Transdermal Drug Delivery of Tazarotene: Determining Tazarotene’s Potential in Local Transdermal Therapy
Abstract
:1. Introduction
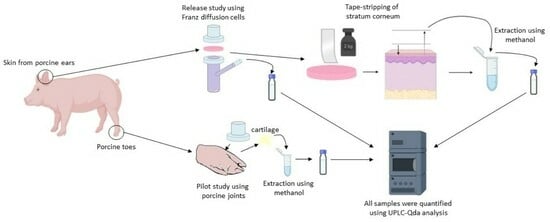
2. Materials and Methods
2.1. Materials
2.2. Preparation of Topical Formulations
2.3. Release Studies by In Vitro Experiments
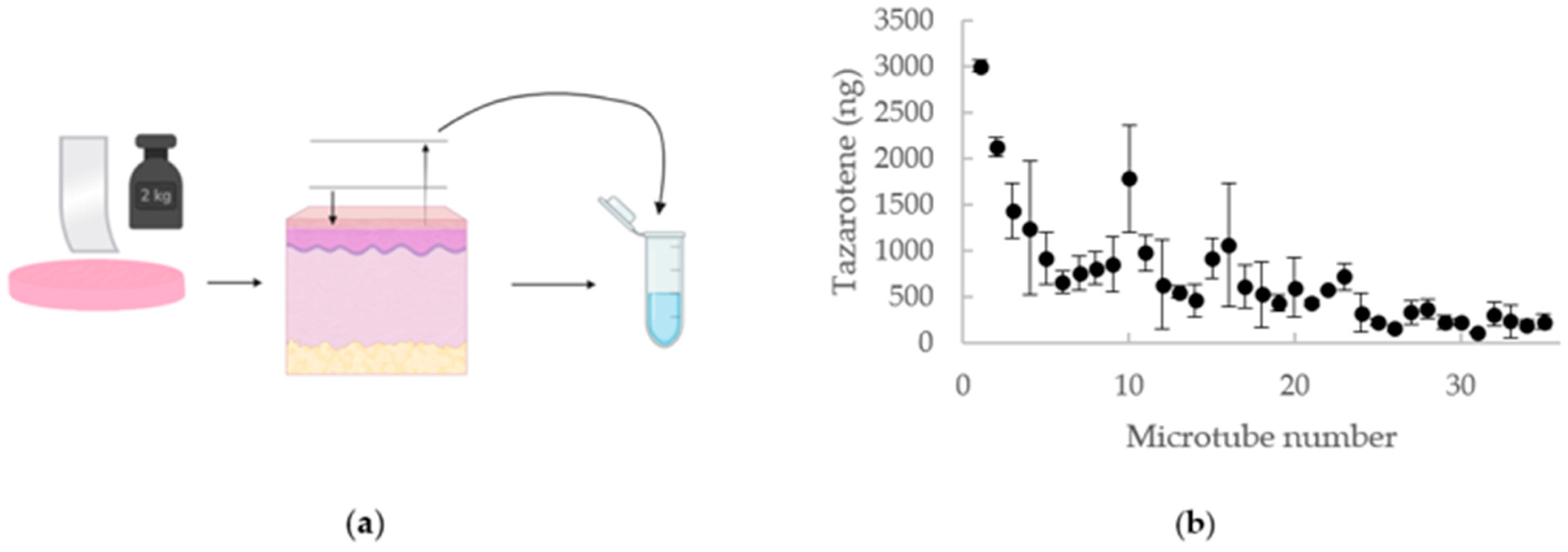
2.4. Tape-Stripping
2.5. UPLC-QDa Analysis
2.6. Pilot Study Using Porcine Joints
3. Results
3.1. Transdermal Drug Delivery
3.2. Tape Stripping
3.3. Pilot Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jonsson, H. Following the genetic clues towards treatment of hand OA. Nat. Rev. Rheumatol. 2018, 14, 503–504. [Google Scholar] [CrossRef] [PubMed]
- David, M.; Hodak, E.; Lowe, N.J. Adverse effects of retinoids. Med. Toxicol. Advers. Drug Exp. 1988, 3, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Lilley, J.S.; Linton, M.F.; Fazio, S. Oral retinoids and plasma lipids. Dermatol. Ther. 2013, 26, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Nesher, G.; Zuckner, J. Rheumatologic complications of vitamin a and retinoids. Semin. Arthritis Rheum. 1995, 24, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Thielitz, A.; Gollnick, H. Topical retinoids in acne vulgaris: Update on efficacy and safety. Am. J. Clin. Dermatol. 2008, 9, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Antille, C.; Tran, C.; Sorg, O.; Saurat, J.H. Penetration and metabolism of topical retinoids in ex vivo organ-cultured full-thickness human skin explants. Ski. Pharmacol. Physiol. 2004, 17, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Guenther, L.C. Topical tazarotene therapy for psoriasis, acne vulgaris, and photoaging. Ski. Ther. Lett. 2002, 7, 1–4. [Google Scholar]
- Gaikwad, J.; Sharma, S.; Hatware, K.V. Review on Characteristics and Analytical Methods of Tazarotene: An Update. Crit. Rev. Anal. Chem. 2020, 50, 90–96. [Google Scholar] [CrossRef]
- Chandraratna, R.A.S. Tazarotene: The first receptor-selective topical retinoid for the treatment of psoriasis. J. Am. Acad. Dermatol. 1997, 37, S12–S17. [Google Scholar] [CrossRef]
- Tang-Liu, D.D.; Matsumoto, R.M.; Usansky, J.I. Clinical pharmacokinetics and drug metabolism of tazarotene: A novel topical treatment for acne and psoriasis. Clin. Pharmacokinet. 1999, 37, 273–287. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5381, Tazarotene. 2020. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Tazarotene (accessed on 1 November 2023).
- Nasr, M.; Abdel-Hamid, S. Optimizing the dermal accumulation of a tazarotene microemulsion using skin deposition modeling. Drug Dev. Ind. Pharm. 2016, 42, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Dando, T.M.; Wellington, K. Topical Tazarotene. Am. J. Clin. Dermatol. 2005, 6, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Kakita, L. Tazarotene versus tretinoin or adapalene in the treatment of acne vulgaris. J. Am. Acad. Dermatol. 2000, 43, S51–S54. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.K.; Raw, A.; Lionberger, R.; Yu, L. Generic development of topical dermatologic products: Formulation development, process development, and testing of topical dermatologic products. AAPS J. 2013, 15, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Godin, B.; Touitou, E. Transdermal skin delivery: Predictions for humans from in vivo, ex vivo and animal models. Adv. Drug Deliv. Rev. 2007, 59, 1152–1161. [Google Scholar] [CrossRef]
- Gorzalczany, S.B.; Rodriguez Basso, A.G. Strategies to apply 3Rs in preclinical testing. Pharmacol. Res. Perspect. 2021, 9, e00863. [Google Scholar] [CrossRef] [PubMed]
- Mali, A.; Bathe, R.; Patil, M. An updated review on transdermal drug delivery systems. Int. J. Adv. Sci. Res. 2015, 1, 244. [Google Scholar] [CrossRef]
- Kloppenburg, M.; Kwok, W.-Y. Hand osteoarthritis—a heterogeneous disorder. Nat. Rev. Rheumatol. 2012, 8, 22–31. [Google Scholar] [CrossRef]
- Marshall, M.; Watt, F.E.; Vincent, T.L.; Dziedzic, K. Hand osteoarthritis: Clinical phenotypes, molecular mechanisms and disease management. Nat. Rev. Rheumatol. 2018, 14, 641–656. [Google Scholar] [CrossRef]
- Abd, E.; Yousef, S.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Roberts, M.S. Skin models for the testing of transdermal drugs. Clin. Pharmacol. Adv. Appl. 2016, 8, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Dick, I.P.; Scott, R.C. Pig Ear Skin as an In-vitro Model for Human Skin Permeability. J. Pharm. Pharmacol. 2011, 44, 640–645. [Google Scholar] [CrossRef] [PubMed]
- OECD. Skin Absorption: In Vivo Method; Test Guideline No. 427; Series on Testing and Assessment. No. 428; OECD: Paris, France, 2004. [Google Scholar]
- Snorradóttir, B.S.; Gudnason, P.I.; Scheving, R.; Thorsteinsson, F.; Másson, M. Release of anti-inflammatory drugs from a silicone elastomer matrix system. Die Pharm. 2009, 64, 19–25. [Google Scholar]
- Snorradottir, B.; Jonsdottir, F.; Sigurdsson, S.; Thorsteinsson, F.; Másson, M. Numerical modelling and experimental investigation of drug release from layered silicone matrix systems. Eur. J. Pharm. Sci. 2013, 49. [Google Scholar] [CrossRef] [PubMed]
- Kryczyk-Poprawa, A.; Zupkó, I.; Bérdi, P.; Żmudzki, P.; Popiół, J.; Muszyńska, B.; Opoka, W. Photostability Testing of a Third-Generation Retinoid-Tazarotene in the Presence of UV Absorbers. Pharmaceutics 2020, 12, 899. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Sahu, A.; Balhara, A.; Giri, S.; Singh, S. Insights into the degradation chemistry of tazarotene, a third generation acetylenic retinoid: LC-HRMS (Orbitrap), LC-MSn and NMR characterization of its degradation products, and prediction of their physicochemical and ADMET properties. J. Pharm. Biomed. Anal. 2020, 186, 113316. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Skrzydlewska, E. Ascorbic acid as antioxidant. Vitam. Horm. 2023, 121, 247–270. [Google Scholar] [CrossRef]
- Neupane, R.; Boddu, S.H.S.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to Biological Skin in Permeation Studies: Current Trends and Possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef]
- Souto, E.B.; Fangueiro, J.F.; Fernandes, A.R.; Cano, A.; Sanchez-Lopez, E.; Garcia, M.L.; Severino, P.; Paganelli, M.O.; Chaud, M.V.; Silva, A.M. Physicochemical and biopharmaceutical aspects influencing skin permeation and role of SLN and NLC for skin drug delivery. Heliyon 2022, 8, e08938. [Google Scholar] [CrossRef]
- Leite-Silva, V.; Almeida, M.; Fradin, A.; Grice, J.; Roberts, M. Delivery of drugs applied topically to the skin. Expert Rev. Dermatol. 2014, 7, 383–397. [Google Scholar] [CrossRef]
- Matsuda, H.; Arima, H. Cyclodextrins in transdermal and rectal delivery. Adv. Drug Deliv. Rev. 1999, 36, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Amidouche, D.; Montassier, P.; Poelman, M.-C.; Duchêne, D. Evaluation by laser Doppler velocimetry of the attenuation of tretinoin induced skin irritation by β-cyclodextrin complexation. Int. J. Pharm. 1994, 111, 111–116. [Google Scholar] [CrossRef]
- Funke, A.P.; Schiller, R.; Motzkus, H.W.; Günther, C.; Müller, R.H.; Lipp, R. Transdermal delivery of highly lipophilic drugs: In vitro fluxes of antiestrogens, permeation enhancers, and solvents from liquid formulations. Pharm. Res. 2002, 19, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, Y.; Lu, R. Mechanism and Application of Chitosan and Its Derivatives in Promoting Permeation in Transdermal Drug Delivery Systems: A Review. Pharmaceuticals 2022, 15, 459. [Google Scholar] [CrossRef]
- Escobar-Chávez, J.J.; Merino-Sanjuán, V.; López-Cervantes, M.; Urban-Morlan, Z.; Piñón-Segundo, E.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. The tape-stripping technique as a method for drug quantification in skin. J. Pharm. Pharm. Sci. 2008, 11, 104–130. [Google Scholar] [CrossRef]
- Kathuria, H.; Handral, H.K.; Cha, S.; Nguyen, D.T.P.; Cai, J.; Cao, T.; Wu, C.; Kang, L. Enhancement of Skin Delivery of Drugs Using Proposome Depends on Drug Lipophilicity. Pharmaceutics 2021, 13, 1457. [Google Scholar] [CrossRef]


| Gel | Cream | ||
|---|---|---|---|
| Ingredient | Amount | Ingredient | Amount |
| Ascorbic acid | 1.35% | Carbopol | 1.08% |
| Carbopol | 2.16% | Ceta-stearyl alcohol | 10.83% |
| Distilled water | 54.07% | Distilled water | 75.78% |
| Natrium hydroxide 5 M | 1.86% | Natrium hydroxide 5 M | 1.49% |
| Polyethylene glycol 6′000 | 13.52% | Paraffin oil | 10.83% |
| Propylene glycol | 27.03% | ||
| Compound | RT (min) | MW (m/z) | Product Ion (m/z) |
|---|---|---|---|
| Tazarotene | 1.38 | 352.20 | 324.10 |
| Tazarotenic acid | 0.83 | 324.10 | 294.10 |
| Linearity: 0.99885 Accuracy and precision: 97.25–100.98%, CV 3.20–7.43% | |||
| Joint | Tazarotene (ng) | Tazarotenic Acid (ng) | Tazarotene Absorbed into Articular Cartilage (%) * | |
|---|---|---|---|---|
| Gel | First joint | 106.22 ± 95.90 | 38.48 ± 0 ** | 0.011% |
| Second joint | 122.06 ± 65.42 | 38.08 ± 1.47 | 0.012% | |
| Cream | First joint | 101.11 ± 0.12 | 45.59 ± 6.18 | 0.010% |
| Second joint | 270.09 ± 0.09 | 40.77 ± 1.96 | 0.027% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamzehpour, H.; Óskarsdóttir, Á.; Jónsson, H.; Jónsdóttir, F.; Sigurjónsson, Ó.E.; Snorradottir, B.S. Transdermal Drug Delivery of Tazarotene: Determining Tazarotene’s Potential in Local Transdermal Therapy. Pharmaceutics 2024, 16, 64. https://doi.org/10.3390/pharmaceutics16010064
Hamzehpour H, Óskarsdóttir Á, Jónsson H, Jónsdóttir F, Sigurjónsson ÓE, Snorradottir BS. Transdermal Drug Delivery of Tazarotene: Determining Tazarotene’s Potential in Local Transdermal Therapy. Pharmaceutics. 2024; 16(1):64. https://doi.org/10.3390/pharmaceutics16010064
Chicago/Turabian StyleHamzehpour, Helena, Ástrós Óskarsdóttir, Helgi Jónsson, Fjóla Jónsdóttir, Ólafur E. Sigurjónsson, and Bergthora S. Snorradottir. 2024. "Transdermal Drug Delivery of Tazarotene: Determining Tazarotene’s Potential in Local Transdermal Therapy" Pharmaceutics 16, no. 1: 64. https://doi.org/10.3390/pharmaceutics16010064
APA StyleHamzehpour, H., Óskarsdóttir, Á., Jónsson, H., Jónsdóttir, F., Sigurjónsson, Ó. E., & Snorradottir, B. S. (2024). Transdermal Drug Delivery of Tazarotene: Determining Tazarotene’s Potential in Local Transdermal Therapy. Pharmaceutics, 16(1), 64. https://doi.org/10.3390/pharmaceutics16010064