An Improved In Vitro Blood-Brain Barrier Model for the Evaluation of Drug Permeability Using Transwell with Shear Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture
2.3. Culture for Transwell Inserts
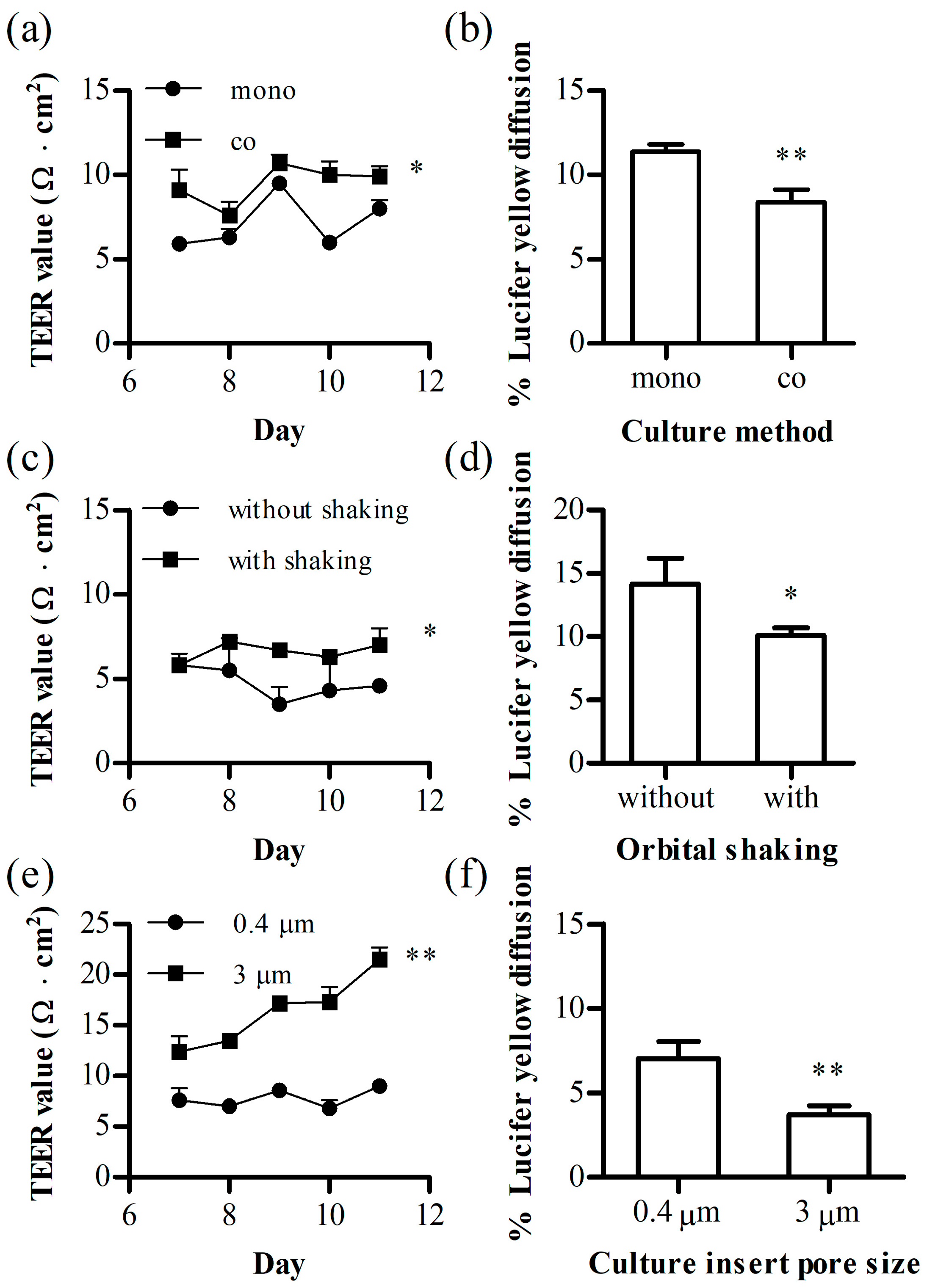
2.4. Optimization of the Transwell Model
2.4.1. Trans-Endothelial Electrical Resistance (TEER)
2.4.2. Lucifer Yellow (LY) Diffusion
- LYBL is the concentration of LY in the basolateral receiver chamber (μM)
- VAP is the volume in the apical receiver chamber (cm3)
- LYAP is the concentration of LY added to the apical donor chamber (μM)
- VBL is the volume in the basolateral donor chamber (cm3)
2.4.3. Rhodamine 123 Efflux
- A is the surface area of the insert filter membrane (cm2)
- C0 is the initial concentration in the donor solution (μM)
- dQ/dt is the amount of the drug transported within a given time period (pmol/s)
2.5. Quantitative Real-Time PCR
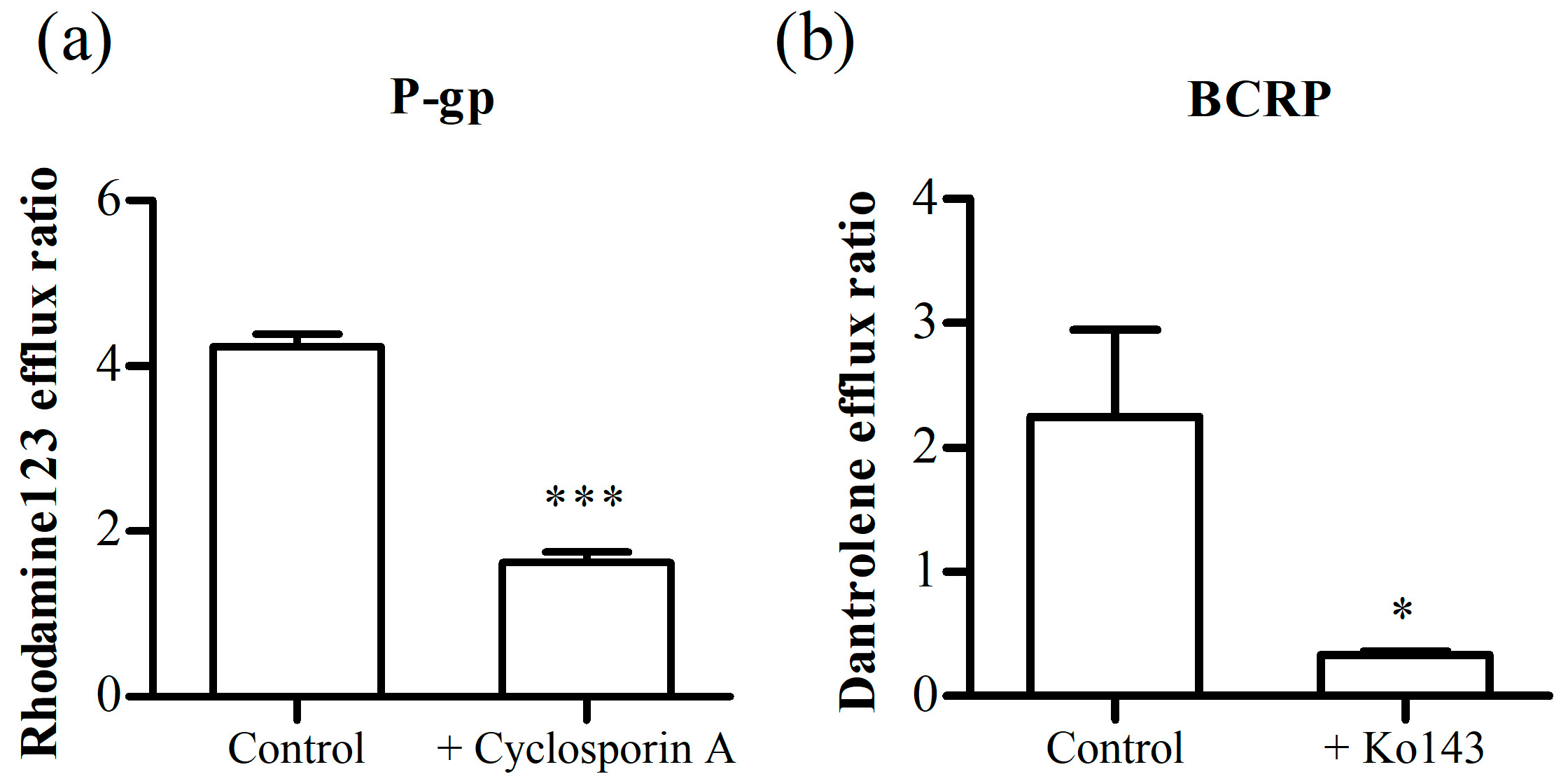
2.6. Efflux Transporter (P-gp, BCRP) Inhibition Assay
2.7. Permeability Test
2.8. Annular Shaking Dish (AS)
2.9. LC-MS/MS
2.10. Statistics
3. Results
3.1. Optimization of the 24-Transwell Model
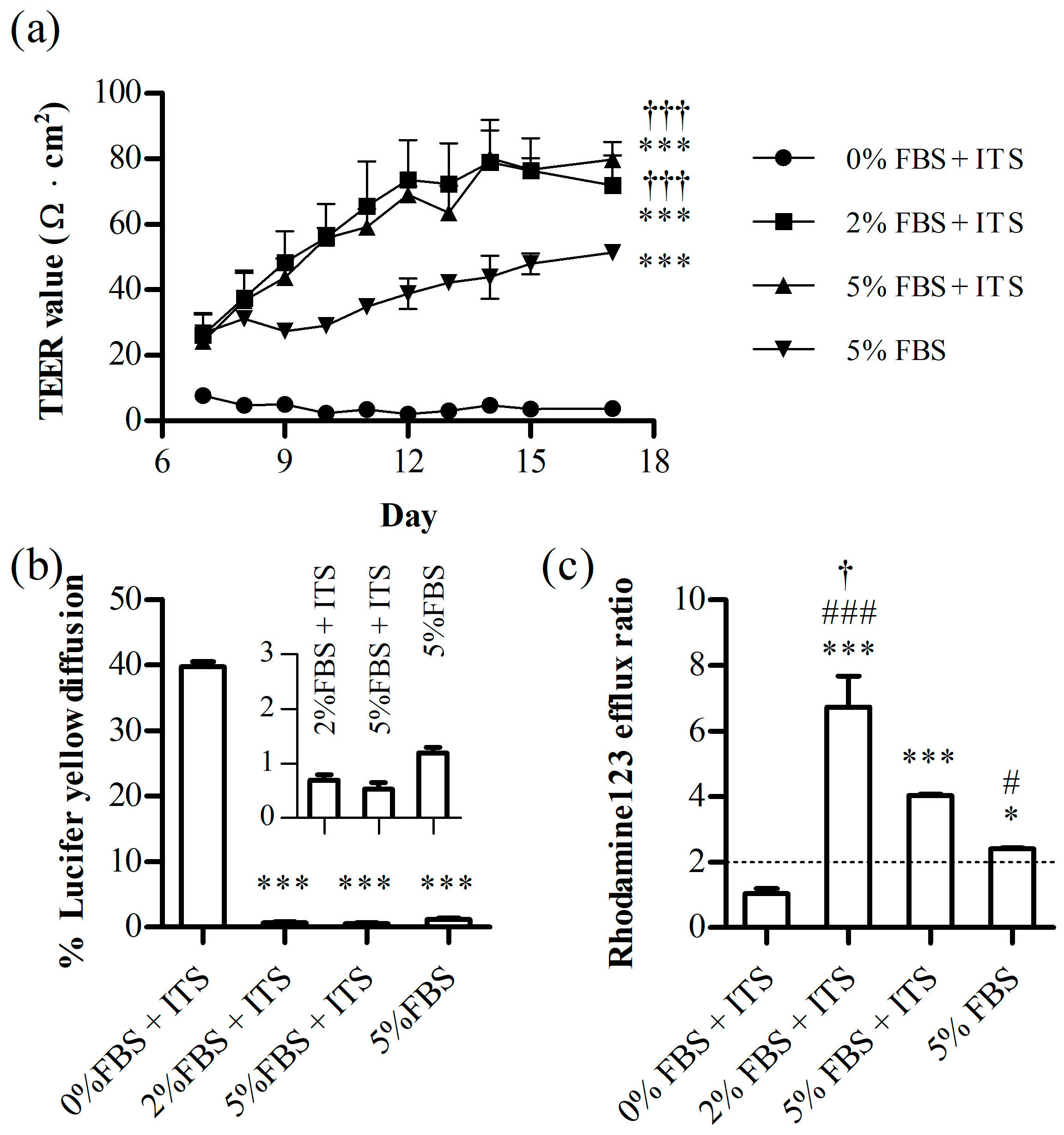
3.2. Annular Shaking Dish (AS)
3.2.1. AS Effect on Cell Layer Integrity
- a: the radius of orbital rotation (1.9 cm)
- ρ: the density of the medium (g/mL)
- η: the viscosity of the medium (7.78 × 10−3 dyn·s/cm2)
- f: the frequency of rotation (rotations/s)
3.2.2. ITS and FBS Effects on the Cell Layer Integrity
3.3. The Evaluation of the Optimized Annular Shaking Dish (AS) BBB Model
3.3.1. mRNA Levels of the Junctional Proteins
3.3.2. Inhibition Assay of P-gp and BCRP
3.3.3. Permeability Assay
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Take, C. 20% Longer to Develop and to Approve vs. Non-CNS Drugs. Tufts CSDD Impact Rep. 2018, 20, 5–8. [Google Scholar]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood-brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- Van Norman, G.A. Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Part 2: Potential Alternatives to the Use of Animals in Preclinical Trials. JACC Basic Transl. Sci. 2020, 5, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Dowden, H.; Munro, J. Trends in clinical success rates and therapeutic focus. Nat. Rev. Drug Discov. 2019, 18, 495–496. [Google Scholar] [CrossRef]
- Sharma, A.; Fernandes, D.C.; Reis, R.L.; Golubczyk, D.; Neumann, S.; Lukomska, B.; Janowski, M.; Kortylewski, M.; Walczak, P.; Oliveira, J.M.; et al. Cutting-edge advances in modeling the blood-brain barrier and tools for its reversible permeabilization for enhanced drug delivery into the brain. Cell Biosci. 2023, 13, 137. [Google Scholar] [CrossRef]
- Chaulagain, B.; Gothwal, A.; Lamptey, R.N.L.; Trivedi, R.; Mahanta, A.K.; Layek, B.; Singh, J. Experimental Models of In Vitro Blood-Brain Barrier for CNS Drug Delivery: An Evolutionary Perspective. Int. J. Mol. Sci. 2023, 24, 2710. [Google Scholar] [CrossRef]
- Shah, B.; Dong, X. Current Status of In vitro Models of the Blood-brain Barrier. Curr. Drug Deliv. 2022, 19, 1034–1046. [Google Scholar] [CrossRef]
- Perez-Lopez, A.; Torres-Suarez, A.I.; Martin-Sabroso, C.; Aparicio-Blanco, J. An overview of in vitro 3D models of the blood-brain barrier as a tool to predict the in vivo permeability of nanomedicines. Adv. Drug Deliv. Rev. 2023, 196, 114816. [Google Scholar] [CrossRef]
- Hajal, C.; Offeddu, G.S.; Shin, Y.; Zhang, S.; Morozova, O.; Hickman, D.; Knutson, C.G.; Kamm, R.D. Engineered human blood-brain barrier microfluidic model for vascular permeability analyses. Nat. Protoc. 2022, 17, 95–128. [Google Scholar] [CrossRef]
- Kurosawa, T.; Sako, D.; Tega, Y.; Debori, Y.; Tomihara, Y.; Aoyama, K.; Kubo, Y.; Amano, N.; Deguchi, Y. Construction and Functional Evaluation of a Three-Dimensional Blood-Brain Barrier Model Equipped with Human Induced Pluripotent Stem Cell-Derived Brain Microvascular Endothelial Cells. Pharm. Res. 2022, 39, 1535–1547. [Google Scholar] [CrossRef]
- Bagchi, S.; Chhibber, T.; Lahooti, B.; Verma, A.; Borse, V.; Jayant, R.D. In-vitro blood-brain barrier models for drug screening and permeation studies: An overview. Drug Des. Dev. Ther. 2019, 13, 3591–3605. [Google Scholar] [CrossRef] [PubMed]
- Sivandzade, F.; Cucullo, L. In-vitro blood-brain barrier modeling: A review of modern and fast-advancing technologies. J. Cereb. Blood Flow Metab. 2018, 38, 1667–1681. [Google Scholar] [CrossRef] [PubMed]
- Prashanth, A.; Donaghy, H.; Stoner, S.P.; Hudson, A.L.; Wheeler, H.R.; Diakos, C.I.; Howell, V.M.; Grau, G.E.; McKelvey, K.J. Are In Vitro Human Blood-Brain-Tumor-Barriers Suitable Replacements for In Vivo Models of Brain Permeability for Novel Therapeutics? Cancers 2021, 13, 955. [Google Scholar] [CrossRef] [PubMed]
- Jagtiani, E.; Yeolekar, M.; Naik, S.; Patravale, V. In vitro blood brain barrier models: An overview. J. Control Release 2022, 343, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Roux, E.; Bougaran, P.; Dufourcq, P.; Couffinhal, T. Fluid Shear Stress Sensing by the Endothelial Layer. Front. Physiol. 2020, 11, 861. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Haga, J.H.; Chien, S. Molecular basis of the effects of shear stress on vascular endothelial cells. J. Biomech. 2005, 38, 1949–1971. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.L.; England, T.J.; O’Sullivan, S.E. A Novel Transwell Blood Brain Barrier Model Using Primary Human Cells. Front. Cell. Neurosci. 2019, 13, 230. [Google Scholar] [CrossRef] [PubMed]
- Sigma-Aldrich, LLC. Technical Bulletin (Caco-2 Wild Type Cell Line, MTOX1000P24). Available online: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/305/116/mtox1000p24bul.pdf (accessed on 27 November 2023).
- Ito, R.; Morio, H.; Baba, T.; Sakaguchi, Y.; Wakayama, N.; Isogai, R.; Yamaura, Y.; Komori, T.; Furihata, T. In Vitro-In Vivo Correlation of Blood-Brain Barrier Permeability of Drugs: A Feasibility Study towards Development of Prediction Methods for Brain Drug Concentration in Humans. Pharm. Res. 2022, 39, 1575–1586. [Google Scholar] [CrossRef]
- Li, N.; Sui, Z.; Liu, Y.; Wang, D.; Ge, G.; Yang, L. A fast screening model for drug permeability assessment based on native small intestinal extracellular matrix. RSC Adv. 2018, 8, 34514–34524. [Google Scholar] [CrossRef]
- Cai, B.; Miao, Y.; Liu, Y.; Xu, X.; Guan, S.; Wu, J.; Liu, Y. Nuclear multidrug-resistance related protein 1 contributes to multidrug-resistance of mucoepidermoid carcinoma mainly via regulating multidrug-resistance protein 1: A human mucoepidermoid carcinoma cells model and Spearman’s rank correlation analysis. PLoS ONE 2013, 8, e69611. [Google Scholar] [CrossRef]
- White, L.A.; Stevenson, E.V.; Yun, J.W.; Eshaq, R.; Harris, N.R.; Mills, D.K.; Minagar, A.; Couraud, P.O.; Alexander, J.S. The Assembly and Application of ‘Shear Rings’: A Novel Endothelial Model for Orbital, Unidirectional and Periodic Fluid Flow and Shear Stress. J. Vis. Exp. 2016, 116, e54632. [Google Scholar] [CrossRef]
- Driessen, R.; Zhao, F.; Hofmann, S.; Bouten, C.; Sahlgren, C.; Stassen, O. Computational Characterization of the Dish-in-a-Dish, a High Yield Culture Platform for Endothelial Shear Stress Studies on the Orbital Shaker. Micromachines 2020, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.D.; Ye, M.; Levy, A.F.; Rothstein, J.D.; Bergles, D.E.; Searson, P.C. The blood-brain barrier: An engineering perspective. Front. Neuroeng. 2013, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Polite, F.; Martorell, J.; Del Rey-Puech, P.; Melgar-Lesmes, P.; O’Brien, C.C.; Roquer, J.; Ois, A.; Principe, A.; Edelman, E.R.; Balcells, M. Pulsatility and high shear stress deteriorate barrier phenotype in brain microvascular endothelium. J. Cereb. Blood Flow Metab. 2017, 37, 2614–2625. [Google Scholar] [CrossRef] [PubMed]
- Ferruzza, S.; Rossi, C.; Sambuy, Y.; Scarino, M.L. Serum-reduced and serum-free media for differentiation of Caco-2 cells. ALTEX 2013, 30, 159–168. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jumarie, C.; Malo, C. Caco-2 cells cultured in serum-free medium as a model for the study of enterocytic differentiation in vitro. J. Cell. Physiol. 1991, 149, 24–33. [Google Scholar] [CrossRef]
- Greene, C.; Hanley, N.; Campbell, M. Claudin-5: Gatekeeper of neurological function. Fluids Barriers CNS 2019, 16, 3. [Google Scholar] [CrossRef]
- Saleh, M.A.A.; Bloemberg, J.S.; Elassaiss-Schaap, J.; de Lange, E.C.M. Drug Distribution in Brain and Cerebrospinal Fluids in Relation to IC(50) Values in Aging and Alzheimer’s Disease, Using the Physiologically Based LeiCNS-PK3.0 Model. Pharm. Res. 2022, 39, 1303–1319. [Google Scholar] [CrossRef]
- Kodaira, H.; Kusuhara, H.; Fujita, T.; Ushiki, J.; Fuse, E.; Sugiyama, Y. Quantitative evaluation of the impact of active efflux by p-glycoprotein and breast cancer resistance protein at the blood-brain barrier on the predictability of the unbound concentrations of drugs in the brain using cerebrospinal fluid concentration as a surrogate. J. Pharmacol. Exp. Ther. 2011, 339, 935–944. [Google Scholar] [CrossRef]
- Sato, S.; Matsumiya, K.; Tohyama, K.; Kosugi, Y. Translational CNS Steady-State Drug Disposition Model in Rats, Monkeys, and Humans for Quantitative Prediction of Brain-to-Plasma and Cerebrospinal Fluid-to-Plasma Unbound Concentration Ratios. AAPS J. 2021, 23, 81. [Google Scholar] [CrossRef]
- Zhao, R.; Kalvass, J.C.; Yanni, S.B.; Bridges, A.S.; Pollack, G.M. Fexofenadine brain exposure and the influence of blood-brain barrier P-glycoprotein after fexofenadine and terfenadine administration. Drug Metab. Dispos. 2009, 37, 529–535. [Google Scholar] [CrossRef]
- de Lange, E.C. Utility of CSF in translational neuroscience. J. Pharmacokinet. Pharmacodyn. 2013, 40, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Balzer, V.; Poc, P.; Puris, E.; Martin, S.; Aliasgari, M.; Auriola, S.; Fricker, G. Re-evaluation of the hCMEC/D3 based in vitro BBB model for ABC transporter studies. Eur. J. Pharm. Biopharm. 2022, 173, 12–21. [Google Scholar] [CrossRef]
- dela Paz, N.G.; Walshe, T.E.; Leach, L.L.; Saint-Geniez, M.; D’Amore, P.A. Role of shear-stress-induced VEGF expression in endothelial cell survival. J. Cell Sci. 2012, 125, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Dardik, A.; Chen, L.; Frattini, J.; Asada, H.; Aziz, F.; Kudo, F.A.; Sumpio, B.E. Differential effects of orbital and laminar shear stress on endothelial cells. J. Vasc. Surg. 2005, 41, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Czupalla, C.J.; Liebner, S.; Devraj, K. In vitro models of the blood-brain barrier. Methods Mol. Biol. 2014, 1135, 415–437. [Google Scholar] [CrossRef]
- Eigenmann, D.E.; Xue, G.; Kim, K.S.; Moses, A.V.; Hamburger, M.; Oufir, M. Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies. Fluids Barriers CNS 2013, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Hinkel, S.; Mattern, K.; Dietzel, A.; Reichl, S.; Muller-Goymann, C.C. Parametric investigation of static and dynamic cell culture conditions and their impact on hCMEC/D3 barrier properties. Int. J. Pharm. 2019, 566, 434–444. [Google Scholar] [CrossRef]
- Beibetaner, N.; Mattern, K.; Dietzel, A.; Reichl, S. DynaMiTES—A dynamic cell culture platform for in vitro drug testing PART 2—Ocular DynaMiTES for drug absorption studies of the anterior eye. Eur. J. Pharm. Biopharm. 2018, 126, 166–176. [Google Scholar] [CrossRef]
- Vestweber, D. VE-cadherin: The major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 223–232. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Begley, D.J. ABC transporters and the blood-brain barrier. Curr. Pharm. Des. 2004, 10, 1295–1312. [Google Scholar] [CrossRef] [PubMed]
- Dauchy, S.; Dutheil, F.; Weaver, R.J.; Chassoux, F.; Daumas-Duport, C.; Couraud, P.O.; Scherrmann, J.M.; De Waziers, I.; Decleves, X. ABC transporters, cytochromes P450 and their main transcription factors: Expression at the human blood-brain barrier. J. Neurochem. 2008, 107, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Nader, E.; Skinner, S.; Romana, M.; Fort, R.; Lemonne, N.; Guillot, N.; Gauthier, A.; Antoine-Jonville, S.; Renoux, C.; Hardy-Dessources, M.D.; et al. Blood Rheology: Key Parameters, Impact on Blood Flow, Role in Sickle Cell Disease and Effects of Exercise. Front. Physiol. 2019, 10, 1329. [Google Scholar] [CrossRef] [PubMed]
- Poon, C. Measuring the density and viscosity of culture media for optimized computational fluid dynamics analysis of in vitro devices. J. Mech. Behav. Biomed. Mater. 2022, 126, 105024. [Google Scholar] [CrossRef] [PubMed]
- Brust, M.; Schaefer, C.; Doerr, R.; Pan, L.; Garcia, M.; Arratia, P.E.; Wagner, C. Rheology of human blood plasma: Viscoelastic versus Newtonian behavior. Phys. Rev. Lett. 2013, 110, 078305. [Google Scholar] [CrossRef]
- Kurosawa, T.; Tega, Y.; Higuchi, K.; Yamaguchi, T.; Nakakura, T.; Mochizuki, T.; Kusuhara, H.; Kawabata, K.; Deguchi, Y. Expression and Functional Characterization of Drug Transporters in Brain Microvascular Endothelial Cells Derived from Human Induced Pluripotent Stem Cells. Mol. Pharm. 2018, 15, 5546–5555. [Google Scholar] [CrossRef]
- Ribecco-Lutkiewicz, M.; Sodja, C.; Haukenfrers, J.; Haqqani, A.S.; Ly, D.; Zachar, P.; Baumann, E.; Ball, M.; Huang, J.; Rukhlova, M.; et al. A novel human induced pluripotent stem cell blood-brain barrier model: Applicability to study antibody-triggered receptor-mediated transcytosis. Sci. Rep. 2018, 8, 1873. [Google Scholar] [CrossRef]




| Compounds | Papp (×10−6 cm/s) | Kp,uu,CSF |
|---|---|---|
| Donepezil | 41.6 ± 7.0 | 1.8 (human) 1 |
| Midazolam | 41.5 ± 2.8 | 1.35 (rat) 2 |
| Carbamazepine | 29.0 ± 4.4 | 0.979 (monkey) 3 |
| Propranolol | 24.6 ± 4.5 | 0.42 (human) 3 |
| Phenytoin | 21.8 ± 1.2 | 0.396 (rat) 3 |
| Metoprolol | 18.1 ± 0.28 | 0.93 (human) 3 |
| Naproxen | 12.3 ± 1.4 | N/A |
| Sulpiride | 10.2 ± 0.82 | 0.049 (rat) 2 |
| Atenolol | 7.6 ± 0.54 | 0.331 (monkey) 3 |
| Dantrolene | 7.1 ± 3.7 | 0.22 (monkey) 3 |
| Fexofenadine | 3.5 ± 0.32 | 0.0012 (mouse) 4 |
| Rhodamine123 | 0.67 ± 0.14 | N/A |
| FITC-dextran 4 kDa | 0.70 ± 0.15 | N/A |
| FITC-dextran 40 kDa | 0.23 ± 0.062 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Shin, S.-A.; Lee, C.S.; Chung, H.J. An Improved In Vitro Blood-Brain Barrier Model for the Evaluation of Drug Permeability Using Transwell with Shear Stress. Pharmaceutics 2024, 16, 48. https://doi.org/10.3390/pharmaceutics16010048
Kim J, Shin S-A, Lee CS, Chung HJ. An Improved In Vitro Blood-Brain Barrier Model for the Evaluation of Drug Permeability Using Transwell with Shear Stress. Pharmaceutics. 2024; 16(1):48. https://doi.org/10.3390/pharmaceutics16010048
Chicago/Turabian StyleKim, Junhyeong, Seong-Ah Shin, Chang Sup Lee, and Hye Jin Chung. 2024. "An Improved In Vitro Blood-Brain Barrier Model for the Evaluation of Drug Permeability Using Transwell with Shear Stress" Pharmaceutics 16, no. 1: 48. https://doi.org/10.3390/pharmaceutics16010048
APA StyleKim, J., Shin, S.-A., Lee, C. S., & Chung, H. J. (2024). An Improved In Vitro Blood-Brain Barrier Model for the Evaluation of Drug Permeability Using Transwell with Shear Stress. Pharmaceutics, 16(1), 48. https://doi.org/10.3390/pharmaceutics16010048





