better absorption rate than nonfunctionalized carriers Preferential accumulation in human pancreatic cancer xenografts Significant inhibition of COLO357 cell proliferation | [135] |
| Chitosan oligosaccharide | Celastrol | Significantly inhibited tumor growth, induced apoptosis, and suppressed tumor metastasis of pancreatic cancer Lowered hepatic cytotoxicity | [136] |
| Liposomal nanoparticles coated with chitosan–folate | Lawsone | Strong free radical scavenging properties Significant inhibition of pancreatic cancer cell proliferation Increased cellular uptake Upregulated the Caspase 3, 9, and Bax genes | [137] |
| Chitosan-coated solid lipid nanoparticles | Ferulic acid and aspirin | Significantly reduced cell viability in MIA PaCa-2 and Panc-1 cells | [138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najm, A.; Niculescu, A.-G.; Bolocan, A.; Rădulescu, M.; Grumezescu, A.M.; Beuran, M.; Gaspar, B.S. Chitosan and Cyclodextrins—Versatile Materials Used to Create Drug Delivery Systems for Gastrointestinal Cancers. Pharmaceutics 2024, 16, 43. https://doi.org/10.3390/pharmaceutics16010043
Najm A, Niculescu A-G, Bolocan A, Rădulescu M, Grumezescu AM, Beuran M, Gaspar BS. Chitosan and Cyclodextrins—Versatile Materials Used to Create Drug Delivery Systems for Gastrointestinal Cancers. Pharmaceutics. 2024; 16(1):43. https://doi.org/10.3390/pharmaceutics16010043
Chicago/Turabian StyleNajm, Alfred, Adelina-Gabriela Niculescu, Alexandra Bolocan, Marius Rădulescu, Alexandru Mihai Grumezescu, Mircea Beuran, and Bogdan Severus Gaspar. 2024. "Chitosan and Cyclodextrins—Versatile Materials Used to Create Drug Delivery Systems for Gastrointestinal Cancers" Pharmaceutics 16, no. 1: 43. https://doi.org/10.3390/pharmaceutics16010043







